Abstract
Wheat (Triticum spp. L; Gramineae), a self-pollinating crop, is one of the most important cereal crops. Globally, wheat is an economic crop, utilized as food, feed, seed and industrial uses. Gene banks have conserved a large genetic resource collection of wheat germplasm including wild Triticum species. There are numerous species of Triticum with different genomes and chromosome numbers. Triticum harbors significant diversity based on ploidy level, biological status, geographical regions and morpho-agronomic traits. Introgression of novel alleles through crossing between various wheat genetic resources, e.g. modern varieties with locally-adapted varieties, enhances genetic diversity and preselection for traits of interest, which is required to ensure meaningful natural variation at the phenotype level. Improving wheat for biotic and abiotic stress tolerance traits, quality traits and yield attributes are the main objectives of wheat breeders and geneticists. Achieving these objectives can be facilitated by the application the modern genomics tools to augment traditional breeding programs. This chapter presents an overview of wheat germplasm biodiversity and conservation, objectives and stages of wheat breeding programs, cultivation and traditional breeding methods, in addition to modern plant breeding tools including marker-assisted breeding, genetic engineering and genome editing.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Biodiversity
- Genetic improvement
- Genetic map
- Modern wheat breeding
- Traditional breeding
- Triticum spp.
- Wheat
15.1 Introduction
Wheat (Triticum spp. L.), is the oldest cereal crop which is grown under a wide range of climate and soil conditions. It is adapted to the temperate regions with 30–90 cm annual rainfall. South Asia is the center of origin of wheat. There are many species of Triticum with different genome and chromosome numbers. Wheat is a self-pollinated crop, taxonomically, belonging to the genus Triticum (Linnaeus 1753), tribe Triticeae, family Poaceae (Gramineae) and order Cyperales (Briggle and Reitz 1963). Inflorescences consisting of one to several flowered spikelets which are sessile and alternate on both sides of the rachis and form a spike. Wheat has economic value as food, feed, seed and industrial uses (Nachit 1992). It is cultivated by seed under rainfed and irrigated conditions. Traditional plant breeding plays an important role in introgression of novel alleles through crossing genotypes from various plant genetic resources e.g. modern varieties with locally-adapted varieties, to enhance the genetic diversity and selection for the traits of interest such as high grain yield , early maturing, improved grain quality as well as resistance to lodging, biotic and abiotic stresses. Although new wheat biotechnology approaches using advanced DNA sequences and molecular methods have attracted plant breeders and geneticist, traditional plant breeding methods are still the key and first points to develop new wheat cultivars with desirable traits. In the present chapter, we present an overview of the center of origin, objectives and stages of a breeding program, traditional plant breeding methods, germplasm diversity and conservation, modern plant breeding tools for developing new wheat cultivars with desirable traits.
15.1.1 Origin and Distribution
Understanding the origin of wheat is one of the most important steps to improve it through breeding programs. South Asia is the center of origin of wheat. High genetic variability is found in the Fertile Crescent and bordering countries. There are many species of Triticum with different genomes and chromosome numbers (Table 15.1).
Triticum aestivum is the most common wheat species and represents the most widely grown of all crops including other cereals. It is allohexaploid wheat including three different genomes (A, B, D) with 42 chromosomes. It was developed by crossing T. monococum (2n = 14, AA) with an unknown wheat (2n = 14, BB). The F1 (AB, 2n = 14) was spontaneously doubled and became tetraploid wheat (2n = 28, AABB). The later was crossed with Aegilops squarrosa or T. tauschii (Coss.) Schmalh. (2n = 14, DD) and the F1 (2n = 21, ABD) was spontaneously doubled to produce the hexaploid wheat (2n = 42, AABBDD). This evolutionary process has great impact in wheat breeding; (i) it increased the genetic diversity within wheat and it’s relatives and (ii) it increased the genetic redundancy (defined as possessing many genes that code for similar proteins) within wheat. The first impact was very important for wheat plant breeding to improve target traits and has made wheat the most important cereal crop in the world, while, the second impact had a negative effect on diploid species because it makes for difficult chromosomal manipulations and breeding strategies.
15.1.2 Economic Importance
Wheat (Triticum aestivum) is a strategic and important cereal crop for a significant proportion of the world’s population. It is the main food source of carbohydrates for one-third of the world’s population, or more than two billion people worldwide (36%). Wheat (Triticum spp.) provide about 55% of carbohydrates and 20% of the world’s consumed food calories (Breiman and Graur 1995). Wheat is the third crop in terms of cultivated area and production following rice and maize, which are considered the most important grain crops in the world (FAO 2017). Wheat is grown under a wide range of climatic conditions. The Poaceae family also includes several other major crops, such as barley (Hordeum vulgare L.), rye (Secale cereale L.), maize (Zea mays L.) and rice (Oryza sativa L.). Triticeae is one of the tribes that contains more than 15 genera and 300 species including wheat. Wheat (Triticum) and rye (Secale), Aegilops, Agropyron, Eremopyron and Haynalidia form the subtribe Triticineae (Simmonds 1976). Linnaeus (1753) first classified wheat; Sakamura (1918) reported the number of chromosomes of each species. The latter was a turning point in the Triticum classification. Wheat was separated into three groups. The binary diodes are 14 (n = 7), tetraploids 28 (n = 14) and hexaploids 42 (n = 21) chromosomes. Wheat bread is T. aestivum; T. durum and T. compactum are the other two main types. These three are natural hybridization products among ancestors no longer grown commercially (Briggle 1967).
15.2 Cultivation and Traditional Breeding
15.2.1 Cultivation and Use of the Wheat Crop
Wheat is one of the oldest cereal crops. It is grown under a wide range of climates and soils and adapted to temperate regions with annual rainfall of 30–90 cm. There are two major types of wheat: winter and spring wheat. Always, the winter wheat is sown in the fall; however, the spring wheat is sown in the spring. In 2017, the ten leading wheat producing countries were China, India, Russia, USA, France, Australia, Canada, Pakistan, Ukraine and Germany (FAO 2017). Bread wheat cultivars belong to hexaploid wheat (Triticum aestivum). Wheat genotypes which grown in dry zones are generally considered to be hardened, containing 11–15% protein and strong gluten. The strong gluten of bread wheat entraps carbon dioxide (CO2) formed during the process of fermentation of the dough and the fermented dough can rise. Wheat cultivars grown in humid areas are soft, with 8–10% protein content and weak gluten. Soft wheat flour is used in making cakes, biscuits, pastries and flour. Durum wheat (T. durum Desf.) is considered to be one of the best sources of semolina production and is suitable for pasta and other products (Nachit 1992). On the other hand, diploid wheat is not cultivated because it has no economic importance as a crop anywhere in the world. Most wheat is grown for human nutrition and about 10% of the resulting grains are used industrially to produce starch, paste, dextrose and gluten). Chemical analysis of wheat grains shows they contain all the essential nutrients; 12% water, carbohydrates in the form of starch (60–80%), proteins (8–15%) contain sufficient amounts of all essential amino acids (excluding lysine, tryptophan. methionine), minerals (1.5–2%), vitamins (such as complex B, vitamin E) and crude fiber 2.2%.
15.2.2 Traditional Breeding Methodologies and Limitations
Although modern plant breeding utilizing the advances in DNA technology has attracted wheat breeders and geneticist, traditional plant breeding methods are still the key and first points to develop new wheat cultivars with desirable traits. The primary objective of conventional wheat breeding is to have a plant that can grow and thrive in a wide range of different environment easily. Changes in arable land, harsh cropping systems and food security should be highly considered along with emerging global issues (Davis et al. 2004). These global issues can be addressed in wheat by utilizing methods of plant breeding which provide the ability to select genotypes having desirable genes/QTLs (quantitative trait loci) controlling important traits.
15.2.3 Objectives and Stages of Wheat Breeding Programs
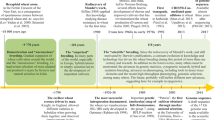
The main challenges for wheat breeders and geneticists are to genetically improve high grain yield, resistance to main diseases (rust, smut, bunt, Fusarium), tolerance to abiotic stresses (drought, salt, heat), early flowering and maturity, response to high doses of fertilizers, dwarf and lodging resistance, etc. (Mohammadi et al. 2012; Mwadzingeni et al. 2016; Salem 2015; Salem et al. 2007; Sallam et al. 2014, 2018b). Wheat can grow in many different environments ranging from temperate irrigated to dry and high rainfall areas and from warm humid to dry cold conditions (Bowne et al. 2012; Sallam et al. 2015). Hence, addressing the problem and setting the objectives are the key points for success in any wheat breeding program. Presence of genetic diversity plays a vital role in improving wheat crop for target traits in breeding programs. Baenziger (2016) determined five main stages for a successful breeding program (Fig. 15.1). Each stage is important and critical to a successful breeding program.
15.2.3.1 Addressing the Problem and Determining the Objectives
Although wheat can be grown in many different environments, wheat breeders work hard to genetically improve wheat crop to solve serious problems that limit wheat production and productivity. Each environment has a specific problem such as drought stress, heat stress, salt stress, diseases, low input environments, insects, etc. Therefore, identifying the problem is very important to determine the appropriate breeding program to improve the target traits. Moreover, the breeding program can also differ based on growth stage. For example, improving drought tolerance in wheat depends on the growth stage of wheat which is exposed to drought (Sallam et al. 2018b). Drought can occur during the seedling stage or grain filling stage (Salem et al. 2004, 2007). Some studies report that there was no correlation between drought tolerance in grain filling stage and drought tolerance in the seedling stage because genotypes can respond differently for their drought tolerance according to the growth stage (Salem et al. 2007; Sallam et al. 2018a).
15.2.3.2 Using a Useful Source of Genetic Variation and Diversity
The next stage after defining the problem and determining the objective is to look for appropriate germplasm that has variation in the target traits to establish how they are inherited. Plant germplasm includes an agronomic description of the material for traits that are useful for breeders and research in crop improvement. There are different types of germplasm including the following:
-
(a)
Landraces which are primitive cultivars selected and cultivated by the farmers for many generations. Landraces have a high level of genetic diversity which is an important source of resistance to various biotic factors and tolerance to abiotic stresses.
