Abstract
Taurine (2-aminoethanesulfonic acid), a sulfur-containing β-amino acid, is found in all animal cells at millimolar concentrations and has been reported to show various health promoting activities including antidiabetic properties. The beneficial effects of taurine in diabetes mellitus have been known. However, the exact mechanism of hypoglycemic action of taurine is not properly defined. In this study, we investigated antidiabetic effect of taurine in the cell culture system using rat skeletal muscle cells. In cultured rat skeletal L6 myotubes, we studied the effect of taurine (0–100 μM) on glucose uptake to plasma membrane from the aspects of AMP-activated protein kinase (AMPK) signaling. Taurine stimulated glucose uptake in a dose-dependent manner by activating AMPK signaling. From these results, it may suggest that taurine show antidiabetic effect by stimulating insulin-independent glucose uptake in rat skeletal muscle.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
- Glucose Uptake
- Wako Pure Chemical Industry
- Antidiabetic Effect
- Increase Glucose Uptake
- Stimulate Glucose Uptake
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Taurine, a sulfur containing beta amino acid, is present in most animal tissues and is essential for the normal functioning of several organs (Brosnan and Brosnan2006). It has been reported that taurine exhibits antioxidative properties, controls blood pressure, membrane stabilizing effect, regulates intracellular Ca2+concentration, inhibits apoptosis, and reduces the levels of pro-inflammatory cytokines in various organs (Aerts and Van Assche2002; Racasan et al.2004; Sinha et al.2007; Das et al.2008,2009; Manna et al.2010). Moreover, taurine is found at high concentrations inside glucagon and somatostatin-containing cells in the pancreatic islets and increases insulin secretion, sensitivity and glucose uptake in different experimental conditions (Cherif et al.1998; De la Puerta et al.2010).
Diabetes is the most common and serious metabolic disease. Several trials have been conducted to reduce the hyperglycemia (Moller2001). Chang (2000) reported that dietary supplementation with taurine was shown to protect pancreatic β-cells in the streptozotocin model of type 1 diabetes. On the other hand, it has been proven that diabetes is associated with a decrease in the levels of endogenous antioxidants, particularly of taurine, so that oxidative damage may be enhanced by the deficiency of taurine, since it frequently becomes depleted in diabetic states (Schaffer et al.2009). The skeletal muscles which account for the majority of insulin-mediated glucose uptake in the postprandial state play an important role in maintaining glucose homeostasis (Saltiel and Kahn2001). In skeletal muscle, especially, insulin increases glucose uptake via a signaling that leads to activation of phosphatidylinositol-3 kinase (PI3K) and AKt, resulting in increased translocation of glucose transporter 4 (GLUT4) to the plasma membrane (Saltiel and Kahn2001). In mammalian cells, the AMP-activated protein kinase (AMPK), which is another GLUT4 translocation promoter, acts as an energy sensor and is activated by an increase in AMP/ATP ratio, by exercise/contraction, and by several compounds including metformin and thiazolidinedione, resulting in stimulation of glucose uptake in skeletal muscles (Zou et al.2004; Towler and Hardie2007). Although a number of studies concerning the effect of taurine on diabetes or hyperglycemia have been reported, little has been validated the effect of taurine on glucose uptake using L6 myotubes. Therefore, this study was conducted to investigate the effects of taurine on glucose uptake in vitro using L6 myotubes and to clarify the mechanisms associated with the enhanced glucose uptake.
2 Methods
2.1 Materials
L6 myoblast cells derived from a rat were purchased from American Type Culture Collection (Rockville, MD, USA; ATCC numbers: CRL-1458). Taurine was obtained from Donga Pharm. (Seoul, Korea). The following items were purchased from the cited commercial sources: Glucose CII Test Kit and compound C from Wako Pure Chemical Industries Ltd. (Osaka, Japan); Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum (FBS) and Gö6983 from Sigma (St. Louis, MO, USA); anti-phospho-AMPK and anti-AMPK antibodies from Cell Signaling Technology (Beverly, MA, USA); horseradish-peroxidase conjugated anti-mouse and anti-rabbit IgG antibodies from Invitrogen (San Diego, CA, USA); ECL Plus Western blotting detection reagents and Hybond ECL nitrocellulose membrane from GE Healthcare (Buckinghamshire, UK).
