Abstract
Taurine protects against tissue damage in a variety of models that share inflammation as a common pathogenic feature. Heavy exercise has been shown to cause inflammation and oxidative stress and to damage muscle tissue. High taurine levels are present in skeletal muscle and may play a role as a cellular defense against free radical-mediated damage. The aim of this study was to determine whether taurine injected in the abdomen alters markers of inflammation and free radical damage after varying degrees of heavy exercise. The effect of intra-abdominal administration of taurine 1 h before heavy exercise was examined. On a daily basis for 10 consecutive days, a speed of 20 m/min for 20 min. Muscle damage was associated with an increase in IL-6 and CD68 of the skeletal muscle. The immunoreactivities for IL-6 and CD68 are shown increase in the 20 min heavy exercise group. The increase in IL-6 and CD68 was suppressed in the 20 min heavy exercise group that received an intra-abdominal injection of taurine. Data from this study show that exercise-induced muscle inflammation is reduced in SOL and EDL of rats treated with taurine prior to exercise.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Taurine (2-aminoethanesulfonic acid) is the most abundant amino acid in the body. It is found in high concentrations in most tissues, including skeletal muscle, heart, nerve, brain, and liver in vertebrates (Jacobsen and Smith 1968). Its cytoprotective activity against tissue damage involves several essential biological, physiological, and pharmacological mechanisms, including membrane stabilization (Pasantes-Morales et al. 1985), antioxidation (Gordon and Heller 1992; Sugiura et al. 2013) and osmoregulation (Thurston et al. 1980). Because of the absence of taurine biosynthesis, the large intracellular taurine pool of skeletal muscle is maintained by a specific transporter (Ramamoorthy et al. 1994). The uptake of taurine differs with muscle type (Kim et al. 1986), as the steady state level of taurine concentration of the slow-twitch fiber is higher than that of the fast-twitch fiber (Iwata et al. 1986). Heavy exercise increases oxidative stress and damages skeletal muscle (Sugiura et al. 2013). The damage is characterized by enhanced muscle protein catabolism and acute inflammation, followed by muscle tissue regeneration (Meeson et al. 2004; Ciciliot and Schiaffino 2010). The progression of muscle repair is modulated by essential and semi-essential amino acids and involves the modulation of inflammation and protein anabolism (Dort et al. 2013; Miyazaki et al. 2004). Taurine protects skeletal muscle against intense exercise-induced nitrosative inflammation and ensuing DNA damage by preventing iNOS expression and the increase in nitrosative stress mediated by heavy exercise (Sugiura et al. 2013). Therefore, the regulation of inflammation is likely to differ with muscle type. To evaluate differences related to muscle type, we observed the effect of taurine administration on inflammation in heavily exercised skeletal muscle of rats.
2 Methods
2.1 Ethics Statement
All experimental protocols were approved by the Animal Ethics Committee of Suzuka University of Medical Science, Japan.
2.2 Animal and Experimental Design
Eight-weeks-old male Wistar rats were obtained from SLC (Hamamatsu, Japan) and weighed 250–260 g on arrival. They were housed in cages (Max, six per cage) and maintained on water and food ad libitum. All animals were maintained at 24.0 °C (45–55 % of humidity) with a 12 h light and 12 h dark cycle. The 18 rats were randomly divided into three groups; Exercise plus saline group (n = 6), Exercise plus taurine group (n = 6), and Control group (n = 6).
2.3 Exercise Protocol and Taurine Supplementation
The exercise protocol of the present study used a widely recognized animal model to study post exercise inflammatory responses. We designed a heavy treadmill exercise protocol on a motor-driven tread-mill (model MK-680; Muromachi Kikai, Tokyo, Japan) in which rats began exercising at 8 m/min for 10 min during the week preceding the actual exercise experiments. The rats became accustomed to locomotion in preparation of the final exercise experiments without stimulating development of skeletal muscle as an adaptation to training. Each group ran on the treadmill at 20 m/min, 25 % grade, for 20 min or until exhaustion. Exhaustion was determined as the point when an animal was unable to right itself when placed on its side. The workload was ~75 % of the rat’s maximal aerobic capacity. Rats subjected to the exercise protocol were either treated with saline or taurine (intra-abdominally) 1 h prior to the onset of the exercise protocol.
2.4 Surgical Method and Muscle Collection
In the control group, extensor digitorum longus and soleus muscles of both legs were removed in the morning of the 10th day. Then, muscle tissue was formalin-fixed and the samples paraffin-embedded.
In the exercise plus saline group and the exercise plus taurine group, the animals were euthanized by cardiac exsanguination under anesthesia 48-h after the 10th day of exercise, and the muscles were immediately excised. The protocol was designed to restore basal conditions prior to sacrifice.
