Abstract
The invasive spotted wing drosophila, Drosophila suzukii Matsumura (Dipt.: Drosophilidae), a native of East Asia, has widely established in North America and Europe, where it is a serious pest of small and stone fruit crops. The lack of effective indigenous parasitoids of D. suzukii in the recently colonized regions prompted the first foreign exploration for co-evolved parasitoids in South Korea during 2013 and 2014. We collected the larval parasitoids Asobara japonica Belokobylskij, A. leveri (Nixon) and A. brevicauda Guerrieri & van Achterberg (Hym.: Braconidae), Ganaspis brasiliensis (Ihering), Leptopilina japonica japonica Novković & Kimura and L. j. formosana Novković & Kimura (Hym.: Figitidae); and the pupal parasitoids Pachycrepoideus vindemiae (Rondani) (Hym.: Pteromalidae) and Trichopria drosophilae Perkins (Hym.: Diapriidae). From UC Berkeley quarantine records, percentage parasitism ranged from 0 to 17.1 % and varied by geography, season, and collection methods. Asobara japonica was the most common parasitoid species. Higher numbers of parasitoids were reared from field-picked fruit as opposed to traps baited with uninfested fruit. Quarantine bioassays confirmed that A. japonica, G. brasiliensis, L. j. japonica, P. vindemiae, and T. drosophilae developed from D. suzukii. Female individuals of the endoparasitoid, A. japonica, were larger when reared on the larger D. suzukii larvae compared with those reared on the smaller larvae of D. melanogaster Meigen. Larger parasitoid size was associated with longer developmental time. Several of the South Korean parasitoid species have the potential for use in classical biological control and may contribute to the suppression of D. suzukii in the newly invaded regions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Key message
-
Spotted wing drosophila is an invasive fly pest that damages soft- and thin-skinned fruit crops.
-
A classical biological control program was initiated by surveys for spotted wing drosophila parasitoids in South Korea during 2013 and 2014.
-
Seven known and one new parasitoid species were reared from spotted wing drosophila, with field percent parasitism by larval parasitoids ranging from 0 to 17.1 %.
-
Quarantine studies are ongoing to select the most suitable natural enemies for release in North America and Europe.
Introduction
Spotted wing drosophila, Drosophila suzukii Matsumura (Diptera: Drosophilidae), is a pomace fly distributed natively across eastern China, Japan, the Korean Peninsula, and other regions in Southeastern Asia (Hauser 2011; Kanzawa 1939). In North America, the fly was first detected in 2008 in California (Bolda et al. 2010) and was subsequently reported in most fruit growing regions in the continental United States (US) and Canada (Asplen et al. 2015; Emiljanowicz et al. 2014; Walsh et al. 2011). Drosophila suzukii was also detected in Spain and Italy in 2008, and soon thereafter reported in other European countries (Calabria et al. 2012; Cini et al. 2014); most recently, the fly was reported in Brazil (Deprá et al. 2014). Drosophila suzukii is considered a key pest of soft- and thin-skin fruits such as blueberries, cherries, figs, raspberries, and strawberries in all of these newly invaded regions (Burrack et al. 2013; Kinjo et al. 2013; Lee et al. 2011; Mitsui et al. 2006; Yu et al. 2013).
The pest status of D. suzukii is attributed to the female’s serrated ovipositor (Atallah et al. 2014) which enables oviposition in ripening, rather than over ripe or rotting fruit. While D. suzukii is unable to oviposit in fruits with thick, hard, or fuzzy skin, it can oviposit and complete development in some of these fruits when the skin is compromised (e.g., pathogen or insect damage, hail) (Lee et al. 2015; Poyet et al. 2014; Steffan et al. 2013; Stewart et al. 2014; Ioriatti et al. 2015). Additionally, D. suzukii’s fast development (a generation in ≈10 days at 22 °C) and high reproductive potential (>600 eggs per female) (Emiljanowicz et al. 2014; Tochen et al. 2014) can result in an explosive population increase (Wiman et al. 2014) and significant economic losses to commercial crops (Beers et al. 2011; Goodhue et al. 2011; Walsh et al. 2011). Control efforts in North America currently rely on the use of insecticides that target adult D. suzukii (Beers et al. 2011; Bruck et al. 2011). However, insecticide-based programs can be limited by the fact that many host fruits in non-crop habitats act as reservoirs for D. suzukii and support its reinvasion into commercial fields (e.g., Klick et al. 2014, 2015).
From this perspective, biological control in non-crop habitats may help suppress regional D. suzukii population densities and provide a new tool for reduced risk and environmentally sound management strategies. Worldwide, over 50 hymenopteran parasitoid species attack drosophilid species. In most cases, these parasitoids attack their host in the late-larval or pupal developmental stages (Carton et al. 1986). The majority of the larval parasitoids are braconids in the genus Asobara and figitids in the genera Leptopilina and Ganaspis, and they predominantly attack host fly species found in fermenting substrates (Asplen et al. 2015). There are also two common pupal parasitoids, Pachycrepoideus vindemiae Rondani (Pteromalidae) and Trichopria drosophilae Perkins (Diapriidae). These larval and pupal parasitoid species undoubtedly play a role in population suppression of some drosophilid species. The effectiveness of these parasitoids against D. suzukii in its invaded regions has, however, not been adequately studied and, where surveys have been conducted, there appears to be limited parasitism rates of D. suzukii in terms of economic suppression (Miller et al. 2015).
