Abstract
The effect of the terpenes α-pinene, eucalyptol, and limonene, individually and as mixtures, on atrazine (ATZ) biodegradation and on biological activity in a biobed biomixture was evaluated. Additionally, terpenes emitted from the biomixture were captured using solid-phase microextraction. Terpenes added individually at relatively low concentrations (50 μg kg−1) significantly enhanced ATZ degradation and biological activity during the first incubation days. No significant effect on ATZ degradation was found from adding the terpene mixture, and, interestingly, an inhibitory effect on phenoloxidase activity was found during the first 20 days of incubation when mixed terpenes were present at 100 μg kg−1. Capturing terpenes demonstrated that during the first hour of incubation a significant fraction of the terpenes was volatilized. These results are the first to demonstrate the feasibility of using terpenes to enhance the degradation of a pesticide. However, successive applications of terpenes or the addition of materials that slowly release terpenes could sustain the ATZ degradation enhancement.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
A factor influencing the success of modern farming and food production is the use of pesticides. However, inadequate pesticide management can lead to surface and groundwater contamination. Pesticides are generally released into the environment from diffuse (nonpoint) or localized (point) sources. Point sources, such as spillage during tank filling or the cleaning of spraying equipment, have been identified as posing major risks for the pesticide contamination of soil and water in agricultural systems (Gan and Koskinen 1998; Schulz 2004).
A biobed is a biopurification system used to minimize point source pesticide contamination in agricultural systems (Torstensson and Castillo 1997). Biobeds have mainly been developed using simple and cheap materials, and a typical biomixture is straw, peat, and soil in volumetric proportions of 50:25:25 % (Torstensson and Castillo 1997). Straw is the main component for ligninolytic fungal growth, soil provides sites for pesticide sorption and favors microbial activity, and peat contributes to the sorption capacity and helps moisture regulation in the biomixture (Torstensson and Castillo 1997; Castillo et al. 2008). Several studies have demonstrated that these biological systems can effectively retain and degrade a wide variety of pesticides (Coppola et al. 2007; Castillo et al. 2008; Vischetti et al. 2008; Tortella et al. 2012). However, there is a lack of studies focused on strategies to allow rapid pesticide degradation in a biomixture when high pesticide concentrations are added as a result of spillages during pesticide handling.
Biostimulation of organic-pollutant-degrading microorganisms by adding volatile organic compounds, such as terpenes, has been used to increase pollutant biodegradation in contaminated soils (Bento et al. 2005; Tyagi et al. 2011; Dudášová et al. 2012). Terpenes are the main volatile components released from plants, and they have similar structures to many organic pollutants. McLoughlin et al. (2009) demonstrated that the monoterpenes limonene, and α-pinene can stimulate the biodegradation of 2,4-dichlorophenol by indigenous soil microorganisms. Dudášová et al. (2012) evaluated the effect of plant materials containing terpenes (orange peel, tangerine peel, pine needles, and ivy leaves) on polychlorinated biphenyl (PCB) degradation by Pseudomonas stutzeri, and demonstrated enhanced PCB degradation when natural terpenes were added. Hernandez et al. (1997) reported that PCB degradation in soil was enhanced by adding plant wastes such as eucalyptus leaves, orange peel, pine needles, and ivy leaves.
The biostimulation of organic-pollutant-degrading microorganisms can possibly be attributed to the terpenes promoting the growth and proliferation of microorganisms and inducing the enzymatic activity involved in pollutant degradation (McLoughlin et al. 2009).
Atrazine (ATZ; 2-chloro-4-ethylamino-6-isopropylamino-s-triazine) is one of the most used agricultural herbicides and is considered to pose a potential risk to water quality because of its intense use and persistence. It has been reported that the main ATZ degradation pathway involves soil microorganisms (Barriuso and Houot 1996; Omotayo et al. 2011).
Although enhanced degradation of several compounds, including PCBs, in soil caused by adding terpenes has been well documented, pesticide biodegradation in complex samples, such as the biomixture of a biobed system, has not been evaluated. The main objective of our investigation was to evaluate the biostimulating effect of terpenes on ATZ degradation when high pesticide concentrations are added to a biomixture. We hypothesized that terpenes, added either individually or as a mixture, would increase biological activity in the biomixture, causing an increase in ATZ biodegradation.
