Abstract
Microorganisms especially bacteria and cyanobacteria have the ability to synthesize polyhydroxyalkanoates (PHAs) granules as carbon and energy storage compounds within their cells. Owing to eco-friendly, biodegradability, modifiable mechanical properties, non-toxicity, biocompatibility, hydrophobicity, cellular growth support, piezoelectricity, attachment without carcinogenic effects, optical purity and desired surface modifications, the PHAs have received substantial attention towards research as well as commercial ventures and comparable to non-biodegradable conventional plastics presently in use. Microbial PHA biosynthetic pathways are grouped into four types, where PHA synthases are the main enzymes. The PHA synthases exploit the hydroxyacyl-CoAs as substrates and catalyze the covalent bond formation among the hydroxyl group of one along with the carboxyl group of other hydroxyalkanoate that result into the formation of PHAs. Depending on the specificity of substrate as well as components of subunit, PHA synthases are grouped into four types, i.e., class I synthesizing Short-Chain-Length (SCL) PHAs (represented by the bacterium Cupriavidus necator), class II synthesizing Medium-Chain-Length (MCL) PHAs (represented by the bacterium Pseudomonas putida), class III (represented by bacterial species such as Allochromatium vinosum), and class IV PHA synthases (so far represented only by Bacillus sp., B. megaterium). Interestingly, these PHA synthases have a preserved cysteine residue as a catalytic active site to which the resulting PHA chain is linked through covalent bond. Overall, this chapter gives an overview on the structure and genes of PHA synthases including PHA biosynthetic routes, mechanism of PHAs polymerization together with biogenesis of PHA granules and phasins as major PHA granule-associated proteins.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Biocompatibility
- Biodegradability
- PHAs
- Phasins
- PHA synthases
- Piezoelectricity
- Polyhydroxyalkanoates
- PHA biosynthetic pathways
- PHA biogenesis
14.1 Introduction
14.1.1 Conventional Petroleum-Based Plastics
Conventional petroleum-based plastics have exceptional light-weight, stability, durability, economic feasibility and desirable material properties with ability to depict a variety of strengths as well as shapes. These unique properties are responsible for their widespread uses in construction, packaging materials, computer equipments, components of automobiles, medical field, printers, etc. (Bernard 2014; Kumar et al. 2015; Sharma et al. 2016; Singh and Mallick 2017a, b; Singh et al. 2017). In 2013, the global plastic production enhanced up to 299 million tons, which was a 3.9% enhancement than the 2012 (Plastics Europe 2015). Plastic production exhibits a little enhancement in 2016, but is still below pre-crisis level. However, in 2017 plastic production is anticipated to continue on a positive trend (Plastics Europe 2016). These plastics are usually manufactured from polyolefins such as polyethylene (PE), polystyrene (PS), polypropylene (PP), polyvinyl chloride (PVC), etc. that are typically synthesized using fossil fuels, used up as well as cast-off into the environment as non-degradable refuses, where they persist as such for many years as a result of largely xenobiotic nature or recalcitrant to microbial attack. The question of what to do with these conventional plastics waste is turning into a worldwide problematic issue pertaining to environment. The environmental apprehensions like solid waste management, plastic waste incineration and safe disposal posed by these plastics resulted into the synthesis of green polymers/biodegradable plastics (Alexander 1981; Ray and Bousmina 2005; Shah et al. 2008; Kumar et al. 2015; Sharma et al. 2016; Singh et al. 2017). Biodegradable plastics are biologically synthesized materials that are environmentally friendly, degradable and lead to mineralization. Such green plastics are relatively new as well as promising due to their actual use and degradation by microbes like bacteria and cyanobacteria. This green plastic can be divided into four classes viz. photodegradable, semi-biodegradable, chemically synthesized and polyhydroxyalkanoates (PHAs) (Ray and Bousmina 2005; Singh et al. 2017). Among these, the microbially originated PHAs depicted unique properties such as biodegradability, modifiable mechanical properties, non-toxicity, biocompatibility, hydrophobicity, cellular growth support, piezoelectricity, attachment without carcinogenic effects, optical purity and desired surface modifications, which make them extremely competitive with conventional plastics for various industrial applications. The industrial applications of PHAs are summarized in Table 14.1.
14.1.2 An Overview on Commercialization Trends and Economics of PHAs Production
The biopolymeric PHAs market is differentiated into linear as well as co-polymerized PHAs in which the co-polymerized PHAs emerged as the foremost revenues producing section because of its desirable material properties akin to conventional petroleum-based plastics. PHAs market based on application is classified into various fields like food services, medical, agriculture, packaging (Table 14.1), etc., where packaging field is the most important revenue making segment due to increasing interest of biodegradable packaging materials. Furthermore, PHAs geography market is divided into North America, Europe, Asia Pacific and Rest of the World. Interestingly, Europe followed by Asia Pacific, is the biggest revenue generating sector because of relative increase in the consumer’s disposable income together with existence of enormous amount of capital by many industries, which triggering more and more investment in research and development (Global Polyhydroxyalkanoate (PHA) Market 2012–2020). There are many top most companies like Metabolix Inc. (USA), Shenzhen Ecomann Technology Co. Ltd. (China), Tianjin GreenBio Materials Co. Ltd. (China), Meredian Inc. (USA) and Biomer (Germany) that are engaged in the biopolymeric PHAs business. Amongst these companies, USA-based Metabolix Inc. and China-based Shenzhen Ecomann Technology depicted the maximum growth and developments relating to PHAs market. Additionally, they have largest expansion plan with novel PHA product developments (as their ultimate growth policies). Such companies already initiated the research and intensifying efforts for industrial production of many different types of PHA biopolymers (Global Trends and Forecasts 2018). For instance, the Cupriavidus necator, a bacterium, is employed by Metabolix, Inc. (USA) for the industrial production of 3-hydroxybutyrate (3HB) and 3-hydroxyvalerate (3HV), i.e., [P(3HB-co-3HV)] co-polymer and marketed in the trade name of BIOPOL® (Luzier 1992). In the near future, these companies are anticipating considerable development as well as growth in PHAs market because of exploring the wide-range of feasible uses and forthcoming inclinations of biodegradable plastics. Table 14.2 shows the current global manufacturers of PHA bioplastics.
PHAs are making pace towards commercialization that is attained by decades of research with hard efforts and commitments. Regardless of commercialization, technology is still at the initial stages for economical large-scale PHAs production (Global Trends and Forecasts 2018; Global Polyhydroxyalkanoate (PHA) Market 2012–2020). The most important limitation of PHAs is their high production price. The commercial PHA biopolymeric materials was reported to be 15–17 times expensive over the conventional plastics and 4–6 times costlier than the commercial polylactic acid in 2004 (Castilho et al. 2009). Nevertheless, metabolic engineering, improved fermentation conditions with higher PHAs accumulation capacities were able to diminish the cost up to US$5 kg−1 in 2009, which was still three times expensive than the cost of polypropylene (DiGregorio 2009). Hence, PHAs still have a limited market, despite their potential to replace 33% of commercial polymers (Castilho et al. 2009). The final price of PHAs are mostly reliant on the cost of organic substrates that supplemented as a carbon source for microbial cultivation. Remarkably, the cost of organic carbon sources found to contribute about 50% of the overall production cost. In addition, the PHA content and yield on organic carbon substrate, PHA productivity as well as downstream prices are the other important factors responsible towards determining their introduction into global market (Choi and Lee 1999). Analysis and economical assessment established that large-scale PHAs synthesis using octane as carbon source would cost approximately US$5–10 kg−1 (Hazenberg and Witholt 1997). It was estimated that the theoretical cost of PHAs accumulated in fed-batch method exploiting waste/cost-effective substances might attain up to 3.51 Eur kg−1 PHAs, while synthetic alternatives such as polypropylene as well as polyethylene cost 1.47 and 1.15 Eur kg−1, respectively (Obruca 2010). Overall, the transformation of raw substances to PHAs appears to be a main factor in the establishment of sustainable biotechnological process and a solution for the price limitations (Możejko-Ciesielska and Kiewisz 2016; Chandel et al. 2018).
As a result of the aforementioned limitations (related to bacterial PHAs production), cyanobacteria are receiving the current attention as alternative hosts for the cost-effective PHAs production because of their little nutrient requirement, their genetic modification is much easier than the higher plants as well as eukaryotic photosynthetic algae, can be stored for long periods of time, ability to transform ‘greenhouse gas’ into PHA biopolymers by oxygenic photosynthesis and low quality/infertile land for their growth. In spite of these advantages, the prominent bottlenecks associated with the cyanobacterial PHAs production involve the lack of an inexpensive mass cultivation technique, unavailability of efficient biomass harvesting system and significantly lower PHA productivity than the bacteria (Singh et al. 2017; Singh and Mallick 2017b). Therefore, on urgent basis, these bottlenecks need to be resolved in order to push cyanobacterial PHAs production towards commercialization phase.
14.2 Occurrence, Structure and Types of PHAs
The exterior portion of PHAs granules possess a large amount of different protein molecules, which signifying that they represent supramolecular complexes having particular roles rather than being merely simple packets possessing abundant amount of carbon and energy. The term ‘carbonosome’ was introduced to specify the multi-functionality of PHA granules (Jendrossek 2009). The prokaryotic water insoluble carbon and energy storage compounds PHAs are a kind of naturally occurring biopolymers with diameter in the range of 0.2 ± 0.5 mm, which are produced as granules in the cytoplasm of a huge and varied forms of microorganisms like bacteria and cyanobacteria under different growth and environmental conditions (Lageveen et al. 1988; Huisman et al. 1989; Stal et al. 1990; Zhang et al. 1994; Kato et al. 1996; Miyake et al. 1996; Lama et al. 1996; Braunegg et al. 1998; Nishioka et al. 2001; Thakor et al. 2003; Tajima et al. 2003; Tian et al. 2005a; Sharma and Mallick 2005a, b; Panda et al. 2006; Sharma et al. 2006; Yezza et al. 2006; Mallick et al. 2007; Sharma et al. 2007; Toh et al. 2008; Panda et al. 2008; Singh and Mallick 2008; Chen 2009, 2010; Sankhla et al. 2010; Li et al. 2011; Singh et al. 2013; Hauf et al. 2013; Osanai et al. 2013; Samantaray and Mallick 2014, 2015; Koller and Maršálek 2015; Singh et al. 2015; Reddy and Mohan 2015; Sharma et al. 2016; Gómez Cardozo et al. 2016; Singh and Mallick 2017a, b, c; Singh et al. 2017). The bacterial strains such as Cupriavidus necator (previously called as Wautersia eutropha/Ralstonia eutropha/Alcaligenes eutrophus) Rhodopseudomonas palustris, Methylobacterium organophilum, etc. synthesized PHAs under limitations of ammonium, sulphate, phosphate, potassium, magnesium, iron and oxygen (Anderson and Dawes 1990; Singh and Mallick 2008, 2009a, b; Singh et al. 2015; Kumar et al. 2015; Singh and Mallick 2017a, b). The bacterial strains viz. Alcaligenes latus, etc. found to produce PHA biopolymers during active cell growth without any nutrient limitation. The occurrence of PHA biopolymers in cyanobacteria was reported in 1966 (Carr 1966; Drosg et al. 2015). Subsequently, numerous cyanobacterial strains are reported to produce biopolymeric PHAs photoautotrophically, while others under chemoheterotrophic conditions using acetate or other organic carbon substrate (Campbell et al. 1982; Sharma et al. 2006, 2007; Mallick et al. 2007; Bhati and Mallick 2012; Samantaray and Mallick 2012, 2014, 2015). The limitation or deficiency of nutrient such as nitrogen and phosphorus usually results in enhanced accumulation of PHAs in cyanobacteria (Takahashi et al. 1998; Nishioka et al. 2001; Bhati and Mallick 2012; Samantaray and Mallick 2012). Table 14.3 summarized the accumulation of PHA biopolymers by different bacterial and cyanobacterial strains/species under diverse environmental conditions.
The first studies on bacterial PHA granules were carried out by Williamson and Wilkinson (1958) followed by Griebel et al. (1968). Interestingly, the extracted PHA granules found to possess nearly 97.5% PHA biopolymer, 2% proteins as well as 0.5% phospholipid molecules (Griebel et al. 1968). The electron microscopic investigations depicted that the exterior portion of PHA granules of B. megaterium as well as B. cereus is coated with a membrane having a thickness of about 15–20 nm (Lundgren et al. 1964). De Koning and Maxwell (1993) suggested the involvement of a single layer of phospholipid covering the PHA granules based on in vitro data of isolated PHA granules. When accumulated by natural PHA producers, an interesting phenomenon occurs, where soluble or membrane-bound PHA synthases catalyze the transformation of hydrophilic monomeric units into hydrophobic polymers (Grage et al. 2009; Nobes et al. 2000). This accountable for the synthesis of intracellular granules made up of a hydrophobic, amorphous polymeric core enclosed by a single layer of phospholipids as well as protein molecules (Jurasek and Marchessault 2004). These proteins involve the PHA synthase, intracellular PHA depolymerases responsible for the depolymerization of PHA polymers that resulting into remobilization of the carbon source (PhaZ family), a regulatory protein (PhaR) and small proteins called phasins, which regulate the size and morphology of granules (York et al. 2002). Various investigations have confirmed the existence of a phospholipid layer in PHA preparations (Parlane et al. 2016). Nevertheless, in vivo occurrence of a phospholipid coat has never been established. Several data have put into question the real occurrence of the lipid layer in vivo (Pötter and Steinbüchel 2006; Beeby et al. 2012; Jendrossek and Pfeiffer 2014), specifically from the studies of electron cryotomography (Wahl et al. 2012) as well as fluorescence microscopy (Bresan et al. 2016), according to which the existence of the lipid coat might develop from an experimental artefact on PHA extraction and preparation.
Merrick and co-workers were initially observed that extracted polyhydroxybutyrate (PHB) granules showed sensitivity towards denaturation phenomenon processes and coined the term native PHB granules (nPHB) so as to specify that such homopolymeric PHB granule preparations relatively similar to the native in vivo condition of PHB granules (Merrick and Doudoroff 1964). Interestingly, merely nPHB granules contain active PHB synthase that are vulnerable to depolymerization with active PHB depolymerases. Once nPHB granules are subjected to chemical treatment like alkali or solvents, physical such as freezing/pelleting through centrifugation or biochemical stresses viz. treatment with enzymes, bioactive compounds etc., the granules quickly denature and remain unaffected towards the action of native PHB depolymerases (Merrick 1965; Merrick et al. 1965; Griebel and Merrick 1971). The polymeric chains are usually in an amorphous form in nPHB, while the denatured PHB associate with a substantially crystalline part.
