Abstract
Adverse health effects of inhaled particulates have been noted for centuries, and studies over the last several decades have linked particle and fiber inhalation to several pulmonary diseases, including fibrosis, silicosis, chronic obstructive pulmonary disease, emphysema, asthma, hypersensitivity pneumonitis, and cancer, as well as cardiovascular disease. Inhaled particles and fibers can produce toxicity through several mechanisms, many mediated by the immune system. Particle and fiber toxicity is influenced by composition, size and shape of the material, as well as surface properties, including surface charge, protein corona, and surface contamination. Dissolution rate within the body can also influence toxicity. Although less extensively studied, some particles and fibers can also produce health effects when ingested and as a result of dermal contact. To help protect against adverse effects of particle and fiber exposure, regulatory agencies in numerous countries have developed exposure limits for airborne particulates in general, as well as for specific particles and fibers of special concern.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Chronic Obstructive Pulmonary Disease
- Inductively Couple Plasma Mass Spectrometry
- Idiopathic Pulmonary Fibrosis
- Inhalation Exposure
- Hypersensitivity Pneumonitis
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
5.1 Introduction
Particulate matter (PM) was in existence long before dinosaurs walked the earth, with volcanoes and forest fires contributing naturally to atmospheric PM levels. Humans have added to the production of particulates through activities such as the combustion of fossil fuels, production of industrial emissions, refuse incineration, and mechanical generation of dusts from agriculture, mining, and motor vehicle traffic. Humans are exposed to PM in air occupationally, at home, and in fact virtually everywhere they go. Recently, human exposure to PM and airborne fibers has expanded to include not only natural, anthropogenic and occupational sources, but also engineered particles and tubes on a nanometer scale (i.e., nanoparticles, NPs).
Human health problems associated with the inhalation of particulates such as silica have been reported since the ancient Greeks [83]. In the modern era, the toxicity of inhaled PM and fibers has been a concern in human health, many believe starting with the discovery in the 1930s that asbestos inhalation causes mesothelioma and fibrosis. In addition to asbestos, there were several air pollution incidents across Europe and the United States in the 1940s and 50s which raised awareness about the toxicity of inhaled PM. As one example, in December of 1952, London was trapped under a thick blanket of smog that lasted for 5 days before changes in the weather caused the air to clear. During this time period, it has been estimated that 100,000 people became ill, leading to the premature death of over 10,000 London residents [21]. This incident was important for the development of the 1956 Clean Air Act in the United States, one of the first environmental protection regulations.
This chapter presents an overview of particulate and fiber toxicology, focusing on inhalation exposure as this is generally considered to be the dominant pathway in terms of health risk for PM. Situations in which oral and dermal exposure to particles or fibers have been shown to cause toxicity will also be covered briefly.
5.2 Diseases Associated with Inhalation of Particles and Fibers
As part of their critical role in oxygenating blood, the lungs are responsible for filtering, humidifying and heating the air that we breathe. The role of filtration is very important, preventing systemic exposure to many toxicants in our environment. However, through the act of filtration, the lungs become uniquely susceptible to injury and disease. It is for this reason that inhalation toxicology has long been a key to understanding human health risks when considering environmental and occupational exposures to substances in air. The following is an overview of the diseases that can be caused by the inhalation of PM and fibers, with examples of exposure leading to illness provided from the epidemiological data.
5.2.1 Fibrosis
The flexibility of the lung is functionally a very important aspect of its structure, and is compromised by fibrosis. When the diaphragm contracts, the chest wall expands, pulling the lung with it and creating negative pressure inside the lung, required for air to flow inside. Fibrosis in the lung is the replacement of healthy tissue with inflexible connective tissue. With increased connective tissue, lung flexibility is compromised leading to decreased breathing efficiency. Severe fibrosis from a chronic exposure cannot only limit activity, but can also cause major organ failure and increased mortality rates.
Asbestos fibers have long been recognized to cause pulmonary fibrosis in humans. More recently, PM from several metals and other materials have also been shown to cause fibrosis in humans during occupational exposure, including aluminum, beryllium, chromium (VI), coal dust, cotton dust, wood dust, mineral dust, and livestock associated particulates [16, 35, 97, 105]. Exposure to metal dusts commonly occurs during welding. Epidemiological studies from five populations in the United Kingdom, the United States and Japan all show significant risk for fibrosis associated with exposure to metal dust from welding [97, 98]. After correcting for age and smoking status, two of these populations showed dose-dependent increases in risk, while one study was only able to show increased risk in the sub-population with the longest metal dust exposure period. Like all epidemiological data, these exposures are complicated by several factors involved in the welding process, including: welding materials, electrodes used, operating temperature, and exposure to gases (CO, CO2, NOx), as well as variation in confinement and ventilation of the work areas [12]. In addition to welding exposure, it has been shown that chronic/occupational exposure to any form of dust is significantly associated with risk for development of idiopathic pulmonary fibrosis [61].
5.2.2 Silicosis
Silicosis is a fibrotic lung disease resulting from inhalation exposure to silica dust. Silica is the most abundant mineral on earth, comprising 67 % of sandstone and up to 40 % of granite. Silica dust is generated during several occupations: quarrying, milling, mining, sand blasting, grinding, excavation, digging, hammering and even ceramics and pottery work. These occupations generate silica dust with particle sizes less than 10 μm, a size sufficiently small enough to be inhaled deep into the lung, leading to disease. The pathology associated with silica inhalation has led to Occupational Safety and Health Administrations (OSHA) to set air quality limits of 0.025–0.35 mg/m3 (varies by country) for silica dust generation to limit occupational exposure [54].
A tunnel digging project in Hawk’s Nest, West Virginia, USA in the 1930s was probably the first major incident to bring attention to the occupational hazards of inhaling silica dust. Of 2,500 underground-exposed workers, 88 % eventually developed silicosis. These events led to 46 of the US states passing occupational safety laws for silica exposure in 1937 [99]. Between 1968 and 2002, the age adjusted mortality rate due to silica exposure has decreased by 93 %; with a mortality rate of 8.9 deaths per million in 1968, dropping to 0.7 deaths per million in 2004 [54, 99]. Despite a decrease in the number of annual deaths due to silicosis, the United States Occupational Safety and Health Administration estimated there were two million industry workers occupationally exposed to silica dust in 2007 [99]. While the annual silica exposure in the United States remains quite high, China leads the way in the number of patients, diagnoses and deaths attributed to silicosis annually. Between 1991 and 1995, there were 500,000 cases of silicosis diagnosed in China, with 6,000 new diagnoses and 24,000 deaths annually [54]. The incidence of silicosis has increased over the last 30 years in some countries; for example, autopsy results from South African gold miners has shown an increase from 3 % to 32 % and 18 % to 22 % for black and white gold miners, respectively, from 1975 to 2007[54]. However, as illustrated with the mortality rate of the United States, the opposite trend is seen in more developed countries due to more stringent occupational safety standards, e.g. use of respirators [99].
5.2.3 Chronic Obstructive Pulmonary Disease and Emphysema
Chronic obstructive pulmonary disease (COPD) is characterized by chronic/progressive irreversible airflow obstruction and is diagnosed by symptoms (e.g., dyspnea, cough, sputum production) and airflow measurement with a spirometer [95]. COPD has been associated with occupational exposures for six centuries and is the fourth leading cause of morbidity and death related to chronic disease [24]. The frequency of COPD has increased over the last 20 years and there are an estimated 16 million people living undiagnosed with COPD in the United States alone [95]. While the leading cause of COPD is cigarette smoking (80–90 % of cases), recognized by “smokers cough”, only about 80 % of COPD deaths can be attributed to smoking [7]. However, Blanc et al. also state that there is a synergistic risk relationship between occupational PM exposures and cigarette smoking with an 18-fold increased risk for COPD for these individuals compared to non-exposed smokers. Sources estimate that at least 15 % of COPD deaths are related to occupational exposures, with an estimated 6 % of all COPD cases being among never smokers [7, 11, 95]. Other groups estimate there is a population attributable risk (PAR) for COPD due to occupational exposure of 31 % for never smokers in the rubber, plastics, leather and textile manufacturing industries for US workers [11]. Also summarized by Boschetto et al., a study looking at Swedish workers shows that 52.6 % of COPD cases among never smokers were attributable to occupational exposure to mineral and wood dusts. Heavy metal fumes, mineral dust (coal, hardrock mining and silica) and organic dust (e.g., cotton and hemp) exposures have also been associated with COPD risk [95]. Ambient air exposure and black smoke levels have been associated with COPD deaths in a population of emergency department admissions in Barcelona [48]. Underground coal mining has shown association with emphysema (a significant aspect of COPD) by other groups, with increased risk for emphysema among both never and ever smokers [53, 103].
Animal models have shown that inhalation exposure to cadmium, coal and silica dust have led to the development of emphysema and COPD-like symptoms [11]. However, animal models for emphysema use an α1-antityrpsin deficient mouse model that is not completely representative of the human condition, and is most appropriate for genetically susceptible humans. Another confounding property of analyzing COPD epidemiological data is the lack of consistency in disease description and diagnosis over the last several decades. Some studies make associations between occupational exposures and specific symptoms, while others only use COPD diagnoses for epidemiological analysis [11, 82].
