Abstract
The sulfur-containing amino acid, taurine (Tau), regulates glucose and lipid homeostasis under normal, pre- and diabetic conditions. Here, we aimed to verify whether Tau supplementation exerts its beneficial effects against obesity, hyperglycemia and alterations in islet functions, in leptin-deficient obese (ob/ob), over a long period of treatment. From weaning until 12 months of age, female ob/ob mice received, or not, 5% Tau in drinking water (obTau group). After this period, a reduction in hypertriglyceridemia and an improvement in glucose tolerance and insulin sensitivity were observed in obTau mice. In addition, the daily metabolic flexibility was restored in obTau mice. In the gastrocnemius muscle of obTau mice, the activation of AMP-activated protein kinase (AMPK) was increased, while total AMPK protein content was reduced. Finally, isolated islets from obTau mice expressed high amounts of pyruvate carboxylase (PC) protein and lower glucose-induced insulin secretion. Taking these evidences together Tau supplementation had long-term positive actions on glucose tolerance and insulin sensitivity, associated with a reduction in glucose-stimulated insulin secretion, in ob/ob mice. The improvement in insulin actions in obTau mice was due, at least in part, to increased activation of AMPK in skeletal muscle, while the increased content of the PC enzyme in pancreatic islets may help to preserve glucose responsiveness in obTau islets, possibly contributing to islet cell survive.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Type 2 diabetes mellitus (T2D) is characterized by insulin resistance and pancreatic β-cell failure (Wang et al. 2017), and the incidence of this disease is increasing worldwide (Lopez-Otin et al. 2013; Palmer et al. 2015). In addition, obesity, insulin resistance and diabetes are major risk factors for the early onset of age-related complications, such as renal dysfunction, cardiovascular disease, stroke, impaired wound healing, infection, depression, and cognitive decline (Lopez-Otin et al. 2013). The hyperphagic leptin-deficient obese (ob/ob) mice display severe obesity, insulin resistance, and massive pancreatic β-cells hyperplasia and hypertrophy (Muzzin et al. 1996; Santos-Silva et al. 2015; Tomita et al. 1992). Moreover, the daily oscillation in energy expenditure is drastically disrupted in ob/ob mice (Hwa et al. 1996, 1997), due to misalignments in several circadian endogenous factors and behaviors (Ando et al. 2011; Laposky et al. 2006; Strohmayer and Smith 1987).
Taurine (Tau; 2-aminoethanesulfonic acid) is found at high levels in mammalian tissues (Huxtable 1992) and this amino acid is reported to decrease adiposity in diet-induced obesity (Batista et al. 2013; Tsuboyama-Kasaoka et al. 2006). Tau also improves insulin secretion and/or sensitivity, and increases glucose and lipid metabolism (Batista et al. 2013; Bonfleur et al. 2015; Nandhini et al. 2005; Ribeiro et al. 2012; Takahashi et al. 2016). Furthermore, Tau supplementation prevents insulin and glucagon hypersecretion, and decreases islet hypertrophy in 3-month-old ob/ob mice (Santos-Silva et al. 2015). However, data concerning the prolonged benefits of Tau treatment for obesity-linked comorbidities are scarce. Tau treatment for 15 months reduces glycemia and mortality in streptozotocin-induced diabetic rats (Di Leo et al. 2004) and Tau supplementation prevented hypercholesterolemia and atherosclerosis in mice fed on a HFD for 6 months (Murakami et al. 2000). However, the mechanisms of action by which Tau regulates body glucose and lipid metabolism in the long term are still unknown. Here, ob/ob mice were supplemented with Tau for 11 months to investigate the effects and cellular mechanisms that long-term Tau treatment has upon lipid and glucose homeostasis, and pancreatic islet function in these genetic and severe obese rodents.
Materials and methods
Experimental groups
All experimental procedures were developed in accordance with the ethics committee in animal experimentation, UNICAMP (certificate no 2018-1), and the methods were conducted in accordance with the approved guidelines. From weaning to 12 months of age, female leptin-deficient obese (ob/ob) mice were supplemented or not with 5% Tau (Ribeiro et al. 2012) in their drinking water (obTau). During the entire experimental period, mice groups were maintained on a 12-h light–dark cycle (lights 6:00–18:00 h) with controlled humidity and temperature (21 ± 2 °C), and allowed free access to standard laboratory chow diet (Nutrilab, Colombo, PR, BRA) and water.
