Abstract
Catharanthus roseus plant is valued for harboring more than 130 bioactive terpenoid indole alkaloids (TIAs) including the two of its leaf derived bisindole alkaloids—vinblastine and vincristine which are indispensible constituents of anti-neoplastic drugs used in metastatic malignancy associated with acute lymphoblastic leukaemia’s and Hodgkin’s/Non-Hodgkin’s lymphomas. The extremely low in planta occurrence of TIAs in C. roseus plants resulting in high commercial demand and exorbitant price have brought this herb in focus of an intense scientific scrutiny in last 30 years. Research efforts have so far advanced in two major directions: towards understanding the enzymology and genetic regulation of the concerned metabolic pathway(s) leading to TIAs biosynthesis in plant and; secondly, exploring the possibility of developing cell/tissue culture based platforms for in vitro TIAs production to meet the industry’s demand. Designing plants, free from such metabolic constraints, can be a possible approach to enhance the production of plant based medicines. This subject of plant metabolic engineering is gaining lot of attention these days. Pathway manipulation using the modern tools of genetic engineering to over-express a limiting enzyme or to suppress the expression of an enzyme using a shared substrate of a branched pathway are attractive options of metabolic engineering for diverting the metabolic flux towards the synthesis of a desired end product. Knowledge, thus gained, indicates that TIAs biogenetic route is characterized by extensive metabolic cross-talk and shuttling of at least 35 intermediates synthesized via 30 enzymatic reactions occurring in four different types of tissues (epidermis, internal phloem parenchyma, idioblasts and leticifers) and five different sub-cellular compartments (cytosol, vacuole, thyllakoid membrane, nucleus and endoplasmic reticulum). The complexity is further compounded by extremely high level of recalcitrancy of C. roseus plant for regeneration and Agrobacterium-mediated genetic transformation for pathway engineering. As a consequence, all genetic modulation efforts so far made in C. roseus are confined to cell suspension and transformed hairy root cultures that lack the required level of cyto- and tissue-differentiation essential for the expression of entire TIAs pathway genes and enzymes. A perusal of published work in C. roseus clearly suggests that inspite of several pathway manipulation/engineering attempts, the level of TIAs production in cell/tissue/hairy root cultures of this herb could never be enhanced to the level of expectations. The enzymatic, developmental and environmental rigidity/complexities associated with the biosynthetic pathway of these alkaloids have often been cited as possible reasons for these disappointing outcomes Therefore, three major areas of investigation are in focused attention of Catharanthus researchers’ the world over are: (1) how to select or design the starting cells or tissue(s) to realize the full potential of applying metabolic engineering tools for up-regulating the TIAs pathway in them; (2) how to overcome the strong recalcitrancy of Catharanthus plant tissues for de novo organogenesis and in vitro plant regeneration for whole plant-level expression of a transgene coding either for a limiting pathway enzyme or a transcription factor that can control the global expression of several pathway genes and; (3) how to overcome the inability of non-differentiated cell cultures to execute those pathway steps that are expressed only in specialized tissues/cells of C. roseus plants. Various biotechnological approaches and generation of novel tissue types have been discussed in the present chapter for the modulation and increased TIAs flux in C. roseus.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Plants have been serving as an extremely valuable source for treatments and therapies since human existence. Thousands of plant species are used as medicines and most of the world population uses them to cure the acute and chronic health problems. Many of the present day modern medicines in the western world have been developed on the basis of our ancient knowledge of traditional plant-based medicines. However, the receptors and mechanisms of their action in human body are being identified only recently. Plants produce a large number of low-molecular weight organic compounds that appear to have little function in plants’ own growth and development. These compounds, designated as “secondary metabolites”, however perform several diverse ecological functions in plant’s defense responses against pathogens, herbivores UV radiations and other environmental extremities (Zhong 2001). Many of these plant secondary metabolites have also been found to possess important biological activities of pharmacological importance for humans. Therefore, intensive studies have been carried out during last six decades to characterize the nature of plant secondary metabolites from the viewpoints of their chemical structures, biosynthesis, bio-activities and clinical utilities.
Attempts to produce these plant based molecules by synthetic chemistry route have largely been found unfruitful and uneconomical due to their complex chirality. Hence, entire commercial demand for several important drug molecules like paclitaxel, vincristine, vinblastine, podophyllotoxin, camptothecin, ergot, digoxin, artemisinin, pilocarpine, morphine, reserpine, hypericine, theophylin etc. is still being met from their source plants (Verma et al. 2012a; Zhong 2001). As majority of the plant secondary metabolites are synthesized in very low amount in a season- and age-dependent manner their agri-based production is often less than the size of their clinical demands resulting in high market cost (Verma et al. 2012a, 2015a). The tools of modern day plant biotechnology are, therefore, finding increasing application to fill these gaps. These advancements are collectively studied under the umbrella of a new subject discipline called ‘metabolic engineering” (Verpoorte and Alfermann 2000). Metabolic engineering is broadly referred to the directed improvement of cellular properties through the modification of a specific biochemical reaction(s) or the introduction of new enzymatic capability in a plant cell, with or without the use of recombinant DNA technology. There are three basic goals of metabolic engineering in plants: more production of a specific desired molecule, less production of a specific unwanted compound, and the production of a novel compound (i.e. a molecule that is produced in nature, but not usually in the target host plant). Strategies adopted for achieving these goals may include the engineering of single steps in a biosynthetic pathway to increase the metabolic flux towards a target compounds, blocking of competitive pathways at branch points of a shared substrate/intermediates, or to introduce short cuts that divert the metabolic flux towards a different route. In addition to these specifically directed gene insertion approaches, strategies like selection of plant genotype with increased density of metabolite producing or accumulating cell types, reduced catabolism of desired molecule, cells with increased availability of precursor molecules drawn from primary metabolite pools and chemical/biotic elicitation of regulatory elements (transcription factors) of a pathway etc. are also studied under the subject of metabolic engineering. The single gene manipulation has only limited value, because the effects of modulating single enzymatic steps are often absorbed by the system in an attempt to restore homeostasis. On the other hand, targeting the multiple steps in the same pathway could help in controlling metabolic flux in a more predictable manner but it is difficult to achieve (Verpoorte and Alfermann 2000). This would involve up-regulating several consecutive enzymes in a pathway or up-regulating enzymes in one pathway while suppressing those in another competing pathway, or using regulatory genes (transcription factors) to establish multipoint control over one or more genes of a given pathway (Verpoorte and Alfermann 2000).
These basic considerations of a plant metabolic engineering programme invariably require the ready availability of several metabolic tools, techniques and material to reach to a successful outcome (Verpoorte and Alfermann 2000). These may include: (1) identification and cloning of a target gene; (2) preparation of a suitable gene construct; (3) development of an efficient method of gene insertion into the host cell; (4) optimization of the regeneration protocol to recover plants from transformed tissue; (5) molecular and functional characterization of transgene to ensure its stable expression; (6) transfer of introduced gene to elite cultivars through conventional breeding, if required and; (7) evaluation of transgene expression for the envisaged task in the backdrop of bio-safety considerations.
Catharanthus roseus—The Madagascar periwinkle plant has a well-documented history of its use in the treatment of several physiological and metabolic disorders, particularly the various types of neoplasmas (Johnson et al. 1963). In traditional systems of medicines, the leaf, seed, flower and root of this plant are frequently used for treating diabetes, hypertension, menorrhagia and tumor growth. In modern systems of medicines the plant is commercially valued for the strong anti-neoplastic efficacy of two of its leaf-derived dimeric terpenoid indole alkaloids (TIAs) namely vincristine and vinblastine and two root-derived monomeric alkaloids—ajmalicine and serpentine that are in wide clinical usage to treat hypertension and other circulatory disorders (Verpoorte et al. 1997, 2002; Van der Heijden et al. 2004; Duarte et al. 2010). Catharanthus alkaloids are chemically classified as “terpenoid indole alkaloids (TIAs)” or “monoterpene indole alkaloids (MIAs)” because of the presence of an indole ring attached to a terpenoidal skeleton (El-Sayed and Verpoorte 2007). Among all the TIAs present in C. roseus plant, maximum clinical attention from a pharmacological point of view has been drawn by vincristine (VCR) and vinblastine (VLB) that occupy an indispensible place in most of the chemotherapy treatments against Hodgkin’s lymphomas, childhood leukaemia, lymphosarcoma, neuroblastoma, and carcinoma of breast and lungs (Neuss and Neuss 1990; Arora et al. 2010). VLB and VCR, sold in the market by the name of velban and oncovin respectively, have so far defied the rules of synthetic chemistry for their chemical synthesis on a commercially and economically viable scale due to their multifaceted structural chirality (Hughes and Shanks 2002) and hence, C. roseus plants represent the sole bio-resource for their production; albeit in very low amount (Kumar et al. 2007). The Catharanthus alkaloids manifest their anti-cancerous activity by arresting tumor cell proliferation by binding to tubulin (“end-capping effect”) and thereby disrupting the spindle assembly (Verma et al. 2012a). Both VCR and VLB are M-phase cell cycle-cycle mitotic inhibitors and are effective at sub-micromolar concentration (10nM–1μM). At higher concentration (>10 μM) they cause tubulin aggregation and results in the formation of tubulin para-crystals (Foye 1995). Due to the lack of cross confrontation with DNA-alkylating drugs, these alkaloids are preferentially included into the combination chemotherapy regimens (Van Der Heijden et al. 2004). Because of their strong cyto-toxic nature the C. roseus plant produces these drug molecules in extremely low amounts (<0.0002%) as a part of its auto-defense mechanism that results in high cost of extraction. Nearly 500–750 kg of dried leaves are required to yield just 1.0 g of VLB.
Vincristine, vinblastine and their derivatives form an essential ingredient of chemotherapy regimens for cancer treatment. They are either used singly or in combination with other medicines. All TIAs in clinical uses are administered intravenously and they are eventually metabolized in liver. Peripheral neuropathy, excessive hair loss, hyponatremia and constipation are some of the harmful side effects associated with these drug molecules. Some of the semi-synthetic derivatives of VLB and VCR such as vinorelbine and vinflunine have been developed to improve their therapeutic index with minimum side effects (Wilson et al. 1999). Both of these derivatives have been found useful in the treatment of non-small cell lung and metastatic breast and bladder cancers (Dipierre et al. 1991; Bennouna et al. 2006; Mano 2006). These alkaloids are frequently incorporated into combination chemotherapy because of their lack of cross resistance with DNA-alkylating drugs and their different mode of action (Van Der Heijden et al. 2004). Some of the clinical attributes of four of the most important TIAs found in Catharanthus roseus plants are described below:
-
VINBLASTINE (C46H56N4O9): Vinblastine (VLB) is the generic name of the alkaloid formerly known as vincaleukoblastine. It is a colorless compound while its sulphate derivative is slightly yellow and hygroscopic. It is soluble in water and methanol. In plants VLB is formed as a chemical analogue of vincristine. It is an integral part of several chemo-therapeutic formulations including the most famous ABVD (Adriamycin, Bleomycin, VLB, Decarbazine) regimen which is highly effective in the treatment of metastatic testicular cancer, Kaposi’s sarcoma and breast carcinoma. VLB can be safely stored at room temperature in an inert environment and is clinically administered in encapsulated form in multilamellar liposomes.
