Abstract
Increase in prevalence of obesity has become a worldwide major health problem in adults, as well as among children and adolescents. Furthermore, total adiposity and truncal subcutaneous fat accumulation during adolescence are positively and independently associated with atherosclerosis at adult ages. Centrally accumulation of body fat is associated with insulin resistance, whereas distribution of body fat in a peripheral pattern is metabolically less important. Obesity is associated with a large decrease in life expectancy. The effect of extreme obesity on mortality is greater among younger than older adults. In this respect, obesity is also associated with increased risk of several cancer types. However, up to 30% of obese patients are metabolically healthy with insulin sensitivity similar to healthy normal weight individuals, lower visceral fat content, and lower intima media thickness of the carotid artery than the majority of metabolically “unhealthy” obese patients.
Abdominal obesity is the most frequently observed component of metabolic syndrome. The metabolic syndrome; clustering of abdominal obesity, dyslipidemia, hyperglycemia and hypertension, is a major public health challenge. The average prevalence of metabolic syndrome is 31%, and is associated with a two-fold increase in the risk of coronary heart disease, cerebrovascular disease, and a 1.5-fold increase in the risk of all-cause mortality.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Metabolic syndrome
- Body mass index
- Metabolically healthy obese
- Insulin resistance
- Obesity-paradox
- Prevalence of obesity
1 Introduction
Increasing prevalence of obesity is a worldwide health concern because excess weight gain causes an increased risk for several diseases, most notably cardiovascular diseases , diabetes , and cancers (Wang et al. 2011). The global food system drivers interact with local environmental and genetic factors to create a wide variation in obesity prevalence between populations. Epidemiologically, in low-income countries, obesity mostly affects middle-aged adults, whereas in high-income countries it affects both sexes and all ages (Swinburn et al. 2011). On the other hand, increased obesity rates lead to a large health and economic burden in all countries (Rtveladze et al. 2014). According to Keaver et al. overweight and obesity are proposed to reach levels of 89% and 85% in males and females, respectively by 2030. This will result in an increase in the obesity-related prevalence of coronary heart disease (CHD) by 97%, cancers by 61% and type 2 diabetes by 21%. Thereupon, the direct healthcare costs will increase significantly. A 5% reduction in population body mass index (BMI) levels by 2030 is estimated to result in €495 million decrease in the expenditures in obesity-related direct healthcare over 20 years (Keaver et al. 2013). Additionally, after adjustment for significant maternal and sociodemographic characteristics, healthcare costs of children with obesity are 1.62 times higher than those of children with healthy weight (Hayes et al. 2016). Cost-effective strategies targeted at reducing the prevalence of obesity during the early years of life can significantly reduce both healthcare and non-healthcare costs over the lifetime (Sonntag et al. 2016). In this respect, the obesity related disease burden on health care expenses should be evaluated with the epidemiological data considering whether the obese population is metabolically healthy or not.
2 Definition and Prevalence of Obesity
Obesity is usually classified by BMI. It is calculated as body weight in kilograms divided by the height in meters squared (kg/m2). Other methods, including waist circumference (WC) and central and peripheral fat mass, have also been used, but currently BMI is continued to be used for the classification of obesity . However, BMI does not give a precise idea about the body composition which affects the health risks of excess weight such as the proportion of body weight which consists of fat or the distribution of fat. These discrepancies will be discussed in later sections. Nevertheless, BMI is now the internationally accepted standard method used by researchers and the others dealing with human health, in spite of its alternatives. According to BMI; individuals are allocated to five different categories as 18.5–24.9 kg/m2: normal range, 25.0–29.9 kg/m2: overweight, 30.0–34.9 kg/m2: class 1-obesity, 35.0–39.9 kg/m2: class 2-obesity, equal or greater 40 kg/m2: class 3-obesity. Morbid obesity is considered to be grade 3 obesity or grade 2 obesity plus significant obesity-related co-morbidities (Ashwell et al. 2014; Dixon et al. 2011). In the past 33 years, 1769 studies from 104 different centers indicated that the established health risks and substantial increase in prevalence of obesity has become a major worldwide health problem. The proportion of adults with a BMI of 25 kg/m2 or greater increased between 1980 and 2013 from 28.8% (95% Uncertainty intervals (UI): 28.4–29.3) to 36.9% (36.3–37.4) in men, and from 29.8% (29.3–30.2) to 38.0% (37.5–38.5) in women (Ng et al. 2014). Moreover, population-based studies in different countries showed that obesity will continue to be a serious health-risk in future. Between 1985 and 2011, the prevalence of adult obesity in Canada increased from 6.1% to 18.3%. Furthermore, since 1985, the prevalence of obesity in classes 1, 2 and 3 increased from 5.1% to 13.1%, from 0.8% to 3.6%, and from 0.3% to 1.6%, respectively. It has been predicted that, by 2019, the prevalence of obesity in classes 1, 2 and 3 will increase to 14.8%, 4.4% and 2.0%, respectively (Twells et al. 2014). By 2030, in the USA up to 86% of adults will be overweight or obese (Ginter and Simko 2014). In Australia, approximately 63% of adults were overweight and obese in between the years 2011–2012. The proportion of the Australian population who are overweight and obese is expected to increase to approximately 66% in 2017 (Sassi et al. 2009; Statistics 2012).
On the other hand the prevalence of overweight and obesity among children and adolescents has also increased worldwide (Ebbeling et al. 2002; Lissau et al. 2004). Thirty-nine articles and one national health report that were undertaken to consideration; in 16 of the 23 countries with national representative data using the International Obesity Task Force (IOTF) cut-off, over-weight and obesity prevalence were found to be higher than 20%, five countries showed prevalence above 30%, and only in two countries prevalence was lower than 10%. Data from the National Health and Nutrition Examination Survey 2009–10 (NHANES) indicated a prevalence of overweight and obesity among 4111 adolescents aged 12 through 19 years of 15.2% and 18.4%, respectively (Bibiloni et al., 2013). IOTF versus BMI cut-offs are widely used to assess the prevalence of child overweight, obesity and thinness. IOTF defines the revised international child cut-offs and they are available corresponding to the following BMI cut-offs at 18 years. 16 kg/m2: thinness grade 3, 17 kg/m2: thinness grade 2, 18.5 kg/m2: thinness grade 1, 23 kg/m2: overweight (unofficial Asian cut-off), 25 kg/m2: overweight, 27 kg/m2: obesity (unofficial Asian cut-off), 30 kg/m2: obesity, 35 kg/m2: morbid obesity (Cole and Lobstein 2012).
The prevalence has increased substantially in children and adolescents in developed countries; 23.8% (BMI; 22.9–24.7) of boys and 22.6% (BMI; 21.7–23.6) of girls were overweight or obese in 2013. The prevalence of overweight and obesity has also increased in children and adolescents in developing countries, from 8.1% (min; 7.7–max; 8.6) to 12.9% (min; 12.3–max; 13.5) for boys and from 8.4% (min; 8.1–max; 8.8) to 13.4% (min; 13.0–max; 13.9) for girls during the same time period (Ng et al. 2014). In an another European country, Spain, the prevalence of overweight and obesity in children was determined by using Spanish reference tables (SPART) , IOTF reference values, and The World Health Organization (WHO) growth standards. The average prevalence of overweight in boys ranged from 14.1% to 26.7%, and in girls from 13.8% to 25.7% (Pérez-Farinós et al. 2013). The WHO Regional Office for Europe analyzed 168,832 children from 12 countries in the context of WHO European Childhood Obesity Surveillance Initiative (COSI). Indeed, COSI routinely measures overweight and obesity prevalence of primary-school children aged 6–9 years. According to COSI data, the prevalence of obesity ranged from 6.0% to 26.6% among boys and from 4.6% to 17.3% among girls. Consequently, overweight among 6–9-year-old children is identified as a serious public health concern (Wijnhoven et al. 2013). BMI is correlated with visceral adipose tissue (VAT) in pediatric populations. Furthermore, BMI may also be converted to BMI percentiles by using Centers for Disease Control and Prevention (CDC) growth charts. BMI percentile is a sensible and useful tool for the prediction of VAT mass, fat mass and cardiovascular disease (CVD) risk in children and adolescents (Harrington et al. 2013). In a series of 14,493 children, increasing waist-to-height ratio (WHtR) was significantly associated with increased cardiometabolic risk in overweight and obese subjects. Obese subjects with WHtR higher than 0.6 should undergo a further cardiometabolic risk assessment (Khoury et al. 2013). However, in 3–5 years old overweight/obese children, WHtR is not found to be superior to WC or BMI in estimating body fat percentage (BF%) and cardiometabolic risk (Sijtsma et al. 2014). Regardless of weight and length at birth, the correlation of higher BMI at preschool age with the ratio of weight gain per cm gain in height may be a better indicator of risk for overweight and obesity (Nascimento et al. 2011). Actually, overweight children often become overweight adolescents and adults later. A child or adolescent with a high BMI percentile may have a high risk of being overweight or obese at 35 years of age, and this risk increases with age (Guo et al. 2002). Furthermore in men, total adiposity and truncal subcutaneous fat accumulation during adolescence, are positively and independently associated with atherosclerosis at age 36 (Ferreira et al. 2004). Indeed, accumulation of body fat centrally is associated with insulin resistance (IR ), whereas distribution of body fat in a peripheral pattern is metabolically less important. Thus, increased visceral or intra-abdominal fat are more insulin resistant than those who have increased quantities of centrally located subcutaneous fat (Kahn et al. 2001). Body fat content and the fat distribution or adiposity is considered as important indicators of health risk. Body adiposity index (BAI) based on the measurements of hip circumference and height is suggested as a useful predictor of obesity (Bergman et al. 2011). Melmer et al. observed that BAI is significantly associated with leptin and hip circumference in 1770 patients from the Salzburg Atherosclerosis Prevention Program in Subjects at High Individual Risk (SAPHIR) study (Melmer et al. 2013). However, a cross-sectional study was conducted in 29,214 men and 21,040 women Spanish Caucasian participants revealed that, WHtR and WC are better adiposity indexes than BAI and BMI (Bennasar-Veny et al. 2013).
