Abstract
In recent times, nanotechnology, which has been one of the main novelties to be developed in the 21st century, has been applied to many sectors, particularly to various industrial sectors including forest-based industry. An output of this is the development of nanomaterials of which nanocelluloses have been studied as high technology biopolymers for application in various materials through the development of films and as reinforcement in papers. With this background, the main objective of this Chapter is to present the use of nanocellulose in the paper making. Accordingly, the Chapter presents characteristics of the most used wood in the world for pulp and paper production, main methods of obtaining cellulose in nature, process of bleaching of pulp, paper making, processes to obtain different types of nanocellulose (microfibrillar nanofiber and cellulose nanocrystals), applications of nanocellulose in the paper making through coating and films as well as by nanocellulose-reinforced pulp and the resulting effects of the use of nanocellulose in paper production. These include increased tensile and burst strengths, weight loss, improved barrier properties for oils, oxygen and moisture, better printing surface, etc. In the end, marketing aspects, possible future opportunities and finally concluding remarks are given. These briefly mention the use of nanocelluloses in papermaking presenting interesting possibilities, which offer improvements in cost-benefit, energy efficiency and biocompatibility, in addition to generating new products with uses are not available today.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Paper has been defined as a material having two dimensions, which is produced from an aqueous suspension of fibers, which in turn are ‘artificially interlaced and subsequently dewatered through mechanical and thermal processes’ [79]. It may be noted that the art of producing paper began more than two millennia ago due to the dire need felt at that time to communicate and record the discoveries in materials that can be transported [53]. Of course, today the paper is being used for a lot of diverse uses, which include printing, hygiene, writing, and packaging.
In a simplified way, modern paper production can be divided into three stages: pulping, bleaching of the pulp and the production of the paper itself. It is possible to disregard the bleaching of the pulp while producing brown pulp, which will be used for papers of packaging applications. One of the earliest milestones for the industrial production of cellulosic pulp was the development of the Kraft process in 1879 [63], starting from this, the paper industry has consistently sought to improve the quality of the paper.
It is interesting to note that like in many fields, the paper industry is characterized by investing a lot in research and development. This includes research in search of new raw materials of the high industrial profile, modification and additives for the Kraft process, reduction or non-use of chlorine compounds in bleaching and, more recently, biorefinery. Van Heiningen [167] defines the term ‘biorefinery’ as an industry that transforms raw materials from renewable sources, such as sugarcane bagasse, wood, forest residues and black liquor into higher value-added products such as biofuels and biomaterials. In this sense obtaining nanocellulose from biomaterials for the most diverse commercial applications fits very well in this concept.
In fact, the credit goes to Wegner et al. [180], who put the use of nanotechnologies in the forest-based industry as one of the main novelties to be developed in the 21st century. Besides, they also suggested two paths for the application of nanotechnology to forest producers, viz., the first path is for nanotechnologies and nanomaterials developed in other industrial sectors to be adopted and deployed in materials, processes, and products used or produced by the forest-based industry. The second path is the development of completely new materials or product platforms using nanoscale structures and properties derived from wood. Although it was still unknown about the exact economic impacts and opportunities for wood as nanomaterials, but it was expected that all nanomaterials and nano-enabled products would grow to exceed one trillion dollars per annum as technology would be developed in the 21st century [64].
While in the last few decades, traditional uses of paper have been found to decline, other new avenues have opened up during the last decades or so. These include incorporation of nanotechnology since the 1990s into papermaking leading to lower energy costs, development of low-cost products with improved paper quality, biocompatible and flexible with sophisticated functionalities [17, 133]. It may not be exaggerating to state that the use of nanotechnology makes it possible to improve the sustainability of paper making processes for the following reasons:
-
More efficient use of resources whereby more resistant papers can be formed with the smaller amount of fibers (by weight);
-
Use of secondary materials such as recycled fibers or fibers with inferior properties, which can be converted into nanofibers and used as additives in papermaking, or produce high-quality papers using secondary fibers with the addition of nanofibers;
-
Development of new materials such as papers with unique properties, such as films with plastic characteristics, good barrier properties for smart packaging, etc.
Considering the above and published reports on the preparation, characterization and various applications of nanomaterials in general and nanocellulose in particular and the latters’ use in paper making, this Chapter will present characteristics of the most used wood in the world for pulp and paper production, main methods of obtaining cellulose in nature, process of bleaching of pulp, paper making, processes to obtain different types of nanocellulose (microfibrillar, nanofiber and cellulose nanocrystals), applications of nanocellulose in the paper making, applications of nanocellulose in paper making through coating and films as well as by nanocellulose-reinforced pulp and the resulting effects of the use of nanocellulose in paper production. The Chapter will also present marketing aspects and possible future opportunities and finally concluding remarks.
2 Wood for Pulp and Paper Production
Although a variety of woods are available, only certain types are used in the paper industry. The basic wood density is considered the most important parameter in its quality evaluation since it has a strong relationship with the other wood properties [61]. Besides, it has a strong effect on the variables of the pulping process and the characteristics of paper pulp [138]. This property is defined by the ratio of the absolute dry weight of the wood to its fully saturated volume. The ideal types of cellulosic pulps for papers for printing and writing and for absorbent papers have been distinguished [134]. According to these authors, the most required criteria for printing and writing papers are lower energy consumption in the mechanical refining, greater specific volume and greater opacity. On the other hand, high capacity for water absorption and increased softness are important in the case of manufacturing of absorbent papers. The authors further state that pulps originating from wood with lower basic densities are ideal for the production of the first type of paper since they have fibers with a smaller thickness and smaller mass per length. For the second type of paper, pulps from denser woods are suitable, because these woods possess fibers with higher thickness than the low-density woods and therefore these would present the greater potential of liquid absorption, and greater mass per length of fibers.
In hardwoods, penetration of liquids occurs rapidly through the vessels, but penetration in the transverse direction practically does not exist. This is because of the fact that the pit membranes of the punctures (Depressions in the secondary cell wall is called ‘pit’) prevent the passage of the cooking liquor (This is a mixture of chemical reagents-NaOH + Na2S). It may be noted that in the case of softwood the penetration of the cooking liquor already occurs through the tracheids cells that, unlike the cellular elements of the hardwoods and therefore have good permeability, even in the transverse direction [166]. Considering the dimensions of the chips, it is emphasized that the thickness should be in the range of 4–6 mm, in different sizes so that both impregnation and diffusion are compromised and, consequently, the delignification rate is decreased [103]. The wood is chemically constituted by polymers that perform the structural functions. These are cellulose, hemicelluloses, and lignins [49]. In addition to these structural components present in wood, there are other constituents, which include starches, proteins, pectins beside other substances soluble in water or other organic solvents called extractives or accidental compounds of wood. From the chemical point of view, the amount and type of lignin directly interfere with the conditions of the pulping process. In general, lignin is classified according to the relative amount of the monomers guaiacila (G), syringyl (S) and p-hydroxyphenyl (H), derived from coniferyl, synapyl and p-coumarilic alcohols respectively. Structures of these are shown in Fig. 1.

Reproduced from Barbosa et al. [15] with the kind permission of the publishers
Precursor alcohols of the phenylprazoid units’ p-hydroxyphenyl (H), guaiacyl (G) and syringyl (S).
In the Eucalyptus wood, lignin is generally formed by the siringila and guaiacila units (SG lignin), while in conifers it is formed by units guaiacila and p-hydroxyphenyl (lignin GH) [15, 49]. The syringyl/guaiac ratio (S/G) significantly affects the degradation and solubilization of lignin from hardwoods, to the point that increasing this ratio allows the use of a lower alkali load, resulting in higher yields [60].
With fibrous structure and length between 2 and 5 mm, the cellulose originated from the conifers, such as species of the genus Pinus, is called ‘long fiber pulp’ and has application in the papers that demand greater resistance, as is the case of paper used in the manufacture of packaging. On the other hand, the short-fiber pulp can range from 0.5 to 2 mm and is produced from hardwoods, such as Eucalyptus, Acacia, Propulos and Betula, and is used for the production of printing and writing papers and tissue paper for sanitary purposes. Table 1 presents the technological characteristics of the main forest species planted in the world for pulp and paper production.
3 Paper Making
3.1 Pulping
The production of cellulosic pulp can occur from processes that use different types of energies, viz., mechanical, thermal and chemical or the combination of these. Despite the great diversity of these pulping processes, the alkaline ones have become the main ones used extensively. With a well known and most used worldwide the ‘Kraft’ process, developed by the German chemist Carl F. Dahl in 1879 [63] and patented in 1884 [150], it is reported that today 90% of the whole cellulose pulp produced in the world comes from this process [111]. The Kraft process was the result of the evolution of the soda process and aimed at dissolving the middle lamella by means of the removal of lignin with consequent individualization of the wood fibers. For this purpose, the wood chips are placed in a digester that is pressurized with the alkaline cooking liquor (NaOH and Na2S). The hegemony of its use stems from its advantages over other processes and this process is adaptable to different types of lignocellulosic materials, producing high-quality pulp and of high bleachability with high efficiency of recovery of chemical reagents and energy.
It is interesting to note that the increase in the rate of delignification and yield are opposite to each other. This is because the reagents used in the pulping processes are not specific for lignin removal; they also remove carbohydrates, which contribute to a reduction in yield [150]. Advantages, the Kraft process demands precise control of its parameters. There are several parameters that affect the delignification rate, the most important are: type of wood, chip quality, alkaline load, cooking time and temperature.
The impregnation of the cooking liquor in the chips aims to distribute the cooking liquor evenly into the wood [184]. The impregnation consists of two different phases: pore penetration and diffusion [84]. These phases are very important for the efficiency of delignification and are directly related to the quality of wood and chip size.
In pulp obtained by chemical processes, the degree of delignification of the pulp is measured by the kappa number, which expresses the residual lignin present in the pulp after cooking [161] and is dependent on the alkaline load, cooking time and temperature. The alkaline charge is applied proportionally to the amount of wood in the digester by seeking a predetermined target kappa number. According to Almeida [7], the increase of alkaline charge leads to greater delignification with consequent reduction of the kappa number. However, greater delignification promotes the greater generation of fines, possibly resulting from the fragmentation of fibers. Time and temperature have been combined into a single control parameter called ‘H factor’, which represents the extent of the reaction [172]. Even though different temperatures can be used, delignification can be estimated accurately by ‘H factor’, provided that the other parameters of the pulping process remain constant. According to Sixta [148] the ‘H factor’ is defined as follows:
where kL is the relative reaction velocity of the pulp. Assuming that the activation energy of the reaction is 134 kJ · mol1, the H-factor can be expressed as:
where t is time and T is temperature, the above equation is valid for temperatures above 100 °C.
According to Sixta [148], the H factor is the area under the relative reaction velocity curve versus time. This parameter is designed to predict the temperature or cooking time required to reach a given kappa number. The result is valid only when the other cooking conditions, such as the effective alkali concentration and the ratio of liquor to wood, remain constant [148]. Figure 2 shows a graph of a conventional Kraft cooking having a maximum temperature of 170 °C, the heating time of 80 min and time of 60 min at a constant temperature, culminating at an H factor of 1100.
H-factor is a useful process control tool for Kraft pulp industries. Even the most modern plants use this parameter to control the degree of delignification of the pulp [136].