-
VINCRISTINE (C46H56N4O10): Vincristine (VCR), also known as leurocristine, is sold under the brand name Oncovin after it was approved by FDA for clinical use in 1963 (Farnsworth 1985). In most commercial preparations VCR appears as a colorless fluid. VCR binds to tubulin dimer and disturbs the microtubule structure which in turn arrests mitosis at metaphase stage. It affects all rapidly dividing cell types including intestinal epithelium and bone marrow. VCR is an essential constituent of drug formulations like CHOP (Cyclophosphhamide, Hydroxydoxorubicin, VCR, Prednisone) and MOPP (Mechlo-rethamine, VCR, Procarbazine, Prednisone) that are strong anti-neoplastic regimens against non-Hodgkin’s lymphoma, Hodgkin’s lymphoma and lymphoblastic leukemia.
-
VINDESINE (C43H55N5O7): Vindesine (VDS) is commercially available as white powder with the trade name Eldisine or Fildesin. It is particularly effective against melanoma, lung cancer, breast cancer and uterine cancers.
-
VINORELBINE (C46H58N4O9): Vinorelbine (VNLB, VRL) was the first semi-synthetic derivative made with vindoline and catharanthine that reached the drug market in the name of Nevelbine). It is a colorless fluid with least side effects amongst all TIAs-based anti-cancer regimens. It is most effective against cell lung cancers.
The extremely low in planta occurrence of TIAs in C. roseus plants resulting in their short supply, high commercial demand and exorbitant price have brought them in focus of intense scientific scrutiny in last 30 years with sole intention of increasing their production. As a consequence, research in this area has proceeded in two major directions. Firstly, towards the understanding of their biosynthesis in plant, including the enzymology and genetic regulation of the concerned metabolic pathway(s) and secondly, towards exploring the possibility of developing cell/tissue culture based platforms for in vitro TIAs production. The wealth of information gathered so far has made TIAs biogenetic pathway as one of the most well dissected and understood metabolic routes at the level of enzymes and corresponding genes in plants.
2 Metabolic Engineering and Emergence of C. roseus as Model System for In Vitro Alkaloid Pathway Modulation
In a nut shell plant metabolic engineering takes into account the understanding of the architecture of a metabolic pathway to identify the major regulatory/limiting steps and then try to overcome these limitation by various biological engineering tools, including the omics approaches. Three conditions must be satisfied in a plant (cell) before a significant increment in the yield of a desired metabolite can be expected to occur. They are: (1) assured supply of starting precursor(s) and/or limiting intermediates, particularly those that lie at the interface of primary and secondary metabolisms; (2) optimal induction of genes/enzymes of an intended pathway at the right time and right place and; (3) availability of a suitable sink to store the synthesized product. Though initial two of these considerations are key to the success of any pathway modulation effort, better understanding of the dynamics of storage and catabolism of a targeted molecules within the plant/cell not only add towards its final productivity but also help in choosing the most suitable downstream process for its extraction and recovery.
Our understanding of metabolic pathway networks in plants, the enzymology and the genes involved at various steps of the metabolite flux within and between the pathways and, the temporal and spatial distribution of a given metabolite(s) within the plant body or its sub-cellular components has now reached to a level where engineering of a target pathway for obtaining higher yields of a given phytomolecule in homo- to hetero-logous expression systems is becoming a theoretical reality. Plant alkaloids (that have provided the maximum numbers of drug molecules to the pharma world), anthocyanins, terpenes and glycosides are occupying the centre stage of these ongoing metabolic engineering efforts. Plant cell and tissue culture techniques are integral components of these engineering enterprises in two major ways: (1) either the cultured tissues are being used as an alternate production platform as such or, (2) they are being used to provide requisite interface for genetic engineering for the hyper-expression of a limiting metabolic step or for the silencing of a branch point sub-way to block the diversion of a limiting pathway intermediate towards non-productive route. Cell or tissue cultures with a congenial physiological and biochemical backgrounds for the expression of a given pathway to produce a desired metabolite are also proving useful in avoiding other issues associated with their agri-based production such as: restricted geographical availability of source plant; seasonal fluctuations in yield; minimization of batch-to-batch variation in quality; avoidance of neighboring cell interference and complications related to long distant transport and segregation of metabolic pools; prolongation of pathway steps that occur for very short time in plants: easy up-scaling in bioreactor and; lesser complications in extractions and industrial downstream processing. Figure 1 shows a spectrum of in vitro approaches that have potential application in enhancing the TIAs flux in C. roseus.
Agri-based production of Catharanthus alkaloids for pharma industry is highly expensive because of their low in planta occurrence leading to very high extraction cost. With a current market price vincristine and vinblastine represents some of the least abundant and most expensive plant natural products in use in the drug sector today. The great pharmaceutical value of C. roseus as a powerful anti-cancerous herb on one hand and high market price and clinical demand of its alkaloids on the other hand, has brought it under intensive scientific scrutiny since 1950s. The initial research efforts made in this plant species were more focused towards elucidation of the TIAs pathway, enzymology associated with their biogenesis and understanding of their pharmacology (Svoboda and Blake 1975; Cordell 1980; Ganapathi and Kargi 1990; Verpoorte et al. 1991, 1993). Research in last two decades has been more directed towards understanding the genetic and developmental regulation of TIAs synthesis and accumulation with an aim to boost their in vitro production in cell, tissue and organ cultures using modern tools of biotechnology (Meijer et al. 1993; Moreno et al. 1995; Verpoorte et al. 1997; St-Pierre et al. 1999; Di Flore et al. 2004; van der Heijden et al. 2004; Seth and Mathur 2005; Mahroug et al. 2006, 2007; Pasquali et al. 2006; Rischer 2006; Shukla et al. 2006, 2010; El-Sayed and Verpoorte 2007; Pietrosuik et al. 2007; Zarate and Verpoorte 2007; Zhao and Verpoorte 2007; Facchini and De Luca 2008; Shukla et al. 2010; Verma and Mathur 2011a, b; Verma et al. 2012a, b, 2013). The wealth of information pertaining to synthesis and regulation of TIAs biogenesis that has gathered in C. roseus, together with its universal occurrence, shorter breeding cycle, low chromosome number, amenability to cell culture approaches and, above all high commercial interest have made this plant a model system for pathway engineering efforts (Zarate and Verpoorte 2007; Facchini and De Luca 2008).
The insights regarding TIAs metabolism in C. roseus has prompted several workers to apply various metabolic engineering approaches to boost their production in cells, tissues and organ cultures in vitro. These developments are advancing in three major directions. These are: (1) selection of high yielding cultures and their growth and production optimizations following precursor feeding and biotic/abiotic elicitations; (2) Standardization of protocols for deliberate pathway gene insertion for hyper-expression of a limiting enzyme; (3) employment of transgenic hairy root cultures and their bioreactor up-scaling for metabolite production (Leckie et al. 1991; Fulzele and Heble 1994; Hirata et al. 1994; Hughes et al. 2004; ten Hoopen et al. 1994; Schlatmann et al. 1995a, b; Canel et al. 1998; van der Fits et al. 2001; Zhao and Zhu Wei-Hua 2001a, b; Whitmer et al. 2002b; Di Flore et al. 2004; Choi et al. 2004; Pasquali et al. 2006; Pietrosuik et al. 2007; Zarate and Verpoorte 2007; Zhao and Verpoorte 2007; Guirimand et al. 2009; Verma and Mathur 2011a, b; Verma et al. 2012a, b, 2013).
Following major gaps in C. roseus research were therefore identified that has been answered through the in vitro biotechnological interventions in recent pasts
-
Majority of pathway modulation work in C. roseus has been carried out with wild type cells and tissue types. Very little attention was paid to generate mutant cell/tissue types with ideal physiological back-ground for pathway modulation, particularly with respect to adequate availability of TIAs precursors drawn from shikimate and terpenoid pathways of primary metabolic pools. Tryptophan availability in particular is crucial because of its least abundance in plant cells and higher demand for synthesis of auxins for growth-sustaining metabolic functions. Failure of efforts to get desired outcomes with exogenous feeding of these precursors in wild type cells might have been a consequence of their inability to accommodate these molecules in cells due to their strong feedback inhibitory actions on other metabolic pathways.
-
Inability of in vitro grown heterotrophic cells/tissue cultures to express NMT enzyme involved in vindoline synthesis from tabersonine because of the absence of a functional chloroplast system under heterotrophic mode of in vitro growth. Efforts to select photo-autotrophic cells were not made.
-
Absence of D4H and DAT transcripts in undifferentiated cell or hairy root cultures because of lack of special laticiferous and idioblast tissues.
-
Poor sink and holding capacity of cells/tissues to store anti-mitotic bisindole alkaloids VLB and VCR.
-
Lack of direct plant regeneration protocols to facilitate whole-plant expression of engineered pathway genes following genetic transformation.
-
Limited know-how’s on bioreactor designing and operation for C. roseus cells and hairy root tissues.
-
Limited understanding of transcription factors and transporter proteins associated with TIAs biosynthesis.
-
Lack of sufficient knowledge on catabolism of TIAs in cells
3 Terpenoid Indole Alkaloids Pathway: A Snap Shot
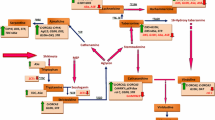
The biosynthesis of MIAs in C. roseus typically represents the elegant complexities of a plant secondary metabolic pathway. The entire multi-step pathway rigidly follows a complex and highly compartmentalized metabolic route which is strictly regulated by several developmental and environmental factors (Fig. 2). From a holistic perspective, the biosynthesis of TIAs in C. roseus proceeds via 30 co-ordinately regulated enzymatic steps involving at least 35 known intermediates (van der Heijden et al. 2004; Facchini and De Luca 2008). Consequently, 30 biogenetic and two regulatory genes have also been identified for their close association with this pathway. Out of a total of 42 cDNA clones identified in major TIAs producing plants, 25 belong to C. roseus (Guirimand et al. 2010). The entire pathway has been shown to precede with involvement of four discrete cell/tissue types (epidermis, internal phloem parenchyma, idioblasts) and participation of five intra-cellular compartments namely cytosol, vacuole, thylakoid membrane, nucleus, and endoplasmic reticulum (De Luca and Cutler 1987; Facchini 2001; El-Sayed and Verpoorte 2007; Mahroug et al. 2007; Facchini and De Luca 2008; Verma et al. 2012a). All TIAs are biosynthesised from a central precursor molecule- strictosidine, which is a condensation product of an indole ring donor tryptamine and a terpenoid moiety donor secologanin. The tryptamine and secologanin undergo a condensation reaction to form strictosidine in the cell vacuole. Strictosidine that heralds the first indication of a switch over of carbon flux from primary to secondary metabolism during TIAs synthesis. Strictosidine, the universal precursor of all subsequent MIAs is further de-glucosylated by the enzyme strictosidine β-glucoxidase to form an unstable aglycon that give rise to cathenamine which is a branch point intermediate that can be routed towards the synthesis of several different types of monomeric alkaloids like catharanthine, ajmalicine, and tabersonine. While ajmalicine is further converted into serpentine in the vacuoles by the action of peroxidase, tabersonine is either converted into lochnericine and then to horhammericine in roots or is diverted towards catharanthine synthesis via dehydrogeissoschizine and stemmadenine as major intermediates.
