Abstract
Like the two important crop legumes soybean and common bean, the model legume Lotus japonicus develops determinate root nodules. L. japonicus is normally infected through root hair infection threads in a process closely synchronised with the progressing primordial cell divisions and organ development. Recent studies of symbiotic mutants have however led to a remarkable and unexpected discovery of two alternative intercellular infection modes, crack entry and infection thread independent single cell infection, in L. japonicus. These results provide genetic support for the origin of rhizobial infection of legumes through direct intercellular epidermal invasion and indicate that this ancient infection process in subsequent evolutionary steps was surpassed by the Nod-factor dependent crack entry and root hair infection thread invasions observed in most extant legumes.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Soybean (Glycine max) and common bean (Phaseolus vulgaris) are the two most important crop legumes for the world’s production of food and feed. Like the model legume Lotus japonicus, both soybean and bean form determinate root nodules following inoculation with their respective rhizobial microsymbionts. A determinate nodule is characterised by a transient meristematic activity and cessation of infection thread growth after the majority of cells in the central zone of the primordia have been infected. The formation of a determinate root nodule is therefore a sequential process where clearly defined developmental stages replace each other and can thus be separated in time. In contrast to determinate nodules, which tend to be round at maturity, indeterminate nodules of for example pea, clover and a second model legume Medicago truncatula retain meristematic activity near the nodule tip and therefore develop into elongate structures. Underlying the tip meristem is the infection zone, where newly produced nodule cells are infected. Following the early stages founding the nodule primordium, an individual indeterminate root nodule will therefore represent spatially – but not temporally – separated stages.
Legume root nodule formation results from two tightly coordinated processes running in parallel. Development of nodule primordia from dedifferentiated root cortical cells initiates a meristem, which will later form the nodule. Simultaneously, a bacterial invasion process targets the primordia. In soybean and bean the outer cortical cells initiate primordia formation. In L. japonicus initial cell divisions were detected in cells of both the outer and middle cortex (Szczyglowski et al. 1998; van Spronsen et al. 2001). Cytoplasmic bridges preparing cells for infection thread passage were observed associated with primordia in L. japonicus, but not in bean (van Spronsen et al. 2001). Like soybean and bean, L. japonicus is normally infected through root hair infection threads, although capacities for crack entry and intercellular invasion were recently uncovered in studies of mutants (Karas et al. 2005; Groth et al. 2010; Madsen et al. 2010). The bacterial infection process starts with rhizobial attachment to plant root hair tips. Subsequent physiological and morphological responses of root hairs result in their curling, and bacteria are entrapped in an infection pocket at the curls’ centre (Szczyglowski et al. 1998; Oldroyd and Downie 2004; Miwa et al. 2006). Starting from the pocket, inwards growing infection threads form and are colonised by rhizobia. These infection threads act as rhizobial conduits from which the Mesorhizobium loti symbiont ultimately will be released and endocytosed into nodule primordia cells. This infection mode targets pre-developed cells of the emerging nodule, and uninfected, so-called interstitial cells, are retained in between the infected cells of the central zone (Imaizumi-Anraku et al. 1997). As a result, infected cells constitute only a subset of cells in the central zone of determinate L. japonicus, bean and soybean nodules. In the infected cells rhizobia differentiate into bacteroids that are surrounded by a peribacteriod membrane derived from the infection thread membrane during endosymbiotic uptake. Together, bacteroids and membrane are referred to as symbiosomes, organelle-like structures where atmospheric dinitrogen is reduced to ammonium. Access to this reduced form of nitrogen provides the host with an ecological advantage over other plants under conditions where soil nitrogen resources are limiting. The M. loti bacteroids contained in L. japonicus nodules are not terminally differentiated and maintain their normal bacterial size, genome content and ability to divide (Van de Velde et al. 2010). This is in contrast to the indeterminate nodules of M. truncatula, where irreversible differentiation of bacteriods is thought to be promoted by an abundance of nodule-specific cysteine-rich anti microbial peptides (Van de Velde et al. 2010). L. japonicus nodules are devoid of these anti microbial peptides, which could be important in maintaining the continuous infection process in the indeterminate nodules (Van de Velde et al. 2010).
2 Recognition Precedes Infection
Several bacterial species belonging to both the α- and the ß-proteobacteria (Moulin et al. 2001; Broughton 2003) can induce root nodules on different legume species. The relationship between the approximately 18,000 legume species and their bacterial microsymbionts is nevertheless selective. L. japonicus can for example develop fully functional nitrogen fixing nodules with M. loti and ineffective or partially effective nodules with NGR234, Rhizobium etli and Bradyrhizobium spp. (Cardenas et al. 1995; Hussain et al. 1999; Banba et al. 2001; Schumpp et al. 2009; Bek et al. 2010). Traditionally these host/non-host relationships were described in the so-called cross-inoculation groups composed of legumes and their compatible rhizobia. However, this catalogue system is inconsistent and far from a perfect reflection of the host/non-host relationships as elucidated using molecular methods.
Ongoing efforts to fine-tune our understanding of host-microbiont specificity and the recognition process at the molecular level discovered a two-way signal exchange as one of the central components of recognition. Rhizobial NodD proteins mediate host recognition by interacting with specific flavonoids or isoflavonoids secreted from host roots (Peters et al. 1986; Spaink et al. 1989). Flavonoid-activated NodD promotes transcription of bacterial nod-genes involved in synthesis and secretion of lipochitin-oligosaccharides (Nod-factors). These molecules serve as major bacterial signals detected by the legume host (Mulligan and Long 1985; Spaink et al. 1991; Truchet et al. 1991). This signalling mechanism was elegantly demonstrated using two bacteria, R. etli and M. loti, from different cross-inoculation groups nodulating bean and Lotus, respectively. Both strains synthesise Nod-factors with the same structure. Artificially bypassing the flavonoid NodD activation by expression of a constitutive nodD transcriptional activator (FITA) in these rhizobial strains extended their host range beyond their cross-inoculation group to encompass both bean and Lotus corniculatus (Cardenas et al. 1995).
3 Nod-Factor Perception and Its Role in Host Determination
In legumes, the lipochitin-oligosaccharide Nod-factors of compatible bacteria initiate a signal cascade leading to nodulin gene activation, root cortical cell dedifferentiation and development of the root nodule (Spaink et al. 1991; Truchet et al. 1991; Hoegslund et al. 2009). The length of the Nod-factor carbohydrate moiety, the size and degree of saturation of the acyl chain and substitutions of the reducing and non-reducing glucosamine residues are characteristic for each rhizobial species. It is these structural features that determine whether the Nod-factor is perceived by the plant (Lerouge et al. 1990; Spaink et al. 1991; Truchet et al. 1991). Mesorhizobium loti strain R7A produces Nod-factors with pentameric GlcNAc backbones. At the reducing end moiety there is a 4-O-fucose with either an acetyl group or a proton in position 3 or 4. The nonreducing moiety is N-methylated and N-acylated (cis-vaccenic acid or stearic acid), and a carbamoyl group is present in position 3 (Lopez-Lara et al. 1995; Bek et al. 2010). Phenotypic characterisation of mutants of M. loti strain R7A show an unchanged infection process and nodule formation in the absence of the N-Methyl and the carbamoyl substitutions. In contrast, the presence of the N-acetylated fatty acid was found to be crucial for nodulation. The acetylated fucosyl group was important for effective Nod-factor signalling and absence of this group led to host dependent nodulation phenotypes when comparing the four Lotus species L. japonicus, L. filicaulis, L. corniculatus and L. burttii (Rodpothong et al. 2009).
Perception of Nod-factor in L. japonicus is mediated by receptor kinases containing LysM modules in their extracellular domains. The two receptor kinases perceiving the Nod-factor signal, NFR1 and NFR5, are predicted to have a topology where single pass transmembrane domains anchor LysM containing extracellular domains and intracellular serine/threonine protein kinases (Madsen et al. 2003; Radutoiu et al. 2003). Combining this prediction with genetic evidence, a heteromeric receptor complex composed of NFR1 and NFR5 was proposed to initiate signal transduction following perception of a correctly decorated Nod-factor (Radutoiu et al. 2003). Biochemical studies have subsequently demonstrated NFR1 to be a dual specificity kinase capable of autophosphorylation and phosphorylation of NFR5 (Madsen et al. 2011). In contrast, no kinase activity could be detected for NFR5, which has domain features characteristic for pseudokinases. Membrane localisation and interaction between NFR1 and NFR5 was shown in vitro by enrichment in purified membrane fractions and in vivo through bimolecular fluorescence complementation (BiFC) in Nicotiana benthamiana and leek cells (Madsen et al. 2011).
