Abstract
Crop plants play an outstanding function in providing food and energy to humans. Rice (Oryza sativa L.) is one of the most important stable crops that have a role in providing the main food to more than half of the world’s people. One of the important factors in increasing yield in rice is the balanced nutrition or supply of the required nutrients in the proper form and ratios. Chemical fertilizers are essential components of modern agriculture by providing essential plant nutrients. However, the overuse of these fertilizers causes serious environmental pollution. But threats of plant pathogens on the attack and damages on the crop productivity cannot be ruled out. Therefore, chemical-based pesticides are thought to be an effective and trustworthy agricultural management measure for repressing pests. Nowadays, the use of beneficial microorganisms and biological control agents are proved as good as synthetic pure/chemicals for the increased plant growth and yield. The diminished utilization of chemical fertilizers for the management of plant pathogens is considered as a secure and maintainable strategy for safe and rewarding agricultural productivity. Based on research conducted until this moment, rice-associated bacteria are encouraging alternatives to chemical fertilizers in an eco-friendly manner. In general, the application of plant growth-promoting bacteria (PGPB) could offer a cheaper and cost-effective approach to overcome the environmental problems caused by chemical fertilizers and their use in the form of biofertilizers and biopesticides could decrease our reliance on synthetic agrochemicals. This chapter highlights the importance of PGPB for enhancing sustainable rice production.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
13.1 Introduction
Cereals are the main source of nutrition for human beings in the world. Among the cereals, rice (Oryza sativa L.) is of great importance. Rice is a staple food in food diet over 40% of the world’s population, especially, in Asia (Naureen et al. 2009a). Of the total energy produced by cereals per person per day, more of it is related to rice. This has made rice as the most important food product in developing countries. According to statistics, the world’s rice cultivation area in 2009 was 153 million hectares, with a production of 585 million tons, which should increase to 800 million tons in 2025. In other words, in order to meet the food needs of this growing population, an increase of 70% in rice production is needed over the next few years. Rice is mostly produced in countries, whose population are growing rapidly and often are limited in terms of land and resources. Therefore, given the limiting factors of production (including decreasing the quality and quantity of agricultural land, reducing water resources and labor shortage), the only rational solution is to increase the yield of rice per unit area of land cultivation or use of high-yielding rice varieties to meet the demand for rice demanded in 2025 (Mishra et al. 2006). However, the use of these varieties requires extensive application of fertilizers such as nitrogen (N) and phosphorus (P) (Hazell 2010). Some of the main constraints on the growth of this crop can be inadequate fertilizer use, pest infestation, and growing of low-yielding traditional varieties, and paucity of water (Datta et al. 2017). In general, one of the most important factors in increasing the rice yield is the balanced nutrition or the supply of essential nutrients. Low-soil fertility is the most important factor which not only seriously affects the rice production but also reduces the quality of the rice (Vaid et al. 2014).
Chemical fertilizers are essential components of modern agriculture due to the provision of essential plant elements. However, the excessive use of these chemical fertilizers for greater production of crop plants including rice can cause unpredictable environmental impacts including leaching and runoff of nutrients, especially N and P, leading to environmental degradation (deterioration in air and water quality) (Gyaneshwar et al. 2002). In addition to essential nutrients, diseases are also among the most significant limiting factors that affect rice production, causing annual yield losses conservatively estimated at five percent (Song and Goodman 2001).
In agricultural systems, the utilization of plant growth-promoting bacteria (PGPB) is of particular consequence in augmenting crop production and preserving sustainable soil fertility (Bagyaraj and Balakrishna 2012). In the past decade, the use of PGPB as a biofertilizer or biological control agent in agriculture has been considered by many researchers. The growth of different crops by these bacteria has been proved in greenhouse and field experiments. Most studies show that these bacteria could have positive and economic effects on crop plants such as corn, wheat, and rice (Freitas and Germida 1990; Çakmakçı et al. 2007; Etesami et al. 2013, 2014a, c, 2015; Ghorchiani et al. 2018; Etesami and Maheshwari 2018) by mechanisms like increasing the availability of soil mineral elements (i.e., through solubilizing insoluble P compounds and potassium (K)-bearing minerals and releasing P and K), producing plant growth-regulating hormones (i.e., indole-3-acetic acid, gibberellin, and cytokinin) and siderophores (increase in availability of Fe, Zn, etc.), producing ACC (1-aminocyclopropane-1-carboxylate) deaminase (decrease of stress ethylene), controlling pathogenic microorganisms (Etesami et al. 2017; Etesami and Maheshwari 2018), and nitrogen fixation (Bhattacharjee et al. 2008; Saharan and Nehra 2011).
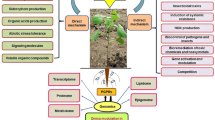
It has been well proven that PGPB could increase plant growth and resistance to environmental stresses (Fig. 13.1) such as salinity (Dimkpa et al. 2009; Egamberdieva and Lugtenberg 2014; Paul and Lade 2014; Choudhary et al. 2016; Qin et al. 2016), drought (Timmusk et al. 2013; Choudhary et al. 2016; Kaushal and Wani 2016; Ngumbi and Kloepper 2016), heavy metal toxicity (Carmen and Roberto 2011; Sessitsch et al. 2013; Ullah et al. 2015), nutritional imbalance (Adesemoye and Kloepper 2009; Yang et al. 2009; Miransari 2013; Chakraborty et al. 2015; Pii et al. 2015; Choudhary et al. 2016), and plant pathogens (bacterium, virus, fungi, etc.) (Compant et al. 2005; Pal and Gardener 2006; Ryan et al. 2008) via miscellaneous mechanisms usually more than one action mechanism (Etesami and Maheshwari 2018). Despite these good reviews, there are a few review studies on PGPB-mediated nutrient availability and biological control of fungal pathogens in rice. Better understanding on interactions of rice with the plant-associated PGPB enhanced nutrient acquisition and controlled fungal rice pathogens is needed for increasing the efficiency of nutrient management and rice disease management in soil and also for promoting eco-friendly low-input sustainable agriculture. Therefore, the aim of this chapter was to reviews advances in research on PGPB capable of increasing the availability of soil insoluble nutrients, their mechanism of action, and their potential use for biofertilization of nutrients in rice and biological control of fungal rice pathogens (Table 13.1).
13.2 Nitrogen-Fixing Bacteria (NFB) in Nitrogen Nutrition
Nitrogen (N) is an important element in the plant and a component of chlorophyll molecules and therefore plays an important role in photosynthesis and in the production of proteins, nucleic acids, and coenzymes. Chemical N-fertilizers are one of the most influential factors in the production and yield of rice. Without the addition of chemical N-fertilizers, the yield of existing varieties is severely limited (Ladha et al. 1997). The excessive use of the chemical fertilizers for greater production of this crop has caused unpredictable environmental impacts. Currently, most of the N-fertilizers are produced through the Haber–Bosch process at chemical fertilizer factories. This process requires a large amount of energy (natural gas or oil), all of which are nonrenewable sources. It also generates carbon dioxide (CO2), which is a greenhouse gas. In developing countries, the cost of purchasing N-fertilizers is usually higher than farmers’ income, which limits yield potential of their crops. Approximately one-third of the applied N (urea-N or nitrate-N, which is applied as fertilizer) is consumed by the plant; the rest of the N can enter as nitrate form into underground waters and are a potential hazard to environmental health. Excess N can also produce nitrous oxide (N2O), an effective greenhouse gas. In addition, since rice grows in an environment susceptible to N loss, more than half of the N-fertilizer used in the paddies is lost through denitrification, ammonia volatilization, and leaching/runoff (Ladha et al. 1997).
In general, the agrosystems that require a lot of N-fertilizers are not sustainable systems because they require the use of nonrenewable natural resources and can endanger the health and the environment (Yanni and Dazzo 2010). Reducing the amount of industrial N production for agricultural systems is one of the important goals of agricultural researchers. In the case of sustainable rice production, an important goal is to replace the industrial N fixation to biological N fixation (Yanni et al. 2001). Two basic ways to solve the problem of N-fertilizer loss in paddy fields can be proposed: One is the regulation of N application time based on rice needs, which increases the efficiency of plant use of applied N and another way is to increase the ability of the rice to biological nitrogen fixation. The second approach is a long-term strategy, but it has multiple environmental benefits and also helps low-income farmers. Additionally, farmers can easily adopt a variety of genotypes with useful features rather than conducting soil and crop management operations that are costly (Ladha et al. 1997).
Recent advances in understanding the legume–rhizobium–symbiotic relationships at the molecular level and the ability to introduce new genes into the rice genome by transformation have made it an excellent opportunity to study the ability of N fixation in rice, although this has remained largely unfinished until now (Dawe 2000). There are such opportunities for cereals including rice. In general, the strategies enabling rice to fix nitrogen are complex and have a long-term nature, but if done, they can increase rice productivity, resource conservation, and environmental security. In addition to the strategies mentioned above, it has been well known that the use of nitrogen fixation technology can reduce the use of N-fertilizers in agricultural land, which can be effective at reducing environmental hazards. Biological nitrogen fixation in rice paddies has significantly contributed to the sustainable yield of these systems. Studies show that biological nitrogen fixation in rice paddies can produce up to 50 kg N per hectare (Elbeltagy et al. 2001). It has been well known that nitrogen fixation through the bacteria associated with rice (associative and free-living bacteria) has a high potential for supply of N for rice. For example, in a study, Mäder et al. (2011) observed an increase of 23% in rice yield obtained upon rice inoculation with N2-fixing Pseudomonas sp. In another study, the co-inoculation of N2-fixing bacteria (i.e., Brevundimonas diminuta PR7, Anabaena oscillarioides CR3, and Ochrobactrum anthropi PR10) remarkably augmented N, P, and K content and bettered rice yield by 21.2%, as compared to the utilization of recommended quantity of N, P, and K fertilizers (Rana et al. 2015). Due to having a very close relationship with the plant, as compared to other bacteria, endophytic bacteria can offer the fixed N to rice without its loss.
Endophyte bacteria seem to be more effective at supplying rice with N than other bacteria. The bacteria isolated from the internal tissues of the plant or isolated from the plants with sterilized surfaces that do not show any symptoms of the disease are regarded as endophytic bacteria (Di Fiore and Del Gallo 1995). It is well documented that a significant diversity of endophytic bacteria such as Pantoea, Burkholderia, Azospirillum, Herbaspirillum, Rhizobium, Methylobacterium, etc., is naturally associated with rice (Carvalho et al. 2014; Mano and Morisaki 2008). Diazotrophs that effectively colonized into rice roots can have a greater potential for N fixation. It has been reported that the contribution of endorhizosphere bacteria to N fixation is much more extensive than the contribution of rhizospheric bacteria because there is no competition in the endorhizosphere with other rhizosphere microorganisms, and carbon sources with low-pressure oxygen oscillations are provided (James et al. 2002). Several endophytic N2-fixing bacteria have been isolated from various rice species including the genera Klebsiella, Citrobacter, Enterobacter, Bacillus, Alcaligenes, Azospirillum, Rhizobium, Sphingomonas, Agrobacterium, Corynebacterium, Herbaspirillum, Azoarcus, Penibacillus, Microbacterium, and Burkholderia (Reinhold-Hurek et al. 2007; Prayitno and Rolfe 2010; Yanni and Dazzo 2010; Gupta et al. 2012; Hongrittipun et al. 2014; Ji et al. 2014).
It has been found that the stimulation of growth of the crop plants (such as rice) inoculated with N2-fixing bacteria may be due to other mechanisms like increasing the availability of soil mineral elements, producing plant growth-regulating hormones, siderophores, and ACC deaminase, and controlling pathogenic microorganisms (Etesami et al. 2017; Etesami and Maheshwari 2018) other than nitrogen fixation (Bhattacharjee et al. 2008; Saharan and Nehra 2011). For example, previous studies have shown that nitrogen accumulation in inoculated non-leguminous plants can be due to either biological N fixation (Elbeltagy et al. 2001; Oliveira et al. 2002) or escalation in nitrogen uptake from soil (Prayitno et al. 1999; Yanni et al. 1997). In another study, Etesami and Alikhani (2016a) showed that bacterial IAA had considerable role in improving use efficiency of N and could increase N content of rice. In other works, Estrada et al. (2013) showed that P-solubilizing diazotrophic bacteria augmented nutrient uptake by rice plants. de Souza et al. (2013) showed that the bacteria (e.g., Herbaspirillum sp., Burkholderia sp., Burkholderia sp., Pseudacidovorax sp., and Rhizobium sp.) unable to solubilize phosphate in in vitro assay and reduce acetylene (low capacity to reduce acetylene) increased levels of N, P, and K in rice shoots. These observations could indicate that growth promotion mechanisms other than N2 fixation such as IAA production and improved nutrient uptake balance (de Souza et al. 2013; Ji et al. 2014). The above studies show that if the purpose of rice inoculation with bacteria is to supply nitrogen to the plant, it is better to use nitrogen-fixing bacteria that have other PGP characteristics (such as IAA, ACC deaminase, siderophores, and phosphate solubilization) as well.