TIAs pathway operating in C. roseus. TDC tryptophan decarboxylase, G10H geraniol-10-hydroxylase, SLS secologanin synthase, STR strictosidine synthase, SGD strictosidine β glucosidase, T16H tabersonine 16-hydroxylase, 16 OMT 16-hydroxytabersonine-16-O-methyltransferase, NMT N-methyltransferase, D4H deacetoxyvindoline-4-hydroxylase, DAT deacetylvindoline 4-O-acetyltransferase, T6,7E tabersonine-6,7-epoxidase, T19H tabersonine 19 hydroxylase, MAT minovincinine-19-O-acetyltransferase, CR cathenamine reductase, PRX1 peroxidase, THAS tetrahydroalstonine reductase
While very little is known about the catharanthine sub-way (Loyola-Vargas et al. 2007), the vindoline route is fairly well dissected at the level of associated enzymes and genes (Schroder et al. 1999; St-Pierre and De Luca 1995; Vazquez-Flota et al. 1997; St-Pierre et al. 1999; Laflamme et al. 2001). Catharanthine has been shown to be exclusively excluded from epidermis and stored in surface wax layer of the leaves (Roepke et al. 2010) as a defense strategy against insect and microbial infestation. Tabersonine is also converted into vindoline in leaves through seven well-characterized enzymatic steps (Dethier and De Luca 1993; Vazquez-Flota et al. 1997; Luca and Laflamme 2001; Pasquali et al. 2006; Campous-Tamayoet al. 2008; Pan et al. 2015). The NMT is the only enzyme of this pathway whose activity is not affected by light, whereas D4H transcript abundance increased significantly upon eposing the plants to light (St. Pierre et al. 1998, Vasquez-Flota and De Luca 1998). Diversion of flux from cathenamine-derived intermediate tabersonine to vindoline is facilitated by seven enzymatic steps in the aerial tissues of C. roseus plants. The biochemistry of this seven-step route involves three hydroxylations and one each of O-methylation, N-methylation and O-acetylation reactions. The tabersonine to 16-hydroxy tabersonine conversion is catalyzed by a cytochrome P450-dependent tabersonine-16-hydroxylase (T16H) enzyme, followed by its methylation by 16-hydroxytabersonine-16-O-methyl transferase (16OMT) which also requires 5-adenosyl-l-methionine as a co-substrate (Levac et al. 2008). The conversion of 16-methoxy tabersonine to 16 methoxy-2, 3-dihydrotabersonine then occurs via an uncharacterised oxidation step. The subsequent step involves a thyllakoid associated N-methyltransferase (NMT) to obtain deacetoxyvindoline. The last two biogenetic reactions are catalyzed by a light-regulated deacetoxyvindoline-4-hydroxylase (D4H) and deacetylvindoline-4-O-acetyltransferase (DAT) enzymes that are expressed only in special idioblast/laticifer cells in leaves. (De Carolis et al. 1990; St-Pierre et al. 1999; Campous-Tamayo et al. 2008; Shukla et al. 2010; Guirimand et al. 2011). In contrast to aerial tissues, the roots of C. roseus plants operate another sub-way from tabersonine to lochnericine by the catalytic action of tabersonine-6, 7 epoxidase (T6,7E; Rodriguez et al. 2003). Lochnericine can then be converted into horhammericine by tabersonine 19-hydroxylase. Alternatively, tabersonine can also be routed towards horhammericine via 6,7 dehydrominovincine and both minovincinine and/or horhammericine can then be acetylated to yield 19-O-acetylhorhammericine by the enzyme minovincinine 19-hydroxy-O-acetyl transferase (MAT) which is localized in cortical cells of growing root tips. Finally, the coupling of monomeric TIA catharanthine and vindoline resulting in the formation of vinblastine and vincristine in leaves mark the termination of TIAs biogenetic pathway in C. roseus. Since vincristine and vinblastine are highly antimitotic molecules due to their inhibitory action on spindle assembly during cell division, this coupling reaction exclusively takes place in the cell vacuole as a measure of cellular containment. The dimerization reaction between catharanthine and vindoline is facilitated by class III basic peroxidase (Prx1; Sottomayor et al. 1998; Sottomayor and Ros Barcelo 2003; Kumar et al. 2007; Costa et al. 2008).
On the basis of cellular involvements during TIAs synthesis, the internal phloem parenchyma (IPAP) cells present in the periphery of stem pith are primary locations for expression of early pathway genes (Burlat et al. 2004) while the leaf epidermis harbors expression of SLS, TDC and STR gene products (St-Pierre et al. 1999) and idioblast and laticifer cells embedded in the palisade tissues of leaves. In root tissue TDC, STR and MAT transcripts are localized in protoderm and cortical cells around root apical meristem (Laflamme et al. 2001; Moreno-Valenzuela et al. 2003). T16H and SGD expressed in the epidermis, whereas ORCA3 and an AP2/ERF type of transcription factor were expressed in all four cell types (Murata and De Luca 2005). G10H expression is confined to vascular tissue (Burlat et al. 2004). TDC activity was maximum in the epidermal cells, while DAT activity was detected in the whole leaf. NMT activity was found in the whole leaf extract.
4 TIA Pathway Engineering: Current Status
-
(a)
The Generation of Novel Tissue with Enhanced Precursor Availability
The strategies to engineer the TIAs pathway in C. roseus revolves around the generation of novel cell and tissue types characterized by sufficient precursor availability, over- expression of limiting enzymes, activation of regulatory transcription factors, silencing of competitive pathways to flux the intermediates in desired direction and optimized growth parameters (Canel et al. 1998; van der Fits and Memelink 2000; Hughes et al. 2004; van der Heijden et al. 2004; Zarate and Verpoorte 2007; Verma et al. 2012c). The materials being generated through these novel approaches now await their up scaling in bioreactors to assess their commercial utility (Zhao and Verpoorte 2007). In attempts to improve the production of the valuable alkaloids such as vincristine and vinblastine, several studies on C. roseus reported also the accumulation of phenolic compounds upon biotic and/or abiotic stress. The accumulation of phenolics may also affect other secondary metabolite pathways including the alkaloid pathways, as plant defence is a complex system. Elucidation of the pathways and understanding their regulation are important for metabolic engineering to improve the production of desired metabolites (Mustafa and Verpoorte 2007). The ability of plant cells to resist 5-MT induced toxicity has often been ascribed to their ability to over synthesize tryptophan to dilute the inhibitory effect of the analogue. This is generally achieved via a relaxed feedback inhibition of the enzyme anthranilate synthase involved in the biosynthesis of tryptophan from chorismate, the end product of shikimate pathway (Meijer et al. 1993; Scott et al. 1979; Radwanski and Last 1995; Seth and Mathur 2005). It is pertinent to recall that tryptophan is one of the least abundant amino acid present in plants but is the sole donor of indole ring for the synthesis of auxins, glucosinolates, phytoalexins and terpenoid indole alkaloids. Exogenous supplementation of tryptophan to enhance alkaloid synthesis in C. roseus has not been successful because it down regulates several other metabolic pathways via activation of another key regulatory enzyme chorismate mutase that push chorismate towards prephenate metabolism (Radwanski and Last 1995; Galili and Hofgen 2002; Whitmer et al. 2002b). Hughes et al. (2004) have shown that TIA accumulation in a transgenic hairy root line of C. roseus could be enhanced through larger tryptophan availability achieved via over-expression of an Arabidopsis feedback resistant anthranilate synthase gene in them.
To generate such novel tissue types with larger availability of tryptophan, the approach of selecting variants resistant to inhibitory stress of the tryptophan analogue namely 5-methyltryptophan was adopted in the studies conducted by Verma et al. (2012c, 2013) . The basis for adopting such an approach was its successful implementation in several earlier studies aimed to enhance the nutritional quality of many agricultural crops in terms of the improved content of essential dietary amino acids like tryptophan, arginine, lysine, phenylalanine, tyrosine, methionine, threonine etc. in them (Brotherton et al. 1996; Kisaka et al. 1996; Kim et al. 2004; Galili et al. 2005). Hyper-accumulation of targeted amino acid in cells resistant to corresponding analogue is a preferred strategy that a variant cell normally evolves to counter-balance the analogue inhibition by competing it out through a dilution effect during protein synthesis. Cultured plant cells selected for resistance to 5-methyltryptophan (5MT) have been shown to have elevated levels of free tryptophan. The key mechanism of 5MT resistance is reported to be the altered (relaxed/reduced) feedback inhibition of anthranilate synthase which is the key feedback control enzyme in the tryptophan biosynthesis from chorismate (Kang and Kameya, 1995; Kisaka et al. 1996). Anthranilate synthase (AS) has two subunits, ASα and ASβ, the former of which catalyzes the conversion of chorismate to anthranilate and is susceptible to feedback inhibition. 5MT resistant cultures have a mutated OASα1gene that code for ASα subunit OASα1D, in which aspartate-323 is replaced with asparagine making it thereby insensitive to feedback inhibition by tryptophan (Wakasa et al. 1999). Though not verified through enzymatic assays, the 5MT tolerant callus and multiple shoot lines selected by Verma et al. (2012c, 2013) have devised this strategy as was evident by hyper tryptophan accumulation in them with comparable growth in comparison to wild line cultures maintained on analogue-free medium, as proposed earlier by Seth and Mathur (2005) for 5MT tolerant callus mutants of C. roseus.
Verma et al. (2013) have selected five cell suspension lines of C. roseus resistant to 5-methyl tryptophan and characterized on the basis of growth, free tryptophan content and terpenoid indole alkaloid accumulation. Experimental parameters to scale up the most productive variant cell lines in 5–7 L air-lift stirred tank bioreactors were also standardized. Crude alkaloid extract of the cells grown in shake flask and this bioreactor batch also showed the formation of yellow-colored crystals which upon 1HNMR and ESI-MS analysis indicated a phenolic identity. This crude alkaloid extract of bioreactor-harvested cells containing this compound at 50 μg/mL concentration registered 65.21, 17.75, 97.0, 100 % more total antioxidant capacity, reducing power, total phenolic content, and ferric-reducing antioxidant power, respectively, when compared with that of extracts of cells grown in shake flask cultures. The latter, however, showed 57.47% better radical scavenging activity (DPPH) than the bioreactor-harvested cells. Another study of Verma et al. (2012c) showed in vitro selection of ten 5-methyltryprophan (5-MT)-resistant multiple shoot culture lines in three genotypes of C. roseus. The variant shoot lines displayed a differential threshold tolerance limit against the analogue stress, ranged from 20 to 70 mg/L 5-MT in the medium. The rooted shoots of 5-MT-tolerant lines were successfully acclimatized under glasshouse environment wherein they grew normally and set seeds. Flowering twigs or leaves excised from 1-year-old glasshouse grown plants of 5-MT variant lines upon postharvest in vivo elicitation with 30 mg/L 5-MT or 5.0 mg/L tryptophan registered an eight-to-tenfold increment in their vindoline content within 24–48 h.
The data obtained with respect to LD50 dose of 5MT stress for the wild line calli or multiple shoots also showed a tissue- and genotype-specific trend which was in agreement with reported doses specified for C. roseus (Seth and Mathur 2005), Oryza sativa (Kisaka et al. 1996; Kim et al. 2004) and Zeal Mays (Kang and Kameya 1995). Derivatization of an efficient selection scheme to isolate 5MT tolerant variant cultures was another highlight of the present study. The scheme has facilitated the recovery of altered phenotype in shortest possible time with efficient elimination of escapees, habituants and fall positives. Though the developed scheme has provided ample scope to isolate variants resistant to sub-lethal to supra-lethal stress of 5MT selection pressure, but it was found that variants selected for resistance to analogue stress around LD50 dose were more stable and showed better capacity to gradually build-up their tolerance level with each subsequent repeat selection or sieving cycle. The employment of callus or multiple shoots as starting material for screening 5MT tolerant variants in the present work was also found to be a better choice over the usually employed cell suspensions in earlier work on in vitro isolation of biochemical mutants. Problems associated with cell suspensions like cell aggregation, chromosomal instability and developmental asynchrony etc. were easily avoided. Though compact callus morphology generally hinders the screening of deep-seated variant cells amongst a larger population of non-variant cells in the wild population, but it could be effectively overcome by dot-plating technique during initial recurrent selection cycles under sustained analogue stress as was done in this study. Shoot cultures in particular also proved a useful starting tissue in case of C. roseus because of their advanced differentiation level (that has more conducive developmental/biochemical levels of specialization for monitoring the flux of the over-produced precursors through TIAs pathway) and the ease with which 5MT tolerant rooted plants could be obtained from them to observe their in vivo behaviour with respect to TIAs productivity and profiles at the whole plant level. A more recent study of Verma et al. (2015b) where 66 plants raised via direct shoot bud organogenesis from pre-plasmolysed leaf explants of C. roseus were assessed under in vivo conditions for their physio-morpho traits, tryptophan metabolism, genetic fidelity and alkaloid profile, showed a strong positive correlation between tryptophan content and 5-methyltryptophan tolerance.
