Abstract
To interpret the fundamental differences in the structure and origin of the braincase sidewalls of monotremes, multituberculates, and therians, we examined MicroCT scans of a mammaliaform, Morganucodon; two non-mammalian cynodonts, Massetognathus and Probainognathus; a stem therian, Vincelestes; a juvenile and adult monotreme, Ornithorhynchus; and two marsupials, Monodelphis and Didelphis. The skull of Morganucodon resembles the pattern predicted for an early mammal: the descending flanges of the frontal and parietal cover the lateral surface of the orbitosphenoid and the palatine forms most of the medial wall of the orbit. In monotremes, the lateral region of the chondrocranium ossifies to form a long presphenoid/orbitosphenoid complex. During the transition from early mammals to extant mammals the height of the alisphenoid decreased drastically, the anterior lamina extended anteriorly to form part of the sidewall while the lateral surface of the orbitosphenoid was exposed by the dorsal withdrawal of the frontal and parietal. By contrast, in multituberculates and therians the lateral edges of the frontals extended further ventrally and the orbitosphenoid was reduced to a smaller orbital exposure below the frontals. In multituberculates the alisphenoid decreased in height, replaced by an anterior extension of the anterior lamina. The palatine withdrew from the orbital wall, replaced by a dorsally directed expansion of the maxilla. Extant therians have lost the anterior lamina. The inferior edges of the frontal followed the further ventral migration of the orbitosphenoid. The alisphenoid and parietal form most of the braincase sidewall.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction (Fig. 1)
In contrast to non-mammalian cynodonts that have partially ossified sidewalls to the braincase and orbital region, the primary sidewall of the braincase in therians is formed by the chondrocranium. A secondary wall consists of ossifications within the chondrocranium plus some dermal bones. Here we use the term “sidewall” to include all ossifications visible in lateral and medial views. Mammals are characterized by a completely ossified sidewall; however, the configuration and makeup of the sidewall differs fundamentally between monotremes, multituberculates, and therian mammals (Kermack 1963; Kermack and Kielan-Jaworowska 1971; Presley and Steel 1976; Hopson and Rougier 1993).
-
In monotremes (Fig. 1a), the presphenoid, orbitosphenoid, parietal, petrosal, and anterior lamina form the sidewall of the braincase, with additional small contributions from the frontal, palatine, pterygoid, and alisphenoid.
-
In multituberculates, various interpretations have been published as to the composition of the braincase sidewall. Kryptobataar (Wible and Rougier 2000) has a reduced alisphenoid and enlarged anterior lamina (Fig. 1b), whereas Lambdopsalis (Miao 1988) retains a large alisphenoid and has a reduced anterior lamina (Fig. 1c). The palatine is absent from the sidewall in both.
-
In therians (Wible 2003), the alisphenoid, frontal, lacrimal, maxilla, palatine, parietal, petrosal, and pterygoid form the sidewall of the braincase with a small contribution from the orbitosphenoid and squamosal (Fig. 1d).
The ontogeny of the skull in extant mammals leads to conflicting views about the homologies of the bones of the sidewall (Bemmelen 1901; Presley 1981; Kuhn and Zeller 1987; Maier 1987; Zeller 1987; Miao 1988; Hopson and Rougier 1993; Averianov and Lopatin 2014). For example, Kuhn and Zeller (1987) claimed that the mammalian alisphenoid is a neomorph, not homologous with the epipterygoid of therapsids; whereas Hopson and Rougier (1993) asserted the homology of these two elements despite their having slightly different patterns of development and relationships to the main branches of the trigeminal nerve (CNV).
Less attention has been devoted to the role and position of the orbitosphenoid in the sidewall of the braincase in monotremes, multituberculates, and therians. Transverse sections of the skulls of therian mammals show the presphenoid/orbitosphenoid as T-shaped, with the presphenoid as the vertical line of the T and the orbitosphenoid its horizontal line (Wible and Hopson 1993). Both result from ossifications within the orbital cartilage, preoptic root, and caudal parts of the trabecular regions of the chondrocranium (Moore 1981; Zeller 1987; Mcbratney-Owen et al. 2008). Appositional bone then provides additional growth (De Beer 1937; Moore 1981; De Beer 1985; Maier 1987; Zeller 1987; Mcbratney-Owen et al. 2008). The presphenoid often develops a sizable sphenoidal sinus, profoundly altering the morphology of this area.
The structure of the braincase of the last common ancestor of monotremes, multituberculates, and therians is unknown and will likely remain so. Mammaliaformes such as Morganucodon watsoni and M. oehleri (Kermack et al. 1981) are closely related to the common ancestor of Mammalia (Luo et al. 2015); consequently, their skull structure probably resembles that of the first mammals. A paucity of fossils document the evolution of the braincase in the major groups of mammals. Exceptions include the numerous skulls of multituberculates (Kielan-Jaworowska et al. 2004), fragmentary remains of triconodonts (Kermack 1963; Crompton and Jenkins 1979), and the early Cretaceous Vincelestes neuquenianus (Hopson and Rougier 1993).
In this paper we review the structure of the braincase of non-mammalian cynodonts (Massetognathus pascuali and Probainognathus jenseni) and provide new information on the skulls of Morganucodon watsoni, Vincelestes neuquenianus, and a late stage of development in the skull of Ornithorhynchus anatinus.
The modern definition of Mammalia—the last common ancestor of monotremes, multituberculates, and therians plus all its descendants—implies that the division between therians and non-therians (Rowe 1987, 1988) represents the earliest stage of the diversification of mammals. Until recently, authors have suggested that monotremes were either closely related to or descended from multituberculates, a long-lived, extinct group of rodent-like early mammals. Luo et al. (2002) reviewed the literature on the relationships between multituberculates and therians or monotremes; and recently Luo (2007), Luo et al. (2015) (see also Rougier et al. 2007; Brusatte and Luo 2016) concluded that monotremes descended from an early branch of the mammalian tree that excludes therians and multituberculates. They suggested that the immediate ancestors of extant monotremes lay within a radiation of mammals (australosphenidians) that existed on Gondwanan continents during the Jurassic/Cretaceous (Rowe et al. 2008; Rich et al. 2016). However, what is known about the relationships of these mammals to one another and extant monotremes is based almost entirely on their molariform teeth, jaw morphology (Kielan-Jaworowska et al. 1987; Rich et al. 1997; Luo et al. 2001, 2002; Pascual et al. 2002; Rougier et al. 2004; Luo 2007), and isolated cranial elements (Rich et al. 2006).
