Abstract
The widespread use of pesticides for agricultural and for nonagricultural purposes worldwide has resulted in the presence of pesticide residues in various environmental matrices. The occurrence of pesticide residues in surface waters, groundwater, and large volumes of soil is mainly due to the inadequate management of these compounds. In this context, a biobed system was developed in Sweden in response to the need for a simple and effective way to minimize environmental contamination from pesticide manipulation, particularly when filling the spraying equipment, a typical point source of contamination. Biobeds are based on the adsorption and degradation potential of organic biomixtures composed of top soil, peat, and straw that fills a deep hole in the ground and a grass layer that covers the surface. Recently, the use of biobeds has expanded to other countries in Europe and Latin America. In Chile, four biobeds similar to the European ones have been installed, making this country a pioneer in this type of decontamination system. This chapter gives a general overview of biobeds technology and the advances in research at laboratory scale related to the treatment of pesticide residues in a biobed system in Chile.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Microbial Community
- Pesticide Residue
- Ligninolytic Enzyme
- Organophosphorus Pesticide
- Atrazine Degradation
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
4.1 Introduction
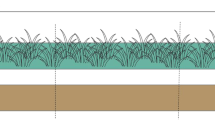
Biobeds are biological biopurification systems developed in Sweden in response to the need for a simple and effective method to minimize environmental contamination from pesticide manipulation, particularly when filling the spraying equipment, a typical point source of contamination (Torstensson and Castillo 1997; Castillo et al. 2008). Biobeds are a low-cost alternative for treating pesticide waste and washings, providing a matrix to absorb the pesticides and facilitate their biodegradation. The biologically active matrix, usually called a biomixture, is composed of straw, peat, and soil in a volumetric proportion of 2:1:1 (respectively) that fills a deep hole (60 cm) in the ground and a grass layer that covers the surface (Torstensson and Castillo 1997; Castillo et al. 2008) (Fig. 4.1).
Each component of a biobed system has a specific function. The biomixture (Fig. 4.1a) is the most important, although each component plays a role in its composition. For example, the straw stimulates the growth of ligninolytic microorganisms such as white-rot fungi and therefore the production of extracellular ligninolytic enzymes, such as peroxidases and phenoloxidases (Tortella et al. 2013a). The peat contributes to high sorption capacity and regulates the humidity of the system, and the soil provides sorption capacity in the biobed and pesticide-degrading microorganisms, including actinobacteria that can act synergistically with the fungi (Briceño et al. 2013a). The grass layer (Fig. 4.1b) that covers the biobed system increases the biobed efficiency, retaining the pesticides on top of biobeds and then controlling the leaching, helps keep the system humid, and promotes evapotranspiration and further pesticide degradation at root level. The gravel layer (Fig. 4.1c) acts as a filter to prevent organic residues from passing out of the biomixture. The waterproofing system (Fig. 4.1d) consists of the lining of the walls and bottom of the bed that prevent the contact of pesticides with adjacent soil. The recirculation system (Fig. 4.1e) consists of a concrete well connected to the biobed and its main functions are receiving the percolate pesticide residues from the biomixture and recirculating them to the biobed. The ceiling (Fig. 4.1f) prevents the entry of precipitation and finally, the support system for application equipment consists of a metallic structure or another material that supports the tractor and application equipment.
Biobed systems can be modified in several different ways according to the amount of pesticide residues to be treated, land available for installation, environmental conditions, geographic location, etc. Therefore, countries incorporating biobed technology have adapted it to local conditions, in some cases modifying the biomixture, construction, and the names.
4.2 Biobeds in the World
The first biobed was built in Sweden in 1993. Today, in Sweden and other parts of the world large numbers of biobeds are functioning on farms and have proven to be efficient at reducing pesticide water-body contamination (Castillo et al. 2008; Vischetti et al. 2008; De Wilde et al. 2010a). Biobeds as an on-farm biopurification systems or “BPS” as described in the literature (Karanasios et al. 2012; Verhagen et al. 2013) have attracted attention in several countries, where work is being conducted to adapt them to local conditions and applications (Antonious 2012).
The biobed system has been evaluated in several countries including the UK, Italy, Belgium, France, Greece, and the USA, where their implementation has led to modifications to the original biobed (Castillo et al. 2008; Antonious 2012; Marinozzi et al. 2013). For example, the depth was modified in the UK to increase the retention time of the pesticide in the bed. In Italy, this technology is known as a biomass bed and utilizes biomixtures as filters through which pesticide-contaminated water is circulated and decontaminated. Because peat is not readily found in Italy, organic materials, such as urban and garden compost, peach stones, and citrus peel, are being tested as replacements (Castillo et al. 2008).
