Abstract
Since the first application of laparoscopic surgery for gastric cancer was performed, radical gastrectomy with minimally invasive approach has gained popularity worldwide. However, due to technically demanding procedures of conventional laparoscopic gastrectomy with D2 lymphadenectomy, adoption of laparoscopic gastrectomy for advanced gastric cancer has been limited. To overcome this technical difficulty, several surgeons started the robotic gastrectomy for gastric cancer using the da Vinci® Surgical System (Intuitive Surgical, Sunnyvale, CA, USA). Advantages of robotic surgery for surgeons such as articulating EndoWrist® instruments, an improved 3D magnified operative view, and tremor filtering facilitate more precise and delicate lymph node dissection compared to conventional laparoscopic surgery. Although several retrospective and small prospective studies revealed the feasibility and safety of robotic gastrectomy for gastric cancer, advantages of robotic gastrectomy from an oncologic point of view are still to be clarified. Here, we documented our current practice of robotic surgery for gastric cancer and review of the literatures, as well as the potential advantages of robotic approach, especially for D2 lymphadenectomy.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
5.1 Introduction
Since the first application of laparoscopic surgery for gastric cancer was performed [1], laparoscopic gastrectomy has been accepted worldwide as a minimally invasive surgery (MIS) which provides reduced postoperative pain and faster recovery after operations [2]. As with open gastric cancer surgery, laparoscopic gastrectomy should be performed in accordance with the oncologic principles: radical lymphadenectomy according to the clinical stage of gastric cancer is required. Due to the technically demanding nature of conventional laparoscopic gastrectomy with D2 lymphadenectomy, the adoption of laparoscopic gastrectomy for advanced gastric cancer has been limited.
To overcome this technical difficulty, several experienced surgeons adopted the robotic gastrectomy for gastric cancer using the da Vinci® Surgical Systems (Intuitive Surgical, Sunnyvale, CA, USA) as a minimally invasive alternative to laparoscopic gastrectomy [3–9]. Robotic surgery system has technical advantages for operators, such as articulating instruments, an improved 3D magnified operative view, tremor filtering, and motion scaling. When compared to conventional laparoscopic gastrectomy, a robotic approach for gastric cancer surgery may facilitate potentially more precise and delicate lymph node dissection, thanks to these advantages. Since the first robotic gastrectomy for gastric cancer was reported in 2003 [10], several retrospective and small prospective studies have revealed that robotic gastrectomy is safe and feasible in terms of the known improved postoperative outcomes of MIS such as reduced blood loss and shorter hospital stay [3–9]. However, because of the lack of randomized controlled trials demonstrating long-term outcomes, advantages of robotic approach from an oncologic point of view are still to be clarified.
In this chapter, we demonstrated procedural details of current practice of robotic surgery for gastric cancer. We also reviewed the literatures regarding robotic gastrectomy, as well as the possible advantages of robotic approach, especially for D2 lymphadenectomy.
5.2 Indications
The indications for robotic gastrectomy for gastric cancer are basically similar to those of the conventional laparoscopic gastrectomy. Patients with early gastric cancer without lymph node metastases (cT1N0M0), which do not meet the criteria for endoscopic resection, are candidates for robotic gastrectomy with limited lymphadenectomy. cT1 cancer with perigastric lymph node involvement and cT2–3 cancer with or without perigastric lymph node involvement are generally accepted indications for robotic gastrectomy with D2 lymphadenectomy. Basically, indications of surgery do not differ between robotic and laparoscopic approaches. Regarding locally advanced gastric cancer with obvious serosal invasion, direct invasion to adjacent organs, and/or bulky extraperigastric lymph node metastases, robotic surgery for these cancers is not generally accepted. However, robotic application for these cancers could be decided according to the surgeon’s experience and expertise. In addition, robotic gastrectomy for those advanced gastric cancers should be carried out within the framework of clinical trials.
5.3 Operative Procedures
5.3.1 Operating Room Setup and Patient Positioning
5.3.1.1 Operating Room Setup
The operating room setup is shown in Fig. 5.1. The patient cart is positioned cephalad to the patient. The vision cart is located at the feet of the patient. The surgeon’s console is placed where the operator is able to see and check the patient. The patient-side assistant is positioned to the left side of the patient. It is recommended for an assistant to have his/her own monitor on the opposite side of the patient. The scrub nurse is at the lower right side of the patient, which is on the opposite side of the patient-side assistant. Operating room configuration is usually dependent on the size of the room as well as the surgeon’s preferences.
