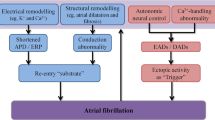
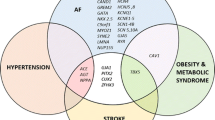
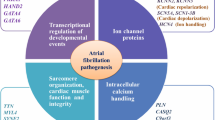
Abstract
Common atrial fibrillation (AF) is a complex disease, and its pathogenesis involves multiple genetic factors, environmental factors, and interactions among these factors. Genetic factors clearly contribute to the risk of common AF. Parental AF increases by more than threefold the risk of AF under age of 75 years in offspring, and first-degree relatives have almost fivefold more risk of developing AF before the age of 60 years. Candidate gene case—control studies investigated the roles of several genes in common AF and thromboembolism in AF, including KCNE1, KCNE4, KCNE5, GNB3, AGT, CETP, coagulation factor II, α-fibrinogen, factor XIII, and IL6. Genomewide single nucleotide polymorphism association studies is the state-of-the art study design for dissecting the common complex AF trait and have successfully identified two SNPs on chromosome 4q25 that are associ ated with risk of atrial fibrillation. Rare families with AF have been reported, and studies of these families identified mutations in several genes for AF, including KCNQ1, KCNE2, KCNE3, and KCNJ2. Two autosomal dominant AF genes were mapped to chromosome 10 q and 6 q, and one autosomal recessive gene for AF was mapped to 5 p, but these genes have not yet been identified. Also, AF can occur in patients with dilated cardiomyopathy with SCN5A and LMNA mutations, long QT syndrome patients with an ankyrin-B mutation, and short QT syndrome patients with a KCNH2 mutation. Genetic studies of AF will continue to provide insight into molecular mechanisms for the pathogenesis of AF and will facilitate realization of genetic testing and genotype-based therapies (personalized medicine) for AF patients.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Atrial fibrillation (AF) is the most common cardiac arrhythmia associated with increased mortality and substantial morbidity. Genetic studies of other types of cardiac arrhythmias, mainly long QT syndrome (LQTS) and Brugada syndrome (BrS), have driven the realization of personalized medicine, known as the right drug/therapy for the right patients, in this specific medical field. To date, disease-causing genes have been identified for an estimated 50% to 75% of LQTS and 25% of BrS cases. The genes identified for LQTS include potas sium channel genes KCNQ1 (LQT1),1 KCNH2 (LQT2),2 KCNE1 (LQT5),3, 4 and KCNE2 (LQT6)5 and the cardiac sodium channel gene SCN5A (LQT3).6, 7 Mutations were also identified in LQTS patients and were associated with other diseases in genes, including a structural ankyrin gene ANK2 (LQT4, sick sinus syndrome with bradycardia); a potassium channel gene KCNJ2 (LQT7, Anderson syndrome); a calcium channel gene CACNA1C (LQT8, Timothy syndrome); and a potassium channel gene HCN4 (LQT9, sick sinus syndrome).8 The LQTS mutations in SCN5A are gain-of-function mutations, whereas loss-of-function mutations in SCN5A cause BrS.9 More than 90% of genotyped LQTS patients belong to LQT1, LQT2, and LQT3 types, and geno-type-phenotype correlation was well defined in these three types of LQTS.
Commercial genetic testing called Familion is now available for LQTS and BrS patients (http://www.familion.com). The testing results have a direct impact on treatment options for individual patients. The LQT1 patients respond to β-blockade, LQT2 patients respond to β-blockade and elevated serum potassium levels, and LQT3 patients respond to sodium channel block-ers.10 Symptomatic LQTS and BrS patients (SCN5A positive) may be managed with implantation of ICDs (implantable cardioverter-defibrillators).10, 11
Genetic studies of AF have made some progress, but much remains to be done. This chapter reviews recent advances and discusses future perspectives in the field of AF genetics. We are optimistic that, in the future, personalized medicine will be a reality for AF patients as it is for LQTS and BrS patients.
Genetic Component of Atrial Fibrillation
Studies have clearly demonstrated that genetic factors contribute to the devel opment of AF. Familial forms of AF were reported but were very rare. The AF in these large families is inherited in a Mendelian fashion. Two forms of inherited AF have been reported, autosomal dominant and autosomal recessive forms. Large families with autosomal dominant AF have been reported,12, 13 but autosomal recessive AF has been reported in only one family to date.14 Simplex cases of AF are common in clinical practice. Some simplex cases may represent autosomal recessive AF, but most of them may simply be sporadic AF. No X-linked AF has been reported.
The common form of AF is most likely to be a complex disease, which is believed to be caused by multiple genetic factors, environmental factors, and interactions among these factors. Indeed, many risk factors have been identified for AF, including advanced age, male gender, valvular heart dis ease, coronary artery disease, hypertension, heart failure, left ventricular dysfunction, hyperthyroidism, and diabetes.14 Studies showed that there was a genetic component in the common form of AF. Fox et al. reported the first population-based study to estimate heritability of AF and found the odds ratios (ORs) for the parent—offspring pair based on a prospective cohort of 2,234 offspring involved in the Framingham Heart Study.15 Seventy of the offspring developed AF during follow-up.15 Atrial fibrillation in at least one parent significantly increased the risk of AF in offspring, with an OR of 1.85, which increased to 3.23 with study participants younger than 75 years or to 3.17 when offspring with overt heart disease were excluded.15 Interestingly, maternal AF was a stronger risk factor than paternal AF. These results demonstrate that there is a genetic predisposition to AF, and parental AF increases the risk of AF in offspring.
