Abstract
Knowledge about the mycorrhizal root traits of plants is critical for understanding ecosystem processes from landscape to global scale. In spite of >130 years of research, information about the mycorrhizal status of plants is scant for multiple taxonomic groups and geographic regions. By critically evaluating published information about mycorrhizal associations from the plant perspective and integrating this information with plant phylogeny, we assigned altogether 335 ectomycorrhizal (EcM) plant genera (ca. 8500 species) into 30 monophyletic lineages. Considering low representation of EcM habit in Gnaphalieae, Myrtoideae, Goodeniaceae and Acacia s.str., we estimate that approx. 6000–7000 plant species from 250 to 300 genera are able to establish EcM symbiosis. We nominated further 22 plant genera (comprising 76 species) that may potentially exhibit EcM habit based on their close phylogenetic proximity to these known EcM groups. EcM plants thus constitute 1.7–2.4% of all accepted higher plant (Embryophyta) species. EcM habit has evolved and persisted two times in various groups of gymnosperms and 28 times in angiosperms over a vast time interval since the Early Jurassic. In addition to these multiple gains, we also recovered several potential losses of EcM habit in Fagales, two groups of Fabales, two groups of Asterales and Myrtoideae that could be attributed to shifts to association with nitrogen-fixing bacteria, shrubby or herbaceous life form or wetland habitat. There is still much confusion about the mycorrhizal status in multiple families where conflicting reports exist and incorrect assignments have rooted themselves deeply in the literature. We also discuss the reasons for conflicting reports and point to further research needs in critical taxa to improve our overall understanding about the evolution of ectomycorrhizal symbiosis in plants.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Ectomycorrhizal plants
- Gains and losses of mutualism
- Nitrogen fixation
- Dual symbiosis
- Pinaceae
- Incorrect determinations
19.1 Introduction
Ectomycorrhizal (EcM) symbiosis has evolved multiple times in plants and fungi (Brundrett 2009; Tedersoo and Smith 2013). Similarly to fungi in general, there is a lot of controversy in understanding the fungal root association of plant species. This can be partly attributed to environmental impact in arbuscular mycorrhizal (AM) associations, where facultatively mycorrhizal plants are common and development of root fungal structures depends on edaphic, floristic and climatic conditions as well as seasonality and ontogeny of plants (Smith and Read 2008). By contrast, the vast majority of EcM plants are obligately mycotrophic, and conflicts in assignment of mycorrhizal status seem to arise more from alternative definitions of the association (Brundrett 2009).
Here we define EcM and EcM-like associations based on the structure, phylogeny and putative function. At least two of these three criteria should be met for considering the associations to be EcM. First, Hartig net and fungal mantle (sheath) are the main structural characteristics of EcM, but these may be incompletely developed or patchy, as often seen in the EcM of herbs and shrubs. EcM associations of the ectendomycorrhiza subtype may additionally exhibit intracellular hyphal development that is characteristic to certain plant-fungal combinations (Yu et al. 2001). Second, EcM plants form phylogenetically distinct groups, although reversal to non-EcM habit (unlike in EcM fungi) or switch to mycoheterotrophy may have occurred. Pyroloid and monotropoid subtypes of EcM in Pyroleae and Monotropeae, respectively, are linked to the arbutoid subtype of Arbuteae within Ericaceae and are considered as EcM (Brundrett 2004). Similarly, mycorrhiza of Pisonia spp. with transfer cells is considered as a subtype of EcM. Third, EcM associations should also be essentially beneficial to both partners, but Monotropeae, Pterosporeae and perhaps Pyroleae do not fulfil this criterion. The phylogeny criterion clearly places Monotropeae and Pterosporeae among EcM associations but keeps orchids separate as they have evolved exclusively intracellular associations with EcM fungi secondarily and even photosynthetic orchids provide little if any benefits to fungi (Cameron et al. 2008). Furthermore, EcM plants also associate with mutualistic fungi from well-known EcM fungal lineages (Tedersoo and Smith 2013), with no known exceptions. The first EcM plant group that evolved certainly developed EcM associations with ‘previously unrecognised’ fungi. Theoretically, newly emerging EcM plants may associate with novel fungal groups, but this has not yet happened or these evolving associations have not persisted (see below). From this perspective, superficial root associations of Entoloma clypeatum group and Rosaceae (and Ulmus) and Helotiales-Graffenrieda (Melastomataceae) are to be considered non-EcM.
The oldest known EcM associations involve Pinaceae. The fossil records of unequivocal mycorrhizal structures of Pinaceae date back to the Eocene, although genera of the family evolved in the Late Jurassic or Early Cretaceous (LePage 2003). Since the Late Cretaceous and throughout Tertiary, many extant EcM groups of plants and fungi have evolved (Chaps. 1 and 20), followed by subsequent radiation and dispersal. In the last decade, several plant families have been deeply studied from the phylogenetic and biogeographic perspective that greatly adds to our understanding of the evolution of functional traits as well as biotrophic interactions with fungi, actinomycetes and other organisms (e.g. Werner et al. 2014).
In this synthesis, we critically assess the EcM status of plant genera based on published literature, personal observations as well as phylogenetic evidence. We also propose a number of genera that are potentially EcM based on their phylogenetic position but with no known root-level study. Finally, we discuss the issues in recognition of EcM symbiosis, ongoing evolution and groups with dual mycorrhiza.
19.2 Data Sources
We have compiled global literature about mycorrhizal status of plants for >10 years, also retrieving decades old literature based on the citations in Harley and Harley (1987), Wang and Qiu (2006), Koele et al. (2012) as well as Google Scholar. We carefully evaluated the descriptions of mycorrhizal status of EcM and putatively EcM plants. We also noted the inoculation and synthesis trials and growth benefits of plants. Based on the methodology, sample size and conflicts with other sources, we determined the reliability of particular studies when interpreting the mycorrhizal status.
Studies on plant phylogeny and molecular dating were searched from Google Scholar by combining names of particular plant genera, families or orders with ‘phylogeny’ and ‘molecular dating’ and ‘biogeography’ as key words. In addition, we searched the Angiosperm Phylogeny Website (www.mobot.org) for additional sources of literature as they often referred to information hidden in supplementary materials and not found by web search engines. We also used the tree file of the most comprehensive vascular plant phylogeny (Zanne et al. 2014). These different sources of phylogenetic information were combined to separate EcM plant genera into monophyletic lineages, allowing reversals to non-EcM habit. We refer to these lineages by taxon names, because in nearly all cases, the lineages fit into particular species, genera, (sub)tribes, (sub)families or orders.
Our consideration of mycorrhizal associations is based on genus level, because members of the same genus usually share the same mycorrhizal status, with multiple notable exceptions in Australian plants (Wang and Qiu 2006; Brundrett 2009). Most economically and ecologically important woody genera have been revised based on molecular phylogenetic tools, which has increased the value of the generic rank. Species-level information is also too sparse for specific conclusions. Plant taxonomy and species richness follows the Plant List (www.theplantlist.org). We follow Werner et al. (2014) and Benson et al. (2004) regarding rhizobial and actinorhizal associations, respectively.
19.3 Evolution of Ectomycorrhizal Habit
Critical evaluation of mycorrhizal and plant phylogenetic literature enabled us to distinguish 30 plant lineages that most probably evolved EcM associations independently (Fig. 19.1; Table 19.1). Searches through plant phylogenies revealed that 335 plant genera can be considered EcM (Table 19.2). Of these groups, 184 (54.9%) plant genera were regarded as ectomycorrhizal based on direct morphological evidence, whereas the remaining 151 genera were considered as belonging to EcM groups based on the monophyly criterion, although only AM has been reported in nine of these genera (see Supporting Information: http://dx.doi.org/10.15156/BIO/587454). The 335 putatively EcM genera were comprised of ca. 8500 species based on the Plant List (except Miller and Seigler 2012 for Acacia s.str.). Since <10% of these species exhibit reports on mycorrhizal status, it is highly possible that several genera and multiple species do not function as ectomycorrhizal (see Sect. 19.4.10). We estimate that approx. 6000–7000 species from 250 to 300 genera are truly capable of forming EcM associations.
Distribution of 30 ectomycorrhizal plant lineages (red branches and names) in the collapsed dated spermatophyte phylogram of Zanne et al. (2014), with improved taxonomic resolution of Fabaceae from LPWG (2017) and de la Estrella et al. (2017). Asterisks indicate major reversal events to predominantly non-mycorrhizal habit according to Brundrett (2009). To illustrate the evolution of EcM symbiosis in tripartite associations with nitrogen-fixing rhizobia and Frankia actinobacteria, these interactions are indicated as branches and clades in blue; F+ and F− (green) denote evolution and loss of N fixation, respectively. Data about N fixation are derived from Werner et al. (2014) and searches of literature for minor uncovered Fabaceae groups. Taxa above the order rank are highlighted in bold. The bar indicates rough age estimates for higher taxa (Zanne et al. 2014), but these are not refined for Detarioideae and relatively recently derived EcM plant groups
Phylogenetic analyses revealed 22 potentially EcM genera (comprising 76 species) that represented sister groups to known EcM plant lineages or critical clades within large EcM groups that had secondarily lost EcM habit in certain occasions (see Sect. 19.7; Fig. 19.2). Thus, the root systems of representative species of these 170 genera nested within or closely affiliated to EcM groups certainly warrant further investigation for better understanding the evolution and distribution of EcM habit.
Native and assisted distribution of top 22 most wanted plant genera that have no information about mycorrhizal status but are placed in sister position to known EcM lineages. Colours are grouped according to phylogenetic affinities as indicated in parentheses, and closely related genera are further distinguished by symbols. Numbers in parentheses indicate the number of species. Note that the European and American distribution of the three Juglandaceae genera represents their introduced range. Similarly, Hawaiian and South African distribution of Syncarpia indicates its introduced range. All data are based on GBIF (accessed 17.12.2016) records that include coordinates
Taxonomic analysis of EcM plant lineages revealed that EcM habit evolved mostly from AM ancestors, which is consistent with Brundrett (2009). However, EcM symbiosis evolved in at least five occasions from non-mycorrhizal or facultatively AM-dependent ancestors (Coccoloba, Persicaria vivipara, Gymnopodium and Pisonieae within Caryophyllales and Kobresia within Poales). The latter finding is in a strong conflict with Maherali et al. (2016) who suggested that only AM habit can be ancestral to EcM habit. However, the authors excluded most of the above-mentioned groups from their final analysis, which must have strongly biased their results.
Of all 30 EcM plant lineages, Gnetum and Pinaceae represent gymnosperms, whereas all others belong to angiosperms The Fabaceae family alone includes seven EcM groups. The Myrtoideae represent the most genus-rich and species-rich EcM plant group (Table 19.2). Two additional Australian groups, viz. Thysanotus and Lobelia, are considered to possess EcM-like root associations, with distinct root anatomy and uncertain mode of nutrition (see Sect. 19.5; Chap. 17).
Integrating the information from community studies of EcM fungi and EcM plant lineages as described here reveals that there are no plant lineage-specific fungal lineages, although certain plant genera may associate with narrow fungal clades. This indicates that the evolution of EcM symbiosis in plants is linked to pre-existing fungal lineages and vice versa. This is a parsimonious scenario that would require critical modification of gene expression in only a single partner to become connected into a mycorrhizal network of a particular type. The lack of unique plant-fungal combinations furthermore indicates that the evolution of the first EcM plant-fungus association was an extremely rare event, which probably occurred and persisted only once or a few times. Unfortunately, there is no information, whether Pinaceae represent the very first EcM plant lineage or whether there was another, now extinct gymnosperm group. Strikingly, all known EcM fungal lineages are much younger than Pinaceae, suggesting that extinct groups of EcM fungi may have primarily associated with plants in the Jurassic period. Divergence times for only a small proportion of EcM fungal lineages have been studied, with oldest groups dating back to the Mid-Cretaceous (Chap. 1). Among the unstudied groups, there are no lineages that could be suspected of being really ancient, although the /cantharellus and /clavulina and some pezizalean lineages such as /tuber-helvella (Bonito et al. 2013) may potentially exceed 100 million years.
Given that certain EcM fungi associate with liverworts, it is possible that the necessary genetic mechanisms of establishing mutualism evolved long before the modern EcM anatomy evolved. However, phylogenetic evidence suggests that the association of Aneuraceae spp. (incl. Aneura = Cryptothallus mirabilis) with EcM Tulasnella sp. and ericoid mycorrhizal (ErM) fungi has evolved secondarily and relatively recently (Pressel et al. 2010). Except for a few instances, most of the potentially ancient associations with Endogonales in liverworts, hornworts and other lower plants are unrelated to endogonaceous EcM lineages (Yamamoto et al. 2015), but certainly more sequence data are required from both liverwort thalli, and roots of vascular plants are needed to understand their role in EcM and AM symbioses (Orchard et al. 2017). Taken together, it is more likely that the partially mycoheterotrophic lower plants switched to EcM plant lineages rather than these were ancestrally present in these bryophytes and then evolved to associate with EcM gymnosperms and angiosperms.
19.4 Ectomycorrhizal Plant Lineages
19.4.1 Pinales
Pinaceae is the oldest extant ectomycorrhizal plant group that consists of 11 extant genera of trees. The genera Pinus and Picea were described as ectomycorrhizal in the pioneering study of Frank (1885), but similar root structures were described several decades earlier. Within Pinaceae, only the narrowly endemic genera Nothotsuga and Pseudolarix remain unconfirmed in terms of EcM habit but are also expected to have EcM. In natural conditions, short roots of Pinaceae are typically fully converted to EcM, but in Cedrus EcM colonisation typically remains <50% in native habitats (L. Tedersoo, unpubl.). In contrast to other EcM plant genera, species of Pinus exhibit characteristic bifurcately branching root tips. Pinaceae serve as hosts for a wide variety of fungi, but the local diversity in Pinaceae habitats tends to be lower than that in temperate deciduous forests (Tedersoo et al. 2012, 2014) probably because of highly acidic needle litter. Several small and recently evolved EcM fungal lineages are associated only with Pinaceae (Tedersoo and Smith 2013), but this could be due to their preference for acidic soils and paucity of studies of angiosperm roots in conifer forests. It is notable that older fungal lineages tend to have included Pinaceae in their host range relatively recently, indicating that the ancient fungal associations were phylogenetically relatively restricted. The family Pinaceae diverged from other extant gymnosperms roughly 340–320 Ma and radiated to extant genera since 198–175 Ma (average estimates: Leslie et al. 2012; Lu et al. 2014), although one conflicting study indicates only half that age (Crisp and Cook 2011).
19.4.2 Gnetales
The genus Gnetum is another gymnosperm group that forms EcM. In contrast to Pinaceae, this group represents mostly climbers, from which two species of trees evolved once in Indo-Malay. Similarly to Pinaceae, the root system of Gnetum is coarse with thick and conspicuous EcM. However, the EcM anatomy of Gnetum is substantially different from those of Pinaceae and any other plant (Brundrett 2009: Fig. 7a). The fungal interface in Gnetum occurs above the epidermis and consists of many fingerlike projections (root hairs) in a matrix of hyphae. Epidermal cells in these roots are also exceptionally narrow and densely packed. The level of EcM colonisation varies strongly, but all plants seem to be EcM (L. Tedersoo, unpubl.). Gnetum is characterised by extremely low EcM fungal richness that is restricted to a few species of Scleroderma in the liana-forming species (Bechem and Alexander 2012). The tree-forming G. gnemon exhibits somewhat greater fungal richness with still a prominent role of Scleroderma (Tedersoo and Põlme 2012). Although Gnetum diverged from the AM Welwitschia 130 Ma, modern groups of Gnetum radiated since 26 Ma, indicating its recent rather than ancient origin (Won and Renner 2006).
