Abstract
Minimally invasive gastric cancer surgery is gaining acceptance, especially in the treatment of patients with early gastric cancer. While offering patients the benefits of minimally invasive surgery, laparoscopic surgery is limited by several disadvantages such as altered operating view and lack of versatility in surgical instrumentation. Robotic surgery offers the surgeon the benefit of superior 3D visualization, the freedom of the EndoWrist function, and the tremble-filtered control of the four robotic arms. Due to the technical advantages of the robotic surgical system, robotic surgery may facilitate the expansion of minimally invasive surgery over laparoscopy. The application of robotic surgery for gastric cancer is increasing in experienced centers. Most reports of the robotic operating methods are only slightly modified from the laparoscopic technique. Robotic gastric cancer surgery including radical subtotal gastrectomy with D2 lymph node dissection is technically feasible and safe and results in similar short-term postoperative outcomes when compared to laparoscopic surgery. The role of robotic surgery in gastric cancer is promising but awaits further comparative studies of long-term results and cost-effectiveness.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The shift in paradigm of gastric cancer treatment is toward an increasingly less invasive approach. Especially for the patients with early stage disease for which surgery is curative, improving the patient’s quality of life after surgery is a close secondary consideration after maintaining strict oncologic principles during surgery. Minimally invasive approaches such as endoscopic mucosal resection/endoscopic submucosal dissection and laparoscopic and robotic gastrectomies with lymph node dissections have safely and effectively treated a select subset of gastric cancer patients with early gastric cancer. Minimally invasive gastric cancer procedures are providing these patients with oncologically sound procedures and improved quality of life [1, 2]. While the advantages of laparoscopy are well established, several inherent limitations of laparoscopic surgery and the complexity of the extended lymph node dissection for gastric cancer have led surgeons to actively investigate robotic surgery as an alternative minimally invasive approach to gastric cancer.
A new level of surgical ingenuity involving robotic computer technology was developed to improve on the strengths and weaknesses of laparoscopy. The first successful robotic gastric cancer surgery was reported from Japan in 2002 and now the role of robotic surgery in the treatment of patients with gastric cancer is growing [3]. However, only a limited number of studies exists evaluating the technique of robotic gastrectomy for gastric cancer [3–12]. We offer a brief review of the indications, the preoperative work-up, and the available methods of robotic gastrectomies with lymph node dissections, and present our institution’s step-by-step technical approach to radical gastrectomy.
Indications and preoperative evaluations
The indications for robotic gastrectomy are the same as those for laparoscopic surgery. Despite a randomized controlled trial regarding the long-term outcome of laparoscopic gastrectomy [13], the oncologic safety of minimally invasive surgery for advanced gastric cancer remains controversial. In all but one study, which included a patient with stage III disease, patients were eligible for robotic gastric cancer operations with the clinical preoperative diagnosis of Stage I or II disease [7]. In most other studies, patients with early gastric cancer as defined by Union Internationale Contre le Cancer and Japanese Gastric Cancer Association classification were eligible for treatment with robotic surgery [14].
Specific exclusion criteria or contraindications for robotic surgery, as for laparoscopic surgery, include those patients with severe COPD or cardiac disease who will not tolerate an extended period of pneumoperitoneum. In general, obvious preoperative findings of T4 cancers and distant metastases are not indicated. Patients who are found on endoscopy to be suitable for endoscopic mucosal resection with small intramucosal early gastric cancers of intestinal histology were first considered for endoscopic mucosal resection.
Operating room configuration
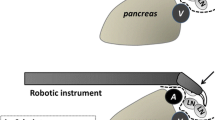
The set-up of the operating room is the preference of the surgeon. Depending on the availability of space and the comfort of the operating team, the set-up may differ slightly. A schematic of the operating room configuration during robotic gastrectomy is shown in Figure 1a. The da Vinci Surgical System (Sunnyvale, CA, USA) was used for all operations described in this report. In general, the anesthesia cart is placed to the immediate left of the patient along with the patient-side assistant who remains on the lower left side. The scrub nurse and the scrub table are located opposite the assistant on the lower right side of the patient with the main assistant monitor cephalad to the scrub nurse. The vision cart where the CO2 insufflator and the vision systems are racked is placed at the foot of the operating table. The surgeon’s master consol is placed towards the lower right corner of the operating room where the surgeon has complete overview of the room including the patient, the robotic instruments and anesthesia cart.
