Abstract
There is an increasing demand for carotenoids, which are fundamental components of the human diet, for example as precursors of vitamin A. Carotenoids are also potent antioxidants and their health benefits are becoming increasingly evident. Protective effects against prostate cancer and age-related macular degeneration have been proposed for lycopene and lutein/zeaxanthin, respectively. Additionally, β-carotene, astaxanthin and canthaxanthin are high-value carotenoids used by the food industry as feed supplements and colorants. The production and consumption of these carotenoids from natural sources, especially from seeds, constitutes an important step towards fortifying the diet of malnourished people in developing nations. Therefore, attempts to metabolically manipulate β-carotene production in plants have received global attention, especially after the generation of Golden Rice (Oryza sativa). The endosperms of Golden Rice seeds synthesize and accumulate large quantities of β-carotene (provitamin A), yielding a characteristic yellow color in the polished grains. Classical breeding efforts have also focused in the development of cultivars with elevated seed carotenoid content, with maize and other cereals leading the way. In this communication we will summarize transgenic efforts and modern breeding strategies to fortify various crop seeds with nutraceutical carotenoids.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Consumers are becoming increasingly aware and interested in healthy foods. In the human diet, carotenoids have been shown to have antioxidant activity which may help to prevent certain kinds of cancers, arthritis and atherosclerosis (Stahl and Sies 2003). Free radicals are by-products of many metabolic reactions in the human body. They can have damaging effects on DNA, proteins and cell membranes and as such have been linked to numerous diseases/aliments. Carotenoids are potent anti-oxidants and might help prevent aliments due to oxidative damage. Although there are over 700 carotenoids identified in nature (Britton et al. 2004; Delgado-Vargas et al. 2000), only the consumption of six of them, α-carotene , β-carotene, lutein , lycopene, zeaxanthin and astaxanthin , have been shown to provide health benefits (Johnson 2002). β-carotene might be the most known of the nutraceutical carotenoids since it is pro-vitamin A and after its consumption there is a conversion of it to its active vitamin A (retinol) form in the intestine (Olson 1989). β-carotene has been shown to alleviate deficiencies leading to night blindness and other related nutritional insufficiencies (Haskell et al. 2005) and may improve gut and immune health (Chew 1993). Lutein and zeaxanthin have been implicated as protective agents against acquired ocular diseases, such as cataracts and age related macular degeneration (Tan et al. 2008). Astaxanthin has also gained attention as a potent antioxidant (Chap. 1). The addition of both ketone and hydroxyl groups to β-carotene by the enzyme β-carotene ketolase produces the red hue nutraceutical carotenoid astaxanthin. Most higher plants do not possess the ability to synthesize it. Thus, manipulation of ketocarotenoid synthesis requires the addition of metabolic steps not usually present in the organs of crop plants.
2 Seed Carotenoids
Seeds are global commodities as much of our food/feed, fuel and fiber are derived from them. As the vehicle to propagate a plant’s next generation, plants sequester energy reserves in the form of carbohydrates, oils and proteins to aid the seed in germination and to ensure propagation of the species. Seeds are high in needed nutrients. They are dry individual entities that can easily be stored or shipped long distance without alteration in the seed content or viability. This represents an advantage over fruits and vegetables which are excellent sources of carotenoids but have shorter shelf lives and may require refrigeration. Undoubtedly, the fortification of staple seed crops is a more cost effective, and thus feasible venue, to cope with malnutrition in developing countries.
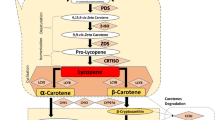
Seed carotenoid composition can vary widely depending on the crop species, cultivar, developmental stage and growing conditions. As seen in Table 13.1, seeds of different crop species primarily accumulate lutein, zeaxanthin and β-carotene (Aluru et al. 2008; Kim et al. 2012; Rodriguez-Suarez et al. 2014; Salas Fernandez et al. 2008 , Yu et al. 2007). However, provitamin A carotenoids are present at low percentages and biofortification of staple crops is an important breeding objective. The role that carotenoids play in seed tissues is less clear than in other plant organs but is emerging (Howitt and Pogson 2006). Interestingly, carotenoid synthesis in seeds is related to abscisic acid (ABA) synthesis which in turn is required for seed dormancy (Maluf et al. 1997). In addition, carotenoids act as antioxidants preventing seed ageing and contributing to seed viability and successful germination (Fig. 13.1) (Havaux et al. 1991; Pinzino et al. 1999).
Phytoene synthase transgenic soybean seed accumulating enhanced levels of β-carotene (Schmidt et al. 2015) display no difficultly in germination
Understanding the biochemical steps of carotenoid synthesis have not resulted yet in the predictable control of the biosynthesis in plants through either breeding or transgenic approaches. Duplication of key biosynthetic genes, pathway bottlenecks, enzyme sub-organelle localization, metabolon assembly and activity are only partially known, species specific and can have a direct effect on seed carotenoid accumulation (Shumskaya et al. 2012; Shumskaya and Wurtzel 2013; Giuliano 2014).
