Abstract
Taurine abundantly contained in the skeletal muscle has been considered as one of essential factors for the differentiation and growth of skeletal muscles. The previous studies in the taurine transporter knockout mice showed that deficiency of taurine content in the skeletal muscle caused incomplete muscular developments, morphological abnormalities, and exercise abilities. In fetal and neonatal periods, taurine must be an essential amino acid due to no biosynthesis capacity, and therefore, taurine should be endogenously supplied through placenta and maternal milk. In general cell culture condition, taurine contained in the culture medium is absent or few, and therefore, most of cultured cells are in taurine-deficient condition. In the present study, we confirmed, in cultured mouse differentiable myoblast, taurine treatment significantly enhanced the differentiation to myotube in a dose-dependent manner, while these effects were abrogated by inhibitions of taurine transport and Ca2+ signaling pathway.
The present study suggested that exogenous taurine might play a key role on the mature differentiation/growth of the skeletal muscle during development period through Ca2+ signaling pathway, and therefore, taurine would contribute the muscle recovery after damages.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
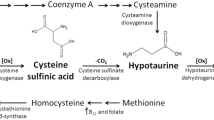
Taurine (2-aminoethanesulfonic acid) is abundantly contained in the skeletal muscles (Jacobsen and Swimth 1968), particularly in the slow-twitch fiber rather than in the fast-twitch fiber (Airaksinen et al. 1990; Iwata et al. 1986). Although taurine is biosynthesized from sulfur-contained amino acids (methionine and cysteine) in the liver and brain via specific enzymes (cysteine sulfinate decarboxylase and cysteine dioxygenase), the biosynthesis ability is very low (Hosokawa et al. 1990; Kaisaki et al. 1995; Ramamoorthy et al. 1994). Therefore, the abundant muscular taurine content depends on the exogenous uptake through a specific transporter, taurine transporter (TAUT). Furthermore, taurine is an essential amino acid in fetus and infant due to lack of taurine biosynthesis ability in the perinatal period, and therefore, a large amount of taurine is endogenously supplied through placenta and maternal milk. It has been reported that a premature baby and incomplete postnatal tissue development and body growth were observed if taurine intake were insufficient in the pregnant and postnatal periods (Aerts and Van Assche 2002; Sturman and Messing 1991; Sturman 1993).
Taurine has been also considered as one of essential factors on the differentiation/growth of skeletal muscles because deficiency of taurine causes incomplete muscular development and exercise abilities. In the TAUT KO mice, the lower body mass and skeletal muscle growth were observed associated with significant deficiency of tissue taurine concentration compared with wild-type mice (Heller-Stilb et al. 2002; Warskulat et al. 2004), and the morphological abnormalities including muscular atrophy and disruption of myofibrillar ultrastructure and the reduction of physical capacity were also found in the TAUT KO mice (Ito et al. 2008). In the TAUT KO mice, the deficiency of tissue taurine innately induced is suggested to influence incomplete development of skeletal muscle tissue.
In general cell culture experiments, the condition should be deficient of taurine because culture medium and serum do not contain taurine at all. Therefore, most of the cells are cultured in the taurine-deficient condition. The present study examined the effect of taurine treatment on the differentiation of mouse myoblast to myotube.
2 Methods
2.1 Culture and Differentiation of Myoblast Cells
Mouse differentiable myoblast (C2C12) was purchased from ATCC (Manassas, VA). C2C12 cells were cultured with growth medium (GM; DMEM supplemented with 10% fetal bovine serum) until confluent, and thereafter, the cells were switched to differentiation medium (DM; DMEM supplemented with 2% horse serum) with or without ∼20 mM taurine for up to a week. Furthermore, the cells were exposed to ∼50 mM taurine transport agonist, β-alanine; 5 μM calcineurin inhibitor, FK-506 (Cayman chemical, MI, USA); or 10 μM Ca2+ chelator, nifedipine (Sigma, MO, USA) in DM with or without 20 mM taurine for 5–6 days.
In C2C12 myoblast, taut mRNA was also silenced using siRNA (HP GenomeWide siRNA duplexes; QIAGEN, Hilden, Germany) by electroporation method (Amaxa™ Nucleofector™ Technology, Lonza, Cologne, Germany). Harvested undifferentiated C2C12 myoblast (1 × 106 cells) was transfected with the siRNA primers of taut or control and pmaxGFP® vector (Lonza), and the cells were grown up to confluent with GM. Thereafter, the cells were differentiated in DM with or without taurine.