Materials and methods
Chemicals
Analytical ATZ standards, 99 % purity, were purchased from Chem Service (West Chester, PA, USA). Formulated ATZ (ATRANEX 500SC) was purchased from Down Agrosciences, Chile. For analysis, a stock ATZ solution (1 g L−1) was prepared from the analytical standards in methanol. Three purified terpene solutions (99 % limonene [mixed isomers], 97 % α-pinene, and 99 % eucalyptol) were obtained from Sigma–Aldrich (Steinheim, Germany). 3-Methyl-2-benzothiazolinone hydrazone (MBTH) and 3-(dimethylamino) benzoic acid (DMAB) were purchased from Sigma–Aldrich. ATZ stock solutions for the degradation experiments were prepared by dissolving aliquots of commercial ATZ in distilled water.
Biomixture preparation
The biomixture was prepared as the traditional Swedish biomixture (Torstensson and Castillo 1997) by mixing top soil (0–20 cm depth, 30.7 % sand, 41.8 % silt, 27.4 % clay, 18 % organic matter, pH 6.1) with no history of ATZ application, commercial peat (39.67 % organic carbon), and winter wheat straw (43 % organic carbon) in volumetric proportions of 1:1:2, respectively. The straw was cut into small pieces (~3 mm) using a food processor, and soil and peat were sieved (to 3 mm). The constituents were mixing vigorously to obtain a homogeneous biomixture (Castillo et al. 2008). The biomixture moisture content was adjusted to 60 % of the water-holding capacity by adding distilled water. The mixture was put into polypropylene bags and stored in the dark at 20 ± 2 °C for 30 days before being used in the experiments.
Degradation studies
A bulk biomixture sample (approximately 7.0 kg dry weight) was separated into 231 subsamples (30 g each) and placed in glass flasks (250 mL). The typical (non-spillage) ATZ field dose is 2 mg kg−1, so, to simulate a pesticide spillage, 210 of the samples were treated with a suspension of formulated ATZ to give a final concentration of approximately 30 mg kg−1 of the active ATZ ingredient. 168 of the samples were then amended with terpenes to give final concentrations of 50 or 100 μg kg−1 of limonene, α-pinene, or eucalyptol, or a total concentration of 50 and 100 μg kg−1 of a mixture of all three terpenes (1:1:1). The remaining 42 samples were used as control samples (21 with ATZ but without terpenes and 21 without either ATZ or terpenes). The flasks were incubated in the dark for 60 days at 25 ± 2 °C, and the moisture level was maintained by periodically applying distilled water. Treated samples and control samples were removed immediately after mixing and at predetermined intervals, and stored at −20 °C until analysis. ATZ was extracted from the biomixture (10 g) by shaking (350 rpm, 2 h) and ultrasonication (30 min) with methanol (20 mL) followed by centrifugation (10,000 rpm). The supernatant (5 mL) was filtered with a PTFE membrane (0.2 μm pore size; Millipore, Billerica, MA, USA) and analyzed by liquid chromatography (HPLC) as described below. ATZ degradation in the biomixture followed first-order kinetics, and the concentration at any time (t) was described by the equation C = C0e−kt. The half-life was obtained from the equation t ½ = ln(2)/k.
Terpene trapping by headspace solid-phase microextraction (HS-SPME)
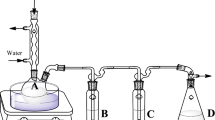
Terpenes released from the biomixture samples were trapped using a modification of the method reported by Leff and Fierer (2008) and Schalchli et al. (2011). Schott flasks (500 mL) were filled with 30 g of biomixture, 30 mg kg−1 of ATZ, and 100 μg kg−1 each of α-pinene, limonene, or eucalyptol, or a mixture of all three (each at 100 μg kg−1). The flasks were covered with silicone rubber seals (42 mm; Duran, Germany) and kept at 25 °C throughout sampling. Terpenes were absorbed by headspace solid-phase microextraction (HS-SPME) with a 100 μm polydimethylsiloxane/divinylbenzene (PDMS/DVB) fiber for 5 min at different incubation times (0.2, 1, 3, 6, 9, 12, and 24 h). Controls consisted of the biomixture without terpenes. Each treatment was performed in triplicate.
