Abstract
Molecular imaging with positron emission tomography (PET) using tumour-seeking radiopharmaceuticals has gained wide acceptance in oncology with many clinical applications. The hybrid imaging modality PET/CT allows assessing molecular as well as morphologic information at the same time. Therefore, PET/CT represents an efficient tool for whole body staging and re-staging within one imaging modality. In oncology the glucose analogue 18F-fluorodeoxyglucose (FDG) is the most widely used PET and PET/CT radiopharmaceutical in clinical routine. FDG PET and PET/CT have been used for staging and re-staging tumour patients in numerous studies. This chapter will discuss the use and the main indications of FDG PET and PET/CT in oncology with special emphasis on lung cancer, oesophageal cancer, colorectal cancer, head and neck cancer, lymphoma and breast cancer (among other tumour entities). A review of the current literature will be given with respect to primary diagnosis, staging and diagnosis of recurrent disease (local, lymph node and distant metastases). Besides its integral role in diagnosis, staging and re-staging of disease in oncology, there is increasing evidence that FDG PET and PET/CT can significantly contribute to therapy response assessment possibly influencing therapeutic management and treatment planning, to therapy tumour control and prediction of prognosis in oncologic patients, which will also be discussed in this chapter.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Positron Emission Tomography
- Standardize Uptake Value
- Invasive Lobular Carcinoma
- Oesophageal Cancer
- Testicular Germ Cell Tumor
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Molecular imaging with positron emission tomography (PET) using tumor-seeking radiopharmaceuticals has gained wide acceptance in oncology with many clinical applications. In oncology the glucose analogue 18F-fluorodeoxyglucose (FDG) is the most widely used radiopharmaceutical. FDG follows a metabolic pathway similar to glucose in vivo, except that it is not metabolised to CO2 and water, but is trapped within cells. Increased consumption of glucose is a characteristic of most tumor cells and is partially related to overexpression of the GLUT-1 glucose transporters and increased hexokinase activity. FDG enables to visualize regional glucose metabolism with high sensitivity and somewhat lower specificity. Despite the high sensitivity of FDG PET false positive findings occur due to physiologic processes, such as brown fat, colonic and gynecologic activity, infectious and inflammatory processes, and rebound thymic hyperplasia. FDG accumulation can be assessed visually, semi-quantitatively, and quantitatively. The most commonly used technique is the standardized uptake value (SUV): a semi-quantitative index of tumor uptake normalized to the injected dose and a measure of the total volume of distribution, such as the patient’s body weight.
The hybrid imaging modality PET/CT has been introduced in the late 1990s. PET/CT imaging allows assessing molecular as well morphologic information at the same time. PET/CT represents an efficient tool for whole-body staging and re-staging within one imaging modality. PET/CT over PET or CT alone improves lesion localization as well as lesion characterization in oncologic imaging.
FDG PET and PET/CT have been used for staging and re-staging tumor patients in numerous studies (Fletcher et al. 2008). This chapter provides an overview of the main clinical applications of FDG PET and PET/CT in oncology.
2 Clinical Applications of FDG PET and PET/CT in Oncology
2.1 Non-Small Cell Lung Cancer
In Europe and the United States, non-small cell lung cancer (NSCLC) is the leading cause of cancer-related deaths in both men and women. Accurate diagnostic work-up and staging of NSCLC is mandatory with respect to mediastinal lymph node involvement and distant metastases, therapy management, and prognosis. A considerable number of studies have provided evidence that FDG PET/CT improves diagnostic accuracy.
In a systematic review by Gould et al. (2001). PET showed a sensitivity of 83–100 % (mean 96 %) with an highly variable specificity of 0–100 % (mean 73 %) for the detection of pulmonary lesions of any size (n = 1.474). Analysis of pulmonary nodules only (n = 450) revealed a mean sensitivity and specificity of 98 and 83 %, respectively (Gould et al. 2001). Another systematic review including four prospective studies assessing the effectiveness of PET in differentiating malignant from benign lesions demonstrated a sensitivity of 86–100 % and a specificity of 40–90 % (Fletcher et al. 2008). As the positive predictive value of PET will be higher in high-risk patients and the negative predictive value will be higher in low-risk patients, PET will be most useful in patients at an intermediate risk of lung cancer. In their review analyzing 800 patients Fischer et al. (2001) reported a sensitivity of 96 % and a specificity of 78 % for FDG PET/CT in the diagnosis and staging of lung cancer (Fischer et al. 2001). Schreyögg et al. evaluated the diagnostic- and cost-effectiveness of integrated PET/CT for staging of NSCLC. The authors reported a diagnostic effectiveness in terms of correct TNM staging of 40 % for CT alone and 60 % for PET/CT. For the assessment of resectability 84 % of patients were staged correctly by PET/CT vs. 70 % by CT alone. The cost-effectiveness analyses showed that costs for PET/CT were within the commonly accepted range for diagnostic tests or therapies (Schreyogg et al. 2010).
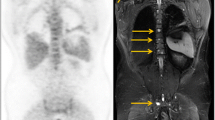
With respect to the detection of mediastinal lymph node metastases comparing PET and CT a systematic review by Birim et al. showed an overall sensitivity and specificity of 83 and 92 %, respectively, for CT 59 and 78 %, respectively (Birim et al. 2005). Lv et al. performed a meta-analysis determining the pooled sensitivity and specificity of FDG PET/CT for mediastinal lymph node staging in NSCLC. The authors reported a pooled sensitivity and specificity of 73 and 92 %, respectively. The authors concluded that integrated FDG PET/CT demonstrated an accurate imaging technique with excellent specificity for mediastinal lymph node staging in patients suffering from NSCLC (Lv et al. 2011) (Fig. 1).
