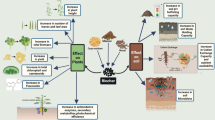
Abstract
Pesticide pollution and soil degradation are two major issues in the agricultural ecosystem. Biochar application is a promising method because it has been shown in numerous previous studies to be highly effective in increasing crop yield and enhancing pesticide degradation. In this section, we will look at the possibilities. Advantages of biochar in increasing fertilizer efficiency by increasing nutrient availability and soil fertility by improving nutrient retention and release. Its role in pesticide chemical degradation and biodegradation is also being studied. Biochar has an inflated surface area with different functional groups, an increased cation exchange capacity, and high stability. The influencing factors and mechanisms for nutrient retention by biochar are discussed (for example, feedstock, pyrolysis temperature, and application rate). Because laboratory experiments and field trials have different conditions, more research should be done on the long-term dynamic function of biochar. In this paper, we examine the potential benefits of biochar in improving fertilizer use efficiency by increasing nutrient availability and soil fertility by improving nutrient retention which means lessening nutrient leaching and gaseous nutrient emission and release. Besides that, the mechanisms of biochar in soil fertility improvement were examined. We have also discussed biochar’s physical and chemical properties, the factors and mechanisms that influence various biochar functions, and identified future prospects and knowledge gaps. We propose a shift from traditional hazardous chemical/pesticidal practices to an eco-friendly method to enhance sustainable practices and also encourage on-farm activities. Through the present review, we want to emphasize microclimatic biochar choices for on-farm ecofriendly input generation by highlighting specific patterns of properties, physiological parameters, and challenges offered in terms of sustainability. This would have a direct impact on using some synergistic approaches towards a sustainable agri-eco environment and building up awareness regarding the advantages and flaws in the field of biochar application. Furthermore, this would encourage farmers, industrialists, and researchers globally to move towards a sustainable approach to fill up the various knowledge gaps.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fertilizers (e.g., nitrogen, phosphorus, and potassium) and pesticide application in agricultural soil have grown increasingly intense as a strategy to boost crop yields. Fertilizer use in India, for example, has risen from 12.4 kgs/ha in 1969 to 175 kgs/ha in 2018, expanding at a 5.96% yearly rate (India fertilizer consumption (1960–2021). And, to establish more sustainable agriculture systems and strengthen rural economies, fundamental reforms in agriculture management are required. Leaching losses of fertilizer and pesticides may occur during intensive application, causing soil fertility to deteriorate and pollution to occur. Furthermore, nutrient leaching from agricultural soils can reduce soil fertility, raise farming costs, hasten soil acidification, and lower crop yields (Laird et al. 2010). Pesticides tend to travel long distances and cross borders, and their ability to bioaccumulate in the food chain can represent a serious threat to human health and the environment (Kuranchie-Mensah et al. 2012). On the one hand, to meet the high demand for food in some countries, people must urgently improve soil fertility and nutrient availability to increase crop yields; on the other hand, pesticide degradation is a critical goal for both soil management and environmental protection. Moreover, some pesticides, such as organochlorine pesticides, are difficult to biodegrade rapidly. Furthermore, microorganisms are sensitive to environmental changes such as heat, desiccation, and ultraviolet radiation (Zhang et al. 2010). Moreover, the competition between different microbial species and other organisms that occurs in the soil is also a challenge.
Sustainable agriculture looks for a variety of agricultural conservation methods that might help to lessen some of the negative effects of land use intensification. Among the many conservation agricultural techniques, biochar may prove to be a crucial and easily available component for sustainable agriculture. Biochar is rich in carbon which is made by heating biomass in the absence of oxygen. It has a porous carbonaceous structure, a functional group, and an aromatic surface. Slow pyrolysis, hydrothermal carbonization, flash carbonization, and gasification are the basic methods for producing biochar (Spokas et al. 2012). Biochar made from biomass pyrolysis can change the soil’s physicochemical qualities, reduce gaseous nitrogen emissions, change soil nutrient availability, minimize nutrient leaching, and boost crop production. Furthermore, biochar made from pig manure can degrade up to 90.6% of carbaryl. Moreover, biochar improved soil microbial properties such as microbial abundance and activity, as well as mycorrhizal associations. These investigations showed that biochar has a lot of potential in terms of preserving soil fertility, inactivating pesticides through abiotic breakdown, and speeding up pesticide biodegradation.
Biochars are biological byproducts of the burning of organic materials (pyrolysis) in the absence or presence of oxygen. Currently, biochar is gaining interest as a possible agricultural input that might increase soil fertility, support sustainable agricultural output, and lessen the negative impacts of various biotic and abiotic pressures. In addition to increasing the amount of nutrients available to plants, microbial activity, organic matter, water-holding capacity, and crop productivity, adding biochar to soil also reduces the need for fertilizer, greenhouse gas emissions, nutrient leaching, and soil erosion (Chen et al. 2019; Thakur, 2022).
Because of the distinctive properties of biochar, such as its large surface area, high stability, ideal pore volume, organic carbon, high cation exchange capacity (CEC), and presence of different functional groups, such as carboxylic, phenolic, hydroxyl, carbonyl, and quinone groups, it exhibits high attraction for contaminants and reduces their bioavailability. Biochar, one of the many conservation agricultural strategies, may prove to be a crucial and affordable input for sustainable agriculture since it may effectively and long-term trap huge amounts of carbon in the soil, enhancing soil fertility and crop yield, and reducing global warming. Additionally, biochar may increase soils’ ability to produce crops under various biotic and abiotic conditions and boost global food security (Kumar et al. 2022, Thakur et al., 2022). This report’s major objective is to provide a comprehensive scientific evaluation of what is currently known about how applying biochar to soils affects the characteristics, processes, and functions of the soil. There are many different types of biochar, and each one can have a different impact on soil quality, crop development, and productivity. Many of the impacts, such as increased growth and production, heavy metal adsorption, water-holding capacity, and plant physiological responses, have been found to be advantageous. However, some mixtures of crops and biochar have shown to be harmful to plant growth. Crop growth and yield are greatly influenced by the rate of biochar input; however, this varies depending on the crop and culture method. The makeup of biochar can also have an impact on soil biota, plant output, and growth. Different studies demonstrated that biochar can be produced from a wide range of biomass resources, including, municipal solid wastes, sewage, energy crops, and agricultural residues. It is estimated that China produces between 600 and 800 tons of crop residues annually, with rice straw, corn straw, wheat straw, and rapeseed straw making up a sizable fraction of this total (Chen et al. 2019). In India, approximately 23% and 58% of total crop residues are generated from fiber and cereal crops. This amounts to around 500 Mt of crop waste produced annually in the country. The yearly global crop residue production was estimated to be at 3700 million dry tons, based on six different crop types including corn rice, wheat, sunflower, barley, rapeseed, and oats (Scarlat et al. 2019). Crop residue burning has negative health impacts on humans, especially on youngsters and pregnant women who are more vulnerable to respiratory and cardiovascular issues. Therefore, converting remaining crop residues into biochar is a viable means of lowering CO2 emissions while increasing other benefits (Kumar et al. 2022). In this paper, we examine the potential benefits of biochar in improving fertilizer use efficiency by increasing nutrient availability and soil fertility by improving nutrient retention which means lessening nutrient leaching and gaseous nutrient emission and release. Besides that, the mechanisms of biochar in soil fertility improvement were examined. We also discussed biochar’s physical and chemical properties, present the factors and mechanisms that influence various biochar functions, and identify prospects and knowledge gaps. This review thus aims at providing microclimatic specificity by defining various parameters, factors, and limitations/challenges for sustainable approaches in the agro-environment section.