-
(b)
Obsolete cultivars which can be defined as earlier-popular varieties that have been changed by new varieties.
-
(c)
Modern cultivars are cultivated high-yielding varieties. They are normally used as parents in a breeding program to improve yield and its attributes.
-
(d)
Advanced breeding lines are highly homozygous lines. They are developed by plant breeders for improving targets traits through plant breeding programs.
-
(e)
Wild forms of cultivated species.
-
(f)
Wild relatives are very important and useful for genetic diversity and they are considered an interesting source of resistance to biotic factors and tolerance to abiotic stresses. They are wild plant species that are genetically related to cultivated crops
-
(g)
Mutants can be obtained from mutation breeding.
In wheat, crossing two or more different genes is the main method of introducing genetic variation. Most of the crosses are between highly homozygous lines (pure lines) to produce a F1 generation. Many studies have focused on the F1 generation to understand the inheritance of important traits using different types of diallele analysis. Others try to achieve offspring with a genetic identity for one or more traits (e.g. early flowering) which is closer to that of the parents by crossing the F1 with the target parent. The F2 population could also be a very interesting target to observe the segmentation of target traits. Recently, most studies have focused on the biparental populations in which highly homozygous lines derived from crossing between parents can be obtained by single seed descent (SSD). These biparental lines are normally used for identifying important QTLs controlling target traits. More interestingly, diverse populations are widely used these days for genome-wide association study (GWAS). This diverse population constitutes different genotypes from different parts on the world. To achieve the goals of any breeding program, germplasm should have a high degree of genetic diversity among plant materials (Eltaher et al. 2018; Salem and Sallam 2016; Salem et al. 2015).
15.2.3.3 Inbreeding and Phenotypic Selection Among the Resulting Variants
The next stage for wheat breeders is to choose which selection and inbreeding methods they will use. Inbreeding transforms genotypes from heterozygosity to homozygous lines. Then, selection chooses a very few of the superior homozygous lines to be integrated into the next stages of the breeding program. Unselected lines are discarded from the breeding program.
Selection is one of the basic methods of traditional plant breeding. It can be artificial (made by human) or natural (by the power of nature). Selection in breeding programs differs by the environment in which the plant will be grown, hence the wheat breeder should be very careful with the plant material tested (Baenziger 2016). For example, if the objective is to select wheat genotypes for winter hardiness, selection should be in environments that allow breeders to have a variation in the traits of interest among the tested genotypes.
15.2.3.4 Screening and Evaluation of Selected Elite Lines for Specific Objectives
Although all the five stages are important for a successful breeding program, screening and evaluating germplasm for the specific objective is the most crucial step before releasing a cultivar. Collecting precise phenotypic data is critical to the selection process. Plant material should be evaluated and screened over years or/and replications or/and locations or/and environments. This step is entirely based on the objectives of the breeding program. To have a fruitful selection and genetic improvement of the wheat crop, a selected trait should have a high heritability probability. The evaluation could also be performed at any growth stage or at many growth stages (Sallam et al. 2016). For example, a biparental population was evaluated for seedling and grain filling stages under drought tolerance to select genotypes having high tolerance at both growth stages (Salem et al. 2004, 2007; Sallam et al. 2018b). The genotype × environment interaction should be highly considered in the selection.
15.2.3.5 Cultivar Release
The last stage of a breeding program is the decision to release a cultivar if it is superior for at least one trait important target trait. In most cases, wheat grain yield should be one of among target traits for each breeding program. The procedures of releasing cultivar differ by country.
15.3 Germplasm Diversity and Conservation
Natural variation including phenotypic and genotypic variation is the fundamental concept of plant breeding , which aims to select useful variation for future generations. The variation can be introduced by crossing, mutation and/or present in nature due to historical recombination of alleles. In wheat, wild relatives, landraces, modern cultivars, breeding materials in addition to gene bank accessions are sources of variation.
15.3.1 Germplasm Diversity
The allopolyploid nature and origin of wheat undoubtedly contribute to its diversity that allowed wheat to grow and adapt to a wide range of environments. Wheat as an allohexaploid crop has different genome levels, e.g. Triticum as diploid 2n = 2x = 14 (T. urartu Than. ex Gand. and T. monococcum, AA genome) and Aegilops (A. speltoides, BB genome) (Marcussen et al. 2014; Rasheed et al. 2018). Due to the hybridization between Triticum and Aegilops, the tetraploid (durum) wheat became in nature (T. turgidum ssp. durum, AABB genome; 2n = 4x = 28). While hexaploid (bread ) wheat developed by the hybridization of tetraploid wheat (AABB) with diploid Aegilops species, (A. tauschii Coss., DD genome; 2n = 2x = 14) to form (T. aestivum, AABBDD genome; 2n = 6x = 42) (International Wheat Genome Sequencing 2014; Rasheed et al. 2018).
Wheat crop improvement relies on genetic diversity and utilizing natural variation for selection using landraces and wild relatives, thus increased the rate of genetic gain in breeding programs. Old wheat germplasm including landraces and wild relatives are an important genetic resource for enhancing modern wheat by capturing new alleles. Wheat diversity bottlenecks that reduced the genetic variation was influenced by many biological processes that started at domestication, which is considered as the first bottleneck in reducing genetic variation. Wheat dissemination from the domesticated Fertile Crescent area to Europe and Asia has slowly adapted to local environments; therefore, the genetic diversity shrank. Further, the genetic diversity in wheat was reduced by depletion of certainly desired alleles from a gene pool as a result of crosses between diploid and tetraploid to produce hexaploid wheat, which occurred naturally a few times in addition to early selection by farmers. Then the diversity within bread wheat was reduced due to breeding procedures by separating environmental from genetic effects and replacing local landraces with newly-improved cultivars. Narrowing the genetic variation is a major concern for plant breeders in wheat genetic improvement progress; a significant decrease in wheat genetic diversity during the last century has been detected (Novoselović et al. 2016). Therefore, protecting wheat genetic diversity is essential for improving yield and adaptation. Introgression of novel alleles through crossing plants from various plant genetic resources e.g. modern varieties with locally adapted varieties, enhances the genetic diversity and preselection for traits of interest which is required to ensure that meaningful natural variation at the phenotypic level. Salem et al. (2015) studied genetic diversity in Egyptian wheat. A dendrogram derived from UPGMA cluster analysis based on the genetic similarity (gs) matrix coefficient for 33 Egyptian wheat genotypes was constructed (Fig. 15.2).
UPGMA cluster analysis-based dendrogram depicting genetic relationships among 33 Egyptian hexaploid wheat genotypes and based on data of 17 microsatellite markers. (Source: Salem et al. 2015)
15.3.2 Cultivar Characterization and Phylogeny
Characterization and evaluation of wheat gene bank collections represent a powerful means for the classification of old and new materials to understand natural variation and its application in breeding. Using the recent advances in technology for characterizing collections at a large scale by applying high-throughput genotyping is applicable. The development of DNA markers makes the genetic diversity in the wheat germplasms, including old collections, highly attractive (Börner et al. 2000a; Salem et al. 2015; Sehgal et al. 2015). Population structure using molecular markers in large diverse collections including wild, landrace and modern varieties can help in understanding and monitoring genetic diversity. By applying the analysis that accurately calculates the relatedness among the individuals and clusters them based on their genetic information is commonly used in association mapping studies. Phylogeny analysis helps in understanding the complex history of wheat dissemination and genetic diversity. Many phylogeny studies have been conducted which were able to characterize wheat collections based on the ploidy level, biological status and geographical regions (Demir et al. 2015; Golovnina et al. 2007; Goncharov et al. 2009). Allelic variation and its distribution over historical time using wild relatives, landraces and cultivars, originating from different geographical regions, and genotyped by high-density single nucleotide polymorphism (SNP) arrays, has long been a main goal of phylogeny studies. Genetic diversity characterization of more than a half million wheat genetic accessions at the level of a collection is a huge challenge that aims to redesign the exploitation of wheat genetic resources. An ex situ gene bank should begin strategies for the exploitation and valorization of wheat genetic resources to unlock their hidden favorable genetic diversity for breeding as a pivotal step for enhancing yield.
15.3.3 Genetic Resources Conservation Approaches
Ex situ collections of wheat held in gene banks are important genetic resources that need to be managed securely in a cost-effective manner and easily accessed by a broad range of users. To maintain plant genetic resource with natural genetic variation, a number of ex situ seed gene banks have been established worldwide. Hundreds of thousands of wheat accessions have been collected since N. I. Vavilov and H. Harlan began seed collection at the beginning of the last century (Börner 2006). Globally, there are over 80 wheat germplasm collections, holding more than 800,000 accessions. The larger wheat collections are maintained at CIMMYT-Mexico (>100,000 accessions); USDA-NSGC, Aberdeen, Idaho, USA (nearly 40,000); Vavilov Research Institute (VIR), Russia; IPK-Gatersleben, Germany; ICARDA, Syria; NBPGR, India and Instituto del Germoplasma, Bari, Italy (each holding approximately 30,000 accessions). These collections represent worldwide geographical regions, biological status and other features like growth habit that need to be phenotypically and genetically characterized appropriately to reveal their potential value in crop improvement and to provide a wider basis for breeding purposes. A list of important world gene banks for plant genetic resources conservation is given in Table 15.2.
The purpose of an ex situ gene bank is to cultivate the accession in a garden or store it in a seed bank; so-called ex situ conservation. In general, the goal of ex situ conservation is to prevent the local, regional or global extinction of a species and to represent, as much as possible, the genetic diversity. Maintaining genetic integrity is one of the major challenges for ex situ conservation, due to contamination by foreign pollen or incorrect handling during multiplication (Börner 2006). Since most of the wheat collections are evolved and delivered by breeders or farmers they consist of predominantly local or regional materials that are most likely duplicate accessions within and between collections. Therefore, characterization and exploitation of the natural variation in wheat germplasms are maintained at the plant genetic resource centers and are essential for future research.
