Abstract
Molecular imaging with positron emission tomography (PET) using tumour-seeking radiopharmaceuticals has gained wide acceptance in oncology with many clinical applications. The hybrid imaging modality PET/CT (computed tomography) allows assessing molecular as well as morphologic information at the same time. Therefore, PET/CT represents an efficient tool for whole-body staging and re-staging within one imaging modality. In oncology, the glucose analogue 18-F-fluorodeoxyglucose (FDG) is the most widely used PET/CT radiopharmaceutical in clinical routine. FDG PET and FDG PET/CT have been used for staging and re-staging of tumour patients in numerous studies. This chapter will discuss the use and the main indications of FDG PET/CT in oncology with special emphasis on lung cancer, lymphoma, head and neck cancer, melanoma and breast cancer (among other tumour entities). A review of the current literature is given with respect to primary diagnosis, staging and diagnosis of recurrent disease. Besides its integral role in diagnosis, staging and re-staging of disease in oncology, there is increasing evidence that FDG PET/CT can be used for therapy response assessment (possibly influencing therapeutic management and treatment planning) by evaluating tumour control, which will also be discussed in this chapter.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Molecular imaging with positron emission tomography (PET) using tumour-seeking radiopharmaceuticals has gained wide acceptance in oncology with many clinical applications. Since its first in vivo application in the 1970s [4, 55] the glucose analogue 18-F-fluorodeoxyglucose (FDG) is the most common PET radiopharmaceutical in clinical routine, especially in oncology. In vivo FDG acts similar to glucose, but being trapped within the cells (‘metabolic trapping’) and not being metabolised any further. Increased consumption of glucose is a characteristic of most tumour cells and is mainly related to overexpression of the GLUT-1 glucose transporters and increased hexokinase activity [102, 159]. FDG enables to visualise regional glucose metabolism with high sensitivity but somewhat lower specificity. Despite the high sensitivity of FDG PET/computed tomography (CT) false-positive findings may occur due to physiologic processes such as brown fat, colonic and gynaecologic activity, infectious and inflammatory processes and rebound thymus hyperplasia. False-negative findings can occur, e.g. in the assessment of brain lesions as a consequence of physiologically high glucose uptake by surrounding normal brain. FDG accumulation can be assessed visually, semi-quantitatively and quantitatively. The most commonly used technique is the standardised uptake value (SUV): a semi-quantitative index of tumour uptake normalised to injected dose and a measure of the total volume distribution, such as the patient’s body weight.
The hybrid imaging modality PET/CT has been clinically introduced in the late 1990s. PET/CT imaging allows assessing molecular as well as morphologic information at the same time. Nowadays, PET/CT represents a state of the art tool for whole-body staging and re-staging in oncology within one imaging modality. PET/CT outperforms PET or CT alone by improving lesion localisation and lesion characterisation in oncology imaging.
2 Clinical Applications of FDG PET/CT in Oncology
This chapter provides an overview of the main clinical applications of FDG PET/CT in oncology.
FDG PET/CT plays an integral role in diagnosis, staging and re-staging of disease in oncology. In addition, there is increasing evidence that FDG PET/CT can also significantly contribute to evaluation of therapy response, tumour control, and prediction of prognosis in oncologic patients (for a review see Herrmann et al. [65]). Conventional imaging modalities are of limited use to assess response to therapy. FDG has been proposed as an imaging surrogate parameter of therapy response. It provides highly reproducible quantitative parameters of tumour glucose metabolism. Changes of tumour consumption can therefore be used to define metabolic response to therapy. For this reason, FDG PET/CT was introduced for the early sequential monitoring of tumour response of breast cancer in 1993 [158].
FDG PET/CT can be used to assess response to therapy early and late in the course of treatment. From the perspective of a clinician, it is very important to differentiate non-responders to therapy early in the course of treatment (especially, if several cycles of a therapy have to be applied, e.g. chemotherapy or immunotherapy) in order to possibly change the therapeutic management. The schedule for PET timing is always dependent on the type of tumour and the treatment plan, often there is no standardised recommendation due to a lack of (randomised, multicentre, prospective) studies. However, a baseline scan is indispensable in any case, followed usually by a second scan after the first or second cycle of therapy. Changes in FDG uptake between the pre-therapeutic and early follow-up scan are used to predict histopathological response and patient outcome. The standardised uptake value is the most widely used FDG PET parameter, and in most studies relative changes (%) are calculated to quantify metabolic response.
Early therapy response as assessed by FDG PET/CT has shown promising results in single-centre studies and should be evaluated in randomised, prospective, multicentre trials in order to implement FDG PET/CT in clinical practice for this concern in the future. In this context, standardisation of patient preparation, data acquisition and processing, and data interpretation are important issues. In order to establish criteria to assess therapy response with the help of PET/CT, e.g. PERCIST criteria have been developed and implemented (PERCIST 1.0, PET Response Criteria In Solid Tumours) [157].
In this chapter, special emphasis will be on the use of FDG PET/CT for therapy response assessment being discussed in separate paragraphs for almost all of the listed tumour entities.
2.1 Non-small Cell Lung Cancer (NSCLC) and Small Cell Lung Cancer (SCLC)
2.1.1 Diagnosis, Staging and Radiation Treatment Planning in NSCLC Patients
Lung cancer is the most common cancer and the leading cause of cancer-related deaths worldwide [38].
Accurate diagnostic work-up and staging of NSCLC is mandatory with respect to mediastinal lymph node involvement and distant metastases affecting therapy management and prognosis. In particular, it is necessary to differentiate patients with potentially curable and resectable disease at early stages from those, who are not suitable for radical surgery, following the currently used staging system based on the tumour–node–metastasis (TNM) classification (description of primary tumour (T), lymph node involvement (N), and presence of distant metastases (M)).
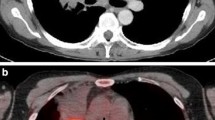
FDG PET/CT allows staging of NSCLC with high diagnostic accuracy [6] and improves staging as compared to CT alone [40, 137] (Fig. 19.1). Several guidelines and professional societies strongly recommend the use of FDG PET/CT for diagnosis/staging (e.g. Interdisciplinary guideline of the German Respiratory Society/German Guidelines Programme Oncology, Cancer Care Ontario (CCO), American Colleges of Chest Physicians and Radiologists (ACP/ACR)) [8, 9, 21, 134] as well as for detection of recurrence/re-staging (e.g. Society of Nuclear Medicine and Molecular Imaging (SNMMI)) [74], partly under special conditions such as planned curatively intended treatment.
47-year old male patient with histopathologically proven non-small cell lung cancer of the left upper lung lobe, referred to FDG PET/CT for primary staging. FDG PET/CT confirmed FDG -positive primary tumour and an already suspected contralateral lung metastasis (cM1a); furthermore, PET/CT revealed an FDG-positive bone metastasis in the Os sacrum (cM1b); (A1-3) CT-Scan, (B1-3) PET scan, (C1-3) PET/CT fused images
The use of PET in the Dutch randomised controlled PLUS trial allowed for avoidance of futile thoracotomies in about 20% of patients with NSCLC (21% as compared to 41% of patients in the group with conventional work-up that did not undergo PET; relative risk reduction of 51% in favour of PET, p = 0.003) [152]. In another trial, Maziak et al. showed that in 337 patients with confirmed clinical stage I, II, or IIIA NSCLC and being considered for surgery (170 assigned to PET/CT and 167 to conventional staging including CT of liver and adrenal glands as well as whole-body bone scan) disease was correctly up-staged in 23 of 167 PET/CT patients and in 11 of 162 conventional staging patients (13.8% vs. 6.8%), thereby sparing these patients from surgery. On the other hand, disease was incorrectly up-staged in 8 PET/CT patients and 1 conventional staging patient (4.8% vs. 0.6%), and it was incorrectly under-staged in 25 PET/CT patients and 48 conventional staging patients, respectively (14.9% vs. 29.6%) [104]. In a recent retrospective study, Kung et al. included 186 potentially operable NSCLC patients, who underwent whole-body FDG PET/CT examination in 2012. Due to disease up-staging 34.9% (65 patients) became inoperable by the results of PET/CT; 102 of the remaining 121 patients received surgery—97 of those proceeded to surgery without further (neoadjuvant) treatment or investigation, 4 received neoadjuvant treatment and 1 had further investigations after PET/CT. As a consequence, changes in management plans occurred in 37.6% due to PET/CT—the authors concluded that PET/CT had great clinical impact with a significant reduction of futile surgery [86].
Schreyögg et al. evaluated the diagnostic accuracy and cost-effectiveness of integrated PET/CT for the staging of NSCLC patients. The authors reported a diagnostic effectiveness in terms of correct TNM staging of 40% for CT alone and 60% for PET/CT. For the assessment of resectability, 84% of patients were staged correctly by PET/CT vs. 70% by CT alone. The cost-effectiveness analysis showed that costs for PET/CT were within the commonly accepted range for diagnostic tests or therapies [129].
With respect to the detection of mediastinal lymph node metastases several studies exist—although FDG PET/CT is more accurate than CT for the staging of mediastinal lymph nodes [137], it is still limited in its ability to differentiate between benign and metastatic lymph nodes. There can be false-negative findings, e.g. due to micrometastases, but false-positive findings seem to be the more decisive problem, e.g. due to inflammation [89]. To solve this problem Rogasch et al. chose an approach similar to the Cheson/Deauville criteria for judging residual lymphoma tumour masses either as vital or not based on a visual scale [28]—after standardised windowing (threshold: 2 × liver SUVmean) they used a 4-point scale as follows: (1) lymph node (LN) uptake ≤ mediastinal blood pool structures (MBPS); (2) MBPS < LN < liver; (3) liver ≤ LN < ‘black’; (4) LN appears ‘black’. As an optimal cut-off to differentiate benign vs. metastatic LNs they found a score >3, reaching high sensitivities (88.9–90.7%), high specificities (92.0–94.6%), high negative predictive values (NPV) (97.2–97.6%), and high accuracies (91.4–93.5%) for different readers with different levels of experience (positive predictive values (PPV) 72.7–80.0%) [125]. There are several other approaches, partly based on SUVmax [89] and on a ratio of lymph node SUVmax to primary tumour SUVmax [30], respectively, or even based on a decision tree model for predicting mediastinal lymph node metastases (including morphologic and functional criteria) [116]. Taken together, more studies applying this or similar decision tree models should be conducted to develop tools for FDG PET/CT readers and to make FDG PET/CT-based nodal staging more reliable.
Distant metastatic spread typically involves the adrenal glands, bones, brain, or liver. Regarding a curative treatment intent, it is of particular interest, whether there are distant metastases present or not, especially if they are unexpected, such as in a low tumour stage. However, the rate of unexpected distant metastases rises with the tumour stage as a prospective Australian trial by MacManus et al. [98] showed including tumour stage I-III patients: at tumour stage I the rate was 7.5%, at stage II 18% and at stage III 24%, which underlines the value of FDG PET/CT also in the staging of (locally) advanced patients, especially with respect to overall survival [144]. FDG PET/CT is suitable for the detection of metastases of the adrenal glands, bones, and liver [6]. For bone metastases, it is superior to conventional bone scintigraphy (with sensitivity, specificity, NPV, PPV and accuracy >90% each) [135], confirmed by similar results of Chang et al. [25]. In contrary, FDG PET/CT is not suitable for the detection of brain metastases due to the high glucose consumption of the normal surrounding brain tissue resulting in an overall poor sensitivity [6]. Thus, additional Magnetic Resonance Imaging (MRI) of the brain should be performed in all symptomatic patients and high-risk patients with curative intention (German S3 guideline, see AWMF [9].
There are further reasons for false-negative findings on FDG PET/CT, mainly due to small lesion size (<5 mm, partial volume effect). Moreover, FDG PET sensitivity may be reduced in specific tumour types showing variable FDG uptake such as bronchiolo-alveolar carcinoma, mucinous forms and neuroendocrine tumours [6].
Finally, in NSCLC patients FDG PET/CT should be used for radiation treatment planning, since it is more accurate in defining the tumour extent than CT alone [16], resulting in a reduction of the dose delivered to normal surrounding tissue (if the PET tumour area is smaller than the one defined on CT) and in the inclusion of areas with viable tumour cells outside the CT-based radiation fields (if PET detects a tumour area more extensive than CT), thus, affecting the target volume definition and the patient`s radiation exposure. In a systematic review and meta-analysis by Hallqvist et al. including 35 cross-sectional studies and one observational study there was a significant change in target delineation in approximately 40% of the patients [61].
2.1.2 Therapy Response Assessment in NSCLC Patients
So far, neoadjuvant chemotherapy is not a standard procedure in NSCLC patients. However, there are many advantages to this treatment modality. First of all, by a neoadjuvant treatment regimen potentially micrometastatic disease should be affected, which accounts for a 5% absolute increase in survival as compared to solely adjuvant chemotherapy [111]. Second, the tissue resected at surgery can serve as a gold standard to evaluate response to a neoadjuvant treatment, as it is already a standard in the assessment of treatment response in early stage breast cancer. And finally, neoadjuvant chemotherapy seems to be better tolerated than adjuvant therapy and the compliance seems to be higher [24].
In a meta-analysis conducted by Zhang et al. evaluating 13 studies with a total of 414 patients a pooled sensitivity, specificity, PPV and NPV for PET-predicted response of 83%, 84%, 74% and 91%, respectively, were reported. All included studies had used pathological outcome as the gold standard. The predictive value of PET in NSCLC patients for evaluating pathological response was significantly higher than that of CT in this meta-analysis [168].
The NEOSCAN trial intended to assess the value of FDG PET/CT for an interim staging in the setting of neoadjuvant chemotherapy in order to guide further neoadjuvant treatment, dependent on response or non-response. 40 stage IB-IIIA lung cancer patients were included, receiving for 2 cycles either cisplatin/carboplatin plus pemetrexed (adenocarcinoma) or cisplatin/carboplatin plus gemcitabine (squamous cell carcinoma). A decrease by at least 35% in SUVpeak in the primary tumour was considered as response, going along with continuation of the same therapy regime for 2 more cycles, whereas therapy of non-responders was switched to vinorelbine plus docetaxel for 2 cycles. In all, 15 patients (38%) were non-responders, of which 10 patients (67%) reacted to vinorelbine plus docetaxel with partial or complete metabolic response as assessed by modified PERCIST criteria, one of those achieving major pathologic response. For all patients, response assessed by PET after 2 and 4 cycles of treatment correlated with major pathologic response (p = 0.016 and 0.034, respectively), whereas CT-based response assessment did not [24].
2.1.3 Staging in SCLC Patients
SCLC is more aggressive than NSCLC and tends to spread with distant metastases much faster. There is a two-staged classification differentiating limited disease (LD) and extensive disease (ED) according to the Veterans Administration Lung Group (VALSG) that was first introduced in the 1950s. Limited disease is defined as disease confined to one hemithorax, the mediastinum and the supraclavicular lymph nodes. All other patients are classified as having ED, including those with malignant pleural effusion [124].
Most of the spare literature concerning FDG PET/CT and SCLC is analysing pre-therapeutic staging. In a systematic review and meta-analysis, Lu et al. showed that among 12 studies with a total of 369 patients the pooled estimated sensitivity and specificity were 97.5% and 98.2%, respectively, for the detection of ED by FDG PET and FDG PET/CT. They regarded FDG PET/CT as a valuable imaging tool for the pre-therapeutic assessment of ED in patients with SCLC [97]. The results of patients with an up-staging after undergoing FDG PET/CT as compared to conventional imaging are similar among several studies, e.g. Zer et al. found a change in the tumour stage up to extensive disease in 6/55 patients (10.9%) [168].
