Abstract
Following the global invasion of Drosophila suzukii (spotted-wing drosophila or SWD), nearly 100 studies have explored biological control of this pest. In 2019, a review summarized 75+ papers covering 57 species of SWD parasitoids, predators, competitors, and pathogens and identified the most promising ones. This review provides an update with recent studies. Since parasitoids are promising natural enemies that can be host-specific and self-disperse, this chapter focuses on SWD parasitoids in its invaded and native ranges, and prospects for classical biological control. To date, six species have been confirmed to attack SWD in the invaded regions including three widely studied generalist pupal parasitoids, Pachycrepoideus vindemiae, Trichopria drosophilae, and T. anastrephae. No locally occurring larval drosophila parasitoids can develop from SWD. In contrast, foreign explorations in China, Japan, and South Korea have revealed 19 species of SWD larval parasitoids. Asobara japonica, Ganaspis brasiliensis, and Leptopilina japonica spp. japonica have been evaluated. Ganaspis brasiliensis is a complex of cryptic species/strains with varying host specificity, some which also occur in regions outside of Asia, but one East Asian strain was found to be the most host-specific to SWD and is currently being petitioned for introduction into North America and Europe.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Drosophila suzukii Matsumura (Diptera: Drosophilidae) (spotted-wing drosophila or SWD) is an invasive pest in North and South America and Europe that threatens the small fruit and cherry industry (Asplen et al. 2015). Although chemical insecticides are effective, this has increased management costs (Farnsworth et al. 2017), led to resistance development (Gress and Zalom 2019), loss of markets (Haviland and Beers 2012), and impacted natural enemies (Whitehouse et al. 2018). Long-term sustainable management relies on effective biological control as well as cultural and behavioral controls. To develop biological control of invasive pests, the classical approach is to use natural enemies that are native to their countries of origin. Introduction of these specialist parasitoids has historically been preferred for controlling exotic pests because these parasitoids are generally more efficient in targeting hosts due to their long-shared history of co-adaption. However, some indigenous generalist parasitoids can adapt to exotic hosts, and such novel interactions may also play an important role in regulating the exotic pests. It is therefore important to evaluate the impacts of indigenous natural enemies while considering imported specialist parasitoids for the management of invasive pests. This chapter review will primarily focus on parasitoids since they are generally more host-specific than predators and entomopathogens, but will also summarize recent work on predators, competitors, and pathogens of SWD since the last review of SWD biological control (Lee et al. 2019). Predators and competitors of SWD are already common in crops and can be further conserved to suppress SWD populations. Pathogens are often commercially available, and if delivered effectively, provide growers additional control options.
8.1 Parasitoids of Drosophila
Parasitoids clearly play an important role in regulating some Drosophila populations, with reported levels of parasitism as high as 80–100% in Europe (e.g., Fleury et al. 2009; Janssen et al. 1988). Over 50 hymenopteran species have been reported to attack frugivorous drosophilids worldwide, including members of Braconidae (Asobara, Aphaereta, Phaenocarpa, Tanycarpa, Aspilota, and Opius), Figitidae (Leptopilina, Ganaspis, Kleidotoma, and Dicerataspis), Diapriidae (Trichopria and Spilomicrus), and Pteromalidae (Pachycrepoideus, Spalangia, Trichomalopsis, and Toxomorpha) (Carton et al. 1986). So far, no drosophilid egg or adult parasitoids have been discovered. All known Drosophila parasitoids are solitary koinobiont larval endoparasitoids in the families of Braconidae and Figitidae (subfamily Eucoilinae), and solitary pupal parasitoids in the families of Pteromalidae and Diapriidae. Larval Drosophila parasitoids attack host larvae, but all emerge from the host puparium formed from the hardened exoskeleton of the fly’s last larval stage. Most known larval parasitoids belong to the genera Asobara, Leptopilina, and Ganaspis. Among them, Asobara tabida (Nees), Leptopilina boulardi Barbotin et al., and L. heterotoma (Thompson) are the three most extensively studied parasitoids attacking common drosophilids such as Drosophila melanogaster (Meigen) breeding in rotting fruit (reviewed in Prévost 2009). The pteromalids are ectoparasitoids that lay their eggs between the host’s puparium case and the pupa, while the diapriids are endoparasitoids. Pachycrepoideus vindemiae (Rondani) and Trichopria drosophilae Perkins are two of the most common and cosmopolitan pupal drosophila parasitoids (Wang et al. 2016a, b).
The parasitoid fauna associated with SWD was poorly understood prior to its worldwide invasion. Since then, a number of studies have discovered and identified effective parasitoids both in Asia and around the world (Daane et al. 2016; Giorgini et al. 2019; Girod et al. 2018b). An earlier review article discussed the practical application potential of parasitoids for a broader audience (Lee et al. 2019). Here, we present a comprehensive review on the complexes of parasitoid species attacking SWD worldwide. We summarize the evaluations for promising parasitoids, and the diversity, dominance, and host specificity of parasitoids native to Asia. Finally, we propose future research directions for promoting parasitoids for the control of SWD by classical, augmentative, or conservation biological control.
8.2 Impact of Parasitoids in the Invaded Ranges
Surveys of locally occurring parasitoids on SWD and closely related frugivorous Drosophila species have been conducted in the USA (Kamiyama et al. 2019; Miller et al. 2015), Mexico (Cancino et al. 2015), Brazil (Wollmann et al. 2016), Spain (Gabarra et al. 2015), France (Kremmer et al. 2017), Switzerland (Knoll et al. 2017), Italy (Mazzetto et al. 2016; Rossi Stacconi et al. 2013), Slovenia (Modic et al. 2019), and Turkey (Zengin and Karaca 2019). These surveys used sentinel traps baited with larval or pupal SWD or D melanogaster in fruit or artificial diet, and occasionally with collections of infested fruits. Exposed materials in the traps were often inevitably contaminated by other drosophilid species. Thus, some reported parasitoid species, especially L. boulardi, may need verification in laboratory tests to confirm the host-parasitoid association (e.g., Garcia-Cancino et al. 2015; Wollmann et al. 2016; Garrido et al. 2018). The three larval parasitoids (A. tabida, L. boulardi, and L. heterotoma) and two pupal parasitoids (P. vindemiae and T. drosophilae) were commonly collected in North America and Europe. These larval parasitoids were, however, exclusively reared from drosophilids other than SWD, whereas the pupal parasitoids were collected from both SWD and other drosophilid species (Table 8.1). In the literature, P. vindemiae is sometime mentioned as P. vindemmiae (e.g., Wollmann et al. 2016). According to Rossi Stacconi et al. (2013), this species was originally described as P. vindemiae by Rondani in 1875, and the latter name should be used thereafter. In several European reports, the Trichopria species was reported as T. cf. drosophilae (e.g., Chabert et al. 2012; Gabarra et al. 2015; Mazzetto et al. 2016). These specimens are likely conspecific to T. drosophilae as reported in other studies. Other pupal parasitoids that were collected from SWD-baited sentinel traps include the pteromalid Spalangia simplex Perkins in Mexico, and the diapriid Trichopria anastrephae Lima in Brazil (Table 8.1).
Various populations of A. tabida, L. boulardi, and L. heterotoma have been tested for their ability to attack and then develop from SWD under laboratory conditions (Table 8.2). To date, none of these larval parasitoids were able to complete development, except for a low percentage of development of L. heterotoma, using populations from northern Italy (Rossi-Stacconi et al. 2015) and France (Iacovone et al. 2018) (Table 8.2). The same larval parasitoid, however, successively parasitized D. melanogaster and other closely related drosophilids in parallel tests. The larval parasitoids’ immature stages failed to develop due to a strong cellular immune response by SWD, causing the fly larvae to increase hemocyte production to encapsulate the immature parasitoids inside the host (Chabert et al. 2012; Iacovone et al. 2018; Kacsoh and Schlenke 2012; Poyet et al. 2013). Nevertheless, this species would still oviposit in SWD larvae which significantly reduced survival of SWD by up to 90%. Variation in mortality was likely due to different experimental procedures with host-parasitoid ratios and exposure times, or geographic variations of resistance and virulence among populations (Kacsoh and Schlenke 2012). Within the SWD’s native range, L. heterotoma and L. boulardi have never been recorded from SWD (Daane et al. 2016; Giorgini et al. 2019; Ideo et al. 2008; Mitsui et al. 2007; Novkovic et al. 2011), while A. tabida has been collected from SWD in Japan possibly from a misidentification (Mitsui et al. 2007). Alternatively, some A. tabida populations in Japan and some L. heterotoma populations in Europe have locally adapted to SWD, explaining the reported parasitism by these species in those areas.
