Abstract
Many species of ferns and lycophytes are threatened by habitat destruction, over-collecting, invasive species, and climate change, and plans to secure their in situ and ex situ conservation are urgently needed. However, there has not been a development of standard methods for fern ex situ storage and conservation, as there has been for seed plants. This is likely due to the fact that ferns have been less studied and are less understood than seed plants and that there has been less funding and institutional focus on them for conservation purposes when compared with domesticated or wild seed plants. Tissues from different stages of the fern and lycophyte life cycle can be used as a ready source of germplasm for ex situ conservation. Spore storage is generally the most efficient method for ex situ fern conservation, but there are also situations where storing gametophytes or sporophytes can be extremely useful. The longevity of spores at −20 °C, the standard seed banking temperature, may vary with the species. Storage in LN should improve longevity in spores and is likely necessary for non-spore tissues. Recent results of a study on the viability of spores, gametophytes, and sporophytes after 20 years of storage in liquid nitrogen is used as a case study to illustrate the potential of these methods. The state of current methods and needs for further research and application are discussed. While there is much more to learn, these tools are available for fern ex situ conservation and should be implemented on a broader scale to preserve rare fern taxa and provide materials for future restoration and research.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Ferns (monilophytes ) and lycophytes are estimated to be about 4% (c. 15,000 species) of all Earth’s plants (Chapman 2009). Within this richness are species with diverse ecological roles, economic importance, and medicinal, food, or biotechnological value (De Long and Prange 2008; Fernández and Revilla 2003; Fernández et al. 2010; Liu et al. 2012; Mannan et al. 2008; Matsuura et al. 2014; Mehltreter et al. 2010; Stamps 2006). However, a large number of fern and lycophyte species are threatened, due to habitat destruction, over-collecting, invasive species, and climate change, and plans to secure their in situ and ex situ conservation are urgently needed (Arcand and Ranker 2008; Ibars and Estrelles 2012). Because of the alternation of life forms, ferns offer several options for ex situ conservation. This chapter will review the current status of fern conservation, and, using a recent case study on long-term cryo-survival, evaluate the current state of fern conservation technology, and outline the potential of current methods and areas for improving future efforts.
2 Current Status of Fern Conservation
2.1 Living Collections
Ferns have been collected and displayed for centuries, particularly in botanic gardens or private collections (e.g. https://ebps.org.uk/ferns/growing/where-to-see-ferns/). There are notable living collections of fern sporophytes, such as at the Royal Botanic Gardens, Edinburgh in the UK (http://www.rbge.org.uk/the-gardens/edinburgh/the-glasshouses/ferns-and-fossils), the Royal Botanic Gardens Victoria in Australia (https://www.rbg.vic.gov.au/visit-melbourne/attractions/plant-collections/fern-gully), or the Lankester Botanical Garden of the University of Costa Rica (http://www.jbl.ucr.ac.cr/helechos). Traditionally, these have been primarily for display, although more recently there are research and conservation missions developing around the collections (e.g. Page et al. 1992). Most species of ferns have not yet been evaluated for their conservation status (IUCN, 2017), but, for example, China alone has 182 species on its Red List, with 79 of those being endemic (Dong et al. 2017). Thus, there may be hundreds of threatened fern species globally. Because the space and labour to conserve living collections are substantial, living collections alone will likely not be able to conserve the wide genetic diversity of the fern species that are threatened. However, banking spores and other alternative propagules can provide a cost-effective supplement to what can be preserved in living collections (Li and Pritchard, 2009).
2.2 Conserving Fern Spores
Spore storage is an efficient and effective method for the ex situ conservation of ferns and lycophytes. Their desiccation tolerance (Chap. 19) and small size offer a simple, compact, and economical ex situ conservation tool for most of the biodiversity of ferns and lycophytes (Ashcroft and Sheffield 2000; Ballesteros 2010; Ballesteros et al. 2011, 2012, 2017; Dyer 1994; Ibars and Estrelles 2012; Mikula et al. 2015; Pence, 2008a, b). In addition, spores are demonstrated to be a ready source of germplasm to aid reestablishment of waning fern populations (Pennisi 2010). Ex situ spore collections were initially created ca. 30 years ago (Dyer 1994), but ex situ spore collections are currently rare if we compare them with the large network of seed banks worldwide (about 1300 throughout the world as of 2006, Rajasekharan 2015). To our knowledge (Alexander et al. 2008; Dyer 1994; Ibars et al. 2011; Pence 2008a several pers. comm. to authors), there are less than ten ex situ spore collections across the world, which represent the majority of the current collections for fern spore ex situ conservation (Table 11.1). In addition, there are spores that are stored for exchange programmes in fern societies (e.g. the British Pteridological Society and the American Fern Society), although the conditions used for these are focused on short-term storage and are not optimal for long-term conservation (Pérez-García and Reyes-Jaramillo 1993).
The scarcity of ex situ spore collections likely represents the fact that (1) ferns have been less studied and are less understood than seed plants and (2) there has been less funding and institutional focus on their conservation when compared with domesticated or wild seed plants. Because of this, there has not been the development of standard methods for fern spore storage as there has been for seed storage, with the development of international best practice standards over the past several decades (e.g. FAO Genebank standards such as FAO 2014). There are only a few publications, most in Spanish and in grey literature, describing procedures for routinely collecting and storing fern spores in germplasm banks (e.g. Bacchetta et al. 2008; Ibars and Estrelles 2015; Ibars et al. 2011; Pence 2008b). A result of this lack of standardized procedures for fern spore storage is the variety of conditions used in the different ex situ spore collections surveyed around the world (Table 11.1). This chapter is not intended to provide a final standard for ex situ spore collections, but, based on the experience of two decades of fern spore storage, aims to contribute to the development of such protocols for fern spore ex situ conservation and to encourage the establishment of fern spore collections within existing germplasm banks.
2.3 Non-spore Options for Fern Conservation
While storing spores is the most efficient method for ex situ fern conservation, there are also situations where storing tissues from gametophytes or sporophytes can be useful conservation tools. If only a few spores are available, they can be multiplied by germinating them into gametophytes and sporophytes, and either of these life forms can be propagated vegetatively, to provide more material for research, restoration, and preservation. There are also fern species for which the only life form known is that of the gametophyte (Farrar 1967, 2016), and thus, no spores are available for storage.
Gametophytes can be maintained as living collections, but because of their diminutive size, they are not as often used for display as sporophytes. Since they are small and easily manipulated, they have often been grown aseptically from spores or gemmae and propagated in vitro (e.g. Kamachi and Noguchi 2012; Marimuthu and Manickam 2011; Raine and Sheffield 1997). Gametophytes are well adapted for in vitro growth, as they consist of a small, photosynthetic thallus, one cell thick, which is highly regenerative and can reproduce clonally by fragmentation or budding (Maeda and Ito 1981; Ong and Ng 1998). In vitro cultures of gametophytes can also serve as a source of tissues for cryopreservation (Barnicoat et al. 2011; Makowski et al. 2015, 2016; Mikula et al. 2009, 2010; Pence 2000a; Wilkinson 2002). Because of the highly regenerative nature of gametophyte tissue and its relatively undifferentiated structure, even if some of the tissues are damaged during cryopreservation, only a small surviving fragment is needed to regenerate the culture (Mikula et al. 2010). In vitro and cryopreservation methods for maintaining gametophytes have been reported for a number of fern species (e.g. Mikula et al. 2010; Pence 2000a), but only a few of these have been endangered ferns (Barnicoat et al. 2011; Pence 2015; Raine and Sheffield 1997; Sara and Manickam 2007).
