Abstract
In the present study, an attempt has been made to determine the leachability of selected trace metals like As, Cr, Co, Se, Ni, Cu, Zn, Cd, Pb and Mn from coal gangue sourced from an underground and open cast mining area(s) of Bhupalpally Coalfields of Telangana State, India. A standard laboratory leaching test (dynamic in nature) developed for combustion residues has been adopted to study the leachability of these trace elements as a function of liquid to solid ratio and pH. A series of static column leaching tests were also conducted to investigate the leachability of these selected heavy metal ions simulating field conditions. The column tests revealed that heavy metals from coal gangue exhibit greater mobility particularly under acidic conditions. Further, relatively higher concentrations were leached for both static and dynamic leaching conditions at low pH levels and is attributed to the difference in solubility product values of respective metal ion complexes. Among the targeted metal ions, with a metal extraction of 30% and 65%, respectively, As and Se showed highest mobility from both static and dynamic leaching tests.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Mining is a primary activity in obtaining minerals and fossil fuels that produce enormous amounts of waste, which is a continuous source of contamination posing a threat to the surrounding environment [1]. In India, coal is the fundamental source of fuel for the production of commercial energy. Coal mining is often associated with the generation of huge volumes of wastes at various stages of its extraction and waste generated during the mineral processing phase is popularly known as ‘coal gangue’.
Of the total mining waste, coal gangue alone accounts to 20–40% and it is characterised by its diversity in grain size and petrographic composition [1,2,3,4]. Among the industrial solid wastes, coal gangue is one of the largest and most harmful wastes generated from the coal production process [5]. Currently, coal gangue is loosely stockpiled at the mining sites and annual accumulative stockpiles of coal gangue in India are to an extent of 550 million tonnes [6]. The disposal of such a large amount of coal gangue induces many environmental and ecological issues like the contamination of soil and underground water bodies.
Coal gangue is used extensively as a raw material in thermal power plants to effectively leverage its calorific value. Though, such utilisation measures of reducing the amount of coal gangue can yield immediate economic benefits but can give rise to serious environmental implications. Exploring avenues for sustainable recycling of coal gangue for economic and environmental concerns is a pressing task for geotechnical and environmental engineers [5].
The significant factor which limits the bulk utilisation of many novel geomaterials is the leaching phenomenon of trace elements into the ground surface. Trace elements due to their cumulative nature and inability to decompose in natural processes pose a special concern [7]. Leaching has proven to be one of the primary pathways for trace elements entry into the ecosystem and studies focused on the leaching behaviour of trace elements from coal gangue are relatively sparse [8]. Compared to coal, coal gangue has shown higher trace element concentrations and subsequently, the emission behaviour of coal gangue trace elements will be in contrast to that of coal [9]. Thus, understanding the type and extent of trace element mobilisation in coal gangue becomes extremely pivotal.
In this context, the present work intends to analyse the leaching phenomenon of trace elements from coal gangue from a sustainability perspective. Based on the review of the literature, As, Cr, Co, Se, Ni, Cu, Zn, Cd, Pb and Mn are the trace metal elements selected for the current study. An attempt has been made to study the effect of testing methodology on the leaching behaviour of trace elements by adopting static and dynamic leaching methods. The effect of liquid to solid ratio on the leaching phenomenon of trace metal elements from coal gangue has also been studied.
2 Materials and Methodology
Coal gangue was procured from Kakatiya Coal mines, Bhupalpally, Telangana. The sourced samples were immediately sealed in plastic bags to prevent contamination. These samples were air-dried and crushed prior to passing through a 2 mm mesh sieve to homogenise them for the subsequent analysis. The powdered coal gangue samples were dried in a temperature-controlled oven at 100 °C for 24 h to remove moisture. Later the content was stored in air-tight polyethylene containers. The samples were sealed for 30 days to reach radioactive equilibrium.
The coal gangue samples were digested using an acid mixture (HCl: HNO3) in the ratio of 3:1 in accordance with the procedure described in USEPA (3050B) [11]. After digestion, the concentrations of toxic elements in coal gangue were determined by inductively coupled plasma atomic emission spectroscopy (ICP-AES). The shake extraction of solids as detailed in ASTM D3987-85 [12] was carried out for dynamic leaching analysis. In this method, a varying liquid to solid ratio of 5–100 has been used and agitated at a rate of 29 rpm for a period of 18 h at a controlled temperature of 27 °C.