3 Regulation of Carotenoid Biosynthesis and Accumulation in Seeds
At least three major mechanisms involved in the regulation of carotenoid biosynthesis and accumulation in higher plants have been described to date. These mechanisms include (i) transcriptional regulation of key carotenoid biosynthetic genes, (ii) the existence of specialized carotenoid-sequestering structures , and (iii) the extent of carotenoid degradation (catabolism).
Several reports indicate that transcript levels of key biosynthetic genes correlate with increased levels of carotenoid content in plants (Chap. 2, Harjes et al. 2008; Vallabhaneni and Wurtzel 2009; Vallabhaneni et al. 2009; Yan et al. 2010; Rodriguez-Suarez et al. 2014). A positive correlation of PSY gene expression and total carotenoid seed content has been the best described example (Li et al. 2008a, b; Vallabhaneni and Wurtzel 2009; da Silva Messias et al. 2014). In Arabidopsis thaliana, PSY is encoded by a single copy gene (Scolnik and Bartley 1994) but most plant species contain a PSY gene family composed of at least two or three homologous genes (Bartley et al. 1992; Bartley and Scolnik 1993; Busch et al. 2002; Gallagher et al. 2004; Li et al. 2008a; Arango et al. 2010; Cardenas et al. 2012). Therefore, functional characterization of PSY homologues could be essential to understanding carotenoid accumulation in seed tissues (Lopez Emparan et al. 2014). The same is true for other gene families involved in carotenoid biosynthesis, underpinning the importance of genome complexity of individual plant species. In maize, the y1 gene encodes for PSY1, one of the three PSY homologues present in this crop genome and several alleles for this gene have been described (Buckner et al. 1996; Gallagher et al. 2004). PSY1 transcript accumulation, but not that of PSY2 and PSY3, positively correlates with carotenoid accumulation in maize endosperm (Li et al. 2008b; Vallabhaneni and Wurtzel 2009). In addition, PSY1 alleles exhibit expression differences that correlate with endosperm carotenoid levels (Buckner et al. 1996). Elevated seed carotenoid content is also the result of lower expression rates of specific biosynthetic genes. Natural genetic variants of low expression/activity of lycopene ε cyclase (LCYE) in maize are correlated with up to threefold differences in β-carotene (Harjes et al. 2008). Similarly, screening and characterization of genetically diverse maize germplasm revealed that elevated β-carotene levels correlate with low transcription of genes encoding for β-carotene hydroxylases (Vallabhaneni et al. 2009; Yan et al. 2010). These elevated β-carotene levels in the kernels are thought to be explained by the reduced conversion of β-carotene to downstream xanthophylls , such as zeaxanthin.
Carotenoid accumulation is influenced by the presence of structures capable of storing carotenoids (Cazzonelli and Pogson 2010). Depending on the plant organ, carotenoids will be stored in different plastids (Chap. 10). Although carotenoids can be stored in all plastid types, the stability in each of them may vary. For example, carotenoids accumulated in chromoplast plastoglobuli exhibit much higher light stability than carotenoids in chloroplast membranes (Merzlyak and Solovchenko 2002). The Orange (Or) gene mutation in cauliflower (Brassica oleracea var botrytis) produces the accumulation of high levels of β-carotene in tissues that normally do not contain carotenoids by triggering the differentiation of proplastids or non-colored plastids into chromoplasts (Chap. 10, Lu et al. 2006; Li et al. 2001). In seeds, elaioplasts are better seed carotenoid-sequestering structures than amyloplasts (DellaPenna and Pogson 2006). Recently, the study of sub-organelle localization in maize cells, rice and Arabidopsis PSY allelic variants revealed that different PSY1 isozymes localize to distinct plastid compartments, highlighting the importance of enzyme and metabolome localization (Shumskaya et al. 2012). Interestingly, transient expression studies revealed that maize PSY2 and PSY3, rice and Arabidopsis PSYs localize to plastoglobuli, which are mostly attached to thylakoid membranes, while maize PSY1 exhibited a dual localization and was also found in the stroma (Shumskaya et al. 2012). A deep study of maize and other grasses PSY1 coding sequences discovered that 99 % of 79 varieties with yellow endosperm carry a threonine residue at position 257 (T257) of the PSY1 protein. Most other PSY1s from white endosperm varieties and two species of Teosinte (the maize wild ancestor), carried either proline or serine at this position. Different structural variants of PSY were thus described and it was suggested that the combination of an insertion in the PSY1 promoter region (providing endosperm expression to the yellow allele) and a unique structural variation of the PSY1 protein, asparagine (N168) and threonine (T257), resulted in the ideal for amyloplast carotenogenesis (Shumskaya et al. 2012).
Carotenoid catabolism has been shown to be another important aspect of carotenoid content regulation. For example, loss of function mutants of the carotenoid cleavage dioxygenase 1 (CCD1) gene in Arabidopsis exhibit 40 % increased levels of total carotenoids in seeds (Auldridge et al. 2006). This is consistent with a functional characterization of a recombinant maize CCD1 that showed that provitamin A carotenoids in the grains could be cleaved by this enzyme (Sun et al. 2008). In addition, both CCD1 copy number variation and CCD1 transcript accumulation during grain development negatively correlated with seed carotenoid content in maize (Vallabhaneni et al. 2010, da Silva Messias et al. 2014). Expression of CCD1 during grain development varied widely among maize landraces, with a white variety exhibiting the highest CCD1 expression levels (da Silva Messias et al. 2014). Similarly, loss of function of the carotenoid cleavage dioxygenase 4 (CCD4) gene in Arabidopsis greatly reduced carotenoid degradation during seed desiccation, resulting in an 8.4-fold increase of β-carotene levels (Gonzalez-Jorge et al. 2013). Clearly, further characterization of seed crops germplasm is required to fully understand CCD1 and CCD4 gene expression in other crop species to better recognize the implications of carotenoid degradation in breeding for enhanced carotenoid content in seeds.
