Abstract
Taurine ameliorates changes occurring in newborn skeletal muscle as a result of gestational protein restriction in C57BL/6 mice, but taurine supplementation effects may be exaggerated in C57BL/6 mice due to their inherent excessive taurinuria.
We examined if maternal taurine supplementation could ameliorate changes in gene expression levels, properties of mitochondria, myogenesis, and nutrient transport and sensing, in male newborn skeletal muscle caused by a maternal low protein (LP) diet in Wistar rats.
LP diet resulted in an 11% non-significant decrease in birth weight, which was not rescued by taurine supplementation (LP-Tau). LP-Tau offspring had significantly lower birth weight compared to controls. Gene expression profiling revealed 895 significantly changed genes, mainly an LP-induced down-regulation of genes involved in protein translation. Taurine fully or partially rescued 32% of these changes, but with no distinct pattern as to which genes were rescued.
Skeletal muscle taurine content in LP-Tau offspring was increased, but no changes in mRNA levels of the taurine synthesis pathway were observed. Taurine transporter mRNA levels, but not protein levels, were increased by LP diet.
Nutrient sensing signaling pathways were largely unaffected in LP or LP-Tau groups, although taurine supplementation caused a decrease in total Akt and AMPK protein levels. PAT4 amino acid transporter mRNA was increased by LP, and normalized by taurine supplementation.
In conclusion, gestational protein restriction in rats decreased genes involved in protein translation in newborn skeletal muscle and led to changes in nutrient transporters. Taurine partly rescued these changes, hence underscoring the importance of taurine in development.
Access provided by CONRICYT-eBooks. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Low birth weight is a hallmark of adverse developmental programming (Hales and Barker 1992) and is associated with a higher risk of developing metabolic disease in later life, including increased risk of impaired insulin secretion (Snoeck et al. 1990; Petrik et al. 1999), and insulin resistance in skeletal muscle (Ozanne et al. 2005). A clear association between low birth weight and insulin resistance has been observed, clearly not with a classical genetic causality, as demonstrated in twin studies (Poulsen et al. 1997). However, the exact mechanism by which low birth weight, as a result of intrauterine growth restriction (IUGR), leads to an increased risk of type 2 diabetes and metabolic syndrome later in life, is still largely unknown.
Skeletal muscle is known to play a key role in whole body metabolism, glucose clearance, and insulin sensitivity. Therefore, a reduction in skeletal muscle mass, and thereby altered muscle myogenesis and function as observed after IUGR, may be causally related to the increased risk of developing metabolic disease in later life (Abdul-Ghani and DeFronzo 2010).
Several animal models have been used to mimic impaired fetal growth (Armitage et al. 2004) including the most studied one, gestational protein restriction. In rats, this model displays a low birth weight of the offspring, impaired glucose homeostasis and insulin resistance (Ozanne et al. 1996, 2005), as well as β-cell dysfunction later in life (Boujendar et al. 2002; Reusens et al. 2008).
Taurine is a semi-essential amino sulphonic acid, which is not a protein component. Taurine is known to be involved in physiological functions such as bile acid formation, intracellular volume regulation, heart function, mitochondrial tRNA conjugation, and reproduction (Lambert et al. 2015).
Human IUGR fetuses have decreased plasma taurine levels (Economides et al. 1989; Cetin et al. 1990), which has also been observed in animal models of IUGR (Reusens et al. 1995; Wu et al. 1998). Taurine deficiency during development and in adult life results in several pathological conditions, including dysfunction of skeletal- and heart muscle (Sturman 1993). Furthermore, the taurine transporter (TauT) knockout mice have revealed the importance of maintaining normal taurine concentrations in tissues, especially in skeletal muscle and heart. In these mice, taurine concentration in skeletal muscle and heart are decreased by 98% compared to wild type mice (Warskulat et al. 2004; Ito et al. 2008, 2014a, b) and the mice exhibit lower body weight, decreased skeletal muscle growth, and lower exercise capacity (Heller-Stilb et al. 2002; Warskulat et al. 2004; Ito et al. 2008). These observations indicate that taurine is very important during development and that taurine deficiency during gestation may lead to adverse developmental programming in skeletal muscle of the offspring.
Maternal taurine supplementation during gestation is, at least partially, able to prevent the effects induced by low birth weight in several animal models of low birth weight and can also prevent the adverse offspring phenotype seen as a consequence of maternal obesity (Li et al. 2013) as well as a maternal high fructose intake (Li et al. 2015). Maternal taurine supplementation has, in the context of gestational protein restriction, been shown to stabilize proliferation and vascularization of the pancreas (Boujendar et al. 2002, 2003) as well as prevent all changes in mRNA expression levels in the pancreas of newborn Wistar rats (Reusens et al. 2008). Likewise, maternal taurine supplementation rescued a large portion of the changes in mRNA expression levels caused by gestational protein restriction in both skeletal muscle and liver (Mortensen et al. 2010a) in newborn C57BL/6J mice. However, the C57BL/6J mouse strain has a defect in taurine renal reabsorption (Harris and Searle 1953) that leads to excessive taurinuria (Chesney et al. 1976). Therefore, the effects of taurine supplementation may be exaggerated when using this animal model.
In the present study, we used gene expression microarrays to examine the effect of maternal taurine supplementation on changes in overall gene expression levels, in newborn Wistar rat hind leg skeletal muscle subjected to gestational protein restriction. Furthermore, we examined if taurine supplementation could ameliorate maternal low protein induced changes in taurine biosynthesis, mitochondrial properties, myogenesis, amino acid transport, and nutrient sensing.
2 Materials and Methods
2.1 Animals and Chemicals
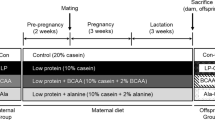
All experimental procedures were approved by The Danish Animal Experiments Inspectorate (license: 2008/561-1515) and were performed according to the ARRIVE (Animal Research: Reporting In vivo Experiments) guidelines. Female Wistar rats (N = 38, 8 weeks of age) were purchased from Taconic Europe A/S (Ejby, Denmark) on day 1 of pregnancy. The animals were housed with a 12 h light/dark cycle, constant temperature (23 °C), and constant humidity (42%) at the University of Copenhagen, Faculty of Health and Medical Sciences, Panum Institute. Unless otherwise stated, chemicals were from Sigma-Aldrich (St. Louis, MO, USA) and were of analytical grade or better.
