Abstract
High-fructose intake has been shown to induce dyslipidemia and other hallmarks of the metabolic syndrome and taurine seems able to ameliorate these effects. However, the effects of high fructose intake on taurine homeostasis are currently unknown. Here we examined the effects of a high-fructose diet with or without oral taurine supplementation (2 % in drinking water) for 6 weeks in Wistar rats.
Fructose significantly decreased hepatic triglyceride content, an increase in plasma triglyceride content but had no effect on plasma non-esterified fatty acids or total cholesterol, with taurine supplementation having no effect on these parameters.
A high fructose diet significantly decreased hepatic taurine content, increased EDL and soleus muscle taurine content and had no effect on plasma taurine content. Fructose increased taurine transporter (TauT) mRNA in liver, but not in EDL or soleus muscle. Interestingly, fructose decreased cysteinesulfinic acid decarboxylase (CSAD) and cysteine dioxygenase (CDO) mRNA levels in EDL muscle, and decreased cysteamine dioxygenase (ADO) mRNA levels in soleus muscle. Fructose did not cause any changes in CSAD, CDO or ADO mRNA levels in liver.
Taurine supplementation elevated plasma and skeletal muscle taurine content, but decreased liver taurine content. Taurine decreased TauT mRNA levels in both EDL and soleus muscle, but increased it in liver. Furthermore, taurine decreased CSAD mRNA levels in liver and soleus muscle as well as decreased CDO mRNA levels in soleus muscle.
These data suggest that taurine transport and biosynthesis are affected by high fructose intake in a tissue specific manner.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Regular food constituents have a profound effect on both development and protection against obesity, type 2 diabetes and other adverse metabolic changes. In the last few decades, dietary fructose has become a major constituent of the modern western diet, with the main source being sucrose from beet- or sugar cane, high fructose corn syrup, fruits, and honey (Tappy et al. 2010).
In human studies, high fructose intake has been shown to induce dyslipidemia and other adverse metabolic changes (Bantle et al. 2000; Crapo and Kolterman 1984). Many studies have reported that a high fructose diet in rodents causes insulin resistance, dyslipidemia and type 2 diabetes (Basciano et al. 2005; Samuel 2011; Tappy et al. 2010). For that reason a high-fructose diet in rodents has become a common model for the development of dyslipidemia, insulin resistance and type 2 diabetes (Tran et al. 2009). Dietary fructose is therefore believed to be a contributing factor for the marked increase in the incidence of the metabolic syndrome in western countries.
Taurine is a semi-essential β-amino acid and has emerged as a participant in the complex network constituting the pathogenesis of the metabolic syndrome and type 2 diabetes. Several studies suggest that during the development of obesity and type 2 diabetes, dysregulation of taurine homeostasis occurs in the organism. Indeed, a high-fructose diet in rodents has been reported to decrease plasma and liver taurine levels. Furthermore, several animal studies have shown that taurine may prevent fructose induced insulin resistance (El Mesallamy et al. 2010; Nandhini and Anuradha 2002; Nandhini et al. 2004).
In the body, taurine is found in relatively high concentrations in tissues (5–50 mM) but about 3 orders of magnitude lower in plasma (10–100 μM) (Hansen 2001; Huxtable 1992). It is known to have a number of physiological functions, such as conjugation with bile acids, intracellular osmolyte for volume regulation and antioxidant properties (Hansen 2001). Taurine homeostasis in the body involves a balance between the rate of biosynthesis from methionine and cysteine, taurine transport, dietary intake and kidney reabsorption/excretion.
Taurine is synthesized from methionine and cysteine in the liver by two different pathways. In the transsulfuration pathway, which is believed to be the primary pathway of taurine biosynthesis, homocysteine is initially converted to cysteine. Cysteine is then oxidized by cysteine dioxygenase (CDO) to generate cysteinesulfinate, which is further decarboxylated by cysteinesulfinic acid decarboxylase (CSAD) forming hypotaurine. CSAD is believed to be the rate-limiting step in the primary pathway. The secondary pathway involves the oxidation of cysteamine by cysteamine dioxygenase (ADO) resulting in hypotaurine. The hypotaurine generated by both pathways is thought to be spontaneously oxidized to taurine (Simmons et al. 2006; Stipanuk and Dominy 2006; Stipanuk et al. 2006; Ueki et al. 2012).