15.3.4 Cytogenetics
Cytogenetic methods such as chromosome banding and in situ hybridization remain relevant in the post-genomic era for molecular characterization of allopolyploid plants such as wheat, where the combination of the different genomes in some cases makes it difficult to assess the reorganization of chromosomes during evolution. The first wheat cytogenetic study was conducted by Sakamura (1918) who discovered polyploid series of the diploid, tetraploid and hexaploid in wheat, with a basic chromosome number of x = 7. The cytogenetic analyses could split the chromosomes of polyploid wheat and their progenitors into tetraploid and hexaploid genomes (Shcherban et al. 2016). Chromosome banding techniques allow for the identification of the chromosome duplication and chromosome polymorphism, as well to understand the evolutionary processes (Friebe and Gill 1996). Hybridization in situ is another technique that directly localizing DNA sequences on chromosomes of which fluorescence in situ hybridization (FISH) is used to show the DNA sequence distribution on chromosomes, whereas genomic in situ hybridization (GISH) is commonly used to identify the genomic composition of wheat amphiploids and hybrids (Cuadrado et al. 2008). The translocations of chromosome segments in wheat varieties have been detected by a cytogenetic method that allowed the breeders to use marker-assisted breeding for selection of the desired genotypes. Such an approach was found useful in breeding aspects to determine the resistance of phytopathogens and increased productivity e.g. Russian wheat varieties which carried intact wheatgrass chromosomes had high resistance to fungal diseases and high grain quality (Salina et al. 2015). There is clear evidence that despite extensive development of high-throughput molecular markers, cytogenetic methods are still imperative for characterizing the genetic diversity and application in breeding research.
15.4 Molecular Breeding
The first step toward the creation and release of a new cultivar is to identify the sources of genetic variation by evaluating a large number of genotypes. After identifying the available genetic variation, superior genotypes can be used as parents in breeding programs. In a highly diverse crop like wheat, the number of evaluated genotypes could reach into the hundreds of thousands. Evaluating such a large number of genotypes in the field is very expensive and time-consuming. Molecular markers are a possible technique which can help to reduce the number of evaluated genotypes and hence save time and expense. For example, molecular markers are typically used during backcrossing programs to track a small number of loci, which helps the breeder identify the germplasm close to the recurrent parent and reduce the required efforts, as compared to the traditional backcrossing programs (Langridge 2003).
15.4.1 Molecular Markers
Due to the size and complexity of the wheat genome, the application of molecular markers is quite complicated compared to other crop genomes. However, many efforts have been made to understand the wheat genome using different types of molecular markers. These efforts led to publication of the first wheat genome map in 1998 using simple sequence repeat (SSR) markers (Röder et al. 1998). That study was followed by many others which mapped many quantitative loci associated with important traits using different types of molecular markers (Bhusal et al. 2017; Börner et al. 2000b, 2002; Echeverry-Solarte et al. 2015; Salem et al. 2007). The different types of molecular breeding tools can be classified into two major types, molecular markers and molecular maps.
15.4.1.1 Molecular Markers in Wheat
Molecular markers have been used routinely in wheat breeding over the last 50 years. Due to continuous advances in biotechnology, new types of molecular markers are typically appearing which are usually faster than the previously developed types. Generally, molecular markers used in wheat can be classified into three general types: hybridization-based DNA markers, polymerase chain reaction (PCR)-based markers and DNA chip and sequencing-based DNA markers.
Hybridization-Based DNA Markers
These marker types are considered as first-generation markers. Developing this type of molecular markers was based on the variation in the DNA fragment lengths which are produced by a specific restriction enzyme. These are commonly used to differentiate between two or more individuals or for fingerprinting purpose. One example is restriction fragment length polymorphism (RFLP). However, using this type of marker in wheat is not very effective due to the low level of polymorphism identified by this marker resulting from the high frequency of monotonous DNA in the wheat genome (Khan et al. 2014).
PCR-Based/Markers
These molecular markers are considered as second-generation markers which were developed to reduce time, effort and cost required for molecular mapping and genotyping. They function depending on developing a primer which can be hybridized to a part of the DNA and produce a new DNA strand. Due to the development of PCRs, many copies of the DNA can be obtained. PCR products are then separated by gel electrophoresis. This type of marker can be used for two main different purposes; to identify the existence of a specific gene and the diversity between the studied genotypes. Different kinds of markers following this type are random amplified polymorphic DNA (RAPD), simple sequence repeats (SSRs), inter-simple sequence repeat (ISSR), amplified fragment length polymorphism (AFLP) and diversity arrays technology (DArT). SSR markers are considered the most common type of PCR-based markers used in wheat. SSR is a very short sequence (1–6 nucleotide) repeated randomly in wheat (Figs. 15.3 and 15.4). Due to the repetition of the SSR in different parts of the genome, it becomes a useful tool to predict a high level of polymorphism (Gupta et al. 1999; Röder et al. 1998). Due to this advantage, SSR markers have been widely used to study the diversity in the wheat genome. Many SSR markers are now identified as specific markers for important genes in wheat. Two of the databases which present specific wheat SSR markers and mapped SSR markers are Grain Genes (https://wheat.pw.usda.gov/ggpages/SSRclub/) and Integrated Breeding Platform (https://www.integratedbreeding.net/104/communities/genomics-crop-info/agricultural-genomics/markers/ssr-markers/wheat).
Eropherogram analysis using fragment analyser software of polymorphic SSR marker in mapping population of two wheat parental lines ATRI 5283 x ATRI 15010 and F2 generation amplified with the fluorescence labels SSR marker locus Xgwm429-2BS. Source: Salem (2004). Dissertation under supervision of Dr. Marion Röder Laboratory, Gene and Genome Mapping Group, IPK, Gatersleben, Germany
DNA Chip and Sequencing-Based DNA Markers
SNP is a single base change in the DNA sequence. Due to the advances in sequencing methods which produce numerous SNPs, SNP markers are now widely used in genetic diversity, population structure, linkage mapping, linkage disequilibrium, whole genome association study, genomic selection and marker-assisted selection. SNPs replaced SSR markers in many plant species because they have the following attributes: low cost, high genomic abundance, codominance inheritance, easy documentation, locus specify and low genotyping error rates. Identifying SNP markers and their availability lead to the development of different SNP genotyping platforms such as Kompetitive Allele Specific PCR (KASP).
KASP is a homogenous, non-gel based, fluorescence-based genotyping technology. Genotyping technology in KASP is based on allele-specific oligo extension and fluorescence resonance energy transfer (FRET) for signal generation. KASP genotyping can be carried using 96-, 384- and 1536-well plate formats. This enables the breeder to determine many genotypes in a very short time as no gel is required. Despite the fact that KASP markers are a recent development, many types of research have identified KASP markers for important genes in the wheat genome. Some of the identified markers are listed in Rasheed et al. (2016).
15.4.1.2 Molecular Maps in Wheat
Advances in genome sequencing methods and the appearance of next, third and fourth sequencing techniques, make it possible to identify the sequence of numerous genotypes in a short time. Genotyping-by-sequencing (GBS) is a good example of the revolution in sequencing methods which is used broadly. GBS produces an enormous number of SNP markers distributed on the whole genome. These SNPs can be used in GWAS, genetic diversity, genetic linkage analysis and molecular marker discovery (He et al. 2014). This progress enables wheat breeders to identify the location of QTLs associated with important genes using different analyses such as genome-wide association study and quantitative trait loci.
By applying these new sequence techniques to different wheat populations, the sequence of the 21 chromosomes is available and can be used to assign gene sequence to individual chromosomes, develop physical maps, and identify gene models and the annotation of these models. One of the available databases for this information is International Wheat Genome Sequencing Consortium (IWGSC) (https://www.wheatgenome.org/). This database was first available at 2014 with one milestone chromosome-based genome sequence and will be updated to reach four milestones. The data of this update will be available in 2019.
Genome-Wide Association Study (GWAS)
This type of analysis uses diverse genotypes with known genetic information such as single nucleotide (SNPs ), SSRs or DArT markers. Any number of genotypes can be used to conduct GWAS analysis; however, a minimum of 100 genotypes is required (Kumar et al. 2011). Using the genomic and morphological available data, GWAS detects QTLs responsible for the studied traits (Chang et al. 2018). By studying the linkage disequilibrium (LD) between the identified loci, a number of candidate genes responsible for the studied trait can be identified. Many GWAS studies were done in wheat to detect genes controlling biotic stress resistance. For example, Mourad et al. (2018b) identified SNPs associated with Sr6 stem rust resistance gene. Juliana et al. (2018) identified candidate genes associated with wheat resistance to leaf rust, stripe rust and tan spot. Pariyar et al. (2016) identified candidate genes controlling nematode resistance in wheat using GWAS. Ando et al. (2018) detected the candidate genes controlling the resistance of stripe rust, Septoria blotch and Hessian fly in spring wheat. Mourad et al. (2018a) identified candidate genes controlling common bunt resistance in winter bread wheat. Combining the results of the different GWAS studies will enable wheat breeders to develop maps containing the chromosomal location of the resistance genes. For example, maps of wheat stem rust and stripe rust resistance genes are available (https://maswheat.ucdavis.edu/).
GWAS played an important role to identify candidate genes controlling abiotic stresses. For example, Sukumaran et al. (2018) identified candidate genes controlling drought and heat tolerance in durum wheat. Ayalew et al. (2018) identified 5 candidate genes located on 4 different chromosomes controlling root length under water stress conditions. Liu et al. (2018b) detected 24 candidate genes on 17 chromosomes controlling salt tolerance using SSR markers.
In addition, many studies detected candidate genes controlling important agronomic traits such as spike-related traits (Liu et al. 2018a), grain yield and its related traits (Garcia et al. 2019; Wang et al. 2017) and plant height and 1000-kernel weight (Daba et al. 2018). Identifying the candidate genes which control important traits in wheat will improve breeding, especially if GWAS is followed by deep analysis of the detected loci using haplotype-block analysis. However, the accuracy of GWAS is affected by the accuracy of the available phenotypic data. In this case, high phenotyping platforms and skilled researchers are required. With the advances in sequencing methods, bioinformatics and statistics, the future of GWAS will be very promising in wheat improvements.
