Abstract
Mangrove forests grow on saline, permanently or periodically flooded soils of the tropical and subtropical coasts. The tree species that compose the mangrove are halophytes that have suites of traits that confer differing levels of tolerance of salinity, aridity, inundation and extremes of temperature. Here we review how climate change and elevated levels of atmospheric CO2 will influence mangrove forests. Tolerance of salinity and inundation in mangroves is associated with the efficient use of water for photosynthetic carbon gain which underpins anticipated gains in productivity with increasing levels of CO2. We review evidence of increases in productivity with increasing CO2, finding that enhancements in growth appear to be similar to trees in non-mangrove habitats and that gains in productivity with elevated CO2 are likely due to changes in biomass allocation. High levels of trait plasticity are observed in some mangrove species, which potentially facilitates their responses to climate change. Trait plasticity is associated with broad tolerance of salinity, aridity, low temperatures and nutrient availability. Because low temperatures and aridity place strong limits on mangrove growth at the edge of their current distribution, increasing temperatures over time and changing rainfall patterns are likely to have an important influence on the distribution of mangroves. We provide a global analysis based on plant traits and IPCC scenarios of changing temperature and aridity that indicates substantial global potential for mangrove expansion.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
The trees of mangrove forests have fascinated physiologists for decades. The highly saline, tidally flooded environments of mangrove forests seem unlikely to support tree growth , yet mangroves are some of the most productive forests on the planet (Alongi 2009). Both the number of families and individual species of plants that have evolved the necessary traits to grow in mangrove habitats has been relatively small: 70 species in over 40 million years (Ricklefs et al. 2006), reflecting the complex suite of traits that are required for growth in intertidal environments. The position of these forests in the landscape, on the ecotone between terrestrial and marine habitats, also brings high levels of variation in soil conditions that range over a hierarchy of timescales: daily (e.g., tidal inundation), monthly (e.g., tidal cycles), annual (e.g. seasonal precipitation) and tens to hundreds of years (e.g., sea level rise). Such rhythmic and dynamic conditions require the trees that grow in these intertidal habitats to have high levels of plasticity.
In the present review, we focus on recent insights into the ecophysiological processes that enable mangrove forests to maintain productivity under both saline and anoxic soil conditions, how their physiology is limited by temperature and how these physiological attributes may affect responses of mangrove forests to the complex environmental changes anticipated under future conditions. Enhancements in our understanding of the underlying physiological bases of salinity tolerance in mangroves is important to the development of both salinity tolerant crops and predictive models for management of the wide range of ecosystem services provided by mangrove forests under changing environmental and climatic scenarios (Barbier et al. 2011). However, global climate and atmospheric change do not affect salinity in isolation. Other key environmental factors, namely atmospheric CO2 concentration, temperature and sea level are also changing with far reaching consequences for the structure, function and distribution of mangrove systems.
Water Uptake in Saline Soils
Mangrove tree species tolerate a wide range of soil salinity (Lugo and Snedaker 1974; Odum et al. 1982; Hutchings and Saenger 1987) (Table 1 ) and are highly adapted to salt concentrations in soils that exceed concentrations tolerated by most other plants (Ball 1988a). However, both low and high salinity can limit mangrove growth and productivity (Clough and Sim 1989; Lin and Sternberg 1992; Ball 2002). Saline habitats present physiological challenges for plants because their survival depends on the extraction of almost freshwater from highly saline soils . The low osmotic potentials of saline soil water make water acquisition and transport more difficult than in wet, non-saline soils, leading to high carbon costs of water uptake and transport. These costs are reflected in the typically high water-use efficiency of mangroves which tends to increase with increases in both the salt tolerance of the species and the salinity in which the plants are grown (Ball 1988a). However, such water use characteristics come at the expense of other functions. Table 2 summarizes the traits associated with salinity tolerance in mangroves and indicates some of the putative costs associated with salinity tolerance, including reduced survival in the shade (Ball 2002; Lopez Hoffman et al. 2007), reduced growth rates (Ball 1988a) or loss of mechanical strength (Santini et al. 2012).
Growth in saline environments necessitates adaptations to maintain the low tissue water potentials needed to extract water from highly saline soils, and to limit the loss of extracted water from leaves. To this end, mangrove species can exclude, accumulate, and excrete salts; none of these are salt tolerance strategies per se, although each can be related to water uptake and the requirement for water conservation (Ball 1988a). Mangroves as a broad group are halophytes with a wide range of salinity tolerance among species (Krauss and Ball 2013; Reef and Lovelock 2014) (Table 1). All mangrove species exclude the majority of salt ions during water absorption by the roots (up to 80–95 %; Scholander et al.1962; Scholander et al. 1968; Popp et al. 1993). Casparian bands and suberin lamellae provide barriers to apoplastic water flow through the root endodermis and are well developed close to the root tip (Lawton et al. 1980). Root traits vary among species. For example Bruguiera possesses a large root cap, high levels of phenolic deposits in cells and rapid development of vasculature to prevent salts from entering xylem vessels through this pathway (Lawton et al. 1981). In contrast Avicennia marina has a smaller root cap and vascular development is delayed, which may allow greater salt and water uptake (Fig. 1). Greater development of root apoplastic barriers among species reduces bypass flow, forcing water through the endodermis and enhancing efficient salt exclusion (Krishnamurthy et al. 2014). Indeed, concentration of salts within soils can pose a real dilemma for mangroves; recent stable isotope studies have shown that mangroves utilize less saline water sources when freshwater is available (Sternberg and Swart 1987; Ewe et al. 2007; Lambs et al. 2008; Wei et al. 2013). For example, in Florida R. mangle went from using 100 % shallow soil water in the wet season when that water was fresh to a mix of 55 % shallow soil water and 45 % deeper groundwater during the dry season when deeper groundwater had lower salinity than shallow soil water (Sternberg and Swart 1987; Ewe et al. 2007).
Comparative longitudinal sections of representative Bruguiera gymnorrhiza roots (left) and Avicennia marina roots (right) showing endodermal layers, vascular tissue, and root cap characteristics (after Lawton et al. 1981). Numbers along the pericycle represent approximate distance (cm) from root tip
Reducing Water Loss Under Saline and Arid Conditions
Once water is transported to the leaves, mangroves are highly efficient in the use of water during photosynthesis (Farquhar et al. 1982; Sobrado 2000). Mangroves , which use a C3 photosynthetic pathway, were as much as 35–56 % more efficient in water use than nearby tropical lowland tree species (Ball 1996) and can even surpass stand-level water use efficiency of co-occurring C4 grasses in some settings (Krauss et al. 2014a). Photosynthetic water use efficiency (PWUE) is often reported as the ratio of leaf photosynthetic CO2 assimilation rate to transpiration rate, while the intrinsic PWUE is calculated as the ratio of assimilation rate to stomatal conductance of water vapour. These values can be extremely high in mangroves, with intrinsic PWUE ranging up to 153–212 μmol CO2/mol H2O (Table 3) compared to 40–80 μmol CO2/mol H2O typical in tropical trees, and often increasing with incremental addition of salinity (Ball 1988a; Clough and Sim 1989; Smith et al. 1989; Krauss et al. 2008). In a broad survey of 19 different mangrove species in Australia and Papua New Guinea, Clough and Sim (1989) discovered that intrinsic PWUE did not drop below 49 μmol CO2/mol H2O for any species by site combination measured in the field, and ranged as high as 195 μmol CO2/mol H2O for Avicennia marina where sites were highly saline. Changes in PWUE are also manifest at the stand level; eddy-flux-derived CO2 uptake from a mangrove forest in south Florida decreased 5 % for each 10 parts per thousand (ppt) increment in salinity (Barr et al. 2013). High levels of photosynthetic efficiencies in water use of mangroves are a consequence of structurally imposed limitations on the rates of water supply to the leaves (Sobrado 2000; Lovelock et al. 2006; Hoa et al. 2009; Vandegehuchte et al. 2014), as well as tight regulation of water loss at the leaf level (Ball and Farquhar 1984; Clough and Sim 1989).