2.2 Thyroid Cancer
2.2.1 Diagnosis, Staging and Re-staging
Differentiated thyroid cancer (papillary or follicular) represents about 1% of all malignant tumours and is the most frequent endocrine cancer [14]. It is generally considered to be highly treatable and curable. However, approximately 30% of all patients with recurrence will develop local recurrence or metastases, which cannot be detected by Iodine-131 whole-body scintigraphy due to a loss of the ability of the tumour cells to concentrate iodine in the process of dedifferentiation [52]. According to the in 2015 revised guidelines of the American Thyroid Association (ATA) ‘18FDG PET/CT scanning should be considered in high-risk DTC [differentiated thyroid cancer] patients with elevated serum Tg [thyreoglobulin] (generally >10 ng/mL) with negative RAI [radioiodine] imaging (strong recommendation, moderate-quality evidence)’. Furthermore, it ‘may also be considered as […] a part of initial staging in poorly differentiated thyroid cancers and invasive Hürthle cell carcinomas […] (weak recommendation, low-quality evidence)’ [63]. In the situation of suspected recurrence, elevated Tg levels, and negative RAI scintigraphy FDG PET/CT is also recommended by The Royal College of Radiologists (RCR) [142]. The European Society for Medical Oncology (ESMO) (working group ‘thyroid cancer’) emphasises the main indication of FDG PET/CT in metastatic patients who have lost radioiodine uptake [115].
With respect to Tg level ‘cut-off’ for the use of FDG PET/CT, studies have shown controversial results. However, a ‘cut-off’ of 10 ng/ml seems to be a reasonable value maintaining high accuracy in terms of a good compromise between sensitivity and specificity [14]. The sensitivity depends on the histological subtype and FDG PET is more sensitive in more aggressive subtypes such as poorly differentiated, tall cell, and Hürthle cell thyroid cancer. Thus, the Tg ‘cut-off’ needs to be adapted and lowered in case of aggressive pathological variants of thyroid cancer that may produce low amounts of serum Tg [63]. The diagnostic accuracy of FDG PET/CT is generally high in patients with negative RAI scans and high Tg levels [14] (Fig. 19.2).
53-year old male patient with minimally invasive follicular thyroid cancer, referred to FDG PET/CT for re-staging with known Iodine-131 positive local recurrence and a thyreoglobulin level of 319 µg/l under TSH suppression. Besides the local recurrence FDG PET/CT showed an infracarinal FDG-positive lymph node metastasis and multiple FDG-positive pulmonary metastases; (A1-3) CT-Scan, (B1-3) PET scan, (C1-3) PET/CT fused images
In a recent meta-analysis enrolling 20 studies with 958 thyroid cancer patients with a previous negative RAI scan the combined sensitivity and specificity for conventional FDG PET were both found to be 84%; for FDG PET/CT they were 93% and 81%, respectively, and the overall accuracies were 91% and 93%, respectively [19].
For detecting recurrence with FDG PET/CT Choi et al. reported a sensitivity, specificity, accuracy, PPV and NPV of 64%, 94%, 83%, 86% and 81%, respectively, including 84 papillary thyroid cancer patients with negative RAI scans and high Tg levels. They also investigated the effect of thyroid-stimulating hormone (TSH) stimulation in comparison with TSH suppression and could not find an added value for TSH stimulation [32].
However, the impact of TSH on FDG PET/CT imaging is still an open issue, no consensus has been reached about the usefulness of high TSH levels. Levothyroxine withdrawal or alternatively the use of recombinant TSH might be preferable, especially in cases of relatively low Tg levels (<10 ng/ml) trying to improve sensitivity of FDG PET [14]. According to ATA guidelines ‘To date, there is no evidence that TSH stimulation improves the prognostic value of 18FDG-PET imaging’ [63].
2.3 Head and Neck Cancer
2.3.1 Diagnosis, Staging and Re-staging
Cancers of the oral cavity and lips were the tenth most common cause of death in males living in developing countries in 2012 worldwide, representing the eighth most common cancers in the same population. Smoking, alcohol use, smokeless tobacco use, and human papillomavirus (HPV) infection are the major risk factors for oral cavity cancer, with smoking and alcohol having synergistic effects [146]. The incidence of HPV-associated head and neck cancer is increasing, most commonly arising from the oropharynx [103].
Histopathologically squamous cell carcinomas account for the vast majority of head and neck cancers with >90% [1, 29]. Most common site is the larynx, followed by oral cavity including tongue, lips and salivary glands [1].
A decisive prognostic factor is the metastatic involvement of cervical lymph nodes, crucial for an adequate therapeutic management [139]. For this reason, the role of FDG PET/CT in initial staging of head and neck cancer patients was investigated in many studies.
The use of FDG PET/CT in primary diagnostics/staging is usually not recommended, with the exception of cancers of unknown primary (CUP) with manifestation in cervical lymph node metastases (to learn more about the use of FDG PET/CT in CUPs please note the additional section following below). Cacicedo et al. conducted a prospective study enrolling 84 patients with newly diagnosed, locally advanced head and neck squamous cell carcinoma (stage III–IV disease according to the American Joint Committee on Cancer (AJCC) classification (seventh edition), histologically confirmed). After conventional work-up (physical examination, CT imaging of the head, neck and chest) each patient underwent FDG PET/CT and the results were separately discussed in multidisciplinary tumour boards—TNM stage was determined and afterwards validated by histopathological analysis. The authors found a discordance for 32/84 (38%) patients between the results of conventional work-up and FDG PET/CT with the highest impact on N stage (in 21/32, 65.7%), followed by a change in patient management in 22/84 patients (26%)—PET/CT TNM-classification was significantly more accurate (92.5% vs. 73.7%) than conventional staging with a p-value < 0.001 [18].
There is an ongoing debate, if FDG PET/CT should be implemented in the routine diagnostic work-up of locally advanced head and neck cancers—the National Comprehensive Cancer Network (NCCN) guidelines recommend consideration of FDG PET/CT in the assessment of the initial treatment strategy for loco-regionally advanced disease to evaluate for distant metastases [36]; also RCR recommends the use in this patient population or if initial imaging is inconclusive/with equivocal findings that would preclude radical treatment [142].
However, the main field of FDG PET/CT in head and neck cancer patients is the follow-up and treatment response assessment after chemoradiotherapy.
In routine follow-up, FDG PET/CT is usually recommended after inconclusive conventional imaging to differentiate treatment effects from tumour residuals/recurrence [142] or if there is a high risk for distant metastases due to initial TNM stage, especially in patients considered for major salvage treatment [8]. Recurrence or relapse mostly occurs within the first 2–3 years after first diagnosis [29, 64, 131].
In the management of local recurrence, direct laryngoscopic techniques and physical examination remain key aspects, followed by PET/CT or other imaging modalities as important adjuncts in detecting recurrence in lymph nodes and more distant sites [74] (Fig. 19.3).
55-year old male patient with histopathologically proven second local recurrence of oropharyngeal carcinoma (initially TNM-classification pT2 pN0 L0 V0 R0 cM0, first recurrence rpT3 pN0 L0 V0 Pn1 cM0), referred to FDG PET/CT for re-staging. FDG PET/CT confirmed FDG-positive recurrence in the right oropharynx; furthermore, PET/CT revealed an FDG-positive lung metastasis in the left upper lung lobe (cM1); (A1-2) CT-Scan, (B1-2) PET scan, (C1-2) PET/CT fused images
A recent meta-analysis evaluating 23 studies (constituting 2,247 PET/CT examinations) found a pooled sensitivity and specificity of 92% and 87%, respectively, for detection of recurrence in general (local, regional and distant) in head and neck cancer patients by FDG PET/CT. The pooled sensitivity and specificity of scans performed 4–12 months after treatment were 95% and 78%, respectively, with similar sensitivity but higher specificity for scans performed ≥12 months after treatment with 92% and 91%, respectively [131]. In order to differentiate between the primary site, the loco-regional site and the distant site another meta-analysis including 27 studies (1,195 patients) was conducted recently: the authors found a pooled sensitivity and specificity of 86.2% and 82.3%, respectively, for detecting recurrence at the primary site, of 72.3% and 88.3%, respectively, at the loco-regional (neck) site and of 84.6% and 94.9%, respectively, for distant metastases [29].
2.3.2 Therapy Response Assessment
The prospective randomised PET-Neck trial investigated the role of FDG PET/CT performed 12 weeks after the end of chemoradiotherapy in 564 N2- or N3-patients to decide about the conduction of neck dissection, which was only employed if PET/CT showed an equivocal or incomplete response. PET/CT surveillance resulted in fewer neck dissections than did planned surgery (54 vs. 221), the 2-year overall survival rate was 84.9% in the surveillance group and 81.5% in the planned-surgery group—additionally PET/CT surveillance was more cost-effective over the duration of the trial [105].
The use of FDG PET/CT in follow-up after chemoradiotherapy was also recommended by other authors. There is a broad consensus that a period of at least 12 weeks after the completion of treatment and the scan should be kept to avoid non-specific chemoradiotherapy-related inflammatory FDG uptake [131]. It is still unclear, if HPV-positive patients should undergo FDG PET/CT at a later time after completion of primary treatment, since nodal disease might take longer to respond and regress in these patients [64, 69].
In an effort to standardise image interpretation, Marcus and colleagues proposed the so-called ‘Hopkins criteria’ in order to assess therapy response for head and neck cancers from the results of a post-therapy PET/CT-scan by using a 5-point scale comparing the intensity (as compared to SUV values of the liver and the internal jugular vein) and pattern (focal or diffuse) of FDG PET uptake in primary tumour and neck nodes. This assessment was shown to be highly specific with 92.2% and to carry a high NPV of 91.1% in an evaluated patient collective (57.5% HPV positive) [103].
2.4 Oesophageal Cancer
2.4.1 Diagnosis, Staging and Re-Staging
Oesophageal cancer is one of the most common cancers worldwide being the ninth most common cancer and the sixth most common cause of cancer-related deaths [88]. There are two major histological subtypes of oesophageal cancer: squamous cell carcinoma and adenocarcinoma, the first being previously much more common than the second one. However, a marked increase in the incidence of oesophageal adenocarcinoma in Europe, North America and Australia has been observed during the past four decades, so the incidence of oesophageal adenocarcinoma has surpassed that of oesophageal squamous cell carcinoma in many western countries [88]. Oesophageal adenocarcinoma is mostly originating in the lower third of the oesophagus, often involving the oesophago-gastric junction. The main pathophysiological pathway of oesophageal adenocarcinoma is likely to be chronic gastro-oesophageal reflux, causing a metaplasia known as ‘Barrett’s oesophagus’.
Relevant pre-therapeutic prognostic factors are local tumour invasion, loco-regional lymph node and distant metastases. Endoscopic ultrasound and CT represent the most widely used imaging modalities for the assessment of local tumour invasion (T stage), loco-regional lymph node involvement (N stage) and also distant metastases stage (M stage) [161].
Sensitivity and specificity of FDG PET/CT for staging oesophageal cancer have been investigated in many studies. For instance, Purandare et al. conducted a study enrolling 156 patients with potentially curable oesophageal adenocarcinoma and they found a change in the intent of treatment in 16% of the patients by detecting M1b disease with high sensitivity, specificity, PPV, NPV and accuracy (83.3%, 98.4%, 92.5%, 96.1% and 95.3%, respectively). The authors concluded that FDG PET/CT should be implemented in the initial staging work-up [121].
In their systematic review and meta-analysis Goense et al. assessed the performance of FDG PET/CT for the detection of recurrent oesophageal cancer after curatively intended treatment. Evaluating 8 studies, they found a high sensitivity (96%) and moderate specificity (78%), concluding that histopathologic confirmation of PET/CT-suspected lesions remains necessary due to a considerable false-positive rate [56].
In international guidelines, the recommendation for the use of FDG PET/CT in staging and re-staging of oesophageal cancer differs—in some guidelines the use is recommended for a certain patient cohort, e.g. ESMO where FDG PET/CT should be carried out in candidates for oesophagectomy to exclude distant metastases [94], whereas other guidelines (NCCN) advise FDG PET/CT in initial staging as routine clinical work-up if there is no evidence of M1-disease. Similar to ESMO, NCCN also advises FDG PET/CT in re-staging before surgery for the detection of distant metastases for all patients who receive chemoradiotherapy either as neoadjuvant or definitive treatment [3]. According to the German S3 guideline FDG PET/CT may be used for locally advanced cancers (cT2-4, cN+) to exclude distant metastases prior to curatively intended treatment in anticipation of clinical consequences [120]. The use of FDG PET/CT is also recommended by some societies, e.g. RCR for staging/re-staging of patients suitable for radical treatment, including patients who have received neoadjuvant treatment, and for evaluation of suspected recurrence when other imaging is negative or equivocal [142].
2.4.2 Therapy Response Assessment
The MUNICON I trial was one of the first clinical trials to provide evidence that FDG PET can be used to individualise neoadjuvant therapy—in this trial chemotherapy in patients with locally advanced oesophageal adenocarcinoma. Treatment was adapted based upon PET results conducted 2 weeks after chemotherapy induction in the way that metabolic responders (predefined as decreases of SUV values by 35% or more) received more cycles of chemotherapy for a maximum of 12 weeks, whereas metabolic non-responders underwent surgery immediately [95].
The prospective MUNICON II trial was designed to potentially improve the clinical outcome of metabolic non-responders by applying a salvage neoadjuvant chemoradiotherapy; the authors found an increased histopathologic response rate as compared to the results of the MUNICON I trial, but the primary endpoint of the study—to increase the R0-resection rate—was not met [171].
In contrast, if FDG PET/CT is used to assess therapy response in squamous cell carcinoma patients, it should be conducted after completion of the neoadjuvant therapy [113].
2.5 Gastric Cancer and Gastrointestinal Stromal Tumour (GIST)
2.5.1 Diagnosis, Staging and Re-staging in Gastric Cancer Patients
Gastric cancer is the fourth most common cancer and the second leading cause of cancer-related deaths worldwide [92]. Gastric adenocarcinoma accounts for about 95% of all types of gastric cancer. There are two major subtypes according to Lauren’s classification: intestinal type, which often evolves from the distal stomach in association with chronic Helicobacter pylori infection, and diffuse or signet ring type (non-intestinal type), which commonly evolves from the proximal stomach and is more often found in Western patients combined with chronic reflux and obesity [82].
In the staging of gastric cancer the use FDG PET/CT is limited by a reduced sensitivity. Only about 60% of locally advanced gastric cancers are FDG avid [138]. Especially tumours with non-intestinal type histology are often not FDG avid and can therefore not be imaged by FDG PET/CT. Furthermore, FDG non-avidity is associated with small tumour size, mucinous content and localisation in the distal third of the stomach [65]. For the detection of gastric cancer by FDG PET sensitivities range from 47 to 96% (mean sensitivity 77%, mean specificity 99%) [66]. For detection of loco-regional lymph nodes FDG PET/CT seems to be more specific, but less sensitive as compared to CT alone—FDG PET/CT sensitivity, specificity and accuracy range from 41 to 74%, 75 to 100% and 51 to 76%, respectively, whereas these parameters for contrast-enhanced CT range from 70 to 83%, 62 to 92% and 67 to 80%, respectively [82].