19.4.3 Fagales
The order Fagales is likely to be the oldest angiosperm EcM group that is represented by mostly trees and bushes both in the Northern and Southern Hemispheres. Since >80% of genera of Fagales are EcM, it is likely that EcM habit is ancestral in this group (Larson-Johnson 2015). Nothofagaceae represents the earliest diverging branch with current distribution in relicts of Gondwana. Within Betulaceae (incl. Coryloideae), Alnus is the only genus to associate with N2-fixing Frankia actinobacteria. The monotypic Central American Ticodendraceae family is closely related to Betulaceae, and it has been proven EcM very recently (S. Põlme et al. unpubl.). The Southern Hemisphere Casuarinaceae family represents a sister group to Betulaceae + Ticodendraceae (Larson-Johnson 2015). Within this group, association with Frankia actinobacteria has probably evolved independently. EcM formation is normally present in the genus Allocasuarina but more occasional in Casuarina. AM symbiosis is always present in their roots, but nodules and EcM may be secondarily lacking, depending on species, plant age and soil properties (Reddell et al. 1986). Two additional Casuarinaceae genera, Ceuthostoma and Gymnostoma, have probably fully lost their capacity to form EcM (Duhoux et al. 2001), but certainly more information is needed. Fagaceae are certainly the most widely distributed family of Fagales that comprise only EcM-forming genera such as Quercus and Fagus. Besides the Casuarinaceae family, Juglandaceae represents another group that contains both EcM-forming and non-EcM members. The genera Engelhardia, Oreomunnea, Alfaroa and Carya form a monophyletic group that has been proven to associate with EcM fungi. Besides the EcM groups, Juglandaceae comprise at least one non-EcM genus, i.e. Juglans. Although sporadic reports on EcM exist, the root systems of Juglans have an architecture similar to that of Fraxinus with elongated short roots that is not seen in any EcM groups (except Alnus). There are several genera of Juglandaceae endemic to East Asia (Cyclocarya, Pterocarya, Platycarya) with no information about their mycorrhizal status. Within Fagales, the actinorhizal family Myricaceae seems to have completely lost the capacity to form EcM (but see the probably incorrect report of Sharma et al. 1986). At the family level, there is no information about the mycorrhizal status of Rhoipteleaceae, a narrow endemic of South China. Given the accumulated information, we hypothesise that Fagales gained the EcM-forming ability once, with multiple consequent losses. Development of actinorhizal symbiosis may be one of the causes for these losses (Myricaceae, Casuarinaceae) and for reduced EcM colonisation (Casuarinaceae, Alnus). This does not, however, explain the non-EcM habit of Juglans that typically inhabits EcM-dominated forests. It is possible that the strongly allelopathic biocide juglone has evolved to prevent EcM formation in Juglans spp., in which this substance is particularly abundant. The evolutionary history of Fagales has been relatively well established compared with other plant groups due to outstanding fossil record and economic importance. The EcM Fagales diverged from Hamamelidaceae some 98 Ma. The extant fagalean families diverged between 94 Ma and 72 Ma. The putatively non-EcM groups Gymnostoma, Myricaceae, Juglans and Ceuthostoma diverged from the closest EcM taxa 59 Ma, 56 Ma, 45 Ma and 43 Ma, respectively (Larson-Johnson 2015). Members of Fagales differ strongly in EcM colonisation and the diversity of fungi supported. The actinorhizal genera Alnus and these of Casuarinaceae exhibit relatively low level of colonisation, and these groups harbour a limited set of fungi (Põlme et al. 2013), although molecular data are virtually lacking for Casuarinaceae. Quercus spp. and Juglandaceae spp. are typically moderately colonised by EcM fungi, whereas most groups in Betulaceae (except Alnus), Fagaceae and Nothofagaceae are heavily colonised (>90%). These three families harbour very high diversity of EcM fungi both in Northern and Southern Hemispheres (Tedersoo et al. 2012, 2014). Several EcM fungal lineages are specific to Nothofagus spp. or shared with neighbouring plants in Australia (Tedersoo et al. 2010a).
19.4.4 Caryophyllales
Six phylogenetically distinct groups of EcM plants are recognised within the order of Caryophyllales (Cuenoud et al. 2002; Schuster et al. 2013). The Pisonieae tribe, Achatocarpus and Asteropeia belong to Nyctaginaceae, Achatocarpaceae and Asteropeiaceae families, respectively, whereas Persicaria vivipara, Coccoloba and Gymnopodium belong to Polygonaceae.
Within the Pisonieae tribe, trees and shrubs belonging to Pisonia, Neea and Guapira contain EcM species. All species of the two latter genera are always EcM, but not all species of Pisonia form EcM (Hayward and Hynson 2014). Most of the EcM Pisonia species occur in South and Central America, whereas P. grandis inhabits much of the tropical Oceania (Chap. 20). Except for P. sandwichiensis in Hawaii, several phylogenetically distant endemic species of Pisonia inhabiting the islands of Pacific and Indian oceans are non-ectomycorrhizal and should be transferred to a new genus (Hayward and Hynson 2014). In addition to the genera Pisonia, Neea and Guapira, the monotypic Pisoniella belongs to this group based on phylogenetic analyses (Douglas and Manos 2007). On a morphological basis, Neeopsis, Grajalesia and Cephalomandra may also be related to this EcM group (Douglas and Spellenberg 2010), but no phylogenies are available for these small genera. Species and genera of Pisonieae exhibit extremely specific (P. grandis: Suvi et al. 2010) or strongly specific (Neea, Guapira: Tedersoo et al. 2010b) associations with EcM fungi. In all these genera, the EcM colonisation may be very low, and seedlings are not always associated with EcM fungi (L. Tedersoo, pers. obs.). In P. grandis, specific transfer cells extending from Hartig net to epidermal cells are characteristic anatomic features of EcM (Ashford and Allaway 1982). No specific age estimates exist for the EcM group, but most probably EcM habit evolved between 35 Ma and 20 Ma (Douglas and Manos 2007; Zanne et al. 2014).
Achatocarpus is a small family of trees in Central and South America. The EcM habit of Achatocarpus sp. was convincingly illustrated only recently, and several fungal groups are associated (Alvarez-Manjarrez and Garibay-Orijel 2015; J. Alvarez-Manjarrez, pers. comm.). Phaulothamnus constitutes a sister genus to Achatocarpus, but there is no information about its mycorrhizal status so far. There are no specific phylogenetic or biogeographic studies involving Achatocarpaceae, but Zanne et al. (2014) estimate this group to date back to <12 Ma.
The Polygonaceae is a predominantly non-mycorrhizal family, but there are some conflicting reports (Andrade et al. 2000) that may be derived from attention to AM colonisation. The South and Central American genus Coccoloba contains only EcM species that are among the dominant trees in maritime sand dunes or subcanopy trees, bushes or lianas. The EcM roots of C. uvifera in sand dunes are relatively much broader and more heavily colonised by fungi compared with scattered Coccoloba spp. tree individuals in a rain forest habitat in Ecuador (L. Tedersoo, unpubl.). Fungi associated with Coccoloba spp. in both habitats exhibit relatively greater diversity than in Nyctaginaceae but lower diversity compared with South American Dipterocarpaceae and Fabaceae, suggesting certain level of specificity (Tedersoo et al. 2010b). The genus Coccoloba diverged from AM ancestors around 52 Ma and radiated 24 Ma (Schuster et al. 2013).
The Central American genus Gymnopodium was only recently suggested to be EcM, and so far, published molecular and morphological evidence at the root tip scale is lacking (Bandala et al. 2011). Gymnopodium forms monodominant stands and supports tens of fungal species that are mostly shared with Coccoloba in neighbouring habitats (Bandala et al. 2011). Gymnopodium is a relatively young EcM group since its stem age was estimated to date back 35 Ma (Schuster et al. 2013).
In contrast to these three South and Central American EcM groups, Persicaria vivipara (also known as Polygonum and Bistorta) represents a perennial herb that is distributed throughout the circumarctic habitat and many glacial refugia in the alpine areas of Europe, Asia and North America. Since there is no recent taxonomic work on the genus Persicaria and closely related genus Polygonum, it remains unknown whether any other species of this group exhibit EcM habit as there are only a few and unreliable reports as well as some taxonomic confusion. Besides P. vivipara, the only reliable report on EcM is derived from Polygonum weyrichii in Japan, where all plants exhibited low but consistent colonisation across different habitats (Titus and Tsuyuzaki 2002; Tsuyuzaki et al. 2005) and perhaps P. paronychia (both not transferred to Persicaria) in dunes of Western North America (Zak 1973). In spite of conflicting reports about the EcM status of P. vivipara, we have observed that all individual plants of this species in Estonia and Scandinavia are colonised by EcM fungi, but the level of colonisation usually remains <50% (L. Tedersoo, unpubl.). The root systems and EcM tips of P. vivipara are among the finest and shortest among all EcM groups (Massicotte et al. 1998). P. vivipara associates with multiple fungi and lacks host specificity relative to other arctic and alpine herbs and shrubs (Botnen et al. 2014). P. vivipara seems to be a relatively recently evolved EcM group, with the estimated stem age < 28 million years (Schuster et al. 2013), but probably much less in case of better taxon sampling (<7 million years; Zanne et al. 2014).
Asteropeiaceae represents a monogeneric family of small trees and bushes that is distributed in Madagascar. The EcM status of Asteropeia was first reported in the year 2008 (Ducousso et al. 2004). The roots and EcM tips of A. micraster are extremely narrow and difficult to locate without a stereomicroscope. EcM root tips are sparsely distributed along the long root and contribute to ca. 50% of all root tips. Roots of A. micraster typically inhabit the fermentation horizon, while other EcM plants spread their roots more commonly in mineral soil in SW Madagascar (Tedersoo et al. 2011; unpubl.). Asteropeia associates with a broad range of EcM fungi, most of which are shared with other local EcM plant families (Tedersoo et al. 2011). Asteropeia appears to be an ancient group at the base of Caryophyllales, but no age estimates exist for this genus. According to Zanne et al. (2014), the stem age of Asteropeia dates to around 34 Ma. Asteropeia is sister to Physena (Physenaceae), another Malagasy endemic with no known mycorrhiza information (Cuenoud et al. 2002; Ducousso et al. 2008).
19.4.5 Fabales
The order Fabales represents an extremely large and ecologically important group of herbs, shrubs and trees that has several times independently evolved and multiple times subsequently lost the N2-fixing capacity in association with rhizobial Proteobacteria (Werner et al. 2014). In addition to this rhizobial association, the typically obligately AM Fabaceae have evolved EcM habit at least seven times. The large Detarioideae subfamily itself contains four distantly related EcM clades that we term as the Berlinia group and the Afzelia group, following Bruneau et al. (2008), and Cryptosepalum group and Dicymbe (monogeneric) following the same logic. The distinctness of these four lineages is sufficiently supported in an inclusive and specifically focused phylogenetic study of de la Estrella et al. (2017) but not in earlier studies with less genes and representative taxa (e.g. Bruneau et al. 2008; Smith et al. 2011). The age for the entire Fabaceae and particularly Detarioideae and Acacia is greatly underestimated by Zanne et al. (2014) compared with strictly focused studies of de la Estrella et al. (2017) and Miller et al. (2013).
Acacia s.lat. (Mimosoideae) constitutes a large polyphyletic genus (nearly 1400 species) that has EcM-forming representatives only in the Australian Phyllodina group (Racosperma), known as Acacia s.str. (unfortunately not recognised as such in the Plant List). Acacia s.str. is the largest EcM genus with ca. 1000 accepted species (Miller and Seigler 2012) that represent small trees, bushes and shrubs, which are typically heavily colonised by rhizobia. Partly due to multiple symbiotic partners, certain species of Acacia are among the fastest-growing trees in the world. Most species of Acacia s.str. Seem to be facultatively EcM, because very often individual plants lack EcM and the level of colonisation commonly remains <10%. There is a tendency for larger species of Acacia s.str. (small or large trees) to have both EcM and AM, whereas the shrubs in this genus tend to have AM only (Chap. 17). Only about 50 species have been examined for mycorrhizas, of which about half have AM and the rest have both EcM and AM roots (Ducousso and Thoen 1991; M. Brundrett unpubl.). In some cases, EcM roots are poorly developed and may be nonfunctional. The conditions required for EcM fungi are poorly understood, but these are probably related to soil texture and organic matter or paucity of certain micronutrients. The EcM fungal diversity associated with species of Acacia s.str. Remains unknown, although only a few species from several EcM fungal genera are found under Acacia spp. in Australia and exotic plantations (M. Brundrett, pers. obs.). Acacia s.str. Diverged from other Mimosoideae 27–24 Ma and radiated shortly thereafter (Murphy et al. 2003; Miller et al. 2013).
Aldina is a small genus of South American trees that belongs to the subfamily Papilionoideae (papilionoid legumes). Root systems of Aldina are heavily mycorrhized (>90%) and support a large number of fungal species that are mostly shared with Dicymbe spp. (Smith et al. 2011; L. Tedersoo, unpubl.). The global plant phylogeny suggests that the divergence of Aldina from other legumes dates back >34 Ma (Zanne et al. 2014). Aldina spp. do not associate with rhizobia. The ‘igapó’ riparian forests of Aldina were the main source of mycological collections of R. Singer in the 1970s and 1980s.
The Mirbelieae tribe (sometimes also referred to as Bossiaeeae; papilionoid legumes) represents a group of Australian shrubs and bushes that are most widely distributed in the seasonally dry Mediterranean habitats in P-impoverished soils. Most if not all taxa of the Mirbelieae exhibit root symbiosis with rhizobia. Multiple species have been shown to be EcM, but reports from individual studies are often contradictory. The genera Pultenaea, Gompholobium and Mirbelia are consistently EcM and possess well-developed mantle and Hartig net (Chap. 17). Based on the individual reports of genera, it appears that EcM habit is inherent to the core group of Mirbelieae (Warcup 1980). Published information indicates that EcM habit may have been secondarily lost in certain species and genera. In his pioneering work, J. Warcup inoculated seedlings of Mirbelieae with a number of EcM fungal isolates and demonstrated >tenfold growth benefit of inoculation, although the nature of the control treatment was unspecified. These inoculation trials revealed that at least the tested fungal isolates were not selective among host plant group, allowing us to speculate that some Mirbelieae associate with a broad range of Australian EcM fungi. While there is no information about the colonisation level of Mirbelieae root systems, the EcM structures of most taxa appear poorly developed and only partly matching the morphological EcM definition. The widespread genus Gastrolobium is associated with a wide diversity of fungi, many of which form hypogeous fruit bodies that are an important food source for animals (Lamont et al. 1985). The EcM group radiated around 40 Ma (Crisp and Cook 2003; Schrire et al. 2005). Along with species assigned to Mirbelieae, Warcup (1980) reported EcM on Hardenbergia and Kennedia, but these groups belong to Phaseoleae, and only AM has been found in more recent studies (e.g. Brundrett and Abbott 1991). When referring these groups as Mirbelieae, Warcup (1980) may have misidentified the plants. Furthermore, some species of Daviesia have NM cluster roots, but EcM and/or AM have been reported in others (Table 19.2; Chap. 17).
The Afzelia group (Detarioideae, caesalpinioid legumes) comprises two closely related genera, Afzelia and Intsia. Bruneau et al. (2008) identified the South American genus Brodriguesia as a well-supported sister taxon to these genera within the Afzelia group, but there is no information about the mycorrhizal status of B. santosii that is endemic to E Brazil. The roots of I. bijuga are heavily colonised by EcM fungi (>70%; L. Tedersoo et al. unpubl.), but we have no such data for Afzelia spp. In a few studies, I. bijuga associated with a wide array of fungi with no obvious specificity patterns in the Seychelles and Madagascar (Tedersoo et al. 2007 b, 2011). Species of the Afzelia group do not associate with rhizobia. The age and ancestral distribution of the Afzelia group are not known, and the dating of EcM habit would strongly depend on the mycorrhizal status of Brodriguesia. The stem age of the entire group (incl. Brodriguesia) is 62 Ma (de la Estrella et al. 2017).
The Berlinia group (Detarioideae) represents at least 20 genera of large dominant trees and subcanopy trees in African miombo woodlands and rain forests. Several rain forest taxa of the Berlinia group (e.g. Gilbertiodendron and Microberlinia) form monodominant stands in the mainly AM matrix. Through extremely recalcitrant litter, these trees seem to control the soil conditions that favour proliferation of their symbionts and suppress seedlings of small-seeded arbuscular mycorrhizal plants. The roots are typically heavily colonised (>50%) by EcM fungi, although there are great differences in mycorrhiza density and root branching among tree genera (L. Tedersoo, unpubl.). Individual species and the Berlinia group as a whole establish non-specific associations with EcM fungi (Diedhiou et al. 2010; Tedersoo et al. 2011). Species of the Berlinia group do not associate with rhizobia. The Berlinia group diverged from other Amherstieae 59 Ma and radiated 57 Ma (de la Estrella et al. 2017).
The Cryptosepalum group (Detarioideae) consists of Cryptosepalum spp. and Paramacrolobium coeruleum that represent large and small trees in rain forests and miombo woodlands of Africa. C. exfoliatum forms monodominant stands in the dry deciduous forests biome in NE Zambia (L. Tedersoo, pers. obs.). The roots of C. exfoliatum are heavily colonised (>50%) by EcM fungi (L. Tedersoo, unpubl.). Fungal symbionts of Cryptosepalum spp. and P. coeruleum are shared with species belonging to the Berlinia group (Diedhiou et al. 2010; Tedersoo et al. 2011). Species of the Cryptosepalum group do not associate with rhizobia. The Cryptosepalum group diverged from other Detarioideae 53 Ma and radiated 34 Ma (de la Estrella et al. 2017).
Dicymbe (Detarioideae) is a genus of trees that is distributed in South America. Several species of Dicymbe form monodominant stands that may be codominated with Aldina spp. (Henkel 2003). Roots of Dicymbe species are heavily colonised by EcM fungi, and the mycobionts are shared with Aldina spp. and Pakaraimaea (Smith et al. 2011, 2013). Dicymbe spp. do not associate with rhizobia. The genus Dicymbe diverged from Polystemonanthus dinklagei 24 Ma and radiated 18 Ma (de la Estrella et al. 2017). Treatment of Dicymbe as a separate EcM plant lineage is important, because it is the only EcM Detarioideae group in South America and it does not belong to the large African Berlinia group that was previously hypothesised to have dispersed to South America. Nonetheless, mycorrhizal status of the West African P. dinklagei is not known, and thus it is still possible that EcM ancestors of the Dicymbe group evolved in Africa.
19.4.6 Malpighiales
Several families of Malpighiales contain EcM groups. Unfortunately, those in Phyllanthaceae and Euphorbiaceae have not been dated using a taxonomically focused approach.