The taurine transporter (TauT) is thought to be expressed throughout the body, and belongs to a family of Na+ Cl−-dependent transporters. Taurine uptake across the brush-border membrane of the small intestine is mediated via TauT and H+/amino acid transporter 1 (Anderson et al. 2009). Recent studies have found that the transport of taurine may be upregulated by inflammation (Mochizuki et al. 2004) and decreased by type 2 diabetes (Merheb et al. 2007). In the blood stream taurine is distributed to tissues and cells where it is taken up by TauT and H+/amino acid transporter 1. Knocking out the TauT causes greater than a 90 % reduction in taurine content in some tissues, thereby demonstrating that TauT is the main uptake transporter for taurine (Heller-Stilb et al. 2002). Skeletal muscle accounts for more that 70 % of the total taurine content in the body (Huxtable 1992) and knocking out the TauT leads to skeletal muscle impairment (Warskulat et al. 2004). Taurine excretion is either by the kidney through urine or through taurine-conjugated bile acid excretion in feces (Glass et al. 1992; Odle et al. 1992)
The effects of high fructose intake on taurine content and its metabolism are largely unknown. Thus, we examined the effects of high-fructose diet (60 % energy from fructose) with or without oral taurine supplementation (2 % in drinking water) for 6 weeks on taurine homeostasis in Wistar rats.
2 Methods
2.1 Animals, Study Design and Diet
All experimental procedures described were approved by The Danish Animal Experiments Inspectorate (permit 2013-15-2934-00904) and by the local animal facility at the University of Copenhagen, Denmark. Forty-eight 8-week old male Wistar Hannover GALAS (HanTac:WH) rats were purchased from Taconic (Ejby, Denmark). Food- and water intake were measured bi-weekly. After acclimatization for 1 week, the rats were randomly divided into four groups (n = 12 per group). The animals were fed a control diet or a high fructose diet with or without 2 % taurine supplementation in the drinking water for 6 weeks. The control diet contained 67.3 % energy from carbohydrate (split evenly between corn starch and sucrose), 20 % protein, 12.8 % corn oil. The fructose-rich diet contained 66.8 % energy from fructose, 20.2 % protein, and 12.9 % energy from lard (Harlan Teklad). The taurine used was a chemically synthesized variant (Sigma-Aldrich) and was dissolved directly in the water used in the animal facility. The rats were fed ad libitum, housed two rats per cage, and kept at a 12-h light/dark cycle.
Overnight fasted rats were sedated with a mixture of Hypnorm (active ingredients fentanyl and fluanisone at a concentration of 0.079 mg/ml and 2.5 mg/ml, respectively) and Dormicum (active ingredient midazolam at a concentration of 1.25 mg/ml) in water given as 0.3 ml per 100 g of body weight. Soleus and EDL muscles were dissected from the legs, and the rat was cut open and the liver lobes were dissected. All tissues were quick-frozen in liquid nitrogen and stored at −80 °C for further analysis.