Kacsoh and Schlenke (2012) studied 24 strains or populations of parasitoid species for their effectiveness against D. suzukii, and report that among 12 larval parasitoid species tested, only Asobara japonica Belokobylskij (from Japan), A. citri (from Ivory Coast), and two strains of Ganaspis sp. (from Hawaii and Florida) could complete development on D. suzukii. Of the tested larval parasitoid species, A. japonica had the highest rate of successful development. In most cases, the larval parasitoid species’ failure to develop in D. suzukii was explained by the host’s immune resistance (Kacsoh and Schlenke 2012; Poyet et al. 2013). Similarly, in another laboratory study, Chabert et al. (2012) trialed five parasitoid species that are commonly found in Europe attacking drosophilids (A. tabida Nees, Leptopilina heterotoma (Thomson), L. boulardi Barbotin et al., T. cf drosophilae, and P. vindemiae) and found that only the two pupal parasitoids (T. cf drosophilae and P. vindemiae) successfully developed on D. suzukii. Field surveys also suggest that parasitoids attacking drosophilid species resident in the US and Europe have not readily adapted to the invasive D. suzukii. Typically in field surveys, only the pupal parasitoids T. drosophilae and P. vindemiae have been reported to attack D. suzukii in Italy (Rossi Stacconi et al. 2013), Spain (Gabarra et al. 2015), and the US (Miller et al. 2015; Wang et al. 2016). One exception is a strain of L. heterotoma from northern Italy that was recovered from D. suzukii (Rossi Stacconi et al. 2015).
The lack of effective biological control in the newly invaded range of D. suzukii led to the initiation of a classical biological control program. Genetic analyses suggest that East Asia is the region of origin for D. suzukii populations that invaded North America (Adrion et al. 2014; Chiu et al. 2013; Ometto et al. 2013), and this is the focal region for our initial and planned collections. Information on parasitoids associated with D. suzukii and other drosophilids in Asia is however limited to reports from Japan. For example, Mitsui et al. (2007) reported 15 parasitoid species collected from fruit-baited traps, these included A. japonica, A. tabida, A. rossica Belokobylskij, A. rufescens (Forster), A. pleuralis (Ashmead) A. leveri (Nixon), L. heterotoma, L. victoriae Nordlander, Ganaspis xanthopoda (Ashmead), and P. vindemiae. Among the 15 species, only A. japonica, A. tabida, and G. xanthopoda emerged from D. suzukii. The aim of this study was to determine the presence and biological suitability of Asian parasitoids of D. suzukii to be considered for quarantine examination and potential field release in North America and Europe. In South Korea, no reports of D. suzukii-related damage have been made and this species has not been considered a pest (Asplen et al. 2015). We describe herein the first collection efforts in South Korea and the initial quarantine studies on the imported material.
Materials and methods
Collection sites and methods
The South Korean sample locations were selected based on a pre-collection exploration in 2011 (J. Miller, H. Riedl and Y. Song, pers. comm.). Surveys for frugivorous Drosophila parasitoids in South Korea were conducted at 10 locations and three provinces during August 2013 (J. Miller, B. Miller, H. Riedl and P. Shearer), and at 18 locations and four provinces from June to July 2014 (J. Miller and B. Miller), aided in each year by collaborators in South Korea (Fig. 1).
Collection locations for drosophilid parasitoids in 2013 (black), 2014 (gray) or both years (white) in South Korea: a Namhae Marina; b Boriamsa; c Ungbongsan; d Mangunsan; e Kumosan; f Sacheon; g Guam-Imdo; h Jinju city and Gyeongsang; i Jinju; j Uigoksa and Jinju Heights; k Munsusa; l Jagulsan; m Jeongryeongchi; n Namwon; o Baekyeonsan; p Poyeongsa; q Gamaksan; r Geochang; s Deogyusan; t Jeoksangsan; u Sangju; v Namjangsa; w Gunjae Bong and Ban Suk San. Close locations (<5 km) for both years were merged
During the expeditions, parasitoids were field-surveyed using uninfested-fruit-baited traps deployed for 4–7 days and via the collection of commercial or wild host fruits with a high probability of infestation as determined primarily by the presence of adult flies. The uninfested-fruit-baited traps were based on previous collections by Rossi Stacconi et al. (2013). For each trap, 100–200 g of mixed fruits (banana, blueberry, fig, melon, and peach) was placed in a 162-mL plastic cup. Each cup was fitted with a lid and ten 0.8-cm diameter entrance holes along the cup rim to allow entry of ovipositing flies and parasitoids, but prevent the entry of other animals or larger insects. The plastic cup was placed inside a 500-mL plastic square tub and the ‘cup-in-tub’ set-up was then placed inside an orange delta trap (Suterra, LLC, Bend, OR). The cup-in-tub method provided a dry microhabitat for larvae to pupate when the fruit started decaying. Upon collection of the baited traps, the square tub was covered with a screened lid and the tubs were kept at room temperature (22–28 °C) until pupation. Field-collected fruits were separately placed in 162-mL cups on filter paper inside a 500-mL plastic tub until pupation.
In 2013, we primarily used the uninfested-fruit-baited traps, deployed at inland or coastal forests or along the perimeter of crop fields (Table 1). At each collection site, 8–12 traps were hung at shoulder height (1.5 m) from sturdy branches and deployed for 4–7 days. Commercial blackberries (approximately 10 L), damaged pears, apples, peaches, or split grapes were also sampled in four different locations (Table 1). In 2014, we predominantly used fresh-fruit collections of 2–10 L of wild Rubus fruits per collection site, as these fruits were still available in these unmanaged habitats during June and July (Table 1). In addition, commercial blackberries were collected from one location (Geochang) and 10 uninfested-fruit-baited traps were placed at another location (Boriamsa) where wild Rubus fruits were also collected (Table 1).
Drosophilid pupae that developed from the field-collected larvae were sorted according to collection or trap location and placed on moist filter papers in 5-cm diameter Petri dishes. The dishes were kept at 4 °C before being packaged for hand-carry to the US. During transportation, the puparia were placed inside a Styrofoam box and kept cool with packaged ice.
