Abstract
Wood density is an important plant trait that influences a range of ecological processes, including resistance to damage and growth rates. Wood density is highly dependent on anatomical characteristics associated with the conductive tissue of trees (xylem and phloem) and the fibre matrix in which they occur. Here, we investigated variation in the wood density of the widespread mangrove species Avicennia marina in the Exmouth Gulf in Western Australia and in the Firth of Thames in New Zealand. We assessed how variation in xylem vessel size, fibre wall thickness and proportion of phloem within the wood contributed to variation in wood density and how these characteristics were linked to growth rates. We found the wood density of A. marina to be higher in Western Australia than in New Zealand and to be higher in taller seaward fringing trees than in scrub trees growing high in the intertidal. At the cellular level, high wood density was associated with large xylem vessels and thick fibre walls. Additionally, wood density increased with decreasing proportions of phloem per growth layer of wood. Tree growth rates were positively correlated with xylem vessel size and wood density. We conclude that A. marina can have large xylem vessel sizes and high growth rates while still maintaining high wood density because of the abundance and thickness of fibres in which vessels are found.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Wood density is an ecologically and economically important trait in plants. High wood density increases resistance to physical damage (Niklas 1992) and pathogen resistance (Hillis 1987; Augspurger and Kelly 1984) and thereby contributes to plant survival. Additionally, wood density is an important parameter for estimating the biomass of trees and is used to calculate the carbon stocks in forests (Fearnside 1997). Within a species wood density can vary across environmental gradients, as its variation depends on the anatomy of the wood, which is in turn linked to functions such as water transport, mechanical strength and growth (Chave et al. 2009).
The density of wood in many species is largely determined by fibres, which are thick-walled cells composed of cellulose and lignin, that provide mechanical support (Barnett and Bonham 2004; Beck 2010). Other tissue types can also influence wood density. The abundance of xylem vessels, which have lumen areas for water transport, decreases wood density. Previous studies over a range of species have shown increasing wood density correlates with decreasing xylem vessel lumen area (Hacke et al. 2001; Preston et al. 2006).
Wood density has also been linked to tree growth rates. In a survey of 21 rain forest species from Malaysia King et al. (2006) found tree growth rates decreased with increasing wood density. The relationship between wood density and tree growth rates reflects the presence of large xylem vessels, which allow for high rates of transpiration and photosynthetic carbon gain that supports large canopies and high growth rates (Enquist et al. 1999; King et al. 2006).
Mangrove forests dominate the coasts of tropical and subtropical coastlines, where they are exposed to a range of physical disturbances, including ocean waves, strong winds and tidal currents. Mangroves provide valuable coastal protection services (Mazda et al. 2006) and are increasingly recognized for their role in carbon sequestration (Mcleod et al. 2011). The density of mangrove wood is key to the provision of these ecological services, yet the anatomical basis of variation in wood density in mangroves is poorly known. In this study we assessed variation in wood density of the mangrove species, Avicennia marina.
The Indo-pacific mangrove species A. marina has the widest geographical distribution of all mangroves with a latitudinal distribution from ~25°N to 38°S. Avicennia marina reaches its southernmost distribution at Corner Inlet, Victoria, Australia and in the North Island of New Zealand (Duke 2006). The species tolerates a wide range of soil water salinity and is the dominant species where aridity and/or cool temperatures limit the productivity of mangroves (Morrisey et al. 2010). The wide distribution of A. marina has been linked to its special wood structure, characterized by successive cambia (Tomlinson 1995; Robert et al. 2011). This peculiar wood structure comprises consecutive bands of xylem, interspersed with phloem strands that are connected by a band of parenchyma tissue (Schmitz et al. 2007, 2008). Based on the occurrence of this wood structure in tree species that occur in arid or saline habitats (i.e. only 34 families are known to have this wood structure), it has been proposed that successive cambia are associated with tolerance to water deficits (Robert et al. 2011). The presence of phloem in the successive cambia may have implications for wood density.