13.3 Phosphate-Solubilizing Bacteria (PSB) in Phosphorus Nutrition
After nitrogen (N), phosphorus (P), as a necessary nutrient and a macronutrient, is the most restricting nutrient for the plant (Schachtman et al. 1998; Theodorou and Plaxton 1993). Phosphorus plays several key roles in the plant, including participation in energy transfer reactions, photosynthesis, deformation of sugar into starch, key enzymatic reactions in important metabolic and signaling pathways, and transference of genetic characteristics in plants (Theodorou and Plaxton 1993). There has been an enduring increment in the application of P fertilizers in rice production (Syers et al. 2008) because it is one of the main restricting factors for upland rice production in many regions of the world (Sahrawat et al. 2001). Since water scarcity is becoming a major problem for agriculture, there is a pressing need to cultivate aerobic rice. Aerobic rice requires the same amount of nutrients as flooded rice, but there is a problem of P availability due to its rapid immobilization/fixation with elements such as calcium (Ca2+), iron (Fe3+), and aluminum (Al3+) (Goldstein 1986; Othman and Panhwar 2014). The previous findings also suggest that P deficiency in aerobic crops is quite common (Fageria 2001).
Phosphorus is the most sensitive nutrient to soil pH. The best pH for P uptake by the plant is 6.5. In alkaline condition, P becomes insoluble by reacting with calcium (Ca2+), whereas in acidic soils, it reacts with iron (Fe3+) and aluminum (Al3+) and becomes unavailable to the plants. The amount of P absorbed by the plant in the soil is controlled by several factors such as soil pH, calcium ion concentration, soil organic matter, clay type, and clay amount, root density and exudates, and soil moisture and texture. In order to compensate for the shortage of P, large amounts of P fertilizers are added to the soil annually. The excessive use of P fertilizers and the subsequent accumulation of P in the soil, in addition to increasing costs, have a negative effect on uptake of micronutrients and also contribute to environmental pollution (e.g., eutrophication).
The majority of P fertilizers are adsorbed by solid particles and stored in a solid phase of soil. Most of the P in the fertilizer, after entering the soil, gradually turns into insoluble compounds and is stored as plant unavailable forms in the soil (Dey 1988). It has been reported that the P fertilizer use efficiency in calcareous and alkaline soils does not exceed 20%. The P mobility in the soil is very low and cannot respond to the rapid absorption of the plant. This leads to the emergence and development of phosphate-depleted areas adjacent to the contact surface of roots with soil. Under P-deficient conditions, by modifying root morphology, carbon metabolism; membrane structure, exudation of organic acids, protons, and enzymes; and association with mycorrhizal fungi, and harboring phosphate-solubilizing microorganisms (PSM), some plants have been able to somehow compensate for their lack of P (uptake of adequate P) (Begum and Islam 2005; Islam and Hossain 2012). Among these strategies, secretion of organic acids and association of mycorrhizal fungi are very poor in rice under flooding conditions (Begum et al. 2005; Islam and Hossain 2012). Therefore, the rice plants need an auxiliary system that can easily go beyond these depleted areas and, by developing a wide network around the root system, receive P from an exorbitant volume of adjoining soil.
PGPB such as phosphate-solubilizing bacteria (PSB) are considered to be the most effective plant assistants for the supply of P at the optimal level and seems to be another manner for P nutrition in rice under P-insufficient tropical soils (Islam and Hossain 2012). PSB have been able to dissolve insoluble phosphates through a set of mechanisms such as production of low-molecular-weight organic acids (i.e., gluconic, oxalic, 2-ketogluconic, citric, succinic, lactic, and malic), inorganic acids, siderophores, and exopolysaccharides (EPS), and secretion of hydrolytic enzymes (e.g., phosphatases and phytases, which convert the organic forms of P into P inorganic forms, and thereby increase plant growth under conditions of P deficiency) (Khan et al. 2007, 2014; Sharma et al. 2013). PSB have the ability to dissolve inorganic P in a range of 25–42 µg P ml−1 and organic P between 8 and 18 µg P ml−1 (Guang-Can et al. 2008). Agrobacterium, Pseudomonas, Bacillus, Rhizobium, Flavobacterium, Acinetobacter, Micrococcus, Burkholderia, Achromobacter, Erwinia, Pantoea, and Streptomyces are of the most important bacterial genera of solubilizing insoluble phosphates (Khan et al. 2007, 2014; Sharma et al. 2013).
In addition to improving soil P status, members of the bacterial genera such as Burkholderia, Pseudomonas, Bacillus, Streptomyces, and Pantoea could also suppress soil-borne pathogens (Islam and Hossain 2012; Rodrı́guez and Fraga 1999). PSB, which form less than one percent total bacterial populations in the soils (Kucey 1983), have been isolated from approximately all agricultural soils (both fertile soils and P-deficient ones) (Oehl et al. 2001). Previous studies show that rhizosphere and endorhiza of rice plants also harbor the bacteria with a good potential for solubilizing insoluble phosphates such as Bacillus spp., Pantoea agglomerans, Streptomyces anthocysnicus, Pseudomonas pieketti, P. aeroginosa, Acinetobacter sp., Klebsiella sp., Acinetobacter sp., Enterobacter sp., Microbacterium sp., Pseudomonas sp., B. megaterium, B. firmus, Erwinia, Serratia, and Staphylococcus epidermidis (Etesami et al. 2014a; Islam and Hossain 2012; Naik et al. 2008; Panhwar et al. 2011a; Sapsirisopa et al. 2009; Thakuria et al. 2004; Zeng et al. 2012). Previous studies show that PSB alone or in combination with varying doses of P fertilizers could also increase soil available P and P content in the rice plant tissue (Duarah et al. 2011; Othman and Panhwar 2014; Panhwar et al. 2011a, b, 2013). There are reports that PSB also have the ability to increase the efficiency of P fertilizer and diminish about 25–50% of the required P to plants (Attia et al. 2009; Islam and Hossain 2012; Yildirim et al. 2011). In addition to increasing the efficiency of P fertilizer, PSB also increased total N, K, Ca, S, P, Mg, Fe, Mn, Zn, and Cu contents in plant tissues (Duarah et al. 2011; Gyaneshwar et al. 2002; Islam and Hossain 2012; Yildirim et al. 2011).
It is well known that PSB can increase plant seed germination (Duarah et al. 2011; Sapsirisopa et al. 2009), plant growth and development (i.e., augmented leaf chlorophyll content, leaf area index, tiller numbers, plant height, photosynthesis rate, root morphology, and plant biomass of aerobic rice genotypes) (Duarah et al. 2011; Panhwar et al. 2011a, b), and plant yield and quality (Islam and Hossain 2012), through other mechanisms such as phytohormone production, nitrogen fixation, urease activity, siderophore production, ACC deaminase, and/or antagonisms against phytopathogens, in addition to by solubilizing insoluble phosphates (Islam and Hossain 2012). In general, the above studies show that PSB have been found to have the ability to solubilize P in soil and could reduce fertilizers inputs in rice fields.
13.4 Plant Growth-Promoting Bacteria (PGPB) in Micronutrient Nutrition
Similar to macronutrients, micronutrients are also required for optimum plant growth. Micronutrient deficiencies are omnipresent (Das 2014). For example, 50% of world cereal soils are deficient in zinc (Zn) and 30% of cultivated soils globally are deficient in iron (Fe). Fe deficiency is common in upland, high pH, and aerobic soil due to the low solubility of the oxidized ferric form in aerobic environments (Das 2014; Samaranayake et al. 2012; Zuo and Zhang 2011). Rice is also substantially deficient in Fe (Bouis and Welch 2010). Toxicity of Fe is one of the major constraints to lowland rice production (Das 2014). Manganese (Mn) deficiency is also very common in upland rice (Das 2014). In general, micronutrients-deficient soils hamper the growth of many plants including staple foods such as wheat, rice, corn and sugarcane (Kamran et al. 2017).
Use of micronutrients fertilizers may not be cost-effective in alleviating deficiency of these nutrients and increasing yield of these crop plants. It has been known that bacteria can cause a substantial increase in concentration of micronutrients in crop plants (Etesami and Maheshwari 2018) including in rice grains (Mäder et al. 2011; Pooniya et al. 2012) through various mechanisms such as acidification, production of organic acids/organic anions in soil, which sequester the cations of micronutrients and decrease the pH of the adjacent soil as well as chelate micronutrients and enhance the solubility of these nutrients, and production of siderophores, which mainly form the complexes with Fe(III) (Alexander 1977; Etesami and Maheshwari 2018; Jones and Darrah 1994; Kamran et al. 2017; Saravanan et al. 2007). For example, Zn-solubilizing bacteria such as Pseudomonas fragi, Pantoea dispersa, Pantoea agglomerans, E. cloacae, and Rhizobium sp. are potential alternatives for Zn supplementation and convert applied inorganic Zn to available forms (Kamran et al. 2017). In a study, Vaid et al. (2014) showed that Zn-solubilizing Burkholderia and Acinetobacter caused significant increase in productive tillers per plant (15.1%), number of panicles per plant (13.3%), total Zn uptake of rice (52.5%), the mean dry matter-yield per pot (12.9%), yield of straw (12.4%), yield of grain (17.0%), and number of grains per panicle (12.8%) relative to rice plants non-inoculated with the bacterial isolates in a Zn-deficient soil. It was reported that this increment might be due to solubilization of insoluble soil Zn via generating gluconic acid by these bacteria. In another study, co-inoculation of rice with Providencia sp. PR3, Brevundimonas diminuta PR7, and Ochrobactrum anthropi PR10 recorded an increment of 13–16% in Fe, Zn, Cu, and Mn concentrations, respectively, in rice grains (Rana et al. 2015). Adak et al. (2016) also observed 13–46% enhancement of Fe and 15–41% enhancement of Zn in rice grains through the use of cyanobacterial inoculants, under different modes of rice cultivation. The above studies indicate the potential of the PGPB associated with rice to be used as biofertilizer and overcome deficiency of micronutrients.