Quarantine of imported puparia
Once the Styrofoam box passed through US Homeland Security at San Francisco International Airport, and inspection at the USDA, APHIS, PPQ Plant Inspection Station (permit: P526P-11-03867), imported puparia were processed under controlled conditions (22 ± 3 °C, 12L: 12D, 40–70 % RH, and natural light) in quarantine facilities at the University of California, Berkeley. Hosts for imported parasitoid colonies and quarantine evaluation of imported parasitoid species were obtained from laboratory colonies of D. suzukii and D. melanogaster Meigen (a common host for most parasitoid species attacking drosophilids). The fly colonies originated from field collections in Parlier, California and were maintained on standard corn-meal diets using methods similar to Dalton et al. (2011).
Upon arrival into quarantine, the puparia were sorted to separate those bearing visible signs of parasitism, i.e., visible parasitoid pupae inside the fly puparia. Individual puparia were placed in Eppendorf tubes with a streak of honey-water provided for emerging adults. Emerged flies were killed and preserved with 95 % alcohol along with empty pupal cases in Eppendorf tubes for later species identification. Emerging female parasitoids were individually placed into vials containing artificial diet and larvae or pupae of D. suzukii or D. melanogaster. Male wasps emerging from the same location-based samples were coupled with their respective females. Honey-water droplets were provided for the parasitoids and host larvae/pupae were renewed every 2–3 days. Upon death, individuals of the parental generation of parasitoids were preserved in 95 % alcohol along with their pupal case. Pupae of D. suzukii were distinguishable from other Drosophila pupae by the presence of a pair of distinct respiratory tubes on the anterior end that was used as a diagnostic character (Kanzawa 1939). Vouchers of the figitids are deposited with the U.S. National Entomological Collection (Washington, D. C.), while other species are deposited in the Institute for Sustainable Plant Protection (Portici, Italy) or in the Essig Museum at the University of California (Berkeley, California). Guerrieri et al. (2016) identified all Asobara species using both morphological and molecular methods, while all other species were confirmed or identified by ML Buffington.
Additionally, all dead (no adult emergence) pupae were first reconstituted in water and then dissected under the microscope to assess the presence or absence of recognizable fly or parasitoid cadavers. However, it was often difficult to distinguish parasitized pupae from unparasitized pupae when they died at early stages, and in these cases the pupae were classified as ‘unknown.’ The parasitism rate was estimated as the sum of emerged parasitoids and dead parasitized pupae divided by the sum of emerged flies and parasitoids plus dead parasitized and unparasitized pupae.
Quarantine bioassays
Three parasitoid species, later identified as A. japonica, T. drosophilae, and P. vindemiae, from the collections in 2013, and two additional larval parasitoid species, Leptopilina japonica japonica Novković & Kimura and G. brasiliensis, obtained during the 2014 sampling (see Results section), were briefly tested to confirm their ability to attack and develop from D. suzukii or from D. melanogaster, chosen as a common alternative host. Two or three-day old larvae (for A. japonica, L. j. japonica, and G. brasiliensis) or two or three-day old pupae (for T. drosophilae and P. vindemiae) were offered to the parasitoids for 2 days. For each parasitoid species, one female and one male were released into ventilated plastic vials (40 mL) containing hosts infesting artificial diet, or on plastic cups (330 mL) with three D. suzukii-infested fruits (blueberries or cherries). Fruits were infested by exposing them, in groups of ten, for 3 h into fly rearing cages containing several adult flies; fruits were then observed under the microscope and the number of eggs per fruit was standardized to three by removing excess eggs using a needle probe. The host density in diet vials varied between 10 and 30 for D. suzukii and 40–80 for D. melanogaster. Exposed hosts were reared until the emergence of flies or parasitoids.
The most common parasitoid species (see “Results” section), A. japonica, was subjected to a no-choice test to determine the parasitoid’s performance on two different-sized hosts. One-week-old mated female wasps were individually exposed to 15 young larvae of either host species in vials with artificial diet for 2 days. The number, sex, and developmental time of emerged parasitoids were recorded. A sub-sample of emerged females was also measured for body size (ovipositor and hind tibia length). Tests consisted of 14 and 24 replicates for D. suzukii and D. melanogaster, respectively.
Data analyses
Infestation percentages by D. suzukii or other drosophilids (estimated as number of emerged adults), and parasitism rates in sampled fruits or in fruit-baited traps were subjected to Analysis of Variance (ANOVA) separately for each collection year. The effect of host species on A. japonica’s juvenile development, fertility, and body size was also compared with ANOVA. Prior to the Analysis of Variance (ANOVA), percentage data were logit transformed as needed to normalize the data distribution. All analyses were performed using JMP V11 (SAS 2011, Cary, NC, USA).
Results
Field collections
In 2013, a total of 3266 Drosophila puparia were collected in South Korea and imported to the quarantine facilities at Berkeley (Table 1). Drosophila suzukii adults emerged mainly from collected blackberries, whereas the uninfested-fruit-baited traps primarily captured other drosophilid species (Table 1). There were 97 dead parasitized puparia, 728 dead unparasitized puparia, and 1,085 dead puparia for which parasitism status was unknown. Seven parasitoid species (44 individuals) were recovered from drosophilids collected during 2013: A. japonica (45.5 % of the recovered parasitoids), A. leveri (25 %), L. j. japonica (9.1 %), L. j. formosana Novković & Kimura (2.3 %), L. boulardi (2.3 %), P. vindemiae (6.8 %), and T. drosophilae (9.1 %). Among these, L. j. formosana and L. j. japonica emerged only from D. suzukii; A. japonica, A. leveri, and T. drosophilae emerged from both D. suzukii and other drosophilids; and L. boulardi and P. vindemiae emerged from other drosophilids (Table 2).