QTL Mapping
To apply QTL mapping for a specific trait, a biparental mapping population (Fig. 15.5) such as double haploid lines (DHLs), backcross mapping population (BC), F2:3 mapping population, F6:8 or recombinant inbred lines (RILs) should be used. Parents used to produce any type of these populations should be different in their alleles which affect the phenotypic value of the target trait. QTLs are mapped based on the distance between it and the genetic marker. Based on the number of markers used in genotyping the studied population, a different type of QTL mapping can be used such as single-marker, double-marker or multiple-marker mappings. Different statistics could be applied to map the QTL such as: single interval mapping, multiple interval mapping, QTL-composite interval mapping (CIM), multi-interval mapping (MIM), multiple QTL mapping and multi-trait mapping (MTM) (Tian et al. 2015). A number of genotypes in the studied population vary based on the purpose of the QTL study and the type of population. However, the more genotypes studied, the higher the mapping precision.
Many QTL mappings have been done in wheat using different types of markers. Examples of QTL mapping for different wheat traits are listed in Table 15.3. Applying QTL mapping on the different traits will accelerate plant breeding as it helps the breeder in i) identifying a number of genes controlling the trait, ii) understanding the effect of the genes controlling the trait, iii) determining the location of the gene/s and iv) studying the linkage between the different genes of interest. All of these help in pyramiding many target genes in one genotype to understand the diversity of the studied germplasm (Khan 2015).
15.4.2 Genomic Selection in Wheat
Genomic selection (GS) is used broadly in both animal and plant breeding programs. The main object of GS is to shorten breeding time by predicting the performance of genotypes based on genomic data and evaluating a few genotypes to predict the performance of the rest of the population. GBS produces abundant numbers of SNP markers distributed all over the genome. These SNPs can be used to develop accurate genomic prediction (GP) methods, even for complex genomes like wheat. However, before applying GP, many factors which affect its accuracy should be taken into account. Some of these factors are population size, population structure and marker density. It is reported that the accuracy of GP improved when the tested set is highly diverse, used markers of high density and a maximum of 2000 genotypes are used (Norman et al. 2018).
Many efforts have been made to identify the best percentage of genomic selection in wheat. Belamkar et al. (2018) reported that 50% genomic prediction could be applied in preliminary yield trials in winter bread wheat. For grain yield, it was reported that genomic prediction accuracy reached 0.28–0.45 (Poland et al. 2012). GP accuracy showed a range of 50–80% for grain yield, protein content, gluten index and alveograph measures using different prediction models (Haile et al. 2018).
Fusarium head blight (FHB) was found to be controlled by a limited number of loci with low effects based on GWAS study. The accuracy of GS for this trait was reported to reach 60% (Dong et al. 2018). It seems that using GS in wheat breeding programs will increase breeding progress and lead to new eras in breeding and functional genomics .
15.5 Genetic Engineering
Genetic engineering, or genetic modification , is carried out to manipulate wheat genes directly. It is expected to support conventional breeding for further wheat production by increasing wheat production efficiency and avoid losses due to biotic and abiotic stresses through producing high tolerance lines under diverse conditions.
15.5.1 Methodologies
Genetic engineering offers the opportunity to improve the performance and yield of wheat by using the advances in wheat genome sequencing and molecular breeding. It leads to highly efficient and robust transformation systems targeting sequence-specific nucleases, such as zinc fingers (ZFNs), transcription activator-like effector nucleases (TALENs) and RNA-guided engineered nucleases such as clustered regularly interspaced short palindromic repeats associated protein 9 (Cas9). Transformation systems e.g. Agrobacterium tumefaciens have been successfully applied in wheat using genes of agronomic importance. Since the first report of wheat transformation using the A. tumefaciens approach (Cheng et al. 1997), it has become a worldwide technique that successfully produced transgenic wheat plants with desired gene(s) of agronomic traits (Habib et al. 2014). Such a technique is routinely applied but using the recent advances in engineered nucleases e.g. ZFNs, TALENs and Case9 have been emerging for understanding gene function of valuable traits. Cas9 has shown its applicability and accuracy in producing a wheat line with a trait of interest (Gil-Humanes et al. 2017; Liang et al. 2017). The successful progress in genome editing using technologies such as Cas9 is promising for performance and yield-boosting; therefore, wheat genetic engineering needs to overcome the traditional transformation approaches.
15.5.2 Enhanced Traits
Producing plants carrying multiple desired traits with stable inheritance and expression in the following generations is the aim of genetic engineering. Thus, large increases in yield would be expected if new gene editing e.g. Cas9 were applied to wheat agronomic important traits. There are many agronomic traits that have been improved using transformation and gene editing techniques for the development of stable wheat transgenics, reviewed by (Ishida et al. 2015; Shrawat and Armstrong 2018). Knockout TaGW2, TaLpx-1 and TaMLO genes using Cas9-based multiplexed gene editing (MGE) showed high improvement in 1000-grain weight, grain area, grain width, and grain length, resistance to Fusarium graminearum and powdery mildew, Blumeria graminis f. sp. tritici (Wang et al. 2018). Also, it has been shown that Cas9 in bread wheat improved resistance to infection of powdery mildew by mutated TaMLO genes (Wang et al. 2014). Wheat nutrient value, especially Fe content, has been improved through editing TaVIT2 using Cas9 (Connorton et al. 2017). Kim et al. (2018) showed that Cas9 in wheat helps to improve abiotic stress-related traits through knockout genes, namely wheat dehydration responsive element binding protein 2 (TaDREB2) and wheat ethylene responsive factor 3 (TaERF3). Gene editing techniques face a challenge in wheat due to ploidy levels; for example, in hexaploid wheat. The aforementioned findings show the feasibility of using Cas9 in wheat improvement.
15.5.3 Variation from in Vitro Tissue Culture
Tissue culture techniques are used for many purposes to: i) study the general combining ability (GCA), determine specific combining ability (SCA) and heterosis (Nawara et al. 2017); ii) rescue embryos from wide crosses made to transfer genes from wild relatives to wheat (Tyankova 2000); iii) screen for biotic and abiotic stress in vitro (Nawara et al. 2017); iv) create haploid plants (Santra et al. 2017); v) use as source material for wheat transformation (Waheed et al. 2016); vi) create doubled haploid lines (DHLs) (Srivastava and Bains 2018) and vii) create somaclonal variation (Danci et al. 2010). In order to improve wheat productivity, one of the most significant steps in a breeding program is the select of the right parents. To reach gains in plant biotechnology of wheat using immature embryo culture system, GCA and SCA for in vitro traits is necessary under biotic and abiotic stresses (Nawara et al. 2017). Numerous studies of the genetic control of in vitro traits using immature embryos were also reported in wheat (Barakat and Shehab El-Din 1993; Nawara et al. 2017). Mating designs such as diallel, line x tester have been widely used in genetic investigation to study the inheritance of in vitro traits among some genotypes (Nawara et al. 2017). Analysis of diallel data is usually conducted according to Griffing (1956), who partitioned the total variation of diallel data into GCA of the parents and SCA of the crosses, according to Barakat and Shehab El-Din (1993), Torres and Geraldi (2007) and Nawara et al. (2017). Biotechnology offers several valuable techniques such as cell, tissue and organ culture, which develop the breeding methods to improve the genetic characters including salt tolerance in economic crops. Tissue culture generates a wide range of genetic variation in plant species, which can be incorporated into plant breeding programs. By in vitro selection, mutants with useful agronomic traits, i.e. salt or drought tolerance or disease resistance can be isolated in a short duration. However, the successful use of somaclonal variation is very much dependent on its genetic stability in the subsequent generations (El-Aref 2002; Jain 2001; Mercado et al. 2000). Embryo rescue is more important when it benefits the introduction of genetic material from wild relatives to cultivated species or to produce DHLs. Friebe et al. (1996) reported the importance of genes from wild relatives in wheat breeding programs.
15.5.4 Transgenic Wheat Lines
Transformation systems and gene editing in wheat have produced several transgenic lines showing strong and constitutive transgene expression with improvement in growth performance, yield and stress tolerance. For instance, wheat streak mosaic virus (WSMV), considered as the most destructive viral disease, was engineered and produced independent wheat transgenic lines that showed high resistance through overexpressing the viral coat protein gene (Sivamani et al. 2002). Mackintosh et al. (2007) showed that transgenic wheat lines exhibited resistance against Fusarium graminearum under greenhouse and field trials. It was also confirmed that the transgenic lines showed high tolerance to salt and drought stresses by TaERF3-overexpression (Shavrukov et al. 2016) and significantly higher yield under water stress conditions by transforming TaDREB3 from Bobwhite, a high transformation efficiency cultivar (Rong et al. 2014). In wheat, there are many model cultivars with high transformation efficiency such as Bobwhite, Florida, Chinese Spring, Lunxuan 987, Yumai 66, Kontesa and Fielder, which have been extensively used in genetic studies (Shrawat and Armstrong 2018). Therefore, using targeted genome editing for the development of wheat cultivars with stable performance will potentially revolutionize crop breeding.
15.5.5 Genome Editing
The main objective of plant breeding programs is to produce a new high-yielding cultivar that is also resistant to biotic and abiotic stresses. To achieve this goal, breeders have tried to edit the plant genome by induced random mutations or editing specific gene(s) in the genome using different approaches like ethyl methanosulfonate (EMS) mutagenesis, transfer, T-DNA insertions, zinc finger nucleases (ZFNs) and transcription activator-like effector nucleases (TALENs). However, many difficulties have arisen in applying the previous methods, including protracted time requirements and high costs due to the need for protein engineering (Bortesi and Fischer 2015; Gaj et al. 2013). The new genome-editing technique, CRISPR/CRISPR-associated protein 9 (Cas 9), has been developed to overcome the difficulties of the previous methods. CRISPER/Cas 9 was first discovered as a component of bacterial adaptative immunity (Ishino et al. 1987). Its function was first identified as it when it was observed that Streptococcus thermophilus resists bacteriophage by integrating part of the virus genome into its CRISPR locus (Barrangou et al. 2007). Appropriate restriction enzymes should be used to confirm the targeted gene editing. CRISPR was applied to edit the genome of different plant types like tobacco, Nicotiana tobaccum (Gao et al. 2015); thale cress, Arabidopsis thaliana (Li et al. 2014); rice, Oryza sativa (Shan et al. 2013) and sorghum, Sorghum bicolor (Jiang et al. 2013). Despite, the low cost and time-saving of the CRISPR technique, it is very complicated, especially in dealing with polyploidy germplasm like the wheat genome, which requires editing of each copy of the targeted gene. However, many studies report success in CRISPR editing of some wheat genes. For example, Kim et al. (2018) reported the success of CRISPR/Cas 9 technique in editing some protoplasts like TaDREB2 and TaERF3 which control the stress response of the wheat plant.