5.3.1.2 Patient Positioning
The patient is placed in the supine position with both arms alongside the body to prevent injury to the shoulders and arms. The operation table is tilted up to 15° reverse Trendelenburg position. In order to avoid any shifting of this position, the patient should be carefully secured with gel pads and a strap across the thigh.
5.3.2 Port Placement, Instrumentation, and Docking
5.3.2.1 Port Placement
Two 12 mm trocars (for a camera arm and an assistant) and three 8 mm cannulas (for robot arms) are used for robotic gastrectomy (Fig. 5.2). After the camera port is inserted through the infraumbilical vertical incision with the open method, pneumoperitoneum of 12 mmHg is achieved by insufflation of CO2 gas. Then, surgeons assess the patient’s abdominal cavity and check for optimal port sites under direct vision of the endoscope. The remaining four ports are placed thereafter. The left lower 12 mm port is used by the patient-side assistant. Practically, port placements sometimes require minor adjustments for the patient’s body habitus. Notably, surgeons are recommended to maintain at least 8 cm between all ports. In addition, both ports for the 1st arm (patient’s left side) and the 3rd arm (patient’s right side) should be placed as far lateral as possible.
5.3.2.2 Instrumentations
As shown in Fig. 5.2, the camera arm is docked to the infraumbilical port. The 1st arm holds the Maryland bipolar forceps. The 2nd and the 3rd arms hold the ultrasonic shears or the monopolar curved scissors and the Cadiere forceps, interchangeably. The 3rd arm is employed at the patient’s right side because the 3rd arm should be at the opposite side of the 1st arm, which holds the Maryland forceps, for better countertraction.
5.3.2.3 Docking
After the adjustment of the camera arm setup joint toward the patient’s left side (the side of the patient with just one instrument arm) and confirmation of the sweet spot, the patient cart is rolled in and positioned over the patient’s head. Firstly, the camera arm is docked to the infraumbilical port. Then the three other robotic arms are connected to the ports. Surgeons should be careful to maximize spacing between the 2nd and 3rd arms by spreading these arms as far apart as possible.
5.3.3 Liver Retraction
Various methods of liver retraction have been described, such as the gauze-suspension method [11], suspension using Penrose drains [12], and retraction using Nathanson liver retractor [13]. Surgeons may adopt any retraction methods, as long as sufficient operative view is acquired. The gauze-suspension method is simple and not expensive and causes less damage to the liver than the Nathanson liver retractor [13]. Sufficient preparation of the operative field by appropriate liver retraction is necessary not only for accurate lymph node dissection, especially of the suprapancreatic area, but also for maximal use of instruments for dissection by eliminating the use of instrument for liver retraction.
5.3.4 Distal Subtotal Gastrectomy with D2 Lymphadenectomy
5.3.4.1 Left Side Dissection
The safe division of the greater omentum is achieved by cephalad and ventral retraction of the stomach with the 3rd arm, which creates a draping of the greater omentum. After entering the lesser sac in the area of the mid-transverse colon, the greater omentum is divided at least 3 cm away from the gastroepiploic vessels toward the lower pole of the spleen using the ultrasonic shears (Fig. 5.3a).
Left side dissection of the greater curvature. (a) The greater omentum is divided at least 3 cm away from gastroepiploic vessels toward the lower pole of the spleen. White arrow indicates the direction of retraction by the 3rd arm. (b) The left gastroepiploic vessels (LGEA and V) are ligated by clips and divided
The left gastroepiploic artery and vein are carefully identified, ligated, and divided at its root where lymph node #4sb is located (Fig. 5.3b). The omental branch should be preserved to preserve blood supply to the omentum. For better operative view, it is important to dissect the adhesions between the posterior wall of the stomach and the pancreas. Then, the soft tissue along the greater curvature of the stomach is cleared, which contains part of lymph node #4d, from the proximal resection margin to the short gastric vessel.