It is estimated that there were about 2,800 bioremediation systems such as biobeds in the world and this technology is becoming more. For example, in the UK the number of biobeds grew from 75 in 2007 to 150 in 2010 (Husby 2010). The use of biobeds has expanded to Latin America, in countries such as Peru, Guatemala, and Ecuador, where some pilot/field-scale studies are being developed. Recently, this system has also been developed in Chile, although with significant modifications, and biobeds are currently being built and used at field scale (Diez et al. 2013a; Tortella et al. 2013a).
4.2.1 Evidence of Pesticide Removal in Biobeds
Several studies have demonstrated that biobeds can effectively retain and degrade a wide range of pesticides, either alone or in mixtures (Torstensson and Castillo 1997; Castillo et al. 2007; Fogg et al. 2003, 2004; Vischetti et al. 2004; Castillo and Tortensson 2007; Vischetti et al. 2008). For example, studies with mecoprop and isoproturon have shown that these pesticides can be degraded in biobeds (Henriksen et al. 2003). Niels et al. (2006) evaluated the degradation and leaching of 21 pesticides. They determined that no traces of 10 out of 21 applied pesticides were detected in the percolate. Fogg et al. (2003) evaluated the ability of biobeds to degrade pesticide mixtures (isoproturon and chlorothalonil) and the concentration effect. They found that, with the exception of isoproturon at concentrations greater than 11 mg/kg, degradation was more rapid in the biomix than in topsoil. The degradation of either isoproturon or chlorothalonil was unaffected by the presence of the other pesticides.
Many factors affecting the performance of biobeds as well as adaptation or modification of the original Swedish biobed have been studied by different authors (Castillo et al. 2008; Karanasios et al. 2012). In terms of the biomix composition, laboratory-based studies showed that mixtures of soil-organic waste may be able to degrade high concentrations and complex mixtures of pesticides (Fogg et al. 2004). Castillo and Tortensson (2007) observed that a straw, peat, and soil ratio of 50:25:25 % v/v (respectively) is recommended for the organic mixture composition, because such a mixture favors a low pH, convenient for lignin-degrading fungi and phenoloxidase production and activity. Karanasios et al. (2010) focused their research on identifying various by‐products of the local agricultural practice (either raw or composted), which could be used as alternatives to peat or even straw. They provide the first evidence that straw can be substituted in biomixtures by other lignocellulosic materials readily available in southern Europe.
Vischetti et al. (2004) compared the behavior of chlorpyrifos in two biobed systems: a Swedish biobed and a modified Italian biobed system. They reported that chlorpyrifos half-lives were similar in both biomixtures assessed, but the microbial biomass content was reduced by 25 % and 50 % with 10 mg/kg and 50 mg/kg of chlorpyrifos in the Italian biomix, respectively. Coppola et al. (2007) and Vischetti et al. (2007) studied the biodegradation of chlorpyrifos in a biobed system adapted to Italian conditions. They found that the Italian biomix showed several differences compared to the Swedish biomix in the chlorpyrifos degradation. Vischetti et al. (2008) studied the effect of initial concentration, co-application and repeated applications on chlorpyrifos, and metalaxyl degradation in a biobed. They concluded that both pesticides were degraded relatively quickly due to the presence of the varied microbial community capable of degrading both pesticides.
Spliid and Husby (2010) presented a new closed biobed with recirculation and evaporation for use in colder climates like Denmark. They reported that biobeds used under these climate conditions require special precautions to avoid problems with surplus water. The water is collected from the bottom and recirculated to the biobed. In Belgium, De Wilde et al. (2010a, b, c) studied sorption and degradation processes on an increasing spatial scale (micro- and macroscale). Their main conclusions were the following: sorption coefficients determined in batch sorption experiments are often not suitable for describing solute transport at the column or field scale; matrix composition had no significant influence on pesticide leaching and degradation; however, the addition of cow manure enhanced the degradation of some pesticides; the use of pesticide-primed material significantly enhanced degradation of metalaxyl; an increasing flux had a pernicious influence on sorption and degradation of most of the pesticides studied.
Otherwise, considering that little is known regarding the interactions between biomixture microflora and pesticides, Marinozzi et al. (2013) recently studied the dissipation of the fungicides azoxystrobin, fludioxonil, and penconazole, commonly used in vineyards, in a biomixture composed of pruning residues and straw used in vineyard biopurification systems. The study also examined the impact of fungicides on the microbial community, and the main results showed that fungicides affected the microbial community differently, with penconazole being the compound with the highest adverse effect on both the size and the activity of the biomixture microflora. By contrast, there was a significant change in the structure of the microbial community for penconazole and fludioxonil. High biodegradation and high mineralization capacity are desirable in biobeds. Therefore, attempts have been made to boost their biodegradation capacity via inoculation with pesticide-degrading microorganisms (Karanasios et al. 2012). In this context, Verhagen et al. (2013) demonstrated that bioaugmentation with a mixed degrading enrichment culture can vastly improve the functionality of an on-farm biopurification system. The authors went on to suggest that the use of both plastic carriers and biomix substrata can harbor a stable microbial community that can effectively degrade chlorpropham and 3-chloroaniline.