15.6 Wheat Breeding Methods
Several traditional breeding methods are still in use by plant breeders for crop improvement; the most common methods for wheat are pedigree breeding, pure-line breeding, bulk breeding, mass selection, single seed descent and backcross breeding (Baenziger 2016). The differences in breeding methods depend on two important points: i) the inbreeding of the population and ii) the selection process.
15.6.1 Pedigree Breeding
In this method, wheat breeders evaluate F2 plant for target traits and select the best plants in this generation. The selected seeds are grown in a next generation in a progeny rows. Selection of best plants is done among the grown plants. The selection is performed each generation until some segregation is observed within the progeny row. Segregation within a row is normally based on many factors including the number of segregating traits in the population of study, number of inbred generations and phenotypic data.
15.6.2 Pure-Line Breeding
In this breeding method, a number of selected individual plants from a cultivar are grown in rows and the best rows selected. Finally, the best rows are grown in replicates to make a decision of which selection in most promising (Baenziger 2016).
15.6.3 Bulk Breeding
The wheat breeders grow a bulk of the progeny derived from a specific cross. Then, after harvesting the bulk, a part of the bulk seed is planted again in the next year. The population is considered again when it contains a mixture of predominantly homozygous lines and this takes place after many plantings and harvestings of the bulk (Tee and Qualset 1975).
15.6.4 Mass Selection
Mass selection is the simplest method used for crop improvement. Breeders select large numbers of plants that present a similar phenotype. Seeds of selected plants are mixed together to constitute the new cultivar. The cultivar developed using this method has high genetic variation. Thus, mass selection could be performed again in such a cultivar (Marais and Botes 2009).
15.6.5 Single Seed Descent
The main goal of this method is to rapidly inbreed without artificial or natural selection. Wheat breeders begin with a large number of plants in an F2 population. Then, from each plant a single seed is sown. A single seed is harvested again and sown next year. Wheat breeders continue harvesting and sowing the single seed each year until all plants are predominantly homozygous. At this stage, no selection is normally performed (Pignone et al. 2015).
15.6.6 Backcross Breeding
The main objective of using backcross methods is to incorporate simply target inherited traits from unadapted donor genotypes into recipient genotypes. Backcross methods include repeated cycles of crossing to the recipient genotype (recurrent parent), followed by selection of the target trait of interest being transferred (Kenaschuk 1975). Breeders repeat crossing the F1 to a single cultivar which has a good character that could be added. This method was widely used for transferring many favorable characters such as resistance to disease. For example, many stem, leaf, and stripe rust resistant genes were transferred from Triticum species to common wheat (Marais et al. 2003). Resistance to Fusarium head blight was improved in winter wheat through the backcross method (Clark et al. 2016)
15.7 Mutation Breeding
Mutations or sudden heritable changes in the phenotype and genetic material (DNA bases) can occur by repetition errors during cell division or exposure to mutant chemicals or radiation, such as ultraviolet radiation, ionizing radiation or even the viruses of an individual. The change caused by the mutation is not targeted and may lead to the generation of new materials. Mutations are the novel source of difference of all genes. Mutation breeding induces desirable mutations to exploit for crop improvement. It is commonly used in self-pollinated crops such as wheat to produce traits in crops such as larger seeds. Mutations are the method of generating variations. If a mutation cannot be transmitted across the germ line, it cannot be inherited, it has little value in improving the variety in seed-bearing crops such as wheat. If it is not possible to transfer a mutation to the next generation through a pure line, it cannot be inherited and transmitted from one generation to the next, and therefore has little value in the development of the wheat variety, which multiplies by seed. The mutation line resulting from the mutation can be registered directly and released as a cultivar or benefit from the serious line in the breeding programs of the background and the production of new cultivars.
15.7.1 Mutation Breeding Program
A mutation breeding program begins with the M0 generation in which wheat seed is treated with a mutagen. The M1 generation in grown in the first year of treated seeds and the individual plants are harvested separately. M2 generation (second year), individual plants are grown in a line and the plants that contain the mutations or phenotype changes or the mutant allele are selected and harvested separately. M3 (third year), individual plants are grown and stable lines selected from each row. M4 (fourth year), comparative yield trial in the preliminary yield trial with a suitable check and selected superior lines. M5–7 (5th–7th years), in these generations replicated yield trials at several locations with the outstanding line released as a new cultivar. M8 (8th year) seed multiplication for distribution to farmers. A generalized scheme for a wheat mutation breeding program for high grain yield and its component traits is shown in Fig. 15.6.
As of 2013, 286 cultivars of Triticum ssp. reportedly feature a mutation (https://mvd.iaea.org/#!Search?Criteria[0][val] = wheat/). The first use of mutations in wheat breeding programs began in Japan to introduce dwarfism (Kihara 1984; Nonaka 1984). There were spontaneous mutations, Rht8 on 2D chromosome (Salem 2015) and Rht9 on 7BS chromosome, in the Japanese genotype Akakomugi (Gale and Youssefian 1985). As one feature, induced mutations can be used to understand the role of many genes by applying of targeting induced local lesions in genomes (TILLING ) (McCallum et al. 2000).
15.8 Conclusion and Prospects
15.8.1 An Overview of the Current Status
In view of the great economic importance of wheat due to its uses as food, feed, and seed and for industrial uses, a significant body of scientific research has already been carried out worldwide. Since classical breeding methods are laborious and time-consuming, the introgression of novel alleles through crossing plants from various plant genetic resources e.g. modern varieties with locally adapted varieties, enhance genetic diversity and preselection for traits of interest, which is required to ensure that meaningful natural variation at phenotype level. Although new wheat biotechnology approaches that use the advances in DNA sequences and molecular methods have attracted plant breeders and geneticist, traditional plant breeding methods are still the key and first points to developing new wheat cultivars with desirable traits. However, many promising cultivars adapted to climate change and biotic and abiotic stress conditions have been developed. For that, new breeding approaches in wheat for high grain yield, biotic and abiotic stresses to develop new cultivars are needed.
15.8.2 Current Research Initiatives to Combat Global Climate Change
Overpopulation, and biotic and abiotic stress are the most important challenges facing a wheat breeding program. Pre- and post-flowering stresses are the most important effect in wheat growing. Global climate change i.e. frost or high temperature are the greatest important climatic changes attracting considerable wheat breeders’ attention worldwide. Also, diseases such as Ug99, a new stem rust (Puccinia graminis f. sp. tritici), present in some regions of Africa and the Middle East, is predicted to spread rapidly through these regions and may affect the food security in those counties and worldwide (Singh et al. 2011). This requires more investment in breeding programs and the training of new plant pathologists and breeders. Also, more efforts must be made to breed new cultivars with wide adaptability, to extend wheat cultivation under abiotic stress i.e. drought and salt resistance to diminish the effect of global warming. Recent biotechnology tools have been used to develop promising new wheat cultivars with desirable traits. Also, new wheat genomes have been sequenced and innovative molecular methods developed that have inspired plant breeders and geneticist to develop new wheat cultivars with desirable agronomic traits, along with resistance to biotic and abiotic stress.
15.8.3 Recommendations for Future Research
Because there are no genetically distinct pure lines in wheat of most crop traits that are economically important, it is necessary to obtain pure lines through the production of haploids and doubled-haploid lines (DHLs) that can be exploited in breeding programs. Also, DNA markers must be developed which are closely linked to important biotic and abiotic stresses, physiological and anatomical characters, as well as grain yield and its components traits. Genes or QTLs should be identified for qualitative and quantitative attributes to improve these traits. Furthermore, germplasm and biotechnology should be improved to speed up and facilitate the improvement of new promising lines with high yield and enhanced grain quality.