A systematic review revealed that FDG PET detected 10–20 % more distant metastases in comparison to other imaging modalities and led to a therapy management change in 9–64 % [for more information see (Fletcher et al. 2008)]. The PLUS trial studied the effect of PET in the reduction of futile thoracotomies in patients with suspected NSCLC referred for surgery after conventional work-up. The trial reported a significant higher number of patients with futile surgery in the conventional work-up arm compared to the conventional work-up plus PET arm [relative risk reduction, 51 %, 95 % CI 32–80 % (p = 0.003) in favor of PET] (van Tinteren et al. 2002). Viney et al. addressed the impact of FDG PET on the clinical management and surgical outcome of patients with NSCLC stage I-II examining 92 patients using conventional work-up and 91 patients using conventional work-up plus PET. The sensitivity and specificity of FDG PET for the detection of mediastinal disease was 73 and 90 %, respectively. FDG PET could potentially have an impact on management in 26 % of patients. With a minimum 1-year survival, 77 % of patients were alive in the non-PET arm and 80 % in the PET arm (Viney et al. 2004). FDG PET/CT may also be helpful with respect to radiation treatment planning of NSCLC causing a change in radiation volume in up to 25–60 % [for further information see (Fletcher et al. 2008)].
2.2 Oesophageal Cancer
Preoperative assessment of prognostic factors is important in clinical management of patients suffering from oesophageal cancer. Relevant pre-therapeutic prognostic factors are local tumor invasion, locoregional lymph node stage, and distant metastases. Sensitivity and specificity of FDG PET and PET/CT for staging oesophageal cancer have been investigated in many studies. Endoscopic ultrasound and CT represent the most widely used imaging modalities for the assessment of local tumor invasion (T category) and locoregional lymph node involvement. Precise pre-therapeutic staging is important in choosing the best available therapy for the patient. Van Westreenen et al. published a systematic review and showed that FDG PET had a sensitivity of 51 % and a specificity of 84 % for the detection of lymph node staging (van Westreenen et al. 2004). In a more recent meta-analysis van Vliet et al. concluded that endoscopic ultrasound CT and PET contribute to detect metastases in oesophageal cancer. EUS turned out to be more sensitive for locoregional lymph node staging. In turn CT and FDG PET proved to be more specific (van Vliet et al. 2008).
FDG PET and PET/CT can add clinically relevant information with respect to M staging. It is crucial to be able to differentiate patients with locoregional disease from patients with systemic disease. Distant metastases have to be ruled out before the initiation of therapy. FDG PET has a sensitivity and specificity of 67 and 97 %, respectively, in M staging of oesophageal cancer (Fletcher et al. 2008). In systemic disease, there is no curative option and the patients will receive palliative treatment. After exclusion of distant metastases, the selection of the therapeutic regimen depends on the T stage. Localized tumors (T1/T2) have a high likelihood of R0 resection, and primary oesophagectomy represents the most frequent therapeutic procedure. In cases of locally advanced tumors (T3/T4, N1), surgery remains the mainstay of therapy, preceded by a neoadjuvant chemotherapy or radiochemotherapy.
In conclusion, the use of FDG PET and PET/CT is useful for staging oesophageal cancer for the detection of distant metastases.
2.3 Gastric Cancer
FDG PET and PET/CT for staging gastric cancer is limited by a limited sensitivity. Only 60 % of gastric cancers are FDG avid. Especially tumors with non-intestinal-type histology according to Lauren’s classification (signet ring cell carcinoma, diffuse type) are often not FDG avid and can therefore not be imaged with FDG PET and PET/CT. Sensitivities for FDG PET range from 47 to 96 % (mean sensitivity, 77 %; mean specificity, 99 %) for the detection of gastric cancer (Herrmann et al. 2007). Wu et al. carried out a systematic review and meta-analysis. Across nine studies the overall sensitivity of FDG PET for the detection of recurrent gastric cancer was 78 % and the overall specificity was 82 % (Wu et al. 2011).
2.4 Colorectal Cancer
A number of FDG PET studies on staging, detection of recurrence, and changes of therapy management in patients with colorectal cancer have been published [for reviews see (Brush et al. 2011; Fletcher et al. 2008)]. A systematic review and meta-analysis of studies evaluating the diagnostic test accuracy of FDG PET reported a sensitivity of 94 % and a specificity of 98 % in patients with locally recurrent rectal cancer (Fig. 2). Additional information provided by FDG PET is important for the clinical therapy management of patients with recurrent colorectal carcinoma. Visualization of CT negative lesions by FDG PET potentially allows an earlier change of the therapy regimen. Detection of distant metastases and better preoperative assessment of tumor spread can avoid unnecessary surgery, leading to a reduction of morbidity, and mortality associated with aggressive and futile therapies. When data related to changes in the management of these patients were pooled, 29 % of management decisions were changed. The majority were related to the avoidance of surgery as a result of the upstaging of the patients’ disease classification (Huebner et al. 2000).
An expert panel concluded that the use of FDG PET is not beneficial in the primary diagnosis but recommended the use of FDG PET for the detection of extrahepatic recurrence and local relapse (Fletcher et al. 2008). In a more recent systematic review Brush et al. investigated the value of FDG PET and PET/CT in staging and re-staging of colorectal cancer (Brush et al. 2011). The systematic review found insufficient evidence to support the routine use of FDG PET/CT in primary colorectal cancer and only a small amount of evidence supporting its use in the pre-operative staging of recurrent and metastatic CRC. Although FDG PET/CT was shown to change patient management, the data were divergent and the quality of studies was limited.