2.2 Quantitative Real-Time PCR
RNA was extracted from different tissues using Qiazol (Qiagen, Valencia, CA, USA) as described earlier (Larsen et al. 2013). Total RNA was mixed (at a concentration > 0.15 μg/μl for a total of 2 μg RNA in 20 μl volume) with reverse transcriptase, random hexamer primers and nucleotides and cDNA synthesis performed using the High Capacity cDNA Reverse Transcription Kit with RNase inhibitor (Applied Biosystems, Carlsbad, CA, USA) as described earlier (Larsen et al. 2013)
Amplification mixtures were amplified using a SYBR Green mastermix (Applied Biosystems) according to standard conditions ((95 °C 10 min) × 1, (95 °C 15 s, 60 °C 1 min, 95 °C 15 s, 60 °C 15 s, 95 °C 15 s) × 50 cycles in a total volume of 10 μl with a melting curve from 60 to 100 °C) in 384 well plates in triplicates on an ABI VIIA7 real-time PCR system (Applied Biosystems). TBP mRNA levels were used for normalization between samples. The sequences of primers used are:
-
Primers:TauT-forward: 5′-TGGACAGCCAGTTTGTTGAAG-3′,
-
TauT-reverse: 5′-GCAATGAAGATTTCCCGACGA-3′,
-
CSAD-forward: 5′-TGGTCATGGAGCCCAAGTTC-3′,
-
CSAD-reverse: 5′-CATCATGGTTCCCTTCTTCACC-3′,
-
CDO-forward: 5′-GCCTTCACTTGTACAGTCCAC-3′,
-
CDO-reverse: 5′-CTCCAGTGAACCTGAAGTTGTAAAT-3′,
-
ADO-forward: 5′-CCGGTCACTTACATGCACATC-3′,
-
ADO-reverse: 5′-CGTACAGCACCTTGAGCATAC-3′,
-
TBP-forward: 5′-CCCACCAGCAGTTCAGTA-3′,
-
TBP-reverse: 5′-CAATTCTGGGTTTGATCATTC-3′.
2.3 Biochemical Analysis
Fasting glucose was measured using two different automated Accu-Check Glucometers (Roche, Basal, Switzerland) in duplicate from tail vein blood.
Triglycerides were measured in 50 mg liver tissue, hydrolyzed in 0.5 M KOH/85 % ethanol at 60 °C for 30 min. After cooling, MgSO4 was added to 0.1 M and samples were vortexed and centrifuged at 16,000 × g for 20 min at 4 °C. Glycerol was measured spectrophotometrically at 340 nm as described (Wieland 1984). High Density Lipoprotein (HDL) cholesterol and Low Density Lipoprotein (LDL) cholesterol in rat plasma were measured at 450 nm using an ELISA Kit according to instructions from the manufacturer (Novatein Biosciences, Cambridge, MA, USA) at 37 °C. Total cholesterol was calculated as HDL + LDL. Plasma Non-Esterified Fatty Acids (NEFA) were measured at 546 nm using NEFA-HR (2) Kit according to instructions from the manufacturer (WAKO, Richmond, VA, USA) at 37 °C.
Taurine content were measured in 10 μl plasma or 50 mg liver-, EDL- or soleus tissue, by homogenization in 10 % (w/v) TCA, followed by neutralization with 1 M KOH in 100 mM Imidazole buffer. Taurine content was measured spectrophotometrically as described (Matsuda and Asano 2012).
2.4 Statistic Analysis
Data are presented as means ± standard error of the mean (SEM). Statistical analyses were carried out using mixed model two-way ANOVA. The mRNA data were log-transformed before statistical analysis in order to obtain a normal distribution except for EDL ADO mRNA data that had a normal distribution without log-transformation. All statistical analyses were performed using SAS 9.2 (The SAS Institute, Cary, NC, USA). A p-value less than 0.05 was considered significant and a p-value below 0.1 was considered a tendency. A p-value above 0.1 was considered non-significant (NS).
3 Results
3.1 Body Weight, Food Intake, Water Intake and Taurine Intake
All animals demonstrated a steady weight gain (data not shown). Fructose-fed animals had an increase in food intake, but also an increase in water intake (Table 1 ).
Due to increased water intake the fructose-fed animals consumed significantly more taurine than the controls during all six weeks (Table 1). Despite the increase in calorie intake the fructose-fed animals had no difference in body weight after 42 days compared to the controls. Taurine supplementation had no effect on body weight, food intake or water intake (Table 1).
3.2 Plasma Parameters
Fructose increased plasma triglyceride content, but interestingly, fructose also caused a significant decrease in hepatic triglyceride content (Table 2). However, the fructose diet had no effect on the levels of total cholesterol, HDL, LDL, FFA or fasting glucose. Taurine supplementation did not rescue the plasma parameters or the hepatic changes. However, taurine caused a significant increase in fasting glucose (Table 2).
