Abstract
Four types of novel double-tail trisiloxane surfactants of the general formula Me3SiOSiMeR1OSiMe3 (R 1 = –(CH2)3NR2CH2CH(OH)CH2(OCH2CH2)xOCH3; R 2 = –CH2CH(OH)CH2OCH2(CH2)yCH3, –CH2(CH2)3CH3, –CH2CH2CH(CH3)2; x = 8.4, 12.9, 17.5, 22; y = 2, 6), have been synthesized. Their structures were characterized by proton and carbon nuclear magnetic resonance. Most of them are able to reduce the surface tension of water to less than 24 mN/m at concentration levels of 10−5 mol/L and 10−4 mol/L. The emphasis was on the influence of substructures on their spreading ability and hydrolysis resistance. The results showed that a weaker hydrophilicity of a surfactant molecule, a larger molar ratio of methyl to methylene in the whole hydrophobic groups, more flexible hydrophobic groups and introduction of a methyl group in the spacer can all improve the spreading ability of the double-tail trisiloxane surfactant solutions on low-energy solid surfaces. The double-tail trisiloxane surfactants 1F and 2F are stable for more than 270 days in a neutral environment (pH 7.0). The hydrolysis resistance of the double-tail trisiloxane surfactants can be improved by a weaker hydrophilicity of the surfactant molecule, and a larger volume of the hydrophobic groups.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Trisiloxane surfactants are usually denoted M(D′En)M where M stands for the trimethylsiloxy group (CH3)3SiO1/2-, D′ stands for -O1/2Si(CH3)(R)O1/2-, where R is a polyoxyethylene group attached to the silicon by way of a propyl spacer, and En stands for polyoxyethylene, —(CH2–CH2O)nH—[1]. This type of surfactant is effectively able to reduce the surface tension of water to approximately 21 mN/m. However, conventional hydrocarbon surfactants can only reduce the surface tension of water to about 30 mN/m [2] because of the arrangement of methylene groups on the water surface. Additionally, certain trisiloxane surfactant solutions are able to wet and spread rapidly on low-energy solid surfaces. This is called ‘superwetting’ or ‘superspreading’ [3]. The superspreading performance of the trisiloxane surfactants has found extensive use in agricultural adjuvants. Many studies of the spreading mechanism of trisiloxane surfactants have been carried out since their performance was discovered [2, 4–8]. There are a few different trends on the relationship of the structure with the spreading properties of trisiloxane surfactants. For example, a bulkier and more polar carbohydrate unit and the incorporation of hydrophilic spacer elements can reduce the trisiloxane surfactants spreading ability (SA) [9]; it seems that only the T-shaped trisiloxane surfactants are able to spread rapidly on solid surfaces, while flexible linear chain surfactants cannot do so [10]; their spreading performance is apparently affected by the HLB values and the molecular volume of the surfactants [8] and the structure of the trisiloxanyl units (hammer-like, linear) is not a critical parameter for the spreading of the trisiloxane surfactants as long as surfactant bilayers can be formed [11].
Additionally, the trisiloxane surfactants currently available exhibit a poor resistance to hydrolysis [12, 13]. Some of them hydrolyze rapidly when placed in an aqueous environment where the pH value is below 5 or above 9, and are stable only for 40 days even in a neutral aqueous environment (pH 7.0). This shortcoming limits their application as agricultural adjuvants, because they are likely to lose their efficacy as pesticides emulsifiers during transportation or storage. Therefore, the development of hydrolysis resistant superspreading surfactants is a quite important issue in formulating pesticide products.