13.5 Silicate-Solubilizing Bacteria (SSB) in Silicon Nutrition
Silicon (Si) is known as the second most copious element in soils (Epstein 1994). Utilization of Si is known as an ecologically congenial and environmentally friendly technique to augment plant growth, attenuate miscellaneous environmental stresses (i.e., nutritional imbalance, salinity, drought, heavy metal toxicity, and pathogens) in plants, and enhance the plant resistance to multiple stresses (Etesami and Jeong 2018). Despite these benefits, Si is still not classified as an essential element but considered as a beneficial element. This element is useful for some plants such as monocotyledons and Poaceae species (Etesami and Jeong 2018; Epstein 1999; Ma et al. 2007). Rice is one of the plants that accumulate this element (a Si-accumulator/a siliceous plant-containing Si up to 10% in shoots on a dry weight basis) and requires high Si content (a high Si-accumulating crop) (Ma and Takahashi 2002). Rice is known that the escalation in its yield per unit area is connected with Si depletion, which is a matter of concern (Savant et al. 1997). Due to being removed from the soil to produce every 100 kg brown rice (about 20 kg/hm2 SiO2) (Ma and Takahashi 2002), being exported from fields by removing straw residues with the harvest by farmers, and being connived the exogenous use of Si in rice cultivation, plant accessible Si in paddy fields is usually low (Cuong et al. 2017; Etesami and Jeong 2018; Ma and Takahashi 2002). This suggests that Si may become a yield-limiting element for rice production and its exogenous application may be necessary to Si-deficient paddy soil for an economic and sustainable rice production system (both high rice yield and disease resistance) (Bocharnikova et al. 2010; Ning et al. 2014). At the present time, Si-fertilizers are exerted in many countries for augmenting rice yield (Guntzer et al. 2012). In previous studies, the positive effects of Si on rice growth and yield have been reported (Detmann et al. 2012; Gerami et al. 2013; Etesami and Jeong 2018; Jawahar and Vaiyapuri 2013; Lavinsky et al. 2016; Liang et al. 1994; Pati et al. 2016; Prakash et al. 2011; Singh et al. 2005). For example, in a study, Cuong et al. (2017) showed that application of Si in combination with the recommended dose of N, P, and K fertilizers positively affected agronomic and yield-related traits, yield and nutrient uptakes of rice. Si had also beneficial effects on disease resistance of rice (i.e., brown spot caused by the fungus Bipolaris oryzae, rice blast caused by the fungus Pyricularia grisea, and sheath blight caused by Rhizoctonia solani Kuhn, which are becoming more severe on rice plants are grown in Si-depleted soils) (Abed-Ashtiani et al. 2012; Ashtiani et al. 2012; Cacique et al. 2012; Dallagnol et al. 2014; Fauteux et al. 2005; Hayasaka et al. 2005; Ning et al. 2014; Prabhu et al. 2001; Rodrigues and Datnoff 2005; Sakr 2016; Song et al. 2016; Van Bockhaven 2014) by various mechanisms such as maintaining mesophyll cells relatively intact, increasing the thickness of silicon layer, enhancing physiological or induced resistance to fungal colonization (Si acts as a modulator of host resistance to pathogen), depositing in host cell walls and papillae sites, which is the first physical barricade for fungal penetration (Ning et al. 2014), and accumulating phenolics and phytoalexins as well as with the activation of some PR-genes (Rodrigues and Datnoff 2005).
There are some bacteria like Bacillus globisporus, B. mucilaginosus, B. flexus, B. megaterium, Burkholderia eburnean, and Pseudomonas fluorescens that can mobilize K and Si from silicate minerals (i.e., feldspar, muscovite, and biotite) (Kang et al. 2017; Naureen et al. 2015a; Sheng et al. 2008; Vasanthi et al. 2018; Vijayapriya and Muthukkaruppan 2010) by various mechanisms such as producing excess proton, organic ligands, organic acids (i.e., gluconic acid), hydroxyl anion, extracellular EPS, and enzymes (Meena et al. 2014). Inoculation of rice with silicate-solubilizing bacteria (SSB) also caused a significant increase in growth and yield of this plant. In a study (Kang et al. 2017), when combined with silica fertilization, soil inoculation with Burkholderia eburnean CS4-2 promoted all rice growth attributes over those of the water-treated (control) and insoluble silica-fertilized plants. In addition to solubilizing Si, K, and P (e.g., by competing with P fixation sites in soil and decreasing the availability of Fe and Mn in plants) (Kannan and Raj 1998; Sahebi et al. 2015), SSB were also capable of controlling the growth of fungal pathogens such as Magnaporthae grisae, Rhizoctonia solani, Altarnaria alternata, and Macrophomina pheasolina (Naureen et al. 2015a). In addition to the role of Si in increasing rice resistance to pathogens, SSB can antagonize fungal pathogens by the production of hydrolytic enzymes, HCN (hydrogen cyanide), siderophores, and antibiotics (Hassan et al. 2010; Naureen et al. 2015b, 2009b). In a previous study (Vijayapriya and Muthukkaruppan 2010), B. mucilaginosus, which was efficient in silicate solubilization, showed antagonistic activity against Pyricularia oryzae. The above studies indicate the potential of SSB to be used as biofertilizer for overcoming Si deficiency and as biocontrol agents for controlling fungal rice pathogens.
13.6 Combined Use of PGPB and Chemical Fertilizers for Rice Production
Application of biological fertilizers, in particular, GPGB, combined with the use of fertilizers, is the most important integrated plant nutrition strategy for sustainable agricultural management and increasing their production in a sustainable agricultural system with sufficient input (Bagyaraj and Balakrishna 2012; Etesami and Alikhani 2016b).
Beneficial effects of PGPB in increasing nutrient uptake by rice, including NPK uptake, have been reported in previous studies (Adesemoye and Kloepper 2009; Adesemoye et al. 2009; Biswas et al. 2000; Duarah et al. 2011; Etesami and Alikhani 2016a; Vessey 2003). It has been reported that the PGPB can diminish the exertion of chemical fertilizers without compromising with the growth and yield of rice under nutrient-poor soil conditions (Etesami and Alikhani 2016a; Khan et al. 2017). In a study, Etesami and Alikhani (2016a) showed that co-inoculation with endophytic (Pseudomonas putida REN5) and rhizosphere (Pseudomonas fluorescens REN1) bacteria can reduce application rates of N-fertilizer up to 25% for rice plant. These researchers showed that the compound application of P. putida REN5 and P. fluorescens REN1 and nitrogen fertilizer levels (50, 75, and 100% of the recommended N-fertilizer rate) compared to the application of these bacterial isolates with minimum nitrogen fertilizer (25% of the recommended N-fertilizer rate) and or control (25% of the recommended N-fertilizer rate) significantly increased the rice growth indices. It was found that 75% of the recommended fertilizer rate was the minimum level to diminish N-fertilizer. This indicates that nitrogen plays a key role in the growth of the rice plant, and the plant’s yield decreases without the presence of nitrogen. de Souza et al. (2013, 2016) showed that rice plants inoculated with bacterial strains (Herbaspirillum sp. AG15, Herbaspirillum sp. AC32, Pseudacidovorax sp. AC32, Burkholderia sp. CA21, Azospirillum sp. UR51, and Rhizobium sp. UR51), which were isolated from rice rhizosphere, with 50% of the recommended N-fertilizer rate achieved growth indices (i.e., shoot length and dry matter, the number of panicles, and plant yields) similar to those that received the full-fertilization dose without inoculation. Other researchers also confirmed that single PGPR or combinations of PGPR promoted the growth of rice, increased plant height, dry shoot matter, N, P, and K uptake and grain production even when the recommended amount of nitrogen fertilizer was reduced in half (Biswas et al. 2000; Duarah et al. 2011; Khorshidi et al. 2011; Yanni and Dazzo 2010). In addition, rice plants inoculated with these bacterial strains supplemented with 50% N fertilizer accumulated a higher amount of N and P than those that received 100% of N fertilizer alone (de Souza et al. 2016). Khan et al. (2017) also inoculated rice with Burkholderia sp. BRRh-4 and Pseudomonas aeruginosa BRRh-5 along with 50% of recommended N, P, and K fertilizers. Both bacterial strains generated equivalent or higher grain yield of rice relative to the control––plants grown with full recommended––fertilizer doses. The above studies show that PGPB can interact with rice plant under different nitrogen fertilizer levels, but this interaction can be much more productive when plants are treated with low levels of chemical N-fertilizers (de Souza et al. 2016).
Generally speaking, it is believed that PGPB are more effectual in augmenting plant growth under restricting nutrient conditions. Besides, the colonization of the plant root by PGPR might have been repressed by the augmenting levels of nutrients (i.e., N) in the growth medium (Egamberdiyeva 2007; Shaharoona et al. 2008). It was also reported that these bacteria can be used as a supplement to chemical fertilizers to reduce the use of fertilizers but cannot replace nitrogen fertilizer in rice (Etesami and Alikhani 2016a). Generally speaking, the PGPB-based inoculation technology should be consumed along with desired levels of fertilization to achieve maximal benefits in terms of fertilizer savings, nutrient uptake, and rice plant growth (de Souza et al. 2016).
13.7 Biological Control of Fungal Rice Pathogens
Pathogenic microorganisms affecting plant fitness are an outstanding and chronic threat to food production and ecosystem steadfastness throughout the world (Compant et al. 2005). Diseases of fungal, bacterial, viral origin, and damage brought about by insects and nematodes can be led to a significant diminution in crop production. Diseases are one of the most important limiting factors affecting rice production, which reduces annual rice yield by about 5% (Song and Goodman 2001). More than 70% of the diseases caused by fungi, bacteria, viruses or nematodes have been reported in rice (Manandhar et al. 1998). In other words, rice is susceptible to diseases. Pathogenic fungi can reduce the quality and quantity of rice grain production (Chaiharn et al. 2009) and affliction with these fungi are among the most niggling of these diseases as it may result in remarkable crop yield losses (Chaiharn et al. 2009; Suprapta 2012). In addition, the consumption of mycotoxins (e.g., aflatoxins, citrinin, ochratoxin A, fumonisins, and zearalenone)-polluted rice can be hazardous to human beings (Almaguer et al. 2012; Ferre 2016).
To control fungal diseases, fungal pathogens-resistant rice cultivars and fungicides are commonly used. But, due to the loss of resistance to pathogens, despite the high variability of disease agents of the pathogen population, the useful life of many pathogen-resistant cultivars is only several years. Use of fungicides is also expensive and environmentally unfriendly and has led to risks to human health, environmental pollution, residual toxicity, development of pesticide resistance, and other beneficial organisms in the soil (Komárek et al. 2010; Suprapta 2012; Yoon et al. 2013). These fungicides also reduced soil fertility and quality and damaged to natural ecosystems (Chaiharn et al. 2009). Furthermore, there are a number of painstaking diseases for which chemical solutions are few, unproductive, or nonexistent (Gerhardson 2002). Biocontrol is thus being considered as an alternative or a supplemental way of diminishing the utilization of chemicals in agricultural land (Compant et al. 2005, 2010; Etesami and Alikhani 2016b, 2016d; Gerhardson 2002; Pal and Gardener 2006; Suprapta 2012; Welbaum et al. 2004). Bacterial biocontrol agents can control plant pathogens including fungal pathogens by various mechanisms (Fig. 13.2). Various suitable nutrient-rich niches on/or inside roots attract a great diversity of microorganisms, including phytopathogens. Competition for the nutrients (root exudates including organic acids, amino acids, specific sugars, etc.) and niches is a underlying mechanism by which PGPB preserve plants from phytopathogens (Compant et al. 2005).
Biocontrol PGPB are aggressive root colonizers and play an important role in the biological control of plant diseases caused by soil-borne fungal pathogens (Chaiharn et al. 2009). Another mechanism of biological control by PGPR is production of allelochemicals like (i) iron(III)-chelating siderophores, which deprive pathogenic fungi of Fe since the fungal siderophores have lower affinity to Fe compared to bacterial siderophores (Loper and Henkels 1999; O’sullivan and O’Gara 1992; Van Loon and Bakker 2005); (ii) production of antibiotics such as amphisin, 2,4-diacetylphloroglucinol (DAPG), rhizoxin, oomycin A, phenazines, tensin, pyoluteorin, pyrrolnitrin, tensin, tropolone, oligomycin A, kanosamine, zwittermicin A, xanthobaccin, viscosinamide, and cyclic lipopeptides (Compant et al. 2005; de Souza et al. 2003; Défago 1993; Hashidoko et al. 1999; Joseph et al. 2012; Kai et al. 2009; Kim et al. 1999; Nain et al. 2012; Nielsen et al. 2002; Pal and Gardener 2006); (iii) biocidal volatiles like HCN and ammonia (NH3) (Blumer and Haas 2000; Kai et al. 2009; Pal and Gardener 2006; Zou et al. 2007); (iv) lytic enzymes (Chernin and Chet 2002; Sindhu and Dadarwal 2001) such as chitinase (Ordentlich et al. 1988), which inhibits spore germination and germ-tube elongation (Frankowski et al. 2001), laminarinase, which digests and lyses mycelia of some fungi (Lim et al. 1991), β-1,3-glucanase, which lyses fungal cell walls of some fungi (Fridlender et al. 1993; Singh et al. 1999), glucanases, cellulases, and detoxification enzymes (Abbas-Zadeh et al. 2010; Fridlender et al. 1993; Kai et al. 2009; Nain et al. 2012; Pal and Gardener 2006; Sindhu and Dadarwal 2001; Zhao et al. 2010). ISR (induced systemic resistance) is an consequential mechanism by which PGPR in the rhizosphere prime the whole plant body for augmented defense against a scopious range of pathogens and insect herbivores (Compant et al. 2005; Pal and Gardener 2006; Van Loon and Bakker 2005). Biocontrol PGPR, through different mechanisms such as production of siderophores, lipopolysaccharides (Leeman et al. 1995; Maurhofer et al. 1994; Meziane et al. 2005; Van Loon and Bakker 2005; Van Loon et al. 1998; Van Wees et al. 1997), volatile organic compounds (Ping and Boland 2004; Ryu et al. 2004), cyclic lipopeptides, 2,4-diacetylphloroglucinol, and homoserine lactones (Lugtenberg and Kamilova 2009), sensitize the plant immune system for enhanced defense without directly activating overpriced defenses (Pieterse et al. 2014).