2.5 Gastrointestinal Stromal Tumors
Because sarcomas, including gastrointestinal stromal tumors (GISTs) often show a high FDG avidity, FDG PET and PET/CT can be used to evaluate these tumors and the response to therapy [for a review see (van den Abbeele 2008)]. This is of special importance as morphological changes occur late in the course of therapy. Metabolic response as assessed by FDG PET is closely related to clinical response and changes of FDG uptake occur early in the course of imatinib therapy. New CT criteria for therapy response have been proposed for GISTs combining morphological as well as density criteria. Therefore, tumor response to therapy in GISTs is best monitored by the hybrid imaging modality PET/CT.
2.6 Head and Neck Cancer
FDG PET and PET/CT studies in patients with head and neck cancer on diagnosis and staging have been published [for reviews see (Duet et al. 2010; Fletcher et al. 2008)]. There are not sufficient data to support the use of FDG PET and PET/CT in primary diagnosis of head and neck cancer. In patients with advanced disease FDG PET and PET/CT is useful if metastases or a secondary cancer is suspected. In initial M staging a meta-analysis by Xie et al. showed pooled sensitivity estimates for FDG PET/CT of 88 % and specificity estimates of 95 % (Xie et al. 2011). Kubicek et al. demonstrated in a large cohort of patients that the positive predictive value of N staging for patients was 94 % going along with a negative predictive value of 89 %. FDG PET also revealed prognostic information with respect to overall survival (Kubicek et al. 2010).
A systematic review and meta-analysis of studies evaluating post-treatment FDG PET or PET/CT imaging in head and neck cancer was published by Gupta et al. (2011). The aim of the analysis was to assess the diagnostic performance of FDG PET or PET/CT in post-treatment response assessment and/or surveillance imaging of head and neck squamous cell carcinoma. The pooled sensitivity and specificity of post-treatment PET or PET/CT for the primary site were 80 and 88 %, respectively; for the neck the corresponding values were 72 and 88 %. The authors concluded that overall diagnostic performance of post-treatment FDG PET or PET/CT for response assessment and surveillance imaging of head and neck cancer was good, but the positive predictive value was somewhat limited. However, the negative predictive value was shown to be high and therefore an FDG negative post-treatment scan most probably indicates the absence of viable tumor tissue.
2.7 Melanoma
FDG PET has been extensively studied as imaging modality for the detection of metastatic cutaneous melanoma [for reviews see (Fletcher et al. 2008; Patnana et al. 2011)]. In general, sensitivity and specificity for detecting metastases are higher for FDG PET or PET/CT than for conventional imaging modalities. However, for the detection of regional lymph node metastases, sensitivities were lower compared to sentinel node biopsy and FDG PET also showed a lower sensitivity for the detection of lung metastases. Additionally MRI should be performed to exclude brain metastases.
It has also been proposed that FDG PET or PET/CT could be useful in patients who are at high risk for systemic relapse and as additional imaging tool for the detection of recurrent disease. Essler et al. published a study analyzing the predictive values, sensitivity, and specificity of FDG PET/CT compared with the tumor markers S100B and MIA in the follow-up of high-risk melanoma patients. All three biomarkers detected metastases of malignant melanoma with clinically valuable sensitivity and specificity. Among these, FDG PET/CT was the most effective modality. Sensitivity and specificity of PET/CT were 97 %, respectively (Essler et al. 2011) (Fig. 3).
64 year old patient suffering from pulmonal metastasized malignant melanoma (initial diagnosis thoracal left 07/04) referred for FDG PET/CT for re-staging after resection of a pulmonal metastasis of the right lower lobe 02/11. FDG PET/CT revealed FDG-positive soft tissue lesions (A1) CT scan, (B1) PET scan, (C1) PET/CT fused images, bone metastasis (A2) CT scan, (B2) PET scan, (C2) PET/CT fused images and hepatic metastasis (A3) CT scan, (B3) PET scan, (C3) PET/CT fused images
2.8 Lymphoma
Accurate staging is of critical importance in both, Hodgkin’s disease (HD) and non-Hodgkin’s lymphoma (NHL), as treatment is varying according to the stage of the disease (Fletcher et al. 2008). Sensitivity and specificity of FDG PET is different in the various histological lymphoma subtypes. However, it exhibits a high sensitivity in the major classes of lymphoma in clinical practice: HD, diffuse large B cell lymphoma and follicular lymphoma, usually exhibit high FDG uptake. FDG PET has emerged in the last 15 years as a powerful imaging modality in assessing patients with such lymphoma types, and provides substantial information regarding identification of the most suitable site for biopsy, initial staging, response to therapy, assessment of post-treatment residual masses, identification of recurrent disease and potentially, radiotherapy planning. FDG PET has lower sensitivity in some less common NHL subtypes, such as marginal zone lymphoma, especially concerning extranodal manifestations of this subtype, peripheral T cell lymphoma, small lymphocytic NHL, and the mucosa-associated lymphoid tissue (MALT) subtype (Elstrom et al. 2003). In those subtypes FDG avidity has to be assessed by a pretreatment FDG PET almost mandatory, to be able to apply FDG PET in the further clinical workflow.
FDG PET/CT is superior to CT alone in staging and re-staging nodal disease as well as in detecting extranodal involvement including hepatic, splenic, bone marrow, and gastric involvement (Moog et al. 1998). A criterion for splenic involvement in FDG PET is when the uptake in the spleen is higher than in the liver. Concerning bone marrow involvement because of the high proportion of false-negative results, conventional masked biopsies are still needed (Fletcher et al. 2008).