2.5 Immunofluorescence Study of TNF-α, CD68, and IL-6
Standard immunofluorescence methods were used to examine the distribution of TNF-α, CD68, and IL-6 in muscle tissue and normal controls. After deparaffinization and rehydration, antigen was retrieved in 5 % urea by microwave heating for 5 min and then incubated in 1 % H2O2 for 30 min to block endogenous peroxidase activity. Sections of 5 μm thickness were incubated overnight at room temperature with the following antibodies: TNF-α goat polyclonal antibody, CD68 mouse monoclonal antibody, and IL-6 rabbit polyclonal antibody (1:200, Santa Cruz Biotechnology Co., Ltd). For the goat antibodies (TNF-α), the sections were incubated with Donkey anti-goat IgG-Alexa 594 for 2 h. For mouse antibody (CD68), the sections were incubated with Donkey anti-mouse IgG-Alexa 488 for 2 h. For the rabbit antibodies (IL-6), the sections were incubated with Goat anti-Rabbit IgG-Alexa 594 for 2 h.
2.6 Immunohistochemical Grading
Immunohistochemical (IHC) grading, which is based on intensity and frequency of staining, was performed by two independent investigators. The staining intensity was scored as negative (−), weak (+), moderate (++), strong (+++), or very strong (++++). In addition, we measured the brightness of the image by using ImageJ (Wayne Rasband) (Schneider et al. 2012).
2.7 Statistical Analysis
Data are presented as means ± SEM. The two-tailed Student’s t-test was performed. Differences were considered statistically significant at P < 0.05.
3 Results
3.1 Body Weight Progression and Consumption of Water and Food
Over the duration of the experiments, there was no significant difference in body weight progression between the Control and Taurine groups. All rats were in good health, with no pathological signs observed throughout the period of study.
3.2 Localization of TNF-α, IL-6 and CD68 in Normal and Exercised EDL and SOL Skeletal Muscle
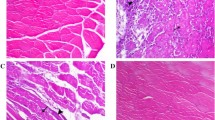
To observe TNF-α, IL-6 and CD68 expression, immunohistochemistry was performed on normal and exercised skeletal muscle. TNF-α and IL-6 immunofluorescence staining shows intense immunoreactivity in the stromal cell of endomysium and blood vessels of EDL and SOL muscle (Figs. 1, 2, 3, and 4). In contrast, CD68 immunoreactivity was localized in some of the muscle fibers of EDL and SOL (Figs. 1, 2, 3, and 4).
3.3 Comparative Quantification of TNF-α, IL-6 and CD68 Immunoreactivities in EDL and SOL Skeletal Muscle
To compare the expression of TNF-α, IL-6 and CD68 in different skeletal muscle of each group, immunoreactive signal intensity was quantified by grading. TNF-α and IL-6 signal intensities exhibited a definitive trend in EDL that was less apparent in SOL. There was no difference in CD68 expression between EDL and SOL (Tables 1 and 2). In the heavy exercise group, TNF-α, IL-6 and CD68 signal intensities significantly increased compared to that of the control group and exercise plus taurine group (p < 0.05) (Figs. 1, 2, 5 and 6).
The average immunoreactivities of IL-6, TNF-α and CD68 in EDL. TNF-α, IL-6 and CD68 immunoreactivities significantly increased in rats undergoing heavy exercise. The immunoreactivities significantly decreased in the taurine exercise group compared to the heavy exercise group. Result show Mean ± SEM, for n = 6 rats in each experimental group. *P < 0.05, exercise group versus exercise + taurine group or control group. The two-tailed Student’s t-test
The average immunoreactivity of IL-6, TNF-α and CD68 in SOL. TNF-α, IL-6 and CD68 immunoreactivities significantly increased in rats performing heavy exercise. Those immunoreactivities significantly decreased in the taurine exercise group compared to that of the heavy exercise group. Result show Mean ± SEM, for n = 6 rats in each experimental group. *P < 0.05, exercise group versus exercise + taurine group or control group. The two-tailed Student’s t-test
4 Discussion
In the present study, we compared the effects of taurine administration on inflammation of various muscle types in rats subjected to a heavy exercise protocol. Our study revealed three findings. First, most of TNF-α and IL-6 preferentially accumulate in stromal cells of skeletal muscle. In contrast, CD68 accumulates within the muscle fibers. Second, signal intensity for TNF-α and IL-6 was observed in some muscle fibers after heavy exercise. Finally, taurine treatment reduced measures of inflammation in rat skeletal muscle after exercise injury. Skeletal muscle damage leads to changes in tissue morphology and function that may last for several weeks (Lapointe et al. 2002; Smith et al. 2008). Heavy exercise is characterized by muscle protein catabolism and acute inflammation, followed by muscle regeneration (Meeson et al. 2004). The progression of muscle repair is modulated by essential and semi-essential amino acids, which alter inflammation and protein anabolism (Miyazaki et al. 2004; Dort et al. 2013). After muscle injury, myocytes and other cells release a number of cytokines, including IL-1β, IL-6, IL-8 and TNF-α, which cause neutrophils to produce a host of cytotoxic substances, including reactive oxygen species, such as superoxide anions, hypochlorite, and hydrogen peroxide (Best et al. 1999; Brickson et al. 2001). The cytokines IL-1, IL-6, and TNF-α stimulate pathways that contribute to activation of the enzyme NADPH-oxidase, which in turn generates reactive oxygen species. Previous research reported that taurine may exert various biological actions that contribute to muscle repair, such as improved outcome of an inflammatory insult, owing to its ability to decrease the production of major pro-inflammatory cytokines (TNF-α, IL-6) (Pilon et al. 2011; Rudkowska et al. 1995). Although the mechanism by which taurine reduces inflammation in skeletal muscle is not fully understood, taurine plays an important role in cytoprotection against ischemia and hypoxia in smooth muscle of stomach (Ma et al. 2003). Taurine efflux may also contribute to the regulatory volume decrease mediated by ion channels and triggered as a response to cell swelling. Released taurine can in turn react with HOCl− produced by activated leukocytes to form taurine chloramine, a strong reactant that inhibits the production of TNF-α and other proinflammatory mediators (Barua et al. 2001).