Biocontrol PGPR-mediated control of several bacterial, fungal, and viral plant diseases in plants by this mechanism (ISR) has been reported (Leeman et al. 1995; Pal and Gardener 2006; Park et al. 2009). It has been also known that the ISR contains ethylene and jasmonate intracellular signaling, and these hormones stimulate host plant defense responses against plant diseases (Glick 2012). Biocontrol PGPR-mediated ISR also fortifies plant cell wall strength (Benhamou et al. 1996, 1998), alters host physiology and metabolic responses (Jeun et al. 2004; Park and Kloepper 2000), and increases accumulation of compounds (i.e., phenylalanine ammonia-lyase, peroxidase, phytoalexins, polyphenol oxidase, and/or chalcone synthase) (Chen et al. 2000; Ongena et al. 2000) that augment synthesis of plant defense chemicals upon challenge by plant pathogens (Compant et al. 2005; Nowak and Shulaev 2003; Ramamoorthy et al. 2001). The total of these changes lead to increased plant resistance to diseases. Generally speaking, the most effectual biological control agents (BCAs) studied to date appear to antagonize pathogens using multitudinous mechanisms (Iavicoli et al. 2003; Pal and Gardener 2006).
The ability of biocontrol PGPR to lessen or prevent the deleterious effects of certain fungal rice pathogens has been well documented (Amruta et al. 2018; Awla et al. 2017; Chaiharn et al. 2009; Etesami and Alikhani 2016b, 2016d, 2018; Velusamy and Gnanamanickam 2008; Verma et al. 2018).
Magnaporthe oryzae (anamorph Pyricularia oryzae), which causes diseases generically called “blast disease” or “blight disease—the most destructive disease of rice (Chaiharn et al. 2009; Dean et al. 2012) and attacks rice plants at all stages of development and infects the aerial parts of the rice plant—including leaves, nodes, stems, and panicles, bringing about annual losses of approximately 10–30% in miscellaneous rice—producing regions (Law et al. 2017), Alternaria sp., which cause leaf spots, Fusarium oxysporum, which cause root rot, Sclerotium sp., which cause stem rot (Chaiharn et al. 2009), Bipolaris oryzae, which causes brown spot disease, Rhizoctonia solani, which causes sheath blight disease, Curvularia oryzae, which causes leaf spot disease, Gibberella fujikuroi, which causes bakanae disease in rice seedlings, and Rhizoctonia oryzae-sativae, which causes aggregate sheath blight disease, have been reported as the most consequential fungal pathogen bringing about diseases in rice (Boukaew et al. 2013; Tamreihao et al. 2016). By a combination of different modes of action such as hydrogen ions and gaseous products including ethylene, HCN and NH3, and siderophore (hydroxamate type), cell wall degrading enzymes (i.e., chitinase, protease, cellulase, β-1,3-glucanase, β-1,4-glucanase, and lipase) and antibiotics, biocontrol PGPB (e.g., Streptomyces sp. S. globisporus, S. sindeneusis, S. flavotricini, S. philanthi, S. vinaceusdrappus, S. corchorusii, Ochrobactrum anthropic, Bacillus sp., B. cereus, B. subtilis, B. methylotrophicus, Enterobacter sp., Pseudomonas aeruginosa, and Pseudomonas sp.) significantly inhibited the mycelia growth of these fungi (Awla et al. 2017; Boukaew et al. 2013; Boukaew and Prasertsan 2014; Chaiharn et al. 2009; Khalil et al. 2014; Li et al. 2011; Ningthoujam et al. 2009; Prapagdee et al. 2008; Shan et al. 2013; Tamreihao et al. 2016; Tokpah et al. 2016; Zarandi et al. 2009).
In previous studies, Etesami and Alikhani (2016d), (2017), and Etesami et al. (2014b) investigated the potential of antifungal activity of the bacterial isolates isolated from rhizosphere and endorhiza of rice, oilseed rape (Brassica napus L.), and berseem clover (Trifolium alexandrinum L.), respectively, against five rice pathogenic fungi (Magnaporthe oryzae, M. salvinii, Fusarium verticillioides, F. fujikuroi, and F. proliferum—the most important pathogenic fungi of rice in Iran) under in vitro conditions. A considerable part of these isolates showed a good percentage of mycelial growth inhibition against all the tested major rice fungal pathogens in dual cultures on solid media (Fig. 13.3) (Etesami and Alikhani 2016d).
Dual culture assay for in vitro inhibition of mycelia of fungal rice pathogens by the endophytic and rhizoshpere strains grown on PDA agar for 5 days. a endophytic strain B. subtilis CEN3; b rhizosphere strain B. cereus CEN5; c endophytic isolate; d rhizosphere isolate; e and f control (pathogen alone); g combination of endophytic and rhizosphere isolates with each other; and h rhizosphere isolate resulted in no inhibition zones
Bacillus species (Bacillus mojavensis, B. amyloliquefaciens, B. subtilis, and B. cereus) were reported as the most propitious bacterial biocontrol agents in rhizosphere and endorhiza of these plants (Etesami and Alikhani 2018). In addition, endophytic bacterial isolates were more effective at mycelial growth inhibition than rhizosphere bacterial isolates. Probably endophytic bacteria use mechanisms similar to PGPR to control plant fungal pathogens. Biocontrol activities of these bacterial strains may be owing to the creation of antifungal metabolites, volatile organic compounds (VOCs), siderophores, and cell wall degrading enzymes (Etesami and Alikhani 2016c).
Among biocontrol bacteria, spore-forming Bacillus bacteria have properties that make them more suitable for development as biocontrol agents, including high resistance to stress, production of various secondary metabolites, induction of ISR in order to reduce the severity of the disease caused by a wide range of pathogens, stimulating plant growth, simplicity in cultivating and maintaining them, as well as use of them as spores on plant or seed inoculation (Alina et al. 2015; Shafi et al. 2017). Besides, Streptomyces bacteria also appear to be auspicious biocontrol agents against a wide range of phytopathogenic fungi due to generating various bioactive compounds such as antibiotics (e.g., Blasticidin S, Kasugamycin, Oligomycin A, geldanamycin, and nigericin) or antifungals which can inhibit or kill the pathogen (Copping and Duke 2007; González-Franco and Robles-Hernandez 2009; Law et al. 2017; Tapadar and Jha 2013; Trejo-Estrada et al. 1998; Yang et al. 2010), the release of extracellular lytic enzymes such as chitinases and glucanases, which play consequential roles in ruination of fungal cell walls (El-Tarabily et al. 2000; González-Franco and Robles-Hernandez 2009; Palaniyandi et al. 2013), and their colonization ability, competitive traits, and survival in various types of soil (ability to produce spores which allow them to survive longer and in various extreme conditions) (González-Franco and Robles-Hernandez 2009; Law et al. 2017; Ningthoujam et al. 2009). Under greenhouse conditions, Streptomyces could result in up to 88.3% disease diminution of rice blast (Law et al. 2017). Approximately, 75% commercially practicable antibiotics were derived from the genus Streptomyces (Kashif et al. 2016). Besides, Streptomyces produces spores that help dissemination and confer resistance to many hostile conditions (Goodfellow and Williams 1983). The biocontrol bacteria not only prevent the growth of pathogens, but also improve plant growth. These bacteria were also positive for different PGP traits such as IAA, ACC deaminase, siderophores, and phosphate solubilization, and could significantly enhance the growth and grain yield production of the plants (Alina et al. 2015; Etesami and Alikhani 2016c, 2017; Shafi et al. 2017; Tamreihao et al. 2016).
There are many studies that show bacterial biocontrol agents can be very promising antagonist candidates against plant pathogens which can be developed for sustainable plant diseases management. Despite these studies and the recent interest in bioassays of plant diseases, it is difficult to find examples of commercial use of biological control agents in controlling pathogens. This can be due to inappropriate screening systems that are used. In general, biocontrol PGPB by colonizing the root system of the plant prevent the establishment of harmful rhizospheric microorganisms on the root of the plant. These rhizobacteria must compete with indigenous microorganisms and effectively colonize the rhizosphere. In other words, the biocontrol agents and PGPB are influenced by native microbial communities. Generally, the antagonistic activity of biocontrol bacteria is tested through in vitro inhibition of fungal pathogens in dual cultures on solid media and then confirmed in bioassays on host plants. It has been reported that in vitro evaluations have some limitations (Compant et al. 2005). Many biological control agents effectively control diseases in vitro conditions, but have not been successful in field conditions. The ineffectiveness of biocontrol bacteria in the field is often attributed to their inability to colonize the roots. Rhizosphere competence and colonization are considered as an important factor in controlling fungal pathogens by biocontrol bacteria because both organisms colonize the same ecology niche and use the same nutrient (Compant et al. 2005). Factors such as temperature, soil moisture, soil texture, and environmental stresses affect the survival and establishment of bacteria.
In general, in many studies, a single biological control agent is usually used to control a pathogen under controlled and greenhouse conditions. This can sometimes result in incompatible performance by the biological control agent under natural conditions because a biological control agent cannot be active in all types of soil environments/agricultural ecosystems (Raupach and Kloepper 1998) or against all pathogens that attack the host plant (there is usually more than one pathogen in the soil). Moreover, this may also be due to inadequate colonization, limited resistance to changes in environmental conditions, and fluctuations in the production of antifungal metabolites by this biological control agent (Dowling and O’Gara 1994). Several solutions have been proposed to overcome these problems including the combined use of two or more isolates in biological control (Raupach and Kloepper 1998). Mixtures of biological control agents with different plant colonization patterns or a biological control agent with antifungal activities against several pathogens (formulation of a biocontrol isolate is simpler and cheaper than that of multiple biocontrol isolates) can be useful for controlling the biological diversity of miscellaneous pathogens via assorted mechanisms of repression of the disease. In general, the use of a combination of bacterial antagonists for biological control of pathogens can expand the range of antifungal activities (protection of the plant against a wide range of fungal pathogens), increase the efficiency, sustainability, and effect of biological control agents, and combine different characteristics without applying genetic engineering. In addition, designing a combination of biocontrol isolates and the use of multiple antifungal properties demonstrated by these isolates can be useful in the sense that at least one of the biological control mechanisms among these isolates may exist under unpredictable field conditions. In addition, mixtures of biocontrol microorganisms can increase the genetic diversity of biological control systems that prolong the stay in the rhizosphere and use a spacious range of biological control mechanisms.
A higher efficiency of several isolates from biocontrol agents against plant pathogens has been reported in previous studies (Etesami and Alikhani 2016c; Lucas et al. 2009; Schisler et al. 1997). In addition to controlling the disease, the combination of biocontrol isolates has also increased plant growth in terms of germination, plant height, and yield. It is noteworthy that the compatibility of biocontrol isolates to be inoculated with each other on plant should be considered. The incompatibility of inoculants (biocontrol isolates) can sometimes prevent the growth of each other and target pathogens. Selection of effective biocontrol isolates of bacteria is also very important for the control of pathogens in plants. The isolation of these bacteria from pathogens repulsive soils can increase the chance of isolating effective isolates. In order to obtain effective isolates, biocontrol bacteria should be isolated from the same environment that they are supposed to be used in it. Formulations and application methods are often of great importance in the effectiveness of biological control, which should pay attention to them. In general, according to the studies conducted on biological control of fungal pathogens in rice, it can be concluded that Streptomyces and Bacillus bacteria may be taken advantage of as a potential bioinoculant agent for biocontrol as well as rice plant growth promoter.