Sporophytes are also grown in vitro for research and horticulture, as well as for conservation (Fernández and Revilla 2003; From 2010; Yu et al. 2017), and in vitro propagation methods for the sporophyte life form are very similar to those used for many species of seed plants (Dolinsek and Camloh 1997; Higuchi et al. 1987; Khan et al. 2008; Rogers and Banister 1992). Such cultures may be initiated through the aseptic germination of spores or from pieces of the sporophyte, sterilized for initiation into culture (Dolinsek and Camloh 1997; Higuchi et al. 1987; Khan et al. 2008; Pence 2014). Many fern species have been propagated in vitro for horticulture (Dolinsek and Camloh 1997; Higuchi et al. 1987; Khan et al. 2008; Rogers and Banister 1992) as a way of providing uniform quality for commercial use. Similarly, a number of rare species of ferns have been propagated in vitro to conserve the species ex situ, as well as to provide material for restoration (Aguraiuja 2010a, b; Ashmore et al. 2011; Houser et al. 2016; Martin et al. 2006; Pence 2014, 2015; Sara and Manickam 2007). However, although there are a few institutions maintaining rare ferns in vitro, including the Lyon Arboretum’s Hawaiian Rare Plant Program, the Center for Conservation and Research at Omaha’s Henry Doorly Zoo, and the Center for Conservation and Research of Endangered Wildlife (CREW) at the Cincinnati Zoo and Botanical Garden, the use of in vitro collections of ferns for conservation has not been widely explored.
Long-term conservation of in vitro fern sporophyte cultures by cryopreservation is possible using growing, meristematic tissues, such as shoot tips or green globular bodies (Pence 2001, 2014, 2015). Cryopreservation of such tissues offers the opportunity to maintain stock lines for future use. It also has potential for conserving pteridophyte species for which spores are difficult to collect or produce in low numbers (Raine and Sheffield 1997). However, as with in vitro collections, there are only a few reports of cryopreserved sporophyte tissues of rare fern taxa (Pence 2014, 2015).
3 Case Study: Long-Term Storage of Ferns in CREW’s Frozen Garden
CREW initiated its Frozen Garden of its CryoBioBank® in the late 1980s (Pritchard et al. 2017). This collection includes propagules that have been stored in liquid nitrogen (LN) (−196 °C) and some also at other temperatures (from about 20 to −20 °C). The Frozen Garden was initiated with the aim of supporting the long-term conservation of native and endangered plant species of the USA but also with the aim of providing resources to contribute to the understanding of long-term storage of plant germplasm in LN. About 25 years after the collection was started, the Institute of Museum and Library Services (IMLS) of the USA funded a complete evaluation of the viability and genetic integrity of CREW’s collection. This evaluation has provided results that can be used to inform current practice for ex situ conservation of ferns.
3.1 Spore Banking
3.1.1 Collection and Storage of Fern Spores
Fern spores stored in the Frozen Garden were collected from several sources over a period of 14 years (from 1992 to 2006). When fronds were collected by CREW’s staff, they were usually air-dried for 5–10 days under ambient conditions in the laboratory in paper envelopes. Subsequently, spores that fell on the paper were collected, dried as indicated below, and cryopreserved. However, if insufficient spores were observed on the paper, sori were scraped, and spores were collected along with sporangia and frond tissues. Some fern spores were sent directly by mail to CREW from several sources (e.g. Hawaii, Alaska, or China), which also included some frond and sporangia tissues. These samples, with spores and non-spore tissues, were dried and cryopreserved together due to the small amount of spores in the samples.
Spores (and other tissues if present) were dried at room temperature and RH < 40% for 2–7 days before storage, using silica gel as a desiccant (RH 20 ± 5%) if room RH was higher than 40%. Samples of the spores were then transferred to 2 ml polypropylene cryovials and immersed directly into LN. For some species, replicates were placed in a mechanical freezer (−20 ± 2 °C), a refrigerator (4 ± 2 °C), or left on the benchtop in the laboratory (21 ± 3 °C) for comparison with the samples stored in LN.
3.1.2 Germination of Fern Spores for Viability Assays
After storage of 9–21 years (e.g. Table 11.2), vials were removed from LN and placed on the benchtop to warm at ambient temperature for 20–60 min. Viability assays were afterward performed by germinating the fern spores in 15 x 150 mm petri dishes on mineral culture medium solidified with agar and prepared with the fungicide nystatin (100 U·ml −1) (Ballesteros et al. 2011, 2012, 2017). Petri dishes were then sealed with Parafilm (Bemis NA, Neenah, WI, USA) and put in a growth chamber set at 20 ± 2 °C with a 16/8 h light/dark cycle (Pence 2000b). Spores were observed using a dissecting microscope at 40× magnification, and germination was considered complete when the outer wall of the spore had ruptured and the rhizoid or the first chlorophyllic cell emerged (Ballesteros et al. 2011, 2012, 2017). Germination was measured regularly up to 30 days in order to calculate total germination and the rate of germination (Ballesteros et al. 2011, 2012, 2017). Then, the normal developmental transition from one-dimensional growth to two-dimensional growth was assessed in gametophytes after 60 days in culture (Ashcroft and Sheffield 2000; Ballesteros et al. 2011, 2012), a sufficient time to observe this transition.
3.1.3 Summary of Results
The original fern spore collection consisted of 162 samples representing 56 species of ferns and 1 species of lycophyte. We tested 93 samples and germination occurred in 45 of the 56 species of ferns tested. Lab notes from the time of banking suggested that the species that did not germinate were likely dead before storage. These were primarily species with green spores (e.g. Matteuccia sp., Equisetum sp., and Onoclea sp.) for which no germination was observed initially and species with nongreen spores for which only a few spores were banked along with sporangia and frond tissues.
The percent germination was variable and depended on the species (Table 11.2). Germination of spores did not seem to be lower for the older species, and spores of most species stored for ≥20 years showed germination >60% (Table 11.2). When germination was measured in replicate samples (cryovials) of a particular species, no large differences were found between them, confirming the repeatability of the results obtained. In most cases, >80% of the spores that germinated developed laminar gametophytes (Table 11.2), except for some accessions for which germination was very low or plates were highly contaminated. Contamination was mostly by fungi growing out of sori and frond tissues.
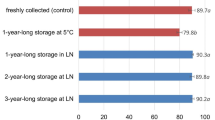
In a few species, the spores were stored at several temperatures (21, 5, −20, and −196 °C) for 10 or 21 years. In these species, spores stored in LN maintained higher rates of germination than spores stored at −20 or 5 °C (Ballesteros et al. in prep.). Actually, for most of these species, germination at 5 or −20 °C was very low (3–10 times lower than germination of spores stored in LN) or nil for both storage times. There was no remaining viability in spores stored at room temperature (21 °C).
3.1.4 Production and Acclimatization of Sporophytes
Gametophytes in the laminar phase from spore cultures were moved to soil by scrapping the surface of the agar medium, washing the gametophytes into a small beaker, and subsequently distributing the gametophytes onto a 1:1 mix of soilless potting mix: pine mulch fines in small plastic boxes (Phytatrays™, Sigma-Aldrich) with lids. When growing on soil, most of the gametophytes developed into the heart-shaped stage after a few weeks. They were sprayed with purified water occasionally to aid in fertilization, and sporophytes usually appeared spontaneously after 3–12 months, depending on the species.
