Abstract
Metabolic engineering has been used to develop Escherichia coli strains that generate d or l-lactic acid as the predominant fermentation product from different carbon sources, including glucose and xylose, which are present in syrups from lignocellulosic hydrolysates. As an introduction, this review presents the relevance that lactic acid has nowadays in several industrial and commercial applications. It also stresses the relevance of producing d or l-lactic acid as pure optical enantiomers for different applications. The second part reviews the metabolic engineering and adaptive evolution efforts developed with E. coli to achieve the production of optically pure d or l-lactic acid using several carbon sources. Furthermore, a set of results using actual mixtures of sugars contained in lignocellulosic hydrolysates is presented and discussed. Even though the efficient conversion of sugars to d or l-lactic acid and high volumetric productivities has been achieved, this review reveals that most work needs to be performed with actual lignocellulosic hydrolysates at the pilot or demonstrative scales to deploy the full potential of this efforts towards industrial production.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction: Renewable (Bio)Chemicals
The world’s population growth is projected to 9.7 billion in 2050 and 11.2 billion by 2100 (United Nations, Department of Economic and Social Affairs, 2015) generates a demand for larger quantities of food, feed, housing, chemicals, energy and goods that are used in everyday life, including a wide array of plastics. Many of these commodities were made from renewable resources at the beginning of 20th century, afterward, they were substituted by petroleum-derived chemicals (Weber et al. 2002); the main driver was the low price of the raw material. However, manufacturing processes and the plastics produced from petroleum generate wastes that are not biodegradable. One way to turn around this situation is to use renewable biological resources to produce biochemical building blocks, which can then be utilized for the manufacture of biodegradable and renewable bioplastics.
Renewable chemicals are chemical compounds that are generated from renewable feedstocks, which are materials from biological origin, also known as bioderived chemicals. According to (Vijayendran 2010) there are three ways to obtain bio-derived chemicals: (1) direct production using conventional thermochemical and catalytic processing of bioderived feedstock; (2) biomass biorefining to obtain bioproducts by biochemical conversion technologies; and (3) bioproducts obtained with the aid of genetically engineered organisms, with designed functionality of monomers as building blocks. Direct production and biomass biorefining are already a reality, and probably many of them are derived from the existing technologies from the beginning of the 20th century. Some examples include the production of lactic acid from bacterial fermentation of simple sugars for the food industry (Tsao et al. 1999) or starch (Vishnu et al. 2000) and the production of bioethanol from sugar cane in Brazil or corn starch in the USA. The second wave of bioproducts involve the conversion of bioderived sugars, cellulosic biopolymers and oils trough biochemical routes that require advanced research and development in the biotechnology and bioprocess fields; some of them are now in the early pilot scale phase. Biochemical processing, using metabolically engineered microorganisms, to improve productivities and separation technologies to produce high-value chemicals have made significant advances in the last years. This may allow the second wave of bioproducts to become a reality shortly, yet some technological advances need to be made to turn it profitable.
According to the European Committee for Standardization (CEN 2011), a product wholly or partly derived from biomass is known as a biobased product. From a technical point of view, almost all industrial materials made from fossil resources could be substituted by their biobased counterparts (Carus et al. 2011). Biobased products include all kinds of biobased chemicals, biobased polymers, biobased plastics, and bioadditives, which are biodegradable. Bio-composites, like wood plastics composites and natural fibers, reinforced plastics and insulation materials, and also the traditional products of the timber industry, are considered biobased products.
In Table 1 some of the most important renewable (bio)chemicals that are used as biobased building blocks, as well as those that will have a potential development shortly are presented. In this table, glucose and other feedstocks are of biological origin such as corn, sugarcane, and sugar beet, among others. According to Aeschelmann and Carus (2015), the European Bioplastics growth projection of bio-based polymers will range from 1.7 million metric tons in 2014 to 7.8 million tons in 2019. The Projection presented in Table 1 from Nova-Institute was adapted from a report made to the Netherlands government (Dammer et al. 2013), and clearly shows that the biochemical building block market will have a positive growth trend. Part of this expected growth results from drivers that are looking at renewable and sustainable materials. An example is the Coca Cola Company’s interest in the “Plant Bottle,” which probably deployed the growth of aforementioned bio-building blocks since they are a precursor of bio-PET bottles. This increase in sustainable materials use is followed by biodegradable polyesters such as polybutylene succinate and poly(butylene adipate-co-terephthalate), which are closely followed by polylactic acid (PLA), biobased polyethylene and starch blends. A dynamic development is foreseen for drop-in bio-based polymers because they can use the same technology as those derived from petrochemicals. Drop-in bio-based polymers are chemically identical to their petrochemical counterparts but at least partially derived from biomass. The use of drop-in biobased PET has a projected production capacity of about 7 million tons by 2020, being polybutylene succinate (PBS) in second place market of drop-in polymers. The biobased polymer market for the biodegradable biopolymers PLA and polyhydroxyalkanoates (PHA) has an impressive growth forecast: between 2014 and 2020, PLA production capacity is expected to almost quadruple, and PHA production capacity is projected to grow tenfold (Aeschelmann and Carus 2015). For a more detailed study of renewable chemicals and markets, the nova-Institute from Germany has launched several reports about this issue (http://www.nova-institut.de).
From the review of various publications about bio-based chemicals and polymers/plastics (de Jong et al. n.d.; Carus et al. 2011) it is clear that one of the main characteristics and advantages of bio-based chemicals is the use of renewable sources as their feedstock. This is relevant in the perspective that the production of many polymers will still be possible even if petroleum depletes in the future. Other social benefits of the manufacture of biobased polymers include the development of rural and forestry activities, the creation of employment and the support for research and development in biotechnology (Golden et al. 2015). However, from the economic point of view, it is necessary to develop stronger policies to support and help the growth of bio-based chemicals. More technological advances are required to make the biobased industry economically viable, the capital risk is high; but there are opportunities to develop new applications in food packaging, promote petroleum independence and generate improved properties to fill new niches or new applications (Aeschelmann and Carus 2015). Furthermore, feedstock availability and the need for large land areas to produce these raw materials are relevant concerns. However, the production of biobased building blocks and polymer derivatives contributes to reducing greenhouse gas emissions, including CO2, increases sustainability in production processes, and can use a wide array of residual biomasses from water or land sources. Before going to the production at high scale, life cycle analysis for biobased products is a requisite to evaluate the environmental, economic, technological and social impacts, either positive or negative (Lammens et al. 2011).