Anatomical Abbreviations
AL–anterior lamina of the petrosal, ALS–alisphenoid, BOC–basioccipital, BS–basisphenoid, D–dentary, df–descending flange of the frontal, ds–dorsum sellae, em–ethmoturbinal, EOC–exoccipital, EP–epipterygoid, ET–ectotympanic, F–frontal, ins–internasal septum, ios–interorbital septum, LAC–lacrimal, lpa–left pila antotica, m–masseter, ME–mesethmoid, MX–maxilla, N–nasal, np–nasopharyngeal passage, ob–olfactory bulb, of–optic foramen, on–olfactory nerve, OS–orbitosphenoid, P–palatine, PA–parietal, pa–pila antotica, PAS–parasphenoid, PET–petrosal, pit–pituitary, pop–preoptic pillar, PS–presphenoid, PT–pterygoid, r–nasoturbinal ridge, SOC–supraoccipital, sof–sphenorbital fissure, SQ–squamosal, t–temporalis, tp–trabecular plate, V–vomer, V 1, V 2 and V 3–ophthalmic, maxillary and mandibular branches, respectively, of the trigeminal nerve.
Institutional Abbreviations
AMNH-American Museum of Natural History, New York, IVPP-Institute of Vertebrate Palaeontology and Palaeoanthropology, Beijing, MACN-Museo Argentino de Ciencias Naturales, Buenos Aires, MCZ-Museum of Comparative Zoology, Harvard University, Cambridge, UFRGS-Universidade Federal do Rio Grande do Sul, Porto Alegre.
Material and Methods
CT-scanning technology has allowed us to examine the structures of the inner and outer sidewalls of the skull in Massetognathus pascuali (MCZ 3790, 3798), Probainognathus jenseni (MCZ 4280), Ornithorhynchus anatinus (AMNH 2013, juvenile; MCZ 17529 adult), Morganucodon watsoni (IVPP 8682), and uncatalogued specimens of Pachygenelus sp., Monodelphis domestica, and Didelphis virginiana. These specimens were scanned at Harvard University’s Center for Nanoscale Systems using an Xtek HMXST Micro-CT x-ray imaging system that provided resolution in our specimens ranging from 0.01 mm per voxel to 0.07 mm per voxel.
The skull of Vincelestes neuquenianus (MACN-N 04) was scanned at the University of Texas’ High-Resolution X-ray Computed Tomography Facility and the lower resolution but still revealing results posted on Digimorph, the Digital Library of the University of Texas. Some of the skull bones of Morganucodon (IVPP 8682) were missing and others had shifted relative to one another before preservation. To reconstruct the skulls of Massetognathus, Probainognathus, Morganucodon, and Ornithorhynchus, image stacks of voxel information were exported from the ct-scanning software, Xtex CT-Pro, processed in VG Studio Max, and imported into Amira, where the bones were segmented and visualized in stereo. A slice movie of Vincelestes was downloaded from the Digimorph digital library (http://digimorph.org/specimens/Vincelestes_neuquenianus/), converted to an image stack, and segmented in Amira. The skulls of the juvenile Ornithorhynchus and adult Monodelphis were bulk stained in Lugols prior to scanning to differentiate soft tissue.
Data Availability
To view three-dimensional reconstructions of Probainognathus, Morganucodon, the infant Ornithorhynchus, or Vincelestes, please refer to Electronic Supplementary Material (ESM) #1–7 in the Online Resources.
Museum of Comparative Zoology specimen stereo images are available for viewing at http://mczbase.mcz.harvard.edu. For access to image stacks or non-MCZ specimen data, please contact the author.
Results
Non-Mammalian Cynodonts (Fig. 2)
For a description of the sidewall of the braincase of non-mammaliaform cynodonts, we have chosen Massetognathus pascuali and Probainognathus jenseni because well-preserved skulls that scanned well were available. In Massetognathus the anterior portion of the presphenoid/orbitosphenoid forms the floor and partial sidewall of the olfactory bulb. The anterior tip is Y-shaped in cross-section, increasing in height posteriorly, with the taller lateral wings and vertical stem of the Y forming a short medial keel that we identify as a presphenoid (Clark and Smith 1993). The potential size and extent of the presphenoid/orbitosphenoid depends upon the degree of ossification within the chondrocranium (Kuhn and Zeller 1987; Maier 1987; Zeller 1987) and perhaps to a larger degree the fossilization process. Probainognathus jenseni has a greater extent of ossification, particularly of the posterior and ventral regions of the orbitosphenoid, and a complete optic foramen is present for the exit of the optic nerve (CNII) (see ESM #5 to view a rotating 3D model of the orbitosphenoid of Probainognathus). This foramen lies ventral to the bulging portion of the orbitosphenoid and close to the midline—as such, right and left optic foramina are confluent and a chiasmatic groove is lacking (Fig. 2d). A groove on each side of the vertical component of the presphenoid leads from the optic foramen. The orbitosphenoid is slightly concave dorsally, immediately rostral to the optic foramina, and becomes convex anteriorly following the overall shape of the anterior cranial cavity, which conforms to the expected shape of the olfactory lobes. The dorsolateral edge of the orbitosphenoid closely follows the domed contour of the ventral surface of the frontal (Fig. 2e: 1). At this point the frontal is narrow and the maximum distance between the wings of the orbitosphenoid is 0.5 cm. Using extant mammal development as a model, we expect that the portion of the presphenoid anterior to the optic foramen ossifies from the embryonic preoptic pillar, while the posterior portion ossifies from the metoptic pillar and becomes part of the orbitosphenoid (Zeller 1987). We suggest that in adult non-mammalian cynodonts much of the floor and sidewall of the braincase and the entire nasal capsule remained either cartilaginous or membranous. Additionally, the bony structures contributing to it were fragile and unlikely to be preserved even in taxa where they were likely extensive. The presphenoid in non-mammaliaform cynodonts probably extended ventrally as a cartilaginous interorbital septum as it does in lizards (Bellairs and Kamal 1981) or an ossified septum as it is in the therocephalians, Microconodon (Abdala et al. 2014) and Theriognathus (Kemp 1972; Huttenlocker and Abdala 2015). The parasphenoid and vomer of Probainognathus had a grooved dorsal edge that held the continuous ventral edges of interorbital and internasal septa. In Massetognathus, the anterior tips of the epipterygoid and parietal overlap the orbitosphenoid wings (Fig. 2c: 2). The maxillary (V2) and mandibular (V3) branches of the trigeminal nerve exit the skull at the border between the petrosal and epipterygoid. The only ossified part of the medial wall of the orbit in non-mammaliaform cynodonts is the small exposure of the orbitosphenoid (Macrini et al. 2007). The antotic pillar ossifies to various extents and forms a pleurosphenoid (Goodrich 1930) that extends anterodorsally from the posterior end of the basisphenoid. Quiroga (1979: fig. 1) illustrated an endocast of the brain of Massetognathus but did not include the orbitosphenoid, or antotic pillars in his figure. However, the dotted line between the pila antotica and the orbitosphenoid we show in Fig. 2a follows the same contour as the ventral surface of the endocast figured by Quiroga. Bonaparte (1962) illustrated an extensive orbital septum, orbitosphenoid and ossified pila antotica in the traversodontid Exaeretodon. Its overall pattern resembles Massetognathus, but with a more robust and complete primary wall and septum. In Massetognathus, the epipterygoid forms a partial sidewall to a space that lacks ossified structures in the midline anterior to the antotic pillar (Fig. 2c: 3). A relatively narrow median gap separates the left and right antotic pillars. As in reptiles (Hopson 1964; Starck 1967; Bellairs and Kamal 1981), the brain extended forward beyond the antotic pillars to reach the pituitary fossa, but probably not into the large space between the epipterygoids. In Massetognathus and other non-mammaliaform cynodonts, the descending wing of the frontal and prefrontal along with the orbital flange of the palatine form part of an oblique posterior wall to the nasal cavity. The endocranial cavity of the non-mammalian cynodont Chiniquodon theotenicus exposed by manual preparation (Kemp 2009) has basically the same structure as that of Probainognathus and Massetognathus.