Sporophytes were acclimatized first by replacing the solid lid with a lid with several holes, then by cracking the lid open, and finally by removing the lids but moving the boxes to a tray under a larger dome (Fig. 11.1g). After several weeks, vents in the domes were gradually opened to allow further acclimatization to the ambient greenhouse conditions. In a few cases, sporophytes appeared spontaneously from gametophytes while they were still on nutrient medium. These sporophytes were similarly moved to soil boxes and acclimatized. Sporophytes that were moved to the field (see section below) were also acclimatized from greenhouse conditions to field conditions (see below). Table 11.2 shows the species for which we obtained sporophytes in the greenhouse during the first months of growth using the methods described above.
Aspect of immature (a) and mature (b) sporangia in Dryopteris sp. Aspect of mature (c-left) sporangia and sporangia after spore dehiscence (c-right) in Osmunda regalis. Mature spores dehisced on paper for their collection (d). Gametophytes of Adiantum tenerum, cryopreserved using the encapsulation dehydration procedure, 2 months after resuming growth in vitro Fig. 11.1 (continued) after 20 years of storage in liquid nitrogen, bar = 0.5 cm (e). Spores of Adiantum melanoleucum germinated on 0.8% agar (left) and half-strength MS medium with 0.25% gellan gum (right), 11 weeks after surface sterilization and plating, bar = 2 cm (f). Sporophytes of Polystichum tsus-simense growing on soil under a dome, spores were stored in LN for 21 years (g). Thelypteris patens outplanted back into their native habitat in Florida (Possley, 2014) to help increase the native populations (F)
3.2 Gametophyte Banking
3.2.1 Growth and Storage of Gametophytes
In vitro cultures of gametophytes of six species of fern were banked in 1995, including Adiantum tenerum, A. trapeziforme, Cibotium glaucum, Davallia fejeensis, Drynaria quercifolia, and Phlebodium aureum. Cultures were initiated in the early 1990s from the aseptic in vitro germination of spores. Spores were germinated on a half-strength Murashige and Skoog (1962) medium and the resulting gametophytes maintained on the same medium (Pence 2000a). Both the encapsulation dehydration method (Fabre and Dereuddre 1990) and drying without encapsulation, with and without a 7-day preculture on abscisic acid (ABA), were tested for their effectiveness in preserving viability of the gametophyte tissue through a 1 h exposure to LN (Pence 2000a). Encapsulation dehydration with preculture on ABA provided the best results, and this protocol was subsequently used for preparing tissues for long-term banking of samples. Dried beads were stored in 2 ml cryovials and rapidly cooled by immersion in LN. Vials were stored submerged in LN. A total of 21 samples from the six species were banked.
3.2.2 Testing the Viability of Gametophytes
Gametophytes were recovered after 20 years of storage with the same methods used to test prestorage viability after 1 h exposure to LN. Tissues were grown on 60 x 15 mm petri plates with half-strength MS medium. Tissues were incubated as for spores (above), although at 26 °C, rather than 20 °C. Prestorage viability was scored as the percent of tissue containing alginate beads showing some tissue growth.
3.2.3 Summary of Results
Eight samples (cryovials) were tested representing all six species, two with replicates banked on different dates. After 20 years of storage in LN, tissues from all 6 species resumed growth in at least one replicate (Pence, unpublished). Survivals ranged from 0% to 90% per vial or 39% to 90% per species, while pre-banking survivals ranged from 7% to 92%. The percent regrowth of a sample was often higher than the original viability recorded for the sample, likely due to the small sample size and the variability of the samples, and thus it was not possible to determine if any deterioration of samples had occurred over time in storage. However, the sample sizes proved large enough to provide material for recovery, and the gametophytic fragments resumed growth into viable cultures (Fig. 11.1e).
3.3 Sporophyte Banking
3.3.1 Growth and Storage of Sporophytes
Shoot cultures of sporophytes of the lycophyte, Selaginella uncinata, had been initiated from surface-sterilized shoots that were grown on half-strength MS medium as for gametophyte cultures. Tips, approximately 1 mm in length, were dissected aseptically from the in vitro-grown shoots, treated for 7 days on half-strength MS medium with and without 10 μM ABA, and cryoprotected using the encapsulation dehydration procedure (Pence 2001). Tissues were banked in 1995.
3.3.2 Summary of Results
The survival of samples exposed to LN for 1 h at the time of banking was higher for tissues treated with ABA than for tissues precultured without ABA (Fig. 11.2) (Pence 2001).
Percent survival of shoot tips of S. uncinata, with and without ABA treatment, over time in cryostorage. Data for 0 and 3.5 years taken from Pence (2001)
After 3.5 years in LN, the shoot tips without ABA preculture showed no survival, while there was still survival, although reduced, in the ABA-treated tissues. However, when samples were removed after 20 years, there was no viability in either the ABA-treated or untreated samples. These results suggest that S. uncinata responded to the encapsulation dehydration procedure with some tolerance to desiccation, but that response was enhanced when the tissues were precultured on a medium with ABA. In addition, the ABA preculture appeared to enhance the ability to survive longer in LN storage than tissues without such treatment. These results are significant, in that they demonstrate that even in LN, viability may decline. Such declines have also been observed in short-lived seeds banked in LN (Ballesteros and Pence 2017), and decreases in longevity appear to be related to stresses on the tissues before banking. The results with S. uncinata also demonstrate that factors which improve the ability of tissues to survive the stress of short-term exposures to LN at the time of banking will likely improve the longevity of the tissue during storage, as well.
4 Practical Considerations for Conserving Ferns
4.1 Spore Banking
4.1.1 Fern Spore Maturity and Quality During Collection
Ideally, spores that are stored should be mature, of good quality, and free from other frond tissues. This is important, for example, to avoid the storage of dead or immature spores (Huang et al. 2014), to avoid the storage of spores that have been aged on the frond after dispersal and likely will show poor longevity during storage (Ballesteros et al. 2011; Li and Shi 2015), and also to avoid fungal and bacterial contamination during fern spore germination and gametophyte growth. Fern spores can be sterilized before storage (Agrawal et al. 1993; Beri and Bir 1993; Constantino et al. 2000; Dyer 1979; Fernández et al. 2010; Ford 1992; Pence 2000b; Simabukuro et al. 1998; Whittier and Pintaud 1999); however, this process can damage fern spores and reduce their germination capacity and gametophyte development (Camloh 1999; Simabukuro et al. 1998). Spores collected properly and sown without frond residues are less likely to become contaminated and more likely to produce normal gametophytes (Aragón and Pangua 2004; Colli Aurea and Perez Sonia 1999; Quintanilla et al. 2002; Whittier 1973).
In order to perform an optimal fern spore harvest, it is very important to plan the collection when the sporangia in the fertile leaves or strobili are mature (Bacchetta et al. 2008; Ibars and Estrelles, 2015; Ibars et al. 2011). A good knowledge of the phenology of the fern species and a few field trips to check its maturity will help to optimize the collection date, as is recommended for fern spores, as well as for seeds and pollen (e.g. Bacchetta et al. 2008; FAO 2014). The identification of mature sporangia can vary among species, but generally they will be found on the youngest healthy leaves presenting closed sporangia that are bright in colour (e.g. Figs 11.1b, c). The sporangia should not be white (nor green for nongreen spores, Fig. 11.1a). Sporangia of green spores generally turn dark green when mature (Fig. 11.1c – right), and sporangia of nongreen spores generally turn brown, yellow, or orange, depending on species (Fig. 11.1b). For species with sori covered by indusia, the colour of this structure (turning from green to yellow or brownish) helps to identify the mature sporangia (Bacchetta et al. 2008; Ibars and Estrelles 2015, Fig. 11.1b).
