Abstract
Drosophila suzukii (Matsumura) has been recently detected causing damage to strawberries in Brazil. Infestation in strawberry culture has often been observed jointly with the presence of Zaprionus indianus Gupta. This study investigated the susceptibility of strawberries at three ripening stages to infestation of D. suzukii and Z. indianus and their interaction. In the laboratory, strawberries cv. Albion at different ripening stages (green, semi-ripe and ripe) were exposed to D. suzukii and Z. indianus for 24 h in choice and no-choice bioassays. Additionally, we evaluated the effects of mechanical damage incurred artificially or by D. suzukii oviposition on Z. indianus infestation. In no-choice bioassay, there were no significant differences in fruit susceptibility to D. suzukii infestation at different ripening stages. However, in choice bioassay, D. suzukii adults preferred to oviposit on R fruit. The presence of mechanical damage did not increase susceptibility of fruit to D. suzukii oviposition. For Z. indianus, there was greater susceptibility of R fruit in relation to SR and G fruit in both the choice and no-choice bioassays. There was a significant and positive interaction of mechanical damage and damage caused by D. suzukii to R fruit and infestation by Z. indianus, which was not observed in SR and G fruit. Although infestation of Z. indianus is related to attack damaged or decaying fruit, this work shows that this species has the ability to oviposit and develop in healthy strawberry fruit with and increased infestation level when the fruit has damage to its epidermis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The spotted wing drosophila (SWD), Drosophila suzukii (Matsumura) (Diptera: Drosophilidae), is one of the major pests associated with the cultivation of small fruits worldwide (Walsh et al 2011, Cini et al 2012, Santos 2014, Asplen et al 2015). This is attributed to its high polyphagia (Dreves et al 2009), rapid population growth (Tochen et al 2014) and dispersion capacity (Walsh et al 2011, Cini et al 2012). This species has spread rapidly across North America, Europe (Walsh et al 2011, Asplen et al 2015, Lee et al 2015) and South America (Deprá et al 2014, Santos 2014), where it has caused significant economic losses in fruit orchards, mainly in small fruits such as blackberry, cherry, raspberry, blueberry and strawberry (Goodhue et al 2011, Bellamy et al 2013, Santos 2014, De Ros et al 2015, Ioriatti et al 2015, Lee et al 2015). It has also recently been reported that D. suzukii has caused economic damage and significant losses in strawberry crops in southern Brazil (Santos 2014).
Fruit damage and economic losses are caused by D. suzukii females, which have a serrated ovipositor with the ability to lay eggs inside healthy ripe fruit (Walsh et al 2011, Cini et al 2012, Lee et al 2015). Injuries caused by external piercing and/or oviposition allow pathogens to penetrate, increasing the losses (Dreves et al 2009, Bolda et al 2010) in addition to causing the release of volatiles (Abraham et al 2015) that attract other drosophilid species such as Zaprionus indianus Gupta (Diptera: Drosophilidae) (Van Timmeren & Isaacs 2013, Joshi et al 2014, Lasa & Tadeo 2015).
The African fig fly, Z. indianus, is considered the primary pest associated with fig culture worldwide (Raga et al 2003, Commar et al 2012), with occurrences in Brazil (Vilela et al 1999), Uruguay (Goní et al 2001), Central America (Van Der Linde et al 2006), North America (Joshi et al 2014, Lasa & Tadeo 2015), Europe (Commar et al 2012) and Asia (Fartyal et al 2014). In figs, females oviposit on the fruit ostiole base and damage is caused by larvae when they penetrate the fruit tissue (Vilela et al 1999). However, Z. indianus is considered a secondary pest to more than 70 other fruit species due to its tendency to attack and feed only on decaying fruit (Joshi et al 2014). This is related to the inability of females to oviposit on ripe fruit without prior injuries or the presence of mechanical damage caused by other insect pests (Fartyal et al 2014).