Properties of biochar
Biochar’s physical and chemical properties influence its adsorption properties. For example, increasing the acidic functional groups in biochar can increase NH4+ adsorption. Biochar has a vast specific surface area with oxygen-containing functional groups in sufficient amounts and it is very stable (Tan et al. 2015). Biochar’s physicochemical properties are primarily determined by the feedstock and the pyrolysis temperature. Many feedstocks which include wood chips, organic wastes, plant residues, and poultry manure left over by the poultry can be used to produce biochar (Steiner et al. 2008). The typical pyrolysis temperature ranges between 200 and 800 C. The biochar framework thus gives a very important indication regarding the content, type, and carbonization conditions of the biomass which finally affect the properties of the resulting biochar. When determining the fundamental characteristics of biochar and forecasting the many application applications, physical and chemical characterizations are required. In place of its surface area, charged surfaces, and functional groups, biochar offers a viable substitute which can be easily harnessed by looking at the properties and microclimatic framework of a specific region.
Biochar production tools and methodologies: from pre-enrichments to post-treatments
Numerous methods are being researched to improve the nutritional content of biochar, boost its efficacy in agriculture, and hence lower application rates. Making fertilizer based on nutrient-rich biochar is one of the possibilities. The pyrolysis of a combination of wheat straw, urea, bentonite clay, phosphate rock, Fe2O3, and FeSO4.7H2O resulted in a biochar-based fertilizer. The final biochar was composed of the following elements: C, 43%; N, 27%; K, 2%; and P, 2.5% (Chew et al. 2020). Furthermore, phosphoric acid, magnesium oxide, and triple superphosphate were added to chicken litter and coffee husk to form P-enriched biochar, according to (da Silva Carneiro et al. 2021), at the moment there are three different types of biochar enrichment techniques: direct treatment, pre-treatment, and post-treatment. In the absence of oxygen, food, horticulture waste, and municipal solid waste are thermally decomposed to form biochar, whereas organic substrates are naturally biodegraded naturally to make compost by the microbial population in an aerobic environment. Compost advantages are also very short-lived because of how quickly it dissolves, in contrast to biochar, which remains in the soil for longer periods of time (Kim et al. 2018).
In some instances, further treatments like re-pyrolysis take place following the post-pyrolysis procedure at low temperatures (Joseph et al. 2015). Pyrolysis of nutrient-rich feedstocks is the only step in the direct treatment process. Some feedstocks are notable for having a very high level of a certain nutrient. P-rich biochar from bone debris (Zwetsloot et al. 2016), with P levels ranging from 12.7% when processed at 350 °C to 15.3% in biochar generated at 750 °C. From the corpses of dairy calves, (Ma and Matsunaka 2013) produced biochar (450 °C) with a total P of 10% (mixture of skin, meat, and bone). By pyrolyzing bacterial biomass waste from Escherichia coli, P-enriched biochar was also created; its P content was 84.7 mg g1, or almost 11 times greater than that of the original biomass (Kim et al. 2018). The authors speculate that the high levels of P might be caused by the K2HPO4 and KH2PO4 present in the culture media. In this process, the initially nutrient-rich feedstock goes through gradual pyrolysis, which encourages nutrient enrichment in the biochar. The pyrolysis temperature, which controls the rate of volatilization or concentration of the nutrients, is the most important aspect of this process after the caliber of the feedstock. N may be enriched in biochar at low temperatures (300–400 °C), whereas P and K can be enriched at higher temperatures (about 700 °C) (Biederman and Harpole 2013). After the pyrolysis process, either at room temperature or under temperature-controlled circumstances, biochars are mixed with a nutrient-rich source, such as soluble mineral fertilizers, clays, pulverized rock, composts, and wastewater to create nutrient-enriched biochar-based fertilizers. Thus, the usage of biochar has various drawbacks, including nutritional deficiencies, high application rates, and high manufacturing costs. One option to get around these drawbacks is to enrich the material. Direct pyrolysis, pre-pyrolysis, and post-pyrolysis are the three major techniques for producing biochar-based fertilizers. In comparison to unenriched biochar, enriched biochar has improved soil biological, physical, and chemical characteristics as well as plant growth and yield.
Surface area
The specific surface area of biochar is significant because it aids in the adsorption of substances (such as heavy metals and organic compounds). Increasing the pyrolysis temperature can increase the specific surface area of biochar as well as the formation of micropores. The surface area of sugarcane bagasse biochar increased from 0.56 to 14.1 m2 g−1 when the pyrolysis temperature was raised from 250 to 600 °C (Huang et al. 2016). Similarly, the surface area of soybean stover biochar produced at 700 °C was 420 m2 g1, which was significantly higher than that of biochar produced at 300 °C (6 m2 g1) (Mohan et al. 2014). One possible explanation is that the release of volatiles within the biochar increases as the pyrolysis temperature rises. Apart from this, the surface area of biochar is directly proportional to the feedstock used. Biochar produced from bagasse and cocopeat had surface areas of 202 and 13.7 m2 g1, respectively. Furthermore, when compared to biomass, volatiles in biochar made from bagasse and cocopeat decreased by 87.1% and 70.1% respectively (Lee et al. 2013). The release of volatile matter, primarily celluloses and hemicelluloses, during pyrolysis increases the formation of vascular bundle structure in biochar and improves its specific surface area and pore structure. In general, the influence of feedstocks and pyrolysis temperatures on biochar’s surface area is mainly described by the release of volatile matter.