FDG PET imaging predicts therapeutic response in lymphoma at various time points after initiation or completion of treatment [for a review see (Czernin et al. 2010; Hutchings and Barrington 2009)]. This has been published by the Consensus of the Imaging Subcommittee of the International Harmonization Project in Lymphoma that has re-defined the roles of PET and CT imaging in assessing therapeutic responses in lymphoma. For more detailed information concerning this issue the reader is directed to the manuscripts by Juweid et al. (2007) and Cheson et al. (2007). In summary FDG PET response is assessed visually by comparing FDG lesion uptake with mediastinal blood pool activity. Lesions with FDG uptake at or below blood pool activity following treatment would strongly suggest a complete response. It has to be kept in mind that lesions with increased FDG uptake do not necessarily represent residual disease because infectious or inflammatory lesions can accumulate the radiotracer. Further, thymic hyperplasia is common after chemotherapy and is a common cause of false positive results. In FDG PET it is often presented as a V-shaped uptake in the anterior mediastinum. Additionally, diffuse bone marrow uptake or splenic uptake post granulocyte colony-stimulating factor therapy can mimic lymphomatous bone marrow or splenic involvement.
2.9 Breast Cancer
Mammography remains the principal imaging tool to screen for breast cancer. There is a high risk of false negative results, when FDG PET is applied to characterize breast lesions. Small lesions cannot be characterized due to the partial volume effects. Histopathological entities including invasive lobular carcinoma, tubular carcinoma, and carcinoma in situ are often not FDG avid enough to be detected by PET.
Local and distant staging has important implications on management and prognosis in patients with breast cancer. Regarding axillary lymph node staging the sensitivity and specificity of FDG PET/CT is higher than that of CT alone; however, the overall accuracy of 79 % was not sufficiently high to suggest that PET/CT imaging could replace the sentinel node biopsy approach (Heusner et al. 2009). Several studies have shown that FDG PET/CT is not sufficiently accurate to permit reliable axillary lymph node staging (Czernin et al. 2010). FDG PET and PET/CT can add substantial diagnostic information in patients clinically suspected to have distant metastases. It is most valuable mainly in patients with locally advanced breast cancer and when neoadjuvant therapy is planned without axillary dissection or sentinel node sampling. For the latter it can be used to evaluate response to treatment.
Concerning tumor recurrence, FDG PET has a high accuracy in detecting locoregional and distant tumor manifestations. It has gained a valuable role in the evaluation of patients with clinical suspicion for recurrence and negative tumor markers as well as in asymptomatic patients with increased tumor markers. Sensitivity and specificity have been reported with a median of 92 % (range, 57–97 %) and 89 % (range, 79–96 %), respectively (Fletcher et al. 2008). However, it has to be noticed that false negative results can be obtained in case of a non FDG avid primary such as the invasive lobular carcinoma.
Regarding the detection of bone metastases, concerning staging and restaging, FDG PET, and bone scintigraphy provide complemental information. Osteolytic metastases are better detected with PET, while osteoblastic metastases often do not exhibit increased glucose utilization and are better detected with bone scintigraphy. Further, FDG PET and PET/CT is helpful to characterize treatment effects in osseous lesions.
2.10 Ovarian Cancer
Ovarian cancer is the leading cause of death from gynecologic cancer in the Western World. Ovarian cancer spreads early by implantation on both the parietal and the visceral peritoneum before spreading through the lymphatics and involving the inguinal, pelvic, paraaortic, and mediastinal lymph nodes. The serum tumor marker CA-125 is widely used to assess the effectiveness of therapy and to detect tumor recurrence.
Unclear ovarian masses are evaluated with ultrasound and/or MRI. FDG PET has been shown to be limited in the diagnosis of cancer of the ovaries. Physiologic FDG uptake observed in the ovaries of women of reproductive age even after hysterectomy is reasonably common and may be mistaken for pathologic uptake. However, focal uptake in the region of the ovaries in postmenopausal women is suspicious of malignancy and should be further evaluated. Surgical exploration remains the standard of reference for the initial staging of ovarian cancer. CT and MRI have been accepted as useful imaging modalities for preoperative staging of ovarian cancer. FDG PET may be useful as an adjunct to diagnostic CT for staging ovarian cancer (Schwarz et al. 2009). FDG PET is more sensitive than CT in detecting small peritoneal deposits and distant metastases. Therefore, it is recommended in the guidelines of the European Society of Urogenital Radiology (ESUR) in suspected stage IV disease (advanced ovarian cancer) and in the presence of indeterminate lymph node appearance (Forstner et al. 2010). FDG PET and PET/CT can be used to monitor response to treatment in advanced ovarian cancer as it is more accurate in characterizing residual disease after chemotherapy than CT alone. However, it cannot replace second-look laparotomy (Schwarz et al. 2009).
Several studies have shown that FDG PET and PET/CT is most useful in the evaluation of tumor recurrence (Schwarz et al. 2009). It has a higher accuracy than conventional imaging in detecting recurrence of ovarian cancer in both rising CA-125 level, and clinical suspicion of recurrence with not elevated CA-125 level (Havrilesky et al. 2005).
2.11 Sarcomas
Sarcomas are a rare heterogeneous group of tumors composing about 1 % of all malignancies, presenting with varied radiologic appearances. With respect to the biologic potential, the World Health Organization (WHO) has classified soft tissue tumors in four categories: benign, intermediate (locally aggressive), intermediate (rarely metastasize), and malignant (Fletcher et al. 2002). A detailed presentation of the different types is beyond the scope of this chapter. A recent meta-analysis of FDG PET studies in patients with sarcoma concluded, that FDG PET can discriminate between benign tumors and low grade tumors and intermediate and high grade tumors. However, using FDG PET it is difficult to distinguish low grade tumors from benign tumors (Bastiaannet et al. 2004). Czernin’s group investigated the baseline glucose metabolic phenotype of sarcoma in more than 100 patients with soft tissue sarcoma. The SUV differed considerably and significantly among the many histological subtypes. Liposarcomas, especially the myxoid variants exhibited low FDG uptake. Sarcomas not otherwise specified (NOS), the most de-differentiated variants, had the highest FDG uptake. Overall, SUVmax was significantly higher in high grade than in low grade sarcomas (11.7 ± 9.1 g/ml vs. 3.7 ± 1.8 g/ml; p < 0.001) (Czernin et al. 2010). FDG PET can be useful in the evaluation of soft tissue tumors, by guiding biopsy, helping to sample in the area with highest glucose utilization. CT or MRI is essential for planning of surgical interventions or radiation treatment. However, in FDG avid sarcomas FDG PET can play a role in staging and restaging (Fig. 4). A limitation of FDG PET that has been alleviated by PET/CT is the detection of pulmonary metastases, presenting as small parenchymal nodules. They are sometimes missed by FDG PET imaging and the CT information is essential to detect them. Furthermore, FDG PET may have a role in assessing response to neoadjuvant chemotherapy, and potentially modify treatment.