Moreover, the connection between BMI and body fat in young people differs among ethnic groups and childhood. CDC and IOTF thresholds showed low sensitivity or low specificity for predicting excess percentage body fat in different ethnic groups. Overweight may not represent an equivalent level of adiposity and ethnic-specific BMI cut-off points (Duncan et al. 2009). The anthropometric results of COSI Round 2 (2009/2010) and changes in BMI showed that the highest significant decrease in BMI-for-age anthropometric-Z-scores was found in countries with higher absolute BMI values and the highest significant increase in countries with lower BMI values. Changes in BMI and prevalence of obesity over a 2-year period varied significantly among European countries (Wijnhoven et al. 2014). As mentioned above the same anthropometric obesity measures cannot be used across all ethnic groups for the assessment of obesity complications. Overall, it is claimed that the central obesity measures, WC and waist-hip ratio (WHpR) are better predictors of cardiovascular risk. Inspite of that WHpR is reported to have a stronger predictive ability than WC and BMI in Caucasian women. However, BMI in Northern European women is a better indicator of risk using in the general CVD and Framingham risk score models. Nonetheless, it has been suggested that WC is the most predictive tool for cardiovascular risk among Asian women (Goh et al. 2014). In fact, Framingham data suggest that obesity and physical inactivity exert adverse effect on development of CHD through the major risk factors. Indeed, the increased risk associated with metabolic syndrome (MS) is reflected by the Framingham scores primarily through high-density lipoprotein (HDL ) cholesterol , blood pressure, and diabetes mellitus. However, some other risk factors for CHD such as triglycerides, low-density lipoprotein (LDL) particles, lipoprotein (a), coagulation factors, and homocysteine are not taken into account. Moreover, the impact of these risk factors may vary in various geographic and ethnic groups (Grundy et al. 1998).
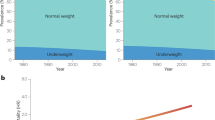
Obesity is associated with large decreases in life expectancy approximately by 3.3–18.7 years and also large increases in healthcare expenditures (Leung et al. 2015). Above BMI 25, each 5 kg/m2 higher BMI is on average associated with about 30% higher overall mortality, which is mainly due to increased risk of cardiovascular death as 40%. While BMI at 30–35 kg/m2, median survival is reduced by 2–4 years; at 40–45 kg/m2, it is reduced by 8–10 years (Prospective Studies Collaboration et al. 2009). However, it is claimed that WHtR is a better predictive risk measure of mortality than BMI (Ashwell 2012; Ashwell et al. 2012). Optimal values of WHtR are calculated as 0.5 for males and 0.46 for females. The years of life lost (YLL) for the different values of WHtR are positively correlated with the life expectancy. In a 30-year-old male non-smoker with a WHtR of 0.7, life expectancy is 7.2 years less than a 30-year-old male with a WHtR of 0.5. The corresponding figure is also valid for a 30-year-old female with WHtR of 0.7 compared to female with WHtR of 0.46. Life expectancy is 4.6 years shorter in patient with higher WHtR. YLL increases dramatically from categories in excess of WHtR 0.52 for both males and females (Ashwell et al. 2014). A pooled data from 11 prospective cohort studies with 650,386 adults aged 20–83 years showed that higher WC was positively associated with higher mortality at all levels of BMI from 20 to 50 kg/m2. In this study median follow-up was 9 years and 78,268 participants died. Life expectancy for highest versus lowest WC was approximately 3 years less for men (WC of ≥110 vs <90 cm) and approximately 5 years less for women (WC of ≥95 vs <70 cm) (Cerhan et al. 2014).
The effect of extreme obesity on mortality is greater among younger than among older adults, greater among men than women, and greater among whites than blacks (Hensrud and Klein 2006). Excess weight related deaths increased by 31% and associated years of potential life loss and quality adjusted life years lost by about 37%, respectively, between 2002 and 2008 in Germany. About 73% of total excess weight related costs are attributable to obesity. The main drivers of direct costs are endocrinological (44%) and cardiovascular (38%) diseases (Lehnert et al. 2015). Amongst 1799 patients with BMI more than or equal to 30 kg/m2, those with either nonspecific or protein-energy malnutrition have increased mortality relative to well-nourished patients, while the odds ratio of 90-day mortality is 1.67 (95% confidence interval (CI), 1.29–2.15; p < 0.0001) (Robinson et al. 2015). In a series of 154,308 intensive care unit patients, Pickkers et al. asserted that hospital mortality risks quickly increase in underweight critically ill patients with BMI < 18.5 kg/m2, whereas obese patients with a BMI of 30–39.9 kg/m2 have the lowest risk of death. Because of the well-known obesity-associated decrease in overall life expectancy, these results could not be explained (Pickkers et al. 2013). Even though the incidence of hypertension, dyslipidemia, type 2 diabetes mellitus , CVD and mortality are directly proportional with the BMI, obese individuals may have better outcomes compared to lean counterparts. This condition is termed as the obesity paradox (Goyal et al. 2014). Obese and morbidly obese patients more frequently develop intensive care unit-acquired infections than patients in lower BMI categories. However, no significant differences were observed among the groups in intensive care unit or hospital mortality rates (Sakr et al. 2008). Indeed multiple statistical reviews have suggested improved outcomes for obese intensive care unit patients. Many articles highlight potential confounders related to metabolic problems that may cause misleading results. Thus, BMI has been traditionally used to stratify risk in obese populations (Kiraly et al. 2011). For instance, obese patients may be at greater risk of developing acute respiratory distress syndrome (ARDS) than normal weight patients (Stapleton and Suratt 2014). Obesity-related factors cause 11% of heart failure cases in men and 14% in women by inducing haemodynamic and myocardial changes due to an increased cardiac lipotoxicity that lead to cardiac dysfunction (Ebong et al. 2014). In 97 studies providing more than 2.88 million individuals and more than 270,000 deaths, relative to normal weight, both obesity (all grades) and grades 2 and 3 obesity are associated with significantly higher all-cause mortality. Grade 1 obesity overall is not associated with higher mortality. Furthermore, overweight is associated with significantly lower all-cause mortality (Flegal et al. 2013). The relative risks of all-cause mortality in overweight and obese patients with type 2 diabetes were 0.81 and 0.72, respectively, compared with the normal or non-overweight patients out of 161,984 participants from nine studies of 13 cohorts (Liu et al. 2015).
Epidemiological studies have shown that obesity is also associated with increased risk of several cancer types. Recently the protein kinase B/phosphatidylinositol 3-kinase/mammalian target of rapamycin (Akt/PI3K/mTOR) cascade has become a focus of the obesity and cancer connection (Vucenik and Stains 2012). On the one hand leptin is positively correlated with adipose stores. On the other hand, it induces cancer progression by activation of PI3K/Akt , mitogen-activated protein kinases (MAPK) , mTOR and signal transducer and activator of transcription 3 (STAT3) pathways as a potential mediator of obesity-related cancer (Chen 2011; Drew 2012). It has been suggested that a close relationship between the metabolic health status and obesity-related cancer mortality. While the risk of cancer mortality decreases depending on the obesity status, it increases depending on the metabolic health status. The mortality rate from cancer rises with the progress of metabolic dysfunction (Oh et al. 2014). Although BMI had no impact on recurrence-free survival in obese women in the low−/intermediate-risk groups, severe obesity (BMI ≥ 35) negatively impacts recurrence-free survival in women with high-risk endometrial cancer (Canlorbe et al. 2015). Eighty-two studies, including 213,075 breast cancer survivors with 23,182 deaths from breast cancer showed that relative risks of mortality are 1.75 for pre-menopausal and 1.34 for post-menopausal breast cancer for obese women. For each 5 kg/m2 increment of BMI before and after 1 year of cancer diagnosis increases risks by 18% and 29% for breast cancer mortality, respectively. In this case obesity is associated with poorer breast cancer survival regardless of BMI ascertainment period (Chan et al. 2014). Similarly, a trend of increased colorectal cancer risk was observed with longer duration of obesity (Peeters et al. 2015), whereas, no association was found between high BMI and risk of prostate cancer incidence in a series of 904 cases (Grotta et al. 2015).