2.2 Culture of L6 Myoblast Cells
L6 myoblast cells were cultured in DMEM containing 10% (vol/vol) FBS, penicillin G (100 U/ml), and streptomycin (100 μg/ml) in a humidified 5% CO2incubator at 37°C. To differentiate into myotubes, the myoblast cells (5 × 104or 7 × 105) were seeded in Falcon 24-place multiwell plates or 60-mM culture dishes and cultured to 90% confluency in DMEM containing 2% FBS for 1 week.
2.3 Determination of Glucose Uptake by Cultured Rat Skeletal L6 Myotubes
Briefly, perfused L6 myoblast cells were subcultured into Falcon 24-place multiwall plates at 5 × 104cells/well and grown for 11 days in 0.4 ml of 2% FBS/DMEM to allow the formation of myotubes. The medium was renewed every 2 days. Subsequently, the 11-day-old myotubes were incubated in filter-sterilized Krebs–Henseleit buffer (141 mg/l MgSO4, 160 mg/l KH2PO4, 350 mg/l KCl, 6,900 mg/l NaCl, 373 mg/l CaCl2·2H2O, 2,100 mg/l NaHCO3, pH 7.4) containing 0.1% bovine serum albumin, 10 mM Hepes, and 2 mM sodium pyruvate (KHH buffer) for 2 h. The myotubes were then cultured for 4 h in KHH buffer containing 11 mM glucose with or without taurine (10–100 μM) and with or without 100 nM insulin or 5 μM compound C, an AMPK inhibitor. The differences in the glucose concentrations in the KHH buffer before and after culture were determined by the absorbance at 505 nM using a microplate reader (Model AD200; Beckman Coulter, Brea, CA, USA) and the Glucose CII Test Kit. The amounts of glucose consumed were calculated.
2.4 Western Blot Analysis
Rat skeletal L6 myotubes were solubilized in a lysis buffer [10 mM Tris–HCl pH 7.4, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate (SDS), 0.5 mM dithiothreitol, 0.2 mg/ml Pefabloc SC, 1 mM Na3VO4] for 30 min at 4°C. The lysates were then sonicated for 10 s and centrifuged at 12,000 × gfor 15 min at 4°C. The protein concentrations of the supernatants were evaluated using a protein assay reagent (Bio-Rad Laboratories). Equal amounts of protein (20 μg/lane) and prestained molecular weight markers (Wako Pure Chemical Industries Ltd.) were loaded onto 10% premade polyacrylamide gels (Wako Pure Chemical Industries Ltd.), separated by electrophoresis and transferred to nitrocellulose membranes. The membranes were then incubated in a blocking solution comprising 3% BSA in Tris-buffered saline (TBS) for 1 h. After the incubation, the membranes were washed in TBS and incubated with anti-phospho-AMPK and anti-AMPK overnight at 4°C. The membranes were then washed in TBS containing 0.1% (vol/vol) Tween-20 for 30 min and incubated with horseradish-peroxidase-conjugated anti-mouse or anti-rabbit IgG antibodies at a dilution of 1:5,000 for 60 min at room temperature. Immunoreactive bands were detected using ECL Plus Western blotting detection reagents. The intensity of each band was analyzed with a lumino-image analyzer (Model LAS-4000 Mini; Fujifilm, Tokyo, Japan) coupled with image analysis software (Multi Gauge Ver. 3.0; Fujifilm).
2.5 Statistical Analysis
All data are presented as the mean ± SEM. The data were evaluated by a one-way analysis of variance. Differences between the mean values were assessed usingTurkey–Kramer multiple comparison test. Statistical significance was considered for values ofP < 0.05.