Here we assessed variation in wood density of A. marina in two locations with different climatic conditions and across an intertidal gradient. Our goals were to (1) assess the variation in wood density between and within locations, (2) investigate the contribution of anatomical features to variation in wood density and (3) test for linkages among variation in wood density, anatomy and tree growth rates in A. marina.
Methods
Site descriptions and sample collection
Our study compares the density and structure of the wood of A. marina and its correlation with tree growth rates at two sampling locations where A. marina is dominant, the Firth of Thames, New Zealand (NZ) and Giralia Bay, Exmouth Gulf in Western Australia (WA).
The Firth of Thames (37.18°S, 175.4°E) is an embayment of about 19-km width in the northern part of the Hauraki Depression, NZ (Hochstein and Nixon 1979). In this site, an extensive mangrove forest with varying forest structure (ranging between ~0.3 to 3 m in height) has developed since the 1950s (Lovelock et al. 2010). The climate is wet, with a mean annual rainfall of 1,400 mm year−1 and cool, with a mean annual air temperature of 15 °C (National Institute of Water and Atmospheric Research (NIWA) 2011). Our second sampling location at Giralia Bay in the Exmouth Gulf, WA (22.4°S, 114.3°E) is arid, with a mean annual rainfall of 300 mm year−1 and warm, with a mean annual air temperature of 25 °C. Cyclones occur every two to three years in the Exmouth region causing short periods of intense rainfall and flooding (Lovelock et al. 2011) and wind gusts in excess of 90 km/h (Australian Bureau of Meteorology 2011).
In January 2007, we collected five mature stems from each of three sites across the intertidal zone in NZ. The sites encompassed trees of differing sizes and ages (Lovelock et al. 2011). The forest at the Firth of Thames, NZ is older towards the landward side (trees recruited in ~1978) and younger on the seaward edge (trees recruited in ~ 1996). In the NZ forests tree size varies in a typical manner for mangroves (Feller et al. 2010), with tall trees on the seaward, more frequently inundated site (NZ-Seaward) to scrub forests ~0.3 m in height in the landward, less frequently inundated site (NZ-Landward-1, 350 m from the seaward edge). The older 1978 forest is a relict fringing forest (NZ-Landward-2, 620 m from the seaward edge) that has tall trees, but is inundated less frequently due to sediment accretion and forest development in a seaward direction over time. At Giralia Bay in WA, we collected wood from six mature stems from the seaward edge of the forest (WA-Seaward) in October 2008. Similar to the fringing site in NZ, the seaward fringing forest at Giralia Bay is inundated by the tide twice daily.
At each site (three at NZ and one in WA), we assessed the porewater salinity. We collected water samples from 30 cm depth using a suction device (McKee et al. 1988) and analysed them with a handheld refractometer (W/ATC 300011, SPER Scientific Scottsdale, USA).
Measurement of wood characteristics
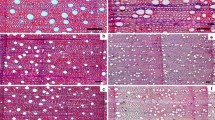
All our measurements were made in the 3–4 outermost layers of wood (Fig. 1a). Wood density was measured for each tree from 1 cm × 1 cm × 2 cm rectangular prisms cut from the outer layers of the wood. Wood density was calculated as the ratio of the oven-dry mass of the wood sample divided by the mass of water displaced by its fresh (green) volume (Chave et al. 2006). Using oven-dried (60 °C) and sanded wood discs (Fig. 1a, b), the number of growth layers, defined as ontogenetic units of phloem and xylem, was counted using an Olympus SZH10 microscope (Tokyo, Japan). A growth layer was counted if it crossed a line drawn from pith to bark along the maximum radius of the stem disc (Schmitz et al. 2007). The proportion of phloem per growth layer was calculated as the width of the phloem band divided by the total width of the growth layer, including both xylem and phloem, measured along the radius of the stem disc.