The second population study to examine the heritability of AF was carried out in Iceland by Arnar et al.16 The risk ratios (RRs) of AF were estimated based on studies of 5,269 AF patients and 10,000 controls (RR is defined as the risk of AF in the relatives divided by the risk in the general population). Familial aggrega tion was demonstrated. The RRs for the first-degree through fifth-degree relatives were 1.77, 1.36, 1.18, 1.10, and 1.05, respectively. Although these RRs are statistically significant values, the risk of AF is very small for the third-degree to fifth-degree relative (RR < 1.18). Interestingly, the RR for first-degree relatives increased to 4.67 in study subjects under the age of 60 years, indicating that the first-degree relatives of AF patients have an almost fivefold higher risk to have AF than the general population. The RRs for second-degree through fifth-degree relatives in subjects under 60 years were 2.13, 1.34, 1.35, and 1.02, respectively. The risk of AF disappeared for the fifth-degree relatives.
Ellinor et al. studied 110 patients with lone AF (AF without structural heart disease). The RRs were very high, 8.1 for sons, 9.5 for daughters, 70 for brothers, 34 for sisters, 4 for mothers, and 2 for fathers.17 Because the population used for this study was an ascertained population for genetic stud ies (not random), selection bias may have augmented the RRs, and precaution should be taken to extrapolate the findings from this study to the general population of AF.
In summary, the studies discussed demonstrated that AF has substantial familial aggregation and strong heritability, indicating that there are genetic variants that predispose to the risk of common AF.
Classification of Atrial Fibrillation Genes: Disease-Causing Genes, Susceptibility Genes, and Disease-Linked Genes
We can classify the genes that are associated with AF into three major catego ries: disease causing, susceptibility, and disease linked. Disease-causing genes are referred to as the genes with mutations that are directly responsible for the pathogenesis of disease, most often single-gene disorders.18 In this case, the mutations are clearly defined as the primary cause of the disease. For example, KCNQ1 mutations cause LQTS,2, 7 and SCN5A mutations cause BrS.9 Disease-causing genes for AF are discussed in detail in a separate section.
Susceptibility genes are more often related to common complex disease traits (e.g., the common form of AF). Variants or single-nucleotide polymor phisms (SNPs) in these genes increase the risk for development of disease and may or may not cause the disease in the context of other genetic and environmental factors.18 The susceptibility genes are commonly identified by a case—control association study showing significantly different allelic or genotypic frequencies of SNPs between control and patient populations. Susceptibility genes for AF are discussed in a separate section.
Disease-linked genes are referred to as the genes with expression or function that is linked to the disease by molecular biology studies or microarray or proteomic analyses.18 Northern blot, Western blot, reverse tran-scriptase polymerase chain reaction (RT-PCR), and other molecular biological techniques can be used to identify candidate genes with expression that dif fers between AF patients and controls. Examples include genes encoding heat shock proteins HSP10, HSP60, and HSP70 19 and sarcolipin (a homologue of phospholamban). At least five studies reported oligonucleotide microarray analysis for profiling expression of thousands of genes from AF tissues and non-AF tissues,20–24 and two complementary DNA (cDNA) microarray studies were also reported for studying AF.25, 26 Each study identified many genes with expression that is associated with AF; however, the relationship of these genes to the disease as a cause or a consequence was not established. Furthermore, each study identified a different set of genes associated with AF. The main problem may be related to the small number of samples used in each study. Future studies with an expanded sample size (e.g., hundreds of patients and controls) may eventually identify a common set of genes with expression that is truly associated with AF (signature pattern). Some of these genes may serve as excellent biomarkers for the disease.
Disease-Causing Genes for Atrial Fibrillation
Disease-causing genes are discussed in detail in Chapter 6. In brief, a gain-of-func-tion mutation (S140G) in KCNQ1 was associated with autosomal dominant AF in a Chinese family.27 More than 50% of the affected members (9/16) in the family are also affected with LQTS, which is caused by loss-of-function or dominant-negative mutations in the KCNQ1 gene.1 Another gain-of- function KCNQ1 muta tion, V141M, was identified in a patient with both AF and short QT syndrome.28 A heterozygous mutation R27C of KCNE2 was identified in two AF patients.29 Mutation R27C did not affect KCNH2—KCNE2 IKr (rapid delayed rectifier potas sium current) current, but it had a gain-of-function effect on the KCNQ1—KCNE2 potassium current. The mutation did not alter the functions of the hyperpolariza-tion-activated cyclic nucleotide-gated (HCN) potassium channel family either.
Mutation R53H in KCNE3 was identified in three patients in a very small Chinese family with AF (note that a 40-year-old normal family member also carried the mutations).30 A mutation in KCNJ2, V93I, was identified in three patients carrying the mutation, as well as two other normal family members aged 42 and 33 years, in one Chinese family.31 Although the V93I mutation increased inward potassium current at −90 to −80 mV and outward potassium current at −60 to −40 mV, the hypothesis that KCNJ2 mutations cause AF needs to be further tested considering the minor change from a valine to isoleucine and the finding that two normal family members also carried the V93I mutation.