The structural similarity between Nod-factors and chitin, a carbohydrate synthesised by different plant pathogens such as fungi, nematodes or insects, raise the possibility of an evolutionary link between chitin and Nod-factor perception, and thus between symbiotic and pathogenetic interactions. In support of this hypothesis, the Arabidopsis chitin receptor CERK1, which was shown to be involved in the perception of fungal pathogens (Miya et al. 2007; Wan et al. 2008), shows high sequence and structural similarity to the NFR1 protein (Radutoiu et al. 2003; Miya et al. 2007; Wan et al. 2008). Subsequent functional studies of the kinase domains further supported this close relationship. Exchange of the NFR1 kinase domain with the corresponding CERK domain and substitution of a portion of the αEF helix in CERK1 with the YAQ amino acid sequence from NFR1 reconstituted symbiotic signalling. Transgenic L. japonicus nfr1 mutant plants carrying this swap construct were functionally complemented and able to develop nodules with M. loti (Nakagawa et al. 2011).
A deeper understanding of the host/non-host relationship between rhizobia and legumes was obtained by transforming the L. japonicus NFR1 and NFR5 genes into M. truncatula. Using this approach it was shown that NFR1 and NFR5 receptors act in concert as host determinants, transforming the non-host M. truncatula into a host able to recognise and be infected by M. loti and an engineered Rhizobium leguminosarum DZL producing a Nod-factor substituted with an acetylated fucosyl on the reducing moiety (Radutoiu et al. 2007). Recognition of these normally non-compatible bacteria triggers root cell dedifferentiation, redifferentiation and initiation of nodule organogenesis as well as infection thread formation. This extended NFR1 and NFR5 mediated signal cascade is dependent on both Nod-factor synthesis, as shown with the M. loti nodC mutant, and structure as shown by the longer infection threads obtained with R. leguminosarum DZL, respectively. In line with these results it was shown that the L. japonicus NFR1 and NFR5 proteins also act in concert if introduced into Lotus filicaulis. Transgenic roots of this species expressing the L. japonicus receptor molecules can be nodulated by the R. leguminosarum DZL strain, which can nodulate L. japonicus, but is otherwise unable to nodulate wild type L. filicaulis roots (Radutoiu et al. 2007).
4 The Role of LysM Domains
Our understanding of the mechanisms involved in Nod-factor perception was further refined through domain swap experiments using receptors from L. japonicus and L. filicaulis. Initially it was observed that L. filicaulis, in contrast to L. japonicus, did not develop root nodules after inoculation with the R. leguminosarum DZL strain (Pacios-Bras 2003). Analysis of transgenic plants subsequently traced this inability back to variations in the amino acid composition of the NFR1 or NFR5 receptors between L. japonicus and L. filicaulis (Radutoiu et al. 2007). Domain swaps combined with substitutions of single amino acids were then used to show that specific recognition of the DZL Nod-factor relied on the LysM containing domains of NFR1 and NFR5 and that the LysM2 domain of NFR5 played a major role in discriminating M. loti and R. leguminosarum DZL Nod-factors in L. filicaulis. A leucine adjacent to a putative Nod-factor binding groove located between the first β-strand and first helix of the L. japonicus NFR5 LysM2 domain, a position filled by a lysine in L. filicaulis, was found to be largely responsible for the recognition of the Nod-factor synthesised by the DZL strain (Radutoiu et al. 2007). However, presence of three LysM domains in the NFR5 and NFR1 receptors (Madsen et al. 2003; Radutoiu et al. 2003; Arrighi et al. 2006), suggests the involvement of more than one LysM domain in Nod-factor perception. Two lines of evidence supports this notion: (1) the non-nodulation phenotype caused by an amino acid substitution in the LysM1 domain of the M. trunctula homolog of NFR5 called NFP (Arrighi et al. 2006) and (2) the involvement of LysM1 of the pea SYM37 NFR1-like receptor in distinguishing “European” and “Middle East” R. leguminosarum bv. viciae strains (Zhukov et al. 2008).
Further insight into the functional role of individual amino acids in Nod-factor perception was obtained in a domain swap study using the extracellular domains of NFR1 and NFR5 from a more distantly related species, Lotus pedunculatus. This Lotus species is normally nodulated by a Bradyrhizobium spp. strain producing a Nod-factor with an additional carbamoyl group at the non-reducing moiety (Bek et al. 2010). It was found that the combined amino acid differences of the NFR1 and NFR5 extracellular domains of L. japonicus and L. pedunculatus were not influencing the recognition of the Nod-factor substituted with one or two carbamoyls at the non-reducing end. Considering that a high number of amino acid variations is found between L. japonicus and L. pedunculatus NFR proteins, this suggests the involvement of only a small fraction of amino acids in deciphering Nod-factor structure.
5 Signal Transduction and Signalling Triggered by the NFR Receptors
In L. japonicus signal transduction following NFR-mediated Nod-factor perception is shared with the arbuscular mycorrhizal symbiosis. Common symbiotic signalling components include at least eight genes encoding the leucine-rich repeat RLK SYMRK (Stracke et al. 2002), the cation channels CASTOR and POLLUX (Imaizumi-Anraku et al. 2005), the nuclear pore proteins NUP133 (Kanamori et al. 2006), NUP85 (Saito et al. 2007) and NENA (Groth et al. 2010), the Ca2+/calmodulin dependent kinase CCaMK (Tirichine et al. 2006) and the nuclear protein CYCLOPS (Yano et al. 2008). Analysis of L. japonicus mutants has shown that the LysM containing Nod-factor receptor(s), SYMRK, CASTOR and POLLUX, and the nuclear pore proteins are required for the induction of calcium oscillations, one of the earliest physiological responses detectable in root hairs after exposure to purified Nod-factor (Miwa et al. 2006; Saito et al. 2007). These calcium oscillations are thought to be interpreted by the CCaMK encoded Ca2+/calmodulin dependent protein kinase (Miwa et al. 2006; Tirichine et al. 2006). A function in cross signalling between infection and organogenesis was proposed for CYCLOPS (Madsen et al. 2010), a direct interactor of CCaMK (Messinese et al. 2007; Yano et al. 2008). Downstream of the shared symbiosis pathway, putative transcription factors encoded by Nin (Schauser et al. 1999), Nsp1 and Nsp2 (Heckmann et al. 2006), and a member of the ERF family (Asamizu et al. 2008; Middleton et al. 2007) are required for regulation of nodule expressed genes and initiation of nodule organogenesis. One of these transcripion factors, NSP2, has been proposed to also contribute to arbuscular mycorrhizal symbiosis in M. truncatula (Maillet et al. 2011). This implies the interesting possibility that common symbiotic signalling could include the level of transcription-factor mediated downstream gene activation.
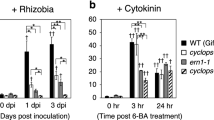
Formation of nodule primordia involves dedifferentiation and reactivation of cortical root cells. A combination of gain and loss of function mutants have shown that cytokinin signalling through the Lhk1 cytokinin receptor is necessary and sufficient for the formation of nodule primordia (Murray et al. 2007; Tirichine et al. 2007). The phenotype of loss of function mutants (hit1) includes a drastic reduction in the number of primordia initiated, suggesting that cytokinin signalling is important for activation of the cell cycle in inner cortical foci that form the starting point of nodule organogenesis (Murray et al. 2007). This phenotype complies with the detection of increased expression of a cytokinin reporter gene in the early phases of nodulation (Lohar et al. 2004). In the dominant snf2 mutants encoding a gain of function LHK1 cytokinin receptor, the opposite phenotype is observed. snf2 mutants develop root nodules spontaneously, i.e. in the absence of any rhizobia or Nod-factor signalling (Tirichine et al. 2007). This demonstrates that cytokinin signalling can induce the nodule meristem directly, presumably by activation of the cell cycle in distinct cell foci along the root. Both of these Lhk1 mutants therefore affect the developmental events in the root cortex, suggesting that cytokinin is a component of the secondary epidermal-cortical signalling triggered by Nod-factor perception. The epidermal developments like root hair deformation and infection thread formation appear to be mainly indirectly affected by cytokinin signalling. In hit1 loss of function mutants, hyperinfection in the form of an increased number of root hair infection threads are observed, and these infection threads are restricted in their progression into the cortex (Murray et al. 2007). This suggests that nodule primordia are involved in regulating infection thread formation through a negatively acting mechanism, and are necessary for their controlled progression into the inner cortex. The disruption of infection thread progression into the cortex in the hit1 loss of function mutant further indicates a positive role for cytokinin signalling. Cytokinin is thus a candidate signal for the coordination of epidermal and cortical processes of infection and primordium formation, respectively.
