Abstract
This is an up-to-date discussion of calcium and phosphorus metabolism and the many regulators both old and new involved in the control of these minerals. The author provides a discussion of dietary sources of these minerals, their intestinal and renal handling. He focuses on the well-established regulators such as parathyroid hormone and vitamin D and also provides new insights into this regulation by the new compounds FGF-23 and Klotho. The author also highlights the molecular sensors for calcium and phosphorus, membrane receptors, and their importance in controlling mineral metabolism at the intestine, kidney, and bone. He ends the chapter with discussions of how macronutrients such as carbohydrates and proteins influence the overall regulation of these minerals.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Metabolism
- Regulation
- Hydroxyapatite
- Calcium
- Phosphorus
- Parathyroid hormone
- PTHrP
- CYP27B1 gene
- Calbindin
- CYP24a1 gene
- Calcitriol
- 1,25(OH)2 vitamin D3
- 24,25(OH)2 vitamin D3
- FGF-23
- Klotho
- Calcium-sensing receptor
- Synthesis
- Secretion
- Galnt3 gene
- TRPV5,6 channels
- Depolarization
- Intestinal transport
- Ionized calcium
- Phosphoric acid
- Phosphate carrier
- Renal handling calcium and phosphorus
- Intracellular calcium function
- Extracellular calcium function
- Bone mineral
- Hypocalcemia
- Hypercalcemia
- Secretion
- Osteoblasts
- Osteoclasts
- Kidney reabsorption
- Intestinal absorption
- 25-Hydroxyvitamin D3
- 1α-Hydroxylase
- IGF-1
- Vitamin D binding protein
- Osteomalacia
- Phosphatonin
- Mutations calcium-sensing receptor
- Phosphate-sensing mechanisms
- Calcium transport mechanisms
- Intestinal calcium transport
- Macronutrients
- Intestinal phosphate absorption
- Renal control mechanism of calcium and phosphate transport
- Hormonal regulation of calcium renal transport
- Calcitonin
- Renal tubular phosphate transport
- Renal proximal tubule
- Cartilage and bone and mineral regulation
- Bone resorption
- Remodeling
- Modulators bone resorption
- Calcitonin
- Estrogen
- Leptin
- β-adrenergic signaling
- Bone formation
- Mineralization
- SIBLING
- Rickets
- Hypophosphatemic rickets
- ADHR
- XLH
- ARHR
- Bone calcium-sensing receptor
- Thick ascending limb
- Phosphate transporters (Npt2a and Npt2c and PiT-2)
- Secreted frizzled-related protein-4
- Na/H exchanger regulatory factors
- Null mice
- Apoptosis
- Cathepsin proteases
- Sclerostin
- Hypocalciuric hypercalcemia
- Cytokine TNF-α
- Rank ligand
- Tissue nonspecific alkaline phosphatase
- Dentin matrix protein-1
- Matrix extracellular phosphoglycoprotein
- PHEX
- Phosphatonin
- Ossification
Introduction
The metabolisms of calcium and inorganic phosphate are intimately connected as, respectively, the cationic and anionic components of crystalline hydroxyapatite, which confers rigidity on the bone matrix (Fig. 2.1). In this chapter, the author considers the biological processes that underlie the balances of Ca2+ and phosphate as well as the key points of exchange in the gut, kidney, and bone, together with their regulation. These balances depend on dietary composition and the effectiveness with which calcium and phosphate are released from ingested food. They also depend on the efficiencies of intestinal absorption, glomerular filtration, and renal tubular reabsorption along with rates of transfer between the blood and the bone mineral store as well as the magnitudes of unavoidable losses associated, for example, with small and large intestinal secretions. Regulation of calcium and phosphate metabolism depends on several important hormones, chiefly parathyroid hormone (PTH), 1,25(OH)2 vitamin D3 (calcitriol), and fibroblast growth factor-23 (FGF-23) as well as molecular sensors for Ca2+, including the so-called calcium-sensing receptor (CaSR), and phosphate. In some cases, these sensors coordinate the synthesis and release of hormones with actions on both Ca2+ and phosphate transfers in targets including intestine, kidney, and bone. In other cases, they directly modulate Ca2+ and/or phosphate transport in key target cells. In addition, macronutrients including carbohydrates and protein are important modulators of calcium and phosphate metabolism with notable positive effects on intestinal absorption.
Partial structure of the bone mineral hydroxyapatite. A view of the repeating structural unit of hydroxyapatite based on the analysis in [269]. The corners of the repeating unit are occupied by hydroxyl ions. The hydroxyl groups are typically carboxylated by reaction with carbon dioxide. Note the presence of two distinct Ca2+ binding sites
Introduction to Calcium and Phosphate
In serum, calcium is present in three main forms: ionized, complexed with small anions such as citrate, and bound to serum proteins, chiefly albumin. After ingestion, calcium is released from foods, in part by the action of gastric acid as well as digestive enzymes in the stomach and small intestine. Calcium absorption is mediated by transport mechanisms in all segments of the small intestine. Even so it is inherently inefficient. Typically, significantly less than 50% of ingested calcium is absorbed (Fig. 2.2).
Whole body calcium and phosphate fluxes in adult humans. Representative fluxes are shown for calcium and phosphate transfers between the extracellular fluid and the gut, kidney, and bone and have been based on previous analyses [208, 270]. The ratio of the fluxes for calcium and phosphate between bone and the extracellular fluid reflects the composition of hydroxyapatite. The fluxes shown between the ECF and kidney refer to glomerular filtration and tubular reabsorption. ECF, extracellular fluid; ICF, intracellular fluid
Phosphoric acid is a strong acid with three pH titratable hydroxyl (OH) groups. For this reason, inorganic phosphate exists in three forms: H2PO −4 , HPO 2−4 , and PO 3−4 . Under standard conditions at 25°C, the pKas that govern the equilibria between the species are (1) 2.13; (2) 7.20; and (3) 12.36 [1]. At physiological ionic strength and temperature, these pKa values shift so that pKa2, for example, falls from 7.2 to around 6.8. Thus, in blood plasma at pH 7.4 with a total inorganic phosphate concentration of around 1.0 mM, the HPO 2−4 concentration is approximately 0.8 mM and the H2PO −4 concentration is around 0.2 mM; the concentrations of other species are negligible. In the acid environment of the distal nephron, where pH values frequently drop below 5.5 as a result of proton secretion [2], H2PO −4 becomes the predominant species. In general, the negative charge on phosphate is balanced in body fluids by the monovalent cations Na+ or K+ with no implications for its water solubility. However, HPO 2−4 forms insoluble precipitates with Ca2+ ions in the presence of water with a solubility product of around 5 × 10−7 (mol/L)2. At the normal ionized concentrations for Ca2+ of 1.2 mM and HPO 2−4 of 0.8 mM in human plasma, this product is exceeded. Nevertheless, precipitation and bone mineral formation do not proceed spontaneously in body fluids or in the interstitium due, in part, to the presence of Mg2+, which competes with Ca2+ [3], and forms phosphate complexes of greater solubility [4], as well as inhibitory polyphosphates and proteins (see below).
Intracellular and Extracellular Roles of Calcium and Phosphate
Ca2+ and inorganic phosphate play multiple physiological roles, many of which are independent of their roles in mineralization. Intracellularly, Ca2+ acts as a pluripotent regulator of biochemical pathways via high affinity interactions with rate-limiting enzymes or protein modulators. Phosphate, on the other hand, acts as a substrate for the synthesis of organic phosphates in biochemical interconversions, molecular recognition, energy storage (e.g., in ATP and creatine phosphate) or as a regulator of protein conformation.
Extracellularly, Ca2+ also supports synaptic transmission, the contractile state of vascular and nonvascular smooth muscle, the final common pathway of coagulation, and platelet aggregation; it also acts as a reservoir for the replenishment of intracellular Ca2+ pools. The requirements of all these processes have led to the establishment of a tight physiological range for the extracellular ionized Ca2+ concentration (1.1–1.3 mM) that is defended at the lower end by the secretory state of the four parathyroid glands under tight feedback control mediated by extracellular Ca2+-sensing receptors (review: [5]) and at the higher end by renal Ca2+-sensing receptors coupled to stimulated calcium excretion [6]. Calcitriol and FGF-23 modulate the parathyroid response: calcitriol by enhancing Ca2+-sensing receptor expression [7] and suppressing PTH expression [8, 9]; FGF-23 by suppressing PTH gene expression [10]. Interestingly, the immediate precursor of calcitriol, 25-hydroxyvitamin D significantly suppresses PTH secretion and mRNA levels at physiologically relevant concentrations [11], suggesting either that 25-hydroxyvitamin D directly activates the parathyroid vitamin D receptor or acts as the substrate for the local generation of calcitriol.
Like Ca2+, the extracellular inorganic phosphate concentration is tightly regulated. However, there is greater variation in its normal range from around 0.7 to 1.4 mM, providing variation in the Ca2+-phosphate solubility product and, thus, in the potential rates of hydroxyapatite formation. Hypophosphatemia is a recognized cause of rickets and osteomalacia (review: [12]) as considered below.
An Overview of Calcium and Phosphate Metabolism
Calcium and phosphate exchange between the blood plasma and extracellular fluid as well as the following major compartments: the intestinal lumen, the intracellular fluid, bone mineral, and renal tubular fluid (Fig. 2.2). In consequence, the overall balances for calcium and phosphate are determined by the rates of intestinal absorption and secretion, the rates of renal filtration and reabsorption, the rates of uptake and extrusion from the cytoplasmic compartment of all tissues, and the rates of bone mineralization and resorption. While the rates of calcium and phosphate uptake and release from bone mineral are matched by the structure of the repeating unit of hydroxyapatite, (Ca10(PO4)6(OH)2), in which the relative proportion of Ca:phosphate is 5:3, the rates of calcium and phosphate transfers between the extracellular fluid and other compartments are less predictable and regulated independently. Nevertheless, key hormonal regulators of calcium metabolism also impact on phosphate metabolism. Notable examples are PTH and, calcitriol. Major relationships between calcium, phosphate, PTH, calcitriol, and FGF-23, the key phosphate regulating hormone, as well as calcitonin are presented in Fig. 2.3 and discussed below.
Relationships between calcium and phosphate with key mineral-regulating hormones. The left-hand panel shows the relationships between calcium and phosphate with calcitriol, the key positive modulator of their intestinal absorption. In addition, the feedback relationships between calcium and PTH (bottom) as well as the phosphate and FGF-23 (top) are shown. Elevated calcium suppresses PTH secretion within minutes; hypocalcemia acutely stimulates PTH secretion. Elevated phosphate, on the other hand, promotes the production of FGF-23 only after a delay of several hours. Also shown in the left-hand panel are the major reciprocal relationships between PTH and calcitriol, and calcitriol and FGF-23. PTH stimulates calcitriol synthesis; calcitriol promotes the production of FGF-23, thereby reducing the risk of hyperphosphatemia following enhanced intestinal phosphate absorption. On the other hand, FGF-23 suppresses calcitriol synthesis and calcitriol suppresses PTH synthesis. These relationships may contribute to a reported positive effect of PTH on FGF-23 synthesis and a reported negative effect of FGF-23 on PTH synthesis. The right-hand panels show the feedback relationships between phosphate and PTH as well as calcium and calcitonin. PTH lowers the serum phosphate concentration by suppressing its reabsorption in the proximal tubule and calcitonin suppresses the serum calcium concentration by suppressing osteoclastic resorption of bone
Parathyroid Hormone
PTH secretion is under the tight inhibitory control of the serum ionized calcium (Ca2+) concentration acting via the CaSR (review: [5]). Thus, PTH secretion is markedly stimulated by hypocalcemia. In addition, PTH secretion is negatively modulated by various factors including l-amino acids [13], which enhance the extracellular Ca2+ sensitivity of the CaSR (reviews: [14, 15]), as well as calcitriol, which upregulates CaSR expression [7] and suppresses PTH expression (review: [16]), 25-hydroxyvitamin D [11], and FGF-23 [10]. Inorganic phosphate promotes parathyroid hyperplasia and PTH secretion and these effects appear to contribute to secondary hyperparathyroidism in chronic kidney disease [17]. Endogenous expression of the single-pass integral membrane protein, α-klotho, in the parathyroid has been reported to facilitate PTH secretion [18].
Consistent with the stimulatory impact of hypocalcemia on its secretion, key actions of PTH are directed to elevating the serum Ca2+ concentration and restraining or suppressing the inorganic phosphate concentration to prevent inappropriate crystallization. Thus, PTH promotes osteoclastic bone resorption via type-I PTH/PTHrP receptors (PTH1Rs) on cells of the osteoblast–osteocyte lineage as well as T lymphocytes [19]. It also modulates renal tubular transport via independent effects to stimulate Ca2+ reabsorption and inhibit phosphate reabsorption. Finally, it promotes 1α-hydroxylase activity in the renal proximal tubule to boost serum calcitriol levels [20] and, thus, stimulate intestinal calcium absorption. In consequence, endogenous PTH is best viewed as a surveillance and emergency-response hormone directed to the prevention and reversal of hypocalcemia ([6]; review: [21]).
PTH stimulates calcium reabsorption in the distal nephron [22]; thereby, enhancing total renal calcium reabsorption from around 95% to 98.5% and thus suppressing renal calcium excretion from around 5% to 1.5% of a largely fixed, filtered load [23]. On the other hand, PTH suppresses phosphate reabsorption in the proximal tubules [24], markedly elevating renal phosphate excretion from around 15–20% to greater than 50% of the filtered load. Correspondingly, parathyroidectomy suppresses fractional phosphate excretion to around 10% [25]. PTH-induced renal phosphate wasting would appear to be an appropriate compensatory response to calcitriol-stimulated small intestinal phosphate uptake and osteoclastic release of phosphate from resorbed hydroxyapatite in bone. Evidence that PTH [26] and, indeed, calcitriol [27] promote the production of the phosphatonin FGF-23 in bone provides additional mechanisms for the activation of renal phosphate wasting under conditions of PTH-stimulated bone resorption and calcitriol synthesis.
Although prolonged elevations in PTH induce osteoclastic bone resorption, acute administration of PTH, in pharmacological doses and in the context of normal serum calcium levels, promotes bone formation (review: [28]), possibly simulating some of the effects of bone cell-derived PTHrP (review: [29]). Thus, acute repetitive stimulation of PTH1Rs on osteoblasts and/or osteocytes for 1–2 h promotes bone formation and mineralization. Perhaps consistent with this, the immediate response to acute administration of PTH is a fall rather than a rise in serum Ca2+ concentration associated with calcium uptake by bone [30, 31].
The outcome of PTH action is, thus, dependent upon context. The duration of exposure, the serum level of calcitriol and its precursor 25-hydroxyvitamin D, the dietary intakes and serum levels of calcium and phosphate, together with the relative levels of the responses in bone and kidney all contribute to the outcome. In the context of prolonged elevation of PTH, the serum calcium concentration rises because the effects of PTH, whether direct or indirect, on all key targets promote Ca2+ accumulation in the extracellular fluid whether from bone, kidney, or gut. However, the impact of prolonged elevation of PTH on phosphate is less predictable and depends on whether the primary effect is on the kidney, in which case serum phosphate levels may drop or the gut, via calcitriol, or on bone in which case serum phosphate levels are more likely to be normal. In the setting of chronic kidney disease, in which there is resistance to PTH actions, serum phosphate levels are typically elevated and serum calcium levels are normal or suppressed (review: [32]).
Calcitriol and 25-Hydroxyvitamin D
Calcitriol is synthesized from 25-hydroxyvitamin D by the action of 1α-hydroxylase, the product of the CYP27B1 gene (review: [33]). In serum, calcitriol is a tightly regulated hormone arising from the actions of multiple nutrient and hormonal inputs on 1α-hydroxylase expression in the renal proximal tubules. Its primary actions are to promote intestinal calcium and phosphate absorption via effects on duodenal and jejunal enterocytes, respectively (review: [33]) and failure of these effects can be overcome by diets that are rich in calcium, phosphate, and certain macronutrients including lactose and protein. Its secondary actions include enhanced renal reabsorption of calcium (review: [33]) and, possibly, phosphate [34]. Together, enhanced absorptions of calcium and phosphate ions are detected by local Ca2+ and phosphate sensors in the gastrointestinal tract and, if sufficient to elevate the serum calcium and/or phosphate levels, by sensors strategically positioned in endocrine, bone, and kidney cells. In this way, enhanced calcium absorption, whether arising from calcitriol-stimulated vitamin D receptors (VDRs) in the duodenum or via an alternative small intestinal absorption pathway can shut down PTH secretion via extracellular Ca2+-sensing receptors on parathyroid cells (review: [5]). Together with the elevated serum calcium and phosphate levels, suppressed PTH levels remove an important stimulatory drive for renal calcitriol synthesis (review: [33]).
In company with adequate levels of serum calcium, calcitriol also suppresses the growth of parathyroid glands in 1α-hydroxylase null mice (review: [35]). Nevertheless, the normal physiological significance of these effects is unclear. In part, this uncertainty arises from observations of a lack of correlation between the serum calcitriol and PTH levels in human subjects, and from the primacy of the serum Ca2+ concentration in determining serum PTH levels.
25-Hydroxyvitamin D is both the substrate for the synthesis of calcitriol and a nutritional modulator in its own right. With respect to mineral metabolism, its primary target appears to be the parathyroid where, in concert with the serum Ca2+ concentration, it suppresses PTH synthesis. This explains highly reproducible negative correlations between serum 25-hydroxyvitamin D and PTH levels (review: [36]) and is supported by evidence that 25-hydroxyvitamin D suppresses PTH gene expression and secretion from cultured parathyroid cells in both the absence and presence of a 1α-hydroxylase inhibitor [11]. Furthermore, in CYP27B1 null mice, vitamin D3 supplements sufficient to raise the serum 25-hydroxyvitamin D level to 400 nmol/L prevented hypocalcemia and markedly increased the expressions of the VDR-sensitive targets, intestinal calbindin-D9K, and renal 24-hydroxylase on a standard diet [37]. Whether these effects operate at more commonly observed concentrations in humans (around 30–80 nmol/L) is currently unclear.
The significance of serum calcitriol levels and calcitriol-dependent activation of small intestinal VDRs for whole body mineral metabolism depends on dietary context. It is critical under conditions of low calcium and/or phosphate intake and in the absence of dietary lactose upon weaning in mice (review: [33]). Under these circumstances, 1α-hydroxylase null or VDR null mice develop hypocalcemia, hypophosphatemia, and secondary hyperparathyroidism taking the form of massively elevated serum PTH levels [38, 39]. However, calcium and phosphate absorption is restored, as reported by serum calcium, phosphate, and PTH levels, upon the introduction of a diet containing 2% calcium, 1-2% phosphate, and 20% lactose [40]. These findings are consistent with the notion of a calcitriol-independent absorption pathway linked to macronutrient digestion and absorption (see below). Under conditions in which dietary calcium and phosphate intake is high and intestinal absorption is supported by adequate levels of key macronutrients, as may frequently occur in humans, the impact of serum calcitriol on mineral metabolism may be limited, as extracellular Ca2+-dependent suppression of PTH removes a key drive for renal calcitriol synthesis. Under these conditions, there is no correlation between serum PTH and calcitriol levels and the negative impact of exogenously administered calcitriol on PTH synthesis and secretion (review: [41]) may be primarily of pharmacological significance. Nevertheless, under these conditions, serum 25-hydroxyvitamin D retains significance as a nutritional modulator with the ability to suppress PTH whether alone or in concert with calcium [42].