2.2 Animal Study Design and Diet
The pregnant Wistar dams were randomly assigned to either a low-protein diet (LP, N = 15) with a protein content of 5% or a matching control diet containing 20% protein (CON, N = 15) or an LP diet supplemented with 2% taurine in the drinking water (LP-TAU, N = 8) from day 1 of gestation. Dams were weighed on day 1 of gestation and on day 20 (the day before giving birth). The animals had ad libitum access to food and water. All diets were isocaloric and obtained from Hope Farms, catalog #4400.12 and #4400.00 for 5% and 20% protein, respectively (Woerden, NL). Ten rats were not pregnant or gave birth to very small litters (N of pups <2) and were removed from the study resulting in CON N = 10, LP N = 10, LP-TAU N = 6.
2.3 Animal Sample Collection
At birth, hind leg skeletal muscle was collected from the pups by cutting off the hind legs followed by dissection using a stereomicroscope; the skin was peeled off and the skeletal muscle tissue dissected free of skin and bone after which it was snap-frozen in liquid nitrogen and stored at −80 °C until further analysis. The hind leg skeletal muscle from three male pups from the same dam was pooled resulting in an N of one per dam. Gender was determined visually before dissection by comparing the distance between the anus and the genital papilla (Whishaw and Kolb 2004).
2.4 RNA Purification and Quantitative Real-Time PCR
RNA purification and quantitative real-time PCR were performed as previously described (Larsen et al. 2013). GAPDH mRNA levels were used for normalization between samples. The primers were designed using the National Center for Biotechnology Information “primer design tool” online using Rattus Norvegius (organism 10116) Refseq RNA database and were designed according to the MIQE guidelines. Primer sequences (gene symbol, genbank acession number: Forward & reverse primer 5′-3′): 4f2hc, NM_019283.3: GCTGACATTGTAGCCACCAAA & GCAATCAAAAGCCTGTCCTCA. ADO, NM_001107626.2: CCGGTCACTTACATGCACATC & CGTACAGCACCTTGAGCATAC. ATF4, NM_024403.2: TCAGAATGGCTGGCTATGGAT & AAGCTCATCTGGCATGGTTTC. Cat2a, NM_022619.3: CCTTATCAACAAGTCTTCTGGGTT & GGGTGACTGCCTCTTACTCA. Cat2b, NM_001134686.2: TTCCCAATGCGTCGTGTAATC & GCACCCGATGACAAAGTAGC. CDO, NM_052809.1: GCCTTCACTTGTACAGTCCAC & CTCCAGTGAACCTGAAGTTGTAAAT. CS, NM_130755.1: CACTGTGGACATGATGTACGG & ATACTATAGCCTCGGAAGCGG. CSAD, NM_001134454.1: TGGTCATGGAGCCCAAGTTC & CATCATGGTTCCCTTCTTCACC. GLUT1, NM_138827.1: GTGTATCCTGTTGCCCTTCTG & GCTTTTTCAGCACACTCTTGG. GLUT4, NM_012751.1: ATGGGTTTCCAGTATGTTGCG & TAAGAGAGAAGGTGTCCGTCG. LAT1, NM_017353.1: GGGGAACATTGTGTTGGCATT & TGGGCAAGGAGATGATGATGG. Mef2a, NM_001014035.1: CATAAAATCGCACCTGGCTTG & AACTCCCTGGGTTAGTGTACG. MHCIb, NM_017240.2: CGTTCTGTCAATGACCTCACC & TTTCCTCCTCGTACTGTTCCC. MHCIIa, NM_001135157.1: AACCATTCAGAGCAAAGACGC & GCTCCTGCTTCAGTTTTCACA. MHCIIb, NM_019325.1: ATCAGTGTTTGTGGTGGATGC & TGCACGGTTGCTTTCACATAG. MHCIIx, NM_001135158.1: CTTCAAGTTCAGACCCACGG & GGAGAGTCTGCCTTTAGGGATG. MHCemb, NM_012604.1: CCACAGTCAGAGGTCCCATAG & ATGCCAAACACTTCCATCTCG. MHCper, NM_001100485.1: GTCCTATGTGAAGAGCGTCATAC & TTAACGGTCACTTTCCCTCCT. Myf5, NM_001106783.1: TCAAACGCATGTGCTTCAGATAA & AGATAAGTCTGGAACTGGAGGAC. Myo5a, NM_022178.1: TCCTGAAGAAAAGAGGTGACGA & GGTCAAAATTGGTGAGGCAGT. Myo5b, NM_017083.1: CAGACAAACAGCCAGACTGAG & AGCCGAGTTCTCCATCTTCAT. MyoD1, NM_176079.1: GCCTGAGCAAAGTGAACGA & CAGACCTTCAATGTAGCGGATG. MyoG, NM_017115.2: AGGAAGTCTGTGTCTGTGGAC & GCTCAATGTACTGGATGGCAC. Ndufb6, NM_001106646.1: TGGCACGAAGCCCAGAATA & TCTCCAGTCTCCAGAATTGTATC. Ndufs1, NM_001005550.1: GTGGGAAGTAACATTGTGGTTAG & TGCCGTTTCAGTCCATCATAG. PAT4, NM_001108127.1: CTGTGAGAGGAGAAGTGTGGA & CTATCGGAAGGTTGTGGGGAT. PDK1, NM_053826.2: TTGACTGTGAAGATGAGTGACC & GCCAATCCGTAACCAAATCCA. PDK4, NM_053551.1: ACACATACTCCACTGCTCCAA & TAACCAAAACCAGCCAAAGGG. Pgc1a, NM_031347.1: GAGTACAACAATGAGCCCGC & CAATCCGTCTTCATCCACCG. Pgc1b, NM_176075.2: AAGTAAGAGAGGCCAGAAGCA & ATTGCGTTTTCTCAGGGTAGC. PPARa, NM_013196.1: GCGGACTACCAGTACTTAGGG & GGAGAGAGGGTGTCTGTGATG. PPARg, NM_013124.3: AGAGCCTTCAAACTCCCTCAT & CTTCAATCGGATGGTTCTTCGG. REDD1, NM_080906.2: CATCAGTTCGCTCACCCTTC & GGACGAGAAACGATCCCAAAG. Sirt1, XM_003751934.1: TTCAGAACCACCAAAGCGG & CCCACAGGAAACAGAAACCC. Sirt3, NM_001106313.2: TCTGGTATCCCTGCCTCAAAG & ATCACGTCAGCCCGTATGTC. SNAT1, NM_138832.1: AACTCAAAGACGGTGTACGCT & TGCTCTGGTACTTGTGAAGGA. SNAT2, NM_181090.2: CGAAGGAGGGTCTTTATTATACGAAC & TGACAATGGGAGAATGAGCAC. TauT, NM_017206.1: TGGACAGCCAGTTTGTTGAAG & GCAATGAAGATTTCCCGACGA. TFAM, NM_031326.1: TGACCTTTTGAGCCTTGACAG & CGCACAGTCTTGATTCCAGTT.