2 Lactic Acid Production, Applications, Polylactic Acid and Markets
Lactic acid (LA) is one of the oldest biobased chemicals that exist since the very early stages of human history. The first time that LA was isolated as a chemical compound was from sour milk in 1789. Its name comes from the French “acide lactique” as named by Lavoisier; Pasteur discovered that this was not a component of milk, but instead was a product from fermentation (Vijayakumar et al. 2008). The IUPAC nomenclature for LA is 2-hydroxypropionic acid with the formula CH3CH(OH)CO2H. Since the second carbon is chiral, it may exist in two optical forms: l(+)-LA (l-LA) and d(-)-LA (D-LA). Lactic acid is classified as GRAS (generally recognized as safe) for use as a food additive by the US FDA (Food and Drug Administration). However, d(-)-lactic acid at an elevated concentration is harmful to human metabolism and can result in acidosis and decalcification (Wee et al. 2006; Vijayakumar et al. 2008). The main uses of lactic acid are shown in Table 2.
Humans, animals, plants, and microorganisms produce LA. The first biotechnological production of l-LA was made in 1839 by Fremy, fermenting carbohydrates such as sucrose, lactose, mannitol, starch, and dextrin. The first commercial production was done in the United States of America in 1881 using a microbial process (Vijayakumar et al. 2008).
Probably its first use was related to food, particularly involving natural or processed fermented food preparations, 70–80% of the l-LA produced is used in food processes (John et al. 2009), being the remaining percentage for nonfood applications. The l(+) form of LA is used in the food and pharmaceutical industry because the human body is only adapted to assimilate this isomer (Vijayakumar et al. 2008). l-LA is a valuable chemical in the food industry as a preservative, acidulant, and flavoring agent, it is also used as feedstock for the manufacture of calcium stearoyl-2-lactylates in the baking industry. The water-retaining capacity and the ability to inhibit tyrosinase (the enzyme responsible for melanin formation) of lactic acid makes it suitable for use as moisturizer in cosmetic formulations and as skin lightening and rejuvenation (John et al. 2009). l-LA has numerous uses in medical/pharmaceutical applications, such as electrolytes in many parenteral/intravenous solutions that are intended to replenish bodily fluids. Also, it is used in a wide variety of medical preparations, which include tablets, prostheses, surgical sutures, and controlled drug delivery systems (Wee et al. 2006). In the leather and textile industries, technical-grade LA is extensively used in leather tanning industries as an acidulant for deliming hides and in vegetable tanning (John et al. 2009). Lactic acid as a descaling agent is often used in many decalcification products, such as bathroom cleaners, coffee machines, and toilets.
The chemical synthesis of lactic acid results from the hydrolysis of lactonitrile by strong acids, which produces the racemic mixture of l and d-LA (Wee et al. 2006; Nampoothiri et al. 2010; John et al. 2009). Lactonitrile results from the action of hydrogen cyanide and catalyst on acetaldehyde derived from fossil petroleum (Vijayakumar et al. 2008) and acetaldehyde is obtained from the oxidation of ethylene, also obtained from petroleum (Gupta et al. 2007). The chemical synthesis pathway for commercial LA production was prevalent some decades ago until an inexpensive fermentation process was developed (Gupta et al. 2007). The biochemical pathway produces a desired optically pure l or d-LA (Okano et al. 2010). Advantages and disadvantages of both production pathways are indicated in Table 3. Nowadays, LA comes mainly (90%) from bacterial fermentation of renewable sources (Vijayakumar et al. 2008), it is recyclable, biodegradable and compostable. The polymer obtained from LA, polylactic acid (PLA), has numerous uses in a wide range of applications, such as protective clothing, food packaging, mulch film, trash bags, rigid containers, shrink wrap, and short shelf-life trays, among others (Wee et al. 2006). Besides the uses indicated in Table 2, since it is environmentally friendly, LA is used as a green solvent for epoxy resins. Lactate esters, especially ethyl lactate, are used as green solvents, cleaning agents, and diluents, and as a precursor of herbicides (Vijayakumar et al. 2008; Datta and Henry 2006).
Increasing interest has arised to use LA for the production of the biobased polymer PLA to substitute the poly(ethylene terephthalate) (PET) since PLA can have similar mechanical properties (rigidity and clarity) as PET. Both forms of LA: d and l are employed for the manufacture of PLA. The optical purity of d or l-LA is crucial to the physical properties of PLA, including its thermostability. Hence, the production of pure enantiomeric d- or l-LA is an important goal (Okano et al. 2010). The polymerization of pure forms of LA can produce highly crystalline PLA that are suitable for commercial uses. The use of the racemic form of LA in the production of PLA gives origin to an amorphous structure (Henton et al. 2005). Many commercial PLAs are copolymers of poly(l-LA) (PLLA) and poly(d, l-LA) (PDLLA), which are produced from l-lactides and d, l-lactides (Lim et al. 2008). Table 4 shows some characteristics of pure PLA and with different proportions of d and l forms. The glass transition temperature (Tg) determines the upper-temperature limit for most commercial applications. The melting point (Tm) is necessary to determine the temperatures used across various processes. “Both of these transitions, Tg and Tm, are strongly affected by overall optical composition, primary structure, thermal history, and molecular weight” (Henton et al. 2005).
As mentioned above the global market for PLA is expected to grow during the next years. The number of companies producing PLA is projected to increase to approximately 27 until the year 2020; the production capacity was 205,000 metric tons in 2014 and is expect to quadruple from 2014 to 2020 (Aeschelmann and Carus 2015). NatureWorks, based in Blair, Nebraska, USA, was established by Cargill Inc. and started producing PLA in 2002 (John et al. 2009; Wee et al. 2006) with a plant capacity in 2010 of 140,000 tons. This company produces polydilactide-based resins (Nature-WorksPLA®), used for packaging applications, and the Ingeo™polydilactide based fibers, which are employed in specialty textiles and fiber applications. Purac, in the Netherlands, produces lactide, as well as medical grade PLA (Schut 2008; Juturu and Wu 2015) for a total of 100,000 tons. Other companies that produce lactic acid and PLA are Pyramid Bioplastics Guben GmBH (60,000 tons, Germany), Archer Daniels Midland Company (USA), Henan Jindan (China) and Galactic (EU, USA).
