Abstract
One of the affected tissues in age-related macular degeneration (AMD) is the retinal pigment epithelium (RPE), a tissue that consists of terminally differentiated cells and that accumulates damage over time. In all tissues, mitochondria (mt), which play an essential role in both cell health (energy) and death (initiator of apoptosis), undergo an aging process through the accumulation of mtDNA damage, changes in mitochondrial dynamics, a reduction in biogenesis, and mitophagy, leading to an overall reduction in mitochondrial energy production and other non-energy-related functions. Here we have compared energy metabolism in primary human RPE cells isolated from aborted fetus or aged donor eyes and grown as stable monolayers. H2O2 treatment resulted in the generation of reactive oxygen species and superoxide, an effect that was significantly augmented by age. Mitochondrial metabolism, as analyzed by Seahorse respirometry, revealed reduced mitochondrial oxygen consumption (ATP production) at baseline and a complete loss of reserve capacity in aged cells. Likewise, glycolysis was blunted in aged cells. Taken together, these studies showed that RPE cells derived from aged donor eyes are more susceptible to oxidative stress, and exhibit a loss in mitochondrial respiratory reserve capacity and a reduction in glycolysis. These data suggest that while old cells may have sufficient energy at rest, they cannot mount a stress response requiring additional ATP and reducing agents. In summary, these data support the hypothesis that mitochondria or energy metabolism is a valid target for therapy in AMD.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
AMD is a slowly progressing multifactorial disease involving genetic abnormalities and environmental insults. Inflammation, oxidative stress and single nucleotide polymorphisms (SNPs) in genes in the complement cascade increase the risk for AMD. RPE cells are affected early and in all forms of AMD. The RPE is composed of a single layer of hexagonal highly pigmented cells, located between the retina and the choroid, forming part of the blood-retina barrier. Its many functions [reviewed by (Strauss 2005)] include: transport of molecules between the subretinal space and the choroidal blood supply; spatial ion buffering; secretion of growth factors, proteases, etc., that control the stability of photoreceptors, Bruch’s membrane (BrM) and the choroid; and finally, modulation of the immune response, since the RPE participates in control of immune privilege in the healthy eye or mounting of an immune response in the diseased eye.
The unique phagocytotic function of the RPE, and the need to efficiently recycle the polyunsaturated fatty acid-rich (PUFA) shed outer segments, exposes the RPE to high levels of oxidative stress [reviewed by (Cai et al. 2000)]. Oxidation of PUFA initiates a chain reaction producing many reactive oxygen species (ROS). Furthermore, RPE cells contain many photosensitizers, and exposure to intense visible light induces generation of ROS. To cope with these toxic oxygen intermediates, the RPE has evolved effective defenses against oxidative damage; it is particularly rich in anti-oxidants. Due to this specialization, the RPE can withstand oxidative stress at levels that would typically kill cells. For example, our own work and that published by others has shown that RPE cells grown as monolayers with stable resistance, are resistant to oxidative stress, withstanding H2O2 treatment up to a concentration of 1 mM (Bailey et al. 2004; Thurman et al. 2009). However, with increasing age, the RPE antioxidative capability appears to be reduced (Cai et al. 2000). Likewise, old RPE cells appear to exhibit mitochondrial decay, such as mitochondrial fission and loss of mitochondrial morphology, bioenergetic deficiencies, and weakened antioxidant defenses (He and Tombran-Tink 2010), and the aging process overall is coupled to an increase in mitochondrial DNA mutations and mitochondrial disorganization (Miquel et al. 1980). Thus, it is likely that aged RPE cells are more susceptible to oxidative stress (Zarbin 2004). In support of this notion, the NEI-sponsored AREDS study demonstrated that subjects at risk for AMD and those with early AMD benefited from supplements containing high levels of antioxidants and zinc (Bartlett and Eperjesi 2003). While cellular bioenergetics (i.e., ATP production) have been assessed at baseline in human RPE cells (He and Tombran-Tink 2010), little is known about cellular bioenergetics under stress conditions.
