Abstract
The skeleton and inferred neurosensory system of the ancestral amniote provide a point of departure to trace neurosensory evolution in extinct stem-mammals that culminated in the origin of crown Mammalia. Early stem-mammal evolution mostly involved enhanced integration of skeletal elements for feeding and locomotion as they became apex predators. With the origin of Cynodontia, modifications of the dentition, oropharynx, and braincase suggest that mastication and olfaction had become major influences in stem-mammal evolution, probably through ontogenetic cascades triggered by expression of an expanded olfactory genome. With the origin of Mammaliaformes, brain size nearly doubled in response to further elaboration of the olfactory system, dentition, the elaboration of hair, all in the context of miniaturization of adult body size. One of the keys to understanding major features in stem-mammal evolution and the origin of Mammalia is the emergence of an unsurpassed ability to perceive and process olfactory and dietary information, and to diversify and exploit the fast-changing chemical environments they faced throughout much of their history. Whether through connectional invasions and epigenetic population matching, or some other developmental mechanism, hypertrophy in peripheral sensory arrays produced cascading influences on central organization. These led to emergence of the unique mammalian neocortex and to the physiological and behavioral repertoires that are so distinctive of mammals today.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
10.1 Phylogenetic Context
One of the central features in pan-mammalian evolution is enlargement of the brain relative to body size (encephalization) and emergence of the unique mammalian neocortex (Rowe 1996a; Rowe et al. 2011). This chapter focuses on what can be inferred about pan-mammalian neurosensory evolution, beginning with divergence of the mammalian total clade from the ancestral amniote, and culminating in the origin of crown clade Mammalia (Fig. 10.1). It attempts to summarize contemporary answers to basic questions articulated by Northcutt (2001): what happened, when did it happen, how did it happen, and why did it happen?
Phylogeny of the major clades of Pan-Mammalia discussed here distributed across the geological time scale. (Modified after Rowe 2020a)
The following discussion employs conventions recommended by PhyloCode (Cantino and de Queiroz 2020), as illustrated in practice in its companion volume Phylonyms (de Queiroz et al. 2020) to designate particular subsets in a hierarchy of clades that includes Mammalia and its closest extinct relatives (Fig. 10.2). The Phylogenetic System is rankless and all taxonomic names, including known paraphyla, are italicized. The name Mammalia is used in reference to the ‘crown clade’ (Rowe 1988, 2020a, b; de Queiroz and Gauthier 1990, 1992, 1994; de Queiroz 1994). Fossil taxa more closely related to Mammalia than to other living taxa, that lie outside its crown clade, are considered to be members of the mammalian ‘stem’ or the paraphyletic extinct mammalian ‘stem-group’ while also belonging to the monophyletic ‘total clade’ of Mammalia. The ‘pan-clade’ naming convention attaches the prefix Pan- (for all) to the crown clade name to reflect its total clade (Rowe 2004; de Queiroz 2007). Pan-Mammalia (Rowe 2020c) is the total clade of Mammalia (Rowe 2020a), and the name Pan-Reptilia designates the total clade of Reptilia. Together Pan-Reptilia and Pan-Mammalia and their last common ancestor comprise the crown clade Amniota. A characterization of the ancestral amniote is where our discussion begins.
Categories of clades and groups employed under the Phylogenetic System of taxonomic nomenclature. (Modified from de Queiroz 2007)
The discussion below is based on a series of phylogenetic and developmental analyses, using increasingly sophisticated taxon/character matrices and imaging instruments that are detailed elsewhere (Gauthier et al. 1988a, b, 1989; Donoghue et al. 1989; Rowe 1988, 1993; Rowe et al. 1995, 2005; Rubidge and Sidor 2001; Kielan-Jaworowska et al. 2004; Meng et al. 2006; Ji et al. 2006; Rowe et al. 2011; Kirk et al. 2014; Rowe and Shepherd 2016; Rowe 2020a).
10.2 Historical Background
Evidence from the fossil record has enjoyed a remarkable resurgence from digital endocasts thanks to computed tomography (e.g. Rowe et al. 1995; Macrini 2006; Balanoff et al. 2016; Balanoff and Bever 2020) and similar non-destructive digital imaging technologies, as well as a flurry of new discoveries of fossils lying along the mammalian stem and in basal positions within the crown clade. Data from the fossil record is augmented and extended far beyond what endocasts alone provide by comparative studies on genome, ontogeny, and mature organization of neurosensory systems of living amniotes, using what Witmer (1995) termed the ‘extant phylogenetic bracket’ – a realm that is enjoying its own renaissance.
A basic tenet of vertebrate paleoneurology is that in order to function properly the central nervous system and many peripheral sensory organs require rigid armatures that are provided mostly by the skeleton and associated connective tissues (Rowe and Shepherd 2016; Rowe 2020a). For example, early development of the brain is driven by a combination of tissue growth and a growing volume of cerebrospinal fluid in the ventricular cavities. In effect the ventricles become an expanding hydrostatic reservoir that places considerable loads on the connective tissues surrounding the brain and sensory organs in early ontogeny. Proper intraventricular pressure is required to drive normal brain expansion and normal skull formation. Epigenetic plasticity of the skull during ontogeny is highly responsive to the mechanical force regime imposed by the developing brain (reviewed in Rowe 1996b; Weisbecker et al. 2021). Similar epigenetic responses occur as the developing olfactory epithelium induces ossification of the bony turbinals (or turbinates) of the ethmoid bone (Rowe et al. 2005; Rowe and Shepherd 2016), and in other systems discussed below.
An integrative approach is used here to infer ancestral states of the neurosensory system in Amniota based on its two living clades, Mammalia and Reptilia, and their fossil records. This ancestral character state reconstruction helps to identify the evolution of novel morphological characters and character states preceding the origin of Mammalia. Patterns of successive correlated transformations identify potential driving factors behind the evolution of mammalian neurosensory systems that extend into genetic and epigenetic controls of development. We will see support for the idea that elaboration of peripheral sensory arrays, including olfactory receptors, teeth, and hair, influenced central organization with a cascade of new inputs. Through epigenetic population matching (Katz and Lasek 1978; Krubitzer and Kaas 2005; Streidter 2005) or some other mechanism, peripheral innovations were important drivers in central reorganization and successive increases in encephalization (Rowe and Shepherd 2016; Shepherd and Rowe 2017; Rowe 2020a).
A corollary is that peripheral sensory structures are not independent; they are parts of larger, integrated neurosensory systems. Generations of paleontologists have speculated on whether certain extinct stem-mammals had evolved whiskers, turbinals, endothermy, etc. (Broom 1932; Brink 1957; Crompton et al. 1978). These studies launched the exciting field of ‘paleobiology’ but hypotheses about soft structures, physiology, and behavior in extinct taxa are often difficult to test. However, in cases where the neurosensory system is implicated or directly involved, tying hypothesized peripheral sensory structures into the larger systems of which they are a part can serve as a test. For example, as detailed below, expression of the huge olfactory receptor (OR) gene family in mammals induces growth of the expansive olfactory receptor epithelium, which in turn induces ossification of its scaffold of turbinals. The expanded number of olfactory neuron axons induces expansion of the olfactory bulb, whose axons in turn induce expansion of the olfactory (piriform) cortex. Hence, hypotheses that an unpreserved system of cartilaginous turbinals was present in early stem-mammal (e.g. Hillenius 1992, 1994) implicitly predict corresponding expansion of olfactory bulb and olfactory cortex that leave corresponding impressions in bones surrounding the endocranial cavity. The hypothesis of cartilaginous nasal turbinals in stem-mammals can be corroborated or falsified by evidence from the braincase and endocasts of the other components of the system.
Additional insights can be gained from Günter Wagner’s (2014) conceptualization of two basic types of morphological innovation or novelty in animal evolution. Type I novelties involve the origin of a novel ‘character identity’, and as examples Wagner cites the vertebrate head and the insect wing. The emergence of Type I innovations is not predicted by conventional Darwinian natural selection, and instead Wagner recognizes a special role for cascading effects of gene duplication and new gene regulatory networks. Pan-mammalian history reveals effects by the brain on skull morphogenesis from inferred gene duplications, particularly in the olfactory receptor sub-genome (Niimura 2012), and in genes regulating the radial units of cortical organization (Rakic 1988, 2000, 2007, 2009).
Type II innovations involve the origin of novel ‘character-states’ and as examples Wagner cites emergence of the tetrapod limb from paired fins, and the emergence of feathers from epidermal scales. In an added level of complexity, Wagner also identifies novel ‘variational modality’ in systems of repeated structures. We will see evidence of Type II innovations and transformations of variational modality in regionalization of the tetrapod vertebral column, differentiation and accelerated evolution in the occlusal dentition and inferred elaboration of olfactory receptors in cynodonts, each with its own special relationship to the neurosensory system.
Finally, the contours of pan-mammal history raise the provocative question of whether the mammalian neocortex, and possibly the masticatory apparatus, qualify as Type I innovations. The heuristic value of asking this question lies in the intricate dissection necessary for such a determination, and may be more informative than arriving at a final answer by advancing our understanding of the remarkable balance between individuation of novel character identities, new character states, and transformed variational modalities, with their functional integration into individual organisms and clades (Fig. 10.3).
Jerison’s (1973) innovative ‘encephalization quotients’ (EQs) are commonly used to quantify the relationships between brain (or endocast) size and body size, but caveats should be acknowledged. Different authors have used different landmarks in fossils to delimit the floor and sides of the anterior half of the endocranial cavity where a bony enclosure is lacking, leading to different endocast reconstructions for individual specimens (Kemp 2009). Estimates of body size have uncertainties that are difficult to calibrate. Different formulas are available to describe the brain-body size relationship, including Jerison (1973), Eisenberg (1981), Manger (2006) and Hurlburt et al. (2013). Different assumptions apply when estimating how much of the endocranial volume was actually filled by brain vs. vascular structures and meninges (Balanoff et al. 2016; Balanoff and Bever 2020). Surprisingly, neuronal cell sizes and densities, generally assumed to be constant across mammalian taxa, are now known to vary in different amniote and mammalian sub clades (Herculano-Houzel et al. 2014). Even today it is rare for authors to document skeletal features in fossils that offer an indication of maturity at time of death, leading to spurious comparisons of EQs in juveniles and adults. In the context of the present review, the most significant caveat is that the oldest taxa discussed below had such tiny brains and unossified braincases that few attempts at reconstructing endocasts have been made (Fig. 10.4; Cope 1886; Baur and Case 1899; Case 1907; Romer and Edinger 1942). Small differences in EQ are probably meaningful only towards crown Mammalia. I assume these issues do not affect the broad trends discussed below.
Endocast of the stem amniote Diadectes (see Fig. 10.3) (From Cope 1886). Edinger (1975: 34) notes that this reconstruction “is not the endocast of one cranium, but a composite; that is, Cope’s introductory sentences stating that observations were made on a part of one skull, and a few other characters derived from two other skulls, apply also to the “brain” specimen.” (1) Dorsal view of endocast. (2) Left lateral view of endocast. (3) Posterior view of endocast. (4) Ventral view of semicircular canals. (5) Anterior view of semicircular canal. (6) Ventral view of semicircular canals. Abbreviations (from Cope)
Fig. 10.4 (continued) Figures 1, 2 and 3 cast of cranial cavity, natural size. As the basicranial axis is lost, the inferior outline posteriorly is provisional only. Figure 1, from above. Figure 2, from the left side. Figure 3, from behind
The letters signify as follows: m. medulla, cb. cerebellum, opl. optic lobe, ep. epiphysis, ppe. posterior process of epiphysis, If. lateral foramen, h. region of cerebral hemispheres, v. cast of vestibule, hap. do. of orifice of horizontal anteroposterior semicircular canal, vt. do. of vertical transverse canal, oc. do of os commune of vertical anteroposterior and vertical transverse canals, aa. do. of anterior ampulla, V. cast of foramen of fifth pair of nerves
Figures 4, 5 and 6 diagrams of the semicircular canals, natural size. Figure 4, interior view. Figure 5, anterior view. Figure 6, inferior view
10.3 The Ancestral Amniote
Pan-Reptilia (including birds) and Pan-Mammalia diverged from the ancestral amniote (Figs. 10.1 and 10.2) during the early Carboniferous, between 340 and 322 million years ago (Didier and Laurin 2020). The latest census of Amniota includes 6399 extant mammal species (Burgin et al. 2018), and more than 20,000 extant reptile species, a number that could rise by 5000–10,000 more, depending on ongoing reassessments of avian subspecies (Barrowclough et al. 2016). The ancestral amniote was a small predatory quadruped, about a half-meter in length, nearly half of which was the tail. The Carboniferous Limnoscelis paludis (Fig. 10.5) is either a basal amniote or a close relative on the amniote stem (Gauthier et al. 1988a), and provides an informative comparison for understanding subsequent amniote history. Early amniote fossils are generally found in deposits formed by what were then circumequitorial forests along rivers and deltas. The early terrestrial ecosystem would seem bizarre from today’s vantage point, consisting mostly of predatory tetrapods who preyed on each other, and on non-vertebrates that were intermediates to the base of the food pyramid (Olson 1966).
10.3.1 The Amniote Skeleton
Whereas aquatic vertebrates are in effect neutrally buoyant, those who successfully moved onto land faced the effects of gravity and this underlies many skeletal innovations in basal amniotes. Because kinetic energy scales to the fifth power of linear dimension (McMahon and Bonner 1983), gravitational challenges increase exponentially with increase in body size. This probably explains why the first amniotes were small, and how similar strategies in strengthening the skeleton enabled different amniote clades to independently evolve large body sizes (Romer 1956, 1966). Amniotes initiated a trend towards simplification of the skeleton by consolidating primitively compound structures into single stronger elements (Sidor 2001). This occurred through ontogenetic re-patterning of regions of the skeleton in which primitively separate ossification centers failed to differentiate and a single element grew in their place, or where separate bones differentiated earlier in ontogeny and quickly fused.
Amniotes abandoned a larval stage and functional gills, and ventilation was achieved through two different systems. The first probably began in stem tetrapods, who co-opted the former pharyngeal skeleton into a branchial pump as lungs became the main site of metabolic gas exchange. The former gill arches were modified through reductions in their numbers, and in the number of elements per arch (Goodrich 1930). Some of these bones would later be co-opted to augment mobility of a fleshy tongue and unique swallowing behaviors (Crompton and Parker 1978; Crompton et al. 2018), and in both stem-mammals and stem-reptiles some were independently co-opted into an impedance matching middle ear (Gauthier et al. 1988a; Clack 2012; Kitazawa et al. 2015). The second system involved a musculoskeletal system in the trunk in which hinged ribs and intercostal muscles acted to move the ribs away from the body center, expanding the cavity surrounding the lungs for aspirational breathing (Janis and Keller 2001; Brainerd 2015). This second system probably originated in support of the branchial pump, which gradually gave way to rib-driven aspirational breathing. This system arose in stem-amniotes and had probably become the dominant of the two systems in early amniotes and stem-mammals (Janis and Keller 2001; Brainerd and Owerkowicz 2006).
Like their aquatic ancestors, the first amniotes were macro-predators, but life on land entailed profound change in how they fed (Lemberg et al. 2021). The ancestral mode of gape-and-suck feeding worked in a water column, but terrestrial feeding entailed precise movements of the jaws, head, and neck, as the amniote mouth became a finely tuned prehensile device for biting and seizing prey items (Romer 1956, 1966). Swallowing also posed a new problem. Amniotes initially solved it with a fleshy tongue and by using inertial swallowing, i.e., by lunging the head and mouth forward against the inertia of a subdued, stationary prey item (Heiss et al. 2018). This implies new levels of coordination between vision and actions of the jaws, head and neck. Many such innovations imply neurosensory elaboration that can only be inferred, but nevertheless paint a more vivid picture of evolving neurosensory capacity.
Along with rib-driven aspirational breathing, the amniote craniovertebral joint reflects continuation of a new variational modality begun in early tetrapods involving increased regionalization of the axial skeleton. The amniote skull articulated with two specialized vertebrae – the ‘atlas-axis complex’ - that enhanced stable mobility of the head on a longer neck. A primitive neck enabling the head to be raised can be traced into early stem-tetrapods (Gauthier et al. 1988b, 1989). Early amniotes further modified this joint to facilitate prey capture and inertial swallowing. It also raised the head somewhat, broadening sensory horizons and directional sensory perception. A design requirement of the craniovertebral joint is to ensure the spinal cord is not stretched or kinked by extended head movements (Jenkins Jr. 1969, 1971; Kemp 2005). At many points in pan-mammalian history, subtle skeletal modifications balanced seemingly conflicting demands of increased head and neck mobility against increases in diameter of the spinal cord that accompanied encephalization and peripheral sensory elaboration (Rowe et al. 2011; Rowe 2020a).
The limbs in early amniotes and stem-mammals were a bit longer than in the first tetrapods, but they were still very short and widely sprawled to the sides of the body. Fossil trackways are wide, showing a short stride, and they must have been quite slow (Romer and Price 1940). The pectoral girdle and forelimbs were heavily built and pulled the body forward by rotating a propeller-shaped humerus at the shoulder. The hindlimb was comparatively short and weakly developed, but strong femoral retractor muscles originating from the base of the tail provided thrust. Alternating lateral undulation of the axial skeleton augmented by the pull-push forces of the limbs also contributed thrust (Romer 1956; Kemp 2005; Hopson 2015). However, asymmetrical axial undulation precluded symmetrical, bilateral expansion of the ribs and must have limited aspirational breathing, and considerably limited metabolic scope during locomotion (Carrier 1987). Some consider the earliest stem-mammals to have been sit-and-wait ambush predators (Hopson 2015).
Compared to their descendants, early amniotes were limited in speed, agility, and gait. They could walk and probably still swim, but it is doubtful they could run, and any locomotion at speed was metabolically limited to short bursts (Carrier 1987). From such an ancestor, running, galloping, jumping, hopping, climbing, gliding, diving, and flying would eventually emerge in pan-mammals, but not without profound skeletal modifications and corresponding neurosensory elaboration (Rowe 2020a). The importance of feeding and locomotion in pan-mammal evolution has long been emphasized by paleontologists (e.g. Goodrich 1930; Romer 1966; Gauthier et al. 1988a). Paleoneurology can now begin to identify correlative neurosensory transformations in response to questions about what, when, how, and why the mammalian neurosensory system evolved (Northcutt 2001).
