Abstract
Eggplants and related germplasm are a barely unveiled genetic treasure, for reasons developed in Chap. 10. Diversity and interspecific crossability researches focused so far on Solanum melongena L., the economic importance of which towers that of the indigenous African S. aethiopicum L. and S. macrocarpon L. and which consequently attracted most of geneticists’ and breeders’ attention. However, as S. melongena shares many connections with eggplant germplasm as a whole, this chapter pays as much attention to this species as to the other cultivated and wild ones. Their genetic and phenotypic diversity is surveyed and critically analysed in order to place the reader at the crossroads between the present knowledge and desirable future researches in terms of both traits of interest to breeders and methods for assessing the diversity. The dense corpus of information about interspecific crossability is organised across several axes. Conventional sexual crosses and somatic hybridisations are presented separately, given both methods yield genetically different interspecific material. The section devoted to sexual crosses begins with a survey of the interspecific barriers, and with an overview of the crossing results that are discussed in their methodological dimensions, in particular the criteria assessing the success or failure of the crossing experiments. Then, the crossing results are structured according to the combinations of crosses within and between cultivated and wild material. Species crossability is discussed with regard to the genepool concept and to relationship between species assessed by phylogenetics. The section ends up with interspecific hybrid by-products such as male sterilities and information on traits genetics. The chapter turns then to somatic hybridisations; this part is structured according to groups of species (e.g. New World species) used as fusion partners of S. melongena, the pivotal taxon for most of the fusion experiments. The conclusions outline the limits of the present knowledge on eggplants germplasm diversity and crossability and suggest potential new research routes on these topics.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
11.1 Introduction
Most diversity and crossability researches have focused so far on Solanum melongena L., the worldwide economically most important eggplant, for which a wide germplasm is available in several genebanks (c.f. Chap. 10); eggplant breeding is rather dynamic in public as well as in seed companies. The mostly indigenous African germplasm of S. aethiopicum L. and S. macrocarpon L., less collected and less available in genebanks, has been characterised and bred to a much lesser extent than in the case of S. melongena. However, this situation is evolving, given that European and Asian seed companies are beginning to focus on the African vegetable market; also, researchers of the public sector are getting increasingly conscious of the potential of this wide source of poorly known diversity. Until now, crossability between cultivated eggplants and relatives has been focused on crosses involving S. melongena; the material was chosen mostly on the basis of criteria such as (1) known or expected relationship with S. melongena, and/or (2) resistance to various pests and diseases affecting S. melongena. The blurred understanding by geneticists and breeders of the complex world of eggplants relatives in terms of range and identity of the species involved, as well as in terms of relatedness degree with the cultivated eggplants, has strongly limited so far the characterisation of wild species and their use in breeding programmes. As seen in Chap. 10, even taxonomists and phylogeneticists had and still have the utmost difficulties to outline a general picture of the part of genus Solanum eggplants belong to, i.e. the subgenus Leptostemonum, also known as “spiny solanums”. Luckily enough and also as seen in Chap. 10, the botanical background is on the way of stepwise clarification and the phylogenetic progresses pave the way for carrying out enlarged and better directed (1) characterisations of eggplants and relative diversity, and (2) investigations of their inter crossability.
First, this chapter summarises the current knowledge on diversity of eggplants and their relatives, from the genetic and phenotypic point of view. We restricted the phenotype to the major morphological and horticultural traits of special interest to breeders. Phenotypic diversity of traits impacted by domestication of Solanum aethiopicum, S. macrocarpon and S. melongena is mentioned in Chap. 12. The second part of the chapter unfolds the rich information provided by interspecific crosses results. Sexual and somatic crosses are analysed separately; sexual crosses results are structured by species groups involving (1) only cultivated eggplants, (2) cultivated eggplants × wild progenitors, (3) cultivated × other wild species, and (4) only wild species. Results are also analysed across several axes including (1) crossability barriers, criteria and predictability, (2) exploitation of male sterilities produced by interspecific crosses, and (3) access to trait genetics. Somatic hybridisation results are summarised and gathered by types of partners, (1) S. melongena + New World Leptostemonum species, (2) S. melongena + Old World Leptostemonum species, (3) other combinations of Leptostemonum species, and (4) S. melongena + distantly related Solanaceae.
11.2 Diversity of Cultivated and Wild Germplasm
Characterisation of diversity is only possible when representative germplasm collections are available in genebanks. As far as eggplants and related species are concerned, several good collections are available for Solanum melongena, whereas those including the African eggplants and wild Leptostemonum species are less numerous and poorly representative of the existing diversity. This is particularly true for the wild species (c.f. Chap. 10). Further, research on germplasm is driven by the economic importance of the crops and consequently by the requirements of breeders which are continuously looking for new traits to be incorporated into their elite germplasm. As a consequence, most available information on diversity is anchored to S. melongena. African eggplants and wild Leptostemonum species have been so far characterised only for a restricted range of traits of interest, mostly disease resistance and fruit biochemical constituents. Here, we limit ourselves to a global survey of the information, in order to indicate the major achievements, as well as the missing information that deserves further research.
11.2.1 Morphological and Genetic Diversity
11.2.1.1 Cultivated Germplasm
Phenotypic diversity for fruit, plant and other traits of interest is described in many papers for Solanum melongena (Prohens et al. 2005; Kumar et al. 2008; Tümbilen et al. 2011b; Cericola et al. 2013), S. aethiopicum (Adeniji et al. 2012; Kouassi et al. 2014) or for two or more eggplant species (Osei et al. 2010; Polignano et al. 2010; Plazas et al. 2014). Morphological diversity of S. melongena, S. aethiopicum and S. macrocarpon has been recently revisited on the basis of large sets of accessions (Kumar et al. 2008; Osei et al. 2010; Polignano et al. 2010; Sunseri et al. 2010; Adeniji et al. 2012; Kouassi et al. 2014; Plazas et al. 2014; Taher et al. 2017). The contribution to the diversity is unequal between traits of breeding interest. On a set of 33 Indian landraces of S. melongena, yield per plant, fruit width, number of long styled flowers per plant, flowering earliness, total phenolic content and ascorbic acid content were the traits which contributed the most to the divergence between accessions (Prabakaran et al. 2015). Of course, the results depend on the set of accessions used and so far no wide range study including accessions representative of the full phenotypical diversity of each cultivated eggplant was carried out. Summaries of the phenotypic diversity of eggplants, together with the Mendelian or quantitative heredity patterns of traits of interest, are available in various chapters (Daunay et al. 2001; Daunay 2008; Daunay and Hazra 2012).
Analyses of the genetic diversity of Solanum melongena using molecular markers provided insights in allelic richness and diversity, for instance among Jordanian (Sadder et al. 2006), Spanish (Prohens et al. 2005), Turkish (Tümbilen et al. 2011b; Demir et al. 2010) and Chinese accessions (Ali et al. 2011). Sampling of S. melongena accessions that originate from wider distribution areas was also used for investigating possible relations between molecular diversity on one hand, and geographical origin, morphological traits or cultivar types on the other hand (Hurtado et al. 2012; Vilanova et al. 2012; Cericola et al. 2013; Naegele et al. 2014). African eggplants’ genetic diversity was also investigated with molecular markers, but to a lesser extent than S. melongena (Sunseri et al. 2010; Tümbilen et al. 2011a). On the whole these publications indicate that molecular markers and morphological traits are complementary tools for assessing diversity.
11.2.1.2 Wild Germplasm
Morphological characterisation of wild Solanum species is common in botanical publications which provide very detailed conventional information, e.g. (Vorontsova and Knapp 2016). Less detailed descriptions can be found in papers comparing parents to their interspecific hybrids (Sect. 11.8). Descriptors derived from IPGRI recommendations for Solanum melongena (IBPGR 1990) were used for comparing morphological traits between S. incanumL., S. insanum L. and S. melongena (Ranil et al. 2017). Phenotypic comparison between accessions of a given wild species of interest is rarely assessed, probably because of the difficulty to access different accessions. However, some examples are available. Indonesian accessions of S. torvum Sw. were compared for morphological traits and resistance to two soil-borne vascular diseases (Gousset et al. 2005). Solanum elaeagnifolium Cav. is mentioned as morphologically variable through its distribution area, in particular for prickliness and leaf shape (Scaldaferro et al. 2012). Genetic diversity for molecular markers between Solanum species has been analysed with the aim to assess (1) genetic distances or (2) phylogenetic relationships between species; only a few publications compared accessions within a single species such as for S. torvum (Clain et al. 2004), and for S. incanum and S. insanum (Tümbilen et al. 2011a).
11.2.2 Pest and Disease Resistances
Pests and disease resistances have a major interest in plant breeding, and resistances have been identified within the cultivated species, as well as among several wild species; see Daunay (2008) for an overview. Pests with major economic importance are root knot nematodes (Meloidogyne spp.), soil-borne diseases (Verticillium dahliae, Fusarium oxysporum f. sp. melongenae and Ralstonia solanacearum species complex-RSSCFootnote 1 (Safni et al. 2014), insects (fruit and shoot borer Leucinodes orbonalis, leaf hopper Amrasca biguttula bigutulla) and mites (Tetranychus spp. and Polyphagotarsonemus latus). The incidence of these pests and diseases on each eggplant species depends on the geographical areas and climatic conditions, but on the whole all cultivated eggplants are susceptible to a similar range of pests and pathogens.
11.2.2.1 Cultivated Germplasm
Resistances to Fusarium wilt (Hébert 1985; Boyaci et al. 2012), bacterial wilt (Daunay 2008; Lebeau et al. 2011) and both pathogens (Daunay et al. 2016) have been identified within Solanum melongena and S. aethiopicum germplasm. Monogenic dominant control has been identified for Fusarium wilt resistance originating from S. melongena (Mutlu et al. 2008; Boyaci et al. 2011) and from S. aethiopicum (Toppino et al. 2008b). Genetic control of resistances to RSSC is very variable (monogenic or polygenic, recessive or dominant) depending on S. melongena accessions (Daunay 2008) and on bacterial strains (Salgon et al. 2017; Salgon et al. 2018). Monogenic dominant resistances to this disease have been recently mapped (Lebeau et al. 2013; Salgon et al. 2017), and their functional characterisation is ongoing (Xiao et al. 2015; Morel et al. 2018). A monogenic resistance of S. melongena to Colletotrichum gloeosporioides (which causes fruit anthracnosis) was also described (Kaan 1973). Search for resistance to viruses has so far concerned a narrow range of viruses towards which some resistances have been identified (Daunay 2008). Resistance to Verticillium wilt (Verticillium dahliae) and root knot nematodes (Meloidogyne spp.) have not been found so far within cultivated eggplant germplasm.
The dense hairiness of some accessions of S. melongena was suggested to be at the origin of their partial resistance to leaf hopper (Daunay 2008). Hairiness of S. aethiopicum Gilo and Aculeatum groups was given as explaining their resistance by antixenosis to mites, whereas the glabrous Kumba group is susceptible (Seck 1997). Contrastingly (and counter-intuitively), the absence of hairs on vegetative parts would confer resistance to leaf hopper and red mites of S. macrocarpon (Daunay 2008) as well as to white fly Trialeurodes vaporariorum (Malausa et al. 1988). Fruit epidermis thickness and biochemical compounds (in sap, glandular hairs or fruits) are also mentioned as possibly interacting with resistance to some pests (Daunay 2008). The publications concerning eggplants resistance to insects and mites are mostly field observations where antixenosis is observable. Very few quantified details on the life cycle of the pests are available; one study revealed the existence of antibiosis towards white fly in S. melongena germplasm (Malausa et al. 1988).
11.2.2.2 Wild Germplasm
Many publications mention the resistance of Solanum species to various pests and pathogens, but the main difficulty in handling the detailed literature on the subject is the frequent unreliability of species identifications. Recent progresses concerning the taxonomy of spiny solanums, together with a better interaction between taxonomists and the community of germplasm holders and geneticists, should solve this issue. Attempts of summing up information are available for instance in (Collonnier et al. 2001a; Robinson et al. 2001; Kashyap et al. 2003; Daunay 2008). Global information indicates that high resistance to major pathogens that are not controlled by Solanum melongena germplasm are available in species so far not crossable (S. sisymbriifolium Lam.) or very difficult to cross with S. melongena (S. torvum); Solanum sisymbriifolium and S. torvum are in particular resistant to Verticillium wilt and to several root knot nematodes.
11.2.3 Diversity for Other Traits
For wild germplasm as well as for cultivated eggplants, much less characterisation researches are focused on other traits than crossability and pest and disease resistance. Graft affinity between cultivated eggplants (scion) and wild species (rootstock) is continually evaluated (Gisbert et al. 2011a, b; Villeneuve et al. 2016). This field of research is of the utmost interest given that grafting is a common worldwide practice for Solanum melongena cultivation. Rootstocks are indeed precious alternatives when resistance to soil-borne pests and diseases is not available in the cultivated germplasm or is not transferable from a resistant wild species because of interspecific cross failure. However, rootstocks may transfer alkaloids to the scion (Villeneuve et al. unpub.) and may also modify soil pathogenic profile (Villeneuve et al. 2014); given their potential side effects, these aspects need to be taken into account in parallel with the evaluation of wild germplasm for graft affinity with cultivated eggplants.
Phenolic acids were analysed in relation to health value (Stommel and Whitaker 2003; Mennella et al. 2010; Plazas et al. 2013; Meyer et al. 2015; Jose et al. 2016; Kaushik et al. 2017) or pest resistance (Prabhu et al. 2009). Glycoalkaloids and furostanol-type steroidal saponins are the major compounds responsible for eggplants bitterness (Aubert et al. 2009a) and diversity among Solanum melongena, S. aethiopicum and S. macrocarpon genotypes is being investigated (Aubert et al. 2009b; Mennella et al. 2010; Sanchez-Mata et al. 2010). Among wild Solanum species, the diversity of alkaloids, both in terms of molecules and content, is wide (Jayakumar and Murugan 2016). These compounds have a strong medicinal and pharmaceutical (Gurbuz et al. 2015; Jayakumar and Murugan 2016), as well as bio-insecticidal interest (Chowanski et al. 2016). Interspecific diversity for phenolic acids and glycoalkaloids was also characterised in order to generate a Solanum metabolic database and look at evolutionary patterns (Wu et al. 2013).
Other wild traits of strong interest, such as root vigour and architecture (Garcia-Fortea et al. 2019) and resistance to drought (Gramazio et al. 2017b), are being looked at, although this approach is so far limited to particular interspecific crosses, between Solanum melongena on one hand and S. elaeagnifolium or S. incanum on the other hand. A detailed phenotyping methodology has been used for a first investigation of root system diversity among accessions of Solanaceae including S. melongena (Bui et al. 2015). Such characterisation should be extended in the future to the cultivated eggplants germplasm and the related wild species, given that climatic changes will unarguably impact yield. Breeders should find a way to face this challenge, in particular by creating varieties (and rootstocks) with vigourous root systems. The many spiny solanums originating from dry (and hot) areas of Africa (Vorontsova and Knapp 2016), Asia (Aubriot et al. 2016) and Australia (Echeverría-Londoño et al. 2018) constitute to this respect an inestimable potential resource of adaptation to dry conditions.
11.3 Crossability Between Eggplants and Relatives
This field of research has attracted many dispersed efforts, limited in many publications to a single or to a few cross partner’s couples, except studies carried out within the frame of taxonomic researches for investigating relationships between species which generally encompass many partner’s couples. Crossability between species has the double interest of (1) informing about their phylogenetic and/or genetic relationships, and (2) identifying germplasm potentially usable as a source of genes controlling traits of interest to be introgressed from one species to another.Footnote 2 The first attempts of interspecific crosses between spiny solanums started from the 1930s and were carried out in particular by Indian and Japanese scientists (Rao 1979; Kirti and Rao 1982a, b). Four Ph.D. theses at the University of Birmingham (Pearce 1975; Niakan 1980; Hasan 1989; Al-Ani 1991) as well as research carried out at INRA in the 1990s (Daunay et al. 1998) achieved large-scale interspecific experiments. The rest of the information is scattered among many publications from the 1960s to now. Results were compiled and updated several times (Hasan 1989; Daunay et al. 1991; Collonnier et al. 2001a; Kashyap et al. 2003; Daunay 2008; Daunay and Hazra 2012).
We provide here the next synthesis, based on a stepwise analysis of the literature. First, we compiled information from references which specify the species used as female or male in the crosses (Al-Ani 1991; Ano et al. 1989, 1991; Ano 1990; Behera and Singh 2002; Bletsos et al. 1998; Bletsos et al. 2004; Bukenya and Carasco 1995; Callano et al. 2015; Cao et al. 2009; Daunay et al. 1998; Garcia-Fortea et al. 2019; Gowda et al. 1990; Isshiki and Kawajiri 2002; Khan and Isshiki 2008, 2009, 2010, 2011; Khan et al. 2017; Kirti and Rao 1980, 1981, 1982a, b, 1983; Kouassi et al. 2016; Kumchai et al. 2013; Lester and Hasan 1991; Lester and Kang 1998; Lester and Niakan 1986; Liu et al. 2015; Mc Cammon and Honma 1983; Olet and Bukenya-Ziraba 2001; Omidiji 1979, 1983, 1982; Oyelana and Ogunwenmo 2009; Oyelana and Ugborogho 2008; Oyelana et al. 2009; Plazas et al. 2016; Prabhu et al. 2009; Prohens et al. 2012; Rajasekaran 1971; Rao and Rao 1984; Rattan et al. 2015; Robinson et al. 2001; Schaff et al. 1982; Sharma et al. 1980; Zhou et al. 2018). The next step aimed at simplifying the information by keeping only the best result obtained for a given cross, whatever the authors or the cross direction. This simplified file was then (1) merged together with the similarly simplified data of Daunay et al. (1991), and (2) sorted in order to keep the best result obtained for each interspecific cross and to eliminate duplicated crosses.
On the whole, 67 spiny species have been used so far in interspecific crosses, including 51 African and Asian species, nine Australian and seven American. When compared to the over 500 spiny species inventoried presently (Chap. 10), it is clear that the knowledge about crossability between spiny solanums is a research field barely investigated, which deserves strong efforts in the future, in particular for crosses involving eggplants and their African and Asian closest relatives (see 11.4.2 and 11.4.3).
Surveying interspecific crossability in spiny solanums is challenging for many reasons, in particular because of the large number of species and crosses involved, of frequent inappropriate use of nomenclature and of occasional species misidentification. Further, a wide range of crossability criteria is found in the literature, given that the expression of pre- or post-zygotic barriers induces a diversity of effects. Lastly, results obtained by different authors for a given interspecific cross are often conflicting, because of the influence of cross direction (partner used as female or male), genotype of parental accessions, as well as environmental conditions. Hence, before entering into a summary of the interspecific crosses achieved so far, we first review the prezygotic and post-zygotic barriers that contribute to the complexity of the results published. We will also emphasise the interest of cytogenetic studies (1) for understanding F1 fertility troubles, together with (2) assessing genetic relationships between the parental species. We then provide examples illustrating the heterogeneity of the information found in the literature, before summarising the best results obtained for the over 200 interspecific crosses attempted so far and structured into four types of crosses:
-
1.
Crosses between cultivated eggplants (Solanum aethiopicum, S. macrocarpon, S. melongena);
-
2.
Crosses between cultivated eggplants and their wild progenitors S. anguivi Lam., S. dasyphyllum Schumach. & Thonn. and S. insanum, respectively, as well as crosses between these wild progenitors;
-
3.
Crosses between cultivated eggplants and (non-progenitor) wild species;
-
4.
Crosses between wild species.
Phenotypes of interspecific hybrids will be discussed in relation to trait heredity patterns. We will continue by reviewing the occasional use of artificial tetraploidisation for restoring male fertility of interspecific hybrids. Next, a special section is dedicated to the cytoplasmic male sterilities obtained by crossing Solanum melongena with several wild species.
Given the wealth of information we provide, we skipped presenting the control data obtained on the parental species, in particular for pollen stainability, given this one is generally above 80% throughout all publications reviewed. Apart some exceptions for which we provide accurate figures, hybrid fertility has been categorised on the basis of pollen stainability values as virtual sterility (<10% pollen stainability), partial fertility (10–50%) and fertility (>50%). The relationships between pollen stainability, viability and fertility are a subject of debate, but as all publications use pollen stainability as a measure of viability or fertility, we kept this criterion. Some publications mention also pollen in vitro germination as a complementary measurement of pollen fertility; this criterion yields generally smaller values than stainability.
