Abstract
In this study, I revise three aspects of the socioecology of woolly monkeys (genus Lagothrix) that might give us a better understanding of the patterns found in this species: (1) the association between temporal variation in fruit abundance and diet, activity, and ranging patterns; (2) the individual trade-offs associated with living in small or large groups, and (3) the relationship between social dominance and foraging success. Using behavioral and ecological data collected during 3 years in Tinigua Park, Colombia, I found that woolly monkeys tend to avoid open-degraded forests, where fruit production is generally lower than it is in mature forests. Diet and activity budgets were highly associated with temporal patterns of fruit production. Daily path length was positively correlated with group size and monthly fruit abundance, and negatively correlated with habitat quality. I found differences in activity budgets and the diet preferences of different age/sex classes. For example, adult males rest more and juveniles play more than other classes. Juveniles and adult females without infants look for arthropods more often than adult males and females with young infants, who showed the highest frequencies of fruit feeding. Dominant adult males were not consistently the most efficient foragers on fruits according to two different indexes. Most of these results are consistent with the expectations from strong intra-group competition for resources. However, females with infants received benefits during feeding similar to those of dominant adult males, which may be mediated by differential aggression from males to other group members (juveniles and females without infants).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Woolly monkeys (genus Lagothrix) are one of the largest primates in the New World (Peres 1994a) and are widely distributed in areas of central and western Amazonia (Fooden 1963). Group size varies (range 10–49) (Peres 1996), and groups usually include several philopatric males, several adult females, and their offspring. Males are consistently dominant over females (Nishimura 1994) and more than half of all aggressive interactions are observed in fruit-feeding contexts (Stevenson et al. 1994). Woolly monkeys prefer to feed on ripe fruits, but they also consume young leaves, seeds, and animal prey (Izawa 1975; Soini 1986; Stevenson 1992, 2004a; Stevenson et al. 1994; Peres 1994b; Defler and Defler 1996; Di Fiore and Rodman 2001; Di Fiore 2004). They are known to consume more than 100 different species of fruits in all places where they have been extensively studied (see references above) and their highly diverse diet is associated with the efficient role they play as seed dispersers (Yumoto et al. 1999; Stevenson 2000, 2002; Dew 2001). The seed shadows generated by woolly monkeys depend mainly on their ranging patterns (Stevenson 2002). However, we are still uncertain about the factors affecting moving patterns. The main aim of this paper is to associate the temporal patterns of habitat use with spatial variation in fruit abundance.
In theory, differences in daily path length of primate groups are determined by several factors such as diet type, food distribution, group size and cohesion, competition, energetic constrains, cognitive abilities, and predation risk, among others (Janson 1992; Janson and Goldsmith 1995; Boinski and Garber 2000). For instance, daily path length for frugivorous primate groups tends to increase linearly with group size (but not for folivorous animals); this relationship is used as evidence of the importance of intra-group competition when food sources are patchily distributed and inconsistent in time and space (Janson and Goldsmith 1995). In terms of group cohesion, socio-ecological models predict that small group size or temporal subdivision of group members is feasible when predation risk is low, and less cohesion is expected under regimes of high intra-group competition (Janson 1988; van Schaik 1989). Terborgh and Janson (1986) postulated long ago that the fission–fusion social organization was common in the large spider and woolly monkeys because they may be less vulnerable to predation than smaller New World monkeys. Although consistent differences in the social organization of these two species have been pointed out (Peres 1996), qualitative observations suggest that all atelines show some plasticity in group cohesion, which might be related to total community size (Strier et al. 1993). We still lack solid quantitative data on cohesiveness at group spatial scales, which could be explained by socio-ecological factors.