14 year old patient with a penile rhabdomyosarcoma referred for FDG PET/CT two times. 1. For restaging after R2 resection 03/11: (A 1a and A 1b) CT scan, (B 1a and B 1b) PET scan, (C 1a and C 1b) PET/CT fused images. 2. For restaging after chemotherapy 05/11: (A 2a and A 2b) CT scan, (B 2a and B 2b) PET scan, (C 2a und C 2b) PET/CT fused images. FDG PET/CT 03/11 revealed two tumor lesions after R2-resection, FDG PET/CT 05/11 showed a good therapy response after chemotherapy
2.12 Pancreatic Cancer
Pancreatic cancer is the fifth most common cancer in both men and women in the US and is associated with a poor overall survival (< 4 %) as curative therapy is restricted to patients suffering from limited disease referred for surgery.
Commonly used imaging tools for the diagnosis of exocrine pancreatic cancer are ultrasound, endosonography, CT, MRI, magnetic resonance, and endoscopic retrograde cholangio-pancreatography (MRCP and ERCP). As 80–90 % of exocrine pancreatic cancers show a high FDG-uptake FDG PET was introduced to potentially improve detection of pancreatic adenocarcinomas. Fletcher et al. reported that FDG PET should additionally be used in selected patients demonstrating inconclusive conventional imaging findings (Fletcher et al. 2008).
In a meta-analysis performed by Orlando et al. comparing PET/CT and CT for the differentiation between benign and malignant pancreatic lesions sensitivity and specificity of FDG PET was 71–100 % and 53–100 %, respectively (for CT 53–100 % and 0–100 %, respectively) (Orlando et al. 2004). In another review comparing the use of PET and conventional work-up (CT, MRI, ultrasonography, and Tl-201-SPECT) in the diagnosis of pancreatic cancer PET showed a pooled sensitivity and specificity of 91 and 86 % and was superior to the other imaging modalities. With respect to a change in patient management caused by PET findings no conclusion could be made due to considerably differing results of these studies (for further information see (Fletcher et al. 2008)). Schick et al. assessed the diagnostic impact of FDG PET evaluating solid pancreatic lesions vs. endosonography, ERCP with intraductal ultrasonography and abdominal ultrasound. The authors reported that the evaluation of dignity of pancreatic lesions was almost equal between FDG PET and the other modalities (Schick et al. 2008).
Fletcher et al. concluded that PET improved differentiation between benign and malignant pancreatic tissue in the diagnostic work-up of patients with suspected pancreatic lesions and might reduce the need for biopsy and surgery influencing morbidity (Fletcher et al. 2008). The value of FDG PET in imaging pancreatic cancer is limited concerning lymph node staging, detection of peritoneal disease, and liver metastasis (especially < 1 cm). In extratumoral staging FDG PET may play a role in the detection of occult lesions (such as e. g. non-hepatic distant metastases and synchronous tumours) (Reske 2009). With respect to re-staging of pancreatic cancer after pancreatic resection Ruf et al. reported that FDG PET and -PET/CT might be superior compared to CT and MRI (sensitivity of 96 and 39 % for PET and CT/MRI, respectively) (Ruf et al. 2005).
2.13 Thyroid Cancer
Differentiated thyroid cancer (papillary or follicular) is highly treatable and curable. However, 10–30 % of patients will develop recurrence or metastases, which cannot be detected by I-131 whole-body scintigraphy. FDG PET is used in patients treated for well-differentiated thyroid cancer showing a rising thyreoglobulin serum marker (Tg) > 10 ng/ml with negative findings of I-131 whole-body scintigraphy. The diagnostic accuracy of FDG PET is generally high in patients with negative radioiodine scans and high Tg levels (Bertagna et al. 2010). In a systematic review Hoof et al. reported a sensitivity and specificity of 70–95 % and 77–100 %, respectively, for the detection of thyroid cancer recurrence using FDG PET. In 82 % of patients with raised tumor markers and negative findings on I-131 whole-body scintigraphy PET localized FDG-positive foci suggestive for recurrence (Hooft et al. 2001) (Fig. 5). In another review evaluating the detection of thyroid cancer recurrence in previously treated patients with elevated serum markers and negative I-131 scan PET showed a sensitivity and specificity of 84 and 56 %, respectively [for further information see (Fletcher et al. 2008)]. With respect to Tg level “cut off”, studies have shown controversial results. However a “cut off” of 10 ng/ml seems to be a reasonable value maintaining high accuracy in terms of a good compromise between sensitivity and specificity. The impact of thyroid-stimulating hormone (TSH) on FDG PET imaging is still an open issue, no complete consensus has been reached about the usefulness of high TSH levels. Levothyroxine withdrawal or alternatively the use of rTSH might be preferable, especially in cases of relatively low Tg levels (< 10 ng/ml) trying to improve sensitivity of FDG PET (Bertagna et al. 2010).