Pachycrepoideus vindemiae and T. drosophilae are the two most studied pupal SWD parasitoids. Although both species are cosmopolitan and sympatric in many regions, P. vindemiae is more widely distributed than T. drosophilae (Knoll et al. 2017; Miller et al. 2015; Wang et al. 2018b). Pachycrepoideus vindemiae is more of a generalist than T. drosophilae, as the former species also attacks hosts in other families of cyclorrhaphous Diptera (Wang and Messing 2004), while T. drosophilae attacks only Drosophilidae (Carton et al. 1986). A lack of pupal immunity against parasitoids may explain why these pupal parasitoids have broader host ranges than larval parasitoid wasps (Kacsoh and Schlenke 2012). These two pupal parasitoids have been evaluated for their efficiency, host specificity, thermal tolerance, and interspecific interactions (Kaçar et al. 2017; Rossi-Stacconi et al. 2015; Rossi Stacconi et al. 2017; Wang et al. 2016a, b, 2018b; Zhu et al. 2017; Bezerra da Silva et al. 2019a, b). Both species can locate SWD pupae in fruit or soil, but T. drosophilae was more efficient than P. vindemiae at some temperatures (Garcia-Cancino et al. 2020; Kaçar et al. 2017; Wang et al. 2018b). At 23 °C, T. drosophilae females from California and South Korea populations survived 27.5 and 20.2 days, respectively, and produced a total of 63.8 and 52.0 offspring, whereas P. vindemiae females from a California population survived 21.5 days and produced 70.0 offspring (Rossi-Stacconi et al. 2015; Wang et al. 2016a). Pachycrepoideus vindemiae has a wider temperature range than T. drosophilae, which may explain the current distribution of these species in North America (Wang et al. 2018b). Interspecific competition between these two parasitoids may reduce the overall impact on the host population. Trichopria drosophilae seems to have an advantage over P. vindemiae in laboratory tests (Wang et al. 2016b). All other tested pupal parasitoids also readily developed from SWD in laboratory tests (Table 8.2). These include the pteromalids Vrestovia fidenas (Walker) and Spalangia erythromera Förster in Europe (Knoll et al. 2017; Mazzetto et al. 2016; Wolf et al. 2019), Muscidifurax raptorellus Girault & Sanders in Canada (Bonneau et al. 2019), and T. anastrephae in Brazil (Kruger et al. 2019; Vieira et al. 2020). All four parasitoids appear to have the potential to help in the control of SWD. Naturally occurring parasitism of SWD populations by pupal parasitoids is generally low (Table 8.1), but augmentative releases may allow them to be useful. In Italy, T. drosophilae was commercially available, and evaluated for its host location, dispersal, and host suppression capabilities in an augmentative release in netted raspberry fields (Rossi-Stacconi et al. 2018). The parasitoid was able to locate SWD in traps up to 40 m away from the release site, and SWD emergence was significantly reduced within a radius of 10 m of the release within netting environment. Recently, the effectiveness of this parasitoid has been evaluated in releases in unmanaged vegetation surrounding cherry orchards in Italy (Rossi Stacconi et al. 2019) and in commercial berry (Rubus fruticosus L.) crops in Mexico (Gonzalez-Cabrera et al. 2019). In Italy, weekly release of the parasitoid at a rate of 0.33 specimens/m2 for 7 weeks resulted in a 34% reduction in fruit infestation in the unmanaged vegetation surrounding orchards. In Mexico, semiweekly release of the parasitoid at a rate of 4.5 wasps/m2 for 50 weeks resulted a fourfold increase in parasitism and a 50% reduction of SWD in the field. Results from these studies suggest that augmentative release of T. drosophilae can suppress SWD populations in the unmanaged areas surrounding crops, thus lowering the severity of pest outbreaks in the crop (Rossi Stacconi et al. 2019). While no augmentative trials have been made with M. raptorellus, this pupal parasitoid is commonly sold for release in livestock operations, making releases in crops potentially feasible.
A population model predicts the optimal timing for releasing T. drosophilae against SWD would be between late spring and early summer when the host population begins to increase (Pfab et al. 2018). Early releases would help reduce fly populations that would likely move from overwintering unmanaged vegetation into early susceptible fruit crops, and at the same time, those released parasitoids would increase their population (Pfab et al. 2018). However, the timing of release will depend on geographical region. For example, in a warm temperate climate such as Mexico, SWD populations are active year-around, and sufficient pest suppression would require repeated augmentative releases (Gonzalez-Cabrera et al. 2019).
8.3 Exploration for Parasitoids in Asia
Exploration for parasitoids native to South Korea, China, and Japan have discovered at least 19 larval parasitoids associated with SWD, including 12 Asobara and 7 figitids (Table 8.1). In South Korea, eight species, Asobara japonica Belokobylskij, A. leveri (Nixon), A. brevicauda Guerrieri & van Achterberg, A. triangulata van Achterberg and Guerrieri, A. mesocauda van Achterberg and Guerrieri, Ganaspis brasiliensis Ihering, Leptopilina japonica Novković & Kimura, and L. j. formosana Novković & Kimura, and the pupal parasitoid T. drosophilae were collected from SWD and other Drosophilidae (Daane et al. 2016). Leptopilina japonica is further divided into the temperate subspecies (L. j. japonica, thereafter, referred to L. japonica) and the subtropical subspecies (L. j. formosana) (Novkovic et al. 2011). The larval parasitoid L. boulardi and the pupal parasitoid P. vindemiae were collected from other drosophilids. Asobara brevicauda, A. triangulata, and A. mesocauda are newly described species (Guerrieri et al. 2016). Parasitism of SWD by these larval parasitoids varied according to geography, season, and collection methods, ranging from 0 to 28.6% (Daane et al. 2016). Ganaspis brasiliensis and L. japonica were the major parasitoids found in fresh fruits infested by SWD, whereas A. japonica was the major parasitoid collected from fruit bait traps infested predominantly by other drosophilids (Daane et al. 2016). A total of 3266 and 20,358 Drosophila puparia were collected in 2013 and 2014, respectively, from a variety of locations, and A. japonica, G. brasiliensis, and L. japonica accounted for 85.7% of all larval parasitoids emerged (Daane et al. 2016). In 2016, a total of 11,575 SWD puparia were collected from several wild Rubus fruits, and G. brasiliensis and L. japonica accounted for 87.1% of total parasitoids emerged (Daane et al. 2016).
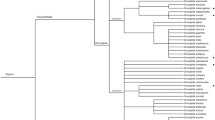
In China, Girod et al. (2018a) conducted surveys in the provinces of Yunnan, Jilin, Beijing, Hubei, and Sichuan by collecting wild and commercial fruits, and Giorgini et al. (2019) conducted surveys in Yunnan Province using banana-baited traps and wild fruit collections. Collected fruits were often co-infested by Drosophila. pulchrella Tan, Hsu & Sheng in Yunnan or by D. subpulchrella Takamori in other regions of China. These two species are also characterized by a serrated ovipositor, like SWD, that allows them to attack fresh fruits. Because the pupae of these three Drosophila spp. are indistinguishable, it was impossible to determine from which host the parasitoids emerged from (Giorgini et al. 2019; Girod et al. 2018a). At least nine larval parasitoids , A. leveri, A. mesocauda, A. unicolorata, A. pleuralis (Ashmead), Areotetes striatiferus Li, G. brasiliensis (or G. cf. brasiliensis), L. japonica, Tanycarpa chors Belokobylskij, and Leptopilina sp., and the pupal parasitoid T. drosophilae were collected in China. The most abundant and frequently collected larval parasitoids were G. brasiliensis and L. japonica. For example, Giorgini et al. (2019) collected a total of 11,683 SWD and D. pulchrella puparia from four wild host fruits (Rubus foliosus Weihe, R. niveus Thunberg, Fragaria moupinensis Cardot, and Sambucus adnate Wallich ex de Candolle) at four different locations during 2016 in Yunnan, China. The majority of emerged parasitoids were G. brasiliensis (63.7%) and L. japonica (33.2%), accounting for 97.1% of total parasitoids. These two parasitoids also accounted for 97.8% of all larval parasitoids emerged from 1792 D. suzukii and D. pulchrella puparia (Hoelmer et al. unpubl. data). The highest parasitism by G. brasiliensis was 47.8% and 42.0% by L. japonica in the 2016 collections in Yunnan, China (Giorgini et al. 2019). The banana traps yielded mainly other Drosophilidae (>99%) and seven Asobara species (primarily A. mesocauda) and six figitids (primarily L. japonica) as well as T. drosophilae and P. vindemiae. Only one A. japonica and one G. xanthopoda were collected, and G. brasiliensis was never collected from banana traps (Giorgini et al. 2019). The surveys showed that most flies emerging from fresh fruits were SWD or the closely related D. pulchrella and D. subpulchrella. This suggests that field collection of fresh fruits is a more reliable method to collect SWD parasitoids (Daane et al. 2016; Giorgini et al. 2019) (Fig. 8.1).