Massetognathus pascuali. a and b, internal and external views of the sidewall of the braincase; c, three transverse sections at the locations labeled as 1–3 in 2b; d, parasagittal section through the braincase of Probainognathus jenseni; e, transverse sections through Probainognathus at locations 1 and 2 in Fig. 2d
Ictidosauria
In this clade we include a number of small Late Triassic and Early Jurassic non-mammaliaform cynodonts, mainly from South America and South Africa, that form a sister group to Mammaliaformes such as Morganucodon (Fig. 3) and Sinoconodon (Sidor and Hancox 2006; Martinelli and Rougier 2007; Martinelli et al. 2009; Bonaparte et al. 2012; Rodrigues et al. 2013; Martinelli and Bento Soares 2016; Meng et al. 2016). Of the ictidosaurs, the sidewall of the cranium has only been partially described in Diarthrognathus (Crompton 1958), Pachygenelus (Wible and Hopson 1993), and Brasilitherium (Bonaparte and Migale 2010; Bonaparte et al. 2012; Rodrigues et al. 2013). These forms share with non-mammalian cynodonts a largely unossified medial wall to the orbit, but differ in that the ventral edge of the frontal extends much farther ventrally, and the palatine contributes to the medial wall of the orbit. The orbitosphenoid is not preserved in an illustrated specimen of Brasilitherium (UFRGS-PV-1043_T) (Rodrigues et al. 2013); but in an uncatalogued, crushed specimen of Pachygenelus (Fig. 4b), the delicate wings of the orbitosphenoid are preserved medial to the inner surface of the descending edges of the frontal. Relative to skull length, the width of the frontal is much greater than in non-mammalian cynodonts, indicating a substantial relative increase in the size of the olfactory bulb. According to Quiroga (1984: 289), one other ictidosaur, Therioherpeton cargnini, “…appears as the most encephalized cynodont.”
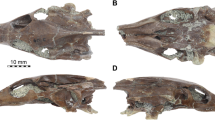
a, reconstruction of the sidewall of the braincase of Morganucodon watsoni. The preserved parts of the orbitosphenoid are indicated with a dotted outline and the presumed extent of the orbitosphenoid is indicated through shading, and its unossified portion with dots; b, four transverse sections through the braincase at the positions indicated in a
a and b, Morganucodon watsoni (IVPP 8682). a, Stereo photograph and annotated figure of the lateral view of the braincase. Dotted horizontal line indicates the positions of the section shown in b; b, stereo photographs and annotated figure of a transverse section through the orbitosphenoid region; c, Pachygenelus sp. Stereo photograph and annotated figure of a section through the orbitosphenoid region
Morganucodon watsoni (Figs. 3, 4 and ESM #Fig. 1)
The specimen of Morganucodon (IVPP 8682) described here is badly fractured, some bones are missing, and those preserved are displaced relative to one another. Consequently, some aspects of the reconstruction of the skull shown in Fig. 3, especially the position of the bones of the anterior orbital wall, are tentative and subject to revision. Foramina for nerves V2 and V3 penetrate a thin anterior lamina continuous with the petrosal. No suture between the anterior lamina and the petrosal could be identified (Kermack et al. 1981). The anterior edge of this lamina is overlapped by the posterior edge of the epipterygoid, which in turn contacts the frontal and parietal. The wings of the orbitosphenoid present in IVPP 8682 (Fig. 3) are also shown in Fig. 4. The preserved portion of the orbitosphenoid is indicated by a dotted outline (OS, Fig. 3), but it probably extended both forward and backward either as bone that was not preserved, as cartilage, or membrane, as indicated by dotted shading, to form more of the base and lateral wall to the olfactory bulb and cerebral hemisphere. A vertical circular ridge on the inner surface of the orbitosphenoid reveals the annular fissure between the olfactory bulb and neocortex (Rowe et al. 2011). The frontal and parietal cover the lateral surface of the thin wing of the orbitosphenoid (Fig. 3b: 3, 4; and Fig. 4) as they do in Pachygenelus (Fig. 4c). The wings of the orbitosphenoid are continuous with the presphenoid, which forms a short median keel barely visible in lateral view, but which probably extended forward as a cartilaginous interorbital septum. Behind the presphenoid the ventral edges of the orbitosphenoid wings diverge away from the midline. The orbital flanges of the palatines (Fig. 3a and b: 2) form large vertical plates that comprise most of the medial walls of the orbits. Our identification of this structure is consistent with the intact orbital flange of the palatine preserved in the other mammaliaform, Sinocodon (Crompton and Luo 1993: fig. 4. 9). Kermack et al. (1981) incorrectly identified these plates as wings of the orbitosphenoid in Morganucodon oehleri. Although it is difficult to establish with certainty, their vertical posterior edges appear parallel to one another, and their dorsoposterior edges contact the lateral surface of the presphenoid. The tall gap between the vertical posterior edges of the palatines below the presphenoid probably contained a cartilaginous septum (shown in Fig. 3b: 3). This septum would have provided a posterior wall to the space between the palatine’s orbital flanges. In an anterior direction, these flanges diverge from the midline and meet the lacrimal and the descending flange of the frontal (Fig. 3b: 2). It is possible that the nasal capsule extended into this space as suggested by Kermack et al. (1981). A dorsal projection of the maxilla and the lacrimal overlap the anteroventral edge of the palatine flange. A narrow gap separates the epipterygoid and palatine—presumably an exit for V1. A separate foramen for the optic nerve could not be identified within the orbitosphenoid, probably because the anterior portion of the orbitosphenoid is not preserved. Behind the presphenoid, the epipterygoids form the lateral walls to an empty space (Fig. 3b: 4). It is not clear how far forward the brain extended into this space. A dorsum sellae and medial clinoid processes extend anterodorsally from the basisphenoid. This specimen lacks ossified antotic pillars, but Kermack et al. (1981) describe the remnants of one emerging from isolated petrosals of Morganucodon.