4 Biotechnological Efforts to Enhance Seed Carotenoid Content
4.1 β-Carotene Increment in Seeds
Due to the high incidence of vitamin A deficiency in developing nations, many crops have been biofortified with β-carotene . With the success of ‘Golden Rice ’ and ‘Golden Rice 2’ (Paine et al. 2005; Enserink 2008) pioneering the way, numerous crops have now been reported to have engineered levels of β-carotene predominantly by the over expression of the phytoene synthase gene from Erwinia uredovora. The use of PSY alone or in combination with other downstream carotenoid enzymes indicates the effectiveness of shuttling the initial substrate in a successful metabolic engineering effort (Schmidt et al. 2015; Fig. 13.1). Emphasizing the importance of PSY gene, emerges the evidence that the Narcise pseudonarcise (daffodil) PSY was a limiting step in the initial enriched Golden Rice (Ye et al. 2000). Subsequently, through systematic testing of different PSY genes, authors selected those belonging to maize and coupled it with the original E.uredovora carotene desaturase (CRTI) gene that resulted in a 23-fold increase in β-carotene in rice grains, giving rise to ‘Golden Rice 2’ (Paine et al. 2005). This, PSY was overexpressed and resulted in elevated β-carotene levels in other seed crops, such as canola ( Brassica napus ) (Fig. 13.2) (Shewmaker et al. 1999), maize (Zea mays) (Aluru et al. 2008), flaxseed (Linum usitatissimum) (Fujisawa et al. 2008), wheat (Triticum aestivum) (Wang et al. 2014) and soybean (Glycine max) (Schmidt et al. 2015; Kim et al. 2012). Efforts to further increase β-carotene in rice endosperm focused on either increasing the up-stream isoprenoid carotenoid precursor or creating a carotenoid storage sink. Firstly, investigators reproduced the ‘Golden Rice 2’ genotype by introducing the same two carotenoid biosynthesis steps in an endosperm-specific manner in rice and then over expressing the 1-deoxy-D-xylose-5-phosphate synthase (DXS) Arabidopsis thaliana gene increasing through this strategy the pool of geranalygeranyl diphosphate (GGPP) carotenoid precursor. This DXS enzyme catalyzes the first, and likely rate-limiting, step of the MEP pathway (2-C-methyl-D-erythritol 4-phosphate) resulting in the synthesis of isoprenoids, and hence carotenoid biosynthesis metabolites. Bai et al. (2016) found that transgenic rice with the overexpressed DXS together with ZmPSY and EuCRTI’ transgenes had a 2.7–5.8-fold increase in total carotenoids, with β-carotene being the most abundant, compared to seeds expressing only the two ‘Golden Rice 2’ gene cassettes. A sink of the carotenoid metabolites was produced by also introducing the A. thaliana ORANGE (OR) gene. The OR gene was originally discovered as a naturally occurring dominant mutant in cauliflower and investigations have shown it results in enhanced β-carotene accumulation through the creation of a storage sink for carotenoids (Li et al. 2001; Lu et al. 2006; Lopez et al. 2008). Bai et al. (2016) overexpressed OR gene in rice endosperm with/without the two transgene cassettes composed by ZmPSY and EuCRTI and found a 2.1–4.7-fold increase in the total carotenoid content, due mostly to β-carotene and lutein over accumulation. Carotenoid enhancement was only detected in the transgenic seeds expressing the ‘Golden Rice 2’ transgenes and the newly introduced seed-specific OR cassette. In contrast to cauliflowers, the overexpression of OR itself is insufficient to result in carotenoid accumulation and chromoplast differentiation in rice seeds. It is likely the presence of the OR protein may enhance chromoplast production only in an environment of sufficient carotenoid metabolites (Bai et al. 2016). It is interesting to note that in ‘Golden Rice 2’ among 75–84 % of total carotenoids corresponds to β-carotene, while Bai et al. (2016) using the same strategy, obtained only 25–39 % of β-carotene in transgenic rice endosperm. In most biofortified transgenic species, variations in carotenoid accumulation using similar carotenoid engineering strategies may be due to variation in the production of transgenics.