3.3 Taurine Content in Plasma, Liver and Skeletal Muscle
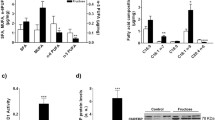
Taurine content in plasma was not affected by fructose. Fructose diet had an opposite effect on liver and muscle, causing increased content of taurine in EDL and soleus muscle and a significant decrease in the hepatic taurine content. Taurine supplementation increased the taurine content in both plasma and skeletal muscle, and surprisingly, taurine supplementation also had a tendency to decrease hepatic taurine content (Fig. 1).
Taurine content in plasma, liver, soleus and EDL. Male Wistar rats (N = 12) per. group, were subjected to four different diet regimes for 6 weeks as described in methods. Taurine contents were measured as described in methods. (Con) Control, (tau) Taurine, (fru) Fructose. Two-way ANOVA statistics: Plasma; diet: NS, taurine: p = 0.0041, diet * taurine: NS. Liver; diet: p = 0.023, taurine: p = 0.09, diet * taurine: NS. EDL; diet: p = 0.0003, taurine: p < 0.0001, diet * taurine: NS. Soleus; diet: p = 0.003, taurine: p < 0.0001, diet * taurine: NS
3.4 Taurine Homeostasis in Liver and Skeletal Muscle
In the liver, fructose significantly increased the level of TauT mRNA and had no effect on ADO, CDO or CSAD mRNA levels. Taurine supplementation also increased the hepatic level of TauT mRNA and decreased the level of CSAD mRNA whereas the mRNA levels of ADO and CDO did not change (Table 3).
The fructose diet had different effects on the taurine synthetic enzymes in the two different skeletal muscles. TauT mRNA levels were decreased in EDL but no effect was seen in soleus with the fructose diet. Fructose decreased the mRNA levels of ADO in soleus muscle and had no effect on ADO mRNA levels in EDL. Both CSAD and CDO mRNA levels were significantly decreased in the in EDL muscle of the fructose fed animals whereas no changes were seen on mRNA levels of CSAD and CDO in soleus muscle (Table 3).
Taurine supplementation significantly decreased the levels of TauT and CSAD mRNA levels in soleus muscle. Taurine also caused a decrease in TauT mRNA levels in EDL muscle. Furthermore, Taurine supplementation of the control fed animals caused a significant decrease in the levels of CDO in soleus muscle and a significant increase in ADO mRNA levels in EDL (Table 3).
4 Discussion
The current study set out to investigate the effects of a high-fructose diet on taurine homeostasis in liver and skeletal muscle of Wistar rats. We saw that the fructose diet affected both taurine content and mRNA levels of taurine synthesizing enzymes in liver and skeletal muscle in a tissue specific manner.
4.1 Body and Plasma Parameters
In this study, there was no difference in weight gain between the experimental groups after 6 weeks of dietary manipulation despite an increased calorie intake in the fructose fed group (Table 1). In addition, an increase in water intake in the fructose group was observed, causing the animals in that group to ingest more taurine compared to the control (Table 1), which may explain some of the experimental differences seen in mRNA levels between the taurine fed, control and taurine fed, fructose fed animals as the fructose + taurine animals had a higher taurine intake than the control + fructose animals.
Fructose did not affect fasting glucose levels after 6 weeks of dietary manipulation as shown in several other studies (Bantle 2009). However, taurine supplementation alone significantly increased fasting glucose levels (Table 2), which is inconsistent with previous findings showing that taurine improves insulin sensitivity and reduces hyperglycemia (Nandhini and Anuradha 2003; Nandhini et al. 2005a). Recently we reported an increase in fasting glucose after 26 weeks of taurine supplementation in male Wistar rats (Larsen et al. 2013). Sub-strain differences in the outbred Wistar rat strain is a possible explanation, as there seem to be some rat strain differences in the response to fructose (Stark et al. 2000). The Wistar sub-strain used in the current study only displayed small, if any, effect of fructose compared to other Wistar sub-strains which become severely glucose intolerant after being fed fructose (El Mesallamy et al. 2010; Nandhini et al. 2005a; Perret et al. 2007).