2.5 Affymetrix Gene Expression Microarray
For gene expression profiling using GeneChip Rat Gene 1.0 ST Arrays (Affymetrix Inc., Santa Clara, CA, USA), four RNA samples, isolated using Qiazol from hindleg skeletal muscle as described above, were chosen at random from each group and analyzed as previously described (Mortensen et al. 2014) followed by analysis using R 3.2.2 (open source: http://www.r-project.org) and Bioconductor 3.1 (open source: http://www.bioconductor.org) with all subsequent calculations carried out on log2 transformed gene expression values. Raw CEL files were normalized using the Robust Multi-array Average method. The expression level threshold (detection limit) was set to the median of the antigenomic (negative controls) probeset expression levels and calculated for each sample and further analyzed as described in the GEO dataset (see below) resulting in 1224 genes changed by the LP diet, of which 895 were known genes. Out of the 895 changed genes, the changes to 88 genes were fully rescued by taurine and the changes to 195 genes were partially rescued by taurine (for calculations and data, see the GEO dataset).
Overrepresentation of gene sets were estimated using DAVID v6.7 (Huang et al. 2009) with probe IDs for the significantly changed genes as input (either all, down-regulated or up-regulated by LP diet) compared against the list of detected probes examining the categories: GOTERM_BP_FAT, GOTERM_CC_FAT, GOTERM_MF_FAT, PANTHER_BP_ALL, PANTHER_MF_ALL, KEGG_PATHWAY, and PANTHER_PATHWAY.
The raw CEL files as well as the analysis results have been deposited in the NCBIs Gene Expression Omnibus (GEO, http://www.ncbi.nlm.nih.gov/geo) with the GEO accession number GSE89054.
2.6 Biochemical Assays
Biochemical assays measuring citrate synthase (CS) activity, creatine kinase (CK) activity as well as the taurine content of skeletal muscle tissues were carried out as described previously (Mortensen et al. 2006, 2010b; Larsen et al. 2015)
2.7 Western Blot Analysis
Western blot analysis of skeletal muscle tissue was carried out as previously described (Larsen et al. 2013) using antibodies purchased from Cell Signaling Technology: total and phosphorylated S6K1 (Thr389) (cat #9234 and #2708); and phosphorylated AMPKα (Thr172) (cat #2603 and #2535); total and phosphorylated Akt (Thr308 and Ser473) (cat #4691, #2965, and #4060). Protein expression of β-actin (ACTB, cat #Sc-47778) and TauT (#166640) were obtained from Santa Cruz Biotechnology. The antibody REDD1 (cat #10638-1-AP) was obtained from Proteintech, OXPHOS obtained from Abcam (cat#ab-110413). Beta-actin (ACTB) protein levels were used for normalization between samples.
2.8 mtDNA
The mtDNA:gDNA ratio was determined as previously described (Gam et al. 2014) using primers specific for either mitochondrial ND1 (mtDNA) or genomic GAPDH (gDNA). The mtDNA:gDNA ratio was calculated as 2*2^-(CtDloop−CtGAPDH).
2.9 Statistics
All data were analyzed using ANOVA followed by post-hoc tests correcting for multiple testing using Tukey-Kramer using SAS 9.4. All mRNA data were log-transformed before statistical analysis in order to obtain a normal distribution. Data are presented as means ± s.e.m. A p-value less than 0.05 was considered significant. NS denotes non-significance, p > 0.1. A p-value between 0.05 and 0.1 was considered a trend.
3 Results
3.1 Birth Weight, Litter Size, Body Weight, and Food Intake
The LP diet group exhibited an 11% decrease in birth weight, but this was not significantly different from CON. However, LP-TAU was significantly lighter at birth compared to CON. Litter size was similar between groups, but LP dams ate significantly less and also gained 17% less weight during pregnancy compared to CON (Table 1).
3.2 Effect of LP Diet and Taurine Supplementation on Taurine Content, Transport, and Synthesis
Taurine content in newborn skeletal muscle increased in the LP-TAU group compared to both CON and LP with 43% and 40% respectively, as seen in Fig. 1a. Changes in taurine content may alter the expression levels of the enzymes involved in taurine biosynthesis (cysteinesulfinic acid decarboxylase (CSAD), cysteine dioxygenase (CDO), and cysteamine dioxygenase (ADO)). However, we found no difference in the mRNA levels of these genes between groups (Fig. 1b). Although the TauT mRNA levels increased 42% in LP compared to CON, the protein levels of TauT were similar between groups (Fig. 1b).