Wu et al. carried out a systematic review and meta-analysis to evaluate the use of FDG PET for the detection of recurrent gastric cancer. Across 9 studies (526 patients) the overall sensitivity of FDG PET was found to be 78% and the overall specificity 82% [162]. A more recent meta-analysis enrolling 14 studies with 828 patients reported a high sensitivity of 85% and a moderate specificity of 78% for FDG PET or FDG PET/CT for diagnosing recurrent gastric cancer—the authors concluded that FDG PET has a great value in the detection of recurrent gastric cancer after surgical resection, which might become more important in the future given the high recurrence rates of gastric cancer [92].
2.5.2 Diagnosis, Staging and Re-staging in GIST Patients
Most of gastrointestinal stromal tumours (GISTs) are found in the stomach (60%), another third is found in the small intestine [126]. Since GISTs often show a high FDG avidity, FDG PET/CT can be used to evaluate these tumours and their response to therapy [150]. A correlation between the FDG uptake and the malignant potential of GISTs presented by the Ki67 labelling index as a mitotic index has been reported by Kamiyama et al. [80].
FDG PET/CT is suitable for the initial staging of GIST patients, since at least half of the patients present with distant metastases at the time of diagnosis [101]. Most common sites for distant metastases are the liver and the peritoneum; loco-regional lymph node metastases are very uncommon [126]. Nevertheless, there is a significant number of patients with GISTs of about 10% that do not show the typically strong FDG uptake on baseline PET scan—this might be related to a high degree of tumour necrosis and myxoid/zystoid degeneration [101, 126].
2.5.3 Therapy Response Assessment in GIST Patients
Metabolic response as assessed by FDG PET/CT is closely related to clinical outcome for GIST patients. Changes of FDG uptake occur early, whereas morphological changes occur late in the course of imatinib therapy [147]. In a systematic review and meta-analysis, Hassanzadeh-Rad et al. found out that the accuracy of FDG PET/CT is higher in detection of treatment failure (e.g. non-responders) than in prediction of good response to therapy [62].
2.6 Colorectal Cancer
2.6.1 Diagnosis, Staging and Re-staging
In the United States in 2017 colorectal cancer was supposed to be the second leading cause of estimated cancer-related deaths in men and the third leading cause in women, even though, its incidence has decreased continually since 2004 by approximately 3% per year until 2013 [132].
At an early stage surgery is a curative treatment approach; however, for locally advanced cancers (pT3-4 or any T N1) usually a multimodality treatment approach is chosen, which includes pre-operative concomitant chemotherapy and radiotherapy [82].
In general, FDG PET/CT is considered not ‘useful’ in the initial staging of colorectal cancer patients, since the added value as compared to conventional imaging seems to be too little [82]. However, in a retrospective study, Petersen et al. analysed the data from 67 patients, who underwent FDG PET/CT additionally to conventional imaging (CT, MRI and/or ultrasound) for initial staging—they found a change in treatment plans in 30% of the cases due to FDG PET/CT, another third of this 30% was either a change from intended curative to palliative therapy or vice versa [118]. Lee and Lee also conducted a retrospective study enrolling 266 colon cancer patients also undergoing both FDG PET/CT and conventional imaging. Multidetector CT and FDG PET/CT showed similar accuracy in detecting lymph node metastases in patients with clinical stage III (36.2% vs. 42%, p = 0.822) and stage IV (60.3% vs. 63.5%, p = 0.509) disease. However, FDG PET/CT results led to a change in management for 1 of 40 (2.5%) with clinical stage I, 0 of 25 (0%) with stage II, 9 of 138 (6.5%) with stage III, and 8 of 63 patients (12.7%) with stage IV disease. The authors concluded that FDG PET/CT might be considered as a routine staging tool for clinical stage III and IV colon cancers [90].
Considering the M stage, liver metastases are most common in colorectal cancer patients with an incidence of approximately 50–60%, of which about one-third are diagnosed at the same time as the primary tumour is detected [82]. A recent meta-analysis by Maffione et al. including a total of 1,059 patients reported a high accuracy for FDG PET and FDG PET/CT for the detection and staging of liver lesions in colorectal cancer (pooled sensitivity and specificity of 93% in patient-based analysis, and 60% and 79%, respectively, in lesion-based analysis). PET showed a lower sensitivity as compared to MRI and CT in patient-based analysis (93%, 100% and 98%, respectively) and lesion-based analysis (66%, 89% and 79%, respectively); however, PET was more specific as compared to MRI and CT in both patient-based and lesion-based analysis (81%, 70% and 70%, respectively, and 86%, 81% and 67%, respectively). FDG PET and PET/CT results led to a change in management in an average of 24% of patients, entailing both exclusion from curative surgery and modification of the surgical approach. The mean incidence of extra-hepatic disease shown by FDG PET or PET/CT, but not detected by conventional imaging was 32% [99].
In the follow-up and detection of recurrent colorectal cancer, FDG PET/CT is regarded as a valuable tool. In a Danish study conducted by Engelmann et al. with a total of 66 prospectively included patients FDG PET/CT detected all relapses occurring within the first 2 years. Cumulative relapse incidences for clinical stages I–III (n = 42 patients) at 6, 12, 18 and 24 months were 7.1%, 14.3%, 19% and 21.4% [45]. A recent meta-analysis including 26 studies (1,794 patients) revealed a pooled sensitivity and specificity of 94% each for FDG PET and PET/CT in the detection of locally recurrent colorectal cancer [166]. Based on rising CEA levels (biochemical recurrence) many studies were conducted examining the usefulness of FDG PET/CT in this situation. In a systematic review and meta-analysis enrolling 11 studies with 510 patients Lu et al. found a pooled sensitivity and specificity of 90.3% and 80.0%, respectively, for FDG PET and a pooled sensitivity and specificity of 94.1% and 77.2%, respectively, for FDG PET/CT in the detection of recurrence in colorectal cancer patients with elevated CEA levels [96].
The use of FDG PET/CT in colorectal cancer patients is recommended by some professional societies such as RCR, seeing the usefulness in many indications, e.g. initial staging of patients with synchronous metastases suitable for resection, re-staging of patients with recurrence (suspected by rising tumour markers and/or clinical suspicion of recurrence with normal or equivocal findings on other imaging), especially if being considered for radical treatment and/or invasive targeted techniques, or assessment of treatment response in patients with rectal carcinoma post (chemo)radiotherapy with indeterminate findings on other imaging [142]. Also SNMMI recommends the use of FDG PET/CT in re-staging for local recurrence and (distant) metastases, especially in the case of rising tumour markers with negative or equivocal findings on conventional imaging [74]. Furthermore, in the initial staging FDG PET/CT ‘may be appropriate’, if distant metastases are suspected in the pre-treatment staging of colorectal cancer according to the ACR Appropriateness Criteria® [51].
According to NCCN guidelines, FDG PET/CT is not indicated for pre-operative staging of rectal cancer in general, and should only be used to evaluate an equivocal finding on a contrast-enhanced CT-scan or in patients with a strong contraindication to intravenous contrast [13]. Following ESMO guidelines, PET/CT ‘may be helpful’ to characterise extra-hepatic disease [149].
2.6.2 Therapy Response Assessment
For therapy response assessment in rectal cancer patients a meta-analysis conducted by Maffione et al. (34 studies, 1,526 patients) found a high pooled accuracy for FDG PET and PET/CT to predict therapy response for the global cohort at the end of treatment (pooled sensitivity 73%, pooled specificity 77%), and especially for early interim PET performed between 1 and 2 weeks after the initiation of neoadjuvant chemoradiotherapy (pooled sensitivity 84%, pooled specificity 81%) [100].
2.7 Pancreatic Cancer
2.7.1 Diagnosis, Staging and Re-Staging
Pancreatic cancer is one of the most lethal cancers with a very poor prognosis and poor overall survival (5-year survival rate of about 4% [154]) as curative therapy is restricted to patients suffering from limited disease referred to surgery.
Commonly used imaging tools for the diagnosis of exocrine pancreatic cancer are ultrasound, endosonography, CT, MRI, and magnetic resonance and endoscopic retrograde cholangiopancreatography (MRCP and ERCP). Since 70–90% of exocrine pancreatic cancers show a high FDG uptake, FDG PET/CT was introduced to potentially improve detection of pancreatic adenocarcinomas.
Tang et al. assessed the diagnostic impact of FDG PET versus FDG PET/CT versus endoscopic ultrasonography in diagnosis of patients with pancreatic carcinoma. The authors reported that FDG PET/CT had a high pooled sensitivity of 90.1%, whereas the specificity was moderate with 80.1% in comparison with endoscopic ultrasonography with a pooled sensitivity and specificity of 81.2% and 93.2%, respectively. They concluded that FDG PET/CT and endoscopic ultrasonography might have different (complementary) roles under different conditions in diagnosing pancreatic carcinoma [141].
Fletcher et al. reported that PET improved differentiation between benign and malignant pancreatic tissue in the diagnostic work-up of patients with suspected pancreatic lesions and might reduce the need for biopsy and surgery influencing morbidity. They recommended FDG PET(/CT) as an additional tool in selected patients demonstrating inconclusive conventional imaging findings [49].
In the situation of potentially operable pancreatic adenocarcinoma an FDG PET/CT-scan is also recommended after inconclusive imaging by RCR [142]. CCO even recommends PET/CT staging in surgery candidates to confirm conventional staging: ‘PET is recommended for staging if a patient is a candidate for potentially curative surgical resection as determined by conventional staging’ [21].
Recently, several studies were conducted to investigate the usefulness of FDG PET/CT in the follow-up after curative surgery and in suspected recurrence, especially if tumour marker levels (CA 19-9) are high and conventional imaging is equivocal. Jung et al. investigated the usefulness of FDG PET/CT in the follow-up of curatively resected pancreatic cancer patients in comparison with CT alone: PET/CT showed a higher sensitivity (84.5% vs. 75.0%) and accuracy (84.5% vs. 74.5%) than CT alone, also in the detection of distant metastases (sensitivities 83.1% vs. 67.7%). In 19 out of 110 patients, recurrences were only seen on PET/CT [79].
In another study, the additional value of FDG PET/CT in unresectable pancreatic carcinoma patients prior to chemoradiotherapy was examined as compared to conventional imaging—in 19 out of 71 (26.8%) PET/CT staging showed distant metastases not detected by conventional staging, entailing a change in the conduction of chemoradiotherapy (or chemotherapy alone, respectively). The authors concluded that PET/CT-based staging might help to select the patients who are suitable for chemoradiotherapy, sparing those patients with metastases from futile radical protocols [145].
2.8 Melanoma
2.8.1 Diagnosis, Staging and Re-staging
Malignant melanoma is one of the most common tumour entities in both sexes worldwide—in the United States there were to be expected an estimated 6% of new cases in males (5th most common) and an estimated 4% in women (6th most common) in 2017, but due to new efficient treatment options like immunotherapies melanoma was not to be expected among the 10 cancer entities with the highest mortality rates [132]. Incidence rates have been rising for many years worldwide by approximately 3% per year since 2004 [123].
Since tumour stage is the most important predictive factor for survival rates, initial staging of the disease is crucial. Surgery remains the gold standard in the treatment of loco-regional disease, but even in stage IV-patients (according to AJCC) survival rates have been improved by metastasectomy [123]. In general, FDG PET/CT has a higher sensitivity and specificity for detecting (distant) metastases as compared to conventional imaging. However, additional MRI of the head should be performed to exclude brain metastases, which might not be detected by PET/CT due to the high physiologic cerebral uptake and a resulting diminished sensitivity. Furthermore, by virtue of limited spatial resolution FDG PET/CT is inferior to sentinel lymph node biopsy in the detection of clinically occult lymph node metastases.
According to NCCN guidelines, PET/CT is recommended in patients equal or higher than stage III with clinically suspicious lymph nodes or with in-transit metastases (IIIB or higher) [35]. ESMO guidelines state that ‘before undertaking additional aggressive local surgical treatments, a detailed staging investigation, that includes high-resolution imaging techniques, such as PET, CT or magnetic resonance imaging is necessary to exclude distant metastases’, and specify that ‘in pT stages > pT3a [i.e. in patients equal or higher than stage IIA], computed tomography (CT) or positron emission tomography (PET) scans are recommended before surgical treatment and sentinel node biopsy’ [43] (Fig. 19.4). The German S3 guideline is not in agreement with ESMO guidelines by stating PET/CT shall not be performed routinely as initial staging procedure up to stage IIA/IIB (suggesting to treat stage IIC patients like high-risk patients); however, in the primary staging of stage III patients and higher it has been shown that PET/CT is superior to the other methods in diagnostic accuracy [10].
78-year old male patient with melanoma of the left shoulder, pT4b, corresponding to AJCC stage IIC, referred to FDG PET/CT for initial staging after primary resection without sentinel lymph node biopsy. FDG PET/CT showed an FDG-positive lymph node metastasis in the left axilla and revealed another suspicious FDG-positive nodule adjacent to the right musculus pectoralis major; (A1) CT-Scan, (B1) PET scan, (C1) PET/CT fused images
A meta-analysis conducted by Xing et al. enrolling 10,528 patients (regardless of stage) investigated the utility of ultrasonography, CT, PET and PET/CT for staging and surveillance of melanoma patients—in the staging of distant metastases the highest sensitivity of 80% was found for PET/CT, going along with a moderate specificity of 87%. Similar results were found in the surveillance of patients with the help of PET/CT to exclude distant metastases [164]. A more recent meta-analysis aimed to review the utility of FDG PET in the detection of systemic metastases in patients with stage III melanoma, including 623 patients. The authors found a sensitivity and specificity of 89.42% and 88.78%, respectively; the area under the operating curve was 0.94. A change in stage and/or management was noted in 22% of patients [123].
In suspected recurrent disease, employment of FDG PET/CT is highly recommended by SNMMI [74], by CCO if just a solitary metastasis is identified at the time of recurrence prior to metastasectomy [21] and by NCCN in the case of nodal recurrence ‘to evaluate specific signs or symptoms’ [35].
A prospective multicentre PET registry study was conducted in Ontario (Canada) to assess the use of PET in advanced or high-risk melanoma as adjunct to clinical and standard radiologic investigation. Approximately 319 patients with potentially resectable high-risk melanoma or recurrent disease under evaluation for isolated metastasis underwent PET/CT, 10 of those already presenting with M1 stage (stage IV). A significant increase in stage to M1 was found after PET/CT in 17.6% of the patients, resulting in significantly more distant surgical interventions in this group as compared to the group without up-staging. None of the already assigned M1 patients was down-staged after PET/CT. The authors concluded that PET/CT has a significant impact on surgical management [136].
Usually, FDG PET/CT is not recommended for routine follow-up; however, according to ESMO guidelines and the German S3 guideline high-risk patients (starting from stage IIC onwards) should routinely undergo cross-sectional imaging, which may include cranial MRI and PET/CT, whole-body MRI or whole-body CT (German S3 guideline, AWMF [10] or ‘ultrasound of lymph nodes, CT or whole-body PET or PET/CT scans’ (ESMO, see Dummer et al. [43], respectively.