High levels of water use efficiency in mangrove tree species are associated with a range of traits, many of which lead to reductions in the leaf to air vapour pressure deficit (VPD). These traits include the presence of leaf pubescence (Reef and Lovelock 2014), the presence of salt on the leaf surface which occurs in salt secreting species and which may increase the humidity around the leaf (Reef and Lovelock 2014), and steep leaf orientations and small, thick leaves both of which affect the thermal balance of leaves (Ball 1988a). The characteristics that minimize VPD are likely to be particularly important in arid environments. Finally, photosynthetic CO2 fixation in non-leaf tissues, which is acquired at lower water costs, could also be an important aspect of salinity tolerance. In A. marina re-fixation of respired CO2 by corticular photosynthesis contributed up to 5 % of the CO2 fixation by the plant (Schmitz et al. 2012). CO2 uptake by roots, although not yet studied in mangroves , has been shown to be significant in other submerged and wetland plants (Raven et al. 1988; Brix 1990; Rich et al. 2008).
Implications of Physiological and Structural Adaptations for Function of the Whole Forest
Much ecophysiological research on mangroves has been directed to leaf-level processes in seedlings, saplings, and occasionally, trees. Saenger (2002) reviews this literature and concludes that with the combination of ecophysiological strategies (e.g., high leaf-level PWUE) and adaptations for living in saline settings (e.g., salt exclusion at the roots, low stomatal conductance ), mangrove forests are likely to be very conservative in water use. Ironically, quantifying the absolute rates of water use in mangroves has not been the central theme of many research programs; however, the available data on sap flux indicate that mangrove trees use water at rates over 3 times less than other forest trees per unit size (Fig. 2a). Individual tree water use ranged from 0.4–64.1 L H2O/day in mangroves (Hirano et al. 1996; Muller et al. 2009; Krauss et al. 2007, 2014a; Lambs and Saenger 2011) compared with 116 ± 16 (SE) L H2O/day from trees in other forest types (Wullschleger et al. 1998). Missing from this analysis are empirical data from large mangrove trees (>55 cm dbh).
a Water use (L/day) versus diameter at breast height (dbh) for individual trees from non-mangrove ecosystems (Wullschleger et al. 1998) versus data currently available from mangrove ecosystems. Bars presented for mangroves represent the absolute range of dbh versus water use values from specific studies (Krauss et al. 2007, 2014a, b; Muller et al. 2009; Lambs and Saenger 2011). b Stand water use (mm; Krauss et al. 2015) versus net ecosystem exchange (-NEE) of atmospheric CO2 (g CO2–C/m2; Barr et al. 2010) from a mangrove forest along the Shark River, Everglades National Park, Florida, USA
Indeed, scaling ecophysiological processes from leaf to stand in mangroves provides insight into ecosystem CO2 and H2O fluxes. Lugo et al. (1975) found that two mangrove forests in Rookery Bay, Florida, USA took up 4.83 and 2.74 g C/m2/day through net ecosystem exchange (-NEE) while using 2.57 and 1.57 mm H2O/day, respectively. Of that transpiration , 95–97 % was associated with the canopy. Estimation of -NEE of carbon using eddy covariance along the Shark River in Everglades National Park, Florida was remarkably similar to Lugo et al.’s estimates, ranging from 2–5 g C/m2/day (Barr et al. 2010). This was despite the fact that trees were nearly 8 m taller and forests had approximately 15 m2/ha greater basal area along the Shark River than in Rookery Bay. During the same period of time, estimated water use of the dominant canopy ranged to 4.22 mm H2O/day for Shark River mangroves, averaging 2.5 mm H2O/day (Krauss et al. 2015; Fig. 2b). Thus, considering that the mangroves along the Shark River registered among the highest rates of carbon uptake among 49 forest types in North America (Amiro et al. 2010), it is truly remarkable that canopy water use was efficient enough in mangroves to represent only 63 % of regional rainfall and 66 % of ET (Krauss et al. 2015). Inherent to this are month-to-month fluctuations in annual rates of canopy-level PWUE that require additional study (Fig. 2b).
Elevated CO2 Effects on Water Use
High levels of water use efficiency in mangroves and the increased PWUE with increasing salinity leads to the expectation that there could be considerable gains in productivity of mangroves with increasing levels of CO2 in the atmosphere as stomatal limitations to CO2 uptake are ameliorated. The effects of elevated CO2 on plant performance have not been studied in mangroves as extensively as in other forest habitats. Only a handful of experimental studies have been conducted on the response of mangrove seedlings to elevated CO2 . Due to the difficulties posed by the intertidal habitat, Free-Air Concentration Enrichment of CO2 (FACE) experiments are yet to be conducted in mangroves and thus we have no experimental data for the effects of elevated CO2 on mature trees. Despite the scarcity of data, it is becoming apparent that while elevated CO2 has a significant effect on PWUE in mangroves, the expected alleviation of salinity stress and subsequent improvement in performance at high salinity does not occur to the extent initially anticipated. The increase in mangrove seedling growth rates observed in response to elevated CO2 ranged from a 12 to a 47 % increase in growth relative to that under ambient CO2 concentrations (Table 4), which is overlapping with the mean and range recorded for well watered tropical tree seedlings of different species experiencing similar elevated CO2 conditions (Cernusak et al. 2011; Krauss et al. 2014b). Studies that have incorporated a salinity treatment in elevated CO2 experiments in mangroves conclude that at supra-optimal salinity conditions, elevated CO2 does not significantly improve seedling growth, despite a significant improvement to PWUE (Ball et al. 1997; Reef et al. 2014) and that a fertilization effect is only observed within the low to mid salinity range. This is likely due to the fact that salinity constrains tree growth in a manner other than water stress (e.g. ion toxicity).
Mangroves respond to elevated CO2 by reducing stomatal conductance and by producing leaves with lower stomatal densities (Farnsworth et al. 1996), but overall productivity of forests may increase. The observed reduction in transpiration rate on a leaf scale can have an effect on forest water use. However, elevated CO2 can also lead to higher leaf area to total biomass ratios. In mangroves, increases in leaf area ratios with increasing [CO2] were observed in most studies (Farnsworth et al. 1996; Ball et al. 1997; Reef et al. 2014). Furthermore, there appears to be a lowering of the light compensation point (LCP) of photosynthesis . In A. germinans grown under elevated CO2 the LCP reduced from 52.3 ± 1.36 µmol m−2 s−1 at (CO2) of 280 ppm to 18.1 ± 9.6 µmol m−2 s−1 at [CO2] of 800 ppm, p = 0.03; R. Reef unpublished data) suggesting that a positive carbon balance in leaves can be maintained under shaded conditions at elevated CO2. Declines in specific leaf area (SLA) were also observed in response to elevated CO2 (Table 4) and in a time series study of herbarium specimens, SLA declined as CO2 has increased over time in A. marina (but not for R. stylosa, Reef and Lovelock 2014). These responses to elevated CO2 could result in increases in leaf level productivity and leaf area index (LAI) at the stand level with rising CO2 levels. Increases in LAI at the stand level could offset the reduction in water use by individual leaves and result in enhanced production, but no overall change to transpiration on larger scales.
An important parameter that can influence the response of mangroves to elevated CO2 in the field is nutrient availability. Under low nutrient conditions, similar to those measured in many scrub mangrove forests, seedling biomass did not increase in response to elevated CO2 (McKee and Rooth 2008). However, when given higher nutrient concentrations, at levels similar to those measured in soils along creek banks, elevated CO2 resulted in a significant 23 % increase in total biomass. Development of nutrient limitations to growth could also be the reason for a down-regulation of photosynthesis and reduced growth rates under elevated CO2 conditions over time in R. mangle grown in pots (Farnsworth et al. 1996). Nutrient distributions are not uniform in mangrove forests due in part to their intertidal nature. Most nutrients are delivered by the tides, thus creating elevation driven gradients in nutrient availability. Furthermore, differences in nutrient inputs from rivers and other landward sources, soil salinity, inundation frequency and faunal activity affect the availability of nutrients (Reef et al. 2010, 2014). The response of mangroves to elevated CO2 will thus likely be dependent on their position in the forest and local nutrient conditions.