5.2.4 Asthma
Rodent models for asthma are not completely predictive of the human condition. For this reason epidemiological data are often used to investigate how factors (e.g., PM) can influence asthma rates in humans. A problem that plagues all epidemiological studies is the number of confounding variables that are inevitably going to exist. The interpretation of epidemiological data relating to asthma and PM exposure can be greatly influenced by environmental variability. PM is just one of six categories of air pollutants generally investigated: SO2, O3, NOx, CO and lead are also assessed for association with disease [101]. Separating the effects caused by each pollution component is often not possible, and it is unlikely that a location with significant contamination from only one category can be found. Factors such as air humidity must also be considered; high humidity, for example, will significantly increase airway permeability and associated risk for a given exposure [107]. For these reasons, finding significant associations between PM and asthma can be challenging.
While air pollution has long been suspected of being associated with asthma, several studies investigating the possible correlation between PM and asthma have failed to find a significant association [4, 40]. When subjects are separated by age, a significant association between PM pollution and asthma in children was found [107]. Several other investigators have found this same association when separating their study group by age [20, 101, 106]. Another study has found significance in a more diverse age group, but only when analyzing with ethnicity as a factor, showing asthma incidence in African Americans to be associated with PM in the air [30].
5.2.5 Hypersensitivity Pneumonitis
Hypersensitivity pneumonitis (HP) in the lung has been observed after inhalation of several biological dusts and fungi during occupational exposures. These reactions require repeat exposures to PM less than 5 μm, with these sizes being able to penetrate deep enough into the lung to elicit an immune response [39]. In some cases HP can be directly induced by PM from birds or animals (e.g. fur and feathers) due to a particular protein allergy; however a majority of HP cases are the result of the Trojan horse effect, rather than direct particle induced toxicity. Materials such as wood and grain dusts can act as carriers for fungus and mycobacteria to invade the lung and induce allergic reactions leading to hypersensitivity reactions after repeat exposures [39]. These kinds of reactions can be controlled if exposure to the allergic agent is limited, however with chronic exposures HP can lead to granuloma development and even pulmonary fibrosis and emphysema [72]. A non-comprehensive list of occupational HP-like diseases is provided in Table 5.1, along with the carrier agent that delivers the fungal/bacterial allergen.
5.2.6 Cancer
Inhalation of PM and fibers has been associated with the development of cancer, particularly of the lung. One confounding variable in the assessment of PM exposure and human lung cancer is the typical latency period of decades between the time of exposure and cancer development in humans [105]. When a long latency period exists, it is difficult to assume causation, and instead only associations can be made between lung cancer in humans and exposure to a given particulate. There are several occupational sources of PM exposure, as well as specific elemental exposures that have been identified as occupational carcinogens: welding fumes, wood dust, asphalt fumes, diesel exhaust, coke oven emissions, asphalt fumes, carbon black, asbestos, cadmium, nickel, silica (quartz), and chromium (VI) [14].
Inhalation of asbestos fibers leading to mesothelioma was probably the first instance in which human cancer was shown to be caused by inhalation of fibers or PM. The carcinogenic potential of asbestos and other fibers is related to their length and biopersistence (Fig. 5.1) [22, 65]. Occupational exposure to welding fumes has also been associated with lung cancer development in several cohorts from countries throughout the world. In 1990, the International Agency for Research on Cancer (IARC) stated that there were 30–50 % more lung cancer incidents among welders compared to a control population [93]. This group analyzed standardized incidence ratios (SIR) of lung cancer in Danish welders working between 1964 and 1984, with more than 21 years of experience, and found a SIR of 1.35 (95 % CI 1.06–1.70). Investigators analyzing cancer risk for welders in Romania, Hungary, Poland, Russia, Slovakia, Czech Republic and the United Kingdom found an odds ratio (OR) of 1.18 (95 % CI 1.01–1.38) when correcting for independent metal exposure to chromium, nickel, cadmium and arsenic [57]. A meta-analysis of risk for specific welding-related jobs was performed for welders in the United States, United Kingdom, Canada, Sweden, New Zealand, Italy, Norway, France, Uruguay, Netherlands, Germany, Argentina, Finland, and Denmark finding no difference in risk between welding jobs, with an overall combined relative risk (CRR) of 1.26 (95 % CI 1.2–1.32) for lung cancer [3]. Investigators analyzing lung cancer risk in Finnish welders born between 1906 and 1945 found significant risk in all exposure groups [90]. For the lowest exposure group, there was an increased RR for all combined cancers (1.09: 95 % CI 1.05–1.14) and for small cell carcinoma (1.15: 95 % CI 1.04–1.27). The medium exposure group was associated with increased risk for all combined cancers (1.16: 95 % CI 1.03–1.31), for squamous cell carcinoma (1.26: 95 % CI 1.04–1.53) and adenocarcinoma (1.42: 95 % CI 1.06–1.91). High exposures were associated with increased risk for squamous cell carcinoma only (1.55: 95 % CI 1.08–2.24) [90]. As mentioned above, specific welding exposure is affected by the materials used, ventilation and operating conditions. Large meta-analyses like these are very important because they include welders from several occupational welding environments, which may help account for these variables.
Unlike most inhalation exposures, breathing leather dust during shoe making or repair has been shown to be associated with risk of sino-nasal (rather than lower respiratory) cancer in five of six analyzed cohorts [10]. Wood dust exposure is also associated with sino-nasal cancers; however, there are several other systemically diffuse cancers associated with wood working as well: digestive, urinary, respiratory, hemopoietic, lymphatic and Hodgkin’s disease [63]. Several studies, summarized by [63], have found significant risks for various cancers with hardwood dust exposure: beech, oak and walnut. Another group found epidemiological associations with both hard and soft woods with differential tumor development. Hardwood exposure was associated with adenocarcinoma while softwood dust exposure was associated with squamous cell carcinoma (SCC) and non-Hodgkin’s lymphoma [25]. In addition, the type of cancer which developed from softwood exposure seemed to differ based on the woodworkers’ occupation; joiners, carpenters and loggers developed SCC, while saw and planing mill workers had an increased risk for non-Hodgkin’s lymphoma. The fact that the specific wood working occupation affects the type of cancer that develops may indicate that the particle size of the wood dust generated is important for pathogenesis and deposition location. For wood dust exposure, it is important to consider whether the wood was treated and how either inherent or surface bound (e.g., fungi) properties of the wood may affect the toxicity. Wood that is intended for outdoor use is often treated with metal-based preservatives (chromium, arsenates, copper) to extend its lifetime; however these metals may cause toxicity after wood dust inhalation. Some of the inherent properties of timber that may conceivably be mutagenic were investigated and found to be innocuous using an in vitro mutagenicity test [62].
In addition to occupational exposures, there is a strong correlation between the development of lung cancer and exposure to atmospheric PM, with an 8 % increase in lung cancer mortality for every increase of 10 μg/m3 of fine PM [77]. Large anthropogenic contributions to atmospheric PM levels occur through the combustion products of fossil fuels, specifically in the form of diesel exhaust (DE). DE is composed mostly of various gases (e.g., CO2, CO, N2, SOx), hydrocarbons and their aldehyde derivatives, polyaromatic hydrocarbons and PM (elemental carbon) [80]. The particle size distribution of the PM fraction in DE has changed over the last several decades as engine types and operating conditions have evolved. Discussed in more detail later in the chapter, particle size affects the deposition location within the respiratory tract and the body’s ability to clear inhaled PM. The surface chemistry of the PM and adsorbed contaminants can also be variable based on post-emission reactions with oxygen and sunlight in the environment [80]. DE was placed on the IARC’s category 1 carcinogen list (known human carcinogens) in June 2012 and there have been several epidemiological studies published that show a significant association between inhalation exposure to DE and lung cancer [34]. One group of investigators found an odds ratio of 7.3 (95 % CI 1.46–36.57) for lung cancer among never-smokers in the highest exposure group of United States underground non-metal miners [91]. An increased risk for lung cancer due to DE exposure has also been observed in a population of Chinese industrial workers with 1–19 years of experience: OR of 12.39 with a 95 % C.I. of 2.4–63.94 [102]. This study did not adjust for smoking status; however, the estimated risk did account for age and familial/personal cancer and lung disease history. While these studies focus on chronic exposure to concentrated DE, it is important to consider that carcinogens may operate on a no-threshold model, with even low levels of DE exposure being capable of inducing carcinogenic effects in susceptible individuals.
5.2.7 Cardiovascular Effects
While an inhalation exposure is likely to target the lungs, there have been cardiovascular effects associated with PM in many inhalation studies. In addition, epidemiological data evaluating risk from air pollution show that increased risk in highly polluted areas can be attributed mostly to additional cardiovascular deaths. It is important to consider how particles are able to translocate from the lungs to the vascular system. It has been shown in rats, using an ex vivo lung perfusion, that nanoparticles administered to the lung are able to translocate to the circulation when lung permeability has increased (for example, due to histamine or H2O2, both of which may result from immune activation) [2].
The extent of particle translocation into the blood stream is size dependent with a large decrease in bioavailability as particle size increases [47]. Demonstrated with polystyrene particles, 240 nm seems to be the maximum diameter for uptake and translocation of particles into the blood stream following an inhalation exposure [46]. In addition to particle size, protein coating of particles is important for uptake. It has been shown that coating particles (of various materials: chitosan, poly-maleic-oleic acid and phosphatidylcholine) with surfactant protein A increases particle uptake into alveolar macrophages when compared to uncoated particles or particles coated with bovine serum albumin (BSA) [85]. Surfactant coating of particles has also been shown to be important for uptake via epithelial cells [47]. As particle size decreases, coating with surfactant becomes more efficient and may contribute to differences observed in translocation of particles based on size [26].