-
(b)
Transgenic Approaches to Enhance the TIAs Flux
-
(i)
Random T-DNA Insertion and Enhanced Alkaloid Profile
Agrobacterium tumefaciens has a unique ability to transfer genes into plant genomes. This ability has been utilized for plant genetic engineering. For successful plant genetic transformations, information regarding interaction of bacterium with host plant proteins and plant genome, plant defense signaling and molecular mechanism of T-DNA transfer is already known. The rol of onco genes corresponding to T-DNA are known to alter morphology of the plant or host plant secondary metabolism. In C. roseus, some reports are there where simple t-DNA insertion leads to alter alkaloid content significantly. Increased level of serpentine and ajmalicine was reported in hairy roots (Parr et al. 1988; Batra et al. 2004; Verma et al. 2012b) as well as whole plant transgenics (Verma et al. 2015c). Bhadra et al. (1993) reported threefold increase in vindoline production in hairy roots while stable vindoline production was also observed in shooty teratomas (O’Keef et al. 1997) and whole plant transgenics (Verma et al. 2015c). Hong et al. (2005) observed increased horhammericine in hairy roots. Vincristine and vinblastine are reported to be rarely found in hairy roots, but it is found in shooty teratomas (Begum 2011, Begum et al. 2009) and hairy roots (Zargar et al. 2010). In due course of transformation events, it is reported that few T-DNA genes are differentially lost. Severe affects on morphology, growth, biosynthetic pathway gene expression and production of specific secondary metabolites have been observed on loss of some ORFs. The injury caused by pathogens enhances the production of defense compounds (secondary metabolites). The mode of action involves the activation of plant defense machinery via following steps: (a) Detection of signal by the pathogen; (b) activation of H+-ATPase; (c) enhanced Ca2+ influx within the cells from the intercellular spaces; (d) activation of calcium dependent protein kinase (CDPK); (e) lastly the activation of NADPH oxidase. NADPH oxidase contributed in the commencement of MAP kinases that in turn produce active oxygen radicals that resulted into the increment of secondary metabolites biosynthesis via enhanced transcription of defense genes. Other important route to enhance secondary metabolite synthesis has been mediated by jasmonic acid and salicylic acid signaling pathway.
-
(ii)
Differentiation based Alkaloid Production
The diversification of TIAs continuum in cultured tissues is linked with differentiation stage essential for the complete TIAs pathway genes and enzymes expression, most specifically those involved in the late steps of the vindoline biosynthetic pathway. The vindoline synthesis in C. roseus shoot cultures could be successfully linked with expression of key enzyme deacetylvindoline acetyl CoA acetyl transferase (DAT) that catalyzes the last step of vindoline biosynthesis. These studies have successfully confirmed the earlier contentions that light dependent vindoline synthesis requires the presence of a particular cellular organization in the form of idioblasts functional thyllakoid system (Murata and De Luca 2005). TIAs biosynthesis in C. roseus was strongly regulated by differentiation of specialized cells and tissues, which the undifferentiated cultures normally lack, hairy roots were considered to be a better candidate for in vitro production because of their higher level of cellular differentiation and improved genetic or biochemical stability in culture. Jung et al. (1995) demonstrated an inter-convertible method of hairy roots and its cell suspensions meant for TIAs production and found that cell suspension initiated from hairy root derived callus had 60% less catharanthine than in the transformed roots. This was restored to original level of 1.5 mg/L when roots were again produced from cell suspension. Moreno-Valenzuela et al. (1998) also observed reduction in TDC and STR activities by 5 and 30% respectively in cell suspension when TIAs production in hairy roots versus cell suspension was compared.
-
(iii)
Efficient Regeneration/Transformation Protocols
Catharanthus roseus is generally considered as a genetically recalcitrant plant species from pathway engineering angle due to the absence of an efficient direct regeneration protocol for transgenic plant production (van der Fits et al. 2001; Di Flore et al. 2004). Most of the genetic modulation efforts so far made in C. roseus (Zarate and Verpoorte 2007) are confined to cell suspension and transformed hairy root cultures due to the fact that they don’t have requisite level of tissue or cyto-differentiation which is necessary for the expression of complete TIAs pathway genes and enzymes (Di Flore et al. 2004). Moreover, the hairy roots and cultured cells of C. roseus are shown to be highly recalcitrant towards de novo regeneration into transgenic plants. Authors (Verma and Mathur 2011a, b) represented the first report on development of protocol for proficient shoot bud induction directly emerging from the leaves of C. roseus.
The most important aspect of a regeneration protocol utilized to genetically modify the species is shoot bud’s de novo direct origin from the leaf tissue (Sharma et al. 2005). Since these de novo shoot buds originates from single cells, they are considered as better prospect for stable transformation leading to non-chimeric transgenic plants in comparison to those arising from pre defined germ lines (somatic embryos) or from a pre-formed axillary or apical meristem (Newell 2000). This work also highlights that a pre-plasmolytic treatment of C. roseus leaves in CPW: 13% mannitol solution. A 60 min pre-plasmolytic treatment was effective for release of the meristematic cells from the developmental block and allowed them to advance through a complete shoot bud regeneration cycle leading to plant formation. This dehydration treatment influenced the organogenesis either through the explants rapid uptake of plant growth promotors or by reducing the cyto-toxic TIAs concentration (through ex-osmosis) in the region of the dividing cells involved in the de novo organogenesis from the leaf explants. The regeneration protocol optimized in this study could also be successfully employed for A. tumefaciens – mediated transgenic plant production in C. roseus. A. tumefaciens (strain-LBA4404) harboring a binary vector pBI121 (with GUS and ntp II genes) with p35SGUS-INT (having GUS Introns) was used. Various parameters such as culture age of bacterial, inoculum density of bacterial suspension, infection method and co-cultivation condition for transformation were successfully optimized for this transgenic protocol development. The flooding approach became more effective if done via a SAAT treatment for 60 s. The Sonication treatments extended more than 60 s were lethal for explants. SAAT is a recently developed technique for efficient A. tumefaciens-based genetic transformation of crops that generally resist the usual manual pricking or vacuum infiltration approaches of infection like cotton, Papaya, Chenopodium, Vigna, Soybean etc. (Trick and Finer 1997). This method involves the short exposure of ultrasound waves to the target plant tissue immersed in an Agrobacterium suspension. The technique efficiently overcomes certain barriers such as the host specificity and the inability of bacterium to reach proper meristematic cells if they are buried deep inside the target tissues (Trick and Finer 1997). Besides, the wounded tissue could also produce more of phenolic substances like acetosyringone that are required for enhancing the binding accessibility of Agrobacterium to the cell surface. Also the extent of physical tissue injury caused during the making of thousands of nano-sized pores by the high energy of ultrasound waves is low in comparison to manual pricking by bacterial filled sterilized needle. This helped in minimizing the callusing response from the explants. Using hypocotyls as explants, Wang et al. (2010) developed genetic transformation method via A. tumefaciens strain EHA105. The construct used was pCAMBIA2301 having GUS reporter gene with a neomycin phosphotransferase II gene (NTPII) as selectable marker. The Best results with 11% transformation frequency were obtained when 10 min sonication was applied to hypocotyls with 80 W. After that explants were subjected to 30 min A. tumefaciens infection and 2 day co-cultivation on 1/2 MS medium having 100 μM acetosyringone. To asses the potential of the given protocol deacetylvindoline-4-O-acetyltransferase (DAT), was over-expressed that lead to nine separate transgenic plants. Vindoline was found to be enhanced in the DAT over-expressing transgenic plants. For optimum transformation frequency, various parameters such as density of Agrobacterium and acetosyringone concentration, duration of co-cultivation, sonication dose and duration and dose of selection pressure i.e. kanamycin were optimized. In a recent report of Weaver et al. (2014), The Fast Agro-mediated Seedling Transformation (FAST) method was developed that involves the co-cultivation and transient transformation of young seedlings. In this particular study, Agrobacterium strongly induced ZCT1 and ORCA3. There are just two reports on regeneration of transgenic plants from transformed hairy roots of C. roseus (Choi et al. 2004; Verma et al. 2012b). The regeneration response was found to be greatly influenced by genotype and varied with different root clones. The regenerated shoots showed prolific rooting with extensive lateral branching and shortened inter-nodes in the transgenic plants. PCR and Southern blotting analysis confirmed the retention of Ri-TL-DNA in these regenerants. Verma et al. (2012b) also found excessive flowering in the regenerated plants.
-
(iv)
Elicitation/Precursor/Inhibition based Studies
Alternation/supplementations in medium recipes of transformed tissue of C. roseus resulted into the altered alkaloid profile. While total alkaloid content was found to be enhanced by low medium nutrients (Toivonen et al. 1992) and penicillin supplementation (Sim et al. 1994) in hairy roots, acetyl salicylic acid increased total alkaloid content in tumor cell suspensions (Godoy-Hernandez and Loyola-Vargas 1997). MES-buffered medium had a negative effect on lochnericine accumulation but enhanced tabersonine synthesis (Morgan and Shanks 2000), whereas fructose supplementation enhanced the catharanthine level in hairy roots (Jung et al. 1992). Effect of oxygenase inhibitors like 1-aminobenzotriazole, clotrimazole (ABT) and 2,5-pyridinedicarboxylic acid was studied to decipher the pathway around tabersonine. The ABT inhibited horhammericine formation while 2,5-pyridinedicarboxylic acid specifically inhibited lochnericine accumulation (Morgan and Shanks 1999). Verapamil and CdCl2, that block the Ca2+ flux across the plasma membrane enhanced the total alkaloid content by 25% in hairy roots and their discharge into the medium by ten times (Moreno-Valenzuela et al. 2003). The specific Ca2+ chelator, EGTA, stimulated 90% of the total alkaloid secretion. Recently, Thakore et al. (2013) reported 98% increment in ajmalicine on treatment with TritonX-100 (0.1% v/v) and n-hexadecane (2% v/v). Metabolic engineering of the biosynthetic pathway of these TIAs have indicated that extremely low yields of the pharmaceutically important alkaloids can be ascribed to the limitation in the availability of these two precursor molecules from the primary pool (Whitmer et al. 2002a). Tryptophan and terpenoid intermediates feeding to transgenic lines over expressing TDC/STR showed high alkaloid production (Whitmer et al. 2002a). It can be clearly interpreted that the terpenoid branch of pathway is limiting. Exogenous feeding of tryptophan and/or terpenoid intermediates like geraniol or loganin, etc in STR over-expressing cell lines has also resulted in higher TIAs production (Whitmer et al. 2003), indicating that it is not only the availability of tryptophan and secologanin, but their actual utilization which is more limiting in guiding the metabolic flux towards TIAs metabolism. Elicitors are known to induce synthesis of valuable secondary metabolites as defense against pathogen and the consequent biosynthetic pathway. Till date so many biotic or abiotic elicitors have been tested to enhance the discharge of secondary metabolites in C. roseus hairy roots. Methyl jasmonates (MeJA) is known to be involved in signal transduction, escorting the reaction of the plant to different environmental signals. One of such reaction is biosynthesis of proteins and secondary metabolites. For some recent years, MeJAs was frequently used as elicitors to induce biosynthesis of secondary metabolism in many plant species, including C. roseus (Loyola-Vargas et al. 2007). TIA genes demonstrate considerable variation in the degree and duration of induction by MeJA. Methyl jasmonate (MeJA) an organic compound used in plant defense caused significant enhancement in the transcript of TIAs pathway genes, particularly catharanthine accumulation while sodium nitroprusside alone or in combination with MeJA caused spectacular reduction in catharanthine accumulation. It is also known to enhance type-1 protein prenyltransferase transcripts in hairy roots of C. roseus. MeJA treated hairy roots induced the disturbance in the integrity of mitochondrial membrane and a reduction in ATP biosynthesis (Ruiz-May et al. 2009). In C. roseus, Octadecanoid-Responsive Catharanthus AP2-domain (ORCA1, ORCA2 and ORCA3) transcription factors play crucial role in up-regulating many TIAs genes in response to MeJA treatment. Goklany et al. (2009) carried out a dose specific study of the MeJA treated hairy roots. Low doses of MeJA were found to be favoring TIAs biosynthesis by keeping ZCT (transcriptional repressors) level low (two to sevenfold) and ORCA (29–40) level high resulting into 8 to 15-fold increment in genes involved in TIA biosynthesis. While at high doses of MeJA, TIAs biosynthesis was inhibited. This was attributed to the increased level of ZCT (40-fold) expression in comparison to ORCA (13 to 19-fold) resulting into and nominal induction of the TIA biosynthetic genes (0 to 6-fold). This study directly pointed out the crucial role of exogenous MeJA supplementation in C. roseus in up-regulating TIA biosynthetic pathway.
