Abstract
α1,4-N-acetylglucosaminyltransferase (α4GnT) is a glycosyltransferase that mediates transfer of GlcNAc from UDP-GlcNAc to βGal residues with α1,4-linkage preferentially present in O-glycans forming GlcNAcα1→4Galβ→R, and cDNA encoding α4GnT was isolated from a human stomach cDNA library by expression cloning. In normal human tissues, O-glycans exhibiting GlcNAcα1→4Galβ→R at nonreducing terminal (αGlcNAc) are exclusively limited to gland mucin secreted from gland mucous cells of the stomach and Brunner’s gland of the duodenum. αGlcNAc in gland mucin is detected by immunohistochemistry with HIK1083 antibody and paradoxical ConA staining. Notably, αGlcNAc inhibits the growth and motility of Helicobacter pylori (H. pylori) by inhibiting the biosynthesis of its cell wall component cholesteryl-α-D-glucopyranoside. In addition, A4gnt-/- mice show no αGlcNAc in gland mucin in the gastroduodenal mucosa, and develop gastric differentiated-type adenocarcinoma through inflammation-associated pathways, even in the absence of H. pylori infection. Thus, αGlcNAc in gland mucin plays a dual role in protecting gland mucous cells from H. pylori infection and also serving as a tumor suppressor of differentiated-type gastric adenocarcinoma.
Access provided by Autonomous University of Puebla. Download reference work entry PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
α1,4-N-acetylglucosaminyltransferase (α4GnT) is a glycosyltransferase that mediates transfer of GlcNAc from UDP-GlcNAc to βGal residues with α1,4-linkage preferentially present in O-glycans forming GlcNAcα1→4Galβ→R (Nakayama et al. 1999). In normal human tissues, O-glycans exhibiting GlcNAcα1→4Galβ→R at nonreducing terminal, which is simply called αGlcNAc, are exclusively limited to gland mucin secreted from gland mucous cells (such as cardiac gland cells, mucous neck cells, and pyloric gland cells) of the stomach and Brunner’s gland of the duodenum (Nakamura et al. 1998) (Fig. 36.1). Thus, α4GnT plays a key role in synthesizing αGlcNAc in gland mucin. αGlcNAc is primarily attached to the mucin core protein MUC6, which acts as a scaffold, but small amounts of αGlcNAc are attached to MUC5AC (Zhang et al. 2001).
cDNA encoding a human α4GnT was cloned by expression cloning (Nakayama et al. 1999). α4GnT has significant homology to α1,4-galactosyltransferase (α4GalT, Gb3/CD77 synthase), with a 35 % overall sequence similarity at the amino acid level (Kojima et al. 2000).
Databanks
EC number: not determined
Alpha-1,4-N-acetylglucosaminyltransferase (A4GNT)
Species | Gene symbol | GenBank accession number | Uniprot ID | PDB accession number |
|---|---|---|---|---|
Homo sapiens | A4GNT | NM_016161.2 | Q9UNA3 | N/A |
Mus musculus | A4gnt | NM_001077424.2 | Q14BT6 | N/A |
Rattus norvegicus | A4gnt | XM_001065156.2 | D3ZG90 | N/A |
Name and History
α1,4-N-acetylglucosaminyltransferase is abbreviated as α4GnT. There are no other synonyms for α4GnT. cDNA encoding human α4GnT was isolated from stomach tissue (Nakayama et al. 1999). Genes encoding orthologous enzymes from other vertebrates including mice and rats have been deposited in databanks. Glycoprotein having αGlcNAc substituents was first isolated from hog gastric mucin (Lloyd et al. 1969). Later, it was demonstrated that the GlcNAcα1→4Galβ→R structure is a characteristic constituent of pig gastric mucin (Kochetkov et al. 1976), rat gastric mucin (Ishihara et al. 1996), and duodenal glands of rat and pig (Van Halbeek et al. 1983). αGlcNAc was also found in mucous neck cells and pyloric gland cells of amphibians and reptiles but has not been detected in fish and birds (Ota et al. 1998). However, the glycosyltransferase responsible for GlcNAcα1→4Galβ→R biosynthesis had not been isolated prior to cloning of α4GnT.