Cation exchange capacity (CEC) and pH values
The CEC measures biochar’s ability to adsorb cations, which are essential nutrients for plants (e.g., NH4+ and Ca2+). Thus, a high biochar CEC can reduce nutrient loss from soil leaching. Increasing the pyrolysis temperature from 200 to 550 °C, the CEC of cordgrass biochar increased from 8.1 to 44.5 comic kg−1, then decreased to 32.4 cmolc kg−1 (Harvey et al. 2011). Similarly, the CEC of sugarcane bagasse biochar rose from 6.40 cmolc kg−1 (pyrolyzed at 250 °C) to 9.66 cmolc kg−1 (pyrolyzed at 500 °C) before falling to 4.19 cmolc kg−1 (pyrolyzed at 600 °C). According to these comparisons, biochar produced at high pyrolysis temperatures (i.e., > 500 °C) has a low CEC. The aromatization of biochar, as well as the disappearance of functional groups on biochar, has been attributed to the decrease in CEC at high pyrolysis temperatures.
The use of biochar can raise soil pH due to the pH of the biochar itself and by improving cation retention in the soil (e.g., Ca2+, Mg2+, and K+). The pH of biochar produced at higher temperatures is higher due to the release of alkali salts from the organic matrix of the feedstock. For example, when the pyrolysis temperature was increased from 300 to 600 °C, the pH value of biochar produced from corn straw increased from 9.37 to 11.32. (Yuan et al. 2011). The pH of swine manure biochar produced at 400 and 800 °C was 7.60 and 11.54, respectively (Tsai et al. 2012). As a result, biochar with a high CEC and pH has a high potential for retaining NH4- and K-fertilizer and increasing their utilization efficacy. Therefore, the physicochemical characteristics of biochar (pH, specific surface area, pore size, CEC, volatile material, ash, and carbon content) alter depending on the kind of feedstock and pyrolysis temperature and finally can be monitored accordingly.
Biochar stability
Although biochar is increasingly recognized as a valuable tool for long-term soil amendment (e.g., carbon sequestration, nutrient retention, and pesticide-contaminated soil remediation), its long-term environmental stability remains unknown. As previously stated, biochar stability is primarily determined by the temperature of pyrolysis and the feedstock. According to one study, certain types of biochar can degrade relatively quickly in some soils, possibly depending on the conditions under which they were produced, implying that pyrolysis could be optimized to produce a more stable biochar. In general, increasing pyrolysis temperatures can improve biochar stability. For example, increasing the pyrolysis temperature from 350 to 550 °C significantly increased the stability of sugarcane bagasse biochar (Cross and Sohi 2013). The amount of recalcitrant carbon substrates affects biochar stability.
Biochar as a nutrient source
Role of biochar as a fertilizer
Humic- and fluvic-like substances come under the category of organic matter and inorganic salts, and a few chemicals like nitrogen, phosphorus, and potassium can be used as fertilizer and can be uptaken up by plants and microorganisms. At 300 °C, Lantana camara biochar contained available P (0.64 mg kg1), available K (711 mg kg1), available Na (1145 mg kg1), available Ca (5880 mg kg1), and available Mg (1010 mg kg1). Similarly, as of Lantana camara biochar, fresh biochar could rise nutrient availability by disposing of huge amounts of N (23–635 mg kg−1) and P (46–1664 mg kg−1) (Mukherjee and Zimmerman 2013). As a result of these findings, biochar appears to have a high potential as a source of restored nutrients. N, P, and K altogether in biochar may not always reflect actual nutrient accessibility to plants. Available N, P, and K (e.g., ammonia (NH4+) nitrate (NO3), phosphate (PO43), and K+) may be related to total N, P, and K. Many recent studies assessed nutrient availability in biochars using rapid column filtration experiments or kinetic models. Total nitrogen, phosphorus, and potassium could be used as indirect indicators for choosing appropriate biochar for use.
Factors influencing biochar nutrient content and availability
The feedstock source and pyrolytic temperature had a significant impact on the nutrient content of biochar. For example, in three woody and four herbaceous biochars, N losses began around 400 °C and then half of the N was lost as volatiles around 750 °C. However, phosphorus was found to be increased in biochar produced at high temperatures as compared to biochar produced at lower temperatures and this is because lower-temperature biochar contains less crystallized phosphorus-associated minerals. Moreover, potassium content increased from 3.7 at 300 °C to 5.02% at 600 °C, while the available potassium which was water-soluble was increased as the pyrolysis temperature was increased (Zheng et al. 2013). Furthermore, the composition of nutrient elements in biochars produced from various feedstocks varies. For example, swine manure biochar produced at 400 °C contained high levels of nitrogen (3.2%) and phosphorus (6.1%) (Tsai et al. 2012). The constituents of Arundo donax biochar produced at 400 °C were low in N (0.69%) and P (0.13%). Furthermore, at 350 °C, the ash content of biochar made from poultry litter (30.7%) was significantly higher than that of biochar made from pine wood chips (30.7%). The pH of the soil plays a vital role in the nutrient availability of biochar as the emission of PO43 and NH4+ was pH-dependent, as compared to the release of K+ and NO3 which is not pH dependent. Similarly, when talking about corn straw biochar, the release of calcium and magnesium is also pH dependent which increases as the pH decreases from 8.9 to 4.5. It is critical to consider the impact of application time on biochar nutrient release. Furthermore, an increase in carbon solidification and nitrogen immobilization of volatile matter in biochar by microorganisms may reduce nutrient breakdown. In practice, these influencing factors may coexist when biochar is applied to the soil. At last, it can be stated that decreasing the pyrolysis temperature and pH of the soil may increase the content of nitrogen and phosphorus but if the pyrolysis temperature is increased it only increases the potassium availability.