2 Results
2.1 Oxidative Stress is Increased in Cells from Aged Donors
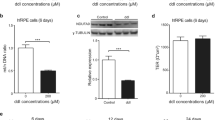
Primary human embryonic RPE cells as well as those isolated from donors (ages 68–72) were grown on Transwell plates as published previously (Bandyopadhyay and Rohrer 2012). Monolayer formation was monitored using transepithelial resistance (TER) measurements to ensure that monolayers of equal levels of differentiation were used (200–300 Ω/cm2, obtained within 2–3 weeks of reaching confluence). At the time of the experiment, fetal bovine serum was removed from the growth media, which had no effect on the TER of established monolayers (Thurman et al. 2009). Monolayers could then be treated with apical application of 0.5 mM H2O2 to induce oxidative stress. Oxidative stress was analyzed by quantifying cytosolic reactive oxygen species (ROS) generation and super oxide production (O2 −) with dichlorofluorescein diacetate and dihydroethedium, respectively (Fig. 106.1).
Oxidative stress is increased in aged RPE cells. Cytosolic a reactive oxygen species (ROS) and b superoxide (O2−) levels was measured using dichlorofluorescein diacetate dye and dihydroethidium, respectively. Both were significantly elevated in aged cells under control condition. Only in embryonic cells could ROS production be increased after exposure to oxidative stress (0.5 mM H2O2). Data are expressed as mean ± SEM (n = 3–4 per condition)
At baseline, in untreated cells, aged RPE cells appear to be under significant oxidative stress since ROS levels were significantly elevated by ~ 6-fold when compared to embryonic cells. Similarly, O2 − are higher by ~ 4-fold. Interestingly, while in embryonic RPE cells, ROS levels increased significantly by ~ 3.5-fold in the H2O2-treated monolayers, no further increase over baseline levels could be observed in the aged RPE cells. In contrast, O2 − levels did not change in cells of either age upon H2O2-treated exposure. Lack of cytotoxic effect was confirmed by monolayer morphology and lack of effect on TER [see (Bandyopadhyay and Rohrer 2012) for embryonic cells; data not shown for aged cells].
2.2 Aged RPE Cells have Reduced Mitochondrial and Glycolytic Metabolic Capacity
Cells take up substrates such as oxygen, glucose, fatty acids, etc., and convert them into energy stored as adenosine-triphosphate (ATP). ATP production requires a number of oxidation/reduction reactions involved in glycolysis (converts glucose into pyruvate), the tricarboxylic acid (TCA) cycle (oxidizes pyruvate-derived acetyl-CoA to generate ATP and reducing agents), and oxidative phosphorylation (utilizes NADH and succinate generated in the TCA cycle to establish a proton gradient to power the ATP synthase). As byproducts, heat, lactic acid and CO2 are released into the extracellular environment. We have published previously on the usefulness of the Seahorse Biosciences XF analyzer (Seahorse Bioscience, Billerica, MD) to track real-time changes in cellular metabolism (Perron et al. 2012). This system uses fluorometric sensors to measure oxygen consumption rates (OCR) and extracellular acidification rates (ECAR) for a single cell layer on the bottom of multi-well plates (Ferrick et al. 2008). Cells were plated in 96-well custom plates and grown in parallel to cells on Transwell plates to determine the time point at which they differentiate and form a monolayer.
Rates were assessed at four stages, basal rate after 15 min of equilibration in the XF instrument, maximal respiratory capacity and mitochondrial oxygen consumption. The latter two parameters were assessed using the following inhibitors: Carbonyl cyanide-4-(trifluoromethoxy)phenylhydrazone (FCCP), a protonophore or an uncoupling agent, since it disrupts ATP synthesis by preventing the buildup of the proton gradient required as the energy source for oxidative phosphorylation; and, sodium azide, a potent inhibitor of mitochondrial respiration that blocks cytochrome c oxidase (complex IV). The normalized OCR (Fig. 106.2a) and ECAR (Fig. 106.2b) values are presented for statistical analysis.