By convention, any interspecific cross is written in the following text as “female x male” when cross direction is known and “partner 1 and partner 2” when it is not specified. We only partially rationalised species nomenclature, given its complexity in the literature, in order to keep close to the names used in the literature together with the accepted names. Hence, we provide the accepted species name together with the name used by the authors (in parentheses), when their correspondence was easy to establish:
-
S. campylacanthum Hochst. ex A.Rich. (S. incanum group A, group B, S. panduriforme Drège ex Dunal, S. delagoense Dunal);
-
S. forskalii Dunal (S. albicaule Kotschy ex Dunal);
-
S. incanum (S. incanum group C);
-
S. insanum (S. melongena group E, group F);
-
S. lichtensteinii Willd. (S. incanum group D);
-
S. multiflorum Roth (S. indicum L. var. multiflorum (Roth) C.B. Clarke;
-
S. viarum Dunal (S. khasianum C.B.Clarke);
-
S. violaceum Ortega (S. indicum L., S. kurzii Brace ex Prain, S. sanitwongsei Craib);
-
S. virginianum L. (S. surattense Burm.f., S. xanthocarpum Willd. ex Walp.Footnote 3).
However, in several cases, the transposition of species names used in the publications to the now accepted names according to recent nomenclature changes could have blurred or mixed up our discussion of interspecific cross results. That is the reason why we decided to keep the species names used in the literature for the following cases:
-
S. capense L. and S. dinteri Bitter (now both under the accepted name S. capense);
-
S. rigescens Jacq., S. rigescentoides Hutch., S. giftbergense Dunal (now all under the name S. humile);
-
S. tomentosum L. and S. coccineum Jacq. (now under the name S. tomentosum);
-
S. sessilistellatum Bitter (now under the name S. nigriviolaceum Bitter).
11.3.1 Prezygotic and Post-zygotic Barriers
Results of interspecific crosses between Solanum species depend on pre- or post-zygotic barriers, the expression of which is assigned to the relationships (genetic or phylogenetic) between parental partners. Prezygotic barriers include absence of pollen germination on the stigma, abnormal or insufficient pollen tube growth through the styleFootnote 4 and as a result absence of fertilisation of polar nuclei (future endosperm) and egg cell (future zygote) by the pollen nuclei. Flowers and fruits’ drops and/or parthenocarpic fruitsFootnote 5 are observed in such cases. Post-zygotic barriers are expressed after fertilisation occurred, and they involve unbalanced collaboration between the parental genomes in the fertilised cells, i.e. the endospermFootnote 6 and/or the zygote. Their expression is visible along different development stages of the F1 embryo, plantlet or adult plant. The genetic imbalance between parental genomes is suggested to explain dysfunction of endosperm growth and of endosperm–embryo metabolic relationships, with consecutive embryo starvation and death, or endosperm autolyse and embryo digestion at an early stage (Lester and Kang 1998). In interspecific crosses between Solanum arcanum Peralta, S. chilense (Dunal) Reiche and S. peruvianum L. (wild tomatoes), endosperm–embryo interactions have been recently investigated at intimate levels (endosperm early cellular stages and maternal and paternal genes expression) for unravelling the genetic parental conflicts at the origin of embryo growth stop and degeneration, resulting in hybrid seed failure (Roth et al. 2018a, b, c). Dysfunction between parental genomes ends up with parthenocarpic fruits, or fruit set with aborted seeds or variable proportion of abnormal seeds. According to Lester and Kang (1998) seed abnormality rate, when used carefully, is a good and easy measure of this early post-zygotic reproductive barrier between species. When this barrier is overcome artificially via careful sowing of the normal seeds or via in vitro embryo rescue (Kharkongar et al. 2013; Sharma et al. 1996), genetic imbalance affecting directly the zygote can lead to seedlings or plantlet death, abnormal, weak interspecific hybrid plants and also rooting difficulties.Footnote 7 When the two parental genomes collaborate relatively correctly, the hybrid plants are vigourous. However, later dysfunctional genetic control of the reproductive process can induce hybrid fertility troubles, frequently observed (next section). This late post-zygotic barrier, that in Nature protects species from gene exchange, is sometimes described as “hybrid breakdown”. The accumulation during lineage divergence of loci interacting negatively and responsible for interspecific hybrids sterility has been theorised on the basis of tomato introgression lines phenotyped for pollen and seed sterility (Moyle and Nakazato 2010).
Another event reported (Rao and Rao 1984) is the occurrence of maternal seeds in a variable proportion, up to 100%, in the fruits set up after an interspecific pollination (examples are provided in Table 11.1). It seems that the foreign pollen induces the development of unfertilised maternal ovules into seeds, instead of, or conjointly with, the fertilisation of these ovules and the development of seeds containing an interspecific embryo. The hypothesis of an apomictic behaviour of the maternal parent was suggested by Rao and Rao (1984). The unexpected and occasional harvest of maternal seeds issued from several interspecific pollinations has also been observed by Daunay et al. (unpubl.).
If species identity is a major factor of the success or failure of any interspecific cross, several authors point out also the influence of parental genotypes (Bletsos et al. 2004; Cao et al. 2009; Daunay 2008; Daunay and Hazra 2012; Devi et al. 2015; Gowda et al. 1990; Kirti and Rao 1982a, b; Lester and Niakan 1986; Omidiji 1979; Plazas et al. 2016; Rajasekaran 1970; Rao 1979; Rao and Rao 1984; Rattan et al. 2015; Schaff et al. 1982; Zhou et al. 2018). The impact of parental genotypes has also been observed in genus Datura and was interpreted as an evidence of the influence of genes or gene complexes. Those genetic factors are distributed throughout the genome and act as a barrier against successful hybridisation, possibly in a complementary way (Rao 1979). Environmental conditions also affect the results of interspecific crosses and, together with the genotypes, are probably at the origin of the heterogeneous results obtained by different authors for a same interspecific cross (for instance with regards to fruit set, hybrid meiosis features or hybrid fertility). Hence in the present state of the art, it is safer not to conclude definitively about the failure of any apparently recalcitrant crosses. For the reasons detailed above and because of the potential continuous improvement in the use of in vitro embryo rescue, tetraploidisation, somatic hybridisation or bridge species, interspecific cross results should be considered as provisional.
11.3.2 Cytogenetic Observations of Late Post-zygotic Barriers
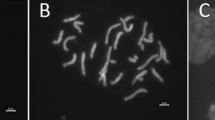
Chromosomes structural repatterning having occurred during the evolutionary process of the species (interchanges, interstitial breakpoints, heteromorphy) maintained the individuality of each taxa (Kirti and Rao 1982b) and is considered as a major factor causing gametic lethality of interspecific hybrids. Hence, chromosome behaviour and shapes during diakinesis (end of prophase I) and metaphase I at the time of F1 pollen mother cell (PMC) meiosis provide information about homologies or homeologiesFootnote 8 between parental chromosomes (Kirti and Rao 1980, 1981, 1982a, b, 1983). As chromosome behaviour differs from one PMC to another and depends also on the meiosis step (diakinesis or metaphase I), cytological observations must be carefully done on several PMC of a given hybrid in order to calculate a reliable estimation of the frequencies of univalent, bivalent and other multivalent occurrence at each meiosis stage. The more univalents, the less homeology between the chromosomes pairs of both parents. The cross between Solanum trilobatum L. and S. virginianum illustrates a case of poor homeology of their chromosomes, with a frequency of bivalents in their F1 varying from 3% to 21%, depending on the cross direction (Table 11.2). Conversely, occurrence of bivalents in hybrids indicates that the concerned chromosome pairs retained sufficient ancestral similarities to allow their pairing. The closer to 12 the number of bivalents, the better the homeology between the parental chromosomes. High chromosome homeology is found between S. melongena and S. violaceum, the reciprocal hybrids of which both display 99% of bivalents during their meiosis (Table 11.2). Hence, frequency of bivalents, or more globally regular or irregular meiosis, depends clearly on cross partners. Cross direction effect on F1 meiosis is less clear, given there are some differences between reciprocal hybrids (e.g. for the F1 S. multiflorum and S. virginianum, with 43 and 56% bivalents) or no differences (e.g. F1 S. aethiopicum and S. macrocarpon, both with irregular meiosis) (Table 11.2). Meiotic behaviour of hybrids S. aethiopicum Aculeatum group (S. integrifolium Poir.) X S. melongena and hybrids S. aethiopicum Aculeatum group x S. insanum (S. melongena var. insanum) was compared (Kirti and Rao 1982b). The high frequency of bivalents in both hybrids led the authors to conclude about homeologies between the three species. Because of differences between both hybrids for types and frequency of chromosomes associations, they also suggested differences “to some extent” between S. melongena and S. insanum.
Pollen stainability is given in most publications as a criterion of interspecific hybrid fertility, and following Daunay et al. (1991), we will reduce hybrid fertility into three classes: (1) F1 virtually sterile with less than 10% pollen stainability, (2) F1 partially fertile (10-50% pollen stainability) and (3) F1 fertile (>50% pollen stainability). On this basis, we state that irregular meiosis can end up either with virtually sterile (e.g. cross S. aethiopicum and S. multiflorum) or partially fertile hybrids (e.g. S. aethiopicum and S. macrocarpon). This means that at least some viable microspores can be produced from abnormal meiosis. On the other hand, a regular or almost regular meiosis, with high bivalents occurrence frequency followed by regular chromosome separation and microspore formation, can end up with fertile or only partially fertile hybrids (e.g. crosses between S. melongenaand S. violaceum and S. melongena and S. viarum), or even with virtually sterile ones (S. melongena and S. aethiopicum). In the two latter cases, post-meiotic degenerative events affecting tetrads or maturing microspores probably occur. In cases of highly sterile F1 pollens, the late expression of the reproductive barrier was attributed either to cryptic chromosomal structural differences or to recombination and segregational events of insufficiently homeologous chromosomes leading to unbalanced gametes (Kirti and Rao 1980, 1982a, b, 1983).
Lastly, one notices that progenies were obtained from interspecific F1, regardless of pollen stainability (Table 11.2), including very poor one as illustrated by the striking case of the virtually sterile hybrids (S. multiflorum x S. aethiopicum), (S. virginianum x S. trilobatum) and (S. virginianum x S. melongena).
Apart from chromosome global pairing at diakinesis and metaphase I, careful cytological observations may reveal abnormal shapes of bivalents (e.g. rods, rings) and of tetravalents (e.g. chains, Y, fish, ring or double-ring types), which are also evidence of multiple homeologies between parental chromosomes and of structural re-organisation/repatterning. For instance, fish-type and double-ring configurations suggest interstitial translocation breakpoints.
Comparative chiasma (crossing over) frequencies per bivalent between a hybrid and its parental species is another indicator of the level of homeology between the chromosomes: the closer the chiasma frequency of the hybrid to that of its parental species, the more homeologous their chromosomes; and the higher the recombination potential between the parental genomes, the more closely related the two parental species. Differences in chiasma frequencies between reciprocal hybrids indicate cytoplasmic influence on meiotic behaviour; this is the case for the cross between S. aethiopicum (S. integrifolium) and S. multiflorum (S. indicum var. multiflorum), with 1.23–1.27 average chiasma frequency per bivalent when S. aethiopicum is the female parent and 1.31–1.34 when it is the male parent (Kirti and Rao 1980). The controls, i.e. the parents, displayed a chiasma frequency of 1.59–1.63.
On the whole, cytogenetic observations reveal the expression of late post-zygotic barriers that are expressed at the time of, or after, F1 flower meiosis. However, the border between impossibility and possibility to go through these late barriers is labile, as exemplified by cases for which progenies are sometimes obtained from virtually sterile hybrids producing a high percentage of sterile pollen (Garcia-Fortea et al. 2019; Kirti and Rao 1980, 1983; Rao and Rao 1984).
11.3.3 Variation of Hybridisation Results
Same species combinations have been used by a number of authors, with either consistent results (e.g. crosses between S. melongena and S. incanum), or with inconsistent results ranging from cross failure to obtaining fertile hybrids (e.g. crosses between S. melongena and S. violaceum; cf. Table 11.3). This could point out that the influence of different parental genotypes and environmental conditions on a crossing result varies with regard to species partnership. Table 11.3 illustrates also the variation of in depth investigation from one author to another; some stopped with the observation of F0 → F1 seed germination, while others went as far as obtaining advanced progenies from the F1.
11.4 Overview of the Best Results Obtained When Crossing Spiny Solanums
For the sake of clarity, as over 200 species combinations have been used in interspecific crosses attempted so far, we decided to split the results into the four crossing categories listed in Sect. 11.3.
The statistical overview of the best results obtained within these four categories of crosses is summarised in Table 11.4. Solanum melongena is by far the cultivated eggplant for which the number of interspecific crosses attempted is the highest (61 crosses, vs. 16 and 3 for S. aethiopicum and S. macrocarpon, respectively). Most of the crosses (116) were attempted between wild species. The best results obtained are distributed along a stepwise scale, from fertile hybrids to no fruit set or setting of parthenocarpic fruits on the maternal parent at the time of the cross. Globally, few publications went as far as attempting to obtain progenies from interspecific hybrids; hence, the data presented in Table 11.4 cannot be used to predict what could be achieved if attempted.
11.4.1 Crosses Between Cultivated Eggplants
Solanum melongena, S. aethiopicum and S. macrocarpon have been crossed in all reciprocal combinations (Table 11.5). The hybrids between S. aethiopicum and S. macrocarpon as well as those between S. aethiopicum and S. melongena are frequently reported as vigourous, whereas those between S. macrocarpon and S. melongena have generally a poor vigour. For this latter species combination, the vigour depends on the parental genotypes (Schaff et al. 1982), regardless of the direction of the cross. Although results differ between authors, all species combinations have produced at best partially fertile of fertile hybrids. In all cases, progenies were obtained from the hybrids, although in the case of S. aethiopicum and S. macrocarpon, observations stopped at the seed set of one of the reciprocal hybrids. Hence, despite some sterility troubles occurring at the level of F1 or of later progenies, the three cultivated eggplants are usable in breeding as sources of traits for each other.
11.4.1.1 Solanum aethiopicum and S. macrocarpon
Partially fertile (10% < pollen stainability < 50%) or virtually sterile hybrids (pollen stainability <10%) with meiotic abnormalities were obtained from this cross (Table 11.5). In the virtually sterile hybrid obtained with Solanum aethiopicum (probably Kumba group) used as the female parent (Omidiji 1983), twelve bivalents were formed in 78% of the F1 pollen mother cells (PMC); however, for other PMC, bivalents were associated to low proportion of univalents, trivalents and tetravalents. Omidiji concluded that the chromosomes of both parental species were sufficiently homeologous for permitting pairing in most PMC, despite cryptic differences (translocations, inversions). Despite metaphase I and later stage meiosis irregularities, the low pollen fertility due to unbalanced gametes did not hamper the hybrid undersized fruits to contain some seeds. In reciprocal hybrids obtained from the cross between S. aethiopicum Gilo group and S. macrocarpon (Oyelana and Ogunwenmo 2009) and displaying partial fertility (21 to 34% pollen stainability), meiotic irregularity was also observed (about 50% bivalents, trivalents, tetravalents, clumps and laggards). Interestingly Omidiji (1983) noticed meiotic irregularities in S. macrocarpon (not mentioned by Oyelana and Ogunwenmo 2009) and questioned a possible hybrid origin of this species.
11.4.1.2 Solanum aethiopicum and S. melongena
Depending on the crosses, hybrids virtually sterile, partially fertile or fertile are described in the literature (Table 11.5). Meiosis of virtually sterile reciprocal F1 is reported as normal (Callano et al. 2015; Kirti and Rao 1982b). Persisting sterility troubles in first backcross (BC) generations are mentioned for a virtually sterile F1 obtained with Solanum aethiopicum Aculeatum group used as female (Ano 1990; Ano et al. 1989, 1991). In BC generations obtained with a similar hybrid and S. melongena used as male recurrent parent, segregation for cytoplasmic male sterility was detected from BC1 onwards (Khan and Isshiki 2010), whereas the male fertile plants still suffered fertility troubles even in BC4 (maximum of 50% stainable pollen). A reciprocal hybrid obtained with S. melongena used as female and S. aethiopicum Kumba group (Prohens et al. 2012) as male, also poorly fertile (0–2% pollen stainability; 28% fruit set) yielded also BC progenies (with each parental species) with limited (but improved) pollen stainability (1–62%) and fruit set (53%).
11.4.1.3 Solanum macrocarpon and S. melongena
F1 meiosis revealed regular chromosome pairing in most pollen mother cells (PMC) with occasional multivalents and univalents in some PMC (Schaff et al. 1982; Wanjari 1976). Hybrid pollen stainability varied from 5 to 21%, depending on the cross direction and parental accessions (Bletsos et al. 2004); it was observed that pollen stainability was better when Solanum melongena was the maternal parent: 10–15% versus 1–9% for S. macrocarpon as the maternal parent (Schaff et al. 1982), but this difference seems arguable. F2, F3 and BC progenies were obtained from reciprocal hybrids, with better pollen stainability than the hybrid, although still lower than that of the parental species (Oyelana and Ugborogho 2008; Schaff et al. 1982).
11.4.2 Crosses Between Cultivated Eggplants and Their Wild Progenitors
Each cultivated eggplant species is fully interfertile with its own wild progenitor, i.e. S. aethiopicum with S. anguivi, S. macrocarpon with S. dasyphyllum and S. melongena with S. insanum (Table 11.6A). This is the case regardless of the direction of the cross, i.e. cultivated species used as female or as male (data not shown).
Crosses between each cultivated eggplant and the wild progenitors of the two other cultivated species were also investigated (Table 11.6B). Data are insufficient to look for a possible difference between reciprocal crosses. A rough comparison of crossability results between partnerships “cultivatedi − cultivatedj” (Table 11.5) and “cultivatedi-wild progenitorj” is possible. The results of such comparisons seem consistent for the crosses involving:
-
S. aethiopicum crossed with S. macrocarpon or S. dasyphyllum (F1 partially fertile);
-
S. aethiopicum crossed with S. melongena (F1 partially fertile) or S. insanum (F1 virtually sterile);
-
S. melongena crossed with S. aethiopicum or S. anguivi (F1 partially fertile);
-
S. melongena crossed with S. macrocarpon or and S. dasyphyllum (F1 partially fertile);
-
S. macrocarpon crossed with S. aethiopicum or S. anguivi (F1 partially fertile);
-
Incomplete data hamper the comparison between S. macrocarpon crossed with S. melongena (F1 partially fertile) or S. insanum (no data).
11.4.3 Crosses Between Cultivated Eggplants and (Non-progenitor) Wild Species
11.4.3.1 Reciprocal Crosses
Many crosses have been attempted by using the parental partners as female and as male parent. We compare the best results obtained so far for reciprocal crosses in the case of three species partnerships for Solanum aethiopicum, one for S. macrocarpon and 52 for S. melongena (see Table 11.7). This table once more illustrates the heterogeneous information available in the literature, as well as the extreme diversity of cases obtained throughout the crosses. Here, we will only discuss the diversity of results obtained in crosses involving S. melongena, since they are numerous enough to provide a general overview. Hybrids virtually sterile, partially fertile or fertile are obtained whether S. melongena is used as female (for six crosses, thirteen and five, respectively) or male parent (four, eight and two, respectively). Hybrid fertility level does not seem to be related to the phylogenetic proximity between S. melongena and the wild species involved. In a number of cases, crosses yielded fertile or partially fertile hybrids regardless of the cross direction, e.g. those involving S. melongena on one hand and S. catombelense Peyr., S. cerasiferum Dunal, S. dinteri, S. incanum, S. rigescentoides, S. sessilistellatum and S. violaceum on the other hand. Several reciprocal crosses produced fertile or partially fertile hybrids for one cross direction only. This is the case for S. melongena used as female and pollinated with S. campylacanthum, S. hastifolium Hochst. ex Dunal, S. lichtensteinii, S. melanospermum F.Muell., S. rigescens Dunal, S. viarum as well as with the nightshade S. scabrum Mill. This is also the case for S. lidii Sunding, S. linnaeanum, Hepper & P.-M.L. Jaeger, S. supinum Dunal (and possibly S. capense and S. cyaneopureum De Wild.Footnote 9) when used as female and pollinated with S. melongena.
One observes also that there are as many as five different types of crossing results (Table 11.8). Fertile (1st type), partially fertile (2nd), virtually sterile (3rd) or unviable interspecific hybrids (4th) together with cross failure (5th type) are obtained for crosses whether S. melongena is used as female or as male parent. On the basis of the available set of reciprocal crosses involving S. melongena and wild species (Table 11.7), it seems that there is no relationship between reciprocal results; indeed, almost every type of result obtained with S. melongena used as female matches with the ones retrieved when S. melongena is used as male and conversely (Table 11.8). Last but not least, progenies can be obtained from any given fertility level (fertile, partially fertile or virtually sterile) of the interspecific hybrids (Table 11.7).