4.3 Biobeds in Chile
The growth in fruit sector exports has increased concern regarding the suitable and safe use of pesticides. At the same time, there is little knowledge or education regarding the effects and use of pesticides in urban or rural populations. Therefore, research and education programs are needed to encourage actions and effective policies that regulate pesticide use.
The point source pollution by pesticides, a result of accidental spillages or inadequate residue management, has been rigorously investigated in the last decade in Europe, finding that it plays a key role in soil and water pollution (Gregoire et al. 2009). In Chile, point source pollution by pesticides has not been evaluated; nevertheless, it is expected that the situation could be similar to Europe, particularly in basins with greater pressure from pesticide use, as in fruit production. Therefore, suitable and efficient management of pesticide residue is a topic that requires further discussion.
As a way to reduce pesticide contamination, the Fund of Scientific and Technological Development (FONDEF) financed the project D09R1006 entitled, “Proper Handling of Pesticide Residues in Fruit Production through the Implementation and Diffusion of Biobeds.” Through this project has developed biobed technology, which Chile is pioneering in Latin America with four units at field scale in the La Araucanía region (38° 44′ 24″ S, 72° 35′ 25″ W) (Diez et al. 2013a). These systems were installed in the INIA-Carillanca Experimental Station, the Maquehue Experimental Station at the Universidad de La Frontera, on Santa Olga farms owned by Agrícola San Clemente, and San José Farms, with these last two being fruit production companies (Fig. 4.2). It should be mentioned that the biobeds were built like Sweden biobeds with modifications, but the biomixture of straw, peat, and soil in the proportion 2:1:1 (respectively) was maintained. For further details see www.lechosbiologicos.cl.
The biobeds installed in at the INIA-Carillanca experimental station were monitored continuously for such parameters as temperature, humidity content, pH modifications, and enzymatic activity in the biomixture. Pesticide degradation was also studied. The evaluated compounds were atrazine, azinphos-methyl, captan, chlorothalonil, chlorpyrifos, diazinon, isoproturon, and methidathion, which were applied at a concentration of 32 mg active ingredient (a.i.)/kg. After 120 days of pesticide application, pesticide residue analysis showed that about 97 % of the captan, chlorothalonil, chlorpyrifos, diazinon, isoproturon, and methidathion were removed, whereas about 89 % of the atrazine and azinphos-methyl were removed (Diez et al. 2013a).
The biomixture is a principal element controlling the degradation efficacy of the biobed (Karanasios et al. 2010). Therefore, during the recent implementation of biobeds in Chile, several studies at laboratory scale were performed to optimize the functionality of this pesticide biopurification system. In this way, the effect of operating conditions, biomixture composition, and stabilization time of the biomixture on pesticide removal was evaluated (Fernández-Alberti et al. 2012; Urrutia et al. 2013). An additional innovation was to use other lignocellulosic residues such as sawdust, barley husks, and oat husk in place of straw and biochar in place of peat (Diez et al. 2013b, c). On the other hand, bearing in mind that the biological decomposition of pesticides is the most important and effective way to remove these compounds from the environment (Dabrowska et al. 2004), biomixture-pesticide-microorganism interactions have been evaluated (Tortella et al. 2013b).
4.3.1 Pesticide Degradation in Biobeds: Studies at Laboratory Scale
The following topic describes the main results obtained from the study at laboratory scale performed by Chilean researchers about pesticide removal in biobed system (Table 4.1). In general, the laboratory studies were performed using 30 cm width × 20 cm height × 50 cm length biobeds which were incubated at room temperature and dark condition. Figure 4.3 shows the biobed model used to evaluate pesticide removal.