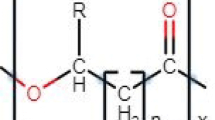
PHAs are a class of linear polyesters of hydroxyalkanoic acids (HA), which are linked together by ester bonds. Figure 14.1 displayed the reaction catalyzed by the PHA synthases, where ‘N’ can gain a value of 35,000. The structure of PHAs synthesized by bacteria/cyanobacteria can be manipulated through physiological or genetic strategies (Steinbüchel 1991). The organic carbon sources are transformed to hydroxyacyl-CoA thioesters within the bacterial/cyanobacterial cell metabolism. In PHAs biosynthesis, the carboxylate group of one monomeric unit establish an ester bond with the hydroxyl group of the adjacent monomeric unit (Philip et al. 2007), where the reaction is catalyzed by the PHA synthase of host. Each PHA monomer involves a side chain R group with ability to vary from methyl (C1) to tridecyl (C13) (Madison and Huisiman 1999; Lu et al. 2009). This alkyl side chain, generally a saturated alkyl group, can also hold the uncommon chemical structure like halogenated, aromatic, unsaturated, branched, epoxidized and substituted alkyl groups (Lageveen et al. 1988; Abe et al. 1990; Doi and Abe 1990; Fritzsche et al. 1990a, b, c; Kim et al. 1991, 1992, 1995; Choi and Yoon 1994; Hazer et al. 1994; Curley et al. 1996; Song and Yoon 1996). In the side chains of biopolymeric PHAs, substituents can be transformed chemically by cross-linking of unsaturated bonds (de Koning et al. 1994; Gagnon et al. 1994a, b). The modifications in the side chains and the ability to change their substituents are responsible for the variation of PHAs family (Madison and Huisiman 1999). Furthermore, the molecular weight of PHA polymeric materials varies from 2 × 105 to 3 × 106 Da that reliant on the microorganism in which the polymer is synthesized and the growth conditions maintained (Byrom 1994; Lee 1995). In these PHA biopolymeric materials, the HA monomeric units are all in the R(-) configuration because of the sterospecificity of the PHA synthase (Philip et al. 2007; Verlinden et al. 2007). Therefore, biosynthesis of PHAs in microorganism assurances sterospecific integration of the R(-) monomeric unit that is important towards the biocompatibility as well as biodegradability of PHAs (Zinn and Hany 2005). The length of the side chain together with its functional group significantly affects the properties of the PHA polymer viz. crystallinity, melting point and glass transition temperature that in turn governs its final uses. The PHAs producers are hopeful and still see potential in this biopolymeric materials claiming that PHAs are new generation of biopolymers and their market requires time to develop. It is projected that demand for PHAs will grow tenfold by 2020 (Aeschelmann et al. 2015).
Reaction catalyzed by PHA synthases (compiled from Rehm 2007)
More than 300 species of microorganisms are recognized so far, which are capable to synthesize PHA biopolymers (Lee et al. 1999). The PHA polymers broadly classified into three types (Table 14.4) depending upon the number of carbon atoms present in the monomeric units, i.e., (a) short-chain-length-PHAs (SCL-PHAs), (b) medium-chain-length-PHAs (MCL-PHAs) and (c) long-chain-length-PHAs (LCL-PHAs) (Anderson and Dawes 1990; Steinbüchel et al. 1992; Vincenzini and De Philippis 1999; Singh and Mallick 2008). Among the various groups of PHA biopolymers, homopolymeric PHB is the most common as well as widespread in different bacteria and cyanobacteria. Interestingly, bacteria have the ability to synthesize SCL-MCL-PHA or SCL-LCL-PHA co-polymers apart from SCL-, MCL- or LCL-PHA polymers (Matsusaki et al. 2000; Lee and Park 2002; Singh and Mallick 2008). Majority of the heterotrophic bacterial species produce either SCL- or MCL- or rarely LCL-PHAs, however, usually failed to synthesize co-polymers of SCL-MCL- or SCL-LCL-PHAs because of PHA synthases substrate specificity that can recognize HAs of a specific range of carbon length (Kato et al. 1996; Singh 2009; Ashby et al. 2002; Singh and Mallick 2017a). Presently, there is increasing attention for investigation and commercial ventures on suitable bacterial species towards the accumulation of PHA co-polymers due to their exceptional physical and mechanical properties (Chen et al. 2001; Lee and Park 2002). A perusal of the literature depicted that except PHB and P(3HB-co-3HV) co-polymers, there is no report traceable towards the synthesis of MCL-/LCL-PHAs or SCL-MCL-PHAs/SCL-LCL-PHAs co-polymers in cyanobacteria. Therefore, intensifying and innovative research is needed for a wide screening of new as well as extremely prolific cyanobacterial strains from diverse environmental conditions and examination for the accumulation of MCL-/LCL-PHAs or SCL-MCL-PHAs/SCL-LCL-PHAs co-polymers, similar to bacteria (Singh et al. 2017).
14.3 Microbial PHA Synthases: Main Enzymes of PHA Biosynthetic Pathways
Microbial PHA synthases of PHA biosynthetic pathways are the key enzymes, where they perform a remarkable role in PHA polymerization process by exploiting the coenzyme A (CoA) thioesters of HAs, i.e., (R)-3-hydroxyacyl-CoA as substrates (Fig. 14.1). The percent yield and the physicochemical properties of PHAs are primarily reliant on the catalytic performance of PHA synthases, i.e., the polymerization of 3-hydroxyacyl-CoA monomeric units into PHA with concomitant liberation of acetyl CoA (Fig. 14.1). Approximately 88 diverse kinds of PHA synthase genes were characterized as well as sequenced from 68 diverse bacterial species (Rehm 2007). During polymerization reactions, they catalyze the covalent bond formation among the hydroxyl group of one and the carboxyl group of another HA (Steinbüchel and Hein 2001). So far, four different groups of PHA synthases (I, II, III and IV) have been reported based on the structure/subunits, amino acid sequence and substrate specificity. The PHA synthases belonging to group I, III and IV polymerize SCL monomers, i.e., C3 to C5, while group II PHA synthase uses MCL monomers ranging from C6 to C14 or more than C14, i.e., LCL monomers. The group I, II, III and IV PHA synthases are signified by C. necator including phototrophic non-sulfur purple bacterial species/strains as well as in majority of heterotrophic bacteria, but excluding the pseudomonads from rRNA homology group I, Pseudomonas putida together with all pseudomonads of rRNA homology group I, Allochromatium vinosum and Bacillus sp. (such as Bacillus megaterium), respectively (Steinbüchel and Hein 2001; Singh and Mallick 2008; Możejko-Ciesielska and Kiewisz 2016; Sharma et al. 2016). Nevertheless, there are few exceptions to this classification as some PHA synthases depict wide-range substrate specificity. For example, PHA synthases of Thiocapsa pfenningii that categorized as group III showed the broad substrate specificity towards CoA thioesters of SCL as well as MCL 3-HA. Similarly, PHA synthases belonging to Aeromonas caviae depict high resemblance to group I with capability to produce a 3-hydroxybutyrate and 3-hydroxyhexanoate co-polymer. Moreover, Pseudomonas sp. 61-3 synthases of group II depict competence to synthesize a co-polymer of 3-hydroxybutyrate and MCL 3-HA. Remarkably, PHA synthase of C. necator belonging to group I capable to produce MCL 3-HA CoA thioesters (Rehm 2003). The group I as well as group II PHA synthases composed of single subunit (PhaC) depicting molecular weights within the range of 61 and 73 kDa (Qi and Rehm 2001). However, group III (e.g. Allochromatium vinosum) as well as group IV PHA synthases (e.g. Bacillus megaterium) need two kinds of subunits, i.e., PhaC (40.3 kDa) and PhaE (20 or 40 kDa), PhaC (41.5 kDa) and PhaR (22 kDa) for their catalytic activities, respectively. The diversity among the group I PHAs synthases was studied signifying that this group depicts more diverse enzymological features. Nevertheless, the data showed that PHA synthases of all groups revealed an identical topology. Interestinly, group I and II PHAs synthases varied at locations 100–130 or 80–110 (Rehm 2003). It was established that PhaE as well as PhaR are participated in the polymerization of PHAs, but their exact function is still not known. Moreover, PhaCR subunits of group IV can be classified into two types, i.e., Bacillus cereus and Bacillus megaterium groups. Recently, it was found that group IV PHA synthases belonging to Bacillus cereus decrease the molecular weights of PHB (Tomizawa et al. 2011). While structures of PhaC are still not clear, but most recent report advocating that the active site of group III PHA synthases could be more polar compared to group I PHA synthases and both are sensitive to the alterations in the alkyl side chain (Jia et al. 2016). Interestingly, these various kinds of PHA synthase found to depict one stringently preserved cysteine residue that is potentially the active site engaged in the polymerization reaction (Griebel et al. 1968).
However, biosynthesis of PHA granules in cyanobacteria has received the consideration since the report of PHB accumulation in N2 fixing cyanobacterium, Chlorogloea fritschii, but identification, cloning, as well as molecular characterization of cyanobacterial PHA synthase has been reported for the first time in the Synechocystis sp. PCC6803, a cyanobacterium (Carr 1966; Hein et al. 1998). It was shown by Hein et al. (1998) that PHA synthase belonging to Synechocystis sp. PCC6803 is a two-component enzyme that represented by two open reading frames (ORFs), i.e., slr1830 as well as slr1829. The ORF slr1830 that encoding a protein having 378 amino acids was designated as phaC (phaCSyn) whereas the other ORF slr1829, located colinear and upstream of phaC was designated to as phaE (phaESyn). The multialignment of the phaE and phaC gene products showed remarkable sequence resemblance with the two subunits viz. PhaE and PhaC of group III PHA synthase belonging to three γ-Proteobacteria namely Chromatium vinosum, Thiocystis violacea and Thiocapsa pfennigii (Hein et al. 1998; Liebergesell et al. 2000; Ansari et al. 2016). Similarly, the cyanobacterium, Synechococcus sp. MA19 also found to possess type III PHA synthases (Steinbüchel and Hein 2001).
14.4 Molecular Basis of PHA Biosynthesis Enzymes
The PHA synthesis genes are usually bunched in the genomes of bacteria. C. necator has been investigated in detail in which the genes encoding PHA synthase (phaC), β-ketothiolase (phaA) and NADPH-linked acetoacetyl-CoA reductase (phaB) form the phaCAB operon (Peoples and Sinskey 1989a, b; Schubert et al. 1988; Slater et al. 1988). In addition, the generally found genetic organization of C. necator amongst PHB producing bacteria, many bacteria depict a diverse gene order, nevertheless, at least the PHB synthase gene is co-localized with other PHB biosynthesis genes. Several bacterial species like P. denitriicans possess next to the PHA synthase additional genes such as phaP (encoding phasin) and phaR (encoding regulator protein) linked to PHA biosynthesis. An operonic organization of PHA biosynthesis genes, linked to the SCL-PHA production (group I PHA synthase gene) was found amongst the β-proteobacteria like C. necator, Burkholderia sp., Delftia acidovorans and Alcaligenes latus.
The pseudomonads that produce MCL-PHAs involve two different genes encoding group II synthases that are parted by the structural gene phaZ encoding a putative intracellular PHA depolymerase (Timm and Steinbüchel 1992; Hoffmann and Rehm 2004, 2005). Merely one of these PHA synthase genes is needed towards the biosynthesis of MCL-PHA (Langenbach et al. 1997; Pham et al. 2004). The phaD gene is positioned directly downstream of the second synthase gene, but upstream of the genes phaI and phaF that are transcribed in opposite direction. PhaI along with PhaF are recognized as structural and regulatory proteins (Prieto et al. 1999; Hoffmann and Rehm 2004, 2005). Remarkably, the genes encoding enzymes like transacylase (PhaG) or the enoyl-CoA hydratase (PhaJ) that are directly involved in the provision of substrate of PHA synthase are not co-localized in pseudomonads (Rehm et al. 1998; Tsuge et al. 2000). The group III PHA synthase genes phaC and phaE are co-localized in the corresponding genomes comprising presumably a single operon (Liebergesell et al. 1992, 1993). The group IV synthase genes are present in the bacterial species belonging to genus Bacillus and consist of phaR and phaC that are separated by phaB (McCool and Cannon 1999, 2001).
The cyanobacterial PHA synthase with its activity was reported in the membrane fractions of a cyanobacterium, Spirulina sp. MA19 for the first time under nitrogen-deficient condition (Miyake et al. 1997; Asada et al. 1999). The genome analysis of Synechocystis sp. PCC6803 that accumulated about 0.1 g PHB per g dry cell weight (dcw) under acetate supplemented condition depicted synthase genes corresponding to the open reading frame (ORF) slr1830 (designated as phaC) and to the collinear ORF slr1829 upstream of phaC (designated as phaE). Interestingly, phaE and phaC genes showed much greater sequence resemblance with the corresponding group III PHA synthase subunits present in the anoxygenic purple sulfur bacterial species viz. Thiocystis violacea, Chromatium vinosum and Thiocapsa pfennigii, which are typical PHA accumulators containing group III PHA synthases. In addition, expression of these genes in Escherichia coli as well as Alcaligenes eutrophus evidently exhibited that co-expression of both phaC and phaE is required to attain an active Spirulina sp. PCC6803 PHA synthase (Hein et al. 1998). Later, it was revealed that Spirulina sp. PCC6803 contains a PHA specific β-ketothiolase encoded by phaASyn and an acetoacetyl-CoA reductase encoded by phaBSyn. Resemblance investigation of the whole genome sequence of this strain showed a cluster of two putative ORFs for these genes, slr1993 (409 amino acids; phaASyn) and slr1994 (240 amino acids; phaBSyn). These ORFs are collinear and co-expressed. The transformation of E. coli cells with phaASyn and phaBSyn along with the PHA synthase of Spirulina sp. PCC6803 led to production of 0.123 g PHA per g dcw under glucose supplemented condition. Furthermore, phylogenetic analysis was carried out to group the origin of phaASyn and phaBSyn genes in the γ-subdivision of Proteobacteria (Taroncher-Oldenburg et al. 2000).
The wide range and generic incidence of cyanobacterial group III PHA synthases was later supported by Hai and colleagues, together with the molecular characterization of PHA synthases of the thermophilic Chlorogloeopsis fritschii PCC 6912 as well as Synechococcus sp. strain MA19. The PHA accumulation potential and the kind of accountable PHA synthases belonging to eleven various cyanobacterial strains were studied by Southern blot analysis with phaC specific probes, Western blot analysis using specific polyclonal antiPhaE antibodies, sequence analysis of PCR products with phaC-specific oligonucleotide primers, cloning techniques, and finally sequence analysis of the PHA synthase structural genes. The presence of group III PHA synthase was evidenced in S. ssp. MA19 and PCC 6715, Chlorogloeopsis fritschii PCC 6912, Anabaena cylindrica SAG 1403-2, Cyanothece ssp. PCC 7424, PCC 8303 and PCC 8801, and Gloeocapsa sp. strain PCC 7428. As a positive control, the screening was compared with crude protein extracts and DNA of Synechocystis sp. strain PCC 6803. No group III PHA synthase was observed in Stanieria sp. strain PCC 7437, Cyanothece sp. strain PCC 8955, and Gloeothece sp. strain PCC 6501 (Hai et al. 2001). Most recently, Numata et al. (2015) reported the specific activity of PHA synthase of the Synechocystis sp. PCC 6803, where both genes, phaC and phaE were co-expressed in a cell-free synthesis system. Specific activity of phaCE was analogous to group I PHA synthases, which usually occurring in most common PHA producers such as C. necator and therefore, contradicting earlier assumptions that inadequate synthase activity could be the cause for modest PHA productivity in cyanobacteria.
14.5 Biosynthetic Routes Involved in the Production of PHAs from Different Carbon Substrates
One of the parameters, which govern the kind of PHA components, is the organic carbon substrates. Microbial world is proficient in accumulating PHAs from different organic carbon substrates varying from cost-effective, complex waste effluents (such as beet and cane molasses) to plant oils and its fatty acids, alkanes and simple carbohydrates (Lageveen et al. 1988; Hängii 1990; Page 1992; Eggink et al. 1993, 1995; Tan et al. 1997; Fukui and Doi 1998). The detections of various PHA monomer units apart from 3-hydroxybutyrate (3HB) more than three decades ago clearly indicated that the PHA synthases depict a wide-range substrate specificity and thus, a wide diversity of PHA monomers can be polymerized (Sudesh et al. 2000). Furthermore, only the existence of a PHA synthase is not enough to allow the accumulation of PHA polymers. PHA production will not take place if the genes that encode enzymes needed towards the production of hydroxyacyl-CoA thioesters are not present or if the routes established through these enzymes are for any kind of reason not functionally active. This is supported by the fact that as soon as there is expression of PHA synthase gene in wild type or ordinary laboratory E. coli strains, even if a functionally active PHA synthase is synthesized, no or merely traces of PHAs are synthesized (Steinbüchel and Hein 2001).