6 Cell to Cell Recognition Mechanisms Regulate Bacterial Entry
Early investigations suggested plant lectin binding of bacterial specific extracellular carbohydrates to be the major determinants of plant-rhizobium host range (Bohlool and Schmidt 1974). Subsequent experiments demonstrated binding of legume lectins to rhizobial surface polysaccharides (Bourne et al. 1994), and indicated that lectins facilitate rhizobial attachment to the root hair tips by binding surface polysaccharides and thereby increasing the local Nod-factor concentration above the required threshold for nodule initiation (van Rhijn et al. 2001). Studies performed with bacterial mutants showed that lectin mediated recognition is particularly important for infection thread progression through cortical cell layers (van Rhijn et al. 1998). Rhizobial host range extension as a result of lectin gene transfer from pea or soybean into clover, alfalfa or L. corniculatus appears also in most cases to be dependent on the Nod-factor structure and/or bacterial exopolysaccharides (van Rhijn et al. 1998, 2001). The mechanism and contribution of plant lectins to the infection process is therefore awaiting further investigation. For a recent review summarising the role of lectins in plant-microbe interactions see De Hoff et al. (2009).
The precise role of the different rhizobial surface polysaccharides for symbiotic partner recognition of bacteria and/or for the complete infection of the plant root tissues is on the other hand, well documented through genetic analysis of rhizobia. cgs mutants of M. loti R7A or strain Ayac 1 BII that are deficient in the synthesis of cyclic ß-glucan induced the formation of empty nodules on L. japonicus (Kelly, personal communication; D’Antuono et al. 2005, 2008). Infection thread development was impaired indicating a role for cyclic ß-glucan during infection thread formation or progression. The lipopolysaccharide (LPS) deficient lps mutants of M. loti tested on L. japonicus so far were only marginally affected in establishing symbiosis, and the phenotypes observed are mainly associated with strain competitiveness (Kelly, personal communication; D’Antuono et al. 2008). Further studies are required in order to determine the influence of LPS on symbiosis in L. japonicus, which in light of the recent report of nitric oxide (NO) release in response to purified LPS may be complex (Murakami et al. 2011). Exopolysaccharides (EPS) appear to be more important for infection of L. japonicus. Nodulation of L. japonicus by eps mutant strains defective in genes that are involved at mid-late stages of EPS biosynthesis (exoU, exoO, exoK and mlr5265) was severely affected. These mutant bacteria induced the formation of small white bumps devoid of bacteria. However, an EPS mutant strain (exoB) disrupted in a very early stage of EPS biosynthesis forms nitrogen-fixing nodules indistinguishable from those induced by wild-type M. loti. Visualisation of the exoU mutant bacteria during the infection process revealed that the EPS-deficient strain was disrupted in its ability to induce normal infection thread formation. The host plant may therefore be able to decipher the EPS structure presented by the invading bacteria most likely through a specialised EPS receptor (Kelly, personal communication). A specific role for bacterial EPS is further supported by an earlier study showing that several eps mutant strains of M. loti strain PN184 lose compatibility with the host Leucaena leucocephala, while retaining full compatibility with a second host, L. pedunculatus (Hotter and Scott 1991).
Strains of M. loti possess either type III or type IV (e.g. MAFF 303099 and R7A, respectively) secretion systems that in several cases have been shown to deliver effector proteins into cells of eukaryotic hosts and to be required for virulence of many Gram-negative bacterial pathogens. In the M. loti strain R7A, expression of genes encoding components of the type IV secretion system is specifically regulated in a symbiosis-dependent manner and linked to the presence of the host (Hubber et al. 2004, 2007). Inactivation of the type III secretion system in the M. loti strain MAFF affects symbiosis neutrally, positively or negatively dependent on the Lotus species tested (Sanchez et al. 2009; Okazaki et al. 2010). L. japonicus was unaffected, while L. filicaulis and L. coniculatus subsp. frondosus formed fewer root nodules in the absence of the secretion system. In contrast, there was a strong negative effect on infection of L. halophilus, which appears capable of detecting a small secreted bacterial effector protein and halting symbiosis development in response (Okazaki et al. 2010). Mutation of the gene encoding this protein reverts the phenotype to fully functional root nodules. Drastic phenotypic changes resulting from mutation of the type III and IV secretion system were found on the alternative host L. leucocephala (Hubber et al. 2004, 2007; Okazaki et al. 2010). Both the R7A and MAFF wild type strains of M. loti induced tumour-like nodules on this host. Inactivation of both the type III and IV protein effector secretion systems in R7A and MAFF lead to the development of fully infected and functional root nodules. Recognition of effector proteins resulting in either suppression of defence responses or induction of the defence response following detection of secreted proteins as pathogen effectors could explain these opposite responses (Hubber et al. 2004, 2007). Interestingly, a gene encoding a Toll-interleukin leucine-rich repeat receptor (TIR-NBS-LRR) suggested to recognise a rhizobial protein effector was identified in soybean (Yang et al. 2010).
7 Invasion Through Root Hair Infection Threads
In L. japonicus the normal infection occurs through infection threads initiated in elongating root hairs of the susceptible region located just behind the root tip. Upon formation of infection pockets, the root hair cell wall dissolves and an infection thread is initiated by invagination and subsequent polar extension of the plasma membrane, which is accompanied by the deposition of new cell wall material (see Gage (2004) for a review). Inward growing infection threads progress through the root hair and are propagated through the cell layers by a reiterated cell autonomous mechanism. Such infection threads are only formed in the presence of rhizobial bacteria. Nod-factor alone elicits the earliest detectable responses such as membrane depolarisation, ion fluxes across the membrane, Ca2+ spiking and cellular alteration in actin and microtubule organisation, but not infection threads (Weerasinghe et al. 2003, 2005; Vassileva et al. 2005; Miwa et al. 2006). Interestingly, a class of mutants characterised by a lack of or reduced infection thread progression have been identified (Schauser et al. 1998; Lombardo et al. 2006; Yano et al. 2006; Murray et al. 2007). In these mutant lines the infection threads were typically arrested either within root hairs/epidermis or within the first cortical cell layers. Furthermore, mutant lines were also identified where infection thread formation was not followed by the release of bacteria into cells of the nodule primordium (Imaizumi-Anraku et al. 1997).