5.3.4.2 Infrapyloric Dissection and Duodenum Division
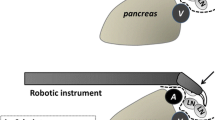
Complete dissection of the infrapyloric area containing lymph node #6 is one of the most difficult steps during radical lymphadenectomy for gastric cancer because of the complicated surgical anatomy of this area and easy bleeding. The transverse mesocolon should be appropriately taken down from the pancreatic head and the gastroepiploic pedicle. In this step, stable and appropriate retraction of the gastroepiploic pedicle by the 3rd arm provides better surgical view in the infrapyloric area. Before dissection of lymph node #6, it is important to have a better understanding of the surgical anatomy to release physiological adhesions between the transverse colon and the duodenum and between the pancreatic head and the posterior wall of the stomach. Note that lymph node #6 is bordered by the anterior superior pancreaticoduodenal vein (ASPDV), right gastroepiploic vein (RGEV), and gastrocolic trunk (GCT) (Fig. 5.4c).
Infrapyloric dissection and duodenum division. (a) The RGEV is identified, ligated by clips, and divided as it joins the ASPDV. White arrow indicates the direction of retraction by the 3rd arm. GCT gastrocolic trunk. (b) The right gastroepiploic artery (RGEA) is divided as it branches from the gastroduodenal artery (GDA). (c) After complete lymphadenectomy of lymph node #6. Note that lymph node #6 is bordered by the ASPDV, RGEV, and GCT. ARCV, accessory right colic vein; arrow head, stump of RGEA; filled star, stump of RGEV. (d) The supraduodenal area is dissected for duodenum division. Supraduodenal vessels are cut using ultrasonic shears (arrow head). (e) Duodenum division using an endoscopic linear stapler
RGEV is identified, ligated, and cut as it joins the ASPDV (Fig. 5.4a). Soft tissues anterior to the ASPDV and GCT should be cleared. On the left side of the RGEV, soft tissues superior to the level of GCT should be retrieved until the pancreatic parenchyma is exposed. On the right side of the REGV, soft tissues superior and anterior to the ASPDV should be dissected. Note that complete detachment of membranous tissues which directly cover the pancreatic parenchyma may cause potential leakage of pancreatic juice.
The right gastroepiploic artery (RGEA) is identified, ligated, and divided as it branches from the gastroduodenal artery (GDA) (Fig. 5.4b). Soft tissues around the root of RGEA should be dissected carefully to avoid injury of the small vessels and pancreatic parenchyma that is sometimes unexpectedly lifted up. The infrapyloric artery is usually encountered after division of RGEA. It may be ligated using clips, if necessary.
The attachments between the duodenum and the pancreatic head are released and the anterior side of the GDA is exposed. The direction of the ultrasonic shears of the 2nd arm fits the dissection of the GDA. After identification of the common hepatic artery (CHA), a 4” × 4” gauze is inserted anterior to the pancreatic head to facilitate the dissection of the supraduodenal area and to avoid injuries to the pancreatic head and major vessels such as the proper hepatic artery (PHA), CHA, and GDA.
The supraduodenal area is dissected for transection of the duodenum (Fig. 5.4d). Supraduodenal vessels are cut using ultrasonic shears. The duodenum is divided approximately 1–2 cm distal to the pylorus using an endoscopic linear stapler by the assistant at the patient’s side (Fig. 5.4e).
5.3.4.3 Right Suprapancreatic Dissection
After transection of the duodenum, the stomach is retracted to the patient’s left and ventral side to identify the right gastric vessels. Note that the hepatoduodenal ligament should be stretched using the Cadiere forceps of the 3rd arm with gauze for appropriate countertraction and better understanding of the surgical anatomy in this area. First, the anterolateral surface of the PHA is exposed to dissect adipose tissues around the root of RGA, which contains lymph node #5. Then, RGA is divided as it branches from PHA (Fig. 5.5a, b).