So far, PHA biosynthetic routes present in microorganisms widely grouped into four kinds (Kumar et al. 2015). The PHB biosynthetic route is best studied in a number of bacterial strains/species such as Azotobacter beijerinckii, C. necator etc. In these groups of bacterial species, the PHB polymer synthesis begin from acetyl-CoA when they supplemented with carbohydrates or acetyl-CoA providing organic carbon substrates. This PHB route includes three enzyme assisted reactions sequences viz. 3-ketothiolase, acetoacetyl-CoA reductase as well as lastly PHB synthase (Oeding and Schlegel 1973; Senior and Dawes 1973). The initial stage of this route includes a reversible reaction catalysed by β-ketothiolase that transformed two acetyl-CoA molecules into acetoacetyl-CoA intermediates (Masamune et al. 1989; Moskowitz and Merrick 1969). The acetoacetyl-CoA molecules so generated are then converted into monomer R-(−)-3-hydroxybutyryl-CoA in the presence of acetoacetyl-CoA reductase and NAD(P)H as reducing power. The last stage includes the PHB synthase, which integrates the monomeric unit, i.e., R-(−)-3-hydroxybutyryl-CoA into the growing polymeric chain of PHB. One general observation regarding the kinds of PHAs synthesize by C. necator is that the integrated monomeric units always consist of merely C3–C5. However, the nature and the ratio of these monomeric units are affected by the kind as well as the relative amount of organic substrates added in the cultivation media (Steinbüchel 1991; Steinbüchel et al. 1993). It has been observed that addition of propionate or valerate with glucose into the growth media of C. necator facilitates the biosynthesis of PHA co-polymer, i.e., [P(3HB-co-3HV)]. This signified that the PHA synthase of C. necator is only active towards polymerizing the monomeric units belonging to SCL HA. Nevertheless, the position of the oxidized carbon in the monomeric unit is seemingly not a critical parameter that supported by the integration of 4- and 5-HA units apart from the more general 3HA units (Doi et al. 1987). C. necator is proficient in producing PHAs from specialized organic carbon substrates viz. 4-hydroxybutyric acid, γ-butyrolactone and 1,4-butanediol, where synthesis of 4HB monomeric units occur together with 3HB (Doi et al. 1989, 1990). The reports show that the polymerizing enzyme of C. necator could really possess a wider range of substrate specificity. This was comprehended when the PHA synthase gene belonging to C. necator was expressed in a heterologous condition that can supply towards a broader variety of HA monomeric units. It was reported that the C. necator PHA synthase can integrate minor quantities of 3-hydroxyhexanoate (3HHx,) 3-hydroxyoctanoate (3HO) and 3-hydroxydodecanoate (3HDD) units (Dennis et al. 1998; Antonio et al. 2000).
Interestingly, a slight modification of aforementioned PHB biosynthetic route has been detected and represented by Rhodospirillum rubrum, where two additional enzymes (enoyl-CoA hydratases) are involved as NADH dependent acetoacetyl-CoA reductase catalyzed the reduction of acetoacetyl-CoA into S-(+)-3-hydroxybutyryl-CoA. These two enoyl-CoA hydratases then transform the resulting S-(+)-3-hydroxybutyryl-CoA isomer into R-(−)-3-hydroxybutyryl-CoA isomer (Moskowitz and Merrick 1969). This constitutes second type of PHA biosynthetic route.
The third kind of PHA biosynthetic route that found to exist in bacterial species/strains like Pseudomonas oleovorans, Pseudomonas aeruginosa as well as the most of pseudomonads from the rRNA homology group I contains the β-oxidation and thiolytic cleavage of fatty acids, i.e., 3-hydroxyacyl-CoA and intermediates of the β-oxidation pathways (Doi 1990; Punrattanasin 2001; Singh and Mallick 2009a). These bacterial species accumulate MCL-PHAs and hardly LCL-PHAs under supplementation of alcohols, alkanes or alkanoates. Also, they are usually not capable of accumulating SCL-PHAs similar to Cupriavidus necator. Under growth environments favouring PHA synthesis, the intermediates of the β-oxidation cycle could be transformed into R-(−)-3-hydroxyacyl-CoA by enoyl-CoA hydratases, epimerases or ketoacyl-CoA reductases and are then polymerized through the catalytic action of PHA synthase (Huisman et al. 1991).
The fourth PHA biosynthetic route occurs in Pseudomonas aeruginosa and PHA is synthesized from acetyl-CoA using fatty acid biosynthetic route (Huijberts et al. 1995; Steinbüchel 1996; Singh and Mallick 2008, 2009b). Some pseudomonads from the rRNA homology group I also synthesize MCL-PHAs including SCL-LCL-PHA co-polymer through this route (Singh et al. 2013). MCL-PHA/SCL-LCL-PHA co-polymer produce through this route includes unrelated organic carbon substrates such as glucose, ethanol, gluconate or acetate. Such organic carbon sources are initially employed towards the synthesis of fatty acids that then produce precursors for PHA polymerase for synthesizing MCL-PHA/SCL-LCL-PHA co-polymer (Singh and Mallick 2017a, b).
Nevertheless, similar to bacteria, cyanobacteria also possess PHA biosynthetic routes for PHAs production. Vincenzini and De Philippis (1999) have widely documented the presence of PHAs in almost ninety strains from four different phylogenetic subdivisions of cyanobacteria. The PHA biosynthetic routes of cyanobacterial species so far only reported to synthesize SCL-PHAs, i.e., PHB homopolymer and P(3HB-co-3HV) co-polymer (Singh et al. 2017). Apart from this, their PHA biosynthetic routes showed similarity with the bacteria (Wang et al. 2013).
14.6 Mechanism of PHAs Polymerization Reaction
The PhaC fits in the α/β-hydrolase family of enzymes, where it catalyzes the stereoselective transformation of the activated precursor (R)-3-hydroxyacyl-CoA into polyoxoesters along with the concomitant liberation of CoA (Peoples and Sinskey 1989b; Schubert et al. 1988; Slater et al. 1988). Commencement of PHA formation needs activation of the thiol group of the cysteine residue of the PhaC active site through the preserved histidine in the same active site, allowing a nucleophilic attack on the thioester bond of the (R)-3-hydroxyacyl-CoA (HA-CoA) substrate, concomitantly liberating CoA and producing a covalent enzyme-substrate intermediate (Fig. 14.2) (Wodzinska et al. 1996; Tian et al. 2005b). PHB synthase of C. necator occurs in aqueous solution as an equilibrium among monomeric and homodimeric enzyme molecules (Gerngross et al. 1994; Zhang et al. 2000). The earlier investigations exhibited that synthase activity was reversibly lost by dilution and the dimer depicts a considerably more specific activity compared to the monomeric form (Zhang et al. 2000). Besides, the experiential relationship between molecular weights of PHB and substrate: enzyme ratios is constant with a single polymeric chain being synthesized by the homodimer synthase that again suggests dimer is the active form of the enzyme (Zhang et al. 2000).
Model depicting the catalytic mechanism of PHA synthase (Rehm 2007)
The active-site model for PHA synthase was initially put forwarded by Ballard et al. (1987), where two thiol groups were proposed to be participated in locating the HA units. This model was later modified by Kawaguchi and Doi (1992) by suggesting that water might function as a chain transfer agent. The present theory of the reaction mechanism of PHA synthases is based on a model in which two thiolates participate towards the covalent catalysis of PHA polymer biosynthesis (Fig. 14.2) (Griebel et al. 1968). Biosynthesis of PHAs commences most possibly at thiolate groups [S1H and S2H] furnished by the PHA synthases [E], where one thiol group acts for a loading site and the second thiol group acts as a priming and elongation. Based upon this present model (Müh et al. 1999; Ballard et al. 1987; Kawaguchi and Doi 1992), the reaction mechanism involves the following steps: one thiol group [S1H] accepts a hydroxyalkanoic acid from CoA thioester with the latter becoming covalently linked to this thiol group [E-S1-CO-Alkyl-OH] and the CoA being liberated, while the developing polymer chain is linked with the second thiol group [E-S2-poly(HA)-OH]. The latter is then transferred to the free hydroxyl group upon a nucleophilic attack of the hydroxyl oxygen atom on the carbonyl carbon atom that give rise to [E-S1-poly(HA)n+1-OH]. A successive transesterification of the lengthened polymer chain from S1 to S2 give rise to [E-S2-poly(HA)n+1-OH] and [E-S1H] and the latter can now receive the next hydroxyalkanoic acid from a CoA thioester. This catalytic reaction cycle essentially is repeated for many thousand times as otherwise the extremely high molecular masses of the synthesized polymers cannot be explained. Furthermore, the catalytic reaction cycle might be interrupted if the developing polymer chain is liberated from [E-S2-poly(HA)-OH] by the nucleophilic attack of a hydroxyl group of a molecule that is not linked to [E] like water. For instance, there is report that hydroxy-compounds such as polyethylene glycol, glycerol or 1,3-propanediol also furnish such a chain terminating hydroxyl group that results in a PHA molecule, which possesses the corresponding hydroxy-compounds covalently connected with the PHAs (Madden et al. 1999; Shah et al. 2000). Report also depicted an enhanced PhaC copy number with a reduction in PHAs chain length, which signifying that the quantity of PhaC in a host cell has a function in regulating chain length of PHA (Sim et al. 1997). This general mechanism illustrated more clearly with the polymerization reaction of PHB, where the histidine (His) deprotonates the cysteine (Cys) to produce the active site thiolate that reacts with (R)-3-hydroxybutyryl-CoA (HB-CoA). This reaction resulted into the acylation of PHB synthase with hydroxybutyrate (HB). A second HB-CoA then binds and the Asp acts as a common base to trigger its hydroxyl group for attack on the HB-PHB synthase thioester, liberating the developing (HB)n-SCoA chain inside the active site. This noncovalent intermediate then quickly reacylates the active site Cys, where the polymerization lasts till the polymer achieves a comparatively uniform molecular mass that varies with organism (Liebergesell and Steinbüchel 1992; Wodzinska et al. 1996; Jia et al. 2000, 2001; Rehm et al. 2002). Experimental evidence showed that chain termination taking place with the transfer of majority of the polymer chain to a second, surface-exposed amino acid that breaks the chain (Tian et al. 2005b).
The preserved residues Cys-319, aspartate (Asp)-480 and His-508 of the group I PHA synthase from C. necator were the first be studied with site-specific mutagenesis that furnished evidence of direct participation in covalent catalysis (Gerngross et al. 1994; Jia et al. 2001). Furthermore, the extremely preserved tryptophan (Trp)-425 was exchanged with alanine strongly decreasing the enzyme activity. Trp-425 has been proposed to be participated in protein-protein interaction through producing a hydrophobic surface towards dimerization of PhaC subunits (Gerngross et al. 1994). Likewise, the homologous amino acid residues Cys-149, His-331 and Asp-302 of PHA synthase from A. vinosum were subjected to site-specific mutagenesis that almost inhibited enzymatic activity. These reports strongly indicated an involvement in covalent catalysis (Jia et al. 2000). Furthermore, the preserved catalytic triad residues of group II PHA synthase of P. aeruginosa were investigated with site-specific mutagenesis in which Cys-296 as well as Asp-452 were reported to be important for the enzyme activity as was observed with the other groups of PHA synthases (Amara and Rehm 2003). Remarkably, substitution of the putative general base catalyst His-480 resulted in strongly impairment of enzymatic activity. However, exchange of the preserved cysteine as well as aspartic acid deactivated the enzyme. Thus, it is anticipated that this cysteine residue supplies one of the thiolate groups towards covalent catalysis. It is the merely cysteine residue that is preserved in any PHA synthase. As the present reaction mechanism of PHA synthase is based on the thoroughly characterized fatty acid synthase, therefore, it is postulated that two thiol groups are essential, where the second thiol was supposed to be available following post-translational modification by a phosphopantethine moiety (Wakil 1989; Chang and Hammes 1990; Gerngross et al. 1994). A likely candidate for this modification is the preserved serine residue at location 260 of the PHA synthase structural gene of C. necator. Nevertheless, existing findings have failed to support this theory for a specific post-translational modification (Rehm and Steinbüchel 1999). The suggested step including chain-transfer was confronted based on the hypothesis that the PHA synthase is not proficient of transferring to a new chain (Kawaguchi and Doi 1992; Gerngross and Martin 1995; Su et al. 2000). However, investigation utilizing the purified C. vinosum PHA synthase indicated a mechanism concerning a chain-transfer step (Liebergesell et al. 1994). Based on kinetic investigations studying the lag phase of the enzyme reaction, it is now suggested that in group I- and group II-PHA synthases, the two thiol groups are furnished by two Cys-319 residues situated on two subunits and that a dimer of PhaC is, hence, the smallest size of these PHA synthases. In this model, Cys-319 alternates between the function of the loading thiol as well as the role of the elongation thiol as aforementioned (Müh et al. 1999). This could certainly also be realistic for the group III PHA synthases. Nevertheless, investigation of the primary structures of all so far sequenced group III PHA synthases depicted a second extremely preserved cysteine residue that is for instance, Cys-130 in PhaC of C. vinosum (Rehm and Steinbüchel 1999). Sinskey and co-workers substituted this cysteine with an alanine in which they achieved an enzyme, which showed merely 0.003% of the activity of the wild-type enzyme (Müh et al. 1999). Overall, the α/β-hydrolase-based catalytic mechanism, mainly considering lipases and cysteine proteases, furnishes a good model for groups I-III of PHA synthases as strengthened by mutational study of the C. necator group I PHA synthase, the A. vinosum group III PHA synthase and the P. aeruginosa group II PHA synthase (Rehm 2003). Currently, no clear final inference can be drawn concerning the provision of the second thiol group towards the catalytic cycle of PHA synthases.