In a previous study by our laboratory, we found that taurine administration significantly reduces iNOS expression, indicating that heavy exercise-induced nitrosative and oxidative stress is modulated by taurine. Up-regulation of iNOS in skeletal muscle is responsible for nitrosative muscle damage induced by heavy exercise, a consequence of inflammation-mediated activation of NF-κB signaling (Sugiura et al. 2013). ATP production and oxygen supply to mitochondria needs to be increased to activate skeletal muscle during exercise. An increase in oxygen consumption likely means an increase in the generation of superoxide radicals (O2 −). Taurine appears to reduce the production of O2 − via redox reaction, particularly in places with high production of O2 −, such as mitochondria (Hansen et al. 2006). This involves the conjugation of taurine with tRNALeu(UUR), which increases the biosynthesis of ND6, a subunit of complex I of the electron transport chain, and prevents the diversion of electrons to oxygen forming O2 − (Jong et al. 2012). Type I muscle fibers contain a large number of mitochondria and perform aerobic energy metabolism by consuming a large number of oxygen molecules to produce ATP during exercise. Therefore, it is likely that type I muscle fibers generate a high level of free radicals from oxygen and are vulnerable to oxidative and nitrosative stress. Compared with type II muscle fibers, type I muscle fibers contain a higher level of polyunsaturated fatty acid and are reportedly vulnerable to lipid peroxidation (Nikolaidis and Mougios 2004; Nikolaidis et al. 2006) as well as to other types of oxidative damage. Because type I muscle fibers also contain a higher number of mitochondria (Moyes 2003) than type II muscle fibers, they may produce a higher level of free radical production during exercise and even at rest.
5 Conclusion
In conclusion, the present study demonstrates that taurine therapy protects against intense exercise-induced inflammation. Taurine may have various biological functions in muscle repair, such as an improved resolution of inflammation, owing to its ability to decrease the production of major pro-inflammatory cytokines.
Abbreviations
- CD68:
-
Cluster of differentiation 68
- EDL:
-
Extensor digitorum longus
- IHC:
-
Immunohistochemistry
- IL-6:
-
Interleukin-6
- iNOS:
-
Inducible nitric oxide synthase
- SOL:
-
Soleus
- Tau:
-
Taurine
- TNF-α:
-
Tumor necrosis factor-α
References
Barua M, Liu Y, Quinn MR (2001) Taurine chloramine inhibits inducible nitric oxide synthase and TNF-alpha gene expression in activated alveolar macrophages: decreased NF-kappaB activation and IkappaB kinase activity. J Immunol 167(4):2275–2281
Best TM, Fiebig R, Corr DT, Brickson S, Ji L (1999) Free radical activity, antioxidant enzyme, and glutathione changes with muscle stretch injury in rabbits. J Appl Physiol (1985) 87(1):74–82
Brickson S, Hollander J, Corr DT, Ji LL, Best TM (2001) Oxidant production and immune response after stretch injury in skeletal muscle. Med Sci Sports Exerc 33(12):2010–2015
Ciciliot S, Schiaffino S (2010) Regeneration of mammalian skeletal muscle. Basic mechanisms and clinical implications. Curr Pharm Des 16(8):906–914
Dort J, Leblanc N, Maltais-Giguere J, Liaset B, Cote CH, Jacques H (2013) Beneficial effects of cod protein on inflammatory cell accumulation in rat skeletal muscle after injury are driven by its high levels of arginine, glycine, taurine and lysine. PLoS One 8(10):e77274
Gordon RE, Heller RF (1992) Taurine protection of lungs in hamster models of oxidant injury: a morphologic time study of paraquat and bleomycin treatment. Adv Exp Med Biol 315:319–328
Hansen SH, Andersen ML, Birkedal H, Cornett C, Wibrand F (2006) The important role of taurine in oxidative metabolism. Adv Exp Med Biol 583:129–135