One of the major possible mechanisms of toxicity leading to cardiovascular effects would be interaction of absorbed particles with clotting factors. It was shown by [84] that TiO2 nanotubes are capable of decreasing blood clotting time through enhancing the formation of the fibrinogen network. In addition, interference with normal clotting behavior was observed for both positively and negatively charged polystyrene particles, capable of causing platelet activation [67]. These investigators hypothesize that positively charged particles are able to bind to sialic acid groups (negatively charged) on platelets, allowing enhanced platelet-platelet aggregation; the mechanism of interaction for negatively charged particles remains unknown.
Myocardial infarction and stroke are the two major risks associated with plaque formation and rupturing in the arteries. Any aspect of a particle or fiber that affects clotting or plaque stability has the potential to cause devastating cardiovascular problems. Most research done in this area is based on relative risk analysis of epidemiological data, with some conflicting trends. For example, some studies indicate that people exposed to air pollution are most susceptible to cardiovascular effects if they have increased fibrinogen blood levels [74, 78], while another study was unable to show such a correlation [89].
While fibrinogen levels have failed to form a solid link between PM air pollution and cardiovascular risks, there is strong evidence to indicate that levels of C-reactive protein (CRP) may be used to expose high cardiovascular risk groups in relation to air pollution. High levels of CRP have been associated with coronary artery disease and may be indicative of an unstable plaque present in the vasculature; more recently, an association between high PM exposure and high CRP levels has been elucidated [21]. Together, these trends may suggest that exposure to air pollution can induce CRP production, which in turn makes arterial plaques more susceptible to rupture, leading to stroke or heart attack. While these associations have shown to be significant when analyzing epidemiological data, the exact mechanisms underlying cardiovascular risks and air pollution are not well understood.
5.3 Mechanisms of Toxicity for Inhaled Particles and Fibers
5.3.1 Immune Mediated Effects
5.3.1.1 Dysregulation of Neutrophil Activity and Reactive Oxygen Species Generation
Polymorphonuclear neutrophils (PMNs) are an important mediator for the initiation of an immune response. Neutrophils are generally the first immune cells to be recruited to sites of injury and make up a majority of immune cells in the blood stream. Upon activation, PMNs will release chemokines and proinflammatory cytokines for the recruitment and activation of additional immune cells. This response is tightly controlled to prevent both inappropriate activation and down regulation of the inflammatory pathway. Upregulation of PMN activity can lead to the development of autoimmune diseases such as systemic lupus erythematosus (SLE) or rheumatoid arthritis (RA), while down regulation of PMN responses can lead to increased susceptibility to bacterial infections. Both activation and down regulation of inflammatory responses have been shown by studies investigating the interaction of PM and fibers with neutrophils.
A variety of responses can be observed with TiO2 particles of various sizes. Small, 1–10 nm, TiO2 particles were shown to induce IL-8 production, but were also shown to prevent PMN apoptosis. Larger TiO2 particles, (345 nm–3 μm) were shown to increase ROS production without causing cytotoxicity, while no response was registered with exposure of PMNs to 10 μm TiO2 particles [31, 52]. In addition to seeing differences in response based on size, material is also an important determinant of inflammatory reaction. Release of lactate dehydrogenase (LDH) from neutrophils was stimulated by 50 nm polymethylmethacrylate (PMMA) particles, while no response was observed for 50 nm polystyrene particles [70]. Differences in response based on material can also be observed with poly (lactic) acid (PLA) particles, in which coating the particle surface with polyethylene glycol (PEG) reduced reactive oxygen species (ROS) production from neutrophils. Neutrophil activation and increased mortality has also been shown to occur with nickel and vanadium particles, leading to LDH and superoxide release, respectively [52].
Despite the fact that many forms of PM and nanoparticles are capable of eliciting an inflammatory response that may mediate their toxicity, interaction with neutrophils can also cause a detoxification reaction. It has been shown that exposure of carbon nanotubes to myeloperoxidase (MPO) released by neutrophils decreases their overall inflammatory nature [45]. Using dynamic light scattering, this group showed that carbon nanotubes exposed to MPO have a decreased length compared to untreated nanotubes. Like asbestos fibers, there is a known positive correlation between nanotube length and toxicity; therefore, [45] hypothesized that the decrease in nanotube size is responsible for the observed decrease in inflammatory response.
5.3.1.2 T-Helper Cells, Type 1 and 2
The human population has extensive genetic variation, which affects individual responses to environmental factors. When investigating an inflammatory endpoint such as asthma, it is important to consider a person’s T helper cell profile. The body has two major types of T-helper cells, types 1 (Th1) and 2 (Th2). Th2 cells produce inflammatory cytokines like interleukins 4 and 5, previously shown to be associated with asthma in humans [33], while Th1 cells produce cytokines that are important for fighting infection. T-helper type 1 cells also help prevent the inflammatory response by releasing interferon-γ which inhibits Th2 cells. All humans have both Th1 and Th2 cells; the ratio between these two cell types can determine whether a person is predisposed to allergic and inflammatory diseases and reactions.
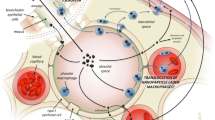
During fetal development we are exposed to maternal Th2 cells and are born with a Th2 dominant profile. Our postnatal environment will determine whether we develop a Th1 or Th2 phenotype as adults. Exposure to mycobacterium in soil has been shown to induce a Th1 type phenotype. This is the basis for the hypothesis that letting your children play outside can help prevent allergies. In contrast, inhalation of diesel exhaust particles and residual oil fly ash has been shown to induce a Th2 profile, exacerbating the inflammatory response and inducing fibrosis (See Fig. 5.2) [9].
5.3.1.3 Inflammatory Response and Fibrosis
Inhaled particles can create a pro-fibrotic environment through several immune-mediated mechanisms (Fig. 5.2). As mentioned above, inhaled particles that lead to a type 2-dominant T helper cell profile will induce an inflammatory response leading to IL-13 release; IL-13 will then induce TGF-β1 release, producing several pro-fibrotic downstream effects. Particles can also create these inflammatory conditions through the induction of TNF-α release, which, like IL-13 will induce TGF-β1. Both collagen deposition and release of connective tissue growth factor are downstream effects of TGF-β1 release, increasing the likelihood of developing fibrosis.
5.3.2 Genotoxic Mechanisms
Animal models can be helpful in assessing general carcinogenicity of a given toxicant; however, inter-species differences in cancer rates and target tissues make it difficult to accurately predict human neoplastic responses based entirely on rodent studies. Specific mechanisms of genotoxicity are investigated in vitro to add to the weight of evidence for carcinogenic potential of a given fiber or particle.
All forms of cancer follow a general developmental pathway, starting with initiation (gene mutation), followed by promotion (expansion of mutated population) and then progression (requires additional mutations) leading to cancer development. Particles and fibers contribute to initiation of carcinogenesis through genotoxic mechanisms. For an initiation event to occur, a genome mutation in a critical gene (cell cycle control or repair mechanisms) must avoid DNA repair and be retained through cell division. There are several possible mechanisms, both direct and indirect, for PM and fibers to cause DNA damage.
Oxidative stress is a common indirect method of DNA damage that can lead to tumor initiation. The DNA base pair guanine is susceptible to oxidation, converting to 8-hydroxy guanine. Guanine traditionally base pairs with cytosine; however, 8-hydroxyguanine does not undergo traditional G-C base pairing. If left unrepaired during cell replication, this mutation may ultimately lead to a base transversion from G-C to T-A pairing (illustrated in Fig. 5.3). If this mutation occurs in the coding region of a gene, there will be changes in the amino acid sequence for that protein, potentially leading to large downstream effects. The ability of asbestos fibers to cause this kind of mutation through oxidative stress has been reported by several groups and summarized by [44].
Direct DNA damage is also a common source of genotoxicity, with chromosomal deletions and strand breaks contributing to DNA mutations. Evidence for asbestos fibers causing this kind of genotoxic damage has also been reported [44]. Although genotoxicity of fibers and particles is a likely mechanism of their carcinogenicity, DNA damage is not required for a particle or fiber to be considered carcinogenic. Non-genotoxic mechanisms can contribute to the promotion of a mutation, rather than its initiation. This mechanism of action can be tested by analyzing the ability of a particle or fiber to cause cancer after administration of a known mutagen.
5.4 Particle and Fiber Properties Influencing Toxicity
When characterizing a given particle/fiber exposure or designing an inhalation study it is particularly important to consider dosimetry issues involved in the exposure. As with chemical exposures, the standard for describing exposure to particles and fibers has been on a mass concentration basis. Inhalation toxicology has studied the effects of micrometer-sized inhalants for several decades, but has only in the last 20 years started to focus on smaller, nanometer-sized particles. As these studies have been completed, it has become apparent that lower mass concentrations of nanometer-sized particles are required to elicit the same responses seen with higher concentrations of micrometer-sized particles; and several studies have suggested that expressing exposure response in terms of particle number or surface area can help to better estimate risk from a given exposure [51, 58, 68]. As discussed in more detail later in this chapter, decreases in particle size show an increase in the efficiency of lung deposition and bioavailability, possibly explaining the observed increased toxicity of smaller particles even at lower number concentrations [79]. One of the largest realistic challenges that we are facing with this consideration is occupational exposure monitoring; testing air quality for mass-based contamination is much more straight forward than testing particle number concentrations in a workplace [37, 58]. Another problem with using mass concentration as a measure of exposure is that an emphasis is placed on larger particles under mixed particle size exposure conditions due to the high mass percentage that large particles will account for [37, 51, 58, 75].