Determination of biological activities in the biomixture
Phenoloxidase activity was determined in all degradation assays using MBTH/DMAB (Castillo et al. 1994). Briefly, samples (10 g) of the biomixture were taken from the degradation assay flasks, agitated (150 rpm, 2 h) with 25 mL of a 100 mM succinate–lactate buffer (pH 4.5), and centrifuged (4,000 rpm, 20 min). The supernatant solution from each sample was collected and filtered through a 0.45 μm membrane and the phenoloxidase activity was measured immediately. The reaction mixture contained 300 μL of 6.6 mM DMAB, 100 μL of 1.4 mM MBTH, 30 μL of 20 mM MnSO4, and 1,560 μL of the filtered sample. The reaction was initiated with the addition of 10 μL of 10 mM H2O2, and was followed in a Spectronic Genesis 2PC spectrophotometer at 590 nm (ε = 0.053 μM−1 cm−1). No correction was made for the possible presence of lignin peroxidase (LiP) and laccase (Lac) activity, so the measurements represent the sum of manganese peroxidase, LiP, and Lac (Castillo and Torstensson 2007) activities and are expressed as phenoloxidase activity.
Hydrolytic activity was determined in all degradation assays by monitoring fluorescein diacetate hydrolysis (FDA), following a method published by Schnürer and Rosswall (1982) with slight modifications. Briefly, 1 g of biomixture taken from a degradation assay was incubated in a 30 mL conical flask with 9.9 mL of sterile 60 mM sodium phosphate buffer (pH 7.8). The reaction was initiated by adding 0.1 mL of a FDA solution (2.0 mg mL−1). After 1 h at 25 ± 1 °C, 10 mL of acetone was added to stop the reaction. A490 was measured spectrophotometrically after removal of the biomixture by centrifugation and filtration. The concentration of the fluorescein released was calculated using a calibration curve produced using standard quantities of FDA, and the results were expressed as μg FDA g−1 h−1. Controls with and without atrazine were included.
Pesticide analysis
HPLC analysis was performed using a Merck Hitachi L-7100 pump, a Rheodyne 7725 injector with a 20 μL loop, and a UV-diode array detector set at 290 nm. Separation was carried out using a Superspher RP-C18 column (5 μm particle size, 4.6 × 150 mm), with a mobile phase of methanol–water buffered with 1 mM ammonium acetate at a flow rate of 1 mL min−1 at room temperature (about 22 ± 1 °C). The mobile phase gradient started at 40:60 methanol:water (v/v), reached 80:20 methanol:water after 15 min, remained constant for 10 min, and returned to 40:60 methanol:water over 5 min. The atrazine recovery was >90 % in the biomixture. The ATZ retention time was 7 min and the detection limit was 0.01 mg L−1 in the aqueous phase.
Analysis of terpenes by GC–MS
The terpenes collected by SPME were analyzed using a Thermo Focus gas chromatograph (GC) (Thermo Electron Corp., Waltham, MA, USA) coupled to a Thermo DSQ mass spectrometer (MS) (Thermo Electron Corp.). Separation was achieved using a DBP-1 capillary column (30 m long, 0.2 mm i.d., 0.33 μm film thickness). The carrier gas was helium, at a flow rate of 1.5 mL min−1. A mass range of 35–500 m/z was monitored, and electron impact ionization was performed at 70 eV with the ion source at 200 °C. The SPME fiber was inserted into the GC injector for thermal desorption in splitless mode for 2 min, with the injector temperature held at 250 °C. The GC oven temperature started at 40 °C then increased to 260 °C at 5 °C min−1, then was held for 5 min (Schalchli et al. 2011). Terpenes were tentatively identified by comparing the experimental mass spectra with the NIST MS Search 2.0 library and commercial standards. The spectra were compared with the library database using a reverse search technique (Pesyna et al. 1976).
Statistical analysis
Experiments were conducted using three independent replicates of each treatment. Data were subjected to a one-way analysis of variance (ANOVA) and the averages were compared using Duncan’s multiple range tests at the 95 % confidence level. A two-way ANOVA was used to test for variation in the volatilization of terpenes from the biomixture.