In order to assess the value of FDG PET/CT for patient management of melanoma patients Mena et al. carried out a retrospective study including 71 patients (regardless of stage) with 4 or more post-operative follow-up PET/CT-scans (246 scans). Recurrence was found in 39.0% of the scans, the examinations resulted in a change of patient’s management in approximately 16.7% of the scans. Change in management was significantly higher in patients with prior clinical suspicion of malignancy recurrence than without prior clinical suspicion. There was a statistically significant difference in the overall survival between patients with at least 1 positive scan and patients with 4 or more negative subsequent follow-up scans at patient level [106]. More recently, a retrospective study analysed the data of 238 patients with 526 scans in Denmark—since 2015 PET/CT has been implemented in the Danish national follow-up programme as a routine examination at 6, 12 and 24 months after treatment for malignant melanoma of stage IIB or higher. However, in the analysis melanoma patients were included regardless of stage (starting from stage IA onwards). The authors found a sensitivity, specificity, PPV and NPV of 89%, 92%, 78% and 97%, respectively. Here there was no significant difference in the diagnostic accuracy of PET/CT between patients referred with or without clinical suspicion of relapse [153]. A prospective study including 170 post-operative stage III patients with a total of 502 scans and a median follow-up of 47 months found relapses in 38% of the patients, the majority of them being asymptomatic. Overall sensitivity and specificity of the approach of sub-stage specific (IIIA/B/C) PET surveillance were 70% and 87%, respectively. PPV and NPV were calculated sub-stage- and time point-dependent—a negative PET at 18 months implied NPVs of 80–84% for true non-recurrence in the follow-up period. The authors considered PET/CT to be suitable to detect (asymptomatic) resectable and potentially curable disease at relapse [91].
2.8.2 Therapy Response Assessment
The therapy of unresectable metastatic melanoma has changed significantly by the introduction of immune check-point inhibitor therapy—the first FDA approved one was ipilimumab in 2011 for the treatment of malignant melanoma.
Sachpekidis et al. studied 41 patients with advanced melanoma prior to and after 2 cycles of ipilimumab treatment with FDG PET/CT (interim PET/CT). Earlier the authors had found that the absolute number of new lesions is a better parameter for prediction of immunotherapy response than changes in SUV and established the so-called PET Response Evaluation Criteria for Immunotherapy (PERCIMT). In this study therapy response assessment was based both on European Organization for Research and Treatment of Cancer (EORTC) criteria from 1999 and the proposed PERCIMT criteria. The patients were dichotomized into those with clinical benefit (CB including stable disease (SD), partial response (PR) and complete response (CR)), accounting for 31 patients, and those without CB (no-CB) showing progressive disease (PD), affecting the remaining 10 patients. According to EORTC criteria interim PET/CT demonstrated PD (meaning no-CB) in 20 patients, according to PERCIMT criteria PD was seen in 9 patients; SD and PR were seen in 19 patients according to EORTC criteria and in 30 patients according to PERCIMT criteria, and CR was seen in 2 patients according to both criteria, resulting in sensitivities (correctly predicting CB), specificities (correctly predicting no-CB), PPV, NPV and accuracies of 64.5%, 90.0%, 95.2%, 45.0% and 70.7%, respectively, for EORTC criteria, and 93.6%, 70.0%, 90.6%, 77.8% and 87.8%, respectively, for PERCIMT criteria [128].
2.9 Lymphoma
2.9.1 Diagnosis, Staging and Re-staging
Lymphomas are a heterogeneous group of malignancies characterised by diverse morphologies, immunophenotypes and cytogenetics. The major subdivision into Hodgkin’s lymphoma (HL) and non-Hodgkin lymphoma (NHL) is following histopathological criteria, with HL presenting ‘Sternberg-Reed cells’ microscopically. Although the World Health Organisation (WHO) does not recommend a ‘grouping’ of lymphomas, there is a further classification dividing NHLs into indolent (low-risk) and aggressive/highly aggressive (intermediate- and high-risk) NHLs, based on terms of biologic behaviour and the subsequently required aggressiveness of the treatment [75].
With an estimated 4% (women) and 5% (men) of new cases in 2017 in the United States, NHLs were supposed to be the 7th most common malignancy in both men and women [132].
In international guidelines and recommendations by professional societies, FDG PET/CT is often recommended in the initial staging work-up of lymphoma patients, especially in HL patients, e.g. RCR [142], NCCN [68] and ESMO [44]. While HL patients usually display a high FDG avidity on PET/CT-scans, the FDG avidity of NHL patients differs. However, almost all histological (nodal) NHL subtypes were reported to be highly FDG avid with the exception of extra-nodal marginal zone lymphoma and small lymphocytic lymphoma [160], and lymphoplasmacytic lymphoma/Waldenström’s macroglobulinemia and mycosis fungoides (unless there is a suspicion of aggressive transformation) [28].
Based on published data, a consensus statement of the Malignant Lymphomas Imaging Working Group (MLIWG) on International Conferences in 2011 and in 2013 (Lugano, Switzerland) recommended the use of PET/CT in the staging of all FDG avid lymphomas, including indolent subtypes [28].
A prospective multicentre PET registry study published in 2017 was conducted to evaluate the clinical impact of pre-treatment FDG PET/CT on the staging and management of apparent limited-stage indolent lymphoma being considered for curative radiation therapy. Significantly, 47 patients (23.9%) of the included 197 patients were up-staged by the PET/CT result from the presumed limited-stage (stage I–II according to Cotswold-modified Ann Arbor classification) to advanced-stage disease (stage III–IV). Vice versa, 10 patients (5.1%) were down-staged, 4 of those patients from advanced-stage to limited-stage disease. After PET/CT (available data of 155 patients), 95 patients (61.3%) were planned to receive active treatment, of whom 59 patients were assigned to undergo the initially planned radiation therapy alone—finally, 34 patients of those received this treatment. The authors concluded that PET/CT should be implemented in the standard work-up of indolent lymphoma patients, as there was a significant impact on further clinical treatment in their study [107].
Instead of TNM staging, the staging of lymphoma patients follows the so-called ‘Ann Arbor classification’, lastly modified in Cotswold in 1988, which takes the site and the dissemination of involved lymphoid and extra-nodal tissue into account. The highest stage IV includes extra-nodal involvement other than contiguous/proximal lesions to the known nodal site, e.g. bone marrow involvement. Since bone marrow biopsy is an invasive method and gives information only about a small part of the bone marrow, many studies were conducted to investigate the sensitivity and specificity of FDG PET/CT in the assessment of bone marrow involvement in lymphoma patients. Recently, in a retrospective analysis evaluating 832 HL patients from the German Hodgkin Study Group trials HD16, HD17 and HD18, the results of bone marrow biopsy and PET scans (either in combination with CT or MRT and in 31 patients (4%) PET alone) were compared. With bone marrow biopsy as reference standard PET showed a sensitivity, specificity, PPV, and NPV of 95.0%, 86.5%, 14.7% and 99.9%, respectively. Considering both bone marrow biopsy and PET as gold standard, sensitivity, specificity, PPV, and NPV of PET were 99.2%, 100%, 100% and 99.9%, respectively. PET found 110 additional patients with suspect focal bone marrow lesions who would have been considered negative by biopsy. Regarding these results, bone marrow biopsy seems to be futile in PET negative HL patients and the authors recommended biopsies only in PET positive patients that would undergo a changed treatment protocol as a result of the suspected bone marrow involvement [155].
Also in diffuse large B-cell lymphoma, the most common subtype with 30–35% of all NHL lymphomas, FDG PET/CT was reported to be more sensitive than bone marrow biopsy for the detection of bone marrow involvement [28]. In their systematic review and meta-analysis, Adams et al. included 7 studies with a total of 654 patients. The pooled estimates for sensitivity and specificity of FDG PET/CT for detecting bone marrow involvement were 88.7% and 99.8%, respectively. The weighted summary proportion of FDG PET/CT negative patients with positive bone marrow biopsy was 3.1%. In conclusion, FDG PET/CT was considered as a complementary tool to bone marrow biopsy for detecting bone marrow involvement, since it cannot be ruled out by a negative FDG PET/CT-scan [2]. However, even if positive PET findings were reported to be highly predictive and are considered usually sufficient to define advanced-stage disease [28], there is no consensus whether diffuse FDG bone marrow uptake should be regarded as positive or negative for involvement [2]. Furthermore, the histological type of lymphomatous cells in the bone marrow (small or large cells meaning discordant or concordant involvement) might have an influence on FDG avidity with FDG PET/CT being more sensitive in concordant bone marrow involvement [2].
Another retrospective study enrolling 185 patients with newly diagnosed lymphoma including indolent subtypes (47 patients, 30 follicular lymphomas and 17 marginal zone lymphomas) found a high concordance rate between bone marrow biopsy and PET/CT in the detection of bone marrow involvement for aggressive B-cell lymphoma (88.1%) and HL (93.8%), as well as high NPVs for PET/CT in the same histological subtypes (93.2% for aggressive B-cell lymphoma and 100% for HL). However, the concordance rate and NPV were much lower in indolent B-NHL with 66.0% and 66.7%, respectively—the authors considered bone marrow biopsy as an indispensable staging procedure in indolent lymphoma patients [31].
In follow-up, routine surveillance scans with FDG PET/CT are not recommended, since there is a high number of false-positive scans (>20%), leading to further unnecessary investigations, such as biopsies, and to increased patient anxiety. Therefore, follow-up scans should be based on clinical indications [28].
2.9.2 Therapy Response Assessment
According to the ‘Lugano Classification’ based on the results of the already mentioned 2 international conferences in Lugano, therapy response assessment is recommended to be performed by FDG PET/CT using the 5-point scale (Cheson/Deauville criteria) both for interim and end-of treatment response for all FDG avid lymphomas within clinical trials [28]. So far, therapy response should be assessed only visually, since there are not enough data for an SUV-based cut-off assessment. However, in NHL patients there are controversies about the interreader agreement after PET/CT applying the 5-point scale [11]. For end-of-treatment assessment, PET/CT is the standard of care for remission assessment in FDG-avid lymphoma (Fig. 19.5). However, for interim assessment, there are ongoing clinical trials evaluating and also questioning the usefulness of interim FDG PET/CT in therapy response assessment, especially during immunochemotherapy in diffuse large B-cell lymphomas [11].
19-year old male patient with Hodgkin lymphoma, referred to FDG PET/CT for initial staging (August 2015; images A1/B1/C1) and for re-staging (January 2016; images A2/B2/C2) after chemotherapy (6 cycles of BEACOPP August-December 2015). Initial FDG PET/CT showed manifestations of lymphoma on both sites of the diaphragm (Ann Arbor stage III), FDG PET/CT after chemotherapy showed vital lymphoma residuals in the mediastinum (visually Cheson/Deauville score 3–4, which means > mediastinal blood pool uptake, but ≤ liver uptake and moderately above liver uptake, respectively). As a consequence the patient was referred to adjuvant radiotherapy; (A1-2) CT-Scan, (B1-2) PET scan, (C1-2) PET/CT fused images
There are a lot of studies investigating the usefulness of FDG PET/CT in interim therapy response assessment in HL patients, also at advanced-stage disease. In an international prospective multicentre trial, 1,119 patients with advanced-stage HL underwent interim PET/CT-scans, of whom 937 patients showed negative scans. Those patients with negative PET findings after 2 cycles of ABVD (doxorubicin, bleomycin, vinblastine and dacarbazine) chemotherapy were randomly assigned to continue ABVD or omit bleomycin in the following 4 cycles. The 3-year progression-free survival rate and overall survival rate in the ABVD group were 85.7% and 97.2%, respectively; the corresponding rates in the AVD group were 84.4% and 97.6%, respectively. In summary, the omission of bleomycin from the ABVD regimen after negative interim PET/CT resulted in a lower incidence of pulmonary toxic effects than with continued ABVD but not significantly lower efficacy [77].
Recently, in an open-label international phase 3 trial 1,013 advanced-stage HL patients were randomised after negative interim FDG PET/CT (after 2 cycles of chemotherapy) to receive either standard eBEACOPP (in all 8 (before June 2011) or 6 cycles (after June 2011)) of bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine and prednisone in escalated doses) or de-escalated protocol with 4 cycles eBEACOPP in all (experimental arm). In PET negative patients the 5-year progression-free survival rates in the standard and experimental arm were 90.8% and 92.2%, respectively. Furthermore, the application of only 4 cycles of eBEACOPP was associated with fewer severe infections (8% vs. 15% after 6/8 cycles) and organ toxicities (8% vs. 18% after 6/8 cycles). The authors concluded that interim PET negativity allows for a reduction to only 4 cycles of eBEACOPP without loss of tumour control [15].
2.10 Sarcoma
2.10.1 Diagnosis, Staging and Re-staging
Sarcomas are a rare heterogeneous group of tumours composing about 1% of all malignancies [156], but carrying a high mortality rate, and presenting with varied radiologic appearances.
Primarily, sarcomas are divided into sarcomas originating from bone and cartilage, respectively, (such as osteosarcoma, Ewing’s sarcoma or chondrosarcoma) or originating from soft tissue (such as liposarcoma, leiomyosarcoma and GIST).
With respect to the biologic potential, WHO has classified soft tissue tumours in four categories: benign, intermediate (locally aggressive), intermediate (rarely metastasising) and malignant [48]. The histological grade of soft tissue sarcomas and chondrosarcomas is supposed to be the most important prognostic factor in those cancer groups [33, 34].
A meta-analysis of FDG PET studies in patients with sarcoma concluded that FDG PET could discriminate between benign tumours and low-grade tumours and intermediate and high-grade tumours [12]. Also other authors found an accurate discrimination at least between low- and high-grade sarcomas employing FDG PET [26]. However, by use of FDG PET it remains difficult to distinguish low-grade tumours from benign tumours, as also confirmed by other studies, e.g. Ioannidis and Lau found FDG PET to be positive in all intermediate and high-grade soft tissue tumours, in 74.4% of low-grade soft tissue tumours and still in 39.3% of benign lesions [71]. Czernin et al. investigated the baseline glucose metabolic phenotype of sarcoma in more than 100 patients with soft tissue sarcoma. The SUV differed considerably and significantly among the many histological subtypes. Liposarcomas, especially the myxoid variants, exhibited low FDG uptake. Sarcomas not otherwise specified (NOS), the most dedifferentiated variants, showed the highest FDG uptake. Overall, SUVmax was significantly higher in high-grade than in low-grade sarcomas (11.7 ± 9.1 g/ml vs. 3.7 ± 1.8 g/ml; p < 0.001) [37].
FDG PET/CT can be useful in the evaluation of soft tissue tumours for guiding biopsy, helping to sample in the area with the highest glucose utilisation [122]. ESMO guidelines also see a role for FDG PET/CT in defining the area a biopsy should be taken from [23]. Furthermore, according to the NCCN guidelines there is a role for FDG PET/CT in the staging, prognostication and therapy response assessment in patients with soft tissue sarcomas of the extremities, the superficial trunk or head and neck, but not in surveillance [156].
A meta-analysis from 2012 enrolling 89 patients focused on the primary staging of high-grade bone and soft tissue sarcomas—the authors found a high sensitivity, specificity, PPV and NPV for the presence of distant metastases detected by FDG PET/CT with 95%, 96%, 87% and 98%, respectively. However, in the detection of lymph node metastases, the PPV was very low with 27%, but there were similarly high rates for sensitivity, specificity and NPV with 100%, 90% and 100%, respectively. The authors considered the use of FDG PET/CT to be ‘feasible’ in the initial assessment of patients with high-grade bone and soft tissue sarcoma. Furthermore, they found the SUVmax of the primary tumour to be a strong prognostic predictor of survival [53].
A more recent review and meta-analysis investigated the role of FDG PET and PET/CT in diagnosis, staging and recurrence assessment of bone sarcoma enrolling 42 trials with a total of 1,530 patients; for discriminating primary bone sarcomas from benign lesions the authors found a pooled sensitivity of 96% and a pooled specificity of 79%, for detecting distant metastases the pooled results on a lesion-based level were 90% and 85% for sensitivity and specificity, respectively, and for detecting recurrence the pooled results on an examination-based level were 92% (sensitivity) and 93% (specificity), respectively (all values referring to PET/CT, even though, results for PET only were comparable) [93].