Right suprapancreatic dissection. (a) Dissection of the avascular area (diamond) among the common hepatic artery (CHA), lymph node #8a (arrow head), and right gastric artery (RGA). (b) The RGA is ligated and cut at its root. Arrow head indicates lymph node #8a. (c) Dissection of lymph node #12a until the left side of the portal vein (filled star) is reached and exposed. Note that the autonomic nerve of the proper hepatic artery (PHA) is grabbed and retracted by the 3rd arm (white arrow) for better surgical view. (d) EndoWrist function enables surgeons to easily retrieve the deep dorsal portion of lymph nodes #8 and #9 (reference mark)
Through the whole process of suprapancreatic lymphadenectomy, it is an important concept to find the right plane between the major arteries and soft tissues containing target lymph nodes for safe and oncologically accurate surgery. Better operative view during dissection of lymph node #12a is obtained by stable retraction of the autonomic nerves along PHA by the Cadiere forceps to the right and caudal side (Fig. 5.5c). For complete D2 lymphadenectomy, soft tissues along the medial and posterior side of the PHA should be dissected to the left side of the portal vein (PV).
The anterior side of the CHA is exposed and soft tissues containing lymph node #8a are dissected from right to left until the bifurcation of the CHA and the splenic artery (SPA). Then, keeping the right plane between CHA and soft tissues, dissection of lymph node #8a proceeds to the cephalad direction of the CHA. To facilitate better operative view in this area, the assistant’s retraction of nerves along the CHA dorsally and caudally is sometimes required (Fig. 5.5d).
The left gastric vein is identified and ligated using clips at the point where it joins the PV or splenic vein (SPV). Sometimes the left gastric vein drains anteriorly to SPV. Surgeons are encouraged to check the variation of the left gastric vein in advance by preoperative contrast-enhanced CT scan.
For retrieval of the right side of lymph node #9, it is required to dissect the soft tissue of the deep portion of the caudal side of the CHA until the celiac axis. Note that preservation of the nerve plexus of celiac axis is recommended in the case of “prophylactic” D2 lymphadenectomy. In general, dissection between the nerve sheath along the artery and soft tissues containing lymph nodes facilitates technically and oncologically safe radical lymphadenectomy.
5.3.4.4 Dissection Around the Left Gastric Artery and Left Suprapancreatic Dissection
To completely dissect the soft tissues around the root of the left gastric artery (LGA) and celiac axis, the retroperitoneal attachments of the lesser curvature and the posterior stomach are divided from right to left side. The avascular area of the left side of the LGA and celiac axis is also exposed and dissected. Then, the LGA is exposed and securely ligated by clips at its origin (Fig. 5.6a).
Division of the left gastric artery (LGA) and left suprapancreatic dissection. (a) The soft tissues along the celiac axis, containing lymph nodes #7 and #9, are dissected and LGA is divided at its root. CHA common hepatic artery, SPA splenic artery, LGV left gastric vein. (b) Exposure of the anterior surface of the splenic vein (SPV) or dorsal side of the pancreatic parenchyma is desirable to completely dissect the deep dorsal portion of lymph node #11p (reference mark). The 3rd arm gently retracts the pancreas dorsally and caudally (white arrow) using gauze
The proximal portion of the SPA is exposed and soft tissues containing lymph node #11p are dissected along the SPA. The anterior and cephalad surfaces of the SPA should be exposed. Stable upward and left lateral retraction of the soft tissues containing lymph node #11p by robotic arm using articulating function allows surgeons to completely dissect the deep portion of lymph node 11p, which is usually one of the most technically demanding steps in conventional laparoscopic gastrectomy. Exposure of the anterior surface of the SPV or dorsal side of the pancreatic parenchyma is desirable for complete retrieval of lymph node #11p (Fig. 5.6b). The distal boundary of lymph node #11p is delineated by the midpoint of the splenic vessels or the posterior gastric artery.
5.3.4.5 Lesser Curvature Dissection
After the gastrohepatic ligament is divided until the right side of the abdominal esophagus, soft tissues along the abdominal esophagus and the lesser curvature of the stomach are cleared until the proximal resection line to dissect lymph nodes #1 and #3 (Fig. 5.7a, b). During this step, the anterior and the posterior vagal nerve should be divided.
Dissection of lymph nodes #1 and #3. (a) The soft tissues along the abdominal esophagus and the lesser curvature side of the stomach (lymph nodes #1 and #3a) are cleared until the proximal resection line from the anterior side. (b) The soft tissues are then dissected from the posterior side of the lesser curvature
The stomach is then transected using two endoscopic linear staplers. Note that a sufficient proximal margin should be ensured.