Indirect calorimetry
At 12 months of age, obTau and ob/ob mice were individually placed in the registration chambers of the calorimetry system and allowed to adapt for 24 h. After adaptation, the volume of carbon dioxide production (VCO2) and the volume of oxygen consumption (VO2) were registered during 24 h using the Oxylet calorimeter system (Pan Lab/Harvard Instruments, Barcelona, Spain). The analysis of the respiratory exchange ratio (RER) was performed using the Metabolism software (Pan Lab/Harvard Instruments, Barcelona, Spain) (Alberts et al. 2006).
Intraperitoneal glucose (ipGTT) and insulin (ipITT) tolerance tests
At the end of experimental period, after overnight fasting, glycemia was measured in obTau and ob/ob mice using a glucose analyzer (Accu-Chek Performa, Roche Diagnostic®, Switzerland), from blood samples collected at the tip of the tail. Afterwards, all the mice received an i.p. injection of glucose (2 g kg−1 body weight). Blood glucose was measured again at 15, 30, 60 and 120 min after glucose administration (Santos-Silva et al. 2015). Two days after the ipGTT, glycemia was measured in 2 h-fasted obTau and ob/ob mice before the administration of an i.p. injection of 10 UI/kg body weight of human insulin (Humulin® R, Lilly’s, São Paulo, SP, BRA) (Santos-Silva et al. 2015). Glycemia was also measured at 3, 6, 9, 12, 15, 18 and 21 min after insulin administration. The values of glycemia were converted to natural logarithmic values (Sugden and Holness) to assess it the decay rate constant (KITT). Using linear regression, the slope was calculated (time × Lnglycemia) and the glycemia decay rate constant (%/min) was obtained multiplying the result by 100.
Obesity evaluation and biochemical nutritional parameters
At 12 months of age, body weights were measured in both mice groups. Subsequently, mice were euthanized by decapitation and the retroperitoneal and perigonadal fat pads and the interscapular brown adipose tissue were collected and weighed. Blood samples were collected and the plasma was used for insulin measurement by radioimmunoassay (Zachariah Tom et al.) (Ribeiro et al. 2010). Plasma glucose concentrations were measured using a glucose analyzer (Accu-Chek Perfoma, Roche Diagnostic, Switzerland). Total cholesterol (CHOL) and triglycerides (TG) were measured using colorimetric standard commercial kits, according to the manufacturer’s instructions (Roche/Hitachi®; Indianopolis, USA, and Wako®; Richmond, USA, respectively).
Static insulin secretion
After euthanasia of 12-month-old obTau and ob/ob mice, a laparotomy was performed and the pancreases were perfused with Hank’s buffer, pH 7.4, containing collagenase type V (Sigma Chemical, St Louis, MO, USA). The pancreases were dissected and incubated at 37 °C for digestion of the exocrine pancreatic tissue. Groups of four isolated islets were incubated for 30 min at 37 °C in Krebs–Ringer bicarbonate (KRB) buffer supplemented with 5.6 mM glucose plus 0.3% BSA (Sigma Chemical, St Louis, MO, USA), pH 7.4, and continuously gassed with 95% O2/5% CO2. This medium was then replaced with fresh KRB buffer containing 2.8, 11.1 and 22.2 mM glucose and the islets were incubated for a further 1 h. After this period, the insulin content of the medium was measured using human insulin radiolabeled with 125I (Perkin Elmer, Waltham, USA) by RIA (Ribeiro et al. 2010). For islet insulin content, groups of four islets were collected and transferred to tubes containing 1 mL of deionized water, and the islet cells were homogenized using an ultrasonic homogenizer (Brinkmann Instruments, Westbury, NY, USA).
Cytoplasmic Ca2+ oscillation measurement by fluorescence
For intracellular Ca2+ concentration ([Ca2+i]) recordings, isolated islets were incubated in KRB medium containing 5.6 mM glucose at 37o C for 2 h. During the last hour of incubation, islets were loaded with 5 µM of the Ca2+-sensitive dye Fura-2 acetoxymethyl ester (AM). Afterwards, single islets were placed inside a thermostatically regulated chamber (37o C) over poly-l-lysine-treated glass coverslips and perifused with a BSA-free KRB buffer containing 2.8 or 11.1 mM glucose. Fura-2AM loaded islets were imaged using an inverted epifluorescence microscope (Nikon Eclipse TE200, Tokyo, Japan). A ratio image was acquired every 3 s with a Cool One camera (Photon Technology International, NJ, USA) using a dual filter wheel equipped with 340, 380 and 10 nm bandpass filters, and a range of neutral density filters (Photon Technology International, NJ, USA). Data were acquired using the Image Master version 5.0 software (Photon Technology International, NJ, USA) (Santos-Silva et al. 2015).