-
(v)
Pathway Gene Over-Expression
Since biosynthesis of all TIAs initiates from a common precursor molecule-strictosidine that is formed by the coupling of tryptophan derived tryptamine and secologanin, limited availability of these precursor molecules from the primary shikimate and secoiridoid pools has often been documented as the most serious problem associated with low TIAs productivity in C. roseus. While over-expression of the gene tryptophan decarboxylase (TDC), which codes for the enzyme that converts tryptophan into tryptamine, has alone not found effective for a concomitant increase in TIAs accumulation, the overexpression of strictosidine synthase (STR), either alone or along with TDC, was found more conducive for enhanced TIAs synthesis (Canel et al. 1998). Tryptophan pool of the cell is maintained by feedback inhibition of the enzyme anthranilate synthase. To avoid a negative feedback regulatory step-the biosynthesis of indole precursor of TIAs i.e. tryptamine, attempts were made to over-express TIA s pathway gene coding enzymes. Tryptophan decarxylase (TDC) mediated catalysis of tryptophan yields tryptamine. The expression of a feedback resistant anthranilate synthase (AS) α-subunit from Arabidopsis thaliana concurrently with the AS β-subunit and TDC gene in the hairy roots of C. roseus improved the metabolic flux of the indole precursors tryptamine towards anti-hypertensive drug ajmalicine with a corresponding decrease in lochnericine, horhammericine and tabersonine (Hughes et al. 2002; Hong et al. 2005; Peebles et al. 2005). Recently C. roseus TDC/STR-TDC over-expression in other Apocynaceae members Rawvolfia serpentina (Mehrotra et al. 2013) and Vinca minor (Verma et al. 2015d) resulted into increased ajmalicine/serpentine and vincamine production respectively. In the mevalonate pathway, HMG-COA is converted to mevalonate by the action of enzyme 3-hydroxy-3-methylglutaryl-COA reductase (HMGR). Mevalonate an IPP precursor, is considered to be a crucial enzyme in the synthesis of cytoplasmic isoprenoids in plants such as fitosterols (campesterol, stigmasterol, and sitosterol) and prenyl chains for someproteins, sugars, and lipids. Ayora-Talavera et al. (2002) reported five to seven times more serpentine in hairy roots on the over-expression of HMGR gene. The coupling of isopentenyl diphosphate (IPP) and dimethylallyl diphosphate (DMAPP) catalysed by GPP synthase (GPPS) to yield Geranyl diphosphate (GPP), the initial point of formation of terpene moiety. The transient over-expression of A. majus AmGPPS.SSU (Geranyl diphosphate smaller sub unit) in C. roseus leaves resulted in the enhancement of vindoline content (Rai et al. 2013). In a study, conducted by Peebles et al. (2011), DXS gene alone as well as along with G10H were over-expressed in hairy roots of C. roseus. Noteworthy enhancement in ajmalicine, serpentine, and lochnericine by 67, 26 and 49%, respectively were observed with concurrent decrease in tabersonine (66%) and hörhammericine (54%). When DXS and G10H were co-over-expressed, ajmalicine, lochnericine and tabersonine were significantly enhanced by 16, 31 and 13%, respectively. Another study conducted by Chang et al. (2014) reported DXR and STR or MECS and STR over-expression in hairy roots. Again it favoured the co-over-expression rather than single gene expression. Higher ajmalicine was registered by co-over-expression of DXR-STR or MECS-STR in comparison to the single gene over-expression of DXR, MECS, and STR. Deacetylvindoline-4-O-acetyltransferase (DAT), the final enzyme in the vindoline biosynthesis that catalyses de-acetylvindoline into vindoline, was also over-expressed with CaMV35S promoter in hairy roots of C. roseus (Magnotta et al. 2007). These workers also found no increment in the vindoline level in the transformed roots but instead, observed fourfolds increase in horhammericine to control lines. Enhanced horhammericine accumulation was attributed to the inhibitory activity of minovincinine-19-O-acetyltransferase (MAT) by the DAT protein as revealed by the enzymatic analysis. Over-expression of DAT gene was found to enhance vindoline production in transgenic plants (Wang et al. 2010). The two peroxidases CrPrx and CrPrx1 are characterized from C. roseus and belong to class III peroxidases. CrPrx and CrPrx1 are known to be apoplastic and vacuoler in nature, respectively. For determining their respective function in planta, these were expressed in Nicotiana tabacum (Kumar et al. 2011). It was interpreted that salt and dehydration stress was preside over by the vacuolar peroxidase while apoplastic peroxidase provide cold stress tolerance. Over-expression of apoplastic peroxidase CrPrx in C. roseus hairy root lines resulted into enhanced ajmalicine and serpentine production. Using biolistic approach, Guirimand et al. (2009) has given a protocol of transient expression in C. roseus cells which was further utilized in subcellular characterization of HDS and G10H to plastids/stromules and to ER membrane. A very recent report showed the D4H like gene but with different transcriptional expression profile (Zhou et al. 2014). The wealth of information gathered so far has made TIAs biogenetic pathway as one of the best dissected and understood metabolic routes at the enzymatic level and corresponding genes level in plants. Over expression of TIAs pathway genes in C. roseus cell cultures has so far not be up to the execution in significantly augmenting sustainable production of the preferred alkaloids because of their lower level of cellular and tissue differentiation. Our understanding of terpenoid indole alkaloids has matured to a much better level of enzymatic and genetic regulations where a cell can now be engineered via incorporation of transgenes for homologous as well as heterologous expression of an indigenous or novel metabolic step(s).
-
(vi)
Transcription Factors and TIA Pathway Regulation
A general understanding of the major routes of biosynthesis for TIAs pathway has been gathered, but the complete expression of pathways is necessary by determining the master regulators i.e. transcription factors that can manipulate whole biosynthetic steps involved in the pathway. ORCAs, ZCTs and WRKY transcription factors are the three major classes that came out in C. roseus pathway modulation. Interaction between the jasmonate- and elicitor-responsive element and two jasmonate-responsive transcription factors known as ORCA2 and ORCA3 takes place in C. roseus. The two ORCAs fit in the APETALA2/ethylene response-factor (AP2/ERF) family of transcription factors. The numerous genes govering primary and secondary metabolism, together with the TDC and STR genes, were shown to be regulated by ORCA3. MeJA induced all metabolite biosynthetic genes, primary and secondary, along with ORCA3 itself, that were tested (Van der Fits and Memelink 2000). Though, ORCA3 did not regulate all MeJA induced genes. For example, MeJA greatly induced expression of G10H, but ORCA3 overexpressing cell lines did not show G10H expression, suggesting that regulation of this gene involves additional jasmonate-responsive transcription factors. Although numerous genes of primary as well as secondary metabolism are regulated by ORCA3, it does control all genes in TIA metabolism. The involvement of additional transcription factors is possible in managing these genes. ORCA2, which is another jasmonate-responsive AP2/ERF-domain transcription factor from C. roseus, is a good applicant for this purpose. As indicated by preliminary results, ORCA2 and ORCA3 have interrelated, but different, assembly of target genes which suggest difference in functions of ORCA proteins, and the necessity of both for the whole range of jasmonate-stimulated metabolic modifications. An increment in AS, TDC, STR and D4H transcripts was generated by ORCA3 over-expression but CRMYC2 and G10H transcription remained unaffected. G10H and ORCA3 co-over-expression induced a significant increase in G10H transcripts. Further, a substantial increase in the production of strictosidine, vindoline, catharanthine and ajmalicine took place due to ORCA3 and G10H over-expression but effects on anhydrovinblastine and vinblastine levels were limited (Pan et al. 2012). Another class of transcription factors i.e. zinc finger proteins (ZCT1, ZCT2, and ZCT3) are known to be transcriptional repressors in C. roseus and belong to the family Cys2/His2-type (transcription factor IIIA-type) ZCT family. They inhibit TDC and STR promoters in vitro by combining in a sequence specific manner (Pauw et al. 2004). Soybean transcription factor GmMYBZ2 inhibits the biosynthesis of catharanthine in hairy roots as stated by Zhou et al. (2011). Bax, a mammalian pro-apoptotic element of the Bcl-2 family when expressed in plants, stimulates oversensitive reactions. It generates transcriptional activation of two important genes TDC and STR in TIAs biosynthetic pathway of C. roseus cells. It induces the production of defense-related protein PR1 in the cells. It can be clearly interpreted that the mouse Bax activates the defense responses in C. roseus cells and stimutes the induction of TIA pathway (Jun and Fang 2007). It is now known that transcription factor CrMYC2 belonging to the family of helix-loop-helix (bHLH) plays crucial role in the activation of ORCA3 (a MeJA-responsive TIA up-regulator transcription factor) gene. The early response of Jasmonates is the activation of the CrMYC2 gene that binds to the sequence of the ORCA3 JRE in vitro. Transient assays showed that it further transactivates expression of reporter gene. Fall in the CrMYC2 expression by RNA interference resulted in a significant decrease in the production of ORCA3 mRNA. A root expressing CrWRKY1, C. roseus WRKY transcription factor, is induced by phytohormones namely jasmonate, ethylene and gibberellic acid. In hairy roots, CrWRKY1 up- regulates some of TIA pathway genes including TDC as well as transcriptional repressors ZCT1 ZCT2, and ZCT3 while down regulates the transcriptional activators ORCA2, ORCA3, and CrMYC2. But when the dominant-repressive form of CrWRKY1 was expressed it resulted into the suppression of TDC and ZCTs and the induction of ORCA3 and CrMYC2. The increased TDC activity, accumulation of tryptamine, resistance against 4-methyl tryptophan inhibition of CrWRKY1 expressing hairy roots were the outcome of up-regulation of TDC gene. The CrWRKY1 hairy roots were showing three times more serpentine as compared to control roots (Suttipanta et al. 2011).