When fertile fronds with mature sporangia are collected, they can be briefly washed to eliminate impurities on the fronds. They can be stored and transported to the laboratory in paper envelopes or, if the transport is done within the same day of collection, in plastic bags (Bacchetta et al. 2008; Ibars and Estrelles 2015; Ibars et al. 2011). Then, in the laboratory, fronds are usually air-dried for a few days under ambient conditions in paper envelopes or sheets, so the spores fall on the surface of the paper (Fig. 11.1d). When fronds have been transported in paper envelopes, these can be used for spores’ collection. Some fern species produce fertile leaves for which spore collection is not as direct as the one indicated above. For example, Matteuccia and Onoclea sp. fertile leaves must be submerged in water for a number of hours, and later the fertile leaves can be dried to release the spores. Without the first hydration step, the fertile leaves do not open (Templeman et al. 1987; Towill and Ikuma 1973).
One of the challenges of dealing with wild species, including ferns, is that it may not always be possible to collect an ideal sample, particularly if the plants are in a location that cannot be visited repeatedly to time the collections to the optimum level of spore maturity. In cases where the sporangia have opened, one may collect any remaining spores from open sporangia (Fig. 11.1c – left) by scraping the frond over paper. However, this can increase the risk of contamination during germination and the high possibility of getting spores of low viability or spores that are negatively affected by LN storage (Li and Shi 2015). If a collection must be made from open sporangia, spores might be germinated in vitro, to grow the gametophytes and then proceed either to gametophyte cryopreservation (described in Sects. 11.3.2 and 11.4.2) or to the growth of sporophytes for spore collection under more controlled conditions (e.g. greenhouse, botanical garden). If spores must be taken while they are still immature, germination may be lower than for mature spores (Huang et al. 2014). However, LN exposure may increase germination (Mikula et al. 2009) or germination rate, as it can with some mature spores (De Brum and Randi 2006; Rogge et al. 2000). Finally, whether the spores are collected at optimal maturity or not, there is the possibility of spores of one or more different species being included in the collection. Such spores may travel by wind to the leaves and be mixed in with the collection. Thus, it is important to monitor the gametophytes and sporophytes produced for the presence of species other than the target species.
4.1.2 Optimal Moisture Content for Fern Spore Storage
Spores in the Frozen Garden were stored dry (open dried at RH about 40% or over silica gel). This desiccation did not seem to affect fern spore viability, whether they were green or nongreen (Pence 2000b). Several authors have recently indicated that both green and nongreen spores are tolerant to desiccation and have greater longevity when stored dry at RHs between 10% and 25% (Ballesteros and Walters 2007a; Ballesteros et al. 2017; Li and Shi 2015; Mikula et al. 2015; Walters et al. 2005). Based on this optimal RH, and taking into account the changes in RH with temperature (and hence to avoid overdrying the spores during LN storage, e.g. Ballesteros and Walters 2011; Vertucci et al. 1994), it is recommended that after spores are collected, they are to be dried for 2–5 days at 20 °C and RHs between 20 and 40% and subsequently stored at the temperature of LN.
4.1.3 Optimal Temperatures for Fern Spore Storage
The results from long-term storage at CREW strongly support an optimal temperature of liquid nitrogen (−196 °C) for long-term storage of fern spores, when compared with −20 °C (e.g. Ballesteros et al. 2011, 2012). Storing in the vapour phase of liquid nitrogen (from −130 to −180 °C) or mechanical freezers providing −80 °C may also be as effective (e.g. Ballesteros et al. 2011, 2012). However, dry storage at −20 °C may be damaging for some species (reviewed in Ballesteros 2010). Poor longevity of spores at −20 °C is probably due to the lipid content and TAG composition of some nongreen spores (Ballesteros and Walters, 2007b), although this may need to be evaluated species by species. For example, Pteris vittata spores stored for about 20 years in both LN and −20 °C showed good viability under both conditions (Ballesteros et al. in prep). Cycles of freezing/defrost (Ballesteros et al. 2012), as would happen if all spores of an accession were stored in one container and it was moved in and out of the freezer each time spores were required, should be avoided at any freezing temperatures. Refrigeration of spores (3–7 °C) or storage of spores at room temperature is not recommended for the long-term conservation of fern spores.
4.2 Gametophytes
Gametophytes are highly adaptable and easily manipulated propagules for ex situ conservation, either in vitro or in cryopreservation.
4.2.1 In Vitro Culture of Gametophytes
Most in vitro cultures of gametophytes have been generated by the aseptic germination of spores, and thus, the considerations described above (Sect. 11.4.1) concerning spore stage, sterilization, and purity are equally important when initiating gametophyte cultures. Media used for in vitro gametophyte growth are usually low in nutrients, and the most commonly used include plain agar or agar or gellan gum with added nutrients, often either Knop’s medium or half-strength Murashige and Skoog (MS) medium (e.g. Marimuthu and Manickam 2011; Mikula et al. 2009; Pence 2000a). For handling during surface sterilization, spores are often enclosed in a paper packet, such as one made by folding filter paper, and the packet is sterilized along with the spores, which are then blotted onto the medium (Pence 2000a). Alternatively, the spores can be suspended in the sterilant and then collected by centrifugation or filtering (de Brum and Randi 2006; Somer et al. 2009). An alternative method for gametophyte culture initiation has been reported for Trichomanes speciosum, in which gemmae were isolated and similarly surface sterilized and cultured (Raine and Sheffield 1997).
The rate of spore germination into gametophytes will vary with the species from days to months, and both germination and growth are influenced by the medium used. In this lab, it has been observed that spores sown on plain agar result in many small individual gametophytes, while spores sown on 0.25% gellan gum containing half-strength MS medium result in fewer spores germinating, but the gametophytes are larger and begin to propagate vegetatively (Fig. 11.1f). Aseptic germination has been reported for many species of leptosporangiate ferns, as well as for several eusporangiate species, including Equisetum spp. (Guillon and Fievet 2003; Kuriyama et al. 1990; Srinivasan and Kaufman 1978) and several Marattiaceae species (Barnicoat et al. 2011). Thus, it appears that gametophyte cultures of many fern species can be readily generated and maintained as in vitro collections, if spores are available. However, while there have been some examples of rare species being conserved as gametophytes in vitro (Ashmore et al. 2011; Marimuthu and Manickam 2011; Pence 2015; Raine and Sheffield 1997), thus far, long-term maintenance of in vitro collections has not been applied extensively to fern ex situ conservation.