10.3.2 Peripheral Sensory System
Many characteristics of the amniote neurosensory system can be explained by a commitment to terrestrial life that altered acuity and balance between individual sensory modalities. For example the lateral line system was present in vertebrates ancestrally to detect electrical impulses transmitted through water, as well as water temperature, chemistry, and turbulence (Rowe 2004). But these signals are not perceptible in air, and in amniotes this entire system was quickly lost; early stem-amniote fossils are recognizable by the absence of lateral line canals on their skulls (Gauthier et al. 1988b, 1989). In contrast, the amniote visual system underwent a vast adaptive radiation in response to a greater diversity of reflective objects on land than in water (Walls 1942). So too, the amniote olfactory system adapted to a more diverse and rapidly changing chemical environment encountered in terrestrial ecosystems (Rowe et al. 2011) and olfactory receptor genes became the fastest evolving gene family in tetrapods (Yohe et al. 2020) and especially pan-mammals.
10.3.2.1 Olfactory system
Amniotes inherited a dual olfactory system consisting of the main olfactory system and the vomeronasal system (accessory olfactory system) (Farbman 1992), that are encoded by separate gene subfamilies (Niimura and Nei 2005, 2006; Niimura 2009). The amniote olfactory system was profoundly transformed as the medium of ventilation and metabolic gas exchange moved from water to air, and it diversified further among the different amniote clades. The following discussion is exclusive to mammals, where genetic and ontogenetic paths are best-known. The vomeronasal system is absent in aquatic mammals, some bats, and platyrhine and anthropoid primates (Bertmar 1981; Bhatnagar and Meisami 1998), but the dual system is present in monotremes, marsupials, as was the case in mammals ancestrally and across the mammalian stem-group.
Differentiation of the main olfactory and vomeronasal systems is induced as a single pair of ectodermal olfactory placodes at the rostral extremity of the neural plate invaginates to contact the rostral end of the developing forebrain (Farbman 1988, 1990; Schlosser 2010, 2017). This contact initiates differentiation and growth of separate main olfactory and vomeronasal epithelia, which together carpet the inner walls of the placode. Once induced, the main olfactory and vomeronasal systems follow separate ontogenetic trajectories, but their divergent synaptic pathways eventually converge in the accessory olfactory bulb (Farbman 1992).
Shortly thereafter, olfactory neurons (OSNs) differentiate in the olfactory epithelium, whose axons induce differentiation of glomeruli in the presumptive olfactory bulb (Figs. 10.6 and 10.7); once contact is made, the expression of a particular olfactory gene is induced, and the expression of other OR genes is suppressed (Chen and Shepherd 2005; Shepherd et al. 2021). Axonal projections from the olfactory bulb in turn induce differentiation of the olfactory cortex (Schlosser 2010; Shepherd et al. 2021). Lying between the olfactory bulb and olfactory cortex is the accessory olfactory bulb; it is probably induced by main olfactory bulb projections and/or vomeronasal receptor axons, but direct evidence is lacking. The rostral position of the olfactory placodes may explain why olfaction is the only peripheral sensory system that projects directly to the telencephalon, whereas the other cranial sensory placodes are positioned lateral or caudal to the presumptive diencephalon and follow different pathways to the telencephalon via the thalamus (Schlosser 2010, 2017; Shepherd et al. 2021).
Circuitry schematic of brain of modern opossum (Didelphis) brain showing (a) sensory inputs and (b) motor outputs. (Modified after Rowe et al. 2011). See anatomical abbreviations
Skull of mature Monodelphis domestica, reconstructed in 3D from computed tomography, in cut-away sagittal (a) and horizontal (b) views. The endocranial cavity was rendered solid beige to show the endocast of the brain in relation to the various bones of the skull, which were individually segmented and colored using VGStudio Max 2.0 software. (Modified after Rowe et al. 2011). See anatomical abbreviations
In aquatic non-tetrapod vertebrates, both the main olfactory receptors, vomeronasal receptors, and the associated terminal nerve (cranial nerve 0) are sensitive to odorant molecules suspended in the water column. In early stem-tetrapods, what formerly were diffusely distributed vomeronasal receptors became organized into an encapsulated vomeronasal organ on the floor of the nasal capsule (Rowe 2004; Rowe et al. 2005). Its receptors are activated primarily by pheromones and other large molecules that are not carried far by air (Baxi et al. 2006; Streidter and Northcutt 2020). Its axons and those from the terminal nerve make their first synapse in the accessory olfactory bulb, where they induce formation of glomeruli that are independent from those of the main olfactory system (Demski 1993; Demski and Schwanzel-Fukuda 1987). Whereas both olfactory systems are important in stem-mammal evolution, unequivocal evidence of transformations in the vomeronasal organ have yet to be recognized in stem-mammal fossils, and our focus now turns to the main olfactory system, which mediates conscious odor perception (Shepherd et al. 2021).
Genes that once coded receptors activated by waterborne molecules were either lost or transformed into new gene families that encode odorant receptors activated by volatile airborne odorants. A great breakthrough in understanding olfactory organization was made by Buck and Axel (1991) in identifying the genes that encode olfactory receptors (ORs), and the finding that each gene codes a receptor that is narrowly tuned to a single odorant molecule, or a narrow family of molecules. Then came the discovery that most vertebrates, including reptiles, have ~100 OR genes, but that the ancestral mammal was inferred to have had ~1200 OR genes based on comparisons among living species (Niimura and Nei 2005, 2006; Niimura 2012; Niimura et al. 2014; Zhou et al. 2021). The discovery that several derived turtle clades have expanded OR genomes (Wang et al. 2013) does not affect the estimated number for amniotes ancestrally, and underscores that the OR genome is the most rapidly evolving subfamily in the tetrapod genome (Yohe et al. 2020). During the evolution of stem-mammals, therefore, a series of OR gene duplications must have increased their numbers by an order of magnitude beyond the numbers inferred present in the ancestral amniote. This was probably a result of multiple tandem gene duplications that led the OR genome to become the largest and most rapidly evolving subfamily in the mammalian genome; this must have occurred by or before the origin of Mammalia (Young et al. 2010; Yohe et al. 2020).
With the origin of Amniota, airflow through the nasal chamber became tied to two distinct functions. Each function is supported by a primary ‘choncha’ or epithelial fold, supported by a low ridge of cartilage protruding into the lumen from the lateral wall of the nasal capsule (Parsons 1967; Gauthier et al. 1988a). The anterior choncha supports mucociliary respiratory epithelium, while the posterior concha supports olfactory epithelium. In Mammalia, (Fig. 10.7) both conchae evolved hypertrophied epithelia supported by elaborate skeletons of paper-thin filigreed scrolls, arbors, and plates of bone known as turbinals (or turbinates), as olfactory and respiratory functions elaborated (Taylor 1977; Rowe et al. 2005; Crompton et al. 2017a).
10.3.2.2 Visual System
There are far more reflective surfaces on land, less light scatter or absorption in air, and more light energy in air than in water (Walls 1942). The ancestral amniote entered a world of new visual information and is inferred to have been diurnal with a retina rich in cones compared to rods (Walls 1942). It may have traded light sensitivity for a marked increase in visual acuity and sharp resolving power because predaceous vertebrates generally require sharp vision to pursue and capture prey, and animals that feed on small objects like insects must be able to resolve them, which is best achieved in a cone-rich eye (Walls 1942). Most genomic accounts suggest the ancestral amniote had tetrachromatic vision (e.g. Streidter and Northcutt 2020). However, the recent discovery that the Tuatara (Sphenodon) has all five of the visual opsin genes found in vertebrates ancestrally (Gemmell et al. 2020), is consistent with the view that the ancestral amniote may have had pentachromatic color vision based on visual pigments of the RhA/Rh1, RhB/Rh2, SWS1, SWS2, and LWS opsin gene families (Collin 2010). Diurnal vision probably led the other senses in the ancestral amniote and in early stem-mammals. However, the RhB/Rh2 opsin genes are absent in Mammalia and must have been lost in its stem group. Further reductions in opsin genes occurred in different clades within Mammalia, and dichromatic crepuscular to nocturnal behaviors in monotremes (Davies et al. 2007; Ashwell 2013) and therians (Ashwell 2010) probably evolved independently (Walls 1942; Collin 2010; Gemmell et al. 2020).
10.3.2.3 Auditory System
The sensitivity and resolving power of hearing in the ancestral amniote and early pan-mammals must have diminished in the transition to airborne acoustic information. Still, the ancestral amniote and its living descendants conserved basic functions of hearing involving frequency discrimination, signal to noise ratio enhancement, and sound localization. They also conserved the plesiomorphic transmission pathway involving transduction of acoustic information by sensory hair cells of the inner ear, which in amniotes involved a basilar papilla and membrane (Streidter and Northcutt 2020), and from there via the auditory nerve to brainstem auditory neurons (Carr and Soares 2002, 2009; Carr and Christiansen-Dalsgaard 2016). The fossil record indicates that an impedance matching middle ear evolved independently in amphibians, stem-reptiles, and stem-mammals (Gauthier et al. 1988a, b, 1989). In each clade, the middle ear has its own distinct anatomical organization and neural mechanisms for sound localization (Carr and Soares 2009). However, in each case, the middle ear develops from elements of the first and/or second branchial arches. Each clade also introduced a tympanic membrane connected via a lever system of bone and/or cartilage that matched airborne sound impedance to the fluid-filled inner ear (Grothe et al. 2005, 2010). Terrestrial hearing was probably limited at first to low frequency vibrations from the ground via the jaws and branchial arches as early amniotes rested their heads on the ground. This may explain the independent derivation of impedance matching middle ears from components of the branchial arches.
10.3.2.4 Peripheral Somatosensory System
Bony scales were lost from the skin in stem amniotes, and in their place are tiny epidermal condensations – body placodes – induced by neural crest cells that would eventually evolve into mammalian hair and reptilian scales and feathers. Amniote body placodes share common spatial expression of placode molecular markers such as Shh, Ctnnb1, and Edar, as well as conserved localized signaling in the dermis underlying the placode by Bmp4, corroborating shared common ancestry (Di-Pöi and Milinkovitch 2016). The appearance of placode-induced epidermal structures began an amazing diversification of integumentary specializations to prevent water loss, protect the skin from solar radiation, enhance sensory perception over the body surface and in the space around it, insulate the body, assist locomotion, provide camouflage, and attract mates. At some late point in stem-mammal history, hair follicles would evolve from body placodes and deliver a deluge of new peripheral information to the brain (Fig. 10.8). Exceptional preservation of a Jurassic stem-mammal indicates that fur evolved before the origin of crown Mammalia (below).
Diagram of a hair follicle and its innervation (Modified after Rowe et al. 2011)
10.3.2.5 The Ancestral Amniote Brain
So little of the endocranial cavity is enclosed by bone that much speculation attends any attempt to reconstruct a basal amniote endocast. Most relevant fossils are badly crushed or incomplete and their state of preservation often defeats CT scanning. As a result, few attempts have been made to reconstruct individual endocasts in a basal or stem-amniote (Fig. 10.4; Cope 1886; Case 1907; Romer and Edinger 1942). Nevertheless, general conclusions can be assembled from fossils and from comparative development of extant amniotes. Anteriorly, the orbitosphenoid formed a thin, Y-shaped ossification that cupped the forebrain from beneath. When preserved, the orbitosphenoid indicates a long narrow forebrain positioned close to the skull roof (Crompton et al. 2017b). The olfactory bulbs were probably closely appressed against the anterior telencephalon, as in extant lissamphibians and turtles (Gauthier et al. 1988a), and in all the later stem-mammal fossils from which endocasts can be extracted (e.g. Macrini 2006; Kemp 2009; Benoit et al. 2016, 2017). Whereas an interhemispheric sulcus divides the cerebral hemispheres in all extant vertebrates, there is no evidence of an interhemispheric ridge along the inferior side of the parietal. This suggests the brain was not strongly inflated in early development and did not exert the profound effect on cranial morphogenesis it would eventually have in some of the later stem-mammals (below). The floor and rear parts of the braincase were ossified and surrounded a cerebellum that was twice as wide as the forebrain. A large pineal stalk was present, and the midbrain was exposed dorsally between the telencephalon and cerebellum (Fig. 10.4).
Telencephalon
Comparative and developmental anatomy in extant amniotes indicate the telencephalon in the ancestral amniote consisted of four basic divisions that surrounded the ventricle. The olfactory (piriform) cortex was positioned laterally, the hippocampus formed the medial wall, the telencephalic roof or dorsal pallium formed the dorsal cortex, and the basal ganglia differentiated in the telencephalic floor. The three cortical areas – dorsal cortex, olfactory cortex and hippocampus – in non-mammalian amniotes (except archosaurs; Briscoe and Ragsdale 2018) have a three-layer construction, consisting of a middle layer of pyramidal neuron bodies and interneurons with an underlying layer of axons and an overlying layer of dendrites of the pyramidal cells and interneurons (Shepherd and Rowe 2017).
The principal cells in the amniote forebrain are pyramidal cells (Shepherd 2011). This cell type is present in amphibians but lacks basal dendrites, whereas in amniotes the basal dendrites are not only present but have become extensively branched and interconnected in a vast synaptic web (Streidter 2005; Shepherd 2011). Pyramidal cells are present in the forebrains of all reptiles except crocodilians and birds, where they were secondarily transformed or lost (Streidter 2005). The amniote cortex surrounded a ventricular zone throughout its extent, and a subventricular zone in its lateral regions from which neurogenesis occurred in an inside-out pattern (Marín and Rubenstein 2001). Neurogenesis proceeded throughout much of ontogeny, and established the basic neurogenerative pattern that gave a degree of radial and columnar organization to the forebrain that was carried to the extreme in Mammalia (Rakic 1988, 2000, 2007, 2009).
In its basic circuitry, the olfactory cortex has a similar neural organization in turtles and lizards (Ulinski 1983; Haberly 1985; Bruce 2007, 2009; Bruce and Braford Jr 2009) and in monotremes (Ashwell 2013), marsupials and placentals (Ashwell 2010; Shepherd 2011), supporting the inference that this organization was present in amniotes ancestrally. Olfactory receptors deliver signals to the olfactory bulb where they form an ‘odor image’. The unique degree of elaboration in mammals involves a chain of more than 20 separate microcircuits (Shepherd et al. 2021). The ‘odor image’ is passed to the olfactory cortex which transforms it into a higher level representation known as an ‘odor object’ with content addressable memory. The ‘odor object’ is passed to the dorsal cortex (or to neocortex in Mammalia) for further associative processing (Shepherd 1991; Wilson and Stevenson 2006). Anatomical and physiological studies in the hippocampus have shown that across amniotes the neurons and circuits are similar to those in the olfactory cortex, with similar long association fibers and interconnections for excitation and inhibition (Connors and Kriegstein 1986; Haberly 2001). In these regards, the intrinsic organization of olfactory cortex and hippocampus are similar to higher association cortical areas, for example the face area of inferotemporal cortex (Haberly 1985; Shepherd and Rowe 2017). There is a close similarity between the intrinsic organization of the hippocampus and the olfactory cortex in terms of layering of inputs on the apical dendrites and long association fibers (Neville and Haberly 2004). Since inputs to the hippocampus consist exclusively of central sites in the limbic regions, it is clear that the three-layered hippocampus was devoted to higher order processing such as learning and memory from the very start of amniote evolution (Rowe and Shepherd 2016; Shepherd and Rowe 2017). In this view, the three-layer dorsal cortex of the ancestral amniote, from which six-layer mammalian neocortex evolved, was not a ‘simple’ cortex for low-level processing, but rather had an organization that subserved high-level association functions analogous to those in olfactory cortex and hippocampus (Rowe and Shepherd 2016; Shepherd and Rowe 2017; Shepherd et al. 2021).
Thalamus
The thalamus switches circuits passing in both directions from the dorsal cortex to the rest of the body. Compared to other tetrapods, amniotes have an expanded and highly differentiated thalamus (Butler 1994; Butler and Hodos 2005; Nieuwenhuys et al. 1998; Streidter and Northcutt 2020). It took on a new level of complex organization in amniotes, one that was further elaborated during stem-mammalian history in association with the emergence of neocortex. Amniotes have an elaborated dorsal thalamus that is larger and contains many more individual cell masses or nuclei than anamniotes (Butler 1994; Butler and Hodos 2005; Nieuwenhuys et al. 1998). Highly characteristic of amniotes is differentiation of discrete specialized nuclei that function as a complex of way-stations for visual, auditory, and somatosensory inputs interposed between the environmental sensory world and dorsal cortex (Butler 1994; Butler and Hodos 2005).
Hypothalamus
The amniote hypothalamus differs from anamniotes in receiving input from those regions with responsibility to memory and the resonance of experience (Butler 1994; Butler and Hodos 2005). Many functions of the hypothalamus are tied to light, to the daily cycle of light from dawn to dusk; the influence of light on the hypothalamus extends to seasonal variability, to the shorter winter days and longer summer days. This is consistent with evidence that the ancestral amniote was diurnal with tetrachromatic or pentachromatic color vision (above). The hypothalamus also regulates water balance by directing kidney function – a crucial process in terrestrial vertebrates. The hypothalamus also controls the production of hormones involved in reproductive physiology, involving the movement of ova in the oviduct, contractions of muscles of the reproductive organs, and many behaviors involved in courtship. Finally, the suprachiasmatic nucleus of the hypothalamus is an autonomous circadian pacemaker. Thus, circadian cycles and seasonality were influential in early amniote and stem-mammal behaviors (Butler and Hodos 2005).
Spinal Cord
The spinal cord is segmented at multiple levels of organization. Each segment forms dorsal (afferent) and ventral (efferent) spinal nerves that correspond in the neck and trunk to the numbers of vertebral segments. The amniote spinal cord is thicker than anamniotes and extends through the entire length of the dorsal vertebral column, and in Mammalia for a variable distance into the tail. It has more different types of cells than anamniotes, and many of these secondary neurons send axons across the midline to the contralateral side for left-right coordination of movement (Butler 1994; Nieuwenhuys et al. 1998). A distinct lateral column of motor neurons provides innervation to the limbs; and there are now expanded cervical enlargements (segments 7 – 10) and lumbosacral enlargements (segments 19 – 22) that represent the initial integrating centers of the brachial and sacral plexi, which innervate muscle complexes during locomotion and control reflexive action in the limbs. Their size is correlated with the lengths of the corresponding extremities (Nieuwenhuys et al. 1998). Another innovation was the aggregation of spinal neurons into discrete ‘motor pools’ that innervate single muscles, probably allowing them to be controlled independently (Streidter and Northcutt 2020). Additionally, the autonomic neuronal groups (i.e. ‘fright and flight reflexes’) of the brainstem and spinal cord were highly developed, indicating that the spinal cord was performing more internal decision-making processes that are independent of the brain (Streidter 2005).