These studies suggest that mutations in ion channels can cause AF. Interestingly, some mutations in non-ion channel genes are also associated with AF in the con text of other diseases. Mutations in the lamin A/C gene (LMNA) were identified in families with both dilated cardiomyopahty and AF.32 A mutation in the ankyrin-B gene was identified in a family with LQT4, sick sinus syndrome with bradycardia, and AF.33 Mutations in SCN5A were identified in families with both dilated car-diomyopathy and AF.34 A mutation in KCNH2, N588K, was identified in a small family with both short QT syndrome and AF.35
Two genetic loci for autosomal dominant AF have been mapped to chromo some 10q22–24,12 and 6q14–1613; however, the specific genes have not been identified yet.
Atrial fibrillation can also inherit as an autosomal recessive trait.14 The first autosomal recessive AF gene has been mapped to 5p13,14 but the specific gene remains to be identified.
Susceptibility Genes for Atrial Fibrillation
The most frequently used method for identifying the susceptibility genes for a common complex disease is the candidate gene case—control association studies. In this study design, a candidate gene is selected based on its poten tial involvement in the disease.18, 36 Single-nucleotide polymorphisms (SNPs) are identified in the candidate gene by searching the HapMap database (www.hapmap.org) and literature or by direct DNA sequence analysis of a panel of patients. The SNPs are genotyped in a group of patients (cases) and matched controls, and the frequencies of SNP alleles or genotypes are then analyzed by a χ2 test or a Fisher exact test. An allele or genotype is associ ated with the disease if its occurrence in the cases is significantly different from that in the controls. Several case—control studies were reported for AF, and are summarized next.
Ion Channel Genes
In a Chinese population in Taiwan, Lai et al. showed a significant association between the KCNE1 SNP G38S and AF.37 The 38G allele increased the risk of AF, with an OR of 2.16 in heterozygotes and 3.58 in homozygotes. The functional effect of this SNP was reported.38 The 38G allele reduced the IKs (slow delayed rectifier potassium current) potassium current, which is consist ent with a finding that KCNE1 null mice developed AF.39 However, this result contradicts the finding that the gain-of-function mutations in KCNQ1 were associated with AF in the familial form of AF.27 Further studies are needed to clarify the discrepancy. Of note is that KCNE1 SNP G38S was not associated with AF in a different Chinese population from mainland China.40
Other members of the KCNE potassium channel subunit genes were also investigated for their association with AF. The SNP E145D in the KCNE4 gene was associated with AF in a Chinese population (OR = 1.66, p = 0.044).40 The SNP T97C in KCNE5 was associated with AF in a population from Denmark.41 The 97T allele was associated with a reduced risk of AF, with an OR of 0.52.
G-Protein Gene
Schreieck et al. showed that the C825T SNP in the G-protein β3 subunit gene (GNB3) was significantly associated with AF in a German popula-tion.42 The TT genotype plays a protective role in AF, with an OR of 0.46 (p = 0.02).
Genes in the Renin—Angiotensin System
In a Chinese population in Taiwan, Tsai et al. reported that three SNPs (M235T, G-6A, and G-217A) in the angiotensinogen (AGT) gene were associated with risk of AF, with ORs ranging from 2.0 to 3.3, but the insertion/dele tion polymorphism in the angiotensin I-converting enzyme (ACE) gene and the A1166C polymorphism of the angiotensin II type I receptor gene (AT I R) were not associated with risk of AF.43 The study implicates involvement of the renin—angiotensin system in the pathogenesis of AF. In a different study in Japanese patients with hypertrophic cardiomyopathy, the insertion/insertion (I/I) genotype of the (ACE) gene was a significant risk factor for AF.44, 45 The earlier study was supported by a report showing that the expression of ACE was threefold increased during chronic persistent AF.46 Furthermore, in a popula tion from the Netherlands, the ACE insertion/deletion polymorphism did not show any significant risk of AF.47
Gene in Lipid Metabolism
The CETP gene encodes the cholesteryl ester transfer protein that enables the transfer of cholesteryl esters from high-density lipoprotein (HDL) to low-density lipoprotein (LDL), which lowers HDL cholesterol. The TaqIB SNP in CETP was associated with AF in a cohort from the Netherlands (OR = 0.35, p = 0.008).47
Genes Involved in Thrombosis and Hemostasis
Because AF is associated with increased risk of stroke and thromboembolic events in AF, several candidate genes involved in thrombosis were analyzed for their association with AF. Factor V Leiden is a SNP in the coagulation factor V gene, R506Q, that produces a resistance to degradation by activated protein C and increases the risk of venous thrombosis. Factor V Leiden was not asso ciated with the risk of AF or with the risk of left atrial thrombus formation in a Japanese population.48 In a large U.S. population with 13,559 adult patients with nonvalvular AF, factor V Leiden was not significantly associated with risk of stroke in AF.49 In a study with 1,531 participants involved in the Stroke Prevention in Atrial Fibrillation III Study (SPAFIII), the factor V Leiden SNP and levels of prothrombin fragment F1.2 (F1.2), ²-thromboglobulin (BTG), and fibrinogen were not associated with thromboembolism in AF.50 This finding was confirmed in two independent Italian populations.51, 52
Factor II is another coagulation factor and a leading risk factor for venous thrombosis. Pengo et al. showed that SNP G20210A in the factor II gene was associated with systemic thromboembolism (OR = 3.0, p < 0.05).51 Poli et al. showed that the same SNP was associated with AF (OR = 2.4, p < 0.05) but not with cerebral or peripheral embolic events in AF.52 Hatzinikolaou-Kotsakou et al.53 showed that both factor II SNP G20210A and factor V Leiden were associated with AF, with ORs of 4.9 and 4.6, respectively, but the study population was small (55 patients and 17 controls).