Due to their high capacity for adaptation to different hosts in temperate regions (Ramniwas et al 2012), several recent studies have reported the occurrence of both Z. indianus and D. suzukii in grape cultivation in the United States (Van Timmeren & Isaacs 2013, Joshi et al 2014), in monitoring traps containing the hydrolysed protein CeraTrap™ in guava culture in Mexico (Lasa & Tadeo 2015) and, directly, in ripe strawberry fruit in southern Brazil (Nava et al 2015). Due to the simultaneous occurrence of D. suzukii and Z. indianus in commercial strawberry crops in southern Brazil (Andreazza et al 2015, Nava et al 2015), there is a need to i) verify the susceptibility of strawberry fruit at different ripening stages to infestation by D. suzukii and Z. indianus to support the management of these pests in the field and ii) determine the interactions between damage caused by oviposition of D. suzukii and by mechanical means on infestation by Z. indianus in strawberry fruit at different ripening stages.
Material and Methods
Insect populations and obtaining fruits
Populations of D. suzukii and Z. indianus were reared in the laboratory on an artificial diet based on corn flour, yeast and sugar (Emiljanowicz et al 2014) and banana and brewer’s yeast (Nava et al 2007). Strawberry fruits of the cultivar Albion at different ripening stages (G- Green, SR – Semi-ripe and R – Ripe) with stems still to attached to the fruits were obtained from a commercial orchard. The strawberry plants were grown under plastic sheeting constructed as a ‘shallow tunnel’ without the application of insecticides during cultivation. The color determination was performed by subjective method based on the intensity of color variations perceptible to the human eye, according to the methodology proposed by Oliveira et al (2005). In the laboratory, the fruit were analysed for pH (AOAC 2005) and total soluble solids (TSS) using the methodology of Pregnolatto (1985). G fruit showed 100% green coloration, pH 3.1 and TSS 6.75 (average weight per fruit was 12.8 g); SR fruit had up to 30% red coloration, pH 3.6 and TSS 7.54 (average weight per fruit was 14.5 g); and R fruit showed 100% red coloration, pH 3.5 and TSS 7.96 (average weight per fruit was 14.3 g). The three fruit types were used to carry out choice and no-choice bioassays at the Laboratory of Entomology of Embrapa Clima Temperado, Pelotas, Rio Grande do Sul State, Brazil, in controlled room conditions of 25 ± 1°C, 60 ± 10% of RH and a 14:10 h (light:dark) photoperiod.
No-choice and choice bioassays
In the first step, we performed in no-choice bioassay. In the laboratory, G, SR and R strawberry fruit were examined under a stereo microscope (40x) to evaluate skin integrity and absence of eggs. Later, the fruit were individually placed in cages composed of transparent plastic cups (300 mL) flipped upside-down on a Petri dish (8 cm diameter) with a 4 m diameter hole cut in the top and sealed with fabric mesh to allow gas exchange and avoiding excess of moisture. Four mated females of D. suzukii or Z. indianus that were 4-5 days old were released into each cage, and distilled water was supplied to the adults via capillarity in cotton wool in 10 mL glass vials. Twenty-four hours after infestation (HAI), the flies were removed and eggs on the fruit (external surface + internal epidermis) were counted with the aid of a stereo microscope (40x). The fruits were then individually placed on a vermiculite layer (1 cm) inside plastic containers (100 mL) that were sealed at the top with Parafilm™ (Bemis Company, Inc.). The plastic containers were assessed daily to quantify fly emergence. The biological variables evaluated per fruit were number of eggs, number of adults and mean developmental time.
In the second step, we performed in choice bioassay. Strawberry fruits at the different ripening stages described above were examined for the presence of lesions and drosophilid eggs. Later, one G, one SR and one R fruit (ratio 1:1:1) were placed in each cage made of a plastic cup (700 mL, 15 cm in diameter) flipped upside-down on a Petri dish (18 cm diameter) with a 4 cm diameter hole at the top and sealed with ‘mesh’ fabric to allow gas exchange and avoid excess moisture. Four mated females of D. suzukii or Z. indianus that were 4-5 days old were placed in each cage and given distilled water. At 24 h after infestation, the flies were removed; from that moment forward, the same methodology as in the no-choice bioassay was followed.