4.2.2 Cryopreservation of Gametophytes
Conservation of in vitro-grown gametophyte tissues has more commonly been done through cryopreservation. Fern gametophyte cultures have been shown to be highly adaptable to cryopreservation, particularly with the encapsulation dehydration procedure (Fabre and Dereuddre 1990). This has been the most widely used cryoprotection method and is the one that successfully maintained viability of gametophytes for 20 years in LN (Pence, unpublished). In this procedure, gametophytes or gametophyte fragments are encapsulated in a sphere of alginate gel and cryoprotected using a combination of osmotic dehydration with concentrated sucrose and drying, before rapid cooling by immersion in LN. This method has worked well for most species reported, although a few have not survived the treatment (Barnicoat et al. 2011). Other cryoprotectant procedures have been tested, particularly encapsulation vitrification, in which chemical cryoprotectants are used, to remove water and protect the tissues (Hirai and Sakai 1999). While some survival of fern gametophytes through this method has been reported, it has generally not been as high as with the encapsulation dehydration method (Makowski et al. 2016; Mikula et al. 2010). The use of vitrification without encapsulation has resulted in extreme plasmolysis of the tissues and no survival after LN exposure. However, dehydration without encapsulation has resulted in some survival, especially when the tissues were pretreated with abscisic acid (ABA) (Pence 2000a). Treatment with ABA may mimic natural signals within the plant that provide desiccation tolerance to the tissues, as has been widely documented in moss gametophytes (Proctor et al. 2007).
Actively growing gametophytes appear to survive cryopreservation better than mature, non-growing tissues (Mikula et al. 2009). Whole gametophytes of some species are too large for encapsulation, but they can either be reduced in size by growth on paclobutrazol (Barnicoat et al. 2011) or cut into smaller fragments for encapsulation (Pence 2015). Whether whole or fragment, tissues from gametophytes often undergo damage during cryoprotection and cryopreservation (Mikula et al. 2009), but the high regenerative capacity of the tissues can reconstitute a culture from only a small portion of the tissue and possibly from single cells (Maeda and Ito 1981).
The application of encapsulation dehydration should be to a much wider range of pteridophyte taxa. Gametophyte cryopreservation has been reported primarily for taxa within the leptosporangiate ferns. Gametophytes of one eusporangiate species, Macroglossum smithii, have shown some survival after LN exposure using encapsulation dehydration, although two other species from the same family (Marattiaceae), Marattia purpurascens and M. werneri, did not show any survival (Barnicoat et al. 2011). To our knowledge, there have been no reports of gametophyte cryopreservation in other eusporangiate genera, such as Psilotum, Equisetum, or Ophioglossum, or in gametophytes of lycophyte genera, such as Lycopodium and Selaginella. In addition, methods are needed for species known only from the gametophyte stage (e.g. Farrar 1967) that would allow routine initiation of gametophyte cultures by direct surface sterilization of gametophytes. Finally, more species should be cryopreserved for long-term studies to more clearly understand the survival of these tissues over the course of decades.
While there are many areas for further research, the recovery of gametophyte growth after 20 years in LN, as well as reports from other labs of survival through short-term LN exposure, indicates that methods are available for the long-term ex situ conservation of gametophytes from likely a large number of fern species. However, these methods have been applied to an only a few rare fern species (Barnicoat et al. 2011; Mikula et al. 2009; Pence 2015; Wilkinson 2002) and could be more widely utilized.
4.3 Sporophytes
Like gametophytes, the sporophytes of ferns and lycophytes can be maintained as in vitro collections, or the in vitro-grown tissues can be cryopreserved for long-term ex situ conservation.
4.3.1 In Vitro Culture of Sporophytes
Most in vitro culture lines of sporophytes are initiated from in vitro-grown gametophytes, which, in turn, are initiated from aseptically germinated spores (see Sect. 11.4.2). When this is possible, it may take a few weeks to several years for the gametophytes to generate sporophytes (Pence 2015). However, additional methods for initiating aseptic sporophyte cultures have been reported, including regeneration from rhizomes, runner tips, leaves, leaf callus, and shoot tips, as well as bud scales and other tissues from in vitro-grown sporophytes (Bertrand et al. 1999; Camloh and Ambrozic-Dolinsek 2010; Higuchi et al. 1987; Jha et al. 2013; Li et al. 2017; Pence 2001; Shukla and Khare 2012; Winarto and Teixeira da Silva 2012), depending on the species and its growth habit. In vitro cultures have been reported for several rare species (Ashmore et al. 2011; Pence 2014, 2015; Taha et al. 2011; Yu et al. 2017), and the potential exists for utilizing such cultures for ex situ conservation as in vitro collections and for using them for propagating plants for restoration projects (Aguraiuja 2010a, b; Possley 2014).
4.3.2 Cryopreservation of Sporophytes
Once established, fern sporophyte cultures can provide materials for cryopreservation in a manner analogous to that of shoot tip cryopreservation in angiosperms (Reed 2008). Species with growing tips can provide shoot tips, and this approach has been successful for Selaginella uncinata and Trichomanes punctatum var. floridanum (Pence 2001; Pence 2015). For other species, such as those that grow from a basal meristem, it may be possible to manipulate in vitro culture conditions to generate the meristematic, green globular bodies (GGBs) (Bertrand et al. 1999; Fernández et al. 1996; Higuchi and Amaki 1989; Higuchi et al. 1987). GGBs of Asplenium scolopendrium var. americanum have been successfully cryopreserved (Pence 2015), and further work is needed to demonstrate this approach in other species.
The long-term survival of fern sporophyte tissues should also be explored further. Shoot tips of Selaginella uncinata precultured with ABA survived 3.5 years of cryostorage, while those without ABA preculture did not. However, even the ABA precultured tissues did not survive 20 years in LN. Thus, questions of longevity, as well as the effects of preculture treatments and of the cryoprotection methods on longevity in cryostorage, must be explored further. In the same study of long-term viability at CREW, shoot tips of several angiosperm species showed good survival after 15–20 years in LN (Pence et al. 2017). As with gametophytes, sporophyte tissue banking has been explored less than spore banking for ferns, although sporophytes of some rare ferns have been cryopreserved in CREW’s Frozen Garden for long-term storage. While there may be fewer situations that require sporophyte banking, it can be important for some species, particularly in the context of conservation, when plants are few and difficult to access and/or produce few spores.
5 Ex Situ Conservation of Ferns: Case Study – Rare Ferns of South Florida
In addition to providing in vitro collections and tissues for cryopreservation, in vitro lines of rare fern species can be used to propagate plants for restoration. In collaboration with the Fairchild Tropical Botanic Garden (Miami, USA), CREW has propagated ferns in vitro of several species that are endangered in Florida, including Thelypteris patens, Adiantum melanoleucum, and Odontosoria clavata. These species were received as spores and germinated in vitro and the gametophytes propagated. After several months, sporophytes were produced, which also could be propagated in vitro. Plants were removed from culture and acclimatized in soil for 2–3 months and then sent to FTBG, where they were grown further into larger plants. Two of the species have been outplanted back into their native habitat to help increase the native populations (Possley 2014) (Fig. 11.1h).
6 Conclusions
Several explants from the life cycle of ferns and lycophytes can be used as a ready source of germplasm for ex situ conservation. Storing spores is the most efficient method, but there are situations where storing gametophytes or sporophytes can be extremely useful, as well. There is a body of work that has shown that current methods available for the storage of spores, gametophytes, and sporophytes in liquid nitrogen are relatively straightforward and similar to protocols employed for seeds and non-seed tissues of flowering plants. Our studies have shown that spores and gametophyte cryostorage can provide viable materials after at least 20 years for the implementation of restoration programmes of endangered fern species, and the potential exists for sporophyte tissues as well. However, given the increasing number of fern and lycophyte species of conservation concern, these ex situ conservation methods have been underutilized. There is a need for the publication of guidelines and protocols to encourage increased attention to these groups and to facilitate best practices in future banking efforts. This would allow current seed and plant tissue banks to utilize their expertise and infrastructure to expand their focus to include ferns and lycophytes and to provide guidance to new efforts, thereby helping to secure the diversity of these species into the future.