Plasma parameters are often used to identify the general health condition in mammals. Studies have demonstrated increased plasma concentrations of triglycerides and cholesterol in rats subjected to a high-fructose diet (Nagai et al. 2009), (Ackerman et al. 2005). However, in this study, no differences were observed in blood cholesterol, LDL or HDL (Table 2), but consistent with the literature, the fructose diet increased plasma triglyceride concentrations (Table 2). Taurine did not significantly attenuates fructose-mediated elevations in plasma triglycerides, which seems inconsistent with previous findings which reported a taurine induced attenuation in dyslipidemia and diabetic complications induced by both fructose and high fat ingestion (Murakami et al. 2000; Sethupathy et al. 2002).
4.2 Taurine Content
Studies have shown that taurine content in plasma and in different tissues is affected by different pathological conditions and also the enzymes involved in taurine synthesis and homeostasis are primarily believed to be regulated by diet (Yamamoto et al. 1995). In humans, the amount of taurine in plasma is lowered by obesity, Type 1 diabetes, and type 2 diabetes (Franconi et al. 1995; Jeevanandam et al. 1991; De Luca et al. 2001). Furthermore, high-fat diet-induced obesity in mice presented with decreased plasma taurine content (Tsuboyama-Kasaoka et al. 2006). In the current study, we found that fructose did not affect plasma taurine content which is in direct contradiction with an earlier study, reporting that Wistar rats had decreased taurine content in plasma as a consequence of a fructose diet (Nandhini et al. 2005a).
Moreover, alloxan-induced type 1 diabetic rats display elevated taurine levels in skeletal muscle, and decreased taurine levels in liver (Reibel et al. 1979). Wijekoon et al. found that the ZDF diabetic rat, a model for type 2 diabetes, display increased taurine content in both skeletal muscle and liver (Wijekoon et al. 2004). This indicates that different models affect taurine homeostasis in different ways. We also found an increase in taurine content in skeletal muscle induced by both fructose and taurine. However, in the liver fructose significantly decreased taurine content with no taurine rescue effect. Surprisingly, taurine supplementation had a tendency to decrease taurine content in the liver. Nandhini et al. have also demonstrated a hepatic decrease in taurine content when subjecting Wistar rats to a high-fructose diet (Nandhini et al. 2005b, 2005c).
4.3 Taurine Transport and Biosynthesis
It has been suggested that during the development of obesity and type 2 diabetes, a dysregulation of taurine homeostasis occurs in the organism, possibly orchestrated by changes in the levels of enzymes involved in taurine transport and synthesis. We therefore measured the mRNA levels of the taurine transporter and the enzymes involved in taurine biosynthesis.
Studies have shown that renal epithelium can adapt to the availability of taurine ingested, as the mRNA level and activity of TauT is responsive to the presence of taurine or precursors of taurine. In this way, the kidney can increase reabsorption or excretion of taurine in response to dietary intake (Chesney et al. 1989). We show in this study that fructose-fed Wistar rats display alterations in mRNA levels of all of the enzymes involved in taurine transport and synthesis. Fructose diet increased mRNA levels of TauT in the liver and suppressed it in EDL muscle. Taurine supplementation had the same effect as fructose on TauT mRNA levels in liver and EDL muscle. In soleus muscle taurine had a suppressing effect, suggesting that dietary fructose is an important regulator of TauT mRNA levels in liver and EDL and a regulator of taurine in all three tissues.
CSAD is believed to be the rate-limiting step in taurine biosynthesis; studies have shown that diet can change CSAD mRNA levels. Jerkins et al. showed that rats fed a high-protein diet contain decreased levels of CSAD mRNA (Jerkins et al. 1998). In the current study, we found a significant decrease in the levels of CSAD mRNA in EDL muscle in the fructose fed animals but no change in soleus muscle and liver. Taurine decreased mRNA levels of CSAD in soleus muscle and liver. This suggests that diet regulates CSAD mRNA levels in EDL muscle and fructose regulates taurine in all three tissues.