In summary, compared to the first stem-tetrapods the ancestral amniote neurosensory system enjoyed an increase in numbers of genes, more neuronal types, and more complex pyramidal cells with greater interconnectivity, faster rates of neuron proliferation that produced a larger forebrain, and elaboration in complexity and computing power on the new world of terrestrial information amniotes had entered. It controlled more highly coordinated body movements using a more complex muscular system. While abandoning the lateral line system, it began a trend to integrate peripheral information from more acute visual and airborne olfactory systems. This underscores that three-layer dorsal cortex of amniotes ancestrally operated at the level of higher order associations underlying analysis, discrimination, learning, and memory (Rowe and Shepherd 2016; Shepherd and Rowe 2017), and a remarkable capacity for detailed analysis of their environment (Nieuwenhuys et al. 1998). Basal amniotes were probably more introspective and reflective of experience, using a more highly developed sense of memory as a guide to action (Butler 1994; Butler and Hodos 2005).
Such was the general organization of the skeleton and neurosensory system in the ancestral amniote. From such an ancestor, we now turn to the fossil record of stem-mammals and the major events in neurosensory evolution culminating with the origin of Mammalia.
10.4 Early Pan-Mammalian History
Pan-Mammalia diverged onto its own evolutionary trajectory in the Early Carboniferous, 340 – 322 million years ago (Didier and Laurin 2020). In most (pre-Phylocode) literature Pan-Mammalia (Rowe 2020c) is referred to by the name ‘Synapsida’ which is used as a synonym for both the paraphyletic stem-group of mammals (e.g. Romer 1956, 1966), and for the total clade of Mammalia (e.g. Gauthier et al. 1988a; Laurin and Reisz 2020). I use the name for an apomorphy-based clade stemming from the fist pan-mammal possessing the synapsid arch (Fig. 10.3, node 1) (Rowe 2020c). The early fossil record of stem-mammals is confined to what were then circumequatorial belts of Pangaea in the Carboniferous and Early Permian. They include several extinct side-branches, including Varanopidae, Caseasauria, Ophiacodontidae, Edaphosauridae, Haptodontidae, and Sphenacodontidae (Fig. 10.3, nodes 1–3; Fig. 10.9) that were long clustered in the paraphylum ‘Pelycosuaria’ (e.g. Romer and Price, 1940; Olson 1959). Beginning in the late nineteenth century, ‘pelycosaurs’ were recognized as representing the most primitive ‘grade’ of evolution involved in the distant ancestry of Mammalia (Rowe 2020a, b), and became known in the vernacular as “mammal-like reptiles”. It was their retention of numerous plesiomorphic amniote characters that persuaded virtually all paleontologists to classify them in what was then conceptualized as ‘paraphylum Reptilia’ which was considered ancestral to all the living amniote clades.
Skulls and skeletons of ‘pelycosaur-grade’ Early Permian stem-mammals: (a) Ophiacodon, (b) Casea, (c) Edaphosaurus, and (d) Dimetrodon. Drawn to same lengths. (Modified after Rowe 2020a)
The endocranial skeleton in early stem-mammals differs little from stem-amniotes and offers few details on brain size and shape. The endocranial cavity is open anteriorly, the forebrain enclosed laterally and ventrally by the (rarely-preserved) orbitosphenoid bone, and only posterior to the hypophysis is the endocranial cavity fully enclosed by bone. The forebrain was a featureless narrow cylinder, and there is no evidence of the interhemispheric sulcus (although it must have been present in life). Comparisons to the lepidosaur Sphenodon are closer than to any living mammal, and indeed these early endocasts only obscured the true relationships of early stem-mammals (e.g. Baur and Case 1899).
Subtle skeleton changes in early stem-mammals with implied neurosensory effects are detailed elsewhere (Rowe and Shepherd 2016; Rowe 2020a). Suffice it here to highlight the main diagnostic feature of Pan-Mammalia currently known, viz. the single temporal fenestra, bounded below by the homolog of the mammalian zygomatic arch (Gauthier et al. 1988a; Laurin and Reisz 2020; Rowe 2020c). The single fenestra and underlying arch comprise the ‘synapsid condition’ (Fig. 10.9), which allowed mandibular adductor musculature room to flex and expand outwards as the jaws snapped together without compressing the brain and blood vessels that lie deep to the adductor muscles. This exemplifies the epigenetic balancing act by the developing skull in supporting both the brain and masticatory system.
The ancestral amniote had small external nostrils that were directed laterally, and the internal nostrils (choanae) formed small openings near the front of the palate (Fig. 10.10). The space between nostril and choana allowed only a small nasal capsule and olfactory epithelium. However, in early stem-mammals the choana were considerably elongated, indicating a larger nasal capsule and expanded olfactory epithelium, beginning a trend in which enhanced olfaction would eventually become a major driver of pan-mammalian evolution (below).
Stages in the evolution of mammalian secondary palate and the ortho-retronasal olfaction duality. (a) Eusthenopteron, a stem-tetrapod; (b) Seymouria, a stem amniote; (c) Dimetrodon, a basal synapsid; (d) Syodon, a more advanced non-cynodontian synapsid; (e) Procynosuchus, the basal-most cynodont with an incipient secondary palate; (f) Thrinaxodon, an early cynodont with a complete secondary palate; (g) Kayentatherium, a basal mammaliamorph with a complex dentition; (h) Morganucodon, a basal mammaliaform, with secondary palate extending to back of tooth row; (i) Didelphis, with secondary extending behind tooth row. (From Rowe and Shepherd 2016). See anatomical abbreviations
At maturity, most of the early stem-mammals had longer faces than other early amniotes, with more than half of the skull lying in front of the orbits, and a jaw articulation displaced to a level behind the occiput that further widened jaw gape. The mouth was lined with a long row of sharp, recurved teeth that were replaced continuously throughout life. Most early stem-mammals had a faster and more powerful bite than other early amniotes. Locomotor evolution involved increased power and speed, with the two sacral ribs attaching to the ilium at a level above the acetabulum, lowering the hip joint beneath the vertebral column and conveying slightly greater stride and lunge capability (Romer and Price 1940; Romer 1956). Some of these taxa, sphenacodontines in particular (Fig. 10.9, top), were the apex predators of the Late Carboniferous and Early Permian (Romer and Price 1940; Romer 1956; Kemp 2005). Indirectly, this implies a greater measure of neural velocity in perception and response to their environmental interactions.
From the start, stem-mammal orbits were large and opened laterally or dorsolaterally, and they held relatively large, mobile eyeballs. The bones enclosing the orbit would undergo multiple evolutionary transformations that redirected the orbits frontally, expanding their fields of stereoscopic vision, and probably altering the range of eyeball movements (Walls 1942; Romer 1956; Kemp 2005; Rowe 2020a).
An auditory innovation arising in Sphenacodontia (Fig. 10.3, node 3) is a notch in the angular bone at the back of the jaw that freed a thin ‘reflected lamina’ that enclosed a narrow air space against the jaw. The ‘reflected lamina’ is the distant transformational homolog of the mammalian ectotympanic, which supports the tympanic membrane. Whether the notch above the reflected lamina held a functional tympanum at this stage is unknown; the delicate reflected lamina itself may have functioned as a crude tympanum. Its significance in audition is clear only in retrospect and its overall mature size and form were unlike any auditory element in living mammals. It probably responded only to loud, low frequency sound, and the sacculus of the inner ear occupied only a shallow depression in the floor of the otic capsule (Olson 1944; Romer and Price 1940; Romer 1956).
Diurnal vision, followed distantly by olfaction, were the leading sensory modalities for much of early stem-mammalian history. Successive subtle changes in the craniovertebral joint and neck raised the head above the body (Jenkins Jr. 1969), and early pan-mammals surveyed broader information horizons than other early amniotes.
10.4.1 Node 4: Therapsida
Therapsida (Rowe 2020d) (Fig. 10.3, node 4) is the clade stemming from the last common ancestor Mammalia shares with the mid-Permian Biarmosuchia, and all its descendants. In its traditional conceptualization as an extinct paraphylum or ‘grade of evolution’, Therapsida included only the extinct side branches Biarmosuchia, Deinocephalia, Gorgonopsia, Dicynodontia, Therocephalia, and a paraphyletic Cynodontia that excluded Mammalia (Fig. 10.11). Kemp (2006) summarized the features separating early Therapsida from more basal stem-mammals: “It has always been recognized that therapsids are in a general way more ‘advanced’, or ‘progressive’ in their biology than their pelycosaurian forebears”. Whether viewed as a grade or a clade, therapsids “... had evolved a higher rate of food assimilation and of ventilatory capacity, a more agile, faster, more energetic mode of locomotion, more elaborate and therefore more sensitive olfaction and hearing, and an increased growth rate” (Kemp 2006:1237).
Skulls and skeletons of Late Permian basal therapsids. (a) Titanophoneus, (b) Moschops, and (c) Lycaenops, drawn to the same lengths. (Modified after Rowe 2020a)
The face in basal therapsids presents an increasingly anterior or frontal axis of attention and activity, and bilateral directional coordination of visual and olfactory fields. The nostrils were redirected anterolaterally, enhancing stereoscopic directional perception of olfactory cues that are important in many mammals (Louis et al. 2008; Catania 2013; Catania and Catania 2015). The choanae are further elongated (Fig. 10.10d) over the condition of the basal-most stem-mammals (Sidor 2003), indicating further expansion of the nasal capsule and olfactory epithelium. The trenchant upper canine is longer than in ‘pelycosaurian grade’ stem-mammals and separates specialized enlarged incisors from unicuspid, recurved postcanine teeth. Early therapsids were increasingly specialized in apprehending and dismembering prey with a bite from their canines and incisors (Gauthier et al. 1988a; Kemp 2005, 2006). The orbits are more frontal in orientation, with an increased field of binocular stereoscopic vision focused in front of the nose and mouth, a characteristic of terrestrial mammalian predators (Walls 1942).
An important new character state in basal therapsids involved their mode of tooth implantation. In the ‘pelycosaur-grade’ stem-mammals, the teeth had shallow implantation and were ankylosed to the jaws. In early therapsids the roots were elongated and held in deep alveoli by the periodontal ligament or ‘gomphosis’ (Osborn 1984; Gaengler and Metzler 1992; Rowe 1993, 2020a; Kemp 2005; LeBlanc et al. 2018). The roots and innervated periodontal ligament signal a new role for neural crest cells in the head that would eventually have a profound impact on mammalian neurosensory systems at multiple levels of organization (Hall 2009). Initially, the dental gomphosis provided a cushion to resist the compressive and shear forces associated with biting (LeBlanc et al. 2018). It would eventually become highly innervated and a key innovation in the evolution of an occlusal dentition and food mastication (see Cynodontia, below).
In the mandible, the reflected lamina of the angular is deeply incised along its dorsal margin, and probably now functioned as a tympanum. However, it remained attached to the mandible along with several other bones in the sound transduction pathway, and any transmitted vibrations had to cross the craniomandibular joint to reach the inner ear. Bones of the middle ear chain had a new measure of individual movement but the sacculus remained little more than a shallow depression (Olson 1944).
An important visual characteristic of living mammals that must have evolved along the mammalian stem involves their manner of eye movement. While the origin of this behavior cannot be pin-pointed, it is expeditious to mention it here. Gordon Walls describes it as follows: “in the matter of eye movements, mammals are at once set off from all other vertebrates by the fact that whenever voluntary movements are possible at all, the two eyes are never independent but are always conjugated. This universal conjugation is associated with the fact that mammals (whales, rabbits, and some others excepted) examine things only binocularly – even the bats, small rodents, insectivores, and other nose- or ear-minded nocturnal forms whose eyes never move even reflexively. Where the eyes are placed laterally as in the rabbits, there usually is no area centralis, let alone a fovea, and there are no spontaneous movements at all. But even the rabbits have the gyroscopic reflex eye movement, including the optomotor reaction. These compensatory movements in mammals are always most extensive in the plane of greatest biological usefulness, which usually means horizontal. The voluntary eye movements of mammals are really best correlated with visual acuity, which, it so happens, does go pretty well with intelligence in this group of vertebrates” (Walls 1942: 310–311).
The early therapsid neck became longer and more flexible, increasing mobility of the head and expanding horizons of the special senses. Basal therapsids had six cervical vertebrae, but soon settled on the seven cervicals almost invariably present in mammals. The mammalian vestibular system helps direct muscles of the neck that are responsible for reflexive compensatory movements of the head and eyes that keep a stereo visual image stable and in focus as the head is otherwise jostled in walking and running (Walls 1942). Maintenance of these reflexes may explain the invariance in number of cervical vertebrae in mammals. We can only speculate that this vestibular feedback traces to early therapsids.
A surprising claim reported that the basal therapsid Kawingasaurus fossilis has an endocast with an EQ that overlaps with the lower range of crown Mammalia and preserves evidence of a ‘neocortex-like structure’ (Laaß and Kaestner 2017). Kawingasaurus is a member of the extinct Permo-Triassic stem-mammal side branch Dicynodontia, and is interested within its highly specialized fossorial clade Cistecephalidae (Cluver 1978). The labeled CT imagery that accompanied this report reveals a fundamental misinterpretation of the bones of the braincase. For example, the structure identified as the ethmoid (Laaß and Kaestner 2017, figs. 2a,b,c,e) is actually the orbitosphenoid, and demonstrates unequivocally a narrow cylindrical forebrain just as in other dicynodonts (e.g. Cluver 1971) and basal therapsids (Rowe et al. 1995; Benoit et al. 2016; Crompton et al. 2018).
In basal Therapsida the vertebral column became more robust and regionalized, and the limbs were longer with the elbows turned back and the knees turned forward. This marks a significant shift from the sprawling sigmoid vertebral propulsion of basal stem-mammals, toward more strident parasagittal gait with limbs playing a more forceful role in locomotion, enhanced aspirational breathing, and enhanced metabolic scope. This implies greater activity levels and more sustained high levels of neurosensory activity. Whether the earliest stem-mammals could run is doubtful, but basal therapsids almost certainly could, implying neurosensory elaboration that sets them apart. Unexpected shape variation was recently documented in endocasts of some early extinct therapsid side branches (Benoit et al. 2016); however, none has obvious bearing on neurosensory events on the direct path to the origin of Mammalia.
10.4.2 Node 9: Cynodontia
Cynodontia (Rowe 2020e) (Fig. 10.3, node 9) arose in the Late Permian ~230 million years ago, and today it includes the 6399 species of extant mammals (Burgin et al. 2018), plus many extinct Mesozoic and Cenozoic side branches. Many unique features of the mammalian skeleton and neurosensory system trace to the first cynodonts, as well as the first of several successive reductions in body size that effected shifts in ecology and life history strategy with profound neurosensory consequences.
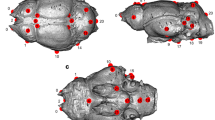
Early cynodonts (Fig. 10.12) manifest the first episode in pan-mammalian history in which the braincase became more fully ossified than in earlier stem-mammals. EQs are slightly higher in basal cynodonts (Benoit et al. 2016), and innovations in brain evolution can be qualitatively appreciated in modifications of the osteocranium in its epigenetic responsiveness to brain development (Rowe 1996a, b; Fabbri et al. 2017). The posterolateral braincase walls became more fully ossified by ventral sheets from the frontal and parietal, and an anterior lamina from the prootic. Most important was the ‘newly formed’ alisphenoid bone. Long thought to be an expanded epipterygoid, it arose as a compound element joining the embryonic ala temporalis (footplate) of the epipterygoid with a new, membranous ossification induced within the spheno-obturator membrane (Presley 1981; Gauthier et al. 1988a). The alisphenoid is thus a compound element. Its ‘new’ portion is induced by expansion of the caudolateral pole of the olfactory cortex in most living mammals (Rowe 1996a, b; Rowe and Shepherd 2016). Given the ontogenetic interdependencies of the different components of the olfactory system (above) this event may reflect the onset of expression of a larger set of OR genes.
Skulls and skeletons of Triassic basal cynodonts. (Bottom) Thrinaxodon; (Middle) Kayentatherium and its clutch of perinates; (Top) Morganucodon. Note the differentiation of thoracic and lumbar vertebrae, indicating presence of the diaphragm. (a, c modified after Rowe 2020a)
In cynodonts a secondary palate appeared, separating the nasopharyngeal passageway from the oral cavity, and displacing the choana to the back of the mouth (Fig. 10.10e). It forms as shelves of the maxillae and palatines grow toward the midline and fuse together to provide a bony floor beneath the nasal capsule and nasopharyngeal passageway, and a bony roof over the oral cavity. An occlusal dentition arose at the same time (Crompton 1963, 1972, 1989; Kemp 2005; Rowe and Shepherd 2016). The new ability to masticate food items yielded faster, enriched caloric return, enabling higher activity levels. Mastication occurs at the posterior (distal) part of the tooth row, where the mandibular adductor musculature was reorganized to exert its greatest force. We may infer that the tongue also took on a new role using the secondary palate as a substrate against which to move food within the oral cavity toward the teeth for mastication (Crompton and Parker 1978). Oral breakdown of food prior to swallowing also enabled more thorough inspection and analysis of food items, and the ability to extract and process new kinds of information from food.
Early cynodont postcanine teeth had ‘triconodont’ crowns in which there are generally three principal cups aligned longitudinally, with the middle cusp the tallest, and with a row of smaller cuspules on a narrow shelf at the base of the inner surface (Crompton 1963; Rowe 2020e; Rowe et al. 1995). Along the rear of the postcanine tooth row, the outer (buccal) surfaces of lower teeth occluded against the inner (lingual) surfaces of the upper teeth and produced irregular wear facets that are evidence of crown-to-crown occlusion (Fig. 10.13). A small degree of jaw rotation and a mobile symphysis facilitated occlusion, which was irregular at first, but eventually became intricately patterned. The rate of tooth replacement in early cynodonts was greatly reduced (Hopson 1971; Osborn and Crompton 1978). This initiated a new ‘variational modality’ involving unprecedented diversification of postcanine crown structure, function, and development that eventually enabled cynodonts to pierce, slice, dice, shred, and grind their food in ever more complex and efficient ways (Rowe and Shepherd 2016; Rowe 2020a). Up to this point, stem-mammal teeth were not subject to much variation, but in cynodonts almost every species has cheek teeth with its own diagnostic crown structure.