The core group of Salicaceae is the most widely recognised EcM lineage within Malpighiales, consisting of Populus and Salix (including the monotypic Chosenia). The EcM Salicaceae are widely distributed from the arctic tundra to temperate forests, extending into tropical areas in riparian habitats. Species of Salix and Populus differ greatly in the structure and size of roots and EcM tips as well as the degree of EcM colonisation. All examined species of Salix and Populus are ectomycorrhizal, although several species include individuals that are non-EcM. Low level of EcM colonisation is characteristic to certain phylogenetic groups as well as individuals inhabiting permanently waterlogged conditions (Lodge 1989; Tedersoo et al. 2013). Populus spp. associate with a highly diverse set of fungi, a few of which are genus specific. Salix spp. associate with fewer fungal species, and the proportion of Salix-specific fungal taxa is greater (Tedersoo et al. 2013). Calibrated phylogenies indicate that EcM Salicaceae diverged from AM groups 45 Ma, whereas Populus and Salix were separated 33 Ma (Davis et al. 2005). Fossil records, however, suggest that modern Salicaceae s.str. Evolved 60–55 Ma (Collinson 1992), which we believe is more likely.
Uapaca (Phyllanthaceae) is a genus of small trees in miombo woodlands and rain forests of Africa and Madagascar. Many rain forest Uapaca spp. have stilted roots. Fine roots of Uapaca are much broader compared with those of other EcM angiosperms. The broad, brittle, red-brown ‘fine’ roots are characteristic to all studied species of Uapaca. Certain large root clusters are heavily mycorrhizal, whereas others are colonised by AM fungi (L. Tedersoo, pers. obs.). Uapaca spp. associate with a diverse community of EcM fungi that is shared with the Berlinia group and Dipterocarpaceae in Africa and Asteropeiaceae, Sarcolaenaceae and Intsia (Afzelia group) in Madagascar (Tedersoo et al. 2011). Uapaca diverged from other Phyllanthaceae <50 Ma and diverged at around 16 Ma (Zanne et al. 2014), but these figures are probably underestimates.
Poranthera (Phyllanthaceae) is a genus of small herbs and shrubs that is distributed in Australia and New Zealand. Several independent authors have consistently interpreted Poranthera as an EcM genus but with low level of colonisation and some individuals uncolonised. Some West Australian material examined did not have EcM roots as these are normally defined (Chap. 17). Inoculated fungi displayed 30–40-fold growth benefit to Poranthera sp. in sterile soils (Kope and Warcup 1986). However, these experiments need to be repeated, since growth responses of this magnitude are only likely in cases where fungi detoxify sterilised soils and control plants die. There is no information about the natural fungal associations of Poranthera, although EcM has been successfully synthesised with fungi from Myrtoideae (Kope and Warcup 1986). There is limited phylogenetic information about Poranthera, although the global analysis of Zanne et al. (2014) suggests they would have split from other Euphorbiaceae around 26 Ma and radiated 19 Ma, which we consider realistic.
19.4.7 Rosales
Pomaderreae is a coherent tribe of Rhamnaceae that is mostly represented by small trees and shrubs in Australia and New Zealand. Unlike some other Rhamnaceae, Pomaderreae spp. do not associate with N2-fixing Frankia actinobacteria. Adolphia californica forms a sister taxon to the Pomaderreae (Onstein et al. 2015), but nothing is known about its mycorrhizal or actinorhizal status. The root system of P. apetala is heavily colonised by EcM fungi (>90%) and associates with a great diversity of mycobionts. The associated fungi display remarkably strong host preference for either Pomaderris or Nothofagus + Eucalyptus (Tedersoo et al. 2008). Molecular studies indicate that Pomaderreae split from other Rhamnaceae 55 Ma and radiated 41 Ma. Phylogenies indicate that the ‘Pomaderreae’ genera Alphitonia and Granitites are placed outside this tribe and are most probably AM (Onstein et al. 2015).
Dryadeae (Rosaceae) represents a tribe of small trees (Cercocarpus) and shrubs (Dryas) that associate with both EcM fungi and Frankia actinobacteria. While Dryas and Cercocarpus are consistently EcM, available information suggest that Chamaebatia is associated with at least Cenococcum (Trappe 1964), but Purshia forms only AM (studies not focused on EcM: Williams 1979; Rose 1980). Information about Cowania is lacking completely. Root systems of Dryas are moderately colonised by EcM fungi (>50%; L. Tedersoo, unpubl.), but such information is lacking for other groups. Both Dryas and Cercocarpus appear to associate with a broad diversity of EcM fungi with no evidence for host specificity (McDonald et al. 2010; Botnen et al. 2014). Dryadeae diverged from other Rosaceae tribes 75 Ma and radiated to currently recognised genera 67 Ma (Chin et al. 2014) that is in a good agreement with a global analysis (Zanne et al. 2014).
Adenostoma is a small genus of bushes not associated with Frankia actinobacteria in Western North America. A. fasciculatum has been reported to form EcM with poorly developed mantle and Hartig net (Cooper 1922; Allen et al. 1999a), but A. sparsifolium has only AM (Allen et al. 1999a). Allen et al. (1999b) observed production of EcM fungal fruit bodies in monospecific Adenostoma patches far from other EcM vegetation, indicating its performance as a functional host. Notably, however, Adenostoma does not facilitate recruitment of tree seedlings that contrasts with local Arbutoideae (Horton et al. 1999). Taken together, we interpret Adenostoma as a facultatively EcM plant genus. We have no information about the root structure, EcM mycobionts or evolutionary history of Adenostoma. The global analysis of Zanne et al. (2014) indicated its separation from extant sister groups <15 Ma.
19.4.8 Malvales
The order Malvales contains two EcM plant groups, viz. Dipterocarpaceae-Cistaceae and Tilia. Malvales is a relatively young group that dates back to 80–70 Ma based on multiple studies focused on the entire angiosperms (e.g. Wikström et al. 2001; Zanne et al. 2014; Tank et al. 2015). Unfortunately, phylogenetic relationships within Malvales are poorly resolved and the divergence estimates accounting for continental disjunctions are strongly conflicting with clock-based estimates.
We define the EcM Dipterocarpaceae-Cistaceae group as a clade that includes all genera of Cistaceae, Pakaraimaeaceae, Dipterocarpaceae s.lat. (incl. Monotoideae and Pseudomonotoideae) and Sarcolaenaceae. Close phylogenetic association of Dipterocarpaceae s.lat., and in particular the genus Pakaraimaea and Cistaceae, has been evident for a long time (Wikström et al. 2001; Ducousso et al. 2004) but considered as an artefact of poor taxon sampling. Strikingly, modern in-depth phylogenetic analyses confirm these early findings (Zanne et al. 2014; Horn et al. 2016, J. Horn, pers. comm.), indicating that the present assumptions about the evolution and biogeography of these groups need to be drastically revised. From the belowground perspective, the monophyly of Dipterocarpaceae-Cistaceae makes sense, because both groups are well known as EcM hosts. Due to great ecological differentiation and the lack of geographic overlap probably within the last 30 My, these subgroups share no fungal species besides Cenococcum geophilum. The roots of all examined species of the Dipterocarpaceae subgroup are of average thickness for angiosperms and appear to be heavily colonised by EcM fungi (>70%), except Monotes which has relatively lower colonisation level (<30%) and low level of branching. Relatively low branching and low level of colonisation is as also characteristic of Cistaceae (L. Tedersoo, pers. obs., but see Massicotte et al. 2010). Furthermore, Cistaceae exhibit relatively fine roots and EcM tips compared with other EcM groups. In Mediterranean Cistus species, the mantle and Hartig net are often poorly developed, but this may be characteristic of pezizalean symbionts that have been frequently studied in this context. Species of the Dipterocarpaceae subgroup associate with multiple mycobionts and display no host specificity in Asia, Africa, Madagascar or South America (Tedersoo et al. 2011; Peay et al. 2015). This also applies to Pakaraimaea dipterocarpacea that is endemic to sandy soils of the Guyana shield (Smith et al. 2013). Little is known about the fungal diversity associated with Cistaceae, but sequence data suggests that Cistaceae associate with a phylogenetically diverse set but species-poor assemblages of EcM fungi (data available in UNITE: www.unite.ut.ee), many of which are Cistaceae specific (e.g. Hebeloma spp., Cortinarius spp.: Comandini et al. 2006). According to early vascular plant phylogenies, the Dipterocarpaceae-Cistaceae group diverged from other taxa 33 Ma and radiated to families since 23 Ma (average values from Wikström et al. 2001), which are anecdotally low values. Later, the stem and crown age of this group was pushed back to 73 and 49 Ma, respectively (Zanne et al. 2014). Given the slow evolution and continental disjunctions in these woody plants, the age of Dipterocarpaceae s.lat., Cistaceae and Pakaraimaea is almost certainly underestimated (Moyersoen 2006; see also Chap. 20).
Tilia is a small genus of bee-pollinated trees that also includes Craigia nested therein. The Central American Mortoniodendron spp. form a sister group to Tilia and Craigia (Nyffeler et al. 2005), but there is no information about the mycorrhizal status of this genus. Roots of Tilia are heavily colonised by EcM fungi (>90%), and fungal richness tends to be among the highest of all EcM plants (Tedersoo et al. 2014, unpubl.), although no Tilia-specific EcM fungal species are known. In contrast to most other EcM trees, litter of Tilia species is nutrient rich and degrades rapidly. Richardson et al. (2015) estimate the stem age and crown age for Tilia + Craigia at 32 and 17 Ma, respectively, but these are certainly underestimates based on the fossil record (Chap. 20).
19.4.9 Asterales
Gnaphalieae (Asteraceae) is a tribe of herbaceous plants (as Inuleae; Warcup and McGee 1983; Warcup 1990) that is comprised of a large number of genera, some of which have been reported as EcM but many others are probably fully non-EcM. Apart from the image of Podolepis by Warcup and McGee (1983), the majority of reported associations lack a Hartig net, and the occurrence of a mantle is inconsistent and may require the presence of companion EcM plants. The same genera, or even species, of Asteraceae examined in Australia were reported to be EcM and AM or AM only in different studies (Table 17.2). It seems most likely that all Asteraceae are predominantly AM plants and the role of EcM-like associations on their roots requires further study. Only the crown group of this tribe with Australian distribution comprises EcM members (clades D-X; cf. Bayer et al. 2002). The taxonomy of Gnaphalieae is poorly resolved, with many currently recognised genera being polyphyletic (Bayer et al. 2002). Especially the genus Helichrysum stands out in terms of polyphyly as certain species belong to the EcM clade, whereas others belong to the neighbouring non-EcM clades (Smissen et al. 2004). Certain species have distributed from Australia to neighbouring islands, but to our knowledge, the mycorrhizal status of the EcM core group of Gnaphalieae has not been addressed outside Australia. Likewise, there is no information about the natural mycobionts of Gnaphalieae. Warcup (1980) also described the genus Isoetopsis as EcM, but this genus is closely related to Aster (Bayer and Cross 2002), and the report is almost certainly incorrect. The EcM status of most genera and vast majority of species remains poorly understood, but the groups that may have EcM evolved in the time frame of 10–16 Ma (Bergh and Linder 2009).
Goodeniaceae represents another Australian-centred family of herbs and shrubs that are reported as EcM or without EcM, sometimes in the same species. The root system of Goodeniaceae has typically low level of superficial fungal colonisation along with AM, and the roots generally lack a Hartig net (see Fig. 17.5); yet, inoculation with EcM fungi was reported to provide plants 10–100x growth benefits in sterile soils (Warcup 1985), but these experimental results have been questioned (Chap. 17). Information about natural mycobionts of Goodeniaceae is lacking, but EcM-like associations were synthesised using fungi from Myrtoideae (Warcup 1985). Many Goodeniaceae spp. are halophytes or hydrophytes, and these are very unlikely to be ectomycorrhizal. Molecular dating studies suggest that Goodeniaceae is an ancient group that separated from its sister groups 78 Ma and radiated 67 Ma (Jabaily et al. 2014). However, the Cretaceous origin of Goodeniaceae is probably overestimated (Zanne et al. 2014 report around 55 Ma for stem age).
19.4.10 Myrtales
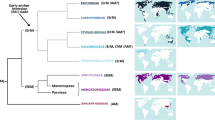
The order Myrtales contains probably a single EcM group—the subsection of Myrtoideae that bear dry seeds. The Myrtoideae subfamily has complex mycorrhizal relationships, especially in Australia. Altogether 95 species of Australian Myrtoideae have been assessed for mycorrhizas: 35% with EcM, 36% with AM and EcM and 29% with AM only (M. Brundrett, unpubl.). To illustrate the present knowledge about Myrtoideae mycorrhizal status from a phylogenetic perspective, we mapped the confirmed lineages on a dated tree (Fig. 19.3). Species within many genera of Myrtoideae differ greatly in their consistency of EcM status, level of EcM colonisation and root morphology, which requires further investigation (Brundrett 2009). It is also common for them to have both AM and EcM in their roots. Despite conflicting evidence or a lack of information about the mycorrhizal status of many Myrtoideae genera, there are well-resolved EcM clades, which are phylogenetically centred around Eucalyptus, Leptospermum and Melaleuca. In the crown group of Myrtoideae, the Myrteae, Syzygeae and Metrosidereae tribes have probably secondarily switched to arbuscular mycorrhizal habit (Thornhill et al. 2015), although conflicting and probably incorrect reports on EcM of Campomanesia and Ugni exist from South America and that of Syzygium kuranda from Australia. Evidence that the Myrtoideae gained many of their EcM symbionts from Nothofagus in the Late Cretaceous is provided by low specificity of fungi between eucalypts and southern beeches (Tedersoo et al. 2008). General observations suggest that large trees such as Eucalyptus s.lat. Host many EcM fungi, whereas bushes and shrubs support relatively low fungal diversity based on fruit-body records (Chap. 17). Interestingly, Myrtoideae are able to associate with indigenous fungi of the Seychelles, Madagascar and continental Africa (Tedersoo et al. 2007b, 2011; Buyck 2008), but not with those of Europe (Pennington et al. 2011). The EcM Myrtoideae diverged from other groups around 85 Ma and radiated 72 Ma. The AM groups evolved probably secondarily between 25 Ma and 65 Ma (Thornhill et al. 2015).
Distribution of mycorrhizal types in the phylogeny of Myrtoideae (Myrtaceae). The backbone tree is adapted from Thornhill et al. (2015). Numbers at nodes and above branches indicate divergence times and Bayesian posterior probabilities, respectively. Names highlighted in red and green denote EcM habit and non-EcM habit, respectively (highlights covering genus name only indicate that different species were assessed for mycorrhiza status). Clades believed to be dominated by EcM habit are shaded. Abbreviated names under major branches indicate tribe names: Back Backhousieae, Cham Chamelaucieae, Euc Eucalypteae, Kan Kanieae, Lept Leptospermeae, Lind Lindsayomyrteae, Loph Lophostemoneae, Mel Melaleuceae, Metr Metrosidereae, Myrt Myrteae, Osb Osbornieae, Sync Syncarpieae, Syz Syzygieae, Tris Tristanieae, Xant Xanthostemoneae
19.4.11 Apiales
Platysace (Apiaceae) represents a single EcM genus in Apiales. The Australian endemic perennial herb genus Platysace forms a sister group to Homalosciadium, another Australian genus (Nicolas 2009), for which there is no available information about mycorrhiza status. Phylogenies suggest that Platysace and Homalosciadium diverged ca. 35 Ma, but these were separated from other subfamilies of Apiaceae some 78–84 Ma (Nicolas 2009) that are probably overestimates. Platysace spp. have been reported to form a well-developed mantle and Hartig net, and individuals exhibit consistent colonisation (Bellgard 1991; Zemunik et al. 2015), but other plants in the same genera have been shown to be AM only (Table 19.2). The occurrence of EcM in this group requires further investigation since the rest of this family seems to be consistently AM. There is no available information about the root structure, EcM colonisation and associated mycobionts.
19.4.12 Ericales
Ericaceae is one of the largest plant families on earth that is particularly well-known for ericoid mycorrhiza (ErM; Chap. 9). At the base of Ericaceae, however, the AM Enkianthoideae forms a successive sister group to the large ErM and relatively small EcM lineage (Schwery et al. 2015). The ErM lineage comprises subfamilies Cassiopoideae, Harrimanelloideae, Ericoideae, Styphelioideae and Vaccinioideae (Chap. 9), whereas the monophyletic EcM group contains Arbutoideae, Pyroloideae and Monotropoideae (tribes Monotropeae and Pterosporeae: Kron and Luteyn 2005) that we refer to collectively as Arbutoideae s.lat. Of these individual subfamilies, Monotropoideae comprises fully non-photosynthetic, so-called mycoheterotrophic plants that form monotropoid subtype of EcM with usually a thick mantle, intensive intracellular root colonisation of hyphae and extensive digestion of hyphal coils (Smith and Read 2008). Most of the Pyroloideae form pyroloid subtype of EcM that has low to moderate intracellular colonisation and usually lacks a mantle (Smith and Read 2008), but mantle development is a function of plant species, fungal species and habitat (L. Tedersoo unpubl.). Nearly all species of Pyrola, Orthilia and Moneses are partially mycoheterotrophic, whereas Chimaphila appears to gain little if any carbon from forest trees via EcM fungi (Tedersoo et al. 2007a; Hynson et al. 2012). Only members of the Arbutoideae subfamily appear fully autotrophic, although they form arbutoid mycorrhiza with intracellular colonisation in addition to a Hartig net and a poorly or fully developed mantle (Smith and Read 2008). Since the fungi and many anatomical features of these specific mycorrhiza types are shared with typical EcM, we continue to consider these as specific subtypes of EcM. While Arbutoideae and Pyrola, Orthilia and Chimaphila from Pyroloideae associate with an extremely wide range of EcM fungi, Moneses (Pyroloideae) and members of the entire Monotropoideae display substantial selectivity for specific fungal groups that are often unrelated (Bidartondo et al. 2015). Moneses associates with Amphinema and Tylospora species (Hynson et al. 2015; L. Tedersoo, unpubl.). Both the ErM and EcM groups diverged from the putatively AM ancestor 110 Ma and radiated further since 102–103 Ma (Schwery et al. 2015). Besides this most recent and comprehensive study, other age estimates for Ericaceae and mycorrhizal groups therein are 1.3–3 times more recent, but these conflict with the fossil record.