5.3.5 Total Gastrectomy with D2 Lymphadenectomy
The options of D2 lymphadenectomy during total gastrectomy (TG) include spleen-preserving TG and TG with splenectomy. To avoid splenectomy-related postoperative complications, several surgeons have adopted spleen-preserving TG with D2 lymphadenectomy [14–16]. However, sufficient dissection of lymph node #10 located in the area of the splenic hilum is very difficult by conventional laparoscopic approach as will be described later. Robotic approach may allow surgeons to perform this technically difficult procedure more accurately with less bleeding without injuries to small vessels at the splenic hilum [17]. In this chapter, we focused on the spleen-preserving lymphadenectomy of station #10.
Dissection of the splenic hilum for lymphadenectomy of station #10 is performed after the division of the left gastroepiploic artery as it branches from the SPA. The branches of splenic vessels are exposed from lower to upper polar vessels. During this step, short gastric vessels are identified, ligated, and divided at its root (Fig. 5.8a). The soft tissues along the branches of splenic vessels should be completely retrieved using ultrasonic shears and Maryland bipolar forceps with articulating function (Fig. 5.8b). After division of the short gastric vessels, the esophagophrenic ligament is dissected and divided for enough mobilization of the abdominal esophagus to make an easier and tension-free anastomosis.
Lymph node dissection of the splenic hilum during spleen-preserving total gastrectomy. (a) It is necessary to exchange the ultrasonic shears and Maryland bipolar forceps when the distance between the port of the 2nd arm and the splenic hilum is too long for ultrasonic shears to reach. (b) The proximal portion of the branches of splenic vessels at the splenic hilar area is exposed. (c). The branches of splenic vessels are exposed from lower to upper polar vessels. (d). Completed lymph node dissection of the splenic hilum during spleen-preserving total gastrectomy. SPA splenic artery
5.3.6 Reconstruction
Several methods for the reconstruction of gastrointestinal continuity have been described as follows [6, 7, 18–20]: gastroduodenostomy, gastrojejunostomy, or Roux-en-Y gastrojejunostomy; intracorporeal or extracorporeal; and linear or circular staplers including transoral anvil placement (Orvil). Because each reconstruction method has its advantages and shortcomings, the selection of the method depends on the resection extent and surgeon’s preference based on their experience. In addition, when an endoscopic linear stapler that can be attached to the robotic arm will be available, robotic approach for intracorporeal anastomosis will be easier and more comfortable.
5.4 Review of Clinical Studies
Robotic approach for surgical treatment of gastric cancer offered ergonomic and technical benefits to surgeons. Using these advantages, surgeons have made efforts to overcome the shortcomings of conventional laparoscopic approach, especially in radical lymph node dissection that is required from the viewpoint of oncologic principles. Several studies comparing robotic gastrectomy to conventional laparoscopic gastrectomy have been reported, which showed acceptable short-term outcomes of robotic gastrectomy. However, because robotic gastrectomy is a relatively novel field for gastric cancer treatment, scientific evidence of the superiority of robotic approach to conventional laparoscopy is still lacking.
One of the largest comparative study published in 2011 [3] showed better short-term outcomes of robotic gastrectomy in terms of reduced intraoperative blood loss compared to conventional laparoscopy. This study also demonstrated its comparable oncologic outcomes to conventional laparoscopic gastrectomy, showing that the number of lymph nodes retrieved did not differ significantly between the two groups. Notably, no tumor involvement was observed in the resection line in the robotic group, while the laparoscopic group did not. The authors concluded that a robotic approach to gastric cancer is a promising alternative to conventional laparoscopic gastrectomy. A meta-analysis published in 2012 comparing robotic and laparoscopic gastrectomy for gastric cancer [21] revealed that robotic gastrectomy was significantly associated with less blood loss with an expense of longer operation time. It also showed comparable overall morbidity and mortality.
In a technical aspect, one single-center prospective case series demonstrated an integrated robotic approach of D2 lymphadenectomy for gastric cancer surgery, which showed no pancreas-related complications, although the number of patients was small [5]. Because pancreas-related complications were mostly associated with radical lymph node dissection, this study suggested the safety of peripancreatic lymphadenectomy using robotic approach.
