Abstract
Nanomaterials, including engineered nano-sized iron oxides, manganese oxides, cerium oxides, titanium oxides, or zinc oxides, provide specific affinity for metal/metalloids adsorption and their application is being rapidly extended for environmental management. Their significant surface area, high number of active surface sites, and high adsorption capacities make them very promising as cost-effective amendments for the remediation of contaminated soils. The alleviation of the toxicities of metal/metalloids by their immobilization in the soil stimulates the growth and development of plants during phytoremediation, but there is a body of evidence indicating that nanomaterials themselves can yield both beneficial and harmful effects in plant systems at the physiological, biochemical, nutritional, and genetic levels. Nanoecotoxicological studies are providing a good understanding of their interactions with plants, and an increasing number of publications have attempted to clarify and quantify their potential risks and consequences for plants. However, many results are contradictory and the safety of engineered nanomaterials still represents a barrier to their wide, innovative use in phytoremediation. Both their positive and negative effects on plants will have to be taken into account to evaluate their applicability, and the scientific community faces a challenge to understand deeply the factors which can determine their relevance in environmental science and technology.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Soil contamination by metals (e.g., Cd, Cu, Mn, Ni, Pb, Zn) and metalloids (e.g., As, Sb) is a current global phenomenon endangering safe agricultural production and groundwater quality. Therefore, society, economics, and science are involved together in the common need for novel and environmentally friendly techniques for soil remediation. Recently, nanotechnology has offered a new generation of environmental remediation technologies that can provide cost-effective solutions to some of the most challenging environmental clean-up problems [1]. While various industrial sectors produce a large number of products containing nanomaterials, nanotechnology is also used in environmental management. Nanoparticles (NPs, materials with at least two dimensions between 1 and 100 nm) and nanomaterials (NMs, materials with at least one dimension smaller than 100 nm) [2,3,4] have the potential to revolutionize agricultural systems, environmental engineering, safety and security, water resources, and numerous other life sciences [5]. The smaller particle sizes, the higher specific surface area, and thus the higher number of reaction sites for metal adsorption represent the main advantages of NMs [6]. Nano-sized metal oxides , including nano-sized iron oxides, manganese oxides, aluminum oxides, zinc oxides, titanium oxides, and cerium oxides, have specific affinities for metal/metalloids adsorption and their application has been rapidly extended for environmental tasks [7,8,9]. Although they can exist naturally in the environment, they can also be produced/engineered intentionally [10], through methods that are becoming simpler, more effective, and cheaper. Concerning the economic aspect, it is clear that the doses of these compounds required for adsorption are lower when applied as engineered NMs because of their huge reactivity, while the contact times needed for the metal/metalloids adsorption are shorter in comparison with conventional adsorbents. In this sense, their application seems to be profitable [11].
2 Nanoparticles for Environmental Remediation
For sites contaminated by metal/metalloids, successful remediation is complicated by the fact that these pollutants do not degrade spontaneously, and it is not usually possible to excavate all the contaminated soil. Therefore, chemical stabilization of the metal/metalloids in these soils, through adsorption, surface precipitation, structural incorporation, or ion exchange, is a viable option for such sites as this technology immobilizes the contaminants in the soils and thus reduces their mobility, bioavailability, and bioaccessibility. In fact, this first step—the reduction of their toxicity—is crucial for the establishment of a vegetation cover on the contaminated sites during phytoremediation. The use of engineered NMs for remediation purposes can then enhance the natural attenuation processes, a key mechanism for the re-establishment of sustainable environmental systems. It has been claimed that nanotechnology has great potential as an environmentally cleaner technology, including alleviation of the toxicities of various metal/metalloids [12]. As a result, several studies have appeared in various journals dealing with the metal-NMs-mediated diminution of metal toxicity [13,14,15]. In general, this remediation technology involves (a) NMs transport along with the solution to the contaminated zone; (b) attachment to soils in the contaminated zone; and (c) reaction with the target contaminants to form less toxic or less mobile products [6]. The principal removal mechanisms for the most common inorganic contaminants can be divided into five categories: (1) adsorption (Cr, As, U, Pb, Ni, Se, Co, Cd, Zn, Ba); (2) reduction (Cr, As, Cu, U, Pb, Ni, Se, Co, Pd, Pt, Hg, Ag); (3) oxidation (As, U, Se, Pb); (4) precipitation (Cu, Pb, Cd, Co, Zn); and (5) co-precipitation (Cr, As, Ni, Se). The overall reaction processes are strongly influenced by a number of factors, in particular the NMs chemical properties and structure, the presence of more than one contaminant species, the pollutant characteristics, and the hydrogeochemistry of the aqueous environment (pH, redox conditions, natural dissolved species, etc.) [6, 16].
Since the amendments applied for the stabilization process need to be cost-efficient and suitable for different soil types and should not pose a risk to environmental compartments, application of engineered NMs in remediation technologies provides a very interesting alternative to soil excavation and dumping, ex situ soil washing, etc., because these are generally disruptive and costly. Nanoparticles have been studied as adsorbents of metals and their characteristics (i.e., large surface area, high number of active surface sites, low intra-particle diffusion rates, and high adsorption capacities) make them very promising for the cost-effective treatment of polluted soils [17, 18]. Then, nanoremediation , defined as the use of nanoparticles for environmental remediation, has the potential not only to reduce the overall costs of cleaning up large-scale contaminated sites, but can also reduce clean-up time, eliminate the need for treatment and disposal of the contaminated soil, and reduce the availability of some contaminants [19,20,21]. This is reflected in the increasing number of publications on this subject and the rising level of funding for remediation projects [6, 22,23,24,25,26].
To date, researchers have mainly focused their attention on the removal of metals from aqueous solutions rather than soil-bound metals, which may be absorbed by plants and subsequently spread into the human food chain. The nanoremediation of contaminated soils is a topic that has been researchedless, compared to the removal of pollutants from water or wastewater [21]. Based on this idea, soil columns can be set up for ex situ remediation, and a liquid suspension of NMs can be added to extract or to immobilize the contaminants (typically metals); the species adsorbed onto the NMs can be removed by applying mild gravitational (centrifugal) or magnetic (in the case of magnetic NMs, such as magnetite) gradients [21]. As a result of these previous leaching experiments with aqueous solutions, and thanks to the demonstrated potential of engineered NMs, they have been gradually incorporated into new in situ strategies for phytoremediation. Most of the information about their behavior in aqueous systems can be extrapolated to the soil solution, which is important since the physical/chemical properties of NMs are one of the most important factors that control their behavior in the environment although obviously it must be modified to take into account the new conditions in the soil. In this context, different hydrochemical parameters—such as pH, Eh, ionic strength, and aqueous chemistry—can change the aggregation kinetics and transformation of engineered NMs and their subsequent behavior. Similarly, natural organic matter alters their stability through electrostatic and steric interactions. The transformation process for NMs is also altered by a confluence of factors, depending on the characteristics of the NMs and of the environmental receptors.
Although the use of plants for phytoremediation and their capacity to accumulate and tolerate high concentrations of metals have been explored, and a significant amount of literature is available, the same is not true regarding NMs, and understanding the response of plants to NMs would be a key element in identifying mechanisms involved in stress tolerance and NMs toxicity [27]. According to Juganson et al. [28], 936 was the total number of publications (as sum of all the materials) found in the Thomson Reuters Web of Science™ for “environmental remediation ” using NMs (for a search done on March 19th, 2015), with 303 publications for nTiO2, 219 for nFeOx, 110 for nAg, 74 for nZnO, 36 for nCuO, 16 for nCeO2, and the rest for other NMs like fullerenes and carbon nanotubes (See chart in Fig. 14.2).
2.1 Main Types of Nanomaterials for the Adsorption of Metals and Metalloids
Metal NMs display size-dependent properties, such as magnetism (magnetic NPs), fluorescence (QDs), or photocatalytic degradation (metal oxide NPs), that have biotechnological applications in sensor development, agrochemical degradation, and soil remediation [29]. Nanoparticles and nanomaterials are mainly classified according to their dimensionality, morphology, or uniformity [30], but the classification according to their chemical properties is the most accepted and useful.
2.1.1 Iron Nanooxides : Nanogoethite, Nanomaghemite , Nanomagnetite
Iron oxides represent natural components of soils and exist in many forms, including mainly goethite (α-FeOOH; prevailing in temperate climatic areas), hematite (α-Fe2O3; prevailing in warm-dry climate zones), maghemite (γ-Fe2O3), and magnetite (Fe3O4). These iron oxides play a crucial role in soil systems due to their ability to adsorb potentially toxic elements such as metals and metalloids [31,32,33]. Synthetic iron-based NMs are thus interesting candidates for the removal of metals and metalloids from contaminated waters and soils, or their stabilization therein, due to their increased specific surface area and modified surface structure, which strongly affect their reactivity and chemistry [34,35,36,37]. During the remediation process, they can be applied directly as nano iron oxides or in the form of their precursors (i.e., nZVI, nano zero-valent iron) ([38] and references therein). The use of iron-based NMs for the in situ immobilization of trace elements limits the potential leachability of metals/metalloids and thus prevents their transport into deeper soil layers and groundwater [39].
Due to its abundance and the presence of surface hydroxyl groups, goethite is significantly involved in the transport and transformation of nutrients and contaminants—including inorganic/organic anions, cations, and some gases [40]. Synthetic nanogoethite (nFeOOH) has been successfully used for the removal of Cu from aqueous solutions, showing photocatalytic activity and a high adsorption capacity for Cu [41].
Maghemite is a common weathering product in soils of temperate, tropical, and subtropical climatic regions, usually formed during the oxidation of magnetite. Synthetic maghemite (nFe2O3) is a promising material for the removal of inorganic contaminants as it is readily available, inexpensive, and can be easily separated and recovered because it is magnetic [42]. Nanomaghemite has been deeply investigated due to its efficient removal of the most-toxic form of As (arsenite, As (III)) [43,44,45,46,47]. Moreover, it has been demonstrated that it is an important scavenger of Cr(VI), Pb(II), Cd(II), Cu(II), and Zn(II) from aqueous solutions and thus could be a useful sorbent for water and soil remediation [4, 42, 48, 49]. The use of nFe2O3 has been reported to be useful for promoting the growth of plants in a contaminated soil [50], mainly due to the immobilization of Zn from the soil pore water (available to plants) with the consequent reduction of its toxicity to the roots and aerial parts. Adsorption of Pb(II) by nFe2O3 occurs mainly through the formation of inner-sphere complexes, while Cd(II) is likely adsorbed as a mixture of inner- and outer-sphere complexes [42]. The effectiveness of the adsorption of these metal/metalloids is affected by the modification of the atomic structure on the particles surface with decreasing size of nanomaghemite [43]. Also, the presence of other components in the soil solution—such as citrate complexes and organic acids [51], or other nutrients [52]—influences the sorption process. For example, PO4 3− has been described as a competitor for arsenite and arsenate immobilization by nanomaghemite [53] due to their similar outer electronic structures.
Magnetite is a mixed-valence magnetic iron oxide, containing Fe2+ and Fe3+, and it can be formed in the soil through (bacteria assisted) weathering of ferrihydrite [54]. Immobilization of As in soils using nanomagnetite (nFe3O4) was performed by Zhang et al. [32], who reported higher stabilization efficiency of nFe3O4 compared to iron sulfide or nZVI. In another study, nFe3O4 proved to be an efficient amendment for the removal of Pb from aqueous solutions, yielding fast adsorption with a maximum capacity of 36 mg Pb g−1 [17]—which was much higher compared to, for example, goethite [55]. Moreover, the behavior of Pb was not affected by the presence of other ions such as Ca, Ni, Co, or Cd. Additionally, desorption and regeneration tests showed that nFe3O4 can be used repeatedly without loss of their adsorption capacity [17]. Shen et al. [56] investigated the influence of pH, temperature, and particle size on the adsorption of metals from aqueous solution by nFe3O4. Under room temperature at pH 4 with an average particle size of 8 nm, ≥85% of Cu2+, Cd2+, Ni2+, and Cr6+ were removed, yielding the maximum at pH > 7 for divalent metals and at pH 2 for hexavalent Cr. In contrast, coarse particles showed values of maximum adsorption capacity about seven-times lower [56].
Recently, the synthesis and utilization of iron NMs (nFeOx) with novel properties and functions have been widely studied, both for their nano size and for their magnetic characteristics [11, 36, 57,58,59]. Typical iron NMs syntheses involve routes including chemical precipitation [60], sol-gel, hydrothermal, dry vapor deposition, surfactant mediation, microemulsion, electro-deposition, and sonochemical methods [36]. Iron oxide composites such as clay–iron oxide magnetic composites and magnetic zeolites can be synthesized and used for the removal of metallic contaminants from water [61]. If the size of the magnetic NPs is reduced to below a few nanometers, they become superparamagnetic. Using an external magnetic field, these particles change their direction. Therefore, the remediation efficiency may be enhanced by combining metal binding and selective adsorption properties with separation of magnetic nano-sorbents from the system, since their magnetic behavior (either ferromagnetic or superparamagnetic) depends on the particle size [36]. Gómez-Pastora et al. [11] illustrated that engineered nFeOx have very high adsorption capacities for metals/metalloids in polluted waters; moreover, their magnetic properties facilitate their collection from the solution—allowing their further reuse. The recovery of magnetic NMs by the use of magnetic gradients [62, 63] represents a promising alternative for sorbent applications.