Taurine concentration, transport and biosynthesis in skeletal muscle of newborn male rats subjected to maternal LP diet with 2% taurine in drinking water. (a) Taurine content in newborn skeletal muscle (ANOVA p = 0.012). (b) Taurine transport and synthesis: Protein levels of TauT (ANOVA NS) and mRNA levels of TauT (ANOVA p = 0.03), CDO (ANOVA NS), CSAD (ANOVA NS), ADO (ANOVA NS). Numbers are mean ± SEM. a.u. (arbitrary units). *Denotes a significant difference in the Tukey-Kramer post hoc test between the indicated groups
3.3 Mitochondrial Properties
The activity of CS tended to increase in LP-TAU compared to CON as shown in Fig. 2a. Transcriptional factor A, mitochondrial (TFAM) mRNA levels decreased 20% in LP-TAU compared to CON (Fig. 2b). LP diet decreased NADH Dehydrogenase (Ubiquinone) Fe–S Protein 1 (Ndufs1) mRNA levels with 19% compared to CON, with no effect on NADH Dehydrogenase (Ubiquinone) 1 beta subcomplex, 6 (Ndufb6) as shown in Fig. 2b. In contrast, CS, pyruvate dehydrogenase kinase, isozyme 4 (PDK4), peroxisome proliferator-activated receptor gamma, coactivator-1α (PGC-1α), PGC-coactivator-1β (PGC-1β), peroxisome proliferator activated receptor α (PPARα) and γ (PPARγ) mRNA levels were similar between groups (Fig. 2b).
Mitochondrial properties in skeletal muscle of newborn male rats subjected to maternal LP diet with 2% taurine in drinking water. (a) CS activity (ANOVA NS) in newborn skeletal muscle. (b) mRNA levels of CS (ANOVA NS), PDK4 (ANOVA NS), NNDUFb6 (ANOVA NS), NDUFs1 (ANOVA p = 0.02) and TFAM (ANOVA p = 0.03), of PGC-1α (ANOVA NS), PGC-1β (ANOVA NS), PPARα (ANOVA NS), PPARγ (ANOVA NS), Sirt1 (ANOVA NS), Sirt3 (ANOVA NS). (c) Protein levels of mitochondrial complex I, II, III and V (ANOVA NS for all). (d) mtDNA:gDNA levels (ANOVA p = 0.02). Numbers are mean ± SEM. a.u. (arbitrary units). *Denotes a significant difference in the Tukey-Kramer post hoc test between the indicated groups
We also measured the protein levels of the complexes in the respiratory chain, but did not see any significant differences between groups (Fig. 2c).
mtDNA (ND1) copy number relative to gDNA were significantly increased by taurine supplementation compared to LP alone—thereby indicating a possible rescue effect of taurine on mtDNA content (Fig. 2d).
3.4 Gene Expression Profiling
The LP diet caused changes in gene expression levels in 895 known genes, with 225 being down-regulated and 670 being up-regulated (Fig. 3a). Taurine supplementation resulted in a partial or full rescue of the LP-induced changes in gene expression levels of 283 known genes (88 fully rescued, 195 partially rescued), corresponding to a 32% rescue (Fig. 3b).
Skeletal muscle gene expression profiling of newborn male rats subjected to maternal LP diet with 2% taurine in drinking water. Hierarchical clustering of genes. (a) Genes changed by maternal LP diet (895 known genes). (b) Genes rescued by taurine (283 genes, 88 fully rescued and 195 partially rescued)
When we examined gene-set over-representation in all the genes changed by LP diet (e.g. both up- and down-regulated by LP) we found that mainly genes involved in protein synthesis were changed (Table 2). When we only examined the genes that were down-regulated by the LP diet, we found that besides changes in genes involved in protein synthesis, there were also changes in genes involved in the response to glucose stimulus (Table 3). Interestingly, when we only examined the genes up-regulated by the LP diet we found no over-representation of genes involved in protein synthesis, but instead we observed changes in cell-communication (Table 4). We found no evidence of taurine rescuing any specific gene sets.
3.5 Changes in System A and PAT Amino Acid Transporters Induced by LP and Taurine
As the gene expression profiling pointed towards major changes in protein translation in newborn skeletal muscle, as an effect of the maternal LP diet, we wondered if the LP diet might have affected amino acid- and glucose transporters in newborn skeletal muscle and measured mRNA levels of several amino acid and glucose transporter systems (System A—sodium-coupled neutral amino acid transporters (SNAT), System L—large neutral amino acid transporters (LAT), PAT—proton-assisted amino acid transporters, CAT—cationic amino acid transporters, GLUT1, and GLUT4—glucose transporters) (Fig. 4).
Nutrient transporters in skeletal muscle of newborn male rats subjected to maternal LP diet with 2% taurine in drinking water. The graph shows mRNA levels of amino acid and glucose transporters: System L; 4F2HC (ANOVA NS), LAT1 (ANOVA NS). System A; SNAT1 (ANOVA p = 0.06), SNAT2 (ANOVA p = 0.002), SNAT4 (ANOVA NS). PAT; PAT1 (ANOVA NS), PAT2 (ANOVA p = 0.07), PAT4 (ANOVA p = 0.0004). CAT; CAT2a (ANOVA NS), CAT2b (ANOVA NS). Glucose transporters; GLUT1 (ANOVA NS), GLUT4 (ANOVA NS). Numbers are mean ± SEM. a.u. (arbitrary units). *Denotes a significant difference in the Tukey-Kramer post hoc test between the indicated groups
In system A, SNAT1 tended to decrease with LP-TAU compared to CON. SNAT2 mRNA levels were significantly lower in the LP-TAU group compared to both LP and CON. SNAT4 mRNA levels were similar between groups. In system L, 4f2hc and LAT1 mRNA levels between groups were similar. LAT2 mRNA levels were not detectable by qPCR. PAT1 mRNA levels were similar between groups. PAT2 mRNA levels tended to decrease with LP diet. PAT4 mRNA levels increased 18% when comparing LP to CON, whereas taurine ameliorated this effect—indicating a rescue effect of taurine. However, Cat2a and Cat2b mRNA levels were similar between groups, whereas Cat1 mRNA could not be detected by qPCR. Furthermore, both GLUT1 and GLUT4 mRNA levels were similar between groups (Fig. 4).