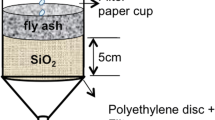
Column apparatus setup described in ASTM D4874-95 [13] has been used for static leaching study. The coal gangue sample was moved into a fixed glass column of 30 mm internal diameter and having a length of 60 cm. The column setup was packed at the top of the sample for uniform dispersion of the solution. The physical properties and chemical composition of the coal gangue are presented in Tables 1 and 2, respectively. And, the particle grain size distribution curve of coal gangue is shown in Fig. 1. It was observed from the grain size distribution curve that no substantial amount of sample passed through the sieve sizes smaller than 0.2 mm mesh sieve.
3 Results and Discussion
3.1 pH of Coal Gangue
The pH of the coal gangue sample was determined using a digital pH metre as detailed in ASTM 4972-13 [14]. The pH was established corresponding to varying liquid to solid (L/S) ratio of 5–100 of deionised double distilled water and coal gangue. From the pH results presented in Fig. 2, it is evident that coal gangue is mostly acidic in nature and the acidity of the sample keeps increasing with L/S ratio up to 80. The highest variation and transition towards the acidic nature from neutral pH was observed at L/S ratio of 20. The continuous decrease in pH with increasing L/S ratio is attributed to an increase in the attack on mineral phases of trace elements by hydrogen ions. Similar observations have been made by Sivapullaiah and Baig [15].
3.2 Static and Dynamic Leaching of Coal Gangue
The cumulative concentration of selected trace metal elements with varying L/S ratio is presented in Fig. 3 and it can be inferred that the leaching phenomenon is complex and distinct to each trace metal element. The variation of cumulative concentration of selected trace metal elements at any L/S ratio is observed to be in the following order: Zn > Mn > Cu > Ni > As > Cr > Se > Co > Pb > Cd. The variation in the cumulative concentration of trace elements with the L/S ratio is may be due to the variations in pH with the L/S ratio.
The metal extraction % (ME) of selected trace elements with varying L/S ratio is presented in Fig. 4. At any L/S ratio, ME of selected trace metal elements was found to be in the order of As > Ni > Zn > Cu > Se > Mn > Cd > Co > Cr > Pb. The oxyanionic elements like As, Cr and pH-sensitive elements like Ni, Cu and Mn exhibited greater leaching under acidic medium with increasing L/S ratio. Whereas, Zn and Se have shown amphoteric leaching phenomenon in both acidic and neutral media. The trace metal elements Pb, Cd and Co have shown a relatively lower amount of extraction even at higher L/S ratio.
To simulate static leaching condition which is usually the case in open cast mines, column studies were performed. A comparison of ME from coal gangue for static leaching and dynamic leaching test was performed at L/S ratio of 20 and the results are presented in Fig. 5. From the results, an inconsistent leaching pattern was observed for both the leaching methods. It was observed that the order of ME is the same for both the tests, but the quantity of ME was relatively higher in static leaching method.
The higher leaching time and greater sample amount in static leaching test are the possible influencing factors for relatively greater mobility of metals [16]. Thus, from this study, it can be concluded that the selected trace metal elements have shown the tendency to leach from the coal gangue. The results obtained are in coherence with the previous studies [8, 16, 17].
3.3 Leaching Mechanism of Selected Trace Metal Elements from Coal Gangue
Arsenic (As) In the current study, As exhibited very high mobility ME of 30%. Being oxyanionic in nature, leaching phenomenon of arsenic is characterised by its pH sensitivity particularly in the acidic medium as it impedes the ability of arsenate to react with other trace elements in forming precipitates [18, 19].
Chromium (Cr (III)) Chromium in bituminous coal is mostly in the trivalent state Cr (III) which is relatively insoluble compared to Cr (VI). Owing to its feebly lower solubility, it remains in insoluble form in the coal gangue matrix.
Cobalt (Co) Though the concentrations of Co in coal gangue are found to be higher, except at L/S of 20, it has largely remained immobile. The greater affinity of Co towards the iron and Fe-bearing species contributes to its poor leaching even under acidic conditions.
Selenium (Se) Coal gangue is highly enriched with Se and among the selected trace metal elements, it exhibited the highest mobility with a ME of 65%. Se usually exists as Selenate in oxidised coal or is bound to Fe oxides. Under both these modes, Se is bound weakly to its respective oxides enabling its easy release [22,23,24].
Nickel (Ni) The release characteristics of Ni are known to be pH-sensitive and the results from this study reconfirm this fact. The leaching phenomenon of Ni is observed to be similar to Se and As.