5.4.1 Size
The initial investigations into nanoparticle toxicity were stimulated by differences in effects that were observed between particles in the nanometer and micrometer size (based on the diameter of spherical particles). One example of size dependent effects can be demonstrated using TiO2 and inflammatory response in neutrophils, as described above with three size groups (1–10 nm, 345 nm–3 μm, and 10 μm) [31]. Differences in response based on particle size have also been observed for ROS generation after in vitro exposure to PM collected from the Los Angeles basin area from November 2001 to March 2002 [55]. When analyzing HO-1 expression in BEAS-2B human lung epithelial cells and RAW 264.7 murine macrophages, the smallest particles (ultrafine particles, < 150 nm) showed the most significant induction of HO-1 expression. While larger particles (coarse, 2.5–10 μm and fine, 0.1–2.5 μm) showed a dose dependent reaction for HO-1 induction, even the highest dose of both coarse and fine particles (100 μg/mL) showed a much smaller induction than the ultrafine particles (< 0.1 μm) at a low dose (8 μg/mL) [55]. HO-1 gene expression can be considered an endpoint for the induction of the antioxidant response element (ARE), indicative of ROS production. This group has also shown that exposure to ultrafine and fine particles lead to a 70 % glutathione (GSH) depletion (based on reduced to oxidized glutathione ratio, GSH:GSSG) in RAW 264.7 cells at 12 and 50 μg/mL respectively, compared to no response for coarse particles at 50 μg/mL.
In addition, it is important to consider the in vivo agglomeration state of PM or NPs, as this is a more significant determinant for clearance, deposition and translocation compared to primary particle size [47]. For example, if particle agglomeration is observed to increase with particle concentration, it is possible that a low concentration exposure may be more toxic than a higher concentration because larger, agglomerated particles are cleared more readily in the upper airways. There is an inverse relationship between particle size and retention. Mucociliary action can be considered a major source of particle clearance for large particles, leading to ingestion. However, as mentioned earlier, small particles are more efficient at penetrating the surfactant layer of the lung and it has been hypothesized that this removes the possibility for mucociliary removal. Micrometer sized particles that enter the lung are generally cleared within 24–48 h after exposure, while particles in the nanometer range show more than 75 % retention 48 h after exposure [64].
5.4.2 Shape (Aspect Ratio for Fibers)
There are a few major factors that influence the toxicity of fibers and carbon nanotubes that have been recognized throughout the literature as important aspects for predicting associated risk. First, the in vivo fiber or tube length is very important for determining toxicity, with long particles being most toxic for two reasons. One reason is that long particles are difficult to clear, penetrating very deep within the lung. Macrophages are very efficient at phagocytizing foreign objects in their environment, and will attempt to clear any fibers or nanotubes. This mechanism of clearance is good for small tubes and fibers because the entire fiber can be engulfed by the cell; the problem arises when the fibers are too long to be fully encapsulated by a macrophage. In addition to clearance, long fibers and nanotubes are more toxic than shorter fibers because they seem to have a higher capacity for the generation of reactive species. Another important aspect determining fiber toxicity is the degree to which fibers dissolve or break down in the lung or other biological environment. The most toxic fiber and nanotube exposures have resulted in a high number of long, biopersistent particles being deposited deep in the lung. The relationship between fiber length, biopersistence and toxicity is illustrated in Fig. 5.1.
5.4.3 Surface Charge
Differences in biological response based on particle surface charge have been observed for several endpoints. One example, mentioned briefly earlier, is the effect of polystyrene particles on platelet activation. [67] investigated neutral, positive and negatively charged polystyrene particles with different effects observed for all particle surface chemistries. This group showed increased platelet activation at 50 μg/kg for positively charged particles and at 100 μg/kg for negatively charged particles, while neutral particles were non-toxic, even at doses of 5,000 μg/kg [67].
In general, positively charged particles seem to have more inherent toxicity compared to neutral or negatively charged particles, as observed by several groups [5, 86, 108]. This is hypothesized to be due to particle interaction with negatively charged cell membrane surfaces. Interactions with the plasma membrane can cause a cell to become leaky and undergo apoptosis or necrosis. If a particle is phagocytized and a phagolysosome is formed, the particle has the potential to disrupt lysosomal membranes, leading to intracellular release of cytotoxic lysosomal contents (see Fig. 5.4). This trend was shown in a study by [15] in which particle toxicity was best correlated with ZP for particles with a charge greater than 14 mV. Furthermore, it was shown that particle toxicity was diminished by protein coating, preventing charge-mediated interaction of particles and membranes.
In addition to the direct effects of zeta potential on toxicity, the role that zeta potential plays on agglomeration behavior must also be considered [47]. A particle or fiber with a high absolute value zeta potential (mV > |30|) will be less likely to agglomerate compared to neutral or weakly charged particles. If toxicity occurs as a surface area mediated effect, then particle agglomeration will decrease toxicity, correlating with decreases in surface area. However, it is important to consider the particle/fiber type and the role that zeta potential may play on toxicity, e.g., cationic particles causing lysosomal membrane disruption.
5.4.4 Protein Corona
It has been widely accepted that particles and fibers that enter a biological environment will quickly become coated with proteins [13, 29, 104], with some exceptions (e.g., PEG surface coating prevents protein binding). In many cases, protein coating appears to lessen observed toxicity compared to particle exposures in a protein-free environment [15]. The exact mechanisms by which proteins alleviate toxicity are not completely understood and vary based on the mode of action for a given particle or fiber. One possible protective mechanism is illustrated in Fig. 5.4. As described above, particles with high positive zeta potentials have been shown to be toxic, possibly through interaction with negatively charged membranes. Figure 5.4 illustrates how particles with a protein corona may be unable to interact with the lysosomal membrane because their charge is masked at the surface.
In addition to mediating toxicity, endogenous protein coating diminishes biological activity and immune recognition of foreign particles [92]. However, there are other studies published that provide evidence that proteins bound to the particle surface undergo changes in conformation [17]. Based on this observation, it can be theorized that an immune-mediated reaction may be generated if new, “non-self”, epitopes are exposed during particle binding, although this has not yet been reported in the literature.
5.4.5 Particle Surface Contamination
PM, engineered nanoparticles, fibers and nanotubes all have the capacity to induce toxicity through what is called the trojan horse effect. This means that rather than the core particulate material itself causing toxicity, contaminants bound to the surface of particles and fibers may be causing toxicity. PM from the combustion of fossil fuels may be contaminated with engine lubricants or unburned gasoline. In support of the trojan horse theory is the knowledge that PM from different sources cause different toxicities [32]. In addition to source, the season in which the particulates are generated has been shown to affect toxicity [36]. These trends suggest that PM toxicity is related not to the core material, but differences in the environment and climate where it was generated.
Engineered nanoparticles, although created in a controlled environment, are not exempt from being contaminated in this fashion. It is important for engineered NPs to be tested for residue surfactant impurities prior to doing any toxicity testing. Surfactants are amphiphilic molecules that are often used for synthesis of nanoparticles, but can disrupt membrane function due to their chemical nature. This form of unintentional toxicity has been reported for CTAB contaminated gold nanorods in cell culture testing [1]. Like engineered NPs, carbon nanotubes (CNT) have several possible sources of contamination from the manufacturing process, including metals and organics [22]. Metals such as cobalt, iron, nickel and molybdenum are used as catalysts for CNT formation. Organics, like carbon dust are used as a raw material for synthesis and may be found bound to nanotubes. In addition to contaminants that originate from synthesis, surfactants are sometimes used to achieve complete dispersion of CNTs before administration to animals or cell culture during toxicity testing [22]. Due to the various sources of particulate and fiber contamination, it is very important to consider how surface bound materials may be confounding your results when conducting or analyzing a toxicity study involving PM or engineered nanomaterials.
5.4.6 Particle Dissolution
Metals are being used extensively in nanomaterials for both electronics and medical supplies to take advantage of several inherent metallic properties, including electromagnetism, conductance, strength and antibacterial function. Metal particles present a unique situation in particle toxicology, in which observed toxicity may be caused directly by the particle or by metal ions that have dissolved from the particle surface. Teasing apart the ion versus particle effects can be challenging, and it is important to consider this dilemma when designing a study involving metal particles.
It has been shown for copper and zinc oxide particles that ion dissolution reaches between 90 % and 100 % after 24 h in a simulated lysosomal environment at pH 5.5 [15]. When analyzing observed toxicity for CuO and ZnO particles it was discovered that ionic dissolution was the particle property which correlated best with the measured inflammatory response. This was not completely surprising, as both copper and zinc ions have been previously shown to be toxic [66, 96]. For metal particles that undergo significant or complete dissolution in a relevant biological environment, toxicity can likely be predicted based on available ion toxicity data.
Recently, it has become apparent that metal particles are generated over time as wear debris in metal based hip replacements. Every year in the United States there are estimated to be 120,000 joint replacement surgeries [76]. The hardware for hip replacement surgeries are made of various materials: ceramic on ceramic (C-O-C), ceramic on plastic (C-O-P), metal on plastic (M-O-P) and metal on metal (M-O-M). Reported metal toxicity from wear debris in hip replacements is rare, with very few reported cases. However, when significant wear has occurred on the metal joint, the effects can be devastating and in some cases permanent. Cobalt, chromium and titanium are typically the metals used for hip replacements [76]. While titanium is biocompatible, there are well known toxicities associated with both cobalt and chromium; cobaltism from non-arthroplasty exposures has exhibited symptoms such as hearing and vision loss [94]. Trivalent chromium (Cr3+) is an essential trace metal, while hexavalent chromium (Cr6+) is a recognized carcinogen and can induce allergic dermal reactions [6]. In at least five of the reported cases of metal toxicity from hip arthroplasty, the toxicity occurred after a revision surgery replacing a fractured ceramic head with either an M-O-M or M-O-P device [41, 69, 73, 81, 94]. Despite careful removal of the ceramic pieces during revision, it is likely that residual ceramic material in the joint is responsible for increased metal wear. It is estimated that under standard post-operative conditions (without residual ceramics in the joint) that 1013 particles per year are generated with an average particle size of less than 50 nm [76]. These wear debris particles can become embedded in the surrounding soft tissue or be transported systemically via the lymphatics after phagocytosis by macrophages. Toxicity probably occurs when the particles dissolve, either in the area of the joint or in the lysosomal environment of a macrophage, exhibiting symptoms similar to Co and Cr ionic toxicity. Symptoms reported for these five individuals include hearing and vision loss (some permanent), dermatitis, several cardiac problems (e.g. fibrosis, atrophy), T cell cytotoxicity, weight loss, dysregulation of iron metabolism and paresthesia. Table 5.2 below summarizes the level of Co and Cr in these five patients for various body fluids, with reference values listed where available.