Nevertheless, the study of hydrolysis resistant silicone surfactants has rarely been reported. A kind of hydrolysis resistant trimethylsiloxane surfactant was mentioned by Wagner et al. [14]. However, its aqueous solutions can only spread effectively on solid surfaces whose solid/vapor interfacial energies (γsv) are no less than 40 mN/m, which indicates that it does not match the agricultural adjuvant requirements. Recently, hydrolysis resistant disiloxane surfactants [12] and trisiloxane surfactants [13] have been synthesized by Leatherman MD et al. Unfortunately, these two types of siloxane surfactant can only reduce the surface tension of water to about 23 mN/m. Moreover, the siloxanes used to synthesize the above hydrolysis resistant surfactants have a very special structure, and they are not easily available on the market. As a consequence, the industrial production of these hydrolysis resistant siloxane surfactants is not easy.
It is known that certain double-tail hydrocarbon chain surfactants, e. g., sodium bis (2-ethylhexyl) sulfosuccinate, exhibit an ability to reduce the surface tension of water, to wet and spread on low-energy surfaces which are superior to those of the corresponding single-tail surfactants [15–18]. It is also known that the incorporation of a methyl group in the spacer of a trisiloxane surfactant is able to improve its hydrolysis resistance [13]. Consequently, to accumulate the previously mentioned features, we synthesized a series of new double-tail trisiloxane surfactants [19]. It was found that their hydrolysis resistant ability (HRA) is greatly improved with respect to original single-tail trisiloxane surfactants, but that their spreading performance is not satisfactory.
As a further contribution, this paper reports on the synthesis and interfacial properties of four types of novel double-tail trisiloxane surfactants, and analyzes the influence of substructure on the SA and HRA.
Experimental Procedures
Materials
Type A single-tail trisiloxane surfactants (1A, 2A, 3A, and 4A), whose structures are shown in Scheme 1, were synthesized as described in our previous report [19]. Paraffin wax was purchased from Shanghai Specimen and Model Factory, China. All of other chemicals were of analytical grade. Water was doubly distilled.
Synthesis
The synthesis route to the double-tail trisiloxane surfactants is shown in Scheme 1. Procedures (a) and (b) were carried out in accordance with references [20] and [21], respectively. One difference is that the used solvent is toluene, rather than methanol in procedure (b). Procedures (c) and (d) were carried out in the light of the reference [19].
Four types (E, F, G and H) and sixteen double-tail trisiloxane surfactants (1E, 2E, 3E, 4E, 1F, 2F, 3F, 4F, 1G, 2G, 3G, 4G, 1H, 2H, 3H and 4H) were prepared. Their molecular structures are shown in Scheme 1.
The 1H- and 13C-NMR spectra of double-tail trisiloxane surfactants prepared were analyzed according to the rule that different chemical environments of H and C result in different chemical shifts, and by comparing the chemical shifts of related compounds [19, 22, 23], The assignments of the chemical shifts in 1H- and 13C-NMR spectra of 1E, IF, 1G and 1H are listed in Tables 1 and 2.
Structural Characterization
1H-and 13C-NMR spectroscopy analyses were carried out with a Varian Mercury-plus 300 spectrometer in CDCl3.
Surface Activity and Hydrolysis Resistant Ability (HRA) Determination
Surface activity experiments were all carried out under constant atmospheric conditions (32 ± 2 °C room temperature, 60 ± 3% relative humidity). Aqueous solution surface tension (γ) values were obtained by the Wilhelmy plate method using a BZY-1 completely automatic surface tensiometer (Shanghai Equity Instruments Factory, China). The critical micelle concentration (CMC) values, the surface tension at CMC (γcmc), the SA and HRA were determined as reported elsewhere [19].
Results and Discussion
Interfacial Properties
The surface excess concentration (Γmax) and the surface area per molecule \( \left( {a^{\text{s}}_{\text{m}} } \right) \) were computed by applying Eqs. 1 and 2 in the steeply downward section of the tension–log concentration plot just below the CMC. The standard free energy of micellization \( \left( {\Updelta G^{0}_{\text{mic}} } \right) \) of the double-tail trisiloxane surfactants was calculated by equation (3) [19, 22].
where R = 8.3144 J/mol K, N A is Avogadro’s number, Γmax and \( a^{\text{s}}_{\text{m}} \) are in mol/cm2 and Å/molecule, respectively. The data of the CMC, γcmc, Γmax, \( a^{\text{s}}_{\text{m}} ,\Updelta G^{0}_{\text{mic}} \) and SA of the double-tail surfactants are listed in Table 3.