5 Roadmap for Future Research
Catharanthus roseus today occupies the central position in ongoing metabolic engineering efforts in medicinal plants. The entire multi-step biogenetic pathway of its extremely priced anti-hypertensive and anti-cancerous alkaloids is fairly very well dissected at biochemical and gene levels. In order to increase the volumetric yield of these pharma molecules for drug industry, cell and tissue cultures of C. roseus are being increasingly tested to provide their alternate production platforms. However, a rigid developmental regulation and involvement of different cell, tissues and organelles in the synthesis of these alkaloids have restricted the utility of these cultures. Concerning its beneficial nature endophytes and allied members have considerable potential as bio-control agents and plant-growth promoters. Special efforts should therefore be made to define the molecular and biochemical bases of symbioses and their physiological effects on plant Endophytes have been extremely valuable in understanding the orchestration of root innate immunity. The root colonization by endophytes results in an increase in plant growth, early flowering, higher seed yield, alteration in the secondary metabolites, and adaptation to abiotic and biotic stresses. So one of the potential area revolve around determining the effect of the biotic and abiotic elicitors in relation with localization and expression of important MIA pathway genes required for the synthesis of the rate limiting products of MIA pathway like Vindoline, Catharanthine, Tabersonine, Vinblastine and Vincristine. Particular emphasis will be over to find out, whether the elicitation affects the cell metabolic machinery via altering the functional site of gene products at different locations in the cell compartments by mapping the location and transfer of gene products. Heterologous gene expression is another emerging trend in recent C. roseus biology. A recent study of Meittinen et al. (2014) reconstituted expression of the eight genes encoding seco-iridoid pathway, together with two genes boosting precursor formation and two downstream alkaloid biosynthesis genes, in Nicotiana benthamiana, allows the heterologous production of the complex MIA strictosidine. VIGS and RNAi-mediated down-regulation of sub-pathways operating at the branch point nodes in the upstream steps of the TIAs metabolism up to tabersonine synthesis will be the potential targets. Photo-autotrophic cell cultures of C. roseus with functional chloroplast system in the idioblast and laticifer networks are one of the future line to be targeted. Such tissue can be potential targets for hyper-expressing vindoline pathway limiting steps and their further condensation with catharanthine to produce antineoplatic alkaloids. Efforts must continue to identify transcriptional activators and transcriptional repressors of TIAs pathway genes to generate novel tissue types with a better understanding of regulatory mechanisms.
References
Arora R, Malhotra P, Mathur AK, Mathur A, Govil CM, Ahuja PS (2010) Anticancer alkaloids of Catharanthus roseus: transition from traditional to modern medicine. In: Arora R (ed) Herbal medicine: a cancer chemopreventive and therapeutic perspective. Jaypee Brothers Medical Publishers, New Delhi, pp 292–310
Ayora-Talavera T, Chappell J, Lozoya-Gloria E, Loyola-Vargas VM (2002) Overexpression of Catharanthus roseus hairy roots of a truncated hamster 3-hydroxy-3-methylglutaryl-CoA reductase gene. Appl Biochem Biotechnol 97:135–145
Batra J, Dutta A, Singh D, Kumar S, Sen J (2004) Growth and terpenoid indole alkaloid production in Catharanthus roseus hairy root clones in relation to left and right termini-linked Ri T-DNA gene integration. Plant Cell Rep 23:148–154
Begum F (2011) Augmented production of vincristine in induced tetraploids of Agrobacterium transformed shooty teratomas of Catharanthus roseus. Med Plants Int J Phytomed Relat Ind 3:59–64
Begum F, Rao SSN, Rao K, Devi YP, Giri A, Giri CC (2009) Increased vincristine production from Agrobacterium tumefaciens C58 induced shooty teratomas of Catharanthus roseus G. Don. Nat Prod Res 23:973–981
Bennouna J, Breton JL, Taurani JM, Ottensmeier C, O’brien M, Kosmidis P, Huat TE, Pinel MC, Colin C, Douillard JY (2006) Vinflunine—an active chemotherapy for treatment of advanced non-small cell lung cancer previously treated with a platinum- based regimen: results of a phase II study. Br J Cancer 94:1383–1388
Bhadra R, Vani S, Shanks J (1993) Production of indole alkaloids by selected hairy root lines of Catharanthus roseus. Biotechnol Bioeng 41:581–592
Brotherton JE, Schechter S, Ranch JP, Widholm JM (1996) Inheritance and stability of 5-methyltryptophan resistance in Datura innoxia selected in vitro. Plant Cell Physiol 37:389–394
Burlat V, Oudin A, Courtois M, Rideau M, St-Pierre B (2004) Co-expression of three MEP pathway genes and geraniol 10-hydroxylase in internal phloem parenchyma of Catharanthus roseus implicates multicellular translocation of intermediates during the biosynthesis of monoterpene indole alkaloids and isoprenoid-derived primary metabolites. Plant J 38:131–141
Campous-Tamayo F, Hernandez-Dominguez E, Vazquez-Flota FA (2008) Vindoline formation in shoot cultures of Catharanthus roseus is synchronously activated with morphogenesis through the last biosynthetic step. Ann Bot 102:409–415
Canel C, Lopes-Cardoso MI, Whitmer S, Van der Fits L, Pasquali G, Van der Heijden R, Hoge JHC, Verpoorte R (1998) Effects of over-expression of strictosidine synthase and tryptophan decarboxylase on alkaloid production by cell cultures of Catharanthus roseus. Planta 205:414–419
Chang K, Qiu F, Chen M, Zeng L, Liu L, Yang C, Lan X, Wang Q, Liao Z (2014) Engineering the MEP pathway enhanced ajmalicine biosynthesis. Int Union Biochem Mol Biol 61:249–255
Choi PS, Kim YD, Choi KM, Chung HJ, Choi DW Jr (2004) Plant regeneration from hairy-root cultures transformed by infection with Agrobacterium rhizogenes in Catharanthus roseus. Plant Cell Rep 22:828–831
Cordell GA (1980) The botanical, chemical, biosynthetic and pharmacological aspects of Catharanthus roseus (L.) G. Don. (Apocynaceae). In: Woo WS, Han BH (eds) Recent advances in natural product research. Seoul National University Press, Seoul, pp 65–72
Costa MMR, Hilliou F, Duarte P, Pereira LG, Almeida I, Leech M, Memelink J, Barcelo AR, Sottomayor M (2008) Molecular cloning and characterization of a vacuolar class III peroxidise involved in the metabolism of anticancer alkaloids in Catharanthus roseus. Plant Physiol 146:403–417
De Carolis E, Chan F, Balsevich J, De Luca V (1990) Isolation and characterization of a 2-oxoglutarate dependent dioxygenase involved in the second-to-last step in vindoline biosynthesis. Plant Physiol 94:1323–1329
De Luca V, Cutler AJ (1987) Subcellular localization of enzymes involved in indole alkaloid biosynthesis in Catharanthus roseus. Plant Physiol 85:1099–1102
Dethier M, De Luca V (1993) Partial purification of an N-methyltransferase involved in vindoline biosynthesis in Catharanthus roseus. Phytochemistry 32:673–678
Di Flore S, Hoppmann V, Fischer R, Schillberg S (2004) Transient gene expression of recombinant terpenoid indole alkaloid enzymes in Catharanthus roseus leaves. Plant Mol Biol Rep 22:15–22
Dipierre A, Lemarie E, Dabouis G, Garnier G, Jacoulet P, Dalphin JC (1991) A phase II study of Navelbine (vinorelbine) in the treatment of non-small cell lung cancer. Am J Clin Oncol 14:115–119
Duarte P, Memelink J, Sottomayor M (2010) Fusions with fluorescent proteins for subcellular localization of enzymes involved in plant alkaloid biosynthesis. In: Fett-Netto A (ed) Methods in molecular biology, metabolic engineering of plant secondary pathways. Humana Press, New York, pp 275–290
El-Sayed M, Verpoorte R (2007) Catharanthus terpenoid indole alkaloids: biosynthesis and regulation. Phytochem Rev 6:277–305
Facchini PJ (2001) Alkaloid biosynthesis in plants: biochemistry, cell biology, molecular regulation, and metabolic engineering applications. Annu Rev Plant Mol Biol 52:29–66
Facchini PJ, De Luca V (2008) Opium poppy and Madagascar periwinkle: model non-model systems to investigate alkaloid biosynthesis in plants. Plant J 54:763–784
Farnsworth NR (1985) Plants and modern medicine: Where science and folklore meet. Traditional medicine. World Health Forum 6:76–80
Foye WO (1995) Cancer chemotherapeutic agents. American Chemical Society, Washington, DC, pp 349–353
Fulzele DP, Heble MR (1994) Large-scale cultivation of Catharanthus roseus cells production of ajmalicine in a 20-l airlift bioreactor. J Biotechnol 35:1–7
Galili G, Hofgen R (2002) Metabolic engineering of amino acids and storage proteins in plants. Metab Eng 4:3–11
Galili G, Amir R, Hoefgen R, Hesse H (2005) Improving the levels of essential amino acids and sulfur metabolites in plants. Biol Chem 386:817–831
Ganapathi G, Kargi F (1990) Recent advances in indole alkaloid production by Catharanthus roseus (Periwinkle). J Exp Bot 41:259–267
Godoy-Hernandez G, Loyola-Vargas VM (1997) Effect of acetylsalicylic acid on secondary metabolism of Catharanthus roseus tumor suspension cultures. Plant Cell Rep 16:287–290
Goklany S, Loring RH, Glick J, Lee-Parsons CWT (2009) Assessing the limitations to terpenoid indole alkaloid biosynthesis in Catharanthus roseus hairy root cultures through gene expression profiling and precursor feeding. Biotechnol Prog 25:1289–1296
Guirimand G, Burlat V, Oudin A, Lanoue A, St-Pierre B, Courdavault V (2009) Optimization of the transient transformation of Catharanthus roseus cells by particle bombardment and its application to the subcellular localization of hydroxymethylbutenyl 4-diphosphate synthase and geraniol 10-hydroxylase. Plant Cell Rep 28:1215–1234
Guirimand G, Courdavault V, St-Pierre B, Burlat V (2010) Biosynthesis and regulation of alkaloids. In: Pua EC, Davey MR (eds) Plant developmental biology—biotechnological perspective, vol 2. pp 139–160
Guirimand G, Guihur A, Pierre P, Hericourt F, Mahroug S, St-Pierre B, Burlat V, Courdavault V (2011) Spatial organization of the vindoline biosynthetic pathway in Catharanthus roseus. J Plant Physiol 168:519–628
Hirata K,Miyamoto K, Miura Y (1994) Catharanthus roseus L (Periwinkle): production of vindoline and catharanthine in multiple shoot culture. In: Bajaj YPS (ed) Biotechnology in Agriculture and Forestry: Medicinal and Aromatic Plants I., vol 4. Springer, Berlin, pp 46–55