19.4.13 Cyperales
The genus Kobresia is a perennial genus of sedges, part of which are EcM in arctic and alpine habitats of the Northern Hemisphere. We consider ectomycorrhizal only the ‘uniseriate’ group (cf. Starr et al. 2004), which is monophyletic within the paraphyletic Carex and contains proven EcM plant species (e.g. K. myosuroides, syn. K. bellardii). Kobresia species outside this core clade are probably non-EcM. However, not all individuals (or perhaps populations) of K. myosuroides are EcM, suggesting the facultative nature of EcM mutualism at least in some habitats. Kobresia is the dominant plant group in the Tibetan Plateau and other Central Asian lowlands, where the EcM habit is consistently reported in several species. The EcM colonisation of individual plants is relatively low, and EcM roots are arranged as unbranched pinnate terminal roots (resembling the structure of Alnus spp.) branching off the main feeder root (L. Tedersoo, unpubl.). Kobresia spp. associate with multiple fungal partners that are not specific to this genus (Gao and Yang 2010). The diversity appears to be, however, relatively low (Tedersoo et al. 2012), but this may result from the tundra and grassland habitat, where EcM plant relative abundance is low compared to forest habitats. Phylogenetic analyses suggest that Kobresia is a relatively recently evolved EcM group as the large paraphyletic genus Carex dates back 21 Ma (Escudero et al. 2012), which pushes the divergence date of EcM Kobresia to <10 Ma (Starr et al. 2004) or <5 Ma (Zanne et al. 2014). The EcM roots of Kobresia seem be derived from dauciform roots, which are swollen lateral roots produced by many members of the Cyperaceae (Chap. 21).
19.5 Groups Forming EcM-Like Associations
We have taken a precautionary approach to assigning EcM status to taxa where such evidence is poor or conflicting and descriptions are lacking or open to multiple ways of interpretation. We briefly discuss these taxonomic groups below.
Multiple groups of orchids form the orchid type of endomycorrhiza with typical EcM fungi that colonise root cells, but these associations are morphologically and functionally distinct from EcM (Dearnaley et al. 2012). Certain thalloid liverworts of Aneuraceae family also establish symbiosis with EcM fungi inside the cells of their belowground and aboveground tissues (Bidartondo and Duckett 2010). In both cases, associations with EcM fungi have evolved secondarily, and EcM fungi and their tree hosts are to a greater or lesser extent exploited by orchids and liverworts as the fungal hyphae are digested inside the root cells, indicating mixotrophic and mycoheterotrophic interactions. Notably, these associations are distributed only in the mycorrhizosphere of EcM plants and never distant from EcM vegetation. There is no evidence that orchids and thalloid liverworts can sustain EcM fungi in the absence of other EcM vegetation (Cameron et al. 2008). As obviously non-mutualistic for the exploited fungi, we do not consider these interactions here. Evolution and biogeography of these mixotrophic and mycoheterotrophic plants has been comprehensively addressed in Merckx (2013).
Lobelia is a paraphyletic cosmopolitan genus of contrasting life forms that evolved in Neotropics 55 Ma and spread further to Africa and Australia (17 Ma; Antonelli 2009). The Australian annual Lobelia spp. have been demonstrated to form a strange form of root symbiosis with both EcM and AM fungi, but perennial species had only AM (Fraser 1931; Warcup 1988). The mycorrhizal isolates displayed 1–100x growth benefits to plants (Warcup 1988), suggesting functional and beneficial associations to their hosts. However, field samples of other Lobelia spp. only had AM in their roots (Brundrett and Abbott 1991). Given the strange seedling development belowground (Fraser 1931), we speculate that some Lobelia species may display mixotrophic lifestyle briefly as seedlings, but their mycorrhizas do not conform to the definition of EcM and require further study.
Thysanotus (Laxmanniaceae, Asparagales) is a genus of monocot herbs endemic to Australia, except two species distributed west to East Asia and Indo-Malay (Sirisena 2010). The Australian species have been reported to form ‘thysanotoid’ mycorrhizal associations with both AM and EcM fungi (Chap. 17). Aseptic synthesis experiments recovered up to twofold growth benefits that were evident in the presence of another mycorrhizal plant (McGee 1988). These experiments suggest that Thysanotus spp. may exhibit mixotrophic associations with EcM or endophytic fungi. Given the association with EcM fungi and formation of EcM-like sheath but not Hartig net, the Australian Lobelia and Thysanotus warrant further mycological, ecological and physiological research to resolve their mycorrhizal status.
19.6 Some Remarkable Examples of Incorrect Reports
The topic of diagnosis and misdiagnosis of mycorrhizal associations is discussed in detail elsewhere (Brundrett 2009), so we present only a few striking cases that have taken root or become influential in mycorrhizal ecology. Both false-positive and false-negative reports about the EcM status of plants are common. False-negative observations are at least partly related to the fact that only AM colonisation has been assessed or insufficient fresh/living material has been studied. False-positive EcM reports may be derived for multiple reasons:
-
(1)
The authors consider any hyphal network on the root surface as a mantle that is indicative of EcM (work of J. Warcup and his followers, early and middle twentieth-century researchers).
-
(2)
Consideration of a weft of dark septate endophytic (DSE) hyphae as poorly developed EcM of Cenococcum (work of T. Dominik and his students).
-
(3)
Careless tracing of roots leading to sample contamination (e.g. EcM reports in ferns, work of early researchers).
-
(4)
Misidentification of plant species (suspected in some reports of J. Warcup).
-
(5)
Careless suggestion of mycorrhiza type based on fruiting habits of fungi without clear belowground evidence (work of B. Peyronel, R. Singer, D. Pegler and that of many other mycologists; summarised in Trappe 1962).
-
(6)
Influence from former publications and wishful thinking.
-
(7)
A general tendency to exaggerate the significance of observed fungal structures in an attempt to publish a ‘more interesting’ story.
Some of these incorrect or incomplete reports have been widely accepted and further cited by other authors without critical reassessment (e.g. Daft et al. 1985; Wang and Qiu 2006; Smith and Read 2008; Phillips et al. 2013; Fisher et al. 2016; Maherali et al. 2016; Lin et al. 2017). In particular, Maherali et al. (2016) assessed the evolution of gains and losses of EcM associations in plants based on mapping mycorrhizal status to phylograms of Zanne et al. (2014). In contrast to this review, they considered Calliandra, Gleditsia, Lonchocarpus, Robinia and Senegalia (all Fabaceae), Cerasus and Padus (both Rosaceae), Graffenrieda (Melastomataceae) and Ceratopetalum (Cunoniaceae) as ectomycorrhizal, representing nine additional EcM lineages. For most of these genera, there is ample evidence for the occurrence of only AM in the literature (members of Fabaceae and Rosaceae), or the described structures cannot be considered EcM (Ceratopetalum, Graffenrieda). Furthermore, Maherali et al. (2016) ignored altogether 11 EcM plant lineages as described here, although many of these are well established.
In Europe, there are multiple reports of EcM occurrence in Rosaceae, especially in the fruit tree genera Malus, Pyrus and Prunus as well as closely related Crataegus, Padus and Sorbus. These reports are particularly evident in the East European and Russian literature published in the 1950s and 1960s. The same authors describe these plants as EcM or non-EcM in their different studies but provide no illustrative evidence. Most commonly, Cenococcum has been reported as a putative symbiont, suggesting that dense colonisation of DSE may have resulted in incorrect assignment of the EcM status. Furthermore, fine roots of Rosaceae exhibit swollen tips; if these become old and turn brown, it is tempting for an inexperienced eye to suspect EcM association. That could be, however, easily checked by examining the squashed root tip under a stereomicroscope. Another example comes from Juniperus communis that is known to be AM, but there are several EcM reports that probably represent misidentification of roots. For example, Reinsvold and Reeves (1986) described a tuberculate EcM of ‘J. osteosperma’ that is clearly donated by a neighbouring Pinus individual. Notably, the roots of pines may distribute >30 m from the trunk even when mature trees are <5 m high. There are several records of EcM in the nitrogen-fixing Elaeagnus angustifolia (Elaeagnaceae) in Russia, although reports of the same species and other Elaeagnus species from Europe and North America have revealed only AM (see Daft et al. 1985).
In North America, Grand (1971) reported tuberculoid EcM from Photinia (Rosaceae), but their images remind us of suilloid mycorrhiza of Pinus. More recent reports suggest AM or NM habit for Photinia spp. Several physiological experiments have been performed based on inoculation of Ulmus americana with EcM fungi. It is anecdotal, because Ulmus spp., incl. U. americana, are non-EcM and form AM based on multiple reports and authors’ personal observations. Morphological studies of these roots by Brundrett et al. (1990) and others have clearly shown that they consistently have AM associations and also have structural features that would make EcM formation unlikely or impossible (suberised epidermis and exodermis). Certain companies (established by former EcM researchers) also promote universal EcM inoculum that supposedly benefits the growth of all trees, including AM trees and Alnus.
Of Asian records, Elaeocarpus (Elaeocarpaceae) has been reported and illustrated to be an EcM genus in Taiwan (Haug et al. 1994), but multiple previous and subsequent studies indicate only AM colonisation for members of this genus. Pimelodendron (often misspelled Pimeleodendron) is a small euphorbiaceous genus of trees that is distributed in the Sunda Islands and New Guinea. P. amboinicum was reported as EcM two decades ago in New Guinea (Verbeken and Walleyn 1999), but these records remain unconfirmed. More recent stable isotope analyses of EcM and AM plant leaves place P. griffithianum deeply into the AM category (Tanaka-Oda et al. 2015). Based on original studies (Tian et al. 2003 and their earlier research), Robinia pseudoacacia has been misinterpreted as EcM by Wang and Qiu (2006). The original descriptions by Bratek et al. (1996) indicated either AM or some intracellular colonisation of Mattirolomyces terfezioides, which is not an EcM fungus.
In South America, many authors have carelessly claimed that certain plant species host putatively EcM fungi. Oft-cited examples include Allophylus (Sapindaceae), Pradosia (syn. Glycoxylon; Sapotaceae), Haematoxylum (syn. Haematoxylon; Fabaceae), Swartzia (Fabaceae) and Inga (Fabaceae). Later it appeared that not all these fungi were in fact ectomycorrhizal (Gyrodon rompelii, Phlebopus spp.); Aldina, Pisonieae and Coccoloba represented local hosts (Meyer 1991; Moyersoen 1993). Other commonly cited South American EcM associations were reported by Thomazini (1974) who claimed that Campomanesia (Myrtoideae) and Bauhinia (Fabaceae) form EcM in Brazil. Furthermore, Frioni et al. (1999) reported EcM associations in Gleditsia, Senegalia (as Acacia bonariensis), Calliandra, Prosopis and Lonchocarpus. However, multiple more recent studies have been unable to confirm these findings, reporting only AM. Graffenrieda (Melastomataceae) has been described to possess a specific type of ectendomycorrhiza (Haug et al. 2004). Given its phylogenetic position, poorly developed mantle-like structure and association with typical root endophytic/fungi related to Rhizoscyphus ericae, we interpret this as somewhat differentiated root endophytic interaction rather resembling ericoid mycorrhiza.
In Africa, Högberg and Piearce (1986) suggested EcM habit for Faurea (Proteaceae) and Pericopsis (Fabaceae), which are commonly cited as examples of African EcM plants. However, several other studies as well as the first author’s observations suggest that these trees are not EcM in Africa or elsewhere. Recently, Bechem et al. (2014) conducted an extensive survey of mycorrhizal status in plants of Cameroon, reporting EcM habit for Angylocalyx, Baikiaea, Baphia, Calpocalyx, Dialium and Hymenostegia (all Fabaceae), Antidesma (Phyllanthaceae), Leptonychia (Malvaceae) and Soyauxia (Peridiscaceae) in addition to known EcM members of the Berlinia group and Uapaca. Roughly half of these findings are not supported by previous studies at genus level, but others lack independent evidence.
In Australia, the floristic distribution of EcM habit is particularly complicated, because commonly accepted EcM plants such as shrubs in the Myrtoideae other than eucalypts may have poorly developed mycorrhiza structures. The pioneering work of Warcup (1980) can be regarded as the most confusing, because he was the primary describer of EcM in multiple plant groups, but he also followed a relaxed criterion for EcM by considering plants with a hyphal weft on a root surface as mycorrhizal. Because he rarely provided illustrations and did not describe the methods used in synthesis trials, his findings have been heavily criticised (Brundrett and Abbott 1991; Brundrett 2009). Nonetheless, subsequent evidence has confirmed some of his striking findings, whereas others appear very unlikely in the context of plant phylogeny and subsequent studies (mycorrhizas.info/ozplants). Therefore, we consider the genera Lasiopetalum (Sterculiaceae), Thomasia (Sterculiaceae), Pimelea (Thymelaeaceae), Opercularia (Rubiaceae) and Isoetopsis (Asteraceae) as insufficiently supported for EcM habit. Based on updated phylogenetic and mycorrhizal information, we also consider doubtful and unlikely the EcM status of the following Australian genera: Ceratopetalum (Cunoniaceae), Astroloma (Ericaceae), Comesperma (Polygalaceae), Erythrophleum (Fabaceae) and Stylidium (Stylidiaceae, Asterales). The latter genus represents a group of perennial herbs in Australia that is reported as EcM with poor mantle and Hartig net development by Warcup and his students. Interestingly, several species of Stylidium are reported to be protocarnivorous, but this is not supported by substantial evidence. Other doubtful examples of EcM in Australian plants, where more recent studies have only found AM, are listed in Table 17.2. Most discrepancies between earlier and more recent studies of Australian plants result because the Hartig net was used to define EcM in recent studies but not in the past.
Multiple putatively incorrect false-positive reports of EcM have propagated themselves across studies and along research projects. Some of these may represent intermediate steps in the AM to EcM evolutionary continuum but in many cases can be more easily explained as the results of misidentification of fungal structures. We acknowledge that there certainly are cases where a continuum of AM to EcM host plants occurs in the same family or genus, and these are worthy of further study. Some of the worst cases of misidentification warrant published corrections for research articles or PhD theses. However, designation of EcM is complex and the status of some plants cannot be fully resolved by us at this time. This complexity arises because evolution of the EcM symbiosis is an ongoing process that is initiated at the level of plant individuals and populations.
19.7 Losses and ‘Facultative’ EcM Associations
Several EcM plant groups stand out as possessing poorly developed mycorrhizal structures and/or inconsistent root colonisation. Furthermore, some groups comprise multiple species with non-EcM populations (Fagales, Myrtoideae, Dryadeae, Acacia s.str., Mirbelieae, Goodeniaceae, Gnaphalieae), which indicates secondary losses of EcM habit. Maherali et al. (2016) found more losses of EcM habit than gains. Although this is probably true, their analysis was based on incorrectly assigned mycorrhizal types and exclusion of many EcM taxa. Our review suggests that there are two floristic features characteristic of such facultative EcM habit and loss of it: herbaceous or shrubby life form and nitrogen-fixing strategy. It is remarkable that EcM evolution—both gains and losses—is closely related to the nitrogen-fixing habit as seen in Fagales, Fabaceae, Rhamnaceae and Rosaceae that altogether comprise nine EcM groups. Furthermore, there are reports about non-EcM habit for nearly all nitrogen-fixing EcM plants. Frankia-associating Myricaceae, some members of Casuarinaceae and perhaps some Dryadeae such as Purshia have lost EcM associations. Similarly, certain Acacia spp. and Mirbelieae spp. associated with rhizobia seem to have lost EcM capacity completely. A deeper look into the Fabaceae phylogeny (Werner et al. 2014; de la Estrella et al. 2017) indicates that the Berlinia group, Cryptosepalum group, Dicymbe and Afzelia group evolved EcM associations before the two major Fabaceae groups evolved rhizobial symbiosis. By contrast, the genus Aldina evolved EcM associations after the nitrogen-fixing trait was lost in its papilionoid ancestors. The Fabaceae phylogeny also suggests that plants either evolved associations with rhizobia first and then evolved EcM associations with subsequent losses of these EcM associations in some groups (Acacia s.str., Mirbeliae). Such losses of EcM are not seen in non-nodulating lineages of Fabaceae (Berlinia group, Afzelia group, Cryptosepalum group, Dicymbe, Aldina; Fig. 19.1). In Fagales, however, the genus Alnus and the whole Casuarinaceae evolved actinorhizal associations when ectomycorrhizal (Larson-Johnson 2015). EcM habit was lost in certain Casuarinaceae, and it was reduced in Alnus as compared to the sister taxa. Within the Rhamnaceae family, EcM habit in Australian Pomaderreae and actinorhizal state in the Chilean Colletiae and NW American Ceanothus is phylogenetically unrelated (Onstein et al. 2015). In Rosaceae, Dryadeae exhibit both EcM and actinorhizal associations, whereas Adenostoma fasciculatum hosts only EcM fungi. Thus, it remains unclear whether the EcM habit or actinorhizal association evolved first in Dryadeae, but it is probable that EcM evolved first considering the pathways in Adenostoma and Alnus. Construction of dated phylogenies of Frankia and evolutionary history of symbiosis-related genes in plants may provide an answer to this question.