The ranging behavior of woolly monkeys varies at both regional and local scales. Populations in the central Amazon region, such as the groups studied by Defler (1987) and Peres (1996), range over larger areas than the groups in upper Amazonia (Stevenson et al. 1994; Di Fiore and Rodman 2001; Di Fiore 2003). These differences in home range size can be up to five times in magnitude, and are associated with variations in population densities, which are negatively correlated with fruit production (Stevenson 2001), which in turn could be influenced by soil fertility (Defler and Defler 1996). Similar differences in home range size and path length have even been documented for groups of woolly monkeys living in the same study site, but with different proportion of good quality habitat (Stevenson and Castellanos 2001). Previous analyses of foraging patterns of woolly monkey groups at Tinigua showed similar fruit-feeding rates and shorter daily path lengths for medium-sized groups (Stevenson and Castellanos 2000). Data from the period of low fruit abundance were not included in these analyses to reduce the variation in path length due to causes other than differences in group size, because daily path length tends to be larger during periods of fruit abundance (Stevenson et al. 1994). These differences have been explained as an energy-saving strategy that seems common in all atelines (Strier 1992; Stevenson et al. 2000; Di Fiore and Rodman 2001). In summary, there is evidence that several ecological and social factors affect the ranging patterns of woolly monkeys, but we lack an analysis that simultaneously takes into account the potential factors affecting daily path length (group size, fruit production, and habitat quality).
Among the atelines, woolly monkeys show the highest degree of sexual dimorphism in body size (Ford and Davis 1992). This dimorphism may be associated with male dominance, but it is unclear why this trait is not present in the related Ateles and Brachyteles. It is possible that in the relatively more cohesive groups of woolly monkeys there is a foraging advantage for dominant individuals. It can be hypothesized that sexual dimorphism is partially related to intragroup competition for preferred resources, such as fruits. If this idea is correct, we should expect a positive association between body size, dominance, and foraging efficiency. Furthermore, if intra-group competition is strong we expect a higher consumption time of defendable resources (fruits and leaves) for dominant than for submissive individuals, and higher budgets devoted to moving for low ranking than for high ranking individuals. The second purpose of this paper is to test these predictions. The specific questions I will address in this paper are: (1) which kinds of forest types are preferred by the woolly monkeys at Tinigua Park; (2) is there an association between fruit production and variables such as habitat use, diet, and activity; (3) are there differences in behavioral patterns between age/sex classes that could be associated with intra-group competition; (4) what socio-ecological factors are most important to explain differences in daily path length; and (5) is male dominance related to foraging efficiency?
Methods
Study site
The study site is located in a tropical lowland forest on the eastern border of Tinigua National Park (201,875 ha), west of Sierra de La Macarena, Departamento del Meta, Colombia (2°40′N, 74°10′W, 350–400 m above sea level). The study site, Centro de Investigaciones Ecológicas La Macarena (CIEM) is on the west margin of the Río Duda. Rainfall is markedly seasonal in the region, with a 2 to 3-month dry period occurring between December and March (Stevenson 2002). Average annual precipitation for the three study years was 2,782 mm.
Data collection
I collected data on activity, diet, and habitat use using a combination of instantaneous and continuous sampling on focal individual woolly monkeys during three yearly cycles (April 1990–March 1991, August 1996–July 1997, and January–December 2000, hereafter referenced as 1990, 1996, and 2000, respectively). I analyzed data from 48 h of focal samples each month, equally distributed throughout the day (0600–1800 hours). I sampled four different age/sex classes [adult males, females with dependent infants (less than 1-year-old), adult females without infants, and immature individuals (juveniles and subadults of both sexes)], sampling one class each day every month between 0600 and 1800 hours. All group members were individually recognized (with the exception of some juveniles and subadult individuals) and samples were not biased for any particular individual. Genital marks, injuries, particular facial expressions, and body size were used to distinguish individuals. I noted the activity of the focal animal every 10 min and classified it into moving, resting, feeding, or social interactions. I also distinguished between different food items such as fruits, unripe fruits and seeds, leaves, flowers, arthropods, and other types rarely ingested (termite nest, water or vertebrate prey). In addition, I counted the number of minutes that focal animals spent feeding on all food types (except for arthropod feeding) in a continuous fashion.