Western blotting
For protein amount evaluation, isolated islets, or fragments of the liver or gastrocnemius muscle were solubilized in antiprotease and antiphophatase buffer containing: 100 mM Tris pH 7.5, 10 mM sodium pyrophosphate, 100 mM sodium fluoride, 10 mM EDTA, 10 mM sodium vanadate, 2 mMPMSF and 1% Triton-X 100. The extracts were then centrifuged at 15.294g at 4 °C for 40 min to remove insoluble material. The protein concentration in the supernatants was assayed using the Bradford dye method (Bradford 1976), using BSA as a standard curve and a commercial Bradford reagent (Bio-Agency Lab., São Paulo, SP, BRA). The samples were homogenized with a loading buffer containing β-mercaptoethanol. After heating at 100 °C for 5 min, the proteins were separated by electrophoresis (30 μg protein/lane in 10% gels) and then transferred to nitrocellulose membranes. Afterwards, nitrocellulose membranes were staining with Ponceau S solution (Sigma-Aldrich, St. Louis, MA, USA). The membranes were incubated with specific primary antibodies against pyruvate kinase (PC, 1:1000; cat.sc 271862), or pyruvate dehydrogenase (PDH, 1:1000; cat sc 377092), phospho (p)-AMPKα Thr172 (1:1000, cat. #2535), or AMPK (1:1000, cat. #2532), or acetyl-CoA carboxylase (ACC, 1:1000; cat. #3662), or p-acetyl-CoA carboxylase Ser79 (pACC, 1:1000; cat. #3661), or carnitine palmitoyl transferase-1a (CPT-1; cat. sc-20669, Santa Cruz Biotechnology Inc., CA, USA). With the exception of the CPT-1 antibody, all primary antibodies were purchased from Cell Signaling Technology, Boston, MA, USA. Anti-glyceraldehyde 3-phosphate dehydrogenase antibody was used as an internal control (GAPDH, 1:1.000, cat.sc25778). Detection of the proteins was performed after a 2 h incubation of the nitrocellulose membranes with horseradish peroxidase-conjugated secondary antibody (1:10000, Invitrogen, São Paulo, SP, BRA) and chemiluminescent reagents, followed by exposure to an ImageQuant LAS 4000 Mini system (GE® Healthcare Bio-Sciences, Uppsala, Sweden). The chemiluminescence band intensities or Ponceau staining of each lane were quantified with the ImageQuant TL 7.0 Software (GE® Healthcare Bio-Sciences, Uppsala, Sweden). To verify if GAPDH protein content differ between groups, the GAPDH protein amount was divided by the respective Ponceau staining in the lane for all samples. The densitometry of each target protein in this study was divided by the respective GAPDH protein. For phosphorylated proteins, the densitometry of bands was divided by the densitometry of the corresponding total protein and GAPDH, and subsequently multiplied by 100 (Ribeiro et al. 2012).
Statistical analysis
Results are presented as mean ± SEM for the number of independent experiments (n) indicated in the table and figure legends. The area under the curve (AUC) was calculated by trapezoidal integration using GraphPad Prism® version 5.00 for Windows (San Diego, CA, USA). Data were prior analyzed using Shapiro–Wilk normality test and subsequently submitted to parametric (unpaired Student’s t test) or non-parametric (Mann–Whitney U test) unpaired tests also using GraphPad Prism® version 5.00 software. The level of significance was set at P < 0.05.
Results
Obesity evaluation and fuel oxidation
Body weight, perigonadal and retroperitoneal fat depots, and interscapular brown adipose tissue accumulation were similar in obTau and ob/ob mice, after 12 months (Table 1). The obTau mice presented a significant increase in RER at night, compared to during the daytime (P < 0.04; Fig. 1a, b). In contrast, RER in ob/ob mice was similar during both night and day periods (Fig. 1a, b).
Tau supplementation increases RER during the light phase in 12-month-old ob/ob mice. a Changes in the respiratory exchange ratio (RER) during 24-h registered in female ob/ob (n = 5) and obTau (n = 6) mice. b Mean ± SEM of RER during the day and night (gray areas) periods. *RER in the night period is different from the day period in obTau mice (Mann–Whitney U test, P < 0.05)
Glucose tolerance, insulin sensitivity and plasma lipids profile
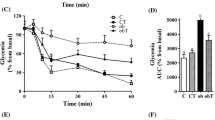
During fasting, obTau and ob/ob mice showed similar glycemia (Fig. 2c). Despite demonstrating a tendency, insulinemia did not differ between obTau and ob/ob mice (Fig. 2d). However, after a glucose challenge, obTau mice displayed better glucose tolerance during the ipGTT, with lower glycemia at 15, 30 and 60 min during the test (P < 0.02, P < 0.01 and P < 0.03, respectively; Fig. 2a). Total glycemia during the ipGTT, expressed as the area under the curve (Fig. 2b), was 66% lower in obTau than registered for ob/ob mice (P < 0.005). In addition, obTau mice presented an enhanced decrease in glycemia after insulin administration, compared to ob/ob mice (Fig. 2e). Tau supplementation also reduced plasma TG concentrations in obTau mice (92 ± 5 mg/dL), compared with ob/ob mice (123 ± 11 mg/dL; P < 0.02). No differences in plasma total CHOL levels were observed between the groups (obTau 160 ± 7 and ob/ob 143 ± 9 mg/dL).