14.7 Mechanism of Biogenesis of PHA Granules
Interestingly, the biosynthesis of PHA granules is somewhat differ over the synthesis of a prokaryotic neutral lipid inclusion such as wax esters or triacylglycerols granules (Wältermann et al. 2005; Wältermann and Steinbüchel 2005). PHA synthase facilitated the template independent polymerization reaction from the corresponding HA-CoA. The PHA synthase is present in the cytoplasm of exponentially cultivated cells that have not yet synthesized PHAs (Haywood et al. 1989). On the other hand, C. necator and Allochromatium vinosum grown cells under environments appropriate for PHA biosynthesis, the PHA synthase is accompanying with the PHA granules as the developing PHA material is covalently associated with the enzymes throughout polymerization reaction, conferring amphiphilic property to the enzyme-polymer complex (Haywood et al. 1989; Gerngross et al. 1993; Liebergesell et al. 1994). Three models namely micelle formation of PHB synthases, budding of PHB from the cytoplasm membrane and scaffold model so far have been proposed for in vivo PHA granule formation (Stubbe and Tian 2003; Tian et al. 2005a, b). They are based on hypothetical considerations as well as experimental data of C. necator. The Micelle Model based on the fact that soluble (cytoplasmic) PHB synthase molecules (PhaC1 dimers) begin to form the hydrophobic PHB polymer if the substrate (HB-CoA) quantity is adequately high (Ellar et al. 1968; Griebel et al. 1968; Gerngross et al. 1993). Owing to the hydrophobic behavior along with lower dissolution of PHB in an aqueous environment, nascent polymeric chains assembled and produce micelle-like structures within the cytoplasm with partly hydrophilic PhaC molecules residing on the polymer surface (Stubbe and Tian 2003; Stubbe et al. 2005). Later, phasins together with other PHB granule-associated proteins (PGAPs) attach with the developing granules. An importance of this model is that commencement of PHB granules should occur at any place within the cytoplasm and these granules usually should be essentially randomly localized within the cell. In budding model, the synthases would be peripheral membrane bound proteins, attached to the inner face of the plasma membrane. Their attachment to the membrane could be assisted by a primer molecule mainly a long chain fatty acid or oligomers of 3-HB covalently bonded to the active site cysteine of the synthase. As the PHB chains grow and the PhaP phasin is formed, budding of a vesicle with a monolayer or partial monolayer of lipid could result, leading to granule formation. At least, the initial step of PHB granules should localize in/at the cytoplasmic membrane if the budding model is correct. This model similar to the model for the biosynthesis of eukaryotic neutral lipid bodies (Wältermann et al. 2005). Nevertheless, this budding model neglects the fact that PhaC as well as phasins are never situated at the cytoplasmic membrane. Tian et al. (2005a, b) proposed a third model for granule formation called scaffold model. This model is based on the fact that PHB synthase of nascent PHB granules is or becomes linked to a yet unknown scaffold molecule within the cell. Also, the subcellular localization of PHB granules in this case would depend on the nature and localization of the scaffold of the PHB producing cell. Jendrossek and Pfeiffer (2014) suggested substituting the earlier micelle mode of PHB granule formation with the scaffold model. This is presently the most accepted model to explain the existing experimental data on PHB carbonosome synthesis in C. necator. For instance, the TEM investigations depicted that PHB granules in the cells, which were cultivated under environments permissive for PHB formation but not for growth (high carbon content, low or no nitrogen source) were often situated relatively at the centre of the cells in close neighbourhood to dark-stained known as mediation elements (Tian et al. 2005a, b). These findings were in agreement with the scaffold model, nevertheless, the nature of the scaffold (mediation element) remained ambiguous. Furthermore, the same group studied the PHB formation in C. necator with cryotomography (Beeby et al. 2012), where it was in agreement with their earlier TEM investigations. The budding model of PHB granule synthesis was excluded in this study owing to the high resolution of cryotomography. Nevertheless, a clear differentiation between the scaffold as well as micelle model was not probable. As the nucleoid area was not evident in Beeby’s cryotomograms, the data do not exclude the probability that the nucleoid signifies the scaffold to which PHB granules are attached. Further studies should be carried out to specify the areas of PHB granule attachment and whether there is a specificity of the DNA locus to which the granules become attached (Jendrossek and Pfeiffer 2014).
14.8 Phasins: Major PHA Granule-Associated Proteins
In PHA synthesizing bacteria, PHA chains are aggregated and form PHA granules within the cytoplasm. These granules are surrounded by different protein molecules known as PHA granule-associated proteins (PGAPs) in which phasins (PhaPs) are composed of small size amphiphilic protein molecules and found to be foremost PGAPs commonly dispersed in different PHA accumulating organisms (Wieczorek et al. 1995, 1996; Pötter and Steinbüchel 2005; Jendrossek and Pfeiffer 2014). The roles of PhaPs are believed to regulate the properties of PHA granules surface apart from the effect upon PHA biosynthesis. The PhaP1Re is the most plentiful phasin of R. eutropha H16 (Wieczorek et al. 1995). The expression level is extremely high because of the strong promoter, i.e., phaP1 through which transcription is controlled as a result of PHA granule binding transcriptional factor PhaRRe (Pötter et al. 2002; Pötter and Steinbüchel 2005; Brigham et al. 2010; Shimizu et al. 2013). The PhaP1Re contributed about 5% of the overall protein molecules of the crude extract of the cells grown on fructose as a carbon source and was anticipated to surround 27–54% of exterior portion of the polymer granules (Wieczorek et al. 1995; Tian et al. 2005c). It was established that PhaP1Re played important role in regulating the size as well as number of polymer granules within the cells including resulting quantity of PHAs (Wieczorek et al. 1995; York et al. 2001b). Remarkably, PhaP1Re failed to bind PhaC1Re straightforwardly in two hybrid assays, nevertheless, produced a high molecular mass complex in the company of PhaC1Re and soluble PHB oligomer in R. eutropha. The PhaC1Re-PhaP1Re-PHB complex exhibited no lag phase in PhaC activity assay that signified that PhaCRe had an active form in the complex (Pfeiffer and Jendrossek 2011; Cho et al. 2012). A comparable reduction in activity was also observed for the synthase belonging to D. acidovorans (PhaCDa) (Ushimaru et al. 2014). In contrast, PhaP1Re enhances the activity for the synthases belonging to A. caviae and P. aeruginosa by decreasing the enzymatic lag phase, though it does not influence the activity of PhaC belonging to C. vinosum (Jossek et al. 1998; Qi et al. 2000; Ushimaru et al. 2014). In addition, various other phasin proteins have been reported in other microorganisms like Sinorhizobium meliloti, Haloferax mediterranii or Herbaspirillum seropedicae. However, there is little information about them apart from their function towards polymer accumulation (Wang et al. 2007; Cai et al. 2012; Tirapelle et al. 2013; Alves et al. 2016). Furthermore, the investigation regarding the secondary structure of the PhaP1Re sequence forecasts a highly α-helical conformation, which is characteristic of phasins. The phasin has been revealed to attain a planar, triangular shaped homotrimeric conformation as depicted by small angle X-ray scattering study. First sequence studies did not reveal a clear, predicted PHA binding motif like long hydrophobic patches (Neumann et al. 2008).
Currently, PHA granule accompanying proteins have received more attention as various research laboratories have revealed that defective or deficient phasins depict considerable impacts on PHA polymer formation. For instance, Tn5-induced phaP1 mutants having defective phasin production are even then capable to form PHB polymer, nevertheless, they produce polymer at a substantially lower rate and nearly the whole PHB is exist in merely one single big granule within the cell (Wieczorek et al. 1995). Likewise, studies conducted with the gene deletion of phaP1 established that the PHB quantity under specific culture cultivation condition was decreased by 50% compared to the wild-type C. necator (York et al. 2001b). However, overexpression of PhaP1Re resulting into enhance granule numbers (Pötter et al. 2002). Furthermore, investigations were also carried out with the phaR deletion mutant strain of C. necator, where PhaP was constitutively expressed at high levels. Nevertheless, no PhaP is formed if phaC is deleted (Wieczorek et al. 1995; York et al. 2001a, b). Notably, deletion of together phaR as well as phaC found to trigger high levels of PhaP expression, even though the organism fails to synthesize PHB polymer.
The R. eutropha phasins also play a role in the stability as well as mobilization of PHB inclusions. Absence of PhaP1Re in a single deletion mutant results a certain degree of PHB autodegradation in vivo, a phenomenon, which is dramatically increased when integrated with the multiple deletion of other phasins, signifying that phasins are important towards stabilization of the granule (Kuchta et al. 2007). Surprisingly, phasins are also essential towards the PHB mobilization triggered by CoA thiolysis that catalyzed by the PhaZ depolymerase. However, PHB lacking phasins is fail to undergo depolymerization by PhaZ, the PhaP1Re alone is enough to help the depolymerase in the process of PHB degradation (Uchino et al. 2007; Eggers and Steinbuchel 2013). In contrast, in the lack of PhaP1Re, the other minor phasins could also play role in PHB mobilization to a variable level (Kuchta et al. 2007; Uchino et al. 2007; Eggers and Steinbuchel 2013). The expression of PhaP1 is stringently controlled at the transcription level by PhaR, therefore, confirming that the phasin is formed merely when environments are permissive towards PHB formation and PhaC is present and in sufficient amount to surround all the surface of biopolymer, but without triggering a protein stock in the cytoplasm (York et al. 2001a; Pötter et al. 2002; York et al. 2002; Wieczorek et al. 1995).
In order to presume a model concerning the regulation of phasin expression, further investigations were carried out with C. necator. It was established that PhaR might attach with artificial PHB granules (Pötter et al. 2002). Moreover, Western immunoblotting as well as immunoelectron microscopic localization with antibodies produced against PhaR undoubtedly revealed that PhaR was attached with the PHB granules. Hence, PhaR has the capability to attach at least to four diverse receptors of the C. necator viz. the promoter regions of phaP1 (1) phaP3 (2) and phaR (3) and the surface of PHB granules (4). These studies support the simple and elegant model towards the regulation of expression of PhaP in C. necator, where PhaR playing the role as a repressor of transcription. Under cultivation environments not appropriate towards PHAs formation or in mutants faulty for PHAs accumulation, PhaR attaches with the phaP promoter region and represses transcription of this gene. The concentration of PhaR in cytoplasm is adequately high to repress transcription of phaP1 and phaP3. However, under physiological environments that are apposite towards PHB accumulation, the constitutively expressed PHA synthase begins to produce PHB polymers that remain covalently associated with this enzyme. At the commencement, tiny micelles are synthesized that become bigger and form the nascent PHB granules. Proteins with a binding capability with the hydrophobic surfaces such as PhaR attach with the granules. However, the concentration of PhaR in cytoplasm from a certain point turn out to be sufficiently low, where it no longer repressed phaP1 as well as phaP3 transcription. Thus, PhaP1 as well as PhaP3 were formed, which then attached with the exterior portion of PHB granules. The PHB granules turn out to be larger and achieved their maximum size. Concurrently, PhaP1 was found to produce uninterruptedly in adequate quantities. Furthermore, little quantities of PhaP3 were also produced. Under to the physiological conditions when the PHB granules have attained the maximum probable size and when majority of the surface of PHB granule is surrounded with PhaP1, no more space will be accessible towards the attachment of additional PhaR or PhaR may even be displaced with PhaP1 (and PhaP3). Therefore, the amount of PhaR in cytoplasm will increase and surpass the threshold amount needed to repress again phaP1 as well as phaP3 transcription. As a result, PhaP1 as well as PhaP3 protein molecules are as no longer formed and these phasins are consequently, not produced too much and therefore, do not surpass the quantity needed to surround the exterior part of PHB granules (Pötter et al. 2005).
Phasins offer a diverse opportunity in the field of Biotechnology (Maestro and Sanz 2017). For instance, the amphiphilic property of phasins makes them appropriate to be exploited as natural biosurfactants. In this regard, pure recombinant PhaPAh belonging to A. hydrophila 4AK4 depicts a strong effect to form emulsions with lubricating oil, soybean oil and diesel when compared to sodium dodecylsulfate, bovine serum albumin, Tween 20 or sodium oleate, even maintaining its activity after heat treatment of the protein or the emulsions themselves (Wei et al. 2011). The most extensively studied application of phasins results from their PHA binding capability. In this sense, the N-terminal, PHA binding domain of PhaF of P. putida GPo1 known as BioF sequence has revealed to be extremely efficient as an affinity tag to immobilize in vivo fusion proteins exploiting mcl-PHA as support (Moldes et al. 2004, 2006). PHA granules having BioF tagged fusion proteins can be simply isolated through centrifugation and utilized directly or if needed, the purification of the adsorbed protein can be attained via gentle elution with detergents, keeping their overall activity in both cases (Moldes et al. 2004). This approach has been shown to be an environment friendly for delivering active proteins to the environment like the Cry1Ab toxin with insecticidal activity (Moldes et al. 2006). Analogous in vivo immobilization approaches have also been established for PhaP1Re exploiting E. coli as heterologous host towards the PHA synthesis (Chen et al. 2014). Here, the gene coding for the D-hydantoinase (DHDT) (enzyme participated in the production of D-amino acids of commercial values like one of the precursors needed towards the formation of semi-synthetic antibiotics) was fused with phaP1. The recombinant fusion protein, PhaP1Re-HDT, resulted to be efficiently attached with the granules and the enzyme depicted to be active and stable (Chen et al. 2014). Apart from this, the specific immobilization of fusion proteins to PHA through phasins is initiated to be used in medicine, both in diagnostic as well as drug delivery uses (Backstrom et al. 2007; Yao et al. 2008; Dong et al. 2010). For instance, two hybrid genes responsible for coding either the mouse interleukin-2 or the myelinoligodendrocyte glycoprotein fused to PhaP1Re were constructed and expressed in a recombinant PHAs producing E. coli strain. The PHA beads achieved from this approach exhibited the eukaryotic proteins appropriately folded and they were then employed for specific and sensitive antibody detection by the fluorescence-activated cell sorting technology (Backstrom et al. 2007). On the other hand, Yao et al. (2008) carried out investigation in which two recombinant fusion proteins with PhaP1Re were produced to attain specific delivery: mannosylated human α1-acid glycoprotein (hAGP), which is capable to bind with the mannose receptor of macrophages and a human epidermal growth factor (hEGF), capable to identify EGF receptors on carcinoma cells. The resulting proteins (rhAGP-PhaP1Re and rhEGF-PhaP1Re) were self-assembled upon the co-polymer of 3-hydroxybutyrate and 3-hydroxyhexanoate [P(3HB-co-3HHx)] nanoparticles, attaining the specific delivery of the payload both in vitro and in vivo. Furthermore, the sequence coding of a peptide having the amino acids Arg-Gly-Asp, the most effective peptide sequence employed to improve cell adhesion on artificial surfaces, was fused to PhaP (Dong et al. 2010). Diverse polyesters like P(3HB-co-3HHx) or 3-hydroxybutyrate and 3-hydroxyvalerate [P(3HB-co-3HV)] co-polymers were coated with purified PhaP-RGD hybrid protein and the complex showed efficient in adhesion as well as improvement of cell growth on two diverse fibroblast cellular lines, signifying feasible uses on implant biomaterials (Dong et al. 2010).
14.9 Conclusion
Polyhydroxyalkanoates (PHAs) signify a complex group of storage polymeric materials, which are produced as insoluble inclusions in the cytoplasm of bacteria and cyanobacteria. Usually, PHA polymer accumulation is stimulated under the supplementation of excess organic carbon substrate together with nutrient limitation/deficiency like phosphorus, nitrogen etc. The key enzymes of PHAs biosynthesis are the PHA synthases that catalyze the enantio-selective polymerization of (R)-hydroxyacyl-CoA thioesters into polyesters. Different metabolic pathways have been identified in bacteria and cyanobacteria to furnish substrate for PHA synthases. PHAs are surrounded with a proteinaceous surface coat known as PHA granule associated proteins (PGAPs), which conforming a network like surface of structural, metabolic as well as regulatory polypeptides including configuring the PHA granules as complex and well organized subcellular structures called ‘carbonosomes’. PGAPs consist of many enzymes like PHA synthases and PHA depolymerases that associated with PHA metabolism. In addition, they also involve small size proteins devoid of catalytic functions called phasins, which surround most of the PHA granule. Phasins play an important role in the physical stabilization of the PHA granule within the cell, ensure the correct distribution of the polymer upon cell division and assist other proteins (synthases and depolymerases) in PHA metabolism. Nevertheless, their specific role is highly dependent both on the microbial strain and on the metabolic state of the cell. Structurally, phasins are amphiphilic proteins which protect the hydrophobic polymer from the cytoplasm. Phasins receiving consideration interest currently as they open up new possibilities of applications such as biosurfactants, diagnostic and drug delivery uses. The most recent investigations revealed that the PHA granules have no phospholipids in vivo and hypothesized that the PHB or PHA granule surface coats in natural producers normally are devoid of phospholipids and merely composed of proteins. In order to draw final conclusion regarding the provision of the second thiol group for the catalytic cycle of PHA synthases, further investigations need to be carried out. The scaffold model, where PHB synthase-PhaM complexes are associated with the bacterial nucleoid and form the PHB granule initiation complex, is presently more accepted model to explain the existing experimental data on PHB carbonosome production in C. necator. However, future studies should be conducted to specify the areas of PHB granule association and whether there is a specificity of the DNA locus to which the granules become associated.