In tritylodontids (Rowe 1986; Sues 1986) the descending flanges of the frontal, orbitosphenoid, and palatine form the medial wall of the orbit. In this aspect they resemble Morganucodon. Some scholars (Rowe 1993; Liu and Olsen 2010) consider tritylodontids more closely related to morganucodontids than to tritheledontids. Others (Zhou et al. 2013; Luo et al. 2015; Martinelli and Bento Soares 2016) have reached the opposite conclusion. We concur with the latter view. If this is correct, it suggests that because the sidewall of the skull in tritheledontids is not complete, closure of the sidewall occurred independently in tritylodontids and morganucodontids.
Ornithorhynchus anatinus (Fig. 5)
The juvenile Ornithorhynchus skull (AMNH 2013) illustrated in Fig. 5 and ESM #2 is slightly older and more ossified than the specimen (MO 39) described and illustrated by Zeller (1989). A single bone (sphenoid) that ossifies in the anterior region of the chondrocranium can be divided into three regions: presphenoid, orbitosphenoid, and mesethmoid—an ossification within the internasal septum. The presphenoid is wide transversely, and ventrally it meets the dorsal tips of the vomer and palatine (Fig. 5c: 2). In the midline the mesethmoid extends forward from the presphenoid and ossifies in the internasal septum. A thick cartilaginous trabecular plate occupies the space anterior to the basisphenoid and below the presphenoid in the trough of the vomer (Fig. 5c: 2). Kesteven and Furst (1929) noted that the basisphenoid ossified before the more anterior portions of the basicranial axis. Within the nasal cavity of the juvenile, the vomer is “U” shaped and follows the contour of the ventral edge of the cartilaginous internasal septum (Fig. 5c: 1). In the orbital region the internasal septum is continuous with the trabecular plate. A divided vomer lies against the lateral surfaces of the trabecular plate right up to the anterior border of the basisphenoid (Fig. 5c: 2). This midline complex divides the nasopharyngeal passage. Behind this point the basisphenoid itself divides the nasopharyngeal passage (Fig. 5c: 3).
Ornithorhynchus anatinus. a and b, internal and external views of the braincase of juvenile; c, three transverse sections through the braincase at the positions indicated in a. c3 includes origins and insertions of adductor muscles. The orbitosphenoid and presphenoid in these sections are shaded black. d, sagittal section through adult skull; e, transverse section at position indicated by vertical line in d
In the adult, the vomer, trabecular plate, and part of the basisphenoid are reduced to a thin vertical plate that divides the nasopharyngeal passage (Fig. 5d, e) and is continuous with the mesethmoid. The broadened presphenoid provides a floor to the olfactory bulb and connects via a wide preoptic pillar to the long orbitosphenoid (Fig. 5c: 2). The portion of the orbitosphenoid anterior to the preoptic pillar forms a lateral wall to the olfactory bulb and olfactory nerve (Fig. 5c: 1) as it did in Probainognathus and likely in Morganucodon as well. The term “orbitosphenoid” in monotremes is misleading because the bone ossifies within the sphenethmoid commissure, preoptic pillar, orbital cartilage, and orbitoparietal commissure; whereas in therians and probably multituberculates the orbitosphenoid ossifies primarily within the orbital cartilage and the preoptic and metoptic roots (Moore 1981; Zeller 1987). The presphenoid and anterior portion of the orbitosphenoid and anterior lamina form a medial wall to the orbit, and the posterior portion of the orbitosphenoid together with the anterior lamina form most of the sidewall of the braincase. As such, the sidewall of the braincase is formed by a composite of the primary wall (sphenoid complex) and the secondary (alisphenoid, anterior lamina).
The frontal of Ornithorhynchus completely overlaps the anterior part of the orbitosphenoid, but the posterior part of the latter has an extensive lateral exposure below the parietal. The temporalis muscle originates on both the orbitosphenoid and anterior lamina (Fig. 5c: 3). In extant monotremes, the temporalis is divided into a larger medial portion, originating on the anterior lamina and inserting on the medial surface of the dentary, and a lateral portion originating mainly on the parietal and orbitosphenoid and inserting on the lateral dorsal surface of the dentary. Ornithorhynchus and Tachyglossus possess a very low ascending process of the dentary and consequently the temporalis fibers that insert on its medial surface and on the transversely wide dorsal surface of the dentary orient vertically (Fig. 5c, 3). The Miocene platypus, Obdurodon (Musser and Archer 1998) and the Early Cretaceous Teinolophos (Rich et al. 2001, 2016) possess a slightly taller coronoid process. The alisphenoid is miniscule, and according to embryological evidence of recent monotremes (De Beer and Fell 1936), likely restricted to the ossified ala temporalis, fused to the basisphenoid, and penetrated by a canal. The brain rests ventrally on a plane connecting the dorsal surface of the presphenoid to the basisphenoid.
In the adult skull (Fig. 5d and e) most of the cartilaginous regions of the juvenile skull are ossified. Fusion between bones makes it difficult to recognize the sutures between them.