70 year old patient with follicular thyroid cancer (pT3acN0cM1/UICC 1987), increasing thyreoglobulin serum marker and negative findings of I-131 whole body scintigraphy referred for FDG PET/CT for restaging. FDG PET/CT revealed multiple FDG-positive pulmonal metastases and cerebral metastasis; (A 1 and 2) CT scan, (B 1 and 2) PET scan, (C 1 and 3) PET/CT fused images
2.14 Cancer of Unknown Primary
About 2–7 % of all malignancies are cancers of unknown primary (CUP) mostly associated with a poor prognosis. As CUPs can often not be identified on conventional imaging, PET might improve the detection rate of CUPs and potentially improve patient outcome by optimizing treatment planning. In a systematic review Delgado-Bolton et al. reported a sensitivity and specificity of 87 and 71 %, respectively, for detecting the primary tumor by PET imaging; overall PET detected 45 % (range, 35–49 %) of the tumors (Delgado-Bolton et al. 2003). Another review evaluated the use of FDG PET for detecting primary tumors in patients with cervical lymph node metastases after conventional imaging (panendoscopy, CT, MRI, or chest radiography). The sensitivity, specificity, and accuracy of PET was 88, 75, and 79 %, respectively. PET was responsible for a therapeutic change in 25 % of patients (Rusthoven et al. 2004). A recent meta-analysis by Kwee et al. reported a primary detection rate of 37 %. Lung, oropharyngeal and pancreatic cancer were reported to represent the most frequently detected primary tumors (Kwee and Kwee 2009).
Further studies are needed to assess the impact that cancer-specific treatments based on PET findings might have on the survival of patients suffering from CUPs.
2.15 Testicular Cancer
Most testicular neoplasms (95 %) are germ cell tumors. The other 5 % include lymphoma and metastases. Testicular germ cell tumors, the commonest tumor in young males (aged 15–35), are divided into seminoma and nonseminomatous germ cell tumors (NSGCT). The NSGCT group includes teratomas of varying degrees of differentiation, tumors containing mixed cell lines of teratomas, and mixed tumors with both teratoma and seminoma components. Pure seminomas may have an elevation of human chorionic gonatotropin-β (β-HCG) but α-fetoprotein (AFP) should be normal. AFP is a tumor marker for NSGCT. An elevation of both β-HCG and AFP reflects different cell lines that accordingly will not necessarily respond the same way to chemotherapy.
CT is usually applied for staging. Pelvic and retroperitoneal lymph node dissection can deliver diagnostic information concerning nodal metastatic disease. FDG PET may be valuable in stage II testicular germ cell tumor (any pT pN1-3) if it is of importance to accurately define the metastatic extent prior to chemotherapy, but it may not be of additional value in patients with stage I tumors (Albers et al. 1999).
FDG PET and PET/CT is most helpful in the setting of tumor recurrence. However, it is important to have information regarding the composition of the primary lesions. Mature teratoma do not show increased FDG uptake. As mature teratoma is present in more than 40 % of resected masses in NSGCT, this is a major source of false negative results. In seminomas the fraction of mature teratoma in residual lesions is much lower (< 5 %). Thus, FDG PET has a greater role in evaluating tumor recurrence in seminoma than in NSGCT (De Santis et al. 2004). The main indications of FDG PET and PET/CT in recurrence of testicular cancer are: (1) differentiation of residual tumor from fibrosis, in particular in lesions > 3 cm; (2) elevation of tumor markers whether or not a residual mass is seen on CT (De Santis et al. 2004; Hain et al. 2000). In the assessment of residual tumor tissue after chemotherapy FDG PET should be performed 4–12 weeks after the end of the treatment, as earlier imaging may result in false-positive results from inflammation following therapy (De Santis et al. 2004).
2.16 Prostate Cancer
FDG PET/CT has shown only limited sensitivity for the detection of differentiated prostate cancer and imaging of recurrent prostate cancer in various studies. Increased FDG uptake and accumulation is regularly only found in dedifferentiated, aggressive, and metastasized prostate cancer (Jadvar 2011; Schoder et al. 2005).
3 Therapy Response Assessment with FDG PET and PET/CT
FDG PET and PET/CT play an integral role in diagnosis, staging, and re-staging of disease in oncology. In addition to that there is increasing evidence that FDG PET and PET/CT can also significantly contribute to evaluation of therapy response, tumor control, and prediction of prognosis in oncologic patients [for a review see (Herrmann et al. 2012)]. Conventional imaging modalities are of limited use to assess response to therapy. FDG has been proposed as imaging surrogate parameter of therapy response. FDG provides several highly reproducible quantitative parameters of tumor glucose metabolism. Changes of glucose consumption can therefore be used to define a metabolic response to therapy. Recently, PET Response Criteria in Solid Tumors (PERCIST 1.0) have been published (Wahl et al. 2009). The authors argued that anatomic imaging alone using standard WHO, and Response Criteria in Solid Tumors (RECIST) criteria have limitations, particularly in assessing the activity of newer cancer therapies that stabilize disease, whereas FDG PET appears particularly valuable in such cases.
FDG PET and PET/CT can be used to assess response to therapy early and late in the course of therapy. From the perspective of a clinician, it is very important to differentiate non-responders to chemotherapy or radiochemotherapy early in the course of treatment to possibly change the therapeutic management. Tumor response can also be assessed early in the course of therapy (i.e., chemotherapy or radio-chemotherapy) with FDG PET and PET/CT. The first scan will take place before therapy, and a second scan is performed 2–4 weeks after initiation of the first therapy cycle (often within the first cycle). Changes in FDG uptake between the pre-therapeutic scan and the early follow-up scan are used to predict histopathological response, and patient survival. The standardized uptake value is the most widely used FDG PET parameter, and in most studies, relative changes (%) are calculated to quantify metabolic response. In summary, numerous studies have investigated post-therapeutic PET scanning in order to define the predictive and prognostic value. Most studies show a clear correlation of metabolic response as assessed by FDG PET on the one hand and response and survival on the other hand (Herrmann et al. 2012).