3.2 Bleaching
This process follows the previous one. It should be noted that the pulp obtained as explained in the previous Section is so intense without the modification, which compromises the mechanical strength of the fiber. Accordingly, bleaching process, which is a chemical process that aims to improve the brightness and cleanliness of cellulosic pulp by removing and/or modifying chromophore and leukoprophic groups are used [55, 72]. After the reactions with the cooking liquor, the lignin in the wood, which is almost colourless, is coloured due to the release of chromophoric groups [55, 72]. Bleaching generally occurs in stages and its progress is always followed by brightness, which is a measure of reflectance of visible blue light at the wavelength of 457 nm, of pulp sheets or paper under standard conditions [31]. The most used method to measure this process is the standard described by ISO 2470: 1999—Paper, board, and pulps. It may be noted that measurement of diffuse blue reflectance factor is expressed in % ISO, while the non-reflective material, absolute black, has a brightness of 0% and a perfect reflectance of light is considered 100%.
Over the years the bleaching chemistry has been changing rapidly, starting with the discovery of bleaching power of chlorine on vegetable fibers, being used in its elemental form (chlorination, C), as calcium hypochlorite (hypochlorination, H) and as chlorine dioxide (dioxidation, D). Table 2 shows the evolution of bleaching sequences over the years. However in the 1990s, due to environmental reasons, the industries had to develop chlorine-free bleaching sequences or sodium hypochlorite [31], as these compounds are the main contributors to the formation of chlorinated organic compounds (Absorbable Organic Halides, AOX) [22]. After this environmental concern, elemental chlorine free (ECF = elemental chlorine free) sequences were created, which is the main technology used today, and totally chlorine free (TCF) sequences, which do not use elemental chlorine or any other chemical reagent that contain chlorine in the molecule. At this stage, oxygen (oxygenation, O), caustic soda (alkaline extraction, E), hydrogen peroxide (perioxidation, P) and ozone (ozonolysis, Z) associated with chelation (Q) and acid hydrolysis (A) are the most commonly used in industries.
The choice of the bleaching sequence will depend on the kappa number after cooking, the type of raw material used in the pulping process, the end use of the bleached pulp and the desired final brightness. As the bleached pulps present on the market have an average brightness of 90% ISO.
3.3 Drying
After bleaching, the cellulosic pulp will be in aqueous suspension, with a consistency of 10–12%. This needs to be transformed, into cellulose bales with final humidity of 10% for the purposes of commercialization and transportation. It may be noted that this step of drying the bleached pulp is the final stage of the manufacturing process of the bleached cellulosic pulp. The process consists of the wet process step followed by the forming step, where the cellulose sheets are formed and finally, the dewatering step. The gradual removal of mater happens through the use of force of gravity, heating, and vacuum. It should be noted that in these steps the pulp is arranged on a permeable forming screen which is also responsible for conducting the pulp along the dryer [48]. The final step of drying the pulp is the removal of the water by the compressor rolls in the pressing step, with water not being withdrawn in the forming step would be removed. Then, a stronger pulp sheet is cut, packed and finally transported.
3.4 Paper Production
3.4.1 Preparation of the Cellulosic Pulp
It may be noted that when there is the production of integrated pulp and paper, the cellulosic pulp will be transported by piping. In pulp industries, which are separate from that of paper, it is necessary to dry the cellulosic pulp to make the transport for the paper mill. Therefore, if the paper mill is integrated with the pulp mill (normally, both paper and pulp are produced using the same mill), the drying step described above does not happen. In that case, the wet cellulosic pulp is pumped through pipes until the production of paper. If there is no integration between the pulp mill and paper mill, the pulp bales will remain dry and packaged for paper production. The first stage of paper production is the mass preparation. At this stage, for non-integrated paper mills, the cellulose bales are placed in the hydrapulper to be disaggregated and reduce the consistency of the mass. In the case of the integrated industries the cellulose used is already moist and are ready for the next stage, i.e., refining. This is a mechanical treatment in which cellulose pulp fibers are broken into fibrils, thus increasing the surface area and, consequently, the binding capacity between the fibers allowing the formation of a strong network [157]. In this way, the refining allows changing the structure of the fibers of the pulp, resulting in modified properties and increasing the mechanical properties of the finished paper. Figure 3 shows the scanning electron micrograph of the refined bleached cellulosic fibers obtained from Eucalyptus wood by one of the authors.
In addition, the energy used in refining causes changes in the fibers, leaving them more prone to collapse during the paper forming process, thereby decreasing the thickness and specific volume of formed sheet. However, refining should not be excessive as it can damage the fibers to the point of reducing the same mechanical properties that have been expected to improve the refining process. It has been recommended that when searching for high brightness pulps, it is necessary to avoid excess during the mechanical refining, as this causes a decrease in the brightness and opacity of the paper [101]. The degree of refining is measured through the Schopper-Riegler grade of drainage, which indicates the ease of the pulp in water shoring and is an important parameter for the evaluation of fiber interweaving—the greater the drainage of the pulp, the lower its capacity to drain water. The most widely used method for determining drainage is described by ISO 5267-1: 1999—Pulps—Determination of drainability—Part 1: Schopper-Riegler method. In order to reach the desired degree of refining, the mass passes through the purification process. This step is aimed at reducing cellulose pulp contamination, and impurities such as plastics, metals, and sand are removed [66] followed by cleaning of pulp to prepare the dough.
In the preparation of the mass, the other components of the paper-making process are added (chemical additives) to improve the mechanical, physical and optical properties of paper as is followed normally in the paper industry [52]. Among the products that may be employed are starches, mineral compounds, vegetable gums, carboxymethylcellulose (CMC) and synthetic polymers [52, 141]. Recently nanocelluloses have also been used as additives in paper production [175].
3.4.2 The Paper Machine
A paper machine consists of different mechanical sections, each of them driven by one motor or an arrangement of one master motor and one or more helping drives, which conventionally speed or torque regulated. Typical sections are represented by fourdrinier, press, dryer, calender, and reel [48, 168]. A schematic drawing of a fourdrinier paper machine is shown in Fig. 4.

Adapted from Lai [87] with the kind permission from publishers
A schematic drawing of a fourdrinier paper machine.
With the recipe of ready-made paper, the mass is transferred to the head box of the paper machine, where a uniform jet of mass is cast on a constantly moving conveyor belt forming screen flame [48]. At this stage, the first control of the paper thickness takes place; more the dough, the thicker would be the paper. The mass thrown on the mat forms a 5% layer of cellulose and additives and 95% water with water being drawn on the flat table. This stage of the process, known as ‘leaf formation’, promotes fiber entanglement and gradual water drainage, giving sufficient strength to the paper, so that it can leave the flat table and run through the various cylinders that make up the rest of the process [150].
In this part of the process, the paper passes through hydraulic presses wherein the excess water is removed, increasing the resistance and reducing the thickness of the paper [48, 150]. With the above process steps, the more resistant the paper reaches the drying step, which is promoted by a series of steam-heated cylinders; water would get evaporated from the pressed sheet, leaving it with the required moisture content for its final application [48]. Upon reaching required moisture content, the paper will receive one more layer of the surface additive according to its final use, and after another drying step, it will proceed to the calendar, which will have uniform thickness together with a better surface finish [48]. After this step, the paper is wound in smaller reels and can be commercialized, both in reels and in the form of sheets of sizes standardized for the final consumer.
4 Cellulose
Cellulose is the most abundant organic polymer on the planet and the largest component of plant biomass [89], with an estimated production of 7.5 × 1010 tons per annum [54]. It can be found in pure form, as in cotton, but is commonly found associated with hemicellulose and lignin in the cell wall [35, 89], as in wood, corresponding to approximately 40 to 45% of mass [149]. In addition to plants, it can also be synthesized by bacteria, algae, and fungi, but in lesser amounts [1]. Figure 5 depicts the main routes of obtaining cellulose in nature. Cellulose can also be obtained by synthesis in vitro and should be highlighted with important development today [82]. The first report of cellulase-catalyzed cellulose formation was based on cellobiosyl fluoride [83] and the first chemosynthesis was performed through polymerization of substituted D-glucose and with open rings followed by deprotection [108].
Figure 6 shows schematically the ultrastructure. It can be seen from the figure that primary and secondary walls differ in the arrangement of cellulose chains. The secondary wall consists of 3 layers, S1, S2, and S3, and the S3 layer has the lowest cellulose content, being composed mainly of xylan. In the primary wall, the fibers are less ordered and essentially composed of chains in all directions within the plane of the wall. At layer S1 showing the very thin lamellae, the arrangement of the fibrils may be visible as is helical (spiral) in nature with a cross-arrangement in certain species. In layer S2 the cellulose chains are grouped in parallel microfibrils, giving a denser arrangement and aligned with the axis of the fiber. About 40–45% of the dry matter of the secondary wall is composed of cellulose [160].

Reproduced from Taiz and Zeiger [160] with the kind permission of the publishers
Ultrastructure of wood.
Cellulose is composed of β-D-anhydroglucopyranose units which bond to each other through the carbons 1–4, forming a basic unit called ‘cellobiose’, which consists of the binding of two molecules of anhydroglucose [54, 145, 149]. The cellulose chain is linear and high molecular weight, which tends to form hydrogen bonds between the molecules [1].
The degree of polymerization (DP) is up to 20.000; however, it varies widely, and the value is around 10.000 in wood [74]. The hydroxyl groups of the cellulose molecules form hydrogen bonds that may be intramolecular or intermolecular. Their ability to form hydrogen bonds play a major role in leading the crystalline packing which also governs the physical properties of cellulose [74] and are these bonds that make cellulose a stable polymer and appreciated as reinforcement in composites [37, 54].
About 36 individual cellulose molecules are brought together by biomass into larger units known as elementary fibrils or microfibrils, which are packed into larger units called microfibrillated cellulose [54, 89]. The latter are in turn assembled into cellulose fibers. All these are shown in Fig. 7. The diameter of elementary fibrils is about 5 nm whereas the microfibrillated cellulose (also called nanofibrillated cellulose-NFC) has diameters ranging from 20 to 60 nm [6, 89]. The microfibrils are formed during the biosynthesis of cellulose and are several micrometres in length. This microfibrillar aggregates which allow the creation of highly ordered regions (i.e., crystalline) form the core alternate with disordered domains (i.e., amorphous) present at the surface [132]. It is these crystalline regions that are extracted, resulting in nanocrystalline cellulose (NCC). The inter- and intra-molecular interactions networks and the molecular orientations of crystalline regions can vary, giving rise to cellulose polymorphs or allomorphs [23, 89].

Reproduced from Rojas et al. [125] with the kind permission of the publishers
Hierarchical structure of cellulose extracted from plants.
As mentioned earlier, the most common way to obtain pulp from wood is through the Kraft chemical pulping process [111], followed by bleaching steps to remove residual lignin on the cellulose surface. This pulp obtained in the paper industry is commonly used to obtain nanocellulose, since pulping and bleaching are characterized as pre-treatments necessary to obtain Nanocellulose, whether microfibrillated, nanofibrillated or nanocrystalline [50, 69, 91, 92, 95, 98, 109, 117, 169, 170, 185, 186].
5 Nanocellulose
The term “nanocellulose” refers to cellulosic materials having at least one of their dimensions in nanometer scale. Nanocelluloses can be produced by different methods and from various lignocellulosic sources [1].
According to Fujisawa et al. [46], so far nanocelluloses can be divided into three groups: cellulose nanocrystals (CNC), micro-fibrillated cellulose (CMF) and nano-fibrillated cellulose (CNF). While the first one (CNC) is produced by a chemical process of acid hydrolysis followed by mechanical agitation of the suspension in water, the second (CMF) is obtained by mechanical disintegration of the cellulosic pulp in water and finally third one CNF is prepared using the combination of chemical oxidation followed by mechanical disintegration in water, or only by the mechanical disintegration method. These mechanisms are shown in Fig. 8.