Hong SB, Peebles CAM, Shanks JV, San KY, Gibson SI (2005) Expression of the Arabidopsis feedback-insensitive anthranilate synthase holoenzyme and tryptophan decarboxylase genes in Catharanthus roseus hairy roots. J Biotechnol 122(1):28–38
Hughes RH, Shanks JV (2002) Metabolic engineering of plants for alkaloid production. Metab Eng 4:41–48
Hughes EH, Hong SB, Shanks JV, San KY, Gibson SI (2002) Characterization of an inducible promoter system in Catharanthus roseus hairy roots. Biotechnol Prog 18:1183–1186
Hughes EH, Hong SB, Gibson SI, Shanks JV, San KY (2004) Expression of a feedback-resistant anthranilate synthase in Catharanthus roseus hairy roots provides evidence for tight regulation of terpenoid indole alkaloid levels. Biotechnol Bioeng 86:718–727
Johnson S, Armstrong I, James G, Marvin G, Burnett JP (1963) The Vinca alkaloids: a new class of oncolytic agents. Lilly Laboratories for Research and Lilly Laboratories for Clinical Research, Indianapolis, IN, pp 1390–1427
Jun XM, Fang DJ (2007) Enhancing terpenoid indole alkaloid production by inducible expression of mammalian Bax in Catharanthus roseus cells. Sci China Ser C Life Sci 50:234–241
Jung KM, Kwak SS, Kim SW, Leo H, Choi CY, Liu JR (1992) Improvement of the catharanthine productivity in hairy root cultures of Catharanthus roseus by using monosaccharides as a carbon source. Biotechnol Lett 14:695–700
Jung KH, Kwak SS, Choi CY, Liu JR (1995) An interchangeable system of hairy root and cell suspension cultures of Catharanthus for indole alkaloid production. Plant Cel Rep 15:51–54
Kang KK, Kameya T (1995) Characterization of anthranilate synthetase and tryptophan synthase in a 5-methyl tryptophan resistant mutant (MR1) of Zea mays L. Breed Sci 45:321–325
Kim DS, Lee IS, Jang CS, Hyun DY, Seo YW, Lee YI (2004) Selection of 5-methyltryptophan resistant rice mutants from irradiated calli derived from embryos. Euphytica 135:9–19
Kisaka H, Kisaka M, Kameya T (1996) Characterization of interfamilier somatic hybrids between 5-methyltryptophan resistant (5-MT-resistant) Rice (Oryza sativa L.) and 5-MT-sensitive Carrot (Daucas carota L.); Expression of resistance to 5-MT by the somatic hybrids. Breed Sci 46:221–226
Kumar S, Dutta A, Sinha AK, Sen J (2007) Cloning, characterization and localization of a novel basic peroxidase gene from Catharanthus roseus. FEBS J 274:1290–1303
Kumar S, Jaggi M, Taneja J, Sinha AK (2011) Cloning and characterization of two new Class III peroxidase genes from Catharanthus roseus. Plant Physiol Biochem 49:404–412
Laflamme P, St Pierre B, De Luca V (2001) Molecular and biochemical analysis of a Madagascar periwinkle root-specific minovincinine-19-hydroxy-O-acetyltransferase. Plant Physiol 125:189–198
Leckie F, Scragg AH, Cliffe KC (1991) Effect of bioreactor design and agitator speed on the growth and alkaloid accumulation by cultures of Catharanthus roseus. Enzyme Microb Technol 13:296–305
Levac D, Murata J, Kim WS, De Luca V (2008) Application of carborundum abrasion for investigating leaf epidermis: molecular cloning of Catharanthus roseus 16 -hydroxy- tabersonine-16-O-methyltransferase. Plant J 53:225–223
Loyola-Vargas VM, Galaz-Avalos RM, Rodríguez-Ku JR (2007) Catharanthus biosynthetic enzymes: the road ahead. Phytochem Rev 6:307–339
Luca D, Laflamme P (2001) The expanding universe of alkaloid biosynthesis. Curr Opin Plant Biol 4:225–233
Magnotta M, Murata J, Chen J, De Luca V (2007) Expression of deacetylvindoline-4-O-acetyltransferase in Catharanthus roseus hairy roots. Phytochemistry 68:1922–1931
Mahroug S, Courdavault V, Thiersault M, St-Pierre B, Burlat V (2006) Epidermis is a pivotal site of at least four secondary metabolic pathways in Catharanthus roseus aerial organs. Planta 223:1191–1200
Mahroug S, Burlat V, St-Pierre B (2007) Cellular and sub-cellular organization of the monoterpenoid indole alkaloid pathway in Catharanthus roseus. Phytochem Rev 6:363–381
Mano M (2006) Vinorelbine in the management of breast cancer: new perspective, revived role in the era of targeted therapy. Cancer Treat Rev 32:106–118
Mehrotra S, Prakash O, Khan F, Kukreja AK (2013) Efficiency of neural network-based combinatorial model predicting optimal culture conditions for maximum biomass yields in hairy root cultures. Plant Cell Rep 32:309–317
Meijer JJ, Ten Hoopen HJG, Luyben KCAM (1993) Effects of hydrodynamic stress on cultured plant cells: a literature survey. Enzyme Microb Technol 15:234–238
Meittinen K et al (2014) The seco-iridoid pathway from Catharanthus roseus. Nat Commun 5:3606. doi:10.1038/ncomms4606
Moreno PRH, Van der Heijden R, Verpoorte R (1995) Cell and tissue cultures of Catharanthus roseus: a literature survey II. Updating from 1988 to 1993. Plant Cell Tiss Org Cult 42:1–25
Moreno-Valenzuela O, Galaz-Avalos RM, Minero-Garcoaa Y, Loyola-Vargas VM (1998) Effect of differentiation on regulation of indole alkaloid production in Catharanthus roseus hairy root. Plant Cell Rep 18:99–104
Moreno-Valenzuela OA, Minero-García Y, Chan W, Mayer-Geraldo E, Carbajal E, Loyola-Vargas VM (2003) Increase in the indole alkaloid production and its excretion into the culture medium by calcium antagonists in Catharanthus roseus hairy roots. Biotechnol Lett 25:1345–1349
Morgan JA, Shanks JV (1999) Inhibitor studies of tabersonine metabolism in C. roseus hairy roots. Phytochemistry 51:61–68
Morgan JA, Shanks JV (2000) Determination of metabolic rate-limitations by precursor feeding in Catharanthus roseus hairy root cultures. J Biotechnol 79:137–145
Murata J, De Luca V (2005) Localization of tabersonine 16-hydroxylase and 16-OH-tabersonine-16-O-methyltransferase to leaf epidermal cells defines them as a major site of precursor biosynthesis in the vindoline pathway in Catharanthus roseus. Plant J 44:581–594
Mustafa NR, Verpoorte R (2007) Phenolic compounds in Catharanthus roseus. Phytochem Rev 6:243–258
Neuss CL, Neuss MN (1990) Therapeutic use of bisindole alkaloids from Catharanthus. In: Brossi A, Suffness M (eds) The alkaloids, vol 37. Academic, San Diego, pp 229–240
Newell CA (2000) Plant transformation technology: developments and applications. Mol Biotechnol 16:53–65
O’Keef BR, Mahady GB, Gills JJ, Beecher CWW (1997) Stable vindoline production in transformed cell culture of Catharanthus roseus. J Nat Prod 60:261–264
Pan Q, Wang Q, Yuan F, Xing S, Zhao J, Choi YH, Verpoorte R, Tian Y, Wang G, Tang K (2012) Overexpression of ORCA3 and G10H in Catharanthus roseus plants regulated alkaloid biosynthesis and metabolism revealed by NMR-metabolomics. PLoS One 7, e43038
Pan Q, Rianika N, Mustafa Tang K, Choi YH, Verpoorte R (2015) Monoterpenoid indole alkaloids biosynthesis and its regulation in Catharanthus roseus: a literature review from genes to metabolites. Phytochem Rev 15:221. doi:10.1007/s111c1-015-9406-4
Parr AJ, Peerless ACJ, Hamill JD, Walton NJ, Robins RJ, Rhodes MJC (1988) Alkaloid production by transformed root cultures of Catharanthus roseus. Plant Cell Rep 7:309–312
Pasquali G, Porto DD, Fett-Neto AG (2006) Metabolic engineering of cell cultures versus whole plant complexity in production of bioactive monoterpene indole alkaloids: recent progress related to old dilemma. J Biosci Bioeng 101:287–296
Pauw B, Hilliou FAO, Martin VS, Chatel G, Wolf CJFD, Champion A, Pre M, Duijn BV, Kijne JW, Fits LVD, Memelink J (2004) Zinc finger proteins act as transcriptional repressors of alkaloid biosynthesis genes in Catharanthus roseus. J Biol Chem 279:52940–52948
Peebles CAM, Hong SB, Gibson SI, Shanks JV, San KY (2005) Transient effects of over-expressing anthranilate synthase a and b subunits in Catharanthus roseus hairy roots. Biotechnol Prog 21:1572–1576
Peebles CAM, Sander GW, Hughes EH, Peacock R, Shanks JV, San KY (2011) The expression of 1-deoxy-D-xylulose synthase and geraniol-10-hydroxylase or anthranilate synthase increases terpenoid indole alkaloid accumulation in Catharanthus roseus hairy roots. Metab Eng 13:234–240
Pietrosuik A, Furmanova M, Lata B (2007) Catharanthus roseus: micropropagation and in vitro techniques. Phytochem Rev 6:459–473
Radwanski ER, Last RL (1995) Tryptophan biosynthesis and metabolism: Biochemical and molecular genetics. Plant Cell 7:921–934
Rai A, Smita SS, Singh AK, Shanker K, Nagegowda DA (2013) Heteromeric and homomeric geranyl diphosphate synthases from Catharanthus roseus and their role in monoterpene indole alkaloid biosynthesis. Mol Plant 6:1531–1549
Rischer H (2006) Gene-to-metabolite networks for terpenoid indole alkaloid biosynthesis in Catharanthus roseus cells. Proc Natl Acad Sci USA 103:5614–5619
Rodriguez S, Compagnon V, Crouch NP, St-Pierre B, De Luca V (2003) Jasmonate- induced epoxidation of tabersonine by a Cytochrome P-450 in hairy root cultures of Catharanthus roseus. Phytochemistry 64:401–409
Roepke J, Salim V, Wu M, Thamm AMK, Murata J, Ploss K, Boland W, De Luca V (2010) Vinca drug components accumulate exclusively in leaf exudates of Madagascar periwinkle. Proc Natl Acad Sci U S A 107:15287–15292
Ruiz-May E, Galaz-Avalos RM, Loyola-Vargas VM (2009) Differential secretion and accumulation of terpene indole alkaloids in hairy roots of Catharanthus roseus treated with methyl jasmonate. Mol Biotechnol 41:278–285
Schlatmann JE, Koolhaas CMA, Vinke JL, Ten Hoopen HJG, Heijnen JJ (1995a) The role of glucose in ajmalicine production by Catharanthus roseus cell cultures. Biotechnol Bioeng 47:525–534
Schlatmann JE, Moreno PRH, Selles M, Vinke JL, Ten Hoopen HJG, Verpoorte R, Heijnen JJ (1995b) Two stage batch process for the production of ajmalicine by Catharanthus roseus: the link between growth and production stage. Biotechnol Bioeng 47:53–59
Schroder G, Unterbusch E, Kaltenbach M, Schmidt J, Strack D, De Luca V, Schroder J (1999) Light-induced cytochrome P450-dependent enzyme in indole alkaloid biosynthesis: Tabersonine 16-hydroxylase. FEBS Lett 458:97–102
Scott AI, Mizukami H, Lee S-L (1979) Characterization of a 5-methyltryptophan resistant strain of Catharanthus roseus cultured cells. Phytochemistry 18:795–798
Seth R, Mathur AK (2005) Selection of 5-methyltryptophan resistant callus lines with improved metabolic flux towards terpenoid indole alkaloid synthesis in Catharanthus roseus. Curr Sci 89:544–548
Sharma KK, Bhatnagar-Mathur PB, Thorpe TA (2005) Genetic transformation technology: status and problems. In Vitro Cell Dev Biol Plant 41:102–112
Shukla AK, Shasany AK, Gupta MM, Khanuja SPS (2006) Transcriptome analysis in Catharanthus roseus leaves and roots for comparative terpenoid indole alkaloid profiles. J Exp Bot 57:3921–3932