3 Escherichia coli Fermentative Metabolism
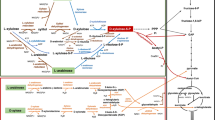
The Gram-negative bacteria Escherichia coli can grow under aerobic and anaerobic conditions. Under fermentative conditions, E. coli transforms carbon substrates into several biochemicals. Using these endogenous organic compounds as terminal electron acceptors, rapid growth and redox balance can be achieved by different fermentative processes (Orencio-Trejo et al. 2010). Because the metabolic products generated by E. coli have different oxidation states, this microorganism can adjust the metabolic pathways to grow on various carbon sources. Glucose is converted to a mixture of fermentation products consisting primarily of acetate and formate, as well as lower amounts of lactate, succinate and ethanol (Fig. 1) (Clark 1989). The fermentation of hexoses, with the same degree of reduction as glucose, generates four extra reducing equivalents, two ATP molecules by substrate-level phosphorylation, and two pyruvate molecules that are available for the formation of different organic acids and ethanol (Orencio-Trejo et al. 2010). It is known that wild-type E. coli strains using glucose as carbon source, under anaerobic conditions, commits only 4.8% of the carbon source to lactate (Yang et al. 1999). If the main acetic acid pathway (AckA-Pta) is deleted in E. coli, this bacterium produces mainly lactate and succinate, with minor amounts of formate, ethanol, and pyruvate (Yang et al. 1999). Moreover, these researchers found that the double mutant ΔackA-pta ΔldhA (ldhA is the gene encoding for the stereospecific d-lactate dehydrogenase) increased the carbon flux to formate and ethanol, with a concomitant reduction in the cellular growth and also in the production of succinate and lactate. Furthermore, when ldhA was overexpressed in the double mutant (ΔackA-pta and ΔldhA), it was found that 90% of the carbon flux went through the lactate pathway (Yang et al. 1999). These results indicate that, whether redox balance is attained, ldhA overexpression can increase the flux to lactate in strains defective in acetate production.
Escherichia coli fermentative metabolism and relevant pathways and modifications for d or l-lactic acid production using metabolic engineering. Nomenclature: Metabolites: Ac Acetate, Ac-CoA Acetyl CoA, AcDhd Acetaldehyde, Ac-P Acetyl-P, D-LA D-lactate, DHA-P Dihydroxyacetone phosphate, DIH Dihydroxyacetone, FORM Formate, EtOH Ethanol, FUM Fumarate, Glc Glucose, G3P Glyceraldehyde 3-phosphate, Gly Glycerol, GLY3P: Glycerol 3-phosphate, L-LA L-lactate, Mgx Methylglyoxal, OAC Oxaloacetate, PEP Phosphoenolpyruvate, PYR Pyruvate; SUC: Succinate, Xyl Xylose. Transporters: PTS phosphotransferase system, mediates uptake of glucose with its concomitant phosphorylation. XylFGH Xylose ABC transporter. Genes: ackA Acetate kinase, adhE Alcohol dehydrogenase, dhaKLM Dihydroxyacetone kinase, frdABCD Fumarate reductase, glpABC Anaerobic glycerol 3-phosphate dehydrogenase, glpD Aerobic glycerol 3-phosphate dehydrogenase, glpK Glycerol kinase, ldhA D-Lactate dehydrogenase, mgsA Methylglyoxal synthase, pflB Pyruvate formate-lyase, ppc Phosphoenolpyruvate carboxylase, poxB Pyruvate oxidase, pta Phosphate acetyltransferase
Under fermentative conditions, E. coli can obtain redox balance when growing on hexoses or pentoses by reducing pyruvate to lactate. However, it is not able to grow on sorbitol or gluconate, because the redox potential cannot be regenerated by the metabolism of sugar alcohols or acid sugars (Orencio-Trejo et al. 2010). Some pyruvate-consuming pathways must be eliminated to increase the carbon flux to lactate (Fig. 1). The reaction catalyzed by pyruvate formate lyase (Pfl) is the major pyruvate-consuming pathway under anaerobic conditions. Hence, most of the strategies to achieve homolactic fermentation include the elimination of the Pfl activity. The pfl knockout in different E. coli strains causes abundant production of d-LA, but the LA volumetric productivity is low, reduced amounts of cell mass are formed, and low growth rates are obtained in comparison to the wild-type strains. Some Δpfl strains are unable to grow on glucose under anaerobic conditions without acetate supplementation, because the Pfl reaction is the main supplier of acetyl-CoA under this conditions (Zhou et al. 2003a; Zhu and Shimizu, 2004; Utrilla et al. 2009, 2012).
4 Metabolic Engineering of E. coli to Produce d or l Lactate
Wild-type E. coli strains have the metabolic pathways to produce lactate (d or l) under anaerobic conditions (Fig. 1). Several strategies have been employed to channel the carbon source mainly to d-lactate. Gupta and Clark (1989) reported that adh and pta double mutants regained the ability to grow anaerobically on hexoses by LA fermentation. Further modifications, deleting the native d-lactate dehydrogenase (ldhA) and expressing heterologous genes encoding l-lactate dehydrogenases, allow the production of optically pure l-LA. The main metabolic engineering strategy has been to increase the pool of pyruvate, through the deletion of genes that consume this metabolite in pathways that channel the carbon source to ethanol, succinate, formate and acetate and increasing the expression of genes with lactate dehydrogenase activity (LDH). One of the first attempts to engineer E. coli strains to metabolize glucose to d-LA as the main fermentation product was reported by Chang et al. (1999). The strain produced high amounts of d-LA as result of the deletion of the gene encoding for phosphotransacetylase (pta). However, succinate was also produced in significant quantities. The gen ppc (encoding phosphoenolpyruvate carboxylase) was deleted, to reduce the production of succinate and to generate a homofermentative lactogenic E. coli (strain RR1), that produced 62 g/L of d-LA from glucose and fed-batch cultivation with a volumetric productivity of 1.03 g/L h. The same research group deleted the ldhA gene and overexpressed the ldh of Lactobacillus casei, producing 45 g/L of optically pure l-lactate and a lower volumetric productivity (0.67 g/L h). Remarkably, these reports showed that when the pathways that compete for pyruvate molecules (succinate, acetate, formate and ethanol) are deleted, lactate, either d or l-LA, with an optical purity that exceed 99% can be produced.