Damage interaction of infestation by D. suzukii and Z. indianus on strawberry fruit
Two bioassays were conducted to determine whether mechanical damage (injuries) to the fruit epidermis facilitated infestation by D. suzukii or Z. indianus and whether damage caused by D. suzukii during oviposition facilitated infestation by Z. indianus. In the first bioassay, G, SR and R fruits were collected in the field and were damaged mechanically in the laboratory (20 perforations in the epidermis at a depth of 2 mm) with the aid of an entomological pin (0.25 mm). Afterwards, the fruits were submitted to infestation by Z. indianus following the same methodologies as previously described.
In the second bioassay, to determine whether injuries caused by D. suzukii facilitated infestation by Z. indianus, intact G, SR and R fruit were collected in the field over the same period as the first bioassay. In the laboratory, the fruits were offered to mated females of D. suzukii (four mated females/cage) that were 4-5 days old and were given distilled water for 24 h following the same procedures as previously described. Afterwards, the fruits were again placed inside the cages, and four mated females of Z. indianus that were 4-5 days old were released inside the cages for 24 h. Fruits were then removed, and eggs of Z. indianus were counted with the aid of a stereo microscope (40x) following the same methodologies as those described above.
Statistical Analysis
In all experiments (no-choice and choice bioassays and damage interaction) the experimental design was completely randomized with 50 fruits (G, SR or R) with each one considered replication per treatment. For the statistical analysis, all data were submitted to a studentized residual analysis to confirm the assumption of normality (Shapiro-Wilk test) using the PROC UNIVARIATE procedure in SAS® 9.1 (SAS Institute 2002). When data did not exhibit a normal distribution they were arcsine square root (x+0.5) transformed prior to the analysis. Afterwards, data were submitted to the variance analysis and the means were compared by the Tukey test (P ≤ 0.05) (PROC ANOVA, SAS Institute 2002).
Results
Strawberry fruit susceptibility to infestation by D. suzukii and Z. Indianus
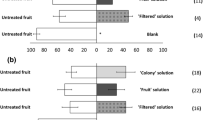
The no-choice bioassays showed no significant differences (F 2, 147 = 3.69, P = 0.07) in susceptibility of strawberry fruit at different ripening stages to infestation by D. suzukii in terms of average number of eggs and adults insects per fruit/female (Fig 1). The choice bioassays showed a greater susceptibility to infestation in strawberry R fruit (F 2, 147 = 112.69, P < 0.0001) compared with SR and G fruit (Fig 1).
Number of Drosophila suzukii eggs and adults emerged per fruit/female (mean ± SE) in strawberry fruit cv. Albion at different ripening stages in choice and no-choice bioassays. Note: Different letters indicate significant differences within each group of bars representing the different combinations of insect developmental stage and bioassay type, according to the Tukey test (P < 0.05).
For Z. indianus, there was an oviposition preference for R fruit in both the no-choice (F 2, 147 = 499.25, P < 0.0001) and choice bioassays in relation to SR and G fruit (Fig 2). No eggs or emerged insects were observed for the SR and G fruits (Fig 2). The mean developmental times (egg to adult) were 11.2 ± 0.07 for D. suzukii (Table 1) and 14.1 ± 0.11 days for Z. indianus (Table 2), with viabilities (egg to adults) greater than 80% for D. suzukii and 70% for Z. indianus.
Number of Zaprionus indianus eggs and adults emerged per fruit/female (mean ± SE) in strawberry fruit cv. Albion at different ripening stages in choice and no-choice bioassays. Note: Different letters indicate significant differences within each group of bars representing the different combinations of insect developmental stage and bioassay type, according to the Tukey test (P < 0.05).