A high-fat diet and genetically (db/db) obese mice show decreased mRNA levels of CDO in white adipose tissue (Tsuboyama-Kasaoka et al. 2006). We saw that fructose suppresses CDO mRNA levels in EDL muscle. In the soleus muscle a decrease was observed in mRNA levels of CDO in the control fed animals compared to that of fructose fed animals, which could be due to the difference in taurine intake between the groups. Fructose had no effect on CDO mRNA levels in the liver. This indicates some kind of dietary regulation of CDO mRNA levels in EDL muscle. Furthermore, we showed regulation of ADO by dietary fructose, which is the enzyme involved in what is believed to be the secondary pathway in taurine biosynthesis (Simmons et al. 2006; Stipanuk and Dominy 2006; Stipanuk et al. 2006; Ueki et al. 2012). In the current study fructose decreases the mRNA levels of ADO in soleus whereas taurine supplementation increases the mRNA levels of ADO in EDL in the control fed animals.
5 Conclusion
In the present study, we show for the first time that tissue and plasma taurine is affected by dietary fructose, with the effect greater in liver than in skeletal muscle. Also, the enzymes involved in taurine biosynthesis and the taurine transporter in liver and skeletal muscle are influenced by a fructose diet. These observations give a clear indication that taurine transport and biosynthesis in liver and skeletal muscle may be dysregulated in the fructose fed rat model and possibly also in specific animal models of malnutrition.
Abbreviations
- ADO:
-
Cysteamine dioxygenase
- CDO:
-
Cysteine dioxygenase
- CSAD:
-
Cysteinesulfinic acid decarboxylase
- EDL:
-
Extensor digitorum longus
- HDL:
-
High density lipoprotein
- LDL:
-
Low density lipoprotein
- NEFA:
-
Non-esterified fatty acids
- NS:
-
Non-significant
- TauT:
-
Taurine transporter
References
Ackerman Z, Oron-Herman M, Grozovski M, Rosenthal T, Pappo O, Link G et al (2005) Fructose-induced fatty liver disease: hepatic effects of blood pressure and plasma triglyceride reduction. Hypertension 45(5):1012–1018
Anderson CMH, Howard A, Walters JRF, Ganapathy V, Thwaites DT (2009) Taurine uptake across the human intestinal brush-border membrane is via two transporters: H + -coupled PAT1 (SLC36A1) and Na + - and Cl(−)-dependent TauT (SLC6A6). J Physiol 587(Pt 4):731–744
Bantle JP (2009) Dietary fructose and metabolic syndrome and diabetes. J Nutr 139(6):1263S–1268S
Bantle JP, Raatz SK, Thomas W, Georgopoulos A (2000) Effects of dietary fructose on plasma lipids in healthy subjects. Am J Clin Nutr 72(5):1128–1134
Basciano H, Federico L, Adeli K (2005) Fructose, insulin resistance, and metabolic dyslipidemia. Nutr Metab 2(1):5
Chesney RW, Jolly K, Zelikovic I, Iwahashi C, Lohstroh P (1989) Increased Na + -taurine symporter in rat renal brush border membranes: preformed or newly synthesized? FASEB J Off Publ Fed Am Soc Exp Biol 3(9):2081–2085
Crapo PA, Kolterman OG (1984) The metabolic effects of 2-week fructose feeding in normal subjects. Am J Clin Nutr 39(4):525–534
El Mesallamy HO, El-Demerdash E, Hammad LN, El Magdoub HM (2010) Effect of taurine supplementation on hyperhomocysteinemia and markers of oxidative stress in high fructose diet induced insulin resistance. Diabetol Metab Syndr 2:46
Franconi F, Bennardini F, Mattana A, Miceli M, Ciuti M, Mian M et al (1995) Plasma and platelet taurine are reduced in subjects with insulin-dependent diabetes mellitus: effects of taurine supplementation. Am J Clin Nutr 61(5):1115–1119
Glass EN, Odle J, Baker DH (1992) Urinary taurine excretion as a function of taurine intake in adult cats. J Nutr 122(5):1135–1142