Root-associated actinobacteria and rhizobia have the potential to render EcM habit redundant for plants, because much of the nutritional benefit of EcM symbiosis is related to nitrogen acquisition. Actinorhizal plants have usually established their niche in early successional habitats that have poorly developed soils with limited nitrogen and little carbon but ample mineral phosphorus supply, except its poor availability at extreme pH values. High phosphorus demand by nitrogen-fixing microbes usually requires assistance of mycorrhizal fungi, probably depending on soil properties and other mycorrhizal benefits. If EcM fungi become too costly for maintenance in terms of carbon energy or phosphorus trade, plants may simply avoid such associations and exploit AM fungi. Except for Myricaceae and Daviesia (Mirbelieae), most rhizobial and actinorhizal plants have high dependency on mycorrhiza.
Low level of EcM formation in non-actinorhizal plants is characteristic to arctic and alpine habitats on the one hand (Persicaria vivipara, Kobresia) and the summer dry Mediterranean biome (Cistaceae in Europe, many plant groups in Australian semidry habitats) on the other hand. Both habitats suffer from severe seasonal drying of soil and paucity of nutrients. The vegetation in these ecosystems is dominated by herbs and shrubs, which may provide insufficient energy to sustain EcM mycobionts. If there are no large EcM trees maintaining the EcM mycelium network, EcM associations may be non-beneficial to plants in AM-dominated communities. Apart from herbs and shrubs, slowly growing trees may also display reduced EcM colonisation in heavily drought-stressed conditions (Lodge 1989; Swaty et al. 2004), further reinforcing the hypothesis of low carbon availability. EcM fungi may not be efficient enough in organic-poor substrates that are derived from low rates of leaf litter accumulation or frequent fires. Over time, Mediterranean and arctic plants may have evolved low colonisation and mycorrhiza biomass to optimise between benefits and costs of EcM mycobionts.
Similarly to seasonally very dry habitats, wetland plants tend to have reduced EcM colonisation. Since EcM fungi have high oxygen demand due to active metabolism, anoxic environments are not optimal for EcM growth. This has been shown experimentally for Salix, Melaleuca and Casuarina species which have both EcM and EM roots and grow in wet habitats but primarily form AM roots when soil is waterlogged (Lodge 1989; Watson et al. 1990; Khan 1993).
Arctic and alpine habitats are dominated by herbs and shrubs, for many of which there are conflicting reports about the mycorrhizal status. Dwarf Betula and Salix as well as Dryas, Bistorta vivipara and certain Kobresia species are nearly always EcM. In addition to these well-established EcM groups, individuals of Potentilla spp., Saxifraga spp., Cassiope tetragona and Pedicularis spp. are strikingly commonly reported as EcM by independent researchers in different regions, although most studies treat these as NM or AM (Table 19.3). Arctic species of Potentilla (Rosaceae) have been reported as EcM in four studies but only AM or NM in 15 studies. Saxifraga oppositifolia (Saxifragaceae) has been considered EcM in three studies but NM or AM in 13 studies. Kohn and Stasovski (1990) reported EcM colonisation in 75% of S. oppositifolia individuals but none of S. tricuspidata individuals in the Canadian Arctic. In Cassiope tetragona, EcM root tips in addition to intracellular colonisation have been recovered in four studies, while six studies report only ErM. In Ellesmere Island, 44% of C. tetragona individuals were considered EcM (Kohn and Stasovski 1990). In the hemiparasitic Pedicularis capitata, EcM was reported in two out of eight individuals, but P. hirsuta was non-mycorrhizal (Kohn and Stasovski 1990). Across all studies, EcM has been reported in Pedicularis spp. three times but AM or NM associations ten times. Many other arctic plant genera have been reported as EcM only once or twice (e.g. Silene, Campanula, Homogyne; Read and Haselwandter 1981), but these are likely to be incorrect. In all these four above-mentioned arctic/alpine EcM groups, the EcM habit has been described for one or a few closely related species. If not systemically incorrect, these results suggest either a recent evolutionary shift to EcM strategy or facultative EcM habit for a group of species. It is possible that in C. tetragona and S. oppositifolia, EcM trait is characteristic of populations and has not become a common trait for a species. Therefore, also population-level analyses are urgently needed to shed further light into the ongoing EcM evolution and adaptive EcM to non-EcM balance in plants. From this perspective, some of the orphan EcM reports may actually represent recent evolutionary trends that cannot be captured in other congeneric species or populations of the same species. The alternative explanation of a highly facultative nature of EcM habit is also likely, because both local and regional processes (soil moisture, pH, limiting nutrients, neighbouring plants, climate) may affect the potential benefits of EcM habit and thus associations with EcM fungi. Nonetheless, in the era of molecular identification technologies, we urge that the authors confirm their unconventional findings of EcM habit with molecular tools or at least voucher the material for such possibility. We also strongly recommend that such novel findings be illustrated for a possibility of alternative interpretation (e.g. Haug et al. 2004).
Besides nitrogen-fixing bacteria, many EcM plants exhibit dual root colonisation with AM fungi. This seems to be a relic of the ancestral AM habit in vascular plants (Cazares and Smith 1996), but it certainly represents an adaptation for nutrition early in ontogeny or at low availability of EcM inoculum. In Salicaceae, much of the EcM colonisation level is phylogenetically determined (Tedersoo et al. 2013), but it depends on soil moisture (as above; Lodge 1989) and nutrient demand (van der Heijden 2001) at the individual and species levels. This indicates that dual mycorrhizal symbiosis may secure the plant host with sufficient nutrients and plants can optimise among the mycorrhiza types or even among fungal individuals (AM fungi: Werner and Kiers 2015) to maximise nutritional benefits. In natural conditions, most dual mycorrhizal plants in Fagaceae, Salicaceae and Myrtoideae become more dominated by EcM fungi at the sapling stage (Dominik 1956; Chen et al. 2000; Egerton-Warburton and Allen 2001), which can be explained by improved carbon availability and accumulation of recalcitrant litter with nutrients in the organic form that favours EcM symbionts over AM mutualists.
19.8 Conclusions
Our study took a critical view on the EcM status of plants and assigned 335 putatively EcM genera with roughly 8500 species into 30 phylogenetically well-delimited lineages. Because of multiple reversals to AM-only habit in several species-rich Australian EcM groups, we believe that around 250–300 genera and 6000–7000 species can be considered consistently ectomycorrhizal, but there is an urgent need for additional analyses especially in Australia and Central America. Based on phylogenetic evidence, the multiple losses of EcM habit in favour to AM (or NM in Myricaceae) and decline in EcM colonisation are related to the evolution of symbiotic nitrogen fixation and reduction of trees and bushes to shrubs and herbs, that is, a common adaptation to harsh Mediterranean and arctic/alpine climate. We also point to multiple potentially erroneous reports, many of which have propagated themselves in the literature, in a hope to better inform subsequent ecological and mycorrhizal studies.
Refining our knowledge about the mycorrhizal status of both fungi and plants will strongly improve our understanding about the evolution of EcM symbiosis. Furthermore, it will have strong implications on our understanding of ecosystem functioning on landscape and global scales due to differential nutritional balance that potentially affects all guilds of soil organisms (Phillips et al. 2013; Averill et al. 2014; Soudzilovskaia et al. 2015; Fisher et al. 2016). Mistakes in mycorrhizal type assignments in modelling studies of ecosystem function may severely bias our understanding of the ecosystem processes and biodiversity. For example, a number of meta-analysis and regional studies of mycorrhizal importance or functioning have included many misallocations of host plants in their datasets, so their results are in doubt. We recommend that an agreed list of EcM hosts be developed as an essential resource for future mycorrhizal and ecological studies. This would be based on the comprehensive summary we have provided here, with resampling and/or reassessing taxa where required.
References
Alexander IJ (1989) Systematics and ecology of ectomycorrhizal legumes. Monogr Syst Bot Mo Bot Gard 29:607–624
Alexander IJ, Högberg P (1986) Ectomycorrhizal tropical angiospermous trees. New Phytol 102:541–549
Allen MF, Egerton-Warburton L, Allen EB, Karen O (1999a) Mycorrhizae of Adenostoma fasciculatum: a combination of unusual ecto- and endo-forms. Mycorrhiza 8:225–228
Allen MF, Trappe JM, Horton TR (1999b) NATS truffle and truffle-like fungi 8: Rhizopogon mengei sp. nov.(Boletaceae, Basidiomycota). Mycotaxon 70:149–152
Alvarez-Manjarrez J, Garibay-Orijel R (2015) Diversity of ectomycorrhizal fungi from a Mexican tropical dry forest. In: Gerhring C (ed) ICOM 8 Paper and poster abstracts. ICOM8, Flagstaff, p 8
Andrade ACS, Queiroz MH, Hermes RAL, Oliveira VL (2000) Mycorrhizal status of some plants of the Araucaria forest and the Atlantic rainforest in Santa Catarina, Brazil. Mycorrhiza 10:131–136
Antonelli A (2009) Have giant lobelias evolved several times independently? Life form shifts and historical biogeography of the cosmopolitan and highly diverse subfamily Lobelioideae (Campanulaceae). BMC Biol 7:1
Asai T (1934) Über das Vorkommen und die Bedeutung der Wurzelpilze in den Landpflanzen. Jpn J Bot 7:107–150
Ashford AE, Allaway WG (1982) A sheathing mycorrhiza on Pisonia grandis (Nyctaginaceae) with development of transfer cells rather than a Hartig net. New Phytol 90:511–519
Ashton DH (1975) The root and shoot development of Eucalyptus regnans. Aust J Bot 23:867–887
Aubriot X, Soulebeau A, Haevermans T, Schatz GE, Cruaud C, Lowry PP (2016) Molecular phylogenetics of Sarcolaenaceae (Malvales), Madagascar’s largest endemic plant family. Bot J Linn Soc 182:729–743
Averill C, Turner BL, Finzi AC (2014) Mycorrhiza-mediated competition between plants and decomposers drives soil carbon storage. Nature 505:543–545
Bai S, Bai Y, Fang L, Liu Y (2003) Mycorrhiza of Cenococcum geophilum (Fr.) formed on Ostryopsis daidiana and mycorrhizal affection on the growth of Ostryopsis davidiana. Sci Silvae Sin 40:194–196
Baikalova AS, Onipchenko VG (1988) Mikosimbiotrofizm alpiiskikh rastenii Teberdinskogo zapovednika. In: Onipchenko VG, Petelin DA (eds) Opyt issledovaniya rastitelnykh soobshchestv v zapovednikakh. CNIL Glavokhoty RSFSR, Moscow, pp 93–107
Bandala VM, Montoya L, Villegas R (2011) Tremelloscypha gelatinosa (Sebacinales) occurring in Gymnopodium forests in the tropical deciduous vegetation from southern Mexico. Mycotaxon 118:147–157
Bayer RJ, Cross EW (2002) A reassessment of tribal affinities of the enigmatic genera Printzia and Isoetopsis (Asteraceae), based on three chloroplast sequences. Aust J Bot 50:677–686
Bayer RJ, Greber DG, Bagnall NH (2002) Phylogeny of Australian Gnaphalieae (Asteraceae) based on chloroplast and nuclear sequences, the trnL intron, trnL/trnF intergenic spacer, matK, and ETS. Syst Bot 27:801–814
Baylis GTS (1962) Rhizophagus: the catholic symbiont. Aust J Sci 25:195–200
Bechem EET, Alexander IJ (2012) Mycorrhiza status of Gnetum spp. in Cameroon: evaluating diversity with a view to ameliorating domestication efforts. Mycorrhiza 22:99–108
Bechem E, Chuyong G, Fon B (2014) A survey of mycorrhizal colonization in the 50-ha Korup Forest dynamic plot in Cameroon. African J Plant Sci 5:1403–1415
Bell T, Yasmeen G (2010) Mycorrhizal associations in the Fabaceae: are they really needed? Australian Flora Foundation, Willoughby
Bellgard SE (1991) Mycorrhizal associations of plant species in Hawkesbury sandstone vegetation. Aust J Bot 39:357–364
Benson DR, Vanden Heuvel BD, Potter D (2004) Actinorhizal symbioses: diversity and biogeography. In: Gillings M, Holmes A (eds) Plant microbiology. BIOS Scientific, Oxford, pp 99–129
Bergh NG, Linder HP (2009) Cape diversification and repeated out-of-southern-Africa dispersal in paper daisies (Asteraceae–Gnaphalieae). Mol Phyl Evol 51:5–18
Bidartondo MI (2005) The evolutionary ecology of mycoheterotrophy. New Phytol 167:335–352
Bidartondo MI, Duckett JG (2010) Conservative ecological and evolutionary patterns in liverwort-fungal symbioses. Proc R Soc Lond B 277:485–492
Bledsoe C, Klein P, Bliss LC (1990) A survey of mycorrhizal plants on Truelove Lowland, Devon Island, NWT, Canada. Can J Bot 68:1848–1856
Bonito G, Smith ME, Nowak M, Healy RA, Guevara G, Cazares E et al (2013) Historical biogeography and diversification of truffles in the Tuberaceae and their newly identified Southern Hemisphere sister lineage. PLoS One 8:e52765
Botnen S, Vik U, Carlsen T, Eidesen PB, Davey ML, Kauserud H (2014) Low host specificity of root-associated fungi at an Arctic site. Mol Ecol 23:975–985
Boursnell JG (1950) The symbiotic seed-borne fungus in the Cistaceae: I. Distribution and function of the fungus in the seedling and in the tissues of the mature plant. Ann Bot 14:217–243
Bratek Z, Jakucs E, Bóka K, Szedlay G (1996) Mycorrhizae between black locust (Robinia pseudoacacia) and Terfezia terfezioides. Mycorrhiza 6:271–274
Breitwieser I, Ward JM (2003) Phylogenetic relationships and character evolution in New Zealand and selected Australian Gnaphalieae (Compositae) inferred from morphological and anatomical data. Bot J Linn Soc 141:183–203
Brundrett MC (2004) Diversity and classification of mycorrhizal associations. Biol Rev 79:473–495
Brundrett MC (2009) Mycorrhizal associations and other means of nutrition of vascular plants: understanding global diversity of host plants by resolving conflicting information and developing reliable means of diagnosis. Plant Soil 320:37–77
Brundrett MC, Abbott LK (1991) Roots of jarrah forest plants. I. Mycorrhizal associations of shrubs and herbaceous plants. Aust J Bot 39:445–457
Brundrett MC, Murase G, Kendrick B (1990) Comparative anatomy of roots and mycorrhizae of common Ontario trees. Can J Bot 68:551–578
Bruneau A, Forest F, Herendeen PS, Klitgaard BB, Lewis GP (2001) Phylogenetic relationships of the Caesalpinioideae (Leguminosae) as inferred from chloroplast trnL intron sequences. Syst Bot 26:487–514