References
Acuna TLB, Rebetzke GJ, He X et al (2014) Mapping quantitative trait loci associated with root penetration ability of wheat in contrasting environments. Mol Breed 34:631–642. https://doi.org/10.1007/s11032-014-0063-x
Ando K, Rynearson S, Muleta KT et al (2018) Genome-wide associations for multiple pest resistances in a Northwestern United States elite spring wheat panel. PLoS One 13(2):e0191305. https://doi.org/10.1371/journal.pone.0191305
Asad MA, Bai B, Lan C et al (2014) Identification of QTL for adult-plant resistance to powdery mildew in Chinese wheat landrace Pinguan 50. Crop J 2:308–314. https://doi.org/10.1016/j.cj.2014.04.009
Ayalew H, Liu H, Börner A et al (2018) Genome-wide association mapping of major root length QTLs under PEG induced water stress in wheat. Front Plant Sci 9:1–9. https://doi.org/10.3389/fpls.2018.01759
Baenziger PS (2016) Wheat breeding and genetics. Ref Mod Food Sci. https://doi.org/10.1016/B978-0-08-100596-5.03001-8
Barrangou R, Fremaux C, Deveau H et al (2007) CRISPR provides acquired resistance against viruses in prokaryotes. Science 315:1709–1712
Barakat MN, Shehab El-Din TM (1993) An in vivo and in vitro analysis of a diallel cross in wheat (Triticum aestivum L.). J Genet Breed 47:211–216
Belamkar V, Guttieri MJ, Hussain W et al (2018) Genomic selection in preliminary yield trials in a winter wheat breeding program. G3 (Bethesda) 8(8):2735–2747. https://doi.org/10.1534/g3.118.200415
Bhusal N, Sarial AK, Sharma P, Sareen S (2017) Mapping QTLs for grain yield components in wheat under heat stress. PLoS One 12(12):e0189594. https://doi.org/10.1371/journal.pone.0189594
Bonneau J, Taylor J, Parent B et al (2013) Multi-environment analysis and improved mapping of a yield-related QTL on chromosome 3B of wheat. Theor Appl Genet 126:747–761. https://doi.org/10.1007/s00122-012-2015-3
Börner A (2006) Preservation of plant genetic resources in the biotechnology era. Biotechnol J 1:1393–1404. https://doi.org/10.1002/biot.200600131
Börner A, Chebotar S, Korzun V (2000a) Molecular characterization of the genetic integrity of wheat (Triticum aestivum L.) germplasm after long-term maintenance. Theor Appl Genet 100:494–497. https://doi.org/10.1007/s001220050064
Börner A, Röder MS, Unger O, Meinel A (2000b) The detection and molecular mapping of a major gene for non-specific adult plant disease resistance against stripe rust (Puccinia striiformis) in wheat. Theor Appl Genet 100:1095–1099
Börner A, Schumann E, Fürste A et al (2002) Mapping of quantitative trait loci determining agronomic important characters in hexaploidy wheat (Triticum aestivum L.). Theor Appl Genet 105:921–936
Bortesi L, Fischer R (2015) The CRISPR/Cas9 system for plant genome editing and beyond. Biotechnol Adv 33:41–52. https://doi.org/10.1016/j.biotechadv.2014.12.006
Bowne JB, Erwin TA, Juttner J et al (2012) Drought responses of leaf tissues from wheat cultivars of differing drought tolerance at the metabolite level. Mol Plant 5:418–429. https://doi.org/10.1093/mp/ssr114
Breiman A, Graur D (1995) Wheat evolution. Isr J Plant Sci 43:85–98
Briggle LW (1967) Morphology of the wheat plant. In: Quisenberry KS, Reitz LP (eds) Wheat and wheat improvement. Amer Soc Agron Inc, Madison, pp 89–116
Briggle LW, Reitz LP (1963) Classification of Triticum species and of wheat varieties grown in the United States, Tech Bull 1278. USDA, Washington DC
Castro AM, Tacaliti MS, Gimenez D et al (2008) Mapping quantitative trait loci for growth responses to exogenously applied stress induced hormones in wheat. Euphytica 164:719–727. https://doi.org/10.1007/s10681-008-9694-5
Chang M, He L, Cai L (2018) An overview of genome-wide association studies. In: Huang T (ed) Computational systems biology. Humana Press, New York, pp 97–108
Cheng M, Fry JE, Pang S et al (1997) Genetic transformation of wheat mediated by Agrobacterium tumefaciens. Plant Physiol 115:971–980. https://doi.org/10.1104/pp.115.3.971
Clark AJ, Sarti-Dvorjak D, Brown-Guedira G et al (2016) Identifying rare FHB-resistant segregants in intransigent backcross and F2 winter wheat populations. Front Microbiol 7:277. https://doi.org/10.3389/fmicb.2016.00277
Connorton JM, Jones ER, Rodríguez-Ramiro I et al (2017) Wheat vacuolar iron transporter TaVIT2 transports Fe and Mn and is effective for biofortification. Plant Physiol 174:2434–2444. https://doi.org/10.1104/pp.17.00672
Cuadrado A, Cardoso M, Jouve N (2008) Physical organization of simple sequence repeats (SSRs) in Triticeae: structural, functional and evolutionary implications. Cytogenet Genome Res 120:210–219. https://doi.org/10.1159/000121069
Daba SD, Tyagi P, Brown-Guedira G, Mohammadi M (2018) Genome-wide association studies to identify loci and candidate genes controlling kernel weight and length in a historical United States wheat population. Front Plant Sci 9:1–14. https://doi.org/10.3389/fpls.2018.01045
Danci M, Danci O, Berbentea F et al (2010) Factors that influence wheat (Triticum aestivum) somaclones and gametoclones regeneration. J Hort Forest Biotech 14(2):243–249
Davis DR, Epp MD, Riordan HD (2004) Changes in USDA food composition data for 43 garden crops, 1950 to 1999. J Am Coll Nutr 23:669–682
Demir P, Onde S, Severcan F (2015) Phylogeny of cultivated and wild wheat species using ATR–FTIR spectroscopy. Spectrochim Acta A Mol Biomol Spectrosc 135:757–763. https://doi.org/10.1016/j.saa.2014.07.025
Dong H, Wang R, Yuan Y et al (2018) Evaluation of the potential for genomic selection to improve spring wheat resistance to fusarium head blight in the Pacific northwest. Front Plant Sci 9:1–15. https://doi.org/10.3389/fpls.2018.00911
Echeverry-Solarte M, Kumar A, Kianian S et al (2015) New QTL alleles for quality-related traits in spring wheat revealed by RIL population derived from supernumerary × non-supernumerary spikelet genotypes. Theor Appl Genet 128(5):893–912. https://doi.org/10.1007/s00122-015-2478-0
El-Aref HM (2002) Employment of maize immature embryo culture for improving drought tolerance. 3rd Scientific Conference of Agriculture Sciences, Fac Agric, Assiut Univ, Assiut, Egypt, 20–22 Oct, pp 463–477
Eltaher S, Sallam A, Belamkar V et al (2018) Genetic diversity and population structure of F3:6 Nebraska winter wheat genotypes using genotyping-by-sequencing. Front Genet 9:76. https://doi.org/10.3389/fgene.2018.00076
FAO (2017) Production year book. FAO, Rome. http://www.fao.org/faostat/en/#rankings/countries_by_commodity
Friebe B, Gill BS (1996) Chromosome banding and genome analysis in diploid and cultivated polyploid wheats. In: Jauhar PP (ed) Methods of genome analysis in plants. CRC Press, Boca Raton, pp 39–60
Friebe B, Jiang J, Raupp WJ, McIntosh RA, Gill BS (1996) Characterization of wheat–alien translocations conferring resistance to diseases and pests: current status. Euph 91:59–87
Gaj T, Gersbach CA, Barbas CFB (2013) ZFN, TALEN and CRISPR/Cas-based methods for genome engineering. Trends Biotechnol 31:397–405. https://doi.org/10.1016/j.tibtech.2013.04.004
Gale MD, Youssefian S (1985) Dwarfing genes in wheat. In: Russell GE (ed) Progress in plant breeding. Butterworth, London, pp 1–35
Gao J, Wang G, Ma S et al (2015) CRISPR/Cas9-mediated targeted mutagenesis in Nicotiana tabacum. Plant Mol Biol 87:99–110. https://doi.org/10.1007/s11103-014-0263-0
Garcia M, Eckermann P, Haefele S et al (2019) Genome-wide association mapping of grain yield in a diverse collection of spring wheat (Triticum aestivum L.) evaluated in southern Australia. PLoS One 14:1–19. https://doi.org/10.25909/5becfa45c176f
Gil-Humanes J, Wang Y, Liang Z et al (2017) High-efficiency gene targeting in hexaploid wheat using DNA replicons and CRISPR/Cas9. Plant J 89:1251–1262
Golovnina KA, Glushkov SA, Blinov AG et al (2007) Molecular phylogeny of the genus Triticum L. Plant Syst Evol 264:195–216
Goncharov NP, Golovnina KA, Kondratenko EY (2009) Taxonomy and molecular phylogeny of natural and artificial wheat species. Breed Sci 59:492–498
Griffing B (1956) Concept of general and specific combining ability in relation to diallel system. Aust J Bio Sci 9:463–493
Gupta PK, Varsheny RK, Sharma PC, Ramesh B (1999) Molecular markers and their applications in wheat breeding. Plant Breed 118:369–390
Habib I, Rauf M, Qureshi J et al (2014) Optimization of somatic embryogenesis and Agrobacterium-mediated transformation of elite wheat (Triticum aestivum) cultivars of Pakistan. Int J Agric Biol 16:1098–1104
Haile JK, Nachit MM, Hammer K, Röder M (2012) QTL mapping of resistance to race Ug99 of Puccinia graminis f. sp. tritici in durum wheat (Triticum durum Desf.). Mol Breed 30:1479–1493. https://doi.org/10.1007/s11032-012-9734-7
Haile JK, Diaye AN, Clarke F et al (2018) Genomic selection for grain yield and quality traits in durum wheat. Mol Breed 38:1–18
He J, Zhao X, Laroche A et al (2014) Genotyping-by-sequencing (GBS), an ultimate marker-assisted selection (MAS) tool to accelerate plant breeding. Front Plant Sci 5:1–8. https://doi.org/10.3389/fpls.2014.00484
International Wheat Genome Sequencing C (2014) A chromosome-based draft sequence of the hexaploid bread wheat (Triticum aestivum) genome. Science 345:1251788. https://doi.org/10.1126/science.1251788