Structure
Human α4GnT consists of 340 amino acids. Its sequence predicts a type II transmembrane topology, consisting of a short, three amino acid cytoplasmic NH2-terminus, a 22 amino acid transmembrane/signal anchoring domain, and a large COOH-terminus exhibiting stem and catalytic domains. Four potential N-glycosylation sites are found in the catalytic domain.
Enzyme Activity Assay and Substrate Specificity
The following reaction is catalyzed by α4GnT:
Its catalytic activity and acceptor specificity were tested using soluble chimeric α4GnT fused with protein A (Nakayama et al. 1999). To do so, a sequence encoding the α4GnT catalytic domain fused with protein A was cloned into the mammalian expression vector pcDNAI and transfected into CHO cells. Chimeric protein was then purified using IgG-Sepharose and incubated with either 1.0 mM or 0.7 mM of various synthetic acceptors together with 1.0 mM UDP-GlcNAc containing 0.5 μCi of UDP-[3H] GlcNAc in the presence of 5 mM MnCl2, pH 7.0. After incubation at 37 °C for 1 h, reaction products purified using a C18 reversed-phase column were subjected to high performance liquid chromatography.
As shown in Fig. 36.2, α4GnT incorporates GlcNAc most efficiently to core 2 branched oligosaccharides, Galβ1→4GlcNAcβ1→6(Galβ1→3)GalNAcα→pNP. In addition, NMR analysis demonstrated that incorporated GlcNAc is actually attached with an α1,4-linkage to Galβ at the nonreducing terminal of the acceptor. Interestingly, α4GnT acts on Galβ1→3(GlcNAcβ1→6)GalNAcα→pNP more efficiently than on the core 1 acceptor, Galβ1→3GalNAcα→pNP, and only GlcNAcα1→4Galβ1→3(GlcNAcβ1→6)GalNAcα→pNP is formed from Galβ1→3(GlcNAcβ1→6)GalNAcα→pNP. These results suggest that addition of β1,6-linked GlcNAc alters acceptor conformation in a manner that makes it a more efficient α4GnT acceptor. α4GnT acts on Galβ1→3GlcNAcβ→pNP slightly better than on Galβ1→4Glcβ1→pNP, but acts much better on Galβ1→4GlcNAcβ1→6(Galβ1→3)GalNAcα→pNP or Galβ1→3GalNAcα→pNP. These results indicate that the enzyme prefers β1,4-linked or β1,3-linked Gal residues on O-glycans. α4GnT does not utilize UDP-GalNAc when these acceptors are tested.
α4GnT substrate specificity. Relative radioactivity of 3H-GlcNAc incorporated into various acceptors by soluble α4GnT is compared with that obtained when Galβ1→4GlcNAcβ1→6(Galβ1→3)GalNAcα→pNP was used as an acceptor. Acceptor concentration was 1.0 mM, except for Galβ1→4GlcNAcβ1→6(Galβ1→3)GalNAcα→pNP, which was used at 0.7 mM. Data represent the mean ± SEM (From Nakayama et al. 1999; Copyright 1999 National Academy of Sciences, USA)
Preparation
α4GnT protein is available only in recombinant form. The expression cloning strategy used to identify human α4GnT is shown in Fig. 36.3 (Nakayama et al. 1999). Briefly, COS-1 cells were co-transfected with a human stomach cDNA library constructed in the mammalian expression vector pcDNAI together with a leukosialin cDNA. Leukosialin is a major membrane-bound sialoglycoprotein from leukocytes that exhibits 80 O-glycans in its extracellular domain (Fukuda and Tsuboi 1999) and we envisaged that leukosialin could be a preferred α4GnT substrate. In fact, leukosialin cDNA co-transfection proved to be critical for α4GnT identification, because expression levels of αGlcNAc on COS-1 cells co-transfected with leukosialin and α4GnT cDNAs were much higher than those in COS-1 cells transfected with α4GnT cDNA alone (Nakayama et al. 1999).