13.8 Conclusions and Future Prospects
Reviews of literature clearly show that rhizosphere and endorhiza of rice harbor bacteria with a potential in promoting rice growth and controlling fungal rice pathogens. The co-inoculation of rice with the PGPB, as an attractive technique for utilization in commercial inoculant formulations than sole-inoculation of these bacteria, could allow declines in the prevalent high rates of fertilizer and the succeeding environmental problems without making compromise plant productivity under in vitro and greenhouse conditions. One of the major challenges encountered during the selection of biocontrol agents and biofertilizers is that biocontrol agents/biofertilizers that appear efficacious based on in vitro and greenhouse experiments might not be effective at controlling plant diseases and increasing rice growth and yield under field conditions. This inefficiency of bacteria can be owing to the variations in environmental conditions in different locations. Therefore, the environmental factors at the location where biocontrol agents/biofertilizers will be applied should be taken into consideration during the selection of suitable biocontrol agents/biofertilizers. Ideally, the biocontrol agents/biofertilizers should be isolated from and applied to locations with similar environmental factors in order to achieve successful biological control/biofertilizers. Besides, the formulation such as liquid, powder, or granule and the method of use of biocontrol agents/biofertilizers such as seed inoculation, soil inoculation, and vegetative part inoculation should be inspected as they are consequential in specifying the outcomes of field experiments. In general, before PGPB can be regarded for agricultural practices, further studies are essential to evaluate the efficacy of PGPB on rice plants under field conditions where there are a variety of constraints such as soil conditions (i.e., pH, soil nutrients status, nutrients sorption capacity, organic matter, and moisture level of the soil, etc.), environmental stresses, and types of autochthonous microorganisms that can affect the survival and growth promotion activities of PGPB/biocontrol agents.
References
Abbas-Zadeh P, Saleh-Rastin N, Asadi-Rahmani H, Khavazi K, Soltani A, Shoary-Nejati AR, Miransari M (2010) Plant growth-promoting activities of fluorescent pseudomonads, isolated from the Iranian soils. Acta Physiol Plan 32:281–288
Abed-Ashtiani F, Kadir J-B, Selamat A-B, Hanif AHB-M, Nasehi A (2012) Effect of foliar and root application of silicon against rice blast fungus in MR219 rice variety. Plant Pathol J 28:164–171
Adak A et al (2016) Micronutrient enrichment mediated by plant-microbe interactions and rice cultivation practices. J Plant Nutr 39:1216–1232
Adesemoye AO, Kloepper JW (2009) Plant–microbes interactions in enhanced fertilizer-use efficiency. Appl Microbiol Biotechnol 85:1–12
Adesemoye AO, Torbert HA, Kloepper JW (2009) Plant growth-promoting rhizobacteria allow reduced application rates of chemical fertilizers. Microb Ecol 58:921–929
Alexander M (1977) Introduction to soil microbiology-2
Alina SO, Constantinscu F, Petruţa CC (2015) Biodiversity of Bacillus subtilis group and beneficial traits of Bacillus species useful in plant protection Romanian. Biotechnol Lett 20:10737
Almaguer M, Rojas TI, Rodríguez-Rajo FJ, Aira MJ (2012) Airborne fungal succession in a rice field of Cuba. Euro J Plant Pathol 133:473–482
Amruta N, Kumar MKP, Puneeth ME, Sarika G, Kandikattu HK, Vishwanath K, Narayanaswamy S (2018) Exploring the potentiality of novel rhizospheric bacterial strains against the rice blast fungus Magnaporthe oryzae. Plant Pathol J 34:126
Ashtiani FA, Kadir J, Nasehi A, Rahaghi SRH, Sajili H (2012) Effect of silicon on rice blast disease pertanika. J Trop Agric Sci 35
Attia M, Ahmed MA, El-Sonbaty MR (2009) Use of biotechnologies to increase growth, productivity and fruit quality of Maghrabi banana under different rates of phosphorus. World J Agric Sci 5:211–220
Awla HK, Kadir J, Othman R, Rashid TS, Hamid S, Wong M-Y (2017) Plant growth-promoting abilities and biocontrol efficacy of Streptomyces sp. UPMRS4 against Pyricularia oryzae. Biol Cont 112:55–63
Bagyaraj DJ, Balakrishna AN (2012) Biofertilizers for sustainable agriculture phytotechnology: Emerg Trends:73
Begum HH, Islam MT (2005) Role of synthesis and exudation of organic acids in phosphorus nutrition in plants in tropical soils. Biotechnology 4:333–340
Begum HH, Osaki M, Shinano T, Miyatake H, Wasaki J, Yamamura T, Watanabe T (2005) The function of a maize-derived phosphoenol pyruvate carboxylase (PEPC) in phosphorus-deficient transgenic rice. Soil Sci Plant Nutr 51:497–506
Benhamou N, Belanger RR, Paulitz TC (1996) Induction of differential host responses by Pseudomonas fluorescens in Ri T-DNA-transformed pea roots after challenge with Fusarium oxysporum f. sp. pisi and Pythium ultimum. Phytopathology 86:1174–1185
Benhamou N, Kloepper JW, Tuzun S (1998) Induction of resistance against Fusarium wilt of tomato by combination of chitosan with an endophytic bacterial strain: ultrastructure and cytochemistry of the host response. Planta 204:153–168
Bhattacharjee RB, Singh A, Mukhopadhyay SN (2008) Use of nitrogen-fixing bacteria as biofertiliser for non-legumes: prospects and challenges. Appl Microbiol Biotechnol 80:199–209
Biswas JC, Ladha JK, Dazzo FB (2000) Rhizobia inoculation improves nutrient uptake and growth of lowland rice
Blumer C, Haas D (2000) Mechanism, regulation, and ecological role of bacterial cyanide biosynthesis. Arch Microbiol 173:170–177
Bocharnikova EA, Loginov SV, Matychenkov VV, Storozhenko PA (2010) Silicon fertilizer efficiency. Russ Agric Sci 36:446–448
Bouis HE, Welch RM (2010) Biofortification—a sustainable agricultural strategy for reducing micronutrient malnutrition in the global south. Crop Sci 50:S-20
Boukaew S, Plubrukam A, Prasertsan P (2013) Effect of volatile substances from Streptomyces philanthi RM-1-138 on growth of Rhizoctonia solani on rice leaf. Biol Cont 58:471–482
Boukaew S, Prasertsan P (2014) Suppression of rice sheath blight disease using a heat stable culture filtrate from Streptomyces philanthi. RM-1-138. Crop Prot 61:1–10
Cacique IS, Domiciano GP, Rodrigues FÁ, Vale FXRd (2012) Silicon and manganese on rice resistance to blast. Bragantia 71:239–244
Çakmakçı R, Erat M, Erdoğan Ü, Dönmez MF (2007) The influence of plant growth–promoting rhizobacteria on growth and enzyme activities in wheat and spinach plants. J Plant Nutr Soil Sci 170:288–295
Carmen B, Roberto D (2011) Soil bacteria support and protect plants against abiotic stresses In: Shaner A (ed) Abiotic stress in plants mechanisms and adaptations, Pub In Tech:143–170
Carvalho TLG, Balsemão-Pires E, Saraiva RM, Ferreira PCG, Hemerly AS (2014) Nitrogen signalling in plant interactions with associative and endophytic diazotrophic bacteria. J Exp Bot 65:5631–5642
Chaiharn M, Chunhaleuchanon S, Lumyong S (2009) Screening siderophore producing bacteria as potential biological control agent for fungal rice pathogens in Thailand. World J Microbiol Biotechnol 25:1919–1928
Chaiharn M, Lumyong S (2011) Screening and optimization of indole-3-acetic acid production and phosphate solubilization from rhizobacteria aimed at improving plant growth. Curr Microbiol 62:173–181
Chakraborty U, Chakraborty B, Dey P, Chakraborty AP (2015) Role of microorganisms in alleviation of abiotic stresses for sustainable agriculture abiotic stresses in crop. Plants:232
Chen C, Belanger RR, Benhamou N, Paulitz TC (2000) Defense enzymes induced in cucumber roots by treatment with plant growth-promoting rhizobacteria (PGPR) and Pythium aphanidermatum. Physiol Mol Plant Pathol 56:13–23
Chernin L, Chet I (2002) Microbial enzymes in biocontrol of plant pathogens and pests Enzymes in the environment: activity, ecology, and applications. Marcel Dekker, New York, pp 171–225
Choudhary DK, Kasotia A, Jain S, Vaishnav A, Kumari S, Sharma KP, Varma A (2016) Bacterial-mediated tolerance and resistance to plants under abiotic and biotic stresses. J Plant Growth Regul 35:276–300
Compant S, Clément C, Sessitsch A (2010) Plant growth-promoting bacteria in the rhizo- and endosphere of plants: their role, colonization, mechanisms involved and prospects for utilization. Soil Biol Biochem 42:669–678. https://doi.org/10.1016/j.soilbio.2009.11.024
Compant S, Duffy B, Nowak J, Clément C, Barka EA (2005) Use of plant growth-promoting bacteria for biocontrol of plant diseases: principles, mechanisms of action, and future prospects. Appl Environ Microbiol 71:4951–4959
Copping LG, Duke SO (2007) Natural products that have been used commercially as crop protection agents. Pest Manag Sci 63:524–554
Cuong TX, Ullah H, Datta A, Hanh TC (2017) Effects of silicon-based fertilizer on growth, yield and nutrient uptake of rice in tropical zone of Vietnam rice. Science 24:283–290
Dallagnol LJ, Rodrigues FA, Mielli MVB, Ma JF (2014) Rice grain resistance to brown spot and yield are increased by silicon. Trop Plant Pathol 39:56–63
Das SK (2014) Role of micronutrient in rice cultivation and management strategy in organic agriculture—a reappraisal. Agric Sci 5:765
Datta A, Ullah H, Ferdous Z (2017) Water management in rice. In: Rice production worldwide. Springer, Cham, pp 255–277
Dawe D (2000) The potential role of biological nitrogen fixation in meeting future demand for rice and fertilizer. The quest for nitrogen fixation in rice, pp 1–9
de Souza JT, de Boer M, de Waard P, van Beek TA, Raaijmakers JM (2003) Biochemical, genetic, and zoosporicidal properties of cyclic lipopeptide surfactants produced by Pseudomonas fluorescens. Appl Environ Microbiol 69:7161–7172
de Souza R et al (2013) The effect of plant growth-promoting rhizobacteria on the growth of rice (Oryza sativa L.) cropped in southern Brazilian fields. Plant Soil 366:585–603
de Souza R, Schoenfeld R, Passaglia LMP (2016) Bacterial inoculants for rice: effects on nutrient uptake and growth promotion. Arch Agron Soil Sci 62:561–569
Dean R et al (2012) The top 10 fungal pathogens in molecular plant pathology. Mol Plant Pathol 13:414–430
Défago G (1993) 2, 4-Diacetylphloroglucinol, a promising compound in biocontrol. Plant Pathol 42:311–312
Detmann KC et al (2012) Silicon nutrition increases grain yield, which, in turn, exerts a feed-forward stimulation of photosynthetic rates via enhanced mesophyll conductance and alters primary metabolism in rice. New Phytol 196:752–762
Dey KB (1988) Phosphate solubilizing organisms in improving fertility status, pp 237–248
Di Fiore S, Del Gallo M (1995) Endophytic bacteria: their possible role in the host plant. In: Azospirillum VI and related microorganisms. Springer, pp 169–187
Dimkpa C, Weinand T, Asch F (2009) Plant–rhizobacteria interactions alleviate abiotic stress conditions. Plant, Cell Environ 32:1682–1694