3 Results
3.1 Taurine Stimulates Glucose Uptake in Cultured Rat Skeletal L6 Myotubes
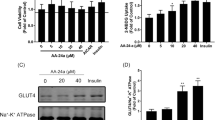
We determined the effect of taurine on glucose uptake under normal (11 mM) glucose condition. In this study, taurine dose-dependently and significantly stimulated glucose uptake at concentrations of 25–100 μM (Fig.26.1). Based on these results, we adopted 100 μM for glucose conditions as the optimal taurine concentrations in the following experiments.
Effect of taurine on glucose uptake in cultured rat skeletal L6 myotubes. L6 myotubes were preincubated in KHH buffer without glucose for 2 h. They were then incubated in KHH buffer containing 11 mM glucose and 0, 25, 50, or 100 μM taurine for 4 h, and the glucose uptake was determined. Each value represents the mean ± SEM for six wells. Values not sharing a common letter are significantly different atP < 0.05 by Turkey–Kramer multiple comparison test
3.2 Influence of Insulin on Taurine Induced Glucose Uptake
To determine the regulatory mechanism by which taurine induced the glucose uptake in rat skeletal L6 myotubes, we performed glucose uptake assays using 100 nM insulin condition. In this study, taurine significantly stimulated glucose uptake at concentrations of 100 μM independently of insulin (Fig.26.2). These results suggest that the stimulatory effect of taurine on glucose uptake is independent on the insulin.
Influence of insulin on taurine induced glucose uptake. L6 myotubes were preincubated in KHH buffer without glucose for 2 h. They were then incubated for 4 h in Krebs–Henseleit buffer with 11 mM glucose in the presence or absence of 0 or 100 μM taurine and 100 nM insulin. Each value represents the mean ± SEM for six wells. Values not sharing a common letter are significantly different atP < 0.05 by Turkey–Kramer multiple comparison test
3.3 Stimulatory Effect of Taurine on Glucose Uptake Is Dependent on the AMPK Pathways
To determine the regulatory mechanism by which taurine induced the glucose uptake in rat skeletal L6 myotubes, we performed glucose uptake assays using kinase inhibitors, namely, compound C, an ATP-competitive inhibitor of AMPK. The promotion of glucose uptake by taurine was completely inhibited by the treatments with compound C (Fig.26.3). These results suggest that the stimulatory effect of taurine on glucose uptake is dependent on the AMPK pathways.
Effects of AMPK inhibitor on taurine-promoted glucose uptake. L6 myotubes were preincubated in KHH buffer without glucose for 2 h. They were then incubated for 4 h in Krebs–Henseleit buffer with 11 mM glucose in the presence or absence of 0 or 100 μM taurine and 5 μM compound C. Each value represents the mean ± SEM for six wells. Values not sharing a common letter are significantly different atP < 0.05 by Turkey–Kramer multiple comparison test
3.4 Taurine Induces the Phosphorylation of AMPK
To examine the activity of AMPK, a well-known main regulator of glucose uptake in skeletal muscle cells, we investigated the temporal expression of phosphorylated AMPK. Taurine time-dependently stimulated the phosphorylation of AMPK under normal glucose conditions (Fig.26.4). Consequently, these data suggest that the main mechanism of glucose uptake by taurine is mediated by the AMPK pathway.