Port placement
The robotic gastrectomy procedure follows the same technical steps as those of laparoscopic gastrectomy. Under general anesthesia, the patient’s arms are tucked on each side to allow for the docking of the robot arms from directly cephalad. The abdomen is then prepared and draped. In general, the port placements for total and subtotal gastrectomy are the same in most reported cases. Minor adjustments are made for patient body habitus and the relative locations of the intraabdominal organs for all cases, and the distance to the esophageal hiatus during total gastrectomies.
An infra-umbilical 12-mm camera port is placed using the open Hasson technique. The patient is then placed in the reverse Trendelenburg position about 15 degrees and a pneumoperitoneum of 12 mmHg is achieved. After adequate insufflation, a total of four additional ports, one 12-mm assistant port and three 8-mm ports for the robotic arms, are placed under direct visualization (Fig. 1b). Minor variations from our port placements have been reported [7]. At this time, the robot surgical cart is brought in from the head of the patient and the four robotic arms are docked (Fig. 1c). The camera arm holds the infra-umbilical port, the 2nd and 3rd robot arms hold an ultrasonic shears and the Cadiere forceps, respectively, on the patient’s right side, while the 1st robot arm on the patient’s left is used for the curved bipolar Maryland forceps.
Technique of distal subtotal gastrectomy with D2 dissection
The first step in a distal subtotal gastrectomy is partial omentectomy. The Cadiere forceps can be used to grab and retract the greater curvature of the stomach upward toward the liver and to the abdominal wall. In this manner, a draping of the greater omentum is created (Fig. 2a). An ultrasonic shears is then used to divide the greater omentum from the mid-transverse colon about 4–5 cm away from the gastroepiploic arteries. The dissection proceeds toward the lower pole of the spleen where the left gastroepiploic vessels are encountered and these vessels are divided at their roots (Fig. 2b). The omental branch is preserved in cases where partial omentectomy is performed (Fig. 2c). After ligation of the left gastroepiploic vessels, the perigastric soft tissues are dissected to the short gastric vessels to clear the greater curvature of the stomach (Fig. 2d). The division of the gastrocolic ligament is continued toward the pylorus.
Left side dissection of the greater curvature. The gastrocolic division begins at least 5 cm from the right gastroepiploic artery (a) toward the left gastroepiploic artery (b). The exposure of the left gastroepiploic artery is performed with preservation of the omental branch (c); the greater curvature is then cleared distally to the resection margin (d)
The right gastroepiploic vessels are encountered as the dissection proceeds to the head of the pancreas. The pancreatic attachments to the duodenum and posterior stomach are released. Then, exposure in the area of the right gastroepiploic vessels is created with a gentle medial and downward retraction of the colonic mesentery provided by the assistant (Fig. 3a). To ensure the adequate dissection of lymph node station #6, the soft tissues above the anterior superior pancreaticoduodenal vein laterally and the middle colic medially should be retrieved (Fig. 3d). The right gastroepiploic vein is divided just proximal to the junction of the anterior superior pancreaticoduodenal vein (Fig. 3c). Afterwards, the right gastroepiploic artery is identified and ligated at its root (Fig. 3d).
Right side dissection of the greater curvature. A medial and downward retraction of the colonic mesentery is provided by the assistant (a) to expose the inferior border of lymph node station #6 at the base of the right gastroepiploic vein (RGEV). The dashed black line indicates the border between lymph node stations #6 and #14v (b) and the right gastroepiploic vein is ligated just above the anteriosuperior pancreaticoduodenal vein (ASPDV) (c). The right gastroepiploic artery (RGEA) is isolated for ligation and division (d). Acc Rt Colic V accessory right colic vein
The dissection continues along the gastroduodenal artery to where it branches from the common hepatic artery. To facilitate the dissection of the supraduodenal soft tissue attachments and to avoid injury of vessels in the hepatoduodenal ligament, a 4 × 4 gauze is inserted anterior to the head of pancreas above the gastroduodenal artery to lie behind the duodenum. When the first portion of the duodenum is fully mobilized by clearing the supraduodenal area (Fig. 4a), the assistant divides the duodenum 1–2 cm distal to the pylorus using an Endo-linear stapler (Fig. 4b).