Damage interaction of infestation by D. suzukii and Z. indianus on strawberry fruit
There were no significant interactions for mechanical damage to the epidermis of G (F 1, 98 = 1.18, P = 0.30), SR (F 1, 98 = 0.27, P = 0.07) or R fruit (F 1, 98 = 3.42, P = 0.07) and the susceptibility to infestation by D. suzukii compared to healthy fruits (Table 1). However, there was a significant (F 2, 144 = 4.83, P = 0.02) interaction of damage caused by oviposition by D. suzukii or mechanical damage to the epidermis of R fruit and infestation by Z. indianus compared to healthy R fruit (Table 2). In the G fruit, this interaction was not significant (F 2, 147 = 1.69, P = 0.12) in regarding to damage of either type compared to healthy G fruit without damage (Table 2). However, in the SR fruit, eggs were observed in the epidermis of fruits damaged mechanically. This result was significantly different (F 2, 144 = 36.92, P < 0.0001) from those obtained for undamaged SR fruit or fruit damaged by D. suzukii, for which no eggs or subsequent adults insects were observed (Table 2). Regarding the period of larval development of Z. indianus, there were no significant differences (F 2, 147 = 4.11, P = 0.17) between larvae that developed on R fruit without damage compared to fruits damaged by D. suzukii or by mechanically damaged fruit (Table 2). For all bioassays, we did not observe eggs or adults insects of D. suzukii or Z. indianus adults in G, SR and R fruit obtained from the field, showing that the fruit were free from the presence of these drosophilids.
Discussion
Oviposition of D. suzukii females occurred during the 24 h period in strawberry G, SR and R fruit in the no-choice bioassay, with no significant differences between the different stages of ripeness. In contrast, in the choice bioassay, females of D. suzukii showed a greater preference for oviposition on R fruits, corroborating previous studies reporting a higher oviposition rate of D. suzukii on ripe fruits of blackberry, blueberry, cherry, raspberry and strawberry (Lee et al 2011) and blueberry with lower skin firmness (Kinjo et al 2013).
Damage caused by D. suzukii is related to the cutting of the fruit skin by the ovipositor of the female and consumption of pulp by the developing larvae (Dreves et al 2009, Bolda et al 2010, Renkema et al 2013). These results and our data confirm that they do not require prior injuries in strawberry to deposit their eggs. However, the piercing of the fruit skin epidermis by mechanical damage or other insects may promote contamination and development of pathogens (Dreves et al 2009, Bolda et al 2010) and can promote infestation by other opportunistic drosophilid (Faucher et al 2013).
In this study, R fruits with previous damage caused by D. suzukii adults or artificial mechanical damage facilitated infestation by Z. indianus. We observed the presence of Z. indianus eggs inside the areas of damage caused by both D. suzukii females and mechanical means, showing the opportunistic ability of Z. indianus adults to infest damaged fruit. However, healthy R strawberry fruit also serve as a substrate for oviposition of Z. indianus because eggs were laid in the strawberry achene region or on the fruit epidermis, and nearly 1.5 days after oviposition, the larvae hatched and penetrated the fruit. This shows the ability of females to oviposit on healthy R strawberry fruit and generate viable off spring, which did not occur in SR and G fruit.
The ability of females to oviposit on healthy R strawberry fruit could be associated with the attraction of D. suzukii and Z. indianus adults to odours released by ripe fruit, as observed in D. suzukii adults and R fruits of raspberry, blackberry, blueberry, cherry and strawberry (Ramniwas et al 2012). In the present study, the higher number of eggs and adults of Z. indianus found in artificially damaged fruits or previously injured by D. suzukii females can be associated with a greater release of volatiles serving as stimuli for orientation, attraction and oviposition. Several studies have shown that insects use volatiles released by plants or fruits for attraction (Bruce & Pickett 2011, Von Arx et al 2011, Revadi et al 2015), orientation in the search for new hosts for feeding (Lebreton et al 2012, Faucher et al 2013) and oviposition (Linz et al 2013). Fact that may have occurred in the present study in which Z. indianus females showed preference for mature fruits or damaged artificially or female of D. suzukii. As well as by the fact that females showed no preference for oviposition on intact SR and G fruit or damaged by D. suzukii (Burrack et al 2013).