Other approaches to elevate β-carotene include a suppression strategy targeting lycopene ε cyclase to exclusively shuttle the conversion of lycopene to β-carotene. This strategy was used successfully in both Brassica seeds (Yu et al. 2007) and potato tubers (Diretto et al. 2007). In an effort to produce high carotenoid and low anti-nutritional Brassica seeds, investigators suppressed the negative regulatory gene of light mediated responses DET1 (DE-ETIOLATED1) both constitutively and seed-specifically (Wei et al. 2009). Sinapate esters, a type of phenolypropanoid metabolites, that produces off flavor and taste in cruciferous seeds, were reduced in both sets of transgenic plants, while total carotenoids, lutein, β-carotene and zeaxanthin, were elevated especially in the constitutively suppressed DET1 lines which exhibited increments of 1.5-fold lutein, 3.9-fold zeaxanthin and 12-fold of β-carotene. Likewise, branching , and hence seed yield, has been associated to seed carotenoid content by the discovery that strigolactones , metabolites derived from carotenoids (Chap. 9), inhibit plant branching (Gomez-Roldan et al. 2008). Specifically the microRNA , AtmiR156b, has been shown to suppress the expression of SPL (SQUAMOSA PROMOTER BINDING PROTEIN LIKE) transcription factor, that regulate leaf primordium initiation and transition from vegetative to reproductive stages (Wang et al. 2009). Wei et al. 2010 overexpressed AtmiR156b both constitutively and seed-specifically in Brassica napus . In addition to the increased flower number, plants also present an increase in β-carotene in the seeds (up to fourfold) only in the constitutive overexpressing transgenic plants. This suggests a complicated and interconnected regulatory network of source / sink components to determine seed carotenoid content. These findings suggest the importance of photosynthate resources availability to determine carotenoid seed composition.
The most important aim in seed fortification is to enhance the health and wellbeing of consumers, humans or animals. The benefits of β-carotene enhanced food crops to children’s eye-health has been well documented (Rao and Rao 2007) and the testing of β-carotene feed on animal health is starting to emerge. Fully oxidized β-carotene may confer anti-inflammatory properties in cattle with respiratory tract disease, such as pneumonia (Duquette et al. 2014). Nogareda et al. (2015), noted a positive impact of a high-carotenoid corn diet on broiler chickens and their resistance to the protozoan parasitic coccidiosis disease. Findings on both cattle and chicken, suggest carotenoids interact beneficially with vaccinations, indicating carotenoids could be used as a complementary strategy to boost disease resistance.
In addition to carotenoid themselves having additional, unexpected, beneficial health impacts, the enhanced accumulation of carotenoids themselves might render the seeds more nutritious. Oil composition analysis of β-carotene fortification efforts in certain seed crops, Brassica and soybean, have shown alterations in fatty acids profiles containing less unsaturated fatty acids (Shewmaker et al. 1999; Schmidt et al. 2015). Oils high in unsaturated fatty acids when used during baking or frying result in the production of heart unhealthy trans fats. Such oil seed crops engineered to have a healthy fatty acid composition will contribute to the reduction of the incidence of coronary heart disease, currently the leading cause of death for Americans (Astrup et al. 2011). Also, engineered oil containing both enhanced β-carotene and decreased levels of unsaturated fatty acids should rival red palm oil’s health and cooking benefits without the destruction of tropical environments and animal habitats (Azhar et al. 2014).
4.2 Zeaxanthin Increments in Seeds
In photosynthetic organisms, zeaxanthin protects cells against photooxidation and membranes against lipid peroxidation by quenching reactive radicals that have been created as toxic byproducts during photosynthesis reactions. The fortification of crops with zeaxanthin gained momentum after reports on the correlation of this carotenoid and lutein in the prevention of age related macular degeneration (AMD) (Tan et al. 2008; Gale et al. 2003). Age-related Macular Degeneration (AMD) is the leading cause of irreversible vision loss in adults age 55 years and older and is currently estimated to affect ten million Americans (Friedman et al. 2004). Due to a combination of an increase in life expectancy and aging ‘Baby Boomers’, studies indicate that there will be 71 million Americans over the age of 65 in 2030 compared to 12 million in 1990. It has been estimated that a 6–10 mg daily intake of zeaxanthin and lutein in Americans would have a $2.5 billion net savings to Medicare system over a 5 years period (Lewin 2009).
Zeaxanthin got its name from the yellow corn, Zea mays L., as it is the principal yellow pigment in corn. It can be found in many fruits and vegetables however its levels in most foodstuff is measured in μg and it needs to be ingested at ∼10 mg levels / day to be beneficial to eye health – a magnitude of 100–1000 fold too low to be biologically relevant. Zeaxanthin-enriched Brassica napus seeds were attempted to be produced by silencing the lycopene ε cyclase enzyme via RNAi technology. Lycopene is a branched point in the carotenoid biosynthesis pathway, suppressing this cyclase enzyme production should inhibit the formation of the ε cycle downstream carotenoids , namely lutein and α carotene , and simultaneously allowing predominantly β rings to form from the other branch pathway giving rise to enhanced levels of β-carotene, zeaxanthin and violaxanthin. The constitutive suppression of the ε cyclase resulted in a notable increase in the desired carotenoids, plus inexplicably lutein, yet levels of zeaxanthin accumulated were very low in maturing seeds (0.26 μg/g) and undetectable in mature and dry seeds. Zeaxanthin levels have been successfully enhanced in non-seed tissues (Romer et al. 2002; Dharmapuri et al. 2002; Wolters et al. 2010) so it might be that zeaxanthin as part of the xanthophyll cycle and a precursor to the phytohormone abscisic acid (ABA) presents unique hurdles to stably accumulate in seeds.