Composition of major Asian parasitoids (Asobara japonica, Ganaspis brasiliensis, and Leptopilina japonica) of frugivorous Drosophilidae collected from (a) fruit-baited traps or (b) via sampling of fresh fruits in South Korea and China during 2013–2018 (for other parasitoid species, see the list on Table 8.1—parasitoid complexes). Data were compiled based on Daane et al. (2016), Giorgini et al. (2019), and unpublished data from recent collections
In Japan, at least six larval parasitoids (A. japonica, A. tabida, L. japonica, G. xanthopoda, T. chors, and Asobara sp.) have been reported to parasitize SWD and other frugivorous Drosophila species (Girod et al. 2018a; Ideo et al. 2008; Kasuya et al. 2013; Mitsui and Kimura 2010; Mitsui et al. 2007; Novkovic et al. 2011). Ganaspis cf. brasiliensis was the most abundant parasitoid collected from SWD in wild fruits with parasitism of 75.6% reported in Nara, Japan (Girod et al. 2018a). Matsuura et al. (2018) showed that G. cf. brasiliensis attacked SWD larvae in fresh fruits in the tree canopy, but rarely in fruits fallen on the ground, suggesting a specific adaptation of a Japanese strain to SWD infesting fresh fruits. An Asobara sp. that was recorded only from SWD in wild fruits (Girod et al. 2018a; Ideo et al. 2008; Nomano et al. 2015) may be more specific; it was speculated to be A. triangulata based on molecular analysis of specimens (Guerrieri et al. 2016). Asobara japonica was the major parasitoid collected in banana traps throughout Japan (Mitsui et al. 2007). Populations of A. japonica in the main islands of Japan and South Korea seem to be parthenogenetic, whereas those in the south-western islands of Japan apparently reproduce sexually (Daane et al. 2016; Murata et al. 2009).
8.4 Prospects for Classical Biological Control
The Asian surveys suggest that G. brasiliensis, L. japonica, and A. japonica are the most dominant and widely distributed larval parasitoids attacking SWD (Fig. 8.1), whereas most other larval parasitoids showed a more restricted distribution and lower parasitism rates (8.1 and 8.2). These three larval parasitoids have been systematically evaluated for their efficiency, host specificity, climatic adaptability as well as potential interaction as classical biological control agents in North America and Europe (Biondi et al. 2017; Daane et al. 2016; Giorgini et al. 2019; Girod et al. 2018b, c; Wang et al. 2018a, 2019, 2020). All three parasitoids readily attack and develop from SWD (Table 8.2) and prefer to attack young host larvae (Wang et al. 2018a). At 23 °C, with SWD larvae in artificial diet, G. brasiliensis adult females survived 17.7 days and produced 98.3 offspring per female, and L. japonica survived 18.7 days and produced 107.2 offspring per female (Wang et al. 2018a), while A. japonica females lived 17.8 days and produced 117.3 offspring per female (Wang et al. unpubl. data). Leptopilina japonica eggs hatched the fastest, followed by A. japonica and then G. brasiliensis and consequently L. japonica outcompeted the other two parasitoids in multi-parasitized hosts (Wang et al. 2019). However, G. brasiliensis discriminated strongly against hosts parasitized by L. japonica, and A. japonica discriminated against hosts parasitized by L. japonica. The combined impacts on host suppression by L. japonica and G. brasiliensis were additive, likely due to the interspecific discrimination by G. brasiliensis. Indeed, both parasitoids coexist in all locations and plants sampled in China or South Korea (Daane et al. 2016; Giorgini et al. 2019), indicating they might synergistically improve the suppression of SWD.
Quarantine tests with a wide range of 24 different drosophila species showed that the South Korean and Yunnan G. brasiliensis populations developed from SWD and several other closely related hosts (D. melanogaster and D. simulans) but did not develop from more distant non-target drosophilid species (Giorgini et al. 2019). Asobara japonica developed from 19 of 24 tested host species, whereas L. japonica developed mainly from species in the melanogaster group (Daane et al. unpubl. data). By comparison, both P. vindemiae and T. drosophilae developed from all 24 tested drosophila species (Wang et al. unpubl. data). Other studies also showed that these two pupal parasitoids develop from nearly all tested hosts, preferentially attacking large hosts with correspondingly large progeny emerging (Chen et al. 2018; Wang et al. 2016a; Wolf et al. 2020; Yi et al. 2020). In Japan, field surveys and laboratory tests also found that A. japonica parasitized various indigenous and exotic drosophilid species (Ideo et al. 2008; Mitsui and Kimura 2010; Mitsui et al. 2007). In Switzerland, Girod et al. (2018b, c) tested six different European non-target fly species with these three larval parasitoids. Similarly, they found that A. japonica developed from all tested drosophilids, and L. japonica successfully parasitized D. melanogaster and D. subobscura. A Japanese population of G. cf. brasiliensis collected from SWD was strictly specific to SWD as reported by Kasuya et al. (2013), whereas another population from China parasitized SWD and D. melanogaster and sporadically parasitized D. subobscura. Thus, A. japonica is more of a generalist, whereas L. japonica appears to be a specialist on melanogaster species group. Currently, G. brasiliensis is considered as the first candidate for classical biological control of SWD due to its demonstrated specificity.
8.5 Diversity of the Ganaspis brasiliensis “Complex”
Buffington and Forshage (2016) first described G. brasiliensis as a new combination based on the specimens collected from SWD in South Korea (Daane et al. 2016) and historical specimens from the Neotropical region. Previously in Japan, Mitsui and Kimura (2010) reported that Ganaspis collected from Drosophila lutescens Okada readily parasitized D. lutescens and other drosophilids tested (>90% parasitism) but rarely accepted SWD (only 3.3% parasitism). These Ganaspis were initially assigned the name G. xanthopoda (Table 8.1). However, Kasuya et al. (2013) showed that SWD was the only drosophilid species infesting fresh wild cherries in Tokyo area, and Ganaspis individuals were the major parasitoids attacking SWD in wild cherry fruits. They reported that this Ganaspis population did not parasitize SWD in Drosophila medium and other Drosophila spp. in fresh cherries; and they identified the population as the D. suzukii-associated G. xanthopoda type. Ganaspis specimens previously assigned as G. xanthopoda are morphologically similar to specimens that were collected from South Korea and identified as G. brasiliensis by Buffington and Forshage (2016) and were thus reassigned to G. brasiliensis (Nomano et al. 2017).
Subsequent molecular analyses of different individuals based on nucleotide sequences of the mitochondrial cytochrome oxidase subunit 1 (CO1) gene , and the inter-transcribed spacers 1 and 2 (ITS1 and ITS2) suggest that individuals thus far morphologically identified as G. brasiliensis could be subdivided into five lineages (Nomano et al. 2017): G1, including individuals collected from SWD from Sendi and Tokyo in Japan; G2, including individuals from a subtropical Japanese island parasitizing Drosophila ficusphila Kikkawa & Peng; G3, including individuals from temperate regions of Japan and high mountains of Southeast Asia (Indonesia, Malaysia) parasitizing different species of Drosophila; G4, including individuals from Indonesia parasitizing Drosophila eugracilis Bock & Wheeler; G5, including individuals previously reported as G. xanthopoda or Ganaspis sp. from Thailand and the Philippines (Schilthuizen et al. 1998), Hawaii and Uganda (Kacsoh and Schlenke 2012), Indonesia (Kimura and Suwito 2012, 2015), Malaysia (Nomano et al. 2017), Benin, Puerto Rico and the Caribbean Sea (Carton et al. 1986), Brazil (Buffington and Forshage 2016), and Mexico (Gonzalez-Cabrera et al. 2020).