Tau supplementation ameliorates glucose tolerance and insulin sensitivity in 12-month-old ob/ob mice. (a) Changes in glycemia during the ipGTT in female ob/ob (n = 6) and obTau (n = 6) mice. Mean ± SEM of total glycemia during the ipGTT (b), fasting glycemia (c) and insulinemia (d), and insulin sensitivity expressed by the KITT (e). *obTau is different from ob/ob mice (Mann–Whitney U test, P < 0.05)
Insulin secretion and cytoplasmic Ca2+ handling in isolated islets
Isolated pancreatic islets from ob/ob Tau-supplemented mice secreted less insulin in response to 11.1 and 22.2 mM glucose, compared with ob/ob islets (P < 0.01; Fig. 3a). No differences in insulin release, at 2.8 mM glucose, were observed between groups (Fig. 3a). In addition, the total islet insulin content was lower in obTau, compared with the ob/ob group (P < 0.05; Fig. 3b). Tau supplementation increased the expression of pyruvate carboxylase (PC) protein in obTau islets (Fig. 3d), compared to ob/ob group (P < 0.04), without modifying the pyruvate dehydrogenase (PDH) protein content (Fig. 3e).
Tau decreases insulin secretion and islet insulin storage, and enhances PC protein content. Mean ± SEM of static insulin secretion in response to increasing glucose concentrations (a) and total islet insulin content (b) in isolated islets from 12-month-old female ob/ob (n = 16–20) and obTau (n = 15–21) mice. Groups of four islets of similar sizes were incubated for 1 h in the presence of different glucose concentrations, as indicated in the figure. *obTau is different from ob/ob group at the same glucose concentration evaluated (Mann–Whitney U test, P < 0.05). GAPDH protein divided by the respective Ponceau staining in the lane (c), as GAPDH/Ponceau protein content did not differ among obTau and ob/ob mice, GAPDH protein was used as internal control. Mean ± SEM of PC (d) and PDH (e) protein content in isolated islets from female ob/ob (n = 6) and obTau (n = 6) mice. *obTau is different from ob/ob mice (Mann–Whitney U test, P < 0.05)
The cytoplasmic Ca2+ concentration increase, in response to 11.1 mM glucose, in isolated islets from ob/ob and obTau mice is shown in Fig. 4a, b, respectively. No alterations in the amplitude (Fig. 4c), number of Ca2+ oscillations (Fig. 4d), or total Ca2+ cytoplasmic concentrations (Fig. 4e) were observed between obTau and ob/ob islets, exposed to 11.1 mM glucose.
Representative curves of 11.1 mM glucose-induced intracellular Ca2+ oscillations in islets from 12-month-old female ob/ob (a, n = 7) and obTau (b, n = 8) mice. Mean ± SEM of amplitude (c), number of [Ca2+]i oscillations (d) and AUC of [Ca2+]i (e) in response to 11.1 mM glucose. The experiments were performed in a perifusion system with KRB containing 2.8 or 11.1 mM glucose (G2.8 and G11.1, respectively). Data were analyzed by unpaired Student t test
AMPK phosphorylation in the gastrocnemius muscle and liver and hepatic protein content of enzymes involved in lipid metabolism
In obTau mice, the pAMPK/AMPK protein content in the gastrocnemius muscle was higher, whereas the total AMPK protein was reduced compared to ob/ob mice (P < 0.02 and P < 0.01, respectively; Fig. 5b, c). However, Tau supplementation did not alter the hepatic protein content of AMPK, pAMPK/AMPK (Fig. 5e, f). Furthermore, no differences in the hepatic protein content of pACC/ACC, ACC (involved in de novo lipogenesis), and CPT-1 (key protein in the fatty acid transport to the mitochondria for β-oxidation) were observed between obTau and ob/ob (Fig. 5g, i, respectively).