2.1.2 Nano Zero-Valent Iron (nZVI)
A vast number of studies have demonstrated the applicability of nano zero-valent iron (nZVI) as an amendment for remediation of metal/metalloid-polluted water systems [6, 11, 64,65,66] or trace elements immobilization in contaminated soil [16, 39, 67,68,69]. The possible mechanisms by which nZVI stabilizes metal/metalloids include adsorption and/or surface precipitation, redox reduction, and co-precipitation in the form of metal iron oxides or oxyhydroxides [6] ([66] and references therein). The particles of nZVI have a core-shell structure, which gives them characteristics typical of both iron oxides (sorption) and elemental Fe0 (reduction) [6]. The iron core (up to 98% Fe) is covered by a shell composed of iron oxides and hydroxides (FeO, Fe2O3, FeOOH). Furthermore, the surface of nZVI particles has a significant influence on their stability and mobility in the environment and it prevents their rapid oxidation. Their increased specific surface area results in much higher reactivity but, on the other hand, the reaction of particles smaller than 20 nm is so fast that their reaction capacity may be depleted before they get to the contaminant. Thus, attaining the optimal balance between the reactivity and lifetime of nZVI needs to be guaranteed for in situ applications [66, 70, 71]. Detailed overviews of nZVI reactivity have been provided by O’Carroll et al. [6] and Yan et al. [72], while the reaction mechanisms have been recently reported by Filip et al. [73].
When nZVI is exposed to air or water, it is oxidized, forming a layer of iron oxides or hydroxides on the surface that is responsible for the subsequent adsorption process [6, 64, 74]. The reaction process is strongly dependent on pH. Under alkaline conditions, a negatively charged surface is favorable to metallic cations adsorption, while the high pH values limit the adsorption of metallic anions. Therefore, nZVI can interact with arsenate and chromate oxyanions at low pH, mainly through electrostatic interaction with the positively charged groups on the surface [45, 56, 75].
Different forms of nZVI are available, including powder, mineral oil suspension, or aqueous suspension. The synthesis methods include formation of the nanomaterial from atoms or molecules through physical/chemical methods (nucleation, vapor condensation, precipitation, agglomeration) and physical/chemical methods to breakdown a bulk material to the nanoscale size (thermal decomposition, thermal reduction of oxide compounds, or pulsed laser ablation) [66, 71]. The synthesis method influences the size, shape, and composition of iron NPs and thus their actual reactivity. Particles of nZVI prepared by the reduction of goethite or hematite are generally bigger (up to 100 nm) and of irregular shape, while formation using NaBH4 provides smaller, regular-shaped particles up to few tens of nm in size [66]. The borohydride reduction of ferrous salts is the most common method of nZVI synthesis for laboratory-scale experiments [71, 76]. However, the industrial application is precluded since highly reactive particles with a significant tendency to agglomerate are produced by this procedure and the overall expenses are high [70, 71]. The easy and cost-effective synthesis of highly reactive NMs, such as nZVI, is a priority of the academic community and nZVI producers. In this context, a method using leaf extracts from different trees was performed to obtain “low-cost” nZVI particles [77].
In order to prevent particle aggregation and to improve nZVI reactivity, various innovative surface modifications/coatings have been developed [6, 66]. Among the traditional agents for nZVI stabilization are poly(acrylic acid), poly(methyl methacrylate), poly(ethylene glycol), polyaspartate, and others. Several stabilizers of natural origin have been tested also, including xanthan gum, guar gum, potato starch, alginate, and chitosan. Bimetallic NPs represent nZVI particles coated with noble metals [6]([78] and references therein). Although the surface properties of nZVI may change, the modifiers generally ensure the transport of stabilized NPs and the reaction with the target contaminant only in the polluted zone. The specific surface of Fe0 can be 4–15 m2 g−1, while up to >40 m2 g−1 can be reached for surface-stabilized particles [6, 71]. The use of several composites such as bentonite-nZVI has been reported also [79].
Due to its abundance, easy accessibility, high reactivity, and efficiency for risk element stabilization, nZVI has become a widespread remediation amendment both on a laboratory scale and for in situ applications. However, as nZVI is a redox-active material, this being important for treatment of redox-sensitive elements (e.g., As, Cr) [14, 80], the impact on soil microbial communities needs to be investigated. According to Li et al. [81], rapid and complete oxidation of Fe0 eliminates its effects on bacteria due to passivation within a few hours. Significant impacts on microbial diversity were reported for nZVI-treated soil contaminated with Pb, whereas there were no effects on microbial activity in Zn-contaminated soil [16].
Efficient treatments of contaminated soils with nZVI have been reported, resulting in Pb and Zn immobilization [16, 69] as well as decreased availability of As [39]. However, nZVI was a less efficient amendment for in situ soil remediation of Cr, compared to other sorbents studied by Chrysochoou et al. [82], while successful remediation of Cr from ore processing residue [83] and wastewater [14, 80] was achieved upon the application of nZVI. Further studies are needed in order to assess the long-term impacts of nZVI on the environment in terms of contaminant stabilization, while minimizing the effects on soil characteristics.
2.1.3 Manganese-Based Materials
Manganese oxides (including hydroxides and oxyhydroxides) together with Fe oxides occur naturally as erosion products in almost all soil types, mainly as coatings on soil particles and pores or in the form of concretions and nodules with poorly crystalline (even amorphous) structure [84]. Compared to Fe oxides, Mn oxides are generally less abundant in soils but appear more efficient in the immobilization of some metals [85, 86]. This is mainly due to their large specific surface and (usually) low value of pH at point of zero charge (pHpzc)—the reason for their negative surface charge in usual soil conditions [87]. Their specific structure, formed by sheets (layers) or tunnels in most cases, allows the accommodation of water molecules or various cations in interlayer or tunnel regions [84]. Manganese oxides possess strong oxidative properties and thus take part in many oxidation-reduction and cation exchange reactions. For this reason, Mn oxides are not suitable amendments for soils contaminated with Cr as they are able to readily oxidize Cr(III) to the more toxic and mobile Cr(VI) [88, 89]. On the other hand, this oxidizing nature can be beneficial in the case of contamination with As; Mn oxides have proved efficient in the oxidization of the more mobile and toxic As(III) to As(V) [90,91,92,93].
Due to their promising properties, many studies focused on the synthesis and testing of engineered Mn nanooxides, which are potential agents for environmental clean-up. Manganese oxide NPs can be prepared both by classical chemical routes [94,95,96,97] and by biotechnological means, using the activity of microorganisms like bacteria or fungi [98,99,100]. In fact, biogenic oxidation of Mn(II) represents also the prevailing route for the formation of Mn oxides in soil. Although thermodynamically favored, Mn(II) oxidation in the environment solely by chemical means is very slow. On the other hand, when the process is mediated microbially, the reaction rate can be increased by several orders of magnitude [101, 102]. As in the case of other nanoadsorbents , the first studies dealing with these materials focused mainly on their synthesis, characterization, or adsorption properties with respect to targeted compounds; in this case, metals/metalloids. Based on these data, possible applications—including remediation—can be proposed. In this context, application of Mn nanooxides for soil remediation appears relatively safe as nanoscale biogenic Mn oxides are natural and ubiquitous soil components. Although numerous studies have been published dealing with the adsorption performance of Mn-based NPs [100, 103,104,105,106,107,108], their application for the direct remediation of contaminated soil, together with assisted phytoremediation, is still rather scarce. Della Puppa et al. [109], together with Michálková et al. [110] and Ettler et al. [111], studied the adsorption properties and stabilizing potential of partially nanoscale amorphous Mn oxide (AMO) with regard to Cd, Cu, Pb, Zn, and As in contaminated soils. In these studies, after application to contaminated soils, AMO was able to decrease significantly the amount of targeted metal/metalloids in the soil solution, even showing a higher sorption capacity for Cd, Cu, and Pb than engineered nanomaghemite [51, 110]. On the other hand, higher dissolution of this agent in acidic conditions—connected with unwanted oxidation and dissolution of soil organic matter—was recorded. For this reason, AMO appears a suitable amendment for neutral and slightly alkaline soils .
2.1.4 Other NMs
In addition to Fe- and Mn-based NPs, there exists a wide variety of novel, engineered NMs potentially usable in the remediation of soil and water contaminated with metals/metalloids (see chart in Fig. 14.2, according to Juganson et al. [28]). To date, one of the most studied NMs is nTiO2, also the most studied photocatalyst worldwide. Besides its applications targeting the decomposition of various organic compounds, dyes, etc., the process of photocatalytic reduction can be used to remove various toxic metal ions as well. Numerous studies have examined the potential of nTiO2 for the reduction of highly mobile and toxic Cr(VI) to Cr(III) [112,113,114] and the immobilization of toxic As(III) species [115]. Other materials (Zn/Al-based nanocomposites) were shown to be very promising as they not only behaved as adsorbents but also had photocatalytic properties, being able to adsorb the highly toxic Cr(VI)—that was subsequently reduced photocatalytically to Cr(III) [116]. Nanoparticles of hydrous Ce oxide were reported as another material suitable for adsorption of Cr(VI) from aqueous solution [117]. Nanoparticles of nMgO, nTiO2, and nZnO were found to be efficient adsorbents for Cr in soil contaminated by leather factory waste, decreasing significantly the exchangeable Cr fraction while increasing the residual fraction [118]. Carbon nanotubes represent another promising type of engineered material, being efficient in the adsorption of various divalent metals from aqueous solution [119]. In the study of Jośko et al. [120], application of multiwalled carbon nanotubes reduced the phytotoxicity of sediment contaminated with various organic and inorganic contaminants.
2.2 Nanomaterials in the Environment
The global production of engineered NMs was estimated to be 260,000–309,000 metric tons in the year 2010; of which about 8–28, 0.4–7, and 0.1–1.5% were estimated to have ended up in soils, water bodies, and the atmosphere, respectively [121]. The use of NMs in environmental remediation will inevitably lead to the release of NMs into the environment and subsequent ecosystems. Once in the environment, NMs may persist for a long time or be taken up by organisms and transferred between organisms of different trophic levels, thus acting as an ecotoxicological hazard, and undergo biodegradation or bioaccumulation in the food chain [121,122,123]. Plants are considered to represent both the first sink for the accumulation of NMs from the surrounding environment and the point of entry for their bioaccumulation in the food chain [124]. For this reason, emerging studies have focused on the general consequences of NMs uptake by plants, as their effects on the biomass production and plant response are very relevant to phytoremediation. Although nanotechnology has the potential to solve problems that cannot be solved by the full-scale products, one important aspect in nanoremediation , the safety of NMs, still represents a barrier to their wide innovative use and is hindering their full application; hence, intensive studies must be done before their use. As concluded recently by Schaumann et al. [125], further assessment of environmental impacts on the fate and effects of NPs is needed.
3 Consequences of Nanomaterials for Plants
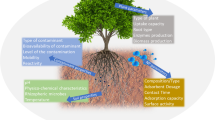
Nanoecotoxicology is a branch within toxicology which focuses on measuring the toxicity of NMs that enter into contact with organisms like plants, bacteria, fish, and invertebrates [126]. A good understanding of the interactions of NMs with the plant system is of paramount importance for assessing their toxicity and trophic transport [127, 128]. To understand and quantify the potential risks for plants, the mobility, bioavailability, toxicity, and persistence of manufactured NMs need to be studied. An increasing number of published studies have attempted to understand the interactions between NMs and plants, and several reviews have already examined the implications of NMs in food crops [8, 129,130,131]. As can be seen in Fig. 14.1, which shows the general trends of the effects of NMs on plants according to the published literature to date, there is sufficient evidence that NMs can yield both beneficial and harmful effects in plant systems at the physiological, biochemical, nutritional, and genetic levels. The interactions between plants and NMs can shed light on the environmental consequences of nanotechnology, but, in contrast to the huge amount of research done on the bulk chemicals as environmental hazards, the research on NMs toxicity is markedly scarce [128] and it needs to be improved.
Matrix of the described effects of NMs on plant physiology during environmental remediation. The matrix has been created using the general effects of each NM from the literature compiled in this book chapter. In some cases, contradictory responses have been detected (two colors in the same box), denoting that the toxicity of NMs to plants is not completely understood
There are many factors which must be taken into account during nanotoxicological studies, and this makes it very complicated to understand the real consequences for plants since even small differences in the design of the experiments can produce different results. For example, for most NMs, relatively high concentrations are needed to cause observable toxicity in plants and the toxicity threshold is species dependent [132, 133]. Owing to their insolubility in water, NMs in general have a limitation for toxicity experiments [128]. Moreover, most plants showed visible signs of recuperation from NMs toxicity—indicating that the toxicity was temporary [4]. Auffan et al. [134] pointed out that chemical stability under physiological redox conditions appears to be a condition for the non-toxicity of metallic NMs. Nevertheless, metallic NMs with strong oxidative or reductive properties can be cytotoxic and genotoxic. Consequently, the nanotoxicological research on the uptake and accumulation of NMs by plants, and their subsequent response, has sometimes generated controversial data [27, 135, 136]. However, when taken together, the apparent differences in the toxicity of NMs to plants may arise from their chemical (reactivity) and physical properties (size, form, aggregation) and the dose (high or low concentrations), exposure time, plants (species, age, physiological status), and experimental conditions (in hydroponics, soils, field, glasshouse, etc.) used. Many of the responses to NMs by plants have been evaluated to find out how NMs improve growth when used as soil amendments to reduce or mitigate the toxicity of contaminants; but their effects must be studied in isolation, to ascertain the effects of the NMs themselves on the plants. If the addition of NMs to a contaminated soil can potentially ameliorate metal-induced damaging effects on growth, by the reduction of metal availability and toxicity, this stimulation of growth may mask the potential negative effects caused by NMs. The effects on key physiological processes in plants of engineered NMs with potential use for phytoremediation, reported to date, are described below.
3.1 Germination
Seed germination tests represent one of the simple and rapid tools for assessing the phytotoxicity of NMs. The recorded effects of NMs on seed germination fall into all possible classes—being negative, nil, or positive, depending on the kind of NM, the species, and the concentration used [10, 27, 129]. For example, strong to total inhibition of germination after seed exposure ton Fe3O4 has been reported for lettuce ( Lactuca sativa ), radish ( Raphanus sativus ), cucumber ( Cucumis sativus ), and spinach ( Spinacia oleracea ) [137, 138]. However, for the same NMs, Barrena et al. [139] reported low to nil toxicity against cucumber and lettuce germination. Nano-CuO did not affect germination, but inhibited growth of Zea mays seedlings [140] and enhanced the seed germination and shoot-to-root ratio of lettuce [141]. Exposure of pea ( Pisum sativum ) seeds to nZnO had no impact on germination [142].The effects of nZVI on germination have been reported to be concentration dependent [143, 144].