Phylogenetic analysis of COI sequences revealed that the G. brasiliensis specimens collected in Yunnan, China (Giorgini et al. 2019), consisted of 77% G1 and 23% G3. Similarly, the G. brasiliensis specimens collected in South Korea in 2017 (and similar sites reported in Daane et al. 2016) consisted of 65% G1 and 35% G3. These results suggest that these two lineages (G1 and G3) appear to be widely distributed in East Asia. They coexist in many locations and attack SWD and the closely related D. pulchrella and D. subpulchrella inhabiting fresh fruits, and have thus been considered sufficiently specific to SWD based on field collections and quarantine evaluations (Daane et al. 2016; Giorgini et al. 2019; Girod et al. 2018b, c; Kasuya et al. 2013). The host range of other lineages is unclear, and they have not been collected from SWD in fresh fruits nor tested in the laboratory with SWD, except that some G5 individuals from Hawaii and Uganda have a capacity to parasitize SWD in laboratory tests but with no or low development (Kacsoh and Schlenke 2012). Thus, G. brasiliensis appears to be a complex of several cryptic strains with varying host specificity and distributions. Given the Asian origin of SWD and the common ancestor of different lineages likely occurs in Asia, the species have likely been introduced to the Neotropics and Africa (Buffington and Forshage 2016; Nomano et al. 2017).
Recent studies further suggest that G1 (called G. cf. brasiliensis in Girod et al. 2018a) and G3 may be two different species. Reeve and Seehausen (2019) compared the acid-soluble insect protein spectra among three different G1 populations collected from Tokyo, Japan, and Dali and Ximing, China and a G1 population collected from Hasuike, Japan, and found that the G3 is significantly different from all G1 specimens. Other ongoing studies indicate the absence of positive crossing between G1 and G3, and different host-searching behaviors. G1 prefers hosts infesting fruits, whereas G3 prefers hosts in rotting substrates (M. Kenis, personal comm.). Further research combining multiple gene analyses and crossing-mating experiments across geographical populations or lineages is clearly needed to fully understand the ecological and genetic diversity of the G. brasiliensis complex.
8.6 Predicted Geographical Ranges of Ganaspis brasiliensis
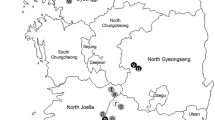
The CLIMEX model (Kriticos et al. 2015) has been used to predict the potential geographical range of G. brasiliensis based on the current known distribution of G1 and G3 lineages in Asia (Daane et al. unpubl. data). Geographical coordinates of 37 collection sites where parasitoids were found in China, South Korea, and Japan were obtained (Kasuya et al. 2013; Daane et al. 2016; Nomano et al. 2017; Matsuura et al. 2018; Giorgini et al. 2019). The model parameters were repetitively adjusted and the function “Compare location,” which describes the potential geographical distribution of species, as controlled by weather variables was subsequently run until the estimated potential G. brasiliensis range coincided best with the known distributions of the species in East Asia. The model predicted that G. brasiliensis would likely establish in the western, southeastern, and east coastal states in North America and most southern European countries where SWD is a major concern of small fruit crops (Fig. 8.2). Indeed, a recent survey in British Columbia, Canada, found that G1 has established in the Vancouver area, possibly through accidental introduction (P. Abram, personal comm.). It remains to be discovered whether G. brasiliensis will be able to colonize all invaded regions by SWD or whether it will be limited by climatic constraints. A comparative study on thermal performance between two populations originally from Yunnan Province of China and Gyeongsangnam-do Province of South Korea revealed the occurrence of a facultative diapause in G. brasiliensis below 17.2 °C (Hougardy et al. 2019). This cold temperature response varied between the populations: South Korean populations entered diapause at 17.2 °C, whereas only a proportion of its Chinese counterpart entered diapause at the same temperature. This suggests that some populations could be a better match for colder climates, or that mixing of populations from different origins could increase plasticity in response to cold seasons.
8.7 Future Directions with Parasitoids
Biological control using parasitoids could be a key component of areawide management programs for SWD by reducing fly populations at the landscape level. To date, three resident pupal parasitoids (P. vindemiae, T. drosophilae, and T. anastrephae) and one Asian larval parasitoid species or species complex (G. brasiliensis or G. cf. brasiliensis) have been identified as potentially promising biological control agents for SWD. A petition for release of the Asian G. brasiliensis in North America and Europe has been submitted, and a regulatory decision is currently pending. The resident pupal parasitoids already adapted to local ecological conditions and which can readily attack SWD could be manipulated either through conservation or augmentation to contribute to SWD suppression. However, the most effective and permanent biological control will likely be achieved by the introduction and augmentation of G. brasiliensis.
Future studies may include (1) the genetic improvement of natural enemies by selecting biological traits among different populations for selection or breeding that are important for effective biological control (Kruitwagen et al. 2018); (2) developing optimal rearing and release strategies for promising parasitoids to maximize establishment potential in different regions; (3) developing strategies to reduce the impacts of non-target control measures such as selective pesticides or cultural management (Cossentine and Ayyanath 2017; Schlesener et al. 2019), (4) introducing different geographic G. brasiliensis strains that are adapted to different climate zones within invaded regions (Hougardy et al. 2019); and if necessary (5) exploration, importation, and evaluation of additional Asian larval parasitoids (such as the unidentified Asobara sp. TK1) that appear to be specific to SWD (Guerrieri et al. 2016; Nomano et al. 2015).
8.8 Predators
Since the 2019 review, earwigs, green lacewings, mirids, and stink bugs have been identified as potential predators. The European earwig, Forficula auricularia L. (Dermaptera, Forficulidae), readily consumed larval and pupal stages of SWD, but could not effectively catch adults in laboratory studies (Englert and Herz 2019). Similarly, F. auricularia reduced the emergence of SWD adults by 45% when confined to infested cherries in a growth chamber, and the reduction was likely due to predation on developing SWD and not removal of parental SWD (Bourne et al. 2019). Green lacewing larvae, Chrysoperla carnea (Stephens) (Neuroptera: Chrysopidae), have reduced emergence of SWD from infested raspberries by 32% (Bonneau et al. 2019), and from infested cherries by 33% (Englert and Herz 2019). The mirid Dicyphushesperus (Knight) (Heteroptera: Miridae) preyed on exposed SWD eggs, and the stink bug Podisus maculiventris (Say) (Hemiptera: Pentatomidae) preyed on exposed larvae that were placed on leaves (Bonneau et al. 2019). Recent studies with minute pirate bugs further support previous work, as Orius insdiosus (Say) (Hemiptera: Anthocoridae) reduced emergence from infested fruit by 49% (Bonneau et al. 2019), and O. majusculus (Reuter) (Hemiptera: Anthocoridae) reduced emergence by 31% (Englert and Herz 2019).
Previous assessments of field predation have revealed 61–100% removal of sentinel pupae on or below the soil surface, and a 19–49% reduction in emerging SWD from infested fruit in fields in Oregon and Maine, USA (Ballman et al. 2017; Woltz and Lee 2017). A recent survey in organic raspberry fields in Wisconsin documented 1–4% predation on sentinel pupae (Kamiyama et al. 2019). Therefore, actual predation levels can vary from field to field depending on the conditions. Predation has also been assessed by molecular analysis as first demonstrated by Wolf et al. (2018) which surveyed predators in organic farms in Germany. In Georgia blueberries, 0.4% of the 1600 collected predators tested positive for predation by molecular analysis (Schmidt et al. 2019). These included hunting spiders, a web-building spider, and one mantid. Both studies using molecular surveys reveal that generalist spiders prey on SWD.
To date, most work on parasitoids and predators has focused on field surveys, measuring natural predation and parasitism rates, or studied the efficacy of agents in enclosed arenas or field releases. More work remains to be done on conserving these natural enemies, and whether specific habitat manipulations will benefit natural enemies and increase SWD control. A landscape-level analysis of blueberry fields revealed that organic systems and fields with vegetation between rows harbored more natural enemies (Schmidt et al. 2019). In their analysis, landscapes with greater composition of non-crop habitats also had higher SWD populations. This may be expected since SWD reproduces on many different wild hosts (Kenis et al. 2016; Lee et al. 2015), and SWD can move from wild fruit to nearby crops (Leach et al. 2018).
8.9 Entomopathogens
In 2019–2020, more studies were published on entomopathogenic nematodes than other pathogens. The recently discovered Oscheius onirici Torrini et al. (Nematoda: Rhabditidae) was sprayed on infested blueberries, reducing pupation by 78% in laboratory trials (Foye and Steffan 2020). Contrary to previous reports (Garriga et al. 2017; Hübner et al. 2017), SWD pupae have appeared to be more susceptible to nematode infections than larvae. For example, Heterorhabditis bacteriophora (Poinar) (Nematoda: Heterorhabditidae) and Steinernema feltiae (Filipjev) (Nematoda: Steinernematidae) caused 72% mortality among SWD pupae, and 20% mortality among larvae in Petri dish assays (Ibouh et al. 2019). Newly tested Heterorhabditis amazonensis (Andalo) and H. indica (Poinar) (Nematoda: Heterorhabditidae), as well as S. carpocapsae (Weiser) and S. feltiae, caused 35, 26, 13, and 43% mortality among SWD pupae, respectively (Brida et al. 2019).