Reproduced from Sofla et al. [151] with the kind permission of the publishers
The mechanism of chemical and mechanical methods for producing CNC and CNF from cellulose.
Reported definition of CMF is fibers with a diameter between 25 and 100 nm, while CNF are nanocelluloses with a diameter between 5 and 30 nm and a variable length between 2 and 10 μm [125, 139]. Both CMF and CNF have amorphous and crystalline zones composing their structure.
According to Samyn et al. [133], the microfibrillated nanocelulose is commonly produced by homogenization, where the fiber shear is performed by a strong pressure drop and impact forces inside the processing chamber. A similar effect is observed by the use of grinding process [91], where processed suspensions generally contain a heterogeneous mixture of CMF and CNF which are characterized by different diameters and aspect ratios (length/diameter) [133]. The CMFs are usually characterized by a smaller aspect ratio than the CNF [86, 85, 107, 177]. Depending on the number of processing steps, or passes through the mill, the geometry of the fibers in a suspension is reduced, generating more CNF, which leaves the suspension more homogeneous [91, 133]. Larger nanocellulose suspensions (CMF) present a large tendency to aggregate and flocculate microfibrils, a fact also justified by the surface charge of nanofibrils [91]. Non-uniformities in the suspending media are constructed by the high tendency of aggregation of single cell microfibrils and/or flocculation with larger fibers. According to Kumar et al. [86, 85] the CMF is generally produced by a single mechanical treatment of the cellulosic pulp, while the CNF is produced by mechanical treatment after the chemical pretreatment of the original pulp fibers [86, 85].
On the other hand, CNC refers to cellulose nanoparticles that underwent hydrolysis under controlled conditions and that lead to the formation of structures in the form of small crystalline cylinders [139]. Depending on the source of extraction, these crystallites will have a diameter of 3–50 nm. The CNFs, as well as the CMFs, exhibit zones with high fibrillation intensity due to the shear forces that the fibers undergo in the production process, whereas the nanocrystals are exclusively from the crystalline regions of the cellulose molecule. Nano-fibrillated cellulose has amorphous and crystalline regions that make up its more elongated chain in the longitudinal direction. In this way, the long length of nano-fibrillated cellulose chains associated to its surface containing a wide range of hydroxyl groups, which exposes the formation of numerous hydrogen bonds [114]. The type of processing and the raw material used results in nanocellulose with different morphologies and dimensions, as presented in Table 3.
Figure 9 shows scanning electron micrographs of cellulose microfibrils (CMFs), cellulose nanofibrils (CNFs), cellulose nanocrystals (CNCs) and others microfibrilatted cellulose that can be applied in papermaking, such as MCC and microfibril.

Reproduced from Liu et al. [97] with the kind permission of the Publishers
TEM images of nanocelluloses extracted by: a—CNC prepared by sulfuric acid hydrolysis, b—cellulose nanocrystals isolated by formic acid hydrolysis, c—CNF prepared by 2,2,6,6- tetramethylpiperidine-1-oxyl (TEMPO)-mediated oxidation, and d—cellulose nanofibrils fabricated by pulp refining.
5.1 Method of CNF and CMF Production
As a semi-crystalline polymer, cellulose allows the extraction of nanostructures with different morphological properties (length, diameter, and aspect ratio), depending on mechanical and physical, depending on the extraction method applied [125]. The methods for producing nanocelluloses can be divided into chemical, physical and biological [44]. Some forms of procurement, various types of equipment and also combinations of chemical, enzymatic and/or mechanical treatments have already been tried for the production of nanocelluloses [57, 71, 114, 130]. The nanocellulose can be produced by mechanical methods such as grinding, cryoencation with high-pressure homogenization with liquid nitrogen, steam explosion, high-intensity ultrasound etc. Some pre-treatments may be used prior to mechanical processes to promote the accessibility of the hydroxyl groups, increase the internal surface, alter the crystallinity, break the hydrogen bonds of the cellulose and thus increase the reactivity of the fibers. The pre-treatments are different chemical hydrolysis (alkali or acid) or enzymatic [125].
5.1.1 Mechanical Methods
According to Rojas et al. [125], the mechanical treatments can isolate nanofibers from the primary and secondary cell wall without severely degrading cellulose. It is reported that depending on the types of mechanical treatment and levels of mechanical force used, inter fibrillar hydrogen bonding is broken [70, 123, 131, 179]. For example, microfluidization and high-intensity ultrasonic treatments produce a high shear degree, causing transverse cleavage along the longitudinal axis of the cellulose fibers. This process tends to damage the microfibrillar structure, reducing the molar mass and the degree of crystallinity of cellulose.
The mechanical methods would involve high production costs, besides they being less efficient and requiring higher energy inputs compared to that of chemical methods [96]. In view of these, it is reported that a chemical pretreatment would be necessary, which reduces energy consumption besides obtaining more hydrophobic surface [125]. Further, the degree of polymerization (DP) is reported to get usually reduced from 1200 DP to 1400 DP between 850 and 500 by the mechanical treatment. It may be noted that a high cellulose DP is desirable because of correlation of cellulose with the tensile strength of the nanofiber, which is reported to be at least 2 GPa [26, 118].
The following are some of the mechanical methods used to produce nanocellulose:
-
(i)
High-Pressure Homogenization (HPH): In this process, first known quantity of the cellulose (2–7% w/v) is passed through slurry at high pressure into a vessel through very small spring-loaded valve assembly using low velocity. This is then exposed to a pressure drop to atmospheric condition with the valve opening and closing in a cyclic motion [45]. This method is reported to be an efficient method for refining of cellulosic fbers in view of its high efficiency, simplicity and without requiring any organic solvents [78]. Nanofibers having 20–100 nm of diameter and several tens of µm long are normally produced by this method. However, clogging of the homogenizer, high energy consumption, and mechanical damage of the crystalline micro fibril structure are some of the limitations of this method [96, 174, 178].
-
(ii)
Microluidizer: This method uses the equipment, which consists of an intensifier pump and an interaction chamber. While the first is for increasing the pressure, the second is for defibrillating the fibers using two types of forces, viz., shear and impact against colliding streams and the channel walls [42]. Dimensions of CNFs produced by this process are of several µm long and less than 100 nm [125].
-
(iii)
Grinding: In this method mechanism involved is fibrillation of cellulose using a suitable equipment say, grinder to break the hydrogen bond and the cell wall structure of the cellulose by shearing force besides individualization of pulp to nanoscale fibers [146]. Accordingly, this method uses grinding equipment consisting of a static and rotating grindstone (1400–3000 rpm). The process involves passing of the pulp slurry between these two stones [92, 125]. Accordingly, the cell wall structure would break down by the shear and compression forces, which generate a gel due to the suspension of nanocelluloses. Therefore, a number of cycles to be passed by the pulp/fibers through a grinder or the amount of energy for processing the fibers is important parameters which affect the quality of resultant NFC produced by this method. The diameters of NFCs produced by this process range from about 5–157 nm [56, 155, 182].
-
(iv)
Cryocrushing: This method involves immersion of water swollen cellulosic fibers into liquid nitrogen followed by its crushing by mortar and pestle [45]. Accordingly, in this method, high impact forces would be applied in order to the freeze the cellulosic fibers leading to rupture of cell wall due to the pressure exerted by ice crystals and thus, liberating nanofibers [146]. Nanofibers of soya beanstalks have been produced by this method by cryocrushing and high-pressure defibrillation procedures [175, 176]. Normally, this method produces CNFs with diameters from 30 to 80 nm [3].
-
(v)
Steam explosion: This method is a thermomechanical process. In this method, cellulose is kept at 200–270 °C is exposed to a high pressure of steam maintained between 14 and 16 bars. Then, the steam penetrates the biomass by diffusion for short periods of time between 20 s to 20 min. This is followed by applying sudden decompression (explosion), which would generate shear forces hydrolyzing the glycosidic and hydrogen bonds, between the glucose chains [77, 125]. The diameter of CNFs produced by this methods lies in the range of 10 µm–50 nm [30, 36].
-
(vi)
High-intensity Ultrasonication: This method is a mechanical process wherein oscillating power is used to isolate cellulose fibrils by hydrodynamic forces of ultrasound [28]. According to Rojas et al. [125], the cavitation during the process leads to a powerful mechanical oscillating power. The gas bubbles formed would expand and explode breaking down the cellulose fibers [27]. The diameter of the nanocellulose produced by this method lies in the range of 5–35 nm [57].
It is reported that the mechanical treatment causes changes in fiber structure [32]. The author suggests following four phenomena can be observed due to the defibrillation process. First one is the internal fibrillation (IF), which is difficult to observe by microscopy techniques. Here, loosening of the fiber bundle takes place, which causes swelling and increased fiber flexibility. The swelling of the cellulose increases its accessibility to reagents, and consequently their reactivity. The second effect is the external fibrillation (EF) at the surface of the fiber. This is basically the defibrillation process of the fibrils, but without their complete removal. It may be noted that when these fibrils extend completely from the fiber there is the generation of the nanofibers (CMF), as the third phenomenon showing the structural alteration. And finally, the fourth one involves the dimensional reduction of the fiber itself by mechanical wear through fiber cutting (FC). The entire phenomenon mentioned above can be observed by microscopy techniques as is evident from Fig. 10, which is a transmission electron micrograph of nanocellulose obtained from Lengowski [91].

Reproduced from Lengowski [91]
Effects of refining on the production of nanofibers by the mechanical process.
5.1.2 Electrospinning
This is a method to form the fibers using an electrical rather than a mechanical driving force and is termed as an ‘electromechanical’ method. Here, the cellulose dispersion is extruded and electrospun under the effect of a high electric field [43], following a 3D spiral trajectory. Once the solvent evaporates, it leaves behind randomly oriented nanofibers in the collector. The CNFs morphology produced by this technical depends on the strength of electric field, solution feed rate and the tip-to-collector distance [125].
5.2 Methods of CNC and MCC Production
5.2.1 Acid Hydrolysis
The mechanism for obtaining CNC by acid hydrolysis involves the removal of the amorphous regions from the cellulose elementary fibrils by hydrolysis, leaving only the crystalline regions [114]. This cellulose is obtained by cutting the elementary fibrils into small fragments followed by bleaching. Subsequently, CNC is extracted from bleached samples by strong acid hydrolysis under strictly controlled conditions of concentration, temperature, agitation, and time [125]. A typical production process involves acid hydrolysis, washing, centrifugation, dialysis, and sonication to form a suspension followed by drying by freeze-drying or heat-drying [54, 92]. CNC is also known as whiskers or cellulose nanocrystals.
The difference between the production of CNC and MCC by acid hydrolysis lies in the reaction time or in the concentration of the reagent, where less time and lower concentrations are used for MCC compared to those to produce CNC [38, 173, 185, 186]. MCC can be characterized as a white powder of fibrous particles with sizes of about 40 µm with a DP 100–200 and about 80% crystallinity, while the CNC has dimensions of 5–10 nm wide, 100–300 nm long with 90% crystallinity when made from cotton and wood cellulose. On the other hand, other sources like bacteria, algae, and tunicin produce nanocrystals with larger size distributions and dimensions comparable to those of CMF (width: 5–60 nm, length: 100 nm to several µm) [10].