Copper (Cu) Cu is observed to leach in a greater degree throughout the range of pH and a rate of mobility was particularly higher in acidic conditions. The Cu ions are not controlled by the dissolution of minerals but are dependent on adsorption [18]. The lack of carbonates and oxy-hydroxides, which resist the mobility of Cu by chemisorption might be the detrimental factor in the higher mobility of copper [20].
Zinc (Zn) The Zn is widely regarded as a mobile element and it is often out-competed by other cations like Pb and Cu adsorption sites [25, 26]. Zn is controlled by its ability to form complexes with organic ligands [27]. In the present study, Zn has shown mobility over a wide range of pH reconfirming its amphoteric nature as observed by Van der Sloot [28].
Cadmium (Cd) The high solubility potential in aquatic conditions makes Cd the element of highest concern for the environment. However, compared to other trace elements, coal gangue is short in Cd concentrations. In addition to this, Cd forms Otavite [CdCO3], a relatively insoluble mineral, which further minimises its leaching [18]. In the present study, Cd exhibited relatively lower mobilisation rates compared to other trace elements (Figs. 4 and 5). Though cadmium has shown considerable mobility in acidic medium, the concentration of it is still nominally.
Lead (Pb) Though the concentration of Pb in coal gangue is relatively higher compared to other trace elements, it remains immobile even in acidic medium. This immobility of Pb is attributed to its mineral form, i.e. pyromorphite which is formed due to precipitation of phosphate-based minerals, which is stable and resists hydronium ion interference in acidic medium [20, 29,30,31,32].
Manganese (Mn) From the results, the pH-sensitive leaching behaviour of Mn is observed and mobility of Mn increases with the decrease in pH. The lack of oxy-hydroxides and carbonates, which resist the mobility of Mn by chemisorption could have contributed to the higher mobility of Mn.
4 Conclusions
In the present study, the static and dynamic leaching behaviour of trace metal elements from coal gangue was analysed under varying pH conditions and liquid to solid ratio. The following conclusions are drawn:
-
With an increase in L/S ratio, the pH value of the coal gangue leachate exhibited consistent decrease. The transition from alkaline to acidic medium occurred at a L/S ratio of 20.
-
The order of variations of cumulative concentrations of selected trace metal elements at any L/S ratio is observed to be: Zn > Mn > Cu > Ni > As > Cr > Se > Co > Pb > Cd.
-
At a given L/S ratio, metal extraction (%) of selected trace metal elements was found to be in the order: As > Ni > Zn > Cu > Se > Mn > Cd > Co > Cr > Pb. The study further revealed that this order remained unchanged with increase in L/S ratio.
-
The oxyanionic elements like As, Cr and other pH-sensitive elements like Ni, Cu and Mn exhibited greater leaching rates under acidic medium particularly with an increase in L/S ratio.
-
Zn and Se exhibited amphoteric leaching behaviour under neutral and acidic ranges.
-
Elements Pb, Cd and Co have shown relatively lower extraction rates with increase in L/S ratio.
References
Karaca O, Cameselle C, Reddy KR (2016) Electrokinetic removal of heavy metals from mine tailings and acid Lake sediments from can basin, turkey. In: GSP 273. ASCE, Chicago, pp 225–234
Plewa F, Mysłek Z (2001) Industrial waste management in underground mining technologies. In: ICEE. Gliwice
Keefer RF, Sajwan K (1993) Trace element in coal and coal combustion residues. Advances in Trace Substances. Research. Florida: Lewis Publishers
Jablonska B, Kityk AV, Busch M, Huber P (2017) The structural and surface properties of natural and modified coal gangue. J Environ Manage 190:80–90
Wu H, Wen Q, Hu L, Gong M, Tang Z (2017) Feasibility study on the application of coal gangue as landfill liner material. Waste Manag 63:161–171
Ministry of Coal: Annual Report. Available online: https://coal.nic.in/content/annual-report-(2017)
Chuncai Z, Guijian L, Dun W, Ting F, Ruwei W, Xiang F (2014) Mobility behaviour and environmental implications of trace elements associated with coal gangue: a case study at the Huainan Coalfield in China. Chemosphere 95:193–199