Results and discussion
Degradation studies
The effects of the terpene applications on ATZ degradation in the biomixture systems are shown in Fig. 1. In general, up to 90 % of the ATZ initially added to the biomixture (30 mg kg−1) was degraded after 60 days of incubation in all treatments and controls. Particularly fast degradation (>50 % of the initial ATZ concentration) was seen during the first 10 days of incubation when limonene or eucalyptol was present at 50 μg kg−1. Significant differences (P < 0.05) in ATZ degradation rates were observed between treatments with the individual terpenes and the controls (Fig. 1a). Adding α-pinene or the terpene mixture caused only a slight increase in ATZ degradation compared with the controls during the first 20 days of incubation, and the increase was not significant (P > 0.05). No significant differences (P > 0.05) were found between the 100 μg kg−1 terpene treatments and the controls at any time (Fig. 1b). Our results are supported by Rhodes et al. (2007), who found low 2,4-dichlorophenol degradation rates in oak woodland and grassland soils amended with a mixture of p-cymene, limonene, and α-pinene, and high degradation rates in soils spiked only with p-cymene. Suttinun et al. (2004) reported that high concentrations of terpenes may be toxic to bacterial cells. The ATZ half-lives in the presence of 50 μg kg−1 of limonene or eucalyptol (9.4 and 9.5 days, respectively) were significantly lower than the half-life in the controls (13.2 days). In contrast, no significant differences in ATZ half-lives were found between 100 μg kg−1 terpene treatments and the controls (Table 1). Our findings demonstrate that stimulation or inhibition of ATZ-degrading microorganisms can occur in the biomixture differently when terpenes are added as a mixture or individually, and at different concentrations. Molecular biology studies of the terpene-amended biomixture might be necessary to assess the effects on ATZ-degrading microorganisms and to evaluate the expression of the genes involved in ATZ degradation (e.g., the atzA gene, demonstrated by Monard et al. (2010)).
Our results clearly show that the efficiency of ATZ degradation was improved quickly after the addition of 50 μg kg−1 of limonene and eucalyptol to the biomixture, but that the improvement was not maintained over time. This could be caused by the quick volatilization of terpenes (Suttinun et al. 2004) or by the terpenes being used up quickly by the microorganisms in the biomixture. Owen et al. (2007) reported that 71 % of 14C-geraniol added to soil was catabolized and used as a carbon source by microorganisms, and only approximately 1 % was volatilized. To evaluate the possibility that terpene volatilization occurred in the biomixture, we captured volatile organic compounds emitted from terpene-amended and control biomixtures during the first 24 h after amendment using SPME, the results of which are shown in Fig. 2.
Terpenes volatilized (% ± SE) from biomixture. The values indicate the percentage of recovery of different terpenes and mixture collected by solid-phase microextraction (SPME) and identified by gas chromatograph coupled to a mass spectrometer (GC–MS) from biomixture at different times (0.2, 1, 3, 6, 9, 12 and 24 h). Absence of letters indicates no significant differences based on the ANOVA (two-way) (P > 0.05)
The recovery of α-pinene at the start of the evaluations (0.2 h) was, at 57.2 ± 6.0 %, higher than that of limonene (48.7 ± 2.7 %) and eucalyptol (31.5 ± 7.2 %). The limonene and α-pinene recovery rates decreased during the first 3 h of the evaluation to 9.6 ± 2.1 %. In contrast, eucalyptol volatilized more slowly, with its recovery rate decreasing to 15.8 ± 1.6 % after 3 h. This behavior is explained by the vapor pressures of the terpenes, α-pinene and limonene having high vapor pressures (399.96 and 266.64 Pa, respectively) compared with eucalyptol (13.33 Pa). The terpene mixture recovery rate decreased from 56.3 ± 16 to 10.2 ± 1.7 % over the first 3 h of the evaluation, which was probably primarily caused by volatilization of α-pinene and limonene. However, no statistically significant differences between the terpene recovery rates over time were found using a two-way ANOVA to compare limonene with the terpene mixture (P = 0.0795), α-pinene with the terpene mixture (P = 0.081), eucalyptol with the terpene mixture (P = 0.2449), limonene with eucalyptol (P = 0.9273), eucalyptol with α-pinene (P = 0.9303), or limonene with α-pinene (P = 0.9999). Several authors have suggested that one of the benefits of using terpenes to stimulate the biodegradation of chemicals is that the soil microorganism population density increases (Suttinun et al. 2004). For example, Hernandez et al. (1997) demonstrated that adding terpene-rich residues to soil increased the population of soil microorganisms that can induce PCB degradation by a factor of 105. A second addition of terpenes during the first 3 h of incubation may, therefore, help improve the efficiency of the ATZ-degrading microorganisms.