11.4.3.2 Global Results for All Types of Crosses
In a number of publications, results are provided without specification of cross direction, or only with a mention of a single cross direction. Therefore, such crosses’ results are excluded from Table 11.7, which gathers only the reciprocal crosses. In order to provide a global overview of the interspecific crosses results (out of the wild progenitors of cultivated eggplants, which are detailed in Sect. 11.4.2), we have gathered the best results obtained from such “one way” crosses as well as “unknown direction” crosses together with the best results obtained from “reciprocal crosses”; we then selected the “top one” results. The global synthesis involving Solanum aethiopicum and S. macrocarpon is provided in Table 11.9 and for S. melongena in Table 11.10.
To date, no fertile hybrids have been obtained when crossing Solanum aethiopicum with any of the 16 wild species tested; however, partially fertile hybrids were obtained with S. incanum and S. violaceum. Progenies were obtained from only one of the virtually sterile hybrids (S. multiflorum). It is worthwhile to retry some of the crosses since they produced a proportion of normal seeds and could perhaps give rise to hybrids. Only one out of the three interspecific crosses attempted so far with S. macrocarpon has yielded a hybrid, the fertility of which is however not known (Robinson et al. 2001).
Interspecific crosses involving Solanum melongena are much more numerous (61) than those involving S. aethiopicum (16) and S. macrocarpon (3). Over half of the crosses yielded hybrids of variable fertility (from fertile to virtually sterile) and from which nine progenies were obtained so far (Table 11.10).
The species yielding fertile or partially fertile hybrids belong either the Melongena clade (Solanum campylacanthum, S. cerasiferum, S. incanum, S. linnaeanum and S. lichtensteinii), to the poorly resolved Old World Anguivi grade (S. burchellii, S. catombelense, S. coccineum, S. cyaneopurpurem, S. dinteri, S. hastifolium, S. lidii, S. rigescens, S. rigescentoides, S. rubetorum Dunal,, S. sessilistellatum (=S. nigriviolaceum), S. supinum, S. tomentosum and S. violaceum), to other Old World clades (S. melanospermum, S. virginianum) as well as to New World clades (S. aculeatissimum Jacq., S. viarum) (Vorontsova et al. 2013; Aubriot et al. 2018). For the hybrid between S. melongena and S. aculeatissimum, information is given only for its tetraploidized form. Unexpectedly, one tetraploid species of subgenus Solanum, S. scabrum, is one of the species yielding partially fertile hybrids when crossed with S. melongena. The species yielding virtually sterile hybrids, or no hybrids at all, display a similar phylogenetic diversity, as those yielding fertile or partially fertile hybrids.
Interestingly, when crossed with Solanum melongena, some species belonging to the New World clade (Stern et al. 2011) yield hybrids. That is the case of S. viarum which produces a fertile hybrid (Sharma et al. 1980), as well as S. elaeagnifolium (Garcia-Fortea et al. 2019) and S. hispidum Pers. (= S. asperolanatum Ruiz & Pav.; Daunay et al. 1991) which produce virtually sterile hybrids. The case of S. aculeatissimum is unclear since the fertility of the diploid hybrid is not indicated (Zhou et al. 2018). That is also the case for the fertile hybrid between S. melongena (female) and S. torvum (Cao et al. 2009) although all other authors having worked on this hybrid report its high sterility (Bletsos et al. 1998, 2004; Daunay unpub.; Mc Cammon and Honma 1983; Plazas et al. 2016; Robinson et al. 2001).
On the whole, this survey of the crossability results between cultivated eggplants and wild relatives indicates that a lot of work has still to be carried out in the future for completing and rationalising the current knowledge, both by extending the range of wild species available (African, Asian and Australian species) and by homogenising of the types of criteria to record. The possibility of obtaining progenies from interspecific hybrids has to be investigated as a priority, because this is the criterion that at the end is essential to breeders for the transfer of wild traits into cultivated germplasm. The apparent loose link between interspecific crosses results and phylogenetic relatedness of the partner species is a questioning matter that constitutes a promising research field for further comparative studies.
11.4.4 Crosses Between Wild Species
One hundred sixteen crosses involving 33 wild species have been attempted between wild Solanum species, out of which 26 crosses were reciprocals. Reciprocal and fertile or partially fertile hybrids were obtained only from the crosses involving S. coccineum on one hand and S. capense or S. violaceum on the other hand (Table 11.11). One cross direction and fertile or partially fertile hybrids were obtained from eight other crosses, involving mostly species of the former Oliganthes section, now included in the Anguivi grade (i.e. S. anguivi, S. capense, S. coccineum, S. rubetorum, S. violaceum) and some species of the Melongena clade (S. campylacanthum crossed with S. cerasiferum and S. incanum). One partially fertile hybrid was unexpectedly obtained when crossing S. violaceum (female) with S. virginianum, two species that are partly in sympatryFootnote 10 but also rather distantly related (Chap. 10).
The global overview of the best results obtained when crossing wild × wild, and that for any cross direction, is provided in Tables 11.12 and 11.13. The global picture is that roughly half (62) of the crosses were “successful” (Table 11.12) and half (54) failed (Table 11.13). Among the species combinations yielding fertile hybrids, one notices members of the Melongena clade that are closely related to each other, namely S. campylacanthum–S. cerasiferum,Footnote 11 S. incanum–S. campylacanthum, S. incanum–S. insanum and S. incanum–S. lichtensteinii. As already mentioned when discussing the reciprocal crosses, members of the former Oliganthes section are also often cross compatible. Detailing the cross failures (Table 11.13) is of limited use given many crosses have been attempted by only one author or with few parental accessions. Some failures are questionable, in particular for crosses between phylogenetically close species of the Melongena clade (Chap. 10), such as S. campylacanthum and S. insanum, S. campylacanthum and S. lichtensteinii and S. incanum and S. linnaeanum.
A few New World species, Solanum sisymbriifolium, S. torvum and S. viarum, have been crossed so far with Old World ones (Tables 11.11, 11.12 and 11.13). Solanum sisymbriifolium was crossed with S. anguivi and S. violaceum (Niakan 1980), as well as with S. incanum (Pearce 1975; Rao 1979). Solanum torvum was also crossed with S. anguivi (Niakan 1980), S. violaceum (Kirti and Rao 1981; Niakan 1980) and S. incanum (Pearce 1975). Solanum torvum was further crossed with S. multiflorum, S. trilobatum and S. virginianum (Rao and Rao 1984). Solanum viarum was crossed with S. anguivi and S. violaceum (Niakan 1980) as well as with S. incanum (Pearce 1975). All these crosses failed except for the cross between S. torvum and S. violaceum which yielded a virtually sterile hybrid (Table 11.12), as did the cross between S. torvum and S. melongena (Table 11.10).
11.5 Is Interspecific Crossability Predictable?
The genepool concept (Harlan and de Wet 1971) was set up for hierarchising the species related to a crop, on the basis of their crossability potential with the crop. Genepools (GP) were conceptualised as GP1 (biological speciesFootnote 12 including wild, weedy and cultivated forms of the crop, all interfertile), GP2 (species that are crossable with GP1 however with some difficulty and hybrids more or less fertile) and GP3 (species that are not crossable with GP1, forming abnormal, lethal or sterile hybrids, or hybrids that request radical techniques for getting success).
Applied to Solanum melongena (Hasan 1989), GP1 was first defined with S. insanum (S. melongena groups E and F sensu Lester) and S. melongena (groups G and H) on the basis of (1) their complete intercrossability (F1 plants with >80% pollen stainability), and (2) of the fact that, at that time, they were belonging to a same biological species. Hasan placed S. incanum (group C) and S. lichtensteinii (S. incanum group D) in GP2; together with S. campylacanthum (S. incanum groups A and B). In later research (Plazas et al. 2016) S. insanum, S. melongena and S. incanum were all (arguably) included in GP1. Solanum lichtensteinii and S. campylacanthum were included in GP2, together with S. linnaeanum, several species of the Anguivi grade (including the cultivated S. aethiopicum and S. macrocarpon and their wild progenitors) as well as species of the Madagascar clade (S. pyracanthos Lam.). Other Old World species, as well as New World species including S. sisymbriifolium, S. torvum and S. elaeagnifolium, were gathered into GP3. These examples illustrate the fluidity in the application of GP definitions for spiny solanums. Also, the global overview of the interspecific results involving S. melongena (see above) shows the limited practical value of the genepool system applied to spiny solanums. The example of S. melongena (Table 11.10) indicates that viable hybrids of various pollen fertilities were obtained when crossed with wild species of any given GP and that progenies can be obtained even from hybrids obtained with GP3 wild species.
Phylogenetic relationships between spiny solanums do not seem to be entirely helpful for predicting interspecific crossability. Indeed, closely related species can yield fertile or partially fertile hybrids when crossed to each other (e.g. S. melongena with other species of the Melongena clade), but species that are far more distant can also yield such hybrids (e.g. S. melongena with the New World S. viarum or the Australian S. melanospermum). Conversely species distantly related to S. melongena can yield hybrids from which progenies were obtained (e.g. S. elaeagnifolium and S. torvum). The ultimate inconsistency is illustrated by the successful cross between two species that are phylogenetically very distant, the tetraploid S. scabrum of subgenus Solanum (Chap. 10) and the diploid eggplant, S. melongena. Indeed, the cross S. melongena (2n = 24) × S. scabrum (2n = 48) yielded a few hexaploid F1 plants, partially fertile. The authors related the unusual ploidy level to the endo-duplication of the triploid zygote (Oyelana et al. 2009). Despite partial pollen stainability (38%), the hybrids produced only parthenocarpic fruits.Footnote 13
Knowledge on crossability combinations between cultivated eggplants and wild species and between wild species is by far very incomplete; this reflects (1) the very rich species diversity in spiny solanums, (2) and the still incomplete knowledge on phylogenetic relationships among Old World spiny solanums. However, the current state of the art and the apparent loose consistency between crossability and phylogenetic relationships seem to indicate that predicting crossability between species is illusory. This has implications on research fields that investigate (1) the biological meaning of current phylogenetic hypotheses and traditional species concept, (2) the range and nature of species chromosomal (and genomic) differentiation making interspecific crosses possible or not, and (3) the identity of the genetic factors that can rock an interspecific cross from impossible with some parents to possible with others.
11.6 Overcoming Interspecific Hybrid Sterility via Tetraploidisation
Several cases of F1 hybrid fertility restauration thanks to chromosome doubling are reported in the literature. Amphidiploids (4x) issued from colchicine treatment of reciprocal hybrids between Solanum melongena and S. aethiopicum Aculeatum group (S. integrifolium) displayed a clear increase of pollen stainability (70–72%), when compared to their diploid counterpart (9–12%); they yielded seeded fruits (86–91% normal seeds), whereas the diploids did not set fruits or set parthenocarpic ones (Ali et al. 1992). Bivalents and quadrivalents were observed at metaphase I in meiosis of a 4x F1 (S. aethiopicum Aculeatum group [S. integrifolium] × S. melongena), which indicates high homeology of the genomes (Isshiki et al. 2000).
F1 (Solanum melongena × S. aethiopicum Gilo group) pollen stainability was improved from 7% (diploid hybrid) up to 67% (tetraploid version) (Isshiki and Taura 2003). The reciprocal hybrid F1 (S. aethiopicum Gilo group x Solanum melongena) whether 2x or 4x did not produce pollen at all. Fruit set was obtained on the reciprocal 4x via selfing or intercross, whereas the diploids did not set fruits. In addition to the interest of chromosome doubling for restoring the fertility of this interspecific hybrid, Isshiki and Taura (2003) demonstrated also that there was a correlation between pollen sterility and cytoplasm donor, but no correlation between ability to set seed and cytoplasm. Contradictory findings on pollen fertility obtained by other authors suggest the existence of intraspecific variations of the cytoplasm between S. aethiopicum cultigroups or accessions, in line with mitochondrial DNA variations previously revealed by RFLPs (Isshiki et al. 2003).
In the case of crosses between Solanum melongena and S. macrocarpon, partial restauration of F1 pollen stainability was achieved by chromosome doubling induced by colchicine treatment (Khan et al. 2013a). The tetraploid hybrids displayed 40% pollen stainability versus 0.9% for its diploid counterpart. Whereas the diploid hybrid did not set fruits, F2 seeds were obtained by selfing the tetraploid F1 and BC1 seeds by backcrossing the tetraploid F1 with the diploid S. macrocarpon (ploidy level of this BC1 progeny was not specified).
Another example is provided by the tetraploidised F1 (Solanum virginianum [S. xanthocarpum] × S. melongena) that produced 78% stainable pollen and its progeny was fertile; on the contrary the diploid (2x) hybrid was highly sterile with 1% stainable pollen (Rajasekaran 1971).
The F1 (S. violaceum [S. indicum] × S. melongena), 2n = 2x, was partially fertile with 49% stainable pollen; after colchicine treatment, its amphidiploid (2n = 4x = 48) was fully fertile (92% stainable pollen) and produced seeds and further fertile progenies (Rajasekaran 1970). The 4x plants were slow in growth, but did not show any gigantism, usually observed in polyploids. Meiosis was normal in the diploid (12 bivalents). The meiosis of tetraploid plants diakinesis and metaphase I yielded more bivalents and tetravalent than univalents and trivalents, but the subsequent stages were mostly normal. Based on chromosome pairing in the F1 and its derived amphidiploid, this latter was classified as a segmental allopolyploid.
The F1 (S. melongena × S. aculeatissimum) hybrid, obtained via embryo rescue (Zhou et al. 2018) was immediately treated with colchicine. The meiotic configuration of the resulting amphidiploid mostly consisted in bivalents, although multivalents were also observed but in low frequency. Lagging chromosomes were observed in later meiosis divisions, and the resulting pollen had 25% stainability.
F1 (S. melongena × S. torvum) has also been tetraploidised with colchicine (Daunay 1987–1988; Cürük and Dayan 2018). Both authors report virtual sterility (pollen stainability <5%) of the hybrids, although Cürük and Dayan (2018) describe two plants (out of 77 obtained) that yielded 8–11% pollen stainability. The tetraploid hybrids displayed improved pollen stainability, although still mediocre (10–15% in Daunay (1987–1987) and less than 3% in Cürük and Dayan (2018)).
These various examples show the interest of doubling the chromosome set for overcoming some F1 hybrid sterility barriers. However, information about the inevitable return, sooner or later, to diploid level is scarcely mentioned by authors. Isshiki and Taura (2003) on the basis of successful production of dihaploids by anther culture of somatic amphidiploids S. aethiopicum Gilo group × S. melongena (Rizza et al. 2002) suggested that anther culture could constitute a promising technique to move tetraploid progenies to the diploid level.
11.7 Disharmonic Interaction Between Wild Cytoplasms and Eggplant Nucleus: An Opportunity for Breeders
Male sterility has an interest for breeding, because it facilitates the production of commercial F1 seeds, given no emasculation of the maternal parent is needed. Cytoplasmic male sterility (CMS) has been found in several interspecific crosses between Solanum species used as females and Solanum melongena. It is explained by an incompatibility between the Solanum cytoplasm and S. melongena nuclear genome. It is a maternally inherited trait that is characterised by a failure to produce or to release functional pollen. In order to be workable for breeding, its expression must be stable regardless of the environmental conditions and must be associated to normal seed set. Cytoplasmic male sterilities of several phenotypes have been obtained from several interspecific crosses involving wild species and S. melongena. They result from unbalanced interactions between wild cytoplasm factor(s), of mitochondrial origin in most cases, and eggplant nuclear factor(s). We detail here two CMS systems. The anther indehiscent type was obtained with cytoplasms of S. violaceum (S. kurzii) and S. virginianum, for which anthers contain normal pollen but do not release it because their terminal pores do not open. The second system is the pollen non-formation type, obtained with cytoplasms of S. aethiopicum Aculeatum group, “S. grandifolium”Footnote 14 and S. anguivi for which the anthers are completely devoid of pollen. Both systems have been summarised (Khan and Isshiki 2016). Other CMS types (Fang et al. 1985; Khan and Isshiki 2008), the petaloid and vestigial anther types, were obtained from a cross between S. aethiopicum Gilo group × S. melongena.
11.7.1 Indehiscent Anthers—Non-release Type
The cross between Solanum violaceum (female) and S. melongena yielded a hybrid with 31% pollen stainability (Isshiki and Kawajiri 2002). When backcrossing it (as female) with S. melongena as recurrent parent, the BC1 and BC2 segregated for anther indehiscence. This trait was fixed in BC3 and BC4, which possessed S. violaceum mitochondrial (mt) and chloroplast (cp) DNAs. All BCs displayed low pollen stainability (0–70%), despite an almost normal meiosis in the advanced BC4 (average chromosome association was 11.6 bivalents + 0.8 univalents, up to 12 bivalents). Similarly, the hybrid between a prickleless form of S. violaceum (S. kurzii) and S. melongena yielded a hybrid with 30% pollen stainability and only 1% in vitro germination (Khan and Isshiki 2009). Segregation for releasing/not releasing the pollen appeared in the BC1 generation, which produced pollen grains regardless of the pollen release ability of the plants. The “not releasing pollen” trait was transmitted to the next BC2 progeny and was fixed without exception in BC3. “Releasing pollen” BC1 and BC2 plants yielded BC2 and BC3 segregating progenies, progressively nearing 100% “not releasing” plants. Average pollen stainability (63–68%) and in vitro germination ability (8–24%) of the BC progenies remained relatively low. Because meiosis of BC3 was normal (complete bivalents at metaphase I), this low pollen quality was attributed to the wild cytoplasm. All BC progenies, regardless of their pollen release type, had the cytoplasm of the wild parent (mtDNA and cpDNA). Fruit set and seed set (after pollination with the recurrent S. melongena parent) increased gradually with successive BC generations, thus indicating the absence of negative effect of the S. kurzii cytoplasm on this trait. This CMS was stable over seasonal climatic changes, but no restorer genes were identified. This is not a problem given that the male sterile plants produce some viable pollen; hence, their maintenance by selfing is potentially feasible.
The hybrid Solanum virginianum × S. melongena is virtually sterile with 5% stainable pollen (Khan and Isshiki 2008). Backcrossed with S. melongena (male parent), all plants of BC1 to BC4 generations displayed indehiscent anthers, although the parents and the F1 had dehiscent ones. The expression of this sterility was shown to be stable over four months, despite warm temperatures varying from 26 to 38 °C. Mitochondrial genomes of F1 and BCs were inherited from S. virginianum (maternal inheritance), while their chloroplast genomes originated from recombination of parental cpDNAs (biparental inheritance). Average chromosome pairing of the F1 at metaphase I was 11.7 bivalents and 0.6 univalents. Despite this ratio reaching 12 bivalents for some plants in the BC generations, microspores degenerated post-meiosis and BC progenies displayed partially stainable pollen, with a tendency to decrease in later generation BCs (67% in BC1, down to 37% in BC4). This research pointed out, for the first time, the presence of recombined cpDNA in progenies of sexual crosses among non-tuberous solanums. If confirmed, this finding would impact the interpretation of phylogenetic trees based on chloroplast markers only, these latter being hypothesised to only reflect maternal inheritance.
Male sterile lines having one or the other of the above-mentioned cytoplasms, S. violaceum (S. kurzii) and S. virginianum, were compared in two studies (Hasnunnahar et al. 2012; Khan et al. 2015). For all of these lines, pollen stainability evaluated with acetocarmine was lower (50–75%) than eggplant control (90–100%) in the first publication. Pollen stainability was even lower for the second study, with 49–56% for lines with Solanum violaceum cytoplasm and 42% for lines with S. virginianum cytoplasm, whereas in vitro pollen germination dropped down to 25% (S. violaceum cytoplasm) and 14% (S. virginianum). Quantitatively, male sterile lines produced as much pollen grains per anther as the S. melongena control, with the exception of those with the S. virginianum cytoplasm that significantly produced less pollen grains (Khan et al. 2015). Fruit set of the lines after manual selfing was correct but variable (53% for lines with S. virginianum cytoplasm, 75–91% for lines with S. violaceum); it was improved (up to 71% and 87–100%, respectively) when the male sterile lines were backcrossed with S. melongena (Hasnunnahar et al. 2012). The average number of seeds per fruit was less than the selfed S. melongena control (784 seeds) for the selfed male sterile lines (362–518 seeds), but similar to it (767–834 seeds) when the lines were backcrossed with S. melongena (Hasnunnahar et al. 2012). The mediocre pollen stainability of the male sterile lines, evaluated with a starch staining solution (Lugol’s), indicated that at the time of pollen maturation their carbohydrate metabolism was abnormal with incomplete starch degradation (Hasnunnahar et al. 2012; Khan et al. 2015). Pollen degeneration in indehiscent CMS lines having S. violaceum or S. virginianum cytoplasms occurs along all stages of pollen development, from unicellular microspores released by the tetrads (29–36%), early bicellular pollen (6–12%) to late bicellular pollen (9–10%).