Ishida Y, Hiei Y, Komari T (2015) High efficiency wheat transformation mediated by Agrobacterium tumefaciens. In: Ogihara Y, Takumi S, Handa H (eds) Advances in wheat genetics: from genome to field. Springer, Tokyo, pp 167–173
Ishino Y, Shinagawa H, Makino K et al (1987) Nucleotide sequence of the iap gene, responsible for alkaline phosphatase isozyme conversion in Escherichia coli and identification of the gene product. J Bacteriol 169:5429–5433
Jain SM (2001) Tissue culture-derived variation in crop improvement. Euph 118:153–166
Jiang W, Zhou H, Bi H et al (2013) Demonstration of CRISPR/Cas9/sgRNA-mediated targeted gene modification in Arabidopsis, tobacco, sorghum and rice. Nucleic Acids Res 41:1–12. https://doi.org/10.1093/nar/gkt780
Juliana P, Singh RP, Singh PK et al (2018) Genome-wide association mapping for resistance to leaf rust, stripe rust and tan spot in wheat reveals potential candidate genes. Theor Appl Genet 131:1405–1422. https://doi.org/10.1007/s00122-018-3086-6
Kenaschuk EO (1975) Flax breeding and genetics. In: Harapiak JT (ed) Oilseed and pulse crops in western Canada – a symposium. Western Co-operative Fertilizers Ltd, Calgary, pp 203–221
Khan S (2015) QTL mapping: a tool for improvement in crop plants. Res J Recent Sci 4:7–12
Khan MK, Pandey A, Choudhary S et al (2014) From RFLP to DArT: molecular tools for wheat (Triticum spp.) diversity analysis. Genet Resour Crop Evol 61:1001–1032. https://doi.org/10.1007/s10722-014-0114-5
Kihara H (1984) Origin and history of ‘Daruma’-a parental variety of Norin 10. Sakamoto S (ed) Proceedings of the International wheat genetics, 6th, Kyoto, Japan 28 Nov–3 Dec 1983, Plant Germ-Plasm Institute, Faculty of Agriculture, Kyoto University, pp 13–19
Kim D, Alptekin B, Budak H (2018) CRISPR/Cas9 genome editing in wheat. Funct Integr Genomics 18:31–41. https://doi.org/10.1007/s10142-017-0572-x
Kulwal P, Kumar N, Khurana P et al (2005) Mapping of a major QTL for pre-harvest sprouting tolerance on chromosome 3A in bread wheat. Theor Appl Genet 111:1052–1059. https://doi.org/10.1007/s00122-005-0021-4
Kumar J, Pretep A, Solanki R et al (2011) Advances in genomics resources for improving food legume crops. J Agric Sci 150:289–318
Langridge P (2003) Molecular breeding of wheat and barley. In: Tuberosa R, Philips RL, Gale M (eds) Proceedings of the international congress, in the wake of the double helix: from the green revolution to the gene revolution, Bologna, Italy, pp 279–286
Li JF, Zhang D, Sheen J (2014) Cas9-based genome editing in Arabidopsis and tobacco. Methods Enzymol 546:459–472. https://doi.org/10.1016/B978-0-12-801185-0.00022-2
Liang Z, Chen K, Li T et al (2017) Efficient DNA-free genome editing of bread wheat using CRISPR/Cas9 ribonucleoprotein complexes. Nat Commun 8:14261. https://doi.org/10.1038/ncomms14261
Linnaeus C (1753) Species plantarum, exhibentes plantas rite cognitas, ad genera relatas, cum differentiis specificus, nominibus trivialibus, synonymis selectis, locis natalibus, secundum systema sexuale digestas. T1 Impensis Laurentii Salvii, Holmiae
Liu J, Xu Z, Fan X et al (2018a) A genome wide association study of wheat spike related traits in China. Front Plant Sci 9:1–14. https://doi.org/10.3389/fpls.2018.01584
Liu Y, Liu Y, Zhang Q et al (2018b) Genome wide association analysis of quantitative trait loci for salinity tolerance related morphological indices in bread wheat. Euphytica 214:176. https://doi.org/10.1007/s10681-018-2265-5
Mackintosh CA, Lewis J, Radmer LE et al (2007) Overexpression of defense response genes in transgenic wheat enhances resistance to Fusarium head blight. Plant Cell Rep 26:479–488
Marais G, Botes W (2009) Recurrent mass selection for routine improvement of common wheat: a review. In: Lichtfouse E (ed) Organic farming, pest control and remediation of soil pollutants, Sustainable agricuture review, vol 1. Springer, Dordrecht, pp 85–105
Marais GF, Pretorius ZA, Marais AS, Wellings CR (2003) Transfer of rust resistance genes from Triticum species to common wheat. S Afr J Plant Soil 20(4):193–198. https://doi.org/10.1080/02571862.2003.10634934
Marcussen T, Sandve SR, Heier L et al (2014) Ancient hybridizations among the ancestral genomes of bread wheat. Science 345(6194):1250092. https://doi.org/10.1126/science.1250092
Mercado JA, Sancho C, Jimenez B, Peran U, Pliego A, Quesada M (2000) Assessment of in vitro growth of apical stem sections and adventitious organogenesis to evaluate salinity tolerance in cultivated tomato. Pl cell Tiss Org Cult 62:101–106
McCallum CM, Comai L, Greene EA, Henikoff S (2000) Targeting induced local lesions in genomes (TILLING). Plant Physiol 123:439–442
Mohammadi M, Sharifi P, Karimizadeh R, Shefazadeh MK (2012) Relationships between grain yield and yield components in bread wheat under different water availability (dryland and supplemental irrigation conditions). Not Bot Hort Agrobo 40:195–200
Mourad AMI, Sallam A, Belamkar V et al (2018a) Genetic architecture of common bunt resistance in winter wheat using genome wide association study. BMC Plant Biol 18:1–14. https://doi.org/10.1186/s12870-018-1435-x
Mourad AMI, Sallam A, Belamkar V et al (2018b) Genome-wide association study for identification and validation of novel SNP markers for Sr6 stem rust resistance gene in bread wheat. Front Plant Sci 9:1–12. https://doi.org/10.3389/fpls.2018.00380
Mwadzingeni L, Shimelis H, Tesfay S, Tsilo TJ (2016) Screening of bread wheat genotypes for drought tolerance using phenotypic and proline analyses. Front Plant Sci 7:1276. https://doi.org/10.3389/fpls.2016.01276
Nachit MM (1992) Durum wheat breeding for Mediterranean dryland of North Africa and West Asia. In: Rajram S, Saari EE, Hetel GP (eds) Durum wheats: challenges and opportunities. CIMMYT, Ciudad Obregon, pp 14–27
Navakode S, Weidner A, Lohwasser U et al (2009) Molecular mapping of quantitative trait loci (QTLs) controlling aluminium tolerance in bread wheat. Euphytica 166:283–290. https://doi.org/10.1007/s10681-008-9845-8
Nawara HM, Mattar MZ, Salem KFM, Eissa OA (2017) Diallel study on some in vitro callus traits of bread wheat (Triticum aestivum L.) under salt stress. Int J Agric Environ Res 3(1):1988–2006
Nonaka S (1984) History of wheat breeding in Japan. In: Sakamoto S (ed) Proceedings of the International wheat genetics, 6th, Kyoto, Japan 28 Nov–3 Dec 1983. Plant Germplasm Institute, Faculty Agriculture, Kyoto University, pp 593–599
Norman A, Taylor J, Edwards J, Kuchel H (2018) Optimising genomic selection in wheat: effect of marker density, population size and population structure on prediction accuracy. G3 (Bethesda) 8:2889–2899. https://doi.org/10.1534/g3.118.200311
Novoselović D, Bentley AR, Šimek R et al (2016) Characterizing Croatian wheat germplasm diversity and structure in a European context by DArT markers. Front Plant Sci 7:184. https://doi.org/10.3389/fpls.2016.00184
Paliwal R, Röder MS, Kumar U et al (2012) QTL mapping of terminal heat tolerance in hexaploid wheat (T. aestivum L.). Theor Appl Genet 125:561–575. https://doi.org/10.1007/s00122-012-1853-3
Pariyar SR, Dababat AA, Sannemann W et al (2016) Genome-wide association study in wheat identifies resistance to the cereal cyst nematode Heterodera filipjevi. Phytopathology 106(10):1128–1138
Pignone D, De Paola D, Rapanà N, Janni M (2015) Single seed descent: a tool to exploit durum wheat (Triticum durum Desf.) genetic resources. Genet Resour Crop Evol 62:1029–1035. https://doi.org/10.1007/s10722-014-0206-2
Poland J, Endelman J, Dawson J et al (2012) Genomic selection in wheat breeding using genotyping-by-sequencing. Plant Genome 5:103–113. https://doi.org/10.3835/plantgenome2012.06.0006
Quarrie S, Gulli M, Calestani C et al (1994) Location of a gene regulating drought-induced abscisic acid production on the long arm of chromosome 5A of wheat. Theor Appl Genet 89:794–800. https://doi.org/10.1007/BF00223721
Rasheed A, Wen W, Gao F et al (2016) Development and validation of KASP assays for genes underpinning key economic traits in bread wheat. Theor Appl Genet 129:1443–1860. https://doi.org/10.1007/s00122-016-2743-x
Rasheed A, Ogbonnaya FC, Lagudah E et al (2018) The goat grass genome’s role in wheat improvement. Nat Plants 4:56–58. https://doi.org/10.1038/s41477-018-0105-1
Röder MS, Korzun V, Wendehake K et al (1998) A microsatellite map of wheat. Genetics 149:2007–2023
Rong W, Qi L, Wang A et al (2014) The ERF transcription factor Ta ERF3 promotes tolerance to salt and drought stresses in wheat. Plant Biotechnol J 12:468–479. https://doi.org/10.1111/pbi.12153
Rosearne GM, Herrera-Foessel SA, Singh R et al (2013) Quantitative trait loci of stripe rust resistance in wheat. Theor Appl Genet 126:2427–2449. https://doi.org/10.1007/s00122-013-2159-9
Sakamura T (1918) Kurze mitteilung über die chromosomenzahlen und die verwandt-schaftsverhältnisse der Triticum arten. Bot Mag Tokyo 32:150–153
Salem KFM (2004) The inheritance and molecular mapping of genes for post-anthesis drought tolerance (PADT) in wheat. Ph.D. Dissertation. Martin Luther University, Halle-Wittenberg, Germany, 124 p
Salem KFM (2015) Allelic state at the microsatellite locus Xgwm261 marking the dwarfing gene Rht8 in Egyptian bread wheat (Triticum aestivum L.) genotypes released from 1947 to 2004. Genetika 47:741–750