3.6 Taurine Supplementation Decreased Both Total AKT and Total AMPK Protein Levels
Due to the changes in both protein translation and amino acid transporters we hypothesized that these changes may be associated with changes in some of the major nutrient sensing signaling pathways.
Phosphoenolpyruvate carboxykinase 1 (PDK1) mRNA and activating transcription factor 4 (ATF4) levels were similar between groups (Fig. 5a). Phosphorylation of AKT p-Thr308 was also similar between groups both when normalized to β-actin and total AKT. Phosphorylation of AKT p-Ser473 tended to increase with LP diet compared to CON when normalized to β-actin and total AKT. Surprisingly, total AKT protein levels decreased in LP-TAU compared to both LP and CON (Fig. 5b).
Nutrient-sensing pathways in skeletal muscle of newborn male rats subjected to maternal LP diet with 2% taurine in drinking water. (a) PDK1, ATF4, and REDD1 mRNA levels and REDD1 protein levels (ANOVA NS for all). (b) Protein levels of AKT p-Thr308 (ANOVA NS) and p-Ser473 NS (ANOVA p = 0.07) and total AKT (ANOVA p = 0.02), AMPK p-Thr172 (ANOVA NS), total AMPK (ANOVA p = 0.02), S6K1 p-Thr389 (ANOVA NS) and total S6K1 (ANOVA NS). Numbers are mean ± SEM. a.u. (arbitrary units). *Denotes a significant difference in the Tukey-Kramer post hoc test between the indicated groups
As shown in Fig. 5b, AMPK p-Thr172 phosphorylation, when normalized to β-actin or total AMPK, was similar between groups. However, total AMPK protein levels also decreased significantly in LP-TAU compared to both LP and CON.
Both DNA-Damage-Inducible Transcript 4 (REDD1) mRNA levels and protein levels were similar between groups (Fig. 5a). Ribosomal Protein S6 Kinase (S6 K1) p-Thr389 phosphorylation tended to increase in LP-TAU compared to LP, when normalized to both β-actin and total S6k1. Total S6k1 protein levels were similar between groups (Fig. 5b).
3.7 Markers of Myogenesis were Unaffected by LP Diet or Taurine Supplementation
The previously discovered LP-induced changes, as well as the observed amelioration by taurine, may have affected changes in skeletal muscle growth and differentiation, therefore, we investigated different markers of myogenesis.
The activity of CK was similar between groups as shown in Fig. 6a. Different markers were measured by qPCR and Myogenin, Myosin heavy chain (MHC) IIX, MHCemb, MHCper, MHCIb, MHCIIa, MHCIIb, Myogenic factor 5 (Myf5), Myosin (Myo5a + 5b), and Myocyte enhancer factor (Mef) 2a mRNA levels were all similar between groups (Fig. 6b). However, Myogenic differentiation 1 (MyoD) mRNA levels tended to decrease with LP diet when taurine was supplemented—indicating some effect of taurine. Mef2d mRNA levels tended to increase with LP diet compared to CON with no effect of taurine (Fig. 6b).
Myogenesis in skeletal muscle of newborn male rats subjected to maternal LP diet with 2% taurine in drinking water. (a) Creatine kinase enzymatic activity (ANOVA NS). (b) mRNA levels of markers of myogenesis; MHCemb, MHCper, MHCIb, MHCIIa, MHCIIb, MHCIIX, myogenin, MyoD (ANOVA p = 0.07), Mef2a, Mef2d (ANOVA p = 0.04), Myo5a, Myo5b, Myf5 (ANOVA NS unless stated otherwise). Numbers are mean ± SEM. a.u. (arbitrary units)
4 Discussion
The aim of this study was to evaluate the effects of taurine supplementation during maternal protein restriction on skeletal muscle of male newborn offspring, with respect to overall gene expression changes, taurine transport and biosynthesis, mitochondrial properties, nutrient transporters and signaling sensing pathways, and fetal skeletal muscle growth and differentiation.
We found that LP-Tau had a lower birth weight compared to CON. Yet maternal protein restriction alone (LP vs. CON) did not lead to a significant decrease in birth weight in the male offspring (Table 1).
Low birth weight is a hallmark feature of developmental programming (Hales and Barker 1992) and maternal protein restriction induced low birth weight has been demonstrated in several animal models (Boujendar et al. 2002; Merezak et al. 2004; Mortensen et al. 2010a). In the present study, however, the low birth weight phenotype was worsened by taurine supplementation. In C57BL/6J mice, maternal low protein diet resulted in a 40% lower birth weight compared to control offspring, and taurine prevented half of the decrease (Mortensen et al. 2010a). The effect of taurine on birth weight in the present study might be caused by differences in taurine homeostasis between species. It is well known, as mentioned, that the mouse strain C57BL/6J has a defect in taurine renal reabsorption (Harris and Searle 1953; Chesney et al. 1976). The rescue effect of taurine in C57BL/6 mice could possibly be interpreted as compensation for this renal absorption defect or as a reflection of species differences.
In the present study, taurine content increased significantly in skeletal muscles of the taurine supplemented group compared to control (Fig. 1c). Furthermore, earlier studies have shown that taurine concentration in plasma decreases with intrauterine growth restriction (Economides et al. 1989; Cetin et al. 1990; Reusens et al. 1995; Wu et al. 1998). However, we saw no effect on taurine content of intrauterine protein restriction in skeletal muscle. To our knowledge, this is the first study to examine the taurine level in newborn skeletal muscle, whereas other studies examined the taurine levels in newborn plasma. Alterations in taurine biosynthesis and transport have been observed with different maternal diet insults (Bagley and Stipanuk 1994; Bella et al. 1999; Tsuboyama-Kasaoka et al. 2006; Stipanuk et al. 2009). In the present study, we saw no changes in mRNA levels of enzymes from the taurine synthesis pathway. Although TauT mRNA levels were increased by gestational protein restriction, the protein levels were unaffected.