The ability of Z. indianus to oviposit and generate offspring in healthy R strawberry fruit and benefit from injuries caused by D. suzukii or mechanical injuries in R strawberries may contribute significantly to the increase in the incidence of Z. indianus in commercial strawberry fields. Similar observations have been done in grape orchards in the United States (Van Timmeren & Isaacs 2013), in sweet orange (Citrus sinensis L.) and guava (Psidium guajava) cultivation in India (Fartyal et al 2014) and Mexico (Lasa & Tadeo 2015), and cattle guava (Psidium cattleianum Sabine), Surinam cherry (Eugenia uniflora L.) and guava fruit in southern Brazil (Andreazza et al 2015). The results from this study help to better understand the behaviour of D. suzukii and Z. indianus in strawberry crop, allowing for the adoption and improvement of management strategies targeting both species.
References
Abraham J, Zhang A, Angeli S, Abubeker S, Michel C, Feng Y, Rodriguez-Saona C (2015) Behavioral and antennal responses of Drosophila suzukii (Diptera: Drosophilidae) to volatiles from fruit extracts. Environ Entomol 44:356–367
Andreazza F, Bernardi D, Botton M, Nava DE (2015) Índice de infestação natural de Drosophila suzukii e Zaprionus indianus (Diptera: Drosophilidae) em frutíferas nativas no município de pelotas. In: XXIV Congresso de Iniciação Científica e XVII Encontro da Pós-Graduação, 2015, Pelotas. Anais… Pelotas: Universidade Federal de Pelotas, Pelotas, Rio Grande do Sul, Brazil, 4p.
Aoac (Association of Official Analytical Chemists) (2005) Official Method of Analysis. 18thed. Washington, D.C.
Arx V, Schmidt-Busser MD, Guerin PM (2011) Host plant volatiles induce oriented flight behaviour in male European grapevine moths, Lobesia botrana. J Insect Physiol 57:1323–1331
Asplen MK, Anfora G, Biondi A, Choi D-S, Chu D, Daane KM, Gibert P, Gutierrez AP, Hoelmer KA, Hutchison WD, Isaacs R, Jiang ZL, Kárpáti Z, Kimura MT, Pascual M, Philips CR, Plantamp C, Ponti L, Vétek G, Vogt H, Walton VM, Yu Y, Zappála L, Desneux N (2015) Invasion biology of spotted wing Drosophila (Drosophila suzukii): a global perspective and future priorities. J Pest Sci 88:469–494
Bellamy DE, Sisterson MS, Walse SS (2013) Quantifying host potentials: indexing postharvest fresh fruits for spotted wing Drosophila, Drosophila suzukii. PLoS ONE 8:e61227
Bolda MP, Goodhue RE, Zalom FG (2010) Spotted wing drosophila: potential economic impact of newly established pest. Agric Resour Econ Update 13:5–8
Bruce TJA, Pickett JA (2011) Perception of plant volatile blends by herbivorous insects–finding the right mix. Phytochemistry 72:1605–1611
Burrack HJ, Fernandez GE, Spivey T, Kraus DA (2013) Variation in selection and utilization of host crops in the field and laboratory by Drosophila suzukii Matsumura (Diptera: Drosophilidae), an invasive frugivore. Pest Manag Sci 69:1173–1180
Cini A, Ioriatti C, Anfora G (2012) A review of the invasion of Drosophila suzukii in Europe and a draft research agenda for integrated pest management. Bull Insectoly 65:149–160
Commar LS, Galego LGD, Ceron CR, Carareto CMA (2012) Taxonomic and evolutionary analysis of Zaprionus indianus and its colonization of Palearctic and Neotropical regions. Genet Mol Biol 35:395–406
De Ros G, Conci S, Pantezzi T, Savini G (2015) The economic impact of invasive pest Drosophila suzukii on berry production in the Province of Trento, Italy. J Berry Res 5:89–96
Deprá M, Poppe JL, Schmitz HJ, Toni DC, Valente VLS (2014) The first records of the invasive pest Drosophila suzukii in the South American continent. J Pest Sci 87:379–383
Dreves AJ, Walton V, Fisher GA (2009) A new pest attacking healthy ripening fruit in Oregon. Spotted Wing Drosophila: Drosophila suzukii (Matsumura). EM 8991 October 2009. Oregon State University, Extension Service.