Given pollen quality of these CMS sources is low and hampers their maintenance by hand selfing and given no restorer genes were identified so far, their use in breeding remains hypothetical.
11.7.2 No Formation of Pollen Grains
The absence of pollen production in the anthers was found in progenies issued from a hybrid between “Solanum grandifolium” (possibly a misidentified germplasm of S. aethiopicum Aculeatum group) and S. melongena (Saito et al. 2009). Genetic study with sterile and fertile progenies led the authors to identify this sterility as a cytoplasmic male sterility (CMS), restorable thanks to a single (Saito et al. 2009) or two (Khan et al. 2013b) dominant gene(s) Rf. This CMS is stable over a range of environments.
A similar expression of male sterility was found in the BC1 progeny issued from the F1 (Solanum aethiopicum Aculeatum group [female] × S. melongena) (Khan and Isshiki 2010). This hybrid (10% pollen stainability) when backcrossed as female with S. melongena produced BC1 plants segregating for male sterility; the male sterile BC1 did not produce pollen. BC2 to BC4 progenies obtained from male sterile plants were fixed for this trait, whereas they still segregated for male sterility and male fertility when obtained from fertile mother plants. Pollen stainability of male fertile BCs remained low (<60%). Genetic analysis showed that the sterility had a cytoplasmic origin and that two independent and dominant genes (Rf) controlled the fertility restoration of this CMS. Whether the BC4 plants were male sterile or male fertile, they displayed the cytoplasm of the wild parent (mt and cpDNA).
Segregation for the absence versus presence of pollen grains within the stamens was observed directly on the F1 (Solanum anguivi × S. melongena) plants (Khan and Isshiki 2011). BC1 progenies obtained from the male sterile F1 plants were all male sterile, whereas the BCs obtained from fertile F1 plants continued to segregate down to BC5. Pollen stainability of the male fertile F1 was 17% and remained low in the BCs (43–56%), although meiosis observed in some BC5 plants was normal (with the exception of rare cases of few univalents). No meiosis at all was detected in the male sterile BC5 plants. All BC progenies possessed S. anguivi cytoplasm. Genetic analysis identified two independent and dominant restorer genes, originating from S. anguivi, each controlling pollen formation in the presence of S. anguivi cytoplasm. Fruit set and seed germination of BC5 were as good as for the S. melongena recurrent parent, although the number of seeds per fruit was lower. The expression of this male sterility being stable, it looks promising for use in breeding.
As we have seen, CMS originating from “S. grandifolium”, S. aethiopicum Aculeatum group and S. anguivi segregate along the successive backcrossing (or selfing) of male fertile plants, given that the restoration of male fertility is under control of either the one or the other or both dominant restorer Rf genes identified in this set of material. In order to speed up the fixation of restorer lines homozygous for the one, the other of both Rf genes, Khan et al. (2013b) experimented anther culture of male fertile plants for producing haploids. They obtained few haploids from two (“S. grandifolium” and S. anguivi) out of the tree cytoplasms tested, thus demonstrating that this technique was workable for fixing eggplant material carrying a wild cytoplasm. Applied to male fertile plants segregating for male sterility, this technique looks promising to produce rapidly homozygous male fertile restorer lines together with male sterile lines. This work opens the path for the use of this CMS in the production of eggplant commercial F1 hybrids.
11.7.3 Towards Genetic Comparisons Between the Two CMS Types
In a wide cross combination experiment, male sterile plants of each cytoplasmic origin were pollinated with male fertile line of their own CMS system and of the other cytoplasms (Khan et al. 2014). The segregation patterns revealed again the occurrence of two independent and dominant restorer genes operating in each CMS system, each Rf gene being able to restore fertility in its own CMS system and also in the other CMS, with similar recovery actions in terms of male and female functionality and seed production. The authors concluded that this similarity was indicative of the close relationships between “S. grandifolium”, S. aethiopicum and S. anguivi. All restorer genes were found to be of wild origin. A single reliable SCAR marker (SCAB101900), linked to Rf genes, was set up and provides the first facility for early and efficient selection in any marker-assisted CMS breeding programme. This marker will facilitate the exploration of CMS and corresponding Rf genes within wild Solanum germplasm, although the authors mention the need for the future to develop further markers more tightly linked than SCAB101900 to Rf genes. The molecular basis of both cytoplasmic male sterilities has been unravelled at the level of mitochondrial genes (Yoshimi et al. 2013).
11.8 Genetic Information Drawn from Interspecific Hybrid Phenotypes
Interspecific hybrids display variable redistributions of parental morphological traits depending on the qualitative or quantitative expression of the traits and on the underlying genetic effects controlling their expression (recessiveness, dominance, additivity, epistasis, etc.). Heterosis for plant vigour, mentioned for a number of interspecific crosses (see Tables 11.3, 11.5, 11.9, 11.10 and 11.12), is observed in hybrids, regardless of pollen fertility. Hence, it seems that the dysfunctioning between parental genomes, expressed at the level of reproductive functions, does not affect development events, as this is exemplified by virtually sterile hybrids that are however vigourous.
11.8.1 Hybrids Between Cultivated Eggplants
11.8.1.1 Solanum aethiopicum and S. macrocarpon
The hybrid obtained with Solanum aethiopicum used as female parent expressed heterosis for plant height and displayed intermediate features between those of the parents for traits such as leaf blade size (Omidiji 1983). The many branched phenotype of the hybrid indicated that this trait is dominant over the less branched one (type of S. macrocarpon). Unexpected prickliness and hairiness absent from both parents were observed in the hybrids issued from this cross (Omidiji 1979, 1983), but the occurrence of this phenotype depends on the parental accessions used (Oyelana and Ogunwenmo 2009). Prickliness was also observed in another hybrid between S. aethiopicum Kumba group and S. macrocarpon (cross direction not specified) as well as unexpected many flowered inflorescences despite the parents having few flowers (Lester 1986). It was hypothesised that the resurgence of these wild or atavic traits (prickliness, hairiness and many flowered inflorescences) in the hybrid was due to loss mutations in the parents and gene complementation in the hybrid.
Also, plants unexpectedly resembling S. macrocarpon were found in the F2 progeny issued from a cross between S. aethiopicum Kumba group (hairless and prickleless) and S dasyphyllum, the wild progenitor of S. macrocarpon (hairy and prickly) (Omidiji 1986).
11.8.1.2 Solanum aethiopicum and S. melongena
The hybrid Solanum melongena × S. aethiopicum Aculeatum group (S. integrifolium) displayed pink flowers and purple fruits (before physiological maturity) as did its S. melongena parent, small fruits as did S. aethiopicum and intermediate plant vigour, leaf and flower sizes (Oyelana and Ugborogho 2008). The single flower observed by these authors (both parents had few or several flowers per inflorescence) is a unique finding since other hybrids, obtained with other S. melongena accessions × S. aethiopicum Kumba group, displayed more flowers than both their parents (Prohens et al. 2012). These hybrid plants were also much taller than each of their parent, but were intermediate for leaf size and flower diameter. They displayed S. melongena traits for anthocyanins on plant parts and S. aethiopicum fruit shape ratio and low fruit phenolic content. They had much smaller fruits than each parental species. Reversion to the wild state was observed for hybrids between S. melongena and S. aethiopicum Kumba group, which displayed prickly leaves although neither of their parents had prickles (Prohens et al. 2012).
11.8.1.3 Solanum macrocarpon and S. melongena
Regardless of the cross direction, hybrids display variable vigour (plant height and number of branches) from very weak to vigourous, depending on the publications (Schaff et al. 1982; Gowda et al. 1990; Bletsos et al. 2004; Oyelana and Ugborogho 2008) or on the parental accessions that were used (Schaff et al. 1982). These hybrids displayed several traits similar to those of Solanum macrocarpon (high number of flowers per inflorescence, accrescent calyx, small round fruits, yellow mature fruit), of S. melongena (presence of prickles on calyx, presence of hairs on leaves, purple fruit), or intermediate between those of both parents (plant height, growth habit, leaf size, petiole length) (Bletsos et al. 2004; Oyelana and Ugborogho 2008; Schaff et al. 1982). Interestingly the hybrids obtained by Schaff et al. (1982) and Bletsos et al. (2004) displayed prickles on their leaves midribs that were absent from both parents. Unexpected prickliness was also observed on other hybrids issued from crosses between other parental accessions (Omidiji 1979). Hence, reversion to the wild prickliness, previously mentioned for hybrids between S. aethiopicum and S. macrocarpon, S. aethiopicum and S. melongena, is also observed for hybrids between S. macrocarpon and S. melongena.
11.8.2 Hybrids Between Cultivated Eggplants and Wild Species
Generally, reciprocal hybrids display identical phenotypes (Kirti and Rao 1982a), although slight differences are sometimes mentioned, such as in the case of the hybrid S. aethiopicum Aculeatum group (S. integrifolium) × S. multiflorum (S. indicum var. multiflorum) which attained a greater height than its reciprocal (Kirti and Rao 1980). When the crosses involve cultivated eggplants and wild species, the hybrid general outline is closer to that of their wild parent than to their cultivated one (Bletsos et al. 1998; Kaushik et al. 2016); Daunay et al. unpub. results). This tendency is explainable by the overall dominance of wild traits over domesticated ones (Lester 1989). However, depending on the quantitatively inherited traits, the phenotype of the hybrid moves closer to one or the other parent and sometimes exceeds them (in the case of transgression).
Although the concept of heterosis is usually used and interpreted only in terms of superiority of the hybrid compared to its parents, it was used as a tool for comparing phenotypes of interspecific hybrids issued from crosses between Solanum melongena and seven wild species,Footnote 15 to those of their parents (Kaushik et al. 2016). Indeed, calculation of heterosis (H) yields values which position the hybrid phenotype by the comparison with its parents. When calculated on the basis of the deviation between the hybrid and its mid parents values for a given trait,Footnote 16 H ranges theoretically from zero (hybrid equals parents average) to +100% (hybrid equals its parent displaying the highest value) or −100% (hybrid equals its parent displaying the lowest value). Positive values intermediate between 0 and 100 mean that the hybrid displays intermediate features that are skewed towards the parent with the highest value, and conversely when negative, values indicate that the hybrid displays intermediate features that are skewed towards the parent with the lowest values. H values over 100% (case of transgressive traits) indicate that the hybrid phenotype is beyond the parent with the highest value (if H is positive) or beyond the parent with the lowest value (if H is negative). Kaushik et al. (2016) showed that, depending on trait types (plant height, stem diameter, leaf size, number of flowers per inflorescence, number of petals, calyx prickliness) and species cross combinations, heterosis displayed variation ranging from −100% up to +91%. For example, for plant height H varied from 2.3% for F1 (S. linnaeanum × S. melongena) to +91% for F1 (S. melongena × S. dasyphyllum). For fruit calyx prickles H varied much more, from −100% for reciprocal F1 between S. melongena and S. anguivi, to +80% for F1 (S. melongena x S. dasyphyllum). Heterosis for the number of petals ranged much less, from −4.8% to +1.9% for the six interspecific hybrids studied. Fruit weight and leaf prickliness behaved differently from the above-mentioned traits. Fruit weight displayed only negative H values, ranging from −6 to −99%, meaning all hybrid combinations bore fruits of a size skewed towards their wild parent. On another extreme, heterosis for leaf prickliness displayed only positive values, some shooting very high for hybrids between S. melongena on one hand and S. incanum (H = 733%) or S. tomentosum (H = 800%) on the other hand. This means that these hybrids were up to seven or eight times pricklier than their prickliest parent.
Partly consistent as well as complementary results about trait heredity pattern were obtained with an F1 (Solanum melongena × S. incanum) (Prohens et al. 2013). This hybrid expressed higher values than its parents, in particular for plant height, leaf length and lobing, prickliness, as well as for fruit browning after being cut. The presence of prickles and of anthocyanins on vegetative parts and fruit epidermis was dominantly expressed (over their absence) in the hybrid. F1 small fruits size was skewed towards the wild parent, which is in favour of dominance of small fruit size over large one. However, it is hazardous to assess the inheritance mode of this trait on the sole basis of interspecific hybrid phenotypes; indeed, the frequently observed absence or reduced number of seeds within the F1 fruits can partly explain the reduction of their sizes. For all the other traits, the hybrid was intermediate between the two parents (incomplete dominance).
Reversion to the wild prickly state was observed in hybrids generated by crosses between an accession of Solanum melongena without prickles and two non- or poorly prickly accessions of S. insanum and S. tomentosum (Plazas et al. 2016).
11.8.3 Hybrids Between Wild Species
The phenotype of interspecific hybrids obtained from the cross between wild species is also informative for accessing trait heredity. Several traits were identified as dominantly expressed (Kirti and Rao 1981; Rao and Rao 1984) such as erect habit over pendant habit, long branches over short branches, hairy brittle leaves over soft textured ones, lengthy many (6–10) flowered inflorescences over short and less (1–3) flowered ones, red or orange mature fruit over yellow ones (Rao and Rao 1984), lobed ovaries over globular ones (Kirti and Rao 1980) and racemose over umbellate inflorescence type (Oyelana et al. 2009). The hybrids express features intermediate to those of their parents for quantitative traits such as dimensions of various plant parts (petioles, leaves, flowers, fruit (e.g. in Oyelana et al., 2009).
11.9 Somatic Interspecific Hybrids
From the 1980s onwards, fusion between protoplasts via polyethylene glycol (PEG) exposure or electrofusion, allied to plant regeneration techniques, allowed for the production of a set of interspecific somatic hybrids (symmetric fusion) or of cybrids (asymmetric fusion) between Solanum species (eggplant, potato, tomato, spiny solanums and black nightshade), as well as of some intergeneric hybrids (Solanum melongena + Nicotiana spp.). Somatic hybridisation was investigated as (1) an alternative route to the sexual crosses for transferring traits of interest (mostly disease resistances) from one species to another, and (2) a method to increase cytoplasmic and nuclear genetic diversity (Sihachakr et al. 1994). Results of hybridisations involving S. melongena were reviewed twice (Collonnier et al. 2001a; Kashyap et al. 2003).
11.9.1 Solanum Melongena + New World Spiny Solanums
Three wild species have been used so far, Solanum sisymbriifolium, S. torvum and S. viarum.
Solanum melongena + S. sisymbriifolium
The first somatic hybrids were obtained by PEG fusion of protoplasts of eggplant (Solanum melongena) and S. sisymbriifolium (Gleddie et al. 1986). They were aneuploid (but close to the 48 expected chromosomes), and plants were smaller than their parents and produced abnormal flowers and pollenless anthers. They segregated for flower colour (purple like the eggplant or white like the wild species) and leaf shape, pubescence and prickliness, but on the whole leaf morphology was closer to that of the eggplant than to S. sisymbriifolium. The hybrids had both the stellate trichomes of eggplant and the glandular ones of S. sisymbriifolium; those having the highest proportion of glandular trichomes displayed resistance and antibiosis to the mite Tetranychus cinnabarinus comparable to that of S. sisymbriifolium (Gleddie et al. 1985). When inoculated with the root knot nematode Meloidogyne incognita, the hybrids developed a few galls, but the nematodes did not reproduce as was observed for S. sisymbriifolium (Gleddie et al. 1985). These observations indicate that trait inheritance in aneuploid hybrids is both conventional and not conventional, depending on the hybrids and on the traits. Later hybrids obtained by electrofusion were tetraploid (2n = 4x = 48) and homogeneous. Their phenotype was intermediate between those of their parents (Collonnier et al. 2003b). Although their pollen stainability ranged from 20 to 30%, they produced fruits with empty seeds. Interestingly, the hybrids inoculated with Verticillium wilt (filtrate of culture medium) and Ralstonia solanacearum (two isolates) displayed resistance levels intermediate between those of the resistant parent, S. sisymbriifolium, and the sensitive one, the eggplant. All hybrids possessed the wild parent chloroplasts (Collonnier et al. 2003b; Gleddie et al. 1986).
Solanum melongena + S. torvum
Solanum torvum was also used for attempting to transfer its pest and disease resistances to eggplant (S. melongena) by the somatic route. The first hybrids, obtained with PEG technique, ranged from possessing 46–48 chromosomes and displayed 5–70% pollen stainability (Guri and Sink 1988a, c). Prickles were present on all but one hybrid, but their colour (purple) differed from the colour of those of S. torvum (green). The long sepals resembled those of eggplant, but petals’ colour was a deeper purple. The hybrids exhibited intermediate morphological characteristics for plant stature, leaf and flower size and shape. Some hybrids had eggplant cpDNA and some had both eggplant and S. torvum cpDNA. The structure of mtDNA was the result of rearrangements between the mtDNA of the parents. Natural infestation with spider mites was strong on eggplant, weak on the wild species and intermediate on the hybrids. When inoculated with Verticillium extracts, hybrid cuttings displayed the resistance of their wild parent. Other authors observed that 15% of somatic hybrids issued from electrofusion had a chromosome number approaching (35 to 46) or reaching tetraploid (48) status (Sihachakr et al. 1989). Leaf shape and flowers number per inflorescence were intermediate to those of the parents, whereas the hybrids expressed the wild parent traits for anthocyanins presence, prickle location and eggplant traits for calyx length and plant height. Interestingly, hairiness was transgressive, with the hybrids displaying a greater hairs density and length. The plants with less chromosomes exhibited a greater morphological variation than those close to 4x = 48.
Another set of somatic hybrids, all tetraploid, acquired the chloroplast from either one parent or the other one; they were vigourous, relatively homogeneous and morphologically intermediate between the parents and displayed 2–20% pollen stainability (Collonnier et al. 2003a). No translocations or recombinations between parental chromosomes could be observed by genomic in situ hybridisation (GISH). Similar to S. torvum, the majority of the hybrids were resistant to Verticillium and bacterial wilt.
Asymmetric hybridisation obtained after irradiation of S. torvum protoplasts (in order to fragment their nuclear DNA) followed by chemical or electrical fusion with normal eggplant protoplasts yielded a wealth of plants, 15% of which were tetraploid, the rest being diploid (Jarl et al. 1999). The majority of the regenerated plants were morphologically similar to eggplant. The tetraploid plants could be distinguished from the diploids by their broad dark green leaves, short internodes, vigourous growth and a slight decrease of pollen stainability. Agronomic and Verticillium tests, performed on hundreds of regenerated plants, identified one highly resistant 4x plant, looking like eggplant with normal fruit and seed set. This plant displayed an EcoRV DNA restriction pattern similar to that of eggplant, except for few bands similar to S. torvum. Although this research did not explain the tetraploid status of this plant, it was the first to indicate that the transfer of a limited amount of DNA of the donor wild parent was possible while keeping eggplant morphological, fertility and agronomical traits.
Solanum melongena + S. viarum
Somatic hybrids issued from S. melongena and S. viarum (S. khasianum) protoplast electrofusion represented 40–50% of the regenerated plants and had a chromosome number ranging from 46 to 48 (Sihachakr et al. 1988). Plants were less vigourous than their parents and relatively homogeneous. Depending on the traits, hybrid phenotype (1) was intermediate (e.g. leaf shape and base blade), (2) expressed dominant traits originating from S. viarum (e.g. anthocyanin presence) or from S. melongena (stem and petiole thickness and shortness), or (3) was transgressive (e.g. higher number of flowers per inflorescence than each of the parents, distribution of prickles over a larger range of plant parts). Pollen stainability ranged 10–15% (it was >98% for the parents), and no fruit set was observed. Sexual hybridisation was more successful (Table 11.10), by yielding a hybrid with c. 62% pollen stainability (Sharma et al. 1980).