CT cross-section through the snout of the early Triassic cynodont Thrinaxodon, showing the deep implantation of postcanine teeth (Therapsida) as well as the occlusal relationship between upper and lower teeth (Cynodontia) on the right. (a) lateral view of skull (reconstructed from CT slices using VGStudio Max) showing slice plane (b), a coronal slice throught the snout. See anatomical abbreviations
The cynodont dentition eventually assembled into a new peripheral sensory array of considerable anatomical and neural complexity (Fig. 10.14), thanks in large part the ‘gomphosis’ mode of tooth implantation inherited from more basal therapsids, and to greatly reduced rates of postcanine replacement (Hopson 1971; Osborn and Crompton 1978). Ontogenetic malleability of the periodontal ligament enabled tooth crowns to establish precise occlusal relationships during eruption (Ten-Cate 1969, 1997). The cynodont periodontal ligament eventually became richly innervated, affording a considerable degree of learning and memory about food items during mastication. Recordings from single nerve fibers demonstrated that human periodontal receptors adapt slowly to maintained tooth loads (Trulsson 2006; Trulsson et al. 2010). Most receptors are broadly tuned to the direction of force application, and about half respond to forces applied to adjacent teeth. Information about the magnitude of tooth loads is made available in the mean firing rate response of periodontal receptors, and they precisely record intensity and spatiotemporal aspects of forces applied to a tooth. These mechanoreceptors are particularly important when biting and chewing because they efficiently encode tooth loading during intraoral food manipulation and are involved in jaw motor control and memory (Trulsson 2006; Trulsson et al. 2010).
In Mammalia, signals from periodontal mechanoreceptors project to separate oral fields of the primary somatosensory cortex (Remple et al. 2003; Kaas et al. 2006; Iyengar et al. 2007; Trulsson et al. 2010; Hlusko et al. 2011). Periodontal receptors encode information about the teeth stimulated and provide a detailed organizational map that adds representation of the dentition to the classic neocortical sensory animunculus (Kubo et al. 2008). There is also strong evidence for bilateral representation of the teeth into the primary sensory cortex coming directly from the thalamus or via transcallosal projections (Kaas et al. 2006; Iyengar et al. 2007; Habre-Hallage et al. 2014). Projections from the somatosensory oral cavity integrate cutaneous stimuli and movements of the tongue and jaws that are important for mastication and for the ability to recognize and discriminate the form of objects by using intraoral or perioral sensors. In the tongue, 80% or more of neurons are tactile, and 2–10% are taste receptors (Iyengar et al. 2007). The connections between the somatosensory representation of the teeth and tongue and adjoining motor and premotor representations of the oral cavity and jaw may help to coordinate motor control in chewing and swallowing (Iyengar et al. 2007), which becomes increasingly complex in the latest stem-mammals and Mammalia (Crompton 1989; Crompton et al. 2018).
Mastication plus a secondary palate liberated an entirely new class of odors and scents from food as it was chewed and broken down, and with this new behavior a new duality was introduced into the main olfactory system, known as ‘ortho-retronasal olfaction’ (Fig. 10.15) (Rowe and Shepherd 2016; Rowe 2020a). The primitive behavior of inhaling external environmental odorant molecules through the naris into the mouth, known as ‘orthonasal’ olfaction, was inherited from early stem-tetrapods. They were the first vertebrates in which the nasal capsule had both an external opening, the naris (nostril), and the internal naris or choana which opened through its floor into the roof of the mouth (Jarvik 1942). The counterpart to orthonasal smell is ‘retronasal’ smell, in which air exhaled from the lungs carries an entirely new information domain of odor molecules liberated through the breakdown of food by chewing, saliva, and actions of the tongue. These molecules pass forward from the caudal part of the oropharynx and via the choana they cross the main olfactory epithelium before being expelled through the nares. Orthonasal smell, retronasal smell, taste, and somatosensory signals from the lips, gums, cheeks, tongue and teeth passed along different pathways, but all eventually evolved convergence onto individual neurons in the neocortical area known as the orbitofrontal cortex that integrate the complex multisensory amalgam called ‘flavor’ (Shepherd 2004, 2006, 2012; De Araujo et al. 2003; Small et al. 2007; Rolls and Grabenhorst 2008; Rowe and Shepherd 2016; Rowe 2020a). The beginnings of this elaborate network trace to the first cynodonts, and its fullest measure of integration occurred as the orbitofrontal region of the neocortex emerged in Mammalia (below).
Diagrammatic representation of orthonasal and retronasal olfactory modes in a dog and human. (Modified from Rowe and Shepherd 2016)
Also apomorphic of Cynodontia is the ‘double occipital condyle’ formed by the right and left exoccipitals positioned at the ventrolateral edges of the foramen magnum. This double articulation expanded the range of stable excursion of the head without impairing passage of an enlarged spinal cord through the foramen magnum (Jenkins Jr. 1969, 1971). The ventrolateral position of the condyles and orientation of the semicircular canals (Berlin et al. 2013; Ekdale 2016) also suggest that the head was habitually held at a tilt with the nose toward the ground.
Separate thoracic and lumbar regions were differentiated such that ribs that encircle the thorax persist anteriorly, while the posterior three to five ribs form attenuated processes that fuse to their respective neural arches (i.e. lumbar ribs). Differentiation of separate thoracic and lumbar regions (Fig. 10.12) marks more symmetrical axial movement during locomotion, and the development of a muscular diaphragm, separating the thoracic and abdominal cavities, and a far more complete decoupling of aspirational breathing from locomotion. The vacuum-chamber or bellows-like tidal diaphragmatic ventilation of Mammalia allows ventilation while moving or at rest, and a sustained supply of oxygen to the brain for greater activity levels (Jenkins Jr. 1971; Gauthier et al. 1988a; Hirasawa and Kuratani 2013; Brainerd 2015). We may speculate that it brought the onset of new olfactory-mediated behaviors such as territorial scent-marking, the rapid sniffing behavior that drives scent tracking (Rowe and Shepherd 2016) and, more speculatively, reproductive behaviors related to parental care of the young.
10.4.3 Node 11 (Unnamed)
Node 11 is the unnamed clade stemming from the last common ancestor that Mammalia shares with Diademodon (Fig. 10.3, node 11). It is diagnosed by further elaboration of the molariform (postcanine) tooth roots, in which each cheek tooth crown has an ‘incipiently divided’ root. That is, there were two separate root canals, each conveying its own dental nerve to the pulp cavity, but a web of bone still connected the roots. This ‘incipient’ division of the roots occurred in Early and Middle Triassic cynodonts, and suggests they were mining more information in the differential loading of individual tooth crowns in mastication of different food types.
10.4.4 Node 12: Probainognathia
Probainognathia designates the clade stemming from the last common ancestor shared by the mid-Triassic Probainognathus and Mammalia (Fig. 10.3, node 12). EQ values in basal probainognathians are about the same as in more basal cynodonts (Quiroga 1979, 1980, 1984, Macrini 2006; Rowe et al. 2011; Benoit et al. 2016). However, EQ values fail to reveal what may be deeper insights into brain evolution based on other features of the endocasts (Wallace 2018).
In early probainognathians (Fig. 10.16) the endocast is more ‘brain-like’ than before, in that it is robustly ‘inflated’ against the braincase walls and embossed into them more vivid details of its external shape. Basal probainognathian endocasts convey the general impression of a much more strongly inflated brain very tightly packaged within a container whose proportions are constrained by competing functions of the skull such as supporting the masticatory system, in the type of relationship demonstrated by Weisbecker et al. (2021) in living and fossil marsupials. We may speculate that this is a time in stem-mammal evolution when the increased numbers and tighter packing of telencephalic neurons progressed, foreshadowing the cellular architecture that became characteristic of mammalian neocortex (Rubenstein and Rakic 1999; Rakic 2000, 2007, 2009; Molnár and Butler 2002; Shepherd and Rowe 2017).
The olfactory bulbs are larger and more distinctly separated by an encircling annular fissure from the rostral end of the cerebral hemispheres. The caudolateral poles of the olfactory (piriform) cortex diverge laterally to a greater degree than in basal cynodonts, and are now approximately as wide as the cerebellum. The forebrain was still long and narrow, but for the first time the interhemispheric sulcus is clearly visible on the endocast, and the cerebral hemispheres are convex and high-domed. Basal probainognathians retain the plesiomorphic absence of an osseous enclosure around the lateral and ventral surfaces of the olfactory bulb and the cerebrum behind the orbitosphenoid (Crompton et al. 2017b), and there remains a measure of subjectivity in reconstructing the complete endocast (Kemp 2009). To be clear, early probainognathians retained primitive endocasts when compared to even the least-encephalized mammal. But from enlarged olfactory bulbs and olfactory cortex, and doming of the dorsal cortex, it seems likely that another increase in expression of duplicated olfactory receptor genes had begun, that olfaction was exerting a far more dominant influence than ever before, and perhaps a new threshold in organization not revealed by the uncertainties in EQ estimates had been crossed. In any event, probainognathian cynodonts with approximately this general state of cerebral organization underwent a significant diversification during the Triassic.
The bones of the jaw lying behind the tooth-bearing dentary are considerably reduced, marking the onset of their negative allometric growth with respect to the skull and mandible (Rowe 1996a, b), and their increasing individuation as components of the auditory chain of the middle ear in a trend toward higher-frequency sound sensitivity.
10.4.5 Node 14: Mammaliamorpha
Mammaliamorpha (Rowe 1988, 2020f) is the clade stemming from the most recent common ancestor Mammalia shares with the extinct side branch Tritylodontidae (Fig. 10.3, Node 14, Fig 10.10g) (Kemp 1983; Rowe 1988). Mammaliamorpha arose ~230 million years ago, diversified into a number of extinct side branches across Pangea in the Late Triassic thru Middle Jurassic. There are several extinct Triassic to Early Jurassic side branches that may lie just within or just outside of Mammaliamorpha, but all share endocasts comparable in most respects to more basal probainognathians (Quiroga 1979, 1980, 1984; Benoit et al. 2016; Rodrigues et al. 2013, 2014, 2019; Wallace 2018; Hoffmann et al. 2019; Pavanatto et al. 2019). These include several taxa referred to as ‘brasilodonts’ (Bonaparte et al. 2005, 2013), a group of uncertain monophyly, Trithelodontidae (Martinelli and Rougier 2007; Sidor and Hancox 2006), and Pseudotherium argentinus (Wallace et al. 2019).
Further reduction in body size may have arisen in basal mammaliamorphs (the last common ancestor of Mammaliaformes unequivocally very small; Rowe 1988, 1993, 2020a; Rowe and Shepherd 2016). The most basal tritylodontid is probably Oligokyphus (Clark and Hopson 1985), and its shrew-sized body is about the same size as Morganucodon and other early mammaliaforms (Fig. 10.17). Miniaturization was attained in part by accelerated maturation of the skeleton at smaller and smaller sizes (Koyabu et al. 2014; Hoffman and Rowe 2018). Numerous descendant clades secondarily attained large body sizes, but most mammaliamorphs remained tiny from the Late Triassic until after the origin of crown Mammalia. Miniaturized mammaliamorphs encountered greater spatial and environmental heterogeneity than their larger ancestors. Entry into new microhabitats promoted dietary diversification, where new food items such as seeds, grains, fungi, small fruiting bodies, and small invertebrates were available for the first time, altering activity patterns and life history strategies (Harvey et al. 1980; Eisenberg 1990; Mace et al. 1981; Hayden et al. 2010). The mammaliamorph postcanine teeth now have two or more fully divided roots, each with its own dental canal and nerve, and molariform crowns occluded in complex patterns. Molariform teeth were not replaced, and their permanence potentially enabled the subtle textural information from different kinds of food to be learned and remembered to an increasing degree. Miniaturization involved greater excursion of the limbs and increased agility moving over complex three-dimensional habitats, implying muscle spindles and joint proprioceptors that were recording more information produced by the greater ranges of movement than before. Agile scampering and climbing were now added to the locomotion repertoire of the mammalian stem group (Kemp 1983, 1988, 2005; Rowe and Shepherd 2016; Rowe 2020a).
Skeletons drawn to scale of Lycaenops (a Late Permian basal therapsid), Thrinaxodon (an Early Triassic basal cynodont), and Morganucodon (a late Triassic basal mammaliaform) showing the reduction in body sizes towards miniaturization. (From: Rowe and Shepherd 2016)
Early mammaliamorph endocasts are generally similar to basal probainognathians. However, the pineal stalk was covered by rapid ontogenetic expansion of the cerebral hemispheres over the midbrain to contact the cerebellum, and the pineal foramen closed. Forebrain expansion may be reflected in ossification of the orbital wall by joined sheets of the frontal and palatine bones (Rowe 1988). The cerebellum has a distinguishable vermis and left and right cerebellar hemispheres bulge on either side (Wallace 2018), but this is probably more a consequence of packaging (Weisbecker et al. 2021) than functional differentiation. In basal mammaliamorphs, the internal auditory meatus is walled medially with separate foramina for the vestibular and cochlear nerves (Kemp 1983; Rowe 1988), and the cochlea underwent a first pulse in elongation, in some cases also curving over an arc of about 70° and suggesting greater sensitivity to a wider range of high frequencies (Luo et al. 2001, 2004; Kielan-Jaworowska et al. 2004; Rodrigues et al. 2013, 2019; Wallace et al. 2019). The angular is now nearly circular, and almost certainly held a tympanic membrane although it was still anchored to the mandible.
A μCT study of the stem-mammaliamorph Brasilitherium (Rodrigues et al. 2014) reported small ossifications in the nasal capsule that were interpreted as primordia of the nasoturbinal and the first ethmoturbinal, which support olfactory epithelium (Rowe et al. 2005). The posterior nasal septum is partly ossified and contributes to an ossified mesethmoid, which also supports olfactory epithelium in mammals. In addition, the nasal cavity expanded posteriorly forming a distinctive ethmoidal recess separated ventrally from the nasopharyngeal duct by an ossified lamina terminalis. Similar structures were reported in the nasal chamber of the closely related mammaliamorph Pseudotherium (Wallace et al. 2019), and possibly in tritylodonts (Kielan-Jaworowska et al. 2004). A primitive, relatively simple skeleton of ossified turbinals in fossils near the mammalian crown should not be surprising. However, in these two cases, the ossifications are very small and are not co-ossified to the wall of the nasal chamber, and other discernible features of the olfactory system leave uncertainty about their identity. Wallace (2018) pointed out that the reconstructed olfactory bulb in Brasilitherium seems excessively large and there is no corresponding expansion of the olfactory cortex. In her study of Pseudotherium, Wallace reconstructed a more conservative flat floor beneath the preserved impressions of the olfactory bulb, reducing endocranial volume by 15%, which placed it within the range of other basal mammaliamorphs. Applying a similar correction to Brasilitherium reduces its endocranial volume into the same cluster. In either case, we may be seeing another incremental increase in expression of OR genes.
Paleontologists have long speculated about whether there may have been an extensive network of cartilaginous turbinals in non-mammalian therapsids (e.g. Brink 1957; Hillenius 1992, 1994; Crompton et al. 2017b). As noted, olfactory gene expression initiates cascading ontogenetic interdependencies of olfactory epithelium surface area, ethmoid turbinal surface area, total area of foramina in the cribriform plate, olfactory bulb size, and olfactory cortex size. The individual components of the olfactory system offer general proxies for the system as a whole (Bird et al. 2018; Garrett and Steiper 2014; Hayden et al. 2010; Pihlström et al. 2005; Rowe et al. 2005; Rowe and Shepherd 2016; Schlosser 2010). However, it is important to recognize that turbinals do not exist as separate parts independent of the rest of the olfactory system. The recent data from endocasts suggests that the degree of olfactory development in basal cynodonts and early mammaliamorphs was still insufficient to induce an extensive scaffold of rigid support that approaches the degree in Mammalia, and the olfactory bulb and olfactory cortex remained relatively small. Moreover, at no time in mammalian ontogeny is there a free-standing extensive network of cartilaginous turbinals in any known mammal (Rowe et al. 2005). Nothing within the ‘extant phylogenetic bracket’ offers support for the hypothesis of an expansive network of cartilaginous turbinals in any stem-mammal. Nevertheless, as imaging technologies improve and larger samples of fossils are scanned, more compelling evidence may yet materialize to document intermediate states in the evolution of an ossified scaffold in late stem-mammals.
In another study based on μCT, Benoit et al. (2016) reported in tritylodontids that the maxillary canal carried the “true” infraorbital nerve and that it supplied vibrissae and a mobile rhinarium. These claims are doubtful because evidence of the other parts of the system to which they communicate is absent. Whiskers and the rhinarium are both parts of the cutaneous field of the trigeminus that develops in mammals in close association with the differentiation of complex facial muscles and a system of intricate circuitry with corresponding representations in the somatosensory area of neocortex, and outputs to the motor cortex (Huber 1930; Grant et al. 2013). Moreover, whiskers are not universally present in therian mammals (Catania and Catania 2015), and ancestral state reconstruction suggests that they evolved independently as many as seven times among therians (Muchlinski et al. 2020) and were never present in monotremes (Huber 1930). Whiskers and the rhinarium are inevitably linked to large numbers of efferent nerve axons, a much thicker infraorbital nerve and an considerably enlarged infraorbital foramen (Muchlinski 2008; Muchlinski et al. 2020). No such enlargement occurs in the “infraorbital canal” illustrated by Benoit et al. (2016). Presence of a mobile rhinarium can probably be dismissed in all stem-mammals because they retain the ossified internasal (prenasal) process of the premaxilla (Rowe 1988, 1993). This process was lost in mammals ancestrally, and a rhinarium seems to have appeared for the first time in therian or stem-therian mammals, along with fully differentiated facial muscles (Huber 1930) associated with a wide repertoire of learned orofacial motor skills (below). Developmental evidence suggests that monotreme facial musculature was apomorphically derived from the ancestral amniote sphincter coli and platysma muscles, and that a limited degree of facial muscle differentiation probably reflects the ancestral state for mammals (Huber 1930; Lightoller 1942). In light of the discovery that a pelt of modern aspect was present in basal mammaliaforms (below), it is conceivable (if speculative) that a primitive cover of innervated hair was present in basal mammaliamorphs. However, the sophisticated cortical barrels that map sensations from whiskers, and other neocortical areas that map sensory stimuli from whiskers, rhinarium, and their associated facial musculature requires cortical computing power for which there is no evidence at this point in stem-mammal evolution.