a Sanded wood disc of Avicennia marina. Inset shows the last layers in which we sampled the wood. b We defined a “layer” as a complete band of xylem (X) and phloem (P) that is composed of conjunctive tissue including phloem strands. c Transverse wood section of Avicennia marina. Insets show how we measured double fibre wall thickness and xylem vessel axes to calculate fibre wall thickness and xylem vessel diameter (Lewis 1992)
Given it can be difficult to resolve small vessels from sanded wood discs, ~1 cm3 cubes were cut from the discs to make transverse microsections (Fig. 1c) that were double stained with Safranin–Alcian blue. We photographed the sections with an Olympus BX61 microscope (Tokyo, Japan). From the photographs we measured 100–200 xylem vessels and 100–200 fibres for each stem. Xylem and phloem area was measured per layer. The number of vessels was counted and the perpendicular axes of the vessel lumina were measured (Fig. 1c). All the measurements were done using Image Pro Plus version 5.0.1 (MediaCybernetics, Maryland, USA).
We calculated the xylem vessel diameter (D) following the ellipse equation of Lewis (1992) (Eq. 1), and the total vessel lumen area (XA) accounting for vessel density (VD) and vessel area (VA) (Eqs. 2, 3, 4).
with vessel density,
and vessel area,
Fibre wall thickness was calculated as the radial distance from fibre lumen 1 to fibre lumen 2 divided by two (Fig. 1c).
Tree growth rates
For the A. marina trees from NZ the pith of each sampled tree was dated using bomb-pulse radio carbon dating with Accelerator Mass Spectrometry (AMS) 14C analysis (Table 1) (Lovelock et al. 2010). Wood samples were dated with a resolution from 1 to 2 years, which is possible given the significant changes in atmospheric 14C after 1955 (when the last bomb-pulse was released) (Hua and Barbetti 2004; Hua 2009). Growth rates were estimated as stem radius divided by tree age. For the trees from the WA-Seaward site, growth rates were estimated from the mean circumference increment in the period April 2008–August 2010, as recorded with 27 mechanical dendrometer bands and converted to radius increment per year.
Data analyses
Differences in wood density, xylem vessel diameter and the proportion of phloem per growth layer between sites were tested using a Kruskal–Wallis test and a Dunn’s post hoc test. These analyses were performed with the software package Prism 5.0 (La Jolla, CA, USA).
To analyse the influence of anatomical characteristics on variation in wood density we used linear regression and stepwise multiple regression analyses. Linear regression analyses were performed using the package lmodel2 (Major Axis) from R (The R Foundation for Statistical Computing). Bootstrap analyses were performed to assess the suitability of the sample size. Confidence intervals of the slope and the intercept were calculated for the linear regressions using lmodel2 and the bias was calculated for the multiple regressions with the R software (bias after bootstrap in all cases was <0.001). These tests indicated that our sample size was adequate and the results were independent of sample size. Prior to analysis we tested for normality using the D’Agostino-Pearson normality test. Independent variables used in the stepwise multiple regression were not correlated.
Results
Wood density was higher in WA than in NZ (Fig. 2; n = 19, p < 0.005). Between the NZ sites wood density was similar in the tall trees from NZ-Seaward and NZ-Landward-2, but wood density was significantly lower in the NZ-Landward-1 scrub trees (Fig. 2).
Our analysis of the anatomical factors that contribute to wood density showed that over all our sites wood density was positively related to mean xylem vessel diameter and total vessel lumen area (Table 2; Fig. 3a, b). Variation in total vessel lumen area can be due to variation in vessel density and/or variation in vessel size. Our examination of xylem vessel density found that vessel density varied from 57 ± 13 vessels mm−2 (mean ± standard deviation) in NZ-Seaward to 64 ± 13 vessels mm−2 in WA-Seaward but these values were not significantly different between sites. Therefore, variation in total vessel lumen area between locations was due to variation in xylem vessel diameter.