Metabolism in RPE cells. Metabolism was assessed using Seahorse Extracellular Flux assays. Basal rate, maximal respiration (FCCP) and mitochondrial oxygen consumption (azide) were assessed in embryonic and aged RPE monolayers. Data are expressed as mean ± SEM (n = 3–5 per condition). a Summary for oxygen consumption rate (OCR); and b extracellular acidification rates (ECAR). Basal mitochondrial metabolism is reduced, but maximal capacity is almost abolished in aged RPE cells, while mitochondrial-dependent O2 consumption was unaffected. ECAR was significantly reduced in aged cells for all three measures; with both age groups exhibiting an increase in glycolysis when mitochondrial respiration was reduced
RPE cells derived from embryonic donor eyes showed a typical behavior in the OCR analysis (Fig. 106.2a), with oxygen consumption rates being maximally stimulated by FCCP (1.7-fold increase when compared to baseline) and significantly inhibited by azide. In comparison, OCR rates in RPE cells derived from aged donor eyes were only slightly inhibited by azide, and maximal respiratory capacity was completely abolished. On average, basal OCR rates of aged RPE cells were within 30 % of those exhibited by embryonic cells, but the maximal respiratory capacity, the additional ATP that can be produced under stress condition, can only be elicited from young but not aged donor cells.
RPE cells, irrespective of the donor age, showed a typical behavior in the ECAR analysis (Fig. 106.2b), in that the glycolytic capacity of the cells increased in response to the agents that interfered with oxidative phosphorylation. In both age-groups, ECAR increased by 60–67 % after FCCP and by 118–128 % after azide application. However, overall, glycolytic capacity was reduced in aged cells by ~ 75 %.
Finally, it was tested whether OCR and ECAR rates are affected by oxidative stress. Basal respiration was significantly decreased in young RPE cells after H2O2-treatment (45 ± 2.8, P < 0.001), while rates were not affected in aged RPE cells when compared to untreated cells (13 ± 17.0, P = 0.6). Likewise, only the embryonic cells exhibited a drop in ECAR after H2O2-exposure (47 ± 7.7, P < 0.01), while the rates of aged RPE cells remained unchanged (basal: 8.0 ± 14.0, P = 0.5).
3 Discussion
Overall, the study was designed to determine the bioenergetics and antioxidant defenses in aged RPE cells. The overall conclusions from this analysis can be summarized as follows: (1) RPE cells from aged donors experience significant oxidative stress at baseline, which cannot be increased after exposure to H2O2; and concomitantly, (2) these aged cells have reduced mitochondrial and glycolytic metabolic capacity that cannot be further reduced by oxidative stress. Taken together, these bioenergetic deficiencies coupled with weakened antioxidant defenses may significantly reduce RPE function and contribute to age-related retinal anomalies.
The OCR and ECAR for a given cell type was correlated with the cells requirement for, or its ability to generate, energy and reducing agents. Here, we analyzed RPE cells in an artificial environment in which most of the normal tissue functions (i.e., retinoid metabolism, phagocytosis of rod outer segments, etc.) were eliminated. Stress was induced artificially by exposure of cells to H2O2 at a concentration known not to cause damage (Bandyopadhyay and Rohrer 2012). H2O2 has been shown previously to reduce state 3 respiration and reduce activity of TCA cycle enzymes (Nulton-Persson and Szweda 2001).