In 2014, a total of 20,358 puparia were collected from a variety of habitats, including wild Rubus located in inland forests and blackberries at one commercial farm (Table 1). Collections of wild Rubus in five of the locations yielded no drosophilid pupae (Table 1). Drosophila suzukii comprised the majority of flies that emerged from Rubus but no D. suzukii emerged from fruit-baited traps in the same localities (Table 1). From this material, there were 116 dead parasitized puparia, 5271 dead unparasitized puparia, and 8383 dead puparia for which parasitism status was unknown. A total of 181 wasps emerged, representing six species of larval parasitoids: A. japonica (60.2 % of the recovered parasitoids), A. leveri (2.2 %), A. brevicauda (2.8 %), L. j. japonica (14.9 %), L. j. formosana (1.7 %), and G. brasiliensis (18.2 %). Among these, A. brevicauda is a new species. Most important for this survey is that A. brevicauda, L. j. japonica, and G. brasiliensis emerged only from D. suzukii, whereas A. japonica and A. leveri emerged from both D. suzukii and other drosophilids, and L. j. formosana emerged only from other drosophilids (Table 2). The two northern Korean locations (Munsusa and Baekyeonsan) yielded no parasitoids (Table 2). Both the braconids and figitids co-existed in seven locations, but the figitids also occurred in another two locations (Table 2). Up to four different larval parasitoids were reared from D. suzukii in a single geographic location.
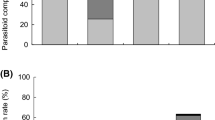
The percentage of D. suzukii among all emerged flies was higher from fruit samples than from fruit-baited traps in both 2013 (F 1,76 = 68.7, P < 0.001) and 2014 (F 1,192 = 1545, P < 0.001) (Fig. 2a). In 2013, mean parasitism of D. suzukii in natural fruit samples (range: 0–17.4 %) was significantly lower than in fruit-baited traps (range: 0.0–33.3 %) (F 1,34 = 4.5, P = 0.042), but mean parasitism of other drosophilids was similar between these two sampling methods (range: 0–33.3 % in fruit samples, and 0–39.1 % in fruit traps) (F 1,46 = 1.3, P = 0.26) (Fig. 2b, c). In 2014, parasitism of D. suzukii was 2.8 ± 0.4 % (n = 171) (range: 0–23.5 %) in fruit samples (no D. suzukii was found in fruit traps). Parasitism of D. suzukii in fruit samples highly varied among locations, but the overall parasitism by braconids (range: 0–10.5 %) was similar to that by figitids (0–8.8 %) (Fig. 3). Mean parasitism of other drosophilids was higher in fruit traps (range: 0–33.3 %) than in fruit samples (range: 0–12.5 %) (F 1,48 = 22.0, P < 0.001) (Fig. 2b, c).
Effect of sampling methods (fruit trap vs. fruit sample) on (a) the percentage of D. suzukii that emerged, (b) parasitism of D. suzukii or (c) other drosophilid species during the 2013 and 2014 exploration in South Korea. Data were pooled from all locations; values are mean ± SE and different letters above the bars indicate significant difference between the two different methods within each year (ANOVA, P < 0.05)
Quarantine rearing and Asobara japonica performance
Of the eight parasitoid species, we were able to establish colonies of five species for further testing in quarantine: A. japonica, L. j. japonica, G. brasiliensis, T. drosophilae, and P. vindemiae. We were unable to develop colonies of A. leveri, L. j. formosana, and A. brevicauda, because we either did not collect any females (A. brevicauda) or collected too few females during any single collection period (A. leveri, and L. j. formosana) (Table 2). We additionally reared some males and one female L. boulardi from drosophilids other than D. suzukii. The limited reared material prevented the development of a colony.
Each of the five tested parasitoid species (A. japonica, L. j. japonica, G. brasiliensis, T. drosophilae, and P. vindemiae) was able to attack and develop from both D. suzukii and D. melanogaster cultured on artificial diet or fruits (Table 3). Detailed quarantine studies are ongoing, but initial screening showed that the numbers of offspring produced per A. japonica female were similar between D. suzukii (8.0 ± 0.7) and D. melanogaster (6.9 ± 0.7) (F 1,37 = 1.1, P = 0.311). However, female parasitoids developed faster on D. melanogaster (27.1 ± 0.2 days) than on D. suzukii (25.9 ± 0.1 days) (F 1,303 = 19.5, P < 0.001), but perhaps as a consequence females reared from D. melanogaster larvae were smaller than those reared from D. suzukii (hind tibia length: F 1,50 = 28.4, P < 0.001; ovipositor length: F 1,50 = 21.6, P < 0.001, Fig. 4).
Discussion
The current study is the first report of a foreign exploration effort for Asian parasitoids of D. suzukii in part of its native range. Six species of larval parasitoids (A. brevicauda, A. japonica, A. leveri, L. j. japonica, L. j. formosana, and G. brasiliensis) and one pupal parasitoid (T. drosophilae) were recorded from D. suzukii in South Korea. Another pupal parasitoid species, P. vindemiae, was field-collected from drosophilid species other than D. suzukii in South Korea, but it was able to attack and develop on D. suzukii in quarantine. Colonies of five out of eight parasitoid species were successfully established using D. suzukii as host. G. brasiliensis was recently re-described as a new combination by Buffington and Forshage (2016).
The estimated parasitism rates varied largely without any clear pattern of location, sampling method, or site. However, parasitism by these larval parasitoids could be underestimated for several reasons. First, at the time when fruits were field-collected, parasitized larvae could have already left the fruits and pupated in the soil, while eggs and young larvae may not have yet been attacked. When these fruits were brought to the South Korean laboratories where the pupae were isolated for shipment to California, there would have already been a bias against pupal parasitoids because many of the larval-pupal parasitoids might have already exited the host fruit. A more precise estimate of the parasitism would be to collect only those pupae that pupated within 1–2 weeks (i.e., when they were at the suitable larval stages at the time of collection) following the collection. Second, mortalities of the imported pupae were high, partly because all collected materials had to be completely sealed during the transposition resulting in mold growth within the Petri dishes and our observation of numerous dead pupae from unknown factors.