Although widely used, germination tests have to be interpreted carefully. When evaluating the influence of NMs on seed germination in relation to the phytoremediation of soil, attention should be paid to the experimental approach as different experimental designs may give different results. Classical ecotoxicological studies dealing with NMs and their effects on seed germination are usually performed with the seeds directly exposed to NMs in the suspension. But, in the context of assisted phytoremediation, this experimental approach appears to be not very suitable. In this case, NMs are applied to soil—the aim being to immobilize contaminating metals, decrease their solubility and toxicity, and promote thus the plant growth. The soil solution from contaminated soil amended with NMs thus represents a system completely different to that of a pure NMs suspension. For this reason, NMs destined for use in assisted phytoremediation should be tested not just directly in suspension; their influence on the soil solution composition and, subsequently, the influence of the soil solution obtained or the amended soil itself on seed germination should be examined too, as the results of these tests could vary significantly.
3.2 Uptake of NMs by the Roots
Most of the available studies on phytotoxicity of NMs have focused mainly on toxicity symptoms of plants, and relatively few have examined the mechanisms of NMs phytotoxicity, uptake, translocation, and bioaccumulation [136]. The roots are the first organ which can suffer from NMs interference in the soil, and for that reason there is an urgent need to evaluate the impacts on plant physiology of NMs, together with their potential ecotoxicity and interactions with the key processes in the rhizosphere [145,146,147]. Possible interactions of NMs with plant roots include adsorption onto the root surface, incorporation into the cell wall, and uptake by the cell [148, 149]. For NMs to enter the root stele, they have to either cross the cell wall and plasma membrane of an endodermal or exodermal cell or cross a root cell wall of a cell exterior to the endodermis/exodermis and move into the stele symplastically [135]. They may be transported from one cell to another through plasmodesmata. However, the exact reasons why only some plant species readily take up several NMs are still unknown and remain to be explored [131]. To cross an intact cell wall, it has been hypothesized that NMs have to move passively through a cell wall pore, suggesting that plant uptake is highly size selective. Even so, NMs could be incorporated passively into the apoplast of the endodermis; they would be then subjected to the highly size-selective permeability of the membranes before reaching the central cylinder [135]. It is generally assumed that it is difficult for NMs bigger than 20 nm to penetrate through the cell wall since the cell wall pore sizes vary from 2 to 20 nm [4, 8, 150,151,152]. According to Burello and Worth [153], NMs with a diameter larger than 20–30 nm act often as bulk materials; thus, the “true nanoeffects” are attributable to NMs with smaller size. Roy and Bhattacharya [4] suggested that NMs can enter plant cells by binding to carrier proteins, through aquaporins and ion channels, by creating new pores, or by binding to organic chemicals in the environmental media. Endocytosis may be also an important pathway by which NMs enter plants [154] since NMs could theoretically activate membrane receptors and induce endocytosis.
The high reactive capacity of NMs—due to their high specific surface area—can stimulate their adhesion to the epithelial root cell wall. Nanomaterials of all compositions also have the potential to aggregate, due to Van der Waals forces or other interactions [155]. As a result, NMs may aggregate along the roots, blocking their proper water uptake and disturbing thus the whole plant physiology and ultimately affecting their growth and development [3]. Aggregation of NMs appears to change the color of the roots surface by covering the epithelial cells [152, 156]. It can affect also the interactions of the plant with the external medium through mechanical disruption of membranes and cell walls, blocking the pores and diminishing the root hydraulic conductivity [156]. Despite their adherence to the surface, due to binding and electrostatic attraction by a limited number of cell surface cation exchange and binding sites on the negatively charged root surface [157], some NMs do not seem to move through the surface of the roots. In this way, inhibition of plant growth may not derive directly from chemical phytotoxicity of NMs. Instead, toxicity may result from the physical interactions between the NMs and plant cell transport pathways [129, 156]. Anyway, even though potential aggregation might dramatically increase the size of the NMs and reduce their mobility [149, 158], Whitley et al. [159] showed that NMs may remain unaggregated in soil pore water for an extended period of time, suggesting that NMs are likely to be bioavailable to plants.
Although some NMs can be found in plant cells and tissues [8], no uptake or toxicity has been reported specifically for nFeOx, which may be due to the adherence of these materials to soil particles. Zhu et al. [124] reported no measurable uptake of nFeOx by pumpkin ( Cucurbita maxima ) grown in either soil or sand, or of nFe3O4 by lima bean plants. Similarly, Wang et al. [160] reported no uptake of nFe3O4 (25 nm in diameter) by pumpkin plants. For poplar, it appeared that some of the nZVI penetrated through the membrane and was internalized in the root cells [161]. Zhou et al. [162] reported the adsorption of nCuO (55 nm in size) onto the Triticum aestivum root surface. As defended by Lü et al. [163], metallic NMs can affect the epithelial root cells but they seem not to have an important effect in the xylem of the plants. In spite of the adsorption of nCeO2 aggregates on the root surface observed by Majumdar et al. [164], Ce accumulation increased linearly with increasing exposure concentrations, corroborating previous studies in other edible plants like tomato [165], rice [166], soybean [167], corn [168], and cucumber [169]. The concentration-dependent linear increase in Ce accumulation in roots suggests uptake through simple diffusion. According to Pradhan et al. [170], nano-sized Mn oxides (nMnOx) were readily taken up from soil by the roots of Vigna radiata and transported to the leaf, where nMnOx acted as a cofactor in a series of enzymatic reactions during the assimilation of nitrate into organic nitrogen compounds. In a nano-ZnO translocation study by Hernandez-Viezcas et al. [171], the use of μXRF images led the authors to conclude that Zn was obtained from nZnO by the roots, but it was not adsorbed on the root surface. This interesting research indicated an important concept because the authors were careful to separate the effects produced by nZnO from those related to and produced by Zn released from the NMs.
The type of roots and their architecture, their age, and the species are also crucial factors in the response to and uptake of NMs by roots. For example, lignin can act as a barrier to reduce the permeability of foreign materials in cells. This could be a reason why, in the study by Ma et al. [161], nZVI was able to enter the root cells of poplar plants, while the relatively high lignin content in the cell wall of Typha latifolia prevented nZVI from passing through it.
3.3 Translocation and Accumulation
The available literature indicates vaguely that NMs are found in plant cells and tissues, and even though some studies report NMs internalization in roots, no translocation to the shoots was found [4, 130]. However, NMs could potentially be taken up by plant roots and transported to shoots through vascular systems, depending upon the composition, shape, size, and plant anatomy [129]. There are many physico-chemical differences between plant species—such as variations in hydraulic conductivity, cell wall pore size, and root exudate chemistry—that could influence NMs bioaccumulation. For example, Zhu et al. [124] observed accumulation of nFe3O4 (min. size 20 nm) in Vigna radiata (mung bean) and pumpkin grown in an aqueous medium, but did not observe bioaccumulation when conducting this same experiment using Phaseolus lunatus (lima bean) or Phaseolus limensis , in either soil or sand. Corredor et al. [172] investigated xylem transport of NMs by injecting graphite-coated iron NMs into the pith cavity of the leaf petiole of pumpkin plants, and a very homogenous population of approximately 46-nm NMs was found in the xylem at a distance from the injection site, suggesting that NMs larger than 46 nm were not transported. Recently, lack of uptake and translocation for nFe2O3 were demonstrated by Martínez-Fernández et al. [156], without the presence of these NMs in the sap of Helianthus annuus , possibly as the result of aggregation of NMs on the root surface. Another study examined the importance of size on uptake by exposing wheat plants to nTiO2 ranging from 14 to 655 nm [173], concluding that NMs greater than 140 nm were not taken up and NMs greater than 36 nm were not translocated into the aerial portions of the plants. The accumulation and translocation of nCeO2 were dose dependent [174], but they accumulated mainly in the root tissue [166, 175]. Birbaium et al. [176] also reported no uptake after exposing 3–5-week-old corn plants to 37-nm-diameter nCeO2 for 14 days. The work by Schwabe et al. [177] showed that nCeO2 with a size range of 17–100 nm is at least partially available for uptake by pumpkin. Translocation of Ce has, however, been documented in previous studies of cucumber [169], corn [178], and beans [164]. Ma et al. [161] reported that nZVI was able to move into the root cells of poplar plants while such internalization was absent in the case of Typha latifolia , maybe because the aggregate of nZVI was too large for the xylem tissues to transport. In both cases, upward transport to the shoots was insignificant. Although Lin and Xing [179] reported accumulation of the nZnO in the protoplast of endodermal cells, they found no evidence that the particles were translocated into the shoots and leaves, possibly as the result of NMs aggregation in the exposure media. Wang et al. [180] observed xylem- and phloem-based transport and biotransformation of nCuO (20–40 nm) as well as nCuO transport from roots to shoots via the xylem and translocation back to roots via the phloem.
In contrast, recent studies show that Au, Ag, CuO, and ZnO NMs are readily taken up and translocated by plants, either as NMs or in their ionic form [181]. Nanomaterials may accumulate and/or increase the concentrations of the component metal in the fruits/grains of agricultural crops, have detrimental or beneficial effects on the agronomic traits, yield, and productivity of plants, induce modifications in the nutritional value of food crops, and transfer within trophic levels. So, it is important to establish whether a more predominating trend of NMs accumulation exists and whether the metals involved follow the same trend as the chemical form available in the soil or in the water [27].
3.4 Water Balance
Because of their relevance to the proper growth, nutrients uptake, stress, and biomass production of plants, more studies of NMs are needed at the root–soil interface, including measurements of plant water relations [182]. As described above, many researchers consider that the observed toxicity exerted by NMs in plants is based on physical plant–NMs interactions. The presence of NMs on the root surface could alter the surface chemistry of the root such that it affects how the roots interact with their environment [183]. For example, it is known that metals/metalloids can reduce the root hydraulic conductivity, with consequent decreases in plant water content, turgor potential, and growth [184, 185], but how metallic NMs influence the transport of water through the roots is not known. Martínez-Fernández et al. [50, 156] found a reduction of the root hydraulic conductivity in plants of H. annuus treated with nFe2O3 in hydroponic culture, but no changes in the internal water status of plants grown in a contaminated soil treated with the same NMs. The work by Trujillo-Reyes et al. [186] suggests that the reduction in dry biomass production in plants exposed to nFeOx is most likely due to the particle aggregation on the surface of the root, which affected water entrance, resulting in growth reduction. Asli and Neuman [187] also found that exposure to nTiO2 (30 nm) caused a reduction of the water transport capacity in sections of primary roots, their effect being proportional to the exposure time in hydroponic culture. The nTiO2 also inhibited transpiration, reduced root hydraulic conductivity in Zea mays root apices, caused cell wall pores to constrict, and resulted in minor inhibition of shoot and root growth. Other work suggests that NMs increase the expression of aquaporins in roots [188, 189], maybe as a response by the plant to compensate a reduction in root hydraulic conductivity. Trujillo-Reyes et al. [190] showed that, although Fe ions/NMs did not affect the water content of lettuce plants, Cu ions/NMs reduced their water content, root length, and dry biomass. Nano-CuO (size: <50 nm) was reported to reduce the transpiration volume in plants [191], also related with an up-regulation of proline-biosynthesis genes under nCuO exposure in Arabidopsis thaliana [192]. Nano-CuO stress induced high accumulation of proline (a water-stress indicator in plants), and the degree of accumulation was associated closely with the nCuO concentration [193, 194]. The accumulative transpiration rate in plants indicated that transpiration was highest for the controls and gradually decreased as the concentration of nZVI increased in poplar, Typha latifolia [161], and peaplants after nZnO exposure [142, 179].
3.5 Nutrients Uptake
A damaged water transport system implies a lower capacity to pass water to the shoot, affecting the transport of all the dissolved elements and causing a deficiency of them in the shoot, according to the plant requirements. Generally speaking, exposure to NMs involves changes in the nutritional status of the plants and development is negatively affected, but positive effects have been documented as well. Studies revealed that the lower uptake of nutrients is related to the fact that NMs clog the root openings and inhibit both hydraulic conductivity and nutrient uptake in roots [156, 187], although NMs with high specific surface areas may also help to sequester nutrients on their surface.
The effects of NMs on plant nutrition have been reported in few studies [195, 196]. Martínez-Fernández et al. [156] detected a significant reduction of trace elements concentrations in shoots and roots of H. annuus exposed to nFe2O3, without changes in their concentrations in the sap. However, the concentration of Mo in the roots increased with the dose of nFe2O3, maybe due to the close relationship between the Fe and Mo uptake systems and because the uptake of Mo can be facilitated under higher Fe concentrations in the external medium [197]. Iron oxide NMs have been reported as facilitators of iron and photosynthates transfer to the leaves of peanut [40]. In bean ( Phaseolus vulgaris ), nCuO decreased the shoot Fe, Zn, and Ca levels, but not that of Mg, while K showed little change and Na increased [195]. Aluminum, Ca, and Zn concentrations in roots and leaves were higher in plants exposed to Cu NMs, compared with the control treatment [190]. Silicon NMs used to mitigate the Cu toxicity increased the contents of Mg, Ca, K, and P in the root and shoot of pea plants [15].
There are different effects related to the nitrogen uptake and metabolism in plants during the interaction with NMs. Manganese NMs affected the assimilatory process by enhancing the net flux of nitrogen assimilation in mung bean plants [170]. Nano-sized TiO2 can also have a positive effect on plants through promotion of the uptake of nitrate, which accelerated the transformation of inorganic nitrogen into organic nitrogen [27], due to increased nitrate reductase activity, and could also protect chloroplasts from aging in soybean and A. thaliana [198, 199]. Exposure to nTiO2 also increased biomass, photosynthesis rate, and enzyme activity in spinach [200,201,202], related to enhanced N2 fixation from nitrogen photoreduction and the stimulation of RuBisCo activity. Cai et al. [203] reported stimulation of the removal of Cu and nitrate from aqueous solution, after the application of bimetallic Fe/Ni NMs. On the other hand, the presence of nZnO in the environment is potentially hazardous to the Rhizobium–legume symbiosis system [142], this interaction being an important factor for plant growth and crop productivity as it provides bioavailable nitrogen to the plants. The presence of nZnO in the rhizosphere affected the early interactions between rhizobia and the host plant as well as nodule development, and subsequently delayed the onset of nitrogen fixation [142].