Early assessment with FDG PET of the response to therapy in oncologic patients has shown promising results in single-center studies and should now be evaluated in randomized, prospective multicenter trials. Such trials are an important step toward possible implementation in clinical practice. Standardization of patient preparation, data acquisition and processing, and data interpretation is an important issue, especially for prospective randomized multicenter studies.
References
Albers P, Bender H, Yilmaz H et al (1999) Positron emission tomography in the clinical staging of patients with Stage I and II testicular germ cell tumors. Urology 53(4):808–811
Bastiaannet E, Groen H, Jager PL et al (2004) The value of FDG-PET in the detection, grading and response to therapy of soft tissue and bone sarcomas; a systematic review and meta-analysis. Cancer Treat Rev 30(1):83–101
Bertagna F, Biasiotto G, Orlando E et al (2010) Role of 18F-fluorodeoxyglucose positron emission tomography/computed tomography in patients affected by differentiated thyroid carcinoma, high thyroglobulin level, and negative 131I scan: review of the literature. Jpn J Radiol 28(9):629–636
Birim O, Kappetein AP, Stijnen T et al (2005) Meta-analysis of positron emission tomographic and computed tomographic imaging in detecting mediastinal lymph node metastases in nonsmall cell lung cancer. Ann Thorac Surg 79(1):375–382
Brush J, Boyd K, Chappell F et al. (2011). The value of FDG positron emission tomography/computerised tomography (PET/CT) in pre-operative staging of colorectal cancer: a systematic review and economic evaluation. Health Technol Assess 15(35): 1–192, iii-iv
Cheson BD, Pfistner B, Juweid ME et al (2007) Revised response criteria for malignant lymphoma. J Clin Oncol 25(5):579–586
Czernin J, Benz MR, Allen-Auerbach MS (2010) PET/CT imaging: the incremental value of assessing the glucose metabolic phenotype and the structure of cancers in a single examination. Eur J Radiol 73(3):470–480
De Santis M, Becherer A, Bokemeyer C et al (2004) 2-18fluoro-deoxy-d-glucose positron emission tomography is a reliable predictor for viable tumor in postchemotherapy seminoma: an update of the prospective multicentric SEMPET trial. J Clin Oncol 22(6):1034–1039
Delgado-Bolton RC, Fernandez-Perez C, Gonzalez-Mate A et al (2003) Meta-analysis of the performance of 18F-FDG PET in primary tumor detection in unknown primary tumors. J Nucl Med 44(8):1301–1314
Duet M, Hugonnet F, Faraggi M (2010) Role of positron emission tomography (PET) in head and neck cancer. Eur Ann Otorhinolaryngol Head Neck Dis 127(1):40–45
Elstrom R, Guan L, Baker G et al (2003) Utility of FDG-PET scanning in lymphoma by WHO classification. Blood 101(10):3875–3876
Essler M, Link A, Belloni B et al (2011) Prognostic value of [18F]-fluoro-deoxy-glucose PET/CT, S100 or MIA for assessment of cancer-associated mortality in patients with high risk melanoma. PLoS ONE 6(9):e24632
Fischer BM, Mortensen J, Hojgaard L (2001) Positron emission tomography in the diagnosis and staging of lung cancer: a systematic, quantitative review. Lancet Oncol 2(11):659–666
Fletcher C, Uni K, Meteus F (eds) (2002) World Health Organization Classification of Tumors, Pathology and Genetics of Tumors of Soft Tissue and Bone. IARC Press, Lyon
Fletcher JW, Djulbegovic B, Soares HP et al (2008) Recommendations on the use of 18F-FDG PET in oncology. J Nucl Med 49(3):480–508
Forstner R, Sala E, Kinkel K et al (2010) ESUR guidelines: ovarian cancer staging and follow-up. Eur Radiol 20(12):2773–2780
Gould MK, Maclean CC, Kuschner WG et al (2001) Accuracy of positron emission tomography for diagnosis of pulmonary nodules and mass lesions: a meta-analysis. JAMA 285(7):914–924
Gupta T, Master Z, Kannan S et al (2011) Diagnostic performance of post-treatment FDG PET or FDG PET/CT imaging in head and neck cancer: a systematic review and meta-analysis. Eur J Nucl Med Mol Imaging 38(11):2083–2095
Hain SF, O’Doherty MJ, Timothy AR et al (2000) Fluorodeoxyglucose positron emission tomography in the evaluation of germ cell tumours at relapse. Br J Cancer 83(7):863–869
Havrilesky LJ, Kulasingam SL, Matchar DB et al (2005) FDG-PET for management of cervical and ovarian cancer. Gynecol Oncol 97(1):183–191
Herrmann K, Benz MR, Krause BJ et al. (2012) F-18-FDG-PET/CT in evaluating response to therapy in solid tumors: where we are and where we can go. Q J Nucl Med Mol Imaging: in press
Herrmann K, Ott K, Buck AK et al (2007) Imaging gastric cancer with PET and the radiotracers 18F-FLT and 18F-FDG: a comparative analysis. J Nucl Med 48(12):1945–1950
Heusner TA, Kuemmel S, Hahn S et al (2009) Diagnostic value of full-dose FDG PET/CT for axillary lymph node staging in breast cancer patients. Eur J Nucl Med Mol Imaging 36(10):1543–1550
Hooft L, Hoekstra OS, Deville W et al (2001) Diagnostic accuracy of 18F-fluorodeoxyglucose positron emission tomography in the follow-up of papillary or follicular thyroid cancer. J Clin Endocrinol Metab 86(8):3779–3786