Additional work has supported the effectiveness of S. carpocapsae, including recent assays with adult SWD. Adults exposed to S. carpocapsae had an infection rate of 65% compared to 4% by S. feltiae and H. bacteriophora (Garriga et al. 2020b). Moreover, when soil with buried SWD pupae was treated with S. carpocapsae, 89% of emerging adults were infected. Teneral adults may be especially vulnerable to infection, and 59% could not move up their plastic cylinder arena. In lab arenas, 21% of infected adults were able to fly, and the authors suggested that this may help with nematode dispersion. As with any pathogen, infected hosts can have defensive responses, and studies of SWD larvae infected with S. carpocapsae and its symbiont bacteria Xenorhabdus nematophila Thomas & Poinar revealed that the pathogen avoided cellular defenses and depressed humoral responses (Garriga et al. 2020a).
The fungal pathogens Beauveria bassiana (Bals.) Vuill and Metarhizium anisopliae (Metch.) Sorok. have been the most widely studied (reviewed in Lee et al. 2019). Recent work has shown them to cause 38% mortality of larvae, and 32–64% of adults when sprayed on SWD in Petri dishes (Ibouh et a. 2019). Interestingly, when grape berries were dipped in fungal suspensions, oviposition by SWD was reduced by 80% compared to the controls. Thus, while fungal treatments may not always directly contact adults when sprayed in the field, and require several days to induce adult mortality, the sprays provide additional protection to the fruit. Assays conducted by Ibouh et al. (2019) exposed flies to grapes for 5 days under standard laboratory conditions, and the duration of fruit protection has still to be determined.
To find bacterial pathogens, Hiebert et al. (2020) collected SWD from infested fruits in the field, isolated, and screened the associated bacteria. Seven isolates were detrimental including the Gram-positive bacteria Brevibacterium frigoritolerans Delaporte & Sasson, Bacillus simplex (exMeyer and Gottheil), Bacillus altitudinis Schivaji et al., Leuconostoc pseudomesenteroides Farrow et al., Paenibacillus dongdonensis Son et al. and Paenibacillus odorifer, and the Gam-negative bacterium Tatumella terrea (Kageyama et al.). The mode of action was explored; Paenibacillus dongdonensis and L. pseudomesenteroides appeared to reduce food uptake in SWD larvae.
8.10 Competitors
Previous laboratory and greenhouse work has shown Drosophila melanogaster to be a promising competitor of SWD. The presence of D. melanogaster is not expected to pose a threat to harvested fruit since it attacks overripe or damaged fruit and could foreseeably compete with SWD during the late season when dropped fruit remains on the ground. The African fig fly, Zaprionus indianus (Gupta), was recently shown to compete with SWD in grapes in laboratory studies, and induce higher SWD mortality (Shrader et al. 2020). Zaprionus indianus generally does not lay eggs in intact fruit but can use the oviposition sites of SWD to lay eggs (Bernardi et al. 2017). Whether co-infestations occur often in the field or could be advantageous for IPM remains to be studied.
8.11 Compatibility of Biological Control
Recent work has investigated the compatibility of biological controls with other control approaches, especially with pesticides commonly used in SWD management. Organophosphates, pyrethroids, and neonicotinoids cause high mortality in the parasitoids T. anastrephae and P. vindemiae in lab bioassays (Schlesener et al. 2019). Spinosad is a commonly used organic insecticide which is unfortunately detrimental to P. vindemiae adults, and female wasps are unable to avoid treated SWD pupae (Cossentine and Ayyanath 2017). The same study also determined that the larval stage of P. vindemiae was susceptible to spinosad when SWD pupae were treated 1 week post-parasitization, but they survived better at the pupal stage when treated 2 weeks post-parasitization. A variety of organic insecticides were tested on two generalist predators of SWD; the green lacewing Chrysoperla rufilabris (Burmeister) was susceptible to spinosad, and the minute pirate bug Orius insidiosus was susceptible to fresh and aged residues of spinosad and sabadilla alkaloids (Sarkar et al. 2019). Moreover, sublethal effects of insecticide exposure resulted in reduced egg hatch of O. insidiosus.
With the variety of pathogens being tested for SWD control, more work is needed to assess compatibility of pathogens with predators and parasitoids, especially if releases are anticipated. Recently, T. drosophilae was unaffected when parasitized SWD pupae were exposed to treatments of B. bassiana, M. anisopliae, H. bacteriophora, or S. feltiae and parasitoid emergence was subsequently monitored in the laboratory (Ibouh et al. 2019). Likewise, adults of T. drosophilae and rove beetle, D. coriaria (Kraatz), were unaffected by H. bacteriophora, S. feltiae, and S. carpocapsae in Petri dish assays (Garriga et al. 2019). However, the predator O. laevigatus (Fieber) experienced reduced survival from exposure to S. carpocapsae in Petri dish assays but not when nematodes were applied to a plant. This suggests that this predator would escape harmful effects in a field situation. Mulching and floor management have been examined as cultural practices to control SWD (Rendon et al. 2020; Rendon and Walton 2019), and specifically target SWD as they often wander to pupate in the soil (Woltz and Lee 2017). Such ground practices to make the soil less hospitable to SWD may however be incompatible with soil drench treatments with nematodes where a moist soil environment is necessary for infective juveniles to survive and find hosts.
8.12 Summary
Many researchers have been dedicated to advancing biological control of SWD as demonstrated by the nearly 100 publications at the time of writing this review. A longer-term approach relies on importing the parasitoid, Ganapsis brasiliensis, to invaded regions. With this parasitoid, there is a need to: breed more effective traits, develop efficient rearing and release strategies, and use geographic strains adapted to various climates. A variety of endemic predators in the field prey on SWD. Augmentative releases of predators have not yet been recommended since their cost-effectiveness and efficacy need determination. As for pathogens, new nematode species have been tested in the laboratory, and nematodes can affect teneral SWD adults as they emerge from treated soil. Moreover, several nematode and fungal pathogens appear to be compatible with common SWD parasitoids and predators. This is promising since many commonly used insecticides for SWD are harmful to these parasitoids and predators. Since most pathogen research has been conducted in the laboratory, field trials are required to develop recommendations. As more information becomes available with biological control agents, additional work is needed to integrate them into SWD management programs.