For the sake of comparison, Table 5 shows a summary of results reported for several studies reported below, which are related to the metabolic engineering of E. coli and fermentation process conditions to produce d or l-LA. Dien et al. (2001) constructed a non-fermentative strain named ND10 (Δpfl and Δldh). This strain was transformed with a plasmid that overexpressed the ldh gene of Streptococcus bovis to produce optically pure l-LA. The strain named FBR11 fermented 100 g/L of glucose in 30 h with a maximum theoretical yield of 93% l-LA. Furthermore, the genes encoding fumarate reductase (frdABCD) for succinate production, alcohol/aldehyde dehydrogenase (adhE) for ethanol production, and pyruvate formate lyase (pflB) for formate and acetyl-CoA production, were deleted in E. coli W3110, showing that it was possible to reach 45 g/L of d-LA with a yield of 94% and an optical purity of 99% (Zhou et al. 2003a). The effect in the production of d-LA was studied in a two stage fermentation. The first stage was performed under aerobic growth and second stage under anaerobiosis. Through deletions in single and multiple genes in an E. coli this study showed that simple deletions in ackA, pta, pflB, dld, poxB, and frdA genes (Fig. 1) improved yield and productivity of d-LA (dld encodes for an aerobic D-lactate dehydrogenase); however, deletions in ppc and adhE had a negative effect on these parameters. In such strain, with multiple mutations, it was observed that the deletion of pps-ackA-pta had not effect in production. However, the strain with deleted ackA-pta, pps, pflB, dld, poxB and adhE genes, increased the lactate yield, volumetric productivity, and reduced the formation of by-products more than 90%, producing 125 g/L of d-LA from 739.5 g of glucose-mineral medium in a 3 L bioreactor (Zhou et al. 2011).
In the past, most studies for d or l-LA production were focused on combining the deletion of pathways that compete for pyruvate and overexpression of different ldh genes; but in recent years the E. coli metabolic pathways have been rationally modified. To regulate the rates of d-LA overproduction without decreasing the cellular growth, the chromosomal upstream region of the ldhA gene was engineering by predicting possible promoter regions. Sequential shortened chromosomal upstream regions were cloned in a ldhA mutant (Zhou et al. 2012). The clones B0013-080C/pTH-rrnB-ldhA6 and B0013-080C/pTH-rrnB-ldhA8 produced d-LA with high efficiency, due to a putative promoter downstream of the −96 site, whose function could be a transcriptional promoter or regulator.
In strains that have been modified by metabolic engineering, the production of optically pure LA has been increased through the expression of heterologous enzymes or modifications in enzymes with LDH activity. The methylglyoxal bypass is a pathway able to produce l and d-LA starting in the dihydroxyacetone phosphate node, and it is induced by low phosphate concentration and an increase in the dihydroxyacetone pool. It is assumed that at a high glycolytic flux this pathway balances ATP production with cellular growth and metabolism by bypassing the ATP production steps in glycolysis. This pathway was inactivated by deleting mgsA in an E. coli B mutant in pflB, frd, adhE, and ackA (strain SZ194). Adaptive evolution mutated such strain (evolved) (Grabar et al. 2006) and the resultant strain (TG114) showed a yield of 0.98 g d-LA/g glucose. Co-products and chiral impurities were below <0.1%. The native gene ldhA, in strain SZ194, was deleted and replaced with the ldhL gene from Pediococcus acidilactici encoding an l-lactate dehydrogenase, to obtain a strain that produces l-LA (Zhou et al. 2003b). The strain was evolved to improve growth and productivity; however, 5% of d-LA was present as a contaminant of l-LA. To remove this contaminant, the mgsA gene was deleted, obtaining strain TG105, which was able to produce only l-LA. After an additional adaptive evolution process, the strain TG108, derivative of TG105, reached a productivity of 2.7 g/L h of l-LA. This study showed that mgsA is a key gen to produce optically pure d or l-LA.
The d-LA isomer has been mainly produced through metabolic modifications and employing the E. coli native genes. However, as shown above, it is possible to replace the ldh gene to produce optically pure l-LA in metabolic engineered E. coli. Furthermore, Zhou et al. (2003b), through a simple adaptive evolution strategy improved the production of l-LA. The strain SZ79 (Zhou et al. 2003b), derivative of E. coli W3310 (focA-pflB, frdBC, adhE, ackA, ldhA) was constructed, replacing the ldhA coding region with the ldhL of P. acidilactici. However, the production of l-LA was poor. Subsequently, the strain was evolved in mineral medium with 5% of glucose, and before 17 days, samples of the culture were spread in mineral medium plates to select potential mutants. A mutant, named SZ85, exhibited a 30-fold increase in the ldhL activity in comparison to the parental strain SZ79. Sequencing revealed mutations in the coding and terminator regions of ldhL, which are presumed to be responsible for the increased activity in the l-lactate dehydrogenase enzyme. On the other hand, Wang et al. (2011a) revealed that amino acid changes in the glycerol dehydrogenase enzyme (GlyDH) found in Bacillus coagulans generate a protein with the capacity to reduce pyruvate to d-LA. B. coagulans produces l-LA as the main fermentation product, at 50 °C and pH 5, conditions that can be used in a simultaneous saccharification and fermentation process of cellulosic materials. A non-fermentative B. coagulans strain was obtained by deleting the genes ldh and alsS (encoding acetolactate synthase), which are essential for 2,3-butanediol production. This strain was unable to growth at pH 5 in anaerobic conditions. The strain was forced to growth in two phases, first in the presence of oxygen and then under oxygen limitation. This adaptive evolution process generated mutants, which were selected growing under fermentative conditions. A mutant strain named QZ19 produced optically pure d-lactate under anaerobic conditions. Through DNA sequencing, the new enzyme responsible for d-lactate dehydrogenase activity was identified as a mutant in glycerol dehydrogenase (glyDH; D121 N and F245S). GlyDH, in its native form, did not show detectable activity with pyruvate; however, when the mutated gene was cloned and expressed in E. coli, pyruvate reduction activity was detected, allowing the production of d-LA (Wang et al. 2011a, b).
5 Fermentation of Glucose, Xylose and Mixtures to Lactic Acid Using Metabolic Engineered E. coli
In comparison to lactic acid bacteria, E. coli, can consume several carbon sources, this advantage can be employed to use sugars from biomass sources such as lignocellulosic hydrolysates, which are rich in hexoses and pentoses. Glucose and xylose are the main sugars contained in syrups from hydrolysates, but they also contain arabinose, mannose, galactose, and galacturonate that E. coli can metabolize.