It may also be noted that CNC and MCC are similar to small cylinders or crystalline characters, isolated from acid hydrolysis of the fibers. This is illustrated in Fig. 11, which shows scanning electron micrographs of CNC obtained from Beauvalet [21] (Fig. 11a) and MCC obtained from Thoorens et al. [162] (Fig. 11b).
It may be noted that the hydrolysis processes rely on the fact that the crystalline regions are insoluble in acids under the conditions in which they are employed. This is due to their inaccessibility because of the high organization of the cellulose molecules in their nanostructure. On the other hand, the natural disorganization of the molecules in the amorphous regions favours the accessibility of the acids and consequently the hydrolysis of the cellulose chains present in these regions [132]. Sulfuric and hydrochloric acids are the most commonly used for acid hydrolysis, but phosphoric and hydrobromic acids have also been used [90].
The most commonly used method for the preparation of CNC is acid hydrolysis of cellulosic materials using sulfuric acid (64% w/w). Cellulose nanofibers have also been produced from hardwood by treatment with the 2,2,6,6-tetramethylpiperidine1-oxyl radical in combination with sodium bromide and NaClO [130].
The CNC has a high aspect ratio, a high modulus and good compatibility with matrix materials [127]. Your morphology is the elongated crystalline rodlike shape and has a limited flexibility because it has no amorphous regions. These CNCs have a degree of crystallinity (55–90%). However, it should be noted that the degree of crystallinity, aspect ratio, and morphology depends on the source of cellulosic material and preparation conditions [144]. The colloidal behaviour and superficial charge of CNCs depend on the acid used for their production [90].
5.2.2 Enzymatic Hydrolysis
It is well known that enzyme is generally used to modify and/or degrade the lignin and hemicelluloses contents in biomass without altering the cellulose portion. It is known that enzyme helps in the restrictive hydrolysis of several elements or selective hydrolysis of specified components in the cellulosic fibers [71]. These enzymes are produced by cellobiohydrolases. There are two types. The first category is A- and B-type cellulases, which are capable of attacking the crystalline portion of cellulose. On the other hand, the second category is C and D type endoglucanases, which are capable of attacking the disordered structure (amorphous) of cellulose [8]. It is reported that enzymatic methods are highly expensive as these methods take long treatment time for a successful hydrolysis and also due to the isolation process of the enzymes [77]. Actually, this process can be used as a pre-treatment for production of nanocellulose by the mechanical method to reduce the energy consumption to produce CNF.
6 Applications of Nanocellulose in Paper Making
6.1 Nanocellulose-Reinforced Pulp
The development of strength in paper is influenced by several factors, viz., length and strength of fiber used, degree of adhesiveness, fiber-fiber contact area and bonding agents in the formation of dry or moist [11, 12, 81, 159]. Due to the unique properties of nanocellulose, there is a growing tendency to use it as a paper reinforcement additive [112]. Many researchers have been using nanocellulose as an additive and films in paper making, either to improve (i) strength properties, barrier properties in food packaging, paper brightness, printability [80] or (ii) to reduce paper weight without loss in mechanical properties, while improving thermal properties and to provide antimicrobial capacity in packages. The functionality of the nano paper emerges from the intrinsic properties of the nano fibrous network, the additional loading of specific nano materials or the additional deposition and modelling of thin films of nanomaterials on the paper surface [17]. According to Zimmermann et al. [187], reactive sites exposed on the surface of the cellulose micro fiber (CMF) perform the formation of a network of nanofibers due to the hydrogen bonds formed. Because of the nanometer scale, the amount of these bonds is enhanced by the larger contact surface between nano and microfibers. This increases the apparent density of the paper, making it more resistant to the passage of air and humidity besides the gain in the mechanical properties [86, 85, 106, 115].
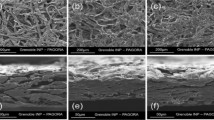
As an additive, the nanocellulose has a similar effect to that produced by the refining of the pulp, reducing paper porosity [50, 91, 117]. This is evident from Fig. 12, which shows scanning electron micrographs of cellulose sheet with and without any additive highlighting the effect of the addition of CMF to the paper [91]. It may be noted that Fig. 12a is micrograph of cellulose sheet without any additive, while Fig. 12b, c are micrographs of cellulose sheet with CMF additive and CMF coating. Besides, such addition of CMF to cellulose gives significant gains in the mechanical properties of the paper, whether produced by virgin fibers [117] or by secondary fibers [116]. It is also reported that wet strength is the main functionality for tissue paper, paper towels, cardboard and other papers [129]. These authors have also reported that the cationic polyelectrolyte (Poly Amideamine Epichlorohydrin—PAE) developed in the 1950s has been used as a wet strength additive in the paper making process. In order to increase the mechanical properties of the paper, the use of nanocellulose and additives such as PAE [4, 5] and amphoteric starch [93] have already been reported.

Reproduced from Lengowski [91]
Effects of nanocellulose on papermaking a cellulose sheet without nay additive; b cellulose sheet with CMF such additive; c cellulose sheet with CMF such coating.
Investigations have also been carried out on the possibility of using CMF and CNF as a reinforcing and filler in a pilot experiment using different degrees of fibrillation and filler mixtures (CMF, CNF, precipitated calcium carbonate, cationic polyacrylamide and two types of starch) [164]. The author has observed that the CNF improved the resistance properties (Scott Index and tensile index) more than the CMF, and also that the CNF associated to the starch presented better resistance to traction being wet. Finally, the author has concluded that in conjunction with CMF or CNF, the filling content could be increased from 30 to 40%, which would imply a potential savings of 3–6.5% compared to conventional sheets. A dose of 6% of applied CNF has been found to promote about 40% energy savings, while the 12% dose reduced energy expenditure by 85% compared to the control to produce pulps at 35 ºSR [34].
Compared with micro-sized cellulose, nanocellulose is more effective as an additive for the paper industry. This is attributed to the interactions between the nanosized elements, which are connected by hydrogen bonds forming a percolated network when the nanocellulose is dispersed in the pulp [3, 73, 75, 76, 80, 183].
Another study has concluded that the drying and wetting process of paper with nanocellulose depends on the amount of added nanocellulose because thenanocellulose clogs the pores and give a greater amount of inter fibrillar connections and preventing fiber-water bonds in the rewetting [120]. It has also been pointed out that 6–12% of CMF could be used in the paper making mix [121], but indices above 5% have been reported to cause a decrease in traction and tear properties [117]. On the other hand, the use of CMF as additives to increase the resistance of thermo-mechanical pulps to produce new types of packaging has also been reported [59]. In addition to improving the mechanical properties, these authors have also observed that the addition of 6 g/m2 of nanocellulose reduced the permeability and drainage of the cellulosic mass. The enzymatic pretreatment combined with mechanical shear forces at high pressure has been found to significantly improve the mechanical strength of the paper without affecting drainage [50, 51]. In another study Gonzalez et al. [50] have concluded that the porosity and mechanical properties were improved when 9% of nanocellulose was added; however the Shopper Riegler degree (This is the ‘degree of refining of a pulp suspension in water and expressing it in terms of the Schooper-Riegler (SR) number, and to determine the de-watering time’) was altered, which becomes a disadvantage in the drainage stage and can cause operational problems in the drying. A similar result was observed by Damásio [34] when 6 and 12% of CNF were added. A decrease in ‘freeness’ (It is ‘a measure of how quickly water is able to drain from a fiber furnish sample. In many cases there is a correlation between freeness values and either (a) a target level of refining of pulp, or (b) the ease of drainage of white water from the wet web, especially in the early sections of a Fourdrinier former’) has been observed while using nanofibers from different sources with increased fibrillation due to water imbibed in the cell cavity and also to internal fibrillation itself, which is consistent with SR behavior [86, 85]. González et al. [50] have also confirmed the tendency to increase the resistance to drainage with the increase of the addition of CNF to the pulp, mainly because of the high surface area of this material.
There are also studies which have reported about the influence of drainage by the size of fibers used, ionic strengths, type of polyelectrolyte and pH of the suspension [156, 159]. Both of the above studies have found that the higher the degree of fibrillation, or higher the concentration of CMF in the mixture, the lower the drainage. However, when the mixture of cationic polyelectrolytes and CMF are used, alteration in drainage can be observed, not to the point of damaging the drying process [159].
The addition of nanocellulose makes the sheet drainage slightly impaired. To overcome this limitation, cationic polymers, such as polyacrylamide, have been used as a fixative for the retention of nanocellulose, as well as for better suspension drain ability [99, 181].
In all the above studies where CMF was added to the sheets, an increase in density of the paper was observed. An increase of 4–30% in density for thermo-mechanical pulp sheets containing 4% CMF and a 10% increase in density to 7% CMF in coniferous Kraft pulp leaves have been observed [39, 102].
While these authors observed a 20% increase in density with the addition of 20% CMF, Sehaqui et al. [140] have observed 30–50% increase in density with the addition of 10% homogenized CMF to softwood Kraft pulp sheets.
Optical properties have also been found to be influenced by the addition of nanocellulose [34]. The increase of nanofibers in the paper composition is reported to cause a significant reduction in the light scattering coefficient of the pulp. The opacity also has also been found to decrease in its values due to the decrease of the light scattering coefficient, with the increase of transparency.
6.2 Coating and Films
Effects of the development of nanocellulosic films and surface deposition of nanocellulose films on paper have been studied by many researchers [50, 58, 91, 122, 124, 135, 149, 153, 154, 158]. An increase in surface density, a decrease in water absorption, reduction in permeability and surface porosity has been observed by Sjöström [149] and Lengowski [91]. Increase in surface porosity has already been shown in Fig. 12. On the other hand, the increase in the surface of the fibers with the micro-fibrillation process has been found to favor a greater number of inter fiber bonds due to the greater availability of OH-groups [50], water retention capacity and increase in Schopper-Riegler grade, implying increased drying cost.
Spence et al. [153] have studied the ability of water retention in nano-cellulose films produced from bleached and unbleached pulp from hardwood and longwood. They have observed that water retention was lower for long-fiber films compared to those of short-fibers and non-bleached pulps compared to bleached pulps. They have also evaluated CMF films with different lignin contents and observed that the samples with higher lignin content had higher rates of water vapour transmission. Besides, significantly higher water retention values and larger surface area were observed by these authors in CMF prepared from unbleached hardwood in comparison to other samples. Another study has reported that the water retention for surface depositions of unbleached nanocellulose when the source of moisture was on the opposite side to that of the surface film [91]. The presence of lignin in the production of its CMF films has been found to provide longer, narrower and more connected pores, thus increasing the rate of water transmission [154]. On the other hand, larger and lignified pores have been found to increase the rate of water vapour transmission because of their lower adsorption capacity [62]. It has also been observed that the specific surface area is strongly correlated with the difficulty of removing water content for the pulps, suggesting that water diffusion is more dependent on pore structure and surface area geometry [154]. On the other hand, Lengowski [91] found that increasing the thickness of the nanocellulosic film on paper caused an increase in mechanical strength (tension, tear and burst index). This is illustrated in Fig. 13, which shows the plots of two different thickness of moisture versus values of tension index, tear index and burst index. On the other hand, this thickness did not influence water absorption properties. While using a bleached and unbleached nano cellulose film, the author observed an increase in the tensile and burst index for bleached CMF deposition, and for the tear, the presence of lignin in CMF caused a gain in this property [91]. These are illustrated in Fig. 14, which shows the plots of mechanical properties with and without any additive on the deposition of surface film (coating) on the sheet. However, the author did not observe any influence of lignin on the thermal stability of the papers. Further, the author has also observed an increase in the mechanical properties of sheets with the addition of CMF (additive) in the production of the sheet compared to that of the sheets without additives. These results are illustrated in Fig. 15, which shows the plots of mechanical properties after surface film deposition on the sheet with and without the addition of CMF [91]. It can be seen from the figure that the gain in the tensile and tear properties are similar to the surface deposition of a nanocellulose film, while the burst index shows a reduction in resistance compared to the use of CMF as an additive. The author observed that the results of the mechanical properties of the papers are enhanced when CMF was used as a reinforcement additive as well as a coating on the paper. It can also be seen that while the values of tensile index, burst index and tear index showed a 134, 50 and 44% increase respectively compared to those values without any addition to the paper [91].