Somatic fusion between eggplant and New World species: uncertain potential for breeding
Regardless of the wild species used, flowering of the somatic hybrids was precocious (Gleddie et al. 1986; Sihachakr et al. 1988, 1989). Ultimately, somatic hybridisation between S. melongena and three New World species is as much hopeful as it is hopeless for introgressing wild resistance traits into Solanum melongena. It is proven that their disease resistances can be transferred into interspecific somatic hybrids, but the improved pollen stainability of these hybrids, when compared to that of their sexual counterparts, is not sufficient for ensuring their reproductive fertility (seed set). Hence, no progenies usable in a breeding programme have been obtained so far. Further, the return of tetraploid somatic hybrids to the diploid status is a supplementary difficulty.
11.9.2 Solanum Melongena + Old World Spiny Solanums
Solanum melongena + S. marginatum
With the aim of transferring the arborescent and perennial characters of Solanum marginatum L.f. into S. melongena, protoplasts of both species were electrofused and somatic hybrids regenerated (Borgato et al. 2007). These hybrids were tetraploid, vigourous and homogeneous, and plants displayed morphological features intermediate to those of the parents, including flower colour (purple-edged petals with central white sector, whereas eggplant has purple flowers and S. viarum has white ones). These plants, grown over three years, displayed the arborescent habit of their wild parent, together with its secondary wood tissues. Cytological observations of the hybrids showed a high frequency of bivalents, together with a low frequency of abnormalities (multivalents, univalents, heteromorphic bivalents and lagging chromosomes). Despite this imperfect homeologous pairing during meiosis division I, the somatic hybrids unexpectedly produced pollen of 85% stainability, a much better score than the virtual sterility obtained with sexual hybrids (Table 11.10); hybrids also set fruits and seeds. The germination of the seeds yielded S1 generation plants that were also arborescent, fertile and similar to the former generation S0 for flower and fruit morphology. Segregation for other traits is not mentioned by the authors; hence, recombination events between the parental chromosomes deserve to be clarified in future.
Solanum melongena + S. violaceum
In order to transfer to eggplant the bacterial wilt (Ralstonia solanacearum) resistance of S. violaceum (S. sanitwongsei in the publication), protoplasts of both species were electrofused and screened on a medium containing bacterial toxins. Plants regenerated from the surviving cells were further screened in contaminated soil and a single one survived (Asao et al. 1994). This plant was tetraploid and expressed intermediate traits (e.g. leaf shape, flower size, colour and diameter, stem anthocyanins), or traits of the cultivated parent (immature fruit black colourFootnote 17), or of the wild parent (mature fruit orange colour,Footnote 18 numerous flowers per inflorescence).Transgressive traits were not observed. Pollen stainability was 82%, i.e. comparable to the score reached by the sexual hybrid (Table 11.10), and hybrids set seeded fruits and the S1 progeny was also tetraploid and fertile. S0 plants as well as S1 progeny were as resistant to bacterial wilt as S. violaceum.
Solanum melongena + S. aethiopicum
This interspecific fusion aimed at transferring S. aethiopicum disease resistances to S. melongena. Iodoacetamide-treated eggplant protoplasts, fused (by dextran method) with S. aethiopicum Aculeatum group (S. integrifolium in the publication) protoplasts, gave rise to vigourous hybrids displaying characters intermediate to those of the parents for flower size and colour, fruit shape and trichome density on the petiole (Kameya et al. 1990). Hybrids were tetraploid (2n = 48) except one which was diploid (2n = 24) and sterile. Progenies issued from selfing of one of the tetraploid plants and tested with Ralstonia solanacearum segregated for resistance; some plants expressed transgression for resistance (higher level than for S. aethiopicum). Other hybrids obtained by electrofusion of the same species displayed also heterosis for plant vigour as a whole: plant height, leaves and stem size (Daunay et al. 1993). All but three plants were intermediate between the parents for morphological traits,Footnote 19 with the exception of prickliness and anthocyanin presence which were similar to S. aethiopicum and dominantly inherited. Most hybrids were tetraploid, and some were hexaploid or mixoploid. Some of the hybrids displayed cpDNA of S. melongena and the others cpDNA of S. aethiopicum. The hybrids segregated for pollen stainability (30–85%Footnote 20) and fruit production (from 3 to >9 kg per plant). The authors noticed that good fertility was mostly associated to tetraploidy and the capture of eggplant chloroplasts. Hybrids obtained again with S. aethiopicum Aculeatum group, as well as with Gilo group (Collonnier et al. 2001b), provided results globally similar to those of Daunay et al. (1993). Tested with Ralstonia solanacearum, most hybrids were as resistant as their S. aethiopicum parents, a few of them being transgressive towards a better resistance (Collonnier et al. 2001b). In vitro anther culture was successful (Rizza et al. 2002; Rotino et al. 2005) in yielding dihaploids (2n = 2x = 24) from the 2n = 4x = 48 somatic hybrids previously obtained by (Collonnier et al. 2001b). The segregation of the dihaploids for flower and fruit traits confirmed that genetic recombination between S. melongena and S. aethiopicum genomes had occurred at the time of the meiosis of the tetraploid somatic hybrids. Return to diploidy was associated to a strong drop of pollen stainability, ranging 8–16% on average for the dihaploids, whereas their tetraploid parents ranged 54–71% (Rotino et al. 2005). Most dihaploids produced parthenocarpic fruits, and the rest of them produced no fruits at all (Rizza et al. 2002). The resistance of S. aethiopicum Gilo and Aculeatum groups to Fusarium wilt was transferred to the dihaploids, which segregated for this trait (Rizza et al. 2002; Rotino et al. 2005). A further biotechnological feat was achieved by producing, with the same anther culture technique, dihaploids from a double somatic hybrid obtained by sexual cross between two simple somatic hybrids (eggplant + S. aethiopicum Aculeatum group) and (eggplant + S. aethiopicum Gilo group) (Rotino et al. 2005). These dihaploids also segregated for Fusarium wilt resistance. Via backcrosses, the resistance of the best dihaploids was further introgressed into S. melongena and integrated into a breeding programme (Rotino et al. 2005). The extent of genetic recombination between the genomes of S. melongena and S. aethiopicum Gilo group was analysed on a population of dihaploids obtained by Rizza et al. (2002), with 280 ISSR markers (71 genotypes) and 3 isozyme systems (70 genotypes) (Toppino et al. 2008a). Disomic and tetrasomic inheritance patterns were identified for ISSR markers. Distorted segregations patterns, not fitting disomic or tetrasomic patterns, were observed for isozymes. These careful analyses confirmed that genes were exchanged between the parental genomes at the time of the meiosis of the somatic hybrid mother plants.
Somatic fusion between eggplant and Old World species: potentials for eggplant breeding
On the whole, somatic hybrids obtained so far between S. melongena and three Old World spiny solanums produce a pollen which stainability is equivalent to that of their sexual counterparts (S. violaceum, see Table 11.10 and S. aethiopicum, see Table 11.5) or a pollen of much better fertility (S. marginatum, see Table 11.10); these hybrids also produce seeded fruits. The transfer of disease resistance was proved successful in the somatic hybrids, as well as in their progenies issued from selfing (S. violaceum) or dihaploidisation (S. aethiopicum). These results, together with segregation events for resistance and morphological traits, as well as genetic analysis with markers (ISSR, isozymes), indicate that recombination between parental genomes occurs at the time of the meiosis of the somatic hybrids. Interestingly for breeders, transgressions towards disease resistance levels that are higher than that of the resistant parent were observed. Importantly, return to diploid status via anther culture and dihaploid production was proved feasible in the case of somatic hybrids obtained with S. aethiopicum; this remains to be demonstrated for the hybrids obtained with other species. In the case of S. aethiopicum, the ploidy status conversion from 2n = 48 to 2n = 24 was associated with an important decrease of pollen fertility. On the whole, the results obtained so far indicate that somatic hybridisation might be complementary to sexual hybridisation, in the specific cases of (1) transgressive resistance, (2) low fertility of sexual hybrids, and (3) if the change of cpDNA and/or mtDNA brings a capital gain over sexual hybrids carrying their maternal cytoplasmic DNA, the agronomic interest of which remains to be demonstrated.
11.9.3 Other Somatic Hybridisations Involving Spiny Solanums
Solanum aethiopicum (Aculeatum group) + S. violaceum
This somatic hybridisation aimed at transferring bacterial wilt tolerance of Solanum violaceum to S. aethiopicum Aculeatum group (S. integrifolium) (Tamura et al. 2002). Despite the low success rate (1.5%) of the electrofusion and plant regeneration, one amphidiploid (2n = 48) hybrid plant grew well. After inoculation, inhibition of bacterial multiplication in the roots and of its spread to plant upperparts was observed in this hybrid as well as in S. violaceum. The hybrid displayed S. aethiopicum anthocyanins pigmentation of stems, prickles and veins, but the general habit and leaf shape of S. violaceum, as well as intermediate flower colour (pale mauve). It bore many small fruits, containing seeds larger than those of each parent and with a germination rate >90%. Another electrofusion experiment (Iwamoto et al. 2007) was carried out with iodoacetamide-treated protoplasts of S. violaceum (S. sanitwongsei, S. kurzii in the publication) and UV-irradiated protoplasts of S. aethiopicum Aculeatum group (S. integrifolium). The putative hybrids, regenerated from some 1000 calli, were classified into three groups, according to their chromosome set and phenotype. One group included amphidiploids (2n = 4x = 48), displaying homogeneous and intermediate morphological features (leaf size, flower colour, fruits shape size and colour). These plants displayed 79% averaged pollen stainability, set fruits and seeds and expressed heterosis for plant vigour and seed size. The two other groups included asymmetric and mostly hexaploid hybrids (2n = 6x = 72), one group with 1-2 S. aethiopicum-S. violaceum parental chromosome dosage and the other group with 2-1 dosage.
Somatic hybridisation between S. aethiopicum Aculeatum group and S. violaceum yielded fertile tetraploid material, whereas sexual hybridisation yielded at best, when S. violaceum is used as female parent (see Table 11.7) a partially fertile diploid hybrid (Lester and Niakan 1986). Given the incompleteness of the available data (possibility to return to the diploid state for the somatic hybrid and obtaining progenies from the sexual hybrid), there is again no clear advantage of somatic hybridisation over sexual hybridisation.
Solanum viarum + S. aculeatissimum
Tetraploid somatic hybrid was regenerated at a rate of 45% from electrofusion of S. viarum (S. khasianum in publication) and S. aculeatissimum protoplasts (Stattmann et al. 1994). Grown in greenhouse, the hybrids were relatively homogeneous, of intermediate phenotype for some traits such as prickliness and leaf shape. They expressed heterosis for plant vigour, leaf and flower size. Flowers were normal, with pollen stainability over 87%, and set fruits with seeds that were germinated. Hence, the somatic hybrids between these two species of the Acanthophora clade are fully fertile.
Solanum torvum + S. tuberosum (potato)
In order to transfer S. torvum resistance to Verticillium dahliae to potato, electrofusion of protoplasts of both species was processed (Jadari et al. 1992). Out of hundreds of calli, four tetraploid hybrids were regenerated. They were vegetatively propagated, in order to be phenotyped in vitro and in greenhouse. Rooting troubles, observed in greenhouse only, were overcome by grafting on parental roots. The plants exhibited intermediate morphology, leaf shape and anthocyanin pigmentation, but their flowers aborted precociously. In vitro inoculation with Verticillium filtrate demonstrated that the hybrids were as resistant as S. torvum.
11.9.4 Solanum Melongena + Distantly Related Solanaceae Crops
A number of somatic hybrids have been regenerated from the fusion of Solanum melongena protoplasts with Solanum species of subgenus Solanum (S. nigrum) and Potatoe (tomato, potato), as well as with other genera (Nicotiana).
Solanum melongena + S. lycopersicum (tomato)
Asymmetric somatic plants were obtained by fusion of gamma irradiated protoplasts of a sexual interspecific tomato hybrid (S. lycopersicum x S. pennellii Correll), together with eggplant protoplasts (Liu et al. 1995). The four plants obtained had abnormal chromosome numbers (42, 45, 57, 60) and were all sterile (flowers drop after self-pollination). Only two of them survived after a few months; they exhibited a branching pattern resembling eggplant and compound leaves as their tomato parent. Other putative asymmetric hybrids obtained with the same partners were close to the expected tetraploidy (2n?=?48) and displayed eggplant morphology (Samoylov and Sink 1996).
Solanum melongena + S. tuberosum (potato)
In order to transfer eggplant bacterial wilt resistance (accession cv.508.3) into a diploid potato (Solanum tuberosum L.), protoplasts of both species were symmetrically fused in a helix fusion chamber (Yu et al. 2013). The hybrids exhibited various ploidy levels (4x, 6x, aneuploidy) with three types of nuclear genomes, potato cpDNA, as well as different phenotypes segregating for parental traits (stem colour), or displaying intermediate features (leaf shape) or trait states similar to or different from their parents (internode length, plant vigour, foliage colour). Screening tests carried out in vitro as well as with potted plants, with the agent of bacterial wilt, revealed segregation of the hybrids for resistance, the best ones having levels of resistance similar to their eggplant-resistant parent. Other hybrids obtained with other parental accessions were obtained via the asymmetric fusion between UV-treated eggplant protoplasts and potato protoplasts (Liu et al. 2016). The potato genome of these hybrids had integrated one to eight eggplant chromosome fragments, in a non-selective manner.Footnote 21 This result demonstrates that breeding potato for resistance to bacterial wilt issued from S. melongena is possible. Some hybrids produced tubers, shaped or not as their potato parent and developed no flowers, abnormal or normal flowers, but none produced pollen. However, as the potato parent unexpectedly did not produce pollen either, the hybrid fertility remains unknown. The authors were very confident in the feasibility of introgressing eggplant bacterial wilt resistance into potato via asymmetric protoplast fusion.
Solanum melongena + Nicotiana sp.
Hybrid plants were obtained by the fusion (dextran method) of protoplasts of a triple tobacco mutant set-up for in vitro selection of the regenerants, together with a “wild type Solanum melongena”, but details about these hybrids were not given (Toki et al. 1990).
Somatic fusion between eggplant and distantly related Solanaceae crops: a field of research insufficiently investigated
The potential of plant breeding using protoplast fusion techniques between distantly related species is far from being sufficiently investigated. The few results obtained so far indicate that transfer of traits is possible, but they also point out recurrent sterility troubles. Asymmetric fusion techniques that allow the transfer of pieces of the donor genome into the recipient species seem to be promising. The transfer of eggplant bacterial wilt resistance into potato seems to be the most promising application of this research domain.
11.10 Conclusions
11.10.1 Germplasm Characterisation
Efficiency of Solanum melongena breeding is on the way to be upgraded thanks to various DNA and RNA technologies (markers, QTLs mapping, sequencing, genes expression, etc.). However, the main challenge of future breeding of this species as well as of the two African eggplants is based on the genetic and phenotypic characterisation of their cultivated germplasm and of the wild relatives, since all this material is entangled in a complex network of relationships (c.f. Chap. 10 and Sect. 11.4). The characterisation carried out so far (Sect. 11.2) was limited by the difficulty of germplasm holders and breeders to outline the species content of eggplants and relatives germplasm, and to access it. Therefore, the phenotypic and genetic potential of subgenus Leptostemonum diversity, far from being unravelled yet, constitutes a promising field of research in many aspects all the more because most traits of interest are common to S. melongena, S. aethiopicum and S. macrocarpon. The breeding of each of these cultivated species will be boosted by the use of an enlarged diversity.
A second challenge relates to the phenotyping methods. Methodologies with improved accuracy that would allow for a better dissection of traits of interest must be set up. Until now phenotyping has been often coarsely carried out; this is the case for graft affinity between rootstock and scion assessed on few genotypes and few criteria (plant survival, growth, earliness, yield and fruit quality) or for resistance to pests, mostly assessed by field observations (degree of infestation). Such traits, based on partner’s interactions, deserve to be more closely looked at from both partner’s sides, at the intimate level of their interaction. For instance, for graft affinity nearly nothing is known so far in terms of histological and biochemical interactions between scion and rootstock, although graft affinity is located at the level of the graft union. Another relevant example concerns the interactions between plants and insects. The influence of plant genotype on insect biotic criteria (e.g. adult longevity, female fecundity, larvae mortality) allows for an accurate identification of possible antibiotic actions of some genotypes towards the insect. Identification of such new and accurate plant traits, unfavourable to the targeted insect, would provide breeders with powerful breeding criteria that should boost forward efficiency of breeding for resistance to insects.
The third promising aspect of future characterisation concerns the traits to be phenotyped. Evaluation for traits currently much sought-after, such as resistance to major pests (root knot nematodes, mites, and most damaging insects such as the fruit and shoot borer and the leaf hopper) as well as pathogens (in particular soil-borne vascular diseases), is a priority. This should allow the discovery of resistances so far unavailable (e.g. resistance to Verticillium wilt and to root knot nematodes within cultivated eggplant germplasm) or impossible to handle because of interspecific cross barriers (resistances to several soil-borne pests and diseases of Solanum torvum). The evaluation of an enlarged germplasm resource should also lead to the identification of different resistance types and genetic systems controlling different strains of a given pathogen, of the utmost breeding interest. An outstanding example is that of S. melongena and the very damaging Ralstonia solanacearum species complex (RSSC) in tropical conditions. Several local S. melongena accessions have been identified as being resistant in their country of origin, but these resistances are rarely effective in other places, likely because the bacterial strains are different. Indeed, strong interactions characterise this host–pathogen couple (Lebeau et al. 2011). Hence, in such a case, a breeder’s utmost dream is to build an “universal resistance”, efficient towards any bacterial strain in any country where the crop has economic importance. When complementary genetic systems (genes and QTLs), originating from different sources and controlling resistance to different strains, are available in the germplasm (and have been characterised), it is theoretically possible to build up, by genetic recombination between the sources, resistance that controls a range of strains wider than the range controlled by each source individually. Such a strategy, involving geneticists and bacteriologists, is ongoing (Salgon et al. 2017, 2018). For other diseases affecting eggplants, if breeders one day face such a case of strong host–pathogen interactions,Footnote 22 they will have to turn to the natural genetic diversity for resistance.
New traits must attract attention of breeders in the near future, such as those directly related to the adaptation to abiotic constraints (e.g. drought). They deserve a special attention, in particular root system structural (e.g. hierarchical ranks between roots, vigour components) and dynamic characteristics (e.g. emission of adventitious roots along plant development steps). Another “new” trait, poorly investigated so far within the germplasm of eggplants and relatives, concerns the alkaloids produced by most of Solanum species. These substances are involved in the bitter taste of the fruits and are toxic at high concentrations. Identifying the chemical diversity of the alkaloids synthetised by Leptostemonum species, quantifying their presence (in particular in the wild germplasm) and unravelling the genetic controls of their biosynthetic pathway are important. Indeed, there is a non-negligible risk of transfer of alkaloids from wild to cultivated eggplants, either by their grafting on wild rootstocks, or by interspecific crosses. Attention should also be turned to a possible resurgence, by genetic complementation, of this wild (atavic) trait when crossing cultivated forms, although this has not been proved yet for alkaloids (Sect. 11.8.1).
Given the expected increasing pressures of abiotic and biotic stresses in a near future, in particular because of the oncoming climatic changes, characterisation of cultivated and wild germplasm is of particular importance for future breeding of eggplants. Genetic and genomic techniques, taking advantage of the syntenic features among solanaceous crops, are complementary tools to phenotyping largely sampled intra- and interspecific germplasm, given they offer another path for mining genes controlling traits of interest and for discovering allelic diversity.
11.10.2 Sexual Crossability
Knowledge on the potential of crossability between species is extremely important for breeders; it gives the information on the basic requirements for transferring traits of interest from one species to another. Also, new traits of interest can arise from interspecific hybridisation, in particular cytoplasmic male sterilities that are of the utmost interest for the production of hybrid seeds (see 11.7). Cultivated eggplant species can be hybridised experimentally to each other and give rise, with some difficulties, to interspecific progenies (see 11.4.1). Although gene transfer from one eggplant species to another is possible, it has been so far barely practised by breeders, since only resistance genes (Fusarium wilt and bacterial wilt) originating from Solanum aethiopicum have been transferred to S. melongena (11.4.1.2 and 11.9.2). Gene transfer from wild species to cultivated eggplants was not carried out for long because the most interesting species carrying breeding strategic traits such as resistance to several soil-borne pests and diseases did not yield hybrids (S. sisymbriifolium) or yielded only virtually sterile ones (S. torvum) when crossed with S. melongena (Table 11.7). The transfer of other wild traits is ongoing, with in particular the transfer of S. elaeagnifolium and S. incanum drought resistance to S. melongena (see 11.2.3). As for S. aethiopicum and S. macrocarpon, the breeding efforts have been much less consistent than for S. melongena, and until now, there has been no attempt of introgressing them with wild traits of interest.