Microarray gene expression analysis of newborn skeletal muscle revealed significant changes in 895 genes (Fig. 3a). Taurine fully or partially rescued 32% of these changes, but with no distinct pattern as to which genes (pathways) were rescued (Fig. 3b). In mice, we have previously demonstrated that taurine supplementation rescued a large portion of the changes in mRNA expression levels in both skeletal muscle and liver caused by maternal protein restriction (Mortensen et al. 2010a). In newborn skeletal muscle, a large fraction of genes involved in amino acid metabolism, protein synthesis, TCA cycle, and energy metabolism genes involved in both Complex I–IV and ATP synthesis showed decreased expression in mice offspring subjected to gestational protein restriction (Mortensen et al. 2010a). In the present study, low protein diet also decreased the mRNA levels Ndufs1 in Complex I and microarray gene expression revealed a decrease in genes involved in protein synthesis, protein translation and genes involved in the response to glucose stimulus (Fig. 2 and Tables 2 and 3).
Interestingly, Reusens et al., showed that taurine supplementation prevented all changes in mRNA expression levels of tricarboxylic acid (TCA) cycle and ATP production in the pancreas in newborn rats caused by gestational protein restriction (Reusens et al. 2008).
Taurine is a constituent of mitochondrial tRNA (Suzuki et al. 2002) and might be essential for normal mitochondrial function. Thus, studies of fetal gene expression profile in pancreas, skeletal muscle and liver suggest that there may be a mitochondrial component in the rescue effect of taurine, due to the distinct pattern of genes that were rescued by taurine supplementation (Reusens et al. 2008; Mortensen et al. 2010a). In the present study, we found no evidence of taurine rescuing in any specific gene sets and we did not see major changes in mitochondrial gene expression as an effect of the LP diet. However, we did observe an increase in mtDNA in offspring of taurine supplemented rats with gestational protein restriction, compared to gestational protein restriction alone, with no difference between CON and LP-TAU. Furthermore, we saw no difference between groups in CS activity, which can be considered a measure of mitochondrial mass (Larsen et al. 2012). In contrast, Park et al., reported a decrease in skeletal muscle mtDNA in gestational protein restricted 5-week old rats (Park et al. 2004).
The concept that intrauterine growth restriction may impair myogenesis has been demonstrated many years ago and has been studied in a variety of species. Effects such as impaired or limited myoblast cell cycle activity, reduced number of myonuclei, and reduced amount of myofibers in the fetus have been reported (Wilson et al. 1988; Dwyer and Stickland 1992; Prakash et al. 1993; Dwyer et al. 1995; Greenwood et al. 1999; Osgerby et al. 2002; Bayol et al. 2004; Costello et al. 2008). In contrast, in the present study, protein restriction throughout gestation had no significant impact on the different markers of myogenesis we measured, other than a trend in MyoD mRNA level towards a more mature myogenesis with taurine supplementation. Furthermore, nutrient sensing pathways were largely unaffected by both gestational protein restriction and taurine supplementation, despite changes seen in system A and PAT amino acid transporters. Taurine supplementation, however, did cause an unexpected decrease in total Akt and AMPK protein levels indicating a possible effect of taurine on growth, insulin signaling, and nutrient sensing.
5 Conclusion
In conclusion, gestational protein restriction in Wistar rats decreased expression of genes involved in protein translation in newborn skeletal muscle and led to a decrease in nutrient transporters in system A and PAT. Taurine was able to rescue parts of these effects, but not as systematic as in C57BL/6J mice. However, the present study further solidifies the importance of taurine availability in fetal development.
Abbreviations
- ACTB:
-
Beta-actin
- ADO:
-
Cysteamine dioxygenase
- ATF4:
-
Activating transcription factor 4
- CDO:
-
Cysteine dioxygenase
- CK:
-
Creatine kinase
- CON:
-
Control
- CS:
-
Citrate synthase
- CSAD:
-
Cysteinesulfinic acid decarboxylase
- GAPDH:
-
Glyceraldehyde-3-Phosphate Dehydrogenase
- gDNA:
-
Genomic DNA
- GLUT:
-
Glucose transporter
- IUGR:
-
Intrauterine growth restriction
- LP:
-
Low protein
- LP-TAU:
-
Low protein diet with taurine supplementation
- MEF:
-
Myocyte enhancer factor
- MHC:
-
Myosin heavy chain
- mtDNA:
-
Mitochondrial DNA
- Myf5:
-
Myogenic factor 5
- Myo:
-
Myosin
- MyoD:
-
Myogenic differentiation 1
- Ndufb6:
-
NADH Dehydrogenase (Ubiquinone) 1 beta subcomplex 6
- Ndufs1:
-
NADH Dehydrogenase (Ubiquinone) Fe–S Protein 1
- PDK1:
-
Phosphoenolpyruvate carboxykinase 1
- PDK4:
-
Pyruvate dehydrogenase kinase 4
- PGC-1α:
-
Peroxisome proliferator-activated receptor γ, coactivator-1α
- PGC-1β:
-
Peroxisome proliferator-activated receptor γ, coactivator-1β
- PPARα:
-
Peroxisome proliferator activated receptor α
- PPARγ:
-
Peroxisome proliferator activated receptor γ
- REDD1:
-
DNA-Damage-Inducible Transcript 4 (DDIT4)
- S6K1:
-
Ribosomal Protein S6 Kinase
- TauT:
-
Taurine transporter
- TFAM:
-
Transcriptional factor A, mitochondrial
References
Abdul-Ghani MA, DeFronzo RA (2010) Pathogenesis of insulin resistance in skeletal muscle. J Biomed Biotechnol 2010:476279. doi:10.1155/2010/476279