Vincelestes neuquenianus (Fig. 6)
Fig. 6a is based on the lateral view figured by Hopson and Rougier (1993) and a stereo view of MACN-05 included in supplementary information (ESM #4a). The palatine, frontal, and tall orbitosphenoid form the medial wall of the orbit. Because the presphenoid/orbitosphenoid is a short deep bone with a complex shape, we include six transverse sections plus anterior and posterior views of it in Fig. 6c and d. The anterior tip of the orbitosphenoid as seen in lateral view lies between the palatine and frontal. Behind this point its dorsal edge contacts the ventral edge of the frontal (Fig. 6c: 1–3) and its posterior edge is covered by the alisphenoid (Fig. 6c: 4–5). The wide horizontal dorsal surface of the presphenoid/orbitosphenoid and its low orbital wings form a floor and partial sidewall to the olfactory bulb with the rest of the sidewall provided by the frontal. In the midline the posterior surface of the presphenoid is nearly vertical but is extended on either side by short wings that reach posterolaterally and are overlapped by the alisphenoid. The posteroventral tip of the presphenoid is connected to the dorsum sellae by a thin strip of bone that appears to be an ossification of the antotic pillar (ESM #4b). According to an endocast reconstructed by Macrini et al. (2007), the brain’s ventral surface lies against the posterior surface of the presphenoid and the dorsal surface of the antotic pillar and basisphenoid. A low medial ridge on the anterior surface of the presphenoid (Fig. 6d) separates shallow depressions on either side. Because of the position of section 2 (Fig. 6c: 2), these shallow depressions appear as open spaces. It is possible that the depressions are early manifestations of sphenoidal sinuses. The posterior edge of each orbital flange of the palatine fits snuggly against the anterolateral edges of the orbitosphenoid. From this point forward the palatine flanges diverge laterally as they do in Morganucodon. The space between them may have been filled with a posterior extension of the nasal capsule. The ventral edge of the palatine contacts the lateral portion of the pterygoid. As can be seen in a ventral view of the palate (ESM #4c), only the lateral part of the pterygoid anterior to the presphenoid is preserved. As seen in sagittal section (Fig. 6b), the anteroventral tip of the orbitosphenoid narrows to a point, but it is broader in an anterior view. Cartilage of the trabecular plate probably filled the midline gap beneath the posteroventral surface of the presphenoid (Fig. 6c: 4) and formed a medial wall to the sphenorbital fissure. The only preserved part of the posterior region of the nasal capsule, a thin vertical mesethmoid, is positioned anterodorsally to the orbitosphenoid (Fig. 6b and ESM #4d). No other structures, such as a cribriform plate or turbinals, can be identified, although small fragments of bone are present in the nasal and cranial cavities. These tend to be larger and more numerous in the nasal cavity.
Extant Therians: Canis familiaris and Didelphis virginiana (Fig. 7)
In therian mammals such as the dog, the presphenoid is a robust bone that forms a prominent part of the cranial base, whereas the orbitosphenoid wings are very small (Miller et al. 1964), and the frontal, alisphenoid, and parietal form the sidewall of the braincase. In Monodelphis (Wible 2003) and Didelphis, the presphenoid/orbitosphenoid is a complex structure. A midline sagittal section through the skull shows an orbitosphenoid connected anteriorly to the presphenoid, while its posterior part lies above the presphenoid. The orbitosphenoid is hollowed out and forms a sphenoidal recess that accommodates endoturbinal V (Rowe et al. 2005). In an adult Didelphis skull it is difficult to determine the anterior borders of the orbitosphenoid because the bone is fused to the ossified nasal capsule. The solid posterior edge of the orbitosphenoid meets the medial surface of the alisphenoid on either side above the sphenorbital fissure as it does in Vincelestes (cf. Figs. 6c: 4–5 and 7c: 3). In an anterior direction the dorsal border of the orbitosphenoid is continuous with the cribriform plate and its lateral border forms a wall to the sphenoidal recess. The presphenoid continues forward beyond the sphenoidal recess to join with the ossified floor of the nasal capsule. In Didelphis, the presphenoid, the ossified floor of the nasal capsule, and the transverse flanges of the palatine form a transverse lamina above the nasopharyngeal passage. The temporalis and masseter (Fig. 7d) hold the dentary in a muscular sling. The temporalis retains the primitive condition and originates on the frontal, parietal, and alisphenoid, and inserts on the ascending ramus of the dentary. In contrast to Ornithorhynchus, the lateral component of the force this muscle generates is significant.
Internal views of the braincase and presphenoid/orbitosphenoid of a, Canis familiaris; and b, Didelphis virginiana; c, three transverse sections through the presphenoid/orbitosphenoid bone of Didelphis at the positions indicated in b; d, transverse section through the temporalis and masseter muscle in Monodelphis domestica
Discussion
Non-mammalian cynodonts lack both an anterior lamina and medial wall to the orbit—other than a small contribution by the dorsally positioned orbitosphenoid. Two significant features of mammaliaforms include: (1) the orbital flange of the palatine forming most of the medial orbital wall, and (2) the presence of an anterior lamina penetrated by two branches of the trigeminal nerve (Kermack et al. 1981). Morganucodon is the only mammaliaform in which the structure of the sidewall of the braincase is now relatively well known. We suggest that it resembled that of the common ancestor of mammals, and discuss how it was modified in therian mammals, multituberculates, and monotremes.
Vincelestes represents an intermediate stage in the transition to extant therian mammals. In contrast to the delicate wings of the orbitosphenoid in Morganucodon, those of Vincelestes are thicker in width and dorsoventrally shorter. The ventral edges of the descending flanges of the frontals and parietals either contact or slightly overlap these wings. The presphenoid/orbitosphenoid seems more robust and dorsoventrally deeper, presumably because of increasing ossification of the interorbital septum. It approaches the trabecular plate on the ventral surface of the palate. The alisphenoid overlaps the posterolateral surface of the presphenoid/orbitosphenoid complex, while the orbital flanges of the palatines contact its deep anteroventral edges. Shallow pockets in the anterior surface of the presphenoid foreshadow deeper sphenoidal sinuses in extant mammals, suggesting that the nasal capsule extended backward between the orbital flanges of the palatines. Numerous bone fragments float in the nasal region. These could be pieces of an ossified nasal capsule or nasal turbinals, but the mesethmoid constitutes the only identifiable bone. Caudal to the presphenoid the braincase wall is composed entirely of the parietal, alisphenoid, anterior lamina, and petrosal. The sphenorbital fissure lies between the anteromedial edge of the alisphenoid and the lateral surface of the orbitosphenoid. Delicate antotic pillars connect the orbitosphenoid to the dorsum sellae, indicating that in comparison with Morganucodon the floor of the brain migrated ventrally (Martinelli and Rougier 2007). In extant therians the presphenoid/orbitosphenoid is reduced in height and usually fused with the cribriform plate and lateral lamina of the nasal capsule. The wings of the orbitosphenoid are either significantly reduced or entirely lost. There is no record of an anterior lamina in extant therian mammals.
Differences in the sidewall of the braincase between multituberculates (Fig. 1b and c; see also Miao 1988; Wible and Rougier 2000) may be more apparent than real, because it is extremely difficult to identify sutures in the numerous skulls of multituberculates that have been described. However, the dorsal part of the alisphenoid is generally accepted to have become smaller in multituberculates, and its exposure on the ventral aspect of the skull greatly increased. In Vincelestes and multituberculates the tall descending flanges of the frontal meet the dorsal edge of the orbitosphenoid. The latter retains its connection with the dorsum sellae. Multituberculates differ from Vincelestes in that their sphenorbital fissure lies between the orbitosphenoid and the anterior lamina (Fig. 1c) rather than between the orbitosphenoid and the alisphenoid. A distinguishing feature of multituberculates is the loss of the orbital flanges of the palatines and their replacement by a dorsal extension of the maxilla, probably related to their derived mastication and powerful musculature (Gambaryan and Kielan-Jaworowska 1995).