Mutant lines known to affect infection thread formation include nin (Schauser et al. 1999), nsp1 and nsp2 (Heckmann et al. 2006), cerberus (Yano et al. 2009), nap1 and pir1 (Yokota et al. 2009), alb1-1 (Imaizumi-Anraku et al. 2000), crinkle (Tansengco et al. 2003), as well as itd1, itd3 and itd4 (Lombardo et al. 2006). Around half of these have been characterised. A functional Nin gene is required for infection thread initiation and for restricting root hair deformation, as well as restricting the size of the infection zone. The domain structure of the NIN protein suggests a function as transcriptional regulator, and the mutant phenotype demonstrates an essential role for the protein in both infection thread formation and organ initiation (Schauser et al. 1999). The phenotype and the transcripts affected in this mutant background also suggest the involvement of NIN in both positive and negative regulation and coordination of infection and organogenesis (Schauser et al. 1999; Hoegslund et al. 2009). Like NIN, NSP1 and NSP2 appear to be involved in activating the gene expression required for infection thread formation. The L. japonicus gene set controlled by these regulators has so far not been identified, but detailed analysis of the putative M. truncatula orthologs of NSP1 and NSP2 suggests that they act as a transcription factor complex activating several downstream genes, including NIN and the symbiosis-induced gene ENOD11 (Hirsch et al. 2009). L. japonicus plants carrying a mutant nap1 or pir1 allele had a significantly diminished ability to capture bacteria within infection pockets and initiating infection threads (Yokota et al. 2009). The rare infection threads that formed disintegrated, and infection threads extending to the base of the root hair cells were only occasionally observed. Lack of infection threads in the root cortex further suggested that NAP1 and PIR1 were essential for the progression of the infection process. Further characterisation showed that the Nap1 and Pir1 genes are essential for establishing the actin organisation in root hairs (Yokota et al. 2009). Molecular analysis revealed the putative Arabidopsis orthologs of these proteins, NAP1 and PIR1, as likely components of the SCAR/WAVE complex that activates the ARP2/3 complex, which binds pre-existing actin polymers and nucleates new actin filaments (Brembu et al. 2004; Deeks et al. 2004; Li et al. 2004). The role of the Cerberus encoded ubiquitin-E3-ligase for infection thread formation is less clear, but its requirement suggests that there is a need for clearing the root hair of particular proteins in order to secure infection thread progression (Yano et al. 2009). A membrane raft associated remorin belonging to a subgroup found so far only in plants of the Rosid I clade, which all nodulators are part of, is upregulated during nodulation (Lefebvre et al. 2010). In M. truncatula this remorin interacts with symbiotic receptors and localises to infection thread- and symbiosome-membranes (Lefebvre et al. 2010). Functionally the remorin was linked to a role in bacterial release and endocytosis, and symbiosis was impaired in remorin mutant plants. So far remorin mutants have not been isolated in L. japonicus, but it will be interesting to investigate the role of this putative membrane anchor for the NFR receptors in determinate nodulation. Similar roles have been suggested for flotillins (Haney and Long 2010) and Rab proteins (Limpens et al. 2009) within indeterminate nodules of M. truncatula. Different Rab protein encoding mRNAs have been shown to be upregulated in L. japonicus nodules (Borg et al. 1997), and analysis of these, as well as of L. japonicus homologs of remorins and flotillins may further contribute to a better understanding of infection during determinate root nodule formation.
8 Parallel Signalling Pathways Regulate Root Hair Infection and Nodule Development
Bacterial infection of legume root nodule primordia is tightly controlled and closely synchronised with the progressing primordial cell divisions and organ development. This coordination has long prevented the separation of the molecular mechanisms underlying these two developmental processes and has limited the identification of plant genes controlling the bacterial infection process, but not nodule organogenesis. As a consequence the genetics of infection thread development is fairly undescribed and among the genes defining this pathway, only Nap1, Pir1 and Cerberus have so far been characterised at the molecular level (Yano et al. 2009; Yokota et al. 2009). Most of the Nod-factor signal transduction pathway mutants mentioned above suffer from simultaneous absence or severe impairment of both organ formation and infection thread development. This has made the dissection of direct and indirect mutational impact on the two processes very difficult. However, combining gain of function mutations with loss of function mutations in trangenes or double, triple and quadruple mutants opened new possibilities for assessing the role of the genes inactivated by loss of function mutations (Hayashi et al. 2010; Madsen et al. 2010). The autoactive versions of CCaMK and LHK1 encoded by the snf1 or snf2 alleles, respectively, were key to this approach. Both snf mutants form spontaneous nodules independent of bacterial presence or infection (Tirichine et al. 2006, 2007). Exploiting the ability of these gain of function alleles to activate the developmental processes from downstream positions defined the backbones of two parallel pathways facilitating (1) infection thread formation and (2) root nodule organogenesis (Hayashi et al. 2010; Madsen et al. 2010). Furthermore, the approach revealed cross-signalling functions between these two pathways for some of the examined genes. It was shown that the LRR receptor kinase SYMRK, the nucleoporins NUP133 and NUP85 and the cation channels CASTOR and POLLUX, all involved in signal transduction down-stream of Nod-factor perception, were dispensable for root hair infection thread development and invasion of nodule primordia in a snf1 genetic background. Since inactivation of the LRR receptor kinase SYMRK, the nucleoporins and the cation channels results in absence of calcium spiking (Miwa et al. 2006), these results further indicate that calcium spiking, apart from activation of CCaMK, is dispensable for infection thread formation (Madsen et al. 2010).
On the other hand, the NFR1 and NFR5 Nod-factor receptors were required for root hair infection thread initiation, and the NAP1 and PIR1 proteins mediating actin rearrangement together with the CERBERUS ubiquitin E3 ligase were required for infection thread progression (Madsen et al. 2010). These observations support an early branching of the Nod-factor signal transduction pathway. The NIN, NSP1 and NSP2 transcriptional regulators were required for both infection thread formation and organogenesis, supporting a simultaneous or sequential role of these proteins in both of these processes. The absence of infection in cyclops snf1 double mutants (Madsen et al. 2010) along with the reported protein-protein interaction between CCaMK and CYCLOPS (Messinese et al. 2007; Yano et al. 2008), indicated a role for CYCLOPS in cross signalling between the organogenic and infection pathways (Madsen et al. 2010).
Nod-factor induced root hair deformation, Ca2+ influx and Ca2+ spiking preceding infection thread formation all require the initial NFR1 and NFR5 mediated perception event (Radutoiu et al. 2003; Miwa et al. 2006). The relation between these phenomena is however not yet fully clarified. Ca2+ spiking following application of Nod-factors is not required for root hair deformation (Miwa et al. 2006) and the presumed Ca2+ dependent activation of CCaMK as mimicked by the snf1 mutation seems insufficient for root hair infection thread formation (Madsen et al. 2010). Separation of root hair deformation and influx was also implied by the observation that three orders of magnitude lower Nod-factor concentration was sufficient to induce root hair deformation compared with that required for the Ca2+ influx (Radutoiu et al. 2003; Miwa et al. 2006). This could suggest that the NFR receptor dependent Ca2+ influx observed in root hairs at high Nod-factor concentration may be a prerequisite for root hair infection thread formation. So far these observations are consistent with the Ca2+ responses occurring in parallel with the NAP1 and PIR1 induced cytoskeletal changes, although the possibility of a low level or localised change in Ca2+ cannot be excluded.
9 Alternative Infection Mechanisms: Crack Entry
Microscopical surveys of infection in legume species representing different clades of the legume family have revealed the existence of a number of alternative infection modes leading to nitrogen fixing root nodules (recently reviewed in Held et al. (2010)). However, with only a few exceptions like Sesbania rostrata (Goormachtig et al. 2004), generally only one infection mode is found in an individual species. L. japonicus is, for example, infected via intracellular root hair infection threads (Szczyglowski et al. 1998; Schauser et al. 1999; Lombardo et al. 2006), while peanut is infected via an intercellular crack entry process (Fabra et al. 2010). One of the surprising outcomes of the experiments combining gain of function and loss of function mutations was the discovery of two additional, although less effective, intercellular infection modes in L. japonicus (Madsen et al. 2010). Loss-of-function alleles of particular genes also indicated the presence of a crack entry based infection mode (Karas et al. 2005; Groth et al. 2010), and mutational abrogation of both root hair and cortical infection thread formation revealed a further mode of infection of presumably more ancient evolutionary origin (Madsen et al. 2010).
A mechanism enabling intercellular infection and formation of trans-cellular infection threads within the root nodules was found to operate in the absence of functional NFR1 and NFR5 receptors. Despite the independence from these proteins known to be required for Nod-factor perception at early stages, the formation of trans-cellular infection threads in nodules did depend on bacterial production of intact Nod-factors, and nodC mutants of M. loti could not induce their development (Madsen et al. 2010). It is yet unclear which molecules are involved in the evident Nod-factor perception in the root cortex. Receptor kinases belonging to the L. japonicus LysM receptor-like kinase (Lys) family, which includes NFR1 and NFR5, are possible candidates (Lohmann et al. 2010). Of these, Lys genes that are expressed predominantly in root and nodule tissues, namely Lys2, Lys3, Lys7, Lys12, Lys15 and Lys20 (Lohmann et al. 2010), are the most likely to be involved. A role of alternative epidermal or cortical Nod-factor receptors is further supported by observations in the tropical legume S. rostrata. Root hair infection was strictly dependent on an intact Nod-factor structure, while root-hair independent crack entry at lateral root bases was less stringently controlled in this species (D’Haeze et al. 2000; Goormachtig et al. 2004).