Effect of preincubation and water-holding capacity. Apart from the composition of the biomixture, an important factor for biobed efficacy is the age or maturity of the biomixture prior to its use in pesticide degradation. The progressive biodegradation of the biomixture component generates a series of microbial communities and enzymatic activities that enables the efficient dissipation of pesticides in the biobed system and avoids metabolite accumulation (Castillo et al. 2008). The moisture level in the biobed is also a relevant parameter to promote different microbial environments that can influence the oxygen level, the microbial activity, and the amount of pesticide in the solution (Fernández-Alberti et al. 2012). In this light, Fernández-Alberti et al. (2012) evaluated the degradation and adsorption of chlorpyrifos (160 mg a.i./kg) and formation 3,5,6-trichloro-2-pyrinidol (TCP) in a biomixture prepared with an Andisol, peat, and straw in a volumetric proportion of 1:1:2 at different preincubation times (0, 15, and 30 days) and with different moisture contents (40, 60, and 80 % of water-holding capacity). Moreover, ligninolytic enzyme activity and microbial respiration in the biomixture were periodically analyzed. The main results of this study showed that the biomix had a greater capacity to retain chlorpyrifos than topsoil. Moreover, the preincubation period, water-holding capacity, and concentration of the chlorpyrifos in the biomix influenced degradation of the contaminant and TCP formation as well as the biological activities in the biomix. Finally, the author concluded that a biomixture with an Andisol, peat, and straw (1:1:2), preincubated for 15 days and incubated with 60 % of water-holding capacity, is capable of degrading chlorpyrifos efficiently. In another study, Tortella et al. (2012) evaluated the effect of using a typical composition of Swedish biomixture at different maturity stages on the degradation of chlorpyrifos. The study was conducted using a biomixture at three maturity stages: 0, 15, and 30 days, where chlorpyrifos was added to the biobeds at a final concentration of 200, 320, and 480 mg/kg. Chlorpyrifos degradation in the biomixture as well as formation of TCP and hydrolytic and phenoloxidase activities were measured. The results showed that the biomixture efficiently degraded chlorpyrifos (degradation efficiency >50 %) in all the maturity stages evaluated. However, chlorpyrifos degradation decreased as the pesticide concentrations increased. 3,5,6-trichloro-2-pyrinidol formation occurred in all biomixtures, but a major accumulation was observed in the biomixture with 30 days of preincubation. Moreover, significant differences were found in both phenoloxidase and hydrolytic activities in the three maturity stages evaluated. These two biological activities were also affected by the increase in pesticide concentration. As a conclusion the authors reported that chlorpyrifos can be degraded efficiently in all the maturity stages evaluated.
Effect of biomixture composition. The composition of the Swedish biomixture has been efficient in degrading several pesticides (Vischetti et al. 2004; Castillo and Tortensson 2007). However, the biomixture had to be adapted due to the greater availability of other lignocellulosic wastes in some countries. Urrutia et al. (2013) evaluated the potential use of readily available wastes as barley husk, sawdust, and oat husk, as total or partial substitutes for straw in a biomixture for pesticide degradation studies. The results showed that a biomixture composed of oat husk was highly efficient in pesticide degradation, with half-life (t 1/2) values of 28, 58, and 26 days for atrazine, chlorpyrifos, and isoproturon, respectively. On the other hand, comparable for degrading capacities with the straw based biomixture were obtained with sawdust and barley husk, but only as partial replacement. By contrast, high t ½ values (more than 100 days) were obtained in biomixtures with total substitution of straw with sawdust or barley husk. Moreover, high and stable biological activity was observed in the biomixtures composed of oat husk. Therefore, the authors reported that straw can be partially or totally replaced by oat husk, thereby permitting an efficient degradation of pesticide mixture, and that straw can be only partially replaced by barley husk and sawdust in the biomixture to allow efficient pesticide degradation. Recently, Diez et al. (2013b) assessed two alternate lignocellulosic materials (barley husks and pine sawdust) as partial substitutes for straw in a biomixture on the degradation of a repeatedly applied mixture of six pesticides (atrazine, isoproturon, iprodione, chlorpyrifos, diazinon, and carbendazim). The results showed that the highest degradation efficiency was found in mixtures containing straw and barley husks. Each biomixture tested achieved a high degradation (50–90 %) of all the pesticides used except iprodione. Moreover, repeated applications of pesticides resulted in a slowing of the degradation rate of all pesticide types in all biomixtures. The study concluded that the straw in the traditional biomixture can be partially replaced by other lignocellulosic materials to efficiently degrade a mixture of pesticides even when the pesticides are added in successive applications and high concentrations. Finally, in the study by Diez et al. (2013c) biochar was evaluated as a partial replacement of peat in pesticide-degrading biomixtures formulated with different soil types. Each biomixture was prepared with one type of soil (clay, trumao, and sandy), straw, peat, and biochar in different volumetric proportions. In each biomixture, the residual pesticide (atrazine, carbendazim, chlorpyrifos, isoproturon, iprodione, and diazinon) concentrations were measured at 0 day and after 40 days. The results showed that at the end of the pesticide degradation assay, changes were observed in the biomixtures that demonstrated differences among their pesticide degradation abilities. In general, pesticide degradation was higher in the control biomixtures (without biochar) than in biomixtures prepared with biochar. One exception was iprodione, which presented higher degradation efficiency when biochar was included in the biomixture. The author indicated that although the use of biochar to replace peat in the biomixtures did not significantly improve pesticide degradation, a decrease in the initial residue concentration of the pesticides was observed. Therefore, biochar may represent an interesting material to replace peat in biomixtures designed to degrade and/or adsorb pesticides.