References
Agrawal DC, Pawar SS, Mascarenhas AF (1993) Cryopreservation of spores of Cyathea spinulosa wall. Ex. Hook. F., an endangered tree fern. J Plant Physiol 142:124–126
Aguraiuja R (2010a) Reinforcement of the populations of the critically endangered endemic fern, Diellia pallid, Kaua’I, Hawwaiian Islands, U.S.A. In: Soorae PS (ed) Global re-introduction perspectives: additional case-studies from around the globe. IUCN/SSC Re-introduction Specialist Group. Abu Dhabi, UAE, pp 319–323
Aguraiuja R (2010b) Conservation introduction of a locally extinct fern species in Estonia during 1998–2008. In: Soorae PS (ed) Global re-introduction perspectives: additional case-studies from around the globe. IUCN/SSC Re-introduction Specialist Group. Abu Dhabi, UAE, pp 345–348
Alexander A, Dudas G, Herren G, Kolben G, Kuhara Y, Murdoch L, Slaven J, Paultre D, Rae N, Rayner J, Vileitaite J, Chamberlain M (2008) Testing the viability of spores held in the fern spore bank at the Royal Botanic Garden, Edinburgh. Newsletter of the Botanical Society of Scotland 91:29–31
Aragón CF, Pangua E (2004) Spore viability under different storage conditions in four rupicolous Asplenium L. taxa. Am Fern J 941:28–38
Arcand NN, Ranker TA (2008) Conservation biology. In: Ranker TA, Haufler CH (eds) Biology and evolution of ferns and lycophytes. Cambridge University Press, Cambridge, pp 257–283
Ashcroft CJ, Sheffield E (2000) The effect of spore density on germination and development in Pteridium, monitored using a novel culture technique. Am Fern J 90:91–99
Ashmore SE, Hamilton KN, Offord CA (2011) Conservation technologies for safeguarding and restoring threatened flora: case studies from Eastern Australia. In Vitro Cell Dev Biol Plant 47:99–109
Bacchetta G, Bueno Sánchez A, Fenu G, Jiménez-Alfaro B, Mattana E, Piotto B, Virevaire M (eds) (2008) Conservación ex situ de plantas silvestres. Principado de Asturias/La Caixa, Principado de Asturias, Spain
Ballesteros D (2010) Conservation of fern spores. In: Kumar A, Fernández H, Revilla-Bahillo A (eds) Working with ferns. Issues and applications. Springer, New York, pp 165–172
Ballesteros D, Pence VC (2017) Survival and death of seeds during liquid nitrogen storage: a case study on seeds with short lifespans. CryoLetters 38(4):278–289
Ballesteros D, Walters C (2007a) Water properties in fern spores: sorption characteristics relating to water affinity, glassy states and storage stability. J Exp Bot 58:1185–1196
Ballesteros D, Walters C (2007b) Calorimetric properties of water and triacylglycerols in fern spores relating to storage at cryogenic temperatures. Cryobiology 55:1–9
Ballesteros D, Walters C (2011) Detailed characterization of mechanical properties and molecular mobility within dry seed glasses: relevance to the physiology of dry biological systems. The Plant J 68:607–619
Ballesteros D, Estrelles E, Walters C, Ibars AM (2011) Effect of storage temperature on green spore longevity for the ferns Equisetum ramosissimum and Osmunda regalis. CryoLetters 32(2):89–98
Ballesteros D, Estrelles E, Walters C, Ibars AM (2012) Effects of temperature and desiccation on ex situ conservation of non-green fern spores. Am J Bot 99:721–729
Ballesteros D, Hill LM, Walters C (2017) Variation of desiccation tolerance and longevity in fern spores. J Plant Physiol 211:53–62
Barnicoat H, Cripps R, Kendon J, Sarasan V (2011) Conservation in vitro of rare and threatened ferns—case studies of biodiversity hotspot and island species. In Vitro Cell Dev Biol Plant 47:37–45
Beri A, Bir SS (1993) Germination of stored spores of Pteris vittata L. Am Fern J 833:73–78
Bertrand AM, Albuerne MA, Fernández H, González A, Sánchez-Tamés R (1999) In vitro organogenesis of Polypodium cambricum. Plant Cell Tissue Organ Cult 57:65–69
Camloh M (1999) Spore age and sterilization affects germination and early gametophyte development of Platycerium bifurcatum. Am Fern J 89:124–132
Camloh M, Ambrozic-Dolinsek J (2010) In vitro regeneration systems of Platycerium. In: Fernández H, Kumar A, Angeles-Revilla A (eds) Working with ferns: issues and applications. Springer Science & Business Media, New York, pp 111–125
Chapman AD (2009) Numbers of living species in Australia and the world. Australian Biodiversity Information Services, Toowoomba, Australia
Colli Aurea MT, Perez Sonia CJG (1999) The effect of red light on the germination of a brazilian pteridophyte. Brazil Arch Biol Technol 422:169–174
Constantino S, Santamaria LM, Hodson E (2000) Storage and in vitro germination of tree fern spores. Bot Garden Microprop News 24:58–60
De Brum FMR, Randi AM (2006) Germination of spores and growth of gametophytes and sporophytes of Rumohra adiantiformis (Forst.) Ching (Dryopteridaceae) after spore cryogenic storage. Rev Bras Bot 29:489–495
De Long JM, Prange RK (2008) Fiddlehead fronds: nutrient rich delicacy. Chronica Hort 48:12–15
Dolinsek JA, Camloh M (1997) Gametophytic and sporophytic regeneration from bud scales of the fern Platycerium bifurcatum (Cav.) C.Chr. in vitro. Ann Bot 80:23–28
Dong S, Zuo Z, Yan Y, Xiang J (2017) Red list assessment of lycophytes and ferns in China. Biodivers Sci 25(7):765–773
Dyer AF (1979) The culture of fern gametophytes for experimental investigation. In: Dyer AF (ed) The experimental biology of ferns. Academic Press, London
Dyer AF (1994) Natural soil spore banks—can they be used to retrieve lost ferns? Biodivers Conserv 3:160–175
Fabre J, Dereuddre J (1990) Encapsulation-dehydration: a new approach to cryopreservation of Solanum shoot tips. CryoLetters 11:413–426
FAO (2014) Genebank standards for plant genetic resources for food and agriculture, Rev. edn, Rome. Food and Agriculture Organization of the United Nations, Rome
Farrar DR (1967) Gametophytes of four tropical fern genera reproducing independently of their sporophytes in the southern Appalachians. Science 155:1266–1267
Farrar DR (2016) Vittaria appalachiana continues to provide insight into the biology of ferns: a commentary on two studies recently published in American Journal of Botany. Am J Bot 103:593–595
Fernández H, Revilla MA (2003) In vitro culture of ornamental ferns. Plant Cell Tissue Organ Cult 73:1–13
Fernández H, Bertrand A, Sánchez-Tamés R (1996) Micropropagation and phase change in Blechnum spicant and Pteris ensiformis. Plant Cell Tissue Organ Cult 44:261–265
Fernández H, Kumar A, Revilla A (eds) (2010) Working with ferns: issues and applications. Springer Science & Business Media, New York