I recorded the approximate position of the focal animal within the home range every 30 min on a grid of hectare plots, and at the same time noted the forest type. I delineated the route of the focal animals on a map every day, and then estimated path length using a map measurer. I used daily path length estimates from twelve-hour diurnal follows, including information from samples interrupted by the night (e.g. 1200–1800 hours + 0600–1200 hours). The duration of focal samples was variable (but longer than 1 h). If the focal animal was lost for more than 10 min, I searched for another individual of the same age/sex class to continue the observations. If I could not find one in less than 10 min, I postponed observations for another day, and the daily path information was discarded.
During the first 2 years, I concentrated on a single social unit (group CR-12), but during the third year, I divided observations between two groups, so that approximately a quarter of the observations were on focal animals of a different group (CR-D). CR-12 comprised about 20 individuals in the early 1990s, and increased the size to about 30 from the mid-1990s until the end of the century. In 1996, this main study group consisted of 6–7 adult males, 0–1 subadult males, 10–11 adult females, 1–2 subadult females, 5–8 juveniles, and 5–3 infants. Occasional observations of the second group suggest that the CR-D group has remained as a small group of about 14 individuals since it was initially described (3 adult males, 4–5 adult females, 5 juveniles, and 2 infants). Throughout the results section data from the two groups were combined, except for the estimates of daily path length, which vary between groups of different size.
I have not observed between-group transfer of adult males, while female transfer is frequently observed. For instance, I have observed a single female associating with three different social units for periods of several months each. Male transfer has only be reported when adult females with male infants move between groups (n=1). Preliminary density estimates in the study area, including at least six different groups, indicate that woolly monkey population density varied during the study years (mean 1990: 42 ind/km2; 1996: 41; 2000: 50; Stevenson 2002).
I quantified fruit production in the forest as the number of trees producing ripe fruits in 5.6 km of phenological transects (Stevenson et al. 1998, 2004b), which were monitored twice a month during the three study years. The number of fruiting trees bearing fruits in transects varied among years (1990: 933; 1996: 862; 2000: 870). Habitat quality within each home range was defined as the proportion of mature forest it included, which produces almost twice as much fruit as the other main forest types, such as open-degraded and flooded (Table 1; see details in Stevenson et al. 1994, 2000).
Results
Habitat use
The home range size of the main study group had been previously estimated to be 169 ha (Stevenson et al. 1994), using the convex polygon method. Instantaneous samples were collected in 117 different 1-ha quadrants in the first year, and 138 and 118 in subsequent study years. Overall, instantaneous samples have been recorded in 173 ha, which excludes some areas entered for short periods. Summing up these quadrants the total home range area seems to be close to 200 ha. The pattern of spatial use of the habitat is very consistent across years, in the sense that a very small proportion of the one-hectare quadrants are heavily used, compared to the number of quadrants that are infrequently visited (Fig. 1). Some changes in the use of the home range have been evident between years. For example, the study group stopped using a fringe of 20 ha toward the western portion of the home range after the first study period, which was compensated with the expansion of the eastern border. In spite of this shift, the areas of common use tended to remain the same from year to year.
Sleeping sites were scattered through the home range and widely distributed. In fact, 74 1-ha plots were used as sleeping sites. A slight aggregation of sleeping sites was found only in three different areas (comprising 5 ha), with 25% of the total sample points (n=175 sleeping sites).