Potential of biochar for increasing soil fertility
Biochar to improve the efficiency of fertilizer
Improving crop yield by increasing fertilizer use efficiency is a viable option. One study it was investigated green waste biochar on radishes. And they discovered that using biochar did not increase radish yield in the absence of nitrogenous fertilizer. But, in the presence of nitrogenous fertilizer, radish yield increased with biochar application, indicating that biochar could efficiently improve plant nitrogen utilization. For example, with a biochar application rate of 100 t/ha, the increase in radish yield in the presence of nitrogenous fertilizer was 266% when compared to the control (the absence of N fertilizer). In the presence of N fertilizer (100 kg/ha−1), however, the yield (percentage) increased from 42% at 10 t/ha to 96% at 50 t/ha of biochar application, when compared to the control (Chan et al. 2008). Furthermore, biochar also has the potential to increase maize grain yield by 28% and Ca, Mg, K, and P availability by 17–600% in biochar-affected fields. As a result, biochar is thought to have a high potential for improving plant fertilizer use efficiency by increasing nutrient availability in the soil (Fig. 3).
Nutrient retention in biochar-treated soil
Biochar’s heterogeneous composition means that its surface can have hydrophilic (which has a natural affinity towards the water), hydrophobic (which repels water), acidic, and basic properties, all of which affect the biochar’s ability to adsorb soil solution components and hence fertilizer retention. Biochar, on the one hand, can improve nutrient retention through the adsorption process. For example, the total amount of NO3, NH4+, and PO43 in the leachates was reduced by 34.0%, 34.7%, 20.6%, and 34.3%, 14.4%, 39.1% respectively, using peanut hull and pepperwood biochar’s generated at 600 °C (Yao et al. 2012). Furthermore, one study found that spartina biochar prepared at 350 °C may absorb 0.5 mmol g1 of K+. As a result, biochar can be used to address nutrient deficiencies in the soil. N2O emissions were reduced by 80% with the use of biochar, according to one study (Yanai et al. 2007). Indeed, improved soil physicochemical features, such as increased porosity and water storage capacity, and decreased bulk density, may contribute to improved nutrient retention after biochar amendment. Overall, biochar offers a lot of promise for increasing fertilizer efficiency by reducing nutrient leaching and gaseous nitrogen losses (Fig. 1).
Biochar impact on microorganisms and soil fertility
Biochar has been found to change soil biological qualities as well as improve soil physicochemical properties. These modifications could improve the structure of soil by increasing organic/mineral complexes (aggregates) and pore spaces, as well as improve nutrient cycles by improving nutrient retention and immobilization and reducing nutrient leaching thus promoting plant growth. Microbes that are present in the rhizosphere such as rhizosphere bacteria and fungi may directly promote plant growth. In conclusion, biochar-implementation alteration in microbial community structure or activity may have an impact on nutrient cycles, plant growth, and soil organic matter cycling. This section of the study illustrates an overview of the effects of biochar qualities on the microbial community which include organic as well as inorganic structure and surface properties.
Influence of biochar on microorganism community
People are gaining interest in using biochar to control soil nutrients and the latest changes in the soil nutrient caused by biochar implementation are also challenges. Some methods which explain how biochar affects soil microorganisms are (1) alteration in food availability, (2) changes in other microbial populations, (3) changes in plant–microbe signaling, and (4) habitat creation and grazer protection. The soil food web has a significant impact on microbial characteristics. Furthermore, the quantity, quality, and distribution of organic matter have a significant impact on the soil food web structure. Although soil organic matter production is modest in comparison to other carbon cycle processes, its relative firmness for microbial breakdown allows soil organic matter accumulation (Table 1).
Biochar’s effect on microbial abundance
According to one study, after adding 30 t ha1 biochar, microbial abundance increased from 366.1 (control) to 730.5 gCg1. Similarly, for the various maturation period (2–61 days), microbial abundance rose by 5–56% as maize stover biochar rates increased (from 0 to 14%) (Domene et al. 2015). The increased microbial abundance could be due to a variety of factors, including increased nutrition availability or labile organic materials on the biochar surface, improved habitat appropriateness and refuge, and improved water retention and aeration. Microbial abundance can also be influenced by nutrient and carbon availability. With the non-identical forms of biochar and the particular bacteria group, this influence differed substantially. The various demands of the plant may have resulted in symbiotic partnerships with biota created through varying nutrient supplies. Similar arguments may apply to the effect of increased C supply in the rhizosphere due to exudation or root turnover, as well as C as an energy source for heterotrophic microbes. As a result, the effect on microbial abundance varied depending on whether biochar additions were used in the rhizosphere or bulk soil. In nutrient-limited settings, on the other hand, microbial abundance may grow due to an increase in nutrition availability after biochar inclusion. Some recent studies show the relevant factors which influence the impact of nutrient and carbon availability on microbial biomass such as (i) existing nutrient and carbon availability in soil, (ii) the added amount of nutrient and carbon, and (iii) microorganism features. Microbial abundance may rise as microorganisms bind to biochar surfaces, making them less susceptible to soil leaching. The major processes of adsorption to biochar include hydrophobic attraction, electrostatic forces, and the formation of precipitates. Moreover, biochar with a well-evolved pore structure may provide a shelter for microorganisms. By investigating pore habitats in biochar, bacteria and fungus may be better protected from predators or rivals.
Toxins and chemical signals that inhibit microbial development could be absorbed by biochar. Furthermore, increase in temperature have been found to have more adsorption on chemicals that are harmful to microbial growth. Humidity may also have a significant impact on microbial growth. Microorganisms would be stressed in soil that is subjected to periodic drying, causing them to go dormant or even die. Because of its huge surface area, biochar has a high-water holding capacity, which may encourage the growth of microbes. However, the original components and properties of biochar cannot be used to draw any further inferences. According to some studies, bacterial cells or chemicals that regulate growth may play a role in the sorption process.
Enhancing the remediation of pesticide-contaminated soil with biochar
Potential of biochar enhancing the degradation of pesticides in soil
Biochar has been shown to aid pesticide breakdown by soil microorganisms. On the one hand, with the implementation of biochar, pesticide biodegradation may be influenced by both enhanced natural microbial activity in the soil and reduced pesticide bioavailability. Biochar has been shown in numerous studies to improve microbial living conditions, including modifying soil pH, raising soil organic matter, increasing soil water content, providing habitat, and lowering competition from other microbes, all of which increase microbial characteristics (e.g., microbial community composition, abundance, and activities) (Ding et al. 2016). However, biochar addition can improve pesticide sorption in soil, lowering pesticide concentrations in the soil solution and decreasing pesticide bioavailability to microorganisms. Chemical degradation and biodegradation, on the other hand, are the two main mechanisms for pesticide elimination in soil with biochar amendment. Since current pesticides are engineered to be quickly dissolved, chemical hydrolysis is a key path of chemical and abiotic degradation in soil. Some research has indicated that when soil is modified with biochar, pesticide hydrolysis can be catalyzed, which has been linked to the effects of higher pH, delivered dissolved metal ions, and active groups on minerals (Zhang et al. 2013). Overall, biochar addition could be a viable and effective way to improve pesticide bioremediation in damaged soils.