Although no randomized controlled trial has been reported comparing robotic and laparoscopic gastrectomy, these reports support the feasibility of robotic gastrectomy for gastric cancer, provided that these operations are performed by experienced surgeons. Multicenter, randomized controlled trials are unequivocally required to establish the sound evidence of robotic gastrectomy in terms of both short- and long-term outcomes.
5.5 Advantages of Robotic Approach in Gastric Cancer Surgery
Considering radical lymph node dissection during gastrectomy, there are several difficulties in conventional laparoscopic gastrectomy [22, 23]. Especially in suprapancreatic lymph node dissection, it is technically challenging to keep the right plane of dissection between adipose tissues and major suprapancreatic vessels or pancreatic parenchyma because of surgeons’ tremors and 2-D flat views. Furthermore, it is also difficult for surgeons to reach the deep portion of the suprapancreatic area with straight instruments without internal articulation for conventional laparoscopic surgery. Nevertheless, for oncologically complete D2 lymphadenectomy, it is necessary to retrieve lymphatic tissues located in the dorsal side of suprapancreatic vessels. Thus, some experienced surgeons started to employ robotic gastrectomy as a promising alternative to conventional laparoscopic gastrectomy [3–8].
The robotic surgery system is able to facilitate technically and oncologically safe robotic gastrectomy, especially in suprapancreatic lymph node dissection, by offering potential benefits to gastric surgeons, such as steady 3D images, an intuitive movement of robot arm instruments, tremor filtering, motion scaling, and instruments with articulating function with 7 degrees of freedom. Robotic articulated instruments make it easier for surgeons to reach the deep dorsal portion of suprapancreatic area compared to laparoscopic unarticulated instruments. Due to stable retraction of tissues by robotic instruments without tremor, surgeons can reduce potential risk of injury to lymphatic tissues and bleeding from dissection plane.
Robotic approach may also enable surgeons to carry out sufficient dissection of lymph node #10, which is located at the splenic hilum, during spleen-preserving total gastrectomy with D2 lymphadenectomy. Complicated vascular anatomy at the splenic hilum sometimes makes surgeons troubled in dissecting lymph node #10. The small branches of the splenic vessels may compromise sufficient lymphadenectomy in the area of the splenic hilum by frequently causing intraoperative hemorrhage. However, the mechanical advantages of robotic approach listed above allow surgeons to easily dissect along the splenic vessels and to sufficiently clear the lymphatic tissue with minimal intraoperative hemorrhage.
By using a robotic surgery system, gastric cancer surgeons can potentially overcome difficulties of D2 lymphadenectomy during MIS. However, robotic surgery has several disadvantages such as expensive initial cost of robot for hospitals, extra financial burden for patients, and longer operative time [3, 21]. It is still controversial whether these disadvantages of robotic approach can be justified by the advantages in radical lymphadenectomy. Future randomized controlled trials should be warranted to assess whether these robotic advantages are beneficial for long-term clinical outcomes of gastric cancer patients.
5.6 Conclusions
In this chapter, we demonstrated the procedures, current status, and clinical advantages of robotic gastrectomy for gastric cancer. Robotic gastrectomy with radical lymphadenectomy is considered as a safe and feasible alternative to conventional laparoscopy. Although scientific evidence of superiority to conventional laparoscopic surgery is still lacking, robotic gastrectomy for gastric cancer patients may be a promising approach by offering more accurate and delicate lymphadenectomy to the patients, which might improve short- and long-term outcomes of gastric cancer patients.