3 Mortality Risk of Metabolically Healthy Obese Individuals
Although obesity significantly increases the risk of developing metabolic disorders, hypertension, CHD, stroke, and several types of cancer, up to 30% of obese patients are metabolically healthy with insulin sensitivity similar to healthy normal weight individuals, lower visceral fat content, and lower intima media thickness of the carotid artery than the majority of metabolically “unhealthy” obese patients (Blüher 2012). These individuals do not display the “typical” metabolic obesity-associated complications. Severity of IR as well as subclinical inflammation , type 2 diabetes , dyslipidemia, hypertension and cardiovascular disease differentiates the metabolically non-healthy obese from metabolically healthy obese (MHO) (Blüher and Schwarz 2014). MHO approximately consists of 10–25% of the obese (Blüher 2010). Systematic review of the prevalence of MHO in database revealed that 30 different forms of metabolic health have been identified in 27 different publications based on four common criteria; blood pressure, HDL cholesterol , triglycerides and plasma glucose. BMI ≥30 kg/m2 is the main indicator used to define obesity in two thirds of the studies. In these cases, estimated metabolically healthy obesity prevalence is between 10% and 51% (Rey-López et al. 2014). In total of 881 obese subjects, a more detailed MHO was defined by using six sets of criteria including different combinations of WC , blood pressure, total HDL cholesterol or LDL -cholesterol, triglycerides, fasting glucose, homeostasis model, high-sensitivity C-reactive protein (hs-CRP), and personal history of cardiovascular, respiratory or metabolic diseases (Marques-Vidal et al. 2012). Actually the MHO phenotype frequently refers to obese individuals with a favorable metabolic profile. In Whitehall II study, 657 individuals out of 7122 participants were obese and 42.5% of these were classified as MHO. Over the median follow-up of 17.4 years, 828 incident cases of CVD or stroke and 798 incident cases of type 2 diabetes were diagnosed. MHO subjects were at increased risk for CVD (Hazard Ratio (HR): 1.97, 95% CI: 1.38–2.80) and type 2 diabetes (HR: 3.25, 95% CI: 2.32–4.54) when compared with metabolically healthy normal weight individuals. For type 2 diabetes , the MHO phenotype is associated with lower risk than the metabolically unhealthy obese, but CVD risk was high in both obesity phenotypes (Hinnouho et al. 2015). Additionally, MHO individuals have higher prevalence of subclinical coronary atherosclerosis than metabolically-healthy normal-weight subjects (Chang et al. 2014). There are no clear accepted criteria on the definition of MHO, as well as the biological mechanisms to explain this phenotype . Several prospective studies suggested that the MHO individual has been associated with a similar risk of developing type 2 diabetes, CVD and mortality when compared to healthy normal weight subjects (Plourde and Karelis 2014). Thus, Phillips et al. showed that prevalence of metabolically healthy individuals was 6.8–36.6% among the obese, whereas prevalence of metabolically unhealthy subjects was 21.8–87% among the non-obese subjects (Phillips et al. 2013). Moreover, eight studies with 61,386 participants were evaluated for all-cause mortality and/or cardiovascular events. MHO individuals had increased risk for events compared with metabolically healthy normal-weight individuals. Compared with metabolically healthy normal-weight individuals, obese persons are at increased risk for adverse long-term outcomes even in the absence of metabolic abnormalities, suggesting that there is no healthy pattern of increased weight (Kramer et al. 2013). In a population-based study among Mexican Americans and non-Hispanic whites, type 2 diabetes mellitus and CVD were evaluated in 2814 and 3700 participants aged 25–64 years, respectively. The risk of developing type 2 diabetes mellitus and CVD is increased in both metabolically unhealthy normal weight and MHO individuals (Aung et al. 2014). At any time of life all metabolically unhealthy groups; whether normal weight or overweight and obese had a similarly elevated risk (Kramer et al. 2013). Several factors may participate in these discrepancies. In contrast to MHO subjects, elderly individuals with the metabolically obese normal-weight phenotype exhibit greater all-cause mortality during 10 years of follow-up (Choi et al. 2013). Although the obese population defines as being metabolically healthy or not depending on the method used to ascertain metabolic health, obese individuals carry an excess risk of mortality irrespective of their metabolic status (Hinnouho et al. 2013). Consequently, MHO participants were not significantly different from healthy lean individuals by any definition. Nevertheless, they have an intermediate level of risk between healthy lean and unhealthy obese groups (Durward et al. 2012). Since the incidence of obesity continues to rise, importance of MHO phenotype is increasing (Roberson et al. 2014). Furthermore, some evidences indicate that less than 15 years of follow-up data in population based cohort may underestimate the overall mortality risk associated with the metabolic disorders (Sundström et al. 2006).
4 Prevalence of Metabolic Syndrome
In epidemiological studies, MS occurrence varies between 20% and 45% of population. Abdominal obesity is the most frequently observed component of MS . The incidence of MS is expected to increase to approximately 53% at 2035 (Gierach et al. 2014). Abdominal obesity, nuclear peroxisome proliferator-activated (PPAR) modulation, IR with or without glucose intolerance, atherogenic dyslipidemia, elevated blood pressure and proinflammatory states are included in the principal components of MS (Tenenbaum et al. 2004). Adipocyte size of both subcutaneous and omental fat are increased with higher body fat mass. Hyperplasia takes place primarily in the subcutaneous fat tissue, whereas fat cell hypertrophy occurs both in the omental and subcutaneous compartments. Eventually, fat accumulation is progressively increases in the subcutaneous tissue than in the visceral fat compartment (Drolet et al. 2008). It has been suggested that WC and BMI are the most accurate surrogate markers of visceral adiposity in young adults, and are good indicators of IR and powerful predictors of the presence of hepatic steatosis (Borruel et al. 2014). However, overweight individuals with similar BMI values have markedly different levels of VAT (Cartier et al. 2008). Actually high amount of VAT has significantly higher concentrations of soluble tumor necrosis factor-alpha (TNF-alpha) receptor 2 compared with obese men with low VAT and with lean controls (Cartier et al. 2010). Although both subcutaneous abdominal adipose tissue and VAT are correlated with metabolic risk factors, VAT remains more strongly associated with an adverse metabolic risk profile (Fox et al. 2007). Indeed, increased VAT is strongly associated with incident hypertension, but not total or subcutaneous adiposity (Chandra et al. 2014). The rate of visceral fat accumulation is different according to the individuals’ gender and ethnic background. Furthermore, the connection between visceral adiposity and obesity-related cardiometabolic problems has been shown to be independent of age, overall obesity or the amount of subcutaneous fat (Hamdy et al. 2006). Because of the significant contribution of visceral fat accumulation to the development of metabolic disorders, VAT is determined through different imaging techniques (Iannucci et al. 2007). Thus, computerized tomography or magnetic resonance imaging recognizes ethnic differences in susceptibility to visceral adiposity and related metabolic abnormalities. Actually clinical diagnosis of visceral obesity alone, IR , or of the MS is not sufficient to assess global risk of CVD (Alberti et al. 2005; Després et al. 2008). Analysis of visceral adiposity by body composition analyzer and single-scan computerized tomography revealed that 130 cm2 of VAT in both sexes and/or 27 kg of fat mass in women are useful cutoff values. In this respect visceral adiposity in men and body fat mass in women seem to be of greater relevance in cardiometabolic risk (Onat et al. 2010). Even so, independent data analysis of 24,670 individuals from ten autonomous communities aged 35–74 years displayed that the average prevalence of MS was 31% (in women 29% and in men 32%). In this study, while the high blood glucose and triglycerides were more frequent in men with MS, abdominal obesity and low HDL cholesterol was predominant in women. Eventually the increase in coronary risk was larger in women than in men (Fernández-Bergés et al. 2012). Abdominally obese and obese, each have a progressive increase in the odds ratio for hypertension when compared with individuals who had a normal BMI or no abdominal obesity based on the US NHANES 2007–2010 (Ostchega et al. 2012).
In 1998, WHO firstly reported an accepted definition of the MS. Within the scope of this guideline, in addition to diabetes or IR any two of the following criteria; obesity , lipid abnormalities, microalbuminuria and hypertension should be covered (Alberti and Zimmet 1998). Subsequently, visceral adiposity, hypertriglyceridemia, HDL -cholesterol, hypertension and more than 110 mg/dL of fasting blood plasma glucose level were accepted as criteria for the MS by the National Cholesterol Education Program-Adult Treatment Panel III (NCEP-ATP III) (Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults 2001). Later, The European Group for the Study of Insulin Resistance (EGIR) proposed an alternative definition for only non-diabetic subjects with hyperinsulinaemia in 2002. Although this definition refers to the same syndrome, in addition to IR there may be two or more of the other components; hyperglycemia, hypertension, dyslipidaemia or central obesity (Balkau et al. 2002).
In April 2005, International Diabetes Federation (IDF) proposed a new definition of the metabolic syndrome (Ford 2005). This latest concept represents a modification of the WHO and NCEP-ATP III, however the main focus is central adiposity. IDF lists the various ethnic group–specific thresholds for WC to define central adiposity. Essentially, to have the MS, an individual should have two or more of the following four criteria in addition to central adiposity; elevated concentrations of triglycerides, reduced concentrations of HDL -cholesterol, elevated blood pressure, and dysglycemia (Ford 2005). According to the American Heart Association/National Heart, Lung, and Blood Institute (AHA/NHLBI) criteria (Grundy et al. 2005), MS is diagnosed when three or more of the following risk factors are present: abdominal obesity (>102 cm in men, and >88 cm in women), hypertension ≥130/≥85 mmHg or specific medication, level of triglycerides ≥150 mg/dL (1.7 mmol/L) or specific medication, low HDL cholesterol : in men <40 mg/dL (1.03 mmol/L), and in women <50 mg/dL (1.29 mmol/L) or specific medication, and fasting plasma glucose ≥100 mg/dL (5.6 mmol/L) or history of diabetes mellitus or taking antidiabetic medications.
According to the 2005 IDF criteria of MS, subsequently revised in 2009, abdominal obesity is identified as the WC >94 cm in men, and >80 cm in women. MS is responsible for the development of IR which decreases the levels of the HDL -cholesterol fraction, increases the levels of triglycerides, and leads to the development of arterial hypertension. Abdominal obesity plus any two of the same risk factors as in NHLBI/AHA criteria: hypertension ≥130/≥85 mmHg or antihypertensive therapy, level of triglycerides ≥150 mg/dL (1.7 mmol/L) or specific medication, low HDL cholesterol: in men <40 mg/dL (1.03 mmol/L), and in women <50 mg/dL (1.29 mmol/L) or specific medication, and fasting plasma glucose ≥100 mg/dL (5.6 mmol/L) or history of diabetes mellitus or taking antidiabetic medications. Diabetes was defined as fasting blood glucose levels of ≥7 mmol/L and/or treatment with antidiabetic medications (Gierach et al. 2014). Based on the results from 16 cohorts relative risk and incident diabetes were 5.17 (95% CI 3.99–6.69) for the 1999 WHO definition (ten cohorts); 4.45 (2.41–8.22) for the 1999 EGIR definition (four cohorts); 3.53 (2.84–4.39) for the 2001 NCEP definition (13 cohorts); 5.12 (3.26–8.05) for the 2005 AHA/NHLBI definition (five cohorts); and 4.42 (3.30–5.92) for the 2005 IDF definition (nine cohorts) (Ford et al. 2008).
Although not all overweight or obese individuals are metabolically disturbed, the majority are insulin-resistant. Metabolically benign obesity in humans is not accompanied by IR and atherosclerosis (Stefan et al. 2008). Indeed IR significantly increases the risk of developing diabetes mellitus type 2 (Kahn et al. 2001). Abdominal adiposity and IR appear to be central to the MS. In the presence of IR , non-esterified free fatty acids mobilization is accelerated from stored adipose tissue triglycerides. Consequently glucose, triglyceride and very low-density lipoprotein productions are increased (Cornier et al. 2008).
As mentioned above despite of the continuous increase in obesity incidence and paralleled rising rates of MS prevalence, no ideal diagnostic criteria are defined for MS, yet (Tenenbaum and Fisman 2011). Homeostasis Model Assessment (HOMA)-IR scores are higher in MS patients than in subjects without the MS (Vonbank et al. 2013). A relationship between the severity of dysglycemia and long-term mortality is also associated with the highest prevalence of the MS (Bergman et al. 2015).