The downstream components involved in signalling upon Nod-factor perception by presumed cortical receptors are unknown. The complete absence of infection in cyclops snf1 double mutants suggests that CCaMK activation followed by signalling through a CCaMK CYCLOPS complex is required for cortical infection thread formation (Madsen et al. 2010). In contrast, infection studies in recently identified mutants of the L. japonicus nucleoporin NENA suggest that crack entry and cortical infection thread formation can occur independent of CCaMK activation through Ca2+ spiking, or alternatively rely on rare activation events (Groth et al. 2010). In line with the former interpretation, crack infection of the outer cortex was retained in CCaMK knockdown-roots of Sesbania despite the loss of root hair infection and nodulation (Capoen et al. 2009).
10 Alternative Infection Mechanisms: Single Cell Peg Entry
Characterisation of synthetic mutants combining gain of function (snf1) and loss of function mutations lead to a remarkable and unexpected discovery of an infection thread independent single cell entry mechanism in L. japonicus (Madsen et al. 2010). This intracellular infection of individual host cells by a process best described as peg entry may constitute the ground state of bacterial invasion during evolution of nodulation, and may allow for an analysis of the cardinal requirements distinguishing endosymbiotic co-existence from pathogenesis. Surprisingly, this entry mode was independent of both the NFR1 and NFR5 plant receptors and the rhizobial Nod-factor signal molecule (Madsen et al. 2010). In a snf1 gain of function genetic background, a bacterial nodC mutant unable to produce Nod-factors was capable of infecting nfr1 nfr5 single and double mutants although at a 20–100 fold reduced efficiency compared to normal root hair invasion. Infection threads were not observed, but symbiosomes were present in the infected cells, indicating that intercellular infection followed by endocytosis occurred in the absence of Nod-factor and the NFR1 and NFR5 Nod-factor receptors (Madsen et al. 2010). Such a capacity for direct intercellular infection could constitute the ancient invasion path that evolved at the emergence of the legume family or possibly at the emergence of the eurosid I clade containing all nodulating plants. Continued division of the primary infected single cells would lead to the fully infected Nod-factor independent nodulation observed in Aeschynomene type nodules (Sprent 2007). Nod-factor independent stem nodulation of Aeschynomene species (Giraud et al. 2007) appears to be a rare exception, however, and in many legumes the subsequent evolutionary step may have been the Nod-factor and cortical Nod-factor receptor dependent crack entry and subsequent cortical infection thread propagation of invasion as seen in S. rostrata (D’Haeze et al. 2000). The Nod-factor dependent crack entry without infection threads and the fully infected nodules in Arachis and Stylosanthes (Noti et al. 1985; Wilson et al. 1987; Boogerd and van Rossum 1997) can be considered a variant of this infection mode. Fixation thread symbiosis, where rhizobia are not released from the infection thread, as seen in many legume trees and in Parasponia (Trinick 1979; Sprent 2007), is another variant. Interestingly, the Gram-positive Frankia bacteria inducing actinorhizal symbiosis in non-legumes are contained in fixation threads comparable to those seen in legumes belonging to the oldest legume subfamily, the Caesalpinioideae (Pawlowski and Bisseling 1996). Bacterial invasion via crack entry followed by the formation of either fixation threads or infection threads with bacterial release into symbiosomes in different members of the caesalpinoid genus Chamaecrista, support the notion of crack entry as a basal feature (Naisbitt et al. 1992; Sprent 2007). The most highly evolved state suggested by the differential signalling and genetic requirements of entry modes revealed in L. japonicus is root hair infection. This infection mode required Nod-factor, epidermal NFR1 and NFR5 receptors, and is predicted to also involve cortical Nod-factor receptors with less stringency than NFR1 and NFR5. Overall this model predicts an evolutionary process leading from pathogen-like bacterial invasion between cells to the more intimate containment of – and coexistence with – symbiosomes inside plant cells.
Taken together, the advanced steps of bacterial release seen in the more recently evolved legume hosts containing symbiosomes inside their cells have provided these plants with a tighter and more selective control of bacterial proliferation and nutrient exchange within the host. Similarly, the root hair infection mode seen in many evolutionarily young legume groups allows for a tighter control of bacterial passage through the epidermis than crack entry mechanisms. Observations of infection modes in a wide selection of legumes belonging to different subfamilies also indicate that alternative invasion modes have been maintained during evolution and that they are not mutually exclusive. Presence of different infection modes in Sesbania (Goormachtig et al. 2004), Chamaecrista (Sprent 2007) and both a Nod-factor dependent and a Nod-factor independent mechanism for nodulation in Aeschynomene sensitiva (Giraud et al. 2007) is in accordance with this scenario. Further comparison of the infection thread and direct infection pathway(s) seems to provide a prime opportunity to assess the basal genetic components involved in the release and endocytosis of bacteria.
References
Arrighi JF, Barre A, Ben Amor B, Bersoult A, Soriano LC, Mirabella R, de Carvalho-Niebel F, Journet EP, Gherardi M, Huguet T, Geurts R, Denarie J, Rouge P, Gough C (2006) The Medicago truncatula lysin motif-receptor-like kinase gene family includes NFP and new nodule-expressed genes. Plant Physiol 142:265–279
Asamizu E, Shimoda Y, Kouchi H, Tabata S, Sato S (2008) A positive regulatory role for LjERF1 in the nodulation process is revealed by systematic analysis of nodule-associated transcription factors of Lotus japonicus. Plant Physiol 147:2030–2040
Banba M, Siddique AB, Kouchi H, Izui K, Hata S (2001) Lotus japonicus forms early senescent root nodules with Rhizobium etli. Mol Plant Microbe Interact 14:173–180
Bek AS, Sauer J, Thygesen MB, Duus JO, Petersen BO, Thirup S, James E, Jensen KJ, Stougaard J, Radutoiu S (2010) Improved characterization of nod factors and genetically based variation in LysM Receptor domains identify amino acids expendable for nod factor recognition in Lotus spp. Mol Plant Microbe Interact 23:58–66
Bohlool BB, Schmidt EL (1974) Lectins: a possible basis for specificity in the Rhizobium-legume root nodule symbiosis. Science 185:269–271
Boogerd FC, van Rossum D (1997) Nodulation of groundnut by Bradyrhizobium: a simple infection process by crack entry. FEMS Microbiol Rev 21:5–27
Borg S, Brandstrup B, Jensen TJ, Poulsen C (1997) Identification of new protein species among 33 different small GTP-binding proteins encoded by cDNAs from Lotus japonicus, and expression of corresponding mRNAs in developing root nodules. Plant J 11:237–250