Finally, in order to understand the effects of elevated CO2 on forest structure and distribution, competitive interactions must be taken into account. Elevated CO2 can improve mangrove salinity tolerance within the range of salinities already suitable for growth (Reef et al. 2014). This shift in the fundamental niche could translate into a range shift depending on competitive outcomes and could be a contributing factor in the observed mangrove encroachment into saltmarsh habitats observed at many locations (Saintilan et al. 2014). In the only experiment to investigate competitive interactions between mangroves and saltmarshes under elevated CO2, McKee and Rooth (2008) found that elevated CO2 does not improve competitive outcomes for the mangrove Avicennia germinans when grown in mixed culture with the saltmarsh species Spartina alterniflora. Although S. alterniflora is a very fast growing species and may not be representative of many saltmarsh species that have slower growth rates, this study highlights the difficulties in predicting the future of mangrove distributions in response to rising CO2 concentrations.
Adaptations to Inundated Soils
Tolerance of periods of inundation by tidal water, flooding and storm surges are essential for mangrove tree species survival, and differences in species tolerances to flooding influences their distributions relative to changing hydroperiods with sea-level rise. Sea level has been relatively stable for the last five thousand years, but accelerating rates of sea level rise and associated geomorphological adjustments of the coast (Woodroffe 1990) are likely to result in vegetation transitions that are linked to inundation tolerance of species. A range of traits are linked to inundation tolerance (Table 5). Mangroves possess a number of elaborate, aerial root structures including prop roots (Rhizophora), pneumatophores (Avicennia, Sonneratia), knee roots (Bruguiera), and cable roots (Xylocarpus, Heritiera), prompting much early speculation into their role in aerating sub-soil roots and soils . Still, some mangrove species lack aerial roots (e.g., Excoecaria). Prominent on many of the aerial root structures and stems are lenticels , or gas exchange pores positioned above the soil surface. Air diffuses through these pores and via abundant aerenchyma tissues to belowground root structures facilitating aeration of roots embedded in oxygen-free soils (Scholander et al. 1955; Skelton and Allaway 1996; Allaway et al. 2001). The structure of mangrove roots facilitates gas exchange (McKee and Mendelssohn 1987; Youssef and Saenger 1996) which in addition to supporting respiration results in the oxidation of phytotoxic substances within roots (e.g., Fe2+, H2S) (Armstrong et al. 1992; Youssef and Saenger 1998a), although leakage of O2 into the rhizosphere may affect the availability of some essential nutrients, particularly phosphorus which are more available under reduced conditions, and some microbial processes which are also favored under low oxygen concentrations (e.g. nitrogen fixation ) (Reef et al. 2010) (Table 4 ). Early rapid root growth and investment in roots in seedlings are also important for the establishment of seedlings on exposed tidal flats and thus influences recruitment and forest expansion (Delgado et al. 2001; Balke et al. 2011).
There are differences among species in the capacity to withstand inundation. Experiments focused on gas exchange in root systems indicate that A. germinans and Laguncularia racemosa seedlings suffered a decrease in root oxygen concentrations when exposed to experimental hypoxia , while Rhizophora mangle did not (McKee 1996). McKee (1996) discovered that differences among species in response to anoxia were attributed to oxygenation of the roots through diffusive O2 fluxes from the shoot, lower root respiration rates in R. mangle than A. germinans or L. racemosa, and less O2 leakage from R. mangle roots to the surrounding soils. Oxygen tends to leak from Avicennia roots to a much greater degree than Rhizophora roots (Thibodeau and Nickerson 1986). In a multi-species comparison, Avicennia marina and Acanthus ilicifolius had the highest concentrations of aerenchyma air space and the lowest diffusional resistance for O2 to soil among eight mangrove species tested in Hong Kong (Pi et al. 2009) suggesting high levels of variation among Indo-Pacific species in their capacity to transport oxygen to and out of roots. Additionally, greater root porosity was found in pneumatophores than other root types of Sonneratia alba from Okinawa (Purnobasuki and Suzuki 2004) indicating variation in oxygen transport within root systems among wide-ranging species. Rates of oxygen leakage from roots not only vary among species but also with the strength of diffusion gradients between the root and soil (Sorrell and Armstrong 1994). O2 leakage from the apical tips of Kandelia candel roots was higher than from the main root walls (Chiu and Chou 1993). Variation in oxygen leakage was attributed to the structure of the root surface, which is compacted and lignified in Rhizophora and Aegiceras and thinner with only 3–4 exodermal layers in Avicennia and Bruguiera (Youssef and Saenger 1996). The differences in mangrove species in the structure, growth and physiology of roots, including their ability to transport and retain O2 within their roots, are likely to lead to differences in species responses to changing inundation regimes and associated hydrological change with sea level rise. Although many other factors are also likely to influence the distribution and composition of forests, including the space in the landscape for landward expansion, human and natural modifications of the coast (Doyle et al. 2010; Traill et al. 2011), underlying differences in species inundation tolerance are likely to be important.
Adaptations to Temperature Thresholds
Variations in temperature affect many processes in mangrove forests ranging from the fundamental metabolic processes of photosynthesis and respiration (e.g., Andrews and Muller 1985; Lovelock 2008) to carbon cycling (Alongi 2009) and reproductive success (Duke 1990). Temperature regimes greatly influence mangrove forest composition and structure with extreme temperature events playing an especially important role in some locations. Increasing global temperatures are likely to result in changes to growth and distribution patterns of mangrove forests on the edge of their ranges which are currently limited by low temperatures and in some locations aridity.
The effects of low temperature on mangrove physiology (Davis 1940; Stuart et al. 2007; Krauss et al. 2008; Ross et al. 2009) and distributions (e.g., West 1977; Sherrod and McMillan 1985; Woodroffe and Grindrod 1991; de Lange and de Lange 1994; Duke et al. 1998; Saenger 2002) have been widely studied, with recent studies focused on mangrove recruitment into warm-temperate salt marsh habitats (e.g., McKee et al. 2012; Osland et al. 2013; Cavanaugh et al. 2014; Saintilan et al. 2014). Mangrove species differ in sensitivity to low temperatures (Table 1), but none can survive the minimum temperatures that occur in cold-temperate climatic zones.
In general, mangrove forest biomass, structural development, and species richness are higher in wet tropical climatic zones. In colder climatic zones (e.g., subtropical or warm-temperate), low temperature stress typically produces mangrove trees that are short in stature with a shrub-like architecture (Woodroffe 1985; Osland et al. 2014a, b). Physiological stress due to low temperatures can be separated into chilling and freezing stress (Kozlowski and Pallardy 1997; Larcher 2003). Whereas chilling stress occurs at leaf temperatures above freezing (i.e., without ice formation), freezing stress occurs at leaf temperatures below freezing when intra- or extra-cellular ice formation occurs. The physiological effects and symptoms of freezing/chilling stress in mangrove trees include reduced metabolic rates, altered membrane structure and permeability (Markley et al. 1982), disrupted water and nutrient transport (Stuart et al. 2007), partial or complete loss of aboveground biomass (e.g., Osland et al. 2014a, b), reduced reproductive success (Duke 1990) and, in extreme cases, mortality (Ross et al. 2009).
Intra- and inter-specific differences in mangrove sensitivity to low temperature stress greatly influence the structure and composition of mangrove forests. There are many examples of differential species and life stage responses to low temperature stress (e.g., Lugo and Patterson-Zucca 1977; Lonard and Judd 1991; Olmsted et al. 1993; Ross et al. 2009; Chen et al. 2010; Pickens and Hester 2011). Climatic origin greatly influences intraspecific responses to chilling stress with mangrove individuals from colder climates typically being better adapted and more resistant to low temperatures (Markley et al. 1982; Sherrod and McMillan 1985). Species sensitivity to low temperature stress can be gauged using species distribution data in combination with multi-decadal climate data (e.g., Table 1; Quisthoudt et al. 2012; Osland et al. 2013). For example, whereas some mangrove species are highly sensitive to chilling stress and are only found in tropical climates (e.g., Bruguiera sexangula, Sonneratia lanceolata), other species have adaptations that enable them to be resistant to higher levels of chilling or freezing stress (e.g., A. germinans, A. marina, Kandelia obovata, Aegiceras corniculatum). Vulnerability to low-temperature induced xylem cavitation is especially high for mangrove trees due to the low xylem water potentials required for water transport in highly saline intertidal environments (Stuart et al. 2007). In a comparison of the most poleward mangrove species in Australia and the United States , Stuart et al. (2007) highlighted the effects of xylem embolism and show that species adapted to climates with colder mean annual minimum temperatures have smaller vessel diameters which enable them to better avoid embolism; however, narrow vessels also constrain water transport and productivity, possibly limiting mangrove forest structural development in these poleward locations. In addition to narrow vessels, variation in membrane properties also likely enables mangrove individuals and species from colder climates to maintain membrane fluidity during exposure to low temperatures (Markley et al. 1982).