References
Asplen MK, Anfora G, Biondi A et al (2015) Invasion biology of spotted wing drosophila (Drosophila suzukii): a global perspective and future priorities. J Pest Sci 88:469–494. https://doi.org/10.1007/s10340-015-0681-z
Ballman ES, Collins JA, Drummond FA (2017) Pupation behavior and predation on Drosophila suzukii (Diptera: Drosophilidae) pupae in Maine wild blueberry fields. J Econ Entomol 110:2308–2317. https://doi.org/10.1093/jee/tox233
Bernardi D, Andreazza F, Botton M et al (2017) Susceptibility and interactions of Drosophila suzukii and Zaprionus indianus (Diptera: Drosophilidae) in damaging strawberry. Neotrop Entomol 46:1–7. https://doi.org/10.1007/s13744-016-0423-9
Bezerra da Silva CS, Price BE, Soohoo-Hui A et al (2019a) Factors affecting the biology of Pachycrepoideus vindemmiae (Hymenoptera: Pteromalidae), a parasitoid of spotted-wing drosophila (Drosophila suzukii). PLoS One 14:19326203
Bezerra da Silva CH, Price BE, Walton VM (2019b) Water-deprived parasitic wasps (Pachycrepoideus vindemmiae) kill more pupae of a pest (Drosophila suzukii) as a water-intake strategy. Sci Rep 9:3592
Biondi A, Wang XG, Miller JC et al (2017) Innate olfactory responses of Asobara japonica toward fruits infested by the invasive spotted wing drosophila. J Insect Behav 30:495–506. https://doi.org/10.1007/s10905-017-9636-y
Bonneau P, Renkema J, Fournier V et al (2019) Ability of Muscidifurax raptorellus and other parasitoids and predators to control Drosophila suzukii populations in raspberries in the laboratory. Insects 10:68. https://doi.org/10.3390/insects10030068
Bourne A, Fountain MT, Wijnen H, Shaw B (2019) Potential of the European earwig (Forficula auricularia) as a biocontrol agent of the soft and stone fruit pest Drosophila suzukii. Pest Manag Sci 75:3340–3345
Brida A, Wilcken SRS, Garrigós Leite L et al (2019) Virulence of entomopathogenic nematode to pupae and adults of Drosophila suzukii in laboratory. Revista Científica Rural 21:126–138. https://doi.org/10.30945/rcr-v21i2.2725
Buffington ML, Forshage M (2016) Redescription of Ganaspis brasiliensis (Ihering, 1905), new combination (Hymenoptera: Figitidae), a natural enemy of the invasive Drosophila suzukii (Matsumura, 1931) (Diptera: Drosophilidae). Proc Entomol Soc Wash 118:1–13. https://doi.org/10.4289/0013-8797.118.1.1
Cancino MDG, Hernandez AG, Cabrera JG et al (2015) Parasitoids of Drosophila suzukii (Matsumura) (Diptera: Drosophilidae) in Colima, Mexico. Southwest Entomol 40:855–858. https://doi.org/10.3958/059.040.0418
Carton Y, Boulétreau B, van Alphen JJM et al(1986) The Drosophila parasitic wasps. In: Ashburner M, Carson HL, Thompson JN (eds) The genetics and biology of Drosophila, vol 3e. Academic, London, pp. 347–394
Chabert S, Allemand R, Poyet M et al (2012) Ability of European parasitoids (Hymenoptera) to control a new invasive Asiatic pest, Drosophila suzukii. Biol Control 63:40–47. https://doi.org/10.1016/j.biocontrol.2012.05.005
Chen JN, Zhou SC, Wang Y et al (2018) Biocontrol characteristics of the fruit fly pupal parasitoid Trichopria drosophilae (Hymenoptera: Diapriidae) emerging from different hosts. Sci Rep 8:e13323. https://doi.org/10.1038/s41598-018-31718-6
Cossentine JE, Ayyanath MM (2017) Limited protection of the parasitoid Pachycrepoideus vindemiae from Drosophila suzukii host-directed spinosad suppression. Entomol Exp Appl 164:78–86. https://doi.org/10.1111/eea.12592
Daane KM, Wang XG, Biondi A et al (2016) First exploration of parasitoids of Drosophila suzukii in South Korea as potential classical biological agents. J Pest Sci 89:823–835. https://doi.org/10.1007/s10340-016-0740-0
Dancau T, Stemberger TLM, Clarke P, Gillespie DR (2017) Can competition be superior to parasitism for biological control? The case of spotted wing Drosophila (Drosophila suzukii), Drosophila melanogaster and Pachycrepoideus vindemmiae. Biocontrol Sci Tech 27:3–16
Englert C, Herz A (2019) Acceptability of Drosophila suzukii as prey for common predators occurring in cherries and berries. J Appl Entomol 143:387–396. https://doi.org/10.1111/jen.12613
Farnsworth D, Hamby KA, Bolda M et al (2017) Economic analysis of revenue losses and control costs associated with the spotted wing drosophila, Drosophila suzukii (Matsumura), in the California raspberry industry. Pest Manag Sci 73:1083–1090. https://doi.org/10.1002/ps.4497
Fleury F, Gibert P, Ris N et al (2009) Ecology and life history evolution of frugivorous Drosophila parasitoids. In: Prévost G (ed) Advances in Parasitology: Parasitoids of Drosophila, Advances in parasitology, vol 70. Academic, London, pp 3–44. https://doi.org/10.1016/s0065-308x(09)70001-6
Foye S, Steffan SA (2020) A rare, recently discovered nematode, Oscheius onirici (Rhabditida: Rhabditidae), kills Drosophila suzukii (Diptera: Drosophilidae) within fruit. J Econ Entomol 113:1047–1051. https://doi.org/10.1093/jee/toz365
Gabarra R, Riudavets J, Rodríguez GA et al (2015) Prospects for the biological control of Drosophila suzukii. Biocontrol 60:331–339
Garcia-Cancino MD, Gonzalez-Cabrera J, Sanchez-Gonzalez JA et al (2020) Biological and population parameters, as well as oviposition preference, of two pupal parasitoids of Drosophila suzukii (Diptera: Drosophilidae) in Mexico. J Entomol Sci 55:87–97. https://doi.org/10.18474/0749-8004-55.1.87
Garrido SA, Cichón LI, Lago JD et al (2018) First record of Leptopilina boulardi (Hymenoptera: Figitidae) associated to Drosophila suzukii (Diptera: Drosophilidae) in Alto Valle of Río Negro and Neuquén, Patagonia, Argentina. Revista de la Sociedad Entomológica Argentina 77:22–27
Garriga A, Morton A, Garcia-del-Pino F (2017) Is Drosophila suzukii as susceptible to entomopathogenic nematodes as Drosophila melanogaster? J Pest Sci 91:789–798. https://doi.org/10.1007/s10340-017-0920-6
Garriga A, Morton A, García-López D, García-del-Pino F (2019) Compatibility of entomopathogenic nematodes with natural enemies for horticultural pest control. Biol Control 138:104050
Garriga A, Mastore M, Morton A et al (2020a) Immune response of Drosophila suzukii larvae to infection with the nematobacterial complex Steinernema carpocapsae-Xenorhabdus nematophila. Insects 11:210. https://doi.org/10.3390/insects11040210
Garriga A, Morton A, Ribes A, Garcia-del-Pino F (2020b) Soil emergence of Drosophila suzukii adults: a susceptible period for entomopathogenic nematodes infection. J Pest Sci 93(2):639–646
Giorgini M, Wang XG, Wang Y et al (2019) Exploration for native parasitoids of Drosophila suzukii in China reveals a diversity of parasitoid species and narrow host range of the dominant parasitoid. J Pest Sci 92:509–522. https://doi.org/10.1007/s10340-018-01068-3
Girod P, Borowiec N, Buffington M et al (2018a) The parasitoid complex of D. suzukii and other fruit feeding Drosophila species in Asia. Sci Rep 8. https://doi.org/10.1038/s41598-018-29555-8
Girod P, Lierhmann O, Urvois T et al (2018b) Host specificity of Asian parasitoids for potential classical biological control of Drosophila suzukii. J Pest Sci 91:1241–1250. https://doi.org/10.1007/s10340-018-1003-z
Girod P, Rossignaud L, Haye T et al (2018c) Development of Asian parasitoids in larvae of Drosophila suzukii feeding on blueberry and artificial diet. J Appl Entomol 142:483–494. https://doi.org/10.1111/jen.12496
Gonzalez-Cabrera J, Moreno-Carrillo G, Sanchez-Gonzalez JA et al (2019) Single and combined release of Trichopria drosophilae (Hymenoptera: Diapriidae) to control Drosophila suzukii (Diptera: Drosophilidae). Neotrop Entomol 48:949–956. https://doi.org/10.1007/s13744-019-00707-3
Gonzalez-Cabrera J, Cordoba-Urtiz EG, Moreno-Carrillo G et al (2020) First report of the parasitoid Ganaspis brasiliensis Ihering (Hymenoptera: Figitidae) in Mexico. Entomol News 129:67–70. https://doi.org/10.3157/021.129.0110
Gress BE, Zalom FG (2019) Identification and risk assessment of spinosad resistance in a California population of Drosophila suzukii. Pest Manag Sci 75:1270–1276. https://doi.org/10.1002/ps.5240
Guerrieri E, Giorgini M, Cascone P et al (2016) Species diversity in the parasitoid genus Asobara (Hymenoptera: Braconidae) from the native area of the fruit fly pest Drosophila suzukii (Diptera: Drosophilidae). PLoS One 11. https://doi.org/10.1371/journal.pone.0147382