Researchers took advantage of the inherent variation in carotenoid content and composition in corn kernels to produce a zeaxanthin-enriched variety. Naqvi et al. (2011) strategy was to combine an already successful engineering approach, to increase carotenoids and breed the transgenes into a genetic background primed for β ring carotenoid production. They too targeted the splitting of lycopene into either the β:β ring structures or β:ε ring structures by the action of lycopene β cyclase and lycopene ε cylase, respectively. Two cultivars that varied presumably in their lycopene ε cyclase activity that exhibit 0.61 and 1.90 ratios of β:ε carotenoids were chosen to cross with their previously engineered enhanced carotenoid line having a 3.51 β:ε ratio as a result of the endosperm-specific expression of three transgenes: maize phytoene synthase, bacterial phytoene desaturase and Genetiana lutea lycopene β-cyclase (Zhu et al. 2008). The result was the β:ε ratio of the 1.90 cultivar increased to 6.80, translating to 56 μg zeaxanthin/ g dry kernel.
4.3 Astaxanthin Increase in Seeds
Astaxanthin has also gained attention from the plant biotechnology community as it is a dietary antioxidant and colorant in aquaculture industries (Chap. 1). The first attempt to engineer astaxanthin into a plant was the expression of β-carotene ketolase from the algae H. pluvialis under the regulatory control of tomato phytoene sythase promoter (Mann et al. 2000). It was the first demonstration that this complex keto carotenoid could successfully be produced and accumulated in plants. Although there were non-detectable levels of astaxanthin in the leaf tissue, it did constitute 23 % of the carotenoids found in the nectaries of the transgenic tobacco plants. Transgenic canola ( Brassica napus ) seeds were engineered with seven carotenoid genes in an effort to produce astaxanthin and other ketocarotenoids with the best line accumulating 0.2 μg astaxanthin/g of dry weight. Ralley et al. (2004) transformed tobacco with two carotenoid genes from the marine bacteria Paracoccus sp., β-carotene ketolase (BKT) and β-carotene hydroxylase (BHY) under constitutive regulatory control in tobacco with the result of the nectar carotenoid containing 5 % astaxanthin, up to nearly 64 μg astaxanthin/g. Huang et al. 2013 also obtained high amounts of astaxanthin not only in vegetative leaves but also in the fruit of a tomato variety with high synthesis capacity for β-carotene through the expression of the algal BKT and BHY . Moreover, the fruit accumulated fivefold more astaxanthin than the leaves, reaching 16.1 mg/g cell dry weight, similar to Haematoccocus pluvialis. Recently, seed-specific accumulation of up to 7 μg/ g astaxanthin and 52 μg/g canthaxanthin has been achieved in soybean seeds by chloroplast targeted PSY gene from Pantoea ananatis and BKT gene from Brevundimonas (Pierce et al. 2015).
Researchers speculate that the enzyme to convert zeaxanthin into astaxanthin itself varies greatly in its efficiency and that this is largely the rate-limiting step in producing astaxanthin. Zhong et al. 2011 reported the in vivo conversion rate of zeaxanthin to astaxanthin by β-carotene ketolase isolated from three algae sources, namely Chlamydomonas reinhardtii, Chlorella zofingiensis and H. pluvialis, as 85 %, 38 % and 1 %, respectively. They then constituatively expressed these ΒΚΤ genes individually in a chloroplast-targeted manner in Arabidopsis and also found that the levels of astaxanthin in dry leaf tissue varied depending on the source of the gene – high levels when the Chlamydomonas gene was used (2 mg/g), moderate levels when the Chlorella gene was used (0.24 mg/g) and non-detectable levels of astaxanthin when the Haematococcus gene was used. This finding indicates that astaxanthin can be made efficiently if the correct β-carotene ketolase enzyme is used. Zhu et al. (2008) also stressed the importance of the ketolase enzyme in the production of astaxanthin. Their work using a combination of up to five transgenes involved in carotenoid biosynthesis into the white maize kernel naturally mutant in phytoene synthase, demonstrated the competition between β−carotene ketolase and hydroxylase for β-carotene as a substrate. A mechanism to streamline the production of astaxanthin might be the use of a multifunctional enzyme, such as astaxanthin synthase from Xanthophyllomyces dendrohous, what seems to be able to convert β-carotene directly to astaxanthin (Ojima et al. 2006).
Genetic enhancement of carotenoids in crop seeds either through conventional breeding or transgenic approaches has already made significant impacts on human and animal health. The field of research will only continue to grow and move forward as scientific breakthroughs on plant genomes, gene regulation and novel transgenic approaches are honed.