Although a rather high number of Solanum species (67) have been used in interspecific crossability studies (see 11.3), this number is low when compared to the size of Leptostemonum subgenus (over 500 species, see Chap. 10) and hence it is clear that crossability attempts will still keep scientists busy in the future. The apparent inconsistency between interspecific crossability results and phylogenetic relationships of the parental species (see 11.5) suggests that predicting crossability between species is for the present time illusory. It indicates also that interspecific crossability between species provides another insight at species relationships, complementary to phylogenetics and other criteria such as phenotype, genetic distance, geographical and ecological distribution (Chap. 10). Indeed, interspecific zygote formation and growth within the seed, and later hybrid growth provide information about the ability of parental genomes to collaborate and ensure or not a normal plant development. Meiosis patterns at diakinesis and metaphase I of interspecific hybrids provide precious information on parental chromosomal interactions, and hence on their chromosomes homologies, homeologies and/or rearrangements. Full sequences of chromosomes of an increasing number of Solanum species will provide a way complementary to cytogenetics for assessing chromosomal and genetic rearrangements between species.
So far crossability studies have been most often “roughly” carried out for two main reasons. First, only a small proportion of the publications went as far as attempting to obtain progenies from the hybrids, although for an eggplant breeder, this is the ultimate criterion to assess the success (or failure) of a given interspecific cross. Second, crossability has been assessed by nearly as many criteria combinations as the number of publications (11.3). This situation can be explained by the fact that results of any interspecific cross depend on many factors, in particular (1) prezygotic and post-zygotic barriers, (2) cross direction (which species is the female or male), (3) genotypes of the parental species, and (4) environmental conditions. As a result of such combinatory conditions, interspecific crosses yield a great variety of results, from no fruit set on the maternal parent to fully fertile hybrids at the extremes of the possible range of responses. Measurements for assessing cross success or failure are consequently also diverse and range from percentages of fruit set, seed set of the maternal parent, F0-->F1 seed normality and germination rate, F1 characteristics (lethality at embryo or plantlet stages, abnormal features, weakness), F1 male meiosis and pollen stainability or germinability, up to F1 fruit set and seed set. Results of any interspecific cross can also change when various techniques are implemented, such as embryo rescue, hormonal treatment or grafting for boosting weak hybrids, artificial chromosomes doubling and other biotechnologies such as somatic hybridisation. As a consequence, results in the literature are extremely heterogeneous and it is rather difficult to unambiguously characterise a “successful cross”. Also, the use of interspecific F1 pollen fertility as a criterion is questioning for at least two reasons. First there is no strict link between meiosis regularity or irregularity and pollen stainability (11.3.2). For this reason, a statistical approach of PMC meiotic behaviour (in the cases where abnormal meiosis yields some proportion of stainable pollen grains) is necessary, together with the identification of additional post-meiotic factors (for the cases where a regular or almost regular meiosis ends up with a rather poor pollen stainability). Second, the ability of an interspecific hybrid to produce F2 or BC progenies is not clearly related to its (male) fertility, since hybrids virtually sterile (e.g. Solanum melongena × S. elaeagnifolium), partially fertile (e.g. S. melongena × S. tomentosum) and fertile (S. violaceum × S. melongena) can yield such progenies. Definitely, anything seems possible when crossing spiny solanums!
When interspecific crosses fully fail or fail in producing interspecific progenies beyond the F1 crucial step, breeders can nonetheless valorise the wild material. This is the case when the species of interest (1) carries resistances to soil-borne pests and pathogens, (2) displays a vigourous growth in unfavourable conditions (water excess or shortage, drought, cold, salinity) or (3) boosts plant vigor, qualitative and/or qualitative yield. The wild species of interest or the interspecific hybrid itself can then be used as eggplant(s) rootstock, provided it has a good graft affinity with the cultivated eggplant used as scion. Grafting is a technique commonly used for S. melongena, and it is workable for the African eggplants. Hence, breeding innovative rootstocks has agronomic and economic interests.
All this means that for the future, much research is still necessary in the field of interspecific crosses between Leptostemonum species and although crossability and phylogenetic relatedness are not clearly associated, it is probably more secure to begin with the closest relatives of eggplants (species belonging to Melongena clade and Anguivi grade). Internationally collaborative initiatives are needed in order to guaranty full coverage of the crosses, use of shared success criteria and clarification of several pending questions.
11.10.3 Somatic Crossability
Somatic hybridisation experiments between spiny solanums and other Solanaceae had its peak in the 1980s–1990s, and its agronomic motivation was mostly the transfer of disease resistances. The techniques for regenerating amphidiploids or asymmetric hybrids are functional. Although morphological features of the polyploids, aneuploids or introgressed somatic hybrids display both expected and unexpected heredity patterns, their expression of disease resistance levels similar to those of their donor parent is a constant throughout the examples reviewed here. The general trend is that somatic hybridisation yields fertile hybrids when partner species share close phylogenetic relationships and yields sterile hybrids when the sexual cross is either impossible or yields sterile material. However, there are some exceptions for which somatic hybridisation is superior to sexual hybridisation (e.g. Solanum melongena + S. marginatum; S. aethiopicum + S. violaceum). In these cases somatic hybrids display better pollen stainability than their sexual counterparts. Somatic hybrid sterility might be compatible with breeding of a vegetatively propagated crop such as potato, since flower fertility is not indispensable. But genetic recombination between parental genomes and fertility of the progenies is indispensable for breeding sexually reproduced crops, such as S. melongena. In such cases, the next obstacle is the return to the diploid status. This was proved feasible thanks to dihaploids production via anther culture on the single example of somatic hybrids between S. melongena and S. aethiopicum. However, return to diploidy came with a strong reduction in pollen fertility. On the whole, S. melongena-S. aethiopicum progenies were obtained and used in breeding from the hybrids, regardless of their sexual or somatic origin. It would be interesting to know if genetic recombination was different between both kinds of hybrids, because this could be a reason for choosing the best “recombining” technique. With the exception of these somatic hybrids, return to diploidy is neither questioned not solved for all other somatic hybrids involving other species combinations.
11.10.4 Hybrid Phenotypes and Genetics of Morphological Traits
Mendelian and quantitative genetics of traits of interest to breeders are not developed in this chapter because they are beyond its scope. Nonetheless some trait heredity patterns are presented, given that the literature offers information on some interspecific hybrid phenotypes. When differences exist between parents (e.g. prickly vs non prickly, resistant to a given pathogen vs sensitive, etc.), F1 hybrid phenotypes (Sect. 11.8) allow us to determine whether a given trait is dominant, incompletely dominant or recessive. Heterosis, or hybrid vigour, is frequently observed for some traits such as plant height and leaves sizes, whereas resurgence of a few atavic (wild) traits (prickliness in particular) occurs in crosses between cultivated eggplants (c.f. 11.8). However, the interspecific F1 phenotype is sometimes biased, such as in the case of fruit size: this trait depends not only on fruit size genes but also on the presence of seeds. As interspecific hybrids frequently display fertility troubles, F1 fruit size must be interpreted with caution. F2 or backcross generations issued from F1 theoretically provide further information on the genetic control of the segregating traits, but in the case of interspecific hybrids progenies, this information is absent because of the sterility of the hybrids or biased because of distorted segregations. Phenotypes of symmetrical or asymmetrical somatic hybrids are even more difficult to interpret in terms of traits genetics, because of the tetraploid or aneuploid status of such hybrids together with cytoplasmic changes.
Along the successive parts of this chapter, we hope to have convinced our readers that examining the diversity and intercrossability of eggplants and relatives is of key importance for future research programmes.
Notes
- 1.
Agents of the bacterial wilt.
- 2.
Transfer is possible either between cultivated eggplants or from wild species to cultivated eggplant, as well as from wild to wild when relevant.
- 3.
The name S. xanthocarpum is extremely tricky because, depending on the author(s) names associated to it, it matches different accepted species names. In this very case that is S. xanthocarpon Schrad. & Wendl. that matches S. virginianum (Daunay et al. 1991).
- 4.
In some cases, mismatch between constitutive pollen tube length and stigma length explains mechanically the incapability of the pollen of one species to reach the ovules of another species.
- 5.
Parthenocarpic fruits can be the response of the ovary to hormones released through the stimulus of pollination.
- 6.
Endosperm is a triploid tissue issued from the fertilization of two maternal and one paternal nuclei. Hence maternal and paternal genetic dosages differ (2 vs. 1).
- 7.
Both these last troubles can be solved either with hormonal treatment of the hybrid plantlets in vitro (e.g. IAA, gibberellic acid) or by their grafting onto roots of one of their parents.
- 8.
In any given species, chromosomes of each pair share a same genetic structure (homology), which allows their close pairing and the formation of bivalents during diakinesis and metaphase I of meiosis. The word “homeology” was coined for designating, for a given pair, the partial similarity between chromosomes originating from different parental species. When homeology between parental chromosomes is sufficient, the meiosis of an interspecific hybrid is possible, but because chromosomes similarity it incomplete, various abnormalities occur at various frequencies during the course of the meiotic divisions.
- 9.
The fertility of the hybrids S. capense × S. melongena and S. cyaneopurpureum × S. melongena being not indicated (Table 11.7) we hypothesize here that they are fertile or partially fertile.
- 10.
Both are found in the same geographical and ecological areas.
- 11.
Because of this interfertility, Olet and Bukenya-Ziraba (2001) suggested S. campylacanthum and S. cerasiferum belong to the same biological species.
- 12.
The biological species concept is based on successful interbreeding between the members of a given (biological) species, and their reproductive isolation from other species.
- 13.
Interestingly, mature fruit colour of the hybrid between S. melongena (yellow) and S. scabrum (purple-black) was red (Oyelana et al. 2009).
- 14.
This name is a synonym of the accepted name S. sessile, an American species of the Geminata Clade. However the species designated under this name in the publications on male sterility is probably another taxon.
- 15.
Namely S. anguivi, S. dasyphyllum, S. incanum, S. insanum, S. lichtensteinii, S. linnaeanum and S. tomentosum.
- 16.
Given P1 is the value of parent 1, P2 the value of parent 2, F1 the value of the F1 (P1 × P2), Heterosis H is calculated as H = 100 * ((F1 − (P1 + P2)/2)/(P1 − P2)/2).
- 17.
Presence of anthocyanins, which confers purple or black fruit colour, is dominant over their absence.
- 18.
Orange (S. violaceum) is dominant over yellow (S. melongena) mature fruit colour.
- 19.
Mature fruits turned orange, an intermediate state between yellow (eggplant) and red (S. aethiopicum).
- 20.
The sexual hybrid also phenotyped in Daunay et al. (1993) displayed 10–30% pollen stainability, very poor fruit set and parthenocarpic fruits.
- 21.
Solanum melongena + S. nigrum PEG fusion between protoplast of Solanum nigrum and iodoacetate-inactivated eggplant protoplasts aimed at transferring atrazine (herbicide) resistance carried out by the chloroplasts of the wild partner into eggplant (Guri and Sink 1988b). The regenerated plants displayed S. nigrum cpDNA pattern and were resistant to atrazine in vitro. The single plant phenotyped resembled S. nigrum had white flowers (although the purple colour of eggplant flower is usually dominant) and sterile (no stainable pollen grains).
This means that any part of eggplant chromosomes can be integrated.
- 22.
It is possible for instance, that when looked at more closely in the future, eggplants resistance to Fusarium oxysp. f. sp. melongenae will reveal interactions with the fungus diversity, as it is the case for tomato (different genitors control different races of Fusarium oxysp. f. sp. lycopersici).
Literature Cited
Adeniji OT, Kusolwa PM, Reuben S (2012) Genetic diversity among accessions of Solanum aethiopicum L. groups based on morpho-agronomic traits. Plant Genet Resour-Charact Util 10(3):177–185. https://doi.org/10.1017/s1479262112000226
Al-Ani MN (1991) Biosystematic study in the genus Solanum section Oliganthes. Ph.D., Birmingham (UK)
Ali M, Okubo H, Fujieda K (1992) Production and characterization of Solanum amphidiploids and their resistance to bacterial wilt. Sci Hortic 49(3–4):181–196. https://doi.org/10.1016/0304-4238(92)90156-7
Ali Z, Xu ZL, Zhang DY, He XL, Bahadur S, Yi JX (2011) Molecular diversity analysis of eggplant (Solanum melongena) genetic resources. Gen Mol Res 10(2):1141–1155. https://doi.org/10.4238/vol10-2gmr1279
Ano G (1990) Amélioration de l’aubergine (Solanum melongena L.) pour la résistance au flétrissement bactérien causé par Pseudomonas solanacearum E.F.S. Journées maraichères CIRAD-ORSTOM-INRA. INRA
Ano G, Hébert Y, Prior P, Messiaen CM (1991) A new source of resistance to bacterial wilt of eggplant obtained from a cross: Solanum aethiopicum L. x Solanum melongena L. Agronomie 11:555–560
Ano G, Prior P, Manyri J, Vincent C (1989) Stratégies de l’amélioration de l’aubergine (Solanum melongena L.) pour la résistance au fletrissement bactérien causé par Pseudomonas solanacearum E.F.S. In: Degras L (ed) Proceedings of the XXVth annual meeting Caribbean food crop society. Guadeloupe, pp 570–579
Asao H, Arai S, Sato T, Hirai M (1994) Characteristics of a somatic hybrid between Solanum melongena L. and Solanum sanitwongsei Craib. Breed Sci 44(3):301–305
Aubert S, Daunay MC, Pochard E (2009a) Saponosides stéroidiques de l’aubergine (Solanum melongena L.). I. intérêt alimentaire, méthodologie d’analyse, localisation dans le fruit. Agronomie 9:641–651
Aubert S, Daunay MC, Pochard E (2009b) Saponosides stéroidiques de l’aubergine (Solanum melongena L.). II. Variation des teneurs liées aux conditions de récolte, aux génotypes et à la quantité de graines. Agronomie 9:751–758
Aubriot X, Knapp S, Syfert MM, Poczai P, Buerki S (2018) Shedding new light on the origin and spread of the brinjal eggplant (Solanum melongena L.) and its relatives. Am J Bot 105(7):1175–1187. https://doi.org/10.1002/ajb2.1133
Aubriot X, Singh P, Knapp S (2016) Tropical Asian species show that the Old World clade of ‘spiny solanums’ (Solanum subgenus Leptostemonum pro parte: Solanaceae) is not monophyletic. Bot J Linnean Soc 181(2):199–223. https://doi.org/10.1111/boj.12412
Behera TK, Singh N (2002) Inter-specific crosses between eggplant (Solanum melongena L.) with related Solanum species. Sci Hortic 95(1–2):165–172. https://doi.org/10.1016/s0304-4238(02)00019-5
Bletsos F, Roupakias D, Tsaktsira M, Scaltsoyjannes A (2004) Production and characterization of interspecific hybrids between three eggplant (Solanum melongena L.) cultivars and Solanum macrocarpon L. Sci Hortic 101(1–2):11–21. https://doi.org/10.1016/j.scienta.2003.09.011
Bletsos FA, Roupakias DG, Tsaktsira ML, Scaltsoyjannes AB, Thanassoulopoulos CC (1998) Interspecific hybrids between three eggplant (Solanum melongena L.) cultivars and two wild species (Solanum torvum Sw. and Solanum sisymbriifolium Lam.). Plant Breed 117(2):159–164. https://doi.org/10.1111/j.1439-0523.1998.tb01471.x
Borgato L, Conicella C, Pisani F, Furini A (2007) Production and characterization of arboreous and fertile Solanum melongena plus Solanum marginatum somatic hybrid plants. Planta 226(4):961–969. https://doi.org/10.1007/s00425-007-0542-y
Boyaci F, Unlu A, Abak K (2012) Screening for resistance to Fusarium wilt of some cultivated eggplants and wild Solanum accessions. In: Leitao JM (ed) Xxviii international horticultural congress on science and horticulture for people, vol 935. Acta Horticulturae. Int Soc Horticultural Science, Lisbon (Portugal), pp 23–27
Boyaci HF, Unlu A, Abak K (2011) Genetic analysis of resistance to wilt caused by Fusarium (Fusarium oxysporum melongenae) in eggplant (Solanum melongena). Indian J Agric Sci 81(9):812–815
Bui HH, Serra V, Pages L (2015) Root system development and architecture in various genotypes of the Solanaceae family. Botany 93 (8):465–474. https://doi.org/10.1139/cjb-2015-0008
Bukenya ZR, Carasco JF (1995) Crossability and cytological studies in Solanum macrocarpon and Solanum linnaeanum (Solanaceae). Euphytica 86(1):5–13. https://doi.org/10.1007/bf00035933
Callano KJL, Huelgas VC, Mendioro MS (2015) Cytogenetics of Solanum aethiopicum L., S-melongena L. and their F-1 hybrids and the mechanism of hybrid sterility and breakdown. Philipp J Crop Sci 40(2):33–44
Cao BH, Lei JJ, Wang Y, Lü YQ, Chen GH (2009) Inter-specific hybridization between Solanum melongena and S. torvum. Acta Horticulturae Sinica 36(2):209–214
Cericola F, Portis E, Toppino L, Barchi L, Acciarri N, Ciriaci T, Sala T, Rotino GL, Lanteri S (2013) The population structure and diversity of eggplant from Asia and the Mediterranean Basin. Plos One 8(9). https://doi.org/10.1371/journal.pone.0073702