Several studies after the year 2000, related to the production of d or l-LA using alternative carbon sources, have employed xylose as the main sugar. The engineered E. coli strain FBR11 (Dien et al. 2001) was grown in xylose, and the l-LA productivity and yield were lower than when the strain was grown in glucose (Table 5), producing 63.3 g/L of l-LA. This work showed that it was possible to produce l-LA from xylose in a complex medium. On the other hand, the evolved strain SZ85, which expresses the ldhL from P. acidilactici integrated into the chromosome, was evaluated in mineral medium with xylose. The volumetric productivity was half of what was observed with glucose, and a yield of 86% of the theoretical maximum was obtained (Zhou et al. 2003b). The low productivity and yield compared to glucose are due mainly to the ATP balance. When the xylose is transported and the pentoses phosphate pathway is employed to metabolize this carbon source, two molecules of ATP are used for transport and pentose phosphorylation. For glucose and using the phosphotransferase system (PTS), the equivalent of one ATP molecule (as phosphoenol pyruvate) is used for both transport and phosphorylation, increasing the energy available for cellular growth. The PTS system is the main transporter of glucose in E. coli, and it participates in catabolic repression. To increase the pool of phosphoenol pyruvate and reduce catabolic repression effects, the strain FBR19 was generated. This strain has deleted the pfl, ldhA, frdABC genes and also the ptsG component of the PTS system, and, as described above, the ldhL from S. bovis was overexpressed in a plasmid (Dien et al. 2002). When the strain FBR19 was evaluated in mixtures of glucose-xylose (50–50 g/L), it reached a productivity of 1.52 g/L h of l-LA, consuming the total glucose and 75% of xylose present in the mixture. This study showed that the catabolite repression was eliminated with the ptsG deletion, being a precedent to use C5-C6 sugar mixtures to produce l-LA.
Another way to increase the availability of ATP for growth and fermentation when xylose is employed as carbon source was shown by Utrilla et al. (2012). These researchers deleted the XylFGH system, i.e. the main xylose transport system in E. coli, which uses one ATP per molecule of transported xylose, and when this sugar is metabolized trough the pentose phosphates pathway, another ATP is used to phosphorylate the C5 derivative of xylose. The strain JU01 (E. coli MG1655 ΔpflB ΔadhE ΔfrdA ΔxylFGH), a lactogenic strain containing the xylFGH deletion was evaluated in mineral medium with xylose (Utrilla et al. 2012). The strain showed a 95% yield of the theoretical maximum and a volumetric productivity of 0.53 g/L h of d-LA. The strain JU01 was subjected to an adaptive evolution process using xylose as selection pressure in mineral medium. After several transfers at two different xylose concentrations (40 and 120 g/L), an evolved strain called JU15 was selected. The d-LA volumetric productivity of JU15 was two-fold higher when compared to JU01 (Table 5). The analysis of the genome sequence of JU15 revealed gene gatC (reported as galactitol transporter) as a xylose transporter, and the mutation S184L in the GatC protein improved the capacity of this protein by increasing the uptake rate of xylose without the use one ATP molecule for xylose transport (Utrilla et al. 2012). To improve the growth under anaerobic conditions and the production of l-LA, the strain SZ470 (ΔfrdBC ΔldhA ΔackA ΔpflB ΔpdhR::pflBp6-acEF-lpd ΔmgsA) was evolved through serial transfers employing complex medium supplemented with xylose. The resulting strain after evolution, named WL204, produced 62 g/L of l-LA from 70 g/L of xylose with a yield of 97% of the theoretical maximum, and an l-LA purity of 99.5%.
These studies showed that metabolically engineered E. coli strains could metabolize xylose to produce d or l-LA with yields near the theoretical maximum. The modifications to increase yields or productivity consuming xylose, included modifications in specific xylose uptake transporters or disruption of the catabolic repression effect and the use of adaptive evolution process to select strain-specific mutations that favor the transport and rate of xylose metabolism to LA. These characteristics are essential to use lignocellulosic hydrolysates which contain mixtures of hexoses and pentoses.
6 Lactic Acid Production from Lignocellulosic Hydrolysates with Metabolic Engineered E. coli
The production of lactate from lignocellulosic hydrolysates have been studied in recent years (Juturu and Wu 2015; Chen et al. 2013). The lignocellulosic hydrolysates contain high concentrations of some toxic compounds such as furfural and 5-hydroxymethyl furfural, and several phenolic compounds, which inhibit growth of E. coli (Martinez et al. 2001). Diverse metabolic engineering strategies have been developed to resist toxicity. The strain XW068 was metabolically engineered to produce d-LA from xylose and glucose present in lignocellulosic hydrolysates and to be resistant to toxicity by furfural (Wang et al. 2011a, b). The strain was mutated in the NADPH-dependent oxidoreductase YqhD and gene fucO was overexpressed, because previously it was demonstrated that a mutation in yqhD and fucO overexpression increases the resistance to furfural in other engineered E. coli strains. Furthermore, this strain was evolved to improve xylose utilization and productivity; the resultant strain XW068 was tested using a xylose-mineral medium with increased furfural concentrations. The strain produced 75.71 g/L of d-LA with a productivity of 1.03 g/L h and a yield of 85% of the theoretical maximum in the presence of 15 mM furfural, showing that the mutation in yqhD and the overexpression of FucO increased the tolerance of E. coli to furfural.
There are two main strategies to improve the fermentation of sugars present in lignocellulosic hydrolysates: (a) reduce the toxic compounds concentration using overliming or another detoxificación method (Martinez et al. 2000); (b) or using non-severe conditions in the pretreatment of the lignocellulosic materials, i.e. avoiding or minimizing the formation of furans and other toxic compounds during the thermochemical pretreatment of biomass (Vargas-Tah et al. 2015; Avci et al. 2013). Diluted acid hemicellulosic hydrolysates from sugar cane bagasse were detoxified using overliming, and d-LA production was tested using strain JU15. From 70 g/L of quantified sugars (xylose, glucose, and arabinose), a productivity of 0.98 g/L h of d-LA was achieved with an apparent yield of 1.11 g d-LA/g of sugars (Utrilla et al. 2016). On the other hand, the strain AV03 (Table 5), a derivative of JU15 (JU15 in ΔpoxB, ΔackA-pta, ΔmgsA) was grown in corn stover hydrolysate that was obtained using non-severe conditions in the pretreatment (Vargas-Tah et al. 2015). Fermentation of these syrups, containing no more than 0.25 g/L of total furans, and using strain AV03 reached 52.2 g/L of d-lactate from a mixture of glucose (42 g/L), xylose (32 g/L) and arabinose (4 g/L), with a productivity of 1.21 g/L h of d-LA and an apparent yield above 100% of the theoretical. In both cases, an apparent high yield was obtained because not all sugars in the hydrolysates were measured, and other carbon sources were also fermented to d-LA. This study showed that it is possible to grow and produce d-lactate from lignocellulosic hydrolysates without a detoxification process. All E. coli strains obtained by metabolic engineering, to produce d or l-lactate, showed yields higher than 80% of the theoretical from different sugars. Such results indicate that the redirection of carbon flux to LA, through the deletion of several genes and strain improvement by adaptive evolution allows an efficient production at cultivation conditions that favor E. coli growth, 37 °C, pH 7.0 under fermentative conditions.