There are sarcomas that preferably occur in children and adolescents such as Ewing’s sarcoma and rhabdomyosarcoma. In a study conducting 46 PET scans in 25 paediatric patients with Ewing’s sarcoma or neuroectodermal sarcoma or rhabdomyosarcoma a favourable accuracy was found for FDG PET (sensitivity, specificity, PPV and NPV were 86%, 80%, 89% and 67%, respectively) [108].
2.10.2 Therapy Response Assessment
Czernin et al. investigated the evaluation of therapy response in soft tissue sarcomas by determining the degree of FDG uptake decrease before and after treatment: In these studies, decreases of tumour FDG uptake by 35% after a single cycle and by >60% at the end of treatment identified all pathological treatment responders, predicting the amount of necrotic tissue in excised tumour tissue (>95% necrosis) [37].
In osteosarcomas Hongtao et al. carried out a meta-analysis examining the therapy response as assessed by FDG PET/CT—they showed that SUVs measured before and after treatment are valuable for predicting the histological response to chemotherapy [67].
2.11 Breast Cancer
2.11.1 Diagnosis, Staging and Re-staging
Breast cancer is the most common type of cancer in women worldwide [119]. In 2014, breast cancer was the second leading cause of cancer-related deaths in women in the United States, in the group of 20–39 years of age it was even the first reason for cancer-related deaths in women [132].
Invasive ductal carcinoma is the most common histopathological subtype of invasive breast cancer accounting for 50–70% of all cases, followed by invasive lobular carcinoma with 5–15%, and finally mucinous and tubular carcinoma representing 1–6% and 1–2% of the cases, respectively. Invasive lobular, mucinous and tubular carcinomas are considered to be low FDG avid [117]; depending on the size and cell density this often also applies to carcinoma in situ [54].
Mammography remains the principal imaging tool to screen for breast cancer. Small lesions cannot be characterised by FDG PET/CT due to the partial volume effect and limited spatial resolution—sensitivities of 59% in T1a–b (<10 mm) and 98% in T1c (11–20 mm) tumours have been reported [83]. According to NCCN guidelines, FDG PET/CT can be used for the staging from stage IIIA onwards (T3 N1 M0), optionally [57]. In a clinical trial Koolen et al. enrolled 154 patients with primary stage II and III breast cancer to evaluate the role of FDG PET/CT in the staging work-up in comparison with conventional imaging techniques (bone scintigraphy, liver ultrasound and chest radiography) to exclude distant metastases prior to neoadjuvant chemotherapy. They found a sensitivity, specificity, PPV, NPV and accuracy of 100%, 96%, 80%, 100% and 97%, respectively; in 16 of 20 patients additional (distant) lesions, metastatic disease or new primary malignancy, were exclusively seen by PET/CT, leading to a change in treatment in 13 of 154 patients (8%). The authors concluded that FDG PET/CT is superior to conventional imaging techniques in the detection of distant metastases in untreated stage II and III breast cancer patients and may be of additional value prior to neoadjuvant chemotherapy [84]. For a recent retrospective study in a total of 234 stage I, II and III breast cancer patients FDG PET/CT was performed before (114 patients) or after surgery (120 patients)—hypermetabolic extra-axillary lymph nodes were detected in 42/234 patients (17.9%) and hypermetabolic distant metastases in 65/234 patients (27.7%), leading to a modification in the staging in 82/234 patients (35%) and in patient management in 69/234 patients (29.4%) [165]. Thus, FDG PET/seems to be of additional value in the staging starting already from stage II onwards (for more information also see Groheux [58]).
A meta-analysis conducted by Sun et al. including 6 studies with 609 patients at any disease stage and regardless of treatment status concluded that FDG PET/CT outperforms conventional imaging by far and has an excellent diagnostic performance in staging of distant metastasis in breast cancer patients indicated by a sensitivity of 99% and a specificity of 95% as compared to conventional imaging with a sensitivity of 57% and a specificity of 88% [140].
Since about 30–35% of all patients ultimately relapse after initial (radical) treatment [119, 163], surveillance of breast cancer patients with initially advanced tumour stage is of special importance. Two-thirds of breast tumour recurrences are local and detection of local recurrence is a domain of mammography and/or ultrasound and, if inconclusive, MRI [119]. However, the 5-year survival rate in patients with distant metastases (mediastinal lymph nodes, bones, lungs, liver and brain) is markedly lower (25% vs. 80% in patients with loco-regional recurrence) [163]. Especially if there are equivocal findings on conventional imaging (CT, MRI, bone scintigraphy) the use of FDG PET/CT is recommended by some professional societies to identify or exclude a relapse, e.g. by ESMO [22], and also for detection of local recurrence by SNMMI [74], and in particular in patients with dense breasts by RCR/RCP [142].
In a recent meta-analysis and systematic review including 26 studies with 1,752 patients with suspected recurrent breast cancer FDG PET or PET/CT was shown to be a highly sensitive and moderately specific imaging modality for the detection of relapse with a sensitivity of 90% and a specificity of 81% [163]. A retrospective clinical study conducted by Jung et al. enrolling 1,162 patients with 1,819 examinations assessed the role of FDG PET/CT in the detection of loco-regional recurrence or distant metastases as compared to conventional imaging (mammography, breast ultrasound, bone scintigraphy and chest radiography)—the authors found a sensitivity, specificity, PPV and NPV of 97.5%, 98.8%, 95.4% and 99.4%, respectively, for FDG PET/CT, the corresponding values for conventional imaging were 75.4%, 98.7%, 93.4% and 94.3%, respectively; thus, the sensitivity of FDG PET/CT was shown to be significantly higher as compared to conventional imaging. The authors considered FDG PET/CT as an acceptable diagnostic imaging modality for post-operative surveillance [78] (Fig. 19.6).
56-year old female patient with history of breast cancer of the right breast (initially TNM-classification pT1b pN0 L0 V0 R0 G3) after surgery and adjuvant radiation therapy, referred to FDG PET/CT for re-staging; clinically already suspicious focal tumour in the right axilla. FDG PET/CT confirmed FDG-positive lymph node metastasis in the right axilla; furthermore, PET/CT revealed an FDG-positive node contralateral in the left axilla, either contralateral primary tumour or further lymph node metastasis; (A1-2) CT-Scan, (B1-2) PET scan, (C1-2) PET/CT fused images
2.11.2 Therapy Response Assessment
For breast cancer, FDG PET/CT was examined in many studies in the setting of neoadjuvant chemotherapy to assess therapy response. A meta-analysis from 2012 enrolling 17 studies with 781 patients summarised a good pooled sensitivity of 84.0%, but only a moderate pooled specificity of 71.3%; however, the studies included were quite heterogeneous. For instance, only 11 studies had reported the receptor status (estrogen, progesterone and human epidermal growth factor receptor (HER) 2). The authors recommended a combination of FDG PET/CT with other imaging modalities such as MRI, ultrasound and mammography to assess therapy response [27].
More recently, Tian et al. conducted a meta-analysis enrolling 22 studies with 1,119 patients; the pathological response in both breast tissues and in the axillary lymph nodes and the accordance with FDG PET/CT results were evaluated. They found a pooled sensitivity of 81.9% and a better-pooled specificity of 79.3% as compared to the results of Cheng et al. the area under the curve being 0.87. Among the included studies there was also a high heterogeneity, the sensitivities in the different studies ranged from 63 to 100% and the specificities ranged from 38 to 97%—the authors suggested that only tumour subtypes with relevant FDG uptake at baseline can be assessed in the course of chemotherapy. In tumours with low initial FDG avidity change in glucose utilisation cannot reasonably be used for therapy response assessment in the course of chemotherapy, but obviously those tumour subtypes were also evaluated in the included studies [143].
In general, therapy response assessment determined by FDG PET/CT seems to be of special interest in more aggressive subtypes like triple negative and HER2-positive breast cancers. The optimal timing of interim PET/CT is still an ongoing issue of research, but seems to depend on the histological cancer subtype and (chemo-/immuno-) therapy regimen [59].
2.12 Ovarian Cancer
2.12.1 Diagnosis, Staging and Re-staging
Nearly 90% of ovarian cancers arise from the epithelium, the remaining 10% are germ cell and stromal tumours [130]. Ovarian cancer is the leading cause of death from gynaecologic cancer in the Western World [85]. The poor prognosis results from an advanced cancer stage, which affects about 70% of patients at the time of diagnosis [46]. Ovarian cancer spreads mainly by local extension, by early implantation on both the parietal and the visceral peritoneum, by lymphatic dissemination (involving the inguinal, pelvic, paraaortic and mediastinal lymph nodes) and rarely through the blood vessels [46]. The serum tumour marker CA-125 is widely used to assess the effectiveness of therapy and to detect tumour recurrence.
Unclear ovarian masses are evaluated with ultrasound, CT and/or MRI, usually followed by ‘staging laparotomy’. Surgical exploration remains the standard reference for the initial staging of ovarian cancer [46, 130]. In the primary staging FDG PET/CT has no definite role, but ‘may be appropriate’ according to the ACR Appropriateness Criteria® in the staging and follow-up of ovarian cancer [81]. FDG PET/CT was shown to be limited in the diagnosis of cancer of the ovaries due to physiologic FDG uptake observed in the ovaries of women at reproductive age; for this reason the examination should be conducted during the postmenstrual phase in premenopausal women. However, focal uptake in the region of the ovaries in postmenopausal women is suspicious of malignancy and should be further evaluated. False-positive results may also occur, e.g. when physiologic bowel activity is mixed up with peritoneal spread [46]. On the other hand, FDG PET/CT may lead to false-negative results in borderline tumours, in lesions smaller than 5 mm (such as carcinomatosis), in women with cystic or necrotic lesions and in mucinous tumours. Still, FDG PET/CT is more sensitive than CT alone in detecting small peritoneal deposits [50, 85]. Furthermore, women with ovarian cancer confined to the pelvis may benefit from a pre-operative FDG PET/CT in order to identify nodal metastases—in a prospective study enrolling 68 patients FDG PET/CT showed a high sensitivity, specificity, accuracy, PPV and NPV of 83.3%, 98.2%, 95.6%, 90.9% and 96.5%, respectively, in a patient-based analysis. The authors concluded that due to the high NPV systematic pelvic and aortic lymphadenectomy could be avoided in many patients [133]. In addition, FDG PET/CT has the advantage of a whole-body examination (‘one-stop shop’), hence it might also detect supradiaphragmatic involvement at the time of primary staging. Therefore, it is recommended in the guidelines of the European Society of Urogenital Radiology (ESUR) in suspected stage IV disease (advanced ovarian cancer) and in the presence of indeterminate lymph node appearance [50].
The most important role of FDG PET/CT in ovarian cancer patients is the evaluation of tumour recurrence, especially when CA-125 levels are rising and conventional imaging is inconclusive or negative [46, 50, 85, 130]. Referring to the ACR Appropriateness Criteria®, in this situation FDG PET/CT is considered ‘usually appropriate’ [81] and is also recommended by RCR [142].
2.12.2 Therapy Response Assessment
In the future, there might be a further role in monitoring response to treatment in advanced ovarian cancer, as FDG PET/CT is supposed to be more accurate in characterising residual disease after chemotherapy as compared to CT alone and as a molecular imaging technique it might also identify responders and non-responders [127]. However, so far it has not replaced second-look laparotomy, maybe also due to issues of cost-effectiveness [85, 130].
2.13 Testicular Cancer
2.13.1 Diagnosis, Staging and Re-staging
Most testicular neoplasms (90–95%) are germ cell tumours [7]. The other 5–10% include stromal tumours, lymphoma and metastases. Testicular germ cell tumours, representing a rare malignancy and occurring most frequently in Caucasian young males aged 15–40 years [112], are divided into seminoma and non-seminoma(tous germ cell tumours ((NSGCT)) with 50% each [112].
The NSGCT group consists of embryonic cell carcinomas including undifferentiated teratomas, differentiated teratomas (with varying degrees of differentiation, and immature and mature teratomas, respectively), yolk sac tumours such as choriocarcinomas and mixed tumours in every possible combination, also in combination with seminoma components. Mature teratomas do not show increased FDG uptake due to a low proliferation rate [5]. As mature teratoma is present in 30–45% of resected residual masses in NSGCT patients, this is a major source of false-negative results in PET scans prior to resection. In seminomas the fraction of mature teratoma in residual lesions is much lower (<5%). Therefore, FDG PET/CT has a greater role in evaluating patients with residual seminoma as compared to NSGCT patients with residuals [39].
However, FDG PET/CT does not contribute to initial staging and has no role in the routine follow-up (ESMO, see Oldenburg et al. [112]), for both of which usually CT is applied.
In contrary, FDG PET/CT is most helpful in the setting of tumour recurrence (when relapse is suspected, e.g. by elevated tumour marker levels, especially in combination with normal conventional imaging findings) and in the evaluation of chemotherapy response.
2.13.2 Therapy Response Assessment
Therapy response was evaluated in detail for residual tumour masses after chemotherapy in seminoma patients with suspicion of viable tumour residuals. Treglia et al. published a systematic review and meta-analysis referring to this question—pooled sensitivity and specificity of FDG PET or PET/CT were 78% and 86%, respectively; FDG showed a high NPV of 94% with a moderate PPV of 58% and a high accuracy of 84%. In addition, they found a significantly higher sensitivity for patients with residual masses >3 cm as compared to those <3 cm (89% vs. 47%) [148].
Thus, the main indication of FDG PET/CT in testicular cancer is the differentiation of residual tumour from fibrosis in seminoma patients, in particular in lesions >3 cm, as also recommended by CCO Cancer Care Ontario [21] and RCR [142]. In the assessment of residual tumour tissue after chemotherapy FDG PET/CT should be performed with a minimum of 6 weeks after the end of the treatment, as earlier imaging may lead to false-positive results due to inflammation following therapy. In the case of a positive PET scan, the probability of residual seminoma is high and a biopsy might be carried out before re-treatment by irradiation or resection [112].
There might be a future role for FDG PET/CT in the assessment of response to Cisplatin, Etoposide and Bleomycin (PEB) chemotherapy in metastatic seminoma patients, as Nechhi et al. pointed out: after 2 cycles of PEB 72.9% of the included 37 patients achieved a complete response (CR) and none of them relapsed (median follow-up of 18 months)—the authors concluded that patients showing CR on PET/CT-scan do not need additional treatment after (according to risk stratification 3 or 4 cycles of) PEB [110]. Further research in larger cohorts is necessary to confirm these results.
2.14 Penile Cancer
2.14.1 Diagnosis, Staging and Re-staging
In Europe and North America or in general in developed countries, penile cancer has a low incidence (≤1 per 100,000) that increases with age, with a peak during the sixth decade [60, 114]. As there is a strong correlation with human papillomavirus (HPV) (one-third of cases can be attributed to HPV-related carcinogenesis), this might explain regional differences in epidemiology with higher incidence rates in South America, Southeast Asia and parts of Africa [60]. The following section focuses on penile squamous cell carcinoma, which represents the vast majority of penile cancer subtypes [114].