Dowling DN, O’Gara F (1994) Metabolites of Pseudomonas involved in the biocontrol of plant disease. Trend Biotechnol 12:133–141
Duarah I, Deka M, Saikia N, Boruah HPD (2011) Phosphate solubilizers enhance NPK fertilizer use efficiency in rice and legume cultivation. 3 Biotech 1:227–238
Egamberdieva D, Lugtenberg B (2014) Use of plant growth-promoting rhizobacteria to alleviate salinity stress in plants. In: Use of microbes for the alleviation of soil stresses, vol 1. Springer, New York, pp 73–96
Egamberdiyeva D (2007) The effect of plant growth promoting bacteria on growth and nutrient uptake of maize in two different soils. Appl Soil Ecol 36:184–189
El-Tarabily KA, Soliman MH, Nassar AH, Al-Hassani HA, Sivasithamparam K, McKenna F, Hardy GS (2000) Biological control of Sclerotinia minor using a chitinolytic bacterium and actinomycetes. Plant Pathol 49:573–583
Elbeltagy A et al (2001) Endophytic colonization and in planta nitrogen fixation by a Herbaspirillum sp. isolated from wild rice species. Appl Environ Microbiol 67:5285–5293
Epstein E (1994) The anomaly of silicon in plant biology. Proc Natl Acad Sci 91:11–17
Epstein E (1999) Silicon. Ann Rev Plant Biol 50:641–664
Estrada GA, Baldani VLD, de Oliveira DM, Urquiaga S, Baldani JI (2013) Selection of phosphate-solubilizing diazotrophic Herbaspirillum and Burkholderia strains and their effect on rice crop yield and nutrient uptake. Plant Soil 369:115–129
Etesami H, Alikhani HA (2016a) Co-inoculation with endophytic and rhizosphere bacteria allows reduced application rates of N-fertilizer for rice plant. Rhizosphere 2:5–12
Etesami H, Alikhani HA (2016b) Evaluation of Gram-positive rhizosphere and endophytic bacteria for biological control of fungal rice (Oryza sativa L.) pathogens. Euro J Plant Pathol 1–8
Etesami H, Alikhani HA (2016c) Rhizosphere and endorhiza of oilseed rape (Brassica napus L.) plant harbor bacteria with multifaceted beneficial effects. Biol Cont 94:11–24
Etesami H, Alikhani HA (2016d) Suppression of the fungal pathogen Magnaporthe grisea by Stenotrophomonas maltophilia, a seed-borne rice (Oryza sativa L.) endophytic bacterium Arch Agron Soil Sci 1–14
Etesami H, Alikhani HA (2017) Evaluation of Gram-positive rhizosphere and endophytic bacteria for biological control of fungal rice (Oryza sativa L.) pathogens. Euro J Plant Pathol 147:7–14
Etesami H, Alikhani HA (2018) Bacillus species as the most promising bacterial biocontrol agents in rhizosphere and endorhiza of plants grown in rotation with each other. Euro J Plant Pathol 150:497–506
Etesami H, Hosseini HM, Alikhani HA (2014a) Bacterial biosynthesis of 1-aminocyclopropane-1-caboxylate (ACC) deaminase, a useful trait to elongation and endophytic colonization of the roots of rice under constant flooded conditions. Physiol Mol Biol Plant 20:425–434
Etesami H, Hosseini HM, Alikhani HA, Mohammadi L (2014b) Bacterial biosynthesis of 1-aminocyclopropane-1-carboxylate (ACC) deaminase and indole-3-acetic acid (IAA) as endophytic preferential selection traits by rice plant seedlings. J Plant Growth Regul 33:654–670
Etesami H, Alikhani HA, Hosseini HM (2015) Indole-3-acetic acid (IAA) production trait, a useful screening to select endophytic and rhizosphere competent bacteria for rice growth promoting agents. MethodsX 2:72–78
Etesami H, Emami S, Alikhani HA (2017) Potassium solubilizing bacteria (KSB): Mechanisms, promotion of plant growth, and future prospects—a review. J Soil Sci Plant Nutr 17:897–911
Etesami H, Jeong BR (2018) Silicon (Si): review and future prospects on the action mechanisms in alleviating biotic and abiotic stresses in plants. Ecotoxicol Environ Safe 147:881–896. https://doi.org/10.1016/j.ecoenv.2017.09.063
Etesami H, Maheshwari DK (2018) Use of plant growth promoting rhizobacteria (PGPRs) with multiple plant growth promoting traits in stress agriculture: action mechanisms and future prospects. Ecotoxicol Environ Safe 156:225–246. https://doi.org/10.1016/j.ecoenv.2018.03.013
Etesami H, Mirsyed Hosseini H, Alikhani HA (2014c) In planta selection of plant growth promoting endophytic bacteria for rice (Oryza sativa L.). J Soil Sci Plant Nutr 14:491–503
Etesami H, Mirsyedhosseini H, Alikhani HA (2013) Rapid screening of berseem clover (Trifolium alexandrinum) endophytic bacteria for rice plant seedlings growth-promoting agents. ISRN Soil Sci 2013
Fageria NK (2001) Nutrient management for improving upland rice productivity and sustainability. Comm Soil Sci Plant Anal 32:2603–2629
Fauteux F, Rémus-Borel W, Menzies JG, Bélanger RR (2005) Silicon and plant disease resistance against pathogenic fungi. FEMS Microbiol Lett 249:1–6
Ferre FS (2016) Worldwide occurrence of mycotoxins in rice. Food Cont 62:291–298
Frankowski J, Lorito M, Scala F, Schmid R, Berg G, Bahl H (2001) Purification and properties of two chitinolytic enzymes of Serratia plymuthica HRO-C48. Arch Microbiol 176:421–426
Freitas JRD, Germida JJ (1990) Plant growth promoting rhizobacteria for winter wheat. Can J Microbiol 36:265–272
Fridlender M, Inbar J, Chet I (1993) Biological control of soilborne plant pathogens by a β-1, 3 glucanase-producing Pseudomonas cepacia. Soil Biol Biochem 25:1211–1221
Gerami M, Fallah A, Moghadam MRK (2013) Study of potassium and sodium silicate on the morphological and chlorophyll content on the rice plant in pot experiment (Oryza sativa L.). Int J Agric Crop Sci 5:6
Gerhardson B (2002) Biological substitutes for pesticides. Trend Biotechnol 20:338–343
Ghorchiani M, Etesami H, Alikhani HA (2018) Improvement of growth and yield of maize under water stress by co-inoculating an arbuscular mycorrhizal fungus and a plant growth promoting rhizobacterium together with phosphate fertilizers. Agric Ecosys Environ 258:59–70
Glick BR (2012) Plant growth-promoting bacteria: mechanisms and applications. Scientifica
Goldstein AH (1986) Bacterial solubilization of mineral phosphates: historical perspective and future prospects. Am J Alter Agric 1:51–57
González-Franco AC, Robles-Hernandez RY (2009) Actinomycetes as biological control agents of phytopathogenic fungi. Tecno Chihua 3:64–73
Goodfellow M, Williams ST (1983) Ecology of actinomycetes. Ann Rev Microbiol 37:189–216
Guang-Can TAO, Shu-Jun T, Miao-Ying CAI, Guang-Hui XIE (2008) Phosphate-solubilizing and-mineralizing abilities of bacteria isolated from soils. Pedosphere 18:515–523
Guntzer F, Keller C, Meunier J-D (2012) Benefits of plant silicon for crops: a review. Agron Sustain Dev 32:201–213
Gupta G, Panwar J, Akhtar MS, Jha PN (2012) Endophytic nitrogen-fixing bacteria as biofertilizer. In: Sustainable agriculture reviews. Springer, pp 183–221
Gyaneshwar P, Kumar GN, Parekh LJ, Poole PS (2002) Role of soil microorganisms in improving P nutrition of plants. In: Food security in nutrient-stressed environments: exploiting plants’ genetic capabilities. Springer, pp 133–143
Hashidoko Y, Nakayama T, Homma Y, Tahara S (1999) Structure elucidation of xanthobaccin A, a new antibiotic produced from Stenotrophomonas sp. strain SB-K88. Tetrahed Lett 40:2957–2960
Hassan MN, Afghan S, Hafeez FY (2010) Suppression of red rot caused by Colletotrichum falcatum on sugarcane plants using plant growth-promoting rhizobacteria. Biocontrol 55:531–542
Hayasaka T, Fujii H, Namai T (2005) Silicon content in rice seedlings to protect rice blast fungus at the nursery stage. J Gen Plant Pathol 71:169–173
Hazell PBR (2010) Asia’s green revolution: past achievements and future challenges rice in the global economy: strategic research and policy issues for food security. International Rice Research Institute, Los Baños, pp 61–92
Hongrittipun P, Youpensuk S, Rerkasem B (2014) Screening of nitrogen fixing endophytic bacteria in Oryza sativa L. J Agric Sci 6:66
Iavicoli A, Boutet E, Buchala A, Métraux J-P (2003) Induced systemic resistance in Arabidopsis thaliana in response to root inoculation with Pseudomonas fluorescens CHA0. Mol Plant-Microbe Interact 16:851–858
Islam MT, Hossain MM (2012) Plant probiotics in phosphorus nutrition in crops, with special reference to rice. In: Maheshwari DK (ed) Bacteria in agrobiology: plant probiotics. Springer, Berlin, pp 325–363
James EK et al (2002) Infection and colonization of rice seedlings by the plant growth-promoting bacterium Herbaspirillum seropedicae Z67. Mol Plant-Microbe Interact 15:894–906
Jawahar S, Vaiyapuri V (2013) Effect of sulphur and silicon fertilization on yield, nutrient uptake and economics of rice. Int Res J Chem 3:35–43
Jeun YC, Park KS, Kim CH, Fowler WD, Kloepper JW (2004) Cytological observations of cucumber plants during induced resistance elicited by rhizobacteria. Biol Cont 29:34–42
Ji SH, Gururani MA, Chun S-C (2014) Isolation and characterization of plant growth promoting endophytic diazotrophic bacteria from Korean rice cultivars. Microbiol Res 169:83–98
Jones DL, Darrah PR (1994) Role of root derived organic acids in the mobilization of nutrients from the rhizosphere. Plant Soil 166:247–257
Joseph B, Ranjan Patra R, Lawrence R (2012) Characterization of plant growth promoting rhizobacteria associated with chickpea (Cicer arietinum L.). Int J Plant Produc 1:141–152
Kai M, Haustein M, Molina F, Petri A, Scholz B, Piechulla B (2009) Bacterial volatiles and their action potential. Appl Microbiol Biotechnol 81:1001–1012
Kamran S, Shahid I, Baig DN, Rizwan M, Malik KA, Mehnaz S (2017) Contribution of zinc solubilizing bacteria in growth promotion and zinc content of wheat. Fron Microbiol 8:2593
Kang S-M et al (2017) Isolation and characterization of a novel silicate-solubilizing bacterial strain Burkholderia eburnea CS4-2 that promotes growth of japonica rice (Oryza sativa L. cv. Dongjin). Soil Sci Plant Nutr 1–9
Kannan NM, Raj SA (1998) Occurrence of silicate solubilizing bacteria in rice ecosystem. Madras Agric J 85:47–49
Kashif MD, Kumar V, Kalpana VN, Devi Rajeswari V (2016) Phylogenetic diversity and biological activity of actinomycetes isolated from Gulf of Mannar, Tamil Nadu, India. Pharm Lett 8:16–24
Kaushal M, Wani SP (2016) Plant-growth-promoting rhizobacteria: drought stress alleviators to ameliorate crop production in drylands. Ann Microbiol 66:35–42
Khalil MS, Moubasher H, Hasan FF (2014) Biological control of rice blast disease by Streptomyces flavotricini. Res J Pharm Biol Chem Sci 5:1453–1461
Khan MMA, Haque E, Paul NC, Khaleque MA, Al-Garni SMS, Rahman M, Islam MT (2017) Enhancement of growth and grain yield of rice in nutrient deficient soils by rice probiotic bacteria. Rice Sci 24:264–273
Khan MS, Zaidi A, Ahmad E (2014) Mechanism of phosphate solubilization and physiological functions of phosphate-solubilizing microorganisms. In: Khan MS (ed) Phosphate solubilizing microorganisms. Springer, Cham, pp 31–62
Khan MS, Zaidi A, Wani PA (2007) Role of phosphate-solubilizing microorganisms in sustainable agriculture—a review. Agron Sustain Dev 27:29–43