Biological activities
Interestingly, adding terpenes to the biomixture stimulated phenoloxidase activity, with the exception of α-pinene at the highest concentration tested, as shown in Fig. 3. Adding 50 μg kg−1 of terpenes (Fig. 3a) caused a significant increase (P < 0.05) in phenoloxidase activity during the first 20 days of incubation compared with the controls. Adding 50 μg kg−1 terpenes either individually or in a mixture increased phenoloxidase activity by a factor of two compared with the controls after 5 days of incubation, and similar results were found at 10 and 20 days (Fig. 3a). Phenoloxidase activity was similar in all treatments after 30 days of incubation, and no significant differences (P > 0.05) were found. An increase in phenoloxidase activity was observed when the biomixture was biostimulated with 100 μg kg−1 of individual terpenes (Fig. 3b), and high values (between 0.5 and 0.6 U kg−1) were found in the samples amended with α-pinene, eucalyptol, or limonene. However, low phenoloxidase activities were found during the first 20 days of incubation (up to 0.25 U kg−1) when the terpenes were added as a mixture at 100 μg kg−1 (Fig. 3b). After 20 days the phenoloxidase activity in the samples with the terpene mixture added recovered to levels similar to the samples treated with individual terpenes. It is clear that a high concentration of terpenes added as a mixture inhibited phenoloxidase activity during the first days of incubation. However, the precise mechanism for the effect on phenoloxidase activity is unclear, and more detailed studies are necessary to better understand if the terpenes were toxic to the microorganisms or inhibited the enzymatic activity in the biomixture. Some volatile organic compounds, such as monoterpenes and sesquiterpenes, have been identified as fungistatic or bacteriostatic compounds that can affect some enzymatic activities in soil (Insam and Seewald 2010).
Phenoloxidase activity after 60 days of incubation in biomixture contaminated with 30 mg kg −1 and amended with α-pinene, eucalyptol, limonene and mixture of these at a 50 and b 100 μg kg −1. Different letters indicate significant differences between treatment within each sampling time based in Duncan test (P ≤ 0.05; n = 3)
After 40 days of incubation, no significant differences (P > 0.05) in phenoloxidase activities could be seen between samples that had 50 and 100 μg kg−1 of added terpenes, and increased activity was seen in all of the treatments. However, the increase in phenoloxidase activity did not correlate with atrazine degradation:an increase in phenoloxidase activity was seen for both 50 and 100 μg kg−1 of added terpenes, but atrazine degradation was enhanced only when 50 μg kg−1 of terpenes were added. This indicates that other microorganisms and their enzymes, such as atrazine chlorohydrolase (Sene et al. 2010), could be participating in atrazine degradation, and were affected at high terpene concentrations in the biomixture. More detailed studies are necessary to clarify this.
Hydrolytic activity in the biomixture treated with terpenes is shown in Fig. 4. FDA hydrolysis showed similar behavior to the phenoloxidase activity, with a marked increase (P < 0.05) during the first 5 days of incubation at both 50 and 100 μg kg−1 of added terpenes compared with the controls (Fig. 4a, b). Interestingly, FDA hydrolysis decreased noticeably in all treatments after 45 days of incubation. Although terpenes can be used as carbon sources by soil microorganisms (Adamczyk et al. 2011), the decrease in FDA hydrolysis could be caused by depletion of the readily available carbon sources, including the added terpenes, in the biomixture after 45 days of incubation. This hypothesis is based on the behavior of both of the enzymatic activities we evaluated. FDA decreased markedly after 45 days of incubation (Fig. 4a, b) and phenoloxidase activity concomitantly increased (Fig. 3a, b), demonstrating that fungal metabolic activation in the biomixture occurred and, therefore, that more complex carbon sources, such as cellulose and lignin, were being used.