3.6 Oxidative Stress
Nanomaterials can mediate significant elevations in reactive oxygen species (ROS) generation and its subsequent consequences (such as membrane damage), as well as the modulation of antioxidant defense system components and cellular redox homeostasis in plants. Iron nanooxides can significantly increase the antioxidant enzyme activities, but their effects seem to be related more to the changes in the mineral composition in the plant than to the presence of nanoscale forms of Fe [190]. Iron NMs toxicity studies have primarily focused on Fe(II) and its oxides, and little is known about the toxicity specific to others NMs such as nZVI. However, nZVI produces Fe(II) and iron oxides through oxidation, and nZVI can produce free radicals which are highly reactive and cause oxidative stress [204]. This could be one of the mechanisms behind the toxic effects of nZVI on plants. Further studies on A. thaliana evidenced that nZVI triggered high plasma membrane H+-ATPase activity, resulting in stomatal opening that was fivefold higher than in unexposed plants [205]. Nano-MnOx has been reported to increase the activity of the electron transport chain by binding with the CP43 protein chain of photosystem II [206]. The nMnOx enhanced the oxygen evolution process, being a part of the water splitting complex in the light reaction of photosynthesis, hence improving the photophosphorylation capacity [206]. Nano-CuO stress also induced modulation of antioxidant enzymes activity, and nCuO treatment caused oxidative damage to rice seedlings, as evident from high ROS-scavenging antioxidant enzymes activity and enhanced malondialdehyde levels [193], and maximally disrupted the plant-defense system by oxidative stress [207]. The accumulation of nTiO2 in plants does not appear to induce oxidative stress in the leaves [173]. Biochemical assays with nZnO indicated increases in the specific activity of CAT (in the root, stem, and leaves of Prosopis juliflora-velutina), but no evidence of chlorosis, necrosis, stunting, or wilting, even after 30 days of treatment [171]. In ROS formation and release, the conversion of fatty acids to toxic lipid peroxides occurs, leading to the disruption of biological membranes [208] and consequently the entrance of and damage by NMs and metals, causing TBARS (thiobarbituric acid reactive species) formation—which damages the membrane permeability. This specific report showed that, by increasing the concentrations of the nZnO, higher values for the TBARS were observed. Silicon NMs protect pea seedlings against Cr(VI) phytotoxicity, by reducing Cr accumulation and oxidative stress and up-regulating the antioxidant defense system and uptake of nutrient elements [15].
3.7 Chlorophylls
Nano-sized materials may interact with the proteins associated with photosystems, the starch-synthesizing machinery, and/or carbohydrate translocation [209]. Since chlorophyll content is considered as an index of the total light harvesting complex and the electron transport components, present in chloroplast membranes [210], it is used as a stress indicator in plants. Studies of the bioavailability of nFe2O3 in A. thaliana, performed by Marusenko et al. [211], suggested that the Fe-NMs were not used for chlorophyll production. Iron NMs reduced the accumulation of chlorophylls in the leaves of Lactuca sativa [190] and Helianthus annuus [156], this effect being related to the reduction of the root hydraulic conductivity and the transport of dissolved nutrients from the solution, especially for Mg since this nutrient is associated with the synthesis of chlorophylls. In an experiment with nCeO2, Zhang et al. [131] related a reduction in the chlorophylls content with the physical adsorption of the NMs on the root surface, and the consequent blockage of Mg uptake by the roots. Nano-CuO was reported to decrease chlorophyll content significantly in wheat [212], soybean [213], and A. thaliana [192], and in Vigna radiata in an in vitro experiment [194]. On the other hand, A. thaliana plants treated with bulk ZnSO4 had a smaller amount of chlorophyll and were shorter compared with the plants treated with nZnO [211]. In an in vitro experiment carried out with Petroselinum crispum by Dehkourdi and Mosavi [214], nTiO2 caused a significant increase in the chlorophyll content of seedlings. Higher chlorophyll contents were also recorded in leaves of Brassica juncea treated with Ag NMs [215].
3.8 Genotoxicity
Plants have been used as indicator organisms in studies of genotoxicology, facilitating data interpretation for a complete understanding of the effect of NMs [216,217,218]. Genotoxicity may be produced by direct interaction of NMs with the genetic material, by indirect damage from NM-induced ROS, or by toxic ions released from soluble NMs [174, 219, 220]. Nanoparticles that cross intracellular membranes (diameter between 8 and 10 nm) may be able to reach the nucleus, through diffusion across the nuclear membrane or transportation through the nuclear pore complexes, and interact directly with DNA [220] and influence DNA replication and transcription of DNA into RNA. However, as expected, this effect is very conditioned by their size. The NMs aggregates could also mechanically damage the chromosomes. Nano-CuO is able to enter the nucleus of plant cells and mediate direct oxidative damage to DNA [221]. Nano TiO2 (~100 nm in size) was found to be genotoxic as well as cytotoxic in plant systems [222]. Kumari et al. [223] showed a direct relationship between the increase in the number of aberrations and the increase in the concentrations of the NMs, by analysis of changes in the chromosome morphology caused by nZnO in root cells of Allium cepa , and explained these results based on ROS activation. According to López-Moreno et al. [174], the toxicity may rise either due to the interaction of the DNA with the Zn ions leached out from the nZnO or its direct interaction with the nZnO. But the absence of nZnO in plant tissues, as shown by the XANES results, failed to confirm the main reason behind the genotoxic response in soybean. It is not clear from these studies whether the genotoxicity in plants is caused by the NMs themselves or their biotransformation within the plants. Ma et al. [129] pointed out that one of the most urgent needs in plant–NMs interaction studies is to determine the genetic response of the plants and the genes that are up-regulated/down-regulated in plants exposed to NMs, but this knowledge is in its infancy still. More research needs to be focused on the differences in toxicity of NMs in relation to their respective bulk counterparts, and on the effects of the ions produced inside or outside the organism exposed to the NMs.
3.9 Growth and Biomass Production
During recent years, the number of peer-reviewed papers related to nanoecotoxicology has increased exponentially. On the one hand, there are abundant references for positive effects of NMs on growth and biomass production: nTiO2 in spinach [200,201,202]; nCeO2 increased root and stem elongation in cucumber [167]; nCuO enhanced lateral root formation in A. thaliana [192]; nCuO enhanced the lignification of root cells in Glycine max [213]; nFeOx increased root elongation in pumpkin [160]; etc. On the other hand, negative effects also appear very often in the related literature: Al2O3 NMs in tobacco plants [224]; nCuO reduced root length in Landoltia punctate [225]; nCuO in L. sativa and M. sativa [196]; nCeO2 decreased stem elongation in corn and inhibited root elongation in alfalfa and tomato [167].
Many researchers have considered the biomass production as a response to the stress, concluding that some external factors (treatments) affect it positively or negatively according to the stimulation or inhibition of the growth, development, and productivity of plants in comparison to untreated controls. However, the biomass production is only the mere consequence of the huge combination of the positive and negative effects (Fig. 14.1) at different plant physiological levels (Fig. 14.2). In contaminated soils, the application of engineered NMs can decrease the contaminant availability in the soil solution and thus enhance the growth of plants. Nevertheless, as described above, there are many steps previous to the complete understanding of the real consequences for plants exposed to NMs. In fact, and generally speaking, long-term exposure to metallic NMs affects growth negatively [226,227,228]. Only when the individual effects of engineered NMs on plants and their applicability are properly evaluated, can concise conclusions be obtained to decide about their use during phytoremediation tasks.
Once in the soil, the engineered NMs can interact with the metal/metalloids in the soil solution, aggregate, or interact with the roots of the plants. According to the balance among all the positive and negative effects at different plant physiological levels, the plant will show the overall effect on biomass production; but, even when apparent changes in growth are not manifested, intrinsic and important effects can happen in the plants. In accordance with Juganson et al. [28], the chart shows the percentages of each type of NM within the total number of publications in the Thomson Reuters Web of Science™ for “environmental remediation” by NMs
Sunflower plants treated with nFe2O3 in a Zn-contaminated soil showed a 25% increase in shoot biomass, related to the Zn-adsorption capacity of the NM [50]. These results highlight the applicability of this NM as an amendment during phytoremediation due to its immobilization of metals in the soil, stimulating the growth of plants by making the contaminants less available. However, an additional experiment with the same species in hydroponic culture showed that treatments with the same nFe2O3 reduced the functionality of the roots, changed the nutrient status of the plants, and led to reductions in the shoot macronutrient concentrations and chlorophyll content [156].
Aspects like stem and root elongation, root gravitropism, architecture of the root system, and number of lateral roots can help to describe the direct effect that NMs have on the growth of plants. Trujillo-Reyes et al. [190] found that iron NMs (Fe/Fe3O4) reduced root size and changed root architecture, as well as affecting the root water content and the chlorophylls accumulation in the leaves of Lactuca sativa . Exposure of peas to nZnO had an impact on root length [142], decreasing the number of first- and second-order lateral roots. When the concentration of nZnO was increased in the medium, the shoot and root lengths declined. Extended treatments with nZnO also resulted in shorter root length than in controls without the NMs in radish, rape, ryegrass, lettuce, corn, and cucumber during seed incubation [132]. Controversial results were also found for nZnO treatments, which increased the lengths of the shoots and roots compared with the control for peanut [229] and Brassica juncea [230]. Ma et al. [231] observed that, although nCeO2 inhibited the root elongation of lettuce, six other plant species were unaffected. Also, the dose is crucial: Ma et al. [232] noted that nCeO2 at concentrations less than 250 mg L−1 significantly increased A. thaliana biomass, but, above 500 mg L−1, biomass and chlorophyll production were reduced and lipid peroxidation was evident.
According to the Thomson Reuters WoS, 770 peer-reviewed papers on nanoecotoxicology that corresponded to the keywords “nano* AND ecotoxic*” were published between 2006 and March 2015 [28]. The rapidly increasing number of scientific publications on ecotoxicity of NMs over the past decade has inspired several review articles summarizing the existing data in the field. However, each review has focused on specific aspects and parameters of NMs testing; therefore, it is difficult to get an overview of all the factors (and their values) that might influence the toxicity of NMs. All these results will be an important factor to take into account with regard to the applicability of NMs for long-term use in phytoremediation tasks, but they will be especially useful when the causes become clear.
4 Limitations and Drawbacks of the Use of NMs
The use of NMs is not exempt from limitations. For example, the proneness to rapid passivation of some NMs [233], susceptibility to geochemical conditions [234], and possible environmental and human health threats of various NMs [235]. The high reactivity and heterogeneous size distribution of NMs may have adverse impacts on the sorption efficiency, which negatively affects the long-term performance and overall applicability. The mobility and sorption capacity of such particles are limited by three principal mechanisms: (1) nanoparticle aggregation followed by gelation, caused by poor colloidal stability, (2) nanoparticle oxidation/corrosion followed by the formation of corrosion precipitates, and (3) nanoparticle trapping from solution by interaction with other components (i.e., mineral surfaces and organic matter) or via microbial removal [71]. In order to prevent them, the particles can be coated with certain organic or inorganic materials. The development of various surface stabilizers and modifiers—such as biopolymers and alginate—has made the NMs much more versatile [11, 59, 236].
In spite of their nano size, NMs may be able to pervade very small spaces in the subsurface and remain suspended in groundwater, allowing the particles to travel farther than larger, macro-sized particles; in practice, the NMs used currently for remediation do not move very far from their injection point [237]. In fact, the introduction of NMs into soil is one of the most difficult aspects to consider in in situ soil bioremediation [21]. Since NMs have the potential to aggregate [155], either during manufacture or during wastewater treatment, this may dramatically reduce their bioavailability, mobility, and toxicity [158, 238], and consequently limit their effectiveness [233, 239]. The aggregation of some NMs supports the need for polymer or other coatings to modify their surface, in order to improve mobility [240].
The regulatory framework generally assumes that NMs possess toxicity and risk equivalent to those materials with larger particles, but the smaller size of NMs results in entirely different physico-chemical properties. Since knowledge about NMs regarding their interaction with biota and their toxicity is scarce, their full-scale application and usage for soil remediation is still problematic. For example, the report by The Scientific Committee on Emerging and Newly Identified Health Risks does not even mention or define engineered NMs.
5 Prospective Work Plan in Phytoremediation with NMs
As described in this book chapter, NMs appear to offer faster and cheaper remediation solutions, and their use at sites around the world is beginning to be explored. Comprehensive utilization of nanotechnology at the present time and unprecedented application of NMs in products will certainly create significant amounts of new-generation waste in the near future [30]. NMs are a reality, but future research efforts need to be directed towards finding new methods for nanoremediation , recognition of the biological effects of NMs in the environment, and creation of the bases of nanobiomonitoring. Recently, a database working group was established in the framework of the European Union Nano Safety Cluster [241]—which highlights that research efforts are necessary to promote science-based regulations for nanotechnology. Detailed research on the biogeochemical behavior of NMs in soil systems, and on the potential advantages and drawbacks of their use in chemical stabilization combined with phytoremediation, is being undertaken by the scientific community, but a much broader view is still needed about their use. Each step in this research will have the potential to provide better knowledge for the use of engineered NMs during remediation tasks and thus to provide a very significant benefit to society, by evaluating the impacts and safety of NMs application to soils contaminated with metal/metalloids.
Most experimental studies with NMs have been conducted in batch systems using model compounds and model media, instead of natural systems. Understanding the fate of NMs and their effects in natural environments also requires more realistic experimental setups [125]. Mesocosm experiments complement laboratory experiments and, as a best practice, can be combined with laboratory experiments to further develop the process of understanding the aging and functioning of NMs [125] as well as their transformations through their life cycle [242]. So, more realistic and holistic studies are needed in future investigations, including long-term experiments in more complex environmental media. Critical knowledge gaps and incomplete studies mean that the mechanisms for the removal of metals by NMs from contaminated soil proposed by different researchers are often contradictory [14]. These discrepancies in the literature can be primarily related to methodological and experimental shortcomings, such as inadequate NMs characterization, lack of consideration of NM aggregation or dissolution, lack of proper controls, or the use of environmentally irrelevant NM concentrations and/or exposure conditions. However, it is now evident that, under certain circumstances, NMs are bioavailable and toxic to several key terrestrial ecoreceptors [135].