The T312A SNP in the α-fibrinogen gene was associated with poststroke mortality in a U.K. AF population.54 In patients with AF, individuals with the A allele showed decreased survival, whereas in the normal population, it did not affect the survival. SNP T312A is close to the FXIIIa crosslinking site at A328. A common SNP in factor XIII, V34L, was associated with rapid FXIII activation but was not associated with AF.55 However, patients with allele 34L showed higher plasma levels of IL6, which may induce a prothrombotic state in AF patients.55 Patients with AF had higher blood levels of IL6 and fibrino-gen after surgery, and the −174G/C SNP in the promoter of IL6 was associated with postoperative AF (GG genotype, OR = 3.25, p = 0.006).56
It is important to note that the results from the case—control association studies should be interpreted with caution as many of these studies are com pounded by the selection bias of cases and controls, population admixture, imperfect matching of cases with controls, phenotyping errors, and small sample sizes.
Genomewide Single Nucleotide Polymorphism Association Studies
To date, case—control association studies for AF have been limited to candidate genes. Recent technological advances in high-throughput genotyping of SNPs have driven down the costs to perform genomewide case—control associa tion studies and make genomewide SNP association studies practical reality. The genomewide association study has the advantage of identifying novel genes associated with AF. More than 50,000 SNPs are required to provide the genome coverage, and a p value less than 5 × 10−7 was proposed to be the cutoff value for achieving significance (80% power).57
On average, sequence variants occur every 1,000 bp in the human genome,58 and approximately 90% of sequence variants are SNPs.59 Over 5 million SNPs have already been reported60 and can be identified at the National Center for Biotechnology Information (NCBI) database and the HapMap database (http:// www.hapmap.org/). High-throughput genotyping technologies have been developed for genomewide SNP genotyping, and an example is Affymetrix microarrays, with 500,000 SNPs. Successful genomewide SNP association studies were reported for age-related macular degeneration (the complement factor H gene),61 type 1 diabetes (the innate immunity viral RNA receptor gene region),62 and the QT interval trait (the NOS1 regulator NOS1AP).63 In July, 2007, Gudbjartsson et al. reported the results from a genome-wide SNP asso ciation study for 550 patients with atrial fibrillation and 4,476 controls from Iceland.64 Genotyping was carried out using the Illumina Hap300 Beadchip with 316, 515 SNPs. A strong association was identified between two SNPs on chromosome 4q25 and atrial fibrillation.64 The result was replicated in three other European populations and a Chinese population from Hong Kong.64 However, the SNPs were not located in any know or putative gene, thus, the specific gene at the locus was not identified.
Future Perspectives
In the next 5 to 10 years, the field of genetics of AF will witness advances in the following research areas:
-
1.
Genetic studies of rare large families will continue to provide important insights into the pathophysiological mechanisms of AF. In particular, iden tification of novel non-ion channel genes will be critical to pinpoint other signaling pathways involved in AF.
-
2.
Genomewide SNP association studies will be a new innovative tool to iden tify many susceptibility genes for AF.
-
3.
Candidate gene case—control association studies will continue to identify some susceptibility genes for AF.
-
4.
Molecular characterization of disease-causing genes, susceptibility genes, and disease-linked genes for AF using a variety of technologies, including transgenic/knockout/knockin mouse models, will provide insight regarding the pathogenesis of AF.
-
5.
Genotype—phenotype correlation studies will lead to genotype-specific therapy (personalized medicine) in AF and move the basic scientific findings to the clinic.
References
1. Wang Q, Curran ME, Splawski I, Burn TC, Millholland JM, VanRaay TJ, Shen J, Timothy KW, Vincent GM, de Jager T, Schwartz PJ, Toubin JA, Moss AJ, Atkinson DL, Landes GM, Connors TD, Keating MT. Positional cloning of a novel potassium channel gene: KVLQT1 mutations cause cardiac arrhythmias. Nat Genet. 1996;12(1):17–23.
2. Curran ME, Splawski I, Timothy KW, Vincent GM, Green ED, Keating MT. A molecular basis for cardiac arrhythmia: HERG mutations cause long QT syn drome. Cell. 1995;80(5):795–803.