Tau supplementation increases AMPK activation only in the gastrocnemius muscle of 12-month-old ob/ob mice. GAPDH protein divided by the respective Ponceau staining in the lane in muscle and liver (a and d respectively), as GAPDH/Ponceau protein content in the liver and muscle did not differ among obTau and ob/ob mice, GAPDH protein was used as internal control. Mean ± SEM of pAMPK/AMPK and AMPK in gastrocnemius muscle (b and c, respectively) and in the liver (e and f, respectively); and pACC/ACC (g), ACC (h) and CPT1 (i) in the liver of 12-month-old female ob/ob (n = 4–7) and obTau (n = 4–7) mice. *obTau is different from ob/ob (Mann–Whitney U test, P < 0.05)
Discussion
Severe obesity predisposes to insulin resistance, which disrupts whole body metabolism and stimulates β-cells to secrete increased amounts of insulin to compensate hyperglycemia (Palmer et al. 2015; Wang et al. 2017). However, this hyperfunction may culminate in β-cell failure and the establishment of T2D (Wang et al. 2017). This hyperfunction aggravates in aging, since this condition itself is known to lead to a progressive decrease in endocrine pancreatic function and insulin action (Gong and Muzumdar 2012; Lopez-Otin et al. 2013). Therapeutic agents that prevent or treat obesity and insulin resistance need to show lower adverse effects but prolonged actions, making them an adequate strategy against the loss of cellular integrity that occurs with aging and contributes to the manifestation of metabolic comorbidities. Here, we show that treatment with Tau for 12 months improves glucose tolerance, insulin sensitivity and daily oxidative metabolism, without alterations in adiposity, in ob/ob mice. No modifications in body weight and adiposity in obTau are in accordance with our previous report (Santos-Silva et al. 2015). Notably, while at 2 months of Tau supplementation a decreased adiposity in high-fat diet C57Bl/6 mice and in hypothalamic obese rats were reported (Batista et al. 2013; Bonfleur et al. 2015), at 12 months of Tau treatment, no preventive action of the amino acid on obesity was observed (Branco et al. 2015), indicating that the Tau effect against fat deposition may differ from species and prolongation of the supplementation period.
The improvement in glucose tolerance in obTau mice is associated with an amelioration of insulin sensitivity, and a reduction in insulin secretory response. The amelioration of insulin action and glucose tolerance is probably due to increase in the expression of pAMPK/AMPK protein in the skeletal muscle of these mice, since AMPK activation in muscle fibers increases glucose uptake (Bergeron et al. 1999). AMPK inhibits AS160, a GTPase-activating protein, via an insulin-independent mechanism, which in turn increases the activity of Rab, culminating in glucose transporter (GLUT)-4 vesicle movement and fusion with the plasma membrane (Treebak et al. 2006). The acute administration of AICAR (an AMPK activator) has been shown to promptly decrease blood glucose levels in ob/ob mice (Halseth et al. 2002). In addition, an increased expression of GLUT-4 protein has been reported in the gastrocnemius muscle of ob/ob mice treated with AICAR for 8 days (Halseth et al. 2002). Enhanced GLUT-4 skeletal muscle content was also demonstrated in l-arginine:glycine amidinotransferase (Murakami et al.) deficient ob/ob mice which, due to a deletion of AGAT, displayed intracellular energy depletion, but enhancements in AMPK activation only in skeletal muscle (Stockebrand et al. 2013).
In addition to the actions of AMPK on glucose uptake and metabolism in the muscle, this kinase also increases fatty acid utilization, preventing TG deposition in tissue (Stockebrand et al. 2013; Zachariah Tom et al. 2014). In ob/ob mice, a muscle-specific mutation in the γ3 subunit of AMPK enhances its action, inhibiting acetyl-CoA carboxylase, which decreases TG accumulation but enhances palmitate oxidation in the gastrocnemius muscle (Zachariah Tom et al. 2014). This effect of AMPK in the muscle may contribute to the decrease in TG plasma levels in obTau mice, observed herein, since no modifications in hepatic AMPK, or in its target protein, ACC, protein content and activation were observed following Tau supplementation.