On some continents, the frequency and intensity of extreme winter events greatly affect mangroves. For example, near the poleward mangrove limit in China and the southeastern United States, mean winter temperatures are not as cold as mean winter temperatures found near the poleward limit of mangroves in Australia or New Zealand . However, extreme minimum temperature events are more intense in China and the southeastern United States which can result in sudden leaf loss, xylem embolism, branch and stem reductions, and, in the most extreme cases, tree mortality (Davis 1940; Lugo and Patterson-Zucca 1977; West 1977; Lonard and Judd 1991; Everitt et al. 1996). In these areas, the spatial extent of mangrove forests expands and contracts in response to the frequency and intensity of extreme winter events (West 1977; Sherrod and McMillan 1985; Stevens et al. 2006; Giri et al. 2011). In North America, Avicennia germinans is a species that is especially adapted to and resistant to extreme winter events. In parts of northern coastal Florida, Louisiana, and the northern coast of Texas, A. germinans individuals often lose a large portion of their aboveground biomass due to freeze events; however, A. germinans is capable of vigorous resprouting from the base of stems after freeze-damage due to the presence of epicormic buds (Lugo and Patterson-Zucca 1977; Tomlinson 1986; Osland et al. 2014a, b). Using data in Table 1 we graphically show the breadth of tolerance of mangrove species in the biogeographic provinces of the Atlantic and Eastern Pacific Ocean region (Fig. 3a) and the Indian and Western Pacific Ocean region (Fig. 3b) to temperature extremes and to aridity and salinity. Those species that are currently documented as expanding in their range (e.g., A. germinans and A. marina) have the broadest tolerance to low temperatures and to other environmental factors, while species that have restricted distributions (e.g., the palm Nypa fruticans) are less tolerant of low and high temperatures, high salinity and aridity.
Spider plots of the overlapping tolerances of mangrove species to high and low temperature and high and low temperature extremes, salinity and aridity (based on data from Table 1). a Common species from the Atlantic-East Pacific biogeographic region; b common species from the Indo-West Pacific region
In contrast, the effects of high temperatures on mangrove trees have not been as extensively considered. Early research indicates that photosynthesis in tropical species of Rhizophoraceae is depressed as leaf temperatures exceed 34 °C (e.g., Andrews and Muller 1985; Cheeseman et al. 1991). Most of this effect is associated with the strong stomatal closure required to minimize rates of water loss that can increase dramatically if leaf temperatures become higher than air temperatures or if high air temperatures are accompanied by low humidity (i.e. increasing leaf-to-air VPD). However, more research is required to understand how mangroves, particularly those in warm tropical climates that may already be close to their thermal limits, will respond to the projected increases in global temperature of at least 2 °C in the coming century. Clark (2004) suggested that productivity of tropical rainforests could decline with increasing temperature due to increases in respiration and declining photosynthetic rates (Slot and Winter this volume). As yet there has been no assessment of this hypothesis in mangrove forests.
Plasticity of Traits Gives Rise to Different Capacity to Adjust to Climate Change
Plasticity of traits is a key feature of plants that allows them to acclimate to changing conditions, including climate (Jump and Penuelas 2005). Some mangrove species occur over broad ranges of environmental conditions and often assume different forms in different habitats, e.g., scrub and tall fringing forms of R. mangle in the Caribbean (Feller 1995; Medina et al. 2010) or for A. marina in New Zealand and southern Australia (Lovelock 2008; Martin et al. 2010) (Fig. 4), displaying high levels of both physiological and morphological plasticity (Lovelock et al. 2006). In fact, plasticity in PWUE among and within mangrove species is a primary reason why water use characteristics in mangroves have not been fully assessed; water use can depend strongly on site or experimental conditions limiting blanket assessments of water conservation in mangroves (see e.g., Becker et al. 1997 vs. Zimmermann et al. 1994; Krauss et al. 2015). In a comparison of the levels of plasticity in different traits over variation in fertility in R. mangle, whole plant architectural traits (e.g. leaf area index, shoot extension and hydraulic properties of stems) had much higher levels of plasticity than leaf level traits (e.g. photosynthesis, specific leaf area ) (Lovelock et al. 2006). As the most plastic traits are often the ones that determine overall plant fitness (Agrawal 2001, Poorter and Lambers 1986; Callaway et al. 2003) this suggests that plasticity in growth rates, canopy development and hydraulic function are potentially the most important traits for successful dominance of mangrove habitats. A high level of plasticity in belowground growth is also likely, but as yet remains relatively unexplored (e.g., McKee 1996; Casteneda Moya et al. 2011; Lang’at et al. 2013). In Table 6 we contrast plasticity in growth (stem extension, relative growth rate or biomass expressed as a mean coefficient of variation) for a range of species where contrasts have been made over variation in treatments. Plasticity in growth varies significantly among mangrove species and tends to be greater in response to variation in nutrient availability than to other treatments. Species with the highest plasticity tend to be the most salt tolerant and widely distributed and may be favored with global climate change. The fitness cost of high levels of plasticity is difficult to determine, but could be associated with being inferior competitors; although with few competing species in mangrove forests (compared to tropical rainforests) there may be few disadvantages to high levels of plasticity, particularly in the biogeographic province of the Atlantic and East Pacific Ocean region where species diversity is particularly low.
Examples of high levels of morphological plasticity within mangrove tree species. The upper left and right photos are of tall (15 m), forests fringing water ways and short (<2 m), scrub forests of Rhizophora mangle, respectively, from Belize. The lower left and right photos are of tall (5 m), fringing forests and short (<1 m), scrub forests, respectively, in New Zealand
Conclusions: Change in Distribution and Productivity of Mangrove Forests
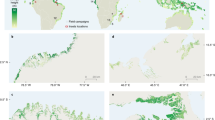
As a group, mangrove species possess many physiological adaptations and life history characteristics that could enable them to adapt positively to future climate change . However, the complex interactions between climatic drivers are only just starting to become clear, and mangrove ecosystem responses to climate change will be highly context dependent (e.g., Saintilan et al. 2009; McKee et al. 2012). In the past, the global distribution and spatial extent of mangrove forests has expanded and contracted in response to changes in sea level, temperature and freshwater availability (Sherrod and McMillan 1985; Woodroffe and Grindrod 1991; Saintilan et al. 2014). In the future, climate change is expected to greatly alter the distribution, composition and ecological properties of mangroves forests and their adjacent ecosystems (i.e., salt marsh, salt flat, seagrass ecosystems) (Fig. 5). On some continents (e.g., North America, Northwest Asia, Australia), warmer winter temperatures will likely lead to poleward mangrove forest range expansion and development at the expense of salt marsh habitat (Osland et al. 2013; Cavanaugh et al. 2014; Saintilan et al. 2014). The southeastern United States is an area where the ecological effects of mangrove migration are expected to be especially large due to the large amount of salt marsh that could be replaced by mangrove forests (Osland et al. 2013). Moreover, increases in sea level, freshwater availability, elevated CO2 and human influences including nutrient enrichment will also affect the productivity , composition and distribution of mangrove forests. In response to sea level rise, mangrove forests are expected to migrate landward where migration corridors exist (Doyle et al. 2010; Traill et al. 2011), although landward coastal wetland migration will be obstructed in some areas by natural and anthropogenic barriers (e.g., sea walls, hydrologic barriers). Species differences in inundation tolerance are likely to influence the composition of forests as sea level rise accelerates. In arid and semi-arid climatic zones, the abundance and composition of mangrove forests and other coastal wetland ecosystems will be greatly influenced by changes in freshwater availability (Smith and Duke 1987; Bucher and Saenger 1994; Saintilan et al. 2009; Semeniuk 2013; Osland et al. 2014a, b). Whereas increased aridity and/or reductions in freshwater input will likely result in reduced mangrove coverage and diversity, the converse is also true; in some areas, increased freshwater inputs, increases in humidity and increases in CO2 could result in changes in communities, higher mangrove coverage, structural development and productivity (Reef and Lovelock 2014; Osland et al. 2014a, b).