7 Lactic Acid Production from Glycerol and Sucrose with Metabolic Engineered E. coli
Other low-cost substrates that have been used to produce d or l-LA, using metabolically engineered E. coli as a biocatalyst include glycerol, sucrose, and molasses. Glycerol has become an attractive carbon source for the production d or l-LA because now it is inexpensive, abundant and has a higher degree of reduction in comparison to sugars, such as glucose and xylose. Glycerol is generated in large amounts as a by-product of the biodiesel and bioethanol industries (Clomburg and Gonzalez 2013). Also, certain microalgae accumulate significant amounts of glycerol (Oren 2005). However, until a decade ago, glycerol has not been considered as a carbon source for E. coli, because the reported inability of this microorganism to ferment glycerol in the absence of external electron acceptors. Moreover, ten years ago a reassessment of glycerol metabolism by E. coli revealed key metabolic factors for glycerol metabolism in a fermentative and respiratory manner (Dharmadi et al. 2006; Gonzalez et al. 2008; Durnin et al. 2009). The two pathways involved in glycerol dissimilation to the glycolytic intermediate dihydroxyacetone phosphate by E. coli are shown in Fig. 1. Both pathways play a significant role in the conversion of glycerol to the glycolytic intermediate dihydroxyacetone phosphate. Those studies evidenced that microaerobic glycerol metabolism turned out to be very efficiently for ethanol production, hydrogen, and formate in a medium containing mineral salts without rich supplements.
Mazumdar et al. (2010) designed an E. coli strain called LA02 Δdld for the efficient production of d-LA by metabolic engineering strategies. The key points for the design of this strain were blocking the production of enzymes leading to the synthesis of competing by-products and overexpressing the enzymes involved in the conversion of glycerol to d-LA (Fig. 1). To minimize the synthesis of succinate, acetate, and ethanol, metabolites that compete with d-LA synthesis from pyruvate, the inactivation of fumarate reductase (frdA), phosphate acetyltransferase (pta), and alcohol/acetaldehyde dehydrogenase (adhE), were carried out. Also, to prevent d-LA from being metabolized, the aerobic d-lactate dehydrogenase (dld) was interrupted. The approach described above allowed the accumulation of d-LA. The increase of the respiratory GlpK-GlpD pathway induced by the introduction in E. coli LA02 Δdld of the plasmid pZSglpKglpD, resulted in the production, in minimal medium, of 32 g/L of d-LA (with 99.9% of chiral purity) from 40 g/L glycerol. The volumetric and specific rates of d-LA production in E. coli LA02 Δdld (pZSglpKglpD) were 1.5 g/L h and 1.25 g/g cell/h, respectively. The redox balance of this pathway also allows the production of 1–2 mol of ATP per mole of d-LA therefore, generating a feasible metabolic pathway.
For the production of d-LA from sucrose, Shukla et al. (2004) engineered E. coli W3110 to provide the capability to ferment sucrose. The E coli strain KO11 (a metabolically engineered ethanologenic strain that metabolizes sucrose) was used as the source of the sucrose gene cluster. The sucrose gene cluster consists of an operon encoding a repressor protein (cscR), an invertase (cscA), and a bicistronic operon (csCKB), encoding fructokinase and an anion symporter for sucrose. A genomic library was used as a source of genomic DNA, and the genes encoding the sucrose gene cluster were cloned in a plasmid called pLOI3501 and expressed in the strain SZ63. The strain SZ63 (pLOI3501) produced, 568 mM d-LA (in 96 h), from 50 g/L sucrose with a 97% yield of the theoretical yield. Furthermore, a mix of glucose, fructose and sucrose, similar to those encountered in molasses and diluted cane molasses (50 g/L total sugars), was also fermented with SZ63(pLOI3501), reaching a concentration of 540 mM of d-LA in 24 h, with a yield of 94%. The longer time to ferment the same amount of sugars in the actual diluted molasses can be attributed to inhibitors that can be generated during the molasses sterilization or during the sugar production process. Finally, the metabolic byproducts synthesized in cultures in diluted molasses were similar to those produced with pure sugars.
8 Concluding Remarks
Several E. coli strains for the production of optically pure d-LA or l-LA have been generated by employing metabolic engineering and laboratory adaptive evolution strategies. As shown in Table 5, as part of the strain generation strategies, there is a common set of deletions on genes that encode enzymes that divert pyruvate to pathways different from LA. These modifications can be considered the core of a metabolic engineering strategy for LA production with E. coli. Reports with engineered E. coli strains indicate that it is possible to produce with high efficiency optically pure d-LA or l-LA (LA yields form carbon source higher that 90% of the theoretical maximum and volumetric productivities higher than 1 g/L h employing mineral salts media). In addition to the rational metabolic engineering strategies employed for production strain generation, the utilization of adaptive evolution schemes has allowed improvement in LA volumetric productivity from a wide range of carbon sources. Such carbon sources can be found in materials derived from residues of several types of industry. Although efforts towards the generation of E. coli strains that efficiently ferment several carbon sources have been successful, this review evidences scarce reports where actual industrial residue materials are employed. Therefore, it is clear that further efforts in research and development must be made to evaluate production strain with carbon sources that originate from residual material that has been proposed for the generation of bio-comodities. This research should be performed under conditions similar to those required in an industrial setting and include fermentation processes (upstream) as well as LA purification (downstream) and scale-up.