3. Schulze-Bahr E, Wang Q, Wedekind H, Haverkamp W, Chen Q, Sun Y, Rubie C, Hordt M, Towbin JA, Borggrefe M, Assmann G, Qu X, Somberg JC, Breithardt G, Oberti C, Funke H. KCNE1 mutations cause Jervell and Lange—Nielsen syndrome. Nat Genet. 1997;17(3):267–268.
4. Splawski I, Tristani-Firouzi M, Lehmann MH, Sanguinetti MC, Keating MT. Mutations in the hminK gene cause long QT syndrome and suppress IKs function. Nat Genet. 1997;17(3):338–40.
5. Abbott GW, Sesti F, Splawski I, Buck ME, Lehmann MH, Timothy KW, Keating MT, Goldstein SA. MiRP1 forms IKr potassium channels with HERG and is associ ated with cardiac arrhythmia. Cell. 1999;97(2):175–187.
6. Wang Q, Shen J, Li Z, Timothy K, Vincent GM, Priori SG, Schwartz PJ, Keating MT. Cardiac sodium channel mutations in patients with long QT syndrome, an inherited cardiac arrhythmia. Hum Mol Genet. 1995;4(9):1603–1607.
7. Wang Q, Shen J, Splawski I, Atkinson D, Li Z, Robinson JL, Moss AJ, Towbin JA, Keating MT. SCN5A mutations associated with an inherited cardiac arrhythmia, long QT syndrome. Cell. 1995;80(5):805–811.
8. Wang Q, Pyeritz RE, Seidman C E, Basson CT. Genetic studies of myocardial and vascular disease. In: Topol EJ, ed. Textbook of cardiovascular medicine. 3rd ed. Philadelphia: Lippincott, Williams and Wilkins; 2006:1967–1989.
9. Chen Q, Kirsch GE, Zhang D, Brugada R, Brugada J, Brugada P, Potenza D, Moya A, Borggrefe M, Breithardt G, Ortiz-Lopez R, Wang Z, Antzelevitch C, O'Brien RE, Schulze-Bahr E, Keating MT, Towbin JA, Wang Q. Genetic basis and molecular mechanism for idiopathic ventricular fibrillation. Nature. 1998;392(6673):293–296.
10. Shimizu W. The long QT syndrome: therapeutic implications of a genetic diagno sis. Cardiovasc Res. 2005;67(3):347–356.
11. Yong S, Tian X, Wang Q. LQT4 gene: the missing ankyrin. Mol. Interv. 2003;3(3):131–136.
12. Brugada R, Tapscott T, Czernuszewicz GZ, Marian AJ, Iglesias A, Mont L, Brugada J, Girona J, Domingo A, Bachinski LL, Roberts R. Identification of a genetic locus for familial atrial fibrillation [see comments]. N Engl J Med. 1997; 336(13):905–911.
13. Ellinor PT, Shin JT, Moore RK, Yoerger DM, MacRae CA. Locus for atrial fibril lation maps to chromosome 6q14–16. Circulation. 2003;107(23):2880–2883.
14. Oberti C, Wang L, Li L, Dong J, Rao S, Du W, Wang Q. Genome-wide linkage scan identifies a novel genetic locus on chromosome 5p13 for neonatal atrial fibril lation associated with sudden death and variable cardiomyopathy. Circulation. 2004;110:3753–3759.
15. Fox CS, Parise H, D' Agostino RB, Sr., Lloyd-Jones DM, Vasan RS, Wang TJ, Levy D, Wolf PA, Benjamin EJ. Parental atrial fibrillation as a risk factor for atrial fibrillation in offspring. JAMA. 2004;291(23):2851–2855.
16. Arnar DO, Thorvaldsson S, Manolio TA, Thorgeirsson G, Kristjansson K, Hakonarson H, Stefansson K. Familial aggregation of atrial fibrillation in Iceland. Eur Heart J. 2006;27(6):708–712.
17. Ellinor PT, Yoerger DM, Ruskin JN, MacRae CA. Familial aggregation in lone atrial fibrillation. Hum Genet. 2005;118(2):179–184.
18. Wang Q. Molecular genetics of coronary artery disease. Curr Opin Cardiol. 2005;20(3):182–188.
19. Kirmanoglou K, Hannekum A, Schafler AE. Expression of mortalin in patients with chronic atrial fibrillation. Basic Res Cardiol. 2004;99(6):404–408.
20. Barth AS, Merk S, Arnoldi E, Zwermann L, Kloos P, Gebauer M, Steinmeyer K, Bleich M, Kaab S, Hinterseer M, Kartmann H, Kreuzer E, Dugas M, Steinbeck G, Nabauer M. Reprogramming of the human atrial transcriptome in permanent atrial fibrillation: expression of a ventricular-like genomic signature. Circ Res. 2005;96(9):1022–1029.
21. Ellinghaus P, Scheubel RJ, Dobrev D, Ravens U, Holtz J, Huetter J, Nielsch U, Morawietz H. Comparing the global mRNA expression profile of human atrial and ventricular myocardium with high-density oligonucleotide arrays. J Thorac Cardiovasc Surg. 2005;129(6):1383–1390.
22. Kim NH, Ahn Y, Oh SK, Cho JK, Park HW, Kim YS, Hong MH, Nam KI, Park WJ, Jeong MH, Ahn BH, Choi JB, Kook H, Park JC, Jeong JW, Kang JC. Altered patterns of gene expression in response to chronic atrial fibrillation. Int Heart J. 2005;46(3):383–395.