Effect on biobed microbial community. The current literature suggests that microbial communities in a pesticide-contaminated biomixture are adversely affected, though recovery is normally observed over time (Tortella et al. 2013c). In this context, and to gain a better understanding of the pesticide-biomixture-microorganism interaction, Tortella et al. (2013b, c) recently investigated carbendazim and atrazine dissipation, and the effect on the microbial community. In the first study, the impact of repeated carbendazim applications on the extent of carbendazim dissipation, microbial diversity, community-level physiological profile, and enzymatic activity within the biomixture was evaluated. After three successive carbendazim applications, the post-application carbendazim dissipation was 87 %, 94 %, and 96 %, respectively. Although microbial enzymatic activity was affected significantly by carbendazim application, it was able to recover after each carbendazim pulse. Likewise, the numbers of culturable bacteria, fungi, and actinobacteria were slightly affected by the addition of the compound, but the inhibitory effect of the pesticide application was temporary. Denaturing gradient gel electrophoresis (DGG) and Biolog Ecoplate™ assays showed that the microbial populations remained relatively stable over time compared to the control. With these results the authors demonstrated the high dissipation capacity of this biomixture and highlighted the microbiological robustness of this biological system. In the second study, the effects of repeated atrazine application (40 mg a.i./kg) on its degradation, microbial communities, and enzyme activities were studied in a peat-based biomixture composed of straw, soil, and peat in the volumetric proportions of 2:1:1 to be used in an on-farm biopurification system. The results showed that atrazine removal efficiency was high (96, 78, and 96 %) after each atrazine application and did not show a lag phase. Microbial enzyme activities were significantly reduced with atrazine application but rapidly recovered. On the other hand, the microbial diversity obtained by Biolog Ecoplate™ was similar after the first and second atrazine applications; however, an inhibitory effect was observed after the third application. After each atrazine application, culturable fungi were reduced, but rapidly recovered with no significant changes in culturable bacteria and actinobacteria compared to the control. Analysis through DGGE patterns revealed that the microbial community structure remained relatively stable over time compared to the controls. The authors concluded that after successive atrazine applications, the peat-based biomixture had a good degradation capacity. Moreover, microbiological assays demonstrated the robustness of the peat-based biomixture from a microbiological point of view to support pesticide degradation (Tortella et al. 2013c).
Effect of biomixture biostimulation. As has been observed, pesticide degradation in biobeds can be limited or improved by several factors. Biostimulation of the indigenous microorganisms through the addition of nutrients is an important aspect to consider because the enrichment of the indigenous microbial populations is the most widely used tool in a bioremediation procedure (Tortella et al. 2010). In this context and in order to ascertain the effect of biomixture stimulation, Tortella et al. (2010) evaluated the degradation of chlorpyrifos (160 a.i. mg/kg) using a biomixture biostimulated with inorganic fertilizer as nitrogen (N), phosphorus (P), and potasio (K) at three concentrations (0.1, 0.5, and 1.0 % w/w). Chlorpyrifos degradation, TCP accumulation, and biological activity of the biomix were evaluated. The results showed that the chlorpyrifos was dissipated efficiently (>75 %) after 40 days of incubation and no additional dissipation was obtained by increasing the NPK concentration after 20 days of incubation. 3,5,6-Trichloro-2-pyrinidol accumulation occurred in all the NPK concentrations evaluated and its concentration increased with the increase of NPK addition, raising the probability of leaching of this compound. Finally, the biological activity in the biomixture increased due to the presence of NPK in all the evaluated concentrations. In conclusion, the results demonstrated that the biomix prepared with an Andisol and biostimulated with NPK nutrient can be recommended in biobeds as a viable alternative to chlorpyrifos dissipation, thereby avoiding the likelihood of soil and water contamination (Tortella et al. 2010). By contrast, and taking into account that biostimulation of organic-pollutant-degrading microorganisms by adding volatile organic compounds such as terpenes has been used to increase pollutant biodegradation in contaminated soils (Bento et al. 2005; Tyagi et al. 2011; Dudášová et al. 2012), Tortella et al. (2013d) studied the effect of the terpenes α-pinene, eucalyptol, and limonene, individually and as mixtures, on atrazine biodegradation and on biological activity in a biobed biomixture. The results showed that terpenes added individually at relatively low concentrations (50 μg/kg) significantly enhanced atrazine degradation and biological activity during the first days of incubation. No significant effect on atrazine degradation was found from adding the terpene mixture, and, interestingly, an inhibitory effect on phenoloxidase activity was found during the first 20 days of incubation when mixed terpenes were present at 100 μg/kg. With this study it was concluded that successive applications of terpenes or the addition of materials that slowly release terpenes could sustain the atrazine degradation enhancement. However a contrary response was observed when natural wastes rich in terpenes as pine needles, eucalyptus leaves, and orange peels are added to the biomixture, where an enhancement of atrazine dissipation can be observed (Tortella et al. 2013e).