Strategy for human α4GnT expression cloning. αGlcNAc-negative COS-1 cells were co-transfected with a human stomach cDNA library and the leukosialin vector, pRcCMV-leu. After 60 h, COS-1 cells expressing GlcNAcα1→4Galβ→R residues were isolated by cell sorting using mixture of HIK1083, PGM36, and PGM37 antibodies. Plasmid DNA isolated from the sorted cells by the Hirt procedure was amplified in E. coli MC1061/P3 cells in the presence of ampicillin and tetracycline. Sibling selection was performed by co-transfecting small pools of plasmids separately into COS-1 cells together with the leukosialin vector. Transfectants stained with the same antibody mixture were screened by immunofluorescence microscopy to isolate a single plasmid encoding a human α4GnT
Transfected cells were then screened using monoclonal antibodies specific for αGlcNAc, namely, HIK1083 (Ishihara et al. 1996), PGM36, and PGM37 (Kurihara et al. 1998), and antibody-positive cells were then enriched by fluorescence-activated cell sorting. Plasmid cDNA was rescued from sorted cells and amplified in bacterial MC1061/P3 cells. As pcDNAI encodes a sup F gene that corrects defects in both ampicillin- and tetracycline-resistance genes in the P3 episome, transformed cells become resistant to both antibiotics, while MC1061/P3 cells transformed by the leukosialin cDNA alone were resistant only to ampicillin. Because of this difference, only plasmids derived from the library were selectively amplified in the presence of ampicillin and tetracycline. Isolation of cDNA encoding human α4GnT was achieved after several rounds of sibling selection. The recombinant enzyme has been expressed in COS-1 cells and in human gastric adenocarcinoma AGS cells. As noted above, a soluble form of the enzyme fused with protein A is used for in vitro GlcNAc transferase assays.
Biological Aspects
α4GnT plays a key role in forming αGlcNAc in gland mucin. Glycan specific for this mucin, also termed class III mucin, was originally identified by paradoxical ConA staining (PCS), a sequential histochemical method that consists of periodate oxidation, sodium borohydride reduction, ConA binding, and a horseradish peroxidase reaction (Katsuyama and Spicer 1978). Molecular cloning of α4GnT allowed us to establish that the carbohydrate moiety recognized by PCS is αGlcNAc (Nakayama et al. 1999).
In human tissues, α4GnT transcripts are found only in stomach and pancreas (Nakayama et al. 1999). Immunohistochemistry using an anti-α4GnT antibody has shown that α4GnT protein is found in the Golgi of mucous cells secreting gland mucin in the gastroduodenal mucosa (Fig. 36.4). αGlcNAc is also expressed in pancreatic ducts showing gastric metaplasia (Nakamura et al. 1998). This lesion is currently regarded as pancreatic intraepithelial neoplasia 1 (PanIN-1) (Hruban et al. 2001), and α4GnT is detected in the Golgi of mucous cells showing PanIN-1 (Zhang et al. 2001). In addition, α4GnT is detected in the Golgi of gallbladder epithelial cells showing gastric metaplasia, which are positive for αGlcNAc (Nakamura et al. 1998, Zhang et al. 2001). Thus, α4GnT expression is regulated in a cell-specific manner. The human gene encoding the enzyme maps to chromosome 3q22.3. Its promoter analysis has not been reported.
Knockout and Transgenic Mice
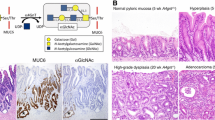
We generated mice deficient in α4GnT by disrupting the A4gnt gene, which encodes mouse α4GnT (Karasawa et al. 2012). A4gnt –/– mice show no αGlcNAc in gland mucin of the gastroduodenal mucosa, establishing that α4GnT is a sole enzyme responsible for the biosynthesis of αGlcNAc. Analyses of A4gnt –/– mice up to 60 weeks of age demonstrated that all the mutant mice develop gastric differentiated-type adenocarcinoma in the antrum of the glandular stomach through a hyperplasia-dysplasia-carcinoma sequence, even in the absence of H. pylori infection (Fig. 36.5). The incidence of adenocarcinoma increases by 50 weeks of age, and all 50-week-old and 60-week-old mice exhibit differentiated-type adenocarcinoma, with cancer cells mostly located in the gastric mucosa. However, gastric undifferentiated-type adenocarcinoma, such as signet ring cell carcinoma, has not developed in the mutant mice. No significant abnormality is found in organs other than the glandular stomach. Genes encoding inflammatory chemokine ligands such as Ccl2, Cxcl1, and Cxcl5; proinflammatory cytokines such as interleukin 11 and interleukin 1β; and growth factors such as hepatocyte growth factor and fibroblast growth factor 7 are upregulated in the gastric mucosa of A4gnt –/– mice, suggestive of tumor-promoting inflammation (Karasawa et al. 2012). In fact, as knockout mice age, inflammatory cell infiltration and angiogenesis in the gastric mucosa progressively increase relative to wild-type mice (Karasawa et al. 2012). Thus, loss of αGlcNAc likely triggers gastric carcinogenesis through inflammation-associated pathways in vivo. Transgenic mice for A4gnt have not been reported.