The relationship between a wood density and xylem vessel diameter, b wood density and total vessel lumen area, c wood density and fibre wall thickness and d wood density and the proportion of phloem per wood layer expressed as a percentage (phloem%/layer) (Details of regression lines are presented in Table 2). Different symbols represent different sites and different positions in the intertidal: WA-Seaward (closed circles), NZ-Seaward (open squares), NZ-Landward-1 (open diamonds) and NZ-Landward-2 (open triangles)
We also observed that A. marina trees with high wood density had thicker fibre walls (Table 2; Fig. 3c) and a lower proportion of phloem per growth layer (Table 2; Fig. 3d). The proportion of phloem per growth layer was highest in landward forests from NZ (Fig. 4). The three components together, total vessel lumen area, fibre wall thickness and the proportion of phloem per growth layer, explained 76 % of the variation in the wood density of A. marina (Table 3).
The proportion of phloem per growth layer expressed as a percentage (Phloem%/Growth Layer) over the sampling sites in New Zealand and Western Australia. Values are the means and standard deviations from 5 to 6 individual trees. Different letters indicate that the means were significantly different (p < 0.05, n = 21)
Tree growth rates increased with larger xylem vessels and were associated with higher wood density over all sites (Table 2; Fig. 5a, b). However, the regression of growth rates and wood density was significant only for the NZ sites. In contrast, over all sites wood density and xylem vessel diameters were significantly positively correlated with tree size (Fig. 6).
The relationship between a tree growth rate and xylem vessel diameter, and b tree growth rate and wood density (Details of regression lines are presented in Table 2). Different symbols represent different sites and different positions in the intertidal: WA-Seaward (closed circles), NZ-Seaward (open squares), NZ-Landward-1 (open diamonds) and NZ-Landward-2 (open triangles)
The relationship between a wood density and stem radius and b xylem vessel diameter and stem radius for the mangrove Avicennia marina (Details of regression lines are presented in Table 2). Different symbols represent different sites and different positions in the intertidal: WA-Seaward (closed circles), NZ-Seaward (open squares), NZ-Landward-1 (open diamonds) and NZ-Landward-2 (open triangles)
Discussion
Over our contrasting sites we found that wood density of A. marina varied (Fig. 2). In the same habitat (seaward fringe) A. marina tended to have higher mean wood density in WA than in NZ. In different sites within the same location in NZ, landward scrub mangroves (NZ-Landward-1) had lower wood density than tall trees in seaward fringing forest (NZ-Seaward) and those in a taller landward forest (NZ-Landward-2). Using the variation in wood density over all sites we found that wood density increased with total vessel lumen area and fibre wall thickness but was negatively correlated with the proportion of phloem per growth layer of wood.
The positive relationship between wood density and total vessel lumen area and xylem vessel diameter observed in A. marina is in contrast with previous studies that have found high wood density is associated with low total vessel lumen areas and small vessel sizes (Preston et al. 2006; Jacobsen et al. 2007). However, by increasing fibre wall thickness, A. marina maintains high wood density even with large xylem diameters (Fig. 3a, c). By investing in thick fibre walls and reducing the proportion of phloem per growth layer in wood, A. marina could maintain both high water conductivity and mechanical strength. Other studies have also observed that high wood density is not always associated with low xylem lumen area, indicating that trees may achieve high wood density using a range of anatomical strategies (Jacobsen et al. 2005; Martínez-Cabrera et al. 2009).