Bourne Y, Ayouba A, Rouge P, Cambillau C (1994) Interaction of a legume lectin with two components of the bacterial cell wall. A crystallographic study. J Biol Chem 269:9429–9435
Brembu T, Winge P, Seem M, Bones AM (2004) NAPP and PIRP encode subunits of a putative wave regulatory protein complex involved in plant cell morphogenesis. Plant Cell 16:2335–2349
Broughton WJ (2003) Roses by other names: taxonomy of the Rhizobiaceae. J Bacteriol 185:2975–2979
Capoen W, Den Herder J, Sun J, Verplancke C, De Keyser A, De Rycke R, Goormachtig S, Oldroyd G, Holsters M (2009) Calcium spiking patterns and the role of the calcium/calmodulin-dependent kinase CCaMK in lateral root base nodulation of Sesbania rostrata. Plant Cell 21:1526–1540
Cardenas L, Dominguez J, Quinto C, Lopez-Lara IM, Lugtenberg BJ, Spaink HP, Rademaker GJ, Haverkamp J, Thomas-Oates JE (1995) Isolation, chemical structures and biological activity of the lipo-chitin oligosaccharide nodulation signals from Rhizobium etli. Plant Mol Biol 29:453–464
D’Antuono AL, Casabuono A, Couto A, Ugalde RA, Lepek VC (2005) Nodule development induced by Mesorhizobium loti mutant strains affected in polysaccharide synthesis. Mol Plant Microbe Interact 18:446–457
D’Antuono AL, Ott T, Krusell L, Voroshilova V, Ugalde RA, Udvardi M, Lepek VC (2008) Defects in rhizobial cyclic glucan and lipopolysaccharide synthesis alter legume gene expression during nodule development. Mol Plant Microbe Interact 21:50–60
De Hoff PL, Brill LM, Hirsch AM (2009) Plant lectins: the ties that bind in root symbiosis and plant defense. Mol Genet Genomics 282:1–15
Deeks MJ, Kaloriti D, Davies B, Malho R, Hussey PJ (2004) Arabidopsis NAP1 is essential for Arp2/3-dependent trichome morphogenesis. Curr Biol 14:1410–1414
D’Haeze W, Mergaert P, Prome JC, Holsters M (2000) Nod factor requirements for efficient stem and root nodulation of the tropical legume Sesbania rostrata. J Biol Chem 275:15676–15684
Fabra A, Castro S, Taurian T, Angelini J, Ibanez F, Dardanelli M, Tonelli M, Bianucci E, Valetti L (2010) Interaction among Arachis hypogaea L. (peanut) and beneficial soil microorganisms: how much is it known? Crit Rev Microbiol 36:179–194
Gage DJ (2004) Infection and invasion of roots by symbiotic, nitrogen-fixing rhizobia during nodulation of temperate legumes. Microbiol Mol Biol Rev 68:280–300
Giraud E, Moulin L, Vallenet D, Barbe V, Cytryn E, Avarre JC, Jaubert M, Simon D, Cartieaux F, Prin Y, Bena G, Hannibal L, Fardoux J, Kojadinovic M, Vuillet L, Lajus A, Cruveiller S, Rouy Z, Mangenot S, Segurens B, Dossat C, Franck WL, Chang WS, Saunders E, Bruce D, Richardson P, Normand P, Dreyfus B, Pignol D, Stacey G, Emerich D, Vermeglio A, Medigue C, Sadowsky M (2007) Legumes symbioses: absence of Nod genes in photosynthetic bradyrhizobia. Science 316:1307–1312
Goormachtig S, Capoen W, James E, Holsters M (2004) Switch from intracellular to intercellular invasion during water stress-tolerant legume nodulation. Proc Natl Acad Sci USA 101:6303–6308
Groth M, Takeda N, Perry J, Uchida H, Draxl S, Brachmann A, Sato S, Tabata S, Kawaguchi M, Wang TL, Parniske M (2010) NENA, a Lotus japonicus homolog of Sec13, is required for rhizodermal infection by arbuscular mycorrhiza fungi and rhizobia but dispensable for cortical endosymbiotic development. Plant Cell 22:2509–2526
Haney CH, Long SR (2010) Plant flotillins are required for infection by nitrogen-fixing bacteria. Proc Natl Acad Sci USA 107:478–483
Hayashi T, Banba M, Shimoda Y, Kouchi H, Hayashi M, Imaizumi-Anraku H (2010) A dominant function of CCaMK in intracellular accommodation of bacterial and fungal endosymbionts. Plant J 63:141–154
Heckmann AB, Lombardo F, Miwa H, Perry JA, Bunnewell S, Parniske M, Wang TL, Downie JA (2006) Lotus japonicus nodulation requires two GRAS domain regulators, one of which is functionally conserved in a non-legume. Plant Physiol 142:1739–1750
Held M, Hossain MS, Yokota K, Bonfante P, Stougaard J, Szczyglowski K (2010) Common and not so common symbiotic entry. Trends Plant Sci 15:540–545
Hirsch S, Kim J, Munoz A, Heckmann AB, Downie JA, Oldroyd GE (2009) GRAS proteins form a DNA binding complex to induce gene expression during nodulation signaling in Medicago truncatula. Plant Cell 21:545–557
Hoegslund N, Radutoiu S, Krusell L, Voroshilova V, Hannah MA, Goffard N, Sanchez DH, Lippold F, Ott T, Sato S, Tabata S, Liboriussen P, Lohmann GV, Schauser L, Weiller GF, Udvardi MK, Stougaard J (2009) Dissection of symbiosis and organ development by integrated transcriptome analysis of Lotus japonicus mutant and wild-type plants. PLoS One 4:e6556
Hotter GS, Scott DB (1991) Exopolysaccharide mutants of Rhizobium loti are fully effective on a determinate nodulating host but are ineffective on an indeterminate nodulating host. J Bacteriol 173:851–859
Hubber A, Vergunst AC, Sullivan JT, Hooykaas PJ, Ronson CW (2004) Symbiotic phenotypes and translocated effector proteins of the Mesorhizobium loti strain R7A VirB/D4 type IV secretion system. Mol Microbiol 54:561–574
Hubber AM, Sullivan JT, Ronson CW (2007) Symbiosis-induced cascade regulation of the Mesorhizobium loti R7A VirB/D4 type IV secretion system. Mol Plant Microbe Interact 20:255–261
Hussain AKMA, Jiang Q, Broughton WJ, Gresshoff PM (1999) Lotus japonicus nodulates and fixes nitrogen with the broad host range Rhizobium sp. NGR234. Plant Cell Physiol 40:894–899
Imaizumi-Anraku H, Kawaguchi M, Koiwa H, Akao S, Syono K (1997) Two ineffective-nodulating mutants of Lotus japonicus – different phenotypes caused by the blockage of endocytotic bacterial release and nodule maturation. Plant Cell Physiol 38:871–881
Imaizumi-Anraku H, Kouchi H, Syono K, Akao S, Kawaguchi M (2000) Analysis of ENOD40 expression in alb1, a symbiotic mutant of Lotus japonicus that forms empty nodules with incompletely developed nodule vascular bundles. Mol Gen Genet 264:402–410
Imaizumi-Anraku H, Takeda N, Charpentier M, Perry J, Miwa H, Umehara Y, Kouchi H, Murakami Y, Mulder L, Vickers K, Pike J, Downie JA, Wang T, Sato S, Asamizu E, Tabata S, Yoshikawa M, Murooka Y, Wu GJ, Kawaguchi M, Kawasaki S, Parniske M, Hayashi M (2005) Plastid proteins crucial for symbiotic fungal and bacterial entry into plant roots. Nature 433:527–531
Kanamori N, Madsen LH, Radutoiu S, Frantescu M, Quistgaard EM, Miwa H, Downie JA, James EK, Felle HH, Haaning LL, Jensen TH, Sato S, Nakamura Y, Tabata S, Sandal N, Stougaard J (2006) A nucleoporin is required for induction of Ca2+ spiking in legume nodule development and essential for rhizobial and fungal symbiosis. Proc Natl Acad Sci USA 103:359–364
Karas B, Murray J, Gorzelak M, Smith A, Sato S, Tabata S, Szczyglowski K (2005) Invasion of Lotus japonicus root hairless 1 by Mesorhizobium loti involves the nodulation factor-dependent induction of root hairs. Plant Physiol 137:1331–1344
Lefebvre B, Timmers T, Mbengue M, Moreau S, Herve C, Toth K, Bittencourt-Silvestre J, Klaus D, Deslandes L, Godiard L, Murray JD, Udvardi MK, Raffaele S, Mongrand S, Cullimore J, Gamas P, Niebel A, Ott T (2010) A remorin protein interacts with symbiotic receptors and regulates bacterial infection. Proc Natl Acad Sci USA 107:2343–2348