Chemical degradation of pesticide in biochar-amended soils
Because of its catalytic properties, biochar can accelerate pesticide hydrolysis in soil. For example, to assess the effects of hydrolysis on carbaryl degradation, researchers autoclaved experimental soils at 120 °C for 30 min and found that the breakdown rate increased from 44.3 for the unamended soil to 55.0% for the biochar-implemented soil. The catalytic effects of biochar on pesticide hydrolysis, on the other hand, are dependent on various factors, which include the feedstock, pyrolysis temperature, and application rate. At 350 °C, carbaryl hydrolysis rates for pig manure biochar and maize straw, biochar were 55.0% and 52.8%, respectively (Ren et al. 2016). Furthermore, at 350 and 700 °C, biochar made from pig manure can hydrolyze 59.1% and 90.6% carbaryl, and 21.2% and 63.4% atrazine, respectively (Zhang et al. 2013). However, according to one study (Ren et al. 2016), the hydrolysis rate of carbaryl reduced from 53.7 to 50.0% as the pyrolysis temperature of rice straw biochar was increased from 350 to 700 °C. Investigations into the biochar application rate yielded the opposite results. The following phenomena could be induced by a variety of changes caused by different biochar applications, such as pH, dissolved metal ions, active groups, and pesticide sorption.
The pH of a pesticide’s chemical hydrolysis can have a significant influence. For example, base-catalyzed hydrolysis of the carbamate ester link in carbaryl, but atrazine is a fairly persistent herbicide that can be hydrolyzed in strong acidic or alkaline solutions. As a result, while an increase in pH caused by biochar may help carbaryl hydrolysis, it does not always help atrazine hydrolysis. Furthermore, the buildup of nucleophiles on the surface of biochar can aid pesticide degradation. Furthermore, hydroxy groups on the biochar surface may operate as nucleophiles, and attached metal atoms on the biochar surface may coordinate a hydrolyzable moiety by building complexes with pesticides, allowing water molecules to attack a nucleophilic ally. Furthermore, one study found that pesticide sorption could be increased in biochar-amended soil, lowering the concentration of free pesticide in the soil solution and potentially slowing the hydrolytic process, and decreasing the breakdown rate (Fig. 2). All of these factors should be considered when determining the pesticide hydrolysis rate. Overall, the catalytic effects of biochar could improve pesticide hydrolysis, but the increased pesticide sorption could diminish it (Jones et al. 2011).
Mechanism related to biochar for removal of contaminants. Biochar’s hypothesized interaction processes with inorganic pollutants. Physical adsorption is depicted as circles on the biochar particle. I, exchange of ions between the target metal and an exchangeable metal in the biochar; II, electrostatic attraction of an anionic metal; III, precipitation of the target metal; IV, electrostatic attraction of a cationic metal
Biodegradation of pesticides in soil treated with biochar
Biochar can influence pesticide biodegradation by affecting microorganism activity and pesticide bioavailability, as well as catalyzing pesticide hydrolysis. With a 0.5% application rate of pig dung biochar produced at 350 °C after 40 days of incubation, carbaryl breakdown efficiency rose from 55.0 to 75.0% in unsterile soil compared to sterile soil indicating enhanced pesticide biodegradation (Ren et al. 2016). Furthermore, the effect of biochar on pesticide biodegradation differed depending on the feedstock, pyrolysis temperature, and application rate. For example, one study found that using a 0.5% application rate, the enhancement of carbaryl biodegradation ranged from 19.5 to 27.3% for three biochar pyrolyzed from rice straw, pig manure, and maize straw at 350 °C, and from 3.1 to 27.3% for maize straw biochar’s produced at 350 and 700 °C, respectively (Ren et al. 2016). Furthermore, with application rates of 0.5% and 5%, the increase of pig dung biochar formed at 350 °C ranged from 13.8 to 20.0%. Biochar’s impacts on microbial characteristics and pesticide bioavailability are largely determined by its qualities. Biochar with high levels of amorphous carbon and liquid organic matter might boost microbial activity since these chemicals are easily digested by microbes as food. The activity of native microorganisms can be greatly affected (usually decreased) by the change in soil pH generated by biochar treatment (Ding et al. 2014). Furthermore, increased pesticide sorption after charcoal addition can reduce pesticide concentration in the soil solution, lowering pesticide bioavailability and biodegradation. According to one study, biochar inhibited microbial atrazine mineralization by interfering with the sorption and desorption processes, lowering atrazine bioavailability. As a result, pesticide sorption in biochar-amended soil could impact biodegradation.
Sorption of pesticides in soil treated with biochar
Pesticide sorption in soil treated with biochar can minimize pesticide mobility, volatilization, leaching, and plant uptake. However, as previously mentioned, the implementation of biochar in the soil may improve pesticide sorption, lowering pesticide concentrations in the soil solution and pesticide bioavailability to microorganisms, reducing chemical and biodegradation of the pesticide. Though both biochar and soil may absorb pesticides, biochar was found to be more effective than soil at absorbing pesticides. Pesticide sorption capacity on biochar is greatly influenced by biochar features such as organic carbon concentration, aromatic nature, specific surface area, and ash content (Fang et al. 2014). For example, one study (Zhang et al. 2013) found that carbaryl and atrazine adsorption was influenced by hydrophobic effects, pore-filling, and π-π electron donor-acceptor interactions. Furthermore, because some pesticides are weak bases and exist as neutral molecules, they can establish weak hydrogen bonds with carboxyl groups or the clay surface via their heterocyclic nitrogen atoms.