As bodily vitamin D stores fall in humans whether due to inadequate diet or intestinal absorption, poor sunlight exposure or skin biosynthesis, or a combination of these factors, as frequently occurs in the elderly (review: [43]), serum 25-hydroxyvitamin D levels fall with attendant increases in serum PTH levels. The serum calcium concentration typically remains in the normal range. Whether elevated serum PTH levels act to maintain or boost serum calcitriol levels under these circumstances is unclear—typically, serum calcitriol levels also remain within the normal reference range. However, there is a cost: accelerated consumption of 25-hydroxyvitamin D. Oral calcium supplements can facilitate calcium absorption in the context of vitamin D deficiency and thereby boost serum ionized Ca2+ levels, typically, still within the normal range. This provides Ca2+-dependent feedback inhibition of renal calcitriol synthesis as well as PTH secretion, further relieving the drive for calcitriol synthesis [44]. Conversely, repletion of vitamin D stores is reflected first in elevated 25-hydroxyvitamin D levels with corresponding falls in serum PTH [45].
25-Hydroxyvitamin D is converted to the key calcium and phosphate-elevating hormone, calcitriol via the action of 1α-hydroxylase. Although the gene CYP27B1 appears to universally encode 1α-hydroxylase in tissues including the kidney, testis, brain, placenta, bone, and skin, thereby catalyzing the local production of calcitriol (review: [46]), only the renal enzyme controls the synthesis of calcitriol for release into the circulation under physiological conditions and is selectively controlled by key micronutrient and hormonal regulators of mineral metabolism (review: [33]). These regulators include elevations in the serum levels of calcium or phosphate (negative), as well as the hormones FGF-23 and calcitriol (also negative), and PTH and IGF-1 (both positive). Downregulation of renal 1α-hydroxylase by calcium, phosphate, FGF-23 (as a delayed reporter of ingested phosphate), and calcitriol itself may all be viewed as physiologically appropriate forms of negative feedback. On the other hand, upregulation of 1α-hydroxylase by PTH and IGF-1 would appear to arise in quite different physiological contexts with distinct implications. Thus, upregulation of 1α-hydroxylase by PTH may be viewed as a component of an “emergency response to hypocalcemia,” whose goal is the restoration of normal serum calcium levels. Upregulation of 1α-hydroxylase by IGF-1, on the other hand, would appear to provide a mechanism by which intestinal calcium and phosphate absorption can be enhanced together with their renal retention in the context of growth and an associated stimulation of bone formation [47–49].
Together, 25-hydroxyvitamin D and calcitriol are carried in the circulation by a 52-kDa vitamin D binding protein (DBP) and both are deactivated by a widely expressed 24-hydroxylase, encoded by the CYP24a1 gene, whose expression is powerfully upregulated by calcitriol [50, 51] and possibly 25-hydroxyvitamin D [52] leading to the generation of 24,25(OH)2 vitamin D or 1α-hydroxy-23-carboxy-24, 25, 26, 27-tetranorvitamin D3 (calcitroic acid). Based on findings in CYP24a1 null mice, 24-hydroxylase prevents inappropriate elevation of serum calcitriol levels as well as attendant hypercalcemia and hypoparathyroidism [53].
In the renal synthesis of calcitriol, the DBP–25-hydroxyvitamin D complex enters the proximal tubular cytoplasm following glomerular filtration and uptake via apical megalin/cubulin-mediated endocytosis [54–56]. DBP null mice exhibit markedly suppressed levels of circulating calcitriol and 25-hydroxyvitamin D, and a demineralizing phenotype [57]. Surprisingly, serum calcium, phosphate, and PTH levels were normal in DBP null mice on control chow but administration of a vitamin D-deficient diet for 4 weeks induced a significant decrease in serum phosphate and a significant increase in serum PTH without a change in serum calcium [57] indicating that the elevated PTH had normalized serum calcium in the context of vitamin D deficiency while suppressing the serum phosphate level. Whether serum FGF-23 levels changed under the conditions of these experiments is unknown. The results indicate that DBP provides a store of circulating vitamin D metabolites and prolongs their serum half-lives but is not necessary for their actions (review: [58]).
The significance of calcitriol for normal mineral homeostasis has been assessed in 1α-hydroxylase (CYP27B1) null mice and VDR null mice. Although the phenotypes of these mice are not identical suggesting the existence of calcitriol-independent actions of VDR in the skin and hair follicles, and VDR-independent actions of calcitriol in the epiphyseal growth plates (review: [33]), they both exhibit severe rickets and osteomalacia after weaning, arising from markedly impaired intestinal calcium and phosphate absorption. The findings indicate that, postweaning, adequate circulating levels of calcitriol together with the expression of its cognate VDRs in the small intestine are required for normal mineral metabolism (review: [33]). Thus, intestinal calcium and phosphate transport was restored in CYP27B1 null mice following administration of exogenous calcitriol [59], and in global VDR null mice by intestine-specific transgenic expression of the VDR [60]. Furthermore, the hypo-mineralizing bone phenotype was clearly dependent upon impaired intestinal calcium and phosphate absorption because it could be overcome by rescue diets containing enhanced contents of calcium and phosphate as well as lactose (review: [33]). The expression of the phenotype only after weaning and the striking impact of lactose in the rescue diet suggests the existence of a calcitriol-independent, macronutrient-associated absorption pathway (see below).
FGF-23 and Klotho
FGF-23 is a “phosphatonin,” i.e., a circulating factor that promotes renal proximal tubular phosphate wasting and thereby lowers serum phosphate levels (reviews: [61, 62]). Although its impact on phosphate metabolism in certain pathological contexts seems clear, its role in the normal surveillance of bodily phosphate metabolism and the time-scale over which it operates are uncertain.
The significance of FGF-23 for the control of renal phosphate excretion and serum phosphate levels was first established in human studies of inherited forms of rickets and/or osteomalacia including autosomal dominant hypophosphatemic rickets (ADHR) [63] and X-linked hypophosphatemia (XLH) [64] as well as tumor-induced osteomalacia [64] in all of which serum FGF-23 levels are elevated in association with renal phosphate wasting, hypophosphatemia, and inappropriately suppressed serum calcitriol levels (review: [41]). Correspondingly, tumoral calcinosis in humans in the context of hyperphosphatemia and elevated serum calcitriol levels typically arises from impaired FGF-23 secretion whether associated with specific FGF-23 mutations [65] or mutations of UDP-GalNAc transferase 3 (GALNT3) that impair FGF-23 processing (review: [66]).
The significance of FGF-23 in the control of phosphate metabolism is supported by transgenic mouse studies, most notably fgf-23 null mice, which exhibit hyperphosphatemia, elevated serum calcitriol levels, hypercalcemia, and widespread calcification [67]. Elevated serum calcitriol levels appear to be critical for the development of both hyperphosphatemia and hypercalcemia in these mice. Consistent with this notion, the double mutant fgf23 −/−/1α-OHase −/− mouse exhibits normal or near-normal serum calcium and phosphate levels as well as normal growth and markedly improved lifespan [68].
FGF-23 is a key negative regulator of serum calcitriol levels. Thus, an acute bolus intravenous injection of FGF-23 in mice suppressed 1α-OHase expression and promoted 24-hydroxylase expression, resulting in markedly suppressed serum calcitriol levels [69]. The effect of FGF-23 on serum calcitriol levels was detected between 3 and 24 h (trough around 9 h) indicating that its effects are likely to be dependent on changes in gene expression. Interestingly, the effect of FGF-23 on the serum calcitriol level in these studies occurred several hours earlier, and was substantially greater, than its effect on the serum phosphate level [69]. Thus, although FGF-23 promotes phosphaturia by downregulating the expressions of Npt2a and Npt2c in the renal proximal tubular luminal membrane [70], and suppresses serum phosphate levels, it is not yet clear whether its effects are mediated directly by FGF receptors in the renal proximal tubule or indirectly via other hormonal factors, e.g., arising from the processing of small integrin-binding ligand N-linked glycoproteins (SIBLINGs) to acidic serine and aspartate-rich motif (ASARM) peptides in bone or via paracrine factors, e.g., from the distal tubule (review: [71]). Complicating these considerations is an observation that FGF-23 suppresses intestinal Npt2b expression in wild-type but not Npt2a or Npt2c null mice [72] independent of serum calcitriol levels, which were markedly suppressed in all three cases. The results point to the existence of, as yet unrecognized, phosphate regulatory signals that operate between the kidney and small intestine and are mediated by Npt2a and 2c and/or one of their partner proteins.
The pronounced negative effect of serum FGF-23 on serum calcitriol levels raised the possibility of a novel feedback loop involving the two hormones. Consistent with this idea, calcitriol activates FGF-23 expression [27, 73, 74]. In addition, prolonged elevations of calcitriol upregulate the expression of FGF-23 and prolonged elevations of FGF-23 coordinately downregulate renal 1α-hydroxylase and upregulate renal 24-hydroxylase (review: [41]). The feedback loop between calcitriol and FGF-23 may contribute to the support of a natural diurnal rhythm in whole body calcium and phosphate metabolism, which in part takes the form of a coordinated trough in the serum levels of calcium (−2%), phosphate (−10%), calcitriol (−10%), and PTH (−20%) at around 0800 [75]. In a recent study, no diurnal rhythm was observed in serum FGF-23 levels, however, there was a pronounced trough in the level of the soluble form of its co-receptor α-klotho (−40%) at around 0000 [76]. The significance of these findings is currently unclear.
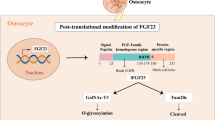
FGF-23 is synthesized in osteocytes and osteoblasts and its secretion depends, in part, on the enzyme GALNT3 which O-glycosylates the peptide (review: [41]). Its expression and serum levels are increased by elevated dietary phosphate intake [77–79] and elevated serum calcitriol levels [27] and these effects have been demonstrated in both mouse and human studies. However, serum FGF-23 levels do not appear to be regulated by acute changes in serum phosphate concentration. Data obtained in acute human studies, for example, suggest that serum FGF-23 levels do not normally respond within 6 h of exposure to a substantial increase in either dietary phosphate intake or the serum phosphate level [79, 80] and possibly not before 12 h [81].
PTH has been reported to promote FGF-23 expression and elevate its serum levels. Thus, PTH administered subcutaneously in mice for 3 days by osmotic mini-pump induced an elevation in serum FGF-23 levels in the context of elevated serum calcium and suppressed serum phosphate levels [26] and in a study in healthy men who received 18-h intravenous infusions of PTH 1–34, serum FGF-23 levels were elevated from 6 to 18 h in association with elevated serum levels of calcitriol and calcium [82]. Whether reported reciprocal interactions between PTH and FGF-23 [10, 26] constitute an additional regulatory loop or are dependent upon the intermediary actions of calcitriol and/or phosphate is currently uncertain.
α-Klotho
α-Klotho is an important partner of FGF-23 and exists in both circulating and integral membrane forms. It contributes to the formation of high affinity FGF-23 receptors by forming ternary complexes with FGF receptors [83] including FGFR1c in the proximal tubule [70]. Consistent with the notion that the actions of α-klotho and FGF-23 are interdependent, the phenotypes of α-klotho null mice and FGF-23 null mice are similar and both include marked elevations of serum phosphate and increased serum levels of calcitriol [84]. In addition, FGF-23 levels are markedly elevated in α-klotho null mice [85] suggesting the existence of a resistance state with dysregulated FGF23 secretion arising from elevated serum calcitriol and/or serum phosphate levels.
More recent work suggests that α-klotho may have additional actions on mineral metabolism that arise, at least in part, from its β-glucuronidase activity. Thus, α-klotho promotes the surface expression of distal nephron TRPV5 [86] by cleaving terminal sialic acid residues to expose an underlying galactose-N-acetylglucosamine, which binds to the plasma membrane lectin, galectin-1 [87]. The outcome is enhanced renal Ca2+ reabsorption (review: [88]). Consistent with these findings the α-klotho null mouse exhibits impaired distal convoluted tubule (DCT) and connecting tubule Ca2+ reabsorption and resistance to the stimulatory action of PTH on Ca2+ reabsorption [89]. In addition, as noted above, α-klotho expression in the parathyroid has also been linked to PTH secretion [18].
Calcium-Sensing Mechanisms: Calcium-Sensing Receptors
Although a number of cellular calcium-sensing mechanisms have been defined based on the activities of ion channels and transporters, one of the best understood calcium-sensing mechanism in human biology is dependent upon the expression of a class C G-protein-coupled receptor known as the CaSR. The CaSR was first cloned from the parathyroid [90], where it mediates feedback control of PTH secretion and thus provides a key defense against hypocalcemia (review: [5]) and subsequently the kidney [91], where it regulates renal calcium excretion, providing an important defense against hypercalcemia [6]. The CaSR also modulates renal phosphate, salt, and water transport (review: [92]). It is expressed in various bodily compartments and organs including the gastro-intestinal tract, kidney, thyroid C-cells, and bone in which it is expressed on cells of both the osteoblast and osteoclast lineages [93]. The roles of the CaSR in the control of renal calcium and phosphate transport as well as bone biology are considered below. In gut biology it has multiple roles that include the promotion of interactions between macronutrient and micronutrient metabolism (review: [94]).
Heterozygous inactivating mutations of the CaSR underlie the uncommon, typically benign, hypercalcemic disorder known as familial hypocalciuric hypercalcemia (FHH) [95] in which there is impaired inhibition of calcium reabsorption in the renal cortical thick ascending limb (TAL) [96]. The primary problem is impaired inhibitory control of renal calcium reabsorption but, in addition, the serum PTH level is typically inappropriately normal [97]. The rare, typically homozygous, form of the disorder presents as a neonatal form of severe hyperparathyroidism with markedly elevated serum calcium levels and pathological fractures within the first weeks of life [98]. The problem arises from extreme resistance to the serum Ca2+ concentration in both the parathyroid and kidney.
In addition to sensing the extracellular Ca2+ concentration, the CaSR is a multimodal chemosensor that responds to l-amino acids and is, thus, sensitive to changes in protein nutrition (review: [99]), as well as ionic strength, pH, Mg2+, neuromodulators including spermine, and glutathione analogs (reviews: [15, 100]). Thus, the CaSR has roles not only in the regulation and modulation of calcium and phosphate metabolism and bone biology but also in various other physiological processes. In the gastro-intestinal tract, these include the modulation of gastrin release and acid secretion (review: [99]) as well as the regulation of cholecystokinin secretion [101–103]. The CaSR may also contribute to well-recognized positive interactions between protein nutrition and calcium and bone metabolism [104].
Phosphate-Sensing Mechanisms
Phosphate-sensing mechanisms have been identified in the intestine, kidney, bone, and growth plate and act to regulate renal phosphate excretion and skeletal development as well as bone mass and mineralization (see below). Biological effects coupled to phosphate-sensing mechanisms include enhanced production of phosphatonins including FGF-23, and MEPE, and suppressed production of calcitriol, both of which are appropriate responses to hyperphosphatemia, as well as apoptosis of hypertrophic chondrocytes in the process of normal growth plate development and osteoblast maturation (review: [105]). In addition, a number of putative phosphate sensors, e.g., based on one or more phosphate transporters, and even elements of potential signaling pathways, e.g., ERK1/2, have been proposed [106]. However, the full significance of these pathways awaits the identification of the molecular sensors and their signaling partners.
Calcium Transport Mechanisms
Epithelial Calcium Transporters in Support of Transcellular Ca2+ Transport
Expression cloning of key epithelial Ca2+ transporters of the small intestine [107] and kidney [108], permitted the identification and detailed analyses of active transport mechanisms in the duodenum and distal nephron (review: [109]). These transporters are now recognized as members of the TRPV family of ion channels, i.e., TRPV6 and TRPV5, respectively (review: [110]). In support of these transport mechanisms are the cytoplasmic Ca2+-binding proteins calbindin-D9k (intestine and kidney) and calbindin-D28k (kidney), which promote calcium uptake and transport by buffering the intracellular Ca2+ concentration, as well as two energy-expending basolateral Ca2+ pump mechanisms: the Ca2+-ATPase isoform Ib and the Na+/Ca2+-exchanger NCX1 coupled to Na+–K+-ATPase activity (review: [109, 110]). The Na+–K+-ATPase maintains a steep electrochemical gradient for Na+ ions across the basolateral membrane favoring Na+ influx from the extracellular fluid to the cytoplasm. The Na+ gradient is used by NCX1 to drive Ca2+ efflux in exchange for Na+ influx (Fig. 2.4; upper cell). These mechanisms support calcitriol-induced Ca2+ absorption in the duodenum (review: [110]) and PTH and calcitriol-induced Ca2+ reabsorption in the distal nephron (review: [22]).
Calcitriol stimulated intestinal absorption pathways for calcium and phosphate. The upper cell presents components of a key duodenal calcium absorptive pathway including the luminal membrane Ca2+ channel, TRPV6, the intracellular Ca2+ binding protein, calbindin D9k, which buffers the cytoplasmic Ca2+ concentration, as well as exit pathways via the Na+/Ca2+ exchanger, NCX1 and the Ca2+-ATPase, PMCAIb (review: [110]). The basolateral Na+/K+-ATPase, which maintains the Na+ gradient across the interstitium–facing membrane is not shown. The lower cell presents components of a key jejunal phosphate absorptive pathway (review: [114]), including the luminal membrane Na+-phosphate co-transporter, Npt2b, basolateral Na+/K+-ATPase, and a basolateral membrane phosphate channel (or transporter). Calcitriol acts via nuclear vitamin D receptors to upregulate the synthesis of molecular components in both pathways. Additional pathways for the small intestinal absorption of calcium, and possibly phosphate, are linked to macronutrient uptake (not shown)
In addition, less well-defined transcellular Ca2+ transport mechanisms provide a link between calcium absorption and the absorption of key macronutrients (see below).
Paracellular Ca2+ Transport Across Epithelia
In addition to transcellular mechanisms of calcium transport, paracellular Ca2+ transport mechanisms support Ca2+ transport in both the intestine and renal tubules. The key elements of these transport mechanisms are a transepithelial electrochemical gradient and a paracellular pathway (Fig. 2.5). A favorable transepithelial electrochemical gradient may arise from either a lumen positive potential difference (expressed with respect to a reference electrode in the interstitium) and/or a transepithelial Ca2+ concentration gradient that favors transport. In addition, paracellular transport requires the presence of a cation-selective intercellular pathway via the junctional complexes. The cloning and identification of claudins, cation-selective proteins that are expressed in tight junctional complexes (review: [111]) have greatly enhanced understandings of these mechanisms and the nature of their regulation. Key sites for paracellular calcium transport include the renal proximal tubule and TAL of Henle’s loop (see below).
Inorganic Phosphate Transport Mechanisms
Inorganic Phosphate Transporters
Three families of inorganic phosphate transporters have been identified: so-called type-I transporters of the SLC17 family, which are now recognized as nonselective organic anion transporters (review: [112]); type-II transporters of the SLC34 family, including NaPi-IIa, -IIb, and -IIc, also known as Npt2a, 2b, and 2c, respectively, which are Na+-dependent, selective for the divalent HPO 2−4 species and expressed in the brush border (luminal) membranes of the renal proximal tubules (2a and 2c) and small intestine (2b); and type-III transporters of the SLC20 family, which are widely expressed, Na+-dependent, selective for the monovalent H2PO −4 species and include PiT-1 and PiT-2 (reviews: [113, 114]).
Despite the identification of transporters on the luminal brush border membranes of transporting epithelia such as small intestine and renal proximal tubule, the identities of phosphate transporters on the basolateral membranes, which are required to deliver inorganic phosphate to the blood, are unclear. Based on energetic considerations, which include a cell-negative membrane potential with respect to the extracellular fluid, and an intracellular inorganic phosphate concentration that is likely to be elevated with respect to the extracellular fluid, phosphate transport across epithelial basolateral membranes is likely to be passive and, thus, mediated by (1) phosphate-permeant anion channels, (2) an electroneutral transporter such as the Band-3 anion exchanger of erythrocytes [115], or (3) a phosphate carrier analogous to mitochondrial SLC25A3 [116].