There is no agreement between the criteria for diagnosis of MS. In 644 consecutive patients with verified carotid disease anthropometric parameters blood pressure, fasting plasma glucose and lipoproteins were measured in order to investigate agreement between AHA/NHLBI and IDF definitions of MS in patients with symptomatic carotid disease and to compare the frequency of cardiovascular risk factor in patients with MS diagnosed by these two sets of criteria. Thus the MS prevalence in patients with symptomatic carotid disease was high regardless of criteria used for its diagnosis (Maksimovic et al. 2012). According to the MS definition of the AHA/NHLBI 2009 Joint Scientific Statement, approximately one-third of the adult U.S. population has MS in 36% of women and 34% of men (Heiss et al. 2014). MS was diagnosed according to IDF and AHA/NHLBI criteria at a rate of 67.9% and 64.9%, respectively. 119 patients out of 644 were categorized differently by the two definitions. The overall agreement of IDF and AHA/NHLBI criteria is 81.5%. Actually IDF and AHA/NHLBI vary in two important aspects. First, in the IDF criteria cut-off values for WC are lower than in modified NCEP criteria (NHLBI/AHA), and the second and crucial, abdominal obesity is required as a prerequisite for diagnosis of MS (Maksimovic et al. 2012). Currently, several different definitions of MS exist, causing substantial confusion as to whether they identify the same individuals or represent a surrogate of risk factors. Therefore, diagnosis, prevention and treatment should better focus on established risk factors rather than the diagnosis of MS (Kassi et al. 2011).
The prevalence of MS in 614 obese children (307 male, 307 female; mean age: 11.3 ± 2.5 years) was found to be 39% and 33% according to the modified WHO and the IDF consensus criteria, respectively (Sangun et al. 2011). In a total of 133 patients, 67 males (50.4%) and 66 females (49.6%) with a mean age of 12.17 ± 3.27 years, the overall prevalence of MS was 19.6%, arterial hypertension and hypertriglyceridemia are the most prevalent metabolic changes. It was recommended that early intervention to control childhood obesity is essential to prevent cardiovascular morbidity and mortality in this series of patients (Guijarro de Armas et al. 2012).
Among the respondents 20 years and older, 3790 women and 4057 men from Turkey, age-adjusted overweight prevalence was 48.4% for women and 46.1% for men (Ergin et al. 2012). The overall prevalence of MS in Turkey was 34.6% (male, 31.2%; female, 37.3%) and 28.8% (male, 23.1%; female, 33.5%) according to IDF criteria and ATP III, respectively. The highest prevalence of MS was detected in 60–69 years of obese people (43.2%) in south district. The prevalence of MS criteria are as follows: type 2 diabetes mellitus , 15%; hypertension, 41.4%; obesity , 44.1%; abdominal obesity, 56.8%; low HDL -cholesterol , 34.1%; hypertriglyceridemia, 35.9%; and high LDL -cholesterol, 27.4% (Gündogan et al. 2009).
In PubMed database and the Cochrane Library originated eight prospective cohort studies, seven cross-sectional studies, and a case-control study, WHO and NCEP are the most popular definitions to describe MS experienced by the elderly. The prevalence of MS varied from 11% to 43% (median 21%) according to the WHO, and 23% to 55% (median 31%) according to NCEP. Obesity and hypertension are the most prevalent individual components of MS. Cardiovascular morbidity is the most serious risk factor in elderly population (Denys et al. 2009).
The Third Report (ATP III) of the NCEP Expert Panel highlighted the importance of identifying and treating patients with the MS and its complications. The US NCEP’s Adult Treatment Panel III requires at least three of five characteristics for MS: (1) abdominal obesity given as WC greater than 102 cm in men and greater than 88 cm in women; (2) hypertriglyceridemia with triglyceride concentration (150 mg/dL or 1.7 mmol/L); (3) abnormal cholesterol profile with HDL cholesterol less than 40 mg/dL or 1 mmol/L in men and less than 50 mg/dL or 1.3 mmol/L in women; (4) blood pressure: 130/85 mm Hg or more; (5) impaired glucose tolerance, i.e. elevated fasting plasma glucose 100 mg/dL or 5.5 mmol/L or more (National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) 2002). A cross-sectional analysis of 4153 Greek adults older than 18 years displayed the age-standardized prevalence of the MS was 23.6%. 61% of those had three components of the MS, 29% had four and only 10% had all five components. Abdominal obesity and arterial hypertension were the most common abnormalities in both sexes with 82% and 78%, respectively (Athyros et al. 2005).
In a multi-stage complex cross-sectional survey designed according to WHO guidelines by using the revised 2005 IDF definition of MS, Davila et al. showed that 64% of 3000 participants from Colombian region had abdominal obesity . Among those, 41% had MS (Davila et al. 2013). The prevalence of MS is 35.73% among all adults in Morocco. MS in women is 2.16 times more frequent than in men. Abdominal obesity is the most common abnormality with 49.15 per centile (El Brini et al. 2014).
The diagnosis of MS by WHO criteria could be made on the basis of IR plus at least two additional risk factors including obesity , hypertension, high triglyceride level, reduced HDL cholesterol level, or micro-albuminuria (Alberti and Zimmet 1998). The IDF dropped the WHO requirement for IR but made abdominal obesity necessary as one of five factors as described above required in the diagnosis (Alberti et al. 2005). Actually WC and insulin resistance estimated by HOMA-IR increases with age. Prevalence of IDF-MS and the Japanese Society of Internal Medicine (JSIM)-MS also increase with age at least until the age of 80, whereas the incidence of MS according to AHA/NHLBI does not show any apparent age changes. Abdominal obesity and IR are mentioned within the sets of criteria by all three institutions. However, significantly elevated linear association between WC and HOMA-IR overlaps only with IDF’s and JSIM’s MS definitions (Sakurai et al. 2010).
34,821 subjects from 12 cohorts from ten European countries and one cohort from USA in the Metabolic syndrome and Arteries REsearch (MARE) Consortium were investigated in accordance with the ATP III criteria (MS was defined as an alteration three or more of the following five components: elevated glucose, fasting glucose ≥110 mg/dL; low HDL cholesterol , <40 mg/dL for men or <50 mg/dL for women; high triglycerides, ≥150 mg/dL; elevated blood pressure, ≥130/≥85 mmHg; abdominal obesity , WC >102 cm for men or >88 cm for women). MS has a 24.3% prevalence (8468 subjects: 23.9% in men vs. 24.6% in women, p < 0.001) with an age-associated increase in its prevalence in all the cohorts. The analysis of the distribution of MS suggested that MS is not a unique entity rather a constellation of cluster of MS components (Scuteri et al. 2015). Prevalence of MS is found to be different according to selected definition and components. In 2051 participants, prevalence of MS was significantly greater when using AHA and IDF compared to the NCEP-ATP III definition (Mancia et al. 2010). Similarly, the prevalence of MS in 867 adults aged 25 years and older from an urban population of Karachi, Pakistan, according to the IDF definition and modified ATP III criteria was 34.8% and 49%, respectively (Hydrie et al. 2009). In 3914 adults aged 35–74 years in Jiangsu province, China, age-standardized prevalence of MS was 30.5% according to the modified NCEP-ATP III. In these patients, high blood pressure was the most prevalent component of MS (45.2%), followed by elevated triglycerides (40.1%) and low HDL cholesterol (40.1%). Multivariate ordinal regression analysis revealed that women had significantly higher risk of MS than men (Zuo et al. 2009).
As appears from the above data, the MS “clustering of abdominal obesity , dyslipidaemia, hyperglycaemia and hypertension” is a major public health challenge worldwide (Eckel et al. 2005). The prevalence of MS increases even more dramatically as BMI increases. More than 6 and 5.5 times more frequently MS occurs in overweight males and females, respectively, compared to under-weight and normal-weight individuals (Ervin 2009). In a total of 21 studies including 372,411 participants with MS, 18,556 deaths from any cause occurred during a mean follow-up of 11.5 years (Wu et al. 2010). Indeed, evaluation of 87 studies including 951,083 patients according to the third NCEP definition showed that MS was associated with a two-fold increase in the risk of coronary heart disease, cerebrovascular disease, all cardiovascular lethal and nonlethal CVD, and a 1.5-fold increase in the risk of all-cause mortality (Cicero and Derosa 2014). Over 12-year follow-up of 2051 individuals, 179 cardiovascular events and 233 deaths were determined for any cause. Risks of fatal and nonfatal cardiovascular events, diabetes mellitus, hypertension and left ventricular hypertrophy were similar for the three definitions of MS. However, the AHA and IDF definitions are more sensitive than that of ATP III in identifying MS condition (Mancia et al. 2010). Nonalcoholic fatty liver disease (NAFLD) and MS frequently coexist and 90% of NAFLD patients have more than one manifestation of the MS. Actually, CVD is the leading cause of death in patients with NAFLD and the MS (Almeda-Valdes et al. 2014).
5 Conclusion
Obesity is associated with large decreases in life expectancy and, also large increases in healthcare expenditures, but the mechanism of obesity -paradox is still needed to be clarified. Although metabolically benign obesity in humans is not associated with IR and atherosclerosis, weight loss is an appropriate treatment outcome in MHO individuals. Thus, from a clinical perspective, MHO is located in the intermediate-risk group. Additionally, there is no agreement between the criteria for diagnosis of MS. Different definitions of MS cause serious confusion as to whether the same individuals are identified. In this context, further epidemiological analysis and investigations are necessary to enlighten the pathological progress of obesity.
References
Alberti, K.G., and P.Z. Zimmet. 1998. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabetic Medicine: A Journal of the British Diabetic Association 15: 539–553. doi:10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S.
Alberti, K.G.M.M., P. Zimmet, J. Shaw, and IDF Epidemiology Task Force Consensus Group. 2005. The metabolic syndrome—A new worldwide definition. Lancet (London, England) 366: 1059–1062. doi:10.1016/S0140-6736(05)67402-8.