In our South Korean collections, A. japonica was the most commonly recovered parasitoid. It was collected in four South Korean provinces and in particular it was recovered in 3 of 10 sampled sites in 2013 and 6 of 18 sampled in 2014. Moreover, 128 A. japonica specimens were obtained out of the 141 total Asobara spp. that emerged in the quarantine laboratory. These results agree with those obtained in the few previous studies of Asian drosophilid parasitoids, A. japonica is the most common and widespread parasitoid and is capable of parasitizing a number of Drosophila species (Ideo et al. 2008; Mitsui et al. 2007; Mitsui and Kimura 2010). Nomano et al. (2015) investigated the host use of eight Japanese Asobara species; six of these species (A. japonica, A. tabida, A. rossica, A. rufescens, and two unidentified Asobara spp.) emerged from D. suzukii breeding on wild Cerasus and Vaccinium fruits. In our survey, A. japonica was commonly collected from D. suzukii in wild Rubus. Interestingly, based on adult emergence in the quarantine, the A. japonica populations collected in our study were 99.8 % female. Similarly, populations of A. japonica on the main islands of Japan were parthenogenetic, whereas those in the subtropical islands were sexually reproducing (Mitsui et al. 2007). However, earlier work by Mitsui et al. (2007) and our study may not be directly comparable as the earlier Japanese survey used banana fruit-baited traps, which tend to attract numerous drosophilids in general, but fewer D. suzukii (XG Wang pers. observ.). On the other hand, recent studies reported variations in genetic, physiological, or ecological traits among different geographic populations of A. japonica, showing a high degree of geographic adaptations to various host habitats (Murata et al. 2009).
With the exception of A. japonica, there is little information on the parasitoid species reared in terms of their geographic distribution in Asia or their host species range. Kasuya et al. (2013) reported three species (L. japonica, Ganaspis sp., and one unidentified Asobara species) from D. suzukii on wild cherry fruit (Prunus donarium Sieb.). The Ganaspis sp. strain obtained from D. suzukii exhibits a high level of specificity for D. suzukii and was referred to suzukii-associated type of G. xanthopoda (Kasuya et al. 2013), while other stains do not appear to have the same level of specificity (Mitsui and Kimura 2010). The two generalist pupal parasitoids T. drosophilae and P. vindemiae are cosmopolitan (Carton et al. 1986; Chabert et al. 2012; Gabarra et al. 2015; Kacsoh and Schlenke 2012; Rossi Stacconi et al. 2015). As expected, these parasitoid species were found in South Korea and readily developed on D. suzukii in the quarantine. In the current study, all three species of figitids were collected in South Korea from D. suzukii collected in fruit samples, especially G. brasiliensis, which was found in multiple locations. We also report one new species (A. brevicauda) and two other species (A. leveri and L. j. formosana) with new host-association records on D. suzukii. It must still be verified whether or not the new species are the same as one of those undescribed species in Japan (Kasuya et al. 2013; Nomano et al. 2015).
Interestingly, we did not recover A. tabida or G. xanthopoda, which were reported to occur over a relatively wide range of the Japanese islands (Mitsui et al. 2007; Nomano et al. 2015). In earlier surveys, a few individuals of A. tabida were collected from D. suzukii in Japan (Mitsui et al. 2007; Nomano et al. 2015) and this host was the focus of our collection efforts in South Korea. However, several laboratory experiments have showed that A. tabida could oviposit but did not survive in D. suzukii (Chabert et al. 2012; Kacsoh and Schlenke 2012). Nomano et al. (2015) suggested several possible causes for this inconsistency in A. tabida and G. xanthopoda host use across different geographic ranges with relatively similar environmental conditions, including misidentification of host species in the earlier collections, host stage influence on parasitoid survival, and kleptoparasitism. Previous studies have shown that host age affected the survival of A. tabida in D. melanogaster (Van Alphen and Janssen 1982), and A. tabida survived better in D. simulans (Sturtevant) previously parasitized by L. boulardi (Kraaijeveld 1999). It is also possible that geographic variations occur in the flies’ immune resistance against parasitoids or in parasitoid species for their preference or effectiveness against flies (Rossi Stacconi et al. 2015).
While uninfested-fruit-baited traps provided a quick means of monitoring the presence of adult parasitoids that attack frugivorous Drosophila, it appeared in our collections to be relatively unattractive to South Korean D. suzukii. We found that the uninfested-fruit-baited traps captured mainly other drosophilid species, thus disrupting the search for specialist parasitoid species. In contrast, our collections of field-infested fresh fruits yielded primarily D. suzukii and with this more host-specific parasitoids, which was similar to previous collections in Japan (Kasuya et al. 2013; Mitsui et al. 2007; Nomano et al. 2015), although the authors did not always emphasize the differences in collection methods. In Japan, Kasuya et al. (2013) reported that D. suzukii was the only fly species breeding on wild cherry fruit. Both the host-specific Asobara species (Nomano et al. 2015) and D. suzukii-associated type of G. xanthopoda (Kasuya et al. 2013) were collected from wild fruits. Fruit traps could also influence the estimated rate of parasitism as both entering flies and parasitoids could reside in the traps for considerable periods of time, permitting repeated oviposition by the same species.