Reproduced from Lengowski [91]
Increase in the mechanical properties after superficial film deposition on the sheet in relation to a sheet without any additive. BUI = burst index; TRI = tear index; TEI = tension index. 1 mm and 2 mm = deposited moisture film thickness.

Reproduced from Lengowski [91]
Increase in the mechanical properties after superficial film deposition (coating) on the sheet in relation to a sheet without any additive. Effect of deposition of unbleached and bleached nanocellulose on mechanical properties of BUI = burst index; TRI = tear index; TEI = tension index. BF = bleached nanocellulose film; UBF = unbleached nanocellulose film.

Reproduced from Lengowski [91]
Increase in the mechanical properties after superficial film deposition on the sheet in relation to a sheet without any additive. Effect of CMF addition, CMF film coating and CMF addition and coating on a paper sheet. SN = sheet with CMF addition; SF = sheet with CMF film coating; SNF = sheet with CMF film coating and addition
A dense surface of the films is an important feature, as it is related to the porosity, which determines the barrier properties of the films [122]. The author has used a paper coating of nanocellulose to improve brightness properties, surface roughness, absorption, and permeability. He noticed an improvement in absorption and porosity and a very small impact on the other properties.
For CMF based films the porosity can be modified by drying from different solvents, creating an adjustable feature that provides an advantage over the melt-formed plastics. Henriksson et al. [58] were able to modify the porosity of water-dried CMF-based films from 28% to porosities of up to 40% with dry films from solvents such as methanol and acetone. In addition, when used as a coat of CMF on paper, the air permeability was found to reduce by 10% as a consequence of surface porosity [158].
Sauders et al. [135] evaluated substitution of CMF in different amounts of acetylation. It was observed that there was no improvement in the contact angle (hydrophobicity) from 15% of substitution and increased crystallinity, while there was a decrease in the rupture stress and in the stress carrying capacity of the material. These have been attributed to acetylation delaying the ability of the fibrils to form bonds during the sheet forming process. Another study has not observed any significant reduction of tensile strength and improvement in the hydrophobicity of the papers after CMF acetylation [124].
Similarly, several studies have also been carried out using CMF as a barrier to gases and liquids [13, 16, 47, 65, 91, 100, 110, 113, 147, 158]. The increase in film thickness, the amount of nanocellulose in the mass [110] reduces the permeability to oxygen, water, oils and carbon dioxide [13, 110, 158, 113] indicating that the use of larger thicknesses or greater percentages in mass, simply acts to increase the tortuous path of oxygen and an improvement of the barrier properties. It is believed that the air permeability of the films decreased due to the disconnection of the surface pores of the films [13]. However, performance is limited by its hygroscopic capacity, and its barrier properties loose efficiency at relatively high humidity [13, 113]. When nanocellulosic samples are in contact with moisture, a reduction of the inter fibrillar forces occurs, thus increasing permeability. However, many methods have been applied for nanocellulose surface modification [142], including acetylation [104], silylation, grafting, use of coupling agent for improving the hydrophobic properties [76, 88, 99], or in the presence of lignin [91]. Recognizing that porosity is an important property for printing, another study has reported that papers with greater porosity may have greater penetration of the ink and may cause printing defects [163].
Considering the applications in food packaging, studies have been reported on the CNC/CNF added to paper [119, 128]. In the study by Rampazzo et al. [119] CNC was used to form a gas barrier film for food packaging. They observed very promising results, providing oxygen and carbon dioxide permeability values hundreds of times smaller than those of equal thickness compared to common barrier synthetic polymers over a wide range of temperatures.
On the other hand, Saini et al. [128] in their study added nisin to CNF and evaluated its potential as an antimicrobial agent in food packaging. Addition of CNF to paper sheets was done in two ways, either directly as an additive or as surface deposition such as coating. The authors found that the latter method (surface deposition) was more efficient than the former (direct addition).
Another property studied is the surface roughness of the composites of CNC-CNF. Damásio [34] produced CNF-CNC nanocomposites by the casting technique, using CNF as the dispersion polymer matrix for different CNC dosages as mechanical reinforcement. The addition of cellulose nanocrystals allowed the reduction of the surface roughness of the nanocomposites produced, increasing the mechanical properties significantly. The incorporation of CNC allowed reduction of the opacity by up to 53%, with consequent gains of transparency of the nanocomposites.
Fang et al. [41] have been reported about its modification by CNF. In this study, CNFs made from TEMPO-oxidized CNFs, where the 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO)/NaBr/NaClO was used to modify the surface properties of the pristine wood fibers by selectively oxidizing the C6 hydroxyl groups of glucose produced transparent nanopapers. This nanopaper exhibited ultrahigh optical transparency (~96%) and ultrahigh haze (~60%), thus delivering an optimal substrate design for solar cell devices, and that may influence a new generation of environmentally.
Another field that is being explored is the use of nanocellulose together with starch in the development of biomaterials, looking for the substitution of polymers derived from petroleum. As starch presents a brittle characteristic, it is necessary to use plasticizers that improve its flexibility [14] although the mechanical and barrier properties have been compromised. Several studies have been reported on the possible applications of biofilms with starch in various sectors such as food, agricultural and pharmaceutical and also in other sectors, where biodegradability is required [18, 19, 105]. Some examples of products under study are garbage bags, films to protect food, diapers, and flexible rods; in agriculture as a film in the ground cover and containers for plants and in the production of slow release fertilizers. Also, biofilms made using starch and nanocellulse can also be used in the preparation of capsules, in the release of medicines, in the substitution of styrofoam, in the protection of equipment during transport and in several other applications [40, 126, 152, 165].
Incorporation of different types of nanocellulose (CNF, CMF, and CNC) as a reinforcing agent in starch films has been studied [29]. All the films presented excellent transparency and increased thermal stability in relation to the mechanical properties; however, the best results were for films reinforced with CMF due to the greater aspect ratio exhibited by this type of fiber. One of the main difficulties of the use of nanocelluloses is its tendency to flocculation and agglomeration in the matrix [94]. While using dispersing agents in the composition Campano et al. [24] have observed a greater homogeneity in the matrix. These authors have also evaluated different retention agents in the mixture, which allowed greater retention of the nanoparticles, causing an increase of traction with lower loads. These authors have suggested that further studies should be carried out with retention and dispersing agents with nanocelluloses in order to industrially apply such an additive. In fact, CNC as a retention agent has already been used for fines in paper production [94]. The highly negative charge of the CNC allowed a strong interaction between cationic polyelectrolytes promoting good drainage and high retention of micro and nanoparticles.
According to Abdul Khalil et al. [2] films based on biodegradable polymer reinforced with nanocellulose present great potential and innumerable benefits for its use in packaging. However, the development of this type of biocomposites is still in the initial stage, and it is necessary to deepen the knowledge in several aspects, such as its quality, cost, and utility.
7 Market and Opportunities
The current demand for sustainability in products is driven by consumers and retailers seeking to differentiate themselves by reducing impacts of product lifecycle on the environment by reducing and minimizing the quantity of packaging and improving the sustainability of their supply chains [25]. The data indicate that this is a growing market with the development of safer and more sustainable products [67]. Also, the cellulose material being a renewable and biodegradable material having low toxicity, this new material can replace petroleum-based packaging, metal components, and other non-renewable materials, which are mostly non-biodegradable and non-renewable. In view of this, cellulose nanomaterials represent an important niche for the design and development of more sustainable products. Nano-cellulose can be used in a variety of products, among the main ones in the cement industry, automotive in the internal and external polymer structure of automobiles, packaging industry whether for food or packaging that demand high strength, waterproof papers, oils and oxygen, special papers, Tissue papers, paper cups, hygienic and absorbent products (disposable diapers), biofilms for plastic replacement, electronics, as well as acting as an excellent stabilizer for suspensions and emulsions such as paints and cosmetics and can be used as a basis for prints 3D of bones and cartilages [68, 143].
According to the “Global Nanocellulose market analysis and trends—Industry forecast to 2020” report, the global nanocellulose market is estimated to reach $295 million by 2020, with a CAGR of 22.15 over the next 5 years due to the expansion of applications and increasing the appeal for green alternatives to oil products. According to the BCC Research Report [20], the global nanocellulose market was $46.8 million in 2014, projected to be $277.7 million in 2019, with a CAGR of 42.8% by 2019. Considering the types of nano-cellulose, cellulose nanofibrillation had a market of 28.2 million in 2014, it is estimated to be 158.3 million in 2019, while nanocrystalline cellulose had a market of $18.0 million in 2014 and is expected to be 116.6 million in 2019.
According to Cowie et al. [33], the annual global market potential for high-volume applications of nanocellulose is estimated at 32.8 million metric tons, based on current markets and middle market penetration estimates. The largest uses for nanocellulose associate a paper and pulp industry are projected to be in packaging coatings (5.3 million metric tons), replacement for the plastic packaging (4.1 million metric tons), plastic film applications (3.3 million metric tons), paper filler (2.4 million metric tons), packaging filler (2.4 million metric tons) and paper coatings (2.2 million metric tons).
8 Final Considerations
The application of nanoscience is an opportunity for greater profitability and autonomy for the pulp and paper industry. This is because it is possible to produce additives and coating for papers using the same raw materials (starch, CMC, synthetic polymers, resins, alkylketene dimer emulsions, alkenyl succinic anhydride) used for paper making without needing other materials to improve the internal bonding of paper fibers, reduce porosity, whiteness, opacity, etc. This opens up possibilities for improving existing products and developing new low-cost, multi-purpose products. There are already different types of nanocellulose that can be used in paper production. However, it is necessary to know the mode of production of the paper and the routes of production of nanocellulose, since the structure or morphology of these different nanocelluloses can be altered according to the routes of and therefore can modify the final properties of the paper. The benefits of nanocelulsoe in pulp and paper industry products include: increased tensile and burst strengths, weight loss, improved barrier properties for oils, oxygen and moisture, better printing surface, optically transparent and/or coloured layer coatings, biodegradability, cost reduction with additives and potentially with drying. The use of nanocelluloses in papermaking presents interesting possibilities, and offers improvements in cost-benefit, energy efficiency and biocompatibility, in addition to generating new products with uses are not available today.