The mature forest was consistently the preferred forest type for the woolly monkeys (Table 1). Although this forest type is the most abundant habitat in the area, the monkeys preferred it disproportionately (G=29.8, P<0.05). It is possible that habitat use samples 30 min apart are not independent of each other, however, the consistent rejection of the open-degraded forest in the three different years suggests that the consistent preference for the mature forest is significant (Table 1, Fig. 2b).
a Temporal variation in fruit abundance in flooded and terra firme (mature and open forests) in terms of the number of plants producing fruits in phenological transects. b Seasonal patterns of habitat use of woolly monkeys in Tinigua Park, as the average for percentage of samples from three study years. The standard error is shown in both cases
The use of different forest types is not constant throughout the year. Usually the proportion of time spent in flooded forests increases during the middle of the year (i.e. rainy season) (Fig. 2b), when several plants that are restricted to those areas produce fruits (e.g. Laetia corymbulosa, Cecropia membranacea), and just when fruit production in the flooded forests reaches similar or higher average values than terra firme forests (Fig. 2a, Stevenson 2004b). In 2 out of 3 years there was a significant correlation between fruit production in the flooded forest (as the number of fruiting trees along transects) and habitat use scores (1996: r=0.90; 2000: r=0.70 n=12; both P<0.05). This was not true during the first study period, but this result might be biased due to the early successional stage of the riparian forest at the time and the lack of an appropriate trail system surrounding the swampy areas of the flooded forest. There was an unusually high use of flooded forest in May 1997 that was probably due to a high production of Inga cylindrica fruits in that particular year (see Stevenson 2005). This plant species produces fruits almost every year, but crop size is highly variable. Habitat use was highly variable during periods of fruit scarcity because it depends in large part on the unpredictable location of particular food sources (e.g. large fruiting fig trees in the flooded forests).
Activity patterns
Activity budgets are relatively constant between years (Table 2). There was a tendency to increase moving time as group size increased during the study periods. Fruit was the main food type ingested in all years and feeding on arthropods was the category with the largest inter-annual variation (Table 2).
Seasonal changes in activity budgets were similar in the three study years. Moving and social interactions were overall more frequent during periods of fruit abundance than in fruit shortage (September–December), while resting showed the opposite pattern (Fig. 3b). The number of fruiting plants in transects was a good predictor of the variations in the main activities of woolly monkeys (Table 3). Feeding activities did not show consistent seasonal differences, but the ingestion of different food types was markedly variable. Ripe fruit feeding was lowest during periods of fruit scarcity, and the same pattern was found for insects (Fig. 3c). However, ripe fruit feeding was not significantly associated with fruit abundance (Table 3), because the monkeys were able to find and ingest ripe fruits from the few trees that produced fruits during the scarcity period. In contrast, the ingestion of unripe fruits, new leaves, and flowers increased significantly during periods of fruit scarcity (Fig. 3c, Table 3).
Temporal variation in a the number of fruiting plants in phenological transects, b activity patterns, and c diet composition of woolly monkeys at Tinigua Park, Colombia. The points show the average for three study periods and the x-axis was artificially arranged to facilitate direct comparison between years
I found large differences among age/sex classes in the frequency of samples devoted to different activities (G=42.8, df=9, P<0.001). The main differences were found in relation to resting activities and social interactions. Adult males tended to rest more frequently than juveniles and females without infants. The opposite pattern was found for moving activities (Fig. 4a). Additionally, the scores of social interactions were very high for juveniles, as they play more often than other age/sex classes, particularly females with infants. There were few differences in overall feeding activities among age/sex classes, but I found differences in the proportion of samples devoted to distinct food types (excluding the “other” category: G=13.1, P=0.04). In this case the main differences were due to the high frequency with which the juveniles and the females without infants were looking for arthropods, in contrast to the adult males (Fig. 4b). Females with infants showed the highest frequency of fruit feeding.
Daily path length
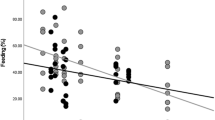
I did not find a significant difference in path lengths between age/sex classes (F=0.7, P=0.97, n=96). In an effort to explain daily path length by socio-ecological variables such as group size, habitat quality and fruit production, I used multiple regression analysis and found that all three variables showed significant partial regression coefficients (group size: F=25.0, P<0.001, r2=0.12; habitat quality: F=7.1, P=0.009, r2=0.04; fruit production: F=21.7, P<0.001, r2=0.13). Habitat quality was negatively related to path length. In contrast, path length was positively related to both group size and fruit production. However, the overall model explained only 26% of the variance in path length, and there are other factors causing a large variation in daily path length among and within years (Table 1).