Ford MV (1992) Growing ferns from spores in sterile culture. In: Ide JM, Jermy AC, Paul AM (eds) Fern horticulture: past, present and future perspectives. Intercept, Andover, pp 295–297
From MM (2010) Conservation status and re-introduction of Bermuda’s Governor Laffan fern—an endangered endemic. In: Sourae PS (ed) Global Re-introduction Perspectives—2010: additional Case Studies from around the Globe. IUCN/SSC Re-introduction Specialist Group, Abu Dhabi, UAE, pp 316–318
Guillon JM, Fievet D (2003) Environmental sex determination in response to light and biased sex ratios in Equisetum gametophytes. J Ecol 91:49–57
Higuchi H, Amaki W (1989) Effects of 6-benzylaminopurine on the organogenesis of Asplenium nidus L. through in vitro propagation. Sci Hortic 37:351–359
Higuchi H, Amaki W, Suzuki S (1987) In vitro propagation of Nephrolepis cordifolia Presl. Sci Hortic 32:105–113
Hirai D, Sakai A (1999) Cryopreservation of in vitro-grown axillary shoot-tip meristems of min (Mentha spicata L.) by encapsulation vitrification. Plant Cell Rep 19:150–155
Houser DC, From M, Landry M, Copeland A, Kellar R (2016) Systematics of Diplazium laffanianum (Athyriaceae), a fern species endemic to Bermuda. Am Fern J 106:206–222
Huang Y-M, Lee P-H, Fu C-H, Chang Y-H (2014) The relation between spore maturity and viability of tree ferns. J Natl Taiwan Mus 67(2):45–58
Ibars AM, Estrelles E (2012) Recent developments in ex situ and in situ conservation of ferns. Fern Gaz 19(3):67–86
Ibars AM, Estrelles E (2015) Protocolo de propagación de helechos. Botanic as PPECTS 1 1:1–5
Ibars AM, Gómez Serrano MA, Mayoral O, Estrelles E (2011) Prioridades para la conservación en el ámbito de los helechos en Castilla-La Mancha. In: Hernandez JE, Herranz JM (eds) Protección de la diversidad vegetal y de los recursos fitogenéticos de Castilla-La Mancha. Instituto de Estudios Albacetenses- Botánico de Castilla-La Mancha. Serie I Estudio, Albacete, Spain, pp 191–213
IUCN (2017) Table 3a: Status category summary by major taxonomic group (plants). In: The IUCN red list of threatened species, version 2017–2. Last updated 14 Sept 2017, http://cmsdocs.s3.amazonaws.com/summarystats/2017-2_Summary_Stats_Page_Documents/2017_2_RL_Stats_Table_3b.pdf. Accessed 29 Sept 2017
Jha TB, Mukherjee S, Basak A, Adhikari J (2013) In vitro morphogenesis in Selaginella microphylla (Kunth.) Spring. Plant Biotechnol Rep 7:239–245
Kamachi H, Noguchi M (2012) Negative gravitropism in dark-grown gametophytes of the fern Ceratopteris richardii. Am Fern J 102:147–153
Khan S, Raziq M, Kayani HA (2008) In vitro propagation of bird’s nest fern (Asplenium nidus) from spores. Pakistan J Bot 40:91–97
Kuriyama A, Sugawara Y, Matsushima H, Takeuchi M (1990) Production of sporophytic structures from gametophytes by cytokinin in Equisetum Arvense. Naturwissenschaften 77(1):31–32
Li D, Pritchard HW (2009) The science and economics of ex situ plant conservation. Trends Plant Sci 14:614–621
Li Y, Shi L (2015) Effect of maturity level and desiccation process on liquid nitrogen storage of green spores of Osmunda japonica. Plant Cell Tissue Organan Cult 120:531–538
Li X, Han J-D, Fang Y-H, Bai S-N, Rao G-Y (2017) Expression analyses of embryogenesis-associated genes during somatic embryogenesis of Adiantum capillus-veneris L. in vitro: new insights into the evolution of reproductive organs in land plants. Front Plant Sci 8:1–12
Liu Y, Wujisguleng W, Long C (2012) Food uses of ferns in China: a review. Acta Soc Bot Pol 81(4):263–270
Maeda M, Ito M (1981) Isolation of protoplasts from fern prothallia and their regeneration to gametophytes. Bot Mag Tokyo 94:35–40
Makowski D, Rybczyński JJ, Mikula A, Klimaszewska K (2015) A simple way to overcome the recalcitrance of the water fern Ceratopteris thalictroides (L.) Brongn. to cryopreservation. Acta Soc Bot Pol 84:385–388
Makowski D, Tomiczak K, Rybczyński JJ, Mikula A (2016) Integration of tissue culture and cryopreservation methods for propagation and conservation of the fern Osmunda regalis L. Acta Physiol Plant 38:1–12
Mannan M, Maridass M, Victor B (2008) A review on the potential uses of ferns. Ethnobotanical Leaflets 12:281–285
Marimuthu J, Manickam VS (2011) Ex situ conservation of two threatened ferns of the Western Ghats through in vitro spore culture. J Threat Taxa 3:1919–1928
Martin KP, Sini S, Zhang CL, Slater A, Madhusoodanan PV (2006) Efficient induction of apospory and apogamy in vitro in silver fern (Pityrogramma calomelanos L.) Plant Cell Rep 25:1300–1307
Matsuura T, Sugimura K, Miyamoto A, Tanaka H, Tanaka N (2014) Spatial characteristics of edible wild fern harvesting in mountainous villages in northeastern Japan using GPS tracks. Forests 5:269–286
Mehltreter K, Walker LR, Sharpe JM (eds) (2010) Fern ecology. Cambridge University Press, Cambridge
Mikula A, Jata K, Rybczynksi JJ (2009) Cryopreservation strategies for Cyathea australis (R.Br.) Domin. CryoLetters 30:429–439
Mikula A, Makowski D, Walters C, Rybczynski JJ (2010) Exploration of cryo-methods to preserve tree and herbaceous fern gametophytes. In: Fernández H, Kumar A, Angeles-Revilla A (eds) Working with ferns: issues and applications. Springer Science & Business Media, New York, pp 173–192
Mikula A, Tomiczak K, Makowski D, Niedzielski M, Rybczynski JJ (2015) The effect of moisture content and temperature on spore aging in Osmunda regalis. Acta Physiol Plant 37:229–240
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497
Ong BL, Ng ML (1998) Regeneration of drought-stressed gametophytes of the epiphytic fern, Pyrrosia piloselloides (L.) price. Plant Cell Rep 18:225–228
Page CN, Dyer AF, Lindsay S, Mann DG (1992) Conservation of Pteridophytes: the ex situ approach. In: Ide JM, Jermy AC, Paul AM (eds) Fern horticulture: past, present and future perspectives. Intercept, Andover, pp 269–278
Pence VC (2000a) Cryopreservation of in vitro grown fern gametophytes. Am Fern J 90:16–23
Pence VC (2000b) Survival of chlorophyllous and nonchlorophyllous fern spores through exposure to liquid nitrogen. Am Fern J 90:119–126
Pence VC (2001) Cryopreservation of shoot tips of Selaginella uncinata. Am Fern J 91:37–40
Pence VC (2004) Ex situ conservation methods for bryophytes and pteridophytes. In: Guerrant Jr. EO, Havens K, Maunder M (eds) Ex situ plant conservation. Supporting species survival in the wild. Island Press,Washington, DC, pp 206–227