Duodenal transection and hepatoduodenal ligament dissection. After creating a supraduodenal window for duodenal transection (a), the duodenum is transected (b). The dissection of the gastroduodenal artery (GDA), the proper hepatic artery (PHA), and the common hepatic artery (CHA) exposes the right gastric artery (RGA) as shown in (c). The hepatoduodenal ligament is dissected for lymph node #8 and #12a clearance (d)
The 3rd robotic arm is used to retract the liver and expose the liver hilum. Following the dissection of the gastroduodenal artery and the proper hepatic artery, the right gastric artery is clearly exposed and ligated at its origin (Fig. 4c). The soft tissues around the proper hepatic artery and medial side of the portal vein area are cleared for lymph node station #12a retrieval (Fig. 4d). Exposure of the soft tissues medial to the proper hepatic artery may be aided by carefully grabbing the nerve fibers on the common hepatic artery and retracting the artery laterally. The left gastric vein is also exposed in this area and divided.
During the dissection of lymph node-bearing soft tissues around the common hepatic artery (lymph node station #8a), the Cadiere forceps may assist by gentle compression downward of the pancreas (Fig. 5a). The gastrohepatic ligament is divided at this time (Fig. 5b). The soft tissues around the left gastric artery are then retrieved for lymph node station #7 by approaching the left gastric artery from various directions. As the retroperitoneal attachments are divided toward the esophageal crus, the left gastric artery is approached from the patient’s right side (Fig. 5c, d).
Approach to suprapancreatic lymph node dissection. The pancreas is compressed with the 3rd robotic arm for improved access to lymph node station #8a around the common hepatic artery (a). The gastrohepatic ligament (b) and the retroperitoneal attachments (c) are detached toward the right esophageal crus to gain access to the left gastric artery from the right side (d)
The dissection also continues along the common trunk of the common hepatic artery and splenic artery to find the root of the left gastric artery (Fig. 6a). Grasping of the tissues encasing the left gastric artery creates a sling with the left gastric artery pedicle, useful for isolating the left gastric artery at its root (Fig. 6b). Angling of camera to view the right side (Fig. 6c) and the left side of the left gastric artery (Fig. 6d) facilitates the complete dissection of the left gastric artery. The left gastric artery is then exposed and transected at the root.
Exposure and dissection of the root of the left gastric artery. As dissection proceeds along the common trunk of the common hepatic artery and splenic artery toward the left gastric artery (a), grasping of the soft tissues encasing the left gastric artery to create a sling of the left gastric artery pedicle (b) ensures complete clearance of lymph node station #7 with the help of back and forth camera rotation between the right (c) and left (d) sides of the left gastric artery
The dissection is continued along the splenic vessels. Exposure around the splenic vessels is created by gentle medial and downward traction of the pancreas (Fig. 7a) and by pushing the stomach to the left side (Fig. 7b). Ultrasonic shears can safely dissect along the splenic artery and vein (Fig. 7c). At times, the EndoWrist function of mono-polar devices can also be used (Fig. 7d).
Exposure and dissection along the splenic vessels. A medial and downward traction on the pancreas by the assistant (a) and the pushing of the stomach to the left side with the 3rd robotic arm (b) exposes lymph nodes #11p to allow dissection along the splenic artery and vein with ultrasonic shears (c) or with an EndoWrist device (c)
The retroperitoneal detachment of the stomach is completed along the lesser curvature up to the right esophageal crus. The soft tissues along the lesser curvature are retrieved up to the esophagocardial junction. During this portion of the procedure, the 3rd robotic arm further retracts the liver to create a larger area of exposure (Fig. 8a). Alternating between anterior and the posterior approaches to the lesser curve dissection allows for complete clearing of the soft tissues in this area (Fig. 8c, d). The extent of dissection along the lesser or greater curvature is designed to maintain at least a 2-cm length of proximal resection margin from the lesion. This allows for full mobilization of the stomach in order to proceed with the gastric resection (Fig. 9a).
Resection and intracorporeal anastomosis for subtotal gastrectomy. Resection of the stomach with the Endo-linear stapler is performed by the assistant (a) and a loop of jejunum about 20 cm from the ligament of Treitz is brought up to the stomach (b) in an antecolic fashion to create an intracorporeal gastrojejunostomy with a 60-mm Endo-linear stapler (c). The closure of the common enterotomy with another 60-mm Endo-linear stapler is done by the assistant from the patient’s right side (d)
After the gastric resection, reconstruction is performed by either the intracorporeal or extracorporeal method with gastroduodenostomy, gastrojejunostomy, or Roux-en-Y gastrojejunostomy.