Feeding efficiency was not consistently higher for the dominant adult males across years. Using the ratio of sampling points feeding on fruits divided by moving scores, both adult males and females with infants showed the highest values (Fig. 5). Feeding efficiency was not consistently higher for adult males across years. Compared to other classes, males tended to show higher scores of feeding efficiency as the population density and group size increased. However the scores in general decreased as population density increased from 1990 to 2000. Using the second index (total feeding time on fruits divided by the moving scores), females with infants were consistently the age/sex class with the highest values of foraging efficiency.
Comparison of two indexes of foraging efficiency estimated for different age/sex classes of woolly monkeys at Tinigua Park in three different study periods. The bars represent the proportion of sampling points feeding on fruits divided by moving scores. The points show the relation between total time in fruit feeding divided by the number of sample points in moving. AM: adult males, FI: female with young infant, AF: adult female, and Juv: juveniles and immature individuals
Discussion
Some of these results on daily movements agree with the findings of previous analyses using data from the first 2 years (Stevenson and Castellanos 2001). We have consistently found that individuals in large groups (≥30) tend to move more to get a similar per capita food intake. In contrast, data collected during the last study period showed that individuals in small groups (≤15) did not necessarily move more than medium-sized groups (≈20), as was found in the preliminary analysis. Overall, the results stress: (1) the importance of intraspecific competition, which predicts a positive relationship between daily path length and group size (when per capita feeding rates are similar between groups); (2) the new results leave some doubts about the role of between-group competition, which predicts that individuals in small groups should move farther than individuals in large groups because small groups are displaced from good quality areas; and (3) the results highlight the relevance of habitat quality in determining ranging patterns. The multiple regression model used to predict ranging patterns showed a positive relationships between daily path length and both group size and fruit production, and a negative relationship with habitat quality. The model including these three variables is able to explain only about a quarter of the variation in daily path length, which suggests there are other variables affecting ranging behaviors that were not taken into account. For example, my observations suggest that movement patterns of adjacent groups are not independent from each other, suggesting that between group interactions play important roles determining the patterns of habitat use.
Daily path length was similar between age/sex classes. On average, females without infants and juveniles show a longer path length than adult males and females with infants, but the difference was not statistically significant. However, I believe that a significant trend would emerge in an analysis using a finer spatial scale. In particular, my observations suggest that both females without infants and juveniles move more than adult males and females with infants at small scales, for example, when they are looking for arthropods within trees. A rigorous test of these observations remains to be done.
There are age/sex differences in the patterns of home range use within atelines. For example, it has been reported that adult male spider monkeys tend to have larger ranges and use peripheral areas of the home range more often than females (Chapman 1988; Symington 1988). In contrast, groups of woolly monkeys are more cohesive (Stevenson 1998; Stevenson et al. 1998), and females visit peripheral areas of the home range as well as males. Only in cases of inter-group agonistic encounters do the adult males tend to congregate at the most peripheral zone.
The patterns of daily path length, some of the differences in diet, activity, and foraging efficiency among age/sex classes support the hypothesis of high intra-group competition in woolly monkeys. In particular, submissive individuals such as juveniles and females without infants showed the highest moving scores, reliance on arthropods, and low foraging efficiency in fruits. A puzzling result is that females with dependent offspring have similar or even higher foraging efficiency scores than adult males. Furthermore, they seem to have good access to fruit sources, low moving scores, and they are seldom attacked by other individuals. Although the high status of females with dependent offspring is difficult to understand, a potential explanation is hypothesized below.