Fluorescein diacetate hydrolysis (FDA) after 60 days of incubation in biomixture contaminated with 30 mg kg −1 and amended with α-pinene, eucalyptol, limonene and mixture of these at a 50 and b 100 μg kg −1. Different letters indicate significant differences between treatment within each sampling time based in Duncan test (P ≤ 0.05; n = 3)
In contrast to the effect on phenoloxidase activity, FDA hydrolysis was not affected by the terpene mixture at either of the concentrations evaluated. This may be because FDA hydrolysis is a less specific enzymatic activity than is peroxidase, and includes the activity of a wide group of soil hydrolases, which are produced by several soil microorganisms. It is, therefore, very difficult to establish if a specific group of microorganisms is affected or stimulated by the presence of terpenes in the biomixture, and molecular tools, such as denaturing gradient gel electrophoresis, are necessary to evaluate this in greater detail. Amaral and Knowles (1998) reported that the effects of monoterpenes on soil microorganisms are complex, with the biological activity of some microbial groups being inhibited and others stimulated.
Conclusion
Adding terpenes to the biomixture enhanced ATZ degradation, especially when eucalyptol and limonene were added individually at a relatively low concentration. The stimulatory effect occurred during the first days of incubation, allowing quick degradation of ATZ and a decrease in its half-life. Both phenoloxidase and FDA activities were temporarily stimulated by the terpenes, but phenoloxidase activity was negatively affected when a mixture of terpenes at 100 μg kg−1 was added.
We demonstrated that ATZ degradation can be enhanced in the short time after a pesticide spillage by the direct application of terpenes. However, successive applications of terpenes could improve the long-term degradation efficiency. Although successive terpene applications could be seen as a way of sustaining microbial activity and improving ATZ degradation in the long-term, it is likely to have a high financial cost. Therefore, an attractive alternative could be using natural residues that have high terpene contents, such as orange peel and eucalyptus leaves, which would cost less. The ability of these alternatives to sustain the biological activity of the ATZ-degrader microorganisms in the biobed system should be evaluated.
References
Adamczyk S, Adamczyk B, Kitunen V, Smolander A (2011) Influence of diterpenes (colophony and abietic acid) and a triterpene (beta-sitosterol) on net N mineralization, net nitrification, soil respiration, and microbial biomass in birch soil. Biol Fertil Soils 47(6):715–720
Amaral JA, Knowles R (1998) Inhibition of methane consumption in forest soils by monoterpenes. J Chem Ecol 24(4):723–734
Barriuso E, Houot S (1996) Rapid mineralization of the s-triazine ring of atrazine in soils in relation to soil management. Soil Biol Biochem 28:1341–1348
Bento FM, Camargo FAO, Okeke BC, Frankenberger WT (2005) Comparative bioremediation of soils contaminated with diesel oil by natural attenuation, biostimulation and bioaugmentation. Bioresour Technol 96(9):1049–1055
Castillo MdP, Torstensson L (2007) Effect of biobed composition, moisture, and temperature on the degradation of pesticides. J Agric Food Chem 55(14):5725–5733
Castillo MdP, Stenström J, Ander P (1994) Determination of manganese peroxidase activity with 3-methyl-2- benzothiazolinone hydrazone and 3-(dimethylamino) benzoic acid. Anal Biochem 218(2):399–404
Castillo MdP, Torstensson L, Stenström J (2008) Biobeds for environmental protection from pesticide use-a review. J Agric Food Chem 56(15):6206–6219
Coppola L, Castillo MdP, Monaci E, Vischetti C (2007) Adaptation of the biobed composition for chlorpyrifos degradation to southern Europe conditions. J Agric Food Chem 55(2):396–401
Dudášová H, Lukáčová L, Murínová S, Dercová K (2012) Effects of plant terpenes on biodegradation of polychlorinated biphenyls (PCBs). Int Biodet Biodeg 69:23–27