Gastric pathology of A4gnt –/– mice. Shown are gastric hyperplasia at 5 weeks of age (a), mild dysplasia at 10 weeks (b), and differentiated-type adenocarcinoma at 50 weeks (c). Normal gastric mucosa of a 5-week-old wild-type mouse is shown as a control (d) (Hematoxylin and eosin staining; scale bar = 100 μm)
Human Disease
Helicobacter pylori (H. pylori) is a causative microbe for various gastric diseases such as chronic active gastritis, gastric adenocarcinoma, and gastric mucosa-associated lymphoid tissue lymphoma (MALT lymphoma) (Peek and Blaser 2002). We demonstrated that growth and motility of H. pylori incubated with recombinant soluble CD43 (sCD43) exhibiting αGlcNAc are significantly suppressed, and that bacteria also show abnormal morphology such as elongation and bending (Fig. 36.6). By contrast, bacteria incubated with control sCD43 without αGlcNAc do not show these effects, indicating that αGlcNAc functions as a natural antibiotic against H. pylori (Kawakubo et al. 2004). Helicobacter species including H. pylori contain a unique glycolipid, cholesteryl-α-d-glucopyranoside (CGL), in their cell wall (Hirai et al. 1995). CGL is indispensable for H. pylori survival, motility, and structural preservation (Kawakubo et al. 2004). CGL biosynthesis is catalyzed by cholesterol α-glucosyltransferase (CHLαGcT) (Lee et al. 2006), and its active form is present in the membrane fraction of H. pylori (Hoshino et al. 2011), suggesting that αGlcNAc in gland mucin is readily accessible to bacterial CHLαGcT. Taken together, studies show that αGlcNAc inhibits CGL biosynthesis in H. pylori by suppressing CHLαGcT, thus protecting the gastric mucosa from infection. Interestingly, it has been shown that the A-A haplotype at the rs2622694-rs397266 locus of the A4GNT gene is associated with a higher risk for H. pylori infection compared with the more frequent G-G haplotype (odds ratio 2.30) (Zheng et al. 2009).
Antimicrobial effects of αGlcNAc on H. pylori. (a) Growth curves of H. pylori cultured in the presence of soluble CD43 with αGlcNAc (αGlcNAc (+)) or soluble CD43 without αGlcNAc (αGlcNAc (−)). 1 milliunit of αGlcNAc (+) corresponds to 1 μg of GlcNAcα-pNP. A600: absorbance at 600 nm. (b) Morphology of H. pylori incubated with 31.2 mU/ml of αGlcNAc (+) or the same protein concentration of αGlcNAc (−) for 3 days (Scanning electron micrograph; scale bar = 1 μm) (From Kawakubo et al. 2004; Copyright 2004 American Association for the Advancement of Science)
α4GnT is detectable in the Golgi of gastric adenocarcinoma cells expressing αGlcNAc but not in peripheral blood cells. Quantitative analysis of α4GnT mRNA in the mononuclear cell fraction of peripheral blood using real-time PCR has been useful to detect circulating gastric cancer cells (Shimizu et al. 2003). This assay indicated that α4GnT mRNA was present in 62.2 % of 37 gastric cancer patients, including those at early disease stages, but not in any of 23 H. pylori-negative healthy volunteers. α4GnT is also detectable in the Golgi of pancreatic adenocarcinoma cells expressing αGlcNAc, and similar α4GnT mRNA detection assays have been used in pancreatic cancer patients (Ishizone et al. 2006).