Wood density of A. marina increased with increasing fibre wall thickness as has been observed in other tree species (Jacobsen et al. 2005; Martínez-Cabrera et al. 2009). The walls of fibres of A. marina are very thick compared with other trees species and their fibre lumina is almost completely lost (International Association of Wood Anatomists (IAWA) 2012). For example, in this study mean fibre wall thickness in WA-Seaward trees was 5.27 ± 0.55 μm (mean ± standard deviation) in A. marina compared with 2 μm in Rollinia rensoniana (Barajas-Morales 1985) The thick-walled fibres of A. marina may increase resistance to breakage by wind and tidal waters during storms, even in rapidly growing trees with large total vessel lumen areas. In WA the large vessels observed in A. marina trees occurred within a dense fibre matrix. In addition to protection from breakage and attack from wood boring invertebrates, fibres add strength to xylem walls that may increase their resistance to collapse under the high tensions that may occur on the xylem water column with water deficits (Jacobsen et al. 2005).
We observed tree growth rates increased with increasing xylem vessel diameter and wood density at the NZ site (Fig. 5). Stronger correlations (over all sites) were observed between xylem vessel size and stem diameter and wood density and stem diameter (Fig. 6), possibly because stem diameter is an integrated measure of tree growth over the entire life time of trees compared with growth rates calculated from changes in stem diameter over 2.3 years of observations, as they were for the WA trees. The relationship between large lumen areas and fast growth rates and tree size is predicted by theoretical analyses of tree growth (Enquist et al. 1999) and has been observed in other species (Poorter et al. 2010; Russo et al. 2010).
Low wood density of A. marina in our study was strongly associated with high proportions of phloem tissue per growth layer in the wood (Fig. 3d). Phloem is a major constituent of A. marina’s wood tissue and results from secondary growth via successive cambia (Fig. 1b, c). Successive cambia have been described in shrubs and tree species of which ~84 % have been found to grow under conditions where drought is common (Robert et al. 2011). Phloem within the wood may be a site for starch depolymerisation, which is the basis of the mechanism for refilling of embolized xylem vessels (Nardini et al. 2010, 2011) and therefore phloem in wood has been proposed to be important in tolerating salinity stress in A. marina (Robert et al. 2011) and drought stress in other species (Salleo et al. 2004). The proportion of phloem per growth layer in the wood varied across the intertidal gradient in NZ, being significantly higher in landward trees compared to seaward trees (Fig. 4). Additionally, previous studies found higher proportions of phloem per growth layer in landward A. marina trees from Kenya (Schmitz et al. 2007, 2008). Our results indicate a high level of phloem in wood is associated with reduced wood density. This may have negative consequences for withstanding damage from wind and waves and also pathogens and pests. Trees growing in suboptimal habitats (high intertidal) with low wood density may have a greater likelihood of physical damage than trees growing in more seaward sites.
Conclusions
Wood density of A. marina varied between locations and across an intertidal gradient in NZ. High wood density of A. marina was associated with large xylem vessel diameters (and total lumen area), thick-walled fibres, low proportion of phloem in the wood, faster growth rates and larger tree sizes. In contrast, low wood density was associated with large areas of phloem within the wood, smaller xylem vessels, thin-walled fibres, slower growth rates and small tree sizes.