Intestinal Calcium Absorption
Intestinal calcium absorption is largely confined to the small intestine and is notably inefficient. Thus, despite the existence of various mechanisms to promote absorption, absorption rates are typically less than 50% of ingested loads (Fig. 2.2). Macronutrients including protein [117, 118] and carbohydrates such as glucose [119] and lactose [40, 120] promote calcium absorption. In addition, calcium absorption is powerfully stimulated by calcitriol, which together with the vitamin D receptor is required to prevent hypocalcemia and secondary hyperparathyroidism in mice after, but not prior to, weaning (review: [33]). Intestinal calcium absorption is positively modulated by estrogens [121–123], as well as prolactin [124, 125] and placental lactogen [126]. Consistent with these effects, intestinal calcium absorption is impaired in vitamin D deficiency (review: [127]), reduced in postmenopausal women (review: [128]), and enhanced in pregnancy [129]. The molecular bases for the effects of calcitriol, estrogens, and prolactin/ placental lactogen are not yet clear. Nevertheless, calcitriol and estrogens (reviews: [109, 110]) as well as prolactin [130] upregulate several recognized molecular components of Ca2+ transport.
Ca2+ ions are absorbed from all three major segments of the small intestine: the duodenum; the jejunum; and the ileum. However, the mechanisms of calcium absorption differ between these segments. In particular, active calcium absorption localizes to the duodenum [131] where it is stimulated by calcitriol [132] and calcium absorption that is coupled to the absorption of glucose or lactose is primarily located in the jejunum and ileum [133, 134]. The location and molecular basis of protein-linked Ca2+ absorption are currently unknown.
Several distinct transepithelial transport pathways support calcium absorption. One of the better recognized pathways operates in the duodenum and comprises: TRPV6 Ca 2+ channels, which mediate Ca2+ uptake from the intestinal lumen across the enterocyte luminal brush border membrane [107]; calbindin-D 9k , an intracellular Ca2+-binding protein, which buffers the cytoplasmic ionized Ca2+ concentration and promotes transcellular Ca2+ transport; and ATP-dependent Ca2+ efflux into the extracellular fluid, including (1) the Na+/Ca2+ exchanger NCX1, coupled to Na+/K+-ATPase activity, or (2) the plasma membrane Ca2+-ATPase, PMCA1b (reviews: [109, 110]).
Calcitriol-Dependent Activation of Calcium Absorption
As noted above, the primary action of calcitriol is to stimulate duodenal calcium absorption. The significance of the pathway is underscored by the phenotypes of CYP27B1 null mice, which lack 1α-hydroxylase required for calcitriol synthesis, as well as VDR null mice, which together exhibit very similar phenotypes including impaired intestinal calcium absorption, hypocalcemia, and rickets (review: [33]). Furthermore, in global VDR null mice intestine-specific expression of VDR normalized intestinal calcium absorption, serum calcium levels, and the bone phenotype [60]. Calcitriol promotes the intestinal expression of key components of duodenal Ca2+ transport including TRPV6, its isoform TRPV5, which is normally expressed at high levels in the kidney, calbindin-D9k, and PMCAIb inviting the conclusion that calcitriol action is wholly dependent upon the upregulation of a duodenal transport “system” based on these components (reviews: [109, 110]). The key components of this transport pathway are presented in Fig. 2.4 (upper cell). Surprisingly, however, recent studies in transgenic mice indicate that the molecular basis of calcitriol action is more complicated. In particular, calcitriol-stimulated intestinal calcium absorption was intact in calbindin-D9k null mice [135] and, indeed, TRPV6 null mice [136]. Furthermore, active calcium absorption was only partially impaired in TRPV6/calbindin-9k double null mice [137]. These results suggest the existence of redundancy in the actions of calcitriol on intestinal calcium transport, e.g., via upregulation of TRPV5 in compensation for TRPV6, or the existence of an alternative pathway. Consistent with the latter notion, recent evidence suggests that calcitriol promotes paracellular calcium transport in the small intestine by modifying the expression of epithelial junction proteins including upregulation of claudins 2 and 12, which support the formation of a divalent cation-permeable pathway [138].
Macronutrient-Dependent Stimulation of Intestinal Calcium Absorption: Carbohydrates
Although vitamin D deficiency or defective VDR status promotes the development of hypocalcemia and secondary hyperparathyroidism, as well as rickets or osteomalacia (review: [33]), rescue diets with increased contents of calcium and phosphate and containing lactose [120] or glucose [119, 134] can prevent and/or restore normal calcium metabolism and the bone phenotype. In addition, the duodenal (i.e., vitamin D-dependent) contribution to Ca2+ absorption appears to be minor, perhaps as low as 15% of the total, when there are plentiful supplies of calcium, phosphate, and macronutrients [134].
Recent work suggests a distinct segmental pattern for macronutrient-dependent calcium absorption based on the jejunum and ileum, and an alternative molecular basis for macronutrient-linked calcium absorption. According to one hypothesis, calcium absorption in the rat jejunum and ileum is dependent upon the expression of Cav1.3 voltage-operated Ca2+ channels in the enterocyte brush-border membrane together with SGLT1 Na+-dependent glucose transporters [119]. SGLT1 mediates membrane depolarization secondary to Na+ influx as well as glucose transport. Depolarization activates Cav1.3 channels to provide a pathway for Ca2+ influx [119]. Thus, the proposed mechanism couples the transports of glucose and Ca2+.
Macronutrient-Dependent Stimulation of Intestinal Calcium Absorption: Protein
The intake of dietary protein, like that of carbohydrates, promotes intestinal calcium absorption [117, 118, 139] and may contribute to the recognized positive impact of dietary protein on bone health (review: [104]). The effect occurs independent of gastric acid production and attendant solubilization of Ca2+ salts, at least in healthy young adults [140]. Whether the effect of dietary protein localizes to a particular intestinal segment and depends on a transcellular or paracellular mechanism is not yet clear. Gaffney-Stomberg et al. demonstrated enhanced Ca2+ uptake into duodenal brush-border membrane vesicles prepared from female rats fed a high (40%) protein diet when compared with vesicles prepared from rats fed a low (5%) protein diet, suggesting changes in the expression of key Ca2+ transport proteins [139]. These results suggest the existence of a dietary protein-modulated transcellular pathway for Ca2+ absorption in the small intestine.
Intestinal Phosphate Absorption
The small intestine is also the primary site of inorganic phosphate absorption. Since organic as well as inorganic phosphate is ingested in the diet, the stomach provides an important site for the nonenzymatic hydrolysis of organic phosphates such as nucleotides and sugar phosphates including phytates as well as inorganic polyphosphates including pyrophosphate, octa-calcium phosphate, and hydroxyapatite. Studies in humans and rats indicate that inorganic phosphate absorption occurs throughout the small intestine, primarily localizes to the duodenum (pH 6–7) and jejunum (pH 7–8) and is mediated by the Na+-dependent type-IIb (Npt2b) and PiT-1 or PiT-2 transporters (review: [114]); on the other hand, inorganic phosphate transport in the mouse primarily localizes to the ileum.
Like intestinal calcium absorption, intestinal phosphate absorption is mediated by all three segments of the small intestine. However, as noted above, important species-related differences have been identified between humans and rats on the one hand, in which phosphate transport is highest in the duodenum, and mice on the other, in which it is highest in the ileum [141]. Like intestinal calcium absorption, intestinal phosphate absorption is stimulated by calcitriol ([142]; review: [143]). Surprisingly, however, at variance with the situation for calcium absorption, the jejunum rather than the duodenum is the primary site of calcitriol-dependent phosphate transport in all three species [141]. In addition, intestinal phosphate transport is markedly enhanced by low dietary phosphate (review: [144]). Whether macronutrients promote inorganic phosphate absorption as observed for calcium absorption is unknown.
Intestinal phosphate transport is mediated by Na+-dependent transcellular as well as Na+-independent, possibly paracellular, mechanisms [145]. However, the Na+-dependent mechanisms are better understood and appear to mediate the effect of calcitriol. Na+-dependent transport is mediated primarily by Npt2b transporters and, to a lesser extent, type-III transporters including PiT-1 and PiT-2 expressed in luminal brush-border membranes (review: [114]). Interestingly, calcitriol upregulates small intestinal expression of Npt2b [141] and PiT-2 [146]. In addition, low dietary phosphate upregulates Npt2b expression [147] independent of calcitriol synthesis or the VDR [148]. Key components of the calcitriol-stimulated Npt2b pathway of intestinal phosphate transport are presented in Fig. 2.4 (lower cell).
Other Modulators of Phosphate Transport
Phosphate transport is also positively modulated by estrogens, which upregulate Npt2b expression [149] consistent with the notion that postmenopausal bone loss may arise from reduced absorption of phosphate as well as calcium (review: [150]). Other hormonal influences on dietary phosphate absorption include epidermal growth factor (EGF), which promotes Npt2b expression [151] as well as glucocorticoids [152, 153] and TNF-α [154], which suppress Npt2b expression and phosphate absorption, suggesting potential explanations of the low bone mass phenotypes associated with prolonged high-dose steroids and inflammatory bowel disease. Other negative influences on intestinal phosphate transport include advancing age as well as matrix extracellular phosphoglycoprotein (MEPE) and FGF-23 (review: [114]).
Renal Mechanisms in the Control of Calcium and Phosphate Transport
Renal Tubular Calcium Transport
Renal Ca2+ reabsorption occurs in the proximal tubule, medullary and cortical TAL, the DCT and connecting tubules (reviews: [96, 155, 156]). As a result, renal calcium excretion in adults typically varies from 2 to 8 mmol/day (around 1.5–5% of the filtered load). The mechanisms that support renal Ca2+ reabsorption differ between tubular segments and include both transcellular and paracellular mechanisms. In addition, they are differentially regulated by hormonal and nutrient-dependent control mechanisms. The primary hormonal regulator of renal Ca2+ reabsorption is PTH, which promotes Ca2+ reabsorption in the distal nephron (i.e., distinct from its proximal tubule site of action on phosphate transport). Other hormonal modulators include calcitriol (review: [157]) and calcitonin [158], both of which promote Ca2+ reabsorption.
Ca2+ Reabsorption Mechanisms in the Proximal Tubule and Thick Ascending Limb
The cellular basis of Ca2+ reabsorption in the proximal tubule and TAL is predominantly paracellular, i.e., via the tight junctions between neighboring cells (review: [155]). It is, thus, governed by the transepithelial electrochemical gradient as well as the ion selectivity and conductance of the intercellular corridor formed by the tight junctional complex (review [159]).
Proximal Tubule
In the proximal tubule, paracellular reabsorption of Ca2+ is favored by both the transepithelial electrical potential difference, which is lumen positive under control conditions [160], as well as the transepithelial concentration gradient in which the luminal Ca2+ concentration is elevated by isosmotic coupling of water transport to NaCl reabsorption. As a result of the high level of water reabsorption, which approximates 75%, the luminal Ca2+ concentration appears to be held above the interstitial Ca2+ concentration throughout the length of the proximal tubule.
Thick Ascending Limb
In the TAL, which is an important site of regulated Ca2+ transport, NaCl reabsorption occurs independent of water reabsorption and is more tightly coupled to the generation of a lumen positive transepithelial potential difference (Fig. 2.5). The luminal membrane components of the TAL mechanism include Na+/K+/2Cl− (NKCC2) co-transporters and inwardly rectifying “ROMK” K+ channels (review: [96]). The basolateral (blood-facing) components include the Na+/K+-ATPase, which provides the driving force for transcellular Na+ reabsorption, and the CLC-KB Cl− channel, which mediates Cl− efflux from the cell to the interstitium in response to a cell-negative membrane potential difference (review: [96]). Control of Ca2+ transport in the TAL arises from regulated expression and transport rates of NKCC2, ROMK, and CLC-KB and is primarily modulated by (1) PTH1Rs, which promote Ca2+ reabsorption in response to elevated serum PTH levels and (2) CaSRs, which suppress Ca2+ reabsorption (review: [96]).
Inhibitory control of paracellular calcium reabsorption in the cortical thick ascending limb by calcium-sensing receptors. Basolateral calcium-sensing receptors are activated by elevated extracellular fluid Ca2+ concentration, e.g., in the context of systemic hypercalcemia or in response to transient local hyper-reabsorption (review: [96]). A signaling pathway linked, in part, to the production of arachidonic acid metabolites suppresses either the transepithelial driving force for reabsorption or Ca2+ permeation via the intercellular junctions
Distal Convoluted Tubules and Connecting Tubules
Unlike the proximal tubule and TAL, Ca2+ reabsorption in the DCTs and connecting tubules, amounting to as much as 15% of the total, is primarily transcellular and dependent on the expression of the TRPV5 Ca2+-selective channel in the luminal membrane, which provides a pathway for Ca2+ influx into the cell, together with PMCA1b or NCX1 on the basolateral membrane, which provide a pathway for Ca2+ efflux to the interstitium (Fig. 2.6; review: [109]). Ca2+ transport is facilitated by expression of the Ca2+ chelators, calbindin-D28k (review: [161]), and calbindin-D9k in the cytoplasm of DCT cells. The chelator properties of these proteins appear to prevent the cytoplasmic ionized Ca2+ concentration from rising to levels (≥ 200 nM) that might otherwise trigger intracellular signaling events and, by maintaining the steep electrochemical gradient for Ca2+ across the apical membrane, facilitate the continuing uptake of Ca2+ ions across the luminal membrane [162]. Interestingly, the cellular uptake of nonprotein Ca2+ chelators such as the EGTA-analog, BAPTA-AM mimics the effect of calbindins on calcium transport [162]. The expression of both Ca2+-binding proteins is promoted by calcitriol [163] and, based on studies in cultured kidney cells, the effect of calcitriol on calbindin-D9k appears to be enhanced by PTH [164].
Positive modulation of transcellular calcium reabsorption in the distal nephron by PTH and calcitriol. The mechanism shown in the cell diagram operates in both distal convoluted tubules and connecting tubules (review: [171]). Ca2+-influx across the luminal membrane is mediated by the Ca2+-selective ion channel, TRPV5. Calbindin-D28k and calbindin-D9k (not shown) buffer the cytoplasmic Ca2+ concentration and thereby promote continuing Ca2+ influx without activating intracellular signaling pathways. Ca2+ exits the cell at the basolateral membrane via PMCA1b and NCX1. PTH and calcitriol both upregulate the expressions of TRPV5, the calbindins, and NCX1 [163, 165]. In addition, PTH activates TRPV5-mediated calcium transport [173]. Although a similar calcium transport mechanism operates in the duodenum, there are key differences between the molecular components (e.g., TRPV6 in the duodenum rather than TRPV5 as here in the distal nephron) and regulation. Thus, although calcitriol promotes calcium transport at both sites, PTH has no direct effect on calcium transport in the duodenum
Hormonal Regulation of Renal Ca2+ Transport
Parathyroid Hormone
PTH regulates renal calcium excretion by promoting Ca2+ reabsorption in the cTAL as well as the DCT and connecting tubule segments of the distal nephron (reviews: [96, 165]). In the cTAL, Ca2+ reabsorption is primarily paracellular, accompanied by Mg2+, and antagonized by the CaSR located on the basolateral membrane. In the DCT and connecting tubule, on the other hand, Ca2+ reabsorption is transcellular and PTH promotes the expression and/or functional activity of proteins that support active transepithelial transport. While PTH provides a key drive for stimulated renal Ca2+ transport in response to hypocalcemia or in the context of primary hyperparathyroidism, suppression of PTH levels plays a relatively minor role in the counter-regulatory response to hypercalcemia. Instead, CaSR-dependent suppression of TAL Ca2+ reabsorption provides a key defense against hypercalcemia [6].
PTH Action in the cTAL
Key determinants of paracellular transport include the concentration gradient between the lumen and interstitium, the transepithelial potential, and the ion permeability of the junctional complex. Although the impact of PTH on NaCl transport in the cTAL is mediated, at least in part, by cAMP-dependent trafficking of NKCC2 to the luminal membrane (review: [96]), PTH is reported to have little or no effect on the transepithelial potential [166] and, thus, on the driving force for transport. Given that TRPV5 and other key components of the DCT transcellular Ca2+ reabsorption mechanism are not expressed in the cTAL (reviews: [96, 155]), it seems reasonable to consider whether PTH stimulation of Ca2+ reabsorption in the cTAL arises instead from enhanced Ca2+ permeability of the junctional complex, e.g., due to increased expression of claudins.
Claudins are a family of four-transmembrane domain, integral membrane proteins that co-localize with occludins in junctional complexes and are widely expressed in epithelial tissues (review: [111]). They are organized so that both their N- and C termini are intracellular, and form homo- and hetero-oligomers with characteristic isoform-specific expression patterns in renal tubular segments (reviews: [111, 167]). Mutations of claudin-16 or -19 have been linked to a rare human disorder of Ca2+ and Mg2+ reabsorption in the TAL known as familial hypercalciuric hypomagnesemia with nephrocalcinosis [168] (review: [167]). Trafficking of claudins to the junctional complexes is regulated, at least in part, by phosphorylation and a PKA site that is required for claudin-16 trafficking has been identified at residue S217 [169].
PTH Action in the DCT and Connecting Tubules
The cloning of TRPV5 (originally identified as ECaC-1) led to its identification as a key component of a transcellular Ca2+ transport apparatus in the distal nephron ([108, 170]; reviews: [156, 171]). The cloning of TRPV6 (originally identified as CaT1) represented a similar advance for understanding transcellular Ca2+ transport in the duodenum [107]. PTH was previously observed to activate Ca2+ uptake across the luminal membrane of distal tubular cells [172], however, the molecular basis for the effect was unknown. It is now clear that PTH promotes both the expression [165] and functional activity [173] of TRPV5. In addition, PTH promotes the expression of calbindin-D28k [165], calbindin-D9k [164], and NCX1 but not PMCA1b [165]. Thus, PTH is a powerful modulator of the expression of key components required for all rate-limiting steps of transcellular transport in the DCT and connecting tubules (see Fig. 2.6).
Calcitriol
The nature of the effect of calcitriol on renal calcium reabsorption was previously confusing due to its elevation of serum calcium levels and, thus, the filtered load. More recently, the availability of mice that are null either for 1α-hydroxylase or the VDR has permitted a more rigorous assessment of the role of calcitriol in renal calcium handling, leading to the conclusion that it promotes calcium conservation. Thus, VDR null mice exhibit inappropriately high renal Ca2+ losses in the context of hypocalcemia, despite the presence of secondary hyperparathyroidism [174]. In addition, 1α-hydroxylase null mice exhibit pronounced decreases in the expressions of key transport proteins of the distal nephron including TRPV5, calbindin D28k, and the basolateral Na+/Ca2+ exchanger, NCX1, which were corrected upon administration of exogenous calcitriol ([163]; see Fig. 2.6).
Calcitonin
Taken together with its well-recognized action in lowering serum Ca2+ concentration [175], the apparently paradoxical positive effect of calcitonin on renal calcium reabsorption suggests a primary role in redirecting calcium to bone and retaining it in established hydroxyapatite stores. Consistent with this notion, osteoclast-specific ablation of the calcitonin receptor in mice attenuated their resistance to hypercalcemia [176].