Armitage JA, Khan IY, Taylor PD et al (2004) Developmental programming of the metabolic syndrome by maternal nutritional imbalance: how strong is the evidence from experimental models in mammals? J Physiol Lond 561:355–377. doi:10.1113/jphysiol.2004.072009
Bagley PJ, Stipanuk MH (1994) The activities of rat hepatic cysteine dioxygenase and cysteinesulfinate decarboxylase are regulated in a reciprocal manner in response to dietary casein level. J Nutr 124:2410–2421
Bayol S, Jones D, Goldspink G, Stickland NC (2004) The influence of undernutrition during gestation on skeletal muscle cellularity and on the expression of genes that control muscle growth. Br J Nutr 91:331–339. doi:10.1079/BJN20031070
Bella DL, Hahn C, Stipanuk MH (1999) Effects of nonsulfur and sulfur amino acids on the regulation of hepatic enzymes of cysteine metabolism. Am J Phys 277:E144–E153
Boujendar S, Reusens B, Merezak S et al (2002) Taurine supplementation to a low protein diet during foetal and early postnatal life restores a normal proliferation and apoptosis of rat pancreatic islets. Diabetologia 45:856–866
Boujendar S, Arany E, Hill D et al (2003) Taurine supplementation of a low protein diet fed to rat dams normalizes the vascularization of the fetal endocrine pancreas. J Nutr 133:2820–2825
Cetin I, Corbetta C, Sereni LP et al (1990) Umbilical amino acid concentrations in normal and growth-retarded fetuses sampled in utero by cordocentesis. Am J Obstet Gynecol 162:253–261
Chesney RW, Scriver CR, Mohyuddin F (1976) Localization of the membrane defect in transepithelial transport of taurine by parallel studies in vivo and in vitro in hypertaurinuric mice. J Clin Invest 57:183–193. doi:10.1172/JCI108258
Costello PM, Rowlerson A, Astaman NA et al (2008) Peri-implantation and late gestation maternal undernutrition differentially affect fetal sheep skeletal muscle development. J Physiol Lond 586:2371–2379. doi:10.1113/jphysiol.2008.150987
Dwyer CM, Stickland NC (1992) Does the anatomical location of a muscle affect the influence of undernutrition on muscle fibre number? J Anat 181(Pt 2):373–376
Dwyer CM, Madgwick AJ, Ward SS, Stickland NC (1995) Effect of maternal undernutrition in early gestation on the development of fetal myofibres in the guinea-pig. Reprod Fertil Dev 7:1285–1292
Economides DL, Nicolaides KH, Gahl WA et al (1989) Plasma amino acids in appropriate- and small-for-gestational-age fetuses. Am J Obstet Gynecol 161:1219–1227
Gam CMBF, Mortensen OH, Qvortrup K et al (2014) Effect of high-fat diet on rat myometrium during pregnancy-isolated myometrial mitochondria are not affected. Pflugers Arch. doi:10.1007/s00424-014-1599-7
Greenwood PL, Slepetis RM, Hermanson JW, Bell AW (1999) Intrauterine growth retardation is associated with reduced cell cycle activity, but not myofibre number, in ovine fetal muscle. Reprod Fertil Dev 11:281–291
Hales CN, Barker DJ (1992) Type 2 (non-insulin-dependent) diabetes mellitus: the thrifty phenotype hypothesis. Diabetologia 35:595–601
Harris H, Searle AG (1953) Urinary amino-acids in mice of different genotypes. Ann Eugenics 17:165–167
Heller-Stilb B, van Roeyen C, Rascher K et al (2002) Disruption of the taurine transporter gene (taut) leads to retinal degeneration in mice. FASEB J 16:231–233. doi:10.1096/fj.01-0691fje
Huang DW, Sherman BT, Zheng X et al (2009) Extracting biological meaning from large gene lists with DAVID. Curr Protoc Bioinformatics Chapter 13:Unit 13.11. doi:10.1002/0471250953.bi1311s27
Ito T, Kimura Y, Uozumi Y et al (2008) Taurine depletion caused by knocking out the taurine transporter gene leads to cardiomyopathy with cardiac atrophy. J Mol Cell Cardiol 44:927–937. doi:10.1016/j.yjmcc.2008.03.001
Ito T, Yoshikawa N, Inui T et al (2014a) Tissue depletion of taurine accelerates skeletal muscle senescence and leads to early death in mice. PLoS One 9:e107409. doi:10.1371/journal.pone.0107409
Ito T, Yoshikawa N, Schaffer SW, Azuma J (2014b) Tissue taurine depletion alters metabolic response to exercise and reduces running capacity in mice. J Amino Acids 2014:964680. doi:10.1155/2014/964680
Lambert IH, Kristensen DM, Holm JB, Mortensen OH (2015) Physiological role of taurine—from organism to organelle. Acta Physiol (Oxford) 213:191–212. doi:10.1111/apha.12365
Larsen S, Nielsen J, Hansen CN et al (2012) Biomarkers of mitochondrial content in skeletal muscle of healthy young human subjects. J Physiol Lond 590:3349–3360. doi:10.1113/jphysiol.2012.230185
Larsen LH, Ørstrup LKH, Hansen SH et al (2013) The effect of long-term taurine supplementation and fructose feeding on glucose and lipid homeostasis in Wistar rats. Adv Exp Med Biol 776:39–50. doi:10.1007/978-1-4614-6093-0_5
Larsen LH, Ørstrup LKH, Hansen SH et al (2015) Fructose feeding changes taurine homeostasis in wistar rats. Adv Exp Med Biol 803:695–706. doi:10.1007/978-3-319-15126-7_55
Li M, Reynolds CM, Sloboda DM et al (2013) Effects of taurine supplementation on hepatic markers of inflammation and lipid metabolism in mothers and offspring in the setting of maternal obesity. PLoS One 8:e76961. doi:10.1371/journal.pone.0076961
Li M, Reynolds CM, Sloboda DM et al (2015) Maternal taurine supplementation attenuates maternal fructose-induced metabolic and inflammatory dysregulation and partially reverses adverse metabolic programming in offspring. J Nutr Biochem 26:267–276. doi:10.1016/j.jnutbio.2014.10.015