In the Berkeley quarantine, A. japonica, L. j. japonica, G. brasiliensis, T. drosophilae, and P. vindemiae readily attacked D. suzukii. This assessment was important to verify that, at least in the laboratory, the North American strain of D. suzukii (from Parlier, CA) did not show complete resistance to the five tested parasitoid species, as was shown in earlier studies (Kacsoh and Schlenke 2012; Poyet et al. 2013). In these preliminary quarantine studies, we also used D. melanogaster as a potential alternative and widespread host. This host was physiologically suitable for all the tested parasitoid species, thus potentially increasing the chances of these parasitoids to survive in the wild even without the availability of the pest. We also found a positive correlation between the host size and the size of emerged A. japonica, thus potentially favoring D. suzukii over other drosophilids as a preferred host. However, a more accurate assessment of the ecological suitability of D. melanogaster for these parasitoid species is needed before predicting its real potential as an alternative host species in the field (Desneux et al. 2012). To this goal of finding specialized parasitoid species that exclusively attack or strongly prefer D. suzukii, future extensive quarantine work is designed to test host preference on a series of non-target fly species. A. japonica was the more common parasitoid reared from D. suzukii (Table 2). If strains of this, or other species, have clearly better performance on or preference for D. suzukii compared with other fly species, an argument could be made for their release in some regions.
In conclusion, some specialized D. suzukii parasitoids in the genera of Asobara, Leptopilina, and Ganaspis seem to be present in South Korea and their specialization may be preferable as biological control candidates for D. suzukii populations that occur in North America and Europe. However, further detailed evaluations are needed to determine their effectiveness and safety with regard to non-target risk in order to obtain release permits in the US and/or in Europe. Moreover, the highest levels of D. suzukii parasitism recorded in South Korea were by the more generalist parasitoid species, particularly A. japonica. Therefore, this initial collection shows promise for improved biological control of D. suzukii in some of its invaded regions. Furthermore, additional collections in Asia will likely result in novel species and species associations with D. suzukii. For example, in 2013 collections in South Korea (K. Hoelmer, E. Guerrieri, M. Giorgini, D.S. Choi) using banana-baited traps infested by D. suzukii yielded A. japonica, A. leveri, G. xanthopoda, and one new species Asobara brevicauda Guerrieri and Van Achterberg (Guerrieri et al. 2016), while collections in Yunnan Province, China using uninfested-banana-baited traps yielded A. japonica, A. leveri, G. xanthopoda, one undescribed Leptopilina sp. and four Asobara species that have been described as new including A. brevicauda, A. elongata Guerrieri and Van Achterberg, A. mesocauda Guerrieri and Van Achterberg, A. unicolorata Guerrieri and Van Achterberg and A. triangulata (Guerrieri et al. 2016). While the material was not processed in quarantine and screened for effectiveness against D. suzukii, these collections highlight the need for further exploration and quarantine screening, as well as more detailed studies of parasitoid-host associations in situ in the native range of D. suzukii. The wide geographic distribution of D. suzukii in Asia also suggests that surveys in other regions of Asia, habitats, and over longer periods and throughout the seasons will be needed to fully discover the diversity of its natural enemies.
Author contribution
KMD, XGW, JCM, PWS, KAH, and VMW conceived and designed the project. KMD and XGW wrote the initial manuscript, all co-authors helped editing the manuscript thereafter. XGW and AB analyzed the data and conducted the quarantine work. JCM, BM, PWS, YS, TK, CJ, DWL, BC, and HR conducted the foreign exploration in South Korea. EG, MG, and KvA identified all Asobara species, while MB identified all other parasitoid species. All authors read, revised, and approved the manuscript.
References
Adrion JR, Kousathanas A, Pascual M, Burrack HJ, Haddad NM, Bergland AO et al (2014) Drosophila suzukii: the genetic footprint of a recent, worldwide invasion. Mol Biol Evol 31:3148–3163
Asplen M, Anfora G, Biondi A, Choi D-S, Chu D, Daane KM et al (2015) Invasion biology of spotted wing drosophila (Drosophila suzukii): a global perspective and future priorities. J Pest Sci 88:469–494
Atallah J, Teixeira L, Salazar R, Zaragoza G, Kopp A (2014) The making of a pest: the evolution of a fruit-penetrating ovipositor in Drosophila suzukii and related species. Proc R Soc Biol Sci Ser B 281:2013–2840
Beers EH, Van Steenwyk RA, Shearer PW, Coates WW, Grant JA (2011) Developing Drosophila suzukii management programs for sweet cherry in the western United States. Pest Manag Sci 67:1386–1395
Bolda MP, Goodhue RE, Zalom FG (2010) Spotted wing drosophila: potential economic impact of a newly established pest. Giannini Foundation of Agricultural Economics, University of California
Bruck DJ, Bolda M, Tanigoshi L, Klick J, Kleiber J, DeFrancesco J et al (2011) Laboratory and field comparisons of insecticides to reduce infestation of Drosophila suzukii in berry crops. Pest Manag Sci 67:1375–1385