Biochar’s negative impact on soil biodiversity
Depending on the biochar and soil type, the effects of biochar on the soil microbial community may be negative, null, or positive. Organic pyrolytic products that are harmful to soil microorganisms, such as phenolics and polyphenolics, may be present in biochar. According to one study, after applying biochar, mycorrhizae and total microbial biomass decreased. According to one study, a decrease in microbial abundance and activity may be expected as a result of increased reservation of toxic substances such as heavy metals, like mercury, cadmium, and arsenic, and pesticides, as well as the release of pollutants from biochar such as bio-oil and polycyclic aromatic hydrocarbons. As a present point, it is unfair to conclude that a specific biochar that is beneficial to one soil biota will also be beneficial to others (Ennis et al. 2012). Several factors, including volatile matter, biochar properties, and salts such as Cl or Na, are most likely to blame for biochar’s negative effects on soil biota. In one study it was found that after using biochar without washing procedures to remove organic and inorganic matter, clover plants’ petioles withered and their leaves discolored. Furthermore, some biochar may show a direct threat to soil micro-organisms, and their functions may lead to lower crop yields as reported in the literature. And these effects may be rapid which must be taken into account for suitability as a soil modification (Fig. 3) .
Limitations/challenges in biochar
Most studies found that biochar amendments were helpful, although there are some limitations. As biochar slows down the soil’s aging process, therefore it is possible that adding new biomass to the ground regularly is necessary for a healthy soil’s nutrient cycling and water balance. In one study by (Anyanwu et al. 2018), it was found that biochar aged in soil inhibits the development of worms and/or fungi. The low thermal diffusivity of biochar has been also shown to be reflected in a decrease in soil thermal diffusivity (Zhao et al. 2016). Additionally, many reports of weed issues following biochar application have been made. Safaei Khorram et al. (2018) showed that a 200% increase in weed growth was observed when biochar was applied at relatively high rates (15 t ha−1) during lentil cultivation, implying that biochar applications may not be beneficial for weed control. Further, plants may experience a delay in flowering as a result of biochar application (Hol et al. 2017). Additionally, biochar’s capacity to adsorb pollutants is selective. For instance, biochar amendment in soil did not inhibit the uptake of the pesticide dichlorodiphenyltrichloroethane (DDT) (Denyes et al. 2016).
Biochar’s ability to absorb nitrogen, in addition to other critical elements like Fe (iron), maybe a detriment to plant growth (Kim et al. 2015). Also, biochar can compete with plant nutrients in the soil after reacting with them (Joseph et al. 2018). For instance, biochar and phosphorous fertilizer applied simultaneously in saline-sodic soil may improve phosphate precipitation/sorption processes. This interaction may have a role in limiting the amount of phosphorus available to plants throughout time (Xu et al. 2016). In terms of the biological effects of applying biochar to soil, it has been found that it can disrupt the breakdown of organic matter, leading to an 11 and 66% decrease in the abundance of fungal species including Ascomycota and Basidiomycota (Zheng et al. 2016). Lastly, feedstock availability might affect the price of biochar manufacturing. After accounting for all costs, Shackley et al. (2011) estimate that producing 1 ton of biochar would set you back between 148 and £389. Furthermore, charges related to regulatory difficulties and testing of the biomass feedstock might add to the total.
Future perspective
The soil properties of biochar-amended filed soils with a long aging time may differ significantly from those of laboratory-based short-term experiments, such as column and leaching studies. Nutrients released from “fresh” biochar are attributed to short-term increases in crop growth. However, one study hypothesized that the constant impact of biochar on soil nutrient availability is due to an increase in surface oxidation and CEC, which intensifies over time, and that this can result in greater nutrient reservation in “aged” biochar compared to “fresh” biochar. This mechanism must be demonstrated in the field over a long duration. Long-term studies determining nutrient dynamics in biochar-amended soil, however, are still required. Further research should be focused on predicting nutrient dynamics in biochar-amended soil by developing and improving available kinetics models in both laboratory and field settings. Understanding the various mechanisms affecting soil nutrient availability and fertility over time is critical for studying nutrient dynamics. According to one study, biochar has a high pesticide sorption capacity and can accumulate pesticide residues in soil. The release of pesticides from biochar, which could act as a new source of pollution, has not been taken into account in most short-term studies. As a result, it is preferable to assess the long-term environmental fate of pesticides that have been sequestered. Currently, the use of biochar for pesticide-polluted soil remediation is primarily based on laboratory, greenhouse, or small-plot short-term experiments. Nonetheless, field conditions are complex, and biochar properties can change over time due to aging, oxidation, or microbial degradation, affecting both pesticide adsorption and hydrolysis capacity. Future studies will therefore require large-scale and long-term field trials.
Conclusion
To increase sustainable agricultural productivity and conserve mineral deposits, integrated nutrient and pest management is required. Fertilizers and pesticides are important plant nutritional and protective agents for increasing crop production in agriculture development. However, fertilizer use efficiency in crop systems is typically very low. Furthermore, indiscriminate pesticide use can lead to severe environmental contamination. Biochar amendment in agricultural soil may be a suitable method for improving plant nutrient uptake and pesticide degradation. Biochar implementation can be done to enhance fertilizer utilization efficiency and soil fertility due to its large surface area, high number of functional groups, and good stability. Biochar can not only improve nutrient adsorption (e.g., NO3−, NH4+, and PO43−) thereby reducing nutrient leaching, but it can also reduce gaseous N losses. Furthermore, the nutrients that have been adsorbed by biochar can be released into the soil later (slow-release fertilizer). Furthermore, biochar has the potential to accelerate pesticide degradation in soil and reduce pesticide uptake by plants. On the one hand, the addition of biochar to soil may improve pesticide removal rates by catalyzing the chemical hydrolysis process. Microbial activities, on the other hand, may be increased after the application of biochar to polluted soil. Notably, pesticide sorption on biochar can reduce the free pesticide concentration in soil solution, thereby impeding the hydrolytic process (chemical degradation) and lowering pesticide bioavailability (biodegradation). As a result, pesticide sorption in biochar-amended soil may be detrimental to degradation. Overall, biochar amendment can improve overall soil health and crop yield by increasing fertilizer use efficiency, soil fertility, and pesticide degradation, resulting in a benefit for sustainable agriculture.
Further research should concentrate on the following identified knowledge gaps: (1) the constant impact of biochar on soil resources and large-scale field trials should be considered; (2) biochar quality varies with various biomass materials and pyrolysis conditions, necessitating the production of biochar specifically designed for soil management based on soil resources and environmental conditions; (3) to maximize the efficiency of pesticide remediation, the dynamic mechanisms of pesticides between microorganisms and biochar should be understood; (4) more research and investigation is needed to thoroughly look over the influencing factors for pesticide degradation by microorganisms and biochar; and (5) the synthesis and application of functionalized biochar as a potential material for soil amendment and remediation should be evaluated.