Chowanski S, Adamski Z, Marciniak P, Rosinski G, Buyukguzel E, Buyukguzel K, Falabella P, Scrano L, Ventrella E, Lelario F, Bufo SA (2016) A review of bioinsecticidal activity of solanaceae alkaloids. Toxins 8(3). https://doi.org/10.3390/toxins8030060
Clain C, Da Silva D, Fock I, Vaniet S, Carmeille A, Gousset C, Sihachakr D, Luisetti J, Kodja H, Besse P (2004) RAPD genetic homogeneity and high levels of bacterial wilt tolerance in Solanum torvum Sw. (Solanaceae) accessions from Reunion Island. Plant Sci 166(6):1533–1540. https://doi.org/10.1016/j.plantsci.2004.02.006
Collonnier C, Fock I, Kashyap V, Rotino GL, Daunay MC, Lian Y, Mariska IK, Rajam MV, Servaes A, Ducreux G, Sihachakr D (2001a) Applications of biotechnology in eggplant. Plant Cell, Tissue Organ Cult 65(2):91–107. https://doi.org/10.1023/a:1010674425536
Collonnier C, Fock I, Mariska I, Servaes A, Vedel F, Siljak-Yakovlev S, Souvannavong V, Sihachakr D (2003a) GISH confirmation of somatic hybrids between Solanum melongena and S-torvum: assessment of resistance to both fungal and bacterial wilts. Plant Physiol Biochem 41(5):459–470. https://doi.org/10.1016/s0981-9428(03)00054-8
Collonnier C, Mulya K, Fock I, Mariska I, Servaes A, Vedel F, Siljak-Yakovlev S, Souvannavong V, Ducreux G, Sihachakr D (2001b) Source of resistance against Ralstonia solanacearum in fertile somatic hybrids of eggplant (Solanum melongena L,) with Solanum aethiopicum L. Plant Sci 160(2):301–313. https://doi.org/10.1016/s0168-9452(00)00394-0
Collonnier U, Fock I, Daunay MC, Servaes A, Vedel F, Sijak-Yakovlev S, Souvannavong V, Sihachakr D (2003b) Somatic hybrids between Solanum melongena and S. sisymbriifolium, as a useful source of resistance against bacterial and fungal wilts. Plant Sci 164(5):849–861. https://doi.org/10.1016/s0168-9452(03)00075-x
Cürük S, Dayan A (2018) Production of diploid and amphidiploid interspecific hybrids of eggplant and Solanum torvum and pollen fertility. J Anim Plant Sci 28(5):1485–1492
Daunay MC (1987–1988) Recherches sur l’aubergine. Rapport d’activité de la station d’amélioration des plantes maraîchères vol 1987–1988. INRA, Montfavet
Daunay MC (2008) Eggplant. In: Prohens J, Nuez F (eds) Handbook of crop breeding, Vegetables: Fabaceae, Liliaceae, Umbelliferae, and Solanaceae. Springer, New York, pp 163–220
Daunay MC, Chaput MH, Sihachakr D, Allot M, Vedel F, Ducreux G (1993) Production and characterization of fertile somatic hybrids of eggplant (Solanum melongena L.) with Solanum aethiopicum L. Theor Appl Genet 85(6–7):841–850
Daunay MC, Hazra P (2012) Eggplant. In: Peter KV, Hazra P (eds) Handbook of vegetables. Studium press LLC, pp 258–322
Daunay MC, Hennart JW, Salgon S, Dintinger J (2016) Eggplant resistance to bacterial wilt and to Fusarium wilt: is there a link? In: Ertsey-Peregi K, Füstös Z, Palotas G, Csillery G (eds) XVI. Eucarpia Capsicum and Eggplant meeting, Kecskemet, Hungary, 12–14 September 2016, pp 99–111
Daunay MC, Lester RN, Ano G (2001) Eggplant. In: Charrier AJ, Hamon S; Nicolas D (eds) Tropical plant breeding. CIRAD and Science Publishers, Inc., pp 199–222
Daunay MC, Lester RN, Dalmon A, Ferri M, Kapilima WMPA, Jullian E (1998) The use of wild genetic resources for eggplant (Solanum melongena) breeding. II. Crossability and fertility of interspecific hybrids. In: Palloix A, Daunay MC (eds) Xth meeting on genetics and breeding of Capsicum and eggplant, Sept 7–11, 1998. INRA, Avignon (France), pp 19–24
Daunay MC, Lester RN, Laterrot H (1991) The use of wild species for the genetic improvement of Brinjal Egpplant (Solanum melongena) and Tomato (Lycopersicon esculentum). In: Hawkes JG, Lester RN, Nee M, Estrada-R N (eds) Solanaceae III, taxonomy, chemistry, evolution. The Royal Botanic Gardens (Kew, U.K.) for the Linnean Society of London, pp 389–412
Demir K, Bakir M, Sarikamis G, Acunalp S (2010) Genetic diversity of eggplant (Solanum melongena) germplasm from Turkey assessed by SSR and RAPD markers. Gen Mol Res 9(3):1568–1576. https://doi.org/10.4238/vol9-3gmr878
Devi CP, Munshi AD, Behera TK, Choudhary H, Vinod Gurung B, Saha P (2015) Cross compatibility in interspecific hybridization of eggplant, Solanum melongena, with its wild relatives. Sci Hortic 193:353–358. https://doi.org/10.1016/j.scienta.2015.07.024
Echeverría-Londoño S, Särkinen T, Fenton IS, Knapp S, Purvis A (2018) Dynamism and context dependency in the diversification of the megadiverse plant genus Solanum L. (Solanaceae). bioRxiv: 348961. https://doi.org/10.1101/348961
Fang M, Mao R, Xie W (1985) Breeding of cytoplasmically inherited male sterile lines of eggplant (Solanum melongena L.). Acta Horticulturae Sinica 12:261–266
Garcia-Fortea E, Gramazio P, Vilanova S, Fita A, Mangino G, Villanueva G, Arrones A, Knapp S, Prohens J, Plazas M (2019) First successful backcrossing towards eggplant (Solanum melongena L.) of a New World species, the silverleaf nightshade (S. elaeagnifolium), and characterization of interspecific hybrids and backcrosses. Sci Hortic 246:563–573
Gisbert C, Prohens J, Nuez F (2011a) Performance of eggplant grafted onto cultivated, wild, and hybrid materials of eggplant and tomato. Int J Plant Prod 5(4):367–380
Gisbert C, Prohens J, Raigon MD, Stommel JR, Nuez F (2011b) Eggplant relatives as sources of variation for developing new rootstocks: effects of grafting on eggplant yield and fruit apparent quality and composition. Sci Hortic 128(1):14–22. https://doi.org/10.1016/j.scienta.2010.12.007
Gleddie S, Keller WA, Setterfield G (1985) Plant regeneration from tissue, cell and protoplast cultures of several wild Solanum species. J Plant Physiol 119(5):405–418. https://doi.org/10.1016/s0176-1617(85)80005-5
Gleddie S, Keller WA, Setterfield G (1986) Production and characterization of somatic hybrids between Solanum melongena L. and Solanum sisymbriifolium Lam. Theor Appl Genet 71(4):613–621
Gousset C, Collonnier C, Mulya K, Mariska I, Rotino GL, Besse P, Servaes A, Sihachakr D (2005) Solanum torvum, as a useful source of resistance against bacterial and fungal diseases for improvement of eggplant (S. melongena L.). Plant Sci 168(2):319–327. https://doi.org/10.1016/j.plantsci.2004.07.034
Gowda PHR, Shivashankar KT, Joshi S (1990) Interspecific hybridization between Solanum melongena and Solanum macrocarpon—study of the F1 hybrid plants. Euphytica 48(1):59–61
Gramazio P, Prohens J, Plazas M, Mangino G, Herraiz FJ, Vilanova S (2017b) Development and genetic characterization of advanced backcross materials and an introgression line population of Solanum incanum in a S. melongena Background. Front Plant Sci 8. https://doi.org/10.3389/fpls.2017.01477
Gurbuz N, Karabey F, Ozturk TK, Kilinc A, Frary A, Doganlar S (2015) Glycoalkaloid isolation from Solanum linnaeanum berries. Fruits 70(6):371-+. https://doi.org/10.1051/fruits/2015037
Guri A, Sink KC (1988a) Interspecific somatic hybrid plants between eggplant (Solanum melongena) and Solanum torvum. Theor Appl Genet 76(4):490–496
Guri A, Sink KC (1988c) Organelle composition in somatic hybrids between an atrazine resistant biotype of Solanum nigrum and Solanum melongena. Plant Sci 58(1):51–58. https://doi.org/10.1016/0168-9452(88)90153-7
Guri A, Sink KC (1988a) Interspecific somatic hybrid plants between eggplant (Solanum melongena L.) and Solanum torvum. Hortscience 23(3):786–786
Harlan JR, de Wet JMJ (1971) Toward a rational classification of cultivated plants. Taxon 20:509–517
Hasan SMZ (1989) Biosystematic study of Solanum melongena L. in Asia and Africa. Ph.D., Birmingham (UK)
Hasnunnahar M, Khan MMR, Isshiki S (2012) Pollen and seed fertility of three functional male-sterile lines of eggplant with the wild Solanum cytoplasms. Sci Hortic 139:58–61. https://doi.org/10.1016/j.scienta.2012.03.004
Hébert Y (1985) résistance comparée de 9 espèces du genre Solanum au flétrissement bactérien (Pseudomonas solanacearum) at au nématode Meloidogyne incognita. Intérêt pour l’Amélioration de l’aubergine (Solanum mlelongena L.) en zone tropicale humide. Agronomie 5(1):27–32. https://doi.org/10.1051/agro:19850104
Hurtado M, Vilanova S, Plazas M, Gramazio P, Fonseka HH, Fonseka R, Prohens J (2012) Diversity and relationships of eggplants from three geographically distant secondary centers of diversity. Plos One 7(7). https://doi.org/10.1371/journal.pone.0041748
IBPGR (ed) (1990) Descriptors for eggplants/Descripteurs pour l’aubergine. International Board for Plant Genetic Resources Rome
Isshiki S, Kawajiri N (2002) Effect of cytoplasm of Solanum violaceum Ort. on fertility of eggplant (S-melongena L.). Sci Hortic 93(1):9–18. https://doi.org/10.1016/s0304-4238(01)00314-4
Isshiki S, Okubo H, Fujieda K (2000) Segregation of isozymes in selfed progenies of a synthetic amphidiploid between Solanum integrifolium and S-melongena. Euphytica 112(1):9–14. https://doi.org/10.1023/a:1003841415838
Isshiki S, Suzuki S, Yamashita K (2003) RFLP analysis of mitochondrial DNA in eggplant and related Solanum species. Genet Resour Crop Evol 50(2):133–137. https://doi.org/10.1023/a:1022954229295
Isshiki S, Taura T (2003) Fertility restoration of hybrids between Solanum melongena L. and S. aethiopicum L. Gilo Group by chromosome doubling and cytoplasmic effect on pollen fertility. Euphytica 134(2):195–201. https://doi.org/10.1023/b:euph.0000003883.39440.6d
Iwamoto Y, Hirai M, Ohmido N, Fukui K, Ezura H (2007) Fertile somatic hybrids between Solanum integrifolium and S. sanitwongsei (syn. S. kurzii) as candidates for bacterial wilt-resistant rootstock of eggplant. Plant Biotechnol 24(2):179–184. https://doi.org/10.5511/plantbiotechnology.24.179
Jadari R, Sihachakr D, Rossignol L, Ducreux G (1992) Transfer of resistance to Verticillium dahliae Kleb. from Solanum torvum Sw. into potato (Solanum tuberosum L.) by protoplast electrofusion. Euphytica 64(1–2):39–47
Jarl CI, Rietveld EM, de Haas JM (1999) Transfer of fungal tolerance through interspecific somatic hybridisation between Solanum melongena and S. torvum. Plant Cell Rep 18(9):791–796. https://doi.org/10.1007/s002990050663
Jayakumar K, Murugan K (2016) Solanum alkaloids and their pharmaceutical roles: a review. J Anal Pharm Res 3(6):00075. https://doi.org/10.15406/japlr.2016.03.00075
Jose RS, Plazas M, Sanchez-Mata MC, Camara M, Prohens J (2016) Diversity in composition of scarlet (S. aethiopicum) and gboma (S. macrocarpon) eggplants and of interspecific hybrids between S. aethiopicum and common eggplant (S. melongena). J Food Compos Anal 45:130–140. https://doi.org/10.1016/j.jfca.2015.10.009
Kaan F (1973) Etude de l’hérédité de la résistance de l’aubergine (Solanum melongena L.) à l’anthracnose des fruits (Colletotrichum gloeosporiodes f. sp. melongenae Penzig Fournet). Annales de l’amélioration des plantes 23(2):127–131
Kameya T, Miyazawa N, Toki S (1990) Production of somatic hybrids between Solanum melongena and S. integrifolium Poir. Jpn J Breed 40(4):429–434
Kashyap V, Kumar SV, Collonnier C, Fusari F, Haicour R, Rotino GL, Sihachakr D, Rajam M (2003) Biotechnology of eggplant. Sci Hortic 97(1):1–25. https://doi.org/10.1016/s0304-4238(02)00140-1
Kaushik P, Gramazio P, Vilanova S, Raigon MD, Prohens J, Plazas M (2017) Phenolics content, fruit flesh colour and browning in cultivated eggplant, wild relatives and interspecific hybrids and implications for fruit quality breeding. Food Res Int 102:392–401. https://doi.org/10.1016/j.foodres.2017.09.028
Kaushik P, Prohens J, Vilanova S, Gramazio P, Plazas M (2016) Phenotyping of eggplant wild relatives and interspecific hybrids with conventional and phenomics descriptors provides insight for their potential utilization in breeding. Front Plant Sci 7. https://doi.org/10.3389/fpls.2016.00677
Khan MMR, Hasnunnahar M, Isshiki S (2013a) Production of amphidiploids of the hybrids between Solanum macrocarpon and eggplant. HortScience 48(4):422–424
Khan MMR, Hasnunnahar M, Iwayoshi M, Isshiki S (2013b) Pollen and seed fertility of the male fertile lines having the fertility restorer gene in three CMS systems of eggplant. Sci Hortic 157:39–44. https://doi.org/10.1016/j.scienta.2013.04.010
Khan MMR, Hasnunnahar M, Iwayoshi M, Isshiki S (2014) Fertility restoration in three CMS systems of eggplant by the Rf genes of each other’s systems and their SCAR marker. Sci Hortic 172:149–154. https://doi.org/10.1016/j.scienta.2014.04.013
Khan MMR, Hasnunnahar M, Iwayoshi M, Ogura-Tsujita Y, Isshiki S (2015) Pollen degeneration in three functional male-sterile lines of eggplant with the wild Solanum cytoplasms. Hortic Environ Biotechnol 56(3):350–357. https://doi.org/10.1007/s13580-015-0015-3
Khan MMR, Hasnunnahar M, Iwayoshi M, Ogura-Tsujita Y, Isshiki S (2017) Pollen and seed fertility differences of the backcross progenies between Solanum virginianum and eggplant with different inheritance pattern of chloroplast DNA. Sci Hortic 218:193–197. https://doi.org/10.1016/j.scienta.2017.02.031
Khan MMR, Isshiki S (2008) Development of a male sterile eggplant by utilizing the cytoplasm of Solanum virginianum and a biparental transmission of chloroplast DNA in backcrossing. Sci Hortic 117(4):316–320. https://doi.org/10.1016/j.scienta.2008.05.006
Khan MMR, Isshiki S (2009) Functional male-sterility expressed in eggplant (Solanum melongena L.) containing the cytoplasm of S. kurzii Brace & Prain. J Horticult Sci Biotechnol 84(1):92–96. https://doi.org/10.1080/14620316.2009.11512486
Khan MMR, Isshiki S (2010) Development of the male-sterile line of eggplant utilizing the cytoplasm of Solanum aethiopicum L. Aculeatum Group. J Jpn Soc Hortic Sci 79(4):348–353. https://doi.org/10.2503/jjshs1.79.348
Khan MMR, Isshiki S (2011) Development of a cytoplasmic male-sterile line of eggplant (Solanum melongena L.) with the cytoplasm of Solanum anguivi. Plant Breed 130(2):256–260. https://doi.org/10.1111/j.1439-0523.2010.01788.x
Khan MMR, Isshiki S (2016) Cytoplasmic male sterility in eggplant. Horticult J 85(1):1–7. https://doi.org/10.2503/hortj.MI-IR03
Kharkongar HP, Khanna VK, Tyagi W, Rai M, Meetei NT (2013) Wide hybridization and embryo-rescue for crop improvement in Solanum. Agrotechnology (11). https://doi.org/10.4172/2168-9881.1000s11-004
Kirti PB, Rao BGS (1980) Chromosome pairing in reciprocal hybrids of Solanum integrifolium and Solanum indicum var. multiflora. Caryologia 33(2):289–294. https://doi.org/10.1080/00087114.1980.10796842
Kirti PB, Rao BGS (1981) Cytogenetic studies on the F1 hybrid Solanum indicum x Solanum torvum. Theor Appl Genet 59(5):303–306. https://doi.org/10.1007/bf00264982
Kirti PB, Rao BGS (1982a) Chromosome relationships of spinous solanums. Proc Indian Acad Sci-Plant Sci 91(2):83–91
Kirti PB, Rao BGS (1982b) Cytological studies on F1 hybrids of Solanum integrifolium with Solanum melongena and Solanum melongena var. insanum. Genetica 59(2):127–131. https://doi.org/10.1007/bf00133296
Kirti PB, Rao BGS (1983) The cytogenetic basis of sterility in spinous Solanum hybrids. Caryologia 36(2):155–164. https://doi.org/10.1080/00087114.1983.10797655
Kouassi A, Beli-Sika E, Tian-Bi TY-N, Alla-N’Nan O, Kouassi AB, N’Zi JC, N’Guetta AS-P, Tio-Toure B (2014) Identification of three distinct eggplant subgroups within the Solanum aethiopicum Gilo group from Côte d’Ivoire by morpho-agronomic characterization. Agriculture 4:260–273
Kouassi B, Prohens J, Gramazio P, Kouassi AB, Vilanova S, Galan-Avila A, Herraiz FJ, Kouassi A, Segui-Simarro JM, Plazas M (2016) Development of backcross generations and new interspecific hybrid combinations for introgression breeding in eggplant (Solanum melongena). Sci Hortic 213:199–207. https://doi.org/10.1016/j.scienta.2016.10.039
Kumar G, Meena BL, Kar R, Tiwari SK, Gangopadhyay KK, Bisht IS, Mahajan RK (2008) Morphological diversity in brinjal (Solanum melongena L.) germplasm accessions. Plant genetic resources: characterization and utilization 6(3):232–236. https://doi.org/10.1017/s1479262108994211
Kumchai J, Wei YC, Lee CY, Chen FC, Chin SW (2013) Production of interspecific hybrids between commercial cultivars of the eggplant (Solanum melongena L.) and its wild relative S. torvum. Gen Mol Res 12(1):755–764. https://doi.org/10.4238/2013.march.13.4
Lebeau A, Daunay MC, Frary A, Palloix A, Wang JF, Dintinger J, Chiroleu F, Wicker E, Prior P (2011) Bacterial wilt resistance in tomato, pepper, and eggplant: genetic resources respond to diverse strains in the Ralstonia solanacearum species complex. Phytopathology 101(1):154–165. https://doi.org/10.1094/phyto-02-10-0048
Lebeau A, Gouy M, Daunay MC, Wicker E, Chiroleu F, Prior P, Frary A, Dintinger J (2013) Genetic mapping of a major dominant gene for resistance to Ralstonia solanacearum in eggplant. Theor Appl Genet 126(1):143–158. https://doi.org/10.1007/s00122-012-1969-5
Lester RN (1986) Taxonomy of scarlet eggplants, Solanum aethiopicum L. Acta Hortic 182:125–132
Lester RN (1989) Evolution under domestication involving disturbance of genic balance. Euphytica 44:125–132
Lester RN, Hasan SMZ (1991) Origin and domestication of the brinjal eggplant, Solanum melongena L., from S. incanum in Africa and Asia. In: Hawkes JG, Lester RN, Nee M, Estrada-R N (eds) Solanaceae III: taxonomy, chemistry, evolution. Royal botanic gardens, Kew, pp 369–387
Lester RN, Kang JH (1998) Embryo and endosperm function and failure in Solanum species and hybrids. Ann Bot 82(4):445–453. https://doi.org/10.1006/anbo.1998.0695
Lester RN, Niakan L (1986) Origin and domestication of the scarlet eggplant, Solanum aethiopicum, from S. anguivi in Africa In: D’Arcy WG (ed) Solanaceae, biology and systematics. Columbia university press, pp 433–456
Liu J, Zheng ZS, Zhou XH, Feng C, Zhuang Y (2015) Improving the resistance of eggplant (Solanum melongena) to Verticillium wilt using wild species Solanum linnaeanum. Euphytica 201(3):463–469. https://doi.org/10.1007/s10681-014-1234-x
Liu KB, Li YM, Sink KC (1995) Asymmetric somatic hybrid plants between an interspecific Lycopersicon hybrid and Solanum melongena. Plant Cell Rep 14(10):652–656. https://doi.org/10.1007/bf00232732
Liu T, Yu Y, Cai XK, Tu W, Xie CH, Liu J (2016) Introgression of bacterial wilt resistance from Solanum melongena to S. tuberosum through asymmetric protoplast fusion. Plant Cell Tissue Organ Cult 125(3):433–443. https://doi.org/10.1007/s11240-016-0958-9
Malausa JC, Daunay MC, Bourgoin T (1988) Resistance of several varieties of eggplant, Solanum melongena L. to the greenhouse whitefly, Trialeurodes vaporariorum Westwood (Homoptera, Aleyrodidae). Agronomie 8(8):693–699. https://doi.org/10.1051/agro:19880804
Mc Cammon KR, Honma S (1983) Morphological and cytogenetic analyses of an interspecific hybrid eggplant. Solanum melongena x Solanum torvum HortScience 18:894–895
Mennella G, Rotino GL, Fibiani M, D’Alessandro A, Francese G, Toppino L, Cavallanti F, Acciarri N, Lo Scalzo R (2010) Characterization of health-related compounds in eggplant (Solanum melongena L.) lines derived from introgression of allied species. J Agric Food Chem 58(13):7597–7603. https://doi.org/10.1021/jf101004z
