Abstract
The pharmaceutical sector is fueled by novel, high-value products that result from research and development. We can all agree that the pharmaceutical sector is the heart of the medical sciences, everything from nourishment to necessary medications, paracetamol to corona vaccine, are all related to the pharmaceutical industry. A relatively new but rapidly developing technique, bioprinting is mostly employed in tissue engineering and regenerative medicine. The advantages of modifying preset tissue forms