References
Augspurger C, Kelly CK (1984) Pathogen mortality of tropical tree seedlings: experimental studies of the effects of dispersal distance, seedling density, and light conditions. Oecologia 61:211–217
Australian Bureau of Meteorology 2011, viewed 28 January 2011. http://www.bom.gov.au
Barajas-Morales J (1985) Wood structural differences between trees of two tropical forests in Mexico. IAWA Bull 6:355–364
Barnett J, Bonham V (2004) Cellulose microfibril angle in the cell wall of wood fibres. Biol Rev 79:461–472
Beck C (2010) An introduction to plant structure and development. Cambridge University Press, Cambridge
Chave J, Muller-Landau HC, Baker TR, Easdale TA, Steege H, Webb CO (2006) Regional and phylogenetic variation of wood density across 2456 neotropical tree species. Ecol Appl 16:2356–2367
Chave J, Coomes D, Jansen S, Lewis S, Swenson N, Zanne A (2009) Towards a worldwide wood economics spectrum. Ecol Lett 12:351–366
Duke NC (2006) Australia′s Mangroves. The authoritative guide to Australian′s mangrove plants. University of Queensland, Brisbane
Enquist B, West G, Charnov E, Brown J (1999) Allometric scaling of production and life-history variation in vascular plants. Nature 401:909–911
Fearnside P (1997) Wood density for estimating forest biomass in Brazilian Amazonia. For Ecol Manage 90:59–87
Feller IC, Lovelock CE, Berger U, McKee KL, Joye SB, Ball MC (2010) Biocomplexity in Mangrove ecosystems. Annu Rev Marine Sci 2:395–417
Hacke U, Sperry JS, Dockman WT, Davis SD, McCulloh KA (2001) Trends in wood density and structure are linked to prevention of xylem implosion by negative pressure. Oecologia 126:457–461
Hillis W (1987) Heartwood and tree exudates, Berlin
Hochstein MP, Nixon IM (1979) Geophysical study of the Hauraki depression, North Island, New Zealand. NZ J Geol Geophys 22:1–19
Hua Q (2009) Radiocarbon: a chronological tool for the recent past. Quat Geochronol 4:378–390
Hua Q, Barbetti M (2004) Review of tropospheric bomb 14C data for carbon cycle modeling and age calibration purposes. Radiocarbon 46:1273–1298
International Association of Wood Anatomists (IAWA) (2012). Viewed 20 January 2012. http://bio.kuleuven.be/sys/iawa/
Jacobsen AL, Ewers FW, Pratt RB, Paddock WA III, Davis SD (2005) Do xylem fibers affect vessel cavitation resistance? Plant Physiol 139:546–556
Jacobsen AL, Agenbag L, Esler KJ, Pratt RB, Ewers FW, Davis SD (2007) Xylem density, biomechanics and anatomical traits correlate with water stress in 17 evergreen shrub species f the Mediterranean-type climate region South Africa. J Ecol 95:171–183
King DA, Davies SJ, Tan S, Noor NSMD (2006) The role of wood density and stem support costs in the growth and mortality of tropical trees. J Ecol 94:670–680
Lewis A (1992) Measuring the hydraulic diameter of a pore or conduit. Am J Bot 79:1158–1161
Lovelock C, Sorrell B, Hancock N, Hua Q, Swales A (2010) Mangrove forest and soil development on a rapidly accreting shore in New Zealand. Ecosystems 13:437–451
Lovelock CE, Feller IC, Adame MF, Reef R, Penrose HM, Wei L, Ball MC (2011) Intense storms and the delivery of materials that relieve nutrient limitations in mangroves of an arid zone estuary. Funct Plant Biol 38:514–522
Martínez-Cabrera H, Jones C, Espino S, Schenk H (2009) Wood anatomy and wood density in shrubs: responses to varying aridity along transcontinental transects. Am J Bot 96:1388–1398
Mazda Y, Magi M, Ikeda Y, Kurokawa T, Asano T (2006) Wave reduction in a mangrove forest dominated by Sonneratia sp. Wetl Ecol Manage 14:365–378
McKee KL, Mendelssohn IA, Hester MW (1988) Reexamination of pore water sulfide concentrations and redox potentials near the aerial roots of Rhizophora mangle and Avicennia germinans. Am J Bot 75:1352–1359
Mcleod E, Chmura GL, Bouillon S, Salm R, Björk M, Duarte CM, Lovelock CE, Schlesinger WH, Silliman BR (2011) A blueprint for blue carbon: toward an improved understanding of the role of vegetated coastal habitats in sequestering CO2. Frontiers Ecol 9:552–560