Although woolly monkeys are highly frugivorous with similar natural history and body size as the closely related spider monkey (Ateles), they do not show the same fluididity in fission–fusion social organization as seen in Ateles. Even when both species live near each other, as they do in Tinigua, sharing the same predators and food supply, groups of woolly monkeys are more cohesive than groups of spider monkeys (Stevenson et al. 1998). The main difference in diet between these two species is the higher consumption of insects by the woolly monkeys (Milton and Nessimian 1984; Stevenson et al. 2000; Dew 2001; Di Fiore and Rodman 2001; Di Fiore 2004). This study confirmed once again the importance of insects in the Tinigua population. We suggested that feeding on arthropods might relax the negative effects of gregariousness by contest competition over fruits (Stevenson et al. 1994). However, in periods when the woolly monkeys eat few insects, they still associate more cohesively than spider monkeys do and it is not clear yet if this seasonal benefit would lead to the evolution of a more cohesive social unit. On the other hand, woolly spider monkeys (Brachyteles) also maintain more cohesive groups than spider monkeys do, possibly because at least some populations seem to rely more on leaves than on fruits (Milton 1984; Strier 1992).
Another difference between woolly monkeys and the other atelines is their greater degree of sexual dimorphism in body size (Rosenberger and Strier 1989). Woolly monkey males are about 45% heavier than females (Ford and Davies 1992). An increase in body weight in adult males does not seem to be favored by female choice because mating is promiscuous (Nishimura 1994, 2003) and infants of a single group are fathered by different males (Escobar-Paramo 1999). Usually all adult and subadult males copulate with each female in estrous and male-male agonistic interactions in mating context are rare (Stevenson 1997). An alternative explanation for increased male size could be the outcome of inter-group and intra-group agonistic interactions. The main result of my analyses on foraging efficiency was that the large adult males are not consistently the most efficient foragers on fruits, in spite of their dominance and their knowledge of the home range (because males are philopatric). In contrast, females with dependent infants (less than 1 year) were in general more efficient foragers than other age/sex classes, an unexpected result given that males are clearly dominant over females, independent of body size (Nishimura 1994; Stevenson et al. 1994). I suggest that the foraging efficiency of females with infants could be mediated by differential aggression by adult males against other females and juveniles, giving priority access to the females that are nursing that male’s offspring. This idea assumes that adult males are able to recognize kin, as was discovered in baboons (Buchan et al. 2003), or that adult males are so closely genetically related that they benefit from taking care of all infants in the group. Similarly, it could be that because all males have mated with the female that is nursing the infant that each male believes that he could be the father and so each male gives her preferential treatment just in case it is his baby. In any case, if adult males allow females with infants to ingest more food than other individuals, such a mechanism could promote a higher degree of group cohesion. More studies will be necessary to test this hypothesis and its assumptions, as well as alternative hypotheses. For instance, it remains to be shown whether between group competition may influence sexual dimorphism in body size. Also, it would be interesting to determine if insect feeding really affects group cohesion.