5.5 Oral Exposures to PM
Ingestion can also be a significant route of exposure to environmental and occupational PM. In addition to direct consumption of PM, inhalation exposures can also lead to ingestion of PM. Large particles or agglomerates can be efficiently cleared in the upper airways or by mucociliary action after inhalation, and are subsequently swallowed. The bioavailability of PM and nanoparticles in the gastrointestinal tract has been shown to be very low for polystyrene and gold particles, with less than 0.1 % of the administered dose found in any tissue for very small, 1.4 nm gold particles [42, 88]. Similar to exposure in the lung, systemic uptake of particles in the GI tract is size dependent with smaller particles being taken up more extensively.
Despite low oral bioavailability of particulates, when considering gastric exposure of metal particles, it is likely that dissolution plays a significant role to increase oral bioavailability. As discussed above, both ZnO and CuO particles have been shown to experience significant dissolution at pH 5.5 after 24 h in a simulated lung environment [15]; gastric exposure at pH 2 would likely cause even more rapid dissolution. When the particles dissolve, their bioavailability is likely to increase significantly; this trend is demonstrated by comparing tissue levels of silver between animals treated with particulate and soluble silver [56]. Oral exposure of both ZnO and Ag nanoparticles in rats has been shown to increase liver enzymes compared to vehicle controls, with accompanying histopathological changes observed [50, 71]. Unfortunately, we cannot say whether the observed toxicities were due to particle or ions. Many methods currently used to quantify metal concentrations in tissues, such as inductively coupled plasma mass spectrometry (ICP-MS) cannot distinguish between particles and ions. Recently, single-particle ICP-MS (SP ICP-MS) has been used to tackle this challenge in aqueous systems [60] and is becoming more common in the analysis of mammalian systems as well.
In addition to particles being absorbed into the blood stream from the GI tract, immune mediated uptake via Peyer’s patches in the lower small intestine is a significant source of exposure, especially for large particles. This form of uptake has been investigated mostly with polystyrene particles [43] which do not show any form of toxicity. However, if an immune-active particle was orally administered, both immune suppression and inflammatory activation are possible outcomes, depending on the material.
Direct damage to the intestinal mucosa caused by particles has not been thoroughly investigated in vivo. There is some evidence of ZnO particles causing intestinal inflammation after oral administration [71], although this could be due to the release of toxic Zn2+ ions. In addition, there have been a few in vitro studies investigating particle toxicity with an intestinal membrane model, caco-2 cells. Mechanisms of toxicity observed with the caco-2 model include generation of ROS and oxidative DNA damage, GSH depletion and disruption of cellular metabolism [27, 28]. These responses are very similar to observed lung cell culture toxicities discussed earlier.
Another possible mechanism of oral toxicity for PM or fibers is interference with the function of intestinal microbiota. Digestion, absorption and digestive immunity are all affected by our intestinal bacteria. Silver and zinc particles have recently been exploited in the nanoparticle industry because of their antibacterial properties [49, 59]; for example, embedded in food packaging materials. If there is release of those particles into food, oral exposure to antibacterial particles is possible. This source of toxicity has been investigated by one group in quail [87] where differences in the amount of gram-positive bacteria were observed.
5.6 Dermal Exposures to PM
Dermal contact with particulates and fibers can cause skin irritation, and in the case of certain metals such as nickel and cobalt, immune mediated contact dermatitis. Generally, risks of systemic effects from dermal exposure to particulates and fibers are considered to be very low. An interesting exception is beryllium. Occupational beryllium dust exposure can lead to chronic beryllium disease (CBD), a lung disease characterized by allergic beryllium sensitivity and granuloma formation in the lung. Sensitization is required, followed by progressive loss of pulmonary function. There is evidence suggesting that genetics may play a role is susceptibility [19, 100]. CBD is devastating disease, and the importance of worker protection has been recognized since the 1940s. A beryllium processing plant in Lorain, OH opened in 1947, only to close a year later due to the high incidence of lung problems and other health complaints in workers, as well as in residents of the surrounding town [8]. These and other early occupational exposures to beryllium lead to the regulation and monitoring of beryllium air levels in factories in 1949, limiting beryllium concentrations at 2 μg/m3 (levels at the Lorain, OH plant were over 43,000 μg/m3).
Due to presentation as a lung disease, it has long been assumed that inhalation exposure to beryllium dust is responsible for both sensitization and development of CBD. As a result, worker protection has historically focused on limiting beryllium dust inhalation. Despite the use of the most effective respiratory protection available, industry workers were still becoming sensitized to beryllium. It was subsequently found that particles were capable of penetrating human skin during simulated activity and, using a mouse model, that dermal contact with beryllium was sufficient to produce sensitization [100]. The development of CBD and the role that dermal sensitization may play is illustrated in Fig. 5.5. Following dermal sensitization to beryllium, even an inhalation exposure less than the standard 2 μg/m3, would be sufficient to lead to the development of CBD later in life. This dermal exposure presents a unique situation due to the downstream systemic effects that can occur. There is some evidence from a beryllium oxide ceramics plant that increased dermal protection can help to reduce sensitization rates among exposed workers, with workers from an old regime being 2.1–8.2 times more likely to become sensitized compared to workers under the new protection rules [18].
5.7 Particle and Fiber Exposure Limits
In an effort to protect the public from adverse health effects from particles and fibers, regulatory agencies in many countries have developed exposure limits. In some cases, these limits apply generally to particulate material in ambient air, but most exposure limits are directed to specific airborne particles and fibers of special concern. These are often developed in the context of occupational exposure standards because the workplace is viewed as where particle and fiber exposures are likely to be the highest. Exposure limits can take the form of recommended guidelines or regulatory agency standards. Although both guidelines and standards are useful in evaluating potential health consequences of inhalation of measured concentrations of particles and fibers in air, standards have the additional weight of being legally enforceable.
Air quality guidelines and standards can vary from one organization or agency to another for a variety of reasons. These can include differences in exposure assumptions or in the interpretation of toxicological data upon which the numerical standards are based, or in the level of protection (i.e., acceptable level of risk) sought. Examples of occupational exposure limits for several particles and fibers from the United States (Occupational Safety and Health Administration and the National Institute for Occupational Safety and Health), Great Britain (Health and Safety Executive), and Japan (Japan Society of Occupational Health) are shown in Table 5.3. Exposure limits can also differ for the same material based on exact occupational activity; see for example cotton dust in Table 5.3. Different exposure limits are sometimes provided for short time periods of exposure (< 1 h) versus a full work day of 8 or 10 h exposure; several examples of this can be found in the table.
Until recently, exposure limits were not explicit for nanomaterials; however, new exposure limits are being developed specifically for very small particles. The National Institute for Public Health and the Environment (RIVM) of the Netherlands has developed exposure limits for nanoparticles: e.g., 20,000 particles/cm3 for TiO2, ZnO, SiO2, Al2O3 and 40,000 particles/cm3 for C60, carbon black and carbon nanotubes.
5.8 Conclusions
Despite increased occupational safety regimes, human exposure to PM and fibers via several exposure routes is unavoidable due to the inherent properties of both the natural and modern environments that we live in. We’ve known for many centuries that inhalation exposure to natural PM can lead to adverse health effects in humans. However, in the last several decades, industrial exposures have broadened our understanding of the vast impact that PM and fiber exposure can have on large numbers of people. Industrial exposures causing disease have led to OSHA guidelines for exposure limits for various materials. Most modern exposures are difficult to fully comprehend due to simultaneous exposure to several compounds. This can be illustrated by the complex analysis of epidemiological data. Estimating risk for particle exposures is also complicated by dosimetry issues: comparing mass, surface area and number concentrations. While analyzing human exposure conditions and outcomes is an intricate process, numerous diseases have been linked to specific PM exposure in several human populations. These associations can in some cases be strengthened with animal exposures; however, human disease progression and presentation is often difficult to simulate in an animal model. Mechanistic investigation on the cellular and molecular levels can be helpful for further elucidation of human disease properties and in many cases involve complex genetic susceptibility. In addition, it is important to consider how intrinsic properties of the exposure material can vastly affect the health impacts observed, as well as how these properties may change over time, altering post-exposure characterization. The prediction and prevention of human disease related to PM exposure will continue to be a challenge, affecting both occupational safety and our everyday lives. As long as technology and human exploration expand, so will our potential exposures to PM and fibers.