10.4.6 Node 15: Mammaliaformes
Mammaliaformes is the clade stemming from the last common ancestor that Mammalia shares with Morganucodonta (Rowe 1988, 2020g) (Fig. 10.3, Node 15). It arose by ~210 million years ago, diversified into a number of extinct side branches across Pangea in the Late Triassic thru Middle Jurassic, and Mammalia arose within it by ~170 million years ago. The most striking feature of early mammaliaforms is that their brains had almost doubled in relative size compared to basal mammaliamorphs, and the endocast is strongly ‘inflated’ and now looks very much like a mammalian brain (Figs. 10.18 and 10.19). Using the Eisenberg (1981) equation, the EQ of non-mammaliaform cynodonts was found to range from ~0.16 to 0.23, whereas the EQ of Morganucodon is ~0.32, reflecting an increase of 30–50% over basal cynodonts (Rowe et al. 2011). The olfactory bulb and olfactory cortex are by far the regions of greatest expansion. A deep annular fissure encircles the olfactory tract, marking a distinctive external division of the brain between the inflated olfactory bulbs and the cortex. The cerebellum is also enlarged, implying expansion of the basal nuclei, thalamus, and medulla.
3D reconstructions of the skull and endocast of Morganucodon, based on high-resolution CT imagery, using false colors to show the bone (tan) and matrix (red). Skull (a1) and endocast (a2) in dorsal view; (b1, b2) ventral view; (c1, c2) right lateral view; (d1, d2) left lateral view; and (e) and skull in occipital view. (Modified from Rowe et al. 2011). See anatomical abbreviations
3D reconstructions of the skull and endocast of Hadrocodium, based on high-resolution CT imagery, using false colors to show the bone (tan) and matrix (red). Skull (a1) and endocast (a2) in dorsal view; (b1, b2) ventral view; (c1, c2) right lateral view; (d1, d2) left lateral view; and (e) and skull in occipital view. (Modified from Rowe et al. 2011). See anatomical abbreviations
The dentition evolved a more complex occlusal pattern. The diphyodont pattern of tooth postcanine tooth replacement characteristic of mammals seems unequivocally established at this point in stem-mammal phylogeny, if not arising earlier in basal mammaliamorphs (Cifelli et al. 1996; Luo et al. 2004). The evolution of non-replacing molars marks a landmark in dental function, learning, and memory. Trulsson et al. (2010) compared the responses to tooth stimulation with those produced by identical vibrotactile stimulation of fingers. The results suggest that the periodontal ligament mechanoreceptors in living mammals play a significant role in specifying forces used to hold and manipulate food between teeth, and in these respects the masticatory system appears analogous to fine finger-control mechanisms used during precision manipulation of small objects. Their fMRI studies revealed activations in posterior insular cortex, leading them to speculate that the dentition, via the periodontal ligament mechanoreceptors, are involved in an important aspect of the feeling of body ownership (Trulsson 2006; Trulsson et al. 2010).
A pelt of modern aspect, with guard hairs and velus underfur, was discovered in the exceptionally preserved Castorocauda lutrasimilis (Ji et al. 2006), a late-surviving non-mammalian member of Mammaliaformes from the Middle Jurassic (~165 million years old) of China. Hair follicles have been called ‘dynamic miniorgans’ owing to their complex patterns of gene expression and complex mesenchymal-epithelial interactions during development, complex innervation (Fig. 10.8), and the many functions they serve, including thermoregulation, physical protection, sensory activity, and social interactions. Hair follicles have large projections to the primary somatosensory area of the neocortex (Fig. 10.6) (Schneider et al. 2009). In mammals, guard hairs are equipped with at least three different kinds of mechanoreceptors that induce the somatotopic sensory maps on the outer layer of neocortex (Sengel 1976; Zelená 1994; Rowe et al. 2011), and each is associated with its own arrector pili musculature and sebaceous glands. In living mammals with small brains (e.g. Monodelphis, Didelphis), the small neocortex is dominated by a single primary somatosensory area that maps sensation from mechanoreceptors in the skin, hair follicles, muscle spindles, and joint receptors. Its conscious component involves body surface monitoring and tactile exploration of the immediate environment. A parallel, underlying neocortical motor map is represented in pyramidal neurons whose axons form the corticospinal (pyramidal) tract that projects directly to the spinal column to program and execute skilled movements requiring precise control of distal musculature. An enlarged foramen magnum in basal mammaliaforms (Figs. 10.18 and 10.19) indicates a thicker spinal cord, possibly an indication that the corticospinal tract had emerged (Rowe et al. 2011; Shepherd and Rowe 2017).
The cochlea in early mammaliaforms, including Hadrocodium (below) is similar to basal mammaliamorphs, curving over about 70°. However, it still lacks the bony lamina which supports the basilar membrane (Graybeal et al. 1989; Kielan-Jaworowska et al. 2004; Luo et al. 2012), and was far less sensitive than the inner ears in Mammalia.
10.4.7 Node 16: Unnamed
The Early Jurassic fossil Hadrocodium wui (Luo et al. 2001), known from a single skull (Figs. 10.19), from the Early Jurassic of China (~190 Ma), is either the closest extinct sister taxon to crown clade Mammalia (Rowe et al. 2011; Luo et al. 2015) (Fig. 10.3, Node 16), or the oldest fossil lying just inside the crown (Rowe et al. 2008). Despite its tiny size, CT scans showed no evidence of un-erupted replacement teeth, suggesting it was mature at time of death. Hadrocodium preserves another pulse in encephalization that raised its EQ to ~0.5, a level within the range of crown mammals (Rowe et al. 2011). This reflects a further increase in relative size of olfactory bulbs and olfactory cortex. Its cerebellum also expanded to such a degree that the occipital plate bulges backwards, where it enclosed a relatively large foramen magnum and thick spinal cord, and possible evidence that the corticospinal tract had emerged.
10.4.8 Node 17: Mammalia
Far more justifiable inferences can be made regarding the ancestral species of Mammalia because we have two major living sister lineages to compare, and thus their most recent common ancestor lies within the ‘extant phylogenetic bracket’ (Rowe 1988, 2020b; Witmer 1995). The fossil record indicates that Monotremata (Rowe et al. 2020) and Theria had diverged by or before the Middle Jurassic, ~170 million years ago (Rowe 1988, 2020a). Perhaps the most remarkable feature in all of pan-mammalian history is the emergence of six-layer neocortex from the three-layer dorsal cortex of amniotes ancestrally, and with it arose the uniquely diverse cognitive and behavioral abilities of mammals (Harris and Shepherd 2015; Rowe and Shepherd 2016; Shepherd and Rowe 2017; Rowe 2020a).
The rhinal fissure is an anatomical boundary between dorsal neocortex and lateral olfactory cortex that is clear in histological samples, and when visible in endocasts it demarcates the two regions. However, in small mammals the meninges are sufficiently thick that they often prevent the inner wall of the parietal from forming a ridge that enters the fissure; the rhinal fissure can be present in life, but not represented in an endocast. In other words, there is no unambiguous anatomical marker for neocortex in endocasts from stem-mammals and many crown mammals. However, histological studies of brains in monotremes (Ashwell 2013) and therians (Ashwell 2010) indicate neocortex is present in both, and its inferred presence in mammals ancestrally is unequivocal.
As noted, the three layer dorsal cortex of basal amniotes functions as an associative network of higher level functions and, over the course of stem-mammal evolution, six-layer neocortex emerged as a further elaboration of this network that enhanced computationally more demanding functions involving multidimensional perceptions, memory, planning, and execution (Shepherd and Rowe 2017). The extinct taxa Morganucodon and Hadrocodium closely approached and then overlapped the lower range of EQ in Mammalia (Fig. 10.20); if neocortex emerged prior to the origin of crown Mammalia, it was more likely present in basal Mammaliaformes than in more distant stem-mammals.
Patterns of brain evolution in phylogeny of basal Triassic cynodonts and selected crown Mammalia. Encephalization Quotient (EQ) is shown as a histogram, and selected endocasts are scaled to EQ. (From Rowe et al. 2011)
The computational power of neocortex derives in part from its subdivisions within and across layers into functionally distinct and specialized regions knowns as ‘fields’ or ‘areas’, and independent elaboration in numbers of neocortical areas is characteristic of different mammalian clades in association with independent evolutionary increases in encephalization (Kaas 2009, 2020). The outputs from cortical areas provide input to other cortical areas where computational functions are reiterated. The increased numbers of cortical areas increase the numbers of computations that are possible, resulting in more sophisticated computations overall (Kaas 2009, 2020; Krubitzer and Hunt 2009). Reconstructing the number and types of areas present in the ancestral mammal is problematic in that most studies have focused on a few model species, and appropriate comparisons between monotremes and therians are limited. That said, estimates are that the ancestral mammal probably had ~20 neocortical areas, including a primary (S1) and secondary (S2) somatosensory areas, and possibly three or four others; primary (V1) and secondary (V2) visual areas, and perhaps one or two others; a primary auditory area (A1), and possibly a second area; a primary motor area (M1); and other areas of limbic, orbitofrontal, and endorhinal cortex (Kaas 2009, 2020; Krubitzer and Hunt 2009; Molnár et al. 2014). The general trend is for larger brains to have more cortical areas, and as many as 200 areas have been tentatively identified in humans (Kaas 2013).
At the cellular level, the pyramidal neuron populations are greatly expanded compared to other tetrapods, and their cell bodies are densely packed in the six-layered neocortex (e.g. Kaas 2009; Molnár et al. 2009). Moreover, during the course of pan-mammalian evolution the basic pyramidal cell present in the ancestral amniote diversified into four main types that lie at different layers in the six-layered neocortex (Shepherd and Rowe 2017). Migration of neuron precursors along radial glial columns generate its columnar organization and increased neocortical thickness (Rakic 1988, 2000, 2007, 2009). Neocortical organization is broadly similar between cortical areas and between species, leading to the idea of a ‘canonical microcircuit’ that employs a similar computational strategy to process multiple types of information (Shepherd 2011; Harris and Shepherd 2015). As the OR genome increased by more than an order of magnitude over the ancestral amniote, and the repertoire of perceptible odorants increased exponentially, the number of microcircuits in the olfactory bulb and olfactory cortex increased correspondingly (Shepherd et al. 2021). The expanded numbers of nuclei in the dorsal thalamus of amniotes (Butler and Hodos 2005; Nieuwenhuys et al. 1998) was carried to extreme degrees in mammals in association with the proliferation of specialized neocortical areas.
In the three-layer dorsal cortex of basal amniotes, peripheral afferent projections to the dorsal and olfactory cortex coursed over the outer layer, while efferents projected from the inner layer to other parts of the brain and body. In mammalian neocortex, peripheral afferents may reach multiple layers of neocortex, efferents may be intratelencephalic projections, corticothalamic projections, or corticospinal projections, effecting a fundamental reorganization of connectivity to, from, and within the primitive three-layer dorsal dorsal cortex (Shepherd 2011; Shepherd and Rowe 2017). In all amniotes, projections from the dorsal cortex innervate the basal ganglia and brainstem, but in mammals (possibly originating in basal Mammaliaformes), neocortical projections can pass directly into the spinal cord as well, forming the unique corticospinal (pyramidal) tract. The uniqueness of neocortex involves not only the elaboration of inherited associative networks, but also new connections through the corticospinal tract that give higher neocortical functions direct access to virtually the entire neuraxis (Shepherd and Rowe 2017).
10.4.8.1 Ossified Ethmoid Complex
Ossification of an elaborate skeleton of ethmoid turbinals occurred by or before the origin of Mammalia. Its beginnings probably extend to early mammaliamorphs or even more basal cynodonts, but so far the evidence in fossils remains open to interpretation (above). The turbinal skeleton in Mammalia afforded a 10-fold or greater increase in the surface area of olfactory epithelium that could be deployed inside the nasal cavity (Rowe et al. 2005). The ethmoid turbinals coalesce around the olfactory nerve fascicles to form the bony cribriform plate, a compound structure that separates the olfactory recess from the cavum cranii. The turbinals grow rostrally from the cribriform plate as the olfactory epithelium matures, and their mature geometry is highly variable among mammals (Rowe et al. 2005; Macrini 2012, 2014). Also ossifying in the nose is the maxillary turbinal (Fig. 10.7), which increases the epithelial surface area by nearly an order of magnitude that is involved in regulating respiratory moisture and heat exchange, (Taylor 1977; Van Valkenburgh et al. 2004; Rowe et al. 2005; Green et al. 2012).
10.4.8.2 The Mammalian Middle Ear
An extraordinary morphogenic consequence of the expanded olfactory cortex in Mammalia is that the auditory chain was disrupted during ontogeny, and those ossicles directly involved in the auditory chain were detached from their ancestral and embryonic position on the mandible, relocated a short distance behind the mandible, and suspended exclusively from beneath the braincase during early ontogeny as the brain grows in circumference (Rowe 1996a, b). The result is that the middle ear was more sensitive and receptive to an extended range of high frequency sound. This left the dentary as the sole element of the mandible in mature Mammalia. Other mechanisms have been hypothesized, and whether detachment is a unique autapomorphy of Mammalia, or Mammalia plus Hadrocodium, or if it represents wide spread convergent evolution among stem-mammals is controversial (Rowe 1988, 1996a, b; Wang et al. 2001; Bever et al. 2005; Luo 2007; Ji et al. 2006; Meng et al. 2006).
Suspension of the middle ear from beneath the cranium offered the mammalian middle ear enhanced sensitivity, and possibly also an extended range of high frequency sound perception. In Mammalia, the cochlea added a bony lamina which supports the basilar membrane and two distinct types of hair cells. Inner hair cells located along the central axis of the cochlea carried efferent signals to cochlear nuclei, as before. But outer hair cells receive efferents from the brain that are thought to amplify sound induced vibrations of the basilar membrane, and in the rodent in which it was first reported, at least, this make the inner hair cells more responsive to sound by a factor of ~100 times (Ren et al. 2011; Streidter and Northcutt 2020). It is doubtful that this degree of amplification was present in the ancestral mammal, since its cochlea was still short, and it surely became a more potent factor in therian mammals that have a long coiled cochlea.
10.4.8.3 Orofacial Motor Skills
Cynodont mastication eventually became linked to a complex of novel orofacial muscles and behaviors involving diverse orofacial motor skills including learned orofacial movements in suckling, chewing, and swallowing (Crompton et al. 2018). Such behaviors were long attributed to brain stem circuits, but it is now apparent from anatomical, electrophysiological imaging, and behavioral studies of the facial sensorimotor cortex in mammals that the face primary motor cortex and the face primary somatosensory cortex make important contributions to the control of these learned movements (Avivi-Arber et al. 2011). Hence, the new function of mastication would eventually be reflected in a large neocortical presence, but these were much later developments that arose within Mammalia and carried to their extreme in therians (Rowe 2020a).
10.4.8.4 Spinal Cord
A double-occipital condyle arose in basal Cynodontia, and in Mammalia the condyles expanded to surround the entire ventral half of the foramen magnum. Correspondingly, the mammalian atlas, or first vertebra, is highly distinctive in forming a bony ring through ontogenetic fusion of the three separate ossification centers (centrum, right & left neural arches) that had remained separate throughout life in all stem-mammals. The limbs and girdles develop secondary ossification centers, the most obvious of which are the cartilaginous epiphyses of the long bones. Sesamoid bones form in tendons of the flexor muscles of the hands and feet, and in the hindlimb a single large sesamoid forms the patella (Rowe 1988, 1993). These modifications correlate with increased thickness and regionalization of the spinal cord, owing in part to the advent of the corticospinal tract, and to increased agility to which the sesamoid bones may contribute.
10.4.8.5 Nocturnality
A popular interpretation is that early mammals and mammaliaforms were nocturnal (e.g. Kermack and Kermack 1984). There is no evidence in extant mammals of RhB/Rh2 opsin genes, which must have been lost somewhere along the mammalian stem. Further reductions in opsin genes occurred in different clades within crown Mammalia, where the SWS1 opsin gene became dysfunctional in monotremates, while the SWS2 opsin gene was lost in therians (Collin 2010, Jacobs 2009, 2013; Wakefield et al. 2008). Thus, as Walls (1942) surmised, the ancestral mammal may have been diurnal with trichromatic vision, and that dichromatic crepuscular to nocturnal behaviors in monotremes and therians evolved independently (with a gene duplication restoring trichromatic vision to some primates). The sclerotic ossicles were also lost in Mammalia (or perhaps Mammaliaformes) ancestrally, allowing the eyeball to become nearly spherical (Walls 1942).
10.5 Discussion
The poorly ossified braincase in basal ‘pelycosaur-grade’ stem-mammals offers little direct evidence of neurosensory organization beyond what can be inferred about the ancestral amniote brain. Diversification in feeding and minor advances in locomotion were the major trends in evolution. Inferred neurosensory elaboration in a few of these taxa, particularly the sphenacodontines, included slightly greater frontality of the orbits, consistent with their inferred role as apex predators. Most show elongation of the choana, suggesting increased size of the olfactory capsule and its olfactory epithelium. In Wagner’s (2014) terms these all qualify as novel character states (Type II innovations).
With the origin of Therapsida, the novel tooth implantation via long roots held in deep alveoli by an innervated periodontal ligament would eventually become a key innovation in evolution of the cynodont masticatory system. Formation of tooth roots and the periodontal ligament marked a new role for neural crest cells in pan-mammalian evolution that eventually had far-reaching neurosensory and morphogenic consequences for stem-mammals.
Increased individuation of regions in the vertebral column occurred in the atlas-axis complex, establishment of seven cervical vertebrae in the neck, and in a shift toward parasagittal movement of the dorsal vertebrae and ribs that may have begun the process of decoupling aspirational breathing from locomotion. Inferences of increased aerobic ventilation and metabolic scope, more agile locomotion, and presumed higher levels of activity are consistent with these anatomical transformations, and with expanded geographic distribution of early therapsids.
Most of the innovations seen in basal therapsids can be categorized as new variational modalities in systems of repeated parts. At this point in stem-mammal history, they probably fit best into Wagner’s category of Type II innovations. In retrospect, however, they foreshadow the later individuation of Type I novelties as the dentition took on a new character identity as an integrated sensory array involved in the novel function of mastication.