Huebner RH, Park KC, Shepherd JE et al (2000) A meta-analysis of the literature for whole-body FDG PET detection of recurrent colorectal cancer. J Nucl Med 41(7):1177–1189
Hutchings M, Barrington SF (2009) PET/CT for therapy response assessment in lymphoma. J Nucl Med 50(Suppl 1):21S–30S
Jadvar H (2011) Prostate cancer: PET with 18F-FDG, 18F- or 11C-acetate, and 18F- or 11C-choline. J Nucl Med 52(1):81–89
Juweid ME, Stroobants S, Hoekstra OS et al (2007) Use of positron emission tomography for response assessment of lymphoma: consensus of the Imaging Subcommittee of International Harmonization Project in Lymphoma. J Clin Oncol 25(5):571–578
Kubicek GJ, Champ C, Fogh S et al (2010) FDG-PET staging and importance of lymph node SUV in head and neck cancer. Head Neck Oncol 2:19
Kwee TC, Kwee RM (2009) Combined FDG-PET/CT for the detection of unknown primary tumors: systematic review and meta-analysis. Eur Radiol 19(3):731–744
Lv YL, Yuan DM, Wang K et al (2011) Diagnostic performance of integrated positron emission tomography/computed tomography for mediastinal lymph node staging in non-small cell lung cancer: a bivariate systematic review and meta-analysis. J Thorac Oncol 6(8):1350–1358
Moog F, Bangerter M, Diederichs CG et al (1998) Extranodal malignant lymphoma: detection with FDG PET versus CT. Radiology 206(2):475–481
Orlando LA, Kulasingam SL, Matchar DB (2004) Meta-analysis: the detection of pancreatic malignancy with positron emission tomography. Aliment Pharmacol Ther 20(10):1063–1070
Patnana M, Bronstein Y, Szklaruk J et al (2011) Multimethod imaging, staging, and spectrum of manifestations of metastatic melanoma. Clin Radiol 66(3):224–236
Reske SN (2009) PET and PET-CT of malignant tumors of the exocrine pancreas. Radiologe 49(2):131–136
Ruf J, Lopez Hanninen E, Oettle H et al (2005) Detection of recurrent pancreatic cancer: comparison of FDG-PET with CT/MRI. Pancreatology 5(2–3):266–272
Rusthoven KE, Koshy M, Paulino AC (2004) The role of fluorodeoxyglucose positron emission tomography in cervical lymph node metastases from an unknown primary tumor. Cancer 101(11):2641–2649
Schick V, Franzius C, Beyna T et al (2008) Diagnostic impact of 18F-FDG PET-CT evaluating solid pancreatic lesions versus endosonography, endoscopic retrograde cholangio-pancreatography with intraductal ultrasonography and abdominal ultrasound. Eur J Nucl Med Mol Imaging 35(10):1775–1785
Schoder H, Herrmann K, Gonen M et al (2005) 2-[18F]fluoro-2-deoxyglucose positron emission tomography for the detection of disease in patients with prostate-specific antigen relapse after radical prostatectomy. Clin Cancer Res 11(13):4761–4769
Schreyogg J, Weller J, Stargardt T et al (2010) Cost-effectiveness of hybrid PET/CT for staging of non-small cell lung cancer. J Nucl Med 51(11):1668–1675
Schwarz JK, Grigsby PW, Dehdashti F et al (2009) The role of 18F-FDG PET in assessing therapy response in cancer of the cervix and ovaries. J Nucl Med 50(Suppl 1):64S–73S
Van den Abbeele AD (2008) The lessons of GIST–PET and PET/CT: a new paradigm for imaging. Oncologist 13(Suppl 2):8–13
van Tinteren H, Hoekstra OS, Smit EF et al (2002) Effectiveness of positron emission tomography in the preoperative assessment of patients with suspected non-small-cell lung cancer: the PLUS multicentre randomised trial. Lancet 359(9315):1388–1393
van Vliet EP, Heijenbrok-Kal MH, Hunink MG et al (2008) Staging investigations for oesophageal cancer: a meta-analysis. Br J Cancer 98(3):547–557
van Westreenen HL, Westerterp M, Bossuyt PM et al (2004) Systematic review of the staging performance of 18F-fluorodeoxyglucose positron emission tomography in esophageal cancer. J Clin Oncol 22(18):3805–3812
Viney RC, Boyer MJ, King MT et al (2004) Randomized controlled trial of the role of positron emission tomography in the management of stage I and II non-small-cell lung cancer. J Clin Oncol 22(12):2357–2362
Wahl RL, Jacene H, Kasamon Y et al (2009) From RECIST to PERCIST: Evolving Considerations for PET response criteria in solid tumors. J Nucl Med 50(Suppl 1):122S–150S
Wu LM, Hu JN, Hua J et al (2012) 18F-Fluorodeoxyglucose Positron Emission Tomography to Evaluate Recurrent Gastric Cancer: A systematic review and meta-analysis. J Gastroenterol Hepatol 27(3):472–480
Xie P, Li M, Zhao H et al (2011) 18F-FDG PET or PET-CT to evaluate prognosis for head and neck cancer: a meta-analysis. J Cancer Res Clin Oncol 137(7):1085–1093
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Krause, B.J., Schwarzenböck, S., Souvatzoglou, M. (2013). FDG PET and PET/CT. In: Schober, O., Riemann, B. (eds) Molecular Imaging in Oncology. Recent Results in Cancer Research, vol 187. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-10853-2_12
Download citation
DOI: https://doi.org/10.1007/978-3-642-10853-2_12
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-10852-5
Online ISBN: 978-3-642-10853-2
eBook Packages: MedicineMedicine (R0)