2.2 Quantifications of Cell Size and Nuclei Number in Myotube
The differentiated C2C12 myotube cultured with and without taurine was fixed with methanol and 4% paraformaldehyde, and then, myosin heavy-chain (MHC) protein as a marker of differentiation in the skeletal muscle was immunohistochemically detected using monoclonal anti-skeletal MHC-fast antibody (Sigma) as the primary antibody and goat anti-mouse IgG antibody conjugated with FITC (Santa Cruz Biotechnology, CA, USA) as the secondary antibody, and the nucleus was labeled with DAPI (KPL, MD, USA). FITC and DAPI were observed using a fluorescence microscopy, and maximal short diameter and apsis length of FITC-positive in differentiated myotube and the number of DAPI-positive nucleus in the FITC-positive myotube were measured, and the number of nucleus per myotube and fusion index as ratio of the number of nucleus to the apsis length of myotube were calculated.
In the C2C12 myotube transfected with taut siRNA, the transfected GFP that is a marker of silenced taut gene was detected using fluorescence microscopy. The efficiency of silencing of taut gene was approximately 70% evaluated as TAUT protein expression by Western blot.
2.3 The mRNA Expression by Quantitative Real-Time PCR Technique
The mRNA expression level of calcineurin inhibitory protein myocyte-enriched calcineurin-interacting protein (MCIP) 1 in the differentiated myotube treated with taurine and nifedipine was quantified by real-time PCR. Total RNA was extracted from the harvested myotube using an RNeasy Plus Mini Kit (QIAGEN). Reverse transcription was performed on 500 ng of total RNA using a PrimeScript® RT reagent Kit (TAKARA Bio. Inc. Shiga, Japan). Real-time quantitative PCR was performed on cDNA aliquots with the FastStart DNA Master SYBR Green I and a LightCycler (Roche Diagnostics, Mannheim, Germany). The sequences of the oligonucleotide primer pairs used to amplify mRNA were as follows: MCIP1 forward primer, 5′-CTTCAGAACATATGACAAGGAC-3′; MCIP1 reverse primer, 5′-AGGTGTGAACTTCCTATGTGTA-3′; β-actin forward primer, 5′-CCTGTATGCCTCTGGTCGTA-3′; and β-actin reverse primer, 5′-AGACTTCGAGCAGGAGATGG-3′. A standard curve for each run was constructed by plotting the crossover point against the log concentration. The concentration of target molecules in each sample was then calculated automatically by reference to this curve (r = −1.00), and results were standardized to the expression of β-actin. The specificity of each PCR product was assessed by melting curve analysis.
2.4 Statistical Analysis
Statistical significances were determined by unpaired Student’s t-test or one-way ANOVA multiple comparison test. Data were expressed as the mean ± SD or the median and plots of individual value. Differences were considered statistically significant when the calculated P value was less than 0.05.
3 Results
3.1 The Effect of Taurine Treatment on the Differentiation of C2C12 Myoblast to Myotube
The effect of taurine treatment on the differentiation of myoblast to myotube in C2C12 cells was evaluated by the measurements of maximal length of short diameter of differentiated myotube, the number of nucleus in the myotube, and the calculation of fusion index which is the number of nucleus per the apsis length of examined myotube. The maximal short diameter in the myotube was increased by taurine treatments compared to that in the untreated control and was significantly higher in 20 mM taurine treatment than the control and 5 mM taurine treatment (Fig. 29.1a). Likewise, the number of nucleus in the differentiated myotube (Fig. 29.1b) and fusion index (Fig. 29.1c) were also significantly increased by 20 mM taurine treatment compared to that in the control and 5 mM taurine treatment.
The effect of taurine treatment on differentiation of C2C12 myoblast to myotube. C2C12 cells were cultured with 0, 5, or 20 mM taurine in the differentiation medium for 7 days. (a) The length of maximum short diameter in myotube, (b) the number of nucleus per myotube, and (c) fusion index was calculated by the number of nucleus per the examined myotube length. Data are shown as the median and value plots. *p < 0.05, **p < 0.01, ‡p < 0.0001 by one-way ANOVA analysis
3.2 The Effects of Inhibitors of Taurine Transport and Ca+2 Signaling on the Taurine-Enhanced C2C12 Differentiation
Figure 29.2 shows the fluorescence images of C2C12 myotube treated with nifedipine or transfected with taut siRNA. During the differentiation period, the cells in both conditions were exposed to 20 mM taurine. The enhanced effect of taurine on the differentiation to myotube evaluated by FITC-positive MHC protein expression was cancelled by the treatment of nifedipine (Fig. 29.2a). The expression of MCIP-1 mRNA in myotube was significantly increased by nifedipine treatment compared to that in undifferentiated myoblast, and the expression level in myotube treated with and without nifedipine was significantly reduced by taurine treatment (Fig. 29.2b). Similarly, FK-506 treatment in the C2C12 cells markedly reduced the enhancement of differentiation to myotube by taurine treatment (data not shown).