References
Abe C, Taima Y, Nakamura Y, Doi Y (1990) New bacterial copolyester of 3-hydroxyalkanoates and 3-hydroxy-ω-fluoroalkanoates produced by Pseudomonas oleovorans. Polym Commun 31:404–406
Aeschelmann F, Carus M, Baltus W (2015) Bio-based building blocks and polymers in the world, capacities, production and applications: status quo and trends towards 2020 (www.biobased.eu/markets)
Alexander M (1981) Biodegradation of chemicals of environmental concern. Science 211:132–138
Ali I, Jamil N (2016) Polyhydroxyalkanoates: current applications in the medical field. Front Biol 11:19–27
Alves LP, Teixeira CS, Tirapelle EF, Donatti L, Tadra-Sfeir MZ, Steffens MB, de Souza EM, de Oliveira Pedrosa F, Chubatsu LS, Müller-Santos M (2016) Backup expression of the PhaP2 phasin compensates for phaP1 deletion in Herbaspirillum seropedicae, maintaining fitness and PHB accumulation. Front Microbiol 7:739
Amara AA, Rehm BHA (2003) Replacement of the catalytic nucleophile cysteine-296 by serine in class II polyhydroxyalkanoate synthase from Pseudomonas aeruginosa-mediated synthesis of a new polyester: identification of catalytic residues. Biochem J 374:413–421
Anderson AJ, Dawes EA (1990) Occurrence, metabolism, metabolic role, and industrial uses of bacterial polyhydroxyalkanoates. Microbiol Rev 54:450–472
Ansari S, Yasin D, Fatma T (2016) Key insights of natural bioplastic polyhyroxybutyrate (PHB) synthesis in cyanobacteria. Am J PharmTech Res
Antonio RV, Steinbüchel A, Rehm BH (2000) Analysis of in vivo substrate specificity of the PHA synthase from Ralstonia eutropha: formation of novel copolyesters in recombinant Escherichia coli. FEMS Microbiol Lett 182:111–117
Asada Y, Miyake M, Miyake J, Kurane R, Tokiwa Y (1999) Photosynthetic accumulation of poly-(hydroxybutyrate) by cyanobacteria- the metabolism and potential for CO2 recycling. Int J Biol Macromol 25:37–42
Ashby RD, Solaiman DKY, Foglia TA (2002) The synthesis of short and medium chain-length poly(hydroxyalkanoate) mixtures from glucose- or alkanoic acid-grown Pseudomonas oleovorans. J Ind Microbiol Biotechnol 28:147–153
Backstrom BT, Brockelbank JA, Rehm BH (2007) Recombinant Escherichia coli produces tailor-made biopolyester granules for applications in fluorescence activated cell sorting: functional display of the mouse interleukin-2 and myelin oligodendrocyte glycoprotein. BMC Biotechnol 7:3. https://doi.org/10.1186/1472-6750-7-3
Ballard DGH, Holmes PA, Senior PJ (1987) Formation of polymers of β-hydroxybutyric acid in bacterial cells and a comparison of the morphology of growth with the formation of polyethylene in the solid state. In: Fontanille M, Guyot A (eds) Recent advances in mechanistic and synthetic aspects of polymerization. Reidel, Kluwer, pp 293–314
Beeby M, Cho M, Stubbe J, Jensen GJ (2012) Growth and localization of polyhydroxybutyrate granules in Ralstonia eutropha. J Bacteriol 194:1092–1099
Bernard M (2014) Industrial potential of polyhydroxyalkanoate bioplastic: a brief review. Univ Saskatchewan Undergraduate Res J 1:1–14
Bhati R, Mallick N (2012) Production and characterization of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) co-polymer by a N2-fixing cyanobacterium, Nostoc muscorum Agardh. J Chem Technol Biotechnol 87:505–512
Bhati R, Mallick N (2015) Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) copolymer production by the diazotrophic cyanobacterium Nostoc muscorum Agardh: process optimization and polymer characterization. Algal Res 7:78–85
Bhati R, Mallick N (2016) Carbon dioxide and poultry waste utilization for production of polyhydroxyalkanoate biopolymers by Nostoc muscorum Agardh: a sustainable approach. J Appl Phycol 28:161–168
Bhati R, Samantaray S, Sharma L, Mallick N (2010) Poly-β-hydroxybutyrate accumulation in cyanobacteria under photoautotrophy. Biotechnol J 5:1181–1185
Bian YZ, Wang Y, Aibaidoula G, Chen GQ, Wu Q (2009) Evaluation of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) conduits for peripheral nerve regeneration. Biomaterials 30:217–225
Borah B, Thakur PS, Nigam JN (2002) The influence of nutritional and environmental conditions on the accumulation of poly-β-hydroxybutyrate in Bacillus mycoides RLJ B-017. J Appl Microbiol 92:776–783
Braunegg G, Gilles L, Klaus F (1998) Polyhydroxyalkanoates biopolyesters from renewable resources: physiological and engineering aspects. J Biotechnol 65:127–161
Bresan S, Sznajder A, Hauf W, Forchhammer K, Pfeiffer D, Jendrossek D (2016) Polyhydroxyalkanoate (PHA) Granules have no phospholipids. Sci Rep 6:26612
Brigham CJ, Sinskey AJ (2012) Applications of polyhydroxyalkanoates in the medical industry. Int J Biotechnol Wellness Ind 1:53–60
Brigham CJ, Budde CF, Holder JW, Zeng Q, Mahan AE, Rha C, Sinskey AJ (2010) Elucidation of β-oxidation pathways in Ralstonia eutropha H16 by examination of global gene expression. J Bacteriol 192:5454–5464
Byrom D (1992) Production of poly-β-hydroxybutyrate and poly-β-hydroxyvalerate copolymers. FEMS Microbiol Rev 103:247–250
Byrom D (1994) Polyhydroxyalkanoate. In: Mobley DP (ed) Plastics from microbes: microbial synthesis of polymers and polymer precursors. Hanser, Munich, pp 5–33
Cai S, Cai L, Liu H, Liu X, Han J, Zhou J, Xiang H (2012) Identification of the haloarchaeal phasin (PhaP) that functions in polyhydroxyalkanoate accumulation and granule formation in Haloferax mediterranei. Appl Environ Microbiol 78:1946–1952
Campbell J, Stevens SE Jr, Bankwill DL (1982) Accumulation of poly-β-hydroxybutyrate in Spirulina platensis. J Bacteriol 149:361–366
Carr NG (1966) The occurrence of poly-β-hydroxybutyrate in the blue-green alga, Chlorogloea fritschii. Biochem Biophys Acta 120:308–310
Castilho LR, Mitchell DA, Freire DMG (2009) Production of polyhydroxyalkanoates (PHAs) from waste materials and by-products by submerged and solid-state fermentation. Biores Technol 100:5996–6009
Chandel AK, Garlapati VK, Singh AK, Antunes FAF, da Silva SS (2018) The path forward for lignocellulose biorefineries: bottlenecks, solutions, and perspective on commercialization. Biores Technol. https://doi.org/10.1016/j.biortech.2018.06.004 (in press)
Chang SI, Hammes GG (1990) Structure and mechanism of action of a multifunctional enzyme: fatty acid synthase. Acc Chem Res 23:363–369
Chen GQ (2009) A microbial polyhydroxyalkanoates (PHA) based bio- and materials industry. Chem Soc Rev 38:2434–2446
Chen G-Q (2010) Plastics completely synthesized by bacteria: polyhydroxyalkanoates. In: Chen G-Q (ed) Plastics from bacteria: natural functions and applications. Microbiology monographs. Springer, Berlin, pp 17–38
Chen GQ, Wu Q (2005a) Microbial production and applications of chiral hydroxyalkanoates. Appl Microbiol Biotechnol 67:592–599
Chen GQ, Wu Q (2005b) The application of polyhydroxyalkanoates as tissue engineering materials. Biomaterials 26:6565–6578
Chen GQ, Zhang G, Park SJ, Lee SY (2001) Industrial scale production of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate). Appl Microbiol Biotechnol 57:50–55
Chen SY, Chien YW, Chao YP (2014) In vivo immobilization of d-hydantoinase in Escherichia coli. J Biosci Bioeng 118:78–81
Cho M, Brigham CJ, Sinskey AJ, Stubbe J (2012) Purification of polyhydroxybutyrate synthase from its native organism, Ralstonia eutropha: implications for the initiation and elongation of polymer formation in vivo. Biochemistry 51:2276–2288
Choi MH, Yoon SC (1994) Polyester biosynthesis characteristics of Pseudomonas citronellolis grown on various carbon sources, including 3-methyl-branched substrates. Appl Environ Microbiol 60:3245–3254
Choi J, Lee SY (1999) Factors affecting the economics of polyhydroxyalkanoate production by bacterial fermentation. Appl Microbiol Biotechnol 51:13–21
Curley JM, Hazer B, Lenz RW (1996) Production of poly(3-hydroxyalkanoates) containing aromatic substituents by Pseudomonas oleovorans. Macromolecules 29:1762–1766
De Koning GJM, Maxwell IA (1993) Biosynthesis of poly-(R)-3-hydroxyalkanoate: an emulsion polymerization. J Environ Polym Degrad 1:223–226
de Koning GJM, van Bilsen HMM, Lemstra PJ, Hazenberg W, Witholt B, Preusting H, van der Galien JG, Schirmer A, Jendrossek D (1994) A biodegradable rubber by crosslinking poly(hydroxyalkanoate) from Pseudomonas oleovorans. Polymer 35:2090–2097
De Morais MG, Stillings C, Roland D, Rudisile M, Pranke P, Costa JAV, Wendorff J (2015) Extraction of poly(3-hydroxybutyrate) from Spirulina LEB 18 for developing nanofibers. Polímeros 25:161–167
Dennis D, McCoy M, Stangl A, Valentin HE, Wu Z (1998) Formation of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) by PHA synthase from Ralstonia eutropha. J Biotechnol 64:177–186
DiGregorio BE (2009) Biobased performance bioplastic. Mirel Chem Biol 16:1–2
Doi Y (1990) Microbial polyesters. VCH Publishers, New York
Doi Y, Abe C (1990) Biosynthesis and characterization of a new bacterial copolyester of 3-hydroxyalkanoates and 3-hydroxy-ω-chloroalkanoates. Macromolecules 23:3705–3707
Doi Y, Tamaki A, Kunioka M, Soga K (1987) Biosynthesis of terpolyesters of 3-hydroxybutyrate, 3-hydroxyvalerate, and 5-hydroxyvalerate in Alcaligenes eutrophus from 5-chloropentanoic and pentanoic acids. Makromol Chem Rapid Commun 8:631–635
Doi Y, Segawa A, Kunioka M (1989) Biodegradable poly(3-hydroxybutyrate-co-4-hydroxybutyrate) produced from gamma-butyrolactone and butyric acid by Alcaligenes eutrophus. Polym Commun 30:169–171
Doi Y, Segawa A, Kunioka M (1990) Biosynthesis and characterization of poly(3-hydroxybutyrate-co-4-hydroxybutyrate) in Alcaligenes eutrophus. Int J Biol Macromol 12:106–111
Doi Y, Kitamura S, Abe H (1995) Microbial synthesis and characterization of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate). Macromolecules 28:4822–4828
Dong Y, Li P, Chen CB, Wang ZH, Ma P, Chen GQ (2010) The improvement of fibroblast growth on hydrophobic biopolyesters by coating with polyhydroxyalkanoate granule binding protein PhaP fused with cell adhesion motif RGD. Biomaterials 31:8921–8930
Doug S (2010) Bioplastics: technologies and global markets. BCC research reports PLS050A
Drosg B, Fritz I, Gattermayr F, Silvestrini L (2015) Photo-autotrophic production of poly(hydroxyalkanoates) in cyanobacteria. Chem Biochem Eng Q 29:145–156
Eggers J, Steinbuchel A (2013) Poly(3-hydroxybutyrate) degradation in Ralstonia eutropha H16 is mediated stereoselectively to (S)-3-hydroxybutyryl coenzyme A (CoA) via crotonyl-CoA. J Bacteriol 195:3213–3223
Eggink G, van der Wal H, Huijberts GNM, de Waard P (1993) Oleic acids as a substrate for poly-3-hydroxyalkanoate formation in Alcaligenes eutrophus and Pseudomonas putida. Ind Crops Prod 1:157–163
Eggink G, de Waard P, Huijberts GNM (1995) Formation of novel poly(hydroxyalkanoates) from long-chain fatty acids. Can J Microbiol (Suppl) 41:14–21
Ellar D, Lundgren DG, Okamura K, Marchessault RH (1968) Morphology of poly-beta-hydroxybutyrate granules. J Mol Biol 35:489–502
Fritzsche K, Lenz RW, Fuller R (1990a) An unusual bacterial polyester with a phenyl pendant group. Macromol Chem 191:1957–1965
Fritzsche K, Lenz RW, Fuller RC (1990b) Production of unsaturated polyesters by Pseudomonas oleovorans. Int J Biol Macromol 12:85–91
Fritzsche K, Lenz WR, Fuller RC (1990c) Bacterial polyesters containing branched poly(β-hydroxyalkanoate) units. Int J Biol Macromol 12:92–101
Fukui T, Doi Y (1998) Efficient production of polyhydroxyalkanates from plant oils by Alcaligenes eutrophus and its recombinant strain. Appl Microbiol Biotechnol 49:333–336
Gagnon KD, Lenz RW, Farris RJ, Fuller RC (1994a) Chemical modification of bacterial elastomers. 1. Peroxide crosslinking. Polymer 35:4358–4367
Gagnon KD, Lenz RW, Farris RJ, Fuller RC (1994b) Chemical modification of bacterial elastomers. 2. Sulfur vulcanization. Polymer 35:4368–4375
Gao X, Yuan XX, Shi ZY, Shen XW, Chen JC, Wu Q, Chen GQ (2012) Production of copolyesters of 3-hydroxybutyrate and medium-chain-length 3-hydroxyalkanoates by E. coli containing an optimized PHA synthase gene. Microb Cell Fact 11:130
Gerngross TU, Martin DP (1995) Enzyme-catalyzed synthesis of poly[(R)-(2)-3-hydroxybutyrate]: formation of macroscopic granules in vitro. Proc Natl Acad Sci 92:6279–6283
Gerngross TU, Reilly P, Stubbe J, Sinskey AJ, Peoples OP (1993) Immunocytochemical analysis of poly-β-hydroxybutyrate (PHB) synthase in Alcaligenes eutrophus H16: localization of the synthase enzyme at the surface of the of PHB granules. J Bacteriol 175:5289–5293
Gerngross TU, Snell KD, Peoples OP, Sinskey AJ, Csuhai E, Masamune S, Stubbe J (1994) Overexpression and purification of the soluble polyhydroxyalkanoate synthase from Alcaligenes eutrophus: evidence for a required posttranslational modification for catalytic activity. Biochemistry 33:9311–9320
Global Polyhydroxyalkanoate (PHA) Market (Sources, Applications, Geography)—Size, Share, Global Trends, Company Profiles, Demand, Insights, Analysis, Research, Report, Opportunities, Segmentation and Forecast, 2012–2020. Retrieved from http://www.reportsandintelligence.com/polyhydroxyalkanoate-market