Digital endocasts of early therapsids (Benoit et al. 2017) provide the earliest models for comparison to later stem-mammals, but at present there is little direct evidence of how they differed from the most basal (pelycosaur grade) stem-mammals. Compared with their living descendants, early therapsids possessed low-resolution olfaction, weak hearing, coarse tactile sensitivity, poorly refined motor coordination, and sensory-motor integration that commanded little cerebral territory. Vision may have been their leading sensory modality.
The origin of Cynodontia signals onset of integration in previously distinct anatomical systems and sensory inputs that were recruited into the masticatory system. The new functions of occlusion and mastication involved further specialization of established incisor, canine, and postcanine regions, and in the complexity and diversity of functions that different parts of the dentition could now perform. A new variational modality ensued in which virtually every species evolved a unique crown structure, whereas rates of tooth replacement slowed (Rowe and Shepherd 2016; Rowe 2020a, e). This was correlated with the appearance of the secondary palate and separation of oral and nasopharyngeal passageways, and initiation of the compound sense of ‘ortho-retronasal olfaction’, which combines with sensory information from the tongue, lips, and cheeks that converges on single neurons in the orbitofrontal region of neocortex. Ossification of the alisphenoid was initiated by expansion of the caudolateral pole of the olfactory cortex, implying elaboration of the olfactory system that was probably induced, ultimately, by expression of a larger number of olfactory receptor genes. The ontogenetic interdependencies that connect the various parts of the olfactory system were probably inherited from the ancestral amniote, but in cynodonts olfaction became sufficiently elaborated to induce visible changes in cranial morphogenesis.
Further individuation of regions of the axial skeleton occurred and, if not from the start, they later gained a surprising degree of integration with the olfactory and masticatory system. The double occipital condyle gave the skull a new kind of articulation to the atlas-axis complex and neck, providing a greater degree of stable dorsoventral and lateral movement by the head and neck and probably refined directional scent detection. At the same time, differentiation of distinct thoracic and lumbar regions indicate the onset of diaphragmatic ventilation, and more complete decoupling of aspirational breathing and sniffing from locomotion.
Basal cynodonts had begun to forge new functional linkages between biting, chewing, swallowing, sniffing and breathing, orthonasal and retronasal olfaction, taste, flavor and, more speculatively, territorial scent marking, scent-tracking, and odorant-moderated reproductive behaviors. The cynodont dentition eventually became individuated into a unique functional unit and sensory array that would eventually project to a large neocortical territory worthy of consideration as a Type 1 novelty. Diversification of the masticatory system became a major feature of cynodont evolution, including major clades within Mammalia. The neural implications are largely unexplored, but it is already clear that the cynodont masticatory system produced a rising tide of new kinds of peripheral information to the brain that imply linkages in the dorsal cortex for the first time of multiple previously independent sensory systems.
With the origin of Mammaliaformes (or possibly earlier, in basal Mammaliamorpha) miniaturization of adult body size occurred. For most of its Late Triassic, Jurassic and Cretaceous history, pan-mammals were mostly shrew-sized animals; a few reached the size of domestic cats, but it was not until the Cenozoic that huge body sizes evolved in crown Mammalia. Miniaturization corresponded with increased precision movements and agility of the skeleton, as well as the volume and kinds of internal information passing between the brain and the musculoskeletal system. Indirect evidence of further encephalization is reflected in ossification of the orbital walls. Ossification of rear parts of the nasal capsule and possible ossified primordia of the ethmoid skeleton suggest expression of another increase in OR genes. The brain in basal Mammaliaformes more than doubled in relative size. Most of this volume increase occurred in the olfactory bulb and olfactory cortex, and in all likelihood their projection to an emerging orbitofrontal region in the dorsal cortex. This probably reflects the largest increase in numbers of expressed olfactory receptor genes yet to occur in stem-mammal history. A pelt of modern aspect was also present. Induced by many thousands of body placodes, the ‘dynamic miniorgans’ (Schneider et al. 2009) that body hair represents must have provided a flood of new peripheral information to dorsal cortex; in Mammalia it has a large presence in somatosensory areas of neocortex. Moreover, from this point onwards the brain as a whole entered a new variational modality in which independent evolutionary increases in encephalization characterize many clades within Mammalia (Fig. 10.20). Instances of secondary reduction in encephalization are rare (Macrini et al. 2006; Kruska 2007; Castiglione et al. 2021).
The discovery of fur in a Jurassic mammaliaform has additional implications for understanding mammalian neurosensory evolution. During ontogeny in mammals, hair performs first as a tactile organ and only later does it insulate as underfur thickens and matures (Zelená 1994; Schneider et al. 2009). Body temperature in newborn mammals is initially regulated by their mothers. This sequence implies that parental care and endothermy may have been present in Mammaliaformes ancestrally. Endothermy may have been an evolutionary consequence of mammaliaform encephalization because a large brain operates properly only within narrow thermal tolerances, and it is metabolically the most expensive organ to maintain. However, metabolism is under hormonal control that does not command large cerebral regions; thus endothermy did not itself drive encephalization (Rowe et al. 2011).
Reproductive strategies may also have been reorganized in basal Mammaliaformes. Fossil evidence was recently discovered of a large clutch of perinates in the Early Jurassic tritylodontid Kayentatherium wellesi (Fig. 10.12b) with a presumed maternal skeleton (Hoffman and Rowe 2018). The single clutch comprises at least 38 individuals, well outside the range of litter sizes documented in extant mammals. This confirms that production of high numbers of offspring represents the ancestral condition for amniotes and also constrains the timing of a reduction in clutch size along the mammalian stem to a late point in stem-mammalian history. Tritylodontids diverged from the mammalian stem just before the pulse of brain expansion that occurred with the origin of Mammaliaformes (Rowe et al. 2011). The association of a high number of offspring and largely isometric cranial growth in Kayentatherium is consistent with a scenario in which increased encephalization, and attendant shifts in metabolism and cranial allometry, drove later changes to reproductive strategy and smaller clutch sizes (Hoffman and Rowe 2018). This was in place in Mammalia ancestrally, but may trace to the origin of Mammaliaformes.
With the origin of Mammalia, we enter the phylogenetic bracket of extant monotremes and therians, which allows a much larger number of justifiable inferences regarding novelties arising in (or before) the last common ancestor of the crown clade. Neocortex, including the corticospinal tract, was undoubtedly present in the ancestral mammal, and the profound integration of the ancestrally distinct structures and systems that neocortex now integrates diagnoses it as a Type I novelty. As we have seen, many of the individual components of the larger system integrated by neocortex can be traced into the mammalian stem-group, to their roots as more-or-less discrete anatomical and functional elements, with plesiomorphic varriational modalities. With such a rich fossil record of intermediate forms all along the mammalian stem, it is doubtful that a precise point of emergence of neocortex as a Type I novelty is susceptible to strict definition, and exactly where along the mammalian stem one draws this somewhat arbitrary boundary depends on one’s research interests and goals (Wagner 2014).
The emergence of neocortex, lying as it does at the integral core of mammalian brain organization, was a central theme in stem-mammal evolution and the origin of Mammalia.
Olfaction, and its integration with other sensory modalities in the orbitoprefrontal region of neocortex, was a central driver in neocortical evolution (Shepherd and Rowe 2017). Olfactory genes form the largest and most rapidly evolving subfamily in the vertebrate (Niimura 2009, 2012), tetrapod (Yohe et al. 2020), and mammalian (Young et al. 2010) genomes. This reflects the selective importance of responding to ever-changing chemical environments that mammals exploited to a degree exceeding other vertebrates. Gene duplication is the primary mechanism of OR gene increases throughout vertebrate history (Bargmann 2006; Niimura 2012; Wagner 2014). In the transition from water onto land, the pace of olfactory receptor gene evolution accelerated into what has been called ‘evolutionary overdrive’ (Yohe et al. 2020), as tetrapods adapted to the more diverse and rapidly changing chemical environment encountered in terrestrial ecosystems. Mammalia carried this trend to its greatest extreme, as measured by the relative size of the mammalian olfactory genome, the complexity of microcircuitry in the mammalian olfactory pathway (Shepherd et al. 2021), and at gross anatomical levels in the size and complexity of epithelial and skeletal structures induced in an ontogenetic cascade that follows olfactory gene expression. The rapid rate of OR pseudogenation observed in many mammalian clades (Young et al. 2010; Niimura 2012) is further evidence of rapid OR evolution, and further emphasizes the rapidity of change in chemical environments successfully occupied by early mammals. As Aboitiz and Montiel (2015) comment: “our hypothesis has common ground with those proposed by Lynch (1986), Rowe et al. (2011) and Rowe and Shepherd (2016) that olfactory systems were key in early mammalian evolution. Here we add to these hypotheses the role of the emergent isocortex [neocortex] as a multimodal interface in the olfactory-hippocampal axis for behavioral navigation”.
The evolving ontogeny of mammalian neocortex proceeded as a surging flood of new peripheral information ascended to the brain (Rowe and Shepherd 2016; Rowe 2020a). Whether through connectional invasions and epigenetic population matching (Katz and Lasek 1978; Krubitzer and Kaas 2005; Streidter 2005), or some other developmental mechanism, hypertrophy of peripheral sensory arrays involving olfaction, dentition, musculoskeletal system, and elaborate integument produced cascading influences on central organization that are so distinctive of mammalian neocortex today. Early mammaliaformes and many early members of crown Mammalia immersed themselves in a wealth of new information in microhabitats dominated to an unprecedented degree by scents, odors, and smells. Their unsurpassed abilities to perceive and process olfactory information and to diversify and exploit the fast-changing chemical environments they faced throughout much of their history is one of the keys to understanding the major features of pan-mammal evolution.
Abbreviations
- Ali :
-
Alisphenoid
- Alv :
-
Alveoli for the dentition
- Ang :
-
Angular
- Art :
-
Articular
- Bs :
-
Basisphenoid
- c :
-
Lower canine
- C :
-
Upper canine
- Cb :
-
Cerebellum
- choa:
-
Choana
- cr :
-
cheek tooth crown
- cve :
-
Cavum epipterycum
- D cond :
-
Condylar process of dentary
- D cor :
-
Coronoid process of dentary
- D ctx :
-
Dorsal cortex (endocast)
- Den :
-
Dentary
- D ang :
-
Angular process of dentary
- D ram :
-
Dentary ramus
- Ec :
-
Ectopterygoid
- Eoc :
-
Exoccipital
- Et 1-5 :
-
Ethmoid turbinals 1-5
- F ann :
-
Annular fissure
- F mag :
-
Foramen magnum
- Fr :
-
Frontal
- Fv :
-
Fenestra vestibuli
- Hyp :
-
Hypophysis (endocast)
- i :
-
1-4 Lower incisors
- I 1-3 :
-
Upper incisors
- iam :
-
Internal auditory meatus (endocast)
- II :
-
Cranial nerve II (endocast)
- Ju :
-
Jugal
- Lac :
-
Lacrimal
- m 1-3 :
-
Lower molars
- M 1-3 :
-
Upper molars
- Max :
-
Maxilla
- Mt :
-
Maxilloturbinal
- Nas :
-
Nasal
- Ncx :
-
Neocortex
- Nt :
-
Nasoturbinal
- Ob :
-
Olfactory bulb
- Ocx :
-
Olfactory (piriform) cortex
- Opl :
-
Optic lobes (endocast)
- p 1-5 :
-
Lower premolars
- P 1-2 :
-
Upper premolars
- Pa :
-
Parietal
- Pal :
-
Palatine
- Pet :
-
Petrosal
- Pin :
-
Pineal body (endocast)
- Pfl :
-
Paraflocculus (endocast)
- Pmx :
-
Premaxilla
- Prom :
-
Promontorium of petrosal
- Pt :
-
Pterygoid
- Qu :
-
Quadrate
- Re lam :
-
Reflected lamina of angular (=ectotympanic)
- Rf :
-
Rhinal fissure
- rt :
-
tooth root
- Smx :
-
Septomaxilla
- Soc :
-
Supraoccipital
- Spc :
-
Spinal cord (endocast)
- Sq :
-
Squamosal
- sss :
-
Superior sagittal sinus (endocast)
- Sv :
-
Sinus venosus
- V :
-
Cranial nerve V (endocast)
- Vo :
-
Vomer
References
Aboitiz F, Montiel JF (2015) Olfaction, navigation, and the origin of isocortex. Front Neurosci 9(402):1–12
Ashwell K (ed) (2010) The neurobiology of Australian marsupials. Cambridge University Press, Cambridge, UK
Ashwell K (ed) (2013) Neurobiology of monotremes: brain evolution in our distant mammalian cousins. CSIRO Publishing, Collingwood
Avivi-Arber L, Martin R, Lee JC et al (2011) Face sensorimotor cortex and its neuroplasticity related to orofacial sensorimotor functions. Arch Oral Biol 56(12):1440–1465
Balanoff AM, Bever GS (2020) The role of endocasts in the study of brain evolution. In: Kaas J (ed) Evolutionary neurosciece, 2nd edn. Academic, New York, pp 223–241
Balanoff AM, Bever GS, Colbert MW et al (2016) Best practices for digitally constructing endocranial casts: examples from birds and their dinosaurian relatives. J Anat 229(2):173–190
Bargmann CI (2006) Comparative chemosensation from receptors to ecology. Nature 444:295–301
Barrowclough GF, Cracraft J, Klicka J et al (2016) How many kinds of birds are there and why does it matter? PLoS One 11(11):e0166307
Baur G, Case EC (1899) The history of the pelycosauria with a description of the genus Dimetrodon. Trans Am Phil Soc 20:5–62
Baxi KN, Dorries KM, Eisthen HL (2006) Is the vomeronasal system really specialized for detecting pheromones? Trends Neurosci 29(1):1–7
Benoit J, Manger PR, Rubidge BS (2016) Palaeoneurological clues to the evolution of defining mammalian soft tissue traits. Sci Rep 6(1):1–10. https://doi.org/10.1038/srep25604
Benoit J, Fernandez V, Manger PR et al (2017) Endocranial casts of pre-mammalian therapsids reveal an unexpected neurological diversity at the deep evolutionary root of mammals. Brain Behav Evol 90(4):311–333
Berlin JC, Kirk EC, Rowe TB (2013) Functional implications of ubiquitous semicircular non-orthogonality in mammals. PLoS One 8(11):e79585. https://doi.org/10.1371/journal.pone.0079585
Bertmar G (1981) Evolution of vomeronasal organs in vertebrates. Evolution 35:359–366
Bever GS, Rowe TB, Ekdale EG et al (2005) Comment on “Independent origins of middle ear bones in monotremes and therians”. Science 309:1492a
Bhatnagar KP, Meisami E (1998) Vomeronasal organ in bats and primates: extremes of structural variability and its phylogenetic implications. Microscopy ResTech 43(6):465–475
Bird DJ, Murphy WJ, Fox-Rosales L et al (2018) Olfaction written in bone: cribriform plate size parallels olfactory receptor gene repertoires in Mammalia. Proc R Soc B 285:20180100. https://doi.org/10.1098/rspb.2018.0100
Bonaparte JF, Martinelli AG, Schultz CL et al (2005) New information on Brasilodon and Brasilitherium (Cynodontia, Probainognathia) from the late Triassic of southern Brazil. Rev Bras Paleontolog 8(1):25–46
Bonaparte JF, Soares MB, Martinelli AG (2013) Discoveries in the Late Triassic of Brazil improves knowledge on the origin of mammals. Hist Nat 3rd Ser 2:5–30
Brainerd EL (2015) Major transformations in vertebrate breathing mechanisms. In: Dial KP, Shubin N, Brainerd EL (eds) Great transformations in vertebrate evolution. University of Chicago Press, Chicago, pp 47–61
Brainerd EL, Owerkowicz T (2006) Functional morphology and evolution of aspirational breathing in tetrapods. Resp Physiol Neeurobi 154:73–88
Brink AS (1957) Speculations on some advanced mammalian characteristics in the higher mammal-like reptiles. Palaeontol Africana 4:77–96
Briscoe SD, Ragsdale CW (2018) Molecular anatomy of the alligator dorsal telencephalon. J Comp Neurol 526(10):1613–1646
Broom R (1932) The mammal-like reptiles of South Africa and the origin of mammals. HF & G Witherby, London
Bruce LL (2007) Evolution of the nervous system in reptiles. In: Bullock TH, Rubenstein LR, Kaas JH (eds) Evolution of nervous systems: a comprehensive reference, The evolution of nervous systems in non-mammalian vertebrates, vol II. Elsevier/Academic, Oxford, pp 125–156
Bruce LL (2009) Evolution of the nervous system in reptiles. In: Kaas JH (ed) Evolutionary nuroscience, 1st edn. Academic, New York, pp 233–265