References
Aluru M, Xu Y, Guo R et al (2008) Generation of transgenic maize with enhanced provitamin A content. J Exp Bot 59:3551–3562
Arango J, Wüst F, Beyer P, Welsch P (2010) Characterization of phytoene synthase from cassava and their involvement in abiotic stress-mediated responses. Planta 232:1251–1262
Astrup A, Dyerberg J, Elwood P et al (2011) The role of reducing intakes of saturated fat in the prevention of cardiovascular disease: where does the evidence stand in 2010? Am J Clin Nutr 93:684–688
Auldridge M, Block A, Vogel JT et al (2006) Characterization of three members of the Arabidopsis carotenoid cleavage dioxygenase family demonstrates the divergent roles of this multifunctional enzyme family. Plant J 45(6):982–93
Azhar B, Lindenmayer DB, Wood J et al (2014) Ecological impacts of oil palm agriculture on forest mammals in plantation estates and smallholdings. Biodivers Conserv 23:1175–1191
Bai C, Capell T, Berman J et al (2016) Bottlenecks in carotenoid biosynthesis and accumulation in rice endosperm are influenced by the precursor-product balance. Plant Biotech J 14:195–205. doi:10.1111/pbi.12373
Bartley GE, Scolnik PA (1993) cDNA cloning, expression during development, and genome mapping of PSY2, a second tomato gene encoding phytoene synthase. J Biol Chem 268:25718–25721
Bartley GE, Viitanen PV, Bacot KO, Scolnik PA (1992) A tomato gene expressed during fruit ripening encodes an enzyme of the carotenoid biosynthesis pathway. J Biol Chem 267:5036–5039
Britton G, Liaaen-Jensen S, Pfander HE (2004) Carotenoids: handbook. Birkhauser, Basel
Buckner B, San Miguel P, Janick-Buckner D, Bennetzen J (1996) The y1 gene of maize codes for phytoene synthase. Genetics 143:479–488
Busch M, Seuter A, Hain R (2002) Functional analysis of the early steps of carotenoid biosynthesis in tobacco. Plant Physiol 128:439–453
Cardenas PD, Gajardo HA, Huebert T et al (2012) Retention of triplicated phytoene synthase (PSY) genes in Brassica napus L. and its diploid progenitors during the evolution of the Brassiceae. Theor Appl Genet 124:1215–1228
Cazzonelli C, Pogson BJ (2010) Source to sink: regulation of carotenoid biosynthesis in plants. Trends Plant Sci 15:266–274
Chew BP (1993) Role of carotenoids in the immune response. J Dairy Sci 76:2804–2811
Da Silva Messias R, Galli V, dos Anjos e Silva SD, Valmor Rombaldi C (2014) Carotenoid biosynthetic and catabolic pathways: gene expression and carotenoid content in grains of maize landraces. Nutrients 6:546–563
Delgado-Vargas F, Jimenez AR, Paredes-Lopez O (2000) Natural pigments: carotenoids, anthocyanins, and betalains – characteristics, biosynthesis, processing and stability. Crit Rev Food Sci Nutr 40:173–289
DellaPenna D, Pogson BJ (2006) Vitamin synthesis in plants: tocopherols and carotenoids. Annu Rev Plant Biol 57:711–38
Dharmapuri S, Rosati C, Pallara P et al (2002) Metabolic engineering of xanthophyll content in tomato fruits. Febs letters 519(1–3):30–34
Diretto G, Welsch R, Tavazza R et al (2007) Silencing of beta-carotene hydroxylase increases total carotenoid and beta-carotene levels in potato tubers. BMC Plant Biol. doi:10.1186/1471-2229-7-11
Duquette SC, Fischer CD, Feener TD et al (2014) Anti-inflammatory effects of retinoids and carotenoid derivatives on caspase-3-dependent apoptosis and efferocytosis of bovine neutrophils. Am J Vet Res 75:1064–1075
Enserink M (2008) Tough lessons from golden rice. Science 320:468–471
Friedman DS, O’Colmain BJ, Tomany SC et al (2004) Prevalence of age-related macular degeneration in the Unites States. Arch Ophthalmol 122:564–572
Fujisawa M, Watanabe M, Choi S et al (2008) Enrichment of carotenoids in flaxseed (Linum usitatissimum) by metabolic engineering with introduction of bacterial phytoene synthase gene crtB. J Biosci Bioeng 105(6):636–641
Gale CR, Hall NF, Phillips DI et al (2003) Lutein and zeaxanthin status and risk of age-related macular degeneration. Invest Opthalmol Vis Sci 44:2461–2465
Gallagher CE, Matthews PD, Li F, Wurtzel ET (2004) Gene duplication in the carotenoid biosynthetic pathway preceded evolution of the grasses. Plant Physiol 135:1776–1783
Giuliano G (2014) Plant carotenoids: genomics meets mutli-gene engineering. Curr Opin Plant Biol 19:111–117
Gomez-Roldan V, Fermas S, Brewer PB, Puech-Pages V, Dun EA, Pillot J-P, Letisse F, Matusova R, Danoun S, Portais J-C, Bouwmeester H, Becard G, Beveridge CA, Rameau C, Rochange SF (2008) Strigolactone inhibition of shoot branching. Nature 455:189–194
Gonzalez-Jorge S, Ha SH, Magallanes-Lundback M et al (2013) Carotenoid cleavage dioxygenase4 is a negative regulator of β-carotene content in Arabidopsis seeds. Plant Cell 25(12):4812–4826