5.9 Definitions, Abbreviations and Symbols
ARE | Antioxidant response element |
BSA | Bovine serum albumin |
CBD | Chronic beryllium disease |
CI | Confidence interval |
CNT | Carbon nanotube |
COC | Ceramic-on-ceramic |
COP | Ceramic-on plastic |
COPD | Chronic obstructive pulmonary disease |
CRP | C-reactive protein |
CRR | Combined relative risk |
CTAB | Cetyltrimethylammonium bromide: a surfactant used in the synthesis of Au nanorods |
DE | Diesel exhaust |
GI | Gastro-intestinal |
GSH | Glutathione |
GSSG | Oxidized glutathione |
HP | Hypersensitivity pneumonitis |
HSE | Health and safety executive UK |
IARC | International agency for research on cancer |
ICP | Inductively coupled plasma |
JSOH | Japan society of occupational health |
MOM | Metal-on-metal |
MOP | Metal-on-plastic |
MS | Mass spectrometry |
NIOSH | National institute for occupational safety and health, USA |
NP | nanoparticle |
OSHA | Occupational safety and health administration, USA |
PAR | Population attributable risk |
PEG | Polyethylene glycol |
PM | Particulate matter |
RIVM | National institute for public health and environment, The Netherlands |
ROS | Reactive oxygen species |
SCC | Squamous cell carcinoma |
SIR | Standardized incidence ratio |
SP | Single particle |
ZP | Zeta-potential |
Bibliography
Alkilany, A.M., Nagaria, P.K., Hexel, C.R., Shaw, T.J., Murphy, C.J., Wyatt, M.D.: Cellular uptake and cytotoxicity of gold nanorods: molecular origin of cytotoxicity and surface effects. Small 5(6), 701–708 (2009)
Alfaro-Moreno, E., Nawrot, T.S., Nemmar, A., Nemery, B.: Particulate matter in the environment: pulmonary and cardiovascular effects. Obstruct. Occup. Environ. Dis. 13, 98–106 (2007)
Ambroise, D., Wild, P., Moulin, J.J.: Update of a meta-analysis on lung cancer and welding. Scand. J. Work Environ. Health 32(1), 22–31 (2006)
Anderson, H.R., Butland, B.K., Donkelaar, A., Brauer, M., Strachan, D.P., Clayton, T., Dingenen, R., Amann, M., Brunekreef, B., Cohen, A., Dentener, F., Lai, C., Lamsal, L.N., Martin, R.V.: Satellite-based estimates of ambient air pollution and global variations in childhood asthma prevalence. Environ. Health Perspect. 120, 1333–1339 (2012)
Asati, A., Santra, S., Kaittanis, C., Perez, J.M.: Surface-charge-dependent cell localization and cytotoxicity of cerium oxide nanoparticles. ACS Nano 4, 5321–5331 (2010)
Barceloux, D.G.: Chromium. J. Toxicol. Clin. Toxicol. 37(2), 173–194 (1999)
Blanc, P.D., Eisner, M.D., Earnest, G., Trupin, L., Balmes, J.R., Yelin, E.H., Gregorich, S.E., Kayz, P.P.: Further exporlation of the links between occupational exposure and chronic obstructive pulmonary disease. J. Occup. Environ. Med. 51(7), 804–810 (2009)
Boffetta, P., Fryzek, J.P., Mandel, J.S.: Occupational exposure to beryllium and cancer risk: a review of the epidemiological evidence. Crit. Rev. Toxicol. 42(2), 107–118 (2012)
Bonner, J.C.: Lung fibrotic responses to particle exposure. Toxicol. Pathol. 35, 148–153 (2007)
Bonneterre, V., Deschamps, E., Persoons, R., Bernardet, C., Liaudy, S., Maitre, A., Gaudemaris, R.: Sino-nasal cancer and exposure to leather dust. Occup. Med. 57, 438–443 (2007)
Boschetto, P., Quintavalle, S., Miotto, D., Cascio, N.L., Zeni, E., Mapp, C.E.: Chronic obstructive pulmonary disease (COPD) and occupational exposures. J. Occup. Med. Toxicol. 1(11), 1–6 (2006)
Buerke, U., Schneider, J., Rosler, J., Woitowitz, H.J.: Interstitial pulmonary fibrosis after severe exposure to welding fumes. Am. J. Ind. Med. 41, 259–268 (2002)
Cedervall, T., Lynch, I., Lindman, S., Berggard, T., Thulin, E., Nilsson, H., Dawson, K.A., Linse, S.: Understanding the nanoparticle–protein corona using methods to quantify exchange rates and affinities of proteins for nanoparticles. PNAS 104(7), 2050–2055 (2007)
Centers for Disease Control and Prevention: . Occupational cancer: carcinogen list. NIOSH and CDC. Retrieved from http://www.cdc.gov/niosh/topics/cancer/npotocca.htm (2012, May 18). 02 June 2012
Cho, W.S., Duffin, R., Thielbeer, F., Bradley, M., Megson, I.L., MacNee, W., Poland, C.A., Tran, C.L., Donaldson, K.: Zeta potential and solubility to toxic ions as mechanisms of lung inflammation caused by metal/metal oxide nanoparticles. Toxicol. Sci. 126(2), 469–477 (2012)
Cohen, R.A.C., Patel, A., Green, F.H.Y.: Lung disease caused by exposure to coal mine and silica dust. Semin. Respir. Crit. Care Med. 29(6), 651–661 (2008)
Cukalevski, R., Lundqvist, M., Oslakovic, C., Dahlbäck, B., Linse, S., Cedervall, T.: Structural changes in apolipoproteins bound to nanoparticles. Langmuir 6(27), 14360–14369 (2011)
Cummings, K.J., Deubner, D.C., Day, G.A., Henneberger, P.K., Kitt, M.M., Kent, M.S., Kreiss, K., Schuler, C.R.: Enhanced preventive programme at a beryllium oxide ceramics facility reduces beryllium sensitization among new workers. Occup. Environ. Med. 64, 134–140 (2006)
Day, G.A., Stefaniak, A.B.., Weston, A., Tinkle, S.S.: Beryllium exposure: dermal and immunological considerations. Int. Arch. Occup. Environ. Health 79, 161–164 (2006)
Delamater, P.L., Finley, A.O., Banerjee, S.: An analysis of asthma hospitalizations, air pollution, and weather conditions in Los Angeles County, California. Sci. Total Environ. 425, 110–118 (2012)
Donaldson, K., Stone, V., Seaton, A., MacNee, W.: Ambient particle inhalation and the cardiovascular system: potential mechanisms. Environ. Health Perspect. 109, 523–527 (2001)
Donaldson, K., Aitken, R., Tran, L., Stone, V., Duffin, R., Forrest, G., Alexander, A.: Carbon nanotubes: a review of their properties in relation to pulmonary toxicology and workplace safety. Toxicol. Sci. 92(1), 5–22 (2006)
Fink, J.N., Lindesmith, L.A., Horvath, E.P.: Hypersensitivity pneumonitis. In: Zenz, C., Dickerson, O.B., Horvath Jr., E.P. (eds.) Occupational Medicine, 3rd edn, pp. 208–209. Mosby, St. Louis (1994)
Fishwick, D., Barber, C.M., Darby, A.C.: Review series: occupational and environmental lung disease; chronis obstructive pulmonary disease and the workplace. Chron. Respir. Dis. 7(2), 113–122 (2010)
Flechsig, R., Nedo, G.: Hazardous health effects of occupational exposure to wood dust. Ind. Health 28, 107–119 (1990)
Geiser, M., Kreyling, W.: Deposition and biokinetics of inhaled nanoparticles. Part. Fibre Toxicol. 20(7), 2 (2010)
Gerloff, K., Albretch, C., Boots, A.W., Förster, I., Schins, R.P.F.: Cytotoxicity and oxidative DNA damage by nanoparticles in human intestinal Caco-2 cells. Nanotoxicology 3(4), 355–364 (2009)
Gerloff, K., Fenoglio, I., Carella, E., Kolling, J., Albrecht, C., Boots, A.W., Förster, I., Schins, R.P.: Distinctive toxicity of TiO2 rutile/anatase mixed phase nanoparticles on Caco-2 cells. Chem. Res. Toxicol. 25(3), 646–655 (2012)
Gessner, A., Lieske, A., Paulke, B.R., Muller, R.H.: Influence of surface charge density on protein adsorption on polymeric nanoparticles: analysis by two-dimensional electrophoresis. Eur. J. Pharm. Biopharm. 54, 165–170 (2002)
Glad, J.A., Brink, L.L., Talbott, E.O., Lee, P.C., Xu, X., Saul, M., Rager, J.: The relationship of ambient ozone and PM(2.5) levels and asthma emergency department visits: possible influence of gender and ethnicity. Arch. Environ. Occup Health 67(2), 103–108 (2012)
Goncalves, D.M., Liz, R., Girard, D.: Activation of neutrophils by nanoparticles. Scienitific World J. 11, 1877–1885 (2011)
Gordon, T.: Linking health effects to PM components, size and sources. Inhal. Toxicol. 19, 3–6 (2007)
Graham, L.M.: All I need is the air I breathe: outdoor air quality and asthma. Paediatr. Respir. Rev. 5, 59–64 (2004)
Gulland, A.: Diesel engine exhaust causes lung cancer, says WHO. BMJ 4174, 1 (2012)
Gustafson, T., Dahlman-Höglund, A., Nilsson, K., Ström, K., Tornling, G., Torén, K.: Occupational exposure and severe pulmonary fibrosis. Respir. Med. 101, 2207–2212 (2007)