α-Klotho
α-klotho contributes to the control of the renal transport of both calcium and phosphate and is expressed at highest levels in the DCT [177] and at much lower levels in the proximal tubule [178]. It supports renal calcium transport via direct and indirect mechanisms. Thus, it directly stabilizes TRPV5 expression in the plasma membrane via its endogenous β-glucuronidase activity [86, 87]. Indirect roles, dependent in part on the modulation of serum calcitriol levels, are suggested by analyses of the α-klotho null mouse, which in addition to enhanced intestinal calcium absorption exhibits enhanced renal calcium excretion and renal tubular calcium-phosphate precipitation associated with down-regulated expression of calbindin-D9k and NCX1 [179].
Modulation of Renal Ca2+ Transport by the CaSR
Elevated extracellular Ca2+ concentration promotes renal calcium excretion independent of its action to suppress PTH levels [6]. Consistent with the notion that the kidney senses and responds to changes in the serum ionized Ca2+ concentration, the CaSR is widely expressed in the renal tubules. Expression in the proximal tubule provides a mechanism for modulating the effects of PTH and dietary phosphate intake on phosphate reabsorption (see below; [180, 181]). Expression in the mTAL provides a mechanism for suppressing NaCl reabsorption that takes the form of reduced expression of NKCC2 and ROMK, thereby disrupting uptake of Na+ and Cl− ions, and K+ recycling across the luminal membrane as well as reduced expression of CLC-KB in the basolateral membrane, thereby disrupting Cl− efflux into the extracellular fluid. Consistent with this notion, activating mutations of the CaSR induce a form of Bartter syndrome (type V) typified by salt wasting and hypokalemia (review: [96]).
Robust expression of the CaSR in the basolateral membrane of the cTAL [182] provides a mechanism for suppressing Ca2+ reabsorption and an important defense against hypercalcemia even in the PTH-null mouse [6]. Although its molecular basis is unclear, the CaSR appears to suppress the activities of NKCC2, ROMK, and Na+/K+-ATPase via the production of arachidonic acid and 20-HETE [183], thereby suppressing the electrical driving force. Alternatively, the CaSR may negatively modulate the insertion of the divalent cation-selective protein, claudin-16 into the junctional complexes thereby reducing the permeability of the paracellular route [184]. CaSR-dependent suppression of PTH-stimulated Ca2+ reabsorption in the cTAL arises, in part, from suppression of cAMP levels [185] either by activation of the heterotrimeric G-protein Gi or intracellular Ca2+-dependent inhibition of type-6 adenylyl cyclase or intracellular Ca2+-dependent activation of type-1 PDE (review: [96]).
Renal Tubular Phosphate Transport
Renal phosphate reabsorption localizes to the proximal tubule and is mediated primarily by the type-II transporters Npt2a and Npt2c, and to a lesser extent, by the type-III transporter, PiT-2 (review: [112]). The significance of Npt2a is underscored by the phenotype of Npt2a null mice, which includes frank renal phosphate wasting, hypophosphatemia, impaired trabecular bone development, elevated serum calcitriol levels, attendant hypercalcemia and hypercalciuria, as well as secondary hypoparathyroidism [186]. By comparison, Npt2c null mice failed to exhibit significant phosphate wasting, hypophosphatemia or a bone phenotype but instead prior to, but not after, weaning exhibited hypercalcemia and hypercalciuria associated with elevated serum calcitriol levels and reduced renal 24-hydroxylase mRNA expression [187]. Consistent with these findings, Npt2a mediates around 70% of Na/Pi co-transport in murine isolated brush border vesicles (review: [112]) and Npt2c mediates around 30% of renal Na/Pi cotransport in mice fed a low phosphate diet. The supporting role of Npt2c in murine proximal tubular phosphate reabsorption is underscored by the phenotype of double null Npt2a/Npt2c mice, which in comparison to either of the single null mice exhibit aggravated hypophosphatemia, exaggerated metabolic disturbances including an elevated calcitriol level, hypercalcemia, suppressed PTH and FGF23 levels, as well as a severe bone phenotype that includes rickets [188].
Initial analyses of human kindreds with the autosomal recessive disorder, hereditary hypophosphatemic rickets with hypercalciuria, indicated that Npt2c plays the primary role in renal phosphate transport in humans [189, 190]. However, a recent report indicates that an inactivating mutation of Npt2a that interferes with its surface expression can also induce a severe phosphate-wasting phenotype [191].
Regulation of Proximal Tubular Phosphate Transport
Proximal tubular phosphate reabsorption is regulated by both dietary and hormonal factors. Thus, low dietary phosphate promotes and high dietary phosphate suppresses the expression of proximal tubular phosphate transporters including Npt2a, Npt2c, and PiT-2 in the luminal brush border membrane (reviews: [112, 192]). Various hormonal factors modulate proximal tubule phosphate transport including the phosphaturic factors: PTH, FGF-23, secreted frizzled-related protein-4 (sFRP-4), and FGF-7 (reviews: [41, 112]). Calcitriol does not appear to modulate proximal tubule phosphate transport (review: [112]).
Regulation of Proximal Tubular Phosphate Transport by PTH
PTH acutely suppresses the surface expression of Npt2a in the luminal brush-border membranes of proximal tubule cells within minutes (see Fig. 2.7) and has more delayed effects on the surface expression of Npt2c and PiT-2 [24, 193]. PTH acts on type-I PTH receptors (PTH1Rs) expressed on both luminal and basolateral membranes of proximal tubule cells and stimulation of both PI-PLC, arising from the activation of luminal receptors, and adenylyl cyclase, arising from the activation of basolateral receptors, appear to contribute to the response (review: [112]). Signaling mechanisms downstream of PTH1R in the proximal tubule are dependent upon Na+/H+-exchanger regulatory factors (NHERFs), which interact via tandem PDZ domains with ion transporters including Npt2a as well as various GPCRs, including PTH1Rs (review: [112]). Consistent with a key role of NHERF-1 in proximal tubular phosphate transport, NHERF-1-null mice exhibit defective expression of Npt2a transporters along with phosphate wasting, hypophosphatemia, hypercalciuria, and osteomalacia [194], a phenotype that closely resembles that of Npt2a null mice [186]. NHERF1 stabilizes luminal membrane expression of Npt2a and modulates PTH action by promoting PTH1R-dependent activation of PI-PLC [195]. Recent work suggests that PTH triggers PKC-dependent phosphorylation of NHERF1, disabling its interaction with Npt2a thereby inducing Npt2a internalization. A recent report indicates that NHERFs may also contribute to the regulation of Npt2c and/or PiT-2 [196]. PTH has indirect as well as direct effects on renal phosphate transport, e.g., by promoting the expression of FGF-23 [26].
PTH suppresses phosphate reabsorption in the proximal tubule via receptors on both the luminal and basolateral membranes. Transcellular phosphate transport is mediated by Npt2a channels as well as Npt2c and PiT-2 channels (neither of the latter are shown) on the luminal membrane coupled to, an as yet, unidentified phosphate channel or transporter on the basolateral membrane. PTH acutely suppresses phosphate transport by disrupting the interaction between Npt2a and NHERF1 at the plasma membrane. In consequence, Npt2a is rapidly internalized and transcellular transport is markedly inhibited (review: [112]). Npt2c and PiT-2 are inhibited by alternative mechanisms with a much slower time-course. Other modulatory influences on phosphate transport are FGF-23, which like PTH markedly inhibits phosphate transport [70] and luminal calcium-sensing receptors, which dampen the inhibitory effect of PTH [181]
Regulation of Proximal Tubular Phosphate Transport by FGF-23 and α-Klotho
FGF-23 induces phosphaturia and impairs the renal synthesis of calcitriol [69, 70] via actions on the proximal tubules. The phosphaturic action of FGF-23 is dependent on FGF1c receptors [70] in the presence of the co-receptor, α-klotho [83, 85] and, like that of PTH, is dependent upon the internalization of Npt2a and Npt2c transporters [70, 72]. However, the actions of FGF-23 on renal phosphate excretion and serum phosphate are delayed in acute studies in humans, i.e., only after 6 h or more [69]. Due to the low level of α-klotho expression in the proximal tubule [177, 178], there has been speculation that the phosphaturic effect of FGF-23 arises indirectly via receptor-dependent expression of paracrine factors from the closely related DCTs ([197]; review: [71]).
Modulation of Proximal Tubular Phosphate Transport by the CaSR
As noted above, the CaSR plays an important role in the inhibitory control of renal calcium reabsorption, which is largely dependent upon its expression in the basolateral membrane of cTAL cells (review: [96]). This mechanism provides a key defense against hypercalcemia [6]. In addition, the CaSR is expressed in the luminal membrane of proximal tubule cells where it reduces the negative impact of PTH on phosphate reabsorption [181]. These results appear to indicate that elevated extracellular Ca2+ levels restrain the phosphaturic response to PTH, thereby limiting the magnitude of phosphate losses and the risk of hypophosphatemia. Interestingly, the introduction of a high phosphate diet or acute exposure to high dose PTH by intraperitoneal injection, both of which induce marked phosphaturia, appear to overcome this restraining mechanism by suppressing proximal tubular expression of the CaSR as well as Npt2a in rats ([180]; review: [92]). The findings suggest that a maximal phosphaturic response requires deactivation of CaSR-dependent inhibition of PTH-dependent signaling as well as decreased surface expression of Npt2a and/or Npt2c.
Novel Hormonal Link: Small Intestinal Phosphate Sensing Drives Renal Phosphate Excretion
Recent work indicates the presence of hitherto unrecognized endocrine interactions between the small intestine and the kidney in the control of phosphate metabolism. In one line of enquiry, instillation of phosphate into the rat small intestinal lumen induced prompt phosphaturia in the absence of an elevation in the serum phosphate level [198]. The phosphaturic effect was unaffected by parathyroidectomy or renal denervation, and occurred independent of changes in the serum levels of FGF-23 or two other phosphatonins, secreted frizzled-related protein-4 (sFRP4) or FGF-7. Consistent with the notion of phosphate-dependent, small intestinal production of a hormonal regulator of renal phosphate transport, infusion of a duodenal extract induced prompt phosphaturia [198]. In a second line of enquiry, homozygous null Npt2b mice, in which small intestinal phosphate absorption was markedly impaired, exhibited only a modest fall in the serum phosphate level in association with upregulated expression of renal proximal tubular Npt2a transporters and reduced serum FGF-23 levels [199]. The results indicate that renal phosphate reabsorption can compensate for impaired intestinal phosphate absorption. Finally, over-expression of a stable mutant of FGF-23, R179Q that retains full biological activity, was recently reported to suppress the expression of small intestinal Npt2b in wild-type mice but not in mice that were null for either of the proximal tubular transporters Npt2a or -2c, despite comparable suppression of serum calcitriol levels by FGF-23 (R179Q) in all three models [72]. The findings suggest dynamic coupling of phosphate transport in the kidney and small intestine, via currently unrecognized phosphate-sensing and associated effector mechanisms.
Roles of Bone and Cartilage in Calcium and Phosphate Metabolism
In addition to conferring mechanical rigidity on bone and cartilage, hydroxyapatite acts as the major bodily reservoir of calcium that is released in response to systemic calcium deficiency. It is also a major site of phosphate storage but, as described below, has lesser significance for the maintenance of serum phosphate levels. This section focuses on systemic factors that either control mineralization at the hydroxyapatite surface, and/or promote bone resorption. Clearly bone formation and mineralization are dependent upon the number, activity, and locations of osteoblasts and osteocytes. However, the burgeoning field of the molecular and cellular biology of bone formation has been excluded from the current discussion, except where it is of direct significance for extracellular fluid calcium and phosphate metabolism. For this reason, the author has omitted discussions of the mechanisms that support the anabolic effect of intermittent PTH including key modulators of osteoblastic bone formation. This extends to negative modulators including serotonin (review: [200]), β-adrenergic agonists (review: [201]), and glucocorticoids (review: [202]) as well as positive modulators including PTHrP (review: [203]), PTH (review: [204]), and IGF-1 (review: [205]) together with their interactions [206]. Thus, the sections that follow focus on the mechanisms that control bone resorption, thereby controlling the release of calcium and phosphate, and mineralization, thereby controlling the transfer of calcium and phosphate to bone.
Significance of the Hydroxyapatite Store in Mineral Metabolism
Although hydroxyapatite is a crystalline phosphate salt of calcium, there is no evidence that it buffers the serum phosphate concentration or corrects hypophosphatemia in the same way that it buffers the extracellular Ca2+ concentration or corrects hypocalcemia [207]. Thus, enhanced bone resorption is a key physiological response to hypocalcemia but there is no comparable resorptive response to hypophosphatemia. Instead, hypophosphatemia markedly impairs growth plate maturation and bone mineralization thereby restricting inorganic phosphate transfer from the extracellular fluid to bone (review: [105]). In addition, soft tissue phosphate stores in liver and muscle, contribute to the buffering of serum phosphate levels via mechanisms that are not well understood (review: [208]).
Significance of the Hydroxyapatite Store in the Context of Calcium Deficiency
The significance of the hydroxyapatite store for calcium metabolism is underscored by the development of acute hypocalcemia in subgroups of patients treated with agents that selectively disable bone resorption including bisphosphonates (review: [209]) or denosumab, a RANK-ligand neutralizing monoclonal antibody [210]. In addition, transgenic mice expressing collagenase-resistant type-I collagen, which disables bone resorption, exhibit a markedly impaired calcemic response to PTH (Fig. 2.8; [211]) indicating that bone resorption is a key element of the PTH-dependent defense against hypocalcemia. Furthermore, persistently elevated PTH in primary hyperparathyroidism promotes bone resorption and elevates the serum Ca2+ concentration in the context of renal Ca2+ retention. Due to its promotion of renal phosphate wasting, however, PTH tends to suppress the serum phosphate concentration in the context of bone resorption. In support of this outcome, PTH also stimulates the production of the phosphatonin FGF-23 [26, 82].
Impaired PTH-induced hypercalcemia in transgenic mice expressing collagenase-resistant type-I collagen. Transgenic rr mice that expressed collagenase-resistant type-I collagen exhibited markedly impaired bone resorption and calcium release in response to intraperitoneal injections of PTH. The data have been redrawn from [211]. Circles, wild-type mice; triangles, transgenic rr mice; open symbols, vehicle controls; closed symbols, PTH-treated. A greater than 50% reduction in PTH-induced hypercalcemia was observed in rr mice. (From [211]. Reprinted with permission)
Significance of the Hydroxyapatite Store in the Context of Phosphate Deficiency
Although the primary, bone-based, hormone response to systemic phosphate deficiency is impaired bone mineral formation [105, 212], hypophosphatemia also stimulates calcitriol synthesis and calcitriol, in turn, stimulates intestinal phosphate absorption. Thus, hypophosphatemia arising in Npt2a null, Npt2c null, and double null mice is associated with rickets and osteomalacia together with suppressed serum FGF-23 and PTH levels, an elevated serum calcitriol level and secondary hypercalciuria [188].
Molecular and Cellular Basis of Bone Resorption and its Regulation
Initial activation of the resorbing compartment at the bone surface requires the action of osteoblast lining cell-derived collagenase on type-I collagen (reviews: [213, 214]). Consistent with this conclusion, collagenase-resistant transgenic mice exhibit markedly impaired bone resorption under both basal and PTH-stimulated conditions [211, 215]. Subsequently, exposed extracellular matrix proteins including fibronectin and osteopontin promote β1-integrin-dependent activation of osteoblasts to express key activators of osteoclast maturation including intercellular adhesion molecule-1 (ICAM-1) and receptor activator of NF-κB ligand (RANK-L) [216]. Osteoblast lining cells also express macrophage colony-stimulating factor (M-CSF; [214]). Bone resorption is completed by the actions of mature osteoclasts, which establish membrane-limited resorption pits into which are released HCl and proteases including cathepsin K and, later, neutral matrix metallo-proteinases (review: [217]). The action of acid on hydroxyapatite releases calcium and phosphate in ionized form into the extracellular fluid.
Two main bone resorption scenarios need to be considered. The first relates to the maintenance of bone mass and architecture. In this scenario, resorption is a necessary component of remodeling and is targeted to the repair of areas of microdamage or to provide mechanical adaptation to altered strain. The outcomes include revised bone architecture together with enhanced bone quality and strength (reviews: [213, 214]). In one proposed mechanism, apoptotic osteocytes signal to osteoclasts via surface osteoblasts [218], e.g., by the withdrawal of tonic, sclerostin-mediated inhibition of osteoblast expression of the osteoclast activators M-CSF and RANK-ligand.
The second scenario is of particular interest to the present discussion and relates to the role of bone as a calcium store. This store is accessed upon a drop in the serum Ca2+ concentration via the release of CaSR-mediated tonic inhibition of PTH secretion leading to elevated serum PTH levels (review: [5]). A similar situation arises in the context of dysregulated PTH secretion, e.g., in primary hyperparathyroidism or FHH (review: [5]). Although hypocalcemia also promotes the synthesis of calcitriol, this effect is generally considered to be of primary significance for intestinal Ca2+ absorption rather than bone resorption (review: [33]). Nevertheless, calcitriol may enhance the responsiveness of osteoblasts and/or osteoclasts to persistent elevation of PTH [35].
PTH-Induced Remodeling
Elevated serum PTH levels induce a general activation of remodeling, which is particularly pronounced on the endocortical surface of cortical bone leading to increased porosity [219] but also affects trabecular bone [220]. Enhanced expression of RANK-L and decreased expression of its soluble receptor osteoprotegerin (OPG) are key elements of the response that include osteoclastogenesis and attendant bone resorption [221].
PTH1Rs, which respond to elevated serum PTH levels as well as locally synthesized PTHrP, are expressed on cells of the osteoblast lineage including marrow stromal cells, osteoblast lining cells, mature osteoblasts, and osteocytes [222]. In addition, PTH1Rs are expressed on T lymphocytes and recent work demonstrates that T-cell-specific deletion of PTH1Rs in mice protects against bone loss via impaired production of the cytokine TNF-α [19]. TNF-α promotes osteoclastogenesis, at least in part, by enhanced stromal cell expression of RANK-L and reduced expression of OPG [19].
Modulation of Bone Remodeling by Calcitriol
Calcitriol has multiple actions on bone mass and mineralization. As noted above, the most important of these actions appear to derive from the promotion of intestinal calcium and phosphate absorption since many of the phenotypic features of 1α-hydroxylase null mice and VDR null mice are corrected by rescue diets rich in calcium, phosphate, and macronutrients (review: [33]). Nevertheless, VDRs are expressed in cells of both the osteoblast and monocyte–macrophage–osteoclast lineages and promote bone formation, at least in part, by enhancing the proliferation of osteoblast progenitors [223], as well as bone resorption, by facilitating the expression of RANK-L [224]. These effects appear to be more pronounced with aging [223] and suggest a role for calcitriol in promoting bone remodeling and turnover (review: [35]). Interestingly, the outcomes of vitamin D receptor function appear to be critically dependent upon the differentiation state of the cells in which it is expressed. Thus, transgenic overexpression of the VDR in mature osteoblasts under the osteocalcin promoter enhanced bone mass and impaired bone resorption [225], suggesting a role for VDRs in the local downregulation of osteoclasts once mature osteoblasts have been established in the remodeling zone.
Bone Remodeling by Osteocytes
A role for osteocyte PTH1Rs in PTH-induced bone-resorption is suggested by the impact of over-expressing a constitutively active PTH1R under the control of the osteocyte-specific dentin matrix protein-1 (DMP-1) promoter [226]. Although such a mechanism might involve enhanced osteoclastogenesis and bone remodeling at the bone surface, recent work suggests that osteocytes may directly participate in a wider remodeling process that includes the perilacunar matrix and canalicular network dependent upon the upregulation of key bone resorption genes in osteocytes themselves (reviews: [227, 228]). Consistent with this concept, the volume of the perilacunar space appears to expand during lactation [228]. Thus, although the mechanisms are not well-defined, hormonal control of “osteocytic osteolysis” and osteocytic bone formation may also contribute to the control of whole body calcium and phosphate.