The CMCs of the double-tail trisiloxane surfactants are in the 10−5–10−4 mol/L range. Most of their γcmc values are below 24.0 mN/m, and some of them are even less than 21 mN/m, which denotes a high effectiveness in reducing the surface tension of water.
In general, the CMC and γcmc values of polyethoxylated surfactants increase with the increase of the number of ethylene oxide units, a trend which has been attributed to a stronger hydrophilicity and a more voluminous hydrophilic group [24]. Basically, except for the unobvious variation of the γcmc values of the H type trisiloxane surfactants (1H, 2H, 3H and 4H), the variations of the CMC, γcmc and \( a^{\text{s}}_{\text{m}} \) values of the other types of the double-tail trisiloxane surfactants obey the above-mentioned rule, and corroborate the trend found for the trisiloxane surfactants [19]. The obviously lower γcmc values of the H type of double-tail trisiloxane surfactants may be attributed to the additional low-energy methyl group in its hydrophobic groups.
Spreading Ability
The Spreading Ability (SA) values of the same type of the double-tail trisiloxane surfactants decrease with the increase of the number of ethylene oxide units (Table 3). The increase in ethylene oxide units make them bulkier and more polar, which results in a poorer SA of the trisiloxane surfactants [9]. We also noted that the SA performance of the double-tail trisiloxane surfactants with a lower γcmc value is not better than other species in the same type of the surfactants. This result is consistent with the literature [9]. As could be seen from Table 3, the SA of the double-tail trisiloxane surfactant, with the lowest CMC value, is the strongest. Maybe it is easier to form special aggregates relevant to the turbidity of a surfactant solution [5–7]. The fact that the CMC value of 4E is lower than that of 3E indicates that the higher hydrophilicity of a double-tail surfactant molecule does not necessarily lead to a higher CMC value, and that other factors are also likely to affect the CMC of the double-tail surfactants.
For the different types of the double-tail trisiloxane surfactants, the γcmc values of 1G, 2G, 3G and 4G are higher than those of 1H, 2H, 3H and 4H, respectively. But the SA of the latter is superior to that of the former (Table 3). This result suggests that the presence of an additional methyl group is able not only to reduce the γcmc value of the H type of double-tail surfactants, but also to decrease the flexibility of the total hydrophobic group of the surfactant. Consequently, the SA of H type of double-tail surfactants is poor.
By comparing the SA of the double-tail surfactants containing the same number of ethylene oxide units of the types E and F, little variations of their SA values are found, although the number of carbon atoms in their hydrophobic groups is not the same (Table 3). Consequently, it may be said that the influence of the number of carbon atoms in the hydrophobic groups of the double-tail trisiloxane surfactants on their SA is rather limited.
However, when compared with the G and H types of double-tail trisiloxane surfactants, the E and F types in which a flexible and hydrophilic spacer has been incorporated, exhibit an obviously improved SA (Table 3). This result demonstrates the impact of a flexible spacer on the SA of double-tail trisiloxane surfactants. Surfactant 1F is found to have the highest SA in all prepared double-tail trisiloxane surfactants. This feature may be attributed to its lowest CMC and the flexible spacer.
In contrast to the SA of the single-tail trisiloxane surfactants [19], the SA of the corresponding double-tail trisiloxane surfactants is relatively poor. This is mainly due to the decrease of the molar ratio of methyl group to methylene group in the latter hydrophobic groups, which leads to the increase in γcmc value. Additionally, the increase of a rigidly hydrophobic group of the latter trends to decrease the flexibility of the total surfactant molecule.