Global Trends and Forecasts to 2018—Polyhydroxyalkanoate (PHA) Market, By Application (Packaging, Food Services, Bio-medical, Agriculture) & Raw Material. Retrieved from http://www.marketsandmarkets.com
Gómez Cardozo JR, Mora Martínez AL, Yepes Pérez M, Correa Londoño GA (2016) Production and characterization of polyhydroxyalkanoates and native microorganisms synthesized from fatty waste. Int J Polym Sci 2016:6541718. https://doi.org/10.1155/2016/6541718
Gouda MK, Swellam AE, Omar SH (2001) Production of PHB by a Bacillus megaterium strain using sugarcane molasses and corn steep liquor as sole carbon and nitrogen sources. Microbiol Res 156:201–207
Grage K, Jahns AC, Parlane N, Palanisamy R, Rasiah IA, Atwood JA, Rehm BH (2009) Bacterial polyhydroxyalkanoate granules: biogenesis, structure, and potential use as nano-/micro-beads in biotechnological and biomedical applications. Biomacromolecules 10:660–669
Griebel RJ, Merrick JM (1971) Metabolism of poly-β-hydroxybutyrate: effect of mild alkaline extraction on native poly-β-hydroxybutyrate granules. J Bacteriol 108:782–789
Griebel R, Smith Z, Merrick JM (1968) Metabolism of poly-β-hydroxybutyrate. I. Purification, composition and properties of native poly-β-hydroxybutyrate granules from Bacillus megaterium. Biochemistry 7:3676–3681
Haase S, Huchzermeyer B, Rath T (2012) PHB accumulation in Nostoc muscorum under different carbon stress situations. J Appl Phycol 24:157–162
Hai T, Hein S, Steinbüchel A (2001) Multiple evidence for widespread and general occurrence of type-III PHA synthases in cyanobacteria and molecular characterization of the PHA synthases from two thermophilic cyanobacteria: Chlorogloeopsis fritschii PCC 6912 and Synechococcus sp. strain MA19. Microbiology 147:3047–3060
Hängii UJ (1990) Pilot scale production of PHB with Alcaligens latus. In: Dawes EA (ed) Novel biodegradable microbial polymers. Kluwer, Dordrecht, pp 60–65
Hauf W, Schlebusch M, Hüge J, Kopka J, Hagemann M, Forchhammer K (2013) Metabolic changes in Synechocystis PCC6803 upon nitrogen-starvation: excess NADPH sustains polyhydroxybutyrate accumulation. Metabolites 3:101–118
Haywood GW, Anderson AJ, Dawes AE (1989) The importance of PHB-synthase substrate specificity in polyhydroxyalkanoate synthesis by Alcaligenes eutrophus. FEMS Microbiol Lett 57:1–6
Hazenberg W, Witholt B (1997) Efficient production of medium chain-length poly(3-hydroxyalkanoates) from octane by Pseudomonas oleovorans: economic considerations. Appl Microbiol Biotechnol 48:588–596
Hazer B, Lenz RW, Fuller RC (1994) Biosynthesis of methylbranched poly(β-hydroxyalkanoate)s by Pseudomonas oleovorans. Macromolecules 27:45–49
Hein S, Tran H, Steinbüchel A (1998) Synechocystis sp. PCC6803 possesses a two-component polyhydroxyalkanoic acid synthase similar to that of anoxygenic purple sulfur bacteria. Arch Microbiol 170:162–170
Hoffmann N, Rehm BH (2004) Regulation of polyhydroxyalkanoate biosynthesis in Pseudomonas putida and Pseudomonas aeruginosa. FEMS Microbiol Lett 237:1–7
Hoffmann N, Rehm BH (2005) Nitrogen-dependent regulation of medium-chain length polyhydroxyalkanoate biosynthesis genes in pseudomonads. Biotechnol Lett 27:279–282
Huijberts GNM, de Rijk TC, de Ward P, Eggink G (1995) 13C nuclear magnetic resonance study of Pseudomonas putida fatty acid metabolic routes involved in poly(3-hydroxyalkanoate) synthesis. J Bacteriol 176:1661–1666
Huisman GW, de Leeuw O, Eggink G, Witholt B (1989) Synthesis of poly-3-hydroxyalkanoates is a common feature of fluorescent pseudomonads. Appl Environ Microbiol 55:1949–1954
Huisman GW, Wonink E, Meima R, Katzemier B, Terpstra P, Witholt B (1991) Metabolism of poly(3-hydroxyalkanoates) by Pseudomonas oleovorans: identification and sequences of genes and function of the encoded proteins in the synthesis and degradation of PHA. J Biol Chem 266:2191–2198
Huong KH, Shantini K, Sharmini R, Amirul AA (2017) Exploring the potential of 1-pentanol and oleic acid for optimizing the production of poly (3-hydroxybutyrate-co-3-hydroxyvalerate) copolymer by Cupriavidus sp. USMAA1020. Arab J Sci Eng 42:2313–2320
Jendrossek D (2009) Polyhydroxyalkanoate granules are complex subcellular organelles (carbonosomes). J Bacteriol 191:3195–3202
Jendrossek D, Pfeiffer D (2014) New insights in the formation of polyhydroxyalkanoate granules (carbonosomes) and novel functions of poly(3-hydroxybutyrate). Environ Microbiol 16:2357–2373
Jia Y, Kappock TJ, Frick T, Sinskey AJ, Stubbe J (2000) Lipases provide a new mechanistic model for polyhydroxybutyrate (PHB) synthases: characterization of the functional residues in Chromatium vinosum PHB synthase. Biochemistry 39:3927–3936
Jia Y, Yuan W, Wodzinska J, Park J, Sinskey AJ, Stubbe J (2001) Mechanistic studies on class I polyhydroxybutyrate (PHB) synthase from Ralstonia eutropha: class I and III synthases share a similar catalytic mechanism. Biochemistry 40:1011–1019
Jia K, Cao R, Hua DH, Li P (2016) Study of class I and class III polyhydroxyalkanoate (PHA) synthases with substrates containing a modified side chain. Biomacromol 17:1477–1485
Jossek R, Reichelt R, Steinbuchel A (1998) In vitro biosynthesis of poly(3-hydroxybutyric acid) by using purified poly(hydroxyalkanoic acid) synthase of Chromatium vinosum. Appl Microbiol Biotechnol 49:258–266
Jurasek L, Marchessault RH (2004) Polyhydroxyalkanoate (PHA) granule formation in Ralstonia eutropha cells: a computer simulation. Appl Microbiol Biotechnol 64:611–617
Kahar P, Tsuge T, Taguchi K, Doi Y (2004) High yield production of polyhydroxyalkanoates from soybean oil by Ralstonia eutropha and its recombinant strain. Polym Degrad Stabil 83:79–86
Kashiwaya Y, Takeshima T, Mori N, Nakashima K, Clarke K, Veech RL (2000) D-beta-hydroxybutyrate protects neurons in models of Alzheimer’s and Parkinson’s disease. Proc Natl Acad Sci USA 97:5440–5444
Kato M, Bao HJ, Kang C-K, Fukui T, Doi Y (1996) Production of novel copolyester of 3-hydroxybutyric acid and medium-chain-length 3-hydroxyalkanoic acids by Pseudomonas sp. 61–3 from sugars. Appl Microbiol Biotechnol 45:363–370
Kawaguchi Y, Doi Y (1992) Kinetics and mechanism of synthesis and degradation of poly(3-hydroxybutyrate) in Alcaligenes eutrophus. Macromolecules 25:2324–2329
Kim YB, Lenz RW, Fuller RC (1991) Preparation and characterization of poly(β-hydroxyalkanoates) obtained from Pseudomonas oleovorans grown with mixtures of 5-phenylvaleric acid and n-alkanoic acids. Macromolecules 24:5256–5360
Kim YB, Lenz RW, Fuller RC (1992) Poly(β-hydroxyalkanoate) copolymers containing brominated repeating units produced by Pseudomonas oleovorans. Macromolecules 25:1852–1857
Kim OY, Gross RA, Rutherford DR (1995) Bioengineering of poly(β-hydroxyalkanoates) for advanced material applications: incorporation of cyano and nitrophenoxy side chain substituents. Can J Microbiol 41:32–43
Koller M, Maršálek L (2015) Cyanobacterial polyhydroxyalkanoate production: status quo and quo vadis?. Curr Biotechnol 4:464–480
Kuchta K, Chi L, Fuchs H, Pötter M, Steinbüchel A (2007) Studies on the influence of phasins on accumulation and degradation of PHB and nanostructure of PHB granules in Ralstonia eutropha H16. Biomacromol 8:657–662
Kumar A, Srivastava JK, Mallick N, Singh AK (2015) Commercialization of bacterial cell factories for the sustainable production of polyhydroxyalkanoate thermoplastics: progress and prospects. Recent Pat Biotechnol 9:4–21
Kyrikou I, Briassoulis D (2007) Biodegradation of agricultural plastic films: a critical review. J Polym Environ 15:125–150
Labuzek S, Radecka I (2001) Biosynthesis of PHB tercopolymer by Bacillus cereus UW85. J Appl Microbiol 90:353–357
Lageveen RG, Huisman GW, Preusting H, Ketelaar P, Eggink G, Witholt B (1988) Formation of polyesters by Pseudomonas oleovorans: effect of substrates on formation and composition of poly(R)-3-hydroxyalkanoates and poly(R)-3-hydroxyalkenoates. Appl Environ Microbiol 54:2924–2932
Lama L, Nicolaus B, Calandrelli V, Maria MC, Romano I, Gambacorta A (1996) Effect of growth conditions on endo- and exopolymer biosynthesis in Anabaena cylindrica 10 C. Phytochemistry 42:655–659
Langenbach S, Rehm BH, Steinbüchel A (1997) Functional expression of the PHA synthase gene phaC1 from Pseudomonas aeruginosa in Escherichia coli results in poly(3-hydroxyalkanoate) synthesis. FEMS Microbiol Lett 150:303–309
Lee SY (1995) Bacterial polydroxyalkanoates. Biotechnol Bioeng 49:1–14
Lee SY, Park SJ (2002) Biosynthesis and fermentative production of SCL-MCL-PHAs. In: Doi Y, Steinbüchel A (eds) Biopolymers Vol. 3a. Wiley/VCH, Weinheim, pp 317–336
Lee SY, Choi J, Wong HW (1999) Recent advances in polyhydroxyalkanoate production by bacterial fermentation: mini-review. Int J Biol Macromol 25:31–36
Li ZJ, Cai L, Wu Q, Chen GQ (2009) Overexpression of NAD kinase in recombinant Escherichia coli harboring the phbCAB operon improves poly(3-hydroxybutyrate) production. Appl Microbiol Biotechnol 83:939–947
Li QA, Chen QA, Li MJ, Wang FS, Qi QS (2011) Pathway engineering results the altered polyhydroxyalkanoates composition in recombinant Escherichia coli. New Biotech 28:92–95
Liebergesell M, Steinbüchel A (1992) Cloning and nucleotide sequences of genes relevant for biosynthesis of poly(3-hydroxybutyric acid) in Chromatium vinosum strain D. Eur J Biochem 209:135–150
Liebergesell M, Mayer F, Steinbüchel A (1993) Analysis of polyhydroxyalkanoic acid-biosynthesis genes of anoxygenic phototrophic bacteria reveals synthesis of a polyester exhibiting an unusual composition. Appl Microbiol Biotechnol 40:292–300
Liebergesell M, Schmidt B, Steinbüchel A (1992) Isolation and identification of granule-associated proteins relevant for poly(3-hydroxyalkanoic acid) biosynthesis in Chromatium vinosum D. FEMS Microbiol Lett 78:227–232
Liebergesell M, Sonomoto K, Madkour M, Mayer F, Steinbuchel A (1994) Purification and characterization of the poly(hydroxyalkanoic acid) synthase from Chromatium vinosum and localization of the enzyme at the surface of poly(hydroxyalkanoic acid) granules. Eur J Biochem 226:71–80
Liebergesell M, Rahalkar S, Steinbüchel A (2000) Analysis of the Thiocapsa pfennigii polyhydroxyalkanoate synthase: subcloning, molecular characterization and generation of hybrid synthases with the corresponding Chromatium vinosum enzyme. Appl Microbiol Biotechnol 54:186–194
Liu KL, Goh SH, Li J (2008) Controlled synthesis and characterizations of amphiphilic poly[(R, S)3-hydroxybutyrate]-poly(ethyleneglycol)-poly[(R, S)-3hydroxybutyrate] triblock copolymers. Polymer 49:732–741
Lu J, Tappel RC, Nomura CT (2009) Mini-review: biosynthesis of poly(hydroxyalkanoates). Polym Rev 49:226–248
Lundgren DG, Pfister RM, Merrick JM (1964) Structure of poly(β-hydroxybutyric acid) granules. J Gen Microbiol 34:441–446
Luo R, Chen J, Zhang L, Chen G (2006) Polyhydroxyalkanoate copolyesters produced by Ralstonia eutropha PHB−4 harboring a low-substrate-specificity PHA synthase PhaC2Ps from Pseudomonas stutzeri 1317. Biochem Engg J 32:218–225
Luzier WD (1992) Materials derived from biomass/biodegradable materials. Proc Natl Acad Sci USA 89:839–842
Madden LA, Anderson AJ, Shah DT, Asrar J (1999) Chain termination in polyhydroxyalkanoate synthesis: involvement of exogenous hydroxy-compounds as chain transfer agents. Int J Biol Macromol 25:43–53
Madison LL, Huisiman GW (1999) Metabolic engineering of poly(3-hydroxyalkanoates): from DNA to plastic. Microbiol Mol Biol Rev 63:21–53
Maestro B, Sanz JM (2017) Polyhydroxyalkanoate-associated phasins as phylogenetically heterogeneous, multipurpose proteins. Microb Biotechnol (in press). https://doi.org/10.1111/1751-7915.12718
Mallick N, Sharma L, Singh AK (2007) Poly-β-hydroxybutyrate accumulation in Nostoc muscorum: effects of metabolic inhibitors. J Plant Physiol 164:312–317
Martin DP, Peoples OP, Williams SF, Zhong LH (1999) Nutritional and therapeutic uses of 3-hydroxyalkanoate oligomers. US Patent Appl 359086
Masamune S, Walsh CT, Sinskey AJ, Peoples OP (1989) Poly-(R)3-hydroxybutyrate (PHB) biosynthesis: mechanistic studies on the biological Claisen condensation catalyzed by β-ketoacyl thiolase. Pure Appl Chem 61:303–312
Massieu L, Haces ML, Montiel T, Hernández-Fonseca K (2003) Acetoacetate protects hippocampal neurons against glutamate-mediated neuronal damage during glycolysis inhibition. Neuroscience 120:365–378
Matsusaki H, Abe H, Doi Y (2000) Biosynthesis and properties of poly(3-hydroxybutyrate-co-3-hydroxyalkanoates) by recombinant strains of Pseudomonas sp. 61-3. Biomacromolecules 1:17–22
McCool GJ, Cannon MC (1999) Polyhydroxyalkanoate inclusion body-associated proteins and coding region in Bacillus megaterium. J Bacteriol 181:585–592
McCool GJ, Cannon MC (2001) PhaC and PhaR are required for polyhydroxyalkanoic acid synthase activity in Bacillus megaterium. J Bacteriol 183:4235–4243