Gan JY, Koskinen WC (1998) Pesticide fate and behavior in soil at evaluated concentrations. In: Kearney PC, Roberts TR (eds) Pesticide remediation in soil and water. Wiley, Chichester, pp 59–84
Hernandez BS, Koh SC, Chial M, Focht DD (1997) Terpene-utilizing isolates and their relevance to enhanced biotransformation of polychlorinated biphenyls in soil. Biodegradation 8(3):153–158
Insam H, Seewald MSA (2010) Volatile organic compounds (VOCs) in soils. Biol Fertil Soils 46(3):199–213
Leff JW, Fierer N (2008) Volatile organic compound (VOC) emissions from soil and litter samples. Soil Biol Biochem 40(7):1629–1636
McLoughlin E, Rhodes AH, Owen SM, Semple KT (2009) Biogenic volatile organic compounds as a potential stimulator for organic contaminant degradation by soil organisms. Environ Pollut 157(1):86–94
Monard C, Martin-Laurent F, Devers-Lamrani M, Lima O, Vandenkoornhuyse P, Binet F (2010) Atz gene expressions during atrazine degradation in the soil drilosphere. Mol Ecol 19(4):749–759
Omotayo AE, Ilori MO, Amund OO, Ghosh D, Roy K, Radosevich MA (2011) Establishment and characterization of atrazine degrading cultures from Nigerian agricultural soil using traditional and Bio-Sep bead enrichment techniques. Appl Soil Ecol 48(1):63–70
Owen SM, Clark S, Pompe M, Semple KT (2007) Biogenic volatile organic compounds as potential carbon sources for microbial communities in soil from the rhizosphere of Populus tremula. FEMS Microbiol Lett 268(1):34–39
Pesyna GM, Venkataraghavan R, Dayringer HE, McLafferty FW (1976) Probability based matching system using a large collection of reference mass spectra. Anal Chem 48(9):1362–1368
Rhodes AH, Owen SM, Semple KT (2007) Biodegradation of 2,4-dichlorophenol in the presence of volatile organic compounds in soils under different vegetation types. FEMS Microbiol Lett 269(2):323–330
Schalchli H, Hormazabal E, Becerra J, Birkett M, Alvear M, Vidal J, Quiroz A (2011) Antifungal activity of volatile metabolites emitted by mycelial cultures of saprophytic fungi. Chem Ecol 27(6):503–513
Schnürer J, Rosswall T (1982) Fluorescein diacetate hydrolysis as a measure of total microbial activity in soil and litter. Appl Environ Microbiol 6:256–1261
Schulz R (2004) Field study on exposure, effects, and risk mitigation of aquatic nonpoint-source insecticide pollution: a review. J Environ Qual 33(2):419–448
Sene L, Converti A, Ribeiro-Secchi GA, Garcia-Simão RdC (2010) New aspect on atrazine biodegradation. Braz Arch Biol Technol 53(2):487–496
Suttinun O, Lederman BP, Luepromchai E (2004) Application of terpene-induced cell for enhancing biodegradation of TCE contaminated soil. Songklanakarin J Sci Technol 26(1):131–142
Torstensson L, Castillo MdP (1997) Use of biobeds in Sweden to minimize environmental spillages from agricultural spraying equipment. Pestic Outlook 8:24–27
Tortella GR, Rubilar O, Castillo MdP, Cea M, Mella-Herrera R, Diez MC (2012) Chlorpyrifos degradation in a biomixture of biobed at different maturity stages. Chemosphere 88(2):224–228
Tyagi M, da Fonseca MMR, de Carvalho CCCR (2011) Bioaugmentation and biostimulation strategies to improve the effectiveness of bioremediation processes. Biodegradation 22(2):231–241
Vischetti C, Monaci E, Cardinali A, Casucci C, Perucci P (2008) The effect of initial concentration, co-application and repeated applications on pesticide degradation in a biobed mixture. Chemosphere 72(11):1739–1743
Acknowledgments
This study was financed by Dirección de Investigación de la Universidad de La Frontera PIA-Project DI10-7004 and partially financed by Fondecyt Project 11100236 and Fondecyt 3110085.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tortella, G.R., Rubilar, O., Stenström, J. et al. Using volatile organic compounds to enhance atrazine biodegradation in a biobed system. Biodegradation 24, 711–720 (2013). https://doi.org/10.1007/s10532-013-9619-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10532-013-9619-4