The inability of ob/ob mice to shift fuel oxidation, according to physiological and nutritional conditions, is restored by Tau, as shown by the increased RER during the night period, which indicates an enhancement in the carbohydrate instead of fat oxidation. In mammals, body metabolism and energy expenditure display daily oscillatory rhythms, which are modulated by several factors, including hormones such as leptin (Alberts et al. 2006; Szewczyk-Golec et al. 2015). Thus, the absence of leptin in ob/ob mice alters daily regulation of the metabolism. Consequently, the treatment of ob/ob mice with leptin decreases insulinemia and enhances glucose and lipid metabolism through the regulation of components that control the circadian clock in peripheral tissues (Ando et al. 2011). In line with this notion, we hypothesize that the lack of leptin, together with the severe insulin resistance in 12-month-old ob/ob mice, disrupts the daily pattern of both the transport of carbohydrates and their utilization by these rodent tissues. Moreover, it has been reported that Tau supplementation attenuates the disruption of molecular clock in pancreatic β-cells induced by high-fat diet. In these rodents, Tau decreases insulin hypersecretion and increases plasma leptin concentrations, as well as, improves the glucose tolerance and insulin peripheral actions (Figueroa et al. 2016). Thus, it is possible that Tau regulates the expression of peripheral and central genes involved in the control of circadian rhythmicity of body metabolism, ameliorating insulin sensitivity and carbohydrate utilization during the daytime period in obTau mice.
Several studies have reported that Tau regulates glucose metabolism (Batista et al. 2013; Nandhini et al. 2005; Ribeiro et al. 2012; Takahashi et al. 2016). Tau is known to increase glycolytic and tricarboxylic acid cycle enzymes in muscle and hepatocytes under normal and pre-diabetic conditions (Nandhini et al. 2005; Takahashi et al. 2016). Here, we observed that Tau enhances the protein amount of PC, but not PDH, in ob/ob islets. PC is a mitochondrial enzyme that converts the carbons of glucose-derived pyruvate into oxaloacetate (Schuit et al. 1997), while PHD is responsible for converting pyruvate into acetyl-CoA (Sugden and Holness 2011). The role of these mitochondrial enzymes, especially PC, in pancreatic β-cell viability and proliferation, has been reported. In pancreatic islets from obese and insulin resistant Zucker Fatty (ZF) rats, the expression of PC is increased, whereas the activity of PDH is reduced (Liu et al. 2002). PC activity and protein content were down-regulated in diabetic db/db mice (Han and Liu 2010). In mild and severe hyperglycemic Agouti-K (AyK) mice, PC activity is enhanced and reduced, respectively (Han and Liu, 2010). Mild hyperglycemic AyK mice display pancreatic islet morphology similar to controls, indicating that increased PC activity is linked to preservation of the endocrine pancreatic structure (Han and Liu 2010). In addition, INS-1 β-cells, incubated with phenylacetic acid, a PC inhibitor, presented decreased cellular viability and increased apoptosis (Lee et al. 2014). All these evidences indicate that reductions in PC expression and activity, in pancreatic islets, are associated with the occurrence of T2D. Thus, we assume that the enhancement in PC protein amount in obTau islets may contribute to maintain β-cell viability, preventing the failure of these cells and their death. This effect, in association with reduced islet hyperfunction, due to the improved insulin sensitivity in the tissues of obTau mice, may represent a long-term protective effect of Tau against disruptions in endocrine pancreatic function.
In summary, our study demonstrates that a prolonged period of Tau supplementation, in ob/ob mice, improves body metabolism and protects β-cells against islet hyperfunction. These effects indicate that this amino acid could represent a good therapeutic strategy against the development of T2D in severe obesity and aging.
References
Alberts P, Johansson BG, McArthur RA (2006) Characterization of energy expenditure in rodents by indirect calorimetry. Curr Protoc Neurosci Chapter 9(9):23. https://doi.org/10.1002/0471142301.ns0923ds36
Ando H, Kumazaki M, Motosugi Y, Ushijima K, Maekawa T, Ishikawa E, Fujimura A (2011) Impairment of peripheral circadian clocks precedes metabolic abnormalities in ob/ob mice. Endocrinology 152:1347–1354. https://doi.org/10.1210/en.2010-1068