Ham, W.A., Ruehl, C.R., Kleeman, M.J.: Seasonal variation of airborne particle deposition efficiency in the human respiratory system. Aerosol Sci. Technol. 45, 795–804 (2011)
Heitbrink, W.A., Evans, D.E., Ku, B.K., Maynard, A.D., Slavin, T.J., Peters, T.M.: Relationships among particle number, surface area, and respirable mass concentrations in automotive engine manufacturing. J. Occup. Environ. Hyg. 6, 19–31 (2009)
Hesterberg, T.W., Miller, W.C., McConnell, E.E., Chevalier, J., Hadley, J.G., Bernstein, D.M., Thevenaz, P., Anderson, R.: Chronic inhalation toxicity of size-separated glass fibers in Fischer 344 rats. Fundam. Appl. Toxicol. 20(4), 464–476 (1993)
Hirschmann, J.V., Pipavath, S.N.J., Godwin, J.D.: Hypersensitivity pneumonitis: a historical. Clinical and radiological review. RadioGraphics 29, 1921–1938 (2009)
Hoek, G., Pattenden, S., Willers, S., Antova, T., Fabianova, E., Braun-Fahrländer, C., Forastiere, F., Gehring, U., Luttmann- Gibson, H., Grizes, L., Heinrich, J., Houthuijs, D., Janssen, N., Katsnelson, B., Kosheleva, A., Moshammer, H., Neuberger, M., Privalova, L., Rudnai, P., Speizer, F., Slachtova, H., Tomaskova, H., Zlotkowskai, R., Fletcher, T.: PM10 and children’s respiratory symptoms and lung function in the PATY study. ERJ Expr. 40(3), 538–547 (2012)
Ikeda, T., Takahashi, K., Kabata, T., Sakagoshi, D., Tomita, K., Yamada, M.: Polyneuropathy caused by cobalt-chromium metallosis after total hip replacement. Muscle Nerve 42, 140–143 (2010)
Jani, P.U., Florence, A., McCarthy, D.E.: Further histological evidence of gastrointestinal absorption of polystyrene nanoparticles in the rat. Int. J. Pharmacol. 84, 245–252 (1992)
Jani, P.U., McCarthy, D.E., Florence, A.T.: Nanosphere and microsphere uptake via Peyer’s patches: observation of the rate of uptake in the rat after a single oral dose. Int. J. Pharmacol. 86, 239–246 (1992)
Jaurand, M.C.: Mechanisms of fiber-induced genotoxicity. Environ. Health Perspect. 105, 1073–1084 (1997)
Kagan, V.E., Konduru, N.V., Feng, W., Allen, B.L., Conroy, J., Volkov, Y., Vlasova, I.I., Belikova, N.A., Yanamala, N., Kapralov, A., Tyurina, Y.Y., Shi, J., Kisin, E.R., Murray, A.R., Franks, J., Stolz, D., Gou, P., Klein-Seetharaman, J., Fadeel, B., Star, A., Shvedova, A.A.: Carbon nanotubes degraded by neutrophil myeloperoxidase induce less pulmonary inflammation. Nat. Nanotechnol. 5(5), 354–359 (2010)
Kato, T., Yashiro, T., Murata, Y., Herbert, D.C., Oshikawa, K., Bando, M., Ohno, S., Sugiyama, Y.: Evidence that exogenous substances can be phagocytized by alveolar epithelial cells and transported into blood capillaries. Cell Tissue Res. 311, 47–51 (2003)
Kendall, M., Holgate, S.: Health impacts and toxicological effects of nanomaterials in the lung. Official J. Asian Pacific Soc. Respirology 17(5), 743–758 (2012)
Kennedy, S.M., Chambers, R., Du, W., Dimich-Ward, H.: Environmental and occupational exposures; do they affect chronic obstructive pulmonary disease differently in women and men? Proc. Am. Thorac. Soc. 4, 692–694 (2007)
Kim, J.S., Kuk, E., Yu, K.N., Kim, J.H., Park, S.J., Lee, H.J., Kim, S.H., Park, Y.K., Park, Y.H., Hwang, C.Y., Kim, Y.K., Lee, Y.S., Leong, D.H., Cho, M.H.: Antimicrobial effects of silver nanoparticles. Nanomedicine 3, 95–101 (2007)
Kim, Y.S., Song, M.Y., Park, J.D., Song, K.S., Ryu, H.R., Chung, Y.H., Chang, H.K., Lee, J.H., Oh, K.H., Kelman, B.J., Hwang, I.K., Yu, I.J.: Subchronic oral toxicity of silver nanoparticles. Particle Fibre Toxicol. 7(20), 1–11 (2010). LOAEL of 125mg/kg, NOAEL 30mg/kg)
Kreyling, W.G., Semmler-Behnke, M., Möller, W.: Health implications of nanoparticles. J. Nanoparticle Res. 8, 543–562 (2006)
Kumazawa, R., Watari, F., Takashi, N., Tanimura, Y., Uo, M., Totsuka, Y.: Effects of Ti ions and particles on neutrophil function and morphology. Biomaterials 23(17), 3757–3764 (2002)
Leigh, J., Driscoll, T.R., Cole, B.D., Beck, R.W., Hull, B.P., Yang, J.: Quantitative relation between emphysema and lung mineral content in coal workers. Occup. Environ. Med. 51, 400–407 (1994)
Leung, C.C., Yu, I.T.S., Chen, W.: Silicosis. Lancet 279, 2008–2018 (2012)
Li, N., Sioutas, C., Cho, A., Schmitz, D., Misra, C., Sempf, J., Wang, M., Oberley, T., Froines, J., Nel, A.: Ultrafine particulate pollultants induce oxidative stress and mitochondrial damage. Environ. Health Perspect. 111(4), 455–460 (2003)
Loechner, K., Hadrup, N., Qvortrup, L.A., Gao, X., Vogel, U., Mortensen, A., Lam, H.R., Larsen, E.H.: Distribution of silver in rats following 28 days of repeated oral exposure to silver nanoparticles or silver acetate. Part. Fibre Toxicol. 8(18), 1–14 (2011)
Mannetje, A., Brennan, P., Zaridze, D., Szeszenia-Dabrowska, N., Rudnai, P., Lissowska, J., Fabiánová, E., Cassidy, A., Mates, D., Bencko, V., Foretova, L., Janout, V., Fevotte, J., Fletcher, T., Boffetta, P.: Welding and lung cancer in Centeral and Eastern Europe and the United Kingdom. Am. J. Epidemiol. 175(7), 706–714 (2012)
Maynard, A.D.: Estimating aerosol surface area from number and mass concentration measurements. Ann. Occup. Hyg. 47(2), 123–144 (2003)
Mirzajani, F., Ghassempour, A., Aliahmadi, A., Esmaeili, M.A.: Antibacterial effect of silver nanoparticles on Staphylococcus aureus. Res. Microbiol. 162(5), 542–549 (2011)
Mitrano, D.M., Lesher, E.K., Bednar, A., Monserud, J., Higgins, C.P., Ranville, J.F.: Detecting nanoparticulate silver using single-particle inductively coupled plasma–mass spectrometry. Environ. Toxicol. Chem. 31(1), 115–121 (2011)
Miyake, Y., Sasaki, S., Yokoyama, T., Chida, K., Azuma, A., Suda, T., Kudoh, S., Sakamoto, N., Okamoto, K., Kobashi, G., Washio, M., Inaba, Y., Tanaka, H.: Occupational and environmental factors and idiopathic pulmonary fibrosis in Japan. Ann. Occup. Hyg. 49(3), 259–265 (2005)
Mohtashamipur, E., Norpoth, K.: Non-mutagenicity of some wood-related compounds in the bacterial/microsome plate incorporation and microsuspension assays. Int. Arch. Occup. Environ. Health 54, 83–90 (1984)
Mohtashamipur, E., Norpoth, K., Lühmann, F.: Cancer epidemiology of woodworking. J. Cancer Res. Clin. Oncol. 115, 503–515 (1989)
Möller, W., Felten, K., Sommerer, K., Scheuch, G., Meyer, G., Meyer, P., Haussinger, K., Kreyling, W.G.: Deposition, retention, and translocation of ultrafine particles from the central airways and lung periphery. Am. J. Respir. Crit. Care Med. 177, 426–432 (2008)
Mossman, B.T., Lippmann, M., Hesterberg, T.W., Kelsey, K.T., Barchowsky, A., Bonner, J.C.: Pulmonary endpoints (lung carcinomas and asbtestosis) following inhalation exposure to asbestos. J. Toxicol. Environ. Health B Crit. Rev. 14, 76–121 (2011)
Nel, A.E., Madler, L., Velegol, D., Xia, T., Hoek, E.M., Somasundaran, P., Klaessig, F., Castranova, V., Thompson, M.: Understanding biophysiochemical interactions at the nano-bio interface. Nat. Mater. 8, 543–557 (2009)
Nemmar, A., Hoylaerts, M., Hoet, P.H.M., Dinsdale, D., Smith, T., Xu, H., Vermylen, J., Nemery, B.: Ultrafine particles affect experimental thrombosis in an in vivo hamster model. Am. J. Respir. Crit. Care Med. 166, 998–1004 (2002)
Oberdoerster, G., Ferin, J., Lehnert, B.E.: Correlation between particle size, in vivo particle persistence, and lung injury. Environ. Health Perspect. 102(Suppl 5), 173–179 (1994)
Oldenburg, M., Wegner, R., Baur, X.: Sever cobalt intoxication due to prosthesis wear in repeated total hip arthroplasty. J. Arthroplasty 24(5), 15–20 (2009)
Papatheofanis, F.J., Barmada, R.: Polymorphonuclear leukocyte degranulation with exposure to polymethylmethacrylate nanoparticles. J. Biomed. Mater. Res. 25(6), 761–771 (1991)
Pasupuleti, S., Alapati, S., Ganapathy, S., Anumolu, G., Pully, N.R., Prakhya, B.M.: Toxicity of zinc oxide nanoparticles through oral route. Toxicol. Ind. Health. 28(8), 675–686 (2012)