Lerouge P, Roche P, Faucher C, Maillet F, Truchet G, Prome JC, Denarie J (1990) Symbiotic host-specificity of Rhizobium meliloti is determined by a sulphated and acylated glucosamine oligosaccharide signal. Nature 344:781–784
Li Y, Sorefan K, Hemmann G, Bevan MW (2004) Arabidopsis NAP and PIR regulate actin-based cell morphogenesis and multiple developmental processes. Plant Physiol 136:3616–3627
Limpens E, Ivanov S, van Esse W, Voets G, Fedorova E, Bisseling T (2009) Medicago N2-fixing symbiosomes acquire the endocytic identity marker Rab7 but delay the acquisition of vacuolar identity. Plant Cell 21:2811–2828
Lohar DP, Schaff JE, Laskey JG, Kieber JJ, Bilyeu KD, Bird DM (2004) Cytokinins play opposite roles in lateral root formation, and nematode and Rhizobial symbioses. Plant J 38:203–214
Lohmann GV, Shimoda Y, Nielsen MW, Jorgensen FG, Grossmann C, Sandal N, Sorensen K, Thirup S, Madsen LH, Tabata S, Sato S, Stougaard J, Radutoiu S (2010) Evolution and regulation of the Lotus japonicus LysM receptor gene family. Mol Plant Microbe Interact 23:510–521
Lombardo F, Heckmann AB, Miwa H, Perry JA, Yano K, Hayashi M, Parniske M, Wang TL, Downie JA (2006) Identification of symbiotically defective mutants of Lotus japonicus affected in infection thread growth. Mol Plant Microbe Interact 19:1444–1450
Lopez-Lara IM, van den Berg JD, Thomas-Oates JE, Glushka J, Lugtenberg BJ, Spaink HP (1995) Structural identification of the lipo-chitin oligosaccharide nodulation signals of Rhizobium loti. Mol Microbiol 15:627–638
Madsen EB, Madsen LH, Radutoiu S, Olbryt M, Rakwalska M, Szczyglowski K, Sato S, Kaneko T, Tabata S, Sandal N, Stougaard J (2003) A receptor kinase gene of the LysM type is involved in legume perception of rhizobial signals. Nature 425:637–640
Madsen LH, Tirichine L, Jurkiewicz A, Sullivan JT, Heckmann AB, Bek AS, Ronson CW, James EK, Stougaard J (2010) The molecular network governing nodule organogenesis and infection in the model legume Lotus japonicus. Nat Commun 1:1–12
Madsen EB, Antolin-Llovera M, Grossmann C, Ye J, Vieweg S, Broghammer A, Krusell L, Radutoiu S, Jensen ON, Stougaard J, Parniske M (2011) Autophosphorylation is essential for the in vivo function of the Lotus japonicus Nod factor receptor 1 and receptor-mediated signalling in cooperation with Nod factor receptor 5. Plant J 65:404–417
Maillet F, Poinsot V, Andre O, Puech-Pages V, Haouy A, Gueunier M, Cromer L, Giraudet D, Formey D, Niebel A, Martinez EA, Driguez H, Becard G, Denarie J (2011) Fungal lipochitooligosaccharide symbiotic signals in arbuscular mycorrhiza. Nature 469:58–63
Messinese E, Mun JH, Yeun LH, Jayaraman D, Rouge P, Barre A, Lougnon G, Schornack S, Bono JJ, Cook DR, Ane JM (2007) A novel nuclear protein interacts with the symbiotic DMI3 calcium- and calmodulin-dependent protein kinase of Medicago truncatula. Mol Plant Microbe Interact 20:912–921
Middleton PH, Jakab J, Penmetsa RV, Starker CG, Doll J, Kalo P, Prabhu R, Marsh JF, Mitra RM, Kereszt A, Dudas B, VandenBosch K, Long SR, Cook DR, Kiss GB, Oldroyd GE (2007) An ERF transcription factor in Medicago truncatula that is essential for Nod factor signal transduction. Plant Cell 19:1221–1234
Miwa H, Sun J, Oldroyd GE, Downie JA (2006) Analysis of Nod-factor-induced calcium signaling in root hairs of symbiotically defective mutants of Lotus japonicus. Mol Plant Microbe Interact 19:914–923
Miya A, Albert P, Shinya T, Desaki Y, Ichimura K, Shirasu K, Narusaka Y, Kawakami N, Kaku H, Shibuya N (2007) CERK1, a LysM receptor kinase, is essential for chitin elicitor signaling in Arabidopsis. Proc Natl Acad Sci USA 104:19613–19618
Moulin L, Munive A, Dreyfus B, Boivin-Masson C (2001) Nodulation of legumes by members of the beta-subclass of Proteobacteria. Nature 411:948–950
Mulligan JT, Long SR (1985) Induction of Rhizobium meliloti nodC expression by plant exudate requires nodD. Proc Natl Acad Sci USA 82:6609–6613
Murakami EI, Nagata M, Shimoda Y, Kucho KI, Higashi S, Abe M, Hashimoto M, Uchiumi T (2011) Nitric oxide production induced in roots of Lotus japonicus by lipopolysaccharide from Mesorhizobium loti. Plant Cell Physiol 52:610–617
Murray JD, Karas BJ, Sato S, Tabata S, Amyot L, Szczyglowski K (2007) A cytokinin perception mutant colonized by Rhizobium in the absence of nodule organogenesis. Science 315:101–104
Naisbitt T, James EK, Sprent JI (1992) The evolutionary significance of the legume genus Chamaecrista, as determined by nodule structure. New Phytol 122:487–492
Nakagawa T, Kaku H, Shimoda Y, Sugiyama A, Shimamura M, Takanashi K, Yazaki K, Aoki T, Shibuya N, Kouchi H (2011) From defense to symbiosis: limited alterations in the kinase domain of LysM receptor-like kinases are crucial for evolution of legume-Rhizobium symbiosis. Plant J 65:169–180
Noti JD, Dudas B, Szalay AA (1985) Isolation and characterization of nodulation genes from Bradyrhizobium sp. (Vigna) strain IRc 78. Proc Natl Acad Sci USA 82:7379–7383
Okazaki S, Okabe S, Higashi M, Shimoda Y, Sato S, Tabata S, Hashiguchi M, Akashi R, Gottfert M, Saeki K (2010) Identification and functional analysis of type III effector proteins in Mesorhizobium loti. Mol Plant Microbe Interact 23:223–234
Oldroyd GED, Downie JA (2004) Calcium, kinases and nodulation signalling in legumes. Nat Rev Mol Cell Biol 5:566–576
Pacios-Bras C (2003). The symbiosis between Lotus japonicus and rhizobia: Function of nod factor structural variation. PhD Thesis, University of Leiden, Leiden, The Netherlands
Pawlowski K, Bisseling T (1996) Rhizobial and actinorhizal symbioses: what are the shared features? Plant Cell 8:1899–1913
Peters NK, Frost JW, Long SR (1986) A plant flavone, luteolin, induces expression of Rhizobium meliloti nodulation genes. Science 233:977–980
Radutoiu S, Madsen LH, Madsen EB, Felle HH, Umehara Y, Groenlund M, Sato S, Nakamura Y, Tabata S, Sandal N, Stougaard J (2003) Plant recognition of symbiotic bacteria requires two LysM receptor-like kinases. Nature 425:585–592
Radutoiu S, Madsen LH, Madsen EB, Jurkiewicz A, Fukai E, Quistgaard EM, Albrektsen AS, James EK, Thirup S, Stougaard J (2007) LysM domains mediate lipochitin-oligosaccharide recognition and Nfr genes extend the symbiotic host range. EMBO J 26:3923–3935
Rodpothong P, Sullivan JT, Songsrirote K, Sumpton D, Cheung KW, Thomas-Oates J, Radutoiu S, Stougaard J, Ronson CW (2009) Nodulation gene mutants of Mesorhizobium loti R7A-nodZ and nolL mutants have host-specific phenotypes on Lotus spp. Mol Plant Microbe Interact 22:1546–1554
Saito K, Yoshikawa M, Yano K, Miwa H, Uchida H, Asamizu E, Sato S, Tabata S, Imaizumi-Anraku H, Umehara Y, Kouchi H, Murooka Y, Szczyglowski K, Downie JA, Parniske M, Hayashi M, Kawaguchi M (2007) NUCLEOPORIN85 is required for calcium spiking, fungal and bacterial symbioses, and seed production in Lotus japonicus. Plant Cell 19:610–624