The maxilla is an expansive bone that ultimately displaces the jugals from the zygomatic arch and is the dominant element in the anterior half of the skull, including the orbit. The presphenoid/orbitosphenoid in multituberculates resembles that of a stem therian such as Vincelestes more closely than it does that of monotremes in both position and structure. This appears to support a view (Rowe 1988; Brusatte and Luo 2016) that monotremes represent an early branch of the mammalian tree, a sister group to the phylogenetic line leading to multituberculates and therians.
Monotremes are distinguished from therians by a greater degree of ossification in the primary wall of the chondrocranium. Their orbitosphenoid is formed from ossifications in the orbitonasal, orbitoparietal commissures, preoptic pillar, and the orbital cartilage—but not the metoptic pillars (Griffiths 1978; Zeller 1989). The pattern of ossification of the chondrocranium in monotremes accounts for the long anteroposterior length of the orbitosphenoid and the reason it forms a partial lateral wall to the olfactory bulb anterior to the preoptic pillar. The orbitosphenoid wings in the specimen of Morganucodon described in this paper are extremely slender and do not extend forward above the orbits. An optic foramen could not be identified either because the anterior portion of the orbitosphenoid was lost or the preoptic and metoptic pillars and orbitonasal commissures remained cartilaginous. In the transition to monotremes, much of the lateral surface of the presphenoid/orbitosphenoid complex became exposed because of a reduction in the height of the descending flanges of the frontal and parietal and of the alisphenoid. As a result, part of the origin of the temporal muscle shifted to the orbitosphenoid and anterior lamina. Monotremes have reduced the ascending ramus of the dentary. The resulting area for the insertion of the temporalis is consequently reduced to the lateral surface of the low coronoid process and dorsal surface of the dentary. In addition, the masseter muscle is smaller and divided into deep and detrahens portions. It is far from clear why in monotremes the alisphenoid shrank and was replaced with the anterior lamina, or why monotremes lost the orbital flanges of the palatine and significantly reduced the size of the pterygoid. One question yet to be explored is whether the unique features of the sidewall of the braincase of monotremes correlate with changes in the organization, orientation, and magnitude of the adductor muscles.
References
Abdala F, Jashashvili T, Rubidge BS, Van Den Heever J (2014) New material of Microgomphodon oligocynus (Eutherapsida, Therocephalia) and the taxonomy of southern African Bauriidae. In: Kammerer CF, Angielczyk KD, Frobisch J (eds) Early Evolutionary History of the Synapsida. Springer, Dordrecht, pp 209–231
Averianov AO, Lopatin AV (2014) On the phylogenetic position of monotremes (Mammalia, Monotremata). Paleontol J 48(4):426–446
Bellairs ADA, Kamal AM (1981) The chondocranium and the development of the skull in recent reptiles. In: Gans C, Parsons TS (eds) Biology of the Reptilia: Morphology F. Academic Press, Toronto, 11, pp 1–263
Bemmelen JFV (1901) Der Schadelbau der Monotremen. Denkschr med-naturw Ges Jena VI:729-798
Bonaparte JF (1962) Descripcion del craneo y mandibula de Exaeretodon frenguelli, Cabrera y su comparacion con Diademodontidae, Tritylodontidae y los Cinodontes sudamericanos. Publ Mus Municip Cienc Nat Mar del Plata 1:135–202
Bonaparte JF, Migale LA (2010) Protomamíferos y mamíferos mesozoicos de América del Sur. Museo Municipal de Ciencias Naturales Carlos Amgehino, Buenos Aires
Bonaparte JF, Soares MB, Martinelli AG (2012) Discoveries in the Late Triassic of Brazil improve knowledge on the origin of mammals. Hist Nat (Corr) 2(2):5–30
Brusatte S, Luo Z-X (2016) The ascent of mammals. Sci Am 314:28–35
Clark CT, Smith KK (1993) Cranial osteogenesis in Monodelphis domestica (Didelphidae) and Macropus eugenii (Macropodidae). J Morphol 215(2):119–149
Crompton AW (1958) The cranial morphology of a new genus and species of ictidosauran. Proc Zool Soc London 130(2):183–216
Crompton AW, Jenkins FA Jr (1979) Origin of mammals. In: Lillegraven JA, Kielan-Jaworowska Z, Clemens WA (eds) Mesozoic Mammals: The First Two-thirds of Mammalian History. University of California Press, Berkeley, Los Angeles & London, pp 59–73
Crompton AW, Luo Z (1993) Relationships of the Liassic mammals Sinoconodon, Morganucodon oehleri, and Dinnetherium. In: Szalay FS, Novacek MJ, McKenna MC (eds) Mammal Phylogeny: Mesozoic Differentiation, Multituberculates, Monotremes, Early Therians, and Marsupials. Springer-Verlag, New York, pp 30–44
De Beer GR (1937) The Development of the Vertebrate Skull. Clarendon Press, Oxford
De Beer GR (1985) The Development of the Vertebrate Skull. The University of Chicago Press, Chicago
De Beer GR, Fell WA (1936) The development of Monotremata. Part III. The development of the skull of Ornithorhynchus. Trans Zool Soc London 28:1–42
Gambaryan PP, Kielan-Jaworowska Z (1995) Masticatory musculature of Asian taeniolabidoid multituberculate mammals. Acta Palaeontol Pol 40(1):45–108
Goodrich ES (1930) Studies on the Structure and Development of Vertebrates. Macmillan and co., limited, London
Griffiths M (1978) The Biology of the Monotremes. Academic Press, New York
Hopson JA (1964) The braincase of the advanced mammal-like reptile Bienotherium. Postilla 87:1–30
Hopson JA, Rougier GW (1993) Braincase structure in the oldest known skull of a therian mammal: implications for mammalian systematics and cranial evolution. Am J Sci 293:268–299
Huttenlocker AK, Abdala F (2015) Revision of the first therocephalian, Theriognathus owen (Therapsida: Whaitsiidae), and implications for cranial ontogeny and allometry in nonmammaliaform eutheriodonts. J Paleontol 89(4):645–664