Yang L, Song J, Bai X, Song B, Wang R, Zhou T, Jia J, Pu H (2016) Leaching behavior and potential environmental effects of trace elements in coal gangue of an open-cast coal mine Area, Inner Mongolia. China. Minerals 6(50):1–18
Zhang YY, Nakano J, Liu LL, Wang XD, Zhang ZT (2015) Trace element partitioning behaviour of coal gangue-fired CFB plant: experimental and equilibrium calculation. Environ Sci Pollut Res 22:15469–15478
Moghal AAB, Shamrani MAA, Zahid WM (2015) Heavy metal desorption studies on the artificially contaminated Al-Qatif soil. Int J Geomate 8(2):1323–1327
U.S. EPA.3050B (1996) Acid Digestion of Sediments, Sludges, and Soils, Revision 2. Washington, DC
ASTM D3987 (2016) Standard practice for shake extraction of solid waste with water. ASTM international, West Conshohocken, PA
ASTM D4874 (2016) Standard test method for leaching solid material in a column apparatus. ASTM international, West Conshohocken, PA
ASTM D4972 (2012) Standard test method for pH of soil. ASTM international, West Conshohocken, PA
Sivapullaiah PV, Baig MAA (2010) Leachability of trace elements from two stabilized low lime Indian fly ashes. Environ Earth Sci 61(8):1734–1735
Guo S (2017) Trace elements in coal gangue: a review. In: Contributions to mineralization, Ali Ismail Al-Juboury, Intech Open, vol 6, pp 127–144
Ashfaq M, Heera LM, Moghal AAB (2018) Characterization of heavy metals from coal gangue. In: Indian geotechnical conference. Bangalore
Komonweeraket K, Cetin B, Aydilek A, Benson CH, Edil TB (2015) Geochemical analysis of leached elements from fly ash stabilized soils. J Geotech Geoenviron Eng 141(5):1–14
Izquierdo M, Querol X (2012) Leaching behaviour of elements from coal combustion fly ash: An overview. Int J Coal Geol 94:54–66
Kumpiene J, Langerkvist A, Mauriice A (2008) Stabilization of As, Cr, Cu, Pb and Zn in soil using amendments–a review. Waste Manag 28:215–225
Huggins FE, Huffman GP (2004) How do lithophile elements occur in organic association in bituminous coals? Int J Coal Geol 58:193–204
Riley KW, French DH, Lambropoulos NA, Farrell OP, Wood RA, Huggins FE (2007) Origin and occurrence of selenium in some Australian coals. Int J Coal Geol 72(2):72–80
Shah P, Strezov V, Prince K, Nelson PF (2008) Speciation of As, Cr, Se and Hg under coal fired power station conditions. Fuel 87(10–11):1859–1869
Yudovich YE, Ketris MP (2005) Arsenic in coal: a review. Int J Coal Geol 61(3–4):141–196
Xinde C, Lena QM, Dean RR, Chip SA (2004) Mechanisms of lead, copper, and zinc retention by phosphate rock. Environ Pollut 131(3):435–444
Kiikkila¨ O (2003) Heavy-metal pollution and remediation of forest soil around the Harjavalta Cu–Ni smelter, in SW Finland. Silva Fennica 37(3):399–415
Kiekens L (1995) Heavy metals in soils. Zink. In: Alloway BJ (ed) 2nd ed. Blackie Academic & Professional, Glasgow, UK
Van der Sloot HA (1990) Leaching behaviour of waste and stabilized waste materials. Characterization for environmental assessment purposes. Waste Manag Res 8:215–228
Cornelis G, Poppe S, Van Gerven T, Van den Broeck E, Ceulemans M, Vandecasteele C (2008) Geochemical modelling of arsenic and selenium leaching in alkaline water treatment sludge from the production of non-ferrous metals. J Hazard Mater 159(2–3):271–279
Sivapullaiah PV, Moghal AAB (2011) Gypsum treated fly ash as a liner for waste disposal facilities. Waste Manag 31:359–369
Moghal AAB (2013) Geotechnical and physico-chemical characterization of low lime fly ashes. Adv Mater Sci Eng 1–11:674306
Moghal AAB (2017) A state-of-the-art review on the role of fly ashes in geotechnical and geoenvironmental applications. J Mater Civil Eng 29(8):04017072
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this paper
Cite this paper
Ashfaq, M., Heera Lal, M., Moghal, A.A.B. (2020). Static and Dynamic Leaching Studies on Coal Gangue. In: Reddy, K.R., Agnihotri, A.K., Yukselen-Aksoy, Y., Dubey, B.K., Bansal, A. (eds) Sustainable Environmental Geotechnics. Lecture Notes in Civil Engineering, vol 89. Springer, Cham. https://doi.org/10.1007/978-3-030-51350-4_28
Download citation
DOI: https://doi.org/10.1007/978-3-030-51350-4_28
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-51349-8
Online ISBN: 978-3-030-51350-4
eBook Packages: EngineeringEngineering (R0)