The global mangrove distribution (green) overlain on: a predicted change in mean temperature (IPCC 2007, A1FI scenario); b predicted changes in annual precipitation (IPCC 2007, A1FI scenario); and c regions where mangrove distribution is sensitive to changes in aridity (red) or low temperatures (blue)
References
Agrawal AA (2001) Phenotypic plasticity in the interactions and evolution of species. Science 294:321–326
Allaway WG, Curran M, Hollington LM, Ricketts MC, Skelton NJ (2001) Gas space and oxygen exchange in roots of Avicennia marina (Forssk.) Vierh. var. australasica (Walp.) Moldenke ex N. C. Duke, the grey mangrove. Wetlands Ecol Manage 9:211–218
Alongi DM (2009) The energetics of mangrove forests. Springer, Dordrecht
Amiro BD, Barr AG, Barr JG, Black TA, Bracho R, Brown M, Chen J, Clark KL, Davis KJ, Desai AR, Dore S, Engel V, Fuentes JD, Goldstein AH, Goulden ML, Kolb TE, Lavigne MB, Law BE, Margolis HA, Martin T, McCaughey JH, Misson L, Montes-Helu M, Noormets A, Randerson JT, Starr G, Xiao J (2010) Ecosystem carbon dioxide fluxes after disturbance in forests of North America. J Geophys Res 115:G00K02
Andrews TJ, Muller GJ (1985) Photosynthetic gas exchange of the mangrove, Rhizophora stylosa Griff. in its natural environment. Oecologia 65:449–455
Armstrong J, Armstrong W, Beckett PM (1992) Phragmites australis: veturi-and humidity-induced pressure flows enhance rhizome aeration and rhizosphere oxidation. New Phytol 120:197–207
Balke T, Bouma TJ, Horstman EM, Webb EL, Erftemeijer PLA, Herman PMJ (2011) Windows of opportunity: thresholds to mangrove seedling establishment on tidal flats. Mar Ecol Prog Ser 440:1–9
Ball MC (1988a) Ecophysiology of mangroves. Trees Struc Funct 2:129–142
Ball MC (1996) Comparative ecophysiology of mangrove forest and tropical lowland moist rainforest. In: Mulkey SS, Chazdon RL, Smith AP (eds) Tropical forest plant ecophysiology. Chapman and Hall, New York, pp 461–496
Ball MC (2002) Interactive effects of salinity and irradiance on growth: implications for mangrove forest structure along salinity gradients. Trees 16:126–139
Ball MC, Farquhar GD (1984) Photosynthetic and stomatal responses of two mangrove species, Aegiceras corniculatum and Avicennia marina, to long term salinity and humidity conditions. Plant Physiol 74:1–6
Ball MC, Pidsley SM (1995). Growth responses to salinity in relation to distribution of two mangrove species, Sonneratia alba and S. lanceolata, in northern Australia. Funct Ecol 77–85
Ball MC, Cochrane MJ, Rawson HM (1997) Growth and water use of the mangroves Rhizophora apiculata and R. stylosa in response to salinity and humidity under ambient and elevated concentrations of atmospheric CO2. Plant Cell Environ 20:1158–1166
Ball MC, Cowan IR, Farquhar GD (1988b) Maintenance of leaf temperature and the optimisation of carbon gain in relation to water loss in a tropical mangrove forest. Australian Journal of Plant Physiology 15:263–276
Barbier EB, Hacker SD, Kennedy C, Koch EW, Stier AC, Silliman BR (2011) The value of estuarine and coastal ecosystem services. Ecol Monogr 81:169–193
Barr JG, Fuentes JD, Engel V, Zieman JC (2009) Physiological responses of red mangroves to the climate in the Florida Everglades. J Geophys Res 114:G02008
Barr JG, Engel V, Fuentes JD, Zieman JC, O’Halloran TL, Smith TJ III, Anderson GH (2010) Controls on mangrove forest-atmosphere carbon dioxide exchanges in western Everglades National Park. J Geophys Res 115:G02020
Barr JG, Engel V, Fuentes JD, Fuller DO, Kwon H (2013) Modeling light use efficiency in a subtropical mangrove forest equipped with CO2 eddy covariance. Biogeoscience 10:2145–2158
Becker P, Asmat A, Mohamad J, Moksin M, Tyree MT (1997) Sap flow rates of mangrove trees are not unusually low. Trees Struc Funct 11:432–435
Brix H (1990) Uptake and photosynthetic utilization of sediment-derived carbon by Phragmites australis (Cav.) Trin. ex Steudel. Aquat Bot 38:377–389
Bucher D, Saenger P (1994) A classification of tropical and subtropical Australian estuaries. Aquat Cons: Mar Freshw Ecosyst 4:1–19
Callaway JC, Sullivan G, Zedler JB (2003) Species-rich plantings increase biomass and nitrogen accumulation in a wetland restoration experiment. Ecol App 13:1626–1639
Camilleri JC, Ribi G (1983) Leaf thickness of mangroves (Rhizophora mangle) growing in different salinities. Biotropica 15:139–141
Cardona-Olarte P, Krauss KW, Twilley RR (2013) Leaf gas exchange and nutrient use efficiency help explain the distribution of two Neotropical mangroves under contrasting flooding and salinity. Int J For Res 524–625
Carlquist S (2007) Successive cambia revisited: ontogeny, histology, diversity, and functional significance. The Journal of the Torrey Botanical Society 134:301–32
Castañeda-Moya E, Twilley RR, Rivera-Monroy VH, Marx BD, Coronado-Molina C, Ewe SM (2011) Patterns of root dynamics in mangrove forests along environmental gradients in the Florida Coastal Everglades, USA. Ecosystems 14:1178–1195
Cavanaugh KC, Kellner JR, Forde AJ, Gruner DS, Parker JD, Rodriguez W, Feller IC (2014) Poleward expansion of mangroves is a threshold response to decreased frequency of extreme cold events. Proc Nat Acad Sci (US) 111:723–727
Cernusak LA, Winter K, Turner BL (2011) Transpiration modulates phosphorus acquisition in tropical tree seedlings. Tree Physiol 31:878–885
Cheeseman JM (1994) Depressions of photosynthesis in mangrove canopies. In: Baker NR, Bowyer JR (eds) Photoinhibition of photosynthesis. Bios Scientific Publishers, Oxford, pp 377–389
Cheeseman JM, Clough BF, Carter DR, Lovelock CE, Eong OJ, Sim RG (1991) The analysis of photosynthetic performance in leaves under field conditions: a case study using Bruguiera mangroves. Photosyn Res 29:11–22
Chen L, Tam NFY, Huang J, Zeng X, Meng X, Zhong C, Wong Y, Lin G (2008) Comparison of ecophysiological characteristics between introduced and indigenous mangrove species in China. Estuar Coast Shelf Sci 79:644–652
Chen L, Wang W, Zhang Y, Huang L, Zhao C, Yang S, Yang Z, Chen Y, Xu H, Zhong C, Su B, Fang B, Chen N, Zeng C, Lin G (2010) Damage to mangroves from extreme cold in early 2008 in southern China. J Plant Ecol (Chinese Version) 34:186–194
Chiu C, Chou C (1993) Oxidation in the rhizosphere of mangrove Kandelia candel seedlings. Soil Sci Plant Nutr 39:725–731
Clark DA (2004) Tropical forests and global warming: slowing it down or speeding it up? Front Ecol Environ 2:73–80
Clough BF (1984) Growth and salt balance of the mangroves Avicennia marina (Forsk.) Vierh. and Rhizophora stylosa Griff. in relation to salinity. Funct Plant Biol 11:419–430
Clough BF (1992) Primary productivity and growth of mangrove forests. Tropical mangrove ecosystems. American Geophysical Union, Washington DC, pp 225–249
Clough BF, Sim RG (1989) Changes in gas exchange characteristics and water use efficiency of mangroves in response to salinity and vapour pressure deficit. Oecologia 79:38–44
Davis JH (1940) The ecology and geologic role of mangroves in Florida, vol 32. Carnegie Institue of Washington Publications. Papers Tortugas Lab, pp 303–412
de Lange WP, de Lange PJ (1994) An appraisal of factors controlling the latitudinal distribution of mangrove (Avicennia marina var. resinifera) in New Zealand. J Coast Res 10:539–548