Kemp TS (1972) Whaitsiid Therocephalia and the origin of cynodonts. Philos Trans R Soc Lond Ser B Biol Sci 264(857):1–53
Kemp TS (2009) The endocranial cavity of a nonmammalian eucynodont, Chiniquodon theotenicus, and its implications for the origin of the mammalian brain. J Vertebr Paleontol 29(4):1188–1198
Kermack KA (1963) The cranial structure of the triconodonts. Philos Trans R Soc Lond Ser B Biol Sci 248B:83–103
Kermack KA, Kielan-Jaworowska Z (1971) Therian and non-therian mammals. In: Kermack DM, Kermack KA (eds) Early Mammals. Academic Press, London, pp 103–115
Kermack KA, Musset F, Rigney HW (1981) The skull of Morganucodon. Zool J Linn Soc 71(1):1–158
Kesteven HL, Furst HC (1929) The skull of Ornithorhynchus, its later development and adult stages. J Anat 63:447–472
Kielan-Jaworowska Z, Cifelli RL, Luo Z-X (2004) Mammals from the Age of Dinosaurs: Origins, Evolution, and Structure. Columbia University Press, New York
Kielan-Jaworowska Z, Crompton AW, Jenkins FA Jr (1987) The origin of egg-laying mammals. Nature 326(6116):871–873
Kuhn HJ, Zeller U (1987) The cavum epiptericum in monotremes and therian mammals. In: Kuhn HJ, Zeller U (eds) Morphogenesis of the Mammalian Skull. Mammalia Depicta. Verlag Paul Parey, Hamburg and Berlin, pp 51–70
Liu J, Olsen P (2010) The phylogenetic relationships of Eucynodontia (Amniota: Synapsida). J Mammal Evol 17(3):151–176
Luo Z-X (2007) Transformation and diversification in early mammal evolution. Nature 450(7172):1011–1019
Luo Z-X, Cifelli RL, Kielan-Jaworowska Z (2001) Dual origin of tribosphenic mammals. Nature 409(6816):53–57
Luo Z-X, Gatesy SM, Jenkins FA Jr, Amaral WW, Shubin NH (2015) Mandibular and dental characteristics of Late Triassic mammaliaform Haramiyavia and their ramifications for basal mammal evolution. Proc Natl Acad Sci U S A 112(51):E7101-E7109
Luo Z-X, Kielan-Jaworowska Z, Cifelli RL (2002) In quest for a phylogeny of Mesozoic mammals. Acta Palaeontol Pol 47(1):1–78
Macrini TE, Rougier GW, Rowe T (2007) Description of a cranial endocast from the fossil mammal Vincelestes neuquenianus (Theriiformes) and its relevance to the evolution of endocranial characters in therians. Anat Rec Adv Integr Anat Evol Biol 290(7):875–892
Maier W (1987) The ontogenetic development of the orbitotemporal region in the skull of Monodelphis domestica (Didelphidae, Marsupialia), and the problem of the mammalian alisphenoid. In: Kuhn HJ, Zeller U (eds) Morphogenesis of the Mammalian Skull. Mammalia Depicta. Verlag Paul Parey, Hamburg and Berlin, pp 71–90
Martinelli AG, Bento Soares M (2016) Evolution of South American non-mammaliaform cynodonts (Therapsida, Cynodontia). In: Agnolin FL, Lio GL, Egli FB, Chimento NR, Novas FE (eds) Contribuciones del MACN: Historia Evolutiva y Paleobiogeográfica de los Vertebrados de América del Sur. Buenos Aires, 6, pp 183–196
Martinelli AG, De La Fuente M, Abdala F (2009) Diademodon tetragonus Seeley, 1894 (Therapsida: Cynodontia) in the Triassic of South America and its biostratigraphic implications. J Vertebr Paleontol 29(3):852–862
Martinelli AG, Rougier GW (2007) On Chaliminia musteloides (Eucynodontia: Tritheledontidae) from the Late Triassic of Argentina, and a phylogeny of Ictidosauria. J Vertebr Paleontol 27(2):442–460
Mcbratney-Owen B, Iseki S, Bamforth SD, Olsen BR, Morriss-Kay GM (2008) Development and tissue origins of the mammalian cranial base. Dev Biol 322(1):121–132
Meng J, Bi S, Zheng X, Wang X (2016) Ear ossicle morphology of the Jurassic euharamiyidan Arboroharamiya and evolution of mammalian middle ear. J Morphol doi: 10.1002/jmor.20565
Miao D (1988) Skull morphology of Lambdopsalis bulla (Mammalia, Multituberculata) and its implications to mammalian evolution. Contrib Geol Univ Wyoming Spec Pap 4:1–104
Miller ME, Christiansen GC, Evans HE (1964) Anatomy of the Dog. W.B. Saunders, Philadelphia
Moore WJ (1981) The Mammalian Skull. Cambridge University Press, Cambridge
Musser AM, Archer M (1998) New information about the skull and dentary of the Miocene platypus Obdurodon dicksoni, and a discussion of ornithorhynchid relationships. Philos Trans R Soc Lond B Biol Sci 353(1372):1063–1079
Pascual R, Goin FJ, Balarino L, Sauthier DEU (2002) New data on the Paleocene monotreme Monotrematum sudamericanum, and the convergent evolution of triangulate molars. Acta Palaeontol Pol 47(3):487–492
Presley R (1981) Alisphenoid equivalents in placentals, marsupials, monotremes and fossils. Nature 294(5842):668–670
Presley R, Steel FLD (1976) On the homology of the alisphenoid. J Anat 121(3):441–459
Quiroga JC (1979) The brain of two mammal-like reptiles (Cynodontia - Therapsida). J Hirnforsch 20(4):341–350
Quiroga JC (1984) The endocranial cast of the advanced mammal-like reptile Therioherpeton cargnini (Therapsida, Cynodontia) from the Middle Triassic of Brazil. J Hirnforsch 25(3):285–290
Rich TH, Hopson JA, Gill PG, Trusler P, Rogers-Davidson S, Morton S, Cifelli RL, Pickering D, Kool L, Siu K, Burgmann FA, Senden T, Evans AR, Wagstaff BE, Seegets-Villiers D, Corfe IJ, Flannery TF, Walker K, Musser AM, Archer M, Pian R, Vickers-Rich P (2016) The mandible and dentition of the Early Cretaceous monotreme Teinolophos trusleri. Alcheringa: 40:1–27
Rich TH, Piper KJ, Pickering D, Wright S (2006) Further Ektopodontidae (Phalangeroidea, Mammalia) from southwestern Victoria. Alcheringa 30(1):133–140
Rich TH, Vickers-Rich P, Constantine A, Flannery TF, Kool L, Van Klaveren N (1997) A tribosphenic mammal from the Mesozoic of Australia. Science 278(5342):1438–1442
Rich TH, Vickers-Rich P, Trusler P, Flannery TF, Cifelli R, Constantine A, Kool L, Van Klaveren N (2001) Monotreme nature of the Australian Early Cretaceous mammal Teinolophos. Acta Palaeontol Pol 46(1):113–118