References
Abdul Khalil HPS, Davoudpour Y, Nazrul Islam MD et al (2014) Production and modification of nanofibrillated cellulose using various mechanical processes: a review. Carbohydr Polym 99:649–665
Abdul Khalil HPS, Tye YY, Leh CP, Saurabh CK et al (2018) Cellulose reinforced biodegradable polymer composite film for packaging applications. In: Jawaid M, Swain S (eds) Bionanocomposites for packaging applications. Springer, Cham, pp 49–64
Abe K, Iwamoto S, Yano H (2007) Obtaining cellulose nanofibers with a uniform width of 15 nm from wood. Biomacromol 8:3276–3278
Ahola S, Turon X, Österberg M et al (2008) Enzymatic hydrolysis of native cellulose nanofibrils and other cellulose model films: effect of surface structure. Langmuir 24(20):11592–11599
Ahola S, Salmi J, Johansson L-S et al (2008b). Model films from native cellulose nanofibrils. Preparation, swelling, and surface interactions. Biomacromol 9(4):1273–1282
Akil HM, Omar MF, Mazuki AAM et al (2011) Kenaf fiber reinforced composites: a review. Mater Des 32:4107–4121
Almeida FS (2003) Influence of alkaline load on the Lo-solids® pulping process for eucalyptus wood. Dissertation, Unisity of São Paulo
Anderson SR, Esposito D, Gillette W et al (2014) Enzymatic preparation of nanocrystalline and microcrystalline cellulose. Tappi J 13(5):35–42
Andrade M (2011) The fiber line of the future for eucalyptus kraft pulp. In: Paper presented at the 5 th international colloquium on eucalyptus pulp, Federal Uniuversity of Viçosa, Porto Seguro, 8–11 may 2011
Angles MN, Dufresne A (2000) Plasticized starch/tunicin whiskers nanocomposites. 1. Structural analysis. Macromol 33(22):8344–8353
Ankerfors M, Duker E, Lindstrom T (2013) Topo-chemical modification of fibres by grafting of carboxymethyl cellulose in pilot scale. Nord Pulp Pap Res J 28(1):6–14
Ankerfors M, Lindström T, Henriksson G (2013b) Method for the manufacture of microfibrillated cellulose. US patent 8,546,558, 8 Feb 2006,
Aulin C, Gallstedt M, Lindstrom T (2010) Oxygen and oil barrier properties of microfibrillated cellulose films and coatings. Cellulose 17:559–574
Azeredo HMC (2012) Fundamentals of food stability. EMBRAPA, Brasília
Barbosa LCA, Maltha CRA, Silva VL et al (2008) Determination of the siringyl/guaiacyl ratio in eucalyptus wood by pyrolysis-gas chromatography/ mass spectrometry (PY–GC/MS) (PI-CG/EM). Quím Nova 31(8):2035–2041
Bardet R, Reverdy C, Belgacem N et al (2015) Substitution of nanoclay in high gas barrier films of cellulose nanofibrils with cellulose nanocrystals and thermal treatment. Cellulose 22(2):1227–1241
Barhoum A, Samyn P, Öhlund T et al (2017) Review of recent research on flexible multifunctional nanopapers. Nanoscale 9:15181–15205
Bastioli C (2005) Handbook of biodegradable polymers. Rapra Technology Limited, Shawbury
Batista JA, Tanada-Palmu PS, Grosso CRF (2005) The effect of addition of fatty acids on pectin films. Ciênc Tecnol Aliment 25:781–788
BCC Research (2015) ‘Biomaterial of the Future’ Nanocellulose to Send Market Booming with 42.8% CAGR. https://www.bccresearch.com/pressroom/avm/biomaterial-of-the-future-nanocellulose-to-send-market-booming-with-42.8-percent-cagr?fbclid=IwAR2AiLB_BSpIdEPCaGGHaEhupzMsL675cwpyPJZakEJGBj30tUTRBMTJxnc. Accessed 9 Dec 2018.
Beuvalet M (2016) Application of cellulose nanomaterials in thermoplastic composites. Univesity of Waterloo, Thesis
Bonfatti EA Jr (2013) Oxygen delignification for kraft pulp with high kappa number. Dissertation, Unisity of São Paulo
Brinchi L, Cotana F, Fourtunati E et al (2013) Production of nanocrystalline cellulose from lignocellulosic biomass: technology and applications. Carbohydr Polym 94:154–169
Campano C, Merayo N, Balea A et al (2017) Mechanical and chemical dispersion of nanocelluloses to improve their reinforcing effect on recycled paper. Cellulose 25(1):269–280
Carbon Disclosure Project (2012) CDP supply chain report. https://www.marriott.com/MarriottInternational/CorporateResponsability/Performance_New_2016/SPG_PDFs/CDP-Supply-Chain-Report-2012.pdf. Accessed 6 Jun 2018
Chaker A, Mutjé P, Vilar MR et al (2014) Agriculture crop residues as a source for the production of nanofibrillated cellulose with low energy demand. Cellulose 21(6):4247–4259
Chen P, Yu H, Liu Y et al (2013) Concentration effects on the isolation and dynamic rheological behavior of cellulose nanofibers via ultrasonic processing. Cellulose 20(1):149–157
Cheng Q, Wang S, Rials TG (2009) Poly(vinyl alcohol) nanocomposites reinforced with cellulose fibrils isolated by high intensity ultrasonication. Compos Part A Appl Sci Manuf 40:218–224
Cheng G, Zhou M, Wei Y-J et al (2017) Comparison of mechanical reinforcement effects of cellulose nanocrystal; cellulose nanofiber; and microfibrillated cellulose in starch composites. Polym Compos https://doi.org/10.1002/pc.24685
Cherian BM, Leão AL, Souza SF, Thomas S, Pothan LA, Kottaisamy M (2010) Isolation of nanocellulose from pineapple leaf fibres by steam explosion. Carbohydr Polym 81:720–725
Colodette JL, Santos VLS (2015) General principles of bleaching. In: Colodette JL, Gomes FJB (eds) Cellulose pulp bleaching. Federal University of Viçosa, Viçosa, pp 173–202
Coutts RSP (2005) A review of Australian research into natural fibre cements composites. Cem Concr Compos 27(5):518–526
Cowie J, Bilek EM, Wegner T et al (2014) Market projections of cellulose nanomaterial-enabled products—part 2: volume estimates. Tappi J 13(6):57–69
Damásio RAP (2015) Characterization and nanoscale applications of nanofibrillated cellulose (NFC) and cellulose nanocrystals (CNC). Dissertation, Federal University of Viçosa
de Souza e Lima MM, Borsali R (2004) Rodlike cellulose microcrystals: structure, properties, and applications. Macromol Rapid Comm 25:771–787
Deep B, Abraham E, Cherian BM et al (2011) Structure, morphology and thermal characteristics of banana nano fibers obtained by steam explosion. Bioresour Technol 102:1988–1997
Dufresne A (2008) Processing of polymer nanocomposites reinforced with polysaccharide nanocrystals. Macromolecules 15(8):4111–4128
Eichhorn SJ (2011) Cellulose nanowhiskers: promising materials for advanced applications. Soft Matter 7(2):303–315
Eriksen Ø, Syverud K, Gregersen Ø (2008) The use of microfibrillated cellulose produced from kraft pulp as strength enhancer in TMP paper. Nord Pulp Pap Res J 23:299–304
Fakhouri FM, Fontes LCB, Gonçalves PVM et al (2007) Films and edible coatings based on native starches and gelatin in the conservation and sensory acceptance of Crimson grapes. J Food Sci Technol 27:369–375
Fang Z, Zhu H, Yuan Y et al (2014) Novel nanostructured paper with ultrahigh transparency and ultrahigh haze for solar cells. Nano Lett 14(2):765–773
Ferrer A, Filpponen I, Rodríguez A et al (2012) Valorization of residual empty palm fruit bunch fibers (EPFBF) by microfluidization: production of nanofibrillated cellulose and EPFBF nanopaper. Bioresour Technol 125:249–255
Frey MW (2008) Electrospinning cellulose and cellulose derivatives. Polym Rev 48(2):378–391
Frone AN, Panaitescu DM, Donescu D (2011) Some aspects concerning the isolation of cellulose micro-and nano-fibers. Sci Bull B Chem Mater Sci UPB 73(2):133–152
Frone AN, Panaitescu DM, Donescu D et al (2011) Preparation and characterization of PVA composites with cellulose nanofibers obtained by ultrasonication. BioResources 6(1):487–512
Fujisawa S, Okita Y, Fukuzumi H et al (2011) Preparation and characterization of TEMPO-oxidized cellulose nanofibrils films with free carboxyl groups. Carbohydr Polym 84(1):579–583
Fukuzumi H, Saito T, Iwata T et al (2009) Transparent and high gas barrier films of cellulose nanofibers prepared by TEMPO-mediated oxidation. Biomacromol 10(1):162–165
Ghosh AK (2011) Fundamentals of paper drying-theory and application from industrial perspective. In: Ahasan A (ed) Evaporation, codensation and heat transfer. InTech, London, pp 535–541
Gomide JL, Gomes FJB (2015) Production and composition of unbleached pulps. In: Colodette JL, Gomes FJB (ed) Cellulose pulp bleaching. Federal University of Viçosa, Viçosa, Brazil. pp 59–115
Gonzalez I, Boufi S, Pèlach M et al (2012) Nanofibrillated cellulose as paper additive in eucalyptus pulps. BioResources 7(4):5167–5180
González I, Vilaseca F, Alcalá M et al (2013) Effect of the combination of biobeating and NFC on the physico-mechanical properties of paper. Cellulose 20(3):1425–1435
Gullichsen J, Paulapuro H (2000) Papermaking science and technology: papermaking chemistry. Fapet Oy, Helsinki
Gunaratne SA (2001) Paper, printing and the printing press: a horizontally integrative machohistory analysis. Gazette 63(6):459–479
Habibi Y, Lucia LA, Rojas OJ (2010) Cellulose nanocrystals: chemistry, self-assembly, and applications. Chem Rev 110(6):3479–3500
Hart PW, Rudie AW (2012) The bleaching of pulp, 5th edn. TAPPI Press, Atlanta
Hassan ML, Mathew AP, Hassan EA et al (2012) Nanofibers from bagasse and rice straw: process optimization and properties. Wood Sci Technol 46(1):193–205
He W, Jiang X, Sun F et al (2014) Extraction and characterization of cellulose nanofibers from Phyllostachys nidularia munro via a combination of acid treatment and ultrasonication. BioResources 9(4):6876–87
Henriksson M, Berglund LA, Isaksson P et al (2008) Cellulose nanopaper structures of high toughness. Biomacromol 9:1579–1585
Hii C, Gregersen ØW, Chinga-Carrasco G et al (2012) The effect of MFC on the pressability and paper properties of TMP and GCC based sheets. Nord Pulp Pap Res J 27(2):388–396
Hinche M, Bassa AGMC, Rottmann W et al (2011) Biotech enhanced levels of syringil lignin improves Eucalyptus pulping efficiency. In: Paper presented at the 5th international colloquium on eucalyptus pulp, Federal Uniuversity of Viçosa, Porto Seguro, Brazil. 8–11 May 2011
Horáček P, Fajstavr M, Stojanović M (2017) The variability of wood density and compression strength of Norway spruce (Picea abies/L./Karst.) within the stem. Beskydy 10(1–2):17–26
Hu Y, Topolkaraev V, Hitner A et al (2000) Measurement of water vapor transmission rate in highly permeable films. J Appl Polym Sci 81(3):1624–1633
Hu J, Zhang Q, Lee D-J (2018) Kraft lignin biorefinery: a proposal. Bioresour Technol 247:1181–1183
Hullmann A (2006). The economic development of nanotechnology—an indicators based analysis. European Commission, DG Research, Unit “Nano S&T—Convergent Science and Technologies”. Staff working paper. http://nanotechnology.cz/storage/nanoarticle_.pdf. Acessed 22 Feb 2018