Pence VC (2008a) Ex situ conservation of ferns and lycophytes – approaches and techniques. In: Ranker TA, Hauffler CH (eds) Biology and evolution of ferns and lycophytes. Cambridge University Press, Cambridge, pp 284–300
Pence VC (2008b) Cryopreservation of bryophytes and ferns. In: Reed BM (ed) Plant cryopreservation: a practical guide. Springer, New York, pp 117–140
Pence VC (2014) In vitro propagation and cryopreservation of the endangered filmy fern, Trichomanes punctatum subsp. floridanum (Hymenophyllaceae). Fern Gaz 19:307–317
Pence VC (2015) Propagation and cryopreservation of Asplenium scolopendrium var. americanum, the American hart’s-tongue fern. Am Fern J 105:211–225
Pence VC, Philpott M, Culley TM, Plair B, Yorke SR, Lindsey K, Vanhove A-C, Ballesteros D (2017) Survival and genetic stability of shoot tips of Hedeoma todsenii R.S. Irving after long-term cryostorage. In Vitro Cellular and Developmental Biology Plant 53(4):328–338
Pennisi E (2010) Tending the global garden. Science 329:1274–1277
Pérez-García B, Reyes-Jaramillo I (1993) Helechos: propagación y conservación. Ciencias 30:10–17
Possley J (2014) Fern conservation in a biodiversity hotspot. The Palmetto 31(2):1–9
Pritchard HW, Nadarajan J, Ballesteros D, Thammasiri K, Prasongsom S, Malik SK, Chaudhury R, Kim H-H, Lin L, Li W-Q, Yang X-Y, Popova E (2017) Cryobiotechnology of tropical seeds – scale, scope and hope. Acta Hortic 1167:37–47
Proctor MCF, Oliver MJ, Wood AJ, Alpert P, Stark LR, Cleavitt NL, Mishler BD (2007) Desiccation-tolerance in bryophytes: a review. Bryologist 110:595–621
Quintanilla LG, Amigo J, Pangua E, Pajaron S (2002) Effect of storage method on spore viability in five globally threatened fern species. Ann Bot 904:461–467
Raine ACA, Sheffield E (1997) Establishment and maintenance of aseptic culture of Trichomanes speciosum gametophytes from gemmae. Am Fern J 87:87–92
Rajasekharan PE (2015) Gene banking for ex situ conservation of plant genetic resources. In: Bahadur B, Rajam MV, Sahijram L, Krishnamurthy KV (eds) Plant biology and biotechnology Vol II: Plant genomics and biotechnology. Springer, New Delhi, pp 445–459
Reed BM (ed) (2008) Plant cryopreservation: a practical guide. Springer, New York
Rogers SMD, Banister S (1992) Micropropagation of Notholaena. Hortscience 27:1224–1225
Rogge GD, Viana AM, Randi AM (2000) Cryopreservation of spores of Dicksonia sellowiana: an endangered tree fern indigenous to South and Central America. CryoLetters 21:223–230
Sara SC, Manickam VS (2007) In vitro developmental ontogeny and life cycle of a rare fern species – Thelypteris confluens (Thunb.) Morton. Indian J Biotechnol 6:372–380
Shukla SP, Khare PB (2012) In vitro shoot regeneration via caulogenesis in fern, Pteris vittata L. J Environ Biol 33:683–687
Simabukuro EA, Dyer AF, Felippe GM (1998) The effect of sterilization and storage conditions on the viability of the spores of Cyathea delgadii. Am Fern J 88:72–80
Somer M, Arbesu R, Menéndez V, Revilla MA, Fernández H (2009) Sporophyte induction studies in ferns in vitro. Euphytica 171:203–210
Srinivasan JC, Kaufman PB (1978). Sex expression in Equisetum scirpoides in vitro. Phytomorphology 28:331–5
Stamps RH (2006) Irrigation and nutrient management practices for commercial leatherleaf fern production in Florida. University of Florida, IFAS, Florida
Taha RM, Haron NW, Wafa SN (2011) Morphological and tissue culture studies of Platycerium coronarium, a rare ornamental fern species from Malaysia. Am Fern J 101:241–251
Templeman TS, DeMaggio AE, Stetler DA (1987) Biochemistry of fern spore germination: globulin storage proteins in Matteuccia struthiopteris L. Plant Physiol 85:343–349
Towill LR, Ikuma H (1973) Photocontrol of the germination of Onoclea spores. Plant Physiol 51:973–978
Vertucci CW, Roos EE, Crane J (1994) Theoretical basis of protocols for seed storage III. Optimum moisture contents for pea seeds stored at different temperatures. Ann Bot 74(5):531–540
Walters C, Hill LM, Wheeler LJ (2005) Dying while dry: kinetics and mechanisms of deterioration in desiccated organisms. Integr Comp Biol 45:751–758
Whittier DP (1973) The effect of light and other factors on spore germination in Botrychium dissectum. Can J Bot 51:1791–1794
Whittier DP, Pintaud JC (1999) Spore germination and early gametophyte development in Stromatopteris. Am Fern J 89:142–146
Wilkinson T (2002) In vitro techniques for the conservation of Hymenophyllum tunbrigense (L.) Sm. Fern Gaz 16:458
Winarto B, Teixeira da Silva JA (2012) Improved micropropagation protocol for leatherleaf fern (Rumohra adiantiformis) using rhizomes as donor explants. Sci Hortic 140:74–80
Yu R, Zhang G, Li H, Cao H, Mo X, Gui M, Zhou X, Jiang Y, Li S, Wang J (2017) In vitro propagation of the endangered tree fern Cibotium barometz through formation of green globular bodies. Plant Cell Tissue Organ Cult 128:369–379
Acknowledgements
We would like to thank Callihan A and Lindsey K for their contribution to the propagation and acclimation of diverse fern species at CREW and Thomas A and Stewart K for the work on the initial banking of spores and gametophytes. Also special thanks to Barber S, Pritchard H, Dickie J, Davies R (UK), Estrelles E (Spain), Mikula A (Poland), Magrini S (Italy), Walters C, Hill L, Wolkis D (USA), Reyes-Jaramillo I (Mexico), Randi M (Brazil), Sershen (South Africa), Huang YM (Taiwan), Cicuzza D, Bin W, Yang X-Y (China), Schnell J, McGill C (New Zealand), and Offord C (Australia) for their contributions in the development of Table 11.1 and Suggii N and From M for information on in vitro sporophyte collections. Fern spore, gametophyte, and sporophyte collections used in this work would not be possible without the help and the donations of multiple collectors and researchers from diverse institutions, including Bannister S, Yoshinaga A, Kawakami K, and staff of the Krohn Conservatory.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Ballesteros, D., Pence, V. (2018). Fern Conservation: Spore, Gametophyte, and Sporophyte Ex Situ Storage, In Vitro Culture, and Cryopreservation. In: Fernández, H. (eds) Current Advances in Fern Research. Springer, Cham. https://doi.org/10.1007/978-3-319-75103-0_11
Download citation
DOI: https://doi.org/10.1007/978-3-319-75103-0_11
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-75102-3
Online ISBN: 978-3-319-75103-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)