Rodrigues PG, Ruf I, Schultz CL (2013) Digital reconstruction of the otic region and inner ear of the non-mammalian cynodont Brasilitherium riograndensis (Late Triassic, Brazil) and its relevance to the evolution of the mammalian ear. J Mammal Evol 20(4):291–307
Rougier GW, Martinelli AG, Forasiepi AM, Novacek MJ (2007) New Jurassic mammals from Patagonia, Argentina: a reappraisal of australosphenidan morphology and interrelationships. Am Mus Novitates 3566:1–54
Rougier GW, Wible JR, Novacek MJ (2004) New specimen of Deltatheroides cretacicus (Metatheria, Deltatheroida) from the Late Cretaceous of Mongolia. Bull Carnegie Mus Nat Hist 36:245–266
Rowe T (1986) Osteological diagnosis of Mammalia, L.1758, and its relationship to extinct Synapsida. PhD dissertation, University of California, Berkeley
Rowe T (1987) Definition and diagnosis in the phylogenetic system. Syst Zool 36(2):208–211
Rowe T (1988) Definition, diagnosis, and origin of Mammalia. J Vertebr Paleontol 8(3):241–264
Rowe T (1993) Phylogenetic systematics and the early history of mammals. In: Szalay FS, Novacek MJ, McKenna MC (eds) Mammal Phylogeny: Mesozoic Differentiation, Multituberculates, Monotremes, Early Therians, and Marsupials. Springer-Verlag, New York, pp 129–145
Rowe TB, Eiting TP, Macrini TE, Ketcham RA (2005) Organization of the olfactory and respiratory skeleton in the nose of the gray short-tailed opossum Monodelphis domestica. J Mammal Evol 12(3–4):303–336
Rowe TB, Macrini TE, Luo Z-X (2011) Fossil evidence on origin of the mammalian brain. Science 332(6032):958–960
Rowe TB, Rich TH, Vickers-Rich P, Springer M, Woodburne MO (2008) The oldest platypus and its bearing on divergence timing of the platypus and echidna clades. Proc Natl Acad Sci U S A 105(4):1238–1242
Sidor CA, Hancox PJ (2006) Elliotherium kersteni, a new tritheledontid from the lower Elliot Formation (Upper Triassic) of South Africa. J Paleontol 80(2):333–342
Starck D (1967) Le crâne des mammifères. In: Grassé PP (ed) Traité de Zoologie, Anatomie, Systématique, Biologie. Masson, Paris, Tome XVI, Fascicule 1, pp 405–549
Sues H-D (1986) The skull and dentition of two tritylodontid synapsids from the Lower Jurassic of western North America. Bull Mus Comp Zool 151(4):217–268
Wible JR (2003) On the cranial osteology of the short-tailed opossum Monodelphis brevicaudata (Didelphidae, Marsupialia). Ann Carnegie Museum 72(3):137–202
Wible JR, Hopson JA (1993) Basicranial evidence for early mammal phylogeny. In: Szalay FS, Novacek MJ, McKenna MC (eds) Mammal Phylogeny: Mesozoic Differentiation, Multituberculates, Monotremes, Early Therians and Marsupials. Springer-Verlag, New York, pp 45–62
Wible JR, Rougier GW (2000) Cranial anatomy of Kryptobaatar dashzevegi (Mammalia, Multituberculata), and its bearing on the evolution of mammalian characters. Bull Am Mus Nat Hist 247:1–124
Zeller U (1987) Morphogenesis of the mammalian skull with special reference to Tupaia. In: Kuhn HJ, Zeller U (eds) Morphogenesis of the Mammalian Skull. Mammalia Depicta. Verlag Paul Parey, Hamburg and Berlin, pp 17–50
Zeller U (1989) Die Entwicklung und Morphologie des Schädels von Ornithorhynchus anatinus (Mammalia: Prototheria: Monotremata). Abh senckenb naturforsch Ges 545:1–138
Zhou C-F, Wu S, Martin T, Luo Z-X (2013) A Jurassic mammaliaform and the earliest mammalian evolutionary adaptations. Nature 500(7461):163–167
Acknowledgements
Thanks to the Museum of Comparative Zoology and the Department of Organismic and Evolutionary Biology at Harvard for their support.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
Morganucodon watsoni (IVPP 8682) a, stereo of the anterior view of the braincase, with frontals, parietals and right petrosal omitted; b, stereo of the internal view of the right side of the braincase (with bones on the left side omitted) and interpretation (PDF 3726 kb)
ESM 2
Ornithorhynchus anatinus (AMNH 2013). Stereo view of the internal view of the right side of the braincase (PDF 2267 kb)
ESM 3
Ornithorhynchus anatinus (AMNH 2013). Video showing half rotation of the orbitosphenoids (shades of pink), presphenoid (purple), palatine (mauve), mesethmoid (green), and pterygoids (yellow orange), basisphenoid (red); followed by a completion of the rotation with additional bones added: anterior lamina, squamosal and parietal (shades of blue), maxilla (yellow), dentary, frontal, supraoccipital, basioccipital, exoccipital and petrosal, (shades of green) (MP4 8052 kb)
ESM 4
Stereo photographs of Vincelestes neuquenianus (MACN-N 04). a, partial view of the left side of the braincase; b. internal view of the left side of the braincase; c, ventral view of braincase; d, dorsal view of the braincase; e, horizontal section through the braincase to illustrate the relation of the orbital flanges of the palatine to the presphenoid/orbitosphenoid (PDF 5391 kb)
ESM 5
Probainognathus jenseni (MCZ 4280). Video showing rotation of the orbitosphenoid and fragment of the frontal (MPG 40415 kb)
ESM 6
Morganucodon watsoni (IVPP 8682). Video showing rotation of segmented bones of the skull (MPG 105554 kb)
ESM 7
Vincelestes neuquenianus (MACN-N05). Video showing rotation of the orbitosphenoids/presphenoid (pink), palatines (red), mesethmoid (light blue), and pterygoids (dark red), followed by rotation of the whole skull with the left side roof and zygomatic arch removed, also partially segmented (MP4 85,647 kb)
Rights and permissions
About this article
Cite this article
Crompton, A., Musinsky, C., Rougier, G. et al. Origin of the Lateral Wall of the Mammalian Skull: Fossils, Monotremes and Therians Revisited. J Mammal Evol 25, 301–313 (2018). https://doi.org/10.1007/s10914-017-9388-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10914-017-9388-7