Delgado P, Hensel PF, Jiménez JA, Day JW (2001) The importance of propagule establishment and physical factors in mangrove distributional patterns in a Costa Rican estuary. Aquat Bot 71:157–178
Doyle TW, Krauss KW, Conner WH, From AS (2010) Predicting the retreat and migration of tidal forests along the northern Gulf of Mexico under sea-level rise. For Ecol Manage 259:770–777
Duke NC (1990) Phenological trends with latitude in the mangrove tree Avicennia marina. J Ecol 78:113–133
Duke NC, Ball MC, Ellison JC (1998) Factors influencing biodiversity and distributional gradients in mangroves. Glob Ecol Biogeogr Lett 7:27–47
Everitt JH, Judd FW, Escobar DE, Davis MR (1996) Integration of remote sensing and spatial information technologies for mapping black mangrove on the Texas gulf coast. J Coast Res 12:64–69
Ewe SML, Sternberg LdSL, Childers DL (2007) Seasonal plant water uptake patterns in the saline Southeast Everglades ecotone. Oecologia 152:607–616
Ewers FW, Lopez-Portillo J, Angeles G, Fisher JB (2004) Hydraulic conductivity and embolism in the mangrove tree Laguncularia racemosa. Tree Physiol 24:1057–1062
Farnsworth EJ, Ellison AM, Gong WK (1996) Elevated CO2 alters anatomy, physiology, growth, and reproduction of red mangrove (Rhizophora mangle L.). Oecologia 108:599–609
Farquhar GD, Ball MC, von Caemmerer S, Roksandic Z (1982) Effect of salinity and humidity on 13C values of halophytes—evidence for diffusional isotopic fractionation determined by the ratio of intercellular/atmospheric CO2 under different environmental conditions. Oecologia 52:121–137
Feller IC (1995) Effects of nutrient enrichment on growth and herbivory of dwarf red mangrove (Rhizophora mangle). Ecological monographs 65:477–505
Giri C, Long J, Tieszen L (2011) Mapping and monitoring Louisiana’s mangroves in the aftermath of the 2010 Gulf of Mexico Oil Spill. J Coast Res 27:1059–1064
Hao G-Y, Jones TJ, Luton C, Zhang Y-J, Manzane E, Scholz FG, Bucci SJ, Cao KF, Goldstein G (2009) Hydraulic redistribution in dwarf Rhizophora mangle trees driven by interstitial soil water salinity gradients: impacts on hydraulic architecture and gas exchange. Tree Physiol 29:697–705
He B, Lai T, Fan H, Wang W, Zheng H (2007) Comparison of flooding-tolerance in four mangrove species in a diurnal tidal zone in the Beibu Gulf. Estuar Coast Shelf Sci 74:254–262
Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A (2005) Very high resolution interpolated climate surfaces for global land areas. Int J Clim 25:1965–1978
Hirano T, Monji N, Hamotani K, Jintana V, Yabuki K (1996) Transpirational characteristics of mangrove species in southern Thailand. Environ Controls Biol 34:285–293
Hutchings P, Saenger P (1987) Ecology of mangroves. Queensland University Press
IPCC (2007) Intergovernmental panel on climate change. Climate change 2007: Synthesis report
Jump A, Penuelas J (2005) Running to stand still: adaptation and the response of plants to rapid climate change. Ecol Lett 8:1010–1020
Kozlowski TT, Pallardy SG (1997) Growth control in woody plants. Academic Press, San Diego
Krauss KW, Allen JA (2003) Influences of salinity and shade on seedling photosynthesis and growth of two mangrove species, Rhizophora mangle and Bruguiera sexangula, introduced to Hawaii. Aquat Bot 77:311–324
Krauss KW, Ball MC (2013) On the halophytic nature of mangroves. Trees Struc Funct 27:7–11
Krauss KW, Twilley RR, Doyle TW, Gardiner ES (2006) Leaf gas exchange characteristics of three Neotropical mangrove species in response to varying hydroperiod. Tree Physiol 26:959–968
Krauss KW, Young PJ, Chambers JL, Doyle TW, Twilley RR (2007) Sap flow characteristics of neotropical mangroves in flooded and drained soils. Tree Physiol 27:775–783
Krauss KW, Lovelock CE, McKee KL, López-Hoffman L, Ewe SML, Sousa WP (2008) Environmental drivers in mangrove establishment and early development: a review. Aquat Bot 89:105–127
Krauss KW, McKee KL, Hester MW (2014a) Water use characteristics of black mangrove (Avicennia germinans) communities along an ecotone with marsh at a northern geographical limit. Ecohydrol 7:354–365
Krauss KW, McKee KL, Lovelock CE, Cahoon DR, Saintilan N, Reef R, Chen L (2014b) How mangrove forests adjust to rising sea level. New Phytol 202:19–34
Krauss KW, Barr JG, Engel V, Fuentes JD, Wang H (2015) Approximations of stand water use versus evapotranspiration from three mangrove forests in southwest Florida, USA. Agric For Meteorol 213: 291–303
Krishnamurthy P, Jyothi-Prakash PA, Qin L, He J, Lin Q, Loh C, Kumar PP (2014) Role of root hydrophobic barriers in salt exclusion of a mangrove plant Avicennia officinalis. Plant Cell Environ 37:1656–1671
Lambs L, Saenger A (2011) Sap flow measurements of Ceriops tagal and Rhizophora mucronata mangrove trees by deuterium tracing and lysimetry. Rapid Commun Mass Spectrom 25:2741–2748
Lambs L, Muller E, Fromard F (2008) Mangrove trees growing in a very saline condition but not using seawater. Rapid Commun Mass Spectrom 22:2835–2843
Lang’at JKS, Kirui BK, Skov MW, Kairo JG, Mencuccini M, Huxham M (2013). Species mixing boosts root yield in mangrove trees. Oecologia 172: 271–278
Larcher W (2003) Physiological plant ecology: ecophysiology and stress physiology of functional groups. Springer, Berlin
Lawton JR, Todd A, Naidoo DK (1981) Preliminary investigations into the structure of the roots of the mangroves, Avicennia marina and Bruguiera gymnorrhiza, in relation to ion uptake. New Phytol 88:713–722
Lin G, Sternberg LDSL (1992) Comparative study of water uptake and photosynthetic gas exchange between scrub and fringe red mangroves, Rhizophora mangle L. Oecologia 90:399–403
Lonard RI, Judd FW (1991) Comparison of the effects of the severe freezes of 1983 and 1989 on native woody plants in the Lower Rio Grande Valley, Texas. Southwestern Nat 36:213–217
Lopez-Hoffman L, Anten NP, Martinez-Ramos M, Ackerly DD (2007) Salinity and light interactively affect neotropical mangrove seedlings at the leaf and whole plant levels. Oecologia 150:545–556
Lovelock CE (2008) Soil respiration and belowground carbon allocation in mangrove forests. Ecosystems 11:342–354
Lovelock CE, Feller IC (2003) Photosynthetic performance and resource utilization of two mangrove species coexisting in a hypersaline scrub forest. Oecologia 134:455–462
Lovelock CE, Ball MC, Choat B, Engelbrecht BM, Holbrook NM, Feller IC (2006) Linking physiological processes with mangrove forest structure: phosphorus deficiency limits canopy development, hydraulic conductivity and photosynthetic carbon gain in dwarf Rhizophora mangle. Plant Cell Environ 29:793–802
Lugo AE, Patterson-Zucca C (1977) The impact of low temperature stress on mangrove structure and growth. Trop Ecol 18:149–161
Lugo AE, Snedaker SC (1974) The ecology of mangroves. Annu Rev Ecol Syst 5:39–64
Lugo AE, Evink G, Brinson MM, Broce A, Snedaker SC (1975) Diurnal rates of photosynthesis, respiration, and transpiration in mangrove forests of south Florida. In: Golley FB, Medina E (eds) Tropical ecological systems. Springer, New York, pp 335–350