New perspectives regarding the combined use of engineered NMs have been proposed and open a huge field for new research. For example, magnetic Fe-NMs have been demonstrated to increase the reactivity and cation exchange capacity of biochar [243, 244], increasing the uptake of nutrients by the plants since the magnetic Fe-NMs can also increase nutrient availability and decomposition of soil organic matter. Also, due to their antimicrobial properties, NMs may increase the resistance of plants to stress and produce indirect plant growth stimulation. Detailed knowledge of NMs ecotoxicity to bacteria and other soil microorganisms is also lacking. It is essential to understand the diversity of the aspects involving engineered NMs and plants if major advances in new fields are to be made.
References
El-Temsah YS, Joner EJ (2013) Effects of nano-sized zero-valent iron (nZVI) on DDT degradation in soil and its toxicity to collembola and ostracods. Chemosphere 92:131–137
Royal Society and The Royal Academy of Engineering, Nanoscience and Nanotechnology: Opportunities and Uncertainties (2004) The Royal Society. http://www.nanotec.org.uk. Accessed 5 Nov 2015
Klaine SJ, Alvarez PJJ, Batley GE, Fernandes TF, Handy RD, Lyon DY, Mahendra S, McLaughlin MJ, Lead JR (2008) Nanomaterials in the environment: behavior, fate, bioavailability and effects. Environ Toxicol Chem 27:1825–1851
Roy A, Bhattacharya J (2012) Removal of Cu(II), Zn(II) and Pb(II) from water using microwave-assisted synthesized maghemite nanotubes. Chem Eng J 211–212:493–500
Baruah S, Dutta J (2009) Nanotechnology applications in sensing and pollution degradation in agriculture. Environ Chem Lett 7:191–204
O’Carroll D, Sleep B, Krol M, Boparai H, Kocur C (2013) Nanoscale zero valent iron and bimetallic particles for contaminated site remediation. Adv Water Resour 51:104–122
Agrawal A, Sahu KK (2006) Kinetic and isotherm studies of cadmium adsorption on manganese nodule residue. J Hazard Mater 137:915–924
Rico CM, Majumdar S, Duarte-Gardea M, Peralta-Videa JR, Gardea-Torresdey JL (2011) Interaction of nanoparticles with edible plants and their possible implications in the food chain. J Agric Food Chem 59:3485–3498
Hua M, Zhang S, Pan B, Zhang W, Lv L, Zhang Q (2012) Heavy metal removal from water/wastewater by nanosized metal oxides: a review. J Hazard Mater 211–212:317–331
Bhatt I, Tripathi BN (2011) Interaction of engineered nanoparticles with various components of the environment and possible strategies for their risk assessment. Chemosphere 82:308–317
Gómez-Pastora J, Bringas E, Ortiz I (2014) Recent progress and future challenges on the use of high performance magnetic nano-adsorbents in environmental applications. Chem Eng J 256:187–204
Dickinson M, Scott TB (2010) The application of zero-valent iron nanoparticles for the remediation of a uranium-contaminated waste effluent. J Hazard Mater 178:171–179
Bommavaram M, Korivi M, Raju Borelli DP, Pabbadhi JD, Nannepag JS (2013) Bacopa monniera stabilized gold nanoparticles (BmGNPs) alleviated the oxidative stress induced by aluminum in albino mice. Drug Invent Today 5:113–118
Wang Y, Fang Z, Liang B, Pokeung Tsang E (2014) Remediation of hexavalent chromium contaminated soil by stabilized nanoscale zero-valent iron prepared from steel pickling waste liquor. Chem Eng J 247:283–290
Tripathi DK, Singh VP, Prasad SM, Chauhan DK, Dubey NK (2015) Silicon nanoparticles (SiNp) alleviate chromium (VI) phytotoxicity in Pisum sativum (L.) seedlings. Plant Physiol Biochem 96:189–198
Fajardo C, Gil-Díaz M, Costa G, Alonso J, Guerrero AM, Nande M, Lobo MC, Martín M (2015) Residual impact of aged nZVI on heavy metal-polluted soils. Sci Total Environ 535:79–84
Nassar NN (2010) Rapid removal and recovery of Pb (II) from wastewater by magnetic nanoadsorbents. J Hazard Mater 184:538–546
Tan Y, Chen M, Hao Y (2012) High efficient removal of Pb (II) by amino functionalized Fe3O4 magnetic nano-particles. Chem Eng J 191:104–111
Zhang WX (2003) Nanoscale iron particles for environmental remediation. A review. J Nanopart Res 5:323–332
Karn B, Kuiken T, Otto M (2009) Nanotechnology and in situ remediation: a review of the benefits and potential risks. Environ Health Perspect 117:1823–1831
Sánchez A, Recillas S, Font X, Casals E, González E, Puntes V (2011) Ecotoxicity of, and remediation with, engineered inorganic nanoparticles in the environment. Trends Anal Chem 30(3):507–516
Joo S, Cheng I (2006) Nanotechnology for environmental remediation. Springer, New York
Singh SN, Tripathi RD (2007) Environmental bioremediation technologies. Springer, Berlin
Klabunde KJ, Erickson L, Koper O, Richards R (2010) Review of nanoscale materials in chemistry: environmental applications. In: ACS Symposium Series 1045. American Chemical Society, Washington, DC
Noubactep C, Caré S, Crane R (2012) Nanoscale metallic iron for environmental remediation: prospects and limitations. Water Air Soil Pollut 223:1363–1382
Tang SCN, Lo IMC (2013) Magnetic nanoparticles: essential factors for sustainable environmental applications. Water Res 47(8):2613–2632
Capaldi Arruda SC, Diniz Silva AL, Moretto Galazzi R, Antunes Azevedo R, Zezzi Arruda MA (2015) Nanoparticles applied to plant science: a review. Talanta 131:693–705
Juganson K, Ivask A, Blinova I, Mortimer M, Kahru A (2015) NanoE-Tox: new and in-depth database concerning ecotoxicity of nanomaterials. Beilstein J Nanotechnol 6:1788–1804
Ghormade V, Deshpande MV, Paknikar KM (2011) Perspectives for nano-biotechnology enabled protection and nutrition of plants. Biotechnol Adv 29:792–803
Bystrzejewska-Piotrowska G, Golimowski J, Urban PL (2009) Nanoparticles: their potential toxicity, waste and environmental management. Waste Manag 29:2587–2595
Kumpiene J, Lagerkvist A, Maurice C (2008) Stabilization of As, Cr, Cu, Pb and Zn in soil using amendments—a review. Waste Manag 28:215–225
Zhang MY, Wang Y, Zhao DY, Pan G (2010) Immobilization of arsenic in soils by stabilized nanoscale zero-valent iron, iron sulfide (FeS), and magnetite (Fe3O4) particles. Chin Sci Bull 55(4–5):365–372
Shipley HJ, Engates KE, Guettner AM (2011) Study of iron oxide nanoparticles in soil for remediation of arsenic. J Nanopart Res 13(6):2387
Deliyanni EA, Lazaridis NK, Peleka EN, Matis KA (2004) Metals removal from aqueous solution by iron-based bonding agents. Environ Sci Pollut Res 11:18–21
Waychunas GA, Kim CS, Banfiled JF (2005) Nanoparticulate iron oxide minerals in soils and sediments: unique properties and contaminant scavenging mechanisms. J Nanopart Res 7:409–433
Mohapatra M, Anand S (2010) Synthesis and applications of nano-structured iron oxides/hydroxides—a review. Int J Eng Sci Technol 2(8):127–146
Mueller NC, Nowack B (2010) Nanoparticles for remediation: solving big problems with little particles. Elements 6:395–400
Komárek M, Vaněk A, Ettler V (2013) Chemical stabilization of metals and arsenic in contaminated soils using oxides—a review. Environ Pollut 172:9–22
Gil-Díaz M, Alonso J, Rodríguez-Valdés E, Pinilla P, Lobo MC (2014) Reducing the mobility of arsenic in brownfield soil using stabilised zero-valent iron nanoparticles. J Environ Sci Health A Tox Hazard Subst Environ Eng 49(12):1361–1369
Liu XM, Zhang FD, Zhang SQ, He XS, Fang R, Feng Z, Wang Y (2010) Effects of nano-ferric oxide on the growth and nutrients absorption of peanut. Plant Nutr Fert Sci 11:14–18
Chen Y-H, Li F-A (2010) Kinetic study on removal of copper(II) using goethite and hematite nano-photocatalysts. J Colloid Interface Sci 347:277–281
Komárek M, Koretsky CM, Stephen KJ, Alessi DS, Chrastný V (2015) Competitive adsorption of Cd(II), Cr(VI), and Pb(II) onto nanomaghemite: a spectroscopic and modeling approach. Environ Sci Technol 49:12851–12859
Auffan M, Rose J, Proux O, Borschneck D, Masion A, Chaurand P, Hazemann JL, Chaneac C, Jolivet JP, Wiesner MR, Van Geen A, Jean-Yves B (2008) Enhanced adsorption of arsenic onto maghemites nanoparticles: As(III) as a probe of the surface structure and heterogeneity. Langmuir 24(7):3215–3222
Prasad B, Ghosh C, Chakraborty A, Bandyopadhyay N, Ray RK (2011) Adsorption of arsenite (As3+) on nano-sized Fe2O3 waste powder from the steel industry. Desalination 274:105–112
Lin S, Lu D, Liu Z (2012) Removal of arsenic contaminants with magnetic c-Fe2O3 nanoparticles. Chem Eng J 211–212:46–52
Tuutijarvi T, Vahala R, Sillanpaa M, Chen G (2012) Maghemite nanoparticles for As(V) removal: desorption characteristics and adsorbent recovery. Environ Technol 33(16):1927–1936
Akhbarizadeh R, Shayestefar MR, Darezereshk E (2014) Competitive removal of metals from wastewater by maghemite nanoparticles: a comparison between simulated wastewater and AMD. Mine Water Environ 33:89–96
Chomoucka J, Drbohlavova J, Huska D, Adam V, Kizek R, Hubalek J (2012) Magnetic nanoparticles and targeted drug delivering. Pharmacol Res 62:144–149
Jiang W, Pelaez M, Dionysiou DD, Entezari MH, Tsoutsou D, O’Shea K (2013) Chromium(VI) removal by maghemite nanoparticles. Chem Eng J 222:527–533
Martínez-Fernández D, Vítková M, Bernal MP, Komárek M (2015) Effects of nano-maghemite on trace element accumulation and drought response of Helianthus annuus L. in a contaminated mine soil. Water Air Soil Pollut 226(4):1–4
Vítková M, Komárek M, Tejnecký V, Šillerová H (2015) Interactions of nano-oxides with low-molecular-weight organic acids in a contaminated soil. J Hazard Mater 293:7–14
Martínez-Fernández D, Bingöl D, Komárek M (2014) Trace elements and nutrients adsorption onto nano-maghemite in a contaminated-soil solution: a geochemical/statistical approach. J Hazard Mater 276:271–277
Chowdhury SR, Yanful EK (2010) Arsenic and chromium removal by mixed magnetite-maghemite nanoparticles and the effect of phosphate on removal. J Environ Manage 91:2238–2247
Sposito G (2008) The chemistry of soils, 2nd edn. Oxford University Press, New York 330 p
Wu Z, Gu X, Wang X, Evans L, Guo H (2003) Effects of organic acids on adsorption of lead onto montmorillonite, goethite and humic acid. Environ Pollut 121:469–475
Shen YF, Tang J, Nie ZH, Wang YD, Ren Y, Zuo I (2009) Preparation and application of magnetic Fe3O4 nanoparticles for wastewater purification. Sep Purif Technol 68:312–319
Afkhami A, Saber-Tehrani M, Bagheri H (2010) Modified maghemite nanoparticles as an efficient adsorbent for removing some cationic dyes from aqueous solution. Desalination 263(1–3):2
Pan BJ, Qiu H, Pan BC, Nie GZ, Xiao LL, Lv L et al (2010) Highly efficient removal of heavy metals by polymer-supported nanosized hydrated Fe(III) oxides: behavior and XPS study. Water Res 44(3):815–824
Dias AM, Hussain A, Marcos AS, Roque AC (2011) A biotechnological perspective on the application of iron oxide magnetic colloids modified with polysaccharides. Biotechnol Adv 29(1):142–155
Nazari M, Ghasemi N, Maddah H, Motlagh MM (2014) Synthesis and characterization of maghemite nanopowders by chemical precipitation method. J Nanostruct Chem 4:99
Oliveira LCA, Petkowicz DI, Smaniotto A, Pergher SBC (2004) Magnetic zeolites: a new adsorbent for removal of metallic contaminants from water. Water Res 38:3699–3704
Yavuz CT, Prakash A, Mayo JT, Colvin VL (2009) Magnetic separations: from steel plants to biotechnology. Chem Eng Sci 64:2510–2521
Andreu JS, Camacho J, Faraudo J, Benelmekki M, Rebollo C, Martínez LM (2011) Simple analytical model for the magnetophoretic separation of superparamagnetic dispersions in a uniform magnetic gradient. Phys Rev E Stat Nonlinear Soft Matter Phys 84:art. no. 021402
Kanel SR, Greneche J-M, Choi H (2006) Arsenic (V) removal from groundwater using nano scale zero-valent iron as a colloidal reactive barrier material. Environ Sci Technol 40:2045–2050
Mueller NC, Braun J, Bruns J, Černík M, Rissing P, Rickerby D, Nowack B (2012) Application of nanoscale zero valent iron (nZVI) for groundwater remediation in Europe. Environ Sci Pollut Res 19:550–558