References
Anyanwu IN, Alo MN, Onyekwere AM, Crosse JD, Nworie O, Chamba EB (2018) Influence of biochar aged in acidic soil on ecosystem engineers and two tropical agricultural plants. Ecotoxicol Environ Saf 153:116–126. https://doi.org/10.1016/j.ecoenv.2018.02.005
Biederman LA, Harpole WS (2013) Biochar and its effects on plant productivity and nutrient cycling: a meta-analysis. GCB Bioenergy 5(2):202–214
Chakoosari MMD (2013) Efficacy of various biological and microbial insecticides. J Biol Today’s World 2(5):249–254
Chan KY, Van Zwieten L, Meszaros I, Downie A, Joseph S (2008) Using poultry litter biochars as soil amendments. Soil Res 46(5):437–444
Chen J, Chen D, Xu Q, Fuhrmann JJ, Li L, Pan G, Li Y, Qin H, Liang C, Sun X (2019) Organic carbon quality, the composition of main microbial groups, enzyme activities, and temperature sensitivity of soil respiration of an acid paddy soil treated with biochar. Biol Fertil Soils 55(2):185–197
Chew J, Zhu L, Nielsen S, Graber E, Mitchell DRG, Horvat J, Mohammed M, Liu M, van Zwieten L, Donne S, Munroe P, Taherymoosavi S, Pace B, Rawal A, Hook J, Marjo C, Thomas DS, Pan G, Li L, … Fan X (2020) Biochar-based fertilizer: supercharging root membrane potential and biomass yield of rice. Sci Total Environ 713:136431. https://doi.org/10.1016/j.scitotenv.2019.136431
Cross A, Sohi SP (2013) A method for screening the relative long-term stability of biochar. Gcb Bioenergy 5(2):215–220
da Silva Carneiro JS, Ribeiro ICA, Nardis BO, Barbosa CF, Lustosa Filho JF, Melo LCA (2021) Long-term effect of biochar-based fertilizers application in tropical soil: agronomic efficiency and phosphorus availability. Sci Total Environ 760:143955. https://doi.org/10.1016/j.scitotenv.2020.143955
Denyes MJ, Rutter A, Zeeb BA (2016) Bioavailability assessments following biochar and activated carbon amendment in DDT-contaminated soil. Chemosphere 144:1428–1434
Ding W, Dong X, Ime IM, Gao B, Ma LQ (2014) Pyrolytic temperatures impact lead sorption mechanisms by bagasse biochars. Chemosphere 105:68–74
Ding Y, Liu Y, Liu S, Li Z, Tan X, Huang X, Zeng G, Zhou L, Zheng B (2016) Biochar to improve soil fertility A review. Agron Sustain Dev 36(2):1–18
Domene X, Hanley K, Enders A, Lehmann J (2015) Short-term mesofauna responses to soil additions of corn stover biochar and the role of microbial biomass. Appl Soil Ecol 89:10–17
Ennis CJ, Evans AG, Islam M, Ralebitso-Senior TK, Senior E (2012) Biochar: carbon sequestration, land remediation, and impacts on soil microbiology. Crit Rev Environ Sci Technol 42(22):2311–2364
Fang Q, Chen B, Lin Y, Guan Y (2014) Aromatic and hydrophobic surfaces of wood-derived biochar enhance perchlorate adsorption via hydrogen bonding to oxygen-containing organic groups. Environ Sci Technol 48(1):279–288
Harvey OR, Herbert BE, Rhue RD, Kuo L-J (2011) Metal interactions at the biochar-water interface: energetics and structure-sorption relationships elucidated by flow adsorption microcalorimetry. Environ Sci Technol 45(13):5550–5556
Hol WHG, Vestergård M, ten Hooven F, Duyts H, van de Voorde TFJ, Bezemer TM (2017) Transient negative biochar effects on plant growth are strongest after microbial species loss. Soil Biol Biochem 115:442–451
Huang X, Liu Y, Liu S, Tan X, Ding Y, Zeng G, Zhou Y, Zhang M, Wang S, Zheng B (2016) Effective removal of Cr (VI) using β-cyclodextrin–chitosan modified biochars with adsorption/reduction bifunctional roles. RSC Adv 6(1):94–104
Jones DL, Edwards-Jones G, Murphy DV (2011) Biochar-mediated alterations in herbicide breakdown and leaching in soil. Soil Biol Biochem 43(4):804–813
Joseph S, Anwar HM, Storer P, Blackwell P, Chia C, Lin Y, Munroe P, Donne S, Horvat J, Wang J, Solaiman ZM (2015) Effects of enriched biochars containing magnetic iron nanoparticles on mycorrhizal colonisation, plant growth, nutrient uptake and soil quality improvement. Pedosphere 25(5):749–760. https://doi.org/10.1016/S1002-0160(15)30056-4
Joseph S, Kammann CI, Shepherd JG, Conte P, Schmidt H-P, Hagemann N, Rich AM, Marjo CE, Allen J, Munroe P (2018) Microstructural and associated chemical changes during the composting of a high temperature biochar: mechanisms for nitrate, phosphate and other nutrient retention and release. Sci Total Environ 618:1210–1223
Kim JA, Vijayaraghavan K, Reddy DHK, Yun Y-S (2018) A phosphorus-enriched biochar fertilizer from bio-fermentation waste: a potential alternative source for phosphorus fertilizers. J Clean Prod 196:163–171. https://doi.org/10.1016/j.jclepro.2018.06.004
Kim H-S, Kim K-R, Kim H-J, Yoon J-H, Yang JE, Ok YS, Owens G, Kim K-H (2015) Effect of biochar on heavy metal immobilization and uptake by lettuce (Lactuca sativa L.) in agricultural soil. Environ Earth Sci 74(2):1249–1259
Kumar A, Singh E, Mishra R, Kumar S (2022) Biochar as environmental armour and its diverse role towards protecting soil, water and air. Sci Total Environ 806:150444
Kuranchie-Mensah H, Atiemo SM, Palm LMN-D, Blankson-Arthur S, Tutu AO, Fosu P (2012) Determination of organochlorine pesticide residue in sediment and water from the Densu river basin, Ghana. Chemosphere 86(3):286–292
Laird D, Fleming P, Wang B, Horton R, Karlen D (2010) Biochar impact on nutrient leaching from a Midwestern agricultural soil. Geoderma 158(3–4):436–442
Lee Y, Park J, Ryu C, Gang KS, Yang W, Park Y-K, Jung J, Hyun S (2013) Comparison of biochar properties from biomass residues produced by slow pyrolysis at 500 C. Biores Technol 148:196–201
Ma YL, Matsunaka T (2013) Biochar derived from dairy cattle carcasses as an alternative source of phosphorus and amendment for soil acidity. Soil Sci Plant Nutr 59(4):628–641