As metastatic involvement of regional lymph nodes is a strong prognostic factor (excellent prognosis in N0-patients with cure in up to 90% [41, 114], invasive lymph node staging is required in patients at intermediate or high risk (intermediate risk: not well-differentiated pT1; high risk: pT2 or higher plus all G3 tumours) of lymphatic spread, i.e. either modified radical inguinal lymphadenectomy (ILND) or dynamic sentinel node biopsy (DSNB) [60]. In enlarged palpable inguinal lymph nodes the presence of metastases is more likely, but even in clinical unsuspicious lymph nodes micrometastases occur with a frequency of about 20–25% [41, 60]. Despite its poorer prognosis, cure is still possible in lymph node-positive disease—for this reason, improved imaging tools are needed to identify those patients. Due to low sensitivities for smaller metastases, FDG PET/CT is not recommended for cN0-patients [114]. Though there was an attempt by a Danish group to combine FDG PET/CT with sentinel node biopsy in cN0-patients and this combined approach showed a sensitivity of 94.4% [76]. In another prospective study, 41 cN0-patients underwent FDG PET/CT prior to bilateral modified or radical ILND—FDG PET/CT showed a high sensitivity but moderate specificity with 80% and 68%, respectively, as well as a high NPV of 91% (although low PPV of 44%). The authors concluded that FDG PET/CT cannot replace invasive nodal staging [42].
In clinically suspicious palpable lymph nodes FDG PET/CT can identify metastases and confirm lymph node involvement [60]. In nodal-positive patients, radical ILND with curative intention is indicated. FDG PET/CT might also help to identify pelvic lymph node metastases, if these are initially suspicious due to enlargement [151].
In follow-up, FDG PET/CT can improve the detection of early regional lymph node involvement and reveals more distant metastases, especially in patients with inguinal node-positive penile cancer (ESMO, see Van Poppel et al. [151]). In a recent study, Zhang et al. showed an overall sensitivity of 82% and specificity of 93% for the detection of distant metastases by FDG PET/CT in a patient-based analysis in 42 stage III- and IV-patients with advanced penile cancer, mostly in the clinical setting of re-staging/evaluation of suspected recurrence (65% of the patients) [169].
2.15 Prostate Cancer
2.15.1 Diagnosis, Staging and Re-staging
FDG PET/CT has shown only a limited sensitivity for the detection of differentiated prostate cancer and imaging of recurrent prostate cancer in various studies. Increased FDG uptake and accumulation is regularly only found in dedifferentiated and aggressive prostate cancer, given a Gleason score >7, and the sensitivity for the detection of metastases increases with increasing serum PSA levels (for more information also see Jadvar [72, 73] and the chapter concerning molecular imaging of prostate cancer in this book).
2.16 Cancer of Unknown Primary
2.16.1 Diagnosis/Detection and Staging
About 3–5% of all malignancies are cancers of unknown primary (CUP) [47], mostly associated with a poor prognosis. By definition, CUPs present with histologically proven metastatic disease for which the site of origin cannot be identified at the time of diagnosis despite extensive diagnostic work-up. The biology of those tumours is still not fully understood [47], although one might hypothesise that the detection of the primary tumour might lead to a more distinctive treatment. The primary tumour can often not be identified on conventional imaging, thus, PET may improve the detection rate of CUPs and potentially improve patient outcome by optimising treatment planning.
A special patient sub-group in the group with those heterogeneous malignancies are patients with neck lymph node metastases from an occult primary who account for up to 10% of all patients with carcinoma of unknown primary site [20]. Since the most frequent histological finding in neck lymph node metastases is squamous cell carcinoma, particularly when the upper neck is involved [20], and squamous cell carcinomas are usually FDG avid, there is a high probability to find the primary tumour by virtue of FDG PET/CT, at least if the tumour size is not below resolution limit. Zhu et al. conducted a meta-analysis including 246 patients and with 44, 97 and 68% for detection rate, sensitivity and specificity, respectively, they found a moderate detection rate, a high sensitivity and a low specificity [170].
Despite the lack of recommendations in official guidelines, a multidisciplinary expert panel of oncologists, radiologists and nuclear physicians with expertise in PET/CT concluded that the use of FDG PET(/CT) is beneficial in the diagnostic work-up of patients with cervical CUPs [49].
At the moment also in extra-cervical CUPs evidence seems to be too rare to recommend the use of FDG PET/CT in guidelines in first line diagnostic work-up with exception of the German Society of Haematology and Oncology, which prefers FDG PET/CT for the detection of the primary tumour instead of CT thorax/abdomen alone [70]. RCR recommends FDG PET/CT for the ‘detection of the primary site when imaging and histopathology has failed to show a primary site, where the site of tumour will influence choice of chemotherapy’ [142].
A meta-analysis by Kwee et al. reported a primary tumour detection rate of 37%. Lung, oropharyngeal and pancreatic cancer were reported to represent the most frequently detected primary tumours [87]. Another meta-analysis was conducted by Moller et al., who found a detection rate of 39.5% for the primary site in patients with extra-cervical CUP, the pooled estimates for sensitivity, specificity and accuracy were 87%, 88% and 87.5%, respectively. The lungs were again the most commonly detected primary tumour site with 50% [109]. A more recent meta-analysis enrolling 1,942 patients came to a similar result with an overall detection rate of 40.93%—the authors concluded that up-front application of FDG PET/CT might have a role in CUP patients by obviating many futile diagnostic procedures [17].
References
Abouzied MM, Fathala A, Alsugair A, Muhaideb AIA, Qahtani MHA (2017) Role of fluorodeoxyglucose-positron emission tomography/computed tomography in the evaluation of head and neck carcinoma. World J Nucl Med. 16(4):257–265
Adams HJ, Kwee TC, de Keizer B, Fijnheer R, de Klerk JM, Nievelstein RA (2014) FDG PET/CT for the detection of bone marrow involvement in diffuse large B-cell lymphoma: systematic review and meta-analysis. Eur J Nucl Med Mol Imaging 41(3):565–574
Ajani JA, D’Amico TA, Almhanna K, Bentrem DJ, Besh S, Chao J et al (2015) Esophageal and esophagogastric junction cancers, version 1.2015. J Natl Compr Canc Netw13(2):194–227
Alavi A, Reivich M (2002) Guest editorial: the conception of FDG-PET imaging. Semin Nucl Med 32(1):2–5
Albers P, Bender H, Yilmaz H, Schoeneich G, Biersack HJ, Mueller SC (1999) Positron emission tomography in the clinical staging of patients with stage I and II testicular germ cell tumors. Urology 53(4):808–811
Ambrosini V, Nicolini S, Caroli P, Nanni C, Massaro A, Marzola MC et al (2012) PET/CT imaging in different types of lung cancer: an overview. Eur J Radiol 81(5):988–1001
Ambrosini V, Zucchini G, Nicolini S, Berselli A, Nanni C, Allegri V et al (2014) 18F-FDG PET/CT impact on testicular tumours clinical management. Eur J Nucl Med Mol Imaging 41(4):668–673
American College of Radiology (ACR) Appropriateness Criteria® (2013). https://acsearch.acr.org/list. Accessed 12 Sept 2018
AWMF (2018) Leitlinienprogramm Onkologie (Deutsche Krebsgesellschaft, Deutsche Krebshilfe, AWMF): Prävention, Diagnostik, Therapie und Nachsorge des Lungenkarzinoms, Langversion 1.0, 2018, AWMF-Registernummer: 020/007OL, http://leitlinienprogramm-onkologie.de/Lungenkarzinom.98.0.html. Accessed 3 Jan 2019
AWMF (2018) Leitlinienprogramm Onkologie (Deutsche Krebsgesellschaft, Deutsche Krebshilfe, AWMF): Diagnostik, Therapie und Nachsorge des Melanoms, Langversion 3.1, 2018, AWMF Registernummer: 032/024OL, http://www.leitlinienprogramm-onkologie.de/leitlinien/melanom/. Accessed 3 Jan 2019
Barrington SF, Mikhaeel NG, Kostakoglu L, Meignan M, Hutchings M, Müeller SP et al (2014) Role of imaging in the staging and response assessment of lymphoma: consensus of the International Conference on Malignant Lymphomas Imaging Working Group. J Clin Oncol 32(27):3048–3058
Bastiaannet E, Groen H, Jager PL, Cobben DC, van der Graaf WT, Vaalburg W et al (2004) The value of FDG-PET in the detection, grading and response to therapy of soft tissue and bone sarcomas; a systematic review and meta-analysis. Cancer Treat Rev 30(1):83–101
Benson AB, Venook AP, Al-Hawary MM, Cederquist L, Chen YJ, Ciombor KK et al (2018) Rectal cancer, version 2.2018, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 16(7):874–901
Bertagna F, Biasiotto G, Orlando E, Bosio G, Giubbini R (2010) Role of 18F-fluorodeoxyglucose positron emission tomography/computed tomography in patients affected by differentiated thyroid carcinoma, high thyroglobulin level, and negative 131I scan: review of the literature. Jpn J Radiol 28(9):629–636
Borchmann P, Goergen H, Kobe C, Lohri A, Greil R, Eichenauer DA et al (2018) PET-guided treatment in patients with advanced-stage Hodgkin’s lymphoma (HD18): final results of an open-label, international, randomised phase 3 trial by the German Hodgkin Study Group. Lancet 390(10114):2790–2802
Bradley J, Thorstad WL, Mutic S, Miller TR, Dehdashti F, Siegel BA et al (2004) Impact of FDG-PET on radiation therapy volume delineation in non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 59(1):78–86
Burglin SA, Hess S, Høilund-Carlsen PF, Gerke O (2017) 18F-FDG PET/CT for detection of the primary tumor in adults with extracervical metastases from cancer of unknown primary: A systematic review and meta-analysis. Medicine (Baltimore) 96(16):e6713
Cacicedo J, Fernandez I, Del Hoyo O, Dolado A, Gómez-Suarez J, Hortelano E et al (2015) Should PET/CT be implemented in the routine imaging work-up of locally advanced head and neck squamous cell carcinoma? A prospective analysis. Eur J Nucl Med Mol Imaging 42(9):1378–1389
Caetano R, Bastos CR, de Oliveira IA, da Silva RM, Fortes CP, Pepe VL et al (2016) Accuracy of positron emission tomography and positron emission tomography-CT in the detection of differentiated thyroid cancer recurrence with negative (131) I whole-body scan results: a meta-analysis. Head Neck 38(2):316–327
Calabrese L, Jereczek-Fossa BA, Jassem J, Rocca A, Bruschini R, Orecchia R et al (2005) Diagnosis and management of neck metastases from an unknown primary. Acta Otorhinolaryngol Ital 25(1):2–12
Cancer Care Ontario (2009). https://www.cancercareontario.ca/en/guidelines-advice/types-of-cancer. Accessed 12 Sept 2018
Cardoso F, Harbeck N, Fallowfield L, Kyriakides S, Senkus E, Group EGW (2012) Locally recurrent or metastatic breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 23 Suppl 7:vii11-9
Casali PG, Abecassis N, Bauer S, Biagini R, Bielack S, Bonvalot S et al (2018) Soft tissue and visceral sarcomas: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol
Chaft JE, Dunphy M, Naidoo J, Travis WD, Hellmann M, Woo K et al (2016) Adaptive neoadjuvant chemotherapy guided by (18)F-FDG PET in resectable non-small cell lung cancers: the NEOSCAN trial. J Thorac Oncol 11(4):537–544
Chang MC, Chen JH, Liang JA, Lin CC, Yang KT, Cheng KY et al (2012) Meta-analysis: comparison of F-18 fluorodeoxyglucose-positron emission tomography and bone scintigraphy in the detection of bone metastasis in patients with lung cancer. Acad Radiol 19(3):349–357
Charest M, Hickeson M, Lisbona R, Novales-Diaz JA, Derbekyan V, Turcotte RE (2009) FDG PET/CT imaging in primary osseous and soft tissue sarcomas: a retrospective review of 212 cases. Eur J Nucl Med Mol Imaging 36(12):1944–1951
Cheng X, Li Y, Liu B, Xu Z, Bao L, Wang J (2012) 18F-FDG PET/CT and PET for evaluation of pathological response to neoadjuvant chemotherapy in breast cancer: a meta-analysis. Acta Radiol 53(6):615–627
Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E et al (2014) Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol 32(27):3059–3068
Cheung PK, Chin RY, Eslick GD (2016) Detecting residual/recurrent head neck squamous cell carcinomas using PET or PET/CT: systematic review and meta-analysis. Otolaryngol Head Neck Surg 154(3):421–432
Cho J, Choe JG, Pahk K, Choi S, Kwon HR, Eo JS et al (2017) Ratio of mediastinal lymph node suv to primary tumor SUV in 18F-FDG PET/CT for nodal staging in non-small-cell lung cancer. Nucl Med Mol Imaging. 51(2):140–146
Cho SF, Chang CC, Liu YC, Chang CS, Hsiao HH, Liu TC et al (2015) Utilization of 18F-FDG PET/CT as a staging tool in patients with newly diagnosed lymphoma. Kaohsiung J Med Sci 31(3):130–137
Choi SJ, Jung KP, Lee SS, Park YS, Lee SM, Bae SK (2016) Clinical usefulness of F-18 FDG PET/CT in papillary thyroid cancer with negative radioiodine scan and elevated thyroglobulin level or positive anti-thyroglobulin antibody. Nucl Med Mol Imaging. 50(2):130–136
Chow WA (2007) Update on chondrosarcomas. Curr Opin Oncol 19(4):371–376
Coindre JM, Terrier P, Guillou L, Le Doussal V, Collin F, Ranchère D et al (2001) Predictive value of grade for metastasis development in the main histologic types of adult soft tissue sarcomas: a study of 1240 patients from the French Federation of Cancer Centers Sarcoma Group. Cancer 91(10):1914–1926
Coit DG, Thompson JA, Algazi A, Andtbacka R, Bichakjian CK, Carson WE et al (2016) Melanoma, version 2.2016, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 14(4):450–473
Colevas AD, Yom SS, Pfister DG, Spencer S, Adelstein D, Adkins D et al (2018) NCCN guidelines insights: head and neck cancers, version 1.2018. J Natl Compr Canc Netw 16(5):479–490
Czernin J, Benz MR, Allen-Auerbach MS (2010) PET/CT imaging: The incremental value of assessing the glucose metabolic phenotype and the structure of cancers in a single examination. Eur J Radiol 73(3):470–480