Tosco T, Papani MP, Viggi CC, Sethi P (2014) Nanoscale zerovalent iron particles for groundwater remediation: a review. J Clean Prod 77:10–21
Alidokht L, Khataee AR, Reyhanitabar A, Oustan S (2011) Cr(VI) immobilization process in a Cr-spiked Soil by zerovalent iron nanoparticles: optimization using response surface methodology. Clean (Weinh) 39(7):633–640
Fajardo C, Ortíz LT, Rodríguez-Membibre ML, Nande M, Lobo MC, Martin M (2012) Assessing the impact of zero-valent iron (ZVI) nanotechnology on soil microbial structure and functionality: a molecular approach. Chemosphere 86:802–808
Gil-Díaz M, Pérez-Sanz A, Vicente MA, Lobo MC (2014) Immobilisation of Pb and Zn in soils using stabilised zero-valent iron nanoparticles: effects on soil properties. Clean Soil Air Water 42(12):1776–1784
Sun YP, Li XQ, Cao J, Zhang WX, Wang HP (2006) Characterization of zero-valent iron nanoparticles. Adv Colloid Interface 120:47–56
Crane RA, Scott TB (2012) Nanoscale zero-valent iron: future prospects for an emerging water treatment technology. J Hazard Mater 211–212:112–125
Yan W, Lien H-L, Koel BE, Zhang W-X (2013) Iron nanoparticles for environmental clean-up: recent developments and future outlook. Evnviron Sci Process Impacts 15(1):63–77
Filip J, Karlický F, Marušák Z, Lazar P, Černík M, Otyepka M, Zbořil R (2014) Anaerobic reaction of nanoscale zerovalent iron with water: mechanism and kinetics. J Phys Chem C 118:13817–13825
Li X-Q, Zhang W-X (2007) Sequestration of metal cations with zerovalent iron nanoparticles—a study with High Resolution X-ray Photoelectron Spectroscopy (HR-XPS). J Phys Chem C 111:6939–6946
Hu J, Chen G, Lo IMC (2005) Removal and recovery of Cr(VI) from wastewater by maghemite nanoparticles. Water Res 39:4528–4536
Wang CB, Zhang WX (1997) Synthesizing nanoscale iron particles for rapid and complete dechlorination of TCE and PCBs. Environ Sci Technol 31:2154–2156
Machado S, Pacheco JG, Nouws HPA, Albergaria JT, Delerue-Matos C (2015) Characterization of green zero-valent iron nanoparticles produced with tree leaf extracts. Sci Total Environ 533:76–81
Soukupová J, Zbořil R, Medřík I, Filip J, Šafářová K, Lédl R, Mashlan M, Nosek J, Černík M (2015) Highly concentrated, reactive and stable dispersion of zero-valent iron nanoparticles: direct surface modification and site application. Chem Eng J 262:813–822
Shi L, Zhang X, Chen Z (2011) Removal of chromium (VI) from wastewater using bentonite-supported nanoscale zero-valent iron. Water Res 45(2):886
Sun X, Yan Y, Liy Y, Han W, Wang L (2014) SBA-15-incorporated nanoscale zero-valent iron particles for chromium(VI) removal from groundwater: mechanism, effect of pH, humic acid and sustained reactivity. J Hazard Mater 266:26–33
Li Z, Greden K, Alvarez PJJ, Gregory KB, Lowry GV (2010) Adsorbed polymer and NOM limits adhesion and toxicity of nanoscale zerovalent iron to E. coli. Environ Sci Technol 44:3162–3167
Chrysochoou M, Johnston CP, Dahal G (2012) A comparative evaluation of hexavalent chromium treatment in contaminated soil by calcium polysulfide and green-tea nanoscale zero-valent iron. J Hazard Mater 201:33–42
Du J, Lu J, Wu Q, Jing C (2012) Reduction and immobilization of chromate in chromite ore processing residue with nanoscale zero-valent iron. J Hazard Mater 215:152–158
Post JE (1999) Manganese oxide minerals: crystal structures and economic and environmental significance. Proc Natl Acad Sci U S A 96:3447–3454
Dong DM, Nelson YM, Lion LW, Shuler ML, Ghiorse WC (2000) Adsorption of Pb and Cd onto metal oxides and organic material in natural surface coatings as determined by selective extractions: new evidence for the importance of Mn and Fe oxides. Water Res 34:427–436
O’Reilly SE, Hochella MF (2003) Lead sorption efficiencies of natural and synthetic Mn and Fe-oxides. Geochim Cosmochim Acta 67:4471–4487
Essington ME (2004) Soil and water chemistry: an integrative approach. CRC Press, Boca Raton, 534 pp. ISBN: 0-8493-1258-2
Feng XH, Zhai LM, Tan WF, Zhao W, Liu F, He JZ (2006) The controlling effect of pH on oxidation of Cr(III) by manganese oxide minerals. J Colloid Interface Sci 298:258–266
Fandeur D, Juillot F, Morin G, Olivi L, Cognigni A, Webb SM, Ambrosi JP, Fritsch E, Guyot F, Brown GE Jr (2009) XANES evidence for oxidation of Cr(III) to Cr(VI) by Mn-oxides in a lateritic regolith developed on serpentinized ultramafic rocks of New Caledonia. Environ Sci Technol 43:7384–7390
Driehaus W, Seith R, Jekel M (1995) Oxidation of arsenate(III) with manganese oxides in water treatment. Water Res 29:297–305
Chen Z, Kim KW, Zhu YG, McLaren R, Liu F, He JZ (2006) Adsorption (AsIII,V) and oxidation (AsIII) of arsenic by pedogenic Fe-Mn nodules. Geoderma 136:566–572
Watanabe J, Tani Y, Chang J, Miyata N, Naitou H, Seyama H (2013) As(III) oxidation kinetics of biogenic manganese oxides formed by Acremonium strictum strain KR21 2. Chem Geol 347:227–232
Villalobos M, Escobar-Quiroz IN, Salazar-Camacho C (2014) The influence of particle size and structure on the sorption and oxidation behavior of birnessite: I. Adsorption of As(V) and oxidation of As(III). Geochim Cosmochim Acta 125:564–581
Singh M, Thanh DN, Ulbrich P, Strnadová N, Štěpánek F (2010) Synthesis, characterization and study of arsenate adsorption from aqueous solution by α- and δ-phase manganese dioxide nanoadsorbents. J Solid State Chem 183:2979–2986
Naderi H, Majles Ara MH, Zebarjadan H, Saydi J, Javidan A (2013) Nonlinear response of nano-particles birnessite-type manganese oxide (γ-MnO2). Optik 124:1560–1563
Simenyuk GY, Zakharov YA, Pavelko NV, Dodonov VG, Pugachev VM, Puzynin AV, Manina TS, Barnakov CN, Ismagilov ZR (2015) Highly porous carbon materials filled with gold and manganese oxide nanoparticles for electrochemical use. Catal Today 249:220–227
Zhang X, Miao W, Li C, Sun X, Wang K, Ma Y (2015) Microwave-assisted rapid synthesis of birnessite-type MnO2 nanoparticles for high performance supercapacitor applications. Mater Res Bull 71:111–115
Villalobos M, Bargar J, Sposito G (2005) Trace metal retention on biogenic manganese oxide nanoparticles. Elements 1:223–226
Miyata N, Tani Y, Sakata M, Iwahori K (2007) Microbial manganese oxide formation and interaction with toxic metal ions. J Biosci Bioeng 104:1–8
Zhou D, Kim DG, Ko SO (2015) Heavy metal adsorption with biogenic manganese oxides generated by Pseudomonas putida strain MnB1. J Ind Eng Chem 24:132–139
Tebo BM, Ghiorse WC, van Waasbergen LG, Siering PL, Caspi R (1997) Bacterially mediated mineral formation: insights into manganese(II) oxidation from molecular genetic and biochemical studies. Rev Mineral Geochem 35:225–266
Morgan JJ (2000) Manganese in natural waters and earth’s crust: its availability to organisms. Met Ions Biol Syst 37:1–34
Nelson YM, Lion LW, Ghiorse WC, Shuler ML (1999) Production of biogenic Mn oxides by Leptothrix discophora SS-1 in a chemically defined growth medium and evaluation of their Pb adsorption characteristics. Appl Environ Microbiol 65:175–180
Gupta K, Bhattacharya S, Chattopadhyay D, Mukhopadhyay A, Biswas H, Dutta J, Ray NR, Ghosh UC (2011) Ceria associated manganese oxide nanoparticles: synthesis, characterization and arsenic(V) sorption behavior. Chem Eng J 172:219–229
Lalhmunsiama, Lee SM, Tiwari D (2013) Manganese oxide immobilized activated carbons in the remediation of aqueous wastes contaminated with copper(II) and lead(II). Chem Eng J 225:128–137
Peña J, Bargar JR, Sposito G (2015) Copper sorption by the edge surfaces of synthetic birnessite nanoparticles. Chem Geol 396:196–207
Yu Z, Zhou L, Huang Y, Song Z, Qiu W (2015) Effects of a manganese oxide-modified biochar composite on adsorption of arsenic in red soil. J Environ Manage 163:155–162
Zhu Q, Li Z (2015) Hydrogel-supported nanosized hydrous manganese dioxide: synthesis, characterization, and adsorption behavior study for Pb2+, Cu2+, Cd2+ and Ni2+ removal from water. Chem Eng J 281:69–80
Della Puppa L, Komárek M, Bordas F, Bollinger JC, Joussein E (2013) Adsorption of copper, cadmium, lead and zinc onto a synthetic manganese oxide. J Colloid Interface Sci 399:99–106
Michálková Z, Komárek M, Šillerová H, Della Puppa L, Joussein E, Bordas F, Vaněk A, Vaněk O, Ettler V (2014) Evaluating the potential of three Fe- and Mn- (nano)oxides for the stabilization of Cd, Cu and Pb in contaminated soils. J Environ Manage 46:226–234
Ettler V, Tomášová Z, Komárek M, Mihaljevič M, Šebek O, Michálková Z (2015) The pH-dependent long-term stability of an amorphous manganese oxide in smelter-polluted soils: implication for chemical stabilization of metals and metalloids. J Hazard Mater 286:386–394
Ku Y, Jung LI (2001) Photocatalytic reduction of Cr(VI) in aqueous solutions by UV irradiation with the presence of titanium dioxide. Water Res 35:135–142
Rengaraj S, Venkataraj S, Yeon JW, Kim Y, Li XZ, Pang GKH (2007) Preparation, characterization and application of Nd–TiO2 photocatalyst for the reduction of Cr(VI) under UV light illumination. Appl Catal 77:157–165
Kim Y, Joo H, Her N, Yoon Y, Lee C, Yoon Y (2013) Self-rotating photocatalytic system for aqueous Cr(VI) reduction on TiO2 nanotube/Ti mesh substrate. Chem Eng J 229:66–71
Yang H, Lin YW, Rajeshwar K (1999) Homogeneous and heterogeneous photocatalytic reactions involving As(III) and As(V) species in aqueous media. J Photochem Photobiol 123:137–143
Hu B, Liu W, Gao W, Han J, Liu H, Lucia LA (2015) Pseudo-Janus Zn/Al-based nanocomposites for Cr(VI) sorption/remediation and evolved photocatalytic functionality. Chem Eng J 277:150–158
Albadarin AB, Yang Z, Mangwandia C, Glocheuxa Y, Walker G, Ahmad MNM (2014) Experimental design and batch experiments for optimization of Cr(VI) removal from aqueous solutions by hydrous cerium oxide nanoparticles. Chem Eng Res Des 92:1354–1362
Taghipour M, Jalali M (2015) Effect of clay minerals and nanoparticles on chromium fractionation in soil contaminated with leather factory waste. J Hazard Mater 297:127–133
Rao GP, Lu C, Su F (2007) Sorption of divalent metal ions from aqueous solution by carbon nanotubes: a review. Sep Purif Technol 58:224–231
Jośko I, Oleszczuk P, Pranagal J, Lehmann J, Xing B, Cornelissen G (2013) Effect of biochars, activated carbon and multiwalled carbon nanotubes on phytotoxicity of sediment contaminated by inorganic and organic pollutants. Ecol Eng 60:50–59
Keller A, McFerran S, Lazareva A, Suh S (2013) Global life cycle releases of engineered nanomaterials. J Nanopart Res 15:1692
Anjum NA, Gill SS, Duarte AC, Pereira E, Ahmad I (2013) Silver nanoparticles in soil–plant systems. J Nanopart Res 15:1–26
Conway JR, Hanna SK, Lenihan HS, Keller AA (2014) Effects and implications of trophic transfer and accumulation of CeO2 nanoparticles in a marine mussel. Environ Sci Technol 48:1517–1524
Zhu H, Han J, Xiao JQ, Jin Y (2008) Uptake, translocation, and accumulation of manufactured iron oxide nanoparticles by pumpkin plants. J Environ Monit 10:713–717
Schaumann GE, Philippe A, Bundschuh M, Metreveli G, Klitzke S, Rakcheev D, Grün A, Kumahor SK, Kühn M, Baumanng T, Lang F, Manz W, Schulz R, Vogel H-J (2015) Understanding the fate and biological effects of Ag- and TiO2-nanoparticles in the environment: the quest for advanced analytics and interdisciplinary concepts. Sci Total Environ 535:3–19
Handy RD, Owen R, Valsami-Jones E (2008) The ecotoxicology of nanoparticles and nanomaterials: current status, knowledge gaps, challenges, and future needs. Ecotoxicology 17:315–325
Sabo-Attwood T, Unrine JM, Stone JW, Murphy CJ, Ghoshroy S, Blom D et al (2012) Uptake, distribution and toxicity of gold nano particles in tobacco (Nicotiana xanthi) seedlings. Nanotoxicology 6:353–360
Anjum NA, Adamb V, Kize R, Duarte AC, Pereira E, Iqbal M, Lukatkin AS, Ahmad I (2015) Nanoscale copper in the soil–plant system toxicity and under lying potential mechanisms. Environ Res 138:306–325