Other Modulators of Bone Resorption
Calcitonin
Calcitonin receptors are class B G-protein coupled receptors [229] expressed by mature osteoclasts [230]. They provide a mechanism by which hypercalcemia-induced calcitonin production impairs bone resorption. Consistent with this concept, both global [175] and osteoclast-specific [176] calcitonin receptor null mice exhibit loss of resistance to induced hypercalcemia. Thus, one key defense against hypercalcemia is based on the activation of the CaSR in thyroid C-cells [231], which promotes calcitonin secretion and, in turn, activates calcitonin receptors in osteoclasts to suppress bone resorption.
Estrogens
Postmenopausal bone loss is responsive to therapy with selective estrogen receptor modulators (SERMs) with a tissue selectivity profile that reduces the risk of major estrogen-related side effects including malignant transformation of mammary epithelial cells (review: [232]). Consistent with this behavior, estrogens negatively modulate bone resorption and bone turnover via effects on osteoclast survival and function, and analyses of transgenic mice indicate that both nuclear receptor subclasses, ER-α and ER-β contribute to the effects (review: [233]). However, the identities of the cell types that coordinate the responses, together with their molecular bases, are not well defined. Estrogens upregulate the expressions of Bcl-2, BMP-2, and OPG in osteoblasts, reduce the expression of cathepsin K in osteoclasts, and suppress the levels of pro-osteoclastogenic cytokines (review: [233]).
β-Adrenergic Signaling Promotes Osteoclastogenesis
In addition to its anti-appetite effect, leptin suppresses bone formation via a hypothalamic relay that activates the sympathetic nervous system [234]. In the periphery, β2-adrenergic receptors on osteoblasts suppress proliferation and promote the expression of RANK-L and, thus, induce osteoclastogenesis and bone resorption ([235]; review: [201]). The full physiological significance of this sympathetic nervous system mechanism and the nature of its primary sensing modalities are currently unknown.
Bone Formation and Local Regulation of Bone Mineralization
Bone formation, including expansion of the bone matrix and mineralization, is dependent upon the actions of mature, differentiated osteoblasts that align to the bone surface. Osteoblast maturation is under the control of signals arising from apoptotic osteocytes including withdrawal of sclerostin, a key inhibitor (review: [213]), together with various systemic signals as noted above.
Mature osteoblasts produce the osteoid collagen scaffold, initiate mineralization by seeding calcium-phosphate-containing matrix vesicles, and promote its nucleation via tissue nonspecific alkaline phosphatase (TNAP), which hydrolyses key local inhibitors including pyrophosphate (review: [236]) and polyphosphates [237]. Inactivating mutants of TNAP underlie the human inherited disorder, hypophosphatasia [238] and TNAP-null mice exhibit similar phenotypic features including rickets due to developmental arrest in epiphyses, as well as osteomalacia [239]. Although matrix vesicle-dependent calcium phosphate seeding occurs normally, extension of nascent hydroxyapatite crystals is arrested in the absence of TNAP (review: [240]).
Whether localized to the intracortical or endochondral surfaces of cortical bone, or to the trabeculae of cancellous bone, mineralization requires net transfers of calcium and phosphate ions from the plasma to the surfaces of growing hydroxyapatite crystals. Under steady-state conditions in adults around 8 mmol of calcium and 5 mmol of phosphate are transferred to hydroxyapatite per day and are balanced by similar fluxes of calcium and phosphate arising from bone resorption (Fig. 2.2; [207, 208]).
Control of Mineralization
Critical to the targeting of mineralization is its restriction to discrete sites that support bone integrity with avoidance of joint capsules and joint spaces. Thus, molecular mechanisms for suppressing inappropriate mineralization and for selectively deactivating them in a spatially and temporally appropriate manner are critical to effective growth and/or repair of bone. In addition to TNAP, the pyrophosphate exporter Ank, which is expressed by chondrocytes [241, 242] and osteoblasts [243] and exports pyrophosphate into the extracellular space, is an important contributor to the local inhibitory mechanism. Interestingly, pyrophosphate appears to have multiple inhibitory effects on mineralization that arise from mineral binding, upregulated expression of osteopontin, and suppressed TNAP activity [244].
Osteoblasts also modulate hydroxyapatite expansion by releasing SIBLINGs that bind to Ca2+ ions at the crystal surface [236]. They include DMP-1, bone sialoprotein, osteopontin, and MEPE. While these proteins typically act as inhibitors of hydroxyapatite formation in their full-length forms, phosphorylation and proteolytic processing yields peptides, which (1) promote mineralization, (2) yield more potent inhibitors of mineralization, and/or (3) enter the circulation to modify phosphate metabolism. Dmp1 provides an example of such an inhibitor, which in an intact phosphorylated form binds to hydroxyapatite and suppresses mineralization [245]. However, upon dephosphorylation and proteolytic processing, a 57-kDa C-terminal peptide is generated that promotes mineralization [245, 246].
SIBLING proteins are also modified by limited proteolysis to generate intensely acidic (i.e., negatively charged) peptides. These so-called ASARM peptides negatively modulate hydroxyapatite crystal growth [247] and upon their release into the blood, promote renal phosphate excretion to lower serum phosphate levels by downregulating the expression of Npt2a (review: [236]). An example of potential clinical importance arises in the context of the cleavage of osteopontin-derived ASARM peptides by the integral membrane endopeptidase, PHEX. Inactivating mutations of PHEX underlie X-linked hypophosphatemic rickets (review: [41]) and failure of PHEX cleavage of phosphorylated osteopontin results in the accumulation of a potent phosphopeptide inhibitor of mineralization [248]. Whether this, or a related, peptide promotes phosphate wasting, another key element of the phenotype, is currently unknown.
Rickets and Osteomalacia: Impact of Low Serum Phosphate
Rickets arises from impaired apoptosis of hypertrophic chondrocytes in the growth plate [249] resulting in a failure of vascularization and ossification. The serum phosphate concentration is critical to the process and hypophosphatemia induces the persistent survival of hypertrophic chondrocytes suggesting the existence of a phosphate-sensing mechanism coupled to the activation of apoptosis (review: [105]).
Osteomalacia arises from impaired mineralization of osteoblast- or osteocyte-derived osteoid and is also highly sensitive to the serum phosphate concentration [250]. In addition to its inhibitory effect on mineralization arising from the lowering of the calcium-phosphate solubility product, low serum phosphate concentration suppresses the maturation of osteoblasts as revealed by reduced expression of osteocalcin and osteopontin (review: [105]).
Hypophosphatemic Rickets
Several human inherited disorders feature hypophosphatemia and rickets. These include X-linked hypophosphatemic rickets (XLH), ADHR, and various forms of autosomal recessive hypophosphatemic rickets (ARHR). Hypophosphatemia has been causally linked to abnormalities of the growth plate and bone in all of these disorders. In part, it arises from impaired proteolytic processing and/or enhanced expression of FGF-23.
ADHR arises typically from gain-of-function mutations in the FGF-23 gene that disable a critical RXXR proteolytic cleavage site (residues 176–179; [63, 251]). Serum FGF-23 levels are elevated and hypophosphatemia results from renal phosphate wasting and an inadequate intestinal absorption response secondary to suppressed serum calcitriol levels. In addition to persistence of the active, full-length form of FGF-23, impaired processing interferes with the production of a potent C-terminal peptide antagonist of full-length FGF-23 [252].
XLH arises from inactivating mutations in the PHEX gene [253–255] and is also associated with elevations of the serum FGF-23 concentration [64]. The Hyp mouse, which has an inactivating mutation of the PHEX gene, exhibits a comparable phenotype to human XLH including elevated serum FGF-23 levels [256]. These phenotypes were initially ascribed to a failure of FGF-23 processing by defective PHEX [64] but PHEX does not cleave FGF-23 and instead appears to negatively modulate its expression [257]. Possibly via the processing of SIBLINGs as described above. Consistent with the notion that enhanced serum FGF-23 levels mediate hypophosphatemia in the Hyp mouse, fgf23/Hyp double null mice, in which FGF-23 production is ablated, exhibit renal phosphate retention and hyperphosphatemia, i.e., a reversal of the Hyp phenotype [258, 259].
ARHR arises from homozygous or compound heterozygous mutations of several genes in humans. These include inactivating mutations of the Npt2c or Npt2a genes (review: [260]) as noted above, as well as inactivating mutations of the Dmp1 gene, which exhibit hypophosphatemic rickets and enhanced serum FGF-23 levels ([189]; review: [261]). Consistent with this, Dmp1 null mice exhibit hypophosphatemic rickets associated with elevated serum FGF-23 levels and renal phosphate wasting [262]. The significance of elevated FGF-23 levels and attendant suppression of the serum phosphate level is underscored by the findings that FGF-23 neutralizing antibodies or a high phosphate diet rescues key features of the phenotype. These include rickets, osteomalacia, and delayed marrow cavity formation, as well as impaired osteoblast to osteocyte differentiation as reported by enhanced expression of Osterix (OSX) and Col-I, and reduced expression of SOST, which encodes the key inhibitory regulator of osteoblast differentiation, sclerostin [263]. Taken together, the results suggest that Dmp1, like PHEX, is a negative regulator of FGF-23 expression. The nature of the interactions between key regulators of osteoblast maturation and mineralization, on the one hand, and enzymes responsible for their proteolytic processing, on the other, remain to be determined. Nevertheless, it seems clear that limited proteolysis of Dmp1 like that of other SIBLING proteins is critical to its actions in bone [246].
Modulation of Bone Formation and Resorption by the Calcium-Sensing Receptor
Early cellular and transgenic mouse studies aimed at exploring a possible role of the CaSR in bone cell biology led to considerable confusion (review: [15]). More recent analyses based on tissue-specific deletion of CaSR exon-7, which encodes the receptor’s heptahelical signaling domain [264] rather than global deletion of CaSR exon-5, which encodes a C-terminal fragment of the receptor’s VFT nutrient-sensing domain [265], has led to a new appreciation of the roles played by the CaSR expressed in the osteoblast, chondrocyte, and monocyte–macrophage–osteoclast lineages with developmental implications for bone and cartilage as well as functional implications for bone remodeling. With respect to bone, homozygous deletion of CaSR exon-7 in osteoblasts under the control of either the Col-I or OSX promoters induced growth retardation and impaired mineralization associated with defective expression of type-I collagen, alkaline phosphatase, and osteocalcin [264]. Consistent with these findings, the CaSR inhibitor (calcilytic) NPS 89636 prevented extracellular Ca2+-dependent upregulation of key markers of osteoblast function in cultured fetal rat calvarial cells [266]. The results indicate that the CaSR, in the presence of physiologically relevant extracellular Ca2+ concentrations, promotes the differentiation of osteoblast progenitors. Furthermore, examination of neonatal global CaSR/PTH double null mice indicates that the CaSR is required for dietary calcium-dependent stimulation of bone turnover [267]. Thus, the CaSR may mediate key nutritional influences on bone development and bone mass (review: [104]). Surprisingly, expression of a constitutively active CaSR under the osteocalcin promoter induced cancellous bone loss in transgenic mice associated with increased osteoclast number and increased osteoclast surface [268]. The physiological significance of these findings requires further study.
The CaSR is also expressed in osteoclasts and their monocyte precursors. Although its significance in osteoclast biology is less well understood, it promotes monocyte differentiation and chemotaxis and provides a mechanism whereby elevated Ca2+ concentrations in the proximity of the bone resorption pit may negatively modulate further bone resorption prior to the recruitment, proliferation, and maturation of osteoblasts in support of bone formation (review: [93]).
Conclusion
When the author agreed to write this chapter, he thought he had undertaken to write an account of the regulation of calcium metabolism alone. That would have simplified life somewhat but would also have been a shame, since this story is really only complete when the metabolisms of both calcium and phosphate are considered together in detail. The story is necessarily complex and involves multiple small intestinal and renal tubular segments, as well as the development and homeostasis of bone and cartilage, together with hormonal and more local molecular signals, some yet undiscovered, that precisely control the functions of these tissues and their interactions. Much of the story points to the roles of micronutrient sensors including the CaSR, which has pluripotent roles in the gut, kidney, bone, and cartilage as well as other sensors, such as those for inorganic phosphate, not yet identified. Ultimately, it provides a picture of an asymmetric economy in which calcium and phosphate are common treated very differently from one another. The positive impact of PTH on serum calcium levels and its neutral or negative impact on serum phosphate levels provides one example. In addition, when their plasma levels are disturbed, calcium and phosphate provoke quite distinct outcomes on bone turnover. The cases of hypocalcemia and hypophosphatemia are particularly striking since the former provokes bone resorption and the latter arrests bone mineralization. There are notable exceptions: the intestinal absorptions of calcium and phosphate are both positively modulated by calcitriol; and calcium and phosphate belong together in the hydroxyapatite crystals in bone. Consideration of all these effects ultimately points to vitamin D and its active metabolites 25-hydroxyvitamin D and calcitriol as central regulators of calcium and phosphate metabolism. The metabolism of calcitriol, in particular, is salutary since it is subject to sensitive and potentially competitive control by all other key players including calcium, phosphate, PTH, and FGF-23. This places the regulation of intestinal calcium and phosphate absorption with its implications for bone mineralization at the very heart of the metabolic web. Clearly, however, there is much still to learn. What are the molecular identities of the phosphate sensors? How does the proteolytic processing of hydroxyapatite binding proteins control phosphate metabolism? What are the mechanisms that underlie the macronutrient modulation of calcium and, perhaps, phosphate transport? How do osteocytes coordinate the actions of bone remodeling cells and participate themselves in the processes of bone turnover? How does the nutritional modulator, 25-hydroxyvitamin D, interact with key control points in calcium and phosphate metabolism? And how can all of the context-specific variations that perturb calcium and phosphate metabolism independently of one another be explained?
References
Aylward GH, Findlay TJV. Dissociation constants of acids and hydrated metal ions. SI chemical data. Australasia: John Wiley and Sons; 1974. p. 122–4.
Wagner C, Devuyst O, Bourgeois S, Mohebbi N. Regulated acid–base transport in the collecting duct. Pflugers Arch. 2009;458:137–56.
Jovancicevic V, Bauer B. Calcium phosphate deposition on iron in oxygen-containing neutral aqueous solutions: an electrochemical approach. Langmuir. 1989;5:261–7.
Verbeeck RMH, Bruyne PAMD, Driessens FCM, Verbeek F. Solubility of magnesium hydrogen phosphate trihydrate and ion-pair formation in the system magnesium hydroxide-phosphoric acid-water at 25 degree C. Inorg Chem. 1984;23:1922–6.
Brown EM, MacLeod RJ. Extracellular calcium sensing and extracellular calcium signaling. Physiol Rev. 2001;81:239–97.
Kantham L, Quinn S, Egbuna O, Baxi K, Butters R, Pang J, Pollak M, Goltzman D, Brown E. The calcium-sensing receptor (CaSR) defends against hypercalcemia independently of its regulation of parathyroid hormone secretion. Am J Physiol Endocrinol Metab. 2009;297:E915–23.
Brown A, Zhong M, Finch J, Ritter C, McCracken R, Morrissey J, Slatopolsky E. Rat calcium-sensing receptor is regulated by vitamin D but not by calcium. Am J Physiol. 1996;270:F454–60.
Silver J, Russell J, Sherwood LM. Regulation by vitamin D metabolites of messenger ribonucleic acid for preproparathyroid hormone in isolated bovine parathyroid cells. Proc Natl Acad Sci USA. 1985;82:4270.
Silver J, Naveh-Many T, Mayer H, Schmelzer H, Popovtzer M. Regulation by vitamin D metabolites of parathyroid hormone gene transcription in vivo in the rat. J Clin Invest. 1986;78:1296–301.
Ben-Dov I, Galitzer H, Lavi-Moshayoff V, Goetz R, Kuro-o M, Mohammadi M, Sirkis R, Naveh-Many T, Silver J. The parathyroid is a target organ for FGF23 in rats. J Clin Invest. 2007;117:4003–8.
Ritter C, Armbrecht H, Slatopolsky E, Brown A. 25-Hydroxyvitamin D(3) suppresses PTH synthesis and secretion by bovine parathyroid cells. Kidney Int. 2006;70:654–9.
Laroche M. Phosphate, the renal tubule, and the musculoskeletal system. Joint Bone Spine. 2001;68: 211–5.
Conigrave AD, Mun H-C, Delbridge L, Quinn SJ, Wilkinson M, Brown EM. L-Amino acids regulate parathyroid hormone secretion. J Biol Chem. 2004;279:38151–9.
Conigrave AD, Quinn SJ, Brown EM. Cooperative multi-modal sensing and therapeutic implications of the extracellular Ca2+-sensing receptor. Trends Pharm Sci. 2000;21:401–7.
Conigrave AD, Hampson DR. Broad-spectrum amino acid-sensing class C G-protein coupled receptors: molecular mechanisms, physiological significance and options for drug development. Pharmacol Ther. 2010;127:252–60.
Hendy G, Goltzman D. Does calcitriol have actions independent from the vitamin D receptor in maintaining skeletal and mineral homeostasis? Curr Opin Nephrol Hypertens. 2005;14:350–4.
Slatopolsky E. The intact nephron hypothesis: the concept and its implications for phosphate management in CKD-related mineral and bone disorder. Kidney Int. 2011;121:S3–8.
Imura A, Tsuji Y, Murata M, Maeda R, Kubota K, Iwano A, Obuse C, Togashi K, Tominaga M, Kita N, Tomiyama K, Iijima J, Nabeshima Y, Fujioka M, Asato R, Tanaka S, Kojima K, Ito J, Nozaki K, Hashimoto N, Ito T, Nishio T, Uchiyama T, Fujimori T, Nabeshima Y. alpha-Klotho as a regulator of calcium homeostasis. Science. 2007;316:1615–8.
Tawfeek H, Bedi B, Li J, Adams J, Kobayashi T, Weitzmann M, Kronenberg H, Pacifici R. Disruption of PTH receptor 1 in T cells protects against PTH-induced bone loss. PLoS One. 2010;5:e12290.
Samadfam R, Xia Q, Miao D, Hendy G, Goltzman D. Exogenous PTH and endogenous 1,25-dihydroxyvitamin D are complementary in inducing an anabolic effect on bone. J Bone Miner Res. 2008;23:1257–66.
Lee M, Partridge N. Parathyroid hormone signaling in bone and kidney. Curr Opin Nephrol Hypertens. 2009;18:298–302.
Boros S, Bindels R, Hoenderop J. Active Ca(2+) reabsorption in the connecting tubule. Pflugers Arch. 2009;458:99–109.
Stewart A, Broadus A. The regulation of renal calcium excretion: an approach to hypercalciuria. Annu Rev Med. 1981;32:457–73.
Picard N, Capuano P, Stange G, Mihailova M, Kaissling B, Murer H, Biber J, Wagner C. Acute parathyroid hormone differentially regulates renal brush border membrane phosphate cotransporters. Pflugers Arch. 2010;460:677–87.
Popovtzer M, Massry S, Villamil M, Kleeman C. Renal handling of phosphorus in oliguric and nonoliguric mercury-induced acute renal failure in rats. J Clin Invest. 1971;50:2347–54.
Lavi-Moshayoff V, Wasserman G, Meir T, Silver J, Naveh-Many T. PTH increases FGF23 gene expression and mediates the high-FGF23 levels of experimental kidney failure: a bone parathyroid feedback loop. Am J Physiol Renal Physiol. 2010;299: F882–9.