Khorshidi YR, Ardakani MR, Ramezanpour MR, Khavazi K, Zargari K (2011) Response of yield and yield components of rice (Oryza sativa L.) to Pseudomonas flouresence and Azospirillum lipoferum under different nitrogen levels. Am -Eurasian J Agric Environ Sci 10:387–395
Kim BS, Moon SS, Hwang BK (1999) Isolation, identification, and antifungal activity of a macrolide antibiotic, oligomycin A, produced by Streptomyces libani. Can J Bot 77:850–858
Komárek M, Čadková E, Chrastný V, Bordas F, Bollinger J-C (2010) Contamination of vineyard soils with fungicides: a review of environmental and toxicological aspects. Environ Int 36:138–151
Kucey RMN (1983) Phosphate-solubilizing bacteria and fungi in various cultivated and virgin Alberta soils. Can J Soil Sci 63:671–678
Ladha JK, Barraquio WL, Revilla L (1997) Isolation of endophytic diazotrophic bacteria from wetland rice. In: Opportunities for biological nitrogen fixation in rice and other non-legumes. Springer, Dordrecht, pp 15–24
Lavinsky AO et al (2016) Silicon improves rice grain yield and photosynthesis specifically when supplied during the reproductive growth stage. J Plant Physiol 206:125–132
Law JW-F et al. (2017) The potential of Streptomyces as biocontrol agents against the rice blast fungus, Magnaporthe oryzae (Pyricularia oryzae). Front Microbiol 8:3
Leeman M, Van Pelt JA, Den Ouden FM, Heinsbroek M, Bakker P, Schippers B (1995) Induction of systemic resistance against Fusarium wilt of radish by lipopolysaccharides of Pseudomonas fluorescens. Phytopathology 85:1021–1027
Li Q et al (2011) Suppression of Magnaporthe oryzae by culture filtrates of Streptomyces globisporus JK-1. Biol Cont 58:139–148
Liang YC, Ma TS, Li FJ, Feng YJ (1994) Silicon availability and response of rice and wheat to silicon in calcareous soils. Comm Soil Sci Plant Anal 25:2285–2297
Lim H-S, Kim Y-S, Kim S-D (1991) Pseudomonas stutzeri YPL-1 genetic transformation and antifungal mechanism against Fusarium solani, an agent of plant root rot. Appl Environ Microbiol 57:510–516
Loper JE, Henkels MD (1999) Utilization of heterologous siderophores enhances levels of iron available to Pseudomonas putida in the rhizosphere. Appl Environ Microbiol 65:5357–5363
Lucas JA, Solano BR, Montes F, Ojeda J, Megias M, Mañero FJG (2009) Use of two PGPR strains in the integrated management of blast disease in rice (Oryza sativa) in Southern Spain. Field Crop Res 114:404–410
Lugtenberg B, Kamilova F (2009) Plant-growth-promoting rhizobacteria. Ann Rev Microbiol 63:541–556
Ma JF, Takahashi E (2002) Soil, fertilizer, and plant silicon research in Japan. Elsevier
Ma JF et al (2007) An efflux transporter of silicon in rice. Nature 448:209
Mäder P et al (2011) Inoculation of root microorganisms for sustainable wheat–rice and wheat–black gram rotations in India. Soil Biol Biochem 43:609–619
Manandhar HK, Lyngs Jørgensen HJ, Mathur SB, Smedegaard-Petersen V (1998) Suppression of rice blast by preinoculation with avirulent Pyricularia oryzae and the nonrice pathogen Bipolaris sorokiniana. Phytopathology 88:735–739
Mano H, Morisaki H (2008) Endophytic bacteria in the rice plant. Microb Environ 23:109–117
Maurhofer M, Hase C, Meuwly P, Metraux JP, Defago G (1994) Induction of systemic resistance of tobacco to tobacco necrosis virus by the root-colonizing Pseudomonas fluorescens strain CHA0: influence of the gacA gene and of pyoverdine production. Phytopathology 84:139–146
Meena VS, Maurya BR, Verma JP (2014) Does a rhizospheric microorganism enhance K+ availability in agricultural soils? Microbiol Res 169:337–347
Meziane H, Van Der Sluis I, Van Loon LC, Höfte M, Bakker PAHM (2005) Determinants of Pseudomonas putida WCS358 involved in inducing systemic resistance in plants. Mol Plant Pathol 6:177–185
Miransari M (2013) Soil microbes and the availability of soil nutrients. Acta Physiol Plant 35:3075–3084
Mishra RPN, Singh RK, Jaiswal HK, Kumar V, Maurya S (2006) Rhizobium-mediated induction of phenolics and plant growth promotion in rice (Oryza sativa L.). Curr Microbiol 52:383–389
Naik PR, Raman G, Narayanan KB, Sakthivel N (2008) Assessment of genetic and functional diversity of phosphate solubilizing fluorescent pseudomonads isolated from rhizospheric soil. BMC Microbiol 8:230
Nain L, Yadav RC, Saxena J (2012) Characterization of multifaceted Bacillus sp. RM-2 for its use as plant growth promoting bioinoculant for crops grown in semi arid deserts. Appl Soil Ecol 59:124–135
Naureen Z et al. (2015a) Isolation and screening of silicate bacteria from various habitats for biological control of phytopathogenic fungi. American J Plant Sci 6:2850
Naureen Z, Hafeez FY, Hussain J, Al Harrasi A, Bouqellah N, Roberts MR (2015b) Suppression of incidence of Rhizoctonia solani in rice by siderophore producing rhizobacterial strains based on competition for iron. Euro Scientific J 11
Naureen Z, Price AH, Hafeez FY, Roberts MR (2009a) Identification of rice blast disease-suppressing bacterial strains from the rhizosphere of rice grown in Pakistan. Crop Protec 28:1052–1060
Naureen Z, Price AH, Wilson MJ, Hafeez FY, Roberts MR (2009b) Suppression of rice blast disease by siderophore-producing bioantagonistic bacterial isolates isolated from the rhizosphere of rice grown in Pakistan. Crop Protec 28:1052–1060
Ngumbi E, Kloepper J (2016) Bacterial-mediated drought tolerance: current and future prospects. Appl Soil Ecol 105:109–125
Nielsen TH, Sørensen D, Tobiasen C, Andersen JB, Christophersen C, Givskov M, Sørensen J (2002) Antibiotic and biosurfactant properties of cyclic lipopeptides produced by fluorescent Pseudomonas spp. from the sugar beet rhizosphere. Appl Environ Microbiol 68:3416–3423
Ning D, Song A, Fan F, Li Z, Liang Y (2014) Effects of slag-based silicon fertilizer on rice growth and brown-spot resistance. PLoS ONE 9:e102681
Ningthoujam S, Sanasam S, Tamreihao K, Nimaich S (2009) Antagonistic activities of local actinomycete isolates against rice fungal pathogens. Afr J Microbiol Res 3:737–742
Nowak J, Shulaev V (2003) Priming for transplant stress resistance in in vitro propagation. Vitro Cell Dev Biol-Plant 39:107–124
O’sullivan DJ, O’Gara F (1992) Traits of fluorescent Pseudomonas spp. involved in suppression of plant root pathogens. Microbiol Rev 56:662–676
Oehl F, Oberson A, Probst M, Fliessbach A, Roth H-R, Frossard E (2001) Kinetics of microbial phosphorus uptake in cultivated soils. Biol Fertil Soil 34:31–41
Oliveira ALMd, Urquiaga S, Döbereiner J, Baldani JI (2002) The effect of inoculating endophytic N2-fixing bacteria on micropropagated sugarcane plants. Plant Soil 242:205–215
Ongena M, Daayf F, Jacques P, Thonart P, Benhamou N, Paulitz TC, Bélanger RR (2000) Systemic induction of phytoalexins in cucumber in response to treatments with fluorescent pseudomonads. Plant Pathol 49:523–530
Ordentlich A, Elad Y, Chet I (1988) The role of chitinase of Serratia marcescens in biocontrol of Sclerotium rolfsii. Phytopathology (USA)
Othman R, Panhwar QA (2014) Phosphate-solubilizing bacteria improves nutrient uptake in aerobic rice. In: Khan MS (ed) Phosphate solubilizing microorganisms. Springer, Cham, pp 207–224
Pal KK, Gardener BM (2006) Biological control of plant pathogens. Plant Health Instr 2:1117–1142
Palaniyandi SA, Yang SH, Zhang L, Suh J-W (2013) Effects of actinobacteria on plant disease suppression and growth promotion. Appl Microbiol Biotechnol 97:9621–9636
Panhwar QA, Jusop S, Naher UA, Othman R, Razi MI (2013) Application of potential phosphate-solubilizing bacteria and organic acids on phosphate solubilization from phosphate rock in aerobic rice. Scientific World J
Panhwar QA, Radziah O, Rahman AZ, Sariah M, Razi IM, Naher UA (2011a) Contribution of phosphate-solubilizing bacteria in phosphorus bioavailability and growth enhancement of aerobic rice. Span J Agric Res 9:810–820
Panhwar QA, Radziah O, Zaharah AR, Sariah M, Razi IM (2011b) Role of phosphate solubilizing bacteria on rock phosphate solubility and growth of aerobic rice
Park KS, Kloepper JW (2000) Activation of PR-1a promoter by rhizobacteria that induce systemic resistance in tobacco against Pseudomonas syringae pv. tabaci. Biol Cont 18:2–9
Park KS, Paul D, Kim JS, Park JW (2009) L-Alanine augments rhizobacteria-induced systemic resistance in cucumber. Folia Microbiol 54:322–326
Pati S, Pal B, Badole S, Hazra GC, Mandal B (2016) Effect of silicon fertilization on growth, yield, and nutrient uptake of rice. Comm Soil Sci Plant Anal 47:284–290
Paul D, Lade H (2014) Plant-growth-promoting rhizobacteria to improve crop growth in saline soils: a review. Agron Sustain Dev 34:737–752
Pieterse CMJ, Zamioudis C, Berendsen RL, Weller DM, Van Wees SCM, Bakker PAHM (2014) Induced systemic resistance by beneficial microbes. Ann Rev Phytopathol 52:347–375
Pii Y, Mimmo T, Tomasi N, Terzano R, Cesco S, Crecchio C (2015) Microbial interactions in the rhizosphere: beneficial influences of plant growth-promoting rhizobacteria on nutrient acquisition process. Rev Biol Fertil Soil 51:403–415
Ping L, Boland W (2004) Signals from the underground: bacterial volatiles promote growth in Arabidopsis. Trend Plant Sci 9:263–266
Pooniya V, Shivay YS, Rana A, Nain L, Prasanna R (2012) Enhancing soil nutrient dynamics and productivity of Basmati rice through residue incorporation and zinc fertilization. Euro J Agron 41:28–37
Prabhu AS, Barbosa Filho MP, Filippi MC, Datnoff LE, Snyder GH (2001) Silicon from rice disease control perspective in Brazil. In: Studies in plant science, vol 8. Elsevier, pp 293–311
Prakash NB, Chandrashekar N, Mahendra C, Patil SU, Thippeshappa GN, Laane HM (2011) Effect of foliar spray of soluble silicic acid on growth and yield parameters of wetland rice in hilly and coastal zone soils of Karnataka, South India. J Plant Nutr 34:1883–1893
Prapagdee B, Kuekulvong C, Mongkolsuk S (2008) Antifungal potential of extracellular metabolites produced by Streptomyces hygroscopicus against phytopathogenic fungi. Int J Biol Sci 4:330
Prayitno J, Rolfe B (2010) Characterization of endophytic diazotroph bacteria isolated from rice HAYATI. J Biosci 17:73–78
Prayitno J et al (1999) Interactions of rice seedlings with bacteria isolated from rice roots. Fun Plant Biol 26:521–535
Qin Y, Druzhinina IS, Pan X, Yuan Z (2016) Microbially mediated plant salt tolerance and microbiome-based solutions for saline agriculture. Biotechnol Adv
Ramamoorthy V, Viswanathan R, Raguchander T, Prakasam V, Samiyappan R (2001) Induction of systemic resistance by plant growth promoting rhizobacteria in crop plants against pests and diseases. Crop Protec 20:1–11
Rana A et al (2015) Prospecting plant growth promoting bacteria and cyanobacteria as options for enrichment of macro-and micronutrients in grains in rice–wheat cropping sequence. Cogent Food Agric 1:1037379
Raupach GS, Kloepper JW (1998) Mixtures of plant growth-promoting rhizobacteria enhance biological control of multiple cucumber pathogens. Phytopathology 88:1158–1164