Ma X, Geisler-Lee J, Deng Y, Kolmakov A (2010) Interactions between engineered nanoparticles (ENPs) and plants: phytotoxicity, uptake and accumulation. Sci Total Environ 408:3053–3061
Miralles P, Church TL, Harris AT (2012) Toxicity, uptake, and translocation of engineered nanomaterials in vascular plants. Environ Sci Technol 46(17):9224–9239
Zhang P, Ma Y, Zhang Z (2015) Interactions between engineered nanomaterials and plants: phytotoxicity, uptake, translocation, and biotransformation. In: Siddiqui MH et al (ed) Nanotechnology and plants sciences. Springer, Basel. doi:10.1007/978-3-319-14502-0_5
Lin D, Xing B (2007) Phytotoxicity of nanoparticles: inhibition of seed germination and root growth. Environ Pollut 150:243–250
Lee W, An Y, Yoon H, Kweon H (2008) Toxicity and bioavailability of copper nanoparticles to the terrestrial plants mung bean (Phaseolus radiatus) and wheat ( Triticum aestivum ): plant uptake for water insoluble nanoparticles. Environ Toxicol Chem 27(9):1915–1921
Auffan M, Rose J, Wiesner MR, Bottero JY (2009) Chemical stability of metallic nanoparticles: a parameter controlling their potential cellular toxicity in vitro. Environ Pollut 157:1127–1133
Judy JD, Bertsch PM (2014) Bioavailability, toxicity, and fate of manufactured nanomaterials in terrestrial ecosystems. Adv Agron 123:1–64
Zhang W, Ebbs SD, Musante C, White JC, Gao C, Ma X (2015) Uptake and accumulation of bulk and nanosized cerium oxide particles and ionic cerium by radish ( Raphanus sativus L.). J Agric Food Chem 63:382–390
García A, Espinosa R, Delgado L, Casals E, González E, Puntes V, Carlos B, Font X, Sánchez A (2011) Acute toxicity of cerium oxide, titanium oxide and iron oxide nanoparticles using standardized tests. Desalination 269:136–141
Wu SG, Huang L, Head J, Chen DR, Kong IC, Tang YJ (2012) Phytotoxicity of metal oxide nanoparticles is related to both dissolved metals ions and adsorption of particles on seed surfaces. J Pet Environ Biotechnol 3:1–6
Barrena R, Casals E, Colón J, Font X, Sánchez A, Puntes V (2009) Evaluation of the ecotoxicity of model nanoparticles. Chemosphere 75:850–857
Wang Q, Ma X, Zhang W, Pei H, Chen Y (2012) The impact of cerium oxide nanoparticles on tomato (Solanum lycopersicum L.) and its implications for food safety. Metallomics 4:1105–1112
Shah V, Belozerova I (2009) Influence of metal nanoparticles on the soil microbial community and germination of lettuce seeds. Water Air Soil Pollut 197:143–148
Huang YC, Fan R, Grusak MA, Sherrier JD, Huang CP (2014) Effects of nano-ZnO on the agronomically relevant Rhizobium–legume symbiosis. Sci Total Environ 497–498:78–90
El-Temsah YS, Joner EJ (2010) Impact of Fe and Ag nanoparticles on seed germination and differences in bioavailability during exposure in aqueous suspension and soil. Environ Toxicol 24(1):42–49
Libralato G, Costa Devoti A, Zanella M, Sabbioni E, Mičetić I, Manodori L, Pigozzo A, Manenti S, Groppi F, Volpi Ghirardini A (2015) Phytotoxicity of ionic, micro and nano-sized iron in three plant species. Ecotoxicol Environ Saf 123:81–88. doi:10.1016/j.ecoenv.2015.07.024
Yang L, Watts DJ (2005) Particle surface characteristics may play an important role in phytotoxicity of alumina nanoparticles. Toxicol Lett 15:122–132
Grieger KD, Fjordbøge A, Hartmann NB, Eriksson E, Bjerg PL, Baun A (2010) Environmental benefits and risks of zero-valent iron nanoparticles (nZVI) for in situ remediation: risk mitigation or trade-off? J Contam Hydrol 118(3–4):165–183
Elsaesser C, Howard V (2012) Toxicology of nanoparticles. Adv Drug Deliv Rev 64:129–137
Nowack B, Bucheli T (2007) Occurrence, behavior and effects of nanoparticles in the environment. Environ Pollut 150(1):5–22
Zhang D, Hua T, Xiao F, Chen C, Gersberg RM, Liu Y, Stuckey D, Ng WJ, Tan SK (2015) Phytotoxicity and bioaccumulation of ZnO nanoparticles in Schoenoplectus tabernaemontani. Chemosphere 120:211–219
Navarro E, Baun A, Behra R, Hartmann NB, Filser J, Miao A-J, Quigg A, Santschi PH, Sigg L (2008) Environmental behavior and ecotoxicity of engineered nanoparticles to algae, plants, and fungi. Ecotoxicology 17:372–387
Rondeau-Mouro C, Defer D, Leboeuf E, Lahaye M (2008) Assessment of cell wall porosity in Arabidopsis thaliana by NMR spectroscopy. Int J Biol Macromol 42:83–92
Dietz K-J, Herth S (2011) Plant nanotoxicology. Trends Plant Sci 16(11):582–589
Burello E, Worth AP (2011) QSAR modeling of nanomaterials. Wiley Interdiscip Rev Nanomed Nanobiotechnol 3:298–306
Liu Q, Chen B, Wang Q, Shi X, Xiao Z, Lin J, Fang X (2009) Carbon nanotubes as molecular transporters for walled plant cells. Nano Lett 9:1007–1010
Pradeep T, Anshup (2009) Noble metal nanoparticles for water purification: a critical review. Thin Solid Films 517:6441–6478
Martínez-Fernández D, Barroso D, Komárek M (2015) Root water transport of Helianthus annuus L. under iron oxide nanoparticle exposure. Environ Sci Pollut Res Int 23(2):1732–1741. doi:10.1007/s11356-015-5423-5
Trakal L, Martínez-Fernández D, Vítková M, Komárek M (2015) Phytoextraction of metals: modeling root metal uptake and associated processes. In: Ansari AA, Gill SS, Gill R, Lanza GR, Lee N (eds) Phytoremediation: management of environmental contaminants. Springer, New York ISBN: 978-3-319-10394-5
Gao J, Youn S, Hovsepyan A, Llaneza VL, Wang Y, Bitton G, Bonzongo JJ (2009) Dispersion and toxicity of selected manufactured nanomaterials in natural river water samples: effects of water chemical composition. Environ Sci Technol 43:3322–3328
Whitley AR, Levard C, Oostveen E, Bertsch PM, Matocha CJ, Kammer FVD, Unrine JM (2013) Behavior of Ag nanoparticles in soil: effects of particle surface coating, aging and sewage sludge amendment. Environ Pollut 182:141–149
Wang H, Kou X, Pei Z, Xiao JQ, Shan X, Xing B (2011) Physiological effects of magnetite (Fe3O4) nanoparticles on perennial ryegrass (Lolium perenne L.) and pumpkin (Cucurbita mixta) plants. Nanotoxicology 5:30–42
Ma X, Gurung A, Deng Y (2013) Phytotoxicity and uptake of nanoscale zero-valent iron (nZVI) by two plant species. Sci Total Environ 443:844–849
Zhou DJS, Li L, Wang Y, Weng N (2011) Quantifying the adsorption and uptake of CuO nanoparticles by wheat root based on chemical extractions. J Environ Sci 23:1852–1857
Lü P, Cao J, He S, Liu J, Li H, Cheng G, Ding Y, Joyce DC (2010) Nano-silver pulse treatments improve water relations of cut rose cv. Movie Star flowers. Postharvest Biol Technol 57(3):196–202
Majumdar S, Almeida IC, Arigi EA, Choi H, VerBerkmoes NC, Trujillo-Reyes J, Flores-Margez JP, White JC, Peralta-Videa JR, Gardea-Torresdey JL (2015) Environmental effects of nanoceria on seed production of common bean ( Phaseolus vulgaris ): a proteomic analysis. Environ Sci Technol 49(22):13283–13293. doi:10.1021/acs.est.5b03452
Wang M, Chen L, Chen S, Ma Y (2012) Alleviation of cadmium-induced root growth inhibition in crop seedlings by nanoparticles. Ecotoxicol Environ Saf 79:48–54
Rico CM, Morales MI, Barrios AC, McCreary R, Hong J, Lee WY, Nunez J, Peralta-Videa JR, Gardea-Torresdey JL (2013) Effect of cerium oxide nanoparticles on the quality of rice (Oryza sativa L.) grains. J Agric Food Chem 61:11278–11285
López-Moreno M, De la Rosa G, Hernández-Viezcas J, Peralta-Videa J, Gardea-Torresdey J (2010) X-ray absorption spectroscopy (XAS) corroboration of the uptake and storage of CeO2 nanoparticles and assessment of their differential toxicity in four edible plant species. J Agric Food Chem 58:3689–3693
Zhao L, Peralta-Videa JR, Varela-Ramirez A, Castillo-Michel H, Li C, Zhang J, Aguilera RJ, Keller AA, Gardea-Torresdey JL (2012) Effect of surface coating and organic matter on the uptake of CeO2NPs by corn plants grown in soil: insight into the uptake mechanism. J Hazard Mater 225–226:131–138
Zhang Z, He X, Zhang H, Ma Y, Zhang P, Ding Y, Zhao Y (2011) Uptake and distribution of ceria nanoparticles in cucumber plants. Metallomics 3:816–822
Pradhan S, Patra P, Mitra S, Dey KK, Jain S, Sarkar S, Roy S, Palit P, Goswami A (2014) Manganese nanoparticles: impact on non-nodulated plant as a potent enhancer in nitrogen metabolism and toxicity study both in vivo and in vitro. J Agric Food Chem 62(35):8777–8785
Hernandez-Viezcas JA, Castillo-Michel H, Servin AD, Peralta-Videa JR, Gardea-Torresdey JL (2011) Spectroscopic verification of zinc absorption and distribution in the desert plant Prosopis juliflora-velutina (velvet mesquite) treated with ZnO nanoparticles. Chem Eng J 170:346–352
Corredor E, Testillano PS, Coronado M, González-Melendi P, Fernández-Pacheco R, Marquina C, Ibarra MR, de la Fuente JM, Rubiales D, Pérez-de-Luque A, Risueño MC (2009) Nanoparticle penetration and transport in living pumpkin plants: in situ subcellular identification. BMC Plant Biol 9(1):45
Larue C, Laurette J, Herlin-Boime N, Khodja H, Fayard B, Flank AM, Brisset F, Carriere M (2012) Accumulation, translocation and impact of TiO2 nanoparticles in wheat ( Triticum aestivum spp.): influence of diameter and crystal phase. Sci Total Environ 431:197–208
López-Moreno ML, de la Rosa G, Hernández-Viezcas JA, Castillo-Michel H, Bote CE, Peralta-Videa JR, Gardea-Torresdey JL (2010) Evidence of the differential biotransformation and genotoxicity of ZnO and CeO2 nanoparticles on soybean ( Glycine max ) plants. Environ Sci Technol 44:7315–7320
Ma Y, Zhang P, Zhang Z, He X, Li Y, Zhang J, Zheng L, Chu S, Yang K, Zhao Y, Chai Z (2014) Origin of the different phytotoxicity and biotransformation of cerium and lanthanum oxide nanoparticles in cucumber. Nanotoxicology 9:262–270
Birbaium K, Brogioli R, Schellenberg M, Stark W, Gunther D, Limbach L (2010) No evidence for cerium dioxide nanoparticle translocation in maize plants. Environ Sci Technol 44(22):8718–8723
Schwabe F, Schulin R, Limbach LK, Stark W, Bürge D, Nowack B (2013) Influence of two types of organic matter on interaction of CeO2 nanoparticles with plants in hydroponic culture. Chemosphere 91:512–520
Zhao L, Peng B, Hernandez-Viezcas JA, Rico C, Sun Y, Peralta-Videa JR et al (2012) Stress response and tolerance of Zea mays to CeO2 nanoparticles: crosstalk among H2O2, heat shock protein, and lipid peroxidation. ACS Nano 6:9615–9622
Lin D, Xing B (2008) Root uptake and phytotoxicity of ZnO nanoparticles. Environ Sci Technol 42:5580–5585
Wang ZXX, Zhao J, Liu X, Feng W, White JC, Xing B (2012) Xylem and phloem based transport of CuO nanoparticles in maize (Zea mays L.). Environ Sci Technol 46:4434–4441
Gardea-Torresdey JL, Rico CM, White JC (2014) Trophic transfer, transformation, and impact of engineered nanomaterials in terrestrial environments. Environ Sci Technol 48:2526–2540
Bolan N, Kunhikrishnan A, Thangarajan R, Kumpiene J, Park J, Makino T, Kirkham MB, Scheckel K (2014) Remediation of heavy metal(loid)s contaminated soils—to mobilize or to immobilize? J Hazard Mater 266:141–166
Canas JE, Long M, Nations S, Vadan R, Dai L, Luo M, Ambikapathi R, Lee EH, Olszyk D (2008) Effects of functionalized and nonfunctionalized single-walled carbon-nanotubes on root elongation of select crop species. Environ Toxicol Chem 27:1922–1931
Carvajal M, Cooke DT, Clarkson DT (1996) Responses of wheat plants to nutrient deprivation may involve the regulation of water-channel function. Planta 199(3):372–381
Martínez-Fernández D, Walker DJ, Romero-Espinar P, Flores P, del Río JA (2011) Physiological responses of Bituminaria bituminosa to heavy metals. J Plant Physiol 168:2206–2211
Trujillo-Reyes J, Vilchis-Nestor AR, Majumdar S, Peralta-Videa JR, Gardea-Torresdey JL (2013) Citric acid modifies surface properties of commercial CeO2 nanoparticles reducing their toxicity and cerium uptake in radish ( Raphanus sativus ) seedlings. J Hazard Mater 263:677–684
Asli S, Neumann PM (2009) Colloidal suspensions of clay or titanium dioxide nanoparticles can inhibit leaf growth and transpiration via physical effects on root water transport. Plant Cell Environ 32:577–584