Kolek O, Hines E, Jones M, LeSueur L, Lipko M, Kiela P, Collins J, Haussler M, Ghishan F. 1alpha,25-Dihydroxyvitamin D3 upregulates FGF23 gene expression in bone: the final link in a renal-gastrointestinal-skeletal axis that controls phosphate transport. Am J Physiol Gastrointest Liver Physiol. 2005;289:G1036–42.
Girotra M, Rubin M, Bilezikian J. The use of parathyroid hormone in the treatment of osteoporosis. Rev Endocr Metab Disord. 2006;7:113–21.
Goltzman D. Studies on the mechanisms of the skeletal anabolic action of endogenous and exogenous parathyroid hormone. Arch Biochem Biophys. 2008;473:218–24.
Parsons J, Neer R, Potts JJ. Initial fall of plasma calcium after intravenous injection of parathyroid hormone. Endocrinology. 1971;89:735–40.
Parsons J, Robinson C. Calcium shift into bone causing transient hypocalcaemia after injection of parathyroid hormone. Nature. 1971;230:581–2.
Kronenberg F. Emerging risk factors and markers of chronic kidney disease progression. Nat Rev Nephrol. 2009;5:677–89.
Bouillon R, Carmeiet G, Verlinden L, van Etten E, Verstuyf A, Luderer HF, Lieben L, Mathieu C, Demay M. Vitamin D and human health: lessons from vitamin D receptor null mice. Endocr Rev. 2008;29:726–76.
Masuda M, Yamamoto H, Kozai M, Tanaka S, Ishiguro M, Takei Y, Nakahashi O, Ikeda S, Uebanso T, Taketani Y, Segawa H, Miyamoto K, Takeda E. Regulation of renal sodium-dependent phosphate co-transporter genes (Npt2a and Npt2c) by all-trans-retinoic acid and its receptors. Biochem J. 2010; 429:583–92.
Goltzman D, Miao D, Panda D, Hendy G. Effects of calcium and of the vitamin D system on skeletal and calcium homeostasis: lessons from genetic models. J Steroid Biochem Mol Biol. 2004;2004:485–9.
Willett A. Vitamin D status and its relationship with parathyroid hormone and bone mineral status in older adolescents. Proc Nutr Soc. 2005;64:193–203.
Rowling M, Gliniak C, Welsh J, Fleet J. High dietary vitamin D prevents hypocalcemia and osteomalacia in CYP27B1 knockout mice. J Nutr. 2007;137:2608–15.
Yoshizawa T, Handa Y, Uematsu Y, Takeda S, Sekine K, Yoshihara Y, Kawakami T, Arioka K, Sato H, Uchiyama Y, Masushige S, Fukamizu A, Matsumoto T, Kato S. Mice lacking the vitamin D receptor exhibit impaired bone formation, uterine hypoplasia and growth retardation after weaning. Nat Genet. 1997;16:391–6.
Panda D, Miao D, Tremblay M, Sirois J, Farookhi R, Hendy G, Goltzman D. Targeted ablation of the 25-hydroxyvitamin D 1alpha-hydroxylase enzyme: evidence for skeletal, reproductive, and immune dysfunction. Proc Natl Acad Sci USA. 2001;98: 7498–503.
Dardenne O, Prud’homme J, Glorieux F, St-Arnaud R. Rescue of the phenotype of CYP27B1 (1alpha-hydroxylase)-deficient mice. J Steroid Biochem Mol Biol. 2004;89–90:327–30.
Bergwitz C, Jüppner H. Regulation of phosphate homeostasis by PTH, vitamin D, and FGF23. Annu Rev Med. 2010;61:91–104.
Heaney R, Dowell M, Hale C, Bendich A. Calcium absorption varies within the reference range for serum 25-hydroxyvitamin D. J Am Coll Nutr. 2003;22:142–6.
Whiting S, Calvo M. Correcting poor vitamin D status: do older adults need higher repletion doses of vitamin D3 than younger adults? Mol Nutr Food Res. 2010;54:1077–84.
Aloia J, Bojadzievski T, Yusupov E, Shahzad G, Pollack S, Mikhail M, Yeh J. The relative influence of calcium intake and vitamin D status on serum parathyroid hormone and bone turnover biomarkers in a double-blind, placebo-controlled parallel group, longitudinal factorial design. J Clin Endocrinol Metab. 2010;95:3216–24.
Björkman M, Sorva A, Tilvis R. Responses of parathyroid hormone to vitamin D supplementation: a systematic review of clinical trials. Arch Gerontol Geriatr. 2009;48:160–6.
Morris HA, Anderson PH. Autocrine and paracrine actions of vitamin D. Clin Biochem Rev. 2010; 31:129–38.
Nesbitt T, Drezner M. Insulin-like growth factor-I regulation of renal 25-hydroxyvitamin D-1-hydroxylase activity. Endocrinology. 1993;132:133–8.
Menaa C, Vrtovsnik F, Friedlander G, Corvol M, Garabédian M. Insulin-like growth factor I, a unique calcium-dependent stimulator of 1,25-dihydroxyvitamin D3 production. Studies in cultured mouse kidney cells. J Biol Chem. 1995;270:25461–7.
Bianda T, Hussain M, Glatz Y, Bouillon R, Froesch E, Schmid C. Effects of short-term insulin-like growth factor-I or growth hormone treatment on bone turnover, renal phosphate reabsorption and 1,25 dihydroxyvitamin D3 production in healthy man. J Intern Med. 1997;241:143–5.
Zierold C, Darwish H, DeLuca H. Two Vitamin D Response Elements Function in the Rat 1,25- Dihydroxyvitamin D 24-Hydroxylase Promoter. J Biol Chem. 1995;270:1675–8.
Meyer M, Goetsch P, Pike J. A downstream intergenic cluster of regulatory enhancers contributes to the induction of CYP24A1 expression by 1alpha,25-dihydroxyvitamin D3. J Biol Chem. 2010;285: 15599–610.
Lou Y-R, Molnar F, Perakyla M, Qiao A, Kalueff AV, St-Arnaud R, Carlberg C, Tuohimaa P. 25-Hydroxyvitamin D3 is an agonistic vitamin D receptor ligand. J Steroid Biochem Mol Biol. 2010;118:162–70.
St-Arnaud R, Arabian A, Travers R, Barletta F, Raval-Pandya M, Chapin K, Depovere J, Mathieu C, Christakos S, Demay M, Glorieux F. Deficient mineralization of intramembranous bone in vitamin D-24-hydroxylase-ablated mice is due to elevated 1,25-dihydroxyvitamin D and not to the absence of 24,25-dihydroxyvitamin D. Endocrinology. 2000;141:2658–66.
Nykjaer A, Dragun D, Walther D, Vorum H, Jacobsen C, Herz J, Melsen F, Christensen E, Willnow T. An endocytic pathway essential for renal uptake and activation of the steroid 25-(OH) vitamin D3. Cell. 1999;96:507–15.
Leheste J, Melsen F, Wellner M, Jansen P, Schlichting U, Renner-Müller I, Andreassen T, Wolf E, Bachmann S, Nykjaer A, Willnow T. Hypocalcemia and osteopathy in mice with kidney-specific megalin gene defect. FASEB J. 2003;17:247–9.
Nykjaer A, Fyfe JC, Kozyraki R, Leheste J-R, Jacobsen C, Nielsen MS, Verroust P, Aminoff M, de la Chapelle A, Moestrup SK, Ray R, Gliemann J, Willnow TE, Christensen EI. Cubilin dysfunction causes abnormal metabolism of the steroid hormone 25(OH) vitamin D3. Proc Natl Acad Sci USA. 2001;98:13895–900.
Safadi F, Thornton P, Magiera H, Hollis B, Gentile M, Haddad J, Liebhaber S, Cooke N. Osteopathy and resistance to vitamin D toxicity in mice null for vitamin D binding protein. J Clin Invest. 1999;103:239–51.
Christakos S, Dhawan P, Liu Y, Peng X, Porta A. New insights into the mechanisms of vitamin D action. J Cell Biochem. 2003;88:695–705.
Dardenne O, Prudhomme J, Hacking S, Glorieux F, St-Arnaud R. Rescue of the pseudo-vitamin D deficiency rickets phenotype of CYP27B1-deficient mice by treatment with 1,25-dihydroxyvitamin D3: biochemical, histomorphometric, and biomechanical analyses. J Bone Miner Res. 2003;18:637–43.
Xue Y, Fleet J. Intestinal vitamin D receptor is required for normal calcium and bone metabolism in mice. Gastroenterology. 2009;136:1317–27.
Hori M, Shimizu Y, Fukumoto S. Minireview: fibroblast growth factor 23 in phosphate homeostasis and bone metabolism. Endocrinology. 2011;152:4–10.
Jüppner H. Phosphate and FGF-23. Kidney Int. 2011;79 Suppl 121:S1–4.
ADHR-Consortium. Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. Nat Genet. 2000;26:345–8.
Jonsson K, Zahradnik R, Larsson T, White K, Sugimoto T, Imanishi Y, Yamamoto T, Hampson G, Koshiyama H, Ljunggren O, Oba K, Yang I, Miyauchi A, Econs M, Lavigne J, Jüppner H. Fibroblast growth factor 23 in oncogenic osteomalacia and X-linked hypophosphatemia. N Engl J Med. 2003;348:1656–63.
Garringer H, Malekpour M, Esteghamat F, Mortazavi S, Davis S, Farrow E, Yu X, Arking D, Dietz H, White K. Molecular genetic and biochemical analyses of FGF23 mutations in familial tumoral calcinosis. Am J Physiol Endocrinol Metab. 2008;295: E929–37.
Chefetz I, Sprecher E. Familial tumoral calcinosis and the role of O-glycosylation in the maintenance of phosphate homeostasis. Biochim Biophys Acta. 2009;1792:847–52.
Shimada T, Kakitani M, Yamazaki Y, Hasegawa H, Takeuchi Y, Fujita T, Fukumoto S, Tomizuka K, Yamashita T. Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J Clin Invest. 2004;113:561–8.
Razzaque M, Sitara D, Taguchi T, St-Arnaud R, Lanske B. Premature aging-like phenotype in fibroblast growth factor 23 null mice is a vitamin D-mediated process. FASEB J. 2006;20:720–2.
Shimada T, Hasegawa H, Yamazaki Y, Muto T, Hino R, Takeuchi Y, Fujita T, Nakahara K, Fukumoto S, Yamashita T. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J Bone Miner Res. 2004;19:429–35.
Gattineni J, Bates C, Twombley K, Dwarakanath V, Robinson M, Goetz R, Mohammadi M, Baum M. FGF23 decreases renal NaPi-2a and NaPi-2c expression and induces hypophosphatemia in vivo predominantly via FGF receptor 1. Am J Physiol Renal Physiol. 2009;297:F282–91.
Jüppner H, Wolf M, Salusky I. FGF-23: more than a regulator of renal phosphate handling? J Bone Miner Res. 2010;25:2091–7.
Tomoe Y, Segawa H, Shiozawa K, Kaneko I, Tominaga R, Hanabusa E, Aranami F, Furutani J, Kuwahara S, Tatsumi S, Matsumoto M, Ito M, Miyamoto K. Phosphaturic action of fibroblast growth factor 23 in Npt2 null mice. Am J Physiol Renal Physiol. 2010;298:F1341–50.
Saito H, Maeda A, Ohtomo S, Hirata M, Kusano K, Kato S, Ogata E, Segawa H, Miyamoto K, Fukushima N. Circulating FGF-23 is regulated by 1alpha,25-dihydroxyvitamin D3 and phosphorus in vivo. J Biol Chem. 2005;280:2543–9.
Saji F, Shigematsu T, Sakaguchi T, Ohya M, Orita H, Maeda Y, Ooura M, Mima T, Negi S. Fibroblast growth factor 23 production in bone is directly regulated by 1{alpha}, 25-dihydroxyvitamin D, but not PTH. Am J Physiol Renal Physiol. 2010;299: F1212–7.
Rejnmark L, Vestergaard P, Heickendorff L, Andreasen F, Mosekilde L. Loop diuretics alter the diurnal rhythm of endogenous parathyroid hormone secretion. A randomized-controlled study on the effects of loop- and thiazide-diuretics on the diurnal rhythms of calcitropic hormones and biochemical bone markers in postmenopausal women. Eur J Clin Invest. 2001;31:764–72.
Carpenter T, Insogna K, Zhang J, Ellis B, Nieman S, Simpson C, Olear E, Gundberg C. Circulating levels of soluble klotho and FGF23 in X-linked hypophosphatemia: circadian variance, effects of treatment, and relationship to parathyroid status. J Clin Endocrinol Metab. 2010;95:E352–7.
Perwad F, Azam N, Zhang M, Yamashita T, Tenenhouse H, Portale A. Dietary and serum phosphorus regulate fibroblast growth factor 23 expression and 1,25-dihydroxyvitamin D metabolism in mice. Endocrinology. 2005;146:5358–64.
Antoniucci D, Yamashita T, Portale A. Dietary phosphorus regulates serum fibroblast growth factor-23 concentrations in healthy men. J Clin Endocrinol Metab. 2006;91:3144–9.
Nishida Y, Taketani Y, Yamanaka-Okumura H, Imamura F, Taniguchi A, Sato T, Shuto E, Nashiki K, Arai H, Yamamoto H, Takeda E. Acute effect of oral phosphate loading on serum fibroblast growth factor 23 levels in healthy men. Kidney Int. 2006;70: 2141–7.
Ito N, Fukumoto S, Takeuchi Y, Takeda S, Suzuki H, Yamashita T, Fujita T. Effect of acute changes of serum phosphate on fibroblast growth factor (FGF)23 levels in humans. J Bone Miner Metab. 2007;25: 419–22.
Vervloet M, van Ittersum F, Büttler R, Heijboer A, Blankenstein M, ter Wee P. Effects of dietary phosphate and calcium intake on fibroblast growth factor-23. Clin J Am Soc Nephrol. 2011;6:383–9.
Burnett-Bowie S, Henao M, Dere M, Lee H, Leder B. Effects of hPTH(1–34) infusion on circulating serum phosphate, 1,25-dihydroxyvitamin D, and FGF23 levels in healthy men. J Bone Miner Res. 2009;24:1681–5.
Kurosu H, Ogawa Y, Miyoshi M, Yamamoto M, Nandi A, Rosenblatt K, Baum M, Schiavi S, Hu M, Moe O, Kuro-o M. Regulation of fibroblast growth factor-23 signaling by klotho. J Biol Chem. 2006;281:6120–3.
Nakatani T, Sarraj B, Ohnishi M, Densmore M, Taguchi T, Goetz R, Mohammadi M, Lanske B, Razzaque M. In vivo genetic evidence for klotho-dependent, fibroblast growth factor 23 (Fgf23)-mediated regulation of systemic phosphate homeostasis. FASEB J. 2009;23:433–41.
Urakawa I, Yamazaki Y, Shimada T, Ijima K, Hasegawa H, Okawa K, Fujita T, Fukumoto S, Yamashita T. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature. 2006;444:770–4.
Chang Q, Hoefs S, van der Kemp A, Topala C, Bindels R, Hoenderop J. The beta-glucuronidase klotho hydrolyzes and activates the TRPV5 channel. Science. 2005;310:490–3.
Cha S, Ortega B, Kurosu H, Rosenblatt K, Kuro-O M, Huang C. Removal of sialic acid involving Klotho causes cell-surface retention of TRPV5 channel via binding to galectin-1. Proc Natl Acad Sci USA. 2008;105:9805–10.
de Groot T, Bindels R, Hoenderop J. TRPV5: an ingeniously controlled calcium channel. Kidney Int. 2008;74:1241–6.
Tsuruoka S, Nishiki K, Ioka T, Ando H, Saito Y, Kurabayashi M, Nagai R, Fujimura A. Defect in parathyroid-hormone-induced luminal calcium absorption in connecting tubules of Klotho mice. Nephrol Dial Transplant. 2006;21:2762–7.
Brown EM, Gamba G, Riccardi D, Lombardi M, Butters R, Kifor O, Sun A, Hediger MA, Lytton J, Hebert SC. Cloning and characterization of an extracellular Ca2+−sensing receptor from bovine parathyroid. Nature. 1993;366:575–80.
Riccardi D, Park J, Lee W, Gamba G, Brown EM, Hebert SC. Cloning and functional expression of a rat kidney extracellular calcium/ polyvalent cation receptor. Proc Natl Acad Sci USA. 1995;92:131–5.
Riccardi D, Brown E. Physiology and pathophysiology of the calcium-sensing receptor in the kidney. Am J Physiol Renal Physiol. 2010;298:F485–99.
Yamaguchi T. The calcium-sensing receptor in bone. J Bone Miner Metab. 2008;26:301–11.
Conigrave AD, Franks AH, Brown EM, Quinn SJ. L-Amino acid sensing by the calcium-sensing receptor: a general mechanism for coupling protein and calcium metabolism? Eur J Clin Nutr. 2002;56:1072–80.
Pollak MR, Brown EM, Chou YW, Hebert SC, Marx SJ, Steinmann B, Levi T, Seidman CE, Seidman JG. Mutations in the human Ca2+-sensing receptor gene cause familial hypocalciuric hypercalcemia and neonatal severe hyperparathyroidism. Cell. 1993;75: 1297–303.
Gamba G, Friedman PA. Thick ascending limb: the Na+: K+: 2Cl– co-transporter, NKCC2, and the calcium-sensing receptor. Pflugers Arch. 2009;458: 61–76.
Brown EM. Clincal lessons from the calcium-sensing receptor. Nat Clin Pract Endocrinol Metab. 2007;3:122–33.
Pollak MR, Chou YW, Marx SJ, Steinmann B, Cole DEC, Brandi ML, Papdopoulos SE, Menko FH, Hendy GN, Brown EM, Seidman CE, Seidman JG. Familial hypocalciuric hypercalcemia and neonatal severe hyperparathyroidism. Effects of mutant gene dosage on phenotype. J Clin Invest. 1994;93:1108–12.
Conigrave AD, Brown EM. l-Amino acid-sensing by calcium-sensing receptors: implications for GI physiology. Am J Physiol. 2006;291:G753–61.
Conigrave AD, Hampson DR. Broad-spectrum amino acid sensing by class 3 G-protein coupled receptors. Trends Endocrinol Metab. 2006;17: 398–407.
Hira T, Nakajima S, Eto Y, Hara H. Calcium-sensing receptor mediates phenylalanine-induced cholecystokinin secretion in enteroendocrine STC-1 cells. FEBS J. 2008;275:4620–6.
Wang Y, Chandra R, Samsa L, Gooch B, Fee B, Cook J, Vigna S, Grant A, Liddle R. Amino acids stimulate cholecystokinin release through the calcium-sensing receptor. Am J Physiol Gastrointest Liver Physiol. 2010;300:G528–37.
Liou A, Sei Y, Zhao X, Feng J, Lu X, Thomas C, Pechhold S, Raybould H, Wank S. The extracellular calcium sensing receptor is required for cholecystokinin secretion in response to l-phenylalanine in acutely isolated intestinal I cells. Am J Physiol Gastrointest Liver Physiol. 2011;300:G538–46.
Conigrave AD, Brown EM, Rizzoli R. Dietary protein and bone health: roles of amino acid-sensing receptors in the control of calcium metabolism and bone homeostasis. Annu Rev Nutr. 2008;28: 131–55.
Tiosano D, Hochberg Z. Hypophosphatemia: the common denominator of all rickets. J Bone Miner Metab. 2009;27:392–401.
Khoshniat S, Bourgine A, Julien M, Weiss P, Guicheux J, Beck L. The emergence of phosphate as a specific signaling molecule in bone and other cell types in mammals. Cell Mol Life Sci. 2011;68:205–18.