Future Perspectives
Expression of αGlcNAc is restricted to gland mucin secreted from the gastroduodenal mucosa. Analysis of A4gnt knockout mice revealed that αGlcNAc serves as a tumor suppressor of differentiated type of gastric adenocarcinoma in the absence of H. pylori infection (Karasawa et al. 2012). In addition, αGlcNAc protects gastric gland mucous cells from H. pylori infection (Kawakubo et al. 2004). Thus, αGlcNAc plays a dual role to prevent the gastric mucosa from gastric tumorigenesis. Future studies are required to elucidate the molecular mechanism mediated by αGlcNAc to block tumor-promoting inflammation and protect gastric mucosa. Those efforts could lead to development of new strategies to prevent, detect, diagnose, and treat gastric cancer.
Cross-References
Further Reading
-
Ishihara et al. (1996): Production of monoclonal antibody HIK1083 directed to αGlcNAc.
-
Katsuyama and Spicer (1978): Development of histochemical technique to detect the carbohydrate moieties (now identified as αGlcNAc) of gland mucin.
-
Karasawa et al. (2012): Phenotypic analysis of knockout mice deficient in α4GnT.
-
Kawakubo et al. (2004): Antimicrobial effect of αGlcNAc on H. pylori.
-
Nakayama et al. (1999): Isolation of human α4GnT cDNA using expression cloning.
References
Fukuda M, Tsuboi S (1999) Mucin-type O-glycans and leukosialin. Biochim Biophys Acta 1455:205–217
Hirai Y, Haque M, Yoshida T, Yokota K, Yasuda T, Oguma K (1995) Unique cholesteryl glucosides in Helicobacter pylori: composition and structural analysis. J Bacteriol 177:5327–5333
Hoshino H, Tsuchida A, Kametani K, Mori M, Nishizawa T, Suzuki T, Nakamura H, Lee H, Ito Y, Kobayashi M, Masumoto J, Fujita M, Fukuda M, Nakayama J (2011) Membrane-associated activation of cholesterol α-glucosyltransferase, an enzyme responsible for biosynthesis of cholesteryl-α-D-glucopyranoside in Helicobacter pylori critical for its survival. J Histochem Cytochem 59:98–105
Hruban RH, Adsay NV, Albores-Saavedra J, Compton C, Garrett ES, Goodman SN, Kern SE, Klimstra DS, Klöppel G, Longnecker DS, Lüttges J, Offerhaus GJ (2001) Pancreatic intraepithelial neoplasia: a new nomenclature and classification system for pancreatic duct lesions. Am J Surg Pathol 25:579–586
Ishihara K, Kurihara M, Goso Y, Urata T, Ota H, Katsuyama T, Hotta K (1996) Peripheral α-linked N-acetylglucosamine on the carbohydrate moiety of mucin derived from mammalian gastric mucous cells: epitope recognized by a newly characterized monoclonal antibody. Biochem J 318:409–416
Ishizone S, Yamauchi K, Kawa S, Shimizu F, Harada O, Sugiyama A, Miyagawa S, Fukuda M, Nakayama J (2006) Clinical utility of quantitative RT-PCR targeted to α1,4-N-acetylglucosaminyltransferase mRNA for detection of pancreatic cancer. Cancer Sci 97:119–126
Karasawa F, Shiota A, Goso Y, Kobayashi M, Sato Y, Masumoto J, Fujiwara M, Yokosawa S, Muraki T, Miyagawa S, Ueda M, Fukuda MN, Fukuda M, Ishihara K, Nakayama J (2012) Essential role of gastric gland mucin in preventing gastric cancer in mice. J Clin Invest 122:923–934
Katsuyama T, Spicer SS (1978) Histochemical differentiation of complex carbohydrates with variants of the concanavalin A – horseradish peroxidase method. J Histochem Cytochem 26:233–250
Kawakubo M, Ito Y, Okimura Y, Kobayashi M, Sakura K, Kasama S, Fukuda MN, Fukuda M, Katsuyama T, Nakayama J (2004) Natural antibiotic function of a human gastric mucin against Helicobacter pylori infection. Science 305:1003–1006
Kochetkov NK, Derevitskaya VA, Arbatsky NP (1976) The structure of pentasaccharides and hexasaccharides from blood group substance H. Eur J Biochem 67:129–136