Hansen SH (2001) The role of taurine in diabetes and the development of diabetic complications. Diabetes Metab Res Rev 17(5):330–346
Heller-Stilb B, van Roeyen C, Rascher K, Hartwig H-G, Huth A, Seeliger MW et al (2002) Disruption of the taurine transporter gene (taut) leads to retinal degeneration in mice. FASEB J Off Publ Fed Am Soc Exp Biol 16(2):231–233
Huxtable RJ (1992) Physiological actions of taurine. Physiol Rev 72(1):101–163
Jeevanandam M, Ramias L, Schiller WR (1991) Altered plasma free amino acid levels in obese traumatized man. Metabolism 40(4):385–390
Jerkins AA, Jones DD, Kohlhepp EA (1998) Cysteine sulfinic acid decarboxylase mRNA abundance decreases in rats fed a high-protein diet. J Nutr 128(11):1890–1895
Larsen LH, Orstrup LKH, Hansen SH, Grunnet N, Quistorff B, Mortensen OH (2013) The effect of long-term taurine supplementation and fructose feeding on glucose and lipid homeostasis in Wistar rats. Adv Exp Med Biol 776:39–50
De Luca G, Calpona PR, Caponetti A, Romano G, Di Benedetto A, Cucinotta D et al (2001) Taurine and osmoregulation: platelet taurine content, uptake, and release in type 2 diabetic patients. Metabolism 50(1):60–64
Matsuda M, Asano Y (2012) A simple assay of taurine concentrations in food and biological samples using taurine dioxygenase. Anal Biochem 427(2):121–123
Merheb M, Daher RT, Nasrallah M, Sabra R, Ziyadeh FN, Barada K (2007) Taurine intestinal absorption and renal excretion test in diabetic patients: a pilot study. Diabetes Care 30(10):2652–2654
Mochizuki T, Satsu H, Nakano T, Shimizu M (2004) Regulation of the human taurine transporter by TNF-alpha and an anti-inflammatory function of taurine in human intestinal Caco-2 cells. BioFactors Oxf Engl 21(1–4):141–144
Murakami S, Kondo Y, Nagate T (2000) Effects of long-term treatment with taurine in mice fed a high-fat diet: improvement in cholesterol metabolism and vascular lipid accumulation by taurine. Adv Exp Med Biol 483:177–186
Nagai Y, Yonemitsu S, Erion DM, Iwasaki T, Stark R, Weismann D et al (2009) The role of peroxisome proliferator-activated receptor gamma coactivator-1 beta in the pathogenesis of fructose-induced insulin resistance. Cell Metab 9(3):252–264
Nandhini ATA, Anuradha CV (2002) Taurine modulates kallikrein activity and glucose metabolism in insulin resistant rats. Amino Acids 22(1):27–38
Nandhini ATA, Thirunavukkarasu V, Anuradha CV (2004) Stimulation of glucose utilization and inhibition of protein glycation and AGE products by taurine. Acta Physiol Scand 181(3):297–303
Nandhini ATA, Thirunavukkarasu V, Anuradha CV (2005a) Taurine modifies insulin signaling enzymes in the fructose-fed insulin resistant rats. Diabetes Metab 31(4 Pt 1):337–344
Nandhini ATA, Thirunavukkarasu V, Anuradha CV (2005b) Taurine prevents collagen abnormalities in high fructose-fed rats. Indian J Med Res 122(2):171–177
Nandhini ATA, Thirunavukkarasu V, Anuradha CV (2005c) Taurine prevents collagen abnormalities in high fructose-fed rats. Indian J Med Res 122:171–177
Nandhini TA, Anuradha CV (2003) Inhibition of lipid peroxidation, protein glycation and elevation of membrane ion pump activity by taurine in RBC exposed to high glucose. Clin Chim Acta Int J Clin Chem 336(1–2):129–135
Odle J, Glass EN, Czarnecki-Maulden GL, Baker DH (1992) Urinary excretion of taurine as a function of taurine intake: potential for estimating taurine bioavailability in the adult cat. Adv Exp Med Biol 315:55–62
Perret P, Slimani L, Briat A, Villemain D, Halimi S, Demongeot J et al (2007) Assessment of insulin resistance in fructose-fed rats with 125I-6-deoxy-6-iodo-D-glucose, a new tracer of glucose transport. Eur J Nucl Med Mol Imaging 34(5):734–744