Hult EL, Iotti M, Lenes M (2010) Efficient approach to high barrier packaging using microfibrillar cellulose and shellac. Cellulose 17(3):575–586
Höglund H (2009) Mchanical pulping. In: Ek M, Gellerstedt G, Henriksson G (eds) Pulp and paper chemistry and technology, vol 2. Pulping chemistry and technology. Walter de Gruyter GmbH & Co., Berlin, pp 57–89
Ianuzzi A (ed) (2012) Greener products: the making and marketing of sustainable brands. CRC Press, Boca Raton
International Organization for Standardization (2017) ISO/TC 6: paper, board and pulps
Ireana Yusra AF, Juahir H, Firdaus NWNA et al (2018) Controlling of Green nanocellulose fiber properties produced by chemo-mechanical treatment process via SEM, TEM, AFM and image analyzer characterization. J Fundam Appl Sci 10(1s):1–17
Isogai A (2013) Wood nanocelluloses: fundamentals and applications as new bio-based nanomaterials. J Wood Sci 59(6):449–459
Janardhnan S, Sain M (2006) Isolation of cellulose microfibrils—an enzymathic approach. BioResources 1:176–188
Jardim CM, Colodette JL(2015) Pulp chromophoric groups. In: Colodette JL, Gomes FJB (eds) Cellulose pulp bleaching. Federal University of Viçosa, Viçosa, Brazil, pp 203–215
Jiang F, Hsieh YL (2014) Super water absorbing and shape memory nanocellulose aerogels from TEMPO-oxidized cellulose nanofibrils via cyclic freezing-thawing. J Mater Chem A 2(2):350–359
John MJ, Thomas S (2008) Biofibres and biocomposites. Carbohydr Polym 71:343–364
Julkapli NM, Bagheri S (2016) Developments in nano-additives for paper industry. J Wood Sci 62:117–130
Kajanto I, Kosonen M (2012) The potential use of micro-and nanofibrillated cellulose as a reinforcing element in paper. J-For 2(6):42–48
Kalia S, Boufi S, Celli A et al (2014) Nanofibrillated cellulose: surface modification and potential applications. Colloid Polym Sci 292(1):5–31
Keerati-u-rai M, Corredig M (2009) Effect of dynamic high pressure homogenization on the aggregation state of soy protein. J Agric Food Chem 57:3556–3562
Keller S (2013) Paper drying in the manufacturing process. In: Banik G, Brückle I (eds) Paper and water, 2nd edn. Butterworth Heinemann, Oxiford, pp p173–211
Kim BY (2014) Investigation of coating color penetration depending on the properties of base paper. J Korea TAPPI 46(2):16–21
Klemm D, Kraner F, Moritz S et al (2011) Nanocelluloses: a new family of nature-based materials. Angew Chem Int Ed 50(2):5438–5466
Kobayashi S, Sakamoto J, Kimura S (2001) In vitro synthesis of cellulose and related polysaccharides. Progr Polym Sci 26(9):1525–1560
Kobayashi S, Uyama H, Masashi O (2001b) Enzymatic polymerization for precision polymer synthesis. Bull Chem Soc Jpn 74(4):635–613
Kolavali R (2013) Diffusion of ions in wood. Thesis, Chalmers University of Technology
Kumar V, Bollström R, Yang A et al (2014) Comparison of nano-and microfibrillated cellulose films. Cellulose 21(5):3443–3456
Kumar A, Singh SP, Singh AK (2014) Preparation and characterization of cellulose nanofibers from bleached pulp using a mechanical treatment method. Tappi J 13(5):25–31
Lai YZ (2012) Wood and wood products. In: Kent J (ed) Handbook of industrial chemistry and biotechnology. Springer, Boston, pp 1057–1115
Laine J, Lindström T, Nordmark GG et al (2002) Studies on topochemical modification of cellulosic fibres-part 2. The effect of carboxymethyl cellulose attachment on fibre swelling and paper strength. Nord Pulp Pap Res J 17(1):50–56
Lavoine N, Desloges I, Dufresne A et al (2012) Microfibrillated cellulose–its barrier properties and applications in cellulosic materials: a review. Carbohydr Polym 90(2):735–764
Lee KY, Tamelin T, Schulter K (2012) High performance cellulose nanocomposites: comparing the reinforcing ability of bacterial cellulose and nanofibrillated cellulose. ACS Appl Mater Interfaces 4(8):4078–86
Lengowski EC (2016) Formation and characterization of films with nanocellulose. Federal University of Paraná, Thesis
Lengowski EC, Muñiz GIB, Nisgoski S et al (2013) Cellulose acquirement evaluation methods with different degrees of crystallinity. Sci Forest 41(98):185–194
Lengowski EC, Bonfatti EA Jr (2017) Incorporation of amphoteric starch and nanocellulose in paper. In: Paper presented at the 1st semana de aperfeiçoamento em engenharia florestal, 17–24 July 2017. Federal University of Paraná, Brazil, Curitiba city
Lenze CJ, Peksa CA, Sun W et al (2016) Intact and broken cellulose nanocrystals as model nanoparticles to promote dewatering and fine-particle retention during papermaking. Cellulose 23(6):3951–3962
Li W, Wang R, Liu S (2011) Nanocrystalline cellulose prepared from softwood kraft pulp via ultrasonic-assisted hydrolysis. BioResources 6(4):4271–4281
Li J, Wei X, Wang Q et al (2012) Homogeneous isolation of nanocellulose from sugarcane bagasse by high pressure homogenization. Carbohydr Polym 90(4):1609–1613
Liu C, Li B, Du H et al (2016) Properties of nanocellulose isolated from corncob residue using sulfuric acid, formic acid, oxidative and mechanical methods. Carbohydr Polym 151:716–724
Liu Y, Sui Y, Liu C et al (2018) A physically crosslinked polydopamine/nanocellulose hydrogel as potential versatile vehicles for drug delivery and wound healing. Carbohydr Polym 188:27–36
Loranger E, Jradi K, Daneault C (2012) Nanocellulose production by ultrasound-assisted TEMPO oxidation of kraft pulp on laboratory and pilot scales. In: IEEE international ultrasonics symposium, IUS, Taipei, Taiwan, article number 6562112, pp 953–995
López-Rubio A, Lagaron JM, Ankerfors M et al (2007) Enhanced film forming and film properties of amylopectin using micro-fibrillated cellulose. Carbohydr Polym 68(4):718–727
MacDonald RG (ed) (1968) The pulping of wood, 2nd edn. Mcgraw-Hill Inc., New York
Manninen M, Kajanto I, Happonen J et al (2011) The effect of microfibrillated cellulose addition on drying shrinkage and dimensional stability of wood-free paper. Nord Pulp Pap Res J 26(3):297–305
Mättänen M, Tikka P (2012) Determination of phenomena involved in impregnation of softwood chips. Part 1: method for calculating the true penetration degree. Nord Pulp Paper Res J 27(3):550–558
Mertaniemi H et al (2012) Functionalized porous microparticles of nanofibrillated cellulose for biomimetic hierarchically structured superhydrophobic surfaces. RSC Adv 2:2882–2886
Missio AL, Mattos BD, Ferreira DF et al (2018) Nanocellulose-tannin films: from trees to sustainable active packaging. J Clean Prod 2:143–151
Mohanty AK, Drzal LT, Misra M (2003) Nano reinforcement of bio-based polymers-the hope and reality. Polym Mater Sci Eng 88:60–61
Moon RJ, Martini A, Naim J et al (2011) Cellulose nanomaterials review: structure, properties and nanocomposites. Chem Soc Rev 40:3941–3994
Nakatsubo F, Kamitakahara H, Hori M (1996) Cationic ring-opening polymerization of 3,6-Di-O-benzyl-α-D-glucose 1,2,4-Orthopivalate and the first chemical synthesis of cellulose. J Am Chem Soc 118(7):1677–1681
Nelson K, Retsina T (2014) Innovative nanocellulose process breaks the cost barrier. Tappi J 13(5):19–23
Nygards S (2011) Nanocellulose in pigment coatings: aspects of barrier properties and printability in offset. Dissertation, Linköping University
Oliveira RCP, Mateus M, Santos DMF (2018) Chronoamperometric and chronopotentiometric investigation of kraft black liquor. Int J Hydrog Energy. https://doi.org/10.1016/j.ijhydene.2018.01.046
Osong SH, Norgren S, Engstrand P (2016) Processing of wood-based microfibrillated cellulose and nanofibrillated cellulose, and applications relating to papermaking: a review. Cellulose 23(1):93–123
Österberg M, Vartiainen J, Lucenius J et al (2013) A fast method to produce strong NFC films as a platform for barrier and functional materials. ACS Appl Mater Interfaces 5(11):4640–4647
Pääkkö M, Ankerfors M, Kosonen H et al (2007) Enzymatic hydrolysis combined with mechanical shearing and high-pressure homogenization for nanoscale cellulose fibrils and strong gels. Biomacromol 8(6):1934–1941
Podsiadlo P, Choi S-Y, Shim B et al (2005) Molecularly engineered nanocomposites: layer-by-layer assembly of cellulose nanocrystals. Biomacromol 6(6):2914–2918
Potulski DC (2016) Influence of nanocellulose on the physical and mechanical properties of primary and recycled paper of Pinus and Eucalyptus. Federal University of Paraná, Thesis
Potulski DC, Muñiz GIB, Klock U et al (2014) The influence of incorporation of microfibrillated cellulose on mechanical strength properties of paper. Sci Forest 42(103):345–351
Rahimi M, Behrooz R (2011) Effect of cellulose characteristic and hydrolyze conditions on morphology and size of nanocrystal cellulose extracted from wheat straw. Int J Polym Mater Po 60(8):529–541
Rampazzo R, Alkan D, Gazzoti S et al (2017) Cellulose nanocrystals from lignocellulosic raw materials; for oxygen barrier coatings on food packaging films. Packag Technol Sci. https://doi.org/10.1002/pts.2308
Rantanen J, Maloney TC (2013) Press dewatering and nip rewetting of paper containing nano-and microfibril cellulose. Nord Pulp Pap Res J 28(4):582–587
Rantanen J, Pirttiniemi J, Kuosmanen P et al (2014) Development of a microfibrillated cellulose composite web forming method. In: Paper presented at TAPPI international conference on nanotechnology for renewable materials, TAPPI, Vancouver, 23–26 June 2014
Richmond F (2014) Cellulose nanofibers use in coated paper. University of Maine, Thesis
Robles NB (2014) Tailoring cellulose nanofibrils for advanced materials. KTH Royal Institute of Technology, Stockholm
Rodionova G, Lenes M, Eriksen Ø et al (2011) Surface chemical modification of microfibrillated cellulose: improvement of barrier properties for packaging applications. Cellulose 18(1):127–134
Rojas J, Bedoya M, Ciro Y (2015) Current trends in the production of cellulose nanoparticles and nanocomposites for biomedical applications. In: Poletto M, Ornaghi HL Jr (eds) Cellulose—fundamental aspects and current trends. InTech, Rijeka, pp 193–228
Róz ALD (2003) The future of plastics: biodegradable and photodegradable. Polymers 13(4):4–5
Sacui IA, Nieuwendaal RC, Burnett DJ et al (2014) Comparison of the properties of cellulose nanocrystals and cellulose nanofibrils isolated from bacteria, tunicate, and wood processed using acid, enzymatic, mechanical, and oxidative methods. ACS Appl Mater Interfaces 6(9):6127–6138