Mendhulkar VD, Shetye LA (2017) Synthesis of biodegradable polymer polyhydroxyalkanoate (PHA) in cyanobacteria Synechococcus elongates under mixotrophic nitrogen- and phosphate-mediated stress conditions. Ind Biotechnol 13:85–93
Merrick JM (1965) Effect of polymyxin B, tyrocidine, gramicidin D, and other antibiotics on the enzymatic hydrolysis of poly-beta-hydroxybutyrate. J Bacteriol 90:965–969
Merrick JM, Doudoroff M (1964) Depolymerization of poly-beta-hydroxybutyrate by an intracellular enzyme system. J Bacteriol 88:60–71
Merrick JM, Lundgren DG, Pfister RM (1965) Morphological changes in poly-beta-hydroxybutyrate granules associated with decreased susceptibility to enzymatic hydrolysis. J Bacteriol 89:234–239
Miyake M, Erata M, Asada Y (1996) A thermophilic cyanobacterium, Synechococcus sp. MA19, capable of accumulating poly-β-hydroxybutyrate. J Ferment Bioengg 82:512–514
Miyake M, Kataoka K, Shirai M, Asada Y (1997) Control of poly-β-hydroxybutyrate synthase mediated by acetyl phosphate in cyanobacteria. J Bacteriol 179:5009–5013
Moldes C, Garcia P, Garcia JL, Prieto MA (2004) In vivo immobilization of fusion proteins on bioplastics by the novel tag BioF. Appl Environ Microbiol 70:3205–3212
Moldes C, Farinos GP, de Eugenio LI, Garcia P, Garcia JL, Ortego F, Hernández-Crespo P, Castañera P, Prieto MA (2006) New tool for spreading proteins to the environment: Cry1Ab toxin immobilized to bioplastics. Appl Microbiol Biotechnol 72:88–93
Moskowitz GJ, Merrick JM (1969) Metabolism of poly-β-hydroxybutyrate II. Enzymatic synthesis of D(-)-β-hydroxybutyryl coenzyme-A by an enoylhydrases from Rhodospirillum rubrum. Biochemistry 8:2748–2755
Możejko-Ciesielska J, Kiewisz R (2016) Bacterial polyhydroxyalkanoates: still fabulous? Microbiol Res 192:271–282
Müh U, Sinskey AJ, Kirby DP, Lane WS, Stubbe J (1999) PHA Synthase from Chromatium vinosum: cysteine 149 is involved in covalent catalysis. Biochemistry 38:826–837
Muller S, Bley T, Babel W (1999) Adaptive responses of Ralstonia eutropha to feast and famine conditions analysed by flow cytometry. J Biotechnol 75:81–97
Neumann L, Spinozzi F, Sinibaldi R, Rustichelli F, Pötter M, Steinbüchel A (2008) Binding of the major phasin, PhaP1, from Ralstonia eutropha H16 to poly(3hydroxybutyrate) granules. J Bacteriol 190:2911–2919
Nishioka M, Nakai K, Miyake M, Asada Y, Taya M (2001) Production of poly-β-hydroyxybutyrate by thermophilic cyanobacterium, Synechococcus sp. MA19, under phosphate limitation. Biotechnol Lett 23:1095–1099
Nobes GAR, Jurasek L, Marchessault RH, Martin DP, Putaux JL, Chanzy H (2000) Growth and kinetics of in vitro poly([R](-)-3-hydroxybutyrate) granules interpreted as particulate polymerization with coalescence. Macromol Rapid Commun 21:77–84
Numata K, Motoda Y, Watanabe S, Osanai T, Kigawa T (2015) Co-expression of two polyhydroxyalkanoate synthase subunits from Synechocystis sp. PCC 6803 by cell free synthesis and their specific activity for polymerization of 3-hydroxybutyryl-CoA. Biochemistry 54:1401–1407
Obruca S (2010) Controlled production and degradation of selected biomaterials. Doctoral thesis, Brno University of Technology
Oeding V, Schlegel HG (1973) Beta-ketothiolase from Hydrogenomonas eutropha HI6 and its significance in the regulation of poly-beta-hydroxybutyrate metabolism. Biochem J 134:239–248
Osanai T, Numata K, Oikawa A, Kuwahara A, Iijima H, Doi Y, Tanaka K, Saito K, Hirai MY (2013) Increased bioplastic production with an RNA polymerase sigma factor SigE during nitrogen starvation in Synechocystis sp. PCC 6803. DNA Res 20:525–535
Page WJ (1992) Production of polyhydroxyalkanoates by Azotobacter vinelandii UWD in beet molasses culture. FEMS Microbiol Rev 103:149–158
Panda B, Mallick N (2007) Enhanced poly-β-hydroxybutyrate accumulation in a unicellular cyanobactrium, Synechocystis sp. PCC 6803. Lett Appl Microbiol 44:194–198
Panda B, Jain P, Sharma L, Mallick N (2006) Optimization of cultural and nutritional conditions for accumulation of poly-β-hydroxybutyrate in Synechocystis sp. PCC 6803. Biores Technol 97:1296–1301
Panda B, Sharma L, Singh AK, Mallick N (2008) Thin layer chromatographic detection of poly-β-hydroxybutyrate (PHB) and poly-β-hydroxybutyrate (PHV) in cyanobacteria. Ind J Biotechnol 7:230–234
Parlane NA, Gupta SK, Rubio Reyes P, Chen S, Gonzalez-Miro M, Wedlock DN, Rehm BH (2016) Self-assembled protein-coated polyhydroxyalkanoate beads: properties and biomedical applications. ACS Biomater Sci Eng (In press). https://doi.org/10.1021/acsbiomaterials.6b00355
Peoples OP, Sinskey AJ (1989a) Poly-β-hydroxybutyrate (PHB) biosynthesis in Alcaligenes eutrophus H16. Identiication and characterization of the PHB polymerase gene (phbC). J Biol Chem 264:15298–15303
Peoples OP, Sinskey AJ (1989b) Poly-β-hydroxybutyrate biosynthesis in Alcaligenes eutrophus H16. Characterization of the genes encoding β-ketothiolase and acetoacetyl-CoA reductase. J Biol Chem 264:15293–15297
Perepelkin KE (2005) Polymeric materials of the future based on renewable plant resources and biotechnologies: fibres, films and plastics. Fibre Chem 37:417–430
Pfeiffer D, Jendrossek D (2011) Interaction between poly(3-hydroxybutyrate) granule-associated proteins as revealed by two-hybrid analysis and identification of a new phasin in Ralstonia eutropha H16. Microbiology 157:2795–2807
Pham TH, Webb JS, Rehm BH (2004) The role of polyhydroxyalkanoate biosynthesis by Pseudomonas aeruginosa in rhamnolipid and alginate production as well as stress tolerance and biofilm formation. Microbiology 150:3405–3413
Philip S, Keshavarz T, Roy I (2007) Polyhydroxyalkanoates: biodegradable polymers with a range of applications. J Chem Technol Biotechnol 82:233–247
Phithakrotchanakoon C, Champreda V, Aiba S, Pootanakit K, Tanapongpipat S (2013) Engineered Escherichia coli for short-chain-length medium-chain-length polyhydroxyalkanoate copolymer biosynthesis from glycerol and dodecanoate. Biosci Biotechnol Biochem 77:1262–1268
Plastics Europe (2015) Plastics-the Facts 2014/2015: an analysis of European plastics production, demand and waste data. https://www.plasticseurope.org/application/files/5515/1689/9220/2014plastics_the_facts_PubFeb2015.pdf
Plastics Europe (2016) Plastics-the Facts 2016: an analysis of European plastics production, demand and waste data. https://www.plasticseurope.org/application/files/4315/1310/4805/plastic-the-fact-2016.pdf
Pötter M, Steinbüchel A (2005) Poly(3-hydroxybutyrate) granule-associated proteins: impacts on poly(3-hydroxybutyrate) synthesis and degradation. Biomacromol 6:552–560
Pötter M, Steinbüchel A (2006) Biogenesis and Structure of polyhydroxyalkanoate granules. Springer, Berlin, pp 109–136
Pötter M, Madkour MH, Mayer F, Steinbüchel A (2002) Regulation of phasin expression and polyhydroxyalkanoate (PHA) granule formation in Ralstonia eutropha H16. Microbiology 148:2413–2426
Pötter M, Muller H, Steinbuchel A (2005) Influence of homologous phasins (PhaP) on PHA accumulation and regulation of their expression by the transcriptional repressor PhaR in Ralstonia eutropha H16. Microbiology 151:825–833
Prieto MA, Buhler B, Jung K, Witholt B, Kessler B (1999) PhaF, a polyhydroxyalkanoate-granule associated protein of Pseudomonas oleovorans GPo1 involved in the regulatory expression system for pha genes. J Bacteriol 181:858–868
Punrattanasin W (2001) The utilization of activated sludge polyhydroxyalkanoates for the production of biodegradable plastics. PhD dissertation, Virginia Polytechnic Institute and State University, Blacksburg, Virginia, USA
Qi Q, Rehm BH (2001) Polyhydroxybutyrate biosynthesis in Caulobacter crescentus: molecular characterization of the polyhydroxybutyrate synthase. Microbiology 147:3353–3358
Qi Q, Steinbuchel A, Rehm BH (2000) In vitro synthesis of poly(3-hydroxydecanoate): purification and enzymatic characterization of type II polyhydroxyalkanoate synthases PhaC1 and PhaC2 from Pseudomonas aeruginosa. Appl Microbiol Biotechnol 54:37–43
Ravenstijn JTJ (2010) The state-of-the art on bioplastics: products, markets, trends and technologies. Polymedia, Lüdenscheid
Ray SS, Bousmina M (2005) Biodegradable polymers and their layered silicate nanocomposites: In greening the 21st century materials world. Prog Mater Sci 50:962–1079
Reddy MV, Mohan SV (2015) Polyhydroxyalkanoates production by newly isolated bacteria Serratia ureilytica using volatile fatty acids as substrate: Bio-electro kinetic analysis. J Microb Biochem Technol 7:26–32
Rehm BH (2003) Polyester synthases: natural catalysts for plastics. Biochem J 37:15–33
Rehm BH (2007) Biogenesis of microbial polyhydroxyalkanoate granules: a platform technology for the production of tailor-made bio-particles. Curr Issues Mol Biol 9:41–62
Rehm BH, Steinbüchel A (1999) Biochemical and genetic analysis of PHA synthases and other proteins required for PHA synthesis. Int J Biol Macromol 25:3–19
Rehm BH, Kruger N, Steinbüchel A (1998) A new metabolic link between fatty acid de novo synthesis and polyhydroxyalkanoic acid synthesis. The PHAG gene from Pseudomonas putida KT2440 encodes a 3-hydroxyacyl-acyl carrier protein-coenzyme a transferase. J Biol Chem 273:24044–24051
Rehm BH, Antonio RV, Spiekermann P, Amara AA, Steinbüchel A (2002) Molecular characterization of the poly(3hydroxybutyrate) (PHB) synthase from Ralstonia eutropha: in vitro evolution, site-specific mutagenesis and development of a PHB synthase protein model. Biochim Biophys Acta 1594:178–190
Samantaray S, Mallick N (2012) Production and characterization of poly-β-hydroxybutyrate (PHB) polymer from Aulosira fertilissima. J Appl Phycol 24:803–814
Samantaray S, Mallick N (2014) Production of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) co-polymer by the diazotrophic cyanobacterium Aulosira fertilissima CCC 444. J Appl Phycol 26:237–245
Samantaray S, Mallick N (2015) Impact of various stress conditions on poly-β-hydroxybutyrate (PHB) accumulation in Aulosira fertilissima CCC 444. Curr Biotechnol 4:366–372
Sangkharak K, Prasertsan P (2012) Screening and identification of polyhydroxyalkanoates producing bacteria and biochemical characterization of their possible application. J Gen Appl Microbiol 58:173–182
Sankhla SS, Bhati R, Singh AK, Mallick N (2010) Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) co-polymer production from a local isolate, Brevibacillus invocatus MTCC 9039. Biores Tecnol 101:1947–1953
Schubert P, Steinbüchel A, Schlegel HG (1988) Cloning of the Alcaligenes eutrophus genes for synthesis of poly-beta-hydroxybutyric acid (PHB) and synthesis of PHB in Escherichia coli. J Bacteriol 170:5837–5847
Senior PJ, Dawes EA (1973) The regulation of poly-β-hydroxybutyrate metabolism in Azotobacter beijerinckii. Biochem J 134:225–238
Shah DT, Tran M, Berger PA, Aggarwal P, Asrar J, Madden LA, Anderson AJ (2000) Synthesis and properties of hydroxy-terminated poly(hydroxyalkanoate)s. Macromolecules 33:2875–2880
Shah AA, Hasan F, Hameed A, Ahmed S (2008) Biological degradation of plastics: a comprehensive review. Biotechnol Adv 26:246–265
Shamala TR, Vijayendra SV, Joshi GJ (2012) Agro-industrial residues and starch for growth and co-production of polyhydroxyalkanoate copolymer and α-amylase by Bacillus sp. CFR-67. Braz J Microbiol 43:1094–1102
Sharma L, Mallick N (2005a) Accumulation of poly-β-hydroxybutyrate in Nostoc muscorum: regulation by pH, light-dark cycles, N and P status and carbon sources. Biores Technol 96:1304–1310
Sharma L, Mallick N (2005b) Enhancement of poly-β-hydroxybutyrate accumulation in Nostoc muscorum under mixotrophy, chemoheterotrophy and limitations of gas-exchange. Biotechnol Lett 27:59–62
Sharma L, Panda B, Singh AK, Mallick N (2006) Studies on poly-β-hydroxybutyrate synthase activity of Nostoc muscorum. J Gen Appl Microbiol 52:209–214
Sharma L, Singh AK, Panda B, Mallick N (2007) Process optimization for poly-β-hydroxybutyrate production in a nitrogen fixing cyanobacterium, Nostoc muscorum using response surface methodology. Biores Technol 98:987–993
Sharma L, Srivastava JK, Singh AK (2016) Biodegradable polyhydroxyalkanoate thermoplastics substituting xenobiotic plastics: a way forward for sustainable environment. In: Singh A, Prasad SM, Singh RP (eds) Plant responses to xenobiotics. Springer, Singapore, pp 317–346
Shimizu R, Chou K, Orita I, Suzuki Y, Nakamura S, Fukui T (2013) Detection of phase-dependent transcriptomic changes and Rubisco-mediated CO2 fixation into poly (3-hydroxybutyrate) under heterotrophic condition in Ralstonia eutropha H16 based on RNA-seq and gene deletion analyses. BMC Microbiol 13:169–183
Shrivastav A, Mishra SK, Mishra S (2010) Polyhydroxyalkanoate (PHA) synthesis by Spirulina subsalsa from Gujarat coast of India. Int J Biol Macromol 46:255–260