Hydrolysis Resistant Ability
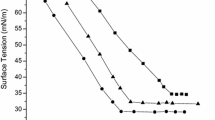
As a whole, the hydrolysis resistant ability (HRA) of the same type of double-tail trisiloxane surfactants tends to decrease with an increase in the number of ethylene oxide units (Figs. 1, 2, 3, 4, 5, 6). However, in the different types of double-tail trisiloxane surfactants, the HRA of those surfactants having more carbon atoms (at constant ethylene oxide number) is higher, for example, the F and E types. The decrease in the number of ethylene oxide units or the increase in the number of carbon atoms in the hydrophobic group tends to weaken the hydrophilicity of a surfactant.
The HRA of G and H types of double-tail trisiloxane surfactants is better than those of the corresponding ones of E and F types in an acidic environment (pH 4.0), while it is poorer than those of the latter in alkaline or neutral pH solutions (Figs. 1, 2, 3, 4, 5, 6). This result suggests that the hydrolysis mechanism of the double-tail trisiloxane surfactants in an acidic environment is different from that in an alkaline or neutral environment.
According to previous results [19], the HRA of double-tail trisiloxane surfactants is obviously superior to that of the single-tail ones. For instance, the double-tail trisiloxane surfactant 1H is stable for 8 days in an acidic environment (pH 4.0), and the surface tension values of aqueous solutions (0.1 wt.%) of the surfactants 1F and 2F are still less than 24.0 mN/m over 270 days in a neutral environment (pH 7.0). Therefore, it may be concluded that the incorporation of an additional hydrophobic group in the double-tail trisiloxane surfactants is able to improve their HRA.
Abbreviations
- HRA:
-
Hydrolysis resistant ability
- SA:
-
Spreading ability
- CMC:
-
Critical micelle concentration
- 1H NMR:
-
Proton nuclear magnetic resonance
- 13C NMR:
-
Carbon nuclear magnetic resonance
- γ:
-
Surface tension
- γsv :
-
Solid/vapour interfacial energies
- γcmc :
-
The surface tension of surfactant solution at CMC
- PTC:
-
Phase transfer catalyst
- HLB:
-
Hydrophile/lipophile balance
- Γmax :
-
Surface excess concentration
- \( a^{\text{s}}_{\text{m}} \) :
-
Surface area per molecule
- \( \Updelta G^{0}_{\text{mic}} \) :
-
Standard free energy of micellization
References
Li X, Washenberger RM, Scriven LE, Davis HT (1999) Phase behavior and microstructure of water/trisiloxane E12 polyoxethylene surfactant/silicone oil systems. Langmuir 15:2267–2277
Hill RM, He M, Davis HT, Scriven LE (1994) Comparison of the liquid crystal phase behavior of four trisiloxane superwetter surfactants. Langmuir 10(6):1724–1734
Hill RM (1998) Superspreading. Curr Opin Colloid Interface Sci 3:247–254
Stoebe T, Lin Z, Hill R, Wavd D, Davis T (1997) Enhanced spreading of aqueous films containing ethoxylated alcohol surfactants on solid substrates. Langmuir 13(26):7270–7275
Stoebe T, Lin Z, Hill R, Wavd D, Davis T (1996) Surfactant-enhanced spreading. Langmuir 12(2):337–344
Wagner R, Wu Y, Czichock G, Berlepsch HV, Weiland B, Rexin F, Perpelittchenke L (1999) Silicon-modified surfactants and wetting: 1. Synthesis of the single components of Silwet L 77 and their spreading performance on a low-energy solid surface. Appl Organometal Chem 13:611–620
Nikolov AD, Wasan DT, Chengara A, Policello GA, Kolossrary I (2002) Superspreading driven by Marangoni flow. Adv Colloid Interface Sci 96(1–3):325–338