Batista TM, Ribeiro RA, da Silva PM, Camargo RL, Lollo PC, Boschero AC, Carneiro EM (2013) Taurine supplementation improves liver glucose control in normal protein and malnourished mice fed a high-fat diet. Mol Nutr Food Res 57:423–434. https://doi.org/10.1002/mnfr.201200345
Bergeron R, Russell RR 3rd, Young LH, Ren JM, Marcucci M, Lee A, Shulman GI (1999) Effect of AMPK activation on muscle glucose metabolism in conscious rats. Am J Physiol 276:E938–944
Bonfleur ML, Borck PC, Ribeiro RA, Caetano LC, Soares GM, Carneiro EM, Balbo SL (2015) Improvement in the expression of hepatic genes involved in fatty acid metabolism in obese rats supplemented with taurine. Life Sci 135:15–21. https://doi.org/10.1016/j.lfs.2015.05.019
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Branco RC et al (2015) Long-term taurine supplementation leads to enhanced hepatic steatosis, renal dysfunction and hyperglycemia in mice fed on a high-fat diet. Adv Exp Med Biol 803:339–351. https://doi.org/10.1007/978-3-319-15126-7_26
Di Leo MA, Santini SA, Silveri NG, Giardina B, Franconi F, Ghirlanda G (2004) Long-term taurine supplementation reduces mortality rate in streptozotocin-induced diabetic rats. Amino Acids 27:187–191. https://doi.org/10.1007/s00726-004-0108-2
Figueroa AL, Figueiredo H, Rebuffat SA, Vieira E, Gomis R (2016) Taurine treatment modulates circadian rhythms in mice fed a high fat diet. Sci Rep 6:36801. https://doi.org/10.1038/srep36801
Gong Z, Muzumdar RH (2012) Pancreatic function, type 2 diabetes, and metabolism in aging. Int J Endocrinol 2012:320482. https://doi.org/10.1155/2012/320482
Halseth AE, Ensor NJ, White TA, Ross SA, Gulve EA (2002) Acute and chronic treatment of ob/ob and db/db mice with AICAR decreases blood glucose concentrations. Biochem Biophys Res Commun 294:798–805. https://doi.org/10.1016/S0006-291X(02)00557-0
Han J, Liu YQ (2010) Reduction of islet pyruvate carboxylase activity might be related to the development of type 2 diabetes mellitus in Agouti-K mice. J Endocrinol 204:143–152. https://doi.org/10.1677/JOE-09-0391
Huxtable RJ (1992) Physiological actions of taurine. Physiol Rev 72:101–163
Hwa JJ, Ghibaudi L, Compton D, Fawzi AB, Strader CD (1996) Intracerebroventricular injection of leptin increases thermogenesis and mobilizes fat metabolism in ob/ob mice. Horm Metab Res 28:659–663. https://doi.org/10.1055/s-2007-979873
Hwa JJ et al (1997) Leptin increases energy expenditure and selectively promotes fat metabolism in ob/ob mice. Am J Physiol 272:R1204–1209
Laposky AD, Shelton J, Bass J, Dugovic C, Perrino N, Turek FW (2006) Altered sleep regulation in leptin-deficient mice. Am J Physiol Regul Integr Comp Physiol 290:R894–903. https://doi.org/10.1152/ajpregu.00304.2005
Lee JH et al (2014) Toxicity generated through inhibition of pyruvate carboxylase and carnitine palmitoyl transferase-1 is similar to high glucose/palmitate-induced glucolipotoxicity in INS-1 beta cells. Mol Cell Endocrinol 383:48–59. https://doi.org/10.1016/j.mce.2013.12.002
Liu YQ, Jetton TL, Leahy JL (2002) beta-Cell adaptation to insulin resistance. Increased pyruvate carboxylase and malate-pyruvate shuttle activity in islets of nondiabetic Zucker fatty rats. J Biol Chem 277:39163–39168. https://doi.org/10.1074/jbc.M207157200
Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G (2013) The hallmarks of aging. Cell 153:1194–1217. https://doi.org/10.1016/j.cell.2013.05.039
Murakami S, Kondo Y, Nagate T (2000) Effects of long-term treatment with taurine in mice fed a high-fat diet: improvement in cholesterol metabolism and vascular lipid accumulation by taurine. Adv Exp Med Biol 483:177–186. https://doi.org/10.1007/0-306-46838-7_19
Muzzin P, Eisensmith RC, Copeland KC, Woo SL (1996) Correction of obesity and diabetes in genetically obese mice by leptin gene therapy. Proc Natl Acad Sci USA 93:14804–14808
Nandhini AT, Thirunavukkarasu V, Anuradha CV (2005) Taurine modifies insulin signaling enzymes in the fructose-fed insulin resistant rats. Diabetes Metab 31:337–344
Palmer AK, Tchkonia T, LeBrasseur NK, Chini EN, Xu M, Kirkland JL (2015) Cellular senescence in type 2 diabetes: a therapeutic opportunity. Diabetes 64:2289–2298. https://doi.org/10.2337/db14-1820