Peng JB, Chen XZ, Berger UV, Vassilev PM, Tsukaguchi H, Brown EM, Hediger MA. Molecular cloning and characterization of a channel-like transporter mediating intestinal calcium absorption. J Biol Chem. 1999;274:22739–46.
Hoenderop JG, van der Kemp AW, Hartog A, van de Graaf SF, van Os CH, Willems PH, Bindels RJ. Molecular identification of the apical Ca2+ channel in 1, 25-dihydroxyvitamin D3-responsive epithelia. J Biol Chem. 1999;274:8375–8.
Hoenderop J, Nilius B, Bindels R. Calcium absorption across epithelia. Physiol Rev. 2005;85:373–422.
Suzuki Y, Landowski C, Hediger M. Mechanisms and regulation of epithelial Ca2+ absorption in health and disease. Annu Rev Physiol. 2008;70: 257–71.
Angelow S, Ahlstrom R, Yu A. Biology of claudins. Am J Physiol Renal Physiol. 2008;295:F867–76.
Biber J, Hernando N, Forster I, Murer H. Regulation of phosphate transport in proximal tubules. Pflugers Arch. 2009;458:39–52.
Murer H, Forster I, Biber J. The sodium phosphate cotransporter family SLC34. Pflugers Arch. 2004; 447:763–7.
Marks J, Debnam ES, Unwin RJ. Phosphate homeostasis and the renal-gastrointestinal axis. Am J Physiol Renal Physiol. 2010;299:F285–96.
Yamaguchi T, Kimoto E. Inhibition of phosphate transport across the human erythrocyte membrane by chemical modification of sulfhydryl groups. Biochemistry. 1992;31:1968–73.
Palmieri F. The mitochondrial transporter family (SLC25): physiological and pathological implications. Pflugers Arch. 2004;447:689–709.
Kerstetter JE, O’Brien KO, Caseria DM, Wall DE, Insogna KL. The impact of dietary protein on calcium absorption and kinetic measures of bone turnover in women. J Clin Endocrinol Metab. 2005;90:26–31.
Kerstetter JE, O’Brien KO, Insogna KL. Dietary protein affects intestinal calcium absorption. Am J Clin Nutr. 1998;68:859–65.
Mace OJ, Lister N, Morgan E, Shepherd E, Affleck J, Helliwell P, Bronk JR, Kellett GL, Meredith D, Boyd R, Pieri M, Bailey PD, Pettcrew R, Foley D. An energy supply network of nutrient absorption coordinated by calcium and T1R taste receptors in rat small intestine. J Physiol. 2009;587:195–210.
Dardenne O, Prud’homme J, Hacking S, Glorieux F, St-Arnaud R. Correction of the abnormal mineral ion homeostasis with a high-calcium, high-phosphorus, high-lactose diet rescues the PDDR phenotype of mice deficient for the 25-hydroxyvitamin D-1alpha-hydroxylase (CYP27B1). Bone. 2003;32: 332–40.
O’Loughlin P, Morris H. Oestrogen deficiency impairs intestinal calcium absorption in the rat. J Physiol. 1998;511:313–22.
Colin E, Van Den Bemd G, Van Aken M, Christakos S, De Jonge H, Deluca H, Prahl J, Birkenhäger J, Buurman C, Pols H, Van Leeuwen J. Evidence for involvement of 17beta-estradiol in intestinal calcium absorption independent of 1,25-dihydroxyvitamin D3 level in the rat. J Bone Miner Res. 1999;14:57–64.
Ten Bolscher M, Netelenbos J, Barto R, Van Buuren L, Van der vijgh W. Estrogen regulation of intestinal calcium absorption in the intact and ovariectomized adult rat. J Bone Miner Res. 1999;14:1197–202.
Pahuja D, DeLuca H. Stimulation of intestinal calcium transport and bone calcium mobilization by prolactin in vitamin D-deficient rats. Science. 1981;214:1038–9.
Charoenphandhu N, Wongdee K, Krishnamra N. Is prolactin the cardinal calciotropic maternal hormone? Trends Endocrinol Metab. 2010;21:395–401.
Takeuchi K, Morikawa H, Ueda Y, Mochizuki M. Studies on the effects of placental lactogen on calcium metabolism during pregnancy. Nippon Naibunpi Gakkai Zasshi. 1988;64:1175–86.
Bronner F. Recent developments in intestinal calcium absorption. Nutr Rev. 2009;67:109–13.
Nordin BE. Calcium and osteoporosis. Nutrition. 1997;13:664–86.
Fudge N, Kovacs C. Pregnancy up-regulates intestinal calcium absorption and skeletal mineralization independently of the vitamin D receptor. Endocrinology. 2010;151:886–95.
Ajibade D, Dhawan P, Fechner A, Meyer M, Pike J, Christakos S. Evidence for a role of prolactin in calcium homeostasis: regulation of intestinal transient receptor potential vanilloid type 6, intestinal calcium absorption, and the 25-hydroxyvitamin D(3) 1alpha hydroxylase gene by prolactin. Endocrinology. 2010;151:2974–84.
Wasserman R, Kallfelz F, Comar C. Active transport of calcium by rat duodenum in vivo. Science. 1961;133:883–4.
Van Cromphaut S, Dewerchin M, Hoenderop J, Stockmans I, Van Herck E, Kato S, Bindels R, Collen D, Carmeliet P, Bouillon R, Carmeliet G. Duodenal calcium absorption in vitamin D receptor-knockout mice: functional and molecular aspects. Proc Natl Acad Sci USA. 2001;98:13324–9.
Chang Y, Hegsted D. Lactose and calcium transport in gut sacs. J Nutr. 1964;82:297–300.
Morgan E, Mace O, Affleck J, Kellett G. Apical GLUT2 and Cav1.3: regulation of rat intestinal glucose and calcium absorption. J Physiol. 2007;580: 593–604.
Akhter S, Kutuzova G, Christakos S, DeLuca H. Calbindin D9k is not required for 1,25-dihydroxyvitamin D3-mediated Ca2+ absorption in small intestine. Arch Biochem Biophys. 2007;460:227–32.
Kutuzova G, Sundersingh F, Vaughan J, Tadi B, Ansay S, Christakos S, Deluca H. TRPV6 is not required for 1alpha,25-dihydroxyvitamin D3-induced intestinal calcium absorption in vivo. Proc Natl Acad Sci USA. 2008;105:19655–9.
Benn B, Ajibade D, Porta A, Dhawan P, Hediger M, Peng J, Jiang Y, Oh G, Jeung E, Lieben L, Bouillon R, Carmeliet G, Christakos S. Active intestinal calcium transport in the absence of transient receptor potential vanilloid type 6 and calbindin-D9k. Endocrinology. 2008;149:3196–205.
Christakos S, Dhawan P, Ajibade D, Benn B, Feng J, Joshi S. Mechanisms involved in vitamin D mediated intestinal calcium absorption and in non-classical actions of vitamin D. J Steroid Biochem Mol Biol. 2010;121:183–7.
Gaffney-Stomberg E, Sun B, Cucchi C, Simpson C, Gundberg C, Kerstetter J, Insogna K. The effect of dietary protein on intestinal calcium absorption in rats. Endocrinology. 2010;151:1071–8.
Wright M, Sullivan R, Gaffney-Stomberg E, Caseria D, O’Brien K, Proctor D, Simpson C, Kerstetter J, Insogna K. Inhibiting gastric acid production does not affect intestinal calcium absorption in young, healthy individuals: a randomized, crossover, controlled clinical trial. J Bone Miner Res. 2010;25:2205–11.
Marks J, Srai SK, Biber J, Murer H, Unwin RJ, Debnam ES. Intestinal phosphate absorption and the effect of vitamin D: a comparison of rats with mice. Exp Physiol. 2006;91:531–7.
Rizzoli R, Fleisch H, Bonjour J. Role of 1,25-dihydroxyvitamin D3 on intestinal phosphate absorption in rats with a normal vitamin D supply. J Clin Invest. 1977;60:639–47.
Jones G, Strugnell S, DeLuca H. Current understanding of the molecular actions of vitamin D. Physiol Rev. 1998;78:1193–231.
Cross HS, Debiec H, Peterlik M. Mechanism and regulation of intestinal phosphate absorption. Miner Electrolyte Metab. 1990;16:115–24.
Williams KB, DeLuca HF. Characterization of intestinal phosphate absorption using a novel method. Am J Physiol Endocrinol Metab. 2007;292: E1917–21.
Katai K, Miyamoto K, Kishida S, Segawa H, Nii T, Tanaka H, Tani Y, Arai H, Tatsumi S, Morita K, Taketani Y, Takeda E. Regulation of intestinal Na+−dependent phosphate co-transporters by a low-phosphate diet and 1,25-dihydroxyvitamin D3. Biochem J. 1999;343:705–12.
Hattenhauer O, Traebert M, Murer H, Biber J. Regulation of small intestinal Na-P(i) type IIb cotransporter by dietary phosphate intake. Am J Physiol. 1999;277:G756–62.
Capuano P, Radanovic T, Wagner CA, Bacic D, Kato S, Uchiyama Y, St-Arnoud R, Murer H, Biber J. Intestinal and renal adaptation to a low-Pi diet of type II NaPi cotransporters in vitamin D receptor- and 1αOHase-deficient mice. Am J Physiol Cell Physiol. 2005;288:C429–34.
Xu H, Uno J, Inouye M, Xu L, Drees J, Collins J, Ghishan F. Regulation of intestinal NaPi-IIb cotransporter gene expression by estrogen. Am J Physiol Gastrointest Liver Physiol. 2003;285:G1317–24.
Heaney R. Phosphorus nutrition and the treatment of osteoporosis. Mayo Clin Proc. 2004;79:91–7.
Xu H, Collins J, Bai L, Kiela P, Ghishan F. Regulation of the human sodium-phosphate cotransporter NaP(i)-IIb gene promoter by epidermal growth factor. Am J Physiol Cell Physiol. 2001;280:C628–36.
Borowitz S, Granrud G. Glucocorticoids inhibit intestinal phosphate absorption in developing rabbits. J Nutr. 1992;122:1273–9.
Arima K, Hines E, Kiela P, Drees J, Collins J, Ghishan F. Glucocorticoid regulation and glycosylation of mouse intestinal type IIb Na-P(i) cotransporter during ontogeny. Am J Physiol Gastrointest Liver Physiol. 2002;283:G426–34.
Chen H, Xua H, Duong J, Li J, Ghishan FK. Tumor necrosis factor-alpha impairs intestinal phosphate absorption in colitis. Am J Physiol Gastrointest Liver Physiol. 2009;296:G775–81.
Dimke H, Hoenderop HJ, Bindels RJ. Hereditary tubular transport disorders: implications for renal handling of Ca2+ and Mg2+. Clin Sci (Lond). 2010;118:1–18.
Markadieu N, Bindels R, Hoenderop J. The renal connecting tubule: resolved and unresolved issues in Ca(2+) transport. Int J Biochem Cell Biol. 2011;43: 1–4.
van de Graaf SF, Boullart I, Hoenderop JG, Bindels RJ. Regulation of the epithelial Ca2+ channels TRPV5 and TRPV6 by 1alpha, 25-dihydroxy Vitamin D3 and dietary Ca2+. J Steroid Biochem Mol Biol. 2004;89–90:303–8.
Hsu Y, Dimke H, Hoenderop J, Bindels R. Calcitonin-stimulated renal Ca2+ reabsorption occurs independently of TRPV5. Nephrol Dial Transplant. 2010;25: 1428–35.
Unwin R, Capasso G, Shirley D. An overview of divalent cation and citrate handling by the kidney. Nephron Physiol. 2004;98:15–20.
Shirley D, Walter S, Folkerd E, Unwin R, Bailey M. Transepithelial electrochemical gradients in the proximal convoluted tubule during potassium depletion in the rat. J Physiol. 1998;513:551–7.
Hemmingsen C. Regulation of renal calbindin-D28K. Pharmacol Toxicol. 2007;87 Suppl 3:5–30.
Koster H, Hartog A, Van Os C, Bindels R. Calbindin-D28K facilitates cytosolic calcium diffusion without interfering with calcium signaling. Cell Calcium. 1995;18:187–96.
Hoenderop J, Dardenne O, Van Abel M, Van Der Kemp A, Van Os C, St-Arnaud R, Bindels R. Modulation of renal Ca2+ transport protein genes by dietary Ca2+ and 1,25-dihydroxyvitamin D3 in 25-hydroxyvitamin D3-1alpha-hydroxylase knockout mice. FASEB J. 2002;16:1398–406.
Cao L, Bolt M, Wei M, Sitrin M, Chun LY. Regulation of calbindin-D9k expression by 1,25-dihydroxyvitamin D(3) and parathyroid hormone in mouse primary renal tubular cells. Arch Biochem Biophys. 2002;400:118–24.
van Abel M, Hoenderop J, van der Kemp A, Friedlaender M, van Leeuwen J, Bindels R. Coordinated control of renal Ca(2+) transport proteins by parathyroid hormone. Kidney Int. 2005;68(4):1708–21.
Suki W, Rouse D, Ng R, Kokko J. Calcium transport in the thick ascending limb of Henle. Heterogeneity of function in the medullary and cortical segments. J Clin Invest. 1980;66:1004–9.
Günzel D, Yu A. Function and regulation of claudins in the thick ascending limb of Henle. Pflugers Arch. 2009;458:77–88.
Simon D, Lu Y, Choate K, Velazquez H, Al-Sabban E, Praga M, Casari G, Bettinelli A, Colussi G, Rodriguez-Soriano J, McCredie D, Milford D, Sanjad S, Lifton R. Paracellin-1, a renal tight junction protein required for paracellular Mg2+ resorption. Science. 1999;285:103–6.
Ikari A, Matsumoto S, Harada H, Takagi K, Hayashi H, Suzuki Y, Degawa M, Miwa M. Phosphorylation of paracellin-1 at Ser217 by protein kinase A is essential for localization in tight junctions. J Cell Sci. 2006;119:1781–9.
Müller D, Hoenderop J, Meij I, van den Heuvel L, Knoers N, den Hollander A, Eggert P, García-Nieto V, Claverie-Martín F, Bindels R. Molecular cloning, tissue distribution, and chromosomal mapping of the human epithelial Ca2+ channel (ECAC1). Genomics. 2000;67:48–53.
Lambers T, Bindels R, Hoenderop J. Coordinated control of renal Ca2+ handling. Kidney Int. 2006;69: 650–4.
Lajeunesse D, Bouhtiauy I, Brunette M. Parathyroid hormone and hydrochlorothiazide increase calcium transport by the luminal membrane of rabbit distal nephron segments through different pathways. Endocrinology. 1994;134:35–41.
de Groot T, Lee K, Langeslag M, Xi Q, Jalink K, Bindels R, Hoenderop J. Parathyroid hormone activates TRPV5 via PKA-dependent phosphorylation. J Am Soc Nephrol. 2009;20:1693–704.
Li Y, Bolt M, Cao L, Sitrin M. Effects of vitamin D receptor inactivation on the expression of calbindins and calcium metabolism. Am J Physiol Endocrinol Metab. 2001;281:E558–64.
Davey R, Turner A, McManus J, Chiu W, Tjahyono F, Moore A, Atkins G, Anderson P, Ma C, Glatt V, MacLean H, Vincent C, Bouxsein M, Morris H, Findlay D, Zajac J. Calcitonin receptor plays a physiological role to protect against hypercalcemia in mice. J Bone Miner Res. 2008;23:1182–93.
Turner A, Tjahyono F, Chiu W, Skinner J, Sawyer R, Moore A, Morris H, Findlay D, Zajac J, Davey R. The role of the calcitonin receptor in protecting against induced hypercalcemia is mediated via its actions in osteoclasts to inhibit bone resorption. Bone. 2011;48:354–61.
Li S, Watanabe M, Yamada H, Nagai A, Kinuta M, Takei K. Immunohistochemical localization of Klotho protein in brain, kidney, and reproductive organs of mice. Cell Struct Funct. 2004;29: 91–9.
Hu M, Shi M, Zhang J, Pastor J, Nakatani T, Lanske B, Razzaque M, Rosenblatt K, Baum M, Kuro-o M, Moe O. Klotho: a novel phosphaturic substance acting as an autocrine enzyme in the renal proximal tubule. FASEB J. 2010;24:3438–50.
Alexander R, Woudenberg-Vrenken T, Buurman J, Dijkman H, van der Eerden B, van Leeuwen J, Bindels R, Hoenderop J. Klotho prevents renal calcium loss. J Am Soc Nephrol. 2009;20:2371–9.
Riccardi D, Traebert M, Ward DT, Kaissling B, Biber J, Hebert SC, Murer H. Dietary phosphate and parathyroid hormone alter the expression of the calcium-sensing receptor (CaR) and the Na+−dependent Pi transporter (NaPi-2) in the rat proximal tubule. Pflugers Arch. 2000;441:379–87.
Ba J, Brown D, Friedman PA. Calcium-sensing receptor regulation of PTH-inhibitable proximal tubule phosphate transport. Am J Physiol. 2003;285: F1233–43.
Riccardi D, Hall AE, Chattopadhyay N, Xu JZ, Brown EM, Hebert SC. Localization of the extracellular Ca2+/polyvalent cation-sensing protein in rat kidney. Am J Physiol. 1998;274:F611–22.
Escalante B, Erlij D, Falck J, McGiff J. Effect of cytochrome P450 arachidonate metabolites on ion transport in rabbit kidney loop of Henle. Science. 1991;251:799–802.
Ikari A, Okude C, Sawada H, Sasaki Y, Yamazaki Y, Sugatani J, Degawa M, Miwa M. Activation of a polyvalent cation-sensing receptor decreases magnesium transport via claudin-16. Biochim Biophys Acta. 2008;1778:283–90.
Takaichi K, Kurokawa K. High Ca2+ inhibits peptide hormone-dependent cAMP production specifically in thick ascending limbs of Henle. Miner Electrolyte Metab. 1986;12:342–6.
Beck L, Karaplis A, Amizuka N, Hewson A, Ozawa H, Tenenhouse H. Targeted inactivation of Npt2 in mice leads to severe renal phosphate wasting, hypercalciuria, and skeletal abnormalities. Proc Natl Acad Sci USA. 1998;95:5372–7.
Segawa H, Onitsuka A, Kuwahata M, Hanabusa E, Furutani J, Kaneko I, Tomoe Y, Aranami F, Matsumoto N, Ito M, Matsumoto M, Li M, Amizuka N, Miyamoto K. Type IIc sodium-dependent phosphate transporter regulates calcium metabolism. J Am Soc Nephrol. 2009;20:104–13.
Segawa H, Onitsuka A, Furutani J, Kaneko I, Aranami F, Matsumoto N, Tomoe Y, Kuwahata M, Ito M, Matsumoto M, Li M, Amizuka N, Miyamoto K. Npt2a and Npt2c in mice play distinct and synergistic roles in inorganic phosphate metabolism and skeletal development. Am J Physiol Renal Physiol. 2009;297:F671–8.
Lorenz-Depiereux B, Benet-Pages A, Eckstein G, Tenenbaum-Rakover Y, Wagenstaller J, Tiosano D, Gershoni-Baruch R, Albers N, Lichtner P, Schnabel D, Hochberg Z, Strom T. Hereditary hypophosphatemic rickets with hypercalciuria is caused by mutations in the sodium-phosphate cotransporter gene SLC34A3. Am J Hum Genet. 2006;78: 193–201.