Sim SJ, Snell KD, Hogan SA, Stubbe J, Rha C, Sinskey AJ (1997) PHA synthase activity controls the molecular weight and polydispersity of polyhydroxybutyrate in vivo. Nature Biotech 15:63–67
Singh A (2009) Accumulation of a novel short-chain-length-long-chain-length polyhydroxyalkanoate co-polymer in a sludge-isolated Pseudomonas aeruginosa MTCC 7925. PhD thesis, Indian Institute of Technology, Kharagpur, India, pp 41–71
Singh AK, Mallick N (2008) Enhanced production of SCL-LCL-PHA co-polymer by sludge-isolated Pseudomonas aeruginosa MTCC 7925. Lett Appl Microbiol 46:350–357
Singh AK, Mallick N (2009a) Exploitation of inexpensive substrates for production of a novel SCL–LCL-PHA co-polymer by Pseudomonas aeruginosa MTCC 7925. J Ind Microbiol Biotechnol 36:347–354
Singh AK, Mallick N (2009b) SCL-LCL-PHA copolymer production by a local isolate, Pseudomonas aeruginosa MTCC 7925. Biotechnol J 4:703–711
Singh AK, Mallick N (2017a) Biological system as a reactor for production of biodegradable thermoplastics, Polyhydroxyalkanoates. In: Thangadurai D, Sangeetha J (eds) Industrial biotechnology: sustainable production and bioresource utilization. CRC Press Taylor and Francis, USA, pp 281–323
Singh AK, Mallick N (2017b) Advances in cyanobacterial polyhydroxyalkanoates production. FEMS Microbiol Lett 364(20). https://doi.org/10.1093/femsle/fnx189
Singh AK, Mallick N (2017c) Pseudomonas aeruginosa MTCC 7925: Biofactory for Novel SCL-LCL-PHA. LAP Lambert Academic Publishing, Germany, pp 1–148
Singh AK, Bhati R, Samantaray S, Mallick N (2013) Pseudomonas aeruginosa MTCC 7925: producer of a novel SCL-LCL-PHA co-polymer. Curr Biotechnol 2:81–88
Singh AK, Ranjana B, Mallick N (2015) Pseudomonas aeruginosa MTCC 7925 as a biofactory for production of the novel SCL-LCL- PHA thermoplastic from non-edible oils. Curr Botechnol 4:65–74
Singh AK, Sharma L, Mallick N, Mala J (2017) Progress and challenges in producing polyhydroxyalkanoate biopolymers from cyanobacteria. J Appl Phycol 29:1213–1232
Slater SC, Voige WH, Dennis DE (1988) Cloning and expression in Escherichia coli of the Alcaligenes eutrophus H16 poly-beta-hydroxybutyrate biosynthetic pathway. J Bacteriol 170:4431–4436
Song JJ, Yoon SC (1996) Biosynthesis of novel aromatic copolyesters from insoluble 11-phenoxyundecanoic acid by Pseudomonas putida BMO1. Appl Environ Microbiol 62:536–544
Stal LJ, Heyer H, Jacobs G (1990) Occurrence and role of poly-hydroxy-alkanoates in the cyanobacterium Oscillatoria limosa. In: Dawes EA (ed) Novel biodegradable microbial polymers. Springer, pp 435–438
Steinbüchel A (1991) Polyhydroxyalkanoic acids. In: Byrom D (ed) Biomaterials: novel materials from biological sources. Stockton, New York, pp 124–213
Steinbüchel A (1996) PHB and other polyhydroxyalkanoic acids. In: Doi Y, Fukuda K (eds) Biodegradable plastics and polymers. Elsevier Science, New York, pp 362–364
Steinbüchel A, Hein S (2001) Biochemical and molecular basis of microbial synthesis of polyhydroxyalkanoates in microorganisms. Adv Biochem Eng Biotechnol 71:81–123
Steinbüchel A, Hustede E, Liebergesell M, Pieper U, Timm A, Valentin H (1992) Molecular basis for biosynthesis and accumulation of polyhydroxyalkanoic acids in bacteria. FEMS Microbiol Rev 103:217–230
Steinbüchel A, Debzi EM, Marchessault RH, Timm A (1993) Synthesis and production of poly(3-hydroxyvaleric acid) homopolymer by Chromobacterium violaceum. Appl Microbiol Biotechnol 39:443–449
Stubbe J, Tian J (2003) Polyhydroxyalkanoate (PHA) homeostasis: the role of the PHA synthase. Nat Prod Rep 20:445–457
Stubbe J, Tian J, He A, Sinskey AJ, Lawrence AG, Liu P (2005) Nontemplate-dependent polymerization processes: polyhydroxyalkanoate synthases as a paradigm. Annu Rev Biochem 74:433–480
Su L, Lenz RW, Takagi Y, Zhang S, Goodwin S, Zhong L, Martin DP (2000) Enzymatic polymerization of (R)-3-hydroxyalkanoates by a bacterial polymerase. Macromolecules 33:229–231
Sudesh K, Abe H, Doi Y (2000) Synthesis, structure and properties of polyhydroxyalkanoates: biological polyesters. Prog Polym Sci 25:1503–1555
Tajima K, Igari T, Nishimura D, Nakamura M, Satoh Y, Munekata M (2003) Isolation and characterization of Bacillus sp. INT005 accumulating polyhydroxyalkanoate (PHA) from gas field soil. J Biosci Bioengg 95:77–81
Takahashi T, Miyake M, Tokiwa Y, Asada Y (1998) Improved accumulation of poly-3-hydroxybutyrate by a recombinant cyanobacterium. Biotechnol Lett 20:183–186
Tan IKP, Sudesh Kumar K, Theanmalar M, Gan SN, Gordon B (1997) Saponified palm kernel oil and its major free fatty acids as carbon substrates for the production of polyhydroxyalkanoates in Pseudomonas putida PGA1. Appl Microbiol Biotechnol 47:207–211
Taroncher-Oldenburg G, Nishina K, Stephanopoulos G (2000) Identification and analysis of the polyhydroxyalkanoate-specific β-ketothiolase and acetoacetyl coenzyme A reductase genes in the cyanobacterium Synechocystis sp. strain PCC6803. Appl Environ Microbiol 66:4440–4448
Thakor NS, Patel MA, Trivedi UB, Patel KC (2003) Production of poly(β-hydroxybutyrate) by Comamonas testosteroni during growth on naphthalene. World J Microbiol Biotechnol 19:185–189
Tian J, He A, Lawrence AG, Liu P, Watson N, Sinskey AJ, Stubbe J (2005a) Analysis of transient polyhydroxybutyrate production in Wautersia eutropha H16 by quantitative western analysis and transmission electron microscopy. J Bacteriol 187:3825–3832
Tian J, Sinskey AJ, Stubbe J (2005b) Detection of intermediates from the polymerization reaction catalyzed by a D302A mutant of class III polyhydroxyalkanoate (PHA) synthase. Biochemistry 44:1495–1503
Tian S-J, Lai W-J, Zheng Z, Wang H-X, Chen G-Q (2005c) Effect of over-expression of phasin gene from Aeromonas hydrophila on biosynthesis of copolyesters of 3-hydroxybutyrate and 3-hydroxyhexanoate. FEMS Microbiol Lett 244:19–25
Timm A, Steinbüchel A (1992) Cloning and molecular analysis of the poly(3-hydroxyalkanoic acid) gene locus of Pseudomonas aeruginosa PAO1. Eur J Biochem 209:15–30
Tirapelle EF, Muller-Santos M, Tadra-Sfeir MZ, Kadowaki MA, Steffens MB, Monteiro RA, Souza EM, Pedrosa FO, Chubatsu LS (2013) Identification of proteins associated with polyhydroxybutyrate granules from Herbaspirillum seropedicae SmR1-old partners, new players. PLoS ONE 8:e75066
Toh PSY, Jau MH, Yew SP, Abed RMM, Sudesh K (2008) Comparison of polyhydroxyalkanoates biosynthesis, mobilization and the effects on cellular morphology in Spirulina platensis and Synechocystis sp. UNIWG. J Biosci 19:21–38
Tomizawa S, Hyakutake M, Saito Y, Agus J, Mizuno K, Abe H, Tsuge T (2011) Molecular weight change of polyhydroxyalkanoate (PHA) caused by the PhaC subunit of PHA synthase from Bacillus cereus YB-4 in recombinant Escherichia coli. Biomacromol 12:2660–2666
Tsuge T, Fukui T, Matsusaki H, Taguchi S, Kobayashi G, Ishizaki A, Doi Y (2000) Molecular cloning of two (R)-specific enoyl-CoA hydratase genes from Pseudomonas aeruginosa and their use for polyhydroxyalkanoate synthesis. FEMS Microbiol Lett 184:193–198
Uchino K, Saito T, Gebauer B, Jendrossek D (2007) Isolated poly(3-hydroxybutyrate) (PHB) granules are complex bacterial organelles catalyzing formation of PHB from acetyl coenzyme A (CoA) and degradation of PHB to acetyl-CoA. J Bacteriol 189:8250–8256
Ushimaru K, Motoda Y, Numata K, Tsuge T (2014) Phasin proteins activate Aeromonas caviae polyhydroxyalkanoate (PHA) synthase but not Ralstonia eutropha PHA synthase. Appl Environ Microbiol 80:2867–2873
Verlinden RA, Hill DJ, Kenward CD, Radecka I (2007) Bacterial synthesis of biodegradable polyhydroxyalkanoates. J Appl Microbiol 102:1437–1449
Vincenzini M, De Philippis R (1999) Polyhydroxyalkanoates. In: Cohen Z (ed) Chemicals from microalgae. Taylor and Francis Inc., USA, pp 292–312
Wahl A, Schuth N, Pfeiffer D, Nussberger S, Jendrossek D (2012) PHB granules are attached to the nucleoid via PhaM in Ralstonia eutropha. BMC Microbiol 12:262
Wakil SJ (1989) Fatty acid synthase, a proficient multifunctional enzyme. Biochemistry 28:4523–4530
Wältermann M, Steinbüchel A (2005) Neutral lipid bodies in prokaryotes: recent insights into structure, formation, and relationship to eukaryotic lipid depots. J Bacteriol 187:3607–3619
Wältermann M, Hinz A, Robenek H, Troyer D, Reichelt R, Malkus U, Galla HJ, Kalscheuer R, Stöveken T, von Landenberg P, Steinbüchel A (2005) Mechanism of lipid-body formation in prokaryotes: how bacteria fatten up. Mol Microbiol 55:750–763
Wang C, Sheng X, Equi RC, Trainer MA, Charles TC, Sobral BW (2007) Influence of the poly-3hydroxybutyrate (PHB) granule-associated proteins (PhaP1 and PhaP2) on PHB accumulation and symbiotic nitrogen fixation in Sinorhizobium meliloti Rm1021. J Bacteriol 189:9050–9056
Wang L, Zhu WF, Wang X, Chen X, Chen G-Q, Xu K (2008) Processability modifications of poly(3-hydroxybutyrate) by plasticizing, blending, and stabilizing. J Appl Polym Sci 107:166–173
Wang B, Pugh S, Nielsen DR, Zhang W, Meldrum DR (2013) Engineering cyanobacteria for photosynthetic production of 3-hydroxybutyrate directly from CO2. Metab Eng 16:68–77
Wei DX, Chen CB, Fang G, Li SY, Chen GQ (2011) Application of polyhydroxyalkanoate binding protein PhaP as a bio-surfactant. Appl Microbiol Biotechnol 91:1037–1047
Weiner RM (1997) Biopolymers from marine prokaryotes. Trends Biotechnol 15:390–394
Wieczorek R, Pries A, Steinbüchel A, Mayer F (1995) Analysis of a 24-kilodalton protein associated with the polyhydroxyalkanoic acid granules in Alcaligenes eutrophus. J Bacteriol 177:2425–2435
Williamson DH, Wilkinson JF (1958) The isolation and estimation of the poly-β-hydroxybutyrate inclusions of Bacillus species. J Gen Microbiol 19:198–209
Wodzinska J, Snell KD, Rhomberg A, Sinskey AJ, Biemann K, Stubbe J (1996) Polyhydroxybutyrate synthase: evidence for covalent catalysis. J Am Chem Soc 118:6319–6320
Xiao XQ, Zhao Y, Chen GQ (2007) The effect of 3-hydroxybutyrate and its derivatives on the growth of glial cells. Biomaterials 28:3608–3616
Yang JE, Choi YJ, Lee SJ, Kang KH, Lee H, Oh YH, Lee SH, Park SJ, Lee SY (2014) Metabolic engineering of Escherichia coli for biosynthesis of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) from glucose. Appl Microbiol Biotechnol 98:95–104
Yao YC, Zhan XY, Zou XH, Wang ZH, Xiong YC, Zhang J, Chen J, Chen GQ (2008) A specific drug targeting system based on polyhydroxyalkanoate granule binding protein PhaP fused with targeted cell ligands. Biomaterials 29:4823–4830
Yezza A, Fournier D, Halasz A, Hawari J (2006) Production of polyhydroxyalkanoates from methanol by a new methylotrophic bacterium Methylobacterium sp. GW2. Appl Microbiol Biotechnol 73:211–218
York GM, Junker BH, Stubbe J, Sinskey AJ (2001a) Accumulation of the PhaP phasin of Ralstonia eutropha is dependent on production of polyhydroxybutyrate in cells. J Bacteriol 183:4217–4226
York GM, Stubbe J, Sinskey AJ (2001b) New insight into the role of the PhaP phasin of Ralstonia eutropha in promoting synthesis of polyhydroxybutyrate. J Bacteriol 183:2394–2397
York GM, Stubbe J, Sinskey AJ (2002) The Ralstonia eutropha PhaR protein couples synthesis of the PhaP phasin to the presence of polyhydroxybutyrate in cells and promotes polyhydroxybutyrate production. J Bacteriol 184:59–66
Zhang H, Obias V, Gonyer K, Dennis D (1994) Production of polyhydroxalkanoates in sucrose-utilizing recombinant Escherichia coli and Klebsiella strains. Appl Environ Microbiol 60:1198–1205
Zhang S, Yasuo T, Lenz RW, Goodwin S (2000) Kinetic and mechanistic characterization of the polyhydroxybutyrate synthase from Ralstonia eutropha. Biomacromol 1:244–251
Zhang JY, Hao N, Chen GQ (2006) Effect of expressing polyhydroxybutyrate synthesis genes (phbCAB) in Streptococcus zooepidemicus on production of lactic acid and hyaluronic acid. Appl Microbiol Biotechnol 71:222–227
Zhang XJ, Luo RC, Wang Z, Deng Y, Chen GQ (2009) Applications of (R)-3-hydroxyalkanoate methyl esters derived from microbial polyhydroxyalkanoates as novel biofuel. Biomacromol 10:707–711
Zinn M, Hany R (2005) Tailored material properties of Polyhydroxyalkanoates through biosynthesis and chemical modification. Adv Eng Mater 7:408–411
Zou XH, Li HM, Wang S, Leski M, Yao YC, Yang XD, Huang QJ, Chen GQ (2009) The effect of 3-hydroxybutyrate methyl ester on learning and memory in mice. Biomaterials 30:1532–1541
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG
About this chapter
Cite this chapter
Singh, A.K., Sharma, L., Srivastava, J.K., Mallick, N., Ansari, M.I. (2018). Microbially Originated Polyhydroxyalkanoate (PHA) Biopolymers: An Insight into the Molecular Mechanism and Biogenesis of PHA Granules. In: Singh, O., Chandel, A. (eds) Sustainable Biotechnology- Enzymatic Resources of Renewable Energy. Springer, Cham. https://doi.org/10.1007/978-3-319-95480-6_14
Download citation
DOI: https://doi.org/10.1007/978-3-319-95480-6_14
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-95479-0
Online ISBN: 978-3-319-95480-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)