Almeda-Valdes, P., N. Aguilar-Olivos, M. Uribe, and N. Méndez-Sánchez. 2014. Common features of the metabolic syndrome and nonalcoholic fatty liver disease. Reviews on Recent Clinical Trials 9: 148–158.
Ashwell, M. 2012. Plea for simplicity: use of waist-to-height ratio as a primary screening tool to assess cardiometabolic risk. Clinical Obesity 2: 3–5. doi:10.1111/j.1758-8111.2012.00037.x.
Ashwell, M., P. Gunn, and S. Gibson. 2012. Waist-to-height ratio is a better screening tool than waist circumference and BMI for adult cardiometabolic risk factors: Systematic review and meta-analysis. Obesity Reviews: An Official Journal of the International Association for the Study of Obesity 13: 275–286. doi:10.1111/j.1467-789X.2011.00952.x.
Ashwell, M., L. Mayhew, J. Richardson, and B. Rickayzen. 2014. Waist-to-height ratio is more predictive of years of life lost than body mass index. PLoS One 9: e103483. doi:10.1371/journal.pone.0103483.
Athyros, V.G., V.I. Bouloukos, A.N. Pehlivanidis, A.A. Papageorgiou, S.G. Dionysopoulou, A.N. Symeonidis, D.I. Petridis, M.I. Kapousouzi, E.A. Satsoglou, D.P. Mikhailidis, and MetS-Greece Collaborative Group. 2005. The prevalence of the metabolic syndrome in Greece: The MetS-Greece Multicentre Study. Diabetes, Obesity & Metabolism 7: 397–405. doi:10.1111/j.1463-1326.2004.00409.x.
Aung, K., C. Lorenzo, M.A. Hinojosa, and S.M. Haffner. 2014. Risk of developing diabetes and cardiovascular disease in metabolically unhealthy normal-weight and metabolically healthy obese individuals. The Journal of Clinical Endocrinology and Metabolism 99: 462–468. doi:10.1210/jc.2013-2832.
Australian Bureau of Statistics 2012. Overweight and obesity. http://www.abs.gov.au/ausstats/abs@.nsf/Lookup/034947E844F25207CA257AA30014BDC7?opendocument. Accessed 26 Aug 2016.
Balkau, B., M.-A. Charles, T. Drivsholm, K. Borch-Johnsen, N. Wareham, J.S. Yudkin, R. Morris, I. Zavaroni, R. van Dam, E. Feskins, R. Gabriel, M. Diet, P. Nilsson, B. Hedblad, and European Group For The Study Of Insulin Resistance (EGIR). 2002. Frequency of the WHO metabolic syndrome in European cohorts, and an alternative definition of an insulin resistance syndrome. Diabetes & Metabolism 28: 364–376.
Bennasar-Veny, M., A.A. Lopez-Gonzalez, P. Tauler, M.L. Cespedes, T. Vicente-Herrero, A. Yañez, M. Tomas-Salva, and A. Aguilo. 2013. Body adiposity index and cardiovascular health risk factors in Caucasians: A comparison with the body mass index and others. PLoS One 8: e63999. doi:10.1371/journal.pone.0063999.
Bergman, R.N., D. Stefanovski, T.A. Buchanan, A.E. Sumner, J.C. Reynolds, N.G. Sebring, A.H. Xiang, and R.M. Watanabe. 2011. A better index of body adiposity. Obesity (Silver Spring, Md.) 19: 1083–1089. doi:10.1038/oby.2011.38.
Bergman, M., A. Chetrit, J. Roth, and R. Dankner. 2015. Dysglycemia and long-term mortality: Observations from the Israel study of glucose intolerance, obesity and hypertension. Diabetes/Metabolism Research and Reviews 31: 368–375. doi:10.1002/dmrr.2618.
Bibiloni, M.D.M., A. Pons, and J.A. Tur. 2013. Prevalence of overweight and obesity in adolescents: A systematic review. ISRN Obesity 2013: 392747. doi:10.1155/2013/392747.
Blüher, M. 2010. The distinction of metabolically “healthy” from “unhealthy” obese individuals. Current Opinion in Lipidology 21: 38–43. doi:10.1097/MOL.0b013e3283346ccc.
———. 2012. Are there still healthy obese patients? Current Opinion in Endocrinology, Diabetes, and Obesity 19: 341–346. doi:10.1097/MED.0b013e328357f0a3.
Blüher, S., and P. Schwarz. 2014. Metabolically healthy obesity from childhood to adulthood—Does weight status alone matter? Metabolism 63: 1084–1092. doi:10.1016/j.metabol.2014.06.009.
Canlorbe, G., S. Bendifallah, E. Raimond, O. Graesslin, D. Hudry, C. Coutant, C. Touboul, G. Bleu, P. Collinet, E. Darai, and M. Ballester. 2015. Severe obesity impacts recurrence-free survival of women with high-risk endometrial cancer: Results of a French Multicenter Study. Annals of Surgical Oncology 22: 2714–2721. doi:10.1245/s10434-014-4295-0.
Cartier, A., I. Lemieux, N. Alméras, A. Tremblay, J. Bergeron, and J.-P. Després. 2008. Visceral obesity and plasma glucose-insulin homeostasis: Contributions of interleukin-6 and tumor necrosis factor-alpha in men. The Journal of Clinical Endocrinology and Metabolism 93: 1931–1938. doi:10.1210/jc.2007-2191.
Cartier, A., M. Côté, J. Bergeron, N. Alméras, A. Tremblay, I. Lemieux, and J.-P. Després. 2010. Plasma soluble tumour necrosis factor-alpha receptor 2 is elevated in obesity: Specific contribution of visceral adiposity. Clinical Endocrinology 72: 349–357. doi:10.1111/j.1365-2265.2009.03671.x.
Cerhan, J.R., S.C. Moore, E.J. Jacobs, C.M. Kitahara, P.S. Rosenberg, H.-O. Adami, J.O. Ebbert, D.R. English, S.M. Gapstur, G.G. Giles, P.L. Horn-Ross, Y. Park, A.V. Patel, K. Robien, E. Weiderpass, W.C. Willett, A. Wolk, A. Zeleniuch-Jacquotte, P. Hartge, L. Bernstein, and A. Berrington de Gonzalez. 2014. A pooled analysis of waist circumference and mortality in 650,000 adults. Mayo Clinic Proceedings 89: 335–345. doi:10.1016/j.mayocp.2013.11.011.
Chan, D.S.M., A.R. Vieira, D. Aune, E.V. Bandera, D.C. Greenwood, A. McTiernan, D. Navarro Rosenblatt, I. Thune, R. Vieira, and T. Norat. 2014. Body mass index and survival in women with breast cancer-systematic literature review and meta-analysis of 82 follow-up studies. Annals of Oncology: Official Journal of the European Society for Medical Oncology 25: 1901–1914. doi:10.1093/annonc/mdu042.
Chandra, A., I.J. Neeland, J.D. Berry, C.R. Ayers, A. Rohatgi, S.R. Das, A. Khera, D.K. McGuire, J.A. de Lemos, and A.T. Turer. 2014. The relationship of body mass and fat distribution with incident hypertension: Observations from the Dallas Heart Study. Journal of the American College of Cardiology 64: 997–1002. doi:10.1016/j.jacc.2014.05.057.
Chang, Y., B.-K. Kim, K.E. Yun, J. Cho, Y. Zhang, S. Rampal, D. Zhao, H.-S. Jung, Y. Choi, J. Ahn, J.A.C. Lima, H. Shin, E. Guallar, and S. Ryu. 2014. Metabolically-healthy obesity and coronary artery calcification. Journal of the American College of Cardiology 63: 2679–2686. doi:10.1016/j.jacc.2014.03.042.
Chen, J. 2011. Multiple signal pathways in obesity-associated cancer. Obesity Reviews: An Official Journal of the International Association for the Study of Obesity 12: 1063–1070. doi:10.1111/j.1467-789X.2011.00917.x.
Choi, K.M., H.J. Cho, H.Y. Choi, S.J. Yang, H.J. Yoo, J.A. Seo, S.G. Kim, S.H. Baik, D.S. Choi, and N.H. Kim. 2013. Higher mortality in metabolically obese normal-weight people than in metabolically healthy obese subjects in elderly Koreans. Clinical Endocrinology 79: 364–370. doi:10.1111/cen.12154.
Cicero, A.F.G., and G. Derosa. 2014. Are there mild and serious metabolic syndromes? The need for a graded diagnosis. Journal of Cardiovascular Medicine (Hagerstown, Md.) 15: 759–760. doi:10.2459/JCM.0000000000000169.
Cole, T.J., and T. Lobstein. 2012. Extended international (IOTF) body mass index cut-offs for thinness, overweight and obesity. Pediatric Obesity 7: 284–294. doi:10.1111/j.2047-6310.2012.00064.x.
Cornier, M.-A., D. Dabelea, T.L. Hernandez, R.C. Lindstrom, A.J. Steig, N.R. Stob, R.E. Van Pelt, H. Wang, and R.H. Eckel. 2008. The metabolic syndrome. Endocrine Reviews 29: 777–822. doi:10.1210/er.2008-0024.
Davila, E.P., M.A. Quintero, M.L. Orrego, E.S. Ford, H. Walke, M.M. Arenas, and M. Pratt. 2013. Prevalence and risk factors for metabolic syndrome in Medellin and surrounding municipalities, Colombia, 2008–2010. Preventive Medicine 56: 30–34. doi:10.1016/j.ypmed.2012.10.027.
Denys, K., M. Cankurtaran, W. Janssens, and M. Petrovic. 2009. Metabolic syndrome in the elderly: An overview of the evidence. Acta Clinica Belgica 64: 23–34. doi:10.1179/acb.2009.006.
Després, J.-P., I. Lemieux, J. Bergeron, P. Pibarot, P. Mathieu, E. Larose, J. Rodés-Cabau, O.F. Bertrand, and P. Poirier. 2008. Abdominal obesity and the metabolic syndrome: contribution to global cardiometabolic risk. Arteriosclerosis, Thrombosis, and Vascular Biology 28: 1039–1049. doi:10.1161/ATVBAHA.107.159228.