Patel, A.M., Ryu, J.H., Reed, C.E.: Hypersensitivity pneumonitis: current concepts and future questions. Curr. Rev. Allergy Clin. Immunol. 108, 661–670 (2001)
Pelcolva, D., Sklensky, M., Janicek, P., Lach, K.: Severe cobalt intoxication following hip replacement revision: clinical features and outcome. Clin. Toxicol. 50, 262–265 (2012)
Peters, A., Doring, A., Wichmann, H.E., Koenig, W.: Increased plasma viscosity during an air pollution episode: a link to mortality? Lancet 349, 1582–1587 (1997)
Peters, A., Wichmann, H.E., Tuch, T., Heinrich, J., Heyder, J.: Respiratory effects are associated with the number of ultrafine particles. Am. J. Respir. Crit. Care Med. 155, 1376–1383 (1997)
Polyzois, I., Nikolopoulos, D., Michos, I., Patsouris, E., Theocharis, S.: Local and systemic toxicity of nanoscale debris particles in total hip arthroplasty. J. Appl. Toxicol. 32, 255–269 (2011)
Pope III, C.A., Burnett, R.T., Thun, J., Calle, E.E., Krewski, D., Ito, K., Thurston, G.D.: Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. JAMA 287(9), 1132–1141 (2002)
Prescott, G.J., Lee, R.J., Cohen, G.R., Elton, R.A., Lee, A.J., Fowkes, F.G., Agius, R.M.: Investigation of factors which might indicate susceptibility to particulate air pollution. Occup. Environ. Med. 57, 53–57 (2000)
Ramachandran, G., Paulsen, D., Watts, W., Kittelson, D.: Mass, surface area and number metrics in diesel occupational exposure assessment. J. Environ. Monit. 7, 728–735 (2005)
Ris, C.: U.S. EPA health assessment for diesel engine exhaust: a review. Inhal. Toxicol. 19, 229–239 (2007)
Rizzetti, M.C., Liberini, P., Zarattini, G., Catalani, S., Pazzaglia, U., Apostoli, P., Padovani, A.: Loss of sight and sound. Could it be the hip? Lancet 373, 1052–1053 (2009)
Rodriguez, E., Ferrer, J., Marti, S., Zock, J.P., Plana, E., Morell, F.: Impact of occupational exposure on severity of COPD. Chest 134(6), 1237–1243 (2008)
Rosen, G.: The History of Miners’ Diseases: A Medical and Social Interpretation, pp. 450–480. Schuman, New York (1943)
Roy, S.C., Oaulosec, M., Grimes, C.A.: The effect of TiO2 nanotubes in the enhancement of blood clotting for the control of hemorrhage. Biomaterials 28, 4667–4672 (2007)
Ruge, C.A., Kirch, J., Cañadas, O., Schneider, M., Perez-Gil, J., Schaefer, U.F., Casals, C., Lehr, C.M.: Uptake of nanoparticles by alveolar macrophages is triggered by surfactant protein A. Nanomedicine 7, 690–693 (2011)
Ruizendaal, L., Bhattacharjee, S., Pournazari, K., Rosso-Vasic, M., de Haan, L.H.J., Alink, G.M., Marcelis, A.T.M., Zuilhof, H.: Synthesis and cytotoxicity of silicon nanoparticles and covalently attached organic monolayers. Nanotoxicology 3(4), 339–347 (2009)
Sawosz, E., Binek, M., Grodzik, M., Ska, M.Z., Sysa, P., Szmidt, M., Niemiec, T., Chwalibog, A.: Influence of hydrocolloidal silver nanoparticles on gastrointestinal microflora and morphology of enterocytes of quails. Arch. Anim. Nutr. 61(6), 444–451 (2007)
Schleh, C., Semmler-Behnke, M., Lipka, J., Wenk, A., Hirn, S., Schäffler, M., Schmid, G., Simon, U., Kreyling, W.G.: Size and surface charge of gold nanoparticles determine absorption across intestinal barriers and accumulation in secondary target organs after oral administration. Nanotoxicology 6(1), 36–46 (2012)
Seaton, A., Soutar, A., Crawford, V., Elton, R., McNerlan, S., Cherrie, J., Watt, M., Agius, R., Stout, R.: Particulate air pollution and the blood. Thorax 54, 1027–1032 (1999)
Siew, S.S., Kauppinen, T., Kyyrönen, P., Heikkilä, P., Pukkala, E.: Exposure to iron and welding fumes and the risk of lung cancer. Scand. J. Work Environ. Health 34(6), 444–450 (2008)
Silverman, D.T., Samanic, C.M., Lubin, J.H., Blair, A.E., Stewart, P.A., Vermeulen, R., Coble, J.B., Rothman, N., Schleiff, P.L., Travis, W.D., Ziegler, R.G., Wachholder, S., Attfield, M.D.: The diesel exhaust in miners study: a nested case–control study of lung cancer and diesel exhaust. J. Natl. Cancer Inst. 104(11), 855–868 (2012)
Smith, M.W., Thomas, N.W., Jenkin, P.G., Miller, N.G.A., Cremaschi, D., Porta, C.: Selective transport of microparticles across Peyer’s patch follicle-associated M cells from mice and rats. Exp. Physiol. 80, 735–743 (1995)
Sørensen, A.R., Thulstrup, A.M., Hansen, J., Ramlai-Hansen, C.H., Meersohn, A., Skytthe, A., Bonde, J.P.: Risk of lung cancer according to mild steel and stainless steel welding. Scand. J. Work Environ. Health 33(5), 379–386 (2007)
Steens, W., von Foerster, G., Katxer, A.: Severe cobalt poisoning with loss of sight after ceramic-metal pairing in a hip—a case report. Acta Orthop. 77(5), 830–832 (2006)
Stephens, M.B., Yew, K.S.: Diagnosis of chronic obstructive pulmonary disease. Am. Fam. Physician 78(1), 87–92 (2008)
Studer, A.M., Limbach, L.K., Van Duc, L., Krumeich, F., Athanassiou, E.K., Gerber, L.C., Moch, H., Stark, W.J.: Nanoparticle cytotoxicity depends on intracellular solubility: comparison of stabilized copper metal and degradable copper oxide nanoparticles. Toxicol. Lett. 197, 169–174 (2010)
Taskar, V.S., Coultas, D.B.: Is idiopathic pulmonary fibrosis an environmental disease? Proc. Am. Thorac. Soc. 3, 293–298 (2006)
Taskar, V.S., Coultas, D.B.: Exposures and idiopathic lung disease. Semin. Respir. Crit. Care Med. 29(6), 670–679 (2008)
Thomas, C.R., Kelley, T.R.: A brief review of silicosis in the United States. Environ. Health Insights 4, 21–26 (2010)
Tinkle, S.S., Antonini, J.M., Rich, B.A., Roberts, J.R., Salmen, R., DePree, K., Adkins, E.J.: Skin as a route of exposure and sensitization in chronic beryllium disease. Environ. Health Perspect. 111(9), 1202–1208 (2003)
Trasande, L., Thurston, G.D.: The role of air pollution in asthma and other pediatric morbidities. J. Allergy Clin. Immunol. 115(4), 689–699 (2005)
Tse, L.A., Yu, I.T., Au, J.S.K., Qiu, H., Wang, X.: Silica dust, diesel exhaust, and painting work are the significant occupational risk factors for lung cancer in nonsmoking Chinese men. Br. J. Cancer 10, 208–213 (2011)
Vallyathan, V., Green, F.H.Y., Brower, P., Attfield, M.: The role of coal mine dust exposure in the development of pulmonary emphysema. Ann. Occup. Hyg. 41(1), 352–357 (1997)
Wasdo, S.C., Barber, D.S., Denslow, N.D., Powers, W.P., Palazuelos, M., Jr, S., Moudgil, B., Roberts, S.: Differential binding of serum proteins to nanoparticles. Int. J. Nanotechnol. 5(1), 92–115 (2008)
Witschi, H.R., Pinkerton, K.E., Van Winkle, L.S., Last, J.A.: Toxic responses of the respiratory system. In: Klaassen, C.D. (ed.) Casarett and Doull’s Toxicology: The Basic Science of Poisons, 7th edn, pp. 609–630. McGraw Hill Medical, New York (2008)
Woodruff, T.J., Parker, J.D., Schoendorf, K.C.: Fine particulate matter (PM2.5) air pollution and selected causes of postneonatal infant mortality in California. Environ. Health Perspect. 114(5), 786–790 (2006)
Yeh, K.W., Chang, C.J., Huang, J.L.: The association of seasonal variations of asthma hospitalization with air pollution among children in Taiwan. Asian Pac. J. Allergy Immunol. 29, 34–41 (2011)
Yin, H., Casey, P.S.: Effects of surface chemistry on cytotoxicity, genotoxicity, and the generation of reactive oxygen species induced by ZnO nanoparticles. Langmuir 26(19), 15399–15408 (2010)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Hinkley, G.K., Roberts, S.M. (2014). Particle and Fiber Toxicology. In: Merkus, H., Meesters, G. (eds) Particulate Products. Particle Technology Series, vol 19. Springer, Cham. https://doi.org/10.1007/978-3-319-00714-4_5
Download citation
DOI: https://doi.org/10.1007/978-3-319-00714-4_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-00713-7
Online ISBN: 978-3-319-00714-4
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)