Iwata H, Obara T, Kim BK, Baba A (1986) Regulation of taurine transport in rat skeletal muscle. J Neurochem 47(1):158–163
Jacobsen JG, Smith LH (1968) Biochemistry and physiology of taurine and taurine derivatives. Physiol Rev 48(2):424–511
Jong CJ, Azuma J, Schaffer S (2012) Mechanism underlying the antioxidant activity of taurine: prevention of mitochondrial oxidant production. Amino Acids 42:2223–2232
Kim BK, Baba A, Iwata H (1986) Taurine transport in chronically stimulated fast- and slow-twitch muscles of the rat. Jpn J Pharmacol 42(3):441–446
Lapointe BM, Frenette J, Cote CH (2002) Lengthening contraction-induced inflammation is linked to secondary damage but devoid of neutrophil invasion. J Appl Physiol (1985) 92(5):1995–2004
Ma N, Ding X, Miwa T, Semba R (2003) Immunohistochemical localization of taurine in the rat stomach. Adv Exp Med Biol 526:229–236
Meeson AP, Hawke TJ, Graham S, Jiang N, Elterman J, Hutcheson K, Dimaio JM, Gallardo TD, Garry DJ (2004) Cellular and molecular regulation of skeletal muscle side population cells. Stem Cells 22(7):1305–1320
Miyazaki T, Matsuzaki Y, Ikegami T, Miyakawa S, Doy M, Tanaka N, Bouscarel B (2004) Optimal and effective oral dose of taurine to prolong exercise performance in rat. Amino Acids 27(3–4):291–298
Moyes CD (2003) Controlling muscle mitochondrial content. J Exp Biol 206(Pt 24):4385–4391
Nikolaidis MG, Mougios V (2004) Effects of exercise on the fatty-acid composition of blood and tissue lipids. Sports Med 34(15):1051–1076
Nikolaidis MG, Petridou A, Mougios V (2006) Comparison of the phospholipid and triacylglycerol fatty acid profile of rat serum, skeletal muscle and heart. Physiol Res 55(3):259–265
Pasantes-Morales H, Wright CE, Gaull GE (1985) Taurine protection of lymphoblastoid cells from iron-ascorbate induced damage. Biochem Pharmacol 34(12):2205–2207
Pilon G, Ruzzin J, Rioux LE, Lavigne C, White PJ, Froyland L, Jacques H, Bryl P, Beaulieu L, Marette A (2011) Differential effects of various fish proteins in altering body weight, adiposity, inflammatory status, and insulin sensitivity in high-fat-fed rats. Metabolism 60(8):1122–1130
Ramamoorthy S, Leibach FH, Mahesh VB, Han H, Yang-Feng T, Blakely RD, Ganapathy V (1994) Functional characterization and chromosomal localization of a cloned taurine transporter from human placenta. Biochem J 300(Pt 3):893–900
Rudkowska I, Marcotte B, Pilon G, Lavigne C, Marette A, Vohl MC (1995) Fish nutrients decrease expression levels of tumor necrosis factor-alpha in cultured human macrophages. Physiol Genomics 40(3):189–194
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9(7):671–675
Smith C, Kruger MJ, Smith RM, Myburgh KH (2008) The inflammatory response to skeletal muscle injury: illuminating complexities. Sports Med 38(11):947–969
Sugiura H, Okita S, Kato T, Naka T, Kawanishi S, Ohnishi S, Oshida Y, Ma N (2013) Protection by taurine against INOS-dependent DNA damage in heavily exercised skeletal muscle by inhibition of the NF-kappaB signaling pathway. Adv Exp Med Biol 775:237–246
Thurston JH, Hauhart RE, Dirgo JA (1980) Taurine: a role in osmotic regulation of mammalian brain and possible clinical significance. Life Sci 26(19):1561–1568
Acknowledgements
This work was partly supported by a grant-in-aid from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this paper
Cite this paper
Kato, T., Okita, S., Wang, S., Tsunekawa, M., Ma, N. (2015). The Effects of Taurine Administration Against Inflammation in Heavily Exercised Skeletal Muscle of Rats. In: Marcinkiewicz, J., Schaffer, S. (eds) Taurine 9. Advances in Experimental Medicine and Biology, vol 803. Springer, Cham. https://doi.org/10.1007/978-3-319-15126-7_62
Download citation
DOI: https://doi.org/10.1007/978-3-319-15126-7_62
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-15125-0
Online ISBN: 978-3-319-15126-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)