Reinhold-Hurek B, Krause A, Leyser B, Miché L, Hurek T (2007) The rice apoplast as a habitat for endophytic N 2-Fixing bacteria. In: The apoplast of higher plants: compartment of storage, transport and reactions. Springer, Dordrecht, pp 427–443
Rodrigues FA, Datnoff LE (2005) Silicon and rice disease management. Fitopatol Brasileira 30:457–469
Rodrı́guez H, Fraga R (1999) Phosphate solubilizing bacteria and their role in plant growth promotion. Biotechnol Adv 17:319–339
Ryan RP, Germaine K, Franks A, Ryan DJ, Dowling DN (2008) Bacterial endophytes: Recent developments and applications. FEMS Microbiol Lett 278:1–9
Ryu C-M, Farag MA, Hu C-H, Reddy MS, Kloepper JW, Paré PW (2004) Bacterial volatiles induce systemic resistance in Arabidopsis. Plant Physiol 134:1017–1026
Saharan BS, Nehra V (2011) Plant growth promoting rhizobacteria: a critical review. Life Sci Med Res 21:30
Sahebi M et al. (2015) Importance of silicon and mechanisms of biosilica formation in plants. BioMed Res Int
Sahrawat KL, Abekoe MK, Diatta S (2001) Application of inorganic phosphorus fertilizer. Sustaining soil fertility in West Africa, pp 225–246
Sakr N (2016) The role of silicon (Si) in increasing plant resistance against fungal diseases. Hellenic Plant Protec J 9:1–15
Samaranayake P, Peiris BD, Dssanayake S (2012) Effect of excessive ferrous (fe 2 +) on growth and iron content in rice (Oryza sativa). Int J Agric Biol 14
Sapsirisopa S, Chookietwattana K, Maneewan K, Khaengkhan P (2009) Effect of salt-tolerant Bacillus inoculum on rice KDML 105 cultivated in saline soil. As J Food Ag-Ind 2:S69–S74
Saravanan VS, Madhaiyan M, Thangaraju M (2007) Solubilization of zinc compounds by the diazotrophic, plant growth promoting bacterium Gluconacetobacter diazotrophicus. Chemosphere 66:1794–1798
Savant NK, Datnoff LE, Snyder GH (1997) Depletion of plant-available silicon in soils: a possible cause of declining rice yields. Comm Soil Sci Plant Anal 28:1245–1252
Schachtman DP, Reid RJ, Ayling SM (1998) Phosphorus uptake by plants: from soil to cell. Plant Physiol 116:447–453
Schisler DA, Slininger PJ, Bothast RJ (1997) Effects of antagonist cell concentration and two-strain mixtures on biological control of Fusarium dry rot of potatoes. Phytopathology 87:177–183
Sessitsch A, Kuffner M, Kidd P, Vangronsveld J, Wenzel WW, Fallmann K, Puschenreiter M (2013) The role of plant-associated bacteria in the mobilization and phytoextraction of trace elements in contaminated soils. Soil Biol Biochem 60:182–194
Sethi SK, Mukherjee AK (2018) Screening of biocontrol potential of indigenous Bacillus spp. isolated from rice rhizosphere against R. solani, S. oryzae, S. rolfsii and response towards growth of rice
Shafi J, Tian H, Ji M (2017) Bacillus species as versatile weapons for plant pathogens: a review. Biotechnol Biotechnol Equip 31:446–459
Shaharoona B, Naveed M, Arshad M, Zahir ZA (2008) Fertilizer-dependent efficiency of Pseudomonads for improving growth, yield, and nutrient use efficiency of wheat (Triticum aestivum L.). Appl Microbiol Biotechnol 79:147–155
Shan H et al (2013) Biocontrol of rice blast by the phenaminomethylacetic acid producer of Bacillus methylotrophicus strain BC79. Crop Protec 44:29–37
Sharma SB, Sayyed RZ, Trivedi MH, Gobi TA (2013) Phosphate solubilizing microbes: sustainable approach for managing phosphorus deficiency in agricultural soils. SpringerPlus 2:587
Sheng XF, Zhao F, He LY, Qiu G, Chen L (2008) Isolation and characterization of silicate mineral-solubilizing Bacillus globisporus Q12 from the surfaces of weathered feldspar. Can J Microbiol 54:1064–1068
Sindhu SS, Dadarwal KR (2001) Chitinolytic and cellulolytic Pseudomonas sp. antagonistic to fungal pathogens enhances nodulation by Mesorhizobium sp. Cicer in chickpea. Microbiol Res 156:353–358
Singh AK, Singh R, Singh K (2005) Growth, yield and economics of rice (Oryza sativa) as influenced by level and time of silicon application. Indian J Agron 50:190–193
Singh PP, Shin YC, Park CS, Chung YR (1999) Biological control of Fusarium wilt of cucumber by chitinolytic bacteria. Phytopathology 89:92–99
Song A et al. (2016) The role of silicon in enhancing resistance to bacterial blight of hydroponic-and soil-cultured rice. Sci Rep 6
Song F, Goodman RM (2001) Molecular biology of disease resistance in rice. Physiol Mol Plant Pathol 59:1–11
Suprapta DN (2012) Potential of microbial antagonists as biocontrol agents against plant fungal pathogens. J ISSAAS 18:1–8
Syers JK, Johnston AE, Curtin D (2008) Efficiency of soil and fertilizer phosphorus use. FAO Fertil Plant Nutrition Bull 18
Tamreihao K, Ningthoujam DS, Nimaichand S, Singh ES, Reena P, Singh SH, Nongthomba U (2016) Biocontrol and plant growth promoting activities of a Streptomyces corchorusii strain UCR208-16 and preparation of powder formulation for application as biofertilizer agents for rice plant. Microbiol Res 192:260–270
Tapadar SA, Jha DK (2013) Disease management in staple crops: a bacteriological approach. In: Maheshwari DK (ed) Bacteria in agrobiology: disease management. Springer, Berlin, pp 111–152
Thakuria D, Talukdar NC, Goswami C, Hazarika S, Boro RC, Khan MR (2004) Characterization and screening of bacteria from rhizosphere of rice grown in acidic soils of Assam. Curr Sci 978–985
Theodorou ME, Plaxton WC (1993) Metabolic adaptations of plant respiration to nutritional phosphate deprivation. Plant Physiol 101:339–344
Timmusk S, Timmusk K, Behers L (2013) Rhizobacterial plant drought stress tolerance enhancement: towards sustainable water resource management and food security. J Food Secur 1:6–9
Tokpah DP, Li H, Wang L, Liu X, Mulbah QS, Liu H (2016) An assessment system for screening effective bacteria as biological control agents against Magnaporthe grisea on rice. Biol Cont 103:21–29
Trejo-Estrada SR, Paszczynski A, Crawford DL (1998) Antibiotics and enzymes produced by the biocontrol agent Streptomyces violaceusniger YCED-9. J Ind Microbiol Biotechnol 21:81–90
Ullah A, Heng S, Munis MFH, Fahad S, Yang X (2015) Phytoremediation of heavy metals assisted by plant growth promoting (PGP) bacteria: a review. Environ Exp Bot 117:28–40
Vaid SK, Kumar B, Sharma A, Shukla AK, Srivastava PC (2014) Effect of Zn solubilizing bacteria on growth promotion and Zn nutrition of rice. J Soil Sci Plant Nutr 14:889–910
Van Bockhaven J (2014) Silicon-induced resistance in rice (Oryza sativa L.) against the brown spot pathogen Cochliobolus miyabeanus
Van Loon LC, Bakker P (2005) Induced systemic resistance as a mechanism of disease suppression by rhizobacteria. In: PGPR: biocontrol and biofertilization. Springer, Dordrecht, pp 39–66
Van Loon LC, Bakker P, Pieterse CMJ (1998) Systemic resistance induced by rhizosphere bacteria. Ann Rev Phytopathol 36:453–483
Van Wees SCM, Pieterse CMJ, Trijssenaar A, Van’t Westende YAM, Hartog F, Van Loon LC (1997) Differential induction of systemic resistance in Arabidopsis by biocontrol bacteria. Mol Plant-Microbe Interact 10:716–724
Vasanthi N, Saleena LM, Raj SA (2018) Silica solubilization potential of certain bacterial species in the presence of different silicate minerals. Silicon 10:267–275
Velusamy P, Gnanamanickam SS (2008) The effect of bacterial secondary metabolites on bacterial and fungal pathogens of rice. In: Secondary metabolites in soil ecology. Springer, Berlin, pp 93–106
Verma SK, Kingsley KL, Bergen MS, Kowalski KP, White JF (2018) fungal disease prevention in seedlings of rice (Oryza sativa) and other grasses by growth-promoting seed-associated endophytic bacteria from invasive Phragmites australis. Microorganisms 6:21
Vessey JK (2003) Plant growth promoting rhizobacteria as biofertilizers. Plant Soil 255:571–586
Vijayapriya M, Muthukkaruppan SM (2010) Isolation and screening of silicate solubilizing bacteria and its biocontrol nature against Pyricularia oryzae. Int J Rec Sci Res 4:87–91
Welbaum GE, Sturz AV, Dong Z, Nowak J (2004) Managing soil microorganisms to improve productivity of agro-ecosystems. Crit Rev Plant Sci 23:175–193
Yang J, Kloepper JW, Ryu C-M (2009) Rhizosphere bacteria help plants tolerate abiotic stress. Trend Plant Sci 14:1–4
Yang PW et al (2010) Oligomycins A and C, major secondary metabolites isolated from the newly isolated strain Streptomyces diastaticus. Folia Microbiol 55:10–16
Yanni YG, Dazzo FB (2010) Enhancement of rice production using endophytic strains of Rhizobium leguminosarum bv. trifolii in extensive field inoculation trials within the Egypt Nile delta. Plant Soil 336:129–142
Yanni YG et al. (1997) Natural endophytic association between Rhizobium leguminosarum bv. trifolii and rice roots and assessment of its potential to promote rice growth. In: Opportunities for biological nitrogen fixation in rice and other non-legumes. Springer, Dordrecht, pp 99–114
Yanni YG et al (2001) The beneficial plant growth-promoting association of Rhizobium leguminosarum bv. trifolii with rice roots. Func Plant Biol 28:845–870
Yildirim E, Karlidag H, Turan M, Dursun A, Goktepe F (2011) Growth, nutrient uptake, and yield promotion of broccoli by plant growth promoting rhizobacteria with manure. Hort Sci 46:932–936
Yoon M-Y, Cha B, Kim J-C (2013) Recent trends in studies on botanical fungicides in agriculture. Plant Pathol J 29:1
Zarandi ME, Bonjar GHS, Dehkaei FP, Moosavi SAA, Farokhi PR, Aghighi S (2009) Biological control of rice blast (Magnaporthe oryzae) by use of Streptomyces sindeneusis isolate 263 in greenhouse. Am J Appl Sci 6:194–199
Zeng QG, Luo F, Zhang ZB, Yan RM, Zhu D (2012) Phosphate solubilizing rhizosphere bacterial T21 isolated from Dongxiang wild rice species promotes cultivated rice growth. Trans Tech Publ 167–175
Zhao J-L, Zhou L-G, Wu J-Y (2010) Promotion of Salvia miltiorrhiza hairy root growth and tanshinone production by polysaccharide–protein fractions of plant growth-promoting rhizobacterium Bacillus cereus. Pro Biochem 45:1517–1522
Zou C-S, Mo M-H, Gu Y-Q, Zhou J-P, Zhang K-Q (2007) Possible contributions of volatile-producing bacteria to soil fungistasis. Soil Biol Biochem 39:2371–2379
Zuo Y, Zhang F (2011) Soil and crop management strategies to prevent iron deficiency in crops. Plant Soil 339:83–95
Acknowledgements
I wish to thank the University of Tehran for providing the necessary facilities for this study.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Ethics declarations
The author has no conflict of interest.
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Etesami, H. (2019). Plant Growth Promotion and Suppression of Fungal Pathogens in Rice (Oryza Sativa L.) by Plant Growth-Promoting Bacteria. In: Maheshwari, D., Dheeman, S. (eds) Field Crops: Sustainable Management by PGPR. Sustainable Development and Biodiversity, vol 23. Springer, Cham. https://doi.org/10.1007/978-3-030-30926-8_13
Download citation
DOI: https://doi.org/10.1007/978-3-030-30926-8_13
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-30925-1
Online ISBN: 978-3-030-30926-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)