Morrisey DA, Swales A, Dittmann S, Morrison MA, Lovelock CE, Beard CM (2010) The ecology and management of temperate mangroves. Oceanogr Mar Biol Annu Rev 48:43–160
Nardini A, Grego F, Trifilo P, Salleo S (2010) Changes of xylem sap ionic content and stem hydraulics in response to irradiance in Laurus nobilis. Tree Physiol 30:628–635
Nardini A, Lo Gullo MA, Salleo S (2011) Refilling embolized xylem conduits: is it a matter of phloem unloading? Plant Sci 180:604–611
National Institute of Water and Atmospheric Research (NIWA) 2011. Viewed 28 Janu 2011. http://cliflo.niwa.co.nz/
Niklas KJ (1992) Plant biomechanics: an engineering approach to plant form and function. University of Chicago Press, Chicago
Poorter L, McDonald I, Alarcón A, Fichtler E, Licona J, Peña-Claros M, Sterck F, Villegas Z, Sass-Klaassen U (2010) The importance of wood traits and hydraulic conductance for the performance and life history strategies of 42 rainforest tree species. New Phytol 185:481–492
Preston AK, Cornwell KW, Denoyer JL (2006) Wood density and vessels traits as distinct correlates of ecological strategy in 51 California coast range angiosperms. New Phytol 170:807–818
Robert E, Schmitz N, Boeren I, Driessens T, Herremands K, De Mey J, De Casteele EV, Beeckman H, Koedam N (2011) Successive cambia: a developmental oddity or an adaptive structure? Public Library Sci ONE 6:e16558. doi:10.1371/journal.pone.0016558
Russo SE, Jenkins KL, Wiser SK, Uriarte M, Duncan RP, Coomes DA (2010) Interspecific relationships among growth, mortality and xylem traits of woody species from New Zealand. Funct Ecol 24:253–262
Salleo S, Lo Gullo MA, Trifilò P, Nardini A (2004) New evidence for a role of vessel-associated cells and phloem in the rapid xylem refilling of cavitated stems of Laurus nobilis L. Plant Cell Environ 27:1065–1076
Schmitz N, Verheyden A, Kairo JG, Beeckman H, Koedman N (2007) Successive cambia development in Avicennia marina (Forssk.) Vierh. climatically driven in the seasonal climate at Gazi Bay, Kenya. Dendrochronologia 25:87–96
Schmitz N, Robert EMR, Verheyden A, Kairo JG, Beeckman H, Koedman N (2008) A patchy growth via successive and simultaneous cambia: key to success of the most widespread mangrove species Avicennia marina. Ann Bot 101:49–58
Tomlinson PB (1995) The botany of Mangroves. Cambridge University Press, Massachusetts
Acknowledgments
This work was supported by The Mexican Council of Science and Technology (CONACYT), The Secretary of Public Education (SEP, Mexico), awards from the Australian Research Council (LP0776680 and DP0774491) and by the National Institute of Water and Atmospheric Research (NIWA), New Zealand. Nele Schmitz was funded by a postdoctoral fellowship and a mobility grant from the Research Foundation-Flanders (FWO). We thank Prof Marilyn Ball and Jack Egerton from the Australian National University, Dr. Ruth Reef and Dr. María Gómez-Cabrera from the University of Queensland, Jennifer and Denver Blake of Giralia Station, Andrew Swales and Nicole Hancock from NIWA. Robert Gould, Richard Webb, Robyn Webb, Wendy Armstrong and Kay Hodge, from the Centre for Microscopy and Microanalysis at The University of Queensland, and Dr. Tim R. Mercer for help with the manuscript and figure preparation. We thank as well Bianca Santini and Edgar Liceaga from the National Autonomous University of Mexico and Prof. Uta Berger, Juliane Vogt and Lin Yue from the Technische Universität Dresden for their help to improve this manuscript. We are thankful to the anonymous reviewers for comments on the previous version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Shane.
Rights and permissions
About this article
Cite this article
Santini, N.S., Schmitz, N. & Lovelock, C.E. Variation in wood density and anatomy in a widespread mangrove species. Trees 26, 1555–1563 (2012). https://doi.org/10.1007/s00468-012-0729-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-012-0729-0