Bruneau A, Mercure M, Lewis GP, Herendeen PS (2008) Phylogenetic patterns and diversification in the caesalpinioid legumes. Botany 86:697–718
Buscardo E, Rodriguez-Echeverria S, Barrico L, Garcia MA, Freitas H, Martin MP, de Angelis P, Muller LAH (2012) Is the potential for the formation of common mycelial networks influenced by fire frequency. Soil Biol Biochem 46:136–144
Buyck B (2008) The edible mushrooms of Madagascar: an evolving enigma. Econ Bot 62:509–520
Buyck B, Duhem B, Eyssartier F, Ducousso B (2012) Podoserpula miranda sp. nov. (Amylocorticiales, Basidiomycota) from New Caledonia. Crypt Mykol 33:453–461
Cameron DD, Johnson I, Read DJ, Leake JR (2008) Giving and receiving: measuring the carbon cost of mycorrhizas in the green orchid, Goodyera repens. New Phytol 180:176–184
Castellano MA, Trappe JM (1985) Mycorrhizal associations of five species of Monotropoideae in Oregon. Mycologia 77:499–502
Cazares E, Smith JE (1996) Occurrence of vesicular-arbuscular mycorrhizae in Pseudotsuga menziesii and Tsuga heterophylla seedlings grown in Oregon coast range soils. Mycorrhiza 6:65–67
Cázares E, Trappe JM, Jumpponen A (2005) Mycorrhiza-plant colonization patterns on a subalpine glacier forefront as a model system of primary succession. Mycorrhiza 15:405–416
Chalermpongse A (1987) Mycorrhizal survey of dry-deciduous and semievergreen dipterocarp forest ecosystems in Thailand. In: Kostermans AJCH (ed) Proceedings of the third round table conference on dipterocarps. UNESCO Regional Office for Science and Technology, Jakarta, pp 81–103
Chen YL, Brundrett MC, Dell B (2000) Effects of ectomycorrhizas and vesicular-arbuscular mycorrhizas, alone or in competition, on root colonization and growth on Eucalyptus globulus and E. urophylla. New Phytol 146:545–556
Chen J-H, Sun H, Wen J, Yang Y-P (2010) Molecular phylogeny of Salix L. (Salicaceae) inferred from three chloroplast datasets and its systematic implications. Taxon 59:29–37
Chevalier G, Mousain D, Couteaudier Y (1975) Associations ectomycorrhiziennes entre Tubéracées et Cistacées. Ann Phytopathol 7:355–356
Chilvers GA, Pryor LD (1965) The structure of eucalypt mycorrhizas. Aust J Bot 13:245–259
Chin SW, Shaw J, Haberle R, Wen J, Potter D (2014) Diversification of almonds, peaches, plums and cherries—molecular systematics and biogeographic history of Prunus (Rosaceae). Mol Phyl Evol 76:34–48
Christoph H (1921) Untersuchungen über die mykotrophen Verhältnisse der “Ericales” und die Keimung von Pirolaceen. Beih Bot Centrabl 38:115–158
Clemmensen KE, Hansen AH (1998) Mykorrhizasymbioser i fire gronlandske plantesamfund i relation tU forskellige jordbundsfaktorer. Arktisk Biologisk Feltkursus, Qeqertarsuaq. University of Copenhagen, Copenhagen
Collinson ME (1992) The early fossil history of Salicaceae: a brief review. Proc R Soc Edinb B 98:155–167
Comandini O, Contu M, Rinaldi AC (2006) An overview of Cistus ectomycorrhizal fungi. Mycorrhiza 16:381–395
Cooper WS (1922) The broad-schlerophyll vegetation of California. Carnegie Inst Publ 319:1–124
Cooper KM (1976) A field survey of mycorrhizas in New Zealand ferns. N Z J Bot 14:169–181
Corrales A, Arnold AE, Ferrer A, Turner BL, Dalling JW (2016) Variation in ectomycorrhizal fungal communities associated with Oreomunnea mexicana (Juglandaceae) in a Neotropical montane forest. Mycorrhiza 26:1–17
Costantin J, Magrou J (1926) Contribution à l’étude des racines des plantes alpines et de leurs mycorhizes. Comp Rend Acad Sci Paris 181(182):26–29
Cripps CL, Eddington LH (2005) Distribution of mycorrhizal types among Alpine vascular plant families on the Beartooth plateau, Rocky Mountains, USA, in reference to large-scale patterns in arctic-alpine habitats. Arct Antarct Alp Res 37:177–188
Crisp MD, Cook LG (2003) Molecular evidence for definition of genera in the Oxylobium group (Fabaceae: Mirbelieae). Syst Bot 28:705–713
Crisp MD, Cook LG (2011) Cenozoic extinctions account for the low diversity of extant gymnosperms compared with angiosperms. New Phytol 192:997–1009
Cuenoud P, Savolainen V, Chatrou LW, Powell M, Grayer RJ, Chase MW (2002) Molecular phylogenetics of Caryophyllales based on nuclear 18S rDNA and plastid RBCL, ATPB and MATK DNA sequences. Am J Bot 89:132–144
Daft MJ, Clelland DM, Gardner IC (1985) Symbiosis with endomycorrhizas and nitrogen-fixing organisms. Proc R Soc Edinb B 85:283–298
Daubenmire RF (1941) Some ecologic features of the subterranean organs of alpine plants. Ecology 22:370–378
Davis CC, Webb CO, Wurdack KJ, Jaramillo CA, Donoghue MJ (2005) Explosive radiation of Malpighiales supports a mid-Cretaceous origin of modern tropical rain forests. Am Nat 165:E36–E65
Dayanandan S, Ashton PS, Williams SM, Primack RB (1999) Phylogeny of the tropical tree family Dipterocarpaceae based on nucleotide sequences of the chloroplast rbcL gene. Am J Bot 86:1182–1190
de Alwis DP, Abeynayake K (1980) A survey of mycorrhizae in some forest trees of Sri Lanka. In: Mikola P (ed) Tropical mycorrhiza research. Clarendon Press, Oxford, pp 146–153
de Campos MC, Pearse SJ, Oliveira RS, Lambers H (2013) Viminaria juncea does not vary its shoot phosphorus concentration and only marginally decreases its mycorrhizal colonization and cluster-root dry weight under a wide range of phosphorus supplies. Ann Bot 111:801–809
de la Estrella M, Forest F, Wieringa JJ, Fougere-Danezan M, Bruneau A (2017) Insights on the evolutionary origin of Detarioideae, a clade of ecologically dominant tropical African trees. New Phytol. doi:10.1111/nph.14523
de Voogd CNA (1933) Cultuurproeven met Shorea platyclados v. Sl in Redjang en Lebong Tectona 26:703–713
Dearnaley JDW, Martos F, Selosse M-A (2012) Orchid mycorrhizas: molecular ecology, physiology, evolution and conservation aspects. Mycotaxon 9:207–230
Diedhiou A, Selosse M-A, Galiana A, Diabate M, Dreyfus B, Ba A, de Faria S, Bena G (2010) Multi-host ectomycorrhizal fungi are predominant in a Guinean tropical rainforest and shared between canopy trees and seedlings. Environ Microbiol 12:2219–2232
Doak KD (1927) Mycorrhiza bearing species in the vicinity of Lafayette, Indiana. Proc Indiana Acad Sci 37:427–440
Dominik T (1956) Mycotrophy of poplars in their natural associations in Poland. Roczn Nauk Lesn 14:247–266
Dominik T, Nespiak A, Pachlewski R (1954) Badanie mykotrofizmu roslinnosci zespolow na skalkach wapiennych w Tatrach. Acta Soc Bot Pol 23:471–485
Douglas NA, Manos PS (2007) Molecular phylogeny of Nyctaginaceae: taxonomy, biogeography, and characters associated with a radiation of the xerophytic genera in North America. Am J Bot 94:856–872
Douglas N, Spellenberg R (2010) A new tribal classification of Nyctaginaceae. Taxon 59:905–910
Ducousso M, Bena G, Bourgeois C, Buyck B, Eyssartier G, Vincelette M, Rabavohitra R, Randrihasipara L, Dreyfus B, Prin Y (2004) The last common ancestor of Sarcolaenaceae and Asian dipterocarp trees was ectomycorrhizal before the India-Madagascar separation, about 88 million years ago. Mol Ecol 13:231–236
Ducousso M, Ramanankierana H, Duponnois R, Rabevohitra R, Randrihasipara L, Vincelette M, Dreyfus B, Prin Y (2008) Mycorrhizal status of native trees and shrubs from eastern Madagascar littoral forests with specific emphasis on one new ectomycorrhizal family, the Asteropeiaceae. New Phytol 178:233–238
Ducousso M, Thoen D (1991) Les types mycorhiziens des Acacieae. In: Riedacker A, Dreyer E, Pafadnam C, Joly H, Bary G (eds) Physiologie des Arbres et Arbustes en zones arides et semi-arides. Paris, John Libbey Eurotext, pp 175–182
Dufrenoy J (1917) The endotrophic mycorrhiza of Ericaceae. New Phytol 16:222–228
Duhoux E, Rinaudo G, Diem HG, Auguy F, Fernandez D, Bogusz D, Franche C, Dommergues Y, Huguenin B (2001) Angiosperm Gymnostoma trees produce root nodules colonized by arbuscular mycorrhizal fungi related to Glomus. New Phytol 149:115–125
Egerton-Warburton L, Allen MF (2001) Endo-and ectomycorrhizas in Quercus agrifolia nee. (Fagaceae): patterns of root colonization and effects on seedling growth. Mycorrhiza 11:283–290
Escudero M, Hipp AL, Waterway MJ, Valente LM (2012) Diversification rates and chromosome evolution in the most diverse angiosperm genus of the temperate zone (Carex, Cyperaceae). Mol Phyl Evol 63:650–655
Fassi B (1957) Ectomycorrhizes chez le Gnetum africanum Welw. Due a Scleroderma sp. Bull Soc Mycol Fr 73:280–286
Fassi B, Fontana A (1962) Micorrize ectotrofiche di Brachystegia laurentii e di alcune altre Caesalpiniaceae minori del Congo. Allionia 8:121–131
Fisher JB, Sweeney S, Brzostek ER, Evans TP, Johnson DJ, Myers JA, Bourg NA, Wolf AT, Howe RW, Phillips RP (2016) Tree-mycorrhizal associations detected remotely from canopy spectral properties. Glob Change Biol 22:2596–2607
Fontana A (1963) Micorrize ectotrofiche in una ciperacea: Kobresia bellardii Degl. G Bot Ital 70:639–641
Fontana A, Giovannetti G (1978) Simbiosi micorrizica fra Cistus incanus L. spp. incanus e Tuber melanosporum Vitt. Allionia 23:5–11
Frank B (1885) Ueber die auf Wurzelsymbiose beruhende Ernährung gewiser Bäume durch unterirdishe Pilze. Ber Deut Bot Ges 3:128–145
Frank B (1887) Ueber neue Mycorhiza-Formen. Ber Deut Bot Ges 5:395–409
Frank B (1888) Ueber die physiologische Bedeutung der Mycorhiza. Ber Deut Bot Ges 6:248–269
Fraser L (1931) An investigation of Lobelia gibbosa and Lobelia dentata I. Mycorrhiza, latex system and general biology. Proc Linn Soc NSW 56:497–525
Frioni L, Minasian H, Volfovicz R (1999) Arbuscular mycorrhizae and ectomycorrhizae in native tree legumes in Uruguay. For Ecol Manag 115:41–47
Gao Q, Yang ZL (2010) Ectomycorrhizal fungi associated with two species of Kobresia in an alpine meadow in the Western Himalaya. Mycorrhiza 20:281–287
Ge Z-W, Smith ME, Zhang Q-Y, Yang ZL (2012) Two species of Asian endemic genus Keteleeria form ectomycorrhizas with diverse fungal symbionts in southwestern China. Mycorrhiza 22:403–408
Gervais GYF, Bruneau A (2002) Phylogenetic analysis of a polyphyletic African genus of Caesalpinioideae (Leguminosae): Monopetalanthus harms. Plant Syst Evol 235:19–34
Grand LF (1971) Tuberculate and Cenococcum mycorrhizae of Photinia (Rosaceae). Mycologia 63:1210–1212
Gunasekara N 2004 Phylogenetic and molecular dating analyses of the tropical tree family Dipterocarpaceae based on chloroplast matK nucleotide sequence data. Thesis. Concordia University, Montreal
Gustafsson MH, Backlund A, Bremer B (1996) Phylogeny of the Asterales Sensu Lato based on rbcL sequences with particular reference to the Goodeniaceae. Plant Syst Evol 199:217–242
Guzmán B, Vargas P (2009) Historical biogeography and character evolution of Cistaceae (Malvales) based on analysis of plastid rbcL and trnL-trnF sequences. Org Div Evol 9:83–99
Harley JL, Harley EL (1987) A check-list of mycorrhiza in the British flora. New Phytol 105(Suppl):1–102
Haselwandter K, Read DJ (1980) Fungal associations of roots of dominant and subdominant plants in high-alpine vegetation systems with special reference to mycorrhiza. Oecologia 45:57–62
Haug I, Lempe J, Homeier J, Weib M, Setaro S, Oberwinkler F, Kottke I (2004) Graffenrieda emarginata (Melastomataceae) forms mycorrhizas with Glomeromycota and with a member of the Hymenoscyphus ericae aggregate in the organic soil of a neotropical mountain rain forest. Can J Bot 82:340–356
Haug I, Weber R, Oberwinkler F, Tschen J (1991) Tuberculate mycorrhizas of Castanopsis borneensis king and Engelhardtia roxburghiana wall. New Phytol 117:25–35
Haug I, Weber R, Oberwinkler F, Tschen J (1994) The mycorrhizal status of Taiwanese trees and the description of some ectomycorrhizal types. Trees 8:237–253
Haug I, Weiß M, Homeier J, Oberwinkler F, Kottke I (2005) Russulaceae and Thelephoraceae form ectomycorrhizas with members of the Nyctaginaceae (Caryophyllales) in the tropical mountain rain forest of southern Ecuador. New Phytol 165:923–936
Hayward J, Hynson NA (2014) New evidence of ectomycorrhizal fungi in the Hawaiian Islands associated with the endemic host Pisonia sandwicensis (Nyctaginaceae). Fungal Ecol 12:62–69
Henkel TW (2003) Monodominance in the ectomycorrhizal Dicymbe corymbosa (Caesalpiniaceae) from Guyana. J Trop Ecol 19:417–437
Henkel TW, Aime MC, Miller SL (2000) Systematics of pleurotoid Russulaceae from Guyana and Japan, with notes on their ectomycorrhizal status. Mycologia 92:1119–1132
Henkel TW, Terborgh J, Vilgalys R (2002) Ectomycorrhizal fungi and their leguminous hosts in the Pakaraima mountains of Guyana. Mycol Res 106:515–531
Hesselman H (1900) Mykorrhizabildningar. Bih Kongl Sv Vet-Akad Handl 26:3–50
Hileman LC, Vasey MC, Parker TV (2001) Phylogeny and biogeography of the Arbutoideae (Ericaceae): implications for the Madrean-Tethyan hypothesis. Syst Bot 26:131–143
Högberg P (1982) Mycorrhizal associations in some woodland forest trees and shrubs in Tanzania. New Phytol 92:407–415
Högberg P, Piearce GD (1986) Mycorrhizas in Zambian trees in relation to host taxonomy, vegetation type and successional patterns. J Ecol 74:775–785
Hong LT (1979) A note on dipterocarp mycorrhizal fungi. Malay For 42:280–283
Horn JW, Wurdack KJ, Dorr LJ (2016) Phylogeny and diversification of Malvales. In: Stogran J (ed) Proceedings in Botany 2016: celebrating our history, Conserving our future. Savannah, p 157
Horton TR, Bruns TD, Parker VT (1999) Ectomycorrhizal fungi associated with Arctostaphylos contribute to Pseudotsuga menziesii establishment. Can J Bot 77:93–102
Hu YS, Wang FH (1984) Anatomical studies of Cathaya (Pinaceae). Am J Bot 71:727–735
Hynson NA, Bidartondo MI, Read DJ (2015) Are there geographic mosaics of mycorrhizal specificity and partial mycoheterotrophy? A case study in Moneses uniflora (Ericaceae). New Phytol 208:1003–1007
Hynson NA, Mambelli SA, Amend AS, Dawson TE (2012) Measuring carbon gains from fungal networks in understory plants from the tribe Pyroleae (Ericaceae):a field manipulation and stable isotope approach. Oecologia 169:307–317
Jabaily RS, Shepherd KA, Gardner AG, Gustafsson MH, Howarth DG, Motley TJ (2014) Historical biogeography of the predominantly Australian plant family Goodeniaceae. J Biogeogr 41:2057–2067
Janos DP (1980) Mycorrhizae and tropical succession. Biotropica 12:56–64
Jenik J, Mensah KOA (1967) Root system of tropical trees. 1. Ectotrophic mycorrhizae of Afzelia africana Sm. Preslia 39:59–65
Jessen K (1914) Rosaceae. Meddel Gronl 37:1–126
Jourand P, Carriconde F, Ducousso M, Majorel C, Hannibal L, Prin Y, Lebrun M (2014) Abundance, distribution and function of Pisolithus albus and other ectomycorrhizal fungi of ultramafic soils in New Caledonia. In: Ba AM, McGuire KL, Diedhiou A (eds) Ectomycorrhizal symbioses in tropical and neotropical forests. CRC Press, Boca Raton, FL, pp 100–125
Kamienski FM (1882) Les organes végétatifs du Monotropa hypopithys L. Mem Soc Nat Sci Nat Math Cherbourg 24:5–40
Katenin AE (1972) Mikoriza rastenii severo-vostoka evropeiskoi chasti USSR. Moscow, Izd Akd Nauk USSR