Markley JL, McMillan C, Thompson GA Jr (1982) Latitidinal differentiation in response to chilling temperatures among populations of three mangroves, Avicennia germinans, Laguncularia racemosa, and Rhizophora mangle, from the western tropical Atlantic and Pacific Panama. Can J Bot 60:2704–2715
Martin CE, Loeschen VS (1993) Photosynthesis in the mangrove species Rhizophora mangle L.: no evidence for CAM-cycling. Photosynthetica 28:391–400
Martin KC, Bruhn D, Lovelock CE, Feller IC, Evans JR, Ball MC (2010) Nitrogen fertilization enhances water-use efficiency in a saline environment. Plant Cell Environ 33:344–357
Maurer EP, Adam JC, Wood AW (2009) Climate model based consensus on the hydrologic impacts of climate change to the Rio Lempa basin of Central America. Hydrol Earth Syst Sci 13:183–194
McKee KL (1996) Growth and physiological responses of Neotropical mangrove seedlings to root zone hypoxia. Tree Physiol 16:883–889
McKee KL, Mendelssohn IA (1987) Root metabolism in the black mangrove (Avicennia germinans (L.)): response to hypoxia. Environ Exp Bot 27:147–156
McKee K, Rooth JE (2008) Where temperate meets tropical: multi-factorial effects of elevated CO2, nitrogen enrichment, and competition on a mangrove-salt marsh community. Glob Change Biol 14:971–984
McKee K, Rogers K, Saintilan N (2012) Response of salt marsh and mangrove wetlands to changes in atmospheric CO2, climate, and sea level. In: Middleton BA (ed) Global change and the function and distribution of wetlands: global change ecology and wetlands. Springer, Dordrecht, pp 63–96
Medina E, Cuevas E, Lugo AE (2010) Nutrient relations of dwarf Rhizophora mangle L. mangroves on peat in eastern Puerto Rico. Plant Ecol 207:13–24
Muller E, Lambs L, Fromard F (2009) Variations in water use by a mature mangrove of Avicennia germinans, French Guiana. Ann Sci For 66:803
Naidoo G, von Willert DJ (1995) Diurnal gas exchange characteristics and water use efficiency of three salt-secreting mangroves at low and high salinities. Hydrobiology 295:13–22
Naidoo G, Rogalla H, von Willert DJ (1998) Field measurements of gas exchange in Avicennia marina and Bruguiera gymnorrhiza. Mangroves Salt Marshes 2:99–107
Naidoo G, Hiralal O, Naidoo Y (2011) Hypersalinity effects on leaf ultrastructure and physiology in the mangrove Avicennia marina. Flora-Morphol, Distrib Funct Ecol Plants 206:814–820
Odum WE, McIvor CC, Smith III TJ (1982) The ecology of the mangroves of south Florida: a community profile. Virginia University Charlottsville Department of Environmental Sciences
Olmsted I, Dunevitz H, Platt WJ (1993) Effects of freezes on tropical trees in Everglades National Park Florida, USA. Trop Ecol 34:17–34
Osland MJ, Enwright N, Day RH, Doyle TW (2013) Winter climate change and coastal wetland foundation species: salt marshes versus mangrove forests in the southeastern US. Glob Change Biol 19:1482–1494
Osland MJ, Day RH, Larriviere JC, From AS (2014a) Aboveground allometric models for freeze-affected black mangroves (Avicennia germinans): equations for a climate sensitive mangrove-marsh ecotone. PLoS ONE 9(6):e99604
Osland MJ, Enwright N, Stagg CL (2014b) Freshwater availability and coastal wetland foundation species: ecological transitions along a rainfall gradient. Ecol 95:2789–2802
Passioura JB, Ball MC, Knight JH (1992) Mangroves may salinize the soil and in so doing limit their transpiration rate. Funct Ecol 6:476–481
Pezeshki SR, DeLaune RD, Patrick WH Jr (1990) Differential response of select mangroves to soil flooding and salinity: gas exchange and biomass partitioning. Can J For Res 20:869–874
Pi N, Tam NFY, Wong MH (2009) Root anatomy and spatial pattern or radial oxygen loss of eight true mangrove species. Aquat Bot 90:222–230
Pickens CN, Hester MW (2011) Temperature tolerance of early life history stages of black mangrove Avicennia germinans: implications for range expansion. Estuaries Coasts 34:824–830
Poorter H, Lambers H (1986) Growth and competitive ability of a highly plastic and a marginally plastic genotype of Plantago major in a fluctuating environment. Physiol Planta 67:217–222
Popp M, Polanía J (1989) Compatible solutes in different organs of mangrove trees. Ann Sci For 46:842s–844s
Popp M, Polanía J, Weiper M (1993) Physiological adaptations to different salinity levels in mangrove. In: Leith H, Al Masoom A (eds) Towards the rationale use of high salinity tolerant plants, vol 1. Kluwer Academic Publishers, Utrecht, The Netherlands, pp 217–224
Purnobasuki H, Suzuki M (2004) Aerenchyma formation and porosity in root of a mangrove plant, Sonneratia alba (Lythraceae). J Plant Res 117:465–472
Quisthoudt K, Schmitz N, Randin CF, Dahdouh-Guebas F, Robert EMR, Koedam N (2012) Temperature variation among mangrove latitudinal range limits worldwide. Trees Struct Funct 26:1919–1931
Raven JA, Handley LL, Macfarlane JJ (1988) The role of CO2 uptake by roots and CAM in acquisition of inorganic C by plants of the isoetid life-form: a review, with new data on Eriocaulon decangulare L. New Phytol 108:125–148
Reef R, Lovelock CE (2014) Regulation of water balance in mangroves. Ann Bot. doi:10.1093/aob/mcu174
Reef R, Feller IC, Lovelock CE (2010) Nutrition of mangroves. Tree Physiol 30:1148–1160
Reef R, Schmitz N, Rogers BA, Ball MC, Lovelock CE (2012) Differential responses of the mangrove Avicennia marina to salinity and abscisic acid. Funct Plant Biol 39:1038–1046
Reef R, Winter K, Morales J, Adame MF, Reef DL, Lovelock CE (2014) The effect of atmospheric carbon dioxide concentrations on the performance of the mangrove Avicennia germinans over a range of salinities. Physiol Planta. doi:10.1111/ppl.12289
Rich SM, Ludwig M, Colmer TD (2008) Photosynthesis in aquatic adventitious roots of the halophytic stem-succulent Tecticornia pergranulata (formerly Halosarcia pergranulata). Plant Cell Environ 31:1007–1016
Ricklefs RE, Schwarzbach AE, Renner SS (2006) Rate of lineage origin explains the diversity anomaly in the world’s mangrove vegetation. Am Nat 168:805–810
Robert EMR, Schmitz N, Boeren I, Driessens T, Herremans K, De Mey J, Van de Casteele E, Beeckman H, Koedam N (2011) Successive cambia: a developmental oddity or an adaptive structure? PloS one 6(1):e16558
Ross MS, Ruiz PL, Sah JP, Hanan EJ (2009) Chilling damage in a changing climate in coastal landscapes of the subtropical zone: a case study from south Florida. Glob Change Biol 15:1817–1832
Saenger P (1982) Morphological, anatomical and reproductive adaptations of Australian mangroves. In: Clough BF (ed) Mangrove ecosystems in Australia. Australian National University Press, Canberra, pp 153–191
Saenger P (2002) Mangrove ecology, silviculture and conservation. Kluwer Academic Publishers, Dordrecht
Saintilan N, Rogers K, McKee K (2009) Saltmarsh-Mangrove interactions in Australasia and the Americas. Chapter 31. In: Perillo GME, Wolanski E, Cahoon DR, Brinson MM (eds) Coastal wetlands; an integrated ecosystems approach. Elsevier, Atlanta, pp 855–883
Saintilan N, Wilson NC, Rogers K, Rajkaran A, Krauss KW (2014) Mangrove expansion and salt marsh decline at mangrove poleward limits. Glob Chan Biol 20:147–157
Santini N, Schmitz N, Lovelock C (2012) Variation in wood density and anatomy in a widespread mangrove species. Trees 26:1555–1563