Salem KFM, Sallam A (2016) Analysis of population structure and genetic diversity of Egyptian and exotic rice (Oryza sativa L.) genotypes. C R Biol 339:1–9. https://doi.org/10.1016/j.crvi.2015.11.003
Salem KFM, Röder MS, Börner A (2004) Molecular mapping of quantitative trait loci (QTLs) determining post-anthesis drought tolerance in hexaploid wheat (Triticum aestivum L.). In: 7 Gesellschaft für Pflanzenzüchtung, GPZ-Tagung, Vortragsveranstaltung zum Thema: Klimatische und edaphische Sortenanpassung und Züchtung für Nachwachsende Rohstoffe. 3–5 March 2004, Halle/Saale, Germany Vort Pflanzenzüchtung 64, pp 21–24
Salem KFM, Röder MS, Börner A (2007) Identification and mapping quantitative trait loci for stem reserve mobilisation in wheat (Triticum aestivum L.). Cereal Res Commun 35:1367–1374
Salem KFM, Röder MS, Börner A (2015) Assessing genetic diversity of Egyptian hexaploid wheat (Triticum aestivum L.) using microsatellite markers. Genet Resour Crop Evol 62:377–385
Salina EA, Adonina IG, Badaeva ED et al (2015) A thinopyrum intermedium chromosome in bread wheat cultivars as a source of genes conferring resistance to fungal diseases. Euphytica 204:91–101
Sallam A, Hamed ES, Hashad M, Omara M (2014) Inheritance of stem diameter and its relationship to heat and drought tolerance in wheat (Triticum aestivum L.). J Plant Breed Crop Sci 6:11–23. https://doi.org/10.5897/JPBCS11.017
Sallam A, Hashad M, Hamed E, Omara M (2015) Genetic variation of stem characters in wheat and their relation to kernel weight under drought and heat stresses. J Crop Sci Biotechnol 18:137–146
Sallam A, Dhanapal AP, Liu S (2016) Association mapping of winter hardiness and yield traits in faba bean (Vicia faba L.). Crop Pasture Sci 67:55–68. https://doi.org/10.1071/CP15200
Sallam A, Amro A, EL-Akhdar A et al (2018a) Genetic diversity and genetic variation in morpho-physiological traits to improve heat tolerance in spring barley. Mol Biol Rep 45:2441–2453. https://doi.org/10.1007/s11033-018-4410-6
Sallam A, Mourad AMI, Hussain W, Baenziger SP (2018b) Genetic variation in drought tolerance at seedling stage and grain yield in low rainfall environments in wheat (Triticum aestivum L.). Euphytica 214:169. https://doi.org/10.1007/s10681-018-2245-9
Santra M, Wang H, Seifert S, Haley S (2017) Doubled haploid laboratory protocol for wheat using wheat-maize wide hybridization. In: Bhalla P, Singh M (eds) Wheat biotechnology. Methods and protocols, methods in molecular biology. Humana Press, New York, pp 235–249
Sehgal D, Vikram P, Sansaloni CP et al (2015) Exploring and mobilizing the GeneBank biodiversity for wheat improvement. PLoS One 10:e0132112. https://doi.org/10.1371/journal.pone.0132112
Shan Q, Wang Y, Li J et al (2013) Targeted genome modification of crop plants using a CRISPR-Cas system. Nat Biotechnol 31:8–10. https://doi.org/10.1038/nbt.2652
Shavrukov Y, Baho M, Lopato S, Langridge P (2016) The Ta DREB 3 transgene transferred by conventional crossings to different genetic backgrounds of bread wheat improves drought tolerance. Plant Biotechnol J 14:313–322
Shcherban AB, Schichkina AA, Salina EA (2016) The occurrence of spring forms in tetraploid Timopheevi wheat is associated with variation in the first intron of the VRN-A1 gene. BMC Plant Biol 16:236
Shrawat AK, Armstrong CL (2018) Development and application of genetic engineering for wheat improvement. Crit Rev Plant Sci 37:335–421
Simmonds NW (1976) Evolution of crop plants. Longman, London
Singh RP, Hodson DP, Huerta-Espino J et al (2011) The emergence of Ug99 races of the stem rust fungus is a threat to world wheat production. Annu Rev Phytopathol 49:465–481
Singh A, Knox RE, DePauw RM et al (2016) Genetic mapping of common bunt resistance and plant height QTL in wheat. Theor Appl Genet 129:243–256. https://doi.org/10.1007/s00122-015-2624-8
Sivamani E, Brey CW, Talbert LE et al (2002) Resistance to wheat streak mosaic virus in transgenic wheat engineered with the viral coat protein gene. Transgenic Res 11:31–41
Srivastava P, Bains NS (2018) Accelerated wheat breeding: doubled haploids and rapid generation advance. In: Gosal S, Wani S (eds) Biotechnologies of crop improvement, vol 1. Springer, Cham, pp 437–461
Sukumaran S, Reynolds MP, Sansaloni C (2018) Genome-wide association genome-wide association analyses identify QTL hotspots for yield and component traits in durum wheat grown under yield potential, drought and heat stress environments. Front Plant Sci 9:1–16. https://doi.org/10.3389/fpls.2018.00081
Tee TS, Qualset CO (1975) Bulk populations in wheat breeding: comparison of single-seed descent and random bulk methods. Euphytica 24:393–405. https://doi.org/10.1007/BF00028206
Tian J, Deng Z, Zhang K et al (2015) Genetic analyses of wheat and molecular marker-assisted breeding, vol 1. Springer, Beijing. https://doi.org/10.1007/978-94-017-7390-4_1
Torres EA, Geraldi IO (2007) Partial diallel analysis of agronomic characters in rice (Oryza sativa L.). Genet Mol Biol 30(3):605–613
Tyankova N (2000) Production and cytogenetic characteristics of wheat-wheat grass hybrids and backcross derivatives. Cereal Res Commun 28:57–64
Vijayalakshmi K, Fritz AK, Paulsen GM et al (2010) Modeling and mapping QTL for senescence-related traits in winter wheat under high temperature. Mol Breed 26:163–175. https://doi.org/10.1007/s11032-009-9366-8
Waheed U, Harwood W, Smedley M et al (2016) Comparison of agrobacterium mediated wheat and barley transformation with nucleoside diphosphate kinase 2 (NDPK2) gene. Pak J Bot 48(6):2467–2475
Wang Y, Cheng X, Shan Q et al (2014) Simultaneous editing of three homoeoalleles in hexaploid bread wheat confers heritable resistance to powdery mildew. Nat Biotechnol 32:947. https://doi.org/10.1038/nbt.2969
Wang S, Zhu Y, Zhang D et al (2017) Genome-wide association study for grain yield and related traits in elite wheat varieties and advanced lines using SNP markers. PLoS One 12:1–14
Wang W, Pan Q, He F et al (2018) Transgenerational CRISPR-Cas9 activity facilitates multiplex gene editing in allopolyploid wheat. CRISPR J 1:65–74. https://doi.org/10.1089/crispr.2017.0010
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Appendices
Appendices
15.1.1 Appendix I: Research Institutes Relevant to Wheat
Institution | Specialization and research activities | Contact information | Website |
|---|---|---|---|
International Maize and Wheat Improvement Center (CIMMYT) | Breeding and molecular breeding program | Wheat Program, The International Maize and Wheat Improvement Center, Mexico | |
International Center for Agricultural Research in the Dry Areas (ICARDA) | Breeding and molecular breeding program | Department of Wheat Breeding/Genetics, International Center for Agricultural Research in the Dry Areas, Rabat, Morocco | |
Leibniz Institute of Plant Genetics and Crop Plant Research (IPK), Gatersleben | Breeding and molecular breeding research | Genbank Department, Leibniz Institute of Plant Genetics and Crop Plant Research, Gatersleben, Germany | |
Wheat Department, Agriculture Research Center (ARC) | Breeding program | Department of Wheat, Agriculture Research Center, Giza, Egypt |
15.1.2 Appendix II: Wheat Genetic Resources
Cultivars | Year of release | Pedigree | Cultivation location / Breeding sites | Important traits |
|---|---|---|---|---|
Mazha | 1940 | Landrace | Shaanxi Province, China | None dwarf genes |
Bima1 | 1951 | Mazha/Biyu | Shaanxi Province, China | None dwarf |
Fengchan3 | 1964 | Danmai 1/Xinong 6028 × Bima1 | Shaanxi Province, China | None dwarf |
Xiaoyan6 | 1981 | (ST2422 × 464)/Xiaoyan96 | Shaanxi Province, China | Rht-B1b + Rht8 |
Changhan58 | 2004 | Changwu112/PH 82–2 | Shaanxi Province, China | Rht-B1b |
Yaqui 50 | – | – | CIMMYT | 4.5 mt/ha yield, none Rht gene |
Pitic 62 | – | – | CIMMYT | 6.5 mt/ha yield, Rht2 Vrn1 + Vrn2 |
Siete Cerros | – | – | CIMMYT | 6.5 T/ha yield, Rht1 Vm1 + Vrn2 |
Yecora 70 | – | – | CIMMYT | 7 T/ha, Rht1 + Rht2 Vrn1 + Vrn3 |
Seri 82 | – | – | CIMMYT | 8 T/ha, Rht1 + Vrn3 |
Opata 85 | – | – | CIMMYT | 8 T/ha, Rht1 |
Baviacora 92 | – | – | CIMMYT | 9 T/ha, Rht1 |
Croc_1/Aegilops tauschii (224)//Opata | – | – | CIMMYT | Primary synthetic wheat, resistance to Pratyulenchus thornei |
Iraq 48 | – | – | Iraq | Possibly identical genetic location as Cre1; also resistant to P. thornei |
AUS4926 | – | – | Australia | Resistance to P. thornei |
Seds 9 | 1994 | Maya“s”/Mon“S”/4//CMH72.428/MRC//jip/3/CMH74A582/5/Giza157∗2SD10003 | Egypt | High yield, susceptible to rust, resistance to smut, long spike |
Giza 168 | 1999 | Mil/Buc//Seri | Egypt | High yield, resistance to rust and smut |
Sakha 94 | 2004 | Opata/Rayon//Kauz | Egypt | High yield, resistance to rust and smut |
Gemmiza 10 | 2004 | Maya74”s”/On//1160147/3/Bb/4/Chat s /5/Ctow | Egypt | High yield, resistance to rust and smut |
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Mourad, A.M.I., Alomari, D.Z., Alqudah, A.M., Sallam, A., Salem, K.F.M. (2019). Recent Advances in Wheat (Triticum spp.) Breeding. In: Al-Khayri, J., Jain, S., Johnson, D. (eds) Advances in Plant Breeding Strategies: Cereals. Springer, Cham. https://doi.org/10.1007/978-3-030-23108-8_15
Download citation
DOI: https://doi.org/10.1007/978-3-030-23108-8_15
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-23107-1
Online ISBN: 978-3-030-23108-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)