Emiljanowicz LM, Ryan GD, Langille A, Newman J (2014) Development, reproductive output and population growth of the fruit fly pest Drosophila suzukii (Diptera: Drosophilidae) on artificial diet. J Econ Entomol 107:1392–1398
Fartyal RS, Sarswat M, Lhamo N, Sati PC, Asha L (2014) Records of Zaprionus indianus and Drosophila suzukii indicus as invasive fruit pests from mid valley region of Garhwal Uttarakhand, India. Dros Inf Serv 97:119–123
Faucher CP, Hilker M, Bruyne M (2013) Interactions of carbon dioxide and food odours in Drosophila: Olfactory hedonics and sensory neuron properties. PLoS ONE 8:e56361
Goní B, Fresia P, Calvino M, Ferreiro MMJ, Valente VLS, Basso da Silva L (2001) First record of Zaprionus indianus Gupta, 1970 (Diptera: Drosophilidae) in southern localities of Uruguay. Dros Inf Serv 84:61–65
Goodhue RE, Bolda M, Farnsworth D, Williams JC, Zalom FG (2011) Spotted-wing drosophila infestation of California strawberries and raspberries: economic analysis of potential revenue losses and control costs. Pest Manag Sci 67:1396–1402
Ioriatti C, Walton V, Dalton D, Anfora G, Grassi A, Maistri S, Mazzoni V (2015) Drosophila suzukii (Diptera: Drosophilidae) and its potential impact to wine grapes during harvest in two cool climate wine grape production regions. J Econ Entomol 4:1–8
Joshi NK, Biddinger DJ, Demchak K, Deppen A (2014) First report of Zaprionus indianus (Diptera: Drosophilidae) in commercial fruits and vegetables in Pennsylvania. J Pest Sci 14:259–263
Kinjo H, Kunimi Y, Ban T, Nakai M (2013) Oviposition efficacy of Drosophila suzukii (Diptera: Drosophilidae) on different cultivars of blueberry. J Econ Entomol 106:1767–1771
Lasa R, Tadeo E (2015) Invasive drosophilid pests Drosophila suzukii and Zaprionus indianus (Diptera: Drosophilidae) in Veracruz, Mexico. Fla Entomol 98:987–988
Lebreton S, Becher PG, Hansson BS, Witzgall P (2012) Attraction of Drosophila melanogaster males to food-related and fly odours. J Insect Physiol 58:125–129
Lee JC, Bruck DJ, Dreves AJ, Ioriatti C, Vogt H, Baufeld P (2011) In focus: spotted wing Drosophila, Drosophila suzukii, across perspectives. Pest Manag Sci 67:1349–1351
Lee JC, Dreves AJ, Cave AM, Kawai S, Isaacs R, Miller JC, Van Timmeren S, Bruck DJ (2015) Infestation of wild and ornamental non crop fruits by Drosophila suzukii (Diptera: Drosophilidae). Ann Entomol Soc Am 3:1–13
Linz J, Baschwitz A, Strutz A, Dweck HKM, Sachse S, Hansson BS, Stensmyr MC (2013) Host plant driven sensory specialization in Drosophila erecta. Proc R Soc B 280:06–26
Nava DE, Nascimento AM, Stein CP, Haddad ML, Bento JMS, Parra JRP (2007) Biology, thermal requirements, and estimation of the number of generations of Zaprionus indianus (Diptera: Drosophilidae) for the main fig producing regions of Brazil. Fla Entomol 90:495–501
Nava DE, Botton M, Bernardi D, Andreazza F, Baronio CA (2015) Bioecologia, monitoramento e controle de Drosophila suzukii na cultura do morangueiro. Embrapa Clima Temperado, Pelotas, Rio Grande do Sul. 28p. (Série documentos 398).