Kojima Y, Fukumoto S, Furukawa K, Okajima T, Wiels J, Yokoyama K, Suzuki Y, Urano T, Ohta M, Furukawa K (2000) Molecular cloning of globotriaosylceramide/CD77 synthase, a glycosyltransferase that initiates the synthesis of globo series glycosphingolipids. J Biol Chem 275:15152–15156
Kurihara M, Ishihara K, Ota H, Katsuyama T, Nakano T, Naito M, Hotta K (1998) Comparison of four monoclonal antibodies reacting with gastric gland mucous cell-derived mucins of rat and frog. Comp Biochem Physiol 121B:315–321
Lee H, Kobayashi M, Wang P, Nakayama J, Seeberger PH, Fukuda M (2006) Expression cloning of cholesterol α-glucosyltransferase, a unique enzyme that can be inhibited by natural antibiotic gastric mucin O-glycans, from Helicobacter pylori. Biochem Biophys Res Commun 349:1235–1241
Lloyd KO, Kabat EA, Beychok S (1969) Immunochemical studies on blood groups. XLIII. The interaction of blood group substances from various sources with a plant lectin, Concanavalin A. J Immunol 102:1354–1362
Nakamura N, Ota H, Katsuyama T, Akamatsu T, Ishihara K, Kurihara K, Hotta K (1998) Histochemical reactivity of normal, metaplastic, and neoplastic tissues to α-linked N-acetylglucosamine residue-specific monoclonal antibody HIK1083. J Histochem Cytochem 46:793–801
Nakayama J, Yeh J-C, Misra AK, Ito S, Katsuyama T, Fukuda M (1999) Expression cloning of a human α1,4-N-acetylglucosaminyltransferase that forms GlcNAcα1→4Galβ→R, a glycan specifically expressed in the gastric gland mucous cell-type mucin. Proc Natl Acad Sci USA 96:8991–8996
Ota H, Nakayama J, Momose M, Kurihara M, Ishihara K, Hotta K, Katsuyama T (1998) New monoclonal antibodies against gastric gland mucous cell-type mucins: a comparative immunohistochemical study. Histochem Cell Biol 110:113–119
Peek RM Jr, Blaser MJ (2002) Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nat Rev Cancer 2:28–37
Shimizu F, Nakayama J, Ishizone S, Zhang MX, Kawakubo M, Ota H, Sugiyama A, Kawasaki S, Fukuda M, Katsuyama T (2003) Usefulness of the real-time reverse transcription-polymerase chain reaction assay targeted to α1,4-N-acetylglucosaminyltransferase for the detection of gastric cancer. Lab Invest 83:187–197
Van Halbeek H, Gerwig GJ, Vliegenthart JG, Smits HL, Van Kerkhof PJ, Kramer MF (1983) Terminal α(1→4)-linked N-acetylglucosamine: a characteristic constituent of duodenal-gland mucous glycoproteins in rat and pig. A high-resolution 1H-NMR study. Biochim Biophys Acta 747:107–116
Zhang MX, Nakayama J, Hidaka E, Kubota S, Yan J, Ota H, Fukuda M (2001) Immunohistochemical demonstration of α1,4-N-acetylglucosaminyltransferase that forms GlcNAcα1,4Galβ residues in human gastrointestinal mucosa. J Histochem Cytochem 49:587–596
Zheng Z, Jia Y, Hou L, Persson C, Yeager M, Lissowska J, Chanock SJ, Blaser M, Chow W, Ye W (2009) Genetic variation in α4GnT in relation to Helicobacter pylori serology and gastric cancer risk. Helicobacter 14:472–477
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Japan
About this entry
Cite this entry
Nakayama, J. (2014). Alpha-1,4-N-Acetylglucosaminyltransferase (A4GNT). In: Taniguchi, N., Honke, K., Fukuda, M., Narimatsu, H., Yamaguchi, Y., Angata, T. (eds) Handbook of Glycosyltransferases and Related Genes. Springer, Tokyo. https://doi.org/10.1007/978-4-431-54240-7_87
Download citation
DOI: https://doi.org/10.1007/978-4-431-54240-7_87
Published:
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-54239-1
Online ISBN: 978-4-431-54240-7
eBook Packages: Biomedical and Life SciencesReference Module Biomedical and Life Sciences