23. Ohki-Kaneda R, Ohashi J, Yamamoto K, Ueno S, Ota J, Choi YL, Koinuma K, Yamashita Y, Misawa Y, Fuse K, Ikeda U, Shimada K, Mano H. Cardiac function-related gene expression profiles in human atrial myocytes. Biochem Biophys Res Commun. 2004;320(4):1328–1336.
24. Ohki R, Yamamoto K, Ueno S, Mano H, Misawa Y, Fuse K, Ikeda U, Shimada K. Gene expression profiling of human atrial myocardium with atrial fibrillation by DNA microarray analysis. Int J Cardiol. 2005;102(2):233–238.
25. Kim YH, Lim dS, Lee JH, Shim WJ, Ro YM, Park GH, Becker KG, Cho-Chung YS, Kim MK. Gene expression profiling of oxidative stress on atrial fibrillation in humans. Exp Mol Med. 2003;35(5):336–349.
26. Lai LP, Lin JL, Lin CS, Yeh HM, Tsay YG, Lee CF, Lee HH, Chang ZF, Hwang JJ, Su MJ, Tseng YZ, Huang SK. Functional genomic study on atrial fibrillation using cDNA microarray and two-dimensional protein electrophoresis techniques and identification of the myosin regulatory light chain isoform reprogramming in atrial fibrillation. J Cardiovasc Electrophysiol. 2004;15(2):214–223.
27. Chen YH, Xu SJ, Bendahhou S, Wang XL, Wang Y, Xu WY, Jin HW, Sun H, Su XY, Zhuang QN, Yang YQ, Li YB, Liu Y, Xu HJ, Li XF, Ma N, Mou CP, Chen Z, Barhanin J, Huang W. KCNQ1 gain-of-function mutation in familial atrial fibrillation. Science. 2003;299(5604):251–254.
28. Hong K, Piper DR, az-Valdecantos A, Brugada J, Oliva A, Burashnikov E, Santos-de -Soto J, Grueso-Montero J, az-Enfante E, Brugada P, Sachse F, Sanguinetti MC, Brugada R. De novo KCNQ1 mutation responsible for atrial fibrillation and short QT syndrome in utero. Cardiovasc Res. 2005;68(3):433–440.
29. Yang Y, Xia M, Jin Q, Bendahhou S, Shi J, Chen Y, Liang B, Lin J, Liu Y, Liu B, Zhou Q, Zhang D, Wang R, Ma N, Su X, Niu K, Pei Y, Xu W, Chen Z, Wan H, Cui J, Barhanin J, Chen Y. Identification of a KCNE2 gain-of-function mutation in patients with familial atrial fibrillation. Am J Hum Genet. 2004;75(5): 899–905.
30. Zhang DF, Liang B, Lin J, Liu B, Zhou QS, Yang YQ. [KCNE3 R53H substitution in familial atrial fibrillation.]. Chin Med J (Engl). 2005;118(20):1735–1738.
31. Xia M, Jin Q, Bendahhou S, He Y, Larroque MM, Chen Y, Zhou Q, Yang Y, Liu Y, Liu B, Zhu Q, Zhou Y, Lin J, Liang B, Li L, Dong X, Pan Z, Wang R, Wan H, Qiu W, Xu W, Eurlings P, Barhanin J, Chen Y. A Kir2.1 gain-of-function mutation underlies familial atrial fibrillation. Biochem Biophys Res Commun. 2005;332(4):1012–1019.
32. Sebillon P, Bouchier C, Bidot LD, Bonne G, Ahamed K, Charron P, Drouin-Garraud V, Millaire A, Desrumeaux G, Benaiche A, Charniot JC, Schwartz K, Villard E, Komajda M. Expanding the phenotype of LMNA mutations in dilated cardiomyopathy and functional consequences of these mutations. J Med Genet. 2003;40(8):560–567.
33. Mohler PJ, Schott JJ, Gramolini AO, Dilly KW, Guatimosim S, duBell WH, Song LS, Haurogne K, Kyndt F, Ali ME, Rogers TB, Lederer WJ, Escande D, Le Marec H, Bennett V. Ankyrin-B mutation causes type 4 long-QT cardiac arrhythmia and sudden cardiac death. Nature. 2003;421(6923):634–639.
34. Olson TM, Michels VV, Ballew JD, Reyna SP, Karst ML, Herron KJ, Horton SC, Rodeheffer RJ, Anderson JL. Sodium channel mutations and susceptibility to heart failure and atrial fibrillation. JAMA. 2005;293(4):447–454.
35. Hong K, Bjerregaard P, Gussak I, Brugada R. Short QT syndrome and atrial fibrillation caused by mutation in KCNH2. J Cardiovasc Electrophysiol. 2005;16(4):394–396.
36. Wang Q, Bond M, Elston RC, Tian X. Molecular genetics. In: Topol EJ, ed. Textbook of cardiovascular medicine. 3rd ed. Philadelphia: Lippincott, Williams and Wilkins; 2006; in press.