Extracorporeal anastomosis
After full mobilization of the stomach, the robot arms are undocked. A 5-cm vertical mini-laparotomy is made in the epigastrium and the stomach is removed for resection and anastomosis. The resection line is determined by palpation of the endoscopically applied clips and confirmation of the location of the lesion. After the gastric resection, gastrointestinal continuity is restored by either a gastroduodenostomy or gastrojejunostomy according to the location of the tumor. Gastroduodenostomy is performed extracorporeally using a circular stapler. In the case of an extracorporeal gastrojejunostomy, a 60 mm linear stapler is used for the transmural inner layer and the enterotomies are closed in two layers. The mini-laparotomy is closed with continuous polydioxanone sutures.
Intracorporeal anastomosis
During an intracorporeal gastrojejunostomy, the proximal margin of the stomach is taken with 60-mm blue load Endo-linear staplers. After the transection of the stomach, the specimen is placed in an Endocatch bag and placed away from the operator’s view. A small gastrostomy is created on the greater curvature. A loop of jejunum about 20 cm from the ligament of Treitz is elevated to the remaining stomach in an antecolic fashion and a small enterotomy is created. The gastrojejunostomy is created using a 60-mm Endo-linear stapler (Fig. 9b, c). The common opening is then closed with another 60-mm linear stapler which is inserted from the right side of the patient (Fig. 9d). In order to do this, the 8-mm trocar which held the 2nd robotic arm is replaced with a 12-mm trocar to allow the Endo-linear stapler to enter the abdomen. The specimen is bagged intracorporeally and retrieved through the extended infra-umbilical trocar site. The trocar sites are closed with 2-0 polydioxanone sutures.
Total gastrectomy
The procedure for total gastrectomy follows the same technique as subtotal gastrectomy with two additional steps. First, the dissection of the short gastric arteries along the gastrosplenic ligament is continued after the ligation of the left gastroepiploic vessels (Fig. 10a). In brief, after dividing the left gastroepiploic vessels at their roots, the short gastric vessels are divided from the surface of the spleen to dissect the 4sa lymph nodes. The stomach is mobilized toward the left side of the esophageal hiatus (lymph node station #2) along the curvature to complete the division of the gastrosplenic ligament. In the case of a D2 lymph node dissection, lymphadenectomy of stations #10 and #11d is performed with preservation of the spleen (Fig. 10b). Once the lesser curve of the stomach is freed from its attachments as previously described under subtotal gastrectomy, the distal esophagus is detached from its hiatus and made mobile along its length.
Total gastrectomy and intracorporeal esophagojejunostomy. The division of the short gastric arteries (a) is followed by complete dissection along the splenic artery and splenic hilum for retrieval of lymph node stations #11d and #10 (b). Vascular clamps are applied on either side of the esophageal resection line (c) and the esophagus is transected. The anvil is placed and secured in the esophagus with a purse-string suture (d). A mini-laparotomy is created for extra-corporeal jejunojejunostomy, the retrieval of the specimen, and the introduction of the circular stapler (e). The insertion of the circular stapler in the jejunum (f) is made ready for intra-abdominal introduction by re-establishment of the pneumoperitoneum with the glove method (g) and an intracorporeal esophagojejunal anastomosis is created (h)
Intracorporeal anastomosis is described as shown in Fig. 10c–h. The proximal margin on the esophagus is determined and vascular clamps are placed on either side of the transection line (Fig. 10c). The esophagus is then divided. The specimen is placed in an Endocatch bag and out of the operating field. A purse-string suture is placed around the distal end of the transected esophagus using robotic instruments. The anvil is placed and securely tied in place (Fig. 10d). The jejunum is then marked with a dye to identify the afferent and efferent loops.
Gastrointestinal continuity is restored laparoscopically after the robot arms are undocked. A mini-laparotomy is used to create an extracorporeal jejunojejunostomy using a linear stapler 50 cm distal to the esophagojejunostomy and also to retrieve the specimen (Fig. 10e). A Roux-en-Y esophagojejunostomy is created using a 25-mm EEA circular stapler introduced via a mini-laparotomy (Fig. 10f). After inserting the EEA stapler in the jejunum, the jejunum and EEA stapler are returned to the intra-abdominal space.
Pneumoperitoneum is re-established using the glove method (Fig. 10g). The esophagojejunostomy is created laparoscopically (Fig. 10h). After confirming the intact anastomoses and the absence of bleeding from the dissected area, all trocars and cannulas are removed under direct laparoscopic visualization. The trocar sites are closed with 2-0 polydioxanone sutures.