Shukla A, Shasany AK, Verma RK, Gupta MM, Mathur AK, Khanuja SPS (2010) Influence of cellular differentiation and elicitation on intermediate and late steps of terpenoid indole alkaloid biosynthesis in Catharanthus roseus. Protoplasma 242:35–47
Sim SJ, Chang HN, Liu JR, Jung KH (1994) Production and secretion of indole alkaloids in hairy root cultures of Catharanthus roseus: effects of in situ adsorption, fungal elicitation and permeabilization. J Ferment Bioeng 78:229–234
Sottomayor M, Ros Barcelo A (2003) Peroxidase from Catharanthus roseus (L.) G. Don and the biosynthesis of alpha-3′,4′-anhydrovinblastine: a specific role for a multifunctional enzyme. Protoplasma 222:97–10
Sottomayor M, Lopez-Serrano M, DiCosmo F, Ros-Barcelo A (1998) Purification and characterization of α-3′,4′-anhydrovinblastine synthase (peroxidase-like) from Catharanthus roseus (L.) G. Don. FEBS Lett 428:299–303
St-Pierre B, De Luca V (1995) A cytochrome P-450 monooxygenase catalyzes the first step in the conversion of tabersonine to vindoline in Catharanthus roseus. Plant Physiol 109:131–139
St-Pierre B, Laflamme P, Alarco AM, De Luca V (1998) The terminal O-acetyltransferase involved in vindoline biosynthesis defines a new class of proteins responsible for coenzyme A-dependent acyl transfer. Plant J 14:703–713
St-Pierre B, Vazquez-Flota FA, De Luca V (1999) Multicellular compartmentation of Catharanthus roseus alkaloid biosynthesis predicts intercellular translocation of a pathway intermediate. Plant Cell 11:887–900
Suttipanta N, Pattanaik S, Kulshrestha M, Patra B, Singh SK, Yuan L (2011) The transcription factor CrWRKY1 positively regulates the terpenoid indole alkaloid biosynthesis in Catharanthus roseus. Plant Physiol 157:2081–2093
Svoboda GH, Blake DA (1975) The phytochemistry and pharmacology of Catharanthus roseus (L.) G. Don. In: Taylor WI, Farnsworth NR (eds) The Catharanthus alkaloids. Marcel Dekker, New York, pp 45–83
Ten Hoopen HJG, Van Gulik WM, Schlatmann JE, Moreno PRH, Vinke JL, Heijnen JJ, Verpoorte R (1994) Ajmalicine production by cell cultures of Catharanthus roseus: from shake flask to bioreactor. Plant Cell Tiss Org Cult 38:85–91
Thakore D, Srivastava AK, Sinha AK (2013) Yield enhancement strategies for enhancement of indole alkaloids in hairy root cultures of Catharanthus roseus. Int J Chem Eng Appl 4:153–156
Toivonen L, Laakso S, Rosenqvist H (1992) The effect of temperature on hairy root cultures of Catharanthus roseus: growth, indole alkaloid accumulation and membrane lipid composition. Plant Cell Rep 11:395–399
Trick HN, Finer JJ (1997) SAAT: sonication assisted Agrobacterium-mediated transformation. Transgenic Res 6:329–336
Van der Fits L, Memelink J (2000) ORCA3, a jasmonate-responsive transcriptional regulator of plant primary and secondary metabolism. Science 289:295–297
Van der Fits L, Hilliou F, Memelink J (2001) T-DNA activation tagging as a tool to isolate regulators of a metabolic pathway from a genetically non-tractable plant species. Transgenic Res 10:513–521
Van der Heijden R, Jabos DJ, Snoeijer W, Hallard D, Verpoorte R (2004) The Catharanthus alkaloids: pharmacognosy and biotechnology. Curr Med Chem 11:1241–1253
Vasquez-Flota F, De Luca V (1998) Jasmonate modulates development and light regulated alkaloid biosynthesis in Catharanthus roseus. Phytochemistry 49:395–402
Vazquez-Flota F, De Carolis E, Alarco A-M, De Luca V (1997) Molecular cloning and characterization of desacetoxyvindoline 4-hydroxylase, a 2-oxoglutarate dependent dioxygenase involved in the biosynthesis of vindoline in Catharanthus roseus (L.) G. Don. Plant Mol Biol 34:935–948
Verma P, Mathur AK (2011a) Direct shoot bud organogenesis and plant regeneration from leaf explants in Catharanthus roseus. Plant Cell Tiss Org Cult 106:401–408
Verma P, Mathur AK (2011b) Agrobacterium tumefaciens mediated transgenic plant production via direct shoot bud organogenesis from from pre-plasmolyzed leaf explants of Catharanthus roseus. Biotechnol Lett 33:1053–1060
Verma P, Mathur AK, Shanker K (2012a) Growth, alkaloid production, rol genes integration, bioreactor up-scaling and plant regeneration studies in hairy root lines of Catharanthus roseus. Plant Biosyst 146:27–40
Verma P, Mathur AK, Shanker K (2012b) Increased availability of tryptophan in 5-methyltryptophan tolerant shoots of Catharanthus roseus and their post-harvest in vivo elicitation induces enhanced vindoline production. Appl Biochem Biotechnol 168:568–579
Verma P, Mathur AK, Srivastava A, Mathur A (2012c) Emerging trends in research on spatial and temporal organization of terpenoid indole alkaloids pathway in Catharanthus roseus: a literature up-date. Protoplasma 249:255–268
Verma P, Mathur AK, Masood N, Luqman S, Shankar K (2013) Tryptophan over producing cell suspensions of Catharanthus roseus (L) G. Don and their up-scaling in stirred tank bioreactor: detection of phenolic compound with antioxidant potential. Protoplasma 250:371–380
Verma P, Mathur AK, Khan SA, Verma N, Sharma A (2015a) Transgenic studies for modulating terpenoid indole alkaloids pathway in Catharanthus roseus: present status and future options. Phytochem Rev. doi:10.1007/s11101-015-9447-8
Verma P, Khan SA, Mathur AK, Srivastava A, Shanker K (2015b) Tryptophan metabolism and evaluation of morphological, biochemical and molecular variations in a field grown plant population derived via direct adventitious shoot bud regeneration from pre-plasmolysed leaves of Catharanthus roseus. Plant Cell Tiss Org Cult 123:357–375
Verma P, Sharma A, Khan SA, Mathur AK, Shanker K (2015c) Morpho-genetic and chemical stability of long term maintained Agrobacterium mediated transgenic Catharanthus roseus plants. Nat Prod Res 29:315–320
Verma P, Sharma A, Khan SA, Shanker K, Mathur AK (2015d) Over-expression of Catharanthus roseus tryptophan decarboxylase and strictosidine synthase in rol gene integrated transgenic cell suspensions of Vinca minor. Protoplasma 252:373–381
Verpoorte R, Alfermann AW (2000) Metabolic engineering of plant secondary metabolism. In: Verpoorte R, Alfermann AW (eds). Kluwer Academic, Dordrecht
Verpoorte R, Van der Heijden R, Van Gulik W M, Ten Hoopen H J G (1991) Plant biotechnology for the production of alkaloids: present status and prospects. In: Brossi A (ed) The alkaloids, vol 40. Academic, San Diego, pp 1–187
Verpoorte R, Van der Heijden R, Schripsema J (1993) Plant cell biotechnology for the production of alkaloids: present status and prospects. J Nat Prod 56:186–207
Verpoorte R, van der Heijden R, Moreno PRH (1997) Biosynthesis of terpenoid indole alkaloids in Catharanthus roseus cells. In: Cordell GA (ed) The alkaloids, vol 49. Academic, San Diego, p 221
Verpoorte R, Contin A, Memelink J (2002) Biotechnology for the production of plant secondary metabolites. Phytochem Rev 1:13–25
Wakasa K, Tozawa Y, Terakawa T, Hasegawa H (1999) Gene encoding a-subunit of rice anthranilate synthase and DNA relating thereto. World Intellectual Property Organization 99/11800
Wang CT, Liu H, Gao XS, Zhang HX (2010) Overexpression of G10H and ORCA3 in the hairy roots of Catharanthus roseus improves catharanthine production. Plant Cell Rep 29:887–894
Weaver J, Goklany S, Rizvi N, Cram EJ, Lee-Parson CWT (2014) Optimizing the transient fast agro-mediated seedling transformation (FAST) method in Catharanthus roseus seedlings. Plant Cell Rep 33:89–97
Whitmer S, Van der Heijden R, Verpoorte R (2002a) Effect of precursor feeding on alkaloid accumulation by a strictosidine synthase over-expressing transgenic cell line S1 of Catharanthus roseus. Plant Cell Tiss Org Cult 69:85–93
Whitmer S, Van der Heijden R, Verpoorte R (2002b) Effect of precursor feeding on alkaloid accumulation by a tryptophan decarboxylase overexpressing transgenic cell line T22 of Catharanthus roseus. J Biotechnol 96:193–203
Whitmer S, Canel C, van der Heijden R, Verpoorte R (2003) Long-term instability of alkaloid production by stably transformed cell lines of Catharanthus roseus. Plant Cell Tiss Org Cult 74:73–80
Wilson L, Pand D, Jordan MA (1999) Modulation of microtubule dynamics by drugs: a paradigm for the actions of cellular regulators. Cell Struct Funct 24:329–335
Zarate R, Verpoorte R (2007) Strategies for the genetic modification of the medicinal plant Catharanthus roseus (L.) G. Don. Phytochem Rev 6:475–491
Zargar M, Farahani F, Nabavi T (2010) Hairy roots production of transgenic Catharanthus roseus L. plants with Agrobacterium rhizogenes under in vitro conditions. J Med Plant Res 4:2199–2203
Zhao J, Verpoorte R (2007) Manipulating indole alkaloid production by Catharanthus roseus cell cultures in bioreactors: from biochemical processing to metabolic engineering. Phytochem Rev 6:435–457
Zhao J, Zhu Wei-Hua HQ (2001a) Selection of fungal elicitors to increase indole alkaloid accumulation in Catharanthus roseus suspension culture. Enzyme Microb Technol 28:666–672
Zhao J, Zhu Wei-Hua HQ (2001b) Enhanced catharanthine production in Catharanthus roseus cell cultures by combined elicitor treatment in shake flasks and bioreactors. Enzyme Microb Technol 28:673–681
Zhong JJ (2001) Biochemical engineering of the production of plant-specific secondary metabolites by cell cultures. Adv Biochem Eng Biotechnol 72:1–26
Zhou ML, Hou HL, Zhu XM, Shao JR, Wu YM, Tang YX (2011) Soybean transcription factor GmMYBZ2 represses catharanthine biosynthesis in hairy roots of Catharanthus roseus. Appl Microbiol Biotechnol 4:1095–1105
Zhou C, Zhang J, Zhao SJ, Hu ZB (2014) An active Catharanthus roseus desacetoxyvindoline-4-hydroxylase-like gene and its transcriptional regulatory profile. Bot Stud 55:29
Acknowledgement
The authors wish to express their sincere thanks to Director CSIR- NCL, for providing the facilities. Grateful appreciation also goes to the Council of Scientific & Industrial Research (CSIR), New Delhi, India, for the financial support in the form of CSIR-SRA (Pool Scientist) to the senior author and DBT-SERB for Fast Track Young Scientist Award to second author.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Verma, P., Khan, S.A., Parasharami, V., Mathur, A.K. (2017). Biotechnological Interventions to Modulate Terpenoid Indole Alkaloid Pathway in Catharanthus roseus Using In Vitro Tools and Approaches. In: Naeem, M., Aftab, T., Khan, M. (eds) Catharanthus roseus. Springer, Cham. https://doi.org/10.1007/978-3-319-51620-2_10
Download citation
DOI: https://doi.org/10.1007/978-3-319-51620-2_10
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-51619-6
Online ISBN: 978-3-319-51620-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)