Fluorescence image of C2C12 myotube treated with 20 mM taurine in the Ca2+ chelator nifedipine treatment (a) and taut siRNA transfection (b) and the expression level of MCIP1 mRNA in the myotube treated with taurine and nifedipine (c). (a, c) C2C12 cells were exposed to differentiation medium containing 20 mM taurine with or without 10 μM nifedipine for 5 days. Myosin heavy-chain protein in myotube was immunohistochemically stained with anti-MHC and FITC-conjugate IgG antibodies (c). C2C12 cells transfected with either control or taut siRNA and GFP vector were differentiated with 20 mM taurine (c). The mRNA expression level of MCIP1 was quantified by real-time PCR and shown as the relative ratio to undifferentiated myoblast. The differentiated myotube was detected by the transfected GFP. Data are shown as the mean ± SD and *P < 0.05, †P < 0.001(vs. myoblast) by the unpaired Student’s t-test and one-way AVOVA multiple comparison test, respectively
In Fig. 29.2c, the silencing taut gene using siRNA technique inhibited the differentiation to myotube in the C2C12 cells treated with 20 mM taurine compared to that in the control siRNA-transfected cells. Furthermore, the effect of taurine on the differentiation to myotube was significantly reduced by β-alanine treatment in a dose-dependent manner (data no shown).
4 Discussion
In the present study, the differentiation of C2C12 fibroblast to myotube was significantly and dose-dependently enhanced by taurine treatment, and the effect of taurine was abrogated by the inhibitions of Ca2+, its signaling pathway, and endogenous taurine transport. Myogenesis consists of multiple processes including ceasing of proliferation, elongation, and fusion into multinucleated myotube, and many factors including insulin growth factor 1 and myocyte-enhanced factor 2 have been reported to enhance these processes in the previous studies (Gossett et al. 1989; Kook et al. 2008; Maeda et al. 2002; Naya and Olson 1999; Semsarian et al. 1999). Taurine might be one of the important factors that regulate muscle maturation in the development period. In the processes of muscle hypertrophy including the fusion of myoblast and the formation of myotube, extracellular Ca2+ and Ca2+-dependent calcineurin (Ca2+/calmodulin-dependent phosphatase) signaling are important pathways (De Arcangelis et al. 2005; Semsarian et al. 1999). In the present study, the enhanced effects of taurine on the differentiation of C2C12 cells were cancelled by treatments of Ca2+ chelator nifedipine and calcineurin inhibitor FK-506, and these results supported the role of taurine on the Ca2+-calcineurin pathway in the differentiation process. The enhanced mRNA expression of MCIP1 by nifedipine treatment was significantly decreased by taurine treatment. The MCIP1 expresses primarily in skeletal muscle and inhibits the activity of calcineurin (Rothermel et al. 2000). Because the gene transcription is potently stimulated by activated calcineurin as negative feedback (Yang et al. 2000), it is possible that taurine might suppress the feedback transcription of MCIP1. Furthermore, it is suggested that intracellular, but not external, taurine uptaken from culture medium might affect on the pathway because the taurine transport inhibitor β-alanine treatment and taut gene silencing significantly inhibited the cell differentiation.
5 Conclusion
In summary, the present study shows that exogenous taurine treatment significantly enhanced the differentiation of C2C12 myoblast to myotube, and the significant effect of taurine might be associated with the Ca2+ signaling pathway. This beneficial effect of taurine would possibly contribute to the muscle recovery after damages.