Conclusion
Robotic gastric cancer surgery is a complex operation which can be performed safely with the expected advantages of minimally invasive surgery. Studies of short-term outcomes demonstrate less blood loss, less pain and shorter hospital stay for robotic gastrectomy when compared to laparoscopic gastric cancer operations [4, 5]. As surgeons become more adept at robotic gastrectomies and the clinical and oncologic outcomes remain favorable, robotic surgery may offer a sound alternative to traditional open or conventional laparoscopic gastric cancer operations.
References
Adachi Y, Suematsu T, Shiraishi N, Katsuta T, Morimoto A, Kitano S, Akazawa K (1999) Quality of life after laparoscopy-assisted Billroth I gastrectomy. Ann Surg 229:49–54
Kim HH, Hyung WJ, Cho GS, Kim MC, Han SU, Kim W, Ryu SW, Lee HJ, Song KY (2010) Morbidity and mortality of laparoscopic gastrectomy versus open gastrectomy for gastric cancer: an interim report–a phase III multicenter, prospective, randomized Trial (KLASS Trial). Ann Surg 251:417–420
Hashizume M, Sugimachi K (2003) Robot-assisted gastric surgery. Surg Clin North Am 83:1429–1444
Kim MC, Heo GU, Jung GJ (2010) Robotic gastrectomy for gastric cancer: surgical techniques and clinical merits. Surg Endosc 24:610–615
Song J, Kang WH, Oh SJ, Hyung WJ, Choi SH, Noh SH (2009) Role of robotic gastrectomy using da Vinci system compared with laparoscopic gastrectomy: initial experience of 20 consecutive cases. Surg Endosc 23:1204–1211
Song J, Oh SJ, Kang WH, Hyung WJ, Choi SH, Noh SH (2009) Robot-assisted gastrectomy with lymph node dissection for gastric cancer: lessons learned from an initial 100 consecutive procedures. Ann Surg 249:927–932
Patriti A, Ceccarelli G, Bellochi R, Bartoli A, Spaziani A, Di Zitti L, Casciola L (2008) Robot-assisted laparoscopic total and partial gastric resection with D2 lymph node dissection for adenocarcinoma. Surg Endosc 22:2753–2760
Giulianotti PC, Coratti A, Angelini M, Sbrana F, Cecconi S, Balestracci T, Caravaglios G (2003) Robotics in general surgery: personal experience in a large community hospital. Arch Surg 138:777–784
Anderson C, Ellenhorn J, Hellan M, Pigazzi A (2007) Pilot series of robot-assisted laparoscopic subtotal gastrectomy with extended lymphadenectomy for gastric cancer. Surg Endosc 21:1662–1666
Anderson C, Hellan M, Kernstine K, Ellenhorn J, Lai L, Trisal V, Pigazzi A (2007) Robotic surgery for gastrointestinal malignancies. Int J Med Robot 3:297–300
Wilson EB (2009) The evolution of robotic general surgery. Scand J Surg 98:125–129
Maeso S, Reza M, Mayol JA, Blasco JA, Guerra M, Andradas E, Plana MN (2010) Efficacy of the Da Vinci surgical system in abdominal surgery compared with that of laparoscopy: a systematic review and meta-analysis. Ann Surg 252:254–262
Hartgrink HH, van de Velde CJ, Putter H, Bonenkamp JJ, Klein Kranenbarg E, Songun I, Welvaart K, van Krieken JH, Meijer S, Plukker JT, van Elk PJ, Obertop H, Gouma DJ, van Lanschot JJ, Taat CW, de Graaf PW, von Meyenfeldt MF, Tilanus H, Sasako M (2004) Extended lymph node dissection for gastric cancer: who may benefit? Final results of the randomized Dutch gastric cancer group trial. J Clin Oncol 22:2069–2077
Nakajima T (2002) Gastric cancer treatment guidelines in Japan. Gastric Cancer 5:1–5
Acknowledgments
This study was supported by a grant from the National R&D Program for Cancer Control, Ministry of Health & Welfare, Republic of Korea (1020410). We thank Dong-Su Jang, our medical illustrator, for Fig. 1a.
Conflict of interest
None disclosed.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hyung, W.J., Woo, Y. & Noh, S.H. Robotic surgery for gastric cancer: a technical review. J Robotic Surg 5, 241–249 (2011). https://doi.org/10.1007/s11701-011-0263-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11701-011-0263-x