References
Kitano S, Iso Y, Moriyama M et al (1994) Laparoscopy-assisted Billroth I gastrectomy. Surg Laparosc Endosc 4:146–148
Kim HH, Hyung WJ, Cho GS et al (2010) Morbidity and mortality of laparoscopic gastrectomy versus open gastrectomy for gastric cancer: an interim report–a phase III multicenter, prospective, randomized trial (KLASS Trial). Ann Surg 251:417–420
Woo Y, Hyung WJ, Pak KH et al (2011) Robotic gastrectomies offer a sound oncologic surgical alternative for the treatment of early gastric cancers comparing favorably with laparoscopic resections. Arch Surg 146:1086–1092
D’Annibale A, Pende V, Pernazza G et al (2011) Full robotic gastrectomy with extended (D2) lymphadenectomy for gastric cancer: surgical technique and preliminary results. J Surg Res 166:e113–e120
Uyama I, Kanaya S, Ishida Y et al (2012) Novel integrated robotic approach for suprapancreatic D2 nodal dissection for treating gastric cancer: technique and initial experience. World J Surg 36:331–337
Kim MC, Heo GU, Jung GJ (2010) Robotic gastrectomy for gastric cancer: surgical techniques and clinical merits. Surg Endosc 24:610–615
Song J, Oh SJ, Kang WH et al (2009) Robot-assisted gastrectomy with lymph node dissection for gastric cancer: lessons learned from an initial 100 consecutive procedures. Ann Surg 249:927–932
Anderson C, Ellenhorn J, Hellan M et al (2007) Pilot series of robot-assisted laparoscopic subtotal gastrectomy with extended lymphadenectomy for gastric cancer. Surg Endosc 21:1662–1666
Kim KM, An JY, Kim HI et al (2012) Major early complications following open, laparoscopic and robotic gastrectomy. Br J Surg 99:1681–1687
Hashizume M, Sugimachi K (2003) Robot-assisted gastric surgery. Surg Clin N Am 83:1429–1444
Woo Y, Hyung WJ, Kim HI et al (2011) Minimizing hepatic trauma with a novel liver retraction method: a simple liver suspension using gauze suture. Surg Endosc 25:3939–3945
Shinohara T, Kanaya S, Yoshimura F et al (2011) A protective technique for retraction of the liver during laparoscopic gastrectomy for gastric adenocarcinoma: using a Penrose drain. J Gastrointest Surg 15:1043–1048
Kinjo Y, Okabe H, Obama K et al (2011) Elevation of liver function tests after laparoscopic gastrectomy using a Nathanson liver retractor. World J Surg 35:2730–2738
Hur H, Jeon HM, Kim W (2008) Laparoscopic pancreas- and spleen-preserving D2 lymph node dissection in advanced (cT2) upper-third gastric cancer. J Surg Oncol 97:169–172
Hyung WJ, Lim JS, Song J et al (2008) Laparoscopic spleen-preserving splenic hilar lymph node dissection during total gastrectomy for gastric cancer. J Am Coll Surg 207:e6–e11
Sakuramoto S, Kikuchi S, Futawatari N et al (2009) Laparoscopy-assisted pancreas- and spleen-preserving total gastrectomy for gastric cancer as compared with open total gastrectomy. Surg Endosc 23:2416–2423
Marano A, Hyung WJ (2012) Robotic gastrectomy: the current state of the art. J Gastric Cancer 12:63–72
Hyung WJ, Woo YH, Noh SH (2011) Robotic surgery for gastric cancer: a technical review. J Robot Surg 5:241–249
Pugliese R, Maggioni D, Sansonna F et al (2009) Outcomes and survival after laparoscopic gastrectomy for adenocarcinoma. Analysis on 65 patients operated on by conventional or robot-assisted minimal access procedures. Eur J Surg Oncol 35:281–288
Pugliese R, Maggioni D, Sansonna F et al (2010) Subtotal gastrectomy with D2 dissection by minimally invasive surgery for distal adenocarcinoma of the stomach: results and 5-year survival. Surg Endosc 24:2594–2602
Xiong B, Ma L, Zhang C et al (2012) Robotic versus laparoscopic gastrectomy for gastric cancer: a meta-analysis of short outcomes. Surg Oncol 21:274–280
Noshiro H, Nagai E, Shimizu S et al (2005) Laparoscopically assisted distal gastrectomy with standard radical lymph node dissection for gastric cancer. Surg Endosc 19:1592–1596
Song KY, Kim SN, Park CH (2008) Laparoscopy-assisted distal gastrectomy with D2 lymph node dissection for gastric cancer: technical and oncologic aspects. Surg Endosc 22:655–659
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Japan
About this chapter
Cite this chapter
Obama, K., Hyung, W.J. (2014). Robotic Gastrectomy for Gastric Cancer. In: Watanabe, G. (eds) Robotic Surgery. Springer, Tokyo. https://doi.org/10.1007/978-4-431-54853-9_5
Download citation
DOI: https://doi.org/10.1007/978-4-431-54853-9_5
Published:
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-54852-2
Online ISBN: 978-4-431-54853-9
eBook Packages: MedicineMedicine (R0)