Effect of biomixture bioaugmentation. Inoculation of microorganisms into biobeds has not been a frequent practice, but the few international studies related to fungal inoculation are promising. In Chile the first approach related to biomixture bioaugmentation was performed by Diez and Tortella (2008), where inoculation with Anthracophyllum discolors Sp4 immobilized in lignocellulose material increased the degradation of pentachlorophenol in two biological systems: biobeds and fixed-bed columns. Recently, Elgueta et al. (2013) studied the formulation of different supports based on agro-forestry waste for the immobilization of Anthracophyllum discolors to be inoculated in a biobed system to increase atrazine degradation. A biomixture composed of an Andisol soil, peat, and straw was contaminated with 60 mg/kg of atrazine and inoculated with 10 % (w/w) of fungal Anthracophyllum discolors. The main results showed that t 1/2 of atrazine decreased from 14.5 days in the non-inoculated biomixture to 6 days in inoculated biomixture. On the other hand, inoculation with A. discolor in the biomixture contaminated with atrazine produced a stimulation in the fungal communities at the end of the experiment. With these results the author concluded that the bioaugmentation using A. discolor improved the atrazine degradation in a biobed system biomixture.
The presence of peat in the biomixture produces a low pH that enhances the growth of lignin-degrading fungi on the straw, which results in the production of ligninolytic enzymes and the subsequent degradation of pesticides. However, in some countries, the peat is replaced by compost for economic and/or environmental reasons (Fogg et al. 2004; Vischetti et al. 2004; Coppola et al. 2007), and the pH in the system can increase to values that are not favorable to the growth of certain fungi (Rousk et al. 2009). Under these conditions, bacterial activity plays a more important role. Briceño et al. (2013a) discussed the feasibility of using actinobacteria as inocula in biobed systems. Two actinobacteria isolated from agricultural soil and characterized as degrading organophosphorus pesticides (Briceño et al. 2012, 2013b) were used in the bioaugmentation of a biomixture contaminated with 25 mg/kg of chlorpyrifos and diazinon. The results showed that inoculation of actinobacteria Streptomyces sp. AC5 improved the enzymatic activity and microbial respiration in the biomixture. In addition, after 45 days of incubation, 48 % and 36 % of residual chlorpyrifos was found in the non-inoculated and inoculated biomixtures, respectively. However, when both strains, Streptomyces sp. AC5 and AC16, were inoculated in the biomixture, no effects on pesticide degradation were observed. Consequently, the authors indicated that future assays are needed to clarify the effect on organophosphorus pesticide degradation in bioaugmented biomixtures with an actinobacteria consortium (Briceño et al. 2013c).
4.4 Concluding Remarks
There is general awareness of the detrimental effects of improper pesticide use on the environment. Soil and water contamination from various sources can be prevented to a large extent by good farming practices, but additional measures are required for point-source contamination from the incorrect handling of pesticides in agriculture. Biobeds are a feasible biotechnological tool proven effective in pesticide removal. Therefore, biobeds have been implemented in several European countries, resulting in a reduction of environmental pollution caused by pesticide residues. Latin America is no stranger to the implementation of biobeds, and recently, four of them were installed in Chile (La Araucanía region), making the country a pioneer in the implementation of this technology in South America. Given the increasing adoption of this technique in different areas and for different purposes, adjustments are needed and consequently a considerable amount of research has been conducted at the laboratory and field scales. In Chile, the investigations to date have validated the efficient removal of pesticides in a biobed system, studying operational parameters to optimize the functioning of the biobeds, to understand the interactions between pesticide degradation, microorganisms, biomixtures, and others. The results obtained over the years have been used to create the “Manual of Construction and Operation of Biobeds,” which has been used to disseminate biobed technology in the diverse public and private agricultural sector. The main goal has been to transmit biobed technology in Chile, giving special attention to the adequate pesticide manipulation to protect the environment and natural resources.
Biobed technology is appropriate for nationwide implementation; however, it must be adapted to local conditions where in many cases the traditional biomixture must be modified by adding different residues according to its availability. In this situation, many questions remain to be answered and research needs to be conducted to obtain an adequate and efficient functioning of biobeds as pesticide biopurification systems. Having knowledge of the efficiency of biobed technology for removing pesticide residues, it is hoped that it can be utilized in various parts of Chile and throughout Latin America.