Haviland DR, Beers EH (2012) Chemical control programs for Drosophila suzukii that comply with international limitations on pesticide residues for exported sweet cherries. J Integr Pest Manag 3:1. https://doi.org/10.1603/ipm11034
Haro-Barchin E, Scheper J, Ganuza C, De Groot GA, Colombari F, van Kats R, Kleijn D (2018) Landscape-scale forest cover increases the abundance of Drosophila suzukii and parasitoid wasps. Basic Appl Ecol 31:33–43
Hiebert N, Carrau T, Bartling M et al (2020) Identification of entomopathogenic bacteria associated with the invasive pest Drosophila suzukii in infested areas of Germany. J Invertebr Pathol 173:107389. https://doi.org/10.1016/j.jip.2020.107389
Hougardy E, BN Hogg, X Wang, Daane KM (2019) Comparsion of thermal performances of two Asian larval parasitoids of Droophila suzukii. Biol Control 136:10400
Hübner A, Englert C, Herz A (2017) Effect of entomopathogenic nematodes on different developmental stages of Drosophila suzukii in and outside fruits. Biocontrol 62:669–680. https://doi.org/10.1007/s10526-017-9832-x
Iacovone A, Ris N, Poirie M et al (2018) Time-course analysis of Drosophila suzukii interaction with endoparasitoid wasps evidences a delayed encapsulation response compared to D. melanogaster. PLoS One 13:e0201573. https://doi.org/10.1371/journal.pone.0201573
Ibouh K, Oreste M, Bubici G et al (2019) Biological control of Drosophila suzukii: Efficacy of parasitoids, entomopathogenic fungi, nematodes and deterrents of oviposition in laboratory assays. Crop Prot 125:104897. https://doi.org/10.1016/j.cropro.2019.104897
Ideo S, Watada M, Mitsui H et al (2008) Host range of Asobara japonica (Hymenoptera : Braconidae), a larval parasitoid of drosophilid flies. Entomol Sci 11:1–6. https://doi.org/10.1111/j.1479-8298.2007.00244.x
Janssen A, Driessen G, Dehaan M et al (1988) The impact of parasitoids on natural populations of temperate woodland Drosophila. Neth J Zool 38:61–73
Kaçar G, Wang XG, Biondi A et al (2017) Linear functional response by two pupal Drosophila parasitoids foraging within single or multiple patch environments. PLoS One 12:e0183525. https://doi.org/10.1371/journal.pone.0183525
Kacsoh BZ, Schlenke TA (2012) High hemocyte load is associated with increased resistance against parasitoids in Drosophila suzukii, a relative of D. melanogaster. PLoS One 7:e34721. https://doi.org/10.1371/journal.pone.0034721
Kamiyama MT, Schreiner Z, Guedot C (2019) Diversity and abundance of natural enemies of Drosophila suzukii in Wisconsin, USA fruit farms. Biocontrol 64:665–676. https://doi.org/10.1007/s10526-019-09966-w
Kasuya N, Mitsui H, Ideo S et al (2013) Ecological, morphological and molecular studies on Ganaspis individuals (Hymenoptera: Figitidae) attacking Drosophila suzukii (Diptera: Drosophilidae). Appl Entomol Zool 48:87–92. https://doi.org/10.1007/s13355-012-0156-0
Kenis M, Tonina L, Eschen R et al (2016) Non-crop plants used as hosts by Drosophila suzukii in Europe. J Pest Sci 89:735–748. https://doi.org/10.1007/s10340-016-0755-6
Kimura MT, Suwito A (2012) Diversity and abundance of frugivorous drosophilids and their parasitoids in Bogor, Indonesia. J Nat Hist 46:1947–1957. https://doi.org/10.1080/00222933.2012.707239
Kimura MT, Suwito A (2015) Altitudinal patterns of abundances and parasitism in frugivorous drosophilids in west Java, Indonesia. J Nat Hist 49:1627–1639. https://doi.org/10.1080/00222933.2015.1005709
Knoll V, Ellenbroek T, Romeis J et al (2017) Seasonal and regional presence of hymenopteran parasitoids of Drosophila in Switzerland and their ability to parasitize the invasive Drosophila suzukii. Sci Rep 7:e40697. https://doi.org/10.1038/srep40697
Kremmer L, Thaon M, Borowiec N et al (2017) Field monitoring of Drosophila suzukii and associated communities in south eastern France as a pre-requisite for classical biological control. Insects 8:124. https://doi.org/10.3390/insects8040124
Kriticos DJ, Maywald GF, Yonow T et al (2015) CLIMEX Version 4: Exploring the effects of climate on plants, animals and diseases. CSIRO, Canberra, 156 pp
Kruger AP, Scheunemann T, Vieira JGA et al (2019) Effects of extrinsic, intraspecific competition and host deprivation on the biology of Trichopria anastrephae (Hymenoptera: Diapriidae) reared on Drosophila suzukii (Diptera: Drosophilidae). Neotrop Entomol 48:957. https://doi.org/10.1007/s13744-019-00705-5
Kruitwagen A, Beukeboom LW, Wertheim B (2018) Optimization of native biocontrol agents, with parasitoids of the invasive pest Drosophila suzukii as an example. Evol Appl 11:1473–1497. https://doi.org/10.1111/eva.12648
Leach H, Hagler JR, Machtley SA et al (2018) Spotted wing drosophila (Drosophila suzukii) utilization and dispersal from the wild host Asian bush honeysuckle (Lonicera spp.). Agri For Entomol 21:149–158. https://doi.org/10.1111/afe.12315
Lee JC, Dreves AM, Cave AM et al (2015) Infestation of wild and ornamental noncrop fruits by Drosophila suzukii (Diptera: Drosophilidae). Ann Entomol Soc Am 108:117–129. https://doi.org/10.1093/aesa/sau014
Lee JC, Wang XG, Daane KM et al (2019) Biological control of spotted-wing drosophila (Diptera: Drosophilidae): current and pending tactics. J Integr Pest Manag 10:13. https://doi.org/10.1093/jipm/pmz012
Matsuura A, Mitsui H, Kimura MT (2018) A preliminary study on distributions and oviposition sites of Drosophila suzukii (Diptera: Drosophilidae) and its parasitoids on wild cherry tree in Tokyo, central Japan. Appl Entomol Zool 53:47–53. https://doi.org/10.1007/s13355-017-0527-7
Mazzetto F, Marchetti E, Amiresmaeili N et al (2016) Drosophila parasitoids in northern Italy and their potential to attack the exotic pest Drosophila suzukii. J Pest Sci 89:837–850. https://doi.org/10.1007/s10340-016-0746-7
Miller B, Anfora G, Buffington M et al (2015) Seasonal occurrence of resident parasitoids associated with Drosophila suzukii in two small fruit production regions of Italy and the USA. Bull Insect 68:255–263
Mitsui H, Kimura MT (2010) Distribution, abundance and host association of two parasitoid species attacking frugivorous drosophilid larvae in central Japan. Eur J Entomol 107:535–540
Mitsui H, Van Achterberg K, Nordlander G et al (2007) Geographical distributions and host associations of larval parasitoids of frugivorous Drosophilidae in Japan. J Nat Hist 41:1731–1738. https://doi.org/10.1080/00222930701504797
Modic S, Žigon P, Razinger J (2019) Trichopria drosophilae (Diapriidae) and Leptopilina heterotoma (Figitidae), native parasitoids of Drosophila suzukii, confirmed in Slovenia. Acta Agri Slovenica 113:181–185
Murata Y, Ideo S, Watada M et al (2009) Genetic and physiological variation among sexual and parthenogenetic populations of Asobara japonica (Hymenoptera: Braconidae), a larval parasitoid of drosophilid flies. Eur J Entomol 106:171–178. https://doi.org/10.14411/eje.2009.020
Nomano FY, Mitsui H, Kimura MT (2015) Capacity of Japanese Asobara species (Hymenoptera; Braconidae) to parasitize a fruit pest Drosophila suzukii (Diptera; Drosophilidae). J Appl Entomol 139:105–113. https://doi.org/10.1111/jen.12141
Nomano FY, Kasuya N, Matsuura A et al (2017) Genetic differentiation of Ganaspis brasiliensis (Hymenoptera: Figitidae) from East and Southeast Asia. Appl Entomol Zool 52:429–437. https://doi.org/10.1007/s13355-017-0493-0
Novkovic B, Mitsui H, Suwito A et al (2011) Taxonomy and phylogeny of Leptopilina species (Hymenoptera: Cynipoidea: Figitidae) attacking frugivorous drosophilid flies in Japan, with description of three new species. Entomol Sci 14:333–346. https://doi.org/10.1111/j.1479-8298.2011.00459.x
Pfab F, Rossi-Stacconi MV, Anfora G et al (2018) Optimized timing of parasitoid release: a mathematical model for biological control of Drosophila suzukii. Theor Ecol 11:489–501. https://doi.org/10.1007/s12080-018-0382-3
Poyet M, Havard S, Prevost G et al (2013) Resistance of Drosophila suzukii to the larval parasitoids Leptopilina heterotoma and Asobara japonica is related to haemocyte load. Physiol Entomol 38:45–53. https://doi.org/10.1111/phen.12002