Dixon, J.B., P. Zimmet, K.G. Alberti, F. Rubino, and International Diabetes Federation Taskforce on Epidemiology and Prevention. 2011. Bariatric surgery: An IDF statement for obese Type 2 diabetes. Arquivos Brasileiros de Endocrinologia e Metabologia 55: 367–382.
Drew, J.E. 2012. Molecular mechanisms linking adipokines to obesity-related colon cancer: Focus on leptin. The Proceedings of the Nutrition Society 71: 175–180. doi:10.1017/S0029665111003259.
Drolet, R., C. Richard, A.D. Sniderman, J. Mailloux, M. Fortier, C. Huot, C. Rhéaume, and A. Tchernof. 2008. Hypertrophy and hyperplasia of abdominal adipose tissues in women. International Journal of Obesity 2005(32): 283–291. doi:10.1038/sj.ijo.0803708.
Duncan, J.S., E.K. Duncan, and G. Schofield. 2009. Accuracy of body mass index (BMI) thresholds for predicting excess body fat in girls from five ethnicities. Asia Pacific Journal of Clinical Nutrition 18: 404–411.
Durward, C.M., T.J. Hartman, and S.M. Nickols-Richardson. 2012. All-cause mortality risk of metabolically healthy obese individuals in NHANES III. Journal of Obesity 2012: 460321. doi:10.1155/2012/460321.
Ebbeling, C.B., D.B. Pawlak, and D.S. Ludwig. 2002. Childhood obesity: Public-health crisis, common sense cure. Lancet (London, England) 360: 473–482. doi:10.1016/S0140-6736(02)09678-2.
Ebong, I.A., D.C. Goff, C.J. Rodriguez, H. Chen, and A.G. Bertoni. 2014. Mechanisms of heart failure in obesity. Obesity Research and Clinical Practice 8: e540–e548. doi:10.1016/j.orcp.2013.12.005.
Eckel, R.H., S.M. Grundy, and P.Z. Zimmet. 2005. The metabolic syndrome. Lancet (London, England) 365: 1415–1428. doi:10.1016/S0140-6736(05)66378-7.
El Brini, O., O. Akhouayri, A. Gamal, A. Mesfioui, and B. Benazzouz. 2014. Prevalence of metabolic syndrome and its components based on a harmonious definition among adults in Morocco. Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy 7: 341–346. doi:10.2147/DMSO.S61245.
Ergin, I., H. Hassoy, and A. Kunst. 2012. Socio-economic inequalities in overweight among adults in Turkey: A regional evaluation. Public Health Nutrition 15: 58–66. doi:10.1017/S1368980011001972.
Ervin, R.B. 2009. Prevalence of metabolic syndrome among adults 20 years of age and over, by sex, age, race and ethnicity, and body mass index: United States, 2003–2006. National Health Statistics Reports 1–7.
Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. 2001. Executive summary of the third report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA 285: 2486–2497.
Fernández-Bergés, D., A. Cabrera de León, H. Sanz, R. Elosua, M.J. Guembe, M. Alzamora, T. Vega-Alonso, F.J. Félix-Redondo, H. Ortiz-Marrón, F. Rigo, C. Lama, D. Gavrila, A. Segura-Fragoso, L. Lozano, and J. Marrugat. 2012. Metabolic syndrome in Spain: Prevalence and coronary risk associated with harmonized definition and WHO proposal DARIOS study. Revista Española de Cardiología (English Edition) 65: 241–248. doi:10.1016/j.recesp.2011.10.015.
Ferreira, I., J.W.R. Twisk, W. van Mechelen, H.C.G. Kemper, J.C. Seidell, and C.D.A. Stehouwer. 2004. Current and adolescent body fatness and fat distribution: Relationships with carotid intima-media thickness and large artery stiffness at the age of 36 years. Journal of Hypertension 22: 145–155.
Flegal, K.M., B.K. Kit, H. Orpana, and B.I. Graubard. 2013. Association of all-cause mortality with overweight and obesity using standard body mass index categories: A systematic review and meta-analysis. JAMA 309: 71–82. doi:10.1001/jama.2012.113905.
Ford, E.S. 2005. Prevalence of the metabolic syndrome defined by the International Diabetes Federation among adults in the U.S. Diabetes Care 28: 2745–2749.
Ford, E.S., C. Li, and N. Sattar. 2008. Metabolic syndrome and incident diabetes: Current state of the evidence. Diabetes Care 31: 1898–1904. doi:10.2337/dc08-0423.
Fox, C.S., J.M. Massaro, U. Hoffmann, K.M. Pou, P. Maurovich-Horvat, C.-Y. Liu, R.S. Vasan, J.M. Murabito, J.B. Meigs, L.A. Cupples, R.B. D’Agostino, and C.J. O’Donnell. 2007. Abdominal visceral and subcutaneous adipose tissue compartments: Association with metabolic risk factors in the Framingham Heart Study. Circulation 116: 39–48. doi:10.1161/CIRCULATIONAHA.106.675355.
Gierach, M., J. Gierach, M. Ewertowska, A. Arndt, and R. Junik. 2014. Correlation between body mass index and waist circumference in patients with metabolic syndrome. ISRN Endocrinology 2014: 514589. doi:10.1155/2014/514589.
Ginter, E., and V. Simko. 2014. Becoming overweight: Is there a health risk? Bratislavske Lékařské Listy 115: 527–531.
Goh, L.G.H., S.S. Dhaliwal, T.A. Welborn, A.H. Lee, and P.R. Della. 2014. Ethnicity and the association between anthropometric indices of obesity and cardiovascular risk in women: A cross-sectional study. BMJ Open 4: e004702. doi:10.1136/bmjopen-2013-004702.
Goyal, A., K.R. Nimmakayala, and J. Zonszein. 2014. Is there a paradox in obesity? Cardiology in Review 22: 163–170. doi:10.1097/CRD.0000000000000004.
Grotta, A., M. Bottai, H.-O. Adami, S.A. Adams, O. Akre, S.N. Blair, D. Mariosa, O. Nyrén, W. Ye, P. Stattin, R. Bellocco, and Y. Trolle Lagerros. 2015. Physical activity and body mass index as predictors of prostate cancer risk. World Journal of Urology 33: 1495–1502. doi:10.1007/s00345-014-1464-5.
Grundy, S.M., G.J. Balady, M.H. Criqui, G. Fletcher, P. Greenland, L.F. Hiratzka, N. Houston-Miller, P. Kris-Etherton, H.M. Krumholz, J. LaRosa, I.S. Ockene, T.A. Pearson, J. Reed, R. Washington, and S.C. Smith. 1998. Primary prevention of coronary heart disease: Guidance from Framingham: A statement for healthcare professionals from the AHA Task Force on Risk Reduction. American Heart Association. Circulation 97: 1876–1887.
Grundy, S.M., J.I. Cleeman, S.R. Daniels, K.A. Donato, R.H. Eckel, B.A. Franklin, D.J. Gordon, R.M. Krauss, P.J. Savage, S.C. Smith, J.A. Spertus, F. Costa, and American Heart Association, National Heart, Lung, and Blood Institute. 2005. Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 112: 2735–2752. doi:10.1161/CIRCULATIONAHA.105.169404.
Guijarro de Armas, M.A.G., S. Monereo Megías, M. Merino Viveros, P. Iglesias Bolaños, and B. Vega Piñero. 2012. Prevalence of metabolic syndrome in a population of obese children and adolescents. Endocrinología y nutrición: Órgano de la Sociedad Española de Endocrinología y Nutrición 59: 155–159. doi:10.1016/j.endonu.2012.01.003.
Gündogan, K., F. Bayram, M. Capak, F. Tanriverdi, A. Karaman, A. Ozturk, H. Altunbas, C. Gökce, A. Kalkan, and C. Yazici. 2009. Prevalence of metabolic syndrome in the Mediterranean region of Turkey: Evaluation of hypertension, diabetes mellitus, obesity, and dyslipidemia. Metabolic Syndrome and Related Disorders 7: 427–434. doi:10.1089/met.2008.0068.
Guo, S.S., W. Wu, W.C. Chumlea, and A.F. Roche. 2002. Predicting overweight and obesity in adulthood from body mass index values in childhood and adolescence. The American Journal of Clinical Nutrition 76: 653–658.
Hamdy, O., S. Porramatikul, and E. Al-Ozairi. 2006. Metabolic obesity: The paradox between visceral and subcutaneous fat. Current Diabetes Reviews 2: 367–373.
Harrington, D.M., A.E. Staiano, S.T. Broyles, A.K. Gupta, and P.T. Katzmarzyk. 2013. BMI percentiles for the identification of abdominal obesity and metabolic risk in children and adolescents: Evidence in support of the CDC 95th percentile. European Journal of Clinical Nutrition 67: 218–222. doi:10.1038/ejcn.2012.203.
Hayes, A., A. Chevalier, M. D’Souza, L. Baur, L.M. Wen, and J. Simpson. 2016. Early childhood obesity: Association with healthcare expenditure in Australia. Obesity (Silver Spring, Md.) 24: 1752–1758. doi:10.1002/oby.21544.
Heiss, G., M.L. Snyder, Y. Teng, N. Schneiderman, M.M. Llabre, C. Cowie, M. Carnethon, R. Kaplan, A. Giachello, L. Gallo, L. Loehr, and L. Avilés-Santa. 2014. Prevalence of metabolic syndrome among Hispanics/Latinos of diverse background: The Hispanic Community Health Study/Study of Latinos. Diabetes Care 37: 2391–2399. doi:10.2337/dc13-2505.
Hensrud, D.D., and S. Klein. 2006. Extreme obesity: A new medical crisis in the United States. Mayo Clinic Proceedings 81: S5–10.
Hinnouho, G.-M., S. Czernichow, A. Dugravot, G.D. Batty, M. Kivimaki, and A. Singh-Manoux. 2013. Metabolically healthy obesity and risk of mortality: Does the definition of metabolic health matter? Diabetes Care 36: 2294–2300. doi:10.2337/dc12-1654.
Hinnouho, G.-M., S. Czernichow, A. Dugravot, H. Nabi, E.J. Brunner, M. Kivimaki, and A. Singh-Manoux. 2015. Metabolically healthy obesity and the risk of cardiovascular disease and type 2 diabetes: The Whitehall II cohort study. European Heart Journal 36: 551–559. doi:10.1093/eurheartj/ehu123.