Harjes CE, Rocheford TR, Bai L, Brutnell TP et al (2008) Natural genetic variation in Lycopene epsilon cyclase tapped for maize biofortification. Science 319(5861):330–333
Haskell MJ, Pandey P, Graham JM et al (2005) Recovery from impaired dark adaptation in nighblind pregnant Napali women who receive small daily does of vitamin A as amaranth leaves, carrots, goat liver, vitaminA-fortified rice, or retinyl palmitate. American J Clin Nutr 81:461–471
Havaux M, Gruszecki W, Dupont I, Leblanc R (1991) Increased heat emission and its relationship to the xanthophyll cycle in pea leaves exposed to strong light stress. J Photochem Photobiol 8:361–370
Howitt CA, Pogson BJ (2006) Carotenoid accumulation and function in seeds and non-green tissues. Plant Cell Environ 29:435–445
Huang J-C, Zhong Y-J, Liu J, Sandmann G, Chen F (2013) Metabolic engineering of tomato for high-yield production of astaxanthin. Metab Eng 17:59–67
Johnson EJ (2002) The role of carotenoids in human health. Nutr Clin Care 5:56–65
Kim M, Kim JK, Kim HJ et al (2012) Genetic modification of the soybean to enhance the β-carotene content through seed-specific expression. PLoS One 7:e48287–e48287
Lewin AS (2009) Geographic atrophy in age-related macular degeneration and TLR3. N Engl J Med 360(21):2251
Li L, Paolillo DJ, Parthasarathy MV et al (2001) A novel gene mutation that confers abnormal patterns of beta-carotene accumulation in cauliflower (Brassica oleracea var. botrytis). Plant J 26(1):59–67
Li F, Vallabhaneni R, Wurtzel ET (2008a) PSY3, a new member of the phytoene synthase gene family conserved in the Poaceae and regulator of abiotic stress-induced root carotenogenesis. Plant Physiol 146:1333–1345
Li F, Vallabhaneni R, Yu J et al (2008b) The maize phytoene synthase gene family: overlapping roles for carotenogenesis in endosperm, photomorphogenesis, and thermal stress tolerance. Plant Physiol 147:1334–1346
Lopez Alex B, Van Eck J, Conlin BJ et al (2008) Effect of the cauliflower or transgene on carotenoid accumulation and chromoplast formation in transgenic potato tubers. J Exp Bot 59:213–223
Lopez Emparan A, Quezada-Martinez D, Zuniga-Bustos M et al (2014) Functional analysis of the Brassica napus L phytoene synthase (PSY) gene family. PLoS One 9:e114878. doi:10.1371/journal.pone.0114878
Lu S, Van Eck J, Zhou X et al (2006) The Cauliflower Or gene encodes a DnaJ Cysteine-Rich domain-containing protein that mediates high levels of β-carotene accumulation. Plant Cell 18:3594–3605
Maluf MP, Saab IN, Wurtzel ET, Sachs MM (1997) The viviparous12 maize mutant is deficient in abscisic acid, carotenoids, and chlorophyll synthesis. J Exp Bot 48:1259–1268
Mann V, Harker M, Pecker I, Hirschberg J (2000) Metabolic engineering of astaxanthin production in tobacco flowers. Nat Biotechnol 18:888–892
Merzlyak M, Solovchenko A (2002) Photostability of pigments in ripening apple fruit: a possible photoprotective role of carotenoids during plant senescence. Plant Sci 163:881–888
Naqvi S, Zhu C, Farre G et al (2011) Synergistic metabolism in hybrid corn indicates bottlenecks in the carotenoid pathway and leads to the accumulation of extraordinary levels of the nutritionally important carotenoid zeaxanthin. Plant Biotechnol J 9:384–393
Nogareda C, Moreno JA, Angulo E et al (2015) Carotenoid-enriched transgenic corn delivers bioavailable carotenoids to poultry and protects them against coccidiosis. Plant Biotech J 14:160–168. doi:10.1111/pbi.12369
Ojima K, Breitenbach J, Visser H et al (2006) Cloning of the astaxanthin synthase gene from Xanthophyllomyces dendrorhous (Phaffia rhodozyma) and its assignment as a B-carotene 3-hydroxylase/4-ketolase. Mol Gen Genomics 275:148–158
Olson JA (1989) Provitam A function of carotenoids: the conversion of beta-carotene into vitamin A. J Nutr 119:105–108
Paine JA, Shipton CA, Sunandha C et al (2005) Improving the nutritional value of golden rice through increased pro-vitamin A content. Nat Biotech 23:482–487
Pierce EC, LaFayette PR, Ortega MA et al (2015) Ketocarotenoid production in soybean seeds through metabolic engineering. PLoS One 10(9):e0138196
Pinzino C, Nanni B, Zandomeneghi M (1999) Aging, free radicals, and antioxidants in wheat seeds. J Agric Food Chem 47:1333–1339
Ralley L, Enfissi EMA, Misawa N et al (2004) Metabolic engineering of ketocarotenoid formation in higher plants. Plant J 39:477–486
Rao AV, Rao LG (2007) Carotenoids and human health. Pharmacol Res 55:207–216
Rodriguez-Suarez C, Mellado-Ortega E, Hornero-Mendez D, Atienza S (2014) Increase in transcript accumulation of Psy1 and e-Lcy genes in grain development is associated with differences in seed carotenoid content between durum wheat and tritordeum. Plant Mol Biol 84:659–673