Mohan D, Sarswat A, Ok YS, Pittman CU Jr (2014) Organic and inorganic contaminants removal from water with biochar, a renewable, low cost and sustainable adsorbent–a critical review. Biores Technol 160:191–202
Mukherjee A, Zimmerman AR (2013) Organic carbon and nutrient release from a range of laboratory-produced biochars and biochar–soil mixtures. Geoderma 193:122–130
Ren X, Zhang P, Zhao L, Sun H (2016) Sorption and degradation of carbaryl in soils amended with biochars: influence of biochar type and content. Environ Sci Pollut Res 23(3):2724–2734
Safaei Khorram M, Fatemi A, Khan MA, Kiefer R, Jafarnia S (2018) Potential risk of weed outbreak by increasing biochar’s application rates in slow-growth legume, lentil (Lens culinaris Medik). J Sci Food Agric 98(6):2080–2088
Scarlat N, Fahl F, Lugato E, Monforti-Ferrario F, Dallemand JF (2019) Integrated and spatially explicit assessment of sustainable crop residues potential in Europe. Biomass Bioenerg 122:257–269. https://doi.org/10.1016/j.biombioe.2019.01.021
Shackley S, Hammond J, Gaunt J, Ibarrola R (2011) The feasibility and costs of biochar deployment in the UK. Carbon Manage 2(3):335–356
Spokas KA, Novak JM, Venterea RT (2012) Biochar’s role as an alternative N-fertilizer: ammonia capture. Plant Soil 350(1):35–42
Steiner C, Glaser B, Geraldes Teixeira W, Lehmann J, Blum WEH, Zech W (2008) Nitrogen retention and plant uptake on a highly weathered central Amazonian ferralsol amended with compost and charcoal. J Plant Nutr Soil Sci 171(6):893–899
Tan X, Liu Y, Zeng G, Wang X, Hu X, Gu Y, Yang Z (2015) Application of biochar for the removal of pollutants from aqueous solutions. Chemosphere 125:70–85
Thakur N (2022) Physicochemical Analysis and Studies on Soil Profile of Prunus Armeniaca Collected from Mid-Hill of Himachal Pradesh. Natl Acad Sci Lett 45:541–548. https://doi.org/10.1007/s40009-022-01167-x
Thakur N, Sharma P, Govender PP et al (2022) Analysis and Extraction of Curcumin at Mid and Late Phase Harvested Curcuma Longa Samples Collected from Western Himalayan Regions. Chemistry Africa 5:1733–1742. https://doi.org/10.1007/s42250-022-00451-z
Tsai W-T, Liu S-C, Chen H-R, Chang Y-M, Tsai Y-L (2012) Textural and chemical properties of swine-manure-derived biochar pertinent to its potential use as a soil amendment. Chemosphere 89(2):198–203
Xu G, Zhang Y, Sun J, Shao H (2016) Negative interactive effects between biochar and phosphorus fertilization on phosphorus availability and plant yield in saline sodic soil. Sci Total Environ 568:910–915
Yanai Y, Toyota K, Okazaki M (2007) Effects of charcoal addition on N2O emissions from soil resulting from rewetting air-dried soil in short-term laboratory experiments. Soil Sci Plant Nutr 53(2):181–188
Yao Y, Gao B, Zhang M, Inyang M, Zimmerman AR (2012) Effect of biochar amendment on sorption and leaching of nitrate, ammonium, and phosphate in a sandy soil. Chemosphere 89(11):1467–1471
Yuan J-H, Xu R-K, Zhang H (2011) The forms of alkalis in the biochar produced from crop residues at different temperatures. Biores Technol 102(3):3488–3497
Zhang A, Cui L, Pan G, Li L, Hussain Q, Zhang X, Zheng J, Crowley D (2010) Effect of biochar amendment on yield and methane and nitrous oxide emissions from a rice paddy from Tai Lake plain, China. Agr Ecosyst Environ 139(4):469–475
Zhang P, Sun H, Yu L, Sun T (2013) Adsorption and catalytic hydrolysis of carbaryl and atrazine on pig manure-derived biochars: impact of structural properties of biochars. J Hazard Mater 244:217–224
Zhao J, Ren T, Zhang Q, Du Z, Wang Y (2016) Effects of biochar amendment on soil thermal properties in the North China Plain. Soil Sci Soc Am J 80(5):1157–1166
Zheng H, Wang Z, Deng X, Zhao J, Luo Y, Novak J, Herbert S, Xing B (2013) Characteristics and nutrient values of biochars produced from giant reed at different temperatures. Biores Technol 130:463–471
Zheng J, Chen J, Pan G, Liu X, Zhang X, Li L, Bian R, Cheng K, Jinwei Z (2016) Biochar decreased microbial metabolic quotient and shifted community composition four years after a single incorporation in a slightly acid rice paddy from southwest China. Sci Total Environ 571:206–217
Zwetsloot MJ, Lehmann J, Bauerle T, Vanek S, Hestrin R, Nigussie A (2016) Phosphorus availability from bone char in a P-fixing soil influenced by root-mycorrhizae-biochar interactions. Plant Soil 408(1):95–105. https://doi.org/10.1007/s11104-016-2905-2
Author information
Authors and Affiliations
Contributions
Both authors have contributed equally.
Corresponding author
Ethics declarations
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: Amjad Kallel
Highlights
1. Biochar has the potential to accelerate pesticide degradation in soil and reduce pesticide uptake by plants.
2. Biochar in soil may improve pesticide removal rates by catalyzing the chemical hydrolysis process.
3. Microbial activities.
On the other hand.
May be increased after the application of biochar to polluted soil.
4. Notably, pesticide sorption on biochar can reduce the free pesticide concentration in soil solution, thereby impeding the hydrolytic process (chemical degradation) and lowering pesticide bioavailability (biodegradation).
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Utkarsh, K., Thakur, N. & Shukla, S.K. Biochar: a feasible and visible solution for agricultural sustainability. Arab J Geosci 16, 239 (2023). https://doi.org/10.1007/s12517-023-11320-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12517-023-11320-5