Effect of taurine on the phosphorylation of AMPK. L6 myotubes were preincubated in Krebs–Henseleit buffer without glucose for 2 h. They were then incubated in KHH buffer containing 11 mM glucose in the presence or absence of 0 or 100 μM taurine for 30, 60, 120, or 240 min. Total lysates were analyzed by immunoblotting with anti-phospho-AMPK and anti-AMPK antibodies
4 Discussion
Taurine, a β-aminosulfonic acid, is the most abundant amino acid and is essential for sustain several structure and function. Recently, several studies have indicated that taurine exhibits beneficial effects in diabetic patients. In addition, it has been reported that taurine influences various biological functions, including antioxidant, brain and retinal development, cell membrane stabilization, osmoregulation, and hypoglycemic action (Thurston et al.1980; Pasantes-Morales et al.1985; El Idrissi and Trenkner2004). In this study, we confirmed the effect of taurine on glucose uptake to the muscle cell, and clarified the regulatory mechanism of glucose uptake by taurine such as AMPK activation in cultured rat skeletal myotubes under normal glucose condition. Our data showed that taurine dose-dependently and significantly stimulated glucose uptake at concentrations from 25 to 100 μM in cultured rat skeletal L6 myotubes. Especially, rat skeletal L6 myotubes are insulin-insensitive cells on glucose uptake and our data showed that the enhancement of glucose uptake by insulin in the L6 myotubes was significantly lower than that by taurine. Also, the effect of taurine was not significantly different in the absence or presence of insulin. Therefore, it was confirmed that the stimulatory effect of taurine on glucose uptake is independent on the action of insulin, even under insulin-insensitive conditions. Several previous studies indicate that taurine shows hypoglycemic effects by enhancing insulin action, as well as by facilitating the interaction of insulin with its receptor (Lampson et al.1983; Maturo and Kulakowski1988). Ribeiro et al. (2009) also reported that plasma taurine level seems be important for β-cell function and insulin action. However, in contrast, it was reported that there was no significant differences after taurine intervention compared to placebo in incremental insulin response, neither during intravenous ted glucose tolerance test (IVGTT) nor in insulin-stimulated glucose disposal during the clamp in type 2 diabetes patients (Brons et al.2004). Also, Doi et al. (2003) reported that among the branched-chain amino acids, leucine and isoleucine increase glucose uptake in an insulin-independent manner in C2C12skeletal muscle cells. Several studies have indicated that taurine is involved in glucose homeostasis; however, the specific molecular mechanisms are unknown (Kulakowski and Maturo1984; Franconi et al.2004).
Insulin-stimulated glucose uptake by skeletal muscle plays an important role in the maintenance of whole-body glucose homeostasis (Herman and Kahn2006). AMPK is an important protein to provide energy in mammalian cells (Towler and Hardie2007). AMPK is activated in the skeletal muscle of mammals by exercise and this activation is associated with an increase in GLUT4-mediated glucose uptake by the tissue (Jessen and Goodyear2005; Magnoni et al.2012). Based on these signaling pathways related to glucose uptake, we investigated the signaling pathways for glucose uptake by taurine using kinase inhibitor. In this study, it was indicated that the stimulatory effect of taurine on glucose uptake is stimulated on the AMPK pathways by promoting the phosphorylation of AMPK (AMPK signaling) under normal glucose condition time-dependently. Recently, Solon et al. (2011) reported that taurine acted similarly to insulin, stimulating the activities of the Akt/FOXO1 and JAK2/STAT3 signaling pathways, while inhibiting the AMPK signaling pathway. On the other hand, Carneiro et al. (2009) reported that mice supplemented with taurine had a significant increased tyrosine phosphorylation of the insulin receptor in skeletal muscle, both at basal and insulin-stimulated states. In another animal experiment, it was reported that taurine increased insulin signal transduction through the phosphatidylinositol 3 kinase (PI3K) pathway, resulting in increased glucose uptake (Colivicchi et al.2004).
These results of this study suggest that taurine has a beneficial effect on glucose uptake in the muscle and that this effect is mediated through a mechanism including the activation of AMPK.
5 Conclusion
Our present study shows that taurine improve the glucose uptake by increasing the AMPK phosphorylation in rat skeletal L6 myotubes. These results may suggest that taurine has an antidiabetic effect by stimulating insulin-independent glucose uptake in skeletal muscle and may have hypoglycemic effects in diabetes.