Kennedy PG, Izzo AD, Bruns TD (2003) There is high potential for the formation of common mycorrhizal networks between understorey and canopy trees in a mixed evergreen forest. J Ecol 91:1071–1080
Khan AG (1993) Occurrence and importance of mycorrhizae in aquatic trees. Mycorrhiza 3:31–38
Klecka A, Vukolov V (1935) Srovnavaci stutlie o mykorrhize drevin. Sb Cesk Akad Zemed 10:443–457
Koele N, Dickie IA, Oleksyn J, Richardson SJ, Reich PB (2012) No globally consistent effect of ectomycorrhizal status on foliar traits. New Phytol 196:845–852
Kohn LM, Stasovski E (1990) The mycorrhizal status of plants at Alexandra fiord, Ellesmere island, Canada, a high arctic site. Mycologia 82:23–35
Kope HH, Warcup JH (1986) Synthesized ectomycorrhizal associations of some Australian herbs and shrubs. New Phytol 104:591–599
Koske RE (1988) Vesicular-arbuscular mycorrhizae of some Hawaiian dune plants. Pac Sci 42:214–226
Kovacs GM, Szigetvari C (2002) Mycorrhizae and other root-associated fungal structures of the plants of a sandy grassland on the great Hungarian plain. Phyton 42:211–224
Kramar U (1901) Studie über die mykorrhiza von Pirola rotundifolia L. Bull Int Acad Sci Prague 6:9–15
Kreisel H (1970) El papel de los hongos en la veetacion forestal de Cuba. Bol Soc Mex Mic 4:39–43
Kron KA, Luteyn JL (2005) Origins and biogeographic patterns in Ericaceae: new insights from recent phylogenetic analyses. Biol Skr 55:479–500
Kühdorf K, Münzenberger B, Begerow D, Gomez-Laurito J, Hüttl RF (2015) Leotia cf. lubrica forms arbutoid mycorrhiza with Comarostaphylis arbutoides (Ericaceae). Mycorrhiza 25:109–120
Ladiges PY, Kellermann J, Nelson G, Humphries CJ, Udovicic F (2005) Historical biogeography of Australian Rhamnaceae, tribe Pomaderreae. J Biogeogr 32:1909–1919
Lam N, Wilson PG, Heslewood MM, Quinn CJ (2002) A phylogenetic analysis of the Chamelaucium alliance (Myrtaceae). Aust Syst Bot 15:535–543
Lamont BB, Ralph CS, Christensen PES (1985) Mycophagous marsupials as dispersal agents for ectomycorrhizal fungi on Eucalyptus calophylla and Gastrolobium bilobum. New Phytol 101:651–656
Langkamp PJ, Dalling MJ (1982) Nutrient cycling in a stand of Acacia holosericea II. Phosphorus and endomycorrhizal associations. Aust J Bot 30:87–106
Largent DL, Sugihara N, Wishner C (1980) Occurrence of mycorrhizae on ericaceous and pyrolaceous plants in northern California. Can J Bot 58:2274–2279
Larson-Johnson K (2015) Phylogenetic investigation of the complex evolutionary history of dispersal mode and diversification rates across living and fossil Fagales. New Phytol 209:418–435
Lavin M, Herendeen PS, Wojciechowski MF (2005) Evolutionary rates analysis of Leguminosae implicates a rapid diversification of lineages during the Tertiary. Syst Biol 54:530–549
Lee SS (1988) Do mycorrhizas play a role in the growth and development of Parashorea densiflora Sloot and Sym. Proc IFS Symp Sci Asia 87:14–17
LePage BA (2003) The evolution, biogeography and palaeoecology of the Pinaceae based on fossil and extant representatives. Acta Hortic 615:29–52
Lesica P, Antibus RK (1986) Mycorrhizal status of hemiparasitic vascular plants in Montana, USA. Trans Br Mycol Soc 86:341–343
Leslie AB, Beaulieu JM, Rai HS, Crane PR, Donoghue MJ, Mathews S (2012) Hemisphere-scale differences in conifer evolutionary dynamics. Proc Natl Acad Sci U S A 109:16217–16221
Li A-R, Guan K-Y (2008) Arbuscular mycorrhizal fungi may serve as another nutrient strategy for some hemiparasitic species of Pedicularis (Orobanchaceae). Mycorrhiza 18:429–436
Lin G, McCormack ML, Ma C, Guo D (2017) Similar below‐ground carbon cycling dynamics but contrasting modes of nitrogen cycling between arbuscular mycorrhizal and ectomycorrhizal forests. New Phytol 213:1440–1451
Lodge DJ (1989) The influence of soil moisture and flooding on formation of VA-endo- and ectomycorrhizae in Populus and Salix. Plant Soil 117:243–253
Lodge DJ (1996) Microorganisms. In: Reagan DP, Waide RB (eds) The food web of a tropical forest. Chicago University Press, Chicago, IL, pp 53–108
Lohman ML (1926) Occurrence of mycorrhiza in Iowa forest plants. Univ Iowa Studies Nat Hist 11:33–58
Longway LJ (2015) Comparing ectomycorrhizal communities of understory giant chinquapin (Chrysolepis chrysophylla) and overstory Pinaceae trees in a mixed conifer forest in Central Oregon. MSc Thesis. Oregon State University, Eugene
LPWG (2017) A new subfamily classification of the Leguminosae based on a taxonomically comprehensive phylogeny. Taxon 66:44–77
Lu Y, Ran J-H, Guo D-M, Yang Z-Y, Wang X-Q (2014) Phylogeny and divergence times of gymnosperms inferred from single-copy nuclear genes. PLoS One 9:e107679
Maeda M (1954) The meaning of mycorrhiza in regard to systematic botany. Kumamoto J Sci B 3:57–84
Maherali H, Oberle B, Stevens PF, Cornwell WK, McGlinn DJ (2016) Mutualism persistence and abandonment during the evolution of the mycorrhizal symbiosis. Am Nat 188:E113–E125
Malloch D, Malloch B (1982) The mycorrhizal status of boreal plants: additional species from northeastern Ontario. Can J Bot 60:1035–1040
Malloch D, Thorn RG (1985) The occurrence of ectomycorrhizae in some species of Cistaceae in North America. Can J Bot 63:872–875
Mangin L (1910) Introduction à l’étude des mycorrhizes des arbres forestières. Nouv Arch Mus Hist Nat Paris 5:213
Massicotte HB, Melville LH, Peterson RL, Luoma DL (1998) Anatomical aspects of field ectomycorrhizas on Polygonum viviparum (Polygonaceae) and Kobresia bellardii (Cyperaceae). Mycorrhiza 7:287–292
Massicotte HB, Melville LH, Tackaberry LE, Peterson RL (2008) A comparative study of mycorrhizas in several genera of Pyroleae (Ericaceae) from western Canada. Botany 86:610–622
Massicotte HB, Peterson RL, Melville LH, Tackaberry LE (2010) Hudsonia ericoides and Hudsonia tomentosa: anatomy of mycorrhizas of two members in the Cistaceae from Eastern Canada. Botany 88:607–616
Masui K (1926) A study of the ectotrophic mycorrhiza of Alnus. Mem Coll Sci, Kyoto Imp Univ 10:190–209
Matsuda Y, Yamada A (2003) Mycorrhizal morphology of Monotropastrum humile collected from six different forests in central Japan. Mycologia 95:993–997
McDonald KR, Pennell J, Frank JL, Southworth D (2010) Ectomycorrhizas of Cercocarpus ledifolius (Rosaceae). Am J Bot 97:1867–1872
McDougall WB (1914) On the mycorhizas of forest trees. Am J Bot 1:51–78
McDougall WB (1928) Mycorhizas from North Carolina and Eastern Tennessee. Bot Gaz 15:141–148
McDougall WB, Jacobs MC (1927) Tree mycorhizas from the central Rocky Mountain region. Bot Gaz 14:258–266
McGee PA (1986) Mycorrhizal associations of plant species in a semiarid community. Aust J Bot 34:585–593
McGee PA (1988) Growth response to and morphology of mycorrhizas of Thysanotus (Anthericaceae: Monocotyledonae). New Phytol 109:459–463
Meers TL, Bell TL, Enright NJ, Kasel S (2010) Do generalisations of global trade-offs in plant design apply to an Australian sclerophyllous flora? Aust J Bot 58:257–270
Merckx V (2013) Mycoheterotrophy: the biology of plants living on fungi. Springer, Berlin
Meyer U (1991) Feinwurzelsysteme und Mykorrhizatypen als Anpassungsmechanismen in Zentralamazonischen Überschwemmungswäldern -Igapó und Vàrzea. PhD Thesis. Universität Hohenheim, Hohenheim
Michelsen A, Schmidt IK, Jonasson S, Quarmby C, Sleep D (1996) Leaf 15N abundance of subarctic plants provides field evidence that ericoid, ectomycorrhizal and non-and arbuscular mycorrhizal species access different sources of soil nitrogen. Oecologia 105:53–63
Mikeladze RM (1960) K poznaniju alpiiskih kovrov Jugo-Ossetii. Probl Bot 5:170–181
Miller OK (1982) Mycorrhizae, mycorrhizal fungi and fungal biomass in subalpine tundra at eagle summit, Alaska. Holarct Ecol 5:125–134
Miller OK, Laursen GA (1978) Ecto-and endomycorrhizae of arctic plants at barrow, Alaska. Ecol Stud 29:229–237
Miller JT, Murphy DJ, Ho SY, Cantrill DJ, Seigler D (2013) Comparative dating of Acacia: combining fossils and multiple phylogenies to infer ages of clades with poor fossil records. Aust J Bot 61:436–445
Miller JT, Seigler D (2012) Evolutionary and taxonomic relationships of Acacia sl (Leguminosae: Mimosoideae). Aust Syst Bot 25:217–224
Morrison TM (1956) Mycorrhiza of silver beech. N Z J For 7:47–60
Morton CM, Dayanandan S, Dissanayake D (1999) Phylogeny and biosystematics of Pseudomonotes (Dipterocarpaceae) based on molecular and morphological data. Plant Syst Evol 216:197–205
Moyersoen B (1993) Ectomicorrizas i micorrizas vesiculo-arbusculares en Caatinga Amazonica del Sur de Venezuela. Sci Guaianae 3:1–82
Moyersoen B (2006) Pakaraimea dipterocarpacea is ectomycorrhizal, indicating an ancient Gondwanaland origin for the ectomycorrhizal habit in Dipterocarpaceae. New Phytol 170:873–883
Murphy DJ, Miller JT, Bayer RJ, Ladiges PY (2003) Molecular phylogeny of Acacia subgenus Phyllodineae (Mimosoideae: Leguminosae) based on DNA sequences of the internal transcribed spacer region. Aust Syst Bot 16:19–26
Nespiak A (1953) Badanie mykotrofizmu roslinnosci alpejeskiej ponad granica kosodrzewiny w granitowych Tatrach. Acta Soc Bot Pol 22:97–125
Newbery DM, Alexander IJ, Thomas DW, Gartlan JS (1988) Ectomycorrhizal rainforest legumes and soil phosphorus in Korup National Park, Cameroon. New Phytol 109:433–450
Nicolas A (2009) Understanding Evolutionary Relationships in the Angiosperm Order Apiales Based on Analyses of Organellar Dna Sequences and Nuclear Gene Duplications. Thesis. Virginia Commonwealth University, Richmond
Noack F (1889) Ueber mykorhizenbildende Pilze. Bot Zeit 24:391–404
Noelle W (1910) Studien zur vergleichenden Anatomie und Morphologie der Koniferenwurzeln mit Rücksicht auf die Systematik. Bot Zeit 68:169–266
Noor S (1981) Mycorrhizas of tropical forest trees. Report on International Foundation for Science Grant No. 173
Nyffeler R, Bayer C, Alverson WS, Yen A, Whitlock BA, Chase MW, Baum DA (2005) Phylogenetic analysis of the Malvadendrina clade (Malvaceae sl) based on plastid DNA sequences. Org Div Evol 5:109–123
O’Brien MM, Quinn CJ, Wilson PG (2000) Molecular systematics of the Leptospermum suballiance (Myrtaceae). Aust J Bot 48:621–628
Onguene NA (2000) Diversity and dynamics of mycorrhizal associations in tropical rain forests with different disturbance regimes in South Cameroon, Tropenbos Cameroon series 3. University of Wageningen, Wageningen
Onipchenko VG, Zobel M (2000) Mycorrhiza, vegetative mobility and responses to disturbance of alpine plants in the Northwestern Caucasus. Fol Geobot 35:1–10
Onstein RE, Carter RJ, Xing Y, Richardson JE, Linder HP (2015) Do Mediterranean‐type ecosystems have a common history?—insights from the buckthorn family (Rhamnaceae). Evolution 69:756–771
Orchard S, Hilton S, Bending GD, Dickie IA, Standish RJ, Gleeson DB, Jeffery RP, Powell JR, Walker C, Bass D, Monk J (2017) Fine endophytes (Glomus tenue) are related to Mucoromycotina, not Glomeromycota. New Phytol 213:481–486
Osmundson TW, Halling RE, Den Bakker HC (2007) Morphological and molecular evidence supporting an arbutoid mycorrhizal relationship in the Costa Rican páramo. Mycorrhiza 17:217–222
Peay KG, Russo SE, McGuire KL, Lim Z, Chan JP, Tan S, Davies SJ (2015) Lack of host specificity leads to independent assortment of dipterocarps and ectomycorrhizal fungi across a soil fertility gradient. Ecol Lett 18:807–816
Pennington HG, Bidartondo MI, Barsoum N (2011) A few exotic mycorrhizal fungi dominate eucalypts planted in England. Fungal Ecol 4:299–302
Perrier N, Amir H, Colin F (2006) Occurrence of mycorrhizal symbioses in the metal-rich lateritic soils of the Koniambo Massif, New Caledonia. Mycorrhiza 16:449–458
Peterson RL, Ashford AE, Allaway WG (1985) Vesicular-arbuscular mycorrhizal associations of vascular plants on heron Island, a great barrier reef coral cay. Aust J Bot 33:669–676
Peyronel B (1922) Nuovi casi di rapprti micorizici tra basidomiceti e fanerogame arboree. Bull Soc Bot Ital 1:7–14
Peyronel B (1930) Simbiosi micorrizica tra piante alpine e Basidiomiceti. Nuov Giorn Bot Ital 37:655–663
Peyronel B, Fassi B (1960) Nuovi casi di simbiosi ectomicorrizica in Leguminose della famiglia delle Cesalpiniacee. Atti Acad Sci Torino 94:36–38
Phillips RP, Brzostek E, Midgley MG (2013) The mycorrhizal-associated nutrient economy: a new framework for predicting carbon–nutrient couplings in temperate forests. New Phytol 199:41–51
Põlme S, Bahram M, Yamanaka T, Nara K, Dai YC, Grebenc T, Kraigher H, Toivonen M, Wang P-H, Matsuda Y, Naadel T, Kennedy PG, Kõljalg U, Tedersoo L (2013) Biogeography of ectomycorrhizal fungi associated with alders (Alnus spp.) in relation to biotic and abiotic variables at the global scale. New Phytol 198:1239–1249
Potter D, Eriksson T, Evans RC, Oh S, Smedmark JEE, Morgan DR, Kerr M, Robertson KR, Arsenault M, Dickinson TA, Campbell CS (2007) Phylogeny and classification of Rosaceae. Plant Syst Evol 266:5–43
Pressel S, Bidartondo MI, Ligrone RO, Duckett JG (2010) Fungal symbioses in bryophytes: new insights in the twenty first century. Phytotaxa 9:238–253
Prin Y, Ducousso M, Tassin J, Béna G, Jourand P, Dumontet V, Moulin L, Contesto C, Ambrosi JP, Chaintreuil C, Dreyfus B (2012) Ectotrophic mycorrhizal symbioses are dominant in natural ultramafic forest ecosystems of New Caledonia. In: Hafidi M, Duponnois R (eds) The mycorrhizal symbiosis in Mediterranean environment: importance in ecosystem stability and in soil rehabilitation strategies. Nova Science Publishers, New York, NY, pp 26–48
Proctor MC (1960) Tuberaria guttata (L.) Fourreau. J Ecol 48:243–253
Pryor LD (1956) Chlorosis and lack of vigor in seedlings of renantherous species of Eucalyptus caused by lack of mycorrhiza. Linn Soc NSW Proc 81:91–96
Ramos G, de Lima HC, Prenner G, de Queiroz LP, Zartman CE, Cardoso D (2016) Molecular systematics of the Amazonian genus Aldina, a phylogenetically enigmatic ectomycorrhizal lineage of papilionoid legumes. Mol Phyl Evol 97:11–18
Read DJ, Haselwandter K (1981) Observations on the mycorrhizal status of some alpine plant communities. New Phytol 88:341–352
Reddell P, Bowen GD, Robson AD (1986) Nodulation of Casuarinaceae in relation to host species and soil properties. Aust J Bot 34:435–444
Reddell P, Hopkins MS, Graham AW (1996) Functional association between apogeotropic aerial roots, mycorrhizas and paper-barked stems in a lowland tropical rainforest in North Queensland. J Trop Ecol 12:763–777
Reddell P, Milnes AR (1992) Mycorrhizas and other specialized nutrient-acquisition strategies: their occurrence in woodland plants from Kakadu and their role in rehabilitation of waste rock dumps at a local uranium mine. Aust J Bot 40:223–242
Redhead JF (1974) Aspects of the biology of mycorrhizal associations occurring on tree species in Nigeria. Thesis. University of Ibadan, Ibadaen
Reinsvold RT, Reeves B (1986) The mycorrhizae of Juniperus osteosperma: identity of the vesicular-arbuscular mycorrhizal symbiont, and resynthesis of VA mycorrhizae. Mycologia 78:108–113