de Groot PM, Wu CC, Carter BW, Munden RF (2018) The epidemiology of lung cancer. Transl Lung Cancer Res 7(3):220–233
De Santis M, Pont J (2004) The role of positron emission tomography in germ cell cancer. World J Urol 22(1):41–46
De Wever W, Ceyssens S, Mortelmans L, Stroobants S, Marchal G, Bogaert J et al (2007) Additional value of PET-CT in the staging of lung cancer: comparison with CT alone, PET alone and visual correlation of PET and CT. Eur Radiol 17(1):23–32
Djajadiningrat RS, Graafland NM, van Werkhoven E, Meinhardt W, Bex A, van der Poel HG et al (2014) Contemporary management of regional nodes in penile cancer—improvement of survival? J Urol 191(1):68–73
Dräger DL, Heuschkel M, Protzel C, Erbersdobler A, Krause BJ, Hakenberg OW et al (2018) [18F]FDG PET/CT for assessing inguinal lymph nodes in patients with penile cancer—correlation with histopathology after inguinal lymphadenectomy. Nuklearmedizin 57(1):26–30
Dummer R, Hauschild A, Lindenblatt N, Pentheroudakis G, Keilholz U, Committee EG (2015) Cutaneous melanoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 26(Suppl 5):v126–v132
Eichenauer DA, Aleman BMP, André M, Federico M, Hutchings M, Illidge T et al (2018) Hodgkin lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 29(Supplement_4):iv19–iv29
Engelmann BE, Loft A, Kjær A, Nielsen HJ, Berthelsen AK, Binderup T et al (2014) Positron emission tomography/computed tomography for optimized colon cancer staging and follow up. Scand J Gastroenterol 49(2):191–201
Fischerova D, Burgetova A (2014) Imaging techniques for the evaluation of ovarian cancer. Best Pract Res Clin Obstet Gynaecol 28(5):697–720
Fizazi K, Greco FA, Pavlidis N, Daugaard G, Oien K, Pentheroudakis G et al (2015) Cancers of unknown primary site: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 26(Suppl 5):v133–v138
Fletcher C, Unni K, Mertens F (2002) WHO classification of tumours: pathology and genetics of tumours of soft tissue and bone. IARC Press, Lyon
Fletcher JW, Djulbegovic B, Soares HP, Siegel BA, Lowe VJ, Lyman GH et al (2008) Recommendations on the use of 18F-FDG PET in oncology. J Nucl Med 49(3):480–508
Forstner R, Sala E, Kinkel K, Spencer JA, Radiology ESoU (2010) ESUR guidelines: ovarian cancer staging and follow-up. Eur Radiol 20(12):2773–2780
Fowler KJ, Kaur H, Cash BD, Feig BW, Gage KL, Garcia EM et al (2017) ACR appropriateness criteria® pretreatment staging of colorectal cancer. J Am Coll Radiol 14(5S):S234–S244
Frilling A, Görges R, Tecklenborg K, Gassmann P, Bockhorn M, Clausen M et al (2000) Value of preoperative diagnostic modalities in patients with recurrent thyroid carcinoma. Surgery 128(6):1067–1074
Fuglø HM, Jørgensen SM, Loft A, Hovgaard D, Petersen MM (2012) The diagnostic and prognostic value of 18F-FDG PET/CT in the initial assessment of high-grade bone and soft tissue sarcoma. A retrospective study of 89 patients. Eur J Nucl Med Mol Imaging 39(9):1416–1424
Fujioka T, Kubota K, Toriihara A, Machida Y, Okazawa K, Nakagawa T et al (2016) Tumor characteristics of ductal carcinoma in situ of breast visualized on [F-18] fluorodeoxyglucose-positron emission tomography/computed tomography: results from a retrospective study. World J Radiol 8(8):743–749
Gallagher BM, Ansari A, Atkins H, Casella V, Christman DR, Fowler JS et al (1977) Radiopharmaceuticals XXVII. 18F-labeled 2-deoxy-2-fluoro-d-glucose as a radiopharmaceutical for measuring regional myocardial glucose metabolism in vivo: tissue distribution and imaging studies in animals. J Nucl Med 18(10):990–996
Goense L, van Rossum PS, Reitsma JB, Lam MG, Meijer GJ, van Vulpen M et al (2015) Diagnostic performance of 18F-FDG PET and PET/CT for the detection of recurrent esophageal cancer after treatment with curative intent: a systematic review and meta-analysis. J Nucl Med 56(7):995–1002
Gradishar WJ, Anderson BO, Balassanian R, Blair SL, Burstein HJ, Cyr A et al (2015) Breast cancer version 2.2015, NCCN Clinical Practice Guidelines in oncology. J Natl Compr Canc Netw 13(4):448–475
Groheux D (2017) FDG-PET/CT for systemic staging of patients with newly diagnosed breast cancer. Eur J Nucl Med Mol Imaging 44(9):1417–1419
Groheux D, Mankoff D, Espié M, Hindié E (2016) 18F-FDG PET/CT in the early prediction of pathological response in aggressive subtypes of breast cancer: review of the literature and recommendations for use in clinical trials. Eur J Nucl Med Mol Imaging 43(5):983–993
Hakenberg OW, Compérat EM, Minhas S, Necchi A, Protzel C, Watkin N (2015) EAU guidelines on penile cancer: 2014 update. Eur Urol 67(1):142–150
Hallqvist A, Alverbratt C, Strandell A, Samuelsson O, Björkander E, Liljegren A et al (2017) Positron emission tomography and computed tomographic imaging (PET/CT) for dose planning purposes of thoracic radiation with curative intent in lung cancer patients: a systematic review and meta-analysis. Radiother Oncol 123(1):71–77
Hassanzadeh-Rad A, Yousefifard M, Katal S, Asady H, Fard-Esfahani A, Moghadas Jafari A et al (2016) The value of (18) F-fluorodeoxyglucose positron emission tomography for prediction of treatment response in gastrointestinal stromal tumors: a systematic review and meta-analysis. J Gastroenterol Hepatol 31(5):929–935
Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE et al (2016) 2015 American Thyroid Association Management Guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the american thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid 26(1):1–133
Helsen N, Van den Wyngaert T, Carp L, Stroobants S (2018) FDG-PET/CT for treatment response assessment in head and neck squamous cell carcinoma: a systematic review and meta-analysis of diagnostic performance. Eur J Nucl Med Mol Imaging 45(6):1063–1071
Herrmann K, Benz MR, Krause BJ, Pomykala KL, Buck AK, Czernin J (2011) (18)F-FDG-PET/CT in evaluating response to therapy in solid tumors: where we are and where we can go. Q J Nucl Med Mol Imaging 55(6):620–632
Herrmann K, Ott K, Buck AK, Lordick F, Wilhelm D, Souvatzoglou M et al (2007) Imaging gastric cancer with PET and the radiotracers 18F-FLT and 18F-FDG: a comparative analysis. J Nucl Med 48(12):1945–1950
Hongtao L, Hui Z, Bingshun W, Xiaojin W, Zhiyu W, Shuier Z et al (2012) 18F-FDG positron emission tomography for the assessment of histological response to neoadjuvant chemotherapy in osteosarcomas: a meta-analysis. Surg Oncol 21(4):e165–e170
Hoppe RT, Advani RH, Ai WZ, Ambinder RF, Aoun P, Bello CM et al (2017) Hodgkin lymphoma version 1.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 15(5):608–638
Huang SH, O’Sullivan B, Xu W, Zhao H, Chen DD, Ringash J et al (2013) Temporal nodal regression and regional control after primary radiation therapy for N2-N3 head-and-neck cancer stratified by HPV status. Int J Radiat Oncol Biol Phys 87(5):1078–1085
Hübner G, Borner M, Neben K, Stöger H (2018) CUP-Syndrom—Krebserkrankungen mit unbekanntem Primärtumor. DGHO (Deutsche Gesellschaft für Hämatologie und Onkologie), Onkopedia. https://www.onkopedia.com/de/onkopedia/guidelines/cup-syndrom-krebserkrankungen-mit-unbekanntem-primaertumor/@@view/html/index.html. Accessed 1 Sept 2018
Ioannidis JP, Lau J (2003) 18F-FDG PET for the diagnosis and grading of soft-tissue sarcoma: a meta-analysis. J Nucl Med 44(5):717–724
Jadvar H (2013) Imaging evaluation of prostate cancer with 18F-fluorodeoxyglucose PET/CT: utility and limitations. Eur J Nucl Med Mol Imaging 40(Suppl 1):S5–10
Jadvar H (2016) Is there use for FDG-PET in prostate cancer? Semin Nucl Med 46(6):502–506
Jadvar H, Colletti PM, Delgado-Bolton R, Esposito G, Krause BJ, Iagaru AH et al (2017) Appropriate Use Criteria for 18F-FDG-PET/CT in Restaging and Treatment Response Assessment of Malignant Disease. J Nucl Med 58(12):2026–2037
Jakić-Razumović J, Aurer I (2002) The World Health Organization classification of lymphomas. Croat Med J 43(5):527–534
Jakobsen JK, Alslev L, Ipsen P, Costa JC, Krarup KP, Sommer P et al (2016) DaPeCa-3: promising results of sentinel node biopsy combined with (18) F-fluorodeoxyglucose positron emission tomography/computed tomography in clinically lymph node-negative patients with penile cancer—a national study from Denmark. BJU Int 118(1):102–111
Johnson P, Federico M, Kirkwood A, Fosså A, Berkahn L, Carella A et al (2016) Adapted Treatment guided by interim PET-CT scan in advanced Hodgkin’s lymphoma. N Engl J Med 374(25):2419–2429
Jung NY, Yoo IR, Kang BJ, Kim SH, Chae BJ, Seo YY (2016) Clinical significance of FDG-PET/CT at the postoperative surveillance in the breast cancer patients. Breast Cancer 23(1):141–148
Jung W, Jang JY, Kang MJ, Chang YR, Shin YC, Chang J et al (2016) The clinical usefulness of 18F-fluorodeoxyglucose positron emission tomography-computed tomography (PET-CT) in follow-up of curatively resected pancreatic cancer patients. HPB (Oxford) 18(1):57–64
Kamiyama Y, Aihara R, Nakabayashi T, Mochiki E, Asao T, Kuwano H et al (2005) 18F-fluorodeoxyglucose positron emission tomography: useful technique for predicting malignant potential of gastrointestinal stromal tumors. World J Surg 29(11):1429–1435
Kang SK, Reinhold C, Atri M, Benson CB, Bhosale PR, Jhingran A et al (2018) ACR Appropriateness Criteria® staging and follow-up of ovarian cancer. J Am Coll Radiol 15(5S):S198–S207
Kitajima K, Nakajo M, Kaida H, Minamimoto R, Hirata K, Tsurusaki M et al (2017) Present and future roles of FDG-PET/CT imaging in the management of gastrointestinal cancer: an update. Nagoya J Med Sci 79(4):527–543
Koolen BB, van der Leij F, Vogel WV, Rutgers EJ, Vrancken Peeters MJ, Elkhuizen PH et al (2014) Accuracy of 18F-FDG PET/CT for primary tumor visualization and staging in T1 breast cancer. Acta Oncol 53(1):50–57
Koolen BB, Vrancken Peeters MJ, Aukema TS, Vogel WV, Oldenburg HS, van der Hage JA et al (2012) 18F-FDG PET/CT as a staging procedure in primary stage II and III breast cancer: comparison with conventional imaging techniques. Breast Cancer Res Treat 131(1):117–126
Kumar Dhingra V, Kand P, Basu S (2012) Impact of FDG-PET and -PET/CT imaging in the clinical decision-making of ovarian carcinoma: an evidence-based approach. Womens Health (Lond) 8(2):191–203
Kung BT, Yong TK, Tong CM (2017) The pearl of FDG PET/CT in preoperative assessment of patients with potentially operable non-small-cell lung cancer and its clinical impact. World J Nucl Med 16(1):21–25
Kwee TC, Kwee RM (2009) Combined FDG-PET/CT for the detection of unknown primary tumors: systematic review and meta-analysis. Eur Radiol 19(3):731–744
Lagergren J, Smyth E, Cunningham D, Lagergren P (2017) Oesophageal cancer. Lancet 390(10110):2383–2396
Lee AY, Choi SJ, Jung KP, Park JS, Lee SM, Bae SK (2014) Characteristics of metastatic mediastinal lymph nodes of non-small cell lung cancer on preoperative F-18 FDG PET/CT. Nucl Med Mol Imaging 48(1):41–46
Lee JH, Lee MR (2014) Positron emission tomography/computed tomography in the staging of colon cancer. Ann Coloproctol 30(1):23–27
Lewin J, Sayers L, Kee D, Walpole I, Sanelli A, Te Marvelde L et al (2018) Surveillance imaging with FDG-PET/CT in the post-operative follow-up of stage 3 melanoma. Ann Oncol 29(7):1569–1574
Li P, Liu Q, Wang C, Wang T, Liu J, Huang G et al (2016) Fluorine-18-fluorodeoxyglucose positron emission tomography to evaluate recurrent gastric cancer after surgical resection: a systematic review and meta-analysis. Ann Nucl Med 30(3):179–187
Liu F, Zhang Q, Zhu D, Li Z, Li J, Wang B et al (2015) Performance of positron emission tomography and positron emission tomography/computed tomography using fluorine-18-fluorodeoxyglucose for the diagnosis, staging, and recurrence assessment of bone sarcoma: a systematic review and meta-analysis. Medicine (Baltimore) 94(36):e1462
Lordick F, Mariette C, Haustermans K, Obermannová R, Arnold D, Committee EG (2016) Oesophageal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 27(suppl 5):v50–v57
Lordick F, Ott K, Krause BJ, Weber WA, Becker K, Stein HJ et al (2007) PET to assess early metabolic response and to guide treatment of adenocarcinoma of the oesophagogastric junction: the MUNICON phase II trial. Lancet Oncol 8(9):797–805
Lu YY, Chen JH, Chien CR, Chen WT, Tsai SC, Lin WY et al (2013) Use of FDG-PET or PET/CT to detect recurrent colorectal cancer in patients with elevated CEA: a systematic review and meta-analysis. Int J Colorectal Dis 28(8):1039–1047
Lu YY, Chen JH, Liang JA, Chu S, Lin WY, Kao CH (2014) 18F-FDG PET or PET/CT for detecting extensive disease in small-cell lung cancer: a systematic review and meta-analysis. Nucl Med Commun 35(7):697–703
MacManus MP, Hicks RJ, Matthews JP, Hogg A, McKenzie AF, Wirth A et al (2001) High rate of detection of unsuspected distant metastases by pet in apparent stage III non-small-cell lung cancer: implications for radical radiation therapy. Int J Radiat Oncol Biol Phys 50(2):287–293
Maffione AM, Lopci E, Bluemel C, Giammarile F, Herrmann K, Rubello D (2015) Diagnostic accuracy and impact on management of (18)F-FDG PET and PET/CT in colorectal liver metastasis: a meta-analysis and systematic review. Eur J Nucl Med Mol Imaging 42(1):152–163
Maffione AM, Marzola MC, Capirci C, Colletti PM, Rubello D (2015) Value of (18)F-FDG PET for predicting response to neoadjuvant therapy in rectal cancer: systematic review and meta-analysis. AJR Am J Roentgenol 204(6):1261–1268
Malle P, Sorschag M, Gallowitsch HJ (2012) FDG PET and FDG PET/CT in patients with gastrointestinal stromal tumours. Wien Med Wochenschr 162(19–20):423–429
Mamede M, Higashi T, Kitaichi M, Ishizu K, Ishimori T, Nakamoto Y et al (2005) [18F]FDG uptake and PCNA, Glut-1, and Hexokinase-II expressions in cancers and inflammatory lesions of the lung. Neoplasia 7(4):369–379
Marcus C, Ciarallo A, Tahari AK, Mena E, Koch W, Wahl RL et al (2014) Head and neck PET/CT: therapy response interpretation criteria (Hopkins Criteria)—interreader reliability, accuracy, and survival outcomes. J Nucl Med 55(9):1411–1416
Maziak DE, Darling GE, Inculet RI, Gulenchyn KY, Driedger AA, Ung YC et al (2009) Positron emission tomography in staging early lung cancer: a randomized trial. Ann Intern Med 151(4):221–228, W-48