Reibel DK, Shaffer JE, Kocsis JJ, Neely JR (1979) Changes in taurine content in heart and other organs of diabetic rats. J Mol Cell Cardiol 11(8):827–830
Samuel VT (2011) Fructose induced lipogenesis: from sugar to fat to insulin resistance. Trends Endocrinol Metab 22(2):60–65
Sethupathy S, Elanchezhiyan C, Vasudevan K, Rajagopal G (2002) Antiatherogenic effect of taurine in high fat diet fed rats. Indian J Exp Biol 40(10):1169–1172
Simmons CR, Liu Q, Huang Q, Hao Q, Begley TP, Karplus PA et al (2006) Crystal structure of mammalian cysteine dioxygenase. J Biol Chem 281(27):18723–18733
Stark AH, Timar B, Madar Z (2000) Adaptation of Sprague Dawley rats to long-term feeding of high fat or high fructose diets. Eur J Nutr 39(5):229–234
Stipanuk MH, Dominy JE (2006) Surprising insights that aren’t so surprising in the modeling of sulfur amino acid metabolism. Amino Acids 30(3):251–256
Stipanuk MH, Dominy JE, Lee J-I, Coloso RM (2006) Mammalian cysteine metabolism: new insights into regulation of cysteine metabolism. J Nutr 136(6 Suppl):1652S–1659S
Tappy L, Lê KA, Tran C, Paquot N (2010) Fructose and metabolic diseases: new findings, new questions. Nutrition 26(11–12):1044–1049
Tran LT, Yuen VG, McNeill JH (2009) The fructose-fed rat: a review on the mechanisms of fructose-induced insulin resistance and hypertension. Mol Cell Biochem 332(1–2):145–159
Tsuboyama-Kasaoka N, Shozawa C, Sano K, Kamei Y, Kasaoka S, Hosokawa Y et al (2006) Taurine (2-aminoethanesulfonic acid) deficiency creates a vicious circle promoting obesity. Endocrinology 147(7):3276–3284
Ueki I, Roman HB, Hirschberger LL, Junior C, Stipanuk MH (2012) Extrahepatic tissues compensate for loss of hepatic taurine synthesis in mice with liver-specific knockout of cysteine dioxygenase. Am J Physiol Endocrinol Metab 302(10):E1292–E1299
Warskulat U, Flögel U, Jacoby C, Hartwig H-G, Thewissen M, Merx MW et al (2004) Taurine transporter knockout depletes muscle taurine levels and results in severe skeletal muscle impairment but leaves cardiac function uncompromised. FASEB J Off Publ Fed Am Soc Exp Biol 18(3):577–579
Wieland O (1984) Methods of enzymatic analysis vol. VI. Verlag Chemie, Weinheim, Dearfield Beach, Florida, Basel. pp. 504–510
Wijekoon EP, Skinner C, Brosnan ME, Brosnan JT (2004) Amino acid metabolism in the Zucker diabetic fatty rat: effects of insulin resistance and of type 2 diabetes. Can J Physiol Pharmacol 82(7):506–514
Yamamoto N, Tanaka T, Noguchi T (1995) Effect of cysteine on expression of cystathionine beta-synthase in the rat liver. J Nutr Sci Vitaminol (Tokyo) 41(2):197–205
Acknowledgements
This research was supported by The Danish Strategic Research Council grant #09-067124 and #09-059921, Danish Medical Research Council grant #271-07-0732, by Købmand i Odense Johann og Hanne Weimann f. Seedorffs Legat, Gangstedfonden, Ernst Fischers mindelegat, Eva og Hans Carl Adolfs Mindelegat, and Direktør Emil Hertz og Hustru Inger Hertz Fond.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer International Publishing Switzerland
About this paper
Cite this paper
Larsen, L.H., Ørstrup, L.K.H., Hansen, S.H., Grunnet, N., Quistorff, B., Mortensen, O.H. (2015). Fructose Feeding Changes Taurine Homeostasis in Wistar Rats. In: Marcinkiewicz, J., Schaffer, S. (eds) Taurine 9. Advances in Experimental Medicine and Biology, vol 803. Springer, Cham. https://doi.org/10.1007/978-3-319-15126-7_55
Download citation
DOI: https://doi.org/10.1007/978-3-319-15126-7_55
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-15125-0
Online ISBN: 978-3-319-15126-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)