References
Antonious G (2012) On-farm bioremediation of dimethazone and trifluralin residues in runoff water from an agricultural field. J Environ Sci Heal B 47:608–621
Bento FM, Camargo FAO, Okeke BC et al (2005) Comparative bioremediation of soils contaminated with diesel oil by natural attenuation, biostimulation and bioaugmentation. Bioresour Technol 96:1049–1055
Briceño G, Fuentes MS, Palma G et al (2012) Chlorpyrifos biodegradation and 3,5,6-trichloro-2-pyridinol production by actinobacterias isolated from soil. Int Biodet Biodeg 73:1–7
Briceño G, Pizzul L, Diez MC (2013a) Biodegradation of Pesticides by Actinobacteria and their Possible Application in Biobed Systems. In: Amoroso MJ, Benimeli CS, Cuozzo SA (eds) Actinobacteria application in bioremediation and production of industrial enzymes. Taylor & Francis, Boca Raton, FL, pp 245–255
Briceño G, Fuentes MS, Rubilar O et al (2013b) Removal of insecticide diazinon from liquid media by free and immobilized Streptomyces sp. isolated from agricultural soil. J Basic Microbiol 53:1–10
Briceño G, Rubilar O, Tortella G et al (2013c) Bioaumentación de una biomezcla con actinobacterias degradadores de residuos de plaguicidas organofosforados. Workshop Internacional y Taller Nacional Valorización de Residuos, oportunidad para la innovación. Universidad de la Frontera, Pucón, Chile
Castillo MP, Tortensson L (2007) Effect of biobed composition, moisture, and temperature on the degradation of pesticides. J Agric Food Chem 55:5725–5733
Castillo MP, Andersson A, Ander P et al (2001) Establishment of the white rot fungus Phanerochaete chrysosporium on unsterile straw of solid substrate fermentation systems intended for degradation of pesticides. World J Microbiol Biotechnol 17:627–633
Castillo MP, Torstensson L, Stenström J (2008) Biobeds for environmental protection from pesticide uses a review. J Agric Food Chem 56:6206–6219
Coppola L, Castillo MP, Monaci E et al (2007) Adaptation of the biobed composition for chlorpyrifos degradation to southern Europe conditions. J Agr Food Chem 55:396–401
Dabrowska D, Kot-Wasik A, Namieoenik J (2004) The Importance of degradation in the fate of selected organic compounds in the environment. Part II. Photodegradation and biodegradation. Pol J Environ Stud 13:617–626
De Wilde T, Spanoghe P, Sniegowksi K et al (2010a) Transport and degradation of metalaxyl and isoproturon in biopurification columns inoculated with pesticide-primed material. Chemosphere 78:56–60
De Wilde T, Spanoghe P, Ryckeboer J et al (2010b) Transport and degradation of pesticides in a biopurification system under variable flux, part I: a microcosm study. Environ Pollut 158: 3309–3316
De Wilde T, Spanoghe P, Ryckeboer J et al (2010c) Transport and degradation of pesticides in a biopurification system under variable flux, part II: a macrocosm study. Environ Pollut 158: 3317–3322
Diez MC, Tortella GR (2008) Pentachlorophenol degradation in two biological systems: biobed and fixed-bed column, inoculated with the fungus Anthracophyllum discolor. The proceeding of the ISMOM, Chile
Diez MC, Palma G, Altamirano C et al (2013a) Manual de construcción y operación de lechos biológicos. Universidad de La Frontera, Temuco
Diez MC, Tortella G, Briceño G et al (2013b) Influence of novel lignocellulosic residues in a biobed biopurification system on the degradation of pesticides applied in repeatedly high doses. Electron J Biotechnol 16:1–11
Diez MC, Levio M, Briceño G et al (2013c) Biochar as a partial replacement of peat in pesticide-degrading biomixtures formulated with different soil types. J Biobased Mater Bioenergy 7:1–7
Dudášová H, Lukáčová L, Murínová S et al (2012) Effects of plant terpenes on biodegradation of polychlorinated biphenyls (PCBs). Int Biodet Biodeg 69:23–27
Elgueta S, Campo M, Diez MC (2013) Formulación de inóculos fúngicos a partir de desechos agroforestales para la degradación de atrazina en un sistema de biopurificación. Workshop Internacional y Taller Nacional Valorización de Residuos, oportunidad para la innovación. Universidad de la Frontera, Pucón, Chile
Fernández-Alberti S, Rubilar O, Tortella G et al (2012) Chlorpyrifos degradation in a Biomix: effect of pre-incubation and water holding capacity. J Soil Sci Plant Nutr 12:785–799
Fogg P, Boxall A, Walker A (2003) Degradation of pesticides in biobeds: the effect of concentration and pesticide mixtures. J Agr Food Chem 51:5344–5349
Fogg P, Boxall A, Walker A et al (2004) Degradation and leaching potential of pesticides in biobed systems. Pest Manag Sci 60:645–654
Gregoire C, Elsaesser D, Huguenot D et al (2009) Mitigation of agricultural nonpoint-source pesticide pollution in artificial wetland ecosystems. Environ Chem Lett 7:205–231