Merezak S, Reusens B, Renard A et al (2004) Effect of maternal low-protein diet and taurine on the vulnerability of adult Wistar rat islets to cytokines. Diabetologia 47:669–675
Mortensen OH, Frandsen L, Schjerling P et al (2006) PGC-1alpha and PGC-1beta have both similar and distinct effects on myofiber switching toward an oxidative phenotype. Am J Physiol Endocrinol Metab 291:E807–E816
Mortensen OH, Olsen HL, Frandsen L et al (2010a) Gestational protein restriction in mice has pronounced effects on gene expression in newborn offspring’s liver and skeletal muscle; protective effect of taurine. Pediatr Res 67:47–53. doi:10.1203/PDR.0b013e3181c4735c
Mortensen OH, Olsen HL, Frandsen L et al (2010b) A maternal low protein diet has pronounced effects on mitochondrial gene expression in offspring liver and skeletal muscle; protective effect of taurine. J Biomed Sci 17(Suppl 1):S38. doi:10.1186/1423-0127-17-S1-S38
Mortensen OH, Larsen LH, Ørstrup LKH et al (2014) Developmental programming by high fructose decreases phosphorylation efficiency in aging offspring brain mitochondria, correlating with enhanced UCP5 expression. J Cereb Blood Flow Metab 34:1205–1211. doi:10.1038/jcbfm.2014.72
Osgerby JC, Wathes DC, Howard D, Gadd TS (2002) The effect of maternal undernutrition on ovine fetal growth. J Endocrinol 173:131–141
Ozanne SE, Smith GD, Tikerpae J, Hales CN (1996) Altered regulation of hepatic glucose output in the male offspring of protein-malnourished rat dams. Am J Phys 270:E559–E564
Ozanne SE, Jensen CB, Tingey KJ et al (2005) Low birthweight is associated with specific changes in muscle insulin-signalling protein expression. Diabetologia 48:547–552. doi:10.1007/s00125-005-1669-7
Park HK, Jin CJ, Cho YM et al (2004) Changes of mitochondrial DNA content in the male offspring of protein-malnourished rats. Ann N Y Acad Sci 1011:205–216
Petrik J, Reusens B, Arany E et al (1999) A low protein diet alters the balance of islet cell replication and apoptosis in the fetal and neonatal rat and is associated with a reduced pancreatic expression of insulin-like growth factor-II. Endocrinology 140:4861–4873. doi:10.1210/endo.140.10.7042
Poulsen P, Vaag AA, Kyvik KO et al (1997) Low birth weight is associated with NIDDM in discordant monozygotic and dizygotic twin pairs. Diabetologia 40:439–446
Prakash YS, Fournier M, Sieck GC (1993) Effects of prenatal undernutrition on developing rat diaphragm. J Appl Physiol 75:1044–1052
Reusens B, Dahri S, Snoech A et al (1995) Long-term consequences of diabetes and its complications may have a fetal origin: experimental and epidemiological evidence. Nestle Nutr Workshop Ser 35:187–198
Reusens B, Sparre T, Kalbe L et al (2008) The intrauterine metabolic environment modulates the gene expression pattern in fetal rat islets: prevention by maternal taurine supplementation. Diabetologia 51:836–845. doi:10.1007/s00125-008-0956-5
Snoeck A, Remacle C, Reusens B, Hoet JJ (1990) Effect of a low protein diet during pregnancy on the fetal rat endocrine pancreas. Biol Neonate 57:107–118
Stipanuk MH, Ueki I, Dominy JE et al (2009) Cysteine dioxygenase: a robust system for regulation of cellular cysteine levels. Amino Acids 37:55–63. doi:10.1007/s00726-008-0202-y
Sturman JA (1993) Taurine in development. Physiol Rev 73:119–147
Suzuki T, Suzuki T, Wada T et al (2002) Taurine as a constituent of mitochondrial tRNAs: new insights into the functions of taurine and human mitochondrial diseases. EMBO J 21:6581–6589
Tsuboyama-Kasaoka N, Shozawa C, Sano K et al (2006) Taurine (2-aminoethanesulfonic acid) deficiency creates a vicious circle promoting obesity. Endocrinology 147:3276–3284. doi:10.1210/en.2005-1007
Warskulat U, Flögel U, Jacoby C et al (2004) Taurine transporter knockout depletes muscle taurine levels and results in severe skeletal muscle impairment but leaves cardiac function uncompromised. FASEB J 18:577–579. doi:10.1096/fj.03-0496fje
Whishaw IQ, Kolb B (2004) The behavior of the laboratory rat: a handbook with tests. Oxford University Press, Oxford
Wilson SJ, Ross JJ, Harris AJ (1988) A critical period for formation of secondary myotubes defined by prenatal undernourishment in rats. Development 102:815–821
Wu G, Pond WG, Ott T, Bazer FW (1998) Maternal dietary protein deficiency decreases amino acid concentrations in fetal plasma and allantoic fluid of pigs. J Nutr 128:894–902
Acknowledgments
We thank Mrs. Bettina Starup Mentz and Mrs. Lis Frandsen, Section for Cellular and Metabolic Research, Dept. of Biomedical Sciences, University of Copenhagen, for expert technical assistance during the conductance of the experiments. This research was supported by Novo Nordisk Fonden, and The Danish Medical Research Council grant #271-07-0732.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer Science+Business Media B.V.
About this paper
Cite this paper
Larsen, L.H., Sandø-Pedersen, S., Ørstrup, L.K.H., Grunnet, N., Quistorff, B., Mortensen, O.H. (2017). Gestational Protein Restriction in Wistar Rats; Effect of Taurine Supplementation on Properties of Newborn Skeletal Muscle. In: Lee, DH., Schaffer, S.W., Park, E., Kim, H.W. (eds) Taurine 10. Advances in Experimental Medicine and Biology, vol 975. Springer, Dordrecht. https://doi.org/10.1007/978-94-024-1079-2_34
Download citation
DOI: https://doi.org/10.1007/978-94-024-1079-2_34
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-024-1077-8
Online ISBN: 978-94-024-1079-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)