Abbreviations
- AMPK:
-
AMP-activated protein kinase
- PI3K:
-
Phosphatidylinositol-3 kinase
- GLUT4:
-
Glucose transporter 4
References
Aerts L, Van Assche FA (2002) Taurine and taurine-deficiency in the perinatal period. J Perinat Med 30:281–286. doi:10.1515/JPM.2002.040
Brons C, Spohr C, Storgaard H, Dyerberg J, Vaag A (2004) Effect of taurine treatment on insulin secretion and action on serum lipid levels in overweighted men with genetic predisposition for type II diabetes mellitus. Eur J Clin Nutr 58:1239–1247. doi:10.1038/sj.ejcn.1601955
Brosnan JT, Brosnan ME (2006) The sulfur-containing amino acids: an overview. J Nutr 136: 1636S–1640S
Carneiro EM, Latorraca MQ, Araujo E, Beltrá M, Oliveras MJ, Navarro M, Berná G, Bedoya FJ, Velloso LA, Soria B, Martin F (2009) Taurine supplementation modulates glucose homeostasis and islet function. J Nutr Biochem 20:503–511. doi:10.1016/j.jnutbio.2008.05.00810.1016/j.jnutbio. 2008.05.008
Chang KJ (2000) Effect of taurine and β-alanine on morphological changes of pancreas in streptozotocin-induced rats. Adv Exp Med Biol 483:571–577. doi:10.1007/0-306-46838-7_61
Cherif H, Reusens B, Ahn MT, Hoet JJ, Remacle C (1998) Effects of taurine on the insulin secretion of rat fetal islets from dams fed a low-protein diet. J Endocrinol 159:341–348. doi:10.1677/joe.0.1590341
Colivicchi MA, Raimondi L, Bianchi L, Tipton KF, Pirisino R, Della Corte L (2004) Taurine prevents streptozotocin impairment of hormone-stimulated glucose uptake in rat adipocytes. Eur J Pharmacol 495:209–215. doi:10.1016/j.ejphar.2004.05.004
Das J, Ghosh J, Manna PM, Sil PC (2008) Taurine provides antioxidant defense against NaF-induced cytotoxicity in murine hepatocytes. Pathophysiology 15:181–190. doi:10.1016/j.pathophys.2008.06.002
Das J, Ghosh J, Manna P, Sinha M, Sil PC (2009) Taurine protects rat testes against NaAsO2-induced oxidative stress and apoptosis via mitochondrial dependent and independent pathways. Toxicol Lett 187:201–210. doi:10.1016/j.toxlet.2009.03.001
De la Puerta C, Arrieta FJ, Balsa JA, Botella-Carretero JI, Zamarron I, Vazquez C (2010) Taurine and glucose metabolism: a review. Nutr Hosp 25:910–919. doi:10.3305/nh.2010.25.6.4815
Doi M, Yamaoka I, Fukunaga T, Nakayama M (2003) Isoleucine, a potent plasma glucose-lowering amino acid, stimulates glucose uptake in C2C12myotubes. Biochem Biophys Res Commun 312:1111–1117. doi:10.1016/j.bbrc.2003.11.039
El Idrissi A, Trenkner E (2004) Taurine as a modulator of excitatory and inhibitory neurotransmission. Neurochem Res 1:189–197. doi:10.1023/B:NERE.0000010448.17740.6e
Franconi F, Di Leo MA, Bennardini F, Ghirlanda G (2004) Is taurine beneficial in reducing risk factors for diabetes mellitus? Neurochem Res 29:143–150
Herman MA, Kahn BB (2006) Glucose transport and sensing in the maintenance of glucose homeostasis and metabolic harmony. J Clin Invest 116:1767–1775. doi:10.1172/JCI29027
Jessen N, Goodyear LJ (2005) Contraction signaling to glucose transport in skeletal muscle. J Appl Physiol 99:330–337. doi:10.1152/japplphysiol.00175.2005
Kulakowski EC, Maturo J (1984) Hypoglycemic properties of taurine: not mediated by enhanced insulin release. Biochem Pharmacol 33:2835–2838. doi:10.1016/0006-2952(84)90204-1
Lampson WG, Kramer JH, Schaffer SW (1983) Potentiation of the actions of insulin by taurine. Can J Physiol Pharmacol 61:457–463. doi:10.1139/y83-070