Reiter N, Lawrie A, Walsh N (2013) The mycorrhizal associations of Borya mirabilis, an endangered Australian native plant. Muelleria 31:81–88
Richards AE, Shapcott A, Playford J, Morrison B, Critchley C, Schmidt S (2003) Physiological profiles of restricted endemic plants and their widespread congenors in the North Queensland wet tropics, Australia. Biol Conserv 111:41–52
Richardson JE, Whitlock BA, Meerow A, Madriñán S (2015) The age of chocolate: a biogeographic history of Theobroma and Malvaceae. Front Ecol Evol 3:120
Rivière T (2004) Biodiversity, molecular ecology and phylogeography of tropical ectomycorrhizal symbiosis. PhD thesis. Université de Montpellier II, Montpellier
Riviere T, Diabaté M, Ducousso M, Prin Y, Dreyfus B, Buyck B, Eyssartier G, Verbeken A, Descheres P, Ba A (2001) Diversity of ectomycorrhizal fungi associated with some native trees in Southern Guinea. In: Abstracts of the ICOM3 conference, Adelaide, Australia
Rose SL (1980) Mycorrhizal associations of some actinomycete nodulated nitrogen-fixing plants. Can J Bot 58:1449–1454
Ruotsalainen AL, Väre H, Oksanen J, Tuomi J (2004) Root fungus colonization along an altitudinal gradient in North Norway. Arct Antarct Alp Res 36:239–243
Samuel G (1926) Note on the distribution of mycorrhiza. Trans R Soc S Aust 50:245–246
Santoso E (1988) The effect of mycorrhizas on the stem diameter and dry weight of dipterocarp seedlings. Buletin Penelitian Hutan 504:11–21
Sarauw GF (1903) Sur les mycorrhizes des arbres forestiers. Rev Mycol 25:157–172
Schlicht A (1889) Beitrag zur Kenntniss der Verbreitung und Bedeutung der Mycorhizen. Landw Jahrb 18:478–506
Schmidt-Lebuhn AN, Bruhl JJ, Telford IR, Wilson PG (2015) Phylogenetic relationships of Coronidium, Xerochrysum and several neglected Australian species of “Helichrysum” (Asteraceae: Gnaphalieae). Taxon 64:96–109
Schrire BD, Lavin M, Lewis GP (2005) Global distribution patterns of the Leguminosae: insights from recent phylogenies. Biol Skr 55:375–422
Schuster TM, Setaro SD, Kron KA (2013) Age estimates for the buckwheat family Polygonaceae based on sequence data calibrated by fossils and with a focus on the amphi-pacific Muehlenbeckia. PLoS One 8:e61261
Schwery O, Onstein RE, Bouchenak‐Khelladi Y, Xing Y, Carter RJ, Linder HP (2015) As old as the mountains: the radiations of the Ericaceae. New Phytol 207:355–367
Sharma SK, Sharma GD, Mishra RR (1986) Status of mycorrhizae in sub-tropical forest ecosystem of Meghalaya. Acta Bot Ind 14:87–92
Singh KG (1966) Ectotrophic mycorrhiza in equatorial rain forests. Malay For 29:13–18
Sirisena UM (2010) Systematic studies on Thysanotus (Asparagales: Laxmanniaceae). Thesis. University of Adelaide, Adelaide
Smissen RD, Breitwieser I, Ward JM (2004) Phylogenetic implications of trans-specific chloroplast DNA sequence polymorphism in New Zealand Gnaphalieae (Asteraceae). Plant Syst Evol 249:37–53
Smith ME, Henkel TW, Aime MC, Fremier AK, Vilgalys R (2011) Ectomycorrhizal fungal diversity and community structure on three co-occurring leguminous canopy tree species in a Neotropical rainforest. New Phytol 192:699–712
Smith ME, Henkel TW, Uehling JK, Fremmier AK, Clarke HD, Vilgalys R (2013) The ectomycorrhizal fungal community in a neotropical forest dominated by the endemic dipterocarp Pakaraimaea dipterocarpacea. PLoS One 8:e55160
Smith SE, Read DJ (2008) Mycorrhizal Symbiosis, 3rd edn. Academic Press, London
Soudzilovskaia N, van der Heijden MGA, Cornelissen JHC, Makarov MI, Onipchenko VG, Maslov MN, Akhmetzanova AA, van Bodegom PM (2015) Quantitative assessment of the differential impacts of arbuscular and ectomycorrhiza on soil carbon cycling. New Phytol 208:280–293
St. John TV (1980) A survey of mycorrhizal infection in an amazonian rain forest. Acta Amaz 10:527–533
Stahl E (1900) Der Sinn der Mycorhizenbildung. Jahrb Wiss Bot 34:539–668
Starr JR, Harris SA, Simpson DA (2004) Phylogeny of the unispicate taxa in Cyperaceae tribe Cariceae I: generic relationships and evolutionary scenarios. Syst Bot 29:528–544
Stutz RC (1972) Survey of mycorrhizal plants. In: Bliss LC (ed) Devon Island IPB project: high arctic ecosystem. Project report 1970 and 1971. University of Alberta, Edmonton, pp 214–216
Suvi T, Tedersoo L, Abarenkov K, Gerlach J, Beaver K, Kõljalg U (2010) Mycorrhizal symbionts of Pisonia grandis and P. sechellarum in Seychelles: identification of mycorrhizal fungi and description of new Tomentella species. Mycologia 102:522–533
Swaty RL, Deckert RJ, Whitham TG, Gehring CA (2004) Ectomycorrhizal abundance and community structure shifts with drought: predictions from tree rings. Ecology 85:1072–1084
Tanaka-Oda A, Kenzo T, Inoue Y, Yano M, Koba K, Ichie T (2015) Variation in leaf and soil δ15N in diverse tree species in a lowland dipterocarp rainforest, Malaysia. Trees 30:509–522
Tandy PA (1975) Studies on sporocarpic Endogonaceae in Australia. Thesis. University of Adelaide, Adelaide
Tank DC, Eastman JM, Pennell MW, Soltis PS, Soltis DE, Hinchliff CE, Brown JW, Sessa EB, Harmon LJ (2015) Nested radiations and the pulse of angiosperm diversification: increased diversification rates often follow whole genome duplications. New Phytol 207:454–467
Tedersoo L, Bahram M, Jairus T, Bechem E, Chinoya S, Mpumba R, Leal M, Randrianjohany E, Razafimandimbison S, Sadam A, Naadel T, Kõljalg U (2011) Spatial structure and the effects of host and soil environments on communities of ectomycorrhizal fungi in wooded savannas and rain forests of continental Africa and Madagascar. Mol Ecol 20:3071–3080
Tedersoo L, Bahram M, Põlme S, Kõljalg U, Yorou NS, Wijesundera R, Villarreal-Ruiz L, Vasco-Palacios A, Quang Thu P, Suija A, Smith ME, Sharp C, Saluveer E, Saitta A, Ratkowsky D, Pritsch K, Riit T, Põldmaa K, Piepenbring M, Phosri C, Peterson M, Parts K, Pärtel K, Otsing E, Nouhra E, Njouonkou AL, Nilsson RH, Morgado LN, Mayor J, May TW, Kohout P, Hosaka K, Hiiesalu I, Henkel TW, Harend H, Guo L, Greslebin A, Grelet G, Geml J, Gates G, Dunstan W, Dunk C, Drenkhan R, Dearnaley J, De Kesel A, Dang T, Chen X, Buegger F, Brearley FQ, Bonito G, Anslan S, Abell S, Abarenkov K (2014) Global diversity and geography of soil fungi. Science 346:1078
Tedersoo L, Bahram M, Toots M, Diédhiou AG, Henkel TW, Kjøller R, Morris MH, Nara K, Nouhra E, Peay KG, Põlme S, Ryberg M, Smith ME, Kõljalg U (2012) Towards global patterns in the diversity and community structure of ectomycorrhizal fungi. Mol Ecol 21:4160–4170
Tedersoo L, Jairus T, Horton BM, Abarenkov K, Suvi T, Saar I, Kõljalg U (2008) Strong host preference of ectomycorrhizal fungi in a Tasmanian wet sclerophyll forest as revealed by DNA barcoding and taxon-specific primers. New Phytol 180:479–490
Tedersoo L, May TW, Smith ME (2010a) Ectomycorrhizal lifestyle in fungi: global diversity, distribution, and evolution of phylogenetic lineages. Mycorrhiza 20:217–263
Tedersoo L, Mett M, Ishida TA, Bahram M (2013) Phylogenetic relationships among host plants explain differences in fungal species richness and community composition in ectomycorrhizal symbiosis. New Phytol 199:822–831
Tedersoo L, Pellet P, Kõljalg U, Selosse M-A (2007a) Parallel evolutionary paths to mycoheterotrophy in understorey Ericaceae and Orchidaceae: ecological evidence for mixotrophy in Pyroleae. Oecologia 151:206–217
Tedersoo L, Põlme S (2012) Infrageneric variation in partner specificity: multiple ectomycorrhizal symbionts associate with Gnetum gnemon (Gnetophyta) in Papua New Guinea. Mycorrhiza 22:663–668
Tedersoo L, Sadam A, Zambrano M, Valencia R, Bahram M (2010b) Low diversity and high host preference of ectomycorrhizal fungi in Western Amazonia, a neotropical biodiversity hotspot. ISME J 4:465–471
Tedersoo L, Smith ME (2013) Lineages of ectomycorrhizal fungi revisited: foraging strategies and novel lineages revealed by sequences from belowground. Fung Biol Rev 27:83–99
Tedersoo L, Suvi T, Beaver K, Kõljalg U (2007b) Ectomycorrhizal fungi of the Seychelles: diversity patterns and host shifts from the native Vateriopsis seychellarum (Dipterocarpaceae) and Intsia bijuga (Caesalpiniaceae) to the introduced Eucalyptus robusta (Myrtaceae), but not Pinus caribaea (Pinaceae). New Phytol 175:321–333
Teste FP, Kardol P, Turner BL, Warldle DA, Zemunik G, Renton M (2017) Laliberte E. Plant-soil feedback and the maintenance of diversity in Mediterranean-climate shrublands Science 355:173–176
Thomas WD Jr (1943) Mycorrhizae associated with some Colorado flora. Phytopathology 33:144–149
Thomazini LI (1974) Mycorrhiza in plants of the ‘Cerrado’. Plant Soil 41:707–711
Thornhill AH, Ho SY, Külheim C, Crisp MD (2015) Interpreting the modern distribution of Myrtaceae using a dated molecular phylogeny. Mol Phyl Evol 93:29–43
Tian C, He X, Zhong Y, Chen J (2003) Effect of inoculation with ecto-and arbuscular mycorrhizae and Rhizobium on the growth and nitrogen fixation by black locust, Robinia pseudoacacia. New For 25:125–131
Titus JH, Tsuyuzaki S (2002) Arbuscular mycorrhizal distribution in relation to microsites on recent volcanic substrates of Mt. Koma, Hokkaido, Japan. Mycorrhiza 12:271–275
Toon A, Cook LG, Crisp MD (2014) Evolutionary consequences of shifts to bird-pollination in the Australian pea-flowered legumes (Mirbelieae and Bossiaeeae). BMC Evol Biol 14:1
Trappe JM (1962) Fungus associates of ectotrophic mycorrhizae. Bot Rev 28:538–606
Trappe JM (1964) Mycorrhizal hosts and distribution of Cenococcum graniforme. Lloydia 27:100–106
Treu R, Laursen GA, Stephenson SL, Landolt JC, Densmore R (1996) Mycorrhizae from Denali national park and preserve, Alaska. Mycorrhiza 6:21–29
Tsuyuzaki S, Hase A, Niinuma H (2005) Distribution of different mycorrhizal classes on mount Koma, northern Japan. Mycorrhiza 15:93–100
Van der Heijden EW (2001) Differential benefits of arbuscular mycorrhizal and ectomycorrhizal infection of Salix repens. Mycorrhiza 10:185–193
van Roosendael J, Thorenaar A (1924) De natuurlijke verjonging van Ngerawan (Hopea mengarawan Miq.) in zuid Sumatra. Tectona 16:519–567
Väre H, Vestberg M, Eurola S (1992) Mycorrhiza and root-associated fungi in Spitsbergen. Mycorrhiza 1:93–104
Väre H, Vestberg M, Ohtonen R (1997) Shifts in mycorrhiza and microbial activity along an oroarctic altitudinal gradient in northern Fennoscandia. Arct Alp Res 29:93–104
Verbeken A, Walleyn R (1999) Is Pterygellus mycorrhizal with a euphorbia? Mycologist 13:37
Vorontsova MS, Hoffmann P, Maurin O, Chase MW (2007) Molecular phylogenetics of tribe Poranthereae (Phyllanthaceae; Euphorbiaceae Sensu Lato). Am J Bot 94:2026–2040
Wang B, Qiu Y-L (2006) Phylogenetic distribution and evolution of mycorrhizas in land plants. Mycorrhiza 16:299–363
Warcup JH (1980) Ectomycorrhizal associations of Australian indigenous plants. New Phytol 85:531–535
Warcup JH (1985) Rhizanthella gardneri (Orchidaceae), its Rhizoctonia endophyte and close association with Melaleuca uncinata (Myrtaceae) in western Australia. New Phytol 99:273–280
Warcup JH (1988) Mycorrhizal associations and seedling development in Australian Lobelioideae (Campanulaceae). Aust J Bot 36:461–472
Warcup JH (1990) The mycorrhizal associations of Australian Inuleae (Asteraceae). Muelleria 7:179–187
Warcup JH, McGee PA (1983) The mycorrhizal associations of some Australian Asteraceae. New Phytol 95:667–672
Watson GW, von der Heide-Spravka KG, Howe VK (1990) Ecological significance of endo−/ectomycorrhizae in the oak sub-genus Erythrobalanus. Arboricult J 14:107–116
Werner GDA, Cornwell WK, Sprent JI, Kattge J, Kiers ET (2014) A single evolutionary innovation drives the deep evolution of symbiotic N2-fixation in angiosperms. Nature Comm 5:4087
Werner GDA, Kiers ET (2015) Order of arrival structures arbuscular mycorrhizal colonization of plants. New Phytol 205:1515–1524
Wikström N, Savolainen V, Chase MW (2001) Evolution of the angiosperms: calibrating the family tree. Proc R Soc Lond B 268:2211–2220
Williams SE (1979) Vesicular-arbuscular mycorrhizae associated with actinomycete-nodulated shrubs, Cercocarpus montanus Raf. and Purshia tridentata (Pursh) DC. Bot Gaz 140:S115–S119
Wilson PG, O’Brien MM, Heslewood MM, Quinn CJ (2005) Relationships within Myrtaceae Sensu Lato based on a matK phylogeny. Plant Syst Evol 251:3–19
Won H, Renner SS (2006) Dating dispersal and radiation in the gymnosperm Gnetum (Gnetales)—clock calibration when outgroup relationships are uncertain. Syst Biol 55:610–622
Wurdack KJ, Hoffmann P, Samuel R, de Bruijn A, van der Bank M, Chase MW (2004) Molecular phylogenetic analysis of Phyllanthaceae (Phyllanthoideae pro parte, Euphorbiaceae Sensu Lato) using plastid rbcL DNA sequences. Am J Bot 91:1882–1900
Xi Z, Ruhfel BR, Schaefer H, Amorim AM, Sugumaran M, Wurdack KJ, Endress PK, Matthews ML, Stevens PF, Mathews S, Davis CC (2012) Phylogenomics and a posteriori data partitioning resolve the Cretaceous angiosperm radiation Malpighiales. Proc Natl Acad Sci 109:17519–17524
Yamamoto K, Degawa Y, Hirose D, Fukuda M, Yamada A (2015) Morphology and phylogeny of four Endogone species and Sphaerocreas pubescens collected in Japan. Mycol Prog 14:86
Yu TE, Egger KN, Peterson RL (2001) Ectendomycorrhizal associations—characteristics and functions. Mycorrhiza 11:167–177
Zainudin SR (1990) Studies on germination and seedling growth of Neobalanocarpus heimii (King) Ashton. Thesis. Universiti Pertanian Malaysia, Kuala Lumpur
Zak B (1973) Classification of ectomycorrizae. In: Marks GC, Kozlowski TT (eds) Ectomycorrhizae: their ecology and physiology. Academic Press, New York, NY, pp 43–78
Zak B (1974) Ectendomycorrhiza of Pacific madrone (Arbutus menziesii). Trans Br Mycol Soc 62:202–204
Zanne AE, Tank DC, Cornwell WK, Eastman JM, Smith SA, FitzJohn RG et al (2014) Three keys to the radiation of angiosperms into freezing environments. Nature 506:89–92
Zemunik G, Laliberte E, Lambers H, Turner BL (2015) Diversity of plant nutrient-acquisition strategies increases during long-term ecosystem development. Nat Plants 1:15050
Acknowledgements
We thank J. Alvarez-Manjarrez for permission to use EcM information about Achatocarpus; A.H. Thornhill for providing a raw phylogram of Myrtaceae; F. Teste, M. de la Estrella and J. Horn for discussing their unpublished results about Australian mycorrhizae, Detarioideae phylogeny and Dipterocarpaceae-Cistaceae phylogeny, respectively; and non-anonymous referees G. Zemunik and J. Trappe for constructive comments. This study was supported by the Estonian Science Foundation grant 1399PUT and MOBERC1.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Tedersoo, L., Brundrett, M.C. (2017). Evolution of Ectomycorrhizal Symbiosis in Plants. In: Tedersoo, L. (eds) Biogeography of Mycorrhizal Symbiosis. Ecological Studies, vol 230. Springer, Cham. https://doi.org/10.1007/978-3-319-56363-3_19
Download citation
DOI: https://doi.org/10.1007/978-3-319-56363-3_19
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-56362-6
Online ISBN: 978-3-319-56363-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)