Buffington ML, Forshage M (2016) Redescription of Ganaspis brasiliensis (Ihering, 1905), new combination (Hymenoptera: Figitidae), a natural enemy of the invasive Drosophila suzukii (Matsumura, 1931) (Diptera: Drosophilidae). Proc Entomol Soc Wash 118(1):1–13
Burrack HJ, Fernandez GE, Spivey T, Kraus DA (2013) Variation in selection and utilization of host crops in the field and laboratory by Drosophila suzukii Matsumara (Diptera: Drosophilidae), an invasive frugivore. Pest Manag Sci 69:1173–1180
Calabria G, Máca J, Bachli G, Serra L, Pascual M (2012) First records of the potential pest species Drosophila suzukii (Diptera: Drosophilidae) in Europe. J Appl Entomol 136:139–147
Carton Y, Boulétreau B, van Alphen JJM, van Lenteren JC (1986) The Drosophila parasitic wasps. In: Ashburner M, Carson HL, Thompson JN (eds) The genetics and biology of Drosophila. Academic Press, London, pp 347–394
Chabert S, Allemand R, Poyet M, Eslin P, Gibert P (2012) Ability of European parasitoids (Hymenoptera) to control a new invasive Asiatic pest, Drosophila suzukii. Biol Control 63:40–47
Chiu JC, Jiang X, Zhao L, Hamm CA, Cridland JM, Saelao P et al (2013) Genome of Drosophila suzukii, the spotted wing drosophila. G3 3:2257–2271
Cini A, Anfora G, Escudero-Colomar LA, Grassi A, Santosuosso U, Seljak G et al (2014) Tracking the invasion of the alien fruit pest Drosophila suzukii in Europe. J Pest Sci 87:559–566
Dalton DT, Walton VM, Shearer PW, Walsh DB, Caprile J, Isaacs R (2011) Laboratory survival of Drosophila suzukii under simulated winter conditions of the Pacific Northwest and seasonal field trapping in five primary regions of small and stone fruit production in the United States. Pest Manag Sci 67:1368–1374
Deprá M, Poppe JL, Schmitz HJ, De Toni DC, Valente VLS (2014) The first records of the invasive pest Drosophila suzukii in the South American continent. J Pest Sci 87:379–383
Desneux N, Blahnik R, Delebecque CJ, Heimpel GE (2012) Host phylogeny and specialisation in parasitoids. Ecol Lett 15:453–460
Emiljanowicz LM, Ryan GD, Langille A, Newman J (2014) Development, reproductive output and population growth of the fruit fly pest Drosophila suzukii (Diptera: Drosophilidae) on artificial diet. J Econ Entomol 107:1392–1398
Gabarra R, Riudavets J, Rodríguez GA, Pujade-Villar J, Arnó J (2015) Prospects for the biological control of Drosophila suzukii. Biocontrol 60:331–339
Goodhue RE, Bolda M, Farnsworth D, Williams JC, Zalom FG (2011) Spotted wing drosophila infestation of California strawberries and raspberries: economic analysis of potential revenue losses and control costs. Pest Manag Sci 67:1396–1402
Guerrieri E, Giorgini M, Cascone P, Carpenito S, van Achterberg C (2016) Species diversity in the parasitoid genus Asobara (Hymenoptera: Braconidae) from the native area of the fruit fly pest Drosophila suzukii (Diptera: Drosophilidae). PLoS One 11:e0147382
Hauser M (2011) A historic account of the invasion of Drosophila suzukii (Matsumura) (Diptera: Drosophilidae) in the continental United States, with remarks on their identification. Pest Manag Sci 67:1352–1357
Ideo S, Watada M, Mitsui H, Kimura MT (2008) Host range of Asobara japonica (Hym.: Braconidae), a larval parasitoid of drosophilid flies. Entomol Sci 11:1–6
Ioriatti C, Walton V, Dalton D, Anfora G, Grassi A, Maistri S, Mazzoni V (2015) Drosophila suzukii (Diptera: Drosophilidae) and its potential impact to wine grapes during harvest in two cool climate wine grape production regions. J Econ Entomol 108:1148–1155
Kacsoh BZ, Schlenke TA (2012) High hemocyte load is associated with increased resistance against parasitoids in Drosophila suzukii, a relative of D. melanogaster. PLoS One 7:e34721
Kanzawa T (1939) Studies on Drosophila suzukii Mats (in Japanese): Yamanashi Agricultural Experimental Station. Kofu, Japan
Kasuya N, Mitsui H, Ideo S, Watada M, Kimura MT (2013) Ecological, morphological and molecular studies on Ganaspis individuals (Hymenoptera: Figitidae) attacking Drosophila suzukii (Diptera: Drosophilidae). Appl Entomol Zool 48:87–92
Kinjo H, Kunimi Y, Ban T, Nakai M (2013) Oviposition efficacy of Drosophila suzukii (Diptera: Drosophilidae) on different cultivars of blueberry. J Econ Entomol 106:1767–1771
Klick J, Lee JC, Hagler JR, Bruck DJ, Yang WQ (2014) Evaluating Drosophila suzukii immunomarking for mark-capture research. Entomol Exp Appl 152:31–41
Klick J, Yang W, Walton V, Dalton D, Hagler J, Dreves A, Lee JC, Bruck D (2015) Distribution and activity of Drosophila suzukii in cultivated raspberry and surrounding vegetation. J Appl Entomol. doi:10.1111/jen.12234
Kraaijeveld AR (1999) Kleptoparasitism as an explanation for paradoxical oviposition decisions of the parasitoid Asobara tabida. J Evol Biol 12:129–133
Lee JC, Bruck DJ, Curry H, Edwards D, Haviland DR, Van Steenwyk RA et al (2011) The susceptibility of small fruits and cherries to the spotted-wing drosophila, Drosophila suzukii. Pest Manag Sci 67:1358–1367
Lee JC, Dreves AJ, Cave AM, Kawai S, Isaacs R, Miller JC et al (2015) Infestation of wild and ornamental noncrop fruits by Drosophila suzukii (Diptera: Drosophilidae). Ann Entomol Soc Am 108:117–129
Miller B, Anfora G, Buffington M, Daane KM, Dalton DT et al (2015) Seasonal occurrence of resident parasitoids associated with Drosophila suzukii in two small fruit production regions of Italy and the USA. Bull Insectol 68:255–263
Mitsui H, Kimura MT (2010) Distribution, abundance and host association of two parasitoid species attacking frugivorous drosophilid larvae in central Japan. Eur J Entomol 107:535–540