Abbreviations
- TAUT:
-
Taurine transporter
- GM:
-
Growth medium
- DM:
-
Differentiation medium
- MHC:
-
Myosin heavy chain
- MCIP:
-
Myocyte-enriched calcineurin-interacting protein
References
Aerts L, Van Assche FA (2002) Taurine and taurine-deficiency in the perinatal period. J Perinat Med 30:281–286
Airaksinen EM, Paljarvi L, Partanen J, Collan Y, Laakso R, Pentikainen T (1990) Taurine in normal and diseased human skeletal muscle. Acta Neurol Scand 81:1–7
De Arcangelis V, Coletti D, Canato M, Molinaro M, Adamo S, Reggiani C, Naro F (2005) Hypertrophy and transcriptional regulation induced in myogenic cell line L6-C5 by an increase of extracellular calcium. J Cell Physiol 202:787–795
Gossett LA, Kelvin DJ, Sternberg EA, Olson EN (1989) A new myocyte-specific enhancer-binding factor that recognizes a conserved element associated with multiple muscle-specific genes. Mol Cell Biol 9:5022–5033
Heller-Stilb B, van Roeyen C, Rascher K, Hartwig HG, Huth A, Seeliger MW, Warskulat U, Haussinger D (2002) Disruption of the taurine transporter gene (taut) leads to retinal degeneration in mice. FASEB J 16:231–233
Hosokawa Y, Matsumoto A, Oka J, Itakura H, Yamaguchi K (1990) Isolation and characterization of a cDNA for rat liver cysteine dioxygenase. Biochem Biophys Res Commun 168:473–478
Ito T, Kimura Y, Uozumi Y, Takai M, Muraoka S, Matsuda T, Ueki K, Yoshiyama M, Ikawa M, Okabe M, Schaffer SW, Fujio Y, Azuma J (2008) Taurine depletion caused by knocking out the taurine transporter gene leads to cardiomyopathy with cardiac atrophy. J Mol Cell Cardiol 44:927–937
Iwata H, Obara T, Kim BK, Baba A (1986) Regulation of taurine transport in rat skeletal muscle. J Neurochem 47:158–163
Jacobsen JG, Smith LH (1968) Biochemistry and physiology of taurine and taurine derivatives. Physiol Rev 48:424–491
Kaisaki PJ, Jerkins AA, Goodspeed DC, Steele RD (1995) Cloning and characterization of rat cysteine sulfinic acid decarboxylase. Biochim Biophys Acta 1262:79–82
Kook SH, Choi KC, Son YO, Lee KY, Hwang IH, Lee HJ, Chung WT, Lee CB, Park JS, Lee JC (2008) Involvement of p38 MAPK-mediated signaling in the calpeptin-mediated suppression of myogenic differentiation and fusion in C2C12 cells. Mol Cell Biochem 310:85–92
Maeda T, Gupta MP, Stewart AF (2002) TEF-1 and MEF2 transcription factors interact to regulate muscle-specific promoters. Biochem Biophys Res Commun 294:791–797
Naya FJ, Olson E (1999) MEF2: a transcriptional target for signaling pathways controlling skeletal muscle growth and differentiation. Curr Opin Cell Biol 11:683–688
Ramamoorthy S, Leibach FH, Mahesh VB, Han H, Yang FT, Blakely RD, Ganapathy V (1994) Functional characterization and chromosomal localization of a cloned taurine transporter from human placenta. Biochem J 15:893–900
Rothermel B, Vega RB, Yang J, Wu H, Bassel-Duby R, Williams RS (2000) A protein encoded within the Down syndrome critical region is enriched in striated muscles and inhibits calcineurin signaling. J Biol Chem 275:8719–8725
Semsarian C, Wu MJ, Ju YK, Marciniec T, Yeoh T, Allen DG, Harvey RP, Graham RM (1999) Skeletal muscle hypertrophy is mediated by a Ca2+-dependent calcineurin signalling pathway. Nature 400:576–581
Sturman JA (1993) Taurine in development. Physiol Rev 73:119–147
Sturman JA, Messing JM (1991) Dietary taurine content and feline reproduction and outcome. J Nutr 121:1195–1203
Warskulat U, Flogel U, Jacoby C, Hartwig HG, Thewissen M, Merx MW, Molojavyi A, Heller-Stilb B, Schrader J, Haussinger D (2004) Taurine transporter knockout depletes muscle taurine levels and results in severe skeletal muscle impairment but leaves cardiac function uncompromised. FASEB J 18:577–579
Yang J, Rothermel B, Vega RB, Frey N, McKinsey TA, Olson EN, Bassel-Duby R, Williams RS (2000) Independent signals control expression of the calcineurin inhibitory proteins MCIP1 and MCIP2 in striated muscles. Circ Res 87:E61–E68
Acknowledgements
This work was supported in part by Kakenhi grants (21790633) from the Japan Society for the Promotion of Science.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media New York
About this paper
Cite this paper
Miyazaki, T., Honda, A., Ikegami, T., Matsuzaki, Y. (2013). The Role of Taurine on Skeletal Muscle Cell Differentiation. In: El Idrissi, A., L'Amoreaux, W. (eds) Taurine 8. Advances in Experimental Medicine and Biology, vol 776. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-6093-0_29
Download citation
DOI: https://doi.org/10.1007/978-1-4614-6093-0_29
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-6092-3
Online ISBN: 978-1-4614-6093-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)