Prévost G (2009) Advances in parasitology, Parasitoids of Drosophila, vol 70. Academic, London
Rendon D, Walton VM (2019) Drip and overhead sprinkler irrigation in blueberry as cultural control for Drosophila suzukii (Diptera: Drosophilidae) in Northwestern United States. J Econ Entomol 112:745–752. https://doi.org/10.1093/jee/toy395
Rendon D, Hamby KA, Arsenault-Benoit AL et al (2020) Mulching as a cultural control strategy for Drosophila suzukii in blueberry. Pest Manag Sci 76:55–66. https://doi.org/10.1002/ps.5512
Reeve MA, Seehausen ML (2019) Discrimination between Asian populations of the parasitoid wasp Ganaspis cf. brasiliensis using a simple MALDI-TOF MS-based method for use with insects. Biology Methods and Protocols 4:bpz002. https://doi.org/10.1093/biomethods/bpz002
Rossi Stacconi MV, Grassi A, Dalton DT et al (2013) First field records of Pachycrepoideus vindemiae as a parasitoid of Drosophila suzukii in European and Oregon small fruit production areas. Entomologia 1:1–15
Rossi Stacconi MV, Panel A, Baser N et al (2017) Comparative life history traits of indigenous Italian parasitoids of Drosophila suzukii and their effectiveness at different temperatures. Biol Control 112:20–27. https://doi.org/10.1016/j.biocontrol.2017.06.003
Rossi Stacconi MV, Grassi A, Ioriatti C et al (2019) Augmentative releases of Trichopria drosophilae for the suppression of early season Drosophila suzukii populations. Biocontrol 64:9–19. https://doi.org/10.1007/s10526-018-09914-0
Rossi-Stacconi MV, Buffington M, Daane KM et al (2015) Host stage preference, efficacy and fecundity of parasitoids attacking Drosophila suzukii in newly invaded areas. Biol Control 84:28–35. https://doi.org/10.1016/j.biocontrol.2015.02.003
Rossi-Stacconi MV, Amiresmaeili N, Biondi A et al (2018) Host location and dispersal ability of the cosmopolitan parasitoid Trichopria drosophilae released to control the invasive spotted wing drosophila. Biol Control 117:188–196. https://doi.org/10.1016/j.biocontrol.2017.11.013
Sarkar N, Rhodes EM, Spies J et al (2019) Evaluation of non-target effects of OMRI-listed insecticides for management of Drosophila suzukii Matsumura in berry crops. J Appl Entomol 144:12–25. https://doi.org/10.1111/jen.12713
Schilthuizen M, Nordlander G, Stouthamer R et al (1998) Morphological and molecular phylogenetics in the genus Leptopilina (Hymenoptera: Cynipoidea: Eucoilidae). Syst Entomol 23:253–264. https://doi.org/10.1046/j.1365-3113.1998.00049.x
Schlesener DCH, Wollmann J, Pazini JB et al (2019) Insecticide toxicity to Drosophila suzukii (Diptera: Drosophilidae) parasitoids: Trichopria anastrephae (Hymenoptera: Diapriidae) and Pachycrepoideus vindemmiae (Hymenoptera: Pteromalidae). J Econ Entomol 112:1197–1206. https://doi.org/10.1093/jee/toz033
Schmidt JM, Whitehouse TS, Green K et al (2019) Local and landscape-scale heterogeneity shape spotted wing drosophila (Drosophila suzukii) activity and natural enemy abundance: implications for trophic interactions. Agri Eco Environ 272:86–94. https://doi.org/10.1016/j.agee.2018.11.014
Shrader M, Burrack HJ, Pfeiffer D (2020) Effects of interspecific larval competition on developmental parameters in nutrient sources between Drosophila suzukii (Diptera: Drosophilidae) and Zaprionus indianus. J Econ Entomol 113:230–238. https://doi.org/10.1093/jee/toz297
Vieira JGA, Kruger AP, Scheuneumann T et al (2020) Some aspects of the biology of Trichopria anastrephae (Hymenoptera: Diapriidae), a resident parasitoid attacking Drosophila suzukii (Diptera: Drosophilidae) in Brazil. J Econ Entomol 113:81–87. https://doi.org/10.1093/jee/toz270
Wang XG, Messing RH (2004) The ectoparasitic pupal parasitoid, Pachycrepoideus vindemmiae (Hymenoptera: Pteromalidae), attacks other primary tephritid fruit fly parasitoids: host expansion and potential non-target impact. Biol Control 31:227–236. https://doi.org/10.1016/j.biocontrol.2004.04.019
Wang XG, Kaçar G, Biondi A et al (2016a) Foraging efficiency and outcomes of interactions of two pupal parasitoids attacking the invasive spotted wing drosophila. Biol Control 96:64–71. https://doi.org/10.1016/j.biocontrol.2016.02.004
Wang XG, Kaçar G, Biondi A et al (2016b) Life-history and host preference of Trichopria drosophilae, a pupal parasitoid of spotted wing drosophila. Biocontrol 61:387–397. https://doi.org/10.1007/s10526-016-9720-9
Wang XG, Nance AH, Jones JML et al (2018a) Aspects of the biology and reproductive strategy of two Asian larval parasitoids evaluated for classical biological control of Drosophila suzukii. Biol Control 121:58–65. https://doi.org/10.1016/j.biocontrol.2018.02.010
Wang XG, Serrato MA, Son Y et al (2018b) Thermal performance of two indigenous pupal parasitoids attacking the invasive Drosophila suzukii (Diptera: Drosophilidae). Environ Entomol 47:764–772. https://doi.org/10.1093/ee/nvy053
Wang XG, Hogg BN, Hougardy E et al (2019) Potential competitive outcomes among three solitary larval endoparasitoids as candidate agents for classical biological control of Drosophila suzukii. Biol Control 130:18–26
Wang XG, Biondi A, Daane KM (2020) Functional responses of three candidate Asian larval parasitoids evaluated for classical biological control of Drosophila suzukii. J Econ Entomol 113:73–80
Whitehouse TS, Sial AA, Schmidt JM (2018) Natural enemy abundance in southeastern blueberry agroecosystems: distance to edge and impact of management practices. Environ Entomol 47:32–38. https://doi.org/10.1093/ee/nvx188
Wolf S, Zeisler C, Sint D et al (2018) A simple and cost-effective molecular method to track predation on Drosophila suzukii in the field. J Pest Sci 91:927–935. https://doi.org/10.1007/s10340-017-0948-7
Wolf S, Baur H, Collatz J (2019) Life history of Vrestovia fidenas, a potential control agent of Drosophila suzukii. Biocontrol 64:263–275. https://doi.org/10.1007/s10526-019-09933-5
Wolf S, Boycheva-Woltering S, Romeis J et al (2020) Trichopria drosophilae parasitizes Drosophila suzukii in seven common non-crop fruits. J Pest Sci 93:627–638. https://doi.org/10.1007/s10340-019-01180-y
Wollmann J, Schlesener DCH, Ferreira MS et al (2016) Parasitoids of Drosophilidae with potential for parasitism on Drosophila suzukii in Brazil. Drosophila Inform Serv 99:38–42
Woltz JM, Lee JC (2017) Pupation behavior and larval and pupal biocontrol of Drosophila suzukii in the field. Biol Control 110:62–69. https://doi.org/10.1016/j.biocontrol.2017.04.007
Yi CD, Cai PM, Lin J et al (2020) Life history and host preference of Trichopria drosophilae from Southern China, one of the effective pupal parasitoids on the Drosophila species. Insects 11:103. https://doi.org/10.3390/insects11020103
Zengin E, Karaca I (2019) Dynamics of trapped adult populations of Drosophila suzukii Matsumura (Diptera: Drosophilidae) and its parasitoids in Uak Province, Turkey. Egypt J Biol Pest Control 29. https://doi.org/10.1186/s41938-019-0147-3
Zhu CJ, Li J, Wang H et al (2017) Demographic potential of the pupal parasitoid Trichopria drosophilae (Hymenoptera: Diapriidae) reared on Drosophila suzukii (Diptera: Drosophilidae). J Asia Pac Entomol 20:747–751. https://doi.org/10.1016/j.aspen.2017.04.008
Acknowledgments
Funding was provided by the National Institute of Food and Agriculture, USDA Specialty Crops Research Initiative under Agreement No. 2015-51181-24252, USDA Organic Research and Extension Initiative under agreement No. 2018-51300-28434, the California Cherry Board, and the University of California Agricultural and Natural Research Grant, and base funds USDA CRIS 8010-22000-028-00D and 2072-22000-040-00D.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Wang, X., Daane, K.M., Hoelmer, K.A., Lee, J.C. (2020). Biological Control of Spotted-Wing Drosophila: An Update on Promising Agents. In: Garcia, F.R.M. (eds) Drosophila suzukii Management. Springer, Cham. https://doi.org/10.1007/978-3-030-62692-1_8
Download citation
DOI: https://doi.org/10.1007/978-3-030-62692-1_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-62691-4
Online ISBN: 978-3-030-62692-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)