References
Aeschelmann F, Carus M (2015) Bio-based building blocks and polymers in the world—capacities, production and applications: status quo and trends toward 2020-short version. http://www.bio-based.eu/market_study/media/files/15-12-03-Bio-based-Building-Blocks-and-Polymers-in-the-World-short-version.pdf. Accessed 15 July 2016
Avci A, Saha BC, Kennedy GJ et al (2013) Dilute sulfuric acid pretreatment of corn stover for enzymatic hydrolysis and efficient ethanol production by recombinant Escherichia coli FBR5 without detoxification. Bioresour Technol 142:312–319. doi:10.1016/j.biortech.2013.05.002
Carus M, Carrez D, Kaeb H, Ravenstijn et al (2011) Level playing field for bio-based chemistry and materials. http://bio-based.eu/downloads/nova-paper-1-level-playing-field/. Accessed 19 July 2016
CEN (2011) Final report of CEN/BT/WG 209 “Bio-based products”. Avaliable via BioBased Economy. ftp://ftp.cen.eu/CEN/Sectors/List/bio_basedproducts/BTWG209finalreport.pdf. Accessed 26 July 2016
Chang D, Jung H, Rhee J (1999) Homofermentative production of d- or l-lactate in metabolically engineered Escherichia coli RR1. Appl Environ Microb 65:1384–1389
Chen X, Zhou L, Tian K, Kumar A et al (2013) Metabolic engineering of Escherichia coli: a sustainable industrial platform for bio-based chemical production. Biotechnol Adv 31:1200–1223. doi:10.1016/j.biotechadv.2013.02.009
Clark DP (1989) The fermentation pathways of Escherichia coli. FEMS Microbiol Rev 63:223–234
Clomburg JM, Gonzalez R (2013) Anaerobic fermentation of glycerol: a platform for renewable fuels and chemicals. Trends Biotechnol 31:20–28. doi:10.1016/j.tibtech.2012.10.006
Dammer L, Carus M, Raschka A et al (2013) Market developments of and opportunities for biobased products and chemicals. Final report. Available via nova-Institute for Ecology and Innovation http://bio-based.eu/publication-search/?wpv_post_search=Market+developments+of+and+opportunities+for+biobased+products+and+chemicals&wpv_filter_submit=. Accessed 11 July 2016
Datta R, Henry M (2006) Lactic acid: recent advances in products, processes and technologies—a review. Chem Technol Biotechnol 1129:1119–1129. doi:10.1002/jctb
de Jong E, Higson A, Walsh P et al (Not date) Bio-based chemicals—value added products from biorefineries. EA Bioenergy, Task 42 Biorefinery. http://www.iea-bioenergy.task42-biorefineries.com/upload_mm/b/a/8/6d099772-d69d-46a3-bbf7-62378e37e1df_Biobased_Chemicals_Report_Total_IEABioenergyTask42.pdf. Accessed 19 July 2016
Dharmadi Y, Murarka A, Gonzalez R (2006) Anaerobic fermentation of glycerol by Escherichia coli: a new platform for metabolic engineering. Biotechnol Bioeng 94:821–829. doi:10.1002/bit.21025
Dien BS, Nichols NN, Bothast RJ (2001) Recombinant Escherichia coli engineered for production of L-lactic acid from hexose and pentose sugars. J Ind Microbiol Biotechnol 27:259–264. doi:10.1038/sj/jim/7000195
Dien BS, Nichols NN, Bothast RJ (2002) Fermentation of sugar mixtures using Escherichia coli catabolite repression mutants engineered for production of L-lactic acid. J Ind Microbiol Biotechnol 29:221–227. doi:10.1038/sj.jim.7000299
Durnin G, Clomburg J, Yeates Z et al (2009) Understanding and harnessing the microaerobic metabolism of glycerol in Escherichia coli. Biotechnol Bioeng 103:148–161. doi:10.1002/bit.22246
Golden JS, Handfield RB, Daystar J, McConnell TE (2015) An economic impact analysis of the U.S. biobased products industry: a report to the Congress of the United States of America. A Joint Publication of the Duke Center for Sustainability & Commerce and the Supply Chain Resource Cooperative at North Carolina State University. https://www.biopreferred.gov/BPResources/files/EconomicReport_6_12_2015.pdf. Accessed 11 Aug 2016
Gonzalez R, Murarka A, Dharmadi Y et al (2008) A new model for the anaerobic fermentation of glycerol in enteric bacteria: trunk and auxiliary pathways in Escherichia coli. Metabol Eng 10:234–245. doi:10.1016/j.ymben.2008.05.001
Grabar TB, Zhou S, Shanmugam KT et al (2006) Methylglyoxal bypass identified as source of chiral contamination in l(+) and d(-)-lactate fermentations by recombinant Escherichia coli. Biotechnol Lett 28:1527–1535. doi:10.1007/s10529-006-9122-7
Gupta S, Clark DP (1989) Escherichia coli derivatives lacking both alcohol dehydrogenase and phosphotransacetylase grow anaerobically by lactate fermentation. J Bacteriol 171:3650–3655
Gupta B, Revagade N, Hilborn J (2007) Poly (lactic acid) fiber: an overview. Prog Polym Sci 32:455–482. doi:10.1016/j.progpolymsci.2007.01.005
Henton DE, Gruber P, Lunt J et al (2005) Polylactic acid technology. In: Mohanty AK, Misra M, Drzal LT (eds) Natural fibers, biopolymers, and biocomposites. CRC Press, Boca Raton, pp 527–578
John RP, Anisha GS, Nampoothiri KM et al (2009) Direct lactic acid fermentation: focus on simultaneous saccharification and lactic acid production. Biotechnol Adv 27:145–152. doi:10.1016/j.biotechadv.2008.10.004
Juturu V, Wu JC (2015) Microbial production of lactic acid: the latest development. Crit Rev Biotechnol 36:967–977. doi:10.3109/07388551.2015.1066305
Lammens TM, Potting J, Sanders JPM et al (2011) Environmental comparison of biobased chemicals from glutamic acid with their petrochemical equivalents. Environ Sci Technol 45(19):8521–8528. doi:10.1021/es201869e
Lim L, Auras R, Rubino M (2008) Progress in polymer science processing technologies for poly (lactic acid). Prog Polym Sci 33:820–852. doi:10.1016/j.progpolymsci.2008.05.004
Liu H, Kang J, Qi Q et al (2011) Production of lactate in Escherichia coli by redox regulation genetically and physiologically. Appl Biochem Biotechnol 164:162–169. doi:10.1007/s12010-010-9123-9
Mäki-Arvela P, Simakova IL, Salmi T et al (2014) Production of lactic acid/lactates from biomass and their catalytic transformations to commodities. Chem Rev 114:1909–1971. doi:10.1021/cr400203v
Martinez A, Rodríguez ME, York SW et al (2000) Effects of Ca(OH)2 treatments (“Overliming”) on the composition and toxicity of bagasse hemicellulose hydrolysate. Biotechnol Bioeng 69:526–536. doi:10.1002/1097-0290(20000905)69:5<526:AID-BIT7>3.0.CO;2-E