Schmitz N, Robert EMR, Verheyden A, Kairo JG, Beeckman H, Koedam N (2008) A patchy growth via successive and simultaneous cambia: key to success of the most widespread mangrove species Avicennia marina? Ann Bot 101:49–58
Schmitz N, Egerton JJG, Lovelock CE, Ball MC (2012) Light-dependent maintenance of hydraulic function in mangrove branches: do xylary chloroplasts play a role in embolism repair? New Phytol 195:40–46
Scholander PF (1968) How mangroves desalinate seawater. Physiol Plant 21:251–261
Scholander PF, van Dam L, Scholander SI (1955) Gas exchange in the roots of mangroves. Am J Bot 42:92–98
Scholander PF, Hammel HT, Hemmingsen EA, Garey W (1962) Salt balance in mangroves. Plant Physiol 37:722–729
Semeniuk V (2013) Predicted response of coastal wetlands to climate changes: a Western Australian model. Hydrobiologia 708:23–43
Sherrod CL, McMillan C (1985) The distributional history and ecology of mangrove vegetation along the northerm Gulf of Mexico coastal region. Contrib Mar Sci 28:129–140
Skelton NJ, Allaway WG (1996) Oxygen and pressure changes measured in situ during flooding in roots of the grey mangrove Avicennia marina (Forssk.) Vierh. Aquat Bot 54:165–175
Smith TJ (1988) Differential distribution between subspecies of the mangrove Ceriops tagal: competitive interactions along a salinity gradient. Aquat Bot 32:79–89
Smith TJ III, Duke NC (1987) Physical determinants of inter-estuary variation in mangrove species richness around the tropical coastline of Australia. J Biogeogr 14:9–19
Smith JAC, Popp M, Lüttge U, Cram WJ, Diaz M, Griffiths H, Lee HSJ, Medina E, Schäfer C, Stimmel K-H, Thonke B (1989) Ecophysiology of xerophytic and halophytic vegetation of a coastal alluvial plain in Northern Venezuela. VI. Water relations and gas exchange of mangroves. New Phytol 111:293–307
Snedaker SC, Araújo RJ (1998) Stomatal conductance and gas exchange in four species of Caribbean mangroves exposed to ambient and increased CO2. Mar Freshw Res 49:325–327
Sobrado MA (2000) Relation of water transport to leaf gas exchange properties in three mangrove species. Trees Struct Funct 14:258–262
Sobrado MA, Ball MC (1999) Light use in relation to carbon gain in the mangrove, Avicennia marina, under hypersaline conditions. Aust J Plant Physiol 26:245–251
Sorrell BK, Armstrong W (1994) On the difficulties of measuring oxygen release by root systems in wetland plants. J Ecol 82:177–183
Spalding MD, Kainuma M, Collins L (2010) World Mangrove Atlas. Earthscan, with International Society for Mangrove Ecosystems, Food and Agriculture Organization of the United Nations, The Nature Conservancy, UNEP World Conservation Monitoring Centre, United Nations Scientific and Cultural Organisation, United Nations University, London. 319 pp
Sternberg LSL, Swart PK (1987) Utilization of freshwater and ocean water by coastal plants of southern Florida. Ecology 68:1898–1905
Stevens PW, Fox SL, Montague CL (2006) The interplay between mangroves and saltmarshes at the transition between temperate and subtropical climate in Florida. Wetland Ecol Manage 14:435–444
Stuart SA, Choat B, Martin KC, Holbrook NM, Ball MC (2007) The role of freezing in setting the latitudinal limits of mangrove forests. New Phytol 173:576–583
Takemura T, Hanagata N, Sugihara K, Baba S, Karube I, Dubinsky Z (2000) Physiological and biochemical responses to salt stress in the mangrove, Bruguiera gymnorrhiza. Aquat Bot 68:15–28
Theuri MM, Kinyamario JI, van Speybroeck D (1999) Photosynthesis and related physiological processes in two mangrove species, Rhizophora mucronata and Ceriops tagal, at Gazi Bay, Kenya. Afr J Ecol 37:180–193
Thibodeau FR, Nickerson NH (1986) Differential oxidation of mangrove substrate by Avicennia germinans and Rhizophora mangle. Am J Bot 73:512–516
Tomlinson PB (1986) The botany of mangroves. Cambridge University Press, New York
Traill LW, Perhans K, Lovelock CE, Prohaska A, McFallan S, Rhodes JR, Wilson KA (2011) Managing for change: wetland transitions under sea-level rise and outcomes for threatened species. Divers Distrib 17:1225–1233
Vandegehuchte MW, Guyot A, Hubau M, De Groote SRE, De Baerdemaeker NJF, Hayes M, Welti N, Lovelock CE, Lockington DA, Steppe K (2014) Long-term versus daily stem diameter variation in co-occurring mangrove species: environmental versus ecophysiological drivers. Agric For Meteor 192–193:51–58
Wang W, Xiao Y, Chen L, Lin P (2007) Leaf anatomical responses to periodical waterlogging in simulated semidiurnal tides in mangrove Bruguiera gymnorrhiza seedlings. Aquat Bot 86:223–228
Wei L, Lockington DA, Poh SC, Gasparon M, Lovelock CE (2013) Water use patterns of estuarine vegetation in a tidal creek system. Oecologia 172:485–494
West RC (1977) Tidal salt-marshes and mangal formations of Middle and South America. In: Chapman VJ (Ed) Ecosystems of the world 1. Wet coastal ecosystems, pp. 193–211. Elsevier Scientific Publishing Co., Amsterdam. 428 pp
Woodroffe CD (1985) Studies of a mangrove basin, Tuff Crater, New Zealand: I. Mangrove biomass and production of detritus. Estuarine, Coastal and Shelf Science 20:265-280
Woodroffe CD, Grindrod J (1991) Mangrove biogeography: the role of Quaternary environmental and sea-level change. J Biogeogr 5:479–492
Wullschleger SD, Meinzer FC, Vertessy RA (1998) A review of whole-plant water use studies in trees. Tree Physiol 18:499–512
Yáñez-Espinosa L, Terrazas T, López-Mata L, Valdez-Hernández J (2004) Wood variation in Laguncularia racemosa and its effect on fibre quality. Wood Sci Technol 38:217–226
Youssef T, Saenger P (1996) Anatomical adaptive strategies to flooding and rhizosphere oxidation in mangrove seedlings. Aust J Bot 44:297–313
Youssef T, Saenger P (1998a) Photosynthetic gas exchange and accumulation of phytotoxins in mangrove seedlings in response to soil physico-chemical characteristics associated with waterlogging. Tree Physiol 18:317–324
Youssef T, Saenger P (1998b) Photosynthetic gas exchange and water use in tropical and subtropical populations of the mangrove Aegiceras corniculatum. Mar Freshw Res 49:329–334
Zimmermann U, Zhu JJ, Meinzer FC, Goldstein G, Schneider H, Zimmermann G, Benkert R, Thürmer F, Melcher P, Webb D, Haase A (1994) High molecular weight osmotic compounds in the xylem sap of mangroves: implications for long-distance water transport. Botanica Acta 107:218–229
Zomer RJ, Trabucco A, van Straaten O, Bossio DA (2006) Carbon, land and water: hydrologic dimensions of climate change mitigation through afforestation and reforestation. IWMI Research Report 101. International Water Management Institute, Colombo
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Lovelock, C.E., Krauss, K.W., Osland, M.J., Reef, R., Ball, M.C. (2016). The Physiology of Mangrove Trees with Changing Climate. In: Goldstein, G., Santiago, L. (eds) Tropical Tree Physiology. Tree Physiology, vol 6. Springer, Cham. https://doi.org/10.1007/978-3-319-27422-5_7
Download citation
DOI: https://doi.org/10.1007/978-3-319-27422-5_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-27420-1
Online ISBN: 978-3-319-27422-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)