Mitsui H, Takahashi KH, Kimura MT (2006) Spatial distributions and clutch sizes of Drosophila species ovipositing on cherry fruits of different stages. Popul Ecol 48:233–237
Mitsui H, Van Achterberg K, Nordlander G, Kimura MT (2007) Geographical distributions and host associations of larval parasitoids of frugivorous Drosophilidae in Japan. J Nat Hist 41:1731–1738
Murata Y, Ideo S, Watada M, Mitsui H, Kimura MT (2009) Genetic and physiological variation among sexual and parthenogenetic populations of Asobara japonica (Hymenoptera: Braconidae), a larval parasitoid of drosophilid flies. Eur J Entomol 106:171–178
Nomano FY, Mitsui H, Kimura MT (2015) Capacity of Japanese Asobara species (Hymenoptera; Braconidae) to parasitize a fruit pest Drosophila suzukii (Diptera; Drosophilidae). J Appl Entomol 139:105–113
Ometto L, Cestaro A, Ramasamy S, Grassi A, Revadi S, Siozios S et al (2013) Linking genomics and ecology to investigate the complex evolution of an invasive Drosophila pest. Genome Biol Evol 5:745–757
Poyet M, Havard S, Prévost G, Chabrerie O, Doury G, Gibert P et al (2013) Resistance of Drosophila suzukii to the larval parasitoids Leptopilina heterotoma and Asobara japonica is related to haemocyte load. Physiol Entomol 38:45–53
Poyet M, Eslin P, Heraude M, Le Roux V, Prévost G, Gibert P et al (2014) Invasive host for invasive pest: when the Asiatic cherry fly (Drosophila suzukii) meets the American black cherry (Prunus serotina) in Europe. Agri For Entomol 16:251–259
Rossi Stacconi MV, Grassi A, Dalton DT, Miller B, Ouantar M, Loni A et al (2013) First field records of Pachycrepoideus vindemiae as a parasitoid of Drosophila suzukii in European and Oregon small fruit production areas. Entomologia 1:1–15
Rossi Stacconi MV, Buffington M, Daane KM, Dalton DT, Grassi A, Kaçar G et al (2015) Host stage preference, efficacy and fecundity of parasitoids attacking Drosophila suzukii in newly invaded areas. Biol Control 84:28–35
Steffan SA, Lee JC, Singleton ME, Vilaire A, Walsh DB, Lavine LS et al (2013) Susceptibility of cranberries to Drosophila suzukii (Diptera: Drosophilidae). J Econ Entomol 106:2424–2427
Stewart TJ, Wang X-G, Molinar A, Daane KM (2014) Factors limiting peach as a potential host for Drosophila suzukii (Diptera: Drosophilidae). J Econ Entomol 107:1771–1779
Tochen S, Dalton DT, Wiman N, Hamm C, Shearer PW, Walton VM (2014) Temperature-related development and population parameters for Drosophila suzukii (Diptera: Drosophilidae) on cherry and blueberry. Environ Entomol 43:501–510
Van Alphen JJM, Janssen ARM (1982) Host selection by Asobara tabida (Braconidae: Alysinae) a larval parasitoids of fruit inhabiting Drosophila species 2: host species selection. Neth J Zool 32:194–214
Walsh DB, Bolda MP, Goodhue RE, Dreves AJ, Lee J, Bruck DJ, Walton VM, O’Neal SD, Zalom FG (2011) Drosophila suzukii (Diptera: Drosophilidae): invasive pest of ripening soft fruit expanding its geographic range and damage potential. J Integr Pest Manag 2:G1–G7
Wang XG, Kaçar G, Biondi A, Daane KM (2016) Life-history and host preference of Trichopria drosophilae, a pupal parasitoid of spotted wing drosophila. BioControl. doi:10.1007/s10526-016-9720-9
Wiman NG, Walton VM, Dalton DT, Anfora G, Burrack HJ, Chiu JC et al (2014) Integrating temperature-dependent life table data into a matrix projection model for Drosophila suzukii population estimation. PLoS One 9:e106909
Yu D, Zalom FG, Hamby KA (2013) Host status and fruit odor response of Drosophila suzukii (Diptera: Drosophilidae) to figs and mulberries. J Econ Entomol 106:1932–1937
Acknowledgments
We thank Riki York and Kyoo Park (Oregon State University), Hyun Jung Kim and Jongwoo Nam (Seoul Women’s University), Uk-Jin Park and Rameswor Maharjan (Andong National University), Oh-Gyeong Kwon (Kyungpook National University), Deuk So Choi (Department of Plant Quarantine, Anyang-si), and Iksoo Kim (Chonnam National University, Gwangju) for assistance with collections in South Korea and Hongyin Chen and Chenxi Liu (Chinese Academy of Agricultural Science), Zon Qi Chen and Yang Wang (Yunnan Academy of Agricultural Science) for collections in China; John Hutchins and Brandy Chavez (University of California, Berkeley) for assistance with insect rearing in the quarantine; and Ken Bloem (Biological Control Coordinator, USDA, APHIS, PPQ) for program design. Antonio Biondi was supported through the People Programme of the European Union’s Seventh Framework Programme FP7/2007–2013 Project ASCII-PIRSES 318246 and from the Italian Ministry of Education, University and Research (PRIN project GEISCA, 2010CXXHJE_004). Funding for research was supported in the US by the USDA-NIFA award # 2010-51181-21167, the USDA APHIS (Farm bill, fund 14-8130-0463), and the California Cherry Board, and in Italy by the UE FP7/2007–2013 project ASCII under Grant agreement PIRSES-GA-2012-318246. Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the USDA. USDA is an equal opportunity provider and employer.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Traugott.
Rights and permissions
About this article
Cite this article
Daane, K.M., Wang, XG., Biondi, A. et al. First exploration of parasitoids of Drosophila suzukii in South Korea as potential classical biological agents. J Pest Sci 89, 823–835 (2016). https://doi.org/10.1007/s10340-016-0740-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10340-016-0740-0