Romer S, Lubeck J, Kauder F et al (2002) Genetic engineering of a zeaxanthin-rich potato by antisense inactivation and co-suppression of carotenoid epoxidation. Metab Eng 4:263–272
Salas Fernandez M, Hamblin M, Li L et al (2008) Quantitative trait loci analysis of endosperm color and carotenoid content in sorghum grain. Crop Sci 48(5):1732–1743
Schmidt MA, Parrott WA, Hildebrand DF et al (2015) Transgenic soya bean seeds accumulating b-carotene exhibit the collateral enhancements of oleate and protein content traits. Plant Biotechnol J 13:590–600
Scolnik PA, Bartley GE (1994) Nucleotide sequence of an Arabidopsis cDNA for phytoene synthase. Plant Physiol 104:1471–1472
Shewmaker CK, Sheehy JA, Daley M et al (1999) Seed-specific overexpression of phytoene synthase: increase in carotenoids and other metabolic effects. Plant J 20:401–412
Shumskaya M, Wurtzel E (2013) The carotenoid biosynthetic pathway: thinking in all dimensions. Plant Sci 208:58–63
Shumskaya M, Bradbury LMT, Monaco RR, Wurtzel ET (2012) Plastid localization of the key carotenoid enzyme phytoene synthase is altered by isozyme, allelic variation, and activity. Plant Cell 24:3725–3741
Stahl W, Sies H (2003) Antioxidant activity of carotenoids. Molec Aspects Med 24:345–351
Sun Z, Hans J, Walter MH et al (2008) Cloning and characterization of a maize carotenoid cleavage dioxygenase (ZmCCD1) and its involvement in the biosynthesis of apocarotenoids with various roles in mutualistic and parasitic interactions. Planta 5:789–801
Tan J, Wang J, Flood V et al (2008) Dietary antioxidants and the long-term incidence of age-related macular degeneration: the Blue Mountains Eye Study. Opthalmology 115:334–314
Vallabhaneni R, Wurtzel ET (2009) Timing and biosynthetic potential for provitamin A accumulation in maize. Plant Physiol 150(2):562–572
Vallabhaneni R, Gallagher CE, Licciardello N et al (2009) Metabolite sorting of germplasm collection reveals the hydroxylase3 locus as a new target for maize provitamin A biofortification. Plant Physiol 151:1635–1645
Vallabhaneni R, Bradbury LMT, Wurtzel ET (2010) The carotenoid dioxygenase gene family in maize, sorghum and rice. Arch Biochem Biophys 504:104–111
Wang JW, Czech B, Weigel D (2009) miR156-regulated SPL transcription factors define an endogenous flowering pathway in Arabidopsis thaliana. Cell 138:738–749
Wang C, Zeng J, Li Y et al (2014) Enrichment of provitamin A content in wheat (Triticum aestivum L.) by introduction of the bacterial carotenoid biosynthetic genes CrtB and CrtI. J Exp Bot 65:2545–2556. doi:10.1093/jxb/eru138
Wei S, Li X, Gruber MY et al (2009) RNAi-mediated suppression of DET1 alters the levels of carotenoids and sinapate esters in seeds of Brassica napus. J Agric Food Chem 57:5326–5333
Wei S, Yu B, Gruber MY et al (2010) Enhanced seed carotenoid levels and braching in transgenic Brassica napus expressing the Arabidopsis miR156b gene. J Agric Food Chem 58:9572–9578
Wolters A-MA, Uitdewilligen JGAML, Kloosterman BA et al (2010) Identification of alleles of carotenoid pathway genes important for zeaxanthin accumulaton in potato tubers. Plant Mol Biol 73:659–671
Yan J, Kandianis CB, Harjes CE et al (2010) Rare genetic variation in Zea mays crtRBI increases β-carotene in maize grain. Nat Genet 42(4):322–329
Ye X, Al-Babili S, Kloti Z et al (2000) Engineering the provitamin A (beta-caroten) biosynthetic pathway into (carotenoid free) rice endosperm. Science 287:303–305
Yu B, Lydiate DJ, Young LW et al (2007) Enhancing the carotenoid content of Brassica napus seeds by downregulating lycopene epsilon cyclase. Transgenic Res 17:573–585
Zhong Y, Huang J, Liu J et al (2011) Functional characterization of various algal carotenoid ketolases reveals that ketolating zeaxanthin efficiently is essential for high production of astaxanthin in transgenic Arabidoposis. J Exp Bot 62:3659–3669
Zhu C, Naqvi S, Breitenbach J et al (2008) Combinatorial genetic transformation generates a library of metabolic phenotypes for the carotenoid pathway in maize. PNAS 105(47):18232–18237
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Federico, M.L., Schmidt, M.A. (2016). Modern Breeding and Biotechnological Approaches to Enhance Carotenoid Accumulation in Seeds. In: Stange, C. (eds) Carotenoids in Nature. Subcellular Biochemistry, vol 79. Springer, Cham. https://doi.org/10.1007/978-3-319-39126-7_13
Download citation
DOI: https://doi.org/10.1007/978-3-319-39126-7_13
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-39124-3
Online ISBN: 978-3-319-39126-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)