Ribeiro RA, Vanzela EC, Oliveira CA, Bonfleur ML, Boschero AC, Carneiro EM (2010) Taurine supplementation: involvement of cholinergic/phospholipase C and protein kinase A pathways in potentiation of insulin secretion and Ca2 + handling in mouse pancreatic islets. Br J Nutr 104:1148–1155. https://doi.org/10.1017/S0007114510001820
Ribeiro RA, Santos-Silva JC, Vettorazzi JF, Cotrim BB, Mobiolli DD, Boschero AC, Carneiro EM (2012) Taurine supplementation prevents morpho-physiological alterations in high-fat diet mice pancreatic beta-cells. Amino Acids 43:1791–1801. https://doi.org/10.1007/s00726-012-1263-5
Santos-Silva JC et al (2015) Taurine supplementation ameliorates glucose homeostasis, prevents insulin and glucagon hypersecretion, and controls beta, alpha, and delta-cell masses in genetic obese mice. Amino Acids 47:1533–1548. https://doi.org/10.1007/s00726-015-1988-z
Schuit F, De Vos A, Farfari S, Moens K, Pipeleers D, Brun T, Prentki M (1997) Metabolic fate of glucose in purified islet cells. Glucose-regulated anaplerosis in beta cells. J Biol Chem 272:18572–18579
Stockebrand M, Sauter K, Neu A, Isbrandt D, Choe CU (2013) Differential regulation of AMPK activation in leptin- and creatine-deficient mice. FASEB J Off Publ Fed Am Soc Exp Biol 27:4147–4156. https://doi.org/10.1096/fj.12-225136
Strohmayer AJ, Smith GP (1987) The meal pattern of genetically obese (ob/ob) mice. Appetite 8:111–123
Sugden MC, Holness MJ (2011) The pyruvate carboxylase-pyruvate dehydrogenase axis in islet pyruvate metabolism: going round in circles? Islets 3:302–319. https://doi.org/10.4161/isl.3.6.17806
Szewczyk-Golec K, Wozniak A, Reiter RJ (2015) Inter-relationships of the chronobiotic, melatonin, with leptin and adiponectin: implications for obesity. J Pineal Res 59:277–291. https://doi.org/10.1111/jpi.12257
Takahashi Y, Tamura Y, Matsunaga Y, Kitaoka Y, Terada S, Hatta H (2016) Effects of taurine administration on carbohydrate metabolism in skeletal muscle during the post-exercise phase. J Nutr Sci Vitaminol 62:257–264. https://doi.org/10.3177/jnsv.62.257
Tomita T, Doull V, Pollock HG, Krizsan D (1992) Pancreatic islets of obese hyperglycemic mice (ob/ob). Pancreas 7:367–375
Treebak JT et al (2006) AMPK-mediated AS160 phosphorylation in skeletal muscle is dependent on AMPK catalytic and regulatory subunits. Diabetes 55:2051–2058. https://doi.org/10.2337/db06-0175
Tsuboyama-Kasaoka N, Shozawa C, Sano K, Kamei Y, Kasaoka S, Hosokawa Y, Ezaki O (2006) Taurine (2-aminoethanesulfonic acid) deficiency creates a vicious circle promoting obesity. Endocrinology 147:3276–3284. https://doi.org/10.1210/en.2005-1007
Wang W, Liu C, Jimenez-Gonzalez M, Song WJ, Hussain MA (2017) The undoing and redoing of the diabetic beta-cell. J Diabetes Comp. https://doi.org/10.1016/j.jdiacomp.2017.01.028
Zachariah Tom R et al (2014) Effects of AMPK activation on insulin sensitivity and metabolism in leptin-deficient ob/ob mice. Diabetes 63:1560–1571. https://doi.org/10.2337/db13-0670
Acknowledgements
The authors thank Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) and Conselho Nacional para o Desenvolvimento Científico e Tecnológico (CNPq) for supporting the research. The authors also thank Marise Carnelossi Brunelli for excellent technical assistance and Nicola Conran for editing English.
Funding
This study was supported by grants from Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP 2014/01717-9; 2015/12611-0), Conselho Nacional para o Desenvolvimento Científico e Tecnológico (CNPq 449794/2014-8).
Author information
Authors and Affiliations
Contributions
PCB, RAR, ECM designed research; PCB, TMB, JFV, RCSB, JCSS, VYN conducted the experiments and acquired data; PCB, RAR analyzed data and performed statistical analysis; ACB, EMC acquired the reagents; PCB, RAR wrote the manuscript; JFV, ACB, EMC revised the manuscript; PCB, RAR, EMC had responsibility for final content.
Corresponding author
Ethics declarations
Conflict of interest
All contributing authors declare no conflicts of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Handling Editor: P. R. Jungblut.
Rights and permissions
About this article
Cite this article
Borck, P.C., Vettorazzi, J.F., Branco, R.C.S. et al. Taurine supplementation induces long-term beneficial effects on glucose homeostasis in ob/ob mice. Amino Acids 50, 765–774 (2018). https://doi.org/10.1007/s00726-018-2553-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-018-2553-3