Bruce LL, Braford MR Jr (2009) Evolution of the limbic system. In: Squire LR (ed) New encyclopedia of neuroscience. Elsevier Academic, Oxford, pp 43–55
Buck L, Axel R (1991) A novel multigene family may encode odorant receptors: a molecular basis for odor recognition. Cell 65:175–187
Burgin CJ, Colella JP, Kahn PL et al (2018) How many species of mammals are there? J Mammal 99(1):1–14
Butler AB (1994) The evolution of the dorsal pallium in the telencephalon of amniotes: cladistic analysis and a new hypothesis. Brain Res Rev 19(1):66–101
Butler AB, Hodos W (2005) Comparative vertebrate neuroanatomy: evolution and adaptation, 2nd edn. Wiley, New York
Cantino PD, De Queiroz K (eds) (2020) PhyloCode: a phylogenetic code of biological nomenclature. CRC Press, Boca Raton
Carr EC, Christiansen-Dalsgaard J (2016) Evolutionary trends in directional hearing. Curr Opin Neurobiol 40:111–117
Carr CE, Soares D (2002) Evolutionary convergence and shared computational principles in the auditory system. Brain Behav Evol 59:294–311
Carr CE, Soares D (2009) Shared and convergent features of the auditory system of vertebrates. In: Kaas JH (ed) Evolutionary neuroscience, 1st edn. Academic, New York, pp 479–493
Carrier DR (1987) The evolution of locomotor stamina in tetrapods: circumventing mechanical constraint. Paleobiology 13:326–341
Case EC (1907) Revision of the pelycosauria of North America, vol 55. Carnegie Inst Washington Publ, Washington, pp 1–176
Castiglione S, Serio C, Piccolo M et al (2021) The influence of domestication, insularity and sociality on the tempo and mode of brain size evolution in mammals. Biol J Linn Soc 132(1):221–231
Catania KC (2013) Stereo and serial sniffing guide navigation to an odour source in a mammal. Nat Commun 4:1441–1449
Catania KC, Catania EH (2015) Comparative studies of somatosensory systems and active sensing. In: Krieger P, Groth A (eds) Sensorimotor integration in the whisker system. Springer, New York, pp 7–30
Chen WR, Shepherd G (2005) The olfactory glomerulus: a cortical module with specific functions. J Neurocytol 34:353–360
Cifelli RL, Rowe TB, Luckett WP et al (1996) Fossil evidence for the origin of the marsupial pattern of tooth replacement. Nature 379:715–718
Clack JA (2012) Gaining ground. Indiana University Press, Bloomington
Clark JM, Hopson JA (1985) Distinctive mammal-like reptile from Mexico and its bearings on the phylogeny of Tritylodontidae. Nature 315:398–400
Cluver MA (1971) The cranial morphology of the Genus Lystrosaurus. Ann S Afr Mus 56:155–273
Cluver MA (1978) The skeleton of the mammal-like reptile Cistecephalus with evidence for a fossorial mode of life. Ann S Afr Mus 76:213–246
Collin SP (2010) Evolution and ecology of retinal photoreception in early vertebrates. Brain Behave Evol 75:174–185
Connors BW, Kriegstein AR (1986) Cellular physiology of the turtle visual cortex: distinctive properties of pyramidal and stellate neurons. J Neurosci 6:164–177
Cope ED (1886) On the structure of the brain and auditory apparatus of a theromorphous reptile of the Permian Epoch. Proc Am Phil Soc 23:234–238
Crompton AW (1963) Tooth replacement in the cynodont Thrinaxodon Liorhinus. Ann S Afr Mus 46:479–521
Crompton AW (1972) Postcanine occlusion in cynodonts and tritylodontids. Bull Br Mus Nat Hist Geol 21:27–71
Crompton AW (1989) The evolution of mammalian mastication. In: Wake DB, Roth G (eds) Complex organismal functions: integration and evolution in vertebrates. Wiley, New York, pp 23–40
Crompton AW, Parker P (1978) Evolution of the mammalian masticatory apparatus. Am Sci 66(2):92–201
Crompton AW, Taylor CR, Jagger JA (1978) Evolution of homeothermy in mammals. Nature 272:333
Crompton AW, Owerkowicz T, Bhullar BAS et al (2017a) Structure of the nasal region of non-mammalian cynodonts and mammaliaforms: speculations on the evolution of mammalian endothermy. J Vert Paleontol 37(1):e1269116
Crompton AW, Musinsky C, Rougier GW et al (2017b) Origin of the lateral wall of the mammalian skull: fossils, monotremes and therians revisited. J Mammal Evol 25(3):301–313. https://doi.org/10.1007/s10914-017-9388-7
Crompton AW, Musinsky C, Bonaparte J et al (2018) Evolution of the mammalian fauces region and the origin of suckling. https://nrs.harvard.edu/URN-3:HUL.INSTREPOS:37364482
Davies WL, Carvalho LS, Cowing JA et al (2007) Visual pigments of the platypus: a novel route to mammalian colour vision. Curr Biol 17(5):R161–R163
De Araujo IE, Rolls ET, Kringelbach ML et al (2003) Taste olfactory convergence, and the representation of the pleasantness of flavour, in the human brain. Eur J Neurosci 18:2059–2068
de Queiroz K (1994) Replacement of an essentialistic perspective on taxonomic definitions as exemplified by the definition of “Mammalia”. Syst Biol 43(4):497–510
de Queiroz K (2007) Toward an integrated system of clade names. Syst Biol 56:956–974
de Queiroz K, Gauthier JA (1990) Phylogeny as a central principle in taxonomy: phylogenetic definitions of taxon names. Syst Biol 39(4):307–322
de Queiroz K, Gauthier JA (1992) Phylogenetic taxonomy. Annu Rev Ecol Evol Syst 23(1):449–480
de Queiroz K, Gauthier JA (1994) Toward a phylogenetic system of biological nomenclature. Trends Ecol Evol 9(1):27–31
de Queiroz K, Cantino PD, Gauthier JA (eds) (2020) Phylonyms: a companion to the PhyloCode. CRC Press, Boca Raton
Demski LS (1993) Terminal nerve complex. Cells Tissues Organs 148(2-3):81–95
Demski LS, Schwanzel-Fukuda M (eds) (1987) The terminal nerve (Nervus Terminalis) structure, function and evolution. Ann N Y Acad Sci 519:1–49
Didier G, Laurin M (2020) Exact distribution of divergence times from fossil ages and tree topologies. Syst Biol 69(6):1068–1087
Di-Pöi N, Milinkovitch MC (2016) The anatomical placode in reptile scale morphogenesis indicates shared ancestry among skin appendages in amniotes. Sci Adv 2016:e1600708
Donoghue MJ, Doyle J, Gauthier JA et al (1989) Importance of fossils in phylogeny reconstruction. Annu Rev Ecol Evol Syst 20:431–460
Edinger T (1975) Paleoneurology 1804–1966: an annotated bibliography. Adv Anat Embryol Cell Biol 49:3–258
Eisenberg JF (1981) The mammalian radiations: an analysis of trends in evolution, adaptation, and behaviour. University of Chicago Press, Chicago
Eisenberg JF (1990) The behavioral/ecological significance of body size in the Mammalia. In: Damuth J, MacFadden B (eds) Body size in mammalian paleobiology: estimation and biological implications. Cambridge University Press, Cambridge, UK, pp 25–37
Ekdale EG (2016) Form and function of the mammalian inner ear. J Anat 228(2):324–337
Fabbri MN, Mongiardino M, Pritchard AC et al (2017) The skull roof tracks the brain during evolution and development of reptiles including birds. Nat Ecol Evol 1(10):1543–1550. https://doi.org/10.1038/s41559-017-0288-2
Farbman AI (1988) Cellular interactions in the development of the vertebrate olfactory system. In: Margolis FL, Getchell TV (eds) Molecular neurobiology of the olfactory system. Plenum Press, New York, pp 319–332
Farbman AI (1990) Olfactory neurogenesis: genetic or environmental controls? Trends Neurosci 13:362–365
Farbman AI (1992) Cell biology of olfaction. Cambridge University Press, CITY
Gaengler P, Metzler E (1992) The periodontal differentiation in the phylogeny of teeth – an overview. J Periodontal Res 27:214–225. https://doi.org/10.1111/j.1600-0765.1992.tb01671.x
Garrett EC, Steiper ME (2014) Strong links between genomic and anatomical diversity in both mammalian olfactory chemosensory systems. Proc B Roy Soc Lond 281:2013–2828
Gauthier JA, Kluge AG, Rowe TB (1988a) Amniote phylogeny and the importance of fossils. Cladistics 4:105–209
Gauthier JA, Kluge AG, Rowe TB (1988b) The early evolution of the Amniota. In: Benton M (ed) The phylogeny and Classification of the Tetrapods, Vol. 1: Amphibians, Reptiles and Birds, Syst Assoc Spec Vol Ser special vol No. 35a. Clarendon Press, Oxford, pp 103–155
Gauthier JA, Cannatella D, de Queiroz K et al (1989) Tetrapod phylogeny. In: Fernholm B, Bremer H, Jornvall H (eds) The hierarchy of life, Nobel sympos 70. Excerpta Medica, Amsterdam, pp 337–353
Gemmell NJ, Rutherford K, Prost S et al (2020) The tuatara genome reveals ancient features of amniote evolution. Nature 584:403–409
Goodrich ES (1930) Studies on the structure and function of vertebrates. Constable & Co, London
Grant R, Haidarliu S, Kennerley NJ et al (2013) The evolution of active vibrissal sensing in mammals: evidence from vibrissal musculature and function in the marsupial opossum Monodelphis domestica. J Exp Biol 216(18):3483–3494
Graybeal A, Rosowski JJ, Ketten DR et al (1989) Inner ear structure in Morganucodon, an early Jurassic mammal. Zool J Linn Soc 96(2):107–117
Green PA, Van Valkenburgh B, Pang B et al (2012) Respiratory and olfactory turbinal size in canid and arctoid carnivorans. J Anat 221(6):609–621
Grothe B, Carr CE, Cassedy JH et al (2005) The evolution of central pathways and their neural processing patterns. In: Manley GA, Popper AN, Fay AN (eds) Evolution of the vertebrate auditory system. Springer, New York, pp 289–359
Grothe B, Pecka M, McAlpine D (2010) Mechanisms of sound localization in mammals. Physiol Rev 90(3):983–1012
Haberly LB (1985) Neuronal circuitry in olfactory cortex: anatomy and functional implications. Chem Senses 10:219–238
Haberly LB (2001) Parallel-distributed processing in olfactory cortex: new insights from morphological and physiological analysis of neuronal circuitry. Chem Senses 26:551–576
Habre-Hallage P, Dricot L, Hermoye L et al (2014) Cortical activation resulting from the stimulation of periodontal mechanoreceptors measured by functional magnetic resonance imaging (fMRI). Clin Oral Investig 18(8):1949–1961
Hall BK (2009) The neural crest and neural crest cells in vertebrate development and evolution. Springer, New York
Harris KD, Shepherd GM (2015) The neocortical circuit: themes and variations. Nat Neurosci 18(2):170–181
Harvey PH, Clutton-Brock TH, Mace GM (1980) Brain size and ecology in small mammals and primates. PNAS 77:4387–4389
Hayden S, Bekaert M, Crider TA et al (2010) Ecological adaptation determines functional mammalian olfactory subgenomes. Genome Res 20:1–9
Heiss E, Aerts P, Van Wassenbergh S (2018) Aquatic–terrestrial transitions of feeding systems in vertebrates: a mechanical perspective. J Exp Biol 221(8):221, jeb154427. https://doi.org/10.1242/jeb.154427
Herculano-Houzel S, Manger PR, Kaas JH (2014) Brain scaling in mammalian evolution as a consequence of concerted and mosaic changes in numbers of neurons and average neuronal cell size. Front Neuroanat 8:1–28
Hillenius WJ (1992) The evolution of nasal turbinates and mammalian endothermy. Paleobiology 18:17–29
Hillenius WJ (1994) Turbinates in therapsids: evidence for late Permian origins of mammalian endothermy. Evolution 48:207–229. https://doi.org/10.1111/j.1558-5646.1994.tb01308.x
Hirasawa T, Kuratani S (2013) A new scenario of the evolutionary derivation of the mammalian diaphragm from shoulder muscles. J Anat 222(5):504–517
Hlusko LJ, Sage RD, Mahaney MC (2011) Modularity in the mammalian dentition: mice and monkeys share a common dental genetic architecture. J Exp Zool B Mol Dev Evol 316(1):21–49
Hoffman EA, Rowe TB (2018) Jurassic stem-mammal perinates and the origin of mammalian reproduction and growth. Nature 561(7721):104–108
Hoffmann CA, Rodrigues PG, Soares MB et al (2019) Brain endocast of two non-mammaliaform cynodonts from southern Brazil: an ontogenetic and evolutionary approach. Hist Biol 2019:1–12
Hopson JA (1971) Postcanine replacement in the gomphodont cynodont Diademodon. In: Kermack DM, Kermack KA (eds) Early Mammals. Zool J Linn Soc 50(suppl 1):1–21
Hopson JA (2015) Fossils, trackways, and transitions in locomotion: a case study of Dimetrodon. In: Dial KP, Shubin N, Brainerd EL (eds) Great transformations in vertebrate evolution. University of Chicago Press, Chicago, pp 125–141
Huber E (1930) Evolution of facial musculature and cutaneous field of Trigeminus. Part I. Quart Rev Biol 5(2):133–188
Hurlburt GR, Ridgely RC, Witmer LM (2013) Relative size of brain and cerebrum in tyrannosaurid dinosaurs: an analysis using brainendocast quantitative relationships in extant alligators. In: Parrish JM, Molnar RE, Currie PJ, Koppelhus EB (eds) Tyrannosaurid paleobiology. Indiana University Press, Bloomington, pp 135–155
Iyengar S, Qi HX, Jain N et al (2007) Cortical and thalamic connections of the representations of the teeth and tongue in somatosensory cortex of new world monkeys. J Compar Neurol 501(1):95–120
Jacobs GH (2009) Evolution of colour vision in mammals. Phil Trans B Royal Soc Lond 364(1531):2957–2967
Jacobs GH (2013) Losses of functional opsin genes, short-wavelength cone photopigments, and color vision – a significant trend in the evolution of mammalian vision. Visual Neurosci 30(1-2):39–53
Janis CM, Keller JC (2001) Modes of ventilation in early tetrapods: costal aspiration as a key feature of amniotes. Acta Palaeontol Pol 46(2):137–170
Jarvik E (1942) On the structure of the snout of crossopterygians and lower gnathostomes in general. Zool Bidr Upps 21:235–675
Jenkins FA Jr (1969) The evolution and development of the dens of the mammalian axis. Anat Rec 164:173–184
Jenkins FA Jr (1971) The postcranial skeleton of African cynodonts: problems in the early evolution of the mammalian postcranial skeleton. Bull Peabody Mus Nat Hist 36:1–216
Jerison H (1973) Evolution of the brain and intelligence. Academic, New York
Ji Q, Luo Z-X, Yuan C et al (2006) A swimming mammaliaform from the Middle Jurassic and ecomorphological diversification of early mammals. Science 311:1123–1127
Kaas JH (2009) The evolution of the dorsal thalamus in mammals. In: Kaas JH (ed) Evolutionary neuroscience, 1st edn. Elsevier, Oxford, pp 569–586
Kaas JH (2013) The evolution of brains from early mammals to humans. Wiley Interdiscip Rev Cogn Sci 4(1):33–45
Kaas JH (2020) The organization of neocortex in early mammals. In: Kaas JH (ed) Evolutionary neuroscience, 2nd edn. Elsevier, Oxford, pp 333–348
Kaas JH, Qi HX, Iyengar S (2006) Cortical network for representing the teeth and tongue in primates. Anat Rec 288(2):182–190
Katz MJ, Lasek RJ (1978) Evolution of the nervous system: role of ontogenetic mechanisms in the evolution of matching populations. PNAS 75(3):1349–1352
Kemp TS (1983) The relationships of mammals. Zool J Linn Soc 77:353–384
Kemp TS (1988) A note on the Mesozoic mammals, and the origin of therians. In: Benton MJ (ed) The phylogeny and classification of the tetrapods, volume 2: mammals, Syst Assoc Spec Vol Ser special vol No. 35b. Clarendon Press, Oxford, pp 23–29
Kemp TS (2005) The origin and evolution of mammals. Oxford University Press, Oxford
Kemp TS (2006) The origin and early radiation of the therapsid mammal like reptiles: a palaeobiological hypothesis. J Evol Biol 19(4):1231–1247
Kemp TS (2009) The endocranial cavity of a nonmammalian cynodonts Chiniquodon theotenicus and its implications for the origin of the mammalian brain. J Vert Paleontol 29(4):1188–1198
Kermack DM, Kermack KE (1984) The evolution of mammalian characters. Springer, New York
Kielan-Jaworowska Z, Cifelli RL, Luo Z-X (2004) Mammals from the age of dinosaurs. Columbia University Press, New York
Kirk EC, Daghighi P, Macrini T et al (2014) Cranial anatomy of the Duchesnean primate Rooneyia viejaensis: New insights from high resolution computed tomography. J Hum Evol 74:82–95
Kitazawa T, Takechi M, Hirasawa T et al (2015) Developmental genetic bases behind the independent origin of the tympanic membrane in mammals and diapsids. Nat Commun 6(1):1–7
Koyabu D, Werneburg I, Morimoto N et al (2014) Mammalian skull heterochrony reveals modular evolution and a link between cranial development and brain size. Nat commun 5(1):1–9
Krubitzer L, Hunt DL (2009) Captured in the new of space and time: understanding cortical field evolution. In: Kaas JH (ed) Evolutionary neuroscience, 1st edn. Elsevier, Oxford, pp 545–568
Krubitzer L, Kaas J (2005) The evolution of the neocortex in mammals: how is phenotypic diversity generated? Curr Opin Neurobiol 15(4):444–453
Kruska DCT (2007) The effects of domestication on brain size. In: Bullock TH, Rubenstein LR, Kaas JH (eds) Evolution of nervous systems: a comprehensive reference, volume II, the evolution of nervous systems in non-mammalian vertebrates. Elsevier Academic, Oxford, pp 143–153
Kubo K, Shibukawa Y, Shintani M et al (2008) Cortical representation area of human dental pulp. J Dental Res 87(4):358–362
Laaß M, Kaestner A (2017) Evidence for convergent evolution of a structure comparable to the mammalian neocortex in a Late Permian therapsid. J Morph 278:1033–1057
Laurin M, Reisz RR (2020) Synapsida. In: de Queiroz K, Cantino PD, Gauthier JA (eds) Phylonyms: a companion to the PhyloCode. CRC Press, Boca Raton, pp 811–814
LeBlanc AR, Brink KS, Whitney MR et al (2018) Dental ontogeny in extinct synapsids reveals a complex evolutionary history of the mammalian tooth attachment system. Proc Royal Soc B 285(1890):20181792. https://doi.org/10.1098/rspb.2018.1792
Lemberg JB, Daeschler EB, Shubin NH (2021) The feeding system of Tiktaalik roseae: an intermediate between suction feeding and biting. PNAS 118(7):1–10. e2016421118
Lightoller GS (1942) Matrices of the facialis musculature: homologization of the musculature in monotremes with that of marsupials and placentals. J Anat 76(3):258–269