Martinez A, Rodríguez ME, Wells ML et al (2001) Detoxification of dilute acid hydrolysates of lignocellulose with lime. Biotechnol Progress 17:287–293. doi:10.1021/bp0001720
Mazumdar S, Clomburg JM, Gonzalez R (2010) Escherichia coli strains engineered for homofermentative production of d-lactic acid from glycerol. Appl Environ Microbiol 76:4327–4336. doi:10.1128/AEM.00664-10
Nampoothiri KM, Nair NR, John RP (2010) An overview of the recent developments in polylactide (PLA) research. Bioresour Technol 101:8493–8501. doi:10.1016/j.biortech.2010.05.092
Okano K, Tanaka T, Ogino C et al (2010) Biotechnological production of enantiomeric pure lactic acid from renewable resources: recent achievements, perspectives, and limits. Appl Microbiol Biotechnol 85:413–423. doi:10.1007/s00253-009-2280-5
Oren A (2005) A hundred years of Dunaliella research: 1905–2005. Saline Syst 1:2. doi:10.1186/1746-1448-1-2
Orencio-Trejo M, Utrilla J, Fernández-Sandoval MT et al (2010) Engineering the Escherichia coli fermentative metabolism. Adv Biochem Eng Biotech 121:71–107. doi:10.1007/10_2009_61
Schut JH (2008) PLA biopolymers-new copolymers, expandable beads, engineering alloys & more. Plast Technol 66–69
Shukla VB, Zhou S, Yomano LP et al (2004) Production of d(-)-lactate from sucrose and molasses. Biotechnol Lett 26:689–693. doi:10.1023/B:BILE.0000024088.36803.4e
Tsao GT, Cao NJ, Du J (1999) Production of multifunctional organic acids from renewable resources. In: Tsao GT (ed) Advances in biochemical engineering/biotechnology, vol 65. Springer, Berlin, pp 243–280
Utrilla J, Gosset G, Martinez A (2009) ATP limitation in a pyruvate formate lyase mutant of Escherichia coli MG1655 increases glycolytic flux to d-lactate. J Ind Microbiol Biotechnol 36:1057–1062. doi:10.1007/s10295-009-0589-9
Utrilla J, Licona-Cassani C, Marcellin E et al (2012) Engineering and adaptive evolution of Escherichia coli for d-lactate fermentation reveals GatC as a xylose transporter. Metab Eng 14:469–476. doi:10.1016/j.ymben.2012.07.007
Utrilla J, Vargas-Tah A, Trujillo-Martínez B et al (2016) Production of d-lactate from sugarcane bagasse and corn stover hydrolysates using metabolic engineered Escherichia coli strains. Bioresour Technol . doi:10.1016/j.biortech.2016.08.067
Vargas-Tah A, Moss-Acosta CL, Trujillo-Martinez B et al (2015) Non-severe thermochemical hydrolysis of stover from white corn and sequential enzymatic saccharification and fermentation to ethanol. Bioresour Technol 198:611–618. doi:10.1016/j.biortech.2015.09.036
Vijayakumar J, Aravindan R, Viruthagiri T (2008) Recent trends in the production, purification and application of lactic acid. Chem Biochem Eng Q 22(2):245–264
Vijayendran B (2010) Bio products from bio refineries-trends, challenges and opportunities. J Bus Chem 7:109–115
Vishnu C, Seenayya G, Reddy G (2000) Direct conversion of starch to l(+)-lactic acid amylase producing Lactobacillus amylophilus GV6. Bioprocess Eng 23:155–158. doi:10.1007/PL00009119
Wang Q, Ingram LO, Shanmugam KT (2011a) Evolution of D-lactate dehydrogenase activity from glycerol dehydrogenase and its utility for D-lactate production from lignocellulose. Proc Natl Acad Sci U S A 108:18920–18925. doi:10.1073/pnas.1111085108
Wang X, Miller EN, Yomano LP et al (2011b) Increased furfural tolerance due to overexpression of NADH-dependent oxidoreductase FucO in Escherichia coli strains engineered for the production of ethanol and lactate. Appl Env Microbiol 77:5132–5140. doi:10.1128/AEM.05008-11
Weber CJ, Haugaard V, Festersen R et al (2002) Production and applications of biobased packaging materials for the food industry. Food Addit Contam 19:172–177. doi:10.1080/0265203011008748
Wee Y, Kim J, Ryu H (2006) Biotechnological production of lactic acid and its recent applications. Food Technol Biotechnol 44:163–172
Yang YT, San KY, Bennett GN (1999) Redistribution of metabolic fluxes in Escherichia coli with fermentative lactate dehydrogenase overexpression and deletion. Metab Eng 1:141–152. doi:10.1006/mben.1998.0111
Zhao J, Xu L, Wang Y et al (2013) Homofermentative production of optically pure L-lactic acid from xylose by genetically engineered Escherichia coli B. Microb Cell Fact 12:57. doi:10.1186/1475-2859-12-57
Zhou S, Causey TB, Hasona A et al (2003a) Production of optically pure D-lactic acid in mineral salts medium by metabolically engineered Escherichia coli W3110. Appl Environ Microbiol 69:399–407. doi:10.1128/AEM.69.1.399-407.2003
Zhou S, Shanmugam KT, Ingram LO (2003b) Functional replacement of the Escherichia coli D-(-)-Lactate dehydrogenase gene (ldhA) with the L-(+)-lactate dehydrogenase gene (ldhL) from Pediococcus acidilactici. Appl Env Microbiol 69:2237–2244. doi:10.1128/AEM.69.4.2237
Zhou L, Zuo Z-R, Chen X-Z et al (2011) Evaluation of genetic manipulation strategies on D-lactate production by Escherichia coli. Curr Microbiol 62:981–989. doi:10.1007/s00284-010-9817-9
Zhou L, Shen W, Niu DD et al (2012) Fine tuning the transcription of ldhA for d-lactate production. J Ind Microbiol Biotechnol 39:209–1217. doi:10.1007/s10295-012-1116-y
Zhu J, Shimizu K (2004) The effect of pfl gene knockout on the metabolism for optically pure D-lactate production by Escherichia coli. Appl Microbiol Biotechnol 64:367–375. doi:10.1007/s00253-003-1499-9
Zhu Y, Eiteman MA, DeWitt K et al (2007) Homolactate fermentation by metabolically engineered Escherichia coli strains. Appl Environ Microbiol 73:456–464. doi:10.1128/AEM.02022-06
Acknowledgements
This work was supported by the Mexican National Council for Science and Technology (CONACYT-Mexico), FONCICYT ERANet-LAC Grant C0013-248192.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Martinez, A., Rodríguez-Alegría, M.E., Fernandes, M.C., Gosset, G., Vargas-Tah, A. (2017). Metabolic Engineering of Escherichia coli for Lactic Acid Production from Renewable Resources. In: Gosset, G. (eds) Engineering of Microorganisms for the Production of Chemicals and Biofuels from Renewable Resources. Springer, Cham. https://doi.org/10.1007/978-3-319-51729-2_5
Download citation
DOI: https://doi.org/10.1007/978-3-319-51729-2_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-51728-5
Online ISBN: 978-3-319-51729-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)