Embryonic RPE cells were found to exhibit a robust increase in oxygen consumption, demonstrating a significant mitochondrial respiratory capacity should additional energy be required. Likewise, embryonic cells appear to consume large amounts of glucose, based on the ECAR levels, which can be elevated under mitochondrial stress conditions. Overall, between glycolysis and the pentose phosphate pathway (generation of reducing equivalents in the form of NADPH; not analyzed here), the embryonic cells appear to have sufficient reducing agents to maintain a non-oxidized environment. Exposure to H2O2 reduced mitochondrial respiration as well as glycolytic capacity, and concomitantly increased the amount of ROS present in the cells. In contrast, old RPE cells have reduced mitochondrial respiration and glycolytic capacity at baseline when compared to embryonic cells, which results in a highly oxidized cellular environment with elevated levels of ROS and O2 −. This level of oxidative stress did not reduce mitochondrial respiration or alter the already elevated levels of increased amounts of ROS and O2 − present in the cells; it did, however, further decrease the glycolytic capacity of the cell.
In future experiments, we wish to examine the possibility of ameliorating these bioenergetic deficiencies to increase energy production and bolster the cell’s antioxidant defenses to improve RPE cell function and reduce its susceptibility to age-related changes and risk factors of age-related macular degeneration.
References
Bailey TA, Kanuga N, Romero IA et al. (2004) Oxidative stress affects the junctional integrity of retinal pigment epithelial cells. Invest Ophthalmol Vis Sci 45:675–684
Bandyopadhyay M, Rohrer B (2012) Matrix metalloproteinase activity creates pro-angiogenic environment in primary human retinal pigment epithelial cells exposed to complement. Invest Ophthalmol Vis Sci 53:1953–1961
Bartlett H, Eperjesi F (2003) Age-related macular degeneration and nutritional supplementation: a review of randomised controlled trials. Ophthalmic Physiol Opt 23:383–399
Cai J, Nelson KC, Wu M et al. (2000) Oxidative damage and protection of the RPE. Prog Retin Eye Res 19:205–221
Ferrick DA, Neilson A, Beeson C (2008) Advances in measuring cellular bioenergetics using extracellular flux. Drug Discov Today 13:268–274
He Y, Tombran-Tink J (2010) Mitochondrial decay and impairment of antioxidant defenses in aging RPE cells. Adv Exp Med Biol 664:165–183
Miquel J, Economos AC, Fleming J et al. (1980) Mitochondrial role in cell aging. Exp Gerontol 15:575–591
Nulton-Persson AC, Szweda LI (2001) Modulation of mitochondrial function by hydrogen peroxide. J Biol Chem 276:23357–23361
Perron NR, Beeson C, Rohrer B (2012) Early alterations in mitochondrial reserve capacity; a means to predict subsequent photoreceptor cell death. J Bioenerg Biomembr 45:101–9
Strauss O (2005) The retinal pigment epithelium in visual function. Physiol Rev 85:845–881
Thurman JM, Renner B, Kunchithapautham K et al. (2009) Oxidative stress renders retinal pigment epithelial cells susceptible to complement-mediated injury. J Biol Chem 284:16939–16947
Zarbin MA (2004) Current concepts in the pathogenesis of age-related macular degeneration. Arch Ophthalmol 122:598–614
Acknowledgments
This work was supported in part by the National Institutes of Health (R01EY019320), Veterans Affairs (I01 RX000444), Foundation Fighting Blindness, and an unrestricted grant to MUSC from Research to Prevent Blindness. The authors have no financial conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this paper
Cite this paper
Rohrer, B., Bandyopadhyay, M., Beeson, C. (2016). Reduced Metabolic Capacity in Aged Primary Retinal Pigment Epithelium (RPE) is Correlated with Increased Susceptibility to Oxidative Stress. In: Bowes Rickman, C., LaVail, M., Anderson, R., Grimm, C., Hollyfield, J., Ash, J. (eds) Retinal Degenerative Diseases. Advances in Experimental Medicine and Biology, vol 854. Springer, Cham. https://doi.org/10.1007/978-3-319-17121-0_106
Download citation
DOI: https://doi.org/10.1007/978-3-319-17121-0_106
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-17120-3
Online ISBN: 978-3-319-17121-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)