References
Boinski S, Garber PA (2000) On the move: How and why animals travel in groups. University of Chicago Press, Chicago
Buchan JC, Alberts SC, Silo JB, Altmann J (2003) True paternal care in a multi-male primate society. Nature 425:179–181
Chapman AC (1988) Patterns of foraging and range use by three species of Neotropical primates. Primates 29:177–194
Defler TR (1987) Ranging and the use of space in a group of woolly monkeys (Lagothrix lagotricha) in the Nw Amazon of Colombia. Int J Primatol 8:420–420
Defler TR, Defler SB (1996) Diet of a group of Lagothrix lagothricha lagothricha in Southeastern Colombia. Int J Primatol 17:161–189
Dew L (2001) Synecology and seed dispersal in woolly monkeys (Lagothrix lagotricha poeppigii) and spider monkeys (Ateles belzebuth belzebuth) in Parque Nacional Yasuni, Ecuador. PhD dissertation, University of California, Davis
Di Fiore A (2003) Ranging behavior and foraging ecology of lowland woolly monkeys (Lagothrix lagotricha poeppigii) in Yasuni National Park, Ecuador. Am J Primatol 59:47–66
Di Fiore A (2004) Diet and feeding ecology of woolly monkeys in a western Amazonian rain forest. Int J Primatol 25:767–801
Di Fiore A, Rodman PS (2001) Time allocation patterns of lowland woolly monkeys (Lagothrix lagotricha poeppigii) in a neotropical terra firma forest. Int J Primatol 22:449–480
Escobar-Paramo P (1999) Inbreeding avoidance and the evolution of mating strategies. PhD dissertation, State University of New York at Stony Brook, New York
Fooden J (1963) A revision of the woolly monkeys (Genus Lagothrix). J Mamm 44:213–247
Ford SM, Davis LC (1992) Systematics and body size: Implications for feeding adaptations in New World monkeys. Am J Phys Anthropol 88:415–468
Izawa K (1975) Foods and feeding behavior of monkeys in the upper Amazon basin. Primates 16:295–316
Janson CH (1988) Intraspecific food competition and primate social-structure—a synthesis. Behaviour 105:1–17
Janson CH, Goldsmith ML (1995) Predicting group-size in primates—foraging costs and predation risks. Behav Ecol 6:326–336
Janson CH (1992) Evolutionary ecology of primate social structure. In: Smith EA, Winterhalder B (eds) Evolutionary ecology and human behavior. Aldine, New York, pp 95–130
Milton K (1984) The role of food processing factors in primate food choice. In: Rodman PS, Cant JGH (eds) Adaptations for foraging in non-human primates: contributions to an organismal biology of prosimians, monkeys and apes. Columbia University Press, New York, pp 249–279
Milton K, Nessimian JL (1984). Evidence for insectivory in two primate species (Callicebus torquatus lugens and Lagothrix lagothricha lagothricha) from Northwestern Amazonia. Am J Primatol 6:367–371
Nishimura A (1994) Social interaction patterns of woolly monkeys (Lagothrix lagotricha): A comparison among Atelines. Sci Eng Rev Doshisha Univ 35:235–254
Nishimura A (2003) Reproductive parameters of wild female Lagothrix lagotricha. Int J Primatol 24:707–722
Peres CA (1994a) Which are the largest new World monkeys? J Hum Evol 26:245–249
Peres CA (1994b) Diet and feeding ecology of gray woolly monkeys (Lagothrix lagothricha cana) in Central Amazonia: comparisons with other Atelines. Int J Primatol 15:333–372
Peres CA (1996) Use of space, spatial group structure, and foraging group size of gray woolly monkeys (Lagothrix lagotricha cana) at Urucu, Brazil. A review of the Atelinae. In: Norconk MA, Garber PA, Rosemberger AF (eds) Adaptive radiations of neotropical primates. Plenum Press, New York, pp 467–488
Rosenberger AL, Strier KB (1989) Adaptive radiation of the ateline primates. J Hum Evol 18:717–750
van Schaik CA (1989) The ecology of social relationships amongst female primates. In: Standen V, Foleyoelds RA (eds) Comparative socioecology. Blackwell, Oxford, pp 195–218
Soini P (1986) A synecological study of a primate community in the Pacaya Samiria National Reserve, Peru. Primate Conserv 7:63–71
Stevenson PR (1992) Diet of woolly monkeys (Lagothrix lagotricha) at La Macarena, Colombia. Field Stud New World Monk Macarena Colomb 6:3–14