37. Lai LP, Su MJ, Yeh HM, Lin JL, Chiang FT, Hwang JJ, Hsu KL, Tseng CD, Lien WP, Tseng YZ, Huang SK. Association of the human minK gene 38G allele with atrial fibrillation: evidence of possible genetic control on the pathogenesis of atrial fibrillation. Am Heart J. 2002;144(3):485–490.
38. Ehrlich JR, Zicha S, Coutu P, Hebert TE, Nattel S. Atrial fibrillation-associated minK38G/S polymorphism modulates delayed rectifier current and membrane localization. Cardiovasc Res. 2005;67(3):520–528.
39. Temple J, Frias P, Rottman J, Yang T, Wu Y, Verheijck EE, Zhang W, Siprachanh C, Kanki H, Atkinson JB, King P, Anderson ME, Kupershmidt S, Roden DM. Atrial fibrillation in KCNE1-null mice. Circ Res. 2005;97(1):62–69.
40. Zeng ZY, Pu JL, Tan C, Teng SY, Chen JH, Su SY, Zhou XY, Zhang S, Li YS, Wang FZ, Gu DF. [The association of single nucleotide polymorphism of slow delayed rectifier K+ channel genes with atrial fibrillation in Han nationality Chinese]. Zhonghua Xin Xue Guan Bing Za Zhi. 2005;33(11):987–991.
41. Ravn LS, Hofman-Bang J, Dixen U, Larsen SO, Jensen G, Haunso S, Svendsen JH, Christiansen M. Relation of 97T polymorphism in KCNE5 to risk of atrial fibrillation. Am J Cardiol. 2005;96(3):405–407.
42. Schreieck J, Dostal S, von BN, Wacker A, Flory M, Weyerbrock S, Koch W, Schomig A, Schmitt C. C825T polymorphism of the G-protein beta3 subunit gene and atrial fibrillation: association of the TT genotype with a reduced risk for atrial fibrillation. Am Heart J. 2004;148(3):545–550.
43. Tsai CT, Lai LP, Lin JL, Chiang FT, Hwang JJ, Ritchie MD, Moore JH, Hsu KL, Tseng CD, Liau CS, Tseng YZ. Renin—angiotensin system gene polymorphisms and atrial fibrillation. Circulation. 2004;109(13):1640–1646.
44. Ogimoto A, Hamada M, Nakura J, Miki T, Hiwada K. Relation between angi-otensin-converting enzyme II genotype and atrial fibrillation in Japanese patients with hypertrophic cardiomyopathy. J Hum Genet. 2002;47(4):184–189.
45. Ogimoto A, Higaki J, Miki T. Polymorphism of inhibitory renin—angiotensin sys tem as a genetic risk factor for atrial fibrillation. Circulation. 2004;110(13):e329.
46. Goette A, Staack T, Rocken C, Arndt M, Geller JC, Huth C, Ansorge S, Klein HU, Lendeckel U. Increased expression of extracellular signal-regulated kinase and angiotensin-converting enzyme in human atria during atrial fibrillation. J Am Coll Cardiol. 2000;35(6):1669–1677.
47. Asselbergs FW, Moore JH, van den Berg MP, Rimm EB, de Boer RA, Dullaart RP, Navis G, van Gilst WH. A role for CETP TaqIB polymorphism in determining susceptibility to atrial fibrillation: a nested case control study. BMC Med Genet. 2006;7:39.
48. Gokce M, Ucar F, Kucukosmanoglu M, Erdogan T, Kaplan S. Factor V Leiden mutation and its relation to left atrial thrombus in chronic nonrheumatic atrial fibrillation. Jpn Heart J. 2003;44(4):481–491.
49. Go AS, Reed GL, Hylek EM, Phillips KA, Liu L, Henault LE, Selby JV, Singer DE. Factor V Leiden and risk of ischemic stroke in nonvalvular atrial fibrillation: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. J Thromb Thrombolysis. 2003;15(1):41–46.
50. Feinberg WM, Pearce LA, Hart RG, Cushman M, Cornell ES, Lip GY, Bovill EG. Markers of thrombin and platelet activity in patients with atrial fibrillation: correlation with stroke among 1531 participants in the stroke prevention in atrial fibrillation III study. Stroke. 1999;30(12):2547–2553.
51. Pengo V, Filippi B, Biasiolo A, Pegoraro C, Noventa F, Iliceto S. Association of the G20210A mutation in the factor II gene with systemic embolism in nonvalvular atrial fibrillation. Am J Cardiol. 2002;90(5):545–547.
52. Poli D, Antonucci E, Cecchi E, Betti I, Valdre L, Mugnaini C, Alterini B, Morettini A, Nozzoli C, Abbate R, Gensini GF, Prisco D. Thrombophilic mutations in high-risk atrial fibrillation patients: high prevalence of prothrombin gene G20210A polymorphism and lack of correlation with thromboembolism. Thromb Haemost. 2003;90(6):1158–1162.
53. Hatzinikolaou-Kotsakou E, Kartasis Z, Tziakas D, Hotidis A, Stakos D, Tsatalas K, Bourikas G, Kotsakou ME, Hatseras DI. Atrial fibrillation and hyperco-agulability: dependent on clinical factors or/and on genetic alterations? J Thromb Thrombolysis. 2003;16(3):155–161.