Mehanna H, Wong WL, McConkey CC, Rahman JK, Robinson M, Hartley AG et al (2016) PET-CT surveillance versus neck dissection in advanced head and neck cancer. N Engl J Med 374(15):1444–1454
Mena E, Taghipour M, Sheikhbahaei S, Mirpour S, Xiao J, Subramaniam RM (2016) 18F-FDG PET/CT and melanoma: value of fourth and subsequent posttherapy follow-up scans for patient management. Clin Nucl Med 41(9):e403–e409
Metser U, Dudebout J, Baetz T, Hodgson DC, Langer DL, MacCrostie P et al (2017) [18 F]-FDG PET/CT in the staging and management of indolent lymphoma: a prospective multicenter PET registry study. Cancer 123(15):2860–2866
Mody RJ, Bui C, Hutchinson RJ, Yanik GA, Castle VP, Frey KA et al (2010) FDG PET imaging of childhood sarcomas. Pediatr Blood Cancer 54(2):222–227
Moller AK, Loft A, Berthelsen AK, Damgaard Pedersen K, Graff J, Christensen CB et al (2011) 18F-FDG PET/CT as a diagnostic tool in patients with extracervical carcinoma of unknown primary site: a literature review. Oncologist 16(4):445–451
Necchi A, Nicolai N, Alessi A, Miceli R, Giannatempo P, Raggi D et al (2016) Interim (18)F-Fluorodeoxyglucose positron emission tomography for early metabolic assessment of response to cisplatin, etoposide, and bleomycin chemotherapy for metastatic seminoma: clinical value and future directions. Clin Genitourin Cancer 14(3):249–254
NSCLC Meta-analysis Collaborative Group (2014) Preoperative chemotherapy for non-small-cell lung cancer: a systematic review and meta-analysis of individual participant data. Lancet 383(9928):1561–1571
Oldenburg J, Fosså SD, Nuver J, Heidenreich A, Schmoll HJ, Bokemeyer C et al (2013) Testicular seminoma and non-seminoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 24 Suppl 6:vi125–vi132
Ott K, Schmidt T, Lordick F, Herrmann K (2014) Importance of PET in surgery of esophageal cancer. Chirurg 85(6):505–512
Ottenhof SR, Leone AR, Horenblas S, Spiess PE, Vegt E (2017) Advancements in staging and imaging for penile cancer. Curr Opin Urol 27(6):612–620
Pacini F, Castagna MG, Brilli L, Pentheroudakis G, Group EGW (2012) Thyroid cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 23 Suppl 7:vii110–vii119
Pak K, Kim K, Kim MH, Eom JS, Lee MK, Cho JS et al (2018) A decision tree model for predicting mediastinal lymph node metastasis in non-small cell lung cancer with F-18 FDG PET/CT. PLoS ONE 13(2):e0193403
Park HL, Yoo IR, O JH, Kim H, Kim SH, Kang BJ (2018) Clinical utility of 18F-FDG PET/CT in low 18F-FDG-avidity breast cancer subtypes: comparison with breast US and MRI. Nucl Med Commun 39(1):35–43
Petersen RK, Hess S, Alavi A, Høilund-Carlsen PF (2014) Clinical impact of FDG-PET/CT on colorectal cancer staging and treatment strategy. Am J Nucl Med Mol Imaging. 4(5):471–482
Piva R, Ticconi F, Ceriani V, Scalorbi F, Fiz F, Capitanio S et al (2017) Comparative diagnostic accuracy of 18F-FDG PET/CT for breast cancer recurrence. Breast Cancer (Dove Med Press) 9:461–471
Porschen R, Buck A, Fischbach W, Gockel I, Görling U, Grenacher L et al (2015) S3-Leitlinie Diagnostik und Therapie der Plattenepithelkarzinome und Adenokarzinome des Ösophagus (Langversion 1.0—September 2015, AWMF-Registernummer: 021/023OL). Z Gastroenterol 53(11):1288–1347
Purandare NC, Pramesh CS, Karimundackal G, Jiwnani S, Agrawal A, Shah S et al (2014) Incremental value of 18F-FDG PET/CT in therapeutic decision-making of potentially curable esophageal adenocarcinoma. Nucl Med Commun 35(8):864–869
Rakheja R, Makis W, Skamene S, Nahal A, Brimo F, Azoulay L et al (2012) Correlating metabolic activity on 18F-FDG PET/CT with histopathologic characteristics of osseous and soft-tissue sarcomas: a retrospective review of 136 patients. AJR Am J Roentgenol 198(6):1409–1416
Rodriguez Rivera AM, Alabbas H, Ramjaun A, Meguerditchian AN (2014) Value of positron emission tomography scan in stage III cutaneous melanoma: a systematic review and meta-analysis. Surg Oncol 23(1):11–16
Rodriguez E, Lilenbaum RC (2010) Small cell lung cancer: past, present, and future. Curr Oncol Rep 12(5):327–334
Rogasch JM, Apostolova I, Steffen IG, Steinkrüger FL, Genseke P, Riedel S et al (2016) Standardized visual reading of F18-FDG-PET in patients with non-small cell lung cancer scheduled for preoperative thoracic lymph node staging. Eur J Radiol 85(8):1345–1350
Ronellenfitsch U, Wängler B, Niedermoser S, Dimitrakopoulou-Strauss A, Hohenberger P (2014) Importance of PET for surgery of gastrointestinal stromal tumors. Chirurg 85(6):493–499
Rubello D, Marzola MC, Colletti PM (2018) The prognostic value of 18F-FDG PET/CT in monitoring chemotherapy in ovarian cancer both at initial diagnosis and at recurrent disease. Clin Nucl Med 43(10):735–738
Sachpekidis C, Anwar H, Winkler J, Kopp-Schneider A, Larribere L, Haberkorn U et al (2018) The role of interim 18F-FDG PET/CT in prediction of response to ipilimumab treatment in metastatic melanoma. Eur J Nucl Med Mol Imaging 45(8):1289–1296
Schreyögg J, Weller J, Stargardt T, Herrmann K, Bluemel C, Dechow T et al (2010) Cost-effectiveness of hybrid PET/CT for staging of non-small cell lung cancer. J Nucl Med 51(11):1668–1675
Schwarz JK, Grigsby PW, Dehdashti F, Delbeke D (2009) The role of 18F-FDG PET in assessing therapy response in cancer of the cervix and ovaries. J Nucl Med 50(Suppl 1):64S–73S
Sheikhbahaei S, Taghipour M, Ahmad R, Fakhry C, Kiess AP, Chung CH et al (2015) Diagnostic accuracy of follow-up FDG PET or PET/CT in patients with head and neck cancer after definitive treatment: a systematic review and meta-analysis. AJR Am J Roentgenol 205(3):629–639
Siegel RL, Miller KD, Jemal A (2017) Cancer statistics, 2017. CA Cancer J Clin 67(1):7–30
Signorelli M, Guerra L, Pirovano C, Crivellaro C, Fruscio R, Buda A et al (2013) Detection of nodal metastases by 18F-FDG PET/CT in apparent early stage ovarian cancer: a prospective study. Gynecol Oncol 131(2):395–399
Silvestri GA, Gonzalez AV, Jantz MA, Margolis ML, Gould MK, Tanoue LT et al (2013) Methods for staging non-small cell lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 143(5 Suppl):e211S–e50S
Silvestri GA, Gould MK, Margolis ML, Tanoue LT, McCrory D, Toloza E et al (2007) Noninvasive staging of non-small cell lung cancer: ACCP evidenced-based clinical practice guidelines (2nd edn). Chest 132(3 Suppl):178S–201S
Singnurkar A, Wang J, Joshua AM, Langer DL, Metser U (2016) 18F-FDG-PET/CT in the staging and management of melanoma: a prospective multicenter ontario PET registry study. Clin Nucl Med 41(3):189–193
Sobic-Saranovic D, Petrusic I, Artiko V, Pavlovic S, Subotic D, Saranovic D et al (2015) Comparison of 18F-FDG PET/CT and MDCT for staging/restaging of non-small cell lung cancer. Neoplasma 62(2):295–301
Stahl A, Ott K, Weber WA, Becker K, Link T, Siewert JR et al (2003) FDG PET imaging of locally advanced gastric carcinomas: correlation with endoscopic and histopathological findings. Eur J Nucl Med Mol Imaging 30(2):288–295
Sun R, Tang X, Yang Y, Zhang C (2015) (18)FDG-PET/CT for the detection of regional nodal metastasis in patients with head and neck cancer: a meta-analysis. Oral Oncol 51(4):314–320
Sun Z, Yi YL, Liu Y, Xiong JP, He CZ (2015) Comparison of whole-body PET/PET-CT and conventional imaging procedures for distant metastasis staging in patients with breast cancer: a meta-analysis. Eur J Gynaecol Oncol 36(6):672–676
Tang S, Huang G, Liu J, Liu T, Treven L, Song S et al (2011) Usefulness of 18F-FDG PET, combined FDG-PET/CT and EUS in diagnosing primary pancreatic carcinoma: a meta-analysis. Eur J Radiol 78(1):142–150
The Royal College Of Radiologists; Royal College Of Physicians Of London; Royal College Of Physicians And Surgeons Of Glasgow; Royal College Of Physicians Of Edinburgh; British Nuclear Medicine Society; Administration Of Radioactive Substances Advisory Committee (2016) Evidence-based indications for the use of PET-CT in the United Kingdom 2016. Clin Radiol 71(7):e171–188
Tian F, Shen G, Deng Y, Diao W, Jia Z (2017) The accuracy of 18F-FDG PET/CT in predicting the pathological response to neoadjuvant chemotherapy in patients with breast cancer: a meta-analysis and systematic review. Eur Radiol 27(11):4786–4796
Tönnies S, Bauer TT, Misch D, Boch C, Blum T, Bittner RC et al (2012) Staging with 18F-FDG-PET/CT influences stage-specific survival in advanced non-small cell lung cancer (NSCLC). Pneumologie 66(4):212–217
Topkan E, Parlak C, Yapar AF (2013) FDG-PET/CT-based restaging may alter initial management decisions and clinical outcomes in patients with locally advanced pancreatic carcinoma planned to undergo chemoradiotherapy. Cancer Imaging 13(3):423–428
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A (2015) Global cancer statistics, 2012. CA Cancer J Clin 65(2):87–108
Treglia G, Mirk P, Stefanelli A, Rufini V, Giordano A, Bonomo L (2012) 18F-Fluorodeoxyglucose positron emission tomography in evaluating treatment response to imatinib or other drugs in gastrointestinal stromal tumors: a systematic review. Clin Imaging 36(3):167–175
Treglia G, Sadeghi R, Annunziata S, Caldarella C, Bertagna F, Giovanella L (2014) Diagnostic performance of fluorine-18-fluorodeoxyglucose positron emission tomography in the postchemotherapy management of patients with seminoma: systematic review and meta-analysis. Biomed Res Int 2014:852681
Van Cutsem E, Cervantes A, Adam R, Sobrero A, Van Krieken JH, Aderka D et al (2016) ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol 27(8):1386–1422
Van den Abbeele AD (2008) The lessons of GIST—PET and PET/CT: a new paradigm for imaging. Oncologist 13(Suppl 2):8–13
Van Poppel H, Watkin NA, Osanto S, Moonen L, Horwich A, Kataja V et al (2013) Penile cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 24 Suppl 6:vi115–24
van Tinteren H, Hoekstra OS, Smit EF, van den Bergh JH, Schreurs AJ, Stallaert RA et al (2002) Effectiveness of positron emission tomography in the preoperative assessment of patients with suspected non-small-cell lung cancer: the PLUS multicentre randomised trial. Lancet 359(9315):1388–1393
Vensby PH, Schmidt G, Kjær A, Fischer BM (2017) The value of FDG PET/CT for follow-up of patients with melanoma: a retrospective analysis. Am J Nucl Med Mol Imaging 7(6):255–262
Vincent A, Herman J, Schulick R, Hruban RH, Goggins M (2011) Pancreatic cancer. Lancet 378(9791):607–620
Voltin CA, Goergen H, Baues C, Fuchs M, Mettler J, Kreissl S et al (2018) Value of bone marrow biopsy in Hodgkin lymphoma patients staged by FDG PET: results from the German Hodgkin Study Group trials HD16, HD17, and HD18. Ann Oncol 29(9):1926–1931
von Mehren M, Randall RL, Benjamin RS, Boles S, Bui MM, Ganjoo KN et al (2018) Soft tissue sarcoma, Version 2.2018, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 16(5):536–563
Wahl RL, Jacene H, Kasamon Y, Lodge MA (2009) From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med 50(Suppl 1):122S–150S
Wahl RL, Zasadny K, Helvie M, Hutchins GD, Weber B, Cody R (1993) Metabolic monitoring of breast cancer chemohormonotherapy using positron emission tomography: initial evaluation. J Clin Oncol 11(11):2101–2111
Warburg O (1928) The chemical constitution of respiration ferment. Science 68(1767):437–443
Weiler-Sagie M, Bushelev O, Epelbaum R, Dann EJ, Haim N, Avivi I et al (2010) (18)F-FDG avidity in lymphoma readdressed: a study of 766 patients. J Nucl Med 51(1):25–30
Wong WL, Chambers RJ (2008) Role of PET/PET CT in the staging and restaging of thoracic oesophageal cancer and gastro-oesophageal cancer: a literature review. Abdom Imaging 33(2):183–190
Wu LM, Hu JN, Hua J, Gu HY, Zhu J, Xu JR (2012) 18F-fluorodeoxyglucose positron emission tomography to evaluate recurrent gastric cancer: a systematic review and meta-analysis. J Gastroenterol Hepatol 27(3):472–480
Xiao Y, Wang L, Jiang X, She W, He L, Hu G (2016) Diagnostic efficacy of 18F-FDG-PET or PET/CT in breast cancer with suspected recurrence: a systematic review and meta-analysis. Nucl Med Commun 37(11):1180–1188
Xing Y, Bronstein Y, Ross MI, Askew RL, Lee JE, Gershenwald JE et al (2011) Contemporary diagnostic imaging modalities for the staging and surveillance of melanoma patients: a meta-analysis. J Natl Cancer Inst 103(2):129–142
Yararbas U, Avci NC, Yeniay L, Argon AM (2018) The value of 18F-FDG PET/CT imaging in breast cancer staging. Bosn J Basic Med Sci. 18(1):72–79
Yu T, Meng N, Chi D, Zhao Y, Wang K, Luo Y (2015) Diagnostic Value of (18)F-FDG PET/CT in detecting local recurrent colorectal cancer: a pooled analysis of 26 individual studies. Cell Biochem Biophys 72(2):443–451
Zer A, Domachevsky L, Rapson Y, Nidam M, Flex D, Allen AM et al (2016) The role of 18F-FDG PET/CT on staging and prognosis in patients with small cell lung cancer. Eur Radiol 26(9):3155–3161
Zhang C, Liu J, Tong J, Sun X, Song S, Huang G (2013) 18F-FDG-PET evaluation of pathological tumour response to neoadjuvant therapy in patients with NSCLC. Nucl Med Commun 34(1):71–77
Zhang S, Li W, Liang F (2016) Clinical value of fluorine-18 2-fluoro-2-deoxy-D-glucose positron emission tomography/computed tomography in penile cancer. Oncotarget 7(30):48600–48606
Zhu L, Wang N (2013) 18F-fluorodeoxyglucose positron emission tomography-computed tomography as a diagnostic tool in patients with cervical nodal metastases of unknown primary site: a meta-analysis. Surg Oncol 22(3):190–194
zum Büschenfelde CM, Herrmann K, Schuster T, Geinitz H, Langer R, Becker K et al (2011) (18)F-FDG PET-guided salvage neoadjuvant radiochemotherapy of adenocarcinoma of the esophagogastric junction: the MUNICON II trial. J Nucl Med 52(8):1189–1196
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Becker, J., Schwarzenböck, S.M., Krause, B.J. (2020). FDG PET Hybrid Imaging. In: Schober, O., Kiessling, F., Debus, J. (eds) Molecular Imaging in Oncology. Recent Results in Cancer Research, vol 216. Springer, Cham. https://doi.org/10.1007/978-3-030-42618-7_19
Download citation
DOI: https://doi.org/10.1007/978-3-030-42618-7_19
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-42617-0
Online ISBN: 978-3-030-42618-7
eBook Packages: MedicineMedicine (R0)