Stevenson PR (1997) Notes on the mating behavior of woolly monkeys (Lagothrix lagotricha) at Tinigua National Park, Colombia. Field Stud Fauna Flora Macarena Colomb 10:13–15
Stevenson PR (1998) Proximal spacing between individuals in a group of woolly monkeys (Lagothrix lagotricha) in Tinigua National Park, Colombia. Int J Primatol 19:299–311
Stevenson PR (2000) Seed dispersal by woolly monkeys (Lagothrix lagothricha) at Tinigua National Park, Colombia: Dispersal distance, germination rates, and dispersal quantity. Am J Primatol 50:275–289
Stevenson PR (2001) The relationship between fruit production and primate abundance in Neotropical forests. Biol J Linn Soc 72:161–178
Stevenson PR (2002) Frugivory and seed dispersal by woolly monkeys (Lagothrix lagothricha) at Tinigua Park, Colombia. PhD dissertation, State University of New York at Stony Brook, New York
Stevenson PR (2004a) Fruit choice by woolly monkeys in Tinigua National Park, Colombia. Int J Primatol 25:367–381
Stevenson PR (2004b) Phenological patterns of woody vegetation at Tinigua National Park, Colombia. Caldasia 26(1):125–150
Stevenson PR (2005) Potential keystone plant species for the frugivore community at Tinigua Park, Colombia. In: Dew L, Boubli JP (eds) Tropical fruits and frugivores: the search for strong interactors. Springer, The Netherlands Berlin Heidelberg pp 38–57
Stevenson PR, Castellanos MC (2000) Feeding rates and daily path range of the Colombian woolly monkeys as evidence for between- and within-group competition. Folia Primatol 71:399–408
Stevenson PR, Castellanos MC (2001) New evidence for large variations in daily path length related to differences in habitat quality in troops of Colombian Woolly Monkeys, Lagothrix lagothricha. XVIII Congress of the International Primatological Society, Adelaide, South Australia, pp 446
Stevenson PR, Quiñones MJ, Ahumada JA (1994) Ecological strategies of woolly monkeys (Lagothrix lagotricha) at La Macarena, Colombia. Am J Primatol 32:123–140
Stevenson PR, Quiñones MJ, Ahumada JA (1998) Effects of fruit patch availability on feeding subgroup size and spacing patterns in four primate species at Tinigua National Park, Colombia. Int J Primatol 19:313–324
Stevenson PR, Quiñones MJ, Ahumada JA (2000) Influence of fruit availability on ecological overlap among four neotropical primates at Tinigua National Park, Colombia. Biotropica 32:533–544
Strier KB (1992) Atelinae adaptations—behavioral strategies and ecological constraints. Am J Phys Anthropol 88:515–524
Strier KB, Mendes FDC, Rimoli J, Rimoli AO (1993) Demography and social-structure of one group of muriquis (Brachyteles arachnoides). Int J Primatol 14:513–526
Symington MM (1988) Demography, ranging patterns, and activity budgets of black spider monkeys (Ateles paniscus chamek) in the Manu-National Park, Peru. Am J Primatol 15:45–67
Terborgh JW, Janson CH (1986) The socioecology of primate groups. Annu Rev Ecol Syst 17:111–135
Yumoto T, Kimura K, Nishimura A (1999) Estimation of the retention times and distances of seed dispersed by two monkey species, Alouatta seniculus and Lagothrix lagotricha, in a Colombian forest. Ecol Res 14:179–191
Acknowledgements
I would like to thank all the field assistants who helped in gathering information, especially Maria Clara Castellanos, Alicia Medina, Carolina García, Monica Pineda, and Tatiana Samper. I thank Charles Janson, Patricia Wright, John G. Fleagle, Anthony DiFiore, Akisato Nishimura, Nicole Gibson, and an anonymous referee for their comments. This study was possible thanks to logistic support of CIEM (Centro de Investigaciones Ecológicas La Macarena) and the permits from Unidad de Parques Nacionales. Financial support came from the following institutions: La Fundación para la Promoción de la Investigación y la Tecnología (Banco de la República), Margot Marsh Foundation, Lincoln Park Zoo, Primate Conservation Inc., and IdeaWild.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Stevenson, P.R. Activity and ranging patterns of Colombian woolly monkeys in north-western Amazonia. Primates 47, 239–247 (2006). https://doi.org/10.1007/s10329-005-0172-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10329-005-0172-6