Meyer RS, Whitaker BD, Little DP, Wu SB, Kennelly EJ, Long CL, Litt A (2015) Parallel reductions in phenolic constituents resulting from the domestication of eggplant. Phytochemistry 115:194–206. https://doi.org/10.1016/j.phytochem.2015.02.006
Morel A, Guinard J, Lonjon F, Sujeeun L, Barberis P, Genin S, Vailleau F, Daunay MC, Dintinger J, Poussier S, Peeters N, Wicker E (2018) The eggplant AG91-25 recognizes the Type III-secreted effector RipAX2 to trigger resistance to bacterial wilt (Ralstonia solanacearum species complex). Mol Plant Pathol 19(11):2459–2472. https://doi.org/10.1111/mpp.12724
Moyle LC, Nakazato T (2010) Hybrid Incompatibility “snowballs” between Solanum species. Science 329(5998):1521–1523. https://doi.org/10.1126/science.1193063
Mutlu N, Boyaci FH, Gocmen M, Abak K (2008) Development of SRAP, SRAP-RGA, RAPD and SCAR markers linked with a Fusarium wilt resistance gene in eggplant. Theor Appl Genet 117(8):1303–1312. https://doi.org/10.1007/s00122-008-0864-6
Naegele RP, Boyle S, Quesada-Ocampo LM, Hausbeck MK (2014) Genetic diversity, population structure, and resistance to phytophthora capsici of a worldwide collection of eggplant germplasm. Plos One 9(5). https://doi.org/10.1371/journal.pone.0095930
Niakan L (1980) Biosystematics of the scarlet eggplant (L.) and related species. Ph.D., Birmingham (UK)
Olet EA, Bukenya-Ziraba R (2001) Variation within the S. incanum complex in Uganda and its relationship with S. cerasiferum In: van den Berg RG, Barendse GWM, Van der Weerden G, Mariani C (eds) Solanaceae V, Advances in taxonomy and utilization. Nijmegen University press, pp 97–108
Omidiji MO (1979) Crossability relationships between some species of Solanum, Lycopersicon and Capsicum cultivated in Nigeria. In: Hawkes JG, Lester RN, Skelding AD (eds) The biology and taxonomy of the Solanaceae. Academic press for the Linnaean Society of London, pp 599–604
Omidiji MO (1982) Interrelationships of Solanum species in different series of the sub-genus Leptostemonum (Dun.) Bitt. Crop Res (Hortic Res) 22:3–12
Omidiji MO (1983) Cytomorphological studies of F1 hybrid Solanum aethiopicum x Solanum macrocarpon. Cytologia 48(1):35–40
Omidiji MO (1986) the role of hybridization in the evolution of species in Solanum subgenus Leptostemonum. In: D’Arcy WG (ed) Solanaceae, biology and systematics. Columbia University Press, pp 468–476
Osei MK, Banful B, Osei CK, Oluoch MO (2010) Characterization of African eggplant for morphological characteristics. J Agric Sci Technol 4(3(serial n°28)):33–37
Oyelana OA, Ogunwenmo KO (2009) Nuclear and non-nuclear interactions in F1 hybrids populations of three Solanum species in the subgenus Leptostemonum, section Melongena (Solanaceae). Turkish J Bot 33:243–255
Oyelana OA, Ogunwenmo KO, Nwagburuka CC, Alabi OA (2009) Cytomorphological analysis of a novel hybrid from Solanum melongena ‘Golden’ x S. scabrum ‘Scabrum’ (Solanaceae). Span J Agric Res 7(2):355–363
Oyelana OA, Ugborogho RE (2008) Phenotypic variation of F-1 and F-2 populations from three species of Solanum L. (Solanaceae). Afr J Biotechnol 7(14):2359–2367
Pearce K (1975) Solanum melongena L. and related species. Ph.D., Birmingham (UK)
Plazas M, Andujar I, Vilanova S, Gramazio P, Herraiz FJ, Prohens J (2014) Conventional and phenomics characterization provides insight into the diversity and relationships of hypervariable scarlet (Solanum aethiopicum L.) and gboma (S. macrocarpon L.) eggplant complexes. Front Plant Sci 5. https://doi.org/10.3389/fpls.2014.00318
Plazas M, Lopez-Gresa MP, Vilanova S, Torres C, Hurtado M, Gramazio P, Andujar I, Herraiz FJ, Belles JM, Prohens J (2013) Diversity and relationships in key traits for functional and apparent quality in a collection of eggplant: fruit phenolics content, antioxidant activity, polyphenol oxidase activity, and browning. J Agric Food Chem 61(37):8871–8879. https://doi.org/10.1021/jf402429k
Plazas M, Vilanova S, Gramazio P, Rodriguez-Burruezo A, Fita A, Herraiz FJ, Ranil R, Fonseka R, Niran L, Fonseka H, Kouassi B, Kouassi A, Prohens J (2016) Interspecific hybridization between eggplant and wild relatives from different genepools. J Am Soc Hort Sci 141(1):34–44
Polignano G, Uggenti P, Bisignano V, Della Gatta C (2010) Genetic divergence analysis in eggplant (Solanum melongena L.) and allied species. Genet Resour Crop Evol 57(2):171–181. https://doi.org/10.1007/s10722-009-9459-6
Prabakaran S, Balakrishnan S, Ramesh Kumar S, Arumugam T, Anandakumar CR (2015) Genetic diversity, trait relationship and path analysis in eggplant landraces. Electron J Plant Breed 6(3):831–837
Prabhu M, Natarajan S, Veeraragavathatham D, Pugalendhi L (2009) The biochemical basis of fruit and shoot borer resistance in interspecific progenies of brinjal (Solanum melongena L.). Eurasian J Biosci 3:50–57. https://doi.org/10.5053/ejobios.2009.3.0.7
Prohens J, Blanca JM, Nuez F (2005) Morphological and molecular variation in a collection of eggplants from a secondary center of diversity: Implications for conservation and breeding. J Am Soc Hort Sci 130(1):54–63
Prohens J, Plazas M, Raigon MD, Segui-Simarro JM, Stommel JR, Vilanova S (2012) Characterization of interspecific hybrids and first backcross generations from crosses between two cultivated eggplants (Solanum melongena and S. aethiopicum Kumba group) and implications for eggplant breeding. Euphytica 186(2):517–538. https://doi.org/10.1007/s10681-012-0652-x
Prohens J, Whitaker BD, Plazas M, Vilanova S, Hurtado M, Blasco M, Gramazio P, Stommel JR (2013) Genetic diversity in morphological characters and phenolic acids content resulting from an interspecific cross between eggplant, Solanum melongena, and its wild ancestor (S. incanum). Ann Appl Biol 162(2):242–257. https://doi.org/10.1111/aab.12017
Rajasekaran S (1970) Cytogenetic studies of F1 hybrid Solanum indicum L. x Solanum melongena L. and its amphidiploid. Euphytica 19(2):217–224. https://doi.org/10.1007/bf01902949
Rajasekaran S (1971) Cytological studies on F1 hybrid (Solanum xanthocarpum Schrad. and Wendl. x Solanum melongena L.) and its amphidiploid. Caryologia 24(3):261–267. https://doi.org/10.1080/00087114.1971.10796434
Ranil RHG, Prohens J, Aubriot X, Niran HML, Plazas M, Fonseka RM, Vilanova S, Fonseka HH, Gramazio P, Knapp S (2017) Solanum insanum L. (subgenus Leptostemonum Bitter, Solanaceae), the neglected wild progenitor of eggplant (S. melongena L.): a review of taxonomy, characteristics and uses aimed at its enhancement for improved eggplant breeding. Genet Resour Crop Evol 64(7):1707–1722. https://doi.org/10.1007/s10722-016-0467-z
Rao NN (1979) The barriers to hybridization between Solanum melongena and some other species of Solanum. In: Hawkes JG, Lester RN, Skelding AD (eds) The biology and taxonomy of the Solanaceae. Academic press for the Linnaean society of London, pp 605–614
Rao SV, Rao BGS (1984) Studies on the crossability relationships of some spinous solanums. Theor Appl Genet 67(5):419–426
Rattan P, Kumar S, Salgotra RK, Samnotra RK, Sharma F (2015) Development of interspecific F-1 hybrids (Solanum melongena x Solanum khasianum) in eggplant through embryo rescue technique. Plant Cell, Tissue Organ Cult 120(1):379–386. https://doi.org/10.1007/s11240-014-0591-4
Rizza F, Mennella G, Collonnier C, Sihachakr D, Kashyap V, Rajam MV, Prestera M, Rotino GL (2002) Androgenic dihaploids from somatic hybrids between Solanum melongena and S. aethiopicum group gilo as a source of resistance to Fusarium oxysporum f. sp melongenae. Plant Cell Rep 20(11):1022–1032. https://doi.org/10.1007/s00299-001-0429-5
Robinson RW, Shail JW, Gao Y (2001) Interspecific hybridization of eggplant for Verticillium wilt resistance and other useful traits. In: Van den Berg RG, Barendse GWM, Van der Weerden G, Mariani C (eds) Solanaceae V, advances in taxonomy and utilization. Nijmegen University Press, pp 279–291
Roth M, Florez-Rueda AM, Griesser S, Paris M, Stadler T (2018a) Incidence and developmental timing of endosperm failure in post-zygotic isolation between wild tomato lineages. Ann Bot 121(1):107–118. https://doi.org/10.1093/aob/mcx133
Roth M, Florez-Rueda AM, Paris M, Stadler T (2018b) Wild tomato endosperm transcriptomes reveal common roles of genomic imprinting in both nuclear and cellular endosperm. Plant J 95(6):1084–1101. https://doi.org/10.1111/tpj.14012
Roth M, Florez-Rueda AM, Stadler T (2018c) Differences in effective ploidy as drivers of genome-wide endosperm expression asymmetries and seed failure in wild tomato hybrids. doi:bioRxiv, 459925
Rotino GL, Sihachakr D, Rizza F, Vale G, Tacconi MG, Alberti P, Mennella G, Sabatini E, Toppino L, D’Alessandro A, Acciarri N (2005) Current status in production and utilization of dihaploids from somatic hybrids between eggplant (Solanum melongena L.) and its wild relatives. Acta Physiologiae Plant 27(4B):723–733. https://doi.org/10.1007/s11738-005-0077-4
Sadder MT, Al-Shareef RM, Hamdan H (2006) Assessment of genetic, morphological, and agronomical diversity among Jordanian eggplant (Solanum melongena L.) landraces using random amplified polymorphic DNA (RAPD). In: Spooner DM, Bohs L, Giovannoni J, Olmstead RG, Shibata D (eds) Solanaceae VI: Genomics meets biodiversity, vol 745. Acta Horticulturae, pp 303–310
Safni I, Cleenwerck I, De Vos P, Fegan M, Sly L, Kappler U (2014) Polyphasic taxonomic revision of the Ralstonia solanacearum species complex: proposal to emend the descriptions of Ralstonia solanacearum and Ralstonia syzygii and reclassify current R. syzygii strains as Ralstonia syzygii subsp syzygii subsp nov., R. solanacearum phylotype IV strains as Ralstonia syzygii subsp indonesiensis subsp nov., banana blood disease bacterium strains as Ralstonia syzygii subsp celebesensis subsp nov and R. solanacearum phylotype I and III strains as Ralstonia pseudosolanacearum sp nov. Int J Syst Evol Microbiol 64:3087–3103. https://doi.org/10.1099/ijs.0.066712-0
Saito T, Matsunaga H, Saito A, Hamato N, Koga T, Suzuki T, Yoshida T (2009) A novel source of cytoplasmic male sterility and a fertility restoration gene in eggplant (Solanum melongena L.) lines. J Jpn Soc Hortic Sci 78(4):425–430. https://doi.org/10.2503/jjshs1.78.425
Salgon S, Jourda C, Sauvage C, Daunay MC, Reynaud B, Wicker E, Dintinger J (2017) Eggplant resistance to the ralstonia solanacearum species complex involves both broad-spectrum and strain-specific quantitative trait loci. Front Plant Sci 8. https://doi.org/10.3389/fpls.2017.00828
Salgon S, Raynal M, Lebon S, Baptiste JM, Daunay MC, Dintinger J, Jourda C (2018) Genotyping by sequencing highlights a polygenic resistance to Ralstonia pseudosolanacearum in eggplant (Solanum melongena L.). Int J Mol Sci 19(2). https://doi.org/10.3390/ijms19020357
Samoylov VM, Sink KC (1996) The role of irradiation dose and DNA content of somatic hybrid calli in producing asymmetric plants between an interspecific tomato hybrid and eggplant. Theor Appl Genet 92(7):850–857. https://doi.org/10.1007/bf00221897
Sanchez-Mata MC, Yokoyama WE, Hong YJ, Prohens J (2010) Alpha-Solasonine and alpha-Solamargine contents of Gboma (Solanum macrocarpon L.) and Scarlet (Solanum aethiopicum L.) Eggplants. J Agric Food Chem 58(9):5502–5508. https://doi.org/10.1021/jf100709g
Scaldaferro M, Chiarini F, Santinaque FF, Bernardello G, Moscone EA (2012) Geographical pattern and ploidy levels of the weed Solanum elaeagnifolium (Solanaceae) from Argentina. Genet Resour Crop Evol 59(8):1833–1847. https://doi.org/10.1007/s10722-012-9807-9
Schaff DA, Jelenkovic G, Boyer CD, Pollack BL (1982) Hybridization and fertility of hybrid derivatives of Solanum melongena L. and S. macrocarpon L. Theor Appl Genet 62(2):149–153. https://doi.org/10.1007/bf00293348
Seck A (1997) Recherche et étude génétique de la résistance de l’aubergine africaine (S. aethiopicum L., spp. Kumba) aux acariens phytophages. RADHORT (FAO). Bulletin de liaison 11:46–53
Sharma DR, Chowdhury JB, Ahuja U, Dhankhar BS (1980) Interspecific hybridization in genus Solanum. A cross between S. melongena and S. khasianum through embryo culture Zeitschrift für Pflanzenzüchtung 85:248–253
Sharma DR, Kaur R, Kumar K (1996) Embryo rescue in plants—a review. Euphytica 89(3):325–337
Sihachakr D, Daunay MC, Serraf I, Chaput MH, Mussio I, Haicour R, Rossignol L, Ducreux G (1994) Somatic hybridization of eggplant (Solanum melongena L.) with its close and wild relatives. In: Bajaj YPS (ed) Biotechnology in agriculture and forestry, somatic hybridization in crop improvement, vol I. Springer, Heidelberg, pp 255–278
Sihachakr D, Haicour R, Chaput MH, Barrientos E, Ducreux G, Rossignol L (1989) Somatic hybrid plants produced by electrofusion between Solanum melongena L. and Solanum torvum Sw. Theor Appl Genet 77(1):1–6. https://doi.org/10.1007/bf00292307
Sihachakr D, Haicour R, Serraf I, Barrientos E, Herbreteau C, Ducreux G, Rossignol L, Souvannavong V (1988) Electrofusion for the production of somatic hybrid plants of Solanum melongena L. and Solanum khasianum Clark, C.B. Plant Sci 57(3):215–223. https://doi.org/10.1016/0168-9452(88)90127-6
Stattmann M, Gerick E, Wenzel G (1994) Interspecific somatic hybrids between Solanum khasianum and S. aculeatissimum produced by electrofusion. Plant Cell Rep 13(3–4):193–196
Stern S, Agra MD, Bohs L (2011) Molecular delimitation of clades within New World species of the “spiny solanums” (Solanum subg. Leptostemonum). Taxon 60(5):1429–1441
Stommel JR, Whitaker BD (2003) Phenolic acid content and composition of eggplant fruit in a germplasm core subset. J Am Soc Hortic Sci 128(5):704–710
Sunseri F, Polignano GB, Alba V, Lotti C, Bisignano V, Mennella G, Alessandro AD, Bacchi M, Riccardi P, Fiore MC, Ricciardi L (2010) Genetic diversity and characterization of African eggplant germplasm collection. Afr J Plant Sci 4(7):231–241
Taher D, Solberg SO, Prohens J, Chou YY, Rakha M, Wu TH (2017) World vegetable center eggplant collection: origin, composition, seed dissemination and utilization in breeding. Front Plant Sci 8. https://doi.org/10.3389/fpls.2017.01484
Tamura N, Murata Y, Mukaihara T (2002) A somatic hybrid between Solanum integrifolium and Solanum violaceum that is resistant to bacterial wilt caused by Ralstonia solanacearum. Plant Cell Rep 21(4):353–358. https://doi.org/10.1007/s00299-002-0524-2
Toki S, Kameya T, Abe T (1990) Production of a triple mutant, chlorophyll deficient, streptomycin resistant, and kanamycin resistant Nicotiana tabacum, and its use in intergeneric somatic hybrid formation with Solanum melongena. Theor Appl Genet 80(5):588–592
Toppino L, Mennella G, Rizza F, D’Alessandro A, Sihachakr D, Rotino GL (2008a) ISSR and isozyme characterization of androgenetic dihaploids reveals tetrasomic inheritance in tetraploid somatic hybrids between Solanum melongena and Solanum aethiopicum group Gilo. J Hered 99(3):304–315. https://doi.org/10.1093/jhered/esm122
Toppino L, Vale G, Rotino GL (2008b) Inheritance of Fusarium wilt resistance introgressed from Solanum aethiopicum Gilo and Aculeatum groups into cultivated eggplant (S-melongena) and development of associated PCR-based markers. Mol Breed 22(2):237–250. https://doi.org/10.1007/s11032-008-9170-x
Tümbilen Y, Frary A, Daunay MC, Doganlar S (2011a) Application of EST-SSRs to examine genetic diversity in eggplant and its close relatives. Turk J Biol 35(2):125–136. https://doi.org/10.3906/biy-0906-57
Tümbilen Y, Frary A, Mutlu S, Doganlar S (2011b) Genetic diversity in Turkish eggplant (Solanum melongena) varieties as determined by morphological and molecular analyses. Int Res J Biotechnol 2(1):16–25
Vilanova S, Manzur JP, Prohens J (2012) Development and characterization of genomic simple sequence repeat markers in eggplant and their application to the study of diversity and relationships in a collection of different cultivar types and origins. Mol Breed 30(2):647–660. https://doi.org/10.1007/s11032-011-9650-2
Villeneuve F, Latour F, Théry T, Erard P, Fournier C, Daunay MC (2016) Screening of solanaceous wild relatives for graft affinity with eggplant (Solanum melongena L.). In: Ertsey-Peregi K, Füstös Z, Palotas G, Csillery G (eds) Proceedings of the XVIth Eucarpia meeting on Capsicum and eggplant (12–14 Sept 2016) Kekckemet, Hungary, pp 152–160
Villeneuve F, Latour F, Théry T, Steinberg C, V. E-H, Pitrat M, Daunay MC (2014) The control of soil borne vascular diseases: limits of genetic resistance of cultivars and rootstocks for controlling Fusarium oxysporum f.sp. melonis (Melon) and Verticillium dahliae (eggplant). In: disinfestation PVisocan-csas (ed) Acta Horticulturae 1044. ISHS, pp 57–65
Vorontsova MS, Knapp S (eds) (2016) A revision of the “spiny solanums”, Solanum subgenus Leptostemonum (Solanaceae), in Africa and Madagascar, vol 99. Systematic botany monographs. The American Society of Plant Taxonomists
Vorontsova MS, Stern S, Bohs L, Knapp S (2013) African spiny Solanum (subgenus Leptostemonum, Solanaceae): a thorny phylogenetic tangle. Bot J Linn Soc 173(2):176–193. https://doi.org/10.1111/boj.12053
Wanjari KB (1976) Cytogenetic studies on F1 hybrids between Solanum melongena L. and Solanum macrocarpon L. Hortic Res 15(2):77–83
Wu SB, Meyer RS, Whitaker BD, Litt A, Kennelly EJ (2013) A new liquid chromatography-mass spectrometry-based strategy to integrate chemistry, morphology, and evolution of eggplant (Solanum) species. J Chromatogr 1314:154–172. https://doi.org/10.1016/j.chroma.2013.09.017
Xiao XO, Cao BH, Li GN, Lei JJ, Chen QH, Jiang J, Cheng YJ (2015) Functional characterization of a putative bacterial wilt resistance gene (RE-bw) in eggplant. Plant Mol Biol Rep 33(4):1058–1073. https://doi.org/10.1007/s11105-014-0814-1
Yoshimi M, Kitamura Y, Isshiki S, Saito T, Yasumoto K, Terachi T, Yamagishi H (2013) Variations in the structure and transcription of the mitochondrial atp and cox genes in wild Solanum species that induce male sterility in eggplant (S. melongena). Theor Appl Genet 126(7):1851–1859. https://doi.org/10.1007/s00122-013-2097-6
Yu Y, Ye WX, He L, Cai XK, Liu T, Liu J (2013) Introgression of bacterial wilt resistance from eggplant to potato via protoplast fusion and genome components of the hybrids. Plant Cell Rep 32(11):1687–1701. https://doi.org/10.1007/s00299-013-1480-8
Zhou XH, Bao SY, Liu J, Yang Y, Zhuang Y (2018) Production and characterization of an amphidiploid derived from interspecific hybridization between Solanum melongena L. and Solanum aculeatissimum Jacq. Sci Hortic 230:102–106. https://doi.org/10.1016/j.scienta.2017.11.024
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Daunay, MC., Salinier, J., Aubriot, X. (2019). Crossability and Diversity of Eggplants and Their Wild Relatives. In: Chapman, M. (eds) The Eggplant Genome. Compendium of Plant Genomes. Springer, Cham. https://doi.org/10.1007/978-3-319-99208-2_11
Download citation
DOI: https://doi.org/10.1007/978-3-319-99208-2_11
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-99207-5
Online ISBN: 978-3-319-99208-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)