Louis M, Huber T, Benton R et al (2008) Bilateral olfactory sensory input enhances chemotaxis behavior. Nat Neurosci 11(2):187–199
Luo Z-X (2007) Transformation and diversification in early mammal evolution. Nature 450:1011–1019
Luo Z-X, Crompton AW, Sun A-L (2001) A new mammal from the Early Jurassic and evolution of mammalian characteristics. Science 292:1535–1540
Luo Z-X, Kielan-Jaworowska Z, Cifelli RL (2004) Evolution of dental replacement in mammals. Bull Carnegie Mus Nat Hist 36:159–175
Luo Z-X, Ruf I, Martin T (2012) The petrosal and inner ear of the Late Jurassic cladotherian mammal Dryolestes leiriensis and implications for ear evolution in therian mammals. Zool J Linn Soc 166(2):433–463
Luo Z-X, Gatesy SM, Jenkins FA Jr et al (2015) Mandibular and dental characteristics of Late Triassic mammaliaform Haramiyavia and their ramifications for basal mammal evolution. PNAS 112(51):E7101–E7109. https://doi.org/10.1073/pnas.1519387112
Lynch G (1986) Synapses, circuits, and the beginnings of memory. MIT Press, Cambridge, MA
Mace GM, Harvey PH, Clutton-Brock TH (1981) Brain size and ecology in small mammals. J Zool 193:333–354
Macrini TE (2006). The evolution of endocranial space in mammals and non-mammalian cynodonts. Dissertation, University of Texas
Macrini TE (2012) Comparative morphology of the internal nasal skeleton of adult marsupials based on x-ray computed tomography. Bull Am Mus Nat Hist 2012:1–91
Macrini TE (2014) Development of the ethmoid in Caluromys philander (Didelphidae, Marsupialia) with a discussion on the homology of the turbinal elements in marsupials. Anat Rec 297(11):2007–2017
Macrini TE, Rowe TB, Archer M (2006) Description of a cranial endocast from a fossil platypus, Obdurodon dicksoni (Monotremata, Ornithorhynchidae), and the relevance of endocranial characters to monotreme monophyly. J Morph 267:1000–1015
Manger PR (2006) An examination of cetacean brain structure with a novel hypothesis correlating thermogenesis to the evolution of a big brain. Biol Rev 81(2):293–338
Marín O, Rubenstein JL (2001) A long, remarkable journey: tangential migration in the telencephalon. Nat Rev Neurosci 2(11):780–790
Martinelli AG, Rougier GW (2007) On Chaliminia musteloides (Eucynodontia: Tritheledontidae) from the Late Triassic of Argentina, and a Phylogeny of Ictidosauria. J Vert Paleontol 27:442–460
McMahon TA, Bonner JT (1983) On size and life. Scientific American Library, New York
Meng J, Hu Y, Wang Y et al (2006) A Mesozoic gliding mammal from northeastern China. Nature 444:889–893
Molnár Z, Butler AB (2002) Neuronal changes during forebrain evolution in amniotes: an evolutionary developmental perspective. Progr Brain Res 136:21–38
Molnár Z, Tavare A, Cheung AFP (2009) The origin of Neocortex: lessons from comparative embryology. In: Kaas JH (ed) Evolutionary neuroscience, 1st edn. Elsevier, Oxford, pp 509–522
Molnár Z, Kaas JH, de Carlos JA et al (2014) Evolution and development of the mammalian cerebral cortex. Brain Behav Evol 83:126–139
Muchlinski MN (2008) The relationship between the infraorbital foramen, infraorbital nerve, and maxillary mechanoreception: implications for interpreting the paleoecology of fossil mammals based on infraorbital foramen size. Anat Rec 291(10):1221–1226
Muchlinski MN, Wible JR, Corfe I et al (2020) Good vibrations: The evolution of whisking in small mammals. Anat Rec 303(1):89–99
Neville KR, Haberly LB (2004) Olfactory cortex. In: Shepherd GM (ed) The synaptic organization of the brain. Oxford University Press, New York, pp 415–454
Nieuwenhuys RHJ, ten Donkelaar HJ, Nicholson C (1998) The central nervous system of vertebrates. Springer, Berlin/Heidelberg
Niimura Y (2009) On the origin and evolution of vertebrate olfactory receptor genes: comparative genome analysis among 23 chordate species. Genome Biol Evol 1:34–44
Niimura Y (2012) Olfactory receptor multigene family in vertebrates: from the viewpoint of evolutionary genomics. Curr Genom 13:103–114
Niimura Y, Nei M (2005) Evolutionary dynamics of olfactory receptor genes in fishes and tetrapods. Proc Natl Acad Sci 102(17):6039–6044
Niimura Y, Nei M (2006) Evolutionary dynamics of olfactory and other chemosensory receptor genes in vertebrates. J Hum Genet 51(6):505–517
Niimura Y, Matsui A, Touhara K (2014) Extreme expansion of the olfactory receptor gene repertoire in African elephants and evolutionary dynamics of orthologous gene groups in 13 placental mammals. Genome Res 24(9):1485–1496
Northcutt RG (2001) Changing views of brain evolution. Brain Res Bull 55(6):663–674
Olson EC (1944) Origin of mammals based upon cranial morphology of the therapsid suborders. Geol Soc Amer Spec Pap 55:1–130
Olson EC (1959) The evolution of mammalian characters. Evolution 13:344–353
Olson EC (1966) Community evolution and the origin of mammals. Ecology 47(2):291–302
Osborn JW (1984) From reptile to mammal: evolutionary considerations of the dentition with emphasis on tooth attachment. Symp Zool Soc Lond 52:549–574
Osborn JW, Crompton AW (1978) The evolution of mammalian from reptilian dentitions. Breviora 399:1–18
Parsons TS (1967) Evolution of the nasal structure in the lower tetrapods. Amer Zool 7:397–413
Pavanatto AE, Kerber L, Dias da Silva S (2019) Virtual reconstruction of cranial endocasts of traversodontid cynodonts (Eucynodontia: Gomphodontia) from the upper Triassic of Southern Brazil. J Morph 280(9):1267–1281
Pihlström H, Fortelius M, Hemilä S et al (2005) Scaling of mammalian ethmoid bones can predict olfactory organ size and performance. Proc B Royal Soc 272:957–962
Presley R (1981) Alisphenoid equivalents in placentals, marsupials, monotremes and fossils. Nature 294:668–670
Quiroga JC (1979) The brain of two mammal-like reptiles (Cynodontia –Therapsida). J Hirnforsch 20:341–350
Quiroga JC (1980) The brain of the mammal-like reptile Probainognathus jenseni (Therapsida, Cynodontia), a correlative paleo-neurological approach to the neocortex at the reptile-mammal transition. J Hirnforsch 21:299–336
Quiroga JC (1984) The endocranial cast of the advanced mammal-like reptile Therioherpeton cargnini (Cynodontia – Therapsida) from the middle Triassic of Brazil. J Hirnforsch 25:285–290
Rakic P (1988) Specification of cerebral cortical areas. Science 241:170–176
Rakic P (2000) Radial unit hypothesis of neocortical expansion. In: Bock G, Cardew G (eds) Evolutionary developmental biology of the cerebral cortex. Novartis found sympos chichester. Wiley, New York, pp 30–52
Rakic P (2007) The radial edifice of cortical architecture: from neuronal silhouettes to genetic engineering. Brain Res Rev 55(2):204–219
Rakic P (2009) Evolution of the neocortex: a perspective from developmental biology. Nature Rev Neurosci 10:724–735
Remple MS, Henry EC, Catania KC (2003) Organization of somatosensory cortex in the laboratory rat (Rattus norvegicus): evidence for two lateral areas joined at the representation of the teeth. J Comp Neurol 467(1):105–118
Ren T, He W, Gillespie PG (2011) Measurement of cochlear power gain in the sensitive gerbil ear. Nat Comm 2(1):1–7
Rodrigues PG, Ruf I, Schultz CL (2013) Digital reconstruction of the otic region and inner ear of the non-mammalian cynodont Brasilitherium riograndensis (Late Triassic, Brazil) and its relevance to the evolution of the mammalian ear. J Mammal Evol 20(4):291–307
Rodrigues PG, Ruf I, Schultz CL (2014) Study of a digital cranial endocast of the non-mammaliaform cynodont Brasilitherium riograndensis (Late Triassic, Brazil) and its relevance to the evolution of the mammalian brain. Paläontol Z 88:329–352
Rodrigues PG, Martinelli AG, Schultz CL et al (2019) Digital cranial endocast of Riograndia guaibensis (Late Triassic, Brazil) sheds light on the evolution of the brain in non-mammalian cynodonts. Hist Biol 31(9):1195–1212
Rolls ET, Grabenhorst F (2008) The orbitofrontal cortex and beyond: from affect to decision-making. Prog Neurobiol 86:216–244
Romer AS (1956) Osteology of the reptilia. University of Chicago Press, Chicago
Romer AS (1966) Vertebrate paleontology. University of Chicago Press, Chicago
Romer AS (1970) The Chanares (Argentina) Triassic reptile fauna: 6. A chiniquodontid cynodont with an incipient squamosal-dentary jaw articulation. Breviora 344:1–18
Romer AS, Edinger T (1942) Endocranial casts and brains of living and fossil Amphibia. J Comp Neurol 77(2):355–389
Romer AS, Price LW (1940) Review of the pelycosauria. Geol Soc Amer Spec Pap 28:1–534
Rowe TB (1988) Definition, diagnosis and origin of Mammalia. J Vertebr Paleontol 8(3):241–264
Rowe TB (1993) Phylogenetic systematics and the early history of mammals. In: Szalay FS, Novacek MJ, McKenna MC (eds) Mammalian phylogeny. Springer, New York, pp 129–145
Rowe TB (1996a) Coevolution of the mammalian middle ear and neocortex. Science 273:651–654
Rowe TB (1996b) Brain heterochrony and evolution of the mammalian middle ear. In: Ghiselin MG, Pinna, G (eds) New perspectives on the history of life. Calif Acad Sci Memoir 20:71–96
Rowe TB (2004) Chordate phylogeny and development. In: Cracraft J, Donoghue MJ (eds) Assembling the tree of life. Oxford University Press, Oxford/New York, pp 384–409
Rowe TB (2020a) The emergence of mammals. In: Kaas JA (ed) Evolutionary neurosciences, 2nd edn. Elsevier, New York, pp 263–319
Rowe TB (2020b) Mammalia. In: de Queiroz K, Cantino PD, Gauthier JA (eds) Phylonyms: a companion to the PhyloCode. CRC Press, Boca Raton, pp 841–847
Rowe TB (2020c) Pan-Mammalia. In: de Queiroz K, Cantino PD, Gauthier JA (eds) Phylonyms: a companion to the PhyloCode. CRC Press, Boca Raton, pp 783–791
Rowe TB (2020d) Therapsida. In: de Queiroz K, Cantino PD, Gauthier JA (eds) Phylonyms: a companion to the PhyloCode. CRC Press, Boca Raton, pp 797–808
Rowe TB (2020e) Cynodontia. In: de Queiroz K, Cantino PD, Gauthier JA (eds) Phylonyms: a companion to the PhyloCode. CRC Press, Boca Raton, pp 813–823
Rowe TB (2020f) Mammaliamorpha. In: de Queiroz K, Cantino PD, Gauthier JA (eds) Phylonyms: a companion to the PhyloCode. CRC Press, Boca Raton, pp 825–831
Rowe TB (2020g) Mammaliaformes. In: de Queiroz K, Cantino PD, Gauthier JA (eds) Phylonyms: a companion to the PhyloCode. CRC Press, Boca Raton, pp 833–839
Rowe TB, Shepherd GM (2016) The role of ortho-retronasal olfaction in mammalian cortical evolution. J Comp Neurol 524:471–495. https://doi.org/10.1002/cne.23802
Rowe TB, Carlson W, Bottorff W (1995) Thrinaxodon: digital atlas of the skull. CD-ROM, 2nd edn. University of Texas Press, Austin
Rowe TB, Eiting TP, Macrini TE et al (2005) Organization of the olfactory and respiratory skeleton in the nose of the gray short-tailed Opossum Monodelphis domestica. J Mammal Evol 12:303–336
Rowe TB, Rich TH, Vickers-Rich P et al (2008) The oldest Platypus, and its bearing on divergence timing of the Platypus and Echidna Clades. PNAS 105:1238–1242
Rowe TB, Macrini TE, Luo Z-X (2011) Fossil evidence on origin of the mammalian brain. Science 332:955–957. https://doi.org/10.1126/science.1203117
Rowe TB, Wallace RVS, Bhullar BAS (2020) Monotremata. In: de Queiroz K, Cantino PD, Gauthier JA (eds) Phylonyms: a companion to the PhyloCode. CRC Press, Boca Raton, pp 833–839
Rubenstein JL, Rakic P (1999) Genetic control of cortical development. Cereb Cortex 9(6):521–523
Rubidge BS, Sidor CA (2001) Evolutionary patterns among Permo-Triassic therapsids. Ann Rev Ecol Syst 32:449–480
Schlosser G (2010) Making senses: development of vertebrate cranial placodes. Int Rev Cell Mol Biol 283:129–234
Schlosser T (2017) Evolution of neural crest and cranial placodes. In: Kaas JA (ed) Evolution of nervous systems, vol 1. Elsevier, New York, pp 25–36
Schneider MR, Schmidt-Ullrich R, Paus R (2009) The hair follicle as a dynamic miniorgan. Curr Biol 19(3):R132–R142
Sengel P (1976) Morphogenesis of skin. Cambridge University Press, Cambridge
Shepherd GM (1991) Computational structure of the olfactory system. In: Eichenbaum HM, Davis J (eds) Olfaction: a model for computational neuroscience. MIT Press, Cambridge, MA, pp 3–42
Shepherd GM (2004) The human sense of smell: are we better than we think? PLoS Biol 2:e146
Shepherd GM (2006) Smell images and the flavour system in the human brain. Nature 444:316–321
Shepherd GM (2011) The microcircuit concept applied to cortical evolution: from three-layer to six-layer cortex. Front Neuroanat 5(30):1–15
Shepherd GM (2012) Neurogastronomy: how the brain creates flavor and why it matters. Columbia University Press, New York
Shepherd GM, Rowe TB (2017) Neocortical lamination: insights from neuron types and evolutionary precursors. Front Neuroanat 11:100. https://doi.org/10.3389/fnana.2017.00100
Shepherd GM, Rowe TB, Greer CA (2021) An evolutionary microcircuit approach to the neural basis of high dimensional sensory processing in olfaction and vision. Front Cell Neurosci 15(658480):1–23
Sidor CA (2001) Simplification as a trend in synapsid cranial evolution. Evolution 55:1419–1442
Sidor CA (2003) The naris and palate of Lycaenodon longiceps (Therapsida: Biarmosuchia), with comments on their early evolution in the Therapsida. J Paleontol 77:977–984
Sidor CA, Hancox PJ (2006) Elliotherium kersteni, a new tritheledontid from the Lower Elliot Formation (Upper Triassic) of South Africa. J Vert Paleontol 80:333–342
Small DM, Bender G, Veldhuizen MG et al (2007) The role of the human orbitofrontal cortex in taste and flavor processing. Ann N Y Acad Sci 1121:136–151
Streidter GF (2005) Principles of brain evolution. Sinauer, Sunderland
Streidter GF, Northcutt G (2020) Brains through time – a natural history of vertebrates. Oxford University Press, Oxford
Taylor CR (1977) Exercise and environmental heat loads: different mechanisms for solving different problems? Internat Rev Physiol 15:119–145
Ten-Cate AR (1969) The mechanism of tooth eruption. In: Melcher AH, Bowen WH (eds) Biology of the Periodontium. Academic, New York, pp 91–103
Ten-Cate AR (1997) The development of the periodontium—a largely ectomesenchymally derived unit. Periodontology 2000 13(1):9–19
Trulsson M (2006) Sensory motor function of human periodontal mechanoreceptors. J Oral Rehabil 33(4):262–273
Trulsson M, Francis ST, Bowtell R et al (2010) Brain activations in response to vibrotactile tooth stimulation: a psychophysical and fMRI study. J Neurophysiol 104(4):2257–2265
Ulinski PS (1983) Dorsal ventricular ridge: a treatise on forebrain organization in reptiles and birds. Wiley, New York
Van Valkenburgh B, Theodor J, Friscia A et al (2004) Respiratory turbinates of canids and felids: A quantitative comparison. J Zool 264:1–13
Wagner G (2014) Homology, genes, and evolutionary innovation. Princeton University Press, Princeton
Wakefield MJ, Anderson M, Chang E et al (2008) Cone visual pigments of monotremes: filling the phylogenetic gap. Visual Neurosci 25(3):257–264
Wallace RVS (2018) A new close mammal relative and the origin and evolution of the mammalian central nervous system. Dissertation, University of Texas at Austin
Wallace RVS, Martínez R, Rowe T (2019) First record of a basal mammaliamorph from the early Late Triassic Ischigualasto Formation of Argentina. PLoS ONE 14(8):e0218791. https://doi.org/10.1371/journal.pone.0218791
Walls GL (1942) The vertebrate eye and its adaptive radiation. Hafner Publishing Company, New York
Wang Y, Hu Y, Meng J et al (2001) An ossified Meckel’s cartilage in two Cretaceous mammals and origin of the mammalian middle ear. Science 294:357–361
Wang Z, Pascual-Anaya J, Zadissa A et al (2013) The draft genomes of soft-shell turtle and green sea turtle yield insights into the development and evolution of the turtle-specific body plan. Nat Genet 45(6):701–706
Weisbecker V, Rowe TB, Wroe S et al (2021) Global elongation and high shape flexibility as an evolutionary hypothesis of accommodating mammalian brains into skulls. Evolution 75(3):625–640
Wilson DA, Stevenson RJ (2006) Learning to smell - olfactory perception from neurobiology to behavior. Johns Hopkins University Press, Baltimore
Witmer L (1995) The extant phylogenetic bracket and the importance of reconstructing soft tissues in fossils. In: Thomason J (ed) Functional morphology in vertebrate paleontology. Cambridge University Press, Cambridge, pp 19–33
Yohe LR, Fabbri M, Hanson M et al (2020) Olfactory receptor gene evolution is unusually rapid across Tetrapoda and outpaces chemosensory phenotypic change. Curr Zool 66(5):505–514
Young JM, Massa HF, Hsu L, Trask BJ (2010) Extreme variability among mammalian V1R gene families. Genome Res 20(1):10–18
Zelená J (1994) Nerves and mechanoreceptors: the role of innervation in the development and maintenance of mammalian mechanoreceptors. Springer, Dordrecht
Zhou Y, Shearwin-Whyatt L, Li J et al (2021) Platypus and echidna genomes reveal mammalian biology and evolution. Nature 2021(1). https://doi.org/10.1038/s41586-020-03039-0
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Rowe, T.B. (2023). Evolution of the Mammalian Neurosensory System: Fossil Evidence and Major Events. In: Dozo, M.T., Paulina-Carabajal, A., Macrini, T.E., Walsh, S. (eds) Paleoneurology of Amniotes . Springer, Cham. https://doi.org/10.1007/978-3-031-13983-3_10
Download citation
DOI: https://doi.org/10.1007/978-3-031-13983-3_10
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-13982-6
Online ISBN: 978-3-031-13983-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)