Landa P, Vankova R, Andrlova J, Hodek J, Marsika P, Storchova H, White JC, Vanek T (2012) Nanoparticle-specific changes in Arabidopsis thaliana gene expression after exposure to ZnO, TiO2, and fullerene soot. J Hazard Mater 241–242:55–62
Martínez-Ballesta MC, Carvajal M (2014) New challenges in plant aquaporin biotechnology. Plant Sci 217–218:71–77
Trujillo-Reyes J, Majumdar S, Botez CE, Peralta-Videa JR, Gardea-Torresdey JL (2014) Exposure studies of core–shell Fe/Fe3O4 and Cu/CuO NPs to lettuce ( Lactuca sativa ) plants: are they a potential physiological and nutritional hazard? J Hazard Mater 267:255–263
Musante C, White JC (2012) Toxicity of silver and copper to Cucurbita pepo: differential effects of nano and bulk-size particles. Environ Toxicol 27:510–517
Nair PG, Chung I (2014) Impact of copper oxide nanoparticles exposure on Arabidopsis thaliana growth, root system development, root lignification, and molecular level changes. Environ Sci Pollut Res 21:12709–12722
Shaw AK, Hossain Z (2013) Impact of nano-CuO stress on rice (Oryza sativa L.) seedlings. Chemosphere 93(6):906–915
Prakash M, Nair G, Kim SH, Chung IM (2014) Copper oxide nanoparticle toxicity in mung bean ( Vigna radiata L.) seedlings: physiological and molecular level responses of in vitro grown plants. Acta Physiol Plant 36:2947–2958
Dimkpa C, McLean J, Britt D, Anderson A (2015) Nano-CuO and interaction with nano-ZnO or soil bacterium provide evidence for the interference of nano-particles in metal nutrition of plants. Ecotoxicology 24:119–129
Hong J, Rico CM, Zhao L, Adeleye AS, Keller AA, Peralta-Videa JR, Gardea-Torresdey JL (2015) Toxic effects of copper-based nanoparticles or compounds to lettuce ( Lactuca sativa ) and alfalfa (Medicago sativa). Evnviron Sci Process Impacts 17:177–185
Bittner F (2014) Molybdenum metabolism in plants and crosstalk to iron. Front Plant Sci 5:28
Lu CM, Zhang CY, Wen JQ, Wu GR, Tao MX (2002) Research on the effect of nanometer materials on germination and growth enhancement of Glycine max and its mechanism. Soybean Sci 21:68–172
Lee CW, Mahendra S, Zodrow K, Li D, Tsai YC, Braam J, Alvarez PJJ (2010) Developmentalphytotoxicity of metal oxide nanoparticles to Arabidopsis thaliana . Environ Toxicol Chem 29:669–675
Gao F, Hong F, Liu C, Zheng L, Su M, Wu X, Yang F, Wu C, Yang P (2006) Mechanism of nano-anatase TiO2 on promoting photosynthetic carbon reaction of spinach. Biol Trace Elem Res 111:239–253
Yang F, Lui C, Gao F, Su M, Wu X, Zheng L, Hong F, Yang P (2007) The improvement of spinach growth by nano-anatase TiO2 treatment is related to nitrogen photoreduction. Biol Trace Elem Res 119:77–88
Linglan M, Chao L, Chunxiang Q, Sitao Y, Jie L, Fengqing G, Fashui H (2008) Rubisco activase mRNA expression in spinach: modulation by nanoanatase treatment. Biol Trace Elem Res 122:168–178
Cai X, Gao Y, Sun Q, Chen Z, Megharaj M, Naidu R (2014) Removal of co-contaminants Cu (II) and nitrate from aqueous solution using kaolin-Fe/Ni nanoparticles. Chem Eng J 244:19–26
Li H, Zhou Q, Wu Y, Wang T, Jiang G (2009) Effects of waterborne nanoiron on medaka (Oryzias latipes): antioxidant enzymatic activity, lipid peroxidation and histopathology. Ecotoxicol Environ Saf 72:684–692
Kim JH, Oh Y, Yoon H, Hwang I, Chang YS (2015) Iron nanoparticle-induced activation of plasma membrane H+-ATPase promotes stomatal opening in Arabidopsis thaliana . Environ Sci Technol 4:1113–1119
Pradhan S, Patra P, Das S, Chandra S, Mitra S, Dey KK, Akbar S, Palit P, Goswami A (2013) Photochemical modulation of biosafe manganese nanoparticles on Vigna radiata : a detailed molecular, biochemical, and biophysical study. Environ Sci Technol 47(22):13122–13131
Shaw AK, Ghosh S, Kalaji HM, Bosa K, Brestic M, Zivcak M et al (2014) Nano-CuO stress induced modulation of antioxidative defense and photosynthetic performance of Syrian barley (Hordeum vulgare L.). Environ Exp Bot 102:37–47
Gratão PL, Polle A, Lea PJ, Azevedo RA (2005) Making the life of heavy metal-stress plants a little easier. Funct Plant Biol 32:481–494
Krishnaraj C, Jagan EG, Ramachandran R, Abirami SM, Mohan N, Kalaichelvan PT (2012) Process Biochem 47:651–658
Terry N (1983) Limiting factors in photosynthesis. IV. Iron stress mediated changes in light-harvesting and electron transport capacity and its effects on photosynthesis in vivo. Plant Physiol 71:855–860
Marusenko Y, Shipp J, Hamilton GA, Morgan JLL, Keebaugh M, Hill H, Dutta A, Zhuo X, Upadhyay N, Hutchings J, Herckes P, Anbar AD, Shock E, Hartnett HE (2013) Bioavailability of nanoparticulate hematite to Arabidopsis thaliana . Environ Pollut 174:150–156
Dimkpa CO, McLean JE, Latta DE, Manangón E, Britt DW, Johnson WP et al (2012) CuO and ananoparticles: phytotoxicity, metal speciation, and induction of oxidative stress in sand-grown wheat. J Nanopart Res 14:1125
Nair P, Chung I (2014) A mechanistic study on the toxic effect of copper oxide nanoparticles in soybean ( Glycine max L.) root development and lignification of root cells. Biol Trace Elem Res 162:342–352
Dehkourdi EH, Mosavi M (2013) Effect of anatase nanoparticles (TiO2) on parsley seed germination ( Petroselinum crispum ) in vitro. Biol Trace Elem Res 155:283–286
Sharma P, Bhatt D, Zaidi MGH, Pardha Saradhi P, Khanna PK, Arora S (2012) Silver nanoparticle-mediated enhancement in growth and antioxidant status of Brassica juncea . Appl Biochem Biotechnol 167:2225–2233
Remedios C, Rosario F, Bastos V (2012) Environmental nanoparticles interactions with plants: morphological, physiological, and genotoxic aspects. J Bot 2012:1–8
Lee S, Chung H, Kim S, Lee I (2013) The genotoxic effect of ZnO and CuO nanoparticles on early growth of buckwheat, Fagopyrum esculentum. Water Air Soil Pollut 224:1–11
Wang H, Wu F, Meng F, White JC, Holden PA, Xing B (2013) Engineered nanoparticles may induce genotoxicity. Environ Sci Technol 47:13212–13214
Barnes CA, Elsaesser A, Arkusz J, Smok A, Palus J et al (2008) Reproducible comet assay of amorphous silica nanoparticles detects no genotoxicity. Nano Lett 8:3069–3074
Magdolenova Z, Collins A, Kumar A, Dhawan A, Stone V, Dusinska M (2014) Mechanisms of genotoxicity. A review of in vitro and in vivo studies with engineered nanoparticles. Nanotoxicology 8(3):233–278
Atha DH, Wang H, Petersen EJ, Cleveland D, Holbrook RD, Jaruga P, Dizdaroglu M, Xing B, Nelson BC (2012) Copper oxide nanoparticle mediated DNA damage in terrestrial plant models. Environ Sci Technol 46:1819–1827
Ghosh M, Bandyopadhyay M, Mukherjee A (2010) Genotoxicity of titanium dioxide (TiO2) nanoparticles at two trophies levels: plant and human lymphocytes. Chemosphere 81(10):1253–1262
Kumari M, Khan SS, Pakrashi S, Mukherjee A, Chandrasekaran N (2011) Cytogenetic and genotoxic effects of zinc oxide nanoparticles on root cells of Allium cepa . J Hazard Mater 190:613–621
Burklew E, Ashlock J, Winfrey WB, Zhang B (2012) Effects of aluminum oxide nanoparticles on the growth, development, and microRNA expression of tobacco (Nicotiana tabacum). PLoS One 7(5):1–10
Shi J, Abid AD, Kennedy IM, Hristova KR, Silk WK (2011) To duckweeds (Landoltia punctata), nanoparticulate copper oxide is more inhibitory than the soluble copper in the bulk solution. Environ Pollut 159:1277–1282
Moon Y-S, Park E-S, Kim T-O, Lee H-S, Lee S-E (2014) SELDI-TOF MS-based discovery of a biomarker in Cucumis sativus seeds exposed to CuO nanoparticles. Environ Toxicol Phar 38(3):922–931
Bandyopadhyay S, Plascencia-Villa G, Mukherjee A, Rico CM, José-Yacamán M, Peralta-Videa JR, Gardea-Torresdey JL (2015) Comparative phytotoxicity of ZnO NPs, bulk ZnO, and ionic zinc onto the alfalfa plants symbiotically associated with Sinorhizobium meliloti in soil. Sci Total Environ 515–516:60–69
Song L, Vijver MG, Peijnenburg WJGM (2015) Comparative toxicity of copper nanoparticles across three Lemnaceae species. Sci Total Environ 518–519:217–224
Prasad TNVKV, Sudhakar P, Sreenivasulu Y, Latha P, Munaswamy V, Reddy KR, Sreeprasad TS, Sajanlal PR, Pradeep T (2012) Effect of nanoscale zinc oxide particles on the germination, growth and yield of peanut. J Plant Nutr 35:905–927
Rao S, Shekhawat GS (2014) Toxicity of ZnO engineered nanoparticles and evaluation of their effect on growth, metabolism and tissue specific accumulation in Brassica juncea . J Environ Chem Eng 2:105–114
Ma Y, Kuang L, He X, Bai W, Ding Y, Zhang Z, Zhao Y, Chai Z (2010) Effects of rare earth oxide nanoparticles on root elongation of plants. Chemosphere 78:273–279
Ma C, Chhikara S, Xing B, Musante C, White JC, Dhankher OP (2013) Physiological and molecular response of Arabidopsis thaliana to nanoparticle cerium and indium oxide exposure. ACS Sustain Chem Eng 1:768–778
Fan G, Cang L, Qin W, Zhou C, Gomes H, Zhou D (2013) Surfactants-enhanced electrokinetic transport of xanthan gum stabilized nano Pd/Fe for the remediation of PCBs contaminated soils. Sep Purif Technol 114:64–72
Shahwan T, Üzüm Ç, Eroğlu AE, Lieberwirth I (2010) Synthesis and characterization of bentonite/iron nanoparticles and their application as adsorbent of cobalt ions. Appl Clay Sci 47:257–262
Ben-Moshe T, Dror I, Berkowitz B (2010) Transport of metal oxide nanoparticles in saturated porous media. Chemosphere 81:387–393
Lim S-F, Zheng Y-M, Zou S-W, Chen JP (2009) Removal of copper by calcium alginate encapsulated magnetic sorbent. Chem Eng J 152:509–513
Tratnyek PG, Johnson RL (2006) Nanotechnologies for environmental cleanup. Nanotoday 1(2):44–48
Phenrat T, Saleh N, Sirk K, Tilton RD, Lowry GV (2007) Aggregation and sedimentation of aqueous nanoscale zerovalent iron dispersions. Environ Sci Technol 41:284–290
Della Vecchia E, Coisson M, Appino C, Vinai F, Sethi R (2009) Magnetic characterization and interaction modeling of zerovalent iron nanoparticles for the remediation of contaminated aquifers. J Nanosci Nanotechnol 9:3210–3218
Nowack B (2008) Pollution prevention and treatment using nanotechnology. In: Krug H (ed) Nanotechnology: environmental aspects, vol 2. Wiley, Weinheim, pp 1–15
EU NanoSafety Cluster-Database WG (2015) http://www.nanosafetycluster.eu/working-groups/4-database-wg.html. Accessed 5 Nov 2015
Mitrano DM, Motellier S, Clavaguera S, Nowack B (2015) Review of nanomaterial aging and transformations through the life cycle of nano-enhanced products. Environ Int 77:132–147
Glaser B, Haumaier L, Guggenberger G, Zech W (2001) The ‘Terra Preta’ phenomenon: a model for sustainable agriculture in the humid tropics. Naturwissenschaften 88:37–41
Joseph S, Anawar HM, Storer P, Blackwell P, Chia C, Lin Y, Munroe P, Donne S, Horvat J, Wang J, Solaiman ZM (2015) Effects of enriched biochars containing magnetic iron nanoparticles on mycorrhizal colonisation, plant growth, nutrient uptake and soil quality improvement. Pedosphere 25(5):749–760
Acknowledgments
Domingo Martínez-Fernández is grateful for financial support from the postdoctoral grant (19835/PD/15) financed by the “Consejería de Educación y Universidades de la CARM”, through the “Fundación Séneca-Agencia de Ciencia y Tecnología de la Región de Murcia”. Michael Komárek is thankful for the support from the Czech Science Foundation (project 15-07117S). The English revision by Dr. David J. Walker is also acknowledged.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Martínez-Fernández, D., Vítková, M., Michálková, Z., Komárek, M. (2017). Engineered Nanomaterials for Phytoremediation of Metal/Metalloid-Contaminated Soils: Implications for Plant Physiology. In: Ansari, A., Gill, S., Gill, R., R. Lanza, G., Newman, L. (eds) Phytoremediation. Springer, Cham. https://doi.org/10.1007/978-3-319-52381-1_14
Download citation
DOI: https://doi.org/10.1007/978-3-319-52381-1_14
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-52379-8
Online ISBN: 978-3-319-52381-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)