Oliveira RP, Nino AFP, Scivittaro WB (2005) Mudas certificadas de morangueiro: maior produção e melhor qualidade da fruta. A lavoura 108:35–38
Pregnolatto W (1985) Normas analíticas do Instituto Adolfo Lutz: Métodos químico-físicos para análise de alimentos, 3rd edn. Instituto Adolfo Lutz. v, São Paulo, p 2
Raga A, Souza Filho MF, Sato ME (2003) Eficiência de protetores de ostíolo do figo sobre a infestação da mosca Zaprionus indianus (Gupta) (Diptera: Drosophilidae) no campo. Arq Inst Biol 70:287–289
Ramniwas S, Kajla B, Parkash R (2012) Extreme physiological tolerance leads the wide distribution of Zaprionus indianus (Diptera: Drosophilidae) in temperate world. Acta Entomol Sin 55:1295–1305
Renkema JM, Miller M, Fraser H, Légaré JPH, Hallett RH (2013) First records of Zaprionus indianus Gupta (Diptera: Drosophilidae) from commercial fruit fields in Ontario and Quebec, Canada. JESO 144:125–130
Revadi S, Vitagliano S, Stacconi RMV, Ramasamy S, Mansourian S, Carlin S, Vrhovsek U, Becher PG, Mazzoni V, Stabelli-Rota O, Angeli S, Dekker T, Gianfranco A (2015) Olfactory responses of Drosophila suzukii to host plant volatiles. Physiol Entomol 40:54–64
Santos RSS dos (2014) Ocorrência de Drosophila suzukii (Matsumura, 1931) (Diptera: Drosophilidae) atacando frutos de morango no Brasil. Embrapa Uva e Vinho, Bento Gonçalves, 4p. 2014. (Comunicado Técnico 159)
Sas Institute (2002) Statistical analysis system: getting 476 started with the SAS learning. SAS 477 Institute, Cary
Timmeren SV, Isaacs R (2013) Control of spotted wind drosophila, Drosophila suzukii, by specific insecticides and by conventional and organic crop protection systems. Crop Prot 54:126–133
Tochen S, Dalton DT, Wiman N, Hamm C, Shearer PW, Walton V (2014) Temperature-related development and population parameters for Drosophila suzukii (Diptera: Drosophilidae) on cherry and blueberry. Environ Entomol 43:501–510
Van Der Linde K, Steck GJ, Hibbard K, Birdsley JS, Alonso LM, Houle D (2006) First records of Zaprionus indianus (Diptera: Drosophilidae), a pest species on commercial fruits from Panama and the United States of America. Fla Entomol 89:402–404
Vilela CR, Teixeira EP, Stein CP (1999) Nova praga nos figos: Zaprionus indianus Gupta, 1970. An Soc Entomol Bras 24:1–2
Walsh DB, Bolda MP, Goodhue RE, Dreves AJ, Lee JC, Bruckd J, Walton VM, O’Neal SD, Zalom FG (2011) Drosophila suzukii (Diptera: Drosophilidae): Invasive pest of ripening soft fruit expanding its geographic range and damage potential. J Pest Manag 106:289–295
Acknowledgments
The authors are grateful to the National Council for the Improvement of Higher Education (CAPES) for granting a scholarship to the first author.
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by Jorge B Torres - UFRPE
Rights and permissions
About this article
Cite this article
Bernardi, D., Andreazza, F., Botton, M. et al. Susceptibility and Interactions of Drosophila suzukii and Zaprionus indianus (Diptera: Drosophilidae) in Damaging Strawberry. Neotrop Entomol 46, 1–7 (2017). https://doi.org/10.1007/s13744-016-0423-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13744-016-0423-9