Magnoni LJ, Vraskou Y, Palstra AP, Planas JV (2012) AMP-activated protein kinase plays an important evolutionary conserved role in the regulation of glucose metabolism in fish skeletal muscle cells. PLoS One 7:e31219. doi:10.1371/journal.pone.0031219
Manna P, Das J, Ghosh J, Sil PC (2010) Contribution of type 1 diabetes to rat liver dysfunction and cellular damage via activation of NOS, PARP, IκBα/NF-κB, MAPKs, and mitochondria-dependent pathways: prophylactic role of arjunolic acid. Free Radic Biol Med 48:1465–1484. doi:10.1016/j.freeradbiomed.2010.02.025
Maturo J, Kulakowski EC (1988) Taurine binding to the purified insulin receptor. Biochem Pharmacol 37:3755–3760. doi:10.1016/0006-2952(88)90411-X
Moller DE (2001) New drug targets for type 2 diabetes and the metabolic syndrome. Nature 414:821–827. doi:10.1038/414821a
Pasantes-Morales H, Wright CE, Gaull GE (1985) Taurine protection of lymphoblastoid cells from iron-ascorbate-induced damage. Biochem Pharmacol 34:2205–2207. doi:10.1016/0006-2952(85)90419-8
Racasan S, Braam B, van der Giezen DM, Goldschmeding R, Boer P, Koomans HA (2004) Perinatal L-arginine and antioxidant supplements reduce adult blood pressure in spontaneously hypertensive rats. Hypertension 44:83–88. doi:10.1161/01.HYP.0000133251.40322.20
Ribeiro RA, Bonfleur ML, Amaral AG, Vanzela EC, Rocco SA, Boschero AC, Carneiro EM (2009) Taurine supplementation enhances nutrient-induced insulin secretion in pancreatic mice islets. Diabetes Metab Res Rev 25:370–379. doi:10.1002/dmrr.959
Saltiel AR, Kahn CR (2001) Insulin signaling and the regulation of glucose and lipid metabolism. Nature 414:799–806. doi:10.1038/414799a
Schaffer SW, Azuma J, Mozaffari M (2009) Role of antioxidant activity of taurine in diabetes. Can J Physiol Pharmacol 87:91–99. doi:10.1139/Y08-110
Sinha M, Manna P, Sil PC (2007) Taurine, a conditionally essential amino acid, ameliorates arsenic-induced cytotoxicity in murine hepatocytes. Toxicol In Vitro 21:1419–1428. doi:10.1016/j.tiv.2007.05.010
Solon CS, Franci D, Ignacio-Souza LM, Romanatto T, Roman EA, Arruda AP, Morari J, Torsoni AS, Carneiro EM, Velloso LA (2011) Taurine enhances the anorexigenic effects of insulin in the hypothalamus of rats. Amino Acids. doi:10.1007/s00726-011-1045-5
Thurston JH, Hauhart RE, Dirgo JA (1980) Taurine: a role in osmotic regulation of mammalian brain and possible clinical significance. Life Sci 26:1561–1568. doi:10.1016/0024-3205(80)90358-6
Towler MC, Hardie DG (2007) AMP-activated protein kinase in metabolic control and insulin signaling. Circ Res 100:328–341. doi:10.1161/01.RES.0000256090.42690.05
Zou MH, Kirkpatrick SS, Davis BJ, Nelson JS, Wiles WG IV, Schlattner U, Neumann D, Brownlee M, Freeman MB, Goldman MH (2004) Activation of the AMP-activated protein kinase by the anti-diabetic drug metformin in vivo. Role of mitochondrial reactive nitrogen species. J Biol Chem 279:43940–43951. doi:10.1074/jbc.M404421200
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media New York
About this paper
Cite this paper
Cheong, S.H., Chang, K.J. (2013). Antidiabetic Effect of Taurine in Cultured Rat Skeletal L6 Myotubes. In: El Idrissi, A., L'Amoreaux, W. (eds) Taurine 8. Advances in Experimental Medicine and Biology, vol 775. Springer, New York, NY. https://doi.org/10.1007/978-1-4614-6130-2_26
Download citation
DOI: https://doi.org/10.1007/978-1-4614-6130-2_26
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4614-6129-6
Online ISBN: 978-1-4614-6130-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)