54. Carter AM, Catto AJ, Grant PJ. Association of the alpha-fibrinogen Thr312Ala polymorphism with poststroke mortality in subjects with atrial fibrillation. Circulation. 1999;99(18):2423–2426.
55. Marin F, Corral J, Roldan V, Gonzalez-Conejero R, del Rey ML, Sogorb F, Lip GY, Vicente V. Factor XIII Val34Leu polymorphism modulates the prothrombotic and inflammatory state associated with atrial fibrillation. J Mol Cell Cardiol. 2004;37(3):699–704.
56. Gaudino M, Andreotti F, Zamparelli R, Di CA, Nasso G, Burzotta F, Iacoviello L, Donati MB, Schiavello R, Maseri A, Possati G. The −174G/C interleukin-6 polymorphism influences postoperative interleukin-6 levels and postoperative atrial fibrillation. Is atrial fibrillation an inflammatory complication? Circulation. 2003;108(suppl 1):II195–II199.
57. Freimer N, Sabatti C. The use of pedigree, sib-pair and association studies of common diseases for genetic mapping and epidemiology. Nat Genet. 2004;36(10): 1045–1051.
58. Bentley DR. Decoding the human genome sequence [In Process Citation]. Hum Mol Genet. 2000;9(16):2353–2358.
59. Wang DG, Fan JB, Siao CJ, Berno A, Young P, Sapolsky R, Ghandour G, Perkins N, Winchester E, Spencer J, Kruglyak L, Stein L, Hsie L, Topaloglou T, Hubbell E, Robinson E, Mittmann M, Morris MS, Shen N, Kilburn D, Rioux J, Nusbaum C, Rozen S, Hudson TJ, Lander ES. Large-scale identification, mapping, and genotyping of single-nucleotide polymorphisms in the human genome. Science. 1998;280(5366):1077–1082.
60. Hinds DA, Stuve LL, Nilsen GB, Halperin E, Eskin E, Ballinger DG, Frazer KA, Cox DR. Whole-genome patterns of common DNA variation in three human populations. Science. 2005;307(5712):1072–1079.
61. Klein RJ, Zeiss C, Chew EY, Tsai JY, Sackler RS, Haynes C, Henning AK, SanGiovanni JP, Mane SM, Mayne ST, Bracken MB, Ferris FL, Ott J, Barnstable C, Hoh J. Complement factor H polymorphism in age-related macular degeneration. Science. 2005;308(5720):385–389.
62. Smyth DJ, Cooper JD, Bailey R, Field S, Burren O, Smink LJ, Guja C, Ionescu-Tirgoviste C, Widmer B, Dunger DB, Savage DA, Walker NM, Clayton DG, Todd JA. A genome-wide association study of nonsynonymous SNPs identifies a type 1 diabetes locus in the interferon-induced helicase (IFIH1) region. Nat Genet. 2006;38(6):617–619.
63. Arking DE, Pfeufer A, Post W, Kao WH, Newton-Cheh C, Ikeda M, West K, Kashuk C, Akyol M, Perz S, Jalilzadeh S, Illig T, Gieger C, Guo CY, Larson MG, Wichmann HE, Marban E, O'Donnell CJ, Hirschhorn JN, Kaab S, Spooner PM, Meitinger T, Chakravarti A. A common genetic variant in the NOS1 regulator NOS1AP modulates cardiac repolarization. Nat Genet. 2006;38(6):644–651.
64. Gudbjartsson DF, Arnar DO, Helgadottir A, Gretarsdottir S, Holm H, Sigurdsson A, Jonasdottir A, Baker A, Thorleifsson G, Kristjansson K, Palsson A, Blondal T, Sulem P, Backman VM, Hardarson GA, Palsdottir E, Helgason A, Sigurjonsdottir R, Sverrisson JT, Kostulas K, Ng MC, Baum L, So WY, Wong KS, Chan JC, Furie KL, Greenberg SM, Sale M, Kelly P, MacRae CA, Smith EE, Rosand J, Hillert J, Ma RC, Ellinor PT, Thorgeirsson G, Gulcher JR, Kong A, Thorsteinsdottir U, Stefansson K. Variants conferring risk of atrial fibrillation on chromosome 4q25. Nature 2007;448(7151):353–357.
Acknowledgments
I thank Susan De Stefano for her assistance. This work was supported by an American Heart Association Established Investigator award, the State of Ohio Wright Center of Innovation grant, and a Biomedical Research and Technology Transfer Partnership Award (BRTT) (Ohio's Third Frontier Project).
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2008 Humana Press, a part of Springer Science+Business Media, LLC
About this chapter
Cite this chapter
Wang, Q.K. (2008). Atrial Fibrillation Genetic Considerations: The Basic Scientist's Perspective. In: Natale, A., Jalife, J. (eds) Atrial Fibrillation. Contemporary Cardiology. Humana Press. https://doi.org/10.1007/978-1-59745-163-5_10
Download citation
DOI: https://doi.org/10.1007/978-1-59745-163-5_10
Publisher Name: Humana Press
Print ISBN: 978-1-58829-856-0
Online ISBN: 978-1-59745-163-5
eBook Packages: MedicineMedicine (R0)