Bergwitz C, Roslin N, Tieder M, Loredo-Osti J, Bastepe M, Abu-Zahra H, Frappier D, Burkett K, Carpenter T, Anderson D, Garabedian M, Sermet I, Fujiwara T, Morgan K, Tenenhouse H, Juppner H. SLC34A3 mutations in patients with hereditary hypophosphatemic rickets with hypercalciuria predict a key role for the sodium-phosphate cotransporter NaPi-IIc in maintaining phosphate homeostasis. Am J Hum Genet. 2006;78:179–92.
Magen D, Berger L, Coady M, Ilivitzki A, Militianu D, Tieder M, Selig S, Lapointe J, Zelikovic I, Skorecki K. A loss-of-function mutation in NaPi-IIa and renal Fanconi’s syndrome. N Engl J Med. 2010;362:1102–9.
Miyamoto K, Segawa H, Ito M, Kuwahata M. Physiological regulation of renal sodium-dependent phosphate cotransporters. Jpn J Physiol. 2004;54: 93–102.
Segawa H, Yamanaka S, Onitsuka A, Tomoe Y, Kuwahata M, Ito M, Taketani Y, Miyamoto K. Parathyroid hormone-dependent endocytosis of renal type IIc Na-Pi cotransporter. Am J Physiol Renal Physiol. 2007;292:F395–403.
Shenolikar S, Voltz J, Minkoff C, Wade J, Weinman E. Targeted disruption of the mouse NHERF-1 gene promotes internalization of proximal tubule sodium-phosphate cotransporter type IIa and renal phosphate wasting. Proc Natl Acad Sci USA. 2002;99: 11470–5.
Capuano P, Bacic D, Roos M, Gisler S, Stange G, Biber J, Kaissling B, Weinman E, Shenolikar S, Wagner C, Murer H. Defective coupling of apical PTH receptors to phospholipase C prevents internalization of the Na+−phosphate cotransporter NaPi-IIa in Nherf1-deficient mice. Am J Physiol Cell Physiol. 2007;292:C927–34.
Villa-Bellosta R, Barac-Nieto M, Breusegem S, Barry N, Levi M, Sorribas V. Interactions of the growth-related, type IIc renal sodium/phosphate cotransporter with PDZ proteins. Kidney Int. 2008; 73:456–64.
Farrow E, Davis S, Summers L, White K. Initial FGF23-mediated signaling occurs in the distal convoluted tubule. J Am Soc Nephrol. 2009;20: 955–60.
Berndt T, Thomas L, Craig T, Sommer S, Li X, Bergstralh E, Kumar R. Evidence for a signaling axis by which intestinal phosphate rapidly modulates renal phosphate reabsorption. Proc Natl Acad Sci USA. 2007;104:11085–90.
Sabbagh Y, O’Brien S, Song W, Boulanger J, Stockmann A, Arbeeny C, Schiavi S. Intestinal npt2b plays a major role in phosphate absorption and homeostasis. J Am Soc Nephrol. 2009;20: 2348–58.
Karsenty G, Yadav VK. Regulation of bone mass by serotonin: molecular biology and therapeutic implications. Annu Rev Med. 2011;62:323–31.
Bonnet N, Pierroz DD, Ferrari S. Adrenergic control of bone remodeling and its implications for the treatment of osteoporosis. J Musculoskelet Neuronal Interact. 2008;8:94–104.
Carpinteri R, Porcelli T, Mejia C, Patelli I, Bilezikian J, Canalis E, Angeli A, Giustina A, Mazziotti G. Glucocorticoid-induced osteoporosis and parathyroid hormone. J Endocrinol Invest. 2010;33(7 Suppl):16–21.
Kronenberg H. PTHrP and skeletal development. Ann N Y Acad Sci. 2006;1068:1–13.
Kousteni S, Bilezikian J. The cell biology of parathyroid hormone in osteoblasts. Curr Osteoporos Rep. 2008;6:72–6.
Yakar S, Courtland H, Clemmons D. IGF-1 and bone: new discoveries from mouse models. J Bone Miner Res. 2010;25:2543–5.
Wang Y, Nishida S, Boudignon B, Burghardt A, Elalieh H, Hamilton M, Majumdar S, Halloran B, Clemens T, Bikle D. IGF-I receptor is required for the anabolic actions of parathyroid hormone on bone. J Bone Miner Res. 2007;22:1329–37.
Parfitt AM. Misconceptions (3): calcium leaves bone only by resorption and enters only by formation. Bone. 2003;33:259–63.
Rizzoli R, Bonjour J-P. Physiology of calcium and phosphate homeostasis. In: Seibel MJ, Robins SP, Bilezikian JP, editors. Dynamics of bone and cartilage metabolism. San Diego: Academic Press; 1999. p. 247–60.
Papapetrou P. Bisphosphonate-associated adverse events. Hormones (Athens). 2009;8:96–110.
Stopeck A, Lipton A, Body J, Steger G, Tonkin K, de Boer R, Lichinitser M, Fujiwara Y, Yardley D, Viniegra M, Fan M, Jiang Q, Dansey R, Jun S, Braun A. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: a randomized, double-blind study. J Clin Oncol. 2010;28:5132–9.
Zhao W, Byrne M, Boyce B, Krane S. Bone resorption induced by parathyroid hormone is strikingly diminished in collagenase-resistant mutant mice. J Clin Invest. 1999;103:517–24.
Lee R, Weber TJ. Disorders of phosphorus homeostasis. Curr Opin Endocrinol Diabet Obes. 2010;17: 561–7.
Martin T, Seeman E. Bone remodelling: its local regulation and the emergence of bone fragility. Best Pract Res Clin Endocrinol Metab. 2008;22:701–22.
Matsuo K, Irie N. Osteoclast–osteoblast communication. Arch Biochem Biophys. 2008;473:201–9.
Chiusaroli R, Maier A, Knight M, Byrne M, Calvi L, Baron R, Krane S, Schipani E. Collagenase cleavage of type I collagen is essential for both basal and parathyroid hormone (PTH)/PTH-related peptide receptor-induced osteoclast activation and has differential effects on discrete bone compartments. Endocrinology. 2003;144:4106–16.
Nakayamada S, Okada Y, Saito K, Tamura M, Tanaka Y. Beta1 integrin/focal adhesion kinase-mediated signaling induces intercellular adhesion molecule 1 and receptor activator of nuclear factor kappaB ligand on osteoblasts and osteoclast maturation. J Biol Chem. 2003;278:45368–74.
Henriksen K, Tanko LB, Qvist P, Delmas PD, Christiansen C, Karsdal MA. Assessment of osteoclast number and function: application in the development of new and improved treatment modalities for bone diseases. Osteoporos Int. 2007;18:681–5.
Hedgecock N, Hadi T, Chen A, Curtiss S, Martin R, Hazelwood S. Quantitative regional associations between remodeling, modeling, and osteocyte apoptosis and density in rabbit tibial midshafts. Bone. 2007;40:627–37.
Lotinun S, Evans G, Bronk J, Bolander M, Wronski T, Ritman E, Turner R. Continuous parathyroid hormone induces cortical porosity in the rat: effects on bone turnover and mechanical properties. J Bone Miner Res. 2004;19:1165–71.
Iida-Klein A, Lu S, Kapadia R, Burkhart M, Moreno A, Dempster D, Lindsay R. Short-term continuous infusion of human parathyroid hormone 1–34 fragment is catabolic with decreased trabecular connectivity density accompanied by hypercalcemia in C57BL/J6 mice. J Endocrinol. 2005;186:549–57.
Ma Y, Cain R, Halladay D, Yang X, Zeng Q, Miles R, Chandrasekhar S, Martin T, Onyia J. Catabolic effects of continuous human PTH (1–38) in vivo is associated with sustained stimulation of RANKL and inhibition of osteoprotegerin and gene-associated bone formation. Endocrinology. 2001;142:4047–54.
Qin L, Raggatt L, Partridge N. Parathyroid hormone: a double-edged sword for bone metabolism. Trends Endocrinol Metab. 2004;15:60–5.
Panda D, Miao D, Bolivar I, Li J, Huo R, Hendy G, Goltzman D. Inactivation of the 25-hydroxyvitamin D 1alpha-hydroxylase and vitamin D receptor demonstrates independent and interdependent effects of calcium and vitamin D on skeletal and mineral homeostasis. J Biol Chem. 2004;279:16754–66.
Xue Y, Karaplis A, Hendy G, Goltzman D, Miao D. Genetic models show that parathyroid hormone and 1,25-dihydroxyvitamin D3 play distinct and synergistic roles in postnatal mineral ion homeostasis and skeletal development. Hum Mol Genet. 2005;14:1515–28.
Gardiner EM, Baldock PA, Thomas GP, Sims NA, Henderson NK, Hollis B, White CP, Sunn KL, Morrison NA, Walsh WR, Eisman JA. Increased formation and decreased resorption of bone in mice with elevated vitamin D receptor in mature cells of the osteoblastic lineage. FASEB J. 2000;14:1908–16.
Rhee Y, Allen M, Condon K, Lezcano V, Ronda A, Galli C, Olivos N, Passeri G, O’Brien C, Bivi N, Plotkin L, Bellido T. PTH receptor signaling in osteocytes governs periosteal bone formation and intra-cortical remodeling. J Bone Miner Res. 2011;26(5):1035–46.
Teti A, Zallone A. Do osteocytes contribute to bone mineral homeostasis? Osteocytic osteolysis revisited. Bone. 2009;44:11–6.
Bonewald LF. The amazing osteocyte. J Bone Miner Res. 2011;26:229–38.
Parthier C, Reedtz-Runge S, Rudolph R, Stubbs M. Passing the baton in class B GPCRs: peptide hormone activation via helix induction? Trends Biochem Sci. 2009;34:303–10.
Lee S, Goldring S, Lorenzo J. Expression of the calcitonin receptor in bone marrow cell cultures and in bone: a specific marker of the differentiated osteoclast that is regulated by calcitonin. Endocrinology. 1995;136:4572–81.
Garrett JE, Tamir H, Kifor O, Simin RT, Rogers KV, Mithal A, Gagel RF, Brown EM. Calcitonin-secreting cells of the thyroid gland express an extracellular calcium-sensing receptor gene. Endocrinology. 1995;136:5202–11.
Bolognese M. SERMs and SERMs with estrogen for postmenopausal osteoporosis. Rev Endocr Metab Disord. 2010;11:253–9.
Krum S. Direct transcriptional targets of sex steroid hormones in bone. J Cell Biochem. 2011;112:401–8.
Takeda S, Elefteriou F, Levasseur R, Liu X, Zhao L, Parker K, Armstrong D, Ducy P, Karsenty G. Leptin regulates bone formation via the sympathetic nervous system. Cell. 2002;111:305–17.
Elefteriou F, Ahn J, Takeda S, Starbuck M, Yang X, Liu X, Kondo H, Richards W, Bannon T, Noda M, Clement K, Vaisse C, Karsenty G. Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature. 2005;434:514–20.
Murshed M, McKee M. Molecular determinants of extracellular matrix mineralization in bone and blood vessels. Curr Opin Nephrol Hypertens. 2010; 19:359–65.
Omelon S, Georgiou J, Henneman Z, Wise L, Sukhu B, Hunt T, Wynnyckyj C, Holmyard D, Bielecki R, Grynpas M. Control of vertebrate skeletal mineralization by polyphosphates. PLoS One. 2009;4:e5634.
Whyte M. Physiological role of alkaline phosphatase explored in hypophosphatasia. Ann N Y Acad Sci. 2010;1192:190–200.
Fedde K, Blair L, Silverstein J, Coburn S, Ryan L, Weinstein R, Waymire K, Narisawa S, Millán J, MacGregor G, Whyte M. Alkaline phosphatase knock-out mice recapitulate the metabolic and skeletal defects of infantile hypophosphatasia. J Bone Miner Res. 1999;14:2015–26.
Orimo H. The mechanism of mineralization and the role of alkaline phosphatase in health and disease. J Nippon Med Sch. 2010;77:4–12.
Ho A, Johnson M, Kingsley D. Role of the mouse ank gene in control of tissue calcification and arthritis. Science. 2000;289:265–70.
Zaka R, Williams CJ. Role of the progressive ankylosis gene in cartilage minerlization. Curr Opin Rheumatol. 2006;18:181–6.
Kim H, Minashima T, McCarthy E, Winkles J, Kirsch T. Progressive ankylosis protein (ANK) in osteoblasts and osteoclasts controls bone formation and bone remodeling. J Bone Miner Res. 2010;25:1771–83.
Addison W, Azari F, Sørensen E, Kaartinen M, McKee M. Pyrophosphate inhibits mineralization of osteoblast cultures by binding to mineral, up-regulating osteopontin, and inhibiting alkaline phosphatase activity. J Biol Chem. 2007;282:15872–83.
Tartaix P, Doulaverakis M, George A, Fisher L, Butler W, Qin C, Salih E, Tan M, Fujimoto Y, Spevak L, Boskey A. In vitro effects of dentin matrix protein-1 on hydroxyapatite formation provide insights into in vivo functions. J Biol Chem. 2004;279:18115–20.
Sun Y, Prasad M, Gao T, Wang X, Zhu Q, D’Souza R, Feng J, Qin C. Failure to process dentin matrix protein 1 (DMP1) into fragments leads to its loss of function in osteogenesis. J Biol Chem. 2010;285:31713–22.
Addison W, Nakano Y, Loisel T, Crine P, McKee M. MEPE-ASARM peptides control extracellular matrix mineralization by binding to hydroxyapatite: an inhibition regulated by PHEX cleavage of ASARM. J Bone Miner Res. 2008;23:1638–49.
Addison W, Masica D, Gray J, McKee M. Phosphorylation-dependent inhibition of mineralization by osteopontin ASARM peptides is regulated by PHEX cleavage. J Bone Miner Res. 2010;25: 695–705.
Sabbagh Y, Carpenter T, Demay M. Hypophosphatemia leads to rickets by impairing caspase-mediated apoptosis of hypertrophic chondrocytes. Proc Natl Acad Sci USA. 2005;102: 9637–42.
Kumar R. New insights into phosphate homeostasis: fibroblast growth factor 23 and frizzled-related protein-4 are phosphaturic factors derived from tumors associated with osteomalacia. Curr Opin Nephrol Hypertens. 2002;11:547–53.
White K, Carn G, Lorenz-Depiereux B, Benet-Pages A, Strom T, Econs M. Autosomal-dominant hypophosphatemic rickets (ADHR) mutations stabilize FGF-23. Kidney Int. 2001;60:2079–86.
Goetz R, Nakada Y, Hu M, Kurosu H, Wang L, Nakatani T, Shi M, Eliseenkova A, Razzaque M, Moe O, Kuro-o M, Mohammadi M. Isolated C-terminal tail of FGF23 alleviates hypophosphatemia by inhibiting FGF23-FGFR-Klotho complex formation. Proc Natl Acad Sci USA. 2010;107:407–12.
HYP-Consortium. A gene (PEX) with homologies to endopeptidases is mutated in patients with X-linked hypophosphatemic rickets. Nat Genet. 1995;11: 130–6.
Holm I, Huang X, Kunkel L. Mutational analysis of the PEX gene in patients with X-linked hypophosphatemic rickets. Am J Hum Genet. 1997;60:790–7.
Dixon P, Christie P, Wooding C, Trump D, Grieff M, Holm I, Gertner J, Schmidtke J, Shah B, Shaw N, Smith C, Tau C, Schlessinger D, Whyte M, Thakker R. Mutational analysis of PHEX gene in X-linked hypophosphatemia. J Clin Endocrinol Metab. 1998;83:3615–23.
Beck L, Soumounou Y, Martel J, Krishnamurthy G, Gauthier C, Goodyer C, Tenenhouse H. Pex/PEX tissue distribution and evidence for a deletion in the 3′ region of the Pex gene in X-linked hypophosphatemic mice. J Clin Invest. 1997;99:1200–9.
Liu S, Guo R, Simpson L, Xiao Z, Burnham C, Quarles L. Regulation of fibroblastic growth factor 23 expression but not degradation by PHEX. J Biol Chem. 2003;278:37419–26.
Sitara D, Razzaque M, Hesse M, Yoganathan S, Taguchi T, Erben R, Jüppner H, Lanske B. Homozygous ablation of fibroblast growth factor-23 results in hyperphosphatemia and impaired skeletogenesis, and reverses hypophosphatemia in Phex-deficient mice. Matrix Biol. 2004;23:421–32.
Liu S, Zhou J, Tang W, Jiang X, Rowe D, Quarles L. Pathogenic role of Fgf23 in Hyp mice. Am J Physiol Endocrinol Metab. 2006;291:E38–49.
Segawa H, Aranami F, Kaneko I, Tomoe Y, Miyamoto K. The roles of Na/Pi-II transporters in phosphate metabolism. Bone. 2009;45 Suppl 1:S2–7.
Qin C, D’Souza R, Feng J. Dentin matrix protein 1 (DMP1): new and important roles for biomineralization and phosphate homeostasis. J Dent Res. 2007;86:1134–41.
Feng JQ, Ward LM, Liu S, Lu Y, Xie Y, Yuan B, Yu X, Rauch F, Davis SI, Zhang S, Rios H, Drezner MK, Quarles LD, Bonewald LF, White KE. Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nat Genet. 2006;38:1310–5.
Zhang R, Lu Y, Ye L, Yuan B, Yu S, Qin C, Xie Y, Gao T, Drezner M, Bonewald L, Feng J. Unique roles of phosphorus in endochondral bone formation and osteocyte maturation. J Bone Miner Res. 2010;26:1047–56.
Chang W, Tu C, Chen TH, Bikle D, Shoback D. The extracellular calcium-sensing receptor (CaSR) is a critical modulator of skeletal development. Sci Signal. 2008;1:1.
Tu Q, Pi M, Karsenty G, Simpson L, Liu S, Quarles LD. Rescue of the skeletal phenotype in CasR-deficient mice by transfer onto the Gcm2 null background. J Clin Invest. 2003;111:1029–37.
Dvorak MM, Siddiqua A, Ward DT, Carter DH, Dallas SL, Nemeth EF, Riccardi D. Physiological changes in extracellular calcium concentration directly control osteoblast function in the absence of calciotropic hormones. Proc Natl Acad Sci USA. 2004;101:5140–5.
Shu L, Ji J, Zhu Q, Cao G, Karaplis A, Pollak M, Brown E, Goltzman D, Miao D. The calcium sensing receptor mediates bone turnover induced by dietary calcium and parathyroid hormone in neonates. J Bone Miner Res. 2011;62(5):1057–71.
Dvorak MM, Chen TH, Orwoll B, Garvey C, Chang W, Bikle DD, Shoback DM. Constitutive activity of the osteoblast Ca2+−sensing receptor promotes loss of cancellous bone. Endocrinology. 2007;148: 3156–63.
Mathew M, Takagi S. Structures of biological minerals in dental research. J Res Natl Inst Stand Technol. 2001;106:1035–44.
Uribarri J. Phosphorus homeostasis in normal health and in chronic kidney disease patients with special emphasis on dietary phosphorus intake. Semin Dial. 2007;20:295–301.
Acknowledgments
The author wishes to thank his colleague Prof. Rebecca Mason for critical comments on the manuscript and the National Health and Medical Research Council of Australia for project grant support in the areas of bone and mineral metabolism and its interactions with macronutrient-sensing mechanisms.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2012 Springer Science+Business Media, LLC
About this chapter
Cite this chapter
Conigrave, A.D. (2012). Regulation of Calcium and Phosphate Metabolism. In: Licata, A., Lerma, E. (eds) Diseases of the Parathyroid Glands. Springer, New York, NY. https://doi.org/10.1007/978-1-4419-5550-0_2
Download citation
DOI: https://doi.org/10.1007/978-1-4419-5550-0_2
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4419-5549-4
Online ISBN: 978-1-4419-5550-0
eBook Packages: MedicineMedicine (R0)