Hydrie, M.Z.I., A.S. Shera, A. Fawwad, A. Basit, and A. Hussain. 2009. Prevalence of metabolic syndrome in urban Pakistan (Karachi): Comparison of newly proposed International Diabetes Federation and modified Adult Treatment Panel III criteria. Metabolic Syndrome and Related Disorders 7: 119–124. doi:10.1089/met.2008.0055.
Iannucci, C.V., D. Capoccia, M. Calabria, and F. Leonetti. 2007. Metabolic syndrome and adipose tissue: New clinical aspects and therapeutic targets. Current Pharmaceutical Design 13: 2148–2168.
Kahn, S.E., R.L. Prigeon, R.S. Schwartz, W.Y. Fujimoto, R.H. Knopp, J.D. Brunzell, and D. Porte. 2001. Obesity, body fat distribution, insulin sensitivity and Islet beta-cell function as explanations for metabolic diversity. The Journal of Nutrition 131: 354S–360S.
Kassi, E., P. Pervanidou, G. Kaltsas, and G. Chrousos. 2011. Metabolic syndrome: Definitions and controversies. BMC Medicine 9: 48. doi:10.1186/1741-7015-9-48.
Keaver, L., L. Webber, A. Dee, F. Shiely, T. Marsh, K. Balanda, I.J. Perry, and I. Perry. 2013. Application of the UK foresight obesity model in Ireland: The health and economic consequences of projected obesity trends in Ireland. PLoS One 8: e79827. doi:10.1371/journal.pone.0079827.
Khoury, M., C. Manlhiot, and B.W. McCrindle. 2013. Role of the waist/height ratio in the cardiometabolic risk assessment of children classified by body mass index. Journal of the American College of Cardiology 62: 742–751. doi:10.1016/j.jacc.2013.01.026.
Kiraly, L., R.T. Hurt, and C.W. Van Way. 2011. The outcomes of obese patients in critical care. JPEN Journal of Parenteral and Enteral Nutrition 35: 29S–35S. doi:10.1177/0148607111413774.
Kramer, C.K., B. Zinman, and R. Retnakaran. 2013. Are metabolically healthy overweight and obesity benign conditions?: A systematic review and meta-analysis. Annals of Internal Medicine 159: 758–769. doi:10.7326/0003-4819-159-11-201312030-00008.
Lehnert, T., P. Streltchenia, A. Konnopka, S.G. Riedel-Heller, and H.-H. König. 2015. Health burden and costs of obesity and overweight in Germany: An update. The European Journal of Health Economics: HEPAC: Health Economics in Prevention and Care 16: 957–967. doi:10.1007/s10198-014-0645-x.
Leung, M.-Y.M., L.M. Pollack, G.A. Colditz, and S.-H. Chang. 2015. Life years lost and lifetime health care expenditures associated with diabetes in the U.S., National Health Interview Survey, 1997–2000. Diabetes Care 38: 460–468. doi:10.2337/dc14-1453.
Lissau, I., M.D. Overpeck, W.J. Ruan, P. Due, B.E. Holstein, M.L. Hediger, and Health Behaviour in School-aged Children Obesity Working Group. 2004. Body mass index and overweight in adolescents in 13 European countries, Israel, and the United States. Archives of Pediatrics & Adolescent Medicine 158: 27–33. doi:10.1001/archpedi.158.1.27.
Liu, X., Y. Liu, J. Zhan, and Q. He. 2015. Overweight, obesity and risk of all-cause and cardiovascular mortality in patients with type 2 diabetes mellitus: A dose-response meta-analysis of prospective cohort studies. European Journal of Epidemiology 30: 35–45. doi:10.1007/s10654-014-9973-5.
Maksimovic, M.Z., H.D. Vlajinac, D.J. Radak, J.M. Marinkovic, and J.B. Jorga. 2012. Prevalence of the metabolic syndrome in patients with carotid disease according to NHLBI/AHA and IDF criteria: A cross-sectional study. BMC Cardiovascular Disorders 12: 2. doi:10.1186/1471-2261-12-2.
Mancia, G., M. Bombelli, R. Facchetti, A. Casati, I. Ronchi, F. Quarti-Trevano, F. Arenare, G. Grassi, and R. Sega. 2010. Impact of different definitions of the metabolic syndrome on the prevalence of organ damage, cardiometabolic risk and cardiovascular events. Journal of Hypertension 28: 999–1006. doi:10.1097/HJH.0b013e328337a9e3.
Marques-Vidal, P., S. Velho, D. Waterworth, G. Waeber, R. von Känel, and P. Vollenweider. 2012. The association between inflammatory biomarkers and metabolically healthy obesity depends of the definition used. European Journal of Clinical Nutrition 66: 426–435. doi:10.1038/ejcn.2011.170.
Melmer, A., C. Lamina, A. Tschoner, C. Ress, S. Kaser, M. Laimer, A. Sandhofer, B. Paulweber, and C.F. Ebenbichler. 2013. Body adiposity index and other indexes of body composition in the SAPHIR study: Association with cardiovascular risk factors. Obesity (Silver Spring, Md.) 21: 775–781. doi:10.1002/oby.20289.
Nascimento, V.G., C.J. Bertoli, and C. Leone. 2011. Ratio of weight to height gain: A useful tool for identifying children at risk of becoming overweight or obese at preschool age. Clinics (São Paulo, Brazil) 66: 1223–1226.
National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). 2002. Third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 106: 3143–3421.
Ng, M., T. Fleming, M. Robinson, B. Thomson, N. Graetz, C. Margono, E.C. Mullany, S. Biryukov, C. Abbafati, S.F. Abera, J.P. Abraham, N.M.E. Abu-Rmeileh, T. Achoki, F.S. AlBuhairan, Z.A. Alemu, R. Alfonso, M.K. Ali, R. Ali, N.A. Guzman, W. Ammar, P. Anwari, A. Banerjee, S. Barquera, S. Basu, D.A. Bennett, Z. Bhutta, J. Blore, N. Cabral, I.C. Nonato, J.-C. Chang, R. Chowdhury, K.J. Courville, M.H. Criqui, D.K. Cundiff, K.C. Dabhadkar, L. Dandona, A. Davis, A. Dayama, S.D. Dharmaratne, E.L. Ding, A.M. Durrani, A. Esteghamati, F. Farzadfar, D.F.J. Fay, V.L. Feigin, A. Flaxman, M.H. Forouzanfar, A. Goto, M.A. Green, R. Gupta, N. Hafezi-Nejad, G.J. Hankey, H.C. Harewood, R. Havmoeller, S. Hay, L. Hernandez, A. Husseini, B.T. Idrisov, N. Ikeda, F. Islami, E. Jahangir, S.K. Jassal, S.H. Jee, M. Jeffreys, J.B. Jonas, E.K. Kabagambe, S.E.A.H. Khalifa, A.P. Kengne, Y.S. Khader, Y.-H. Khang, D. Kim, R.W. Kimokoti, J.M. Kinge, Y. Kokubo, S. Kosen, G. Kwan, T. Lai, M. Leinsalu, Y. Li, X. Liang, S. Liu, G. Logroscino, P.A. Lotufo, Y. Lu, J. Ma, N.K. Mainoo, G.A. Mensah, T.R. Merriman, A.H. Mokdad, J. Moschandreas, M. Naghavi, A. Naheed, D. Nand, K.M.V. Narayan, E.L. Nelson, M.L. Neuhouser, M.I. Nisar, T. Ohkubo, S.O. Oti, A. Pedroza, D. Prabhakaran, N. Roy, U. Sampson, H. Seo, S.G. Sepanlou, K. Shibuya, R. Shiri, I. Shiue, G.M. Singh, J.A. Singh, V. Skirbekk, N.J.C. Stapelberg, L. Sturua, B.L. Sykes, M. Tobias, B.X. Tran, L. Trasande, H. Toyoshima, S. van de Vijver, T.J. Vasankari, J.L. Veerman, G. Velasquez-Melendez, V.V. Vlassov, S.E. Vollset, T. Vos, C. Wang, X. Wang, E. Weiderpass, A. Werdecker, J.L. Wright, Y.C. Yang, H. Yatsuya, J. Yoon, S.-J. Yoon, Y. Zhao, M. Zhou, S. Zhu, A.D. Lopez, C.J.L. Murray, and E. Gakidou. 2014. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet (London, England) 384: 766–781. doi:10.1016/S0140-6736(14)60460-8.
Oh, C.-M., J.K. Jun, and M. Suh. 2014. Risk of cancer mortality according to the metabolic health status and degree of obesity. Asian Pacific Journal of Cancer Prevention: APJCP 15: 10027–10031.
Onat, A., M. Uğur, G. Can, H. Yüksel, and G. Hergenç. 2010. Visceral adipose tissue and body fat mass: Predictive values for and role of gender in cardiometabolic risk among Turks. Nutrition (Burbank, Los Angeles County, Calif.) 26: 382–389. doi:10.1016/j.nut.2009.05.019.
Ostchega, Y., J.P. Hughes, A. Terry, T.H.I. Fakhouri, and I. Miller. 2012. Abdominal obesity, body mass index, and hypertension in US adults: NHANES 2007-2010. American Journal of Hypertension 25: 1271–1278. doi:10.1038/ajh.2012.120.
Peeters, P.J.H.L., M.T. Bazelier, H.G.M. Leufkens, F. de Vries, and M.L. De Bruin. 2015. The risk of colorectal cancer in patients with type 2 diabetes: Associations with treatment stage and obesity. Diabetes Care 38: 495–502. doi:10.2337/dc14-1175.
Pérez-Farinós, N., A.M. López-Sobaler, M.Á. Dal Re, C. Villar, E. Labrado, T. Robledo, and R.M. Ortega. 2013. The ALADINO study: A national study of prevalence of overweight and obesity in Spanish children in 2011. BioMed Research International 2013: 163687. doi:10.1155/2013/163687.