Henriksen VV, Helweg A, Spliid NH et al (2003) Capacity of model biobeds to retain and degrade mecoprop and isoproturon. Pes Manag Sci 59:1076–1082
Husby J (2010) Introduction of the workshop. 3rd European biobed workshop, Piacenza, Italy
Karanasios E, Nikolaos G, Tsiropoulos A et al (2010) Novel biomixtures based on local Mediterranean lignocellulosic materials: evaluation for use in biobed systems. Chemosphere 80:914–921
Karanasios E, Tsiropoulos NG, Karpouzas DG (2012) On-farm biopurification systems for the depuration of pesticide wastewaters: recent biotechnological advances and future perspectives. Biodegradation 23:787–802
Marinozzi M, Laura Coppola L, Elga Monaci E et al (2013) The dissipation of three fungicides in a biobed organic substrate and their impact on the structure and activity of the microbial community. Environ Sci Pollut Res 20:2546–2555
Niels H, Helweg A, Heinrichson K (2006) Leaching and degradation of 21 pesticides in full-scale model biobeds. Chemosphere 65:2223–2232
Rousk J, Brookes P, Bååth E (2009) Contrasting soil pH effects on fungal and bacterial growth suggest functional redundancy in carbon mineralization. Appl Environ Microbiol 6:1589–1596
Spliid NN, Husby J (2010) New closed biobed with recirculation and evaporation for use under colder climates. 3rd European biobed workshop, Piacenza, Italy
Torstensson L, Castillo MP (1997) Use of biobeds in Sweden to minimize environmental spillages from agricultural spraying equipment. Pesticide Outlook 8:24–27
Tortella G, Rubilar O, Cea M et al (2010) Biostimulation of agricultural biobeds with NPK fertilizer on chlorpyrifos degradation to avoid soil and water contamination. J Soil Sci Plant Nutr 10:464–475
Tortella G, Rubilar O, Castillo MP et al (2012) Chlorpyrifos degradation in a biomixture of biobed at different maturity stages. Chemosphere 88:224–228
Tortella GR, Durán N, Rubilar O et al (2013a) Are white-rot fungi a real biotechnological option for the improvement of environmental health? Crit Rev Biotechnol. doi:10.3109/07388551.2013.823597
Tortella GR, Mella-Herrera R, Sousa D et al (2013b) Carbendazim dissipation in the biomixture of on-farm biopurification systems and its effect on microbial communities. Chemosphere 93: 1084–1093
Tortella GR, Mella-Herrera T, Sousa D et al (2013c) Atrazine dissipation and its impact on the microbial communities and community level physiological profiles in a microcosm simulating the biomixture of on-farm biopurification system. J Hazard Mater 260:459–467
Tortella GR, Rubilar O, Stenström J et al (2013d) Using volatile organic compounds to enhance atrazine biodegradation in a biobed system. Biodegradation 24:711–720
Tortella GR, Rubilar O, Cea M et al (2013e) Natural wastes rich in terpenes and their relevance in the matrix of an on-farm biopurification system for the biodegradation of atrazine. Int Biodeter Biodegr 85:8–15
Tyagi M, da Fonseca MMR, de Carvalho CCCR (2011) Bioaugmentation and biostimulation strategies to improve the effectiveness of bioremediation processes. Biodegradation 22:231–241
Urrutia C, Rubilar O, Tortella G et al (2013) Degradation of pesticide mixture on modified matrix of a biopurification system with alternatives lignocellulosic wastes. Chemosphere 92:1361–1366
Verhagen P, De Gelder L, Boon N (2013) Inoculation with a mixed degrading culture improves the pesticide removal of an on-farm biopurification system. Curr Microbiol 67:466–471
Vischetti C, Capri E, Trevisan M et al (2004) Biomassbed: a biological system to reduce pesticide point contamination at farm level. Chemosphere 55:823–828
Vischetti C, Coppola L, Monaci E et al (2007) Microbial impact of the pesticide chlorpyrifos in Swedish and Italian biobeds. Agron Sustain Dev 27:267–272
Vischetti C, Monaci E, Cardinali A et al (2008) The effect of initial concentration, co-application and repeated applications on pesticide degradation in a biobed mixture. Chemosphere 72: 1739–1743
Acknowledgments
The authors gratefully acknowledge the support of the Center of Research and Development for Organic Waste Management (CIDGRO) Cod. 09FC02-6021, Chile.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Briceño, G., Tortella, G., Rubilar, O., Palma, G., Diez, M.C. (2014). Advances in Chile for the Treatment of Pesticide Residues: Biobeds Technology. In: Alvarez, A., Polti, M. (eds) Bioremediation in Latin America. Springer, Cham. https://doi.org/10.1007/978-3-319-05738-5_4
Download citation
DOI: https://doi.org/10.1007/978-3-319-05738-5_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-05737-8
Online ISBN: 978-3-319-05738-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)