Saini S, Sillard C, Belgacem MN et al (2016) Nisin anchored cellulose nanofibers for long term antimicrobial active food packaging. RSC Adv 6:12437–12445
Saito T, Isogai A (2005) A novel method to improve wet strength of paper. Tappi J 4(3):3–8
Saito T, Kimura S, Nishiyama Y, Isogai A (2007) Cellulose nanofibers prepared by TEMPO-mediated oxidation of native cellulose. Biomacromol 8:2485–2491
Saito T, Nishiyama Y, Putaux J-L et al (2006) Homogeneous suspensions of individualized microfibrils from TEMPO-catalyzed oxidation of native cellulose. Biomacromol 7(6):1687–1691
Samir M, Alloin F, Dufresne A (2005) Review of recent research into cellulosic whiskers, their properties and their application in nanocomposite field. Biomacromol 6:612–626
Samyn P, Barhoum A, Öhlund T et al (2018) Review: nanoparticles and nanostructured materials in papermaking. J Mater Sci 53(1):146–184
Santos SD, Sansígolo CA (2007) Wood basic density effect of Eucalyptus grandis x Eucalyptus urophylla clones on bleached pulp quality. Ciên Flor 17(1):53–63
Saunders RE, Pawlak JJ, Lee JM (2014) Properties of surface acetylated microfibrillated cellulose relative to intra- and inter-fibril bonding. Cellulose 21(3):1541–1552
Segura TES, Santos JRS, Sarto C et al (2016) Effect of kappa number variation on modified pulping of Eucalyptus. BioResources 11(4):9842–9855
Segura TES, Zanão M, Santos JRS et al (2012) Kraft pulping of the main hardwoods used around the world for pulp and paper production. In: 2012 TAPPI PEERS CONFERENCE, TAPPI Press, pp 1592–1599
Segura TES, Silva Júnior FG (2016) Potential of C.citriodora for kraft pulp production. TAPPI J 15(3):159–164
Sehaqui H, Allais M, Zhou Q et al (2011) Wood cellulose biocomposites with fibrous structures at micro- and nanoscale. Comp Sci Technol 71(3):382–387
Sehaqui H, Zhou Q, Berglund L (2013) Nanofibrillated cellulose for enhancement of strength in high-density paper structures. Nord Pulp Pap Res J 28(2):182–189
Serviço Nacional De Aprendizagem Industrial (2013) Cellulose. Senai, São Paulo
Sharma S, Zhang X, Nair SS et al (2014) Thermally enhanced high performance cellulose nano fibril barrier membranes. RSC Adv 4:45136–45142
Shatkin JA, Wegner TH, Bilek EM et al (2014) Market projections of cellulose nanomaterial-enabled products—part 1: applications. Tappi J 13(5):9–12
Sinko R, Qin X, Keten S (2015) Interfacial mechanics of cellulose nanocrystals. MRS Bull 40(4):340–348
Siqueira G, Bras J, Dufresne A (2009) Cellulose whiskers versus microfibrils: influence of the nature of the nanoparticle and its surface functionalization on the thermal and mechanical properties of nanocomposites. Biomacromol 10(2):425–432
Siró I, Plackett D (2010) Microfibrillated cellulose and new nanocomposite materials: a review. Cellulose 17(3):459–494
Siró I, Plackett D, Hedenqvist M et al (2011) Highly transparent films from carboxymethylated microfibrillated cellulose: the effect of multiple homogenization steps on key properties. J Appl Polym Sci 119(5):2652–2660
Sixta H (2006) Handbook of pulp. Wiley-VCH Verlag GmbH & Co, KGaA, Weinheim
Sjöström E (2013) Wood chemistry fundamentals and applications. Academic Press, New York
Smook G (2016) Handbook for pulp and paper technologists. TAPPI Press, Atlanta
Sofla MRK, Brown RJ, Tsuzuki T et al (2016) A comparison of cellulose nanocrystals and cellulose nanofibers extracted from bagasse using acid and ball milling methods. Adv Nat Sci Nanosci Nanotech 7:035004
Souza AC, Benze R, Ferrão ES et al (2012) Cassava starch biodegradable films: influence of glycerol and clay nanoparticles content on tensile and barrier properties and glass transition temperature. LWT J Food Sci Technol 46(1):110–117
Spence KL, Venditti RA, Rojas OJ et al (2010) The effect of chemical composition on microfibrillar cellulose films from wood pulps: water interactions and physical properties for packaging applications. Cellulose 17(4):835–848
Spence KL, Venditti RA, Rojas OJ et al (2011) A comparative study of energy consumption and physical properties of microfibrillated cellulose produced by different processing methods. Cellulose 18(4):1097–1111
Stelte W, Sanadi AR (2009) Preparation and characterization of cellulose nanofibers from two commercial hardwood and softwood pulps. Ind Eng Chem Res 48(24):11211–9
Su J, Mosse WKL, Sharman S et al (2013) Effect of tethered and free microfibrillated cellulose (MFC) on the properties of paper composites. Cellulose 20(4):1925–1935
Swinehart D (2012) Fundamentals of refining. MeadWestvaco Center for Packaging Innovation, Rayleigh
Syverud K, Stenius P (2009) Strength and barrier properties of MFC films. Cellulose 16:75–85
Taipale T, Österberg M, Nykänen A et al (2010) Effect of microfibrillated cellulose and fines on the drainage of kraft pulp suspensions and paper strength. Cellulose 17(5):1005–1020
Taiz L, Zeiger E (2017) Plant physiology, 6th edn. Sinauer Associates, Sunderland
Technical Association of Pulp and Paper Industry (2013) T 236 om-13: kappa number of pulp. TAPPI Press, Atlanta
Thoorens G, Krier F, Leclercq B et al (2014) Microcrystalline cellulose, a direct compression binder in a quality by design environment—a review. I J Pharm 473(1–2):64–72
Tognetta L, Santos O, Dragoni O et al (2014) Paper. SENAI, São Paulo
Torvinen K (2014) Binding fillers for high filler content papers by using CNF/CMF. In: Paper presented at international conference on nanotechnology for renewable materials, TAPPI, Vancouver, 23–26 June 2014
Tuovinen L, Peltonen S, Jarvinen K (2003) Drug release from starch-acetate films. J Control Release 91(4):345–354
Usta I (2005) A review of the configuration of bordered pits to simulate the fluid flow. Maderas Cien Tecnol 7(2):121–132
Van Heiningen ARP (2006) Converting a kraft pulp mill into an integrated forest biorefinery. Pulp Pap Canada 107(6):38–43
Valenzuela A, Bentley JM, Lorenz RD (2005) Evaluation of torsional oscillations in paper machine sections. IEEE Trans IndusAppl 41(2):493–501
Viana LC, Muñiz GIB, Hein PRG et al (2016) NIR spectroscopy can evaluate the crystallinity and the tensile and burst strengths of nanocellulosic films. Maderas Cienc Tecnol 18(3):493–504
Viana LC, Muniz GIB, Magalhaes WLE (2017) Physical and mechanical properties of nano-structed films produced from the unbleached Pinus sp. kraft pulp. Sci Forest 45(116): 653–662
Vivian MA, Segura TES, Bonfatti Júnior EA et al (2015) Wood quality of Pinus taeda and Pinus sylvestris for kraft pulp production. Sci Forest 43(105):183–191
Vroom KE (1957) The H factor: a means of expressing cooking times and temperatures as a single variable. Pulp Pap Canada 58(3):228–231
Wang Y, Cao X, Zhang L (2006) Effects of cellulose whiskers on properties of soy protein thermoplastics. Macromol Biosci 6(7):524–531
Wang H, Li D, Zhang R (2013) Preparation of ultralong cellulose nanofibers and optically transparent nanopapers derived from waste corrugated paper pulp. BioResources 8:1374–1384
Wang B, Sain M (2007) Isolation of nanofibers from soybean source and their reinforcing capability on synthetic polymers. Compos Sci Technol 67(11–12):2521–2527
Wang B, Sain M (2007) Dispersion of soybean stock-based nanofiber in a plastic matrix. Polym Int 56(4):538–546
Wang B, Sain M, Oksman K (2007) Study of structural morphology of hemp fiber from the micro to the nanoscale. Appl Compos Mater 14:89–103
Wang Y, Wei Y, Li J et al (2013) Homogeneous isolation of nanocellulose from cotton cellulose by high pressure homogenization. J Mater Sci Chem Eng 1(5):49–52
Wang Q, Zhao X, Zhu JY (2014) Kinetics of strong acid hydrolysis of a bleached kraft pulp for producing cellulose nanocrystals (CNCs). Ind Eng Chem Res 53(27):11007–11014
Wegner T, Skog KE, Ince PJ et al (2010) Uses and desirable properties of wood in the 21st century. J For 108(4):165–173
Wågberg L, Decher G, Norgren M et al (2008) The build-up of polyelectrolyte multilayers of microfibrillated cellulose and cationic polyelectrolytes. Langmuir 24:784–795
Xie C, Liu Z-M, Wu P et al (2013) Optimization of preparation technology of alkali pretreated reed pulp nano-cellulose. Chem Ind For Prod 33(1):32–36
Xu Q, Li W, Cheng Z et al (2014) TEMPO/NaBr/NaClO-mediated surface oxidation of nanocrystalline cellulose and its microparticulate retention system with cationic polyacrylamide. BioResources 9(1):994–1006
Xu Y, Yin X, Lin T et al (2018) Silica retention by the addition of sodium metaaluminate during the impregnation stage of bamboo kraft pulping. J Wood Chem Technol 38(1):35–43
Zeni M et al (2015) Preparation of microcellulose (Mcc) and nanocellulose (Ncc) from eucalyptus kraft ssp pulp. Polym Sci 1:1–5
Zeni M, Favero D, Pacheco K et al (2015) Preparation of microcellulose (Mcc) and nanocellulose (Ncc) from Eucalyptus kraft ssp pulp. Polym Sci 1:1–5
Zimmermann T, Bordeanu N, Strub E (2010) Properties of nanofibrillated cellulose from different raw materials and its reinforcement potential. Carbohydr Polym 79:1086–1093
Acknowledgements
At the outset, the authors express their sincere thanks to the Editors of the book (Inamuddin, Sabu Thomas, Raguvendra Mishra and Abdullah M. Asiri), particularly Prof. Inamuddin for inviting us to contribute this Chapter. The authors place on record and appreciate the kind permission given by some of the authors (who have given permission to use their figures), M/s. Elsevier Inc Publishers, Springer, Sociedade Brasileira de Química—SBQ, Brazil, www.plantphysiol.org or www.plantcell.org—“Copyright American Society of Plant Biologists.” InTech Open Publishers, IOP Publishing and the Vietnam Academy of Science and Technology (VAST) to reproduce some of the figures from their publications free of charges. One of the authors (KGS) would like to thank the PPISR, Bangalore-India with whom he is associated with presently for their encouragement and interest in this collaboration.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Lengowski, E.C., Bonfatti Júnior, E.A., Kumode, M.M.N., Carneiro, M.E., Satyanarayana, K.G. (2019). Nanocellulose in the Paper Making. In: Inamuddin, Thomas, S., Kumar Mishra, R., Asiri, A. (eds) Sustainable Polymer Composites and Nanocomposites. Springer, Cham. https://doi.org/10.1007/978-3-030-05399-4_36
Download citation
DOI: https://doi.org/10.1007/978-3-030-05399-4_36
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-05398-7
Online ISBN: 978-3-030-05399-4
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)