Zhang Y, Zhang G-Y, Han F (2007) Spreading mechanism of new glucosamide-based trisiloxane surfactant on low-energy surface. Acta Chim Sin 65(5):465–469
Wagner R, Richter L, Weiβmüller J, Reiners J, Klein KD, Schaefer D, Stadtmüller S (1997) Silicon-modified carbohydrate surfactants: IV. The impact of substructures on the wetting behaviour of siloxanyl-modified carbohydrate surfactants on low-energy surfaces. Appl Organometal Chem 11:617–632
Sner Y, Couzis A, Koplik J, Maldarelli C, Tomassone MS (2005) Molecular dynamics study of the influence of surfactant structure on surfactant-facilitated spreading of droplets on solid surfaces. Langmuir 21(26):12160–12170
Zhu S, Miller WG, Scriven LE, Davis HT (1994) Superspreading of water–silicone surfactant on hydrophobic surfaces. Colloids Surf A 90(1):63–78
Leatherman MD, Policello GA, Rajaraman SK (2007) Hydrolysis resistant organomodified disiloxane surfactants. US Patent 20,070,088,091
Policello GA, Leatherman MD, Peng WQ, Rajaraman SK, Xia ZJ (2007) Hydrolysis resistant organomodified trisiloxane surfactants. US Patent 20,070,184,005
Wagner R, Wu Y, Berlepsch HV, Zastrow H, Weiland B, Perepelittchenko L (1999) Silicon-modified surfactants and wetting: V. The spreading behaviour of trimethylsilane surfactants on energetically different solid surfaces. Appl Organometal Chem 13:845–855
Wang L, Qiao W, Cao C, Li Z (2008) Synthesis and characterization of a novel double-tailed cationic surfactant. Colloids Surf A 320(1–3):271–274
Simončič B, Rozman V (2007) Wettability of cotton fabric by aqueous solutions of surfactants with different structures. Colloid Surf A 292(2–3):236–245
Nave S, Eastoe F, Penfold J (2000) What is so special about Aerosol-OT? 1. Aqueous systems. Langmuir 16(23):8733–8740
Nave S, Paul A, Eastoe F, Pitt AR, Heenan RK (2005) What is so special about Aerosol-OT? Part IV. Phenyl-tipped surfactants. Langmuir 21(22):10021–10027
Peng Z, Lu C, Lai J (2009) Synthesis and properties of novel double-tail trisiloxane surfactants. J Surf Deterg (in press) doi: 10.1007/s11743-009-1134-6
Guo LM, Wu SX (2005) Synthesis of oligoethylene glycol diglycidyl ether by phase transfer catalysis. Fine Chem 22(supplement):108–111
Zhang GD, Han F, Zhang GY (2006) Synthesis and characterization of a series of trisiloxanes. China Surf Deterg Cosmet 36(2):73–80
Han F, Zhang G (2004) New family of Gemini surfactants with glucosamide-based trisiloxane. Colloid Surf A 237:79–85
Snow SA (1993) Synthesis, characterization, stability, aqueous surface activity, and aqueous solution aggregation of the novel, cationic siloxane surfactants (Me3SiO)2Si(Me)-(CH2) +3 NMe2(CH2)2ORX− (R = H, C(O)Me, C(O)NH(Ph); X = Cl, Br, I, NO3, MeOSO3). Langmuir 9(2):424–430
Zhu YY (2003) The relationship between surfactant structure and properties. Petroleum Industry Press, Beijing, p 46
Acknowledgments
The authors wish to thank Tao Cai and Haiyang Gao who offered helpful assistance in structural characterization. The financial supports of the Natural Science Research Project of the Department of Education of Guangdong Province (No. 04J016) and the Science and Technology Program of Guangdong Province (No. 2005B16001155) are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Peng, Z., Lu, C. & Xu, M. Influence of Substructures on the Spreading Ability and Hydrolysis Resistance of Double-Tail Trisiloxane Surfactants. J Surfact Deterg 13, 75–81 (2010). https://doi.org/10.1007/s11743-009-1144-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11743-009-1144-4