Sanchez C, Iannino F, Deakin WJ, Ugalde RA, Lepek VC (2009) Characterization of the Mesorhizobium loti MAFF303099 type-three protein secretion system. Mol Plant Microbe Interact 22:519–528
Schauser L, Handberg K, Sandal N, Stiller J, Thykjaer T, Pajuelo E, Nielsen A, Stougaard J (1998) Symbiotic mutants deficient in nodule establishment identified after T-DNA transformation of Lotus japonicus. Mol Gen Genet 259:414–423
Schauser L, Roussis A, Stiller J, Stougaard J (1999) A plant regulator controlling development of symbiotic root nodules. Nature 402:191–195
Schumpp O, Crevecoeur M, Broughton WJ, Deakin WJ (2009) Delayed maturation of nodules reduces symbiotic effectiveness of the Lotus japonicus-Rhizobium sp. NGR234 interaction. J Exp Bot 60:581–590
Spaink HP, Okker RJ, Wijffelman CA, Tak T, Goosen-de Roo L, Pees E, van Brussel AA, Lugtenberg BJ (1989) Symbiotic properties of rhizobia containing a flavonoid-independent hybrid nodD product. J Bacteriol 171:4045–4053
Spaink HP, Sheeley DM, van Brussel AA, Glushka J, York WS, Tak T, Geiger O, Kennedy EP, Reinhold VN, Lugtenberg BJ (1991) A novel highly unsaturated fatty acid moiety of lipo-oligosaccharide signals determines host specificity of Rhizobium. Nature 354:125–130
Sprent JI (2007) Evolving ideas of legume evolution and diversity: a taxonomic perspective on the occurrence of nodulation. New Phytol 174:11–25
Stracke S, Kistner C, Yoshida S, Mulder L, Sato S, Kaneko T, Tabata S, Sandal N, Stougaard J, Szczyglowski K, Parniske M (2002) A plant receptor-like kinase required for both bacterial and fungal symbiosis. Nature 417:959–962
Szczyglowski K, Shaw RS, Wopereis J, Copeland S, Hamburger D, Kasiborski B, Dazzo FB, de Bruijn FJ (1998) Nodule organogenesis and symbiotic mutants of the model legume Lotus japonicus. Mol Plant Microb Interact 11:684–697
Tansengco ML, Hayashi M, Kawaguchi M, Imaizumi-Anraku H, Murooka Y (2003) crinkle, a novel symbiotic mutant that affects the infection thread growth and alters the root hair, trichome, and seed development in Lotus japonicus. Plant Physiol 131:1054–1063
Tirichine L, Imaizumi-Anraku H, Yoshida S, Murakami Y, Madsen LH, Miwa H, Nakagawa T, Sandal N, Albrektsen AS, Kawaguchi M, Downie A, Sato S, Tabata S, Kouchi H, Parniske M, Kawasaki S, Stougaard J (2006) Deregulation of a Ca2+/calmodulin-dependent kinase leads to spontaneous nodule development. Nature 441:1153–1156
Tirichine L, Sandal N, Madsen LH, Radutoiu S, Albrektsen AS, Sato S, Asamizu E, Tabata S, Stougaard J (2007) A gain-of-function mutation in a cytokinin receptor triggers spontaneous root nodule organogenesis. Science 315:104–107
Trinick MJ (1979) Structure of nitrogen-fixing nodules formed by Rhizobium on roots of Parasponia andersonii Planch. Can J Microbiol 25:565–578
Truchet G, Roche P, Lerouge P, Vasse J, Camut S, de Billy F, Prome J-C, Denarie J (1991) Sulphated lipo-oligosaccharide signals of Rhizobium meliloti elicit root nodule organogenesis in alfalfa. Nature 351:670–673
Van de Velde W, Zehirov G, Szatmari A, Debreczeny M, Ishihara H, Kevei Z, Farkas A, Mikulass K, Nagy A, Tiricz H, Satiat-Jeunemaitre B, Alunni B, Bourge M, Kucho K, Abe M, Kereszt A, Maroti G, Uchiumi T, Kondorosi E, Mergaert P (2010) Plant peptides govern terminal differentiation of bacteria in symbiosis. Science 327:1122–1126
van Rhijn P, Goldberg RB, Hirsch AM (1998) Lotus corniculatus nodulation specificity is changed by the presence of a soybean lectin gene. Plant Cell 10:1233–1250
van Rhijn P, Fujishige NA, Lim PO, Hirsch AM (2001) Sugar-binding activity of pea lectin enhances heterologous infection of transgenic alfalfa plants by Rhizobium leguminosarum biovar viciae. Plant Physiol 126:133–144
van Spronsen PC, Groenlund M, Bras CP, Spaink HP, Kijne JW (2001) Cell biological changes of outer cortical root cells in early determinate nodulation. Mol Plant Microbe Interact 14:839–847
Vassileva VN, Kouchi H, Ridge RW (2005) Microtubule dynamics in living root hairs: transient slowing by lipochitin oligosaccharide nodulation signals. Plant Cell 17:1777–1787
Wan J, Zhang XC, Neece D, Ramonell KM, Clough S, Kim SY, Stacey MG, Stacey G (2008) A LysM receptor-like kinase plays a critical role in chitin signaling and fungal resistance in Arabidopsis. Plant Cell 20:471–481
Weerasinghe RR, Collings DA, Johannes E, Allen NS (2003) The distributional changes and role of microtubules in Nod factor-challenged Medicago sativa root hairs. Planta 218:276–287
Weerasinghe RR, Bird DM, Allen NS (2005) Root-knot nematodes and bacterial Nod factors elicit common signal transduction events in Lotus japonicus. Proc Natl Acad Sci USA 102:3147–3152
Wilson KJ, Anjaiah V, Nambiar PT, Ausubel FM (1987) Isolation and characterization of symbiotic mutants of Bradyrhizobium sp. (Arachis) strain NC92: mutants with host-specific defects in nodulation and nitrogen fixation. J Bacteriol 169:2177–2186
Yang S, Tang F, Gao M, Krishnan HB, Zhu H (2010) R gene-controlled host specificity in the legume-rhizobia symbiosis. Proc Natl Acad Sci USA 107:18735–18740
Yano K, Tansengco ML, Hio T, Higashi K, Murooka Y, Imaizumi-Anraku H, Kawaguchi M, Hayashi M (2006) New nodulation mutants responsible for infection thread development in Lotus japonicus. Mol Plant Microbe Interact 19:801–810
Yano K, Yoshida S, Muller J, Singh S, Banba M, Vickers K, Markmann K, White C, Schuller B, Sato S, Asamizu E, Tabata S, Murooka Y, Perry J, Wang TL, Kawaguchi M, Imaizumi-Anraku H, Hayashi M, Parniske M (2008) CYCLOPS, a mediator of symbiotic intracellular accommodation. Proc Natl Acad Sci USA 105:20540–20545
Yano K, Shibata S, Chen WL, Sato S, Kaneko T, Jurkiewicz A, Sandal N, Banba M, Imaizumi-Anraku H, Kojima T, Ohtomo R, Szczyglowski K, Stougaard J, Tabata S, Hayashi M, Kouchi H, Umehara Y (2009) CERBERUS, a novel U-box protein containing WD-40 repeats, is required for formation of the infection thread and nodule development in the legume-Rhizobium symbiosis. Plant J 60:168–180
Yokota K, Fukai E, Madsen LH, Jurkiewicz A, Rueda P, Radutoiu S, Held M, Hossain MS, Szczyglowski K, Morieri G, Oldroyd GE, Downie JA, Nielsen MW, Rusek AM, Sato S, Tabata S, James EK, Oyaizu H, Sandal N, Stougaard J (2009) Rearrangement of actin cytoskeleton mediates invasion of Lotus japonicus roots by Mesorhizobium loti. Plant Cell 21:267–284
Zhukov V, Radutoiu S, Madsen LH, Rychagova T, Ovchinnikova E, Borisov A, Tikhonovich I, Stougaard J (2008) The pea Sym37 receptor kinase gene controls infection-thread initiation and nodule development. Mol Plant Microbe Interact 21:1600–1608
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2012 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Markmann, K., Radutoiu, S., Stougaard, J. (2012). Infection of Lotus japonicus Roots by Mesorhizobium loti . In: Perotto, S., Baluška, F. (eds) Signaling and Communication in Plant Symbiosis. Signaling and Communication in Plants, vol 11. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-20966-6_2
Download citation
DOI: https://doi.org/10.1007/978-3-642-20966-6_2
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-20965-9
Online ISBN: 978-3-642-20966-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)