Phillips, C.M., C. Dillon, J.M. Harrington, V.J.C. McCarthy, P.M. Kearney, A.P. Fitzgerald, and I.J. Perry. 2013. Defining metabolically healthy obesity: Role of dietary and lifestyle factors. PLoS One 8: e76188. doi:10.1371/journal.pone.0076188.
Pickkers, P., N. de Keizer, J. Dusseljee, D. Weerheijm, J.G. van der Hoeven, and N. Peek. 2013. Body mass index is associated with hospital mortality in critically ill patients: An observational cohort study. Critical Care Medicine 41: 1878–1883. doi:10.1097/CCM.0b013e31828a2aa1.
Plourde, G., and A.D. Karelis. 2014. Current issues in the identification and treatment of metabolically healthy but obese individuals. Nutrition, Metabolism, and Cardiovascular Diseases: NMCD 24: 455–459. doi:10.1016/j.numecd.2013.12.002.
Prospective Studies Collaboration, G. Whitlock, S. Lewington, P. Sherliker, R. Clarke, J. Emberson, J. Halsey, N. Qizilbash, R. Collins, and R. Peto. 2009. Body-mass index and cause-specific mortality in 900,000 adults: Collaborative analyses of 57 prospective studies. Lancet (London, England) 373: 1083–1096. doi:10.1016/S0140-6736(09)60318-4.
Rey-López, J.P., L.F. de Rezende, M. Pastor-Valero, and B.H. Tess. 2014. The prevalence of metabolically healthy obesity: A systematic review and critical evaluation of the definitions used. Obesity Reviews: An Official Journal of the International Association for the Study of Obesity 15: 781–790. doi:10.1111/obr.12198.
Roberson, L.L., E.C. Aneni, W. Maziak, A. Agatston, T. Feldman, M. Rouseff, T. Tran, M.J. Blaha, R.D. Santos, A. Sposito, M.H. Al-Mallah, R. Blankstein, M.J. Budoff, and K. Nasir. 2014. Beyond BMI: The “Metabolically healthy obese” phenotype & its association with clinical/subclinical cardiovascular disease and all-cause mortality—A systematic review. BMC Public Health 14: 14. doi:10.1186/1471-2458-14-14.
Robinson, M.K., K.M. Mogensen, J.D. Casey, C.K. McKane, T. Moromizato, J.D. Rawn, and K.B. Christopher. 2015. The relationship among obesity, nutritional status, and mortality in the critically ill. Critical Care Medicine 43: 87–100. doi:10.1097/CCM.0000000000000602.
Rtveladze, K., T. Marsh, S. Barquera, L.M. Sanchez Romero, D. Levy, G. Melendez, L. Webber, F. Kilpi, K. McPherson, and M. Brown. 2014. Obesity prevalence in Mexico: Impact on health and economic burden. Public Health Nutrition 17: 233–239. doi:10.1017/S1368980013000086.
Sakr, Y., C. Madl, D. Filipescu, R. Moreno, J. Groeneveld, A. Artigas, K. Reinhart, and J.-L. Vincent. 2008. Obesity is associated with increased morbidity but not mortality in critically ill patients. Intensive Care Medicine 34: 1999–2009. doi:10.1007/s00134-008-1243-0.
Sakurai, T., S. Iimuro, A. Araki, H. Umegaki, Y. Ohashi, K. Yokono, and H. Ito. 2010. Age-associated increase in abdominal obesity and insulin resistance, and usefulness of AHA/NHLBI definition of metabolic syndrome for predicting cardiovascular disease in Japanese elderly with type 2 diabetes mellitus. Gerontology 56: 141–149. doi:10.1159/000246970.
Sangun, Ö., B. Dündar, M. Köşker, Ö. Pirgon, and N. Dündar. 2011. Prevalence of metabolic syndrome in obese children and adolescents using three different criteria and evaluation of risk factors. Journal of Clinical Research in Pediatric Endocrinology 3: 70–76. doi:10.4274/jcrpe.v3i2.15.
Sassi, F., M. Devaux, M. Cecchini, and E. Rusticelli. 2009. The obesity epidemic: Analysis of past and projected future trends in selected OECD countries (OECD Health Working Papers). Paris: Organisation for Economic Co-operation and Development.
Scuteri, A., S. Laurent, F. Cucca, J. Cockcroft, P.G. Cunha, L.R. Mañas, F.U. Mattace Raso, M.L. Muiesan, L. Ryliškytė, E. Rietzschel, J. Strait, C. Vlachopoulos, H. Völzke, E.G. Lakatta, P.M. Nilsson, and Metabolic Syndrome and Arteries Research (MARE) Consortium. 2015. Metabolic syndrome across Europe: Different clusters of risk factors. European Journal of Preventive Cardiology 22: 486–491. doi:10.1177/2047487314525529.
Sijtsma, A., G. Bocca, C. L’abée, E.T. Liem, P.J.J. Sauer, and E. Corpeleijn. 2014. Waist-to-height ratio, waist circumference and BMI as indicators of percentage fat mass and cardiometabolic risk factors in children aged 3–7 years. Clinical Nutrition (Edinburgh, Scotland) 33: 311–315. doi:10.1016/j.clnu.2013.05.010.
Sonntag, D., S. Ali, and F. De Bock. 2016. Lifetime indirect cost of childhood overweight and obesity: A decision analytic model. Obesity (Silver Spring, Md.) 24: 200–206. doi:10.1002/oby.21323.
Stapleton, R.D., and B.T. Suratt. 2014. Obesity and nutrition in acute respiratory distress syndrome. Clinics in Chest Medicine 35: 655–671. doi:10.1016/j.ccm.2014.08.005.
Stefan, N., K. Kantartzis, J. Machann, F. Schick, C. Thamer, K. Rittig, B. Balletshofer, F. Machicao, A. Fritsche, and H.-U. Häring. 2008. Identification and characterization of metabolically benign obesity in humans. Archives of Internal Medicine 168: 1609–1616. doi:10.1001/archinte.168.15.1609.
Sundström, J., U. Risérus, L. Byberg, B. Zethelius, H. Lithell, and L. Lind. 2006. Clinical value of the metabolic syndrome for long term prediction of total and cardiovascular mortality: Prospective, population based cohort study. BMJ 332: 878–882. doi:10.1136/bmj.38766.624097.1F.
Swinburn, B.A., G. Sacks, K.D. Hall, K. McPherson, D.T. Finegood, M.L. Moodie, and S.L. Gortmaker. 2011. The global obesity pandemic: Shaped by global drivers and local environments. Lancet (London, England) 378: 804–814. doi:10.1016/S0140-6736(11)60813-1.
Tenenbaum, A., and E.Z. Fisman. 2011. “The metabolic syndrome... is dead”: These reports are an exaggeration. Cardiovascular Diabetology 10: 11. doi:10.1186/1475-2840-10-11.
Tenenbaum, A., M. Motro, E. Schwammenthal, and E.Z. Fisman. 2004. Macrovascular complications of metabolic syndrome: An early intervention is imperative. International Journal of Cardiology 97: 167–172. doi:10.1016/j.ijcard.2003.07.033.
Twells, L.K., D.M. Gregory, J. Reddigan, and W.K. Midodzi. 2014. Current and predicted prevalence of obesity in Canada: A trend analysis. CMAJ Open 2: E18–E26. doi:10.9778/cmajo.20130016.
Vonbank, A., C.H. Saely, P. Rein, and H. Drexel. 2013. Insulin resistance is significantly associated with the metabolic syndrome, but not with sonographically proven peripheral arterial disease. Cardiovascular Diabetology 12: 106. doi:10.1186/1475-2840-12-106.
Vucenik, I., and J.P. Stains. 2012. Obesity and cancer risk: Evidence, mechanisms, and recommendations. Annals of the New York Academy of Sciences 1271: 37–43. doi:10.1111/j.1749-6632.2012.06750.x.
Wang, Y.C., K. McPherson, T. Marsh, S.L. Gortmaker, and M. Brown. 2011. Health and economic burden of the projected obesity trends in the USA and the UK. Lancet (London, England) 378: 815–825. doi:10.1016/S0140-6736(11)60814-3.
Wijnhoven, T.M.A., J.M.A. van Raaij, A. Spinelli, A.I. Rito, R. Hovengen, M. Kunesova, G. Starc, H. Rutter, A. Sjöberg, A. Petrauskiene, U. O’Dwyer, S. Petrova, V. Farrugia Sant’angelo, M. Wauters, A. Yngve, I.-M. Rubana, and J. Breda. 2013. WHO European Childhood Obesity Surveillance Initiative 2008: Weight, height and body mass index in 6-9-year-old children. Pediatric Obesity 8: 79–97. doi:10.1111/j.2047-6310.2012.00090.x.
Wijnhoven, T.M.A., J.M.A. van Raaij, A. Spinelli, G. Starc, M. Hassapidou, I. Spiroski, H. Rutter, É. Martos, A.I. Rito, R. Hovengen, N. Pérez-Farinós, A. Petrauskiene, N. Eldin, L. Braeckevelt, I. Pudule, M. Kunešová, and J. Breda. 2014. WHO European Childhood Obesity Surveillance Initiative: Body mass index and level of overweight among 6–9-year-old children from school year 2007/2008 to school year 2009/2010. BMC Public Health 14: 806. doi:10.1186/1471-2458-14-806.
Wu, S.H., W.S. Hui, Z. Liu, and S.C. Ho. 2010. Metabolic syndrome and all-cause mortality: A meta-analysis of prospective cohort studies. European Journal of Epidemiology 25: 375–384. doi:10.1007/s10654-010-9459-z.
Zuo, H., Z. Shi, X. Hu, M. Wu, Z. Guo, and A. Hussain. 2009. Prevalence of metabolic syndrome and factors associated with its components in Chinese adults. Metabolism 58: 1102–1108. doi:10.1016/j.metabol.2009.04.008.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Engin, A. (2017). The Definition and Prevalence of Obesity and Metabolic Syndrome. In: Engin, A., Engin, A. (eds) Obesity and Lipotoxicity. Advances in Experimental Medicine and Biology, vol 960. Springer, Cham. https://doi.org/10.1007/978-3-319-48382-5_1
Download citation
DOI: https://doi.org/10.1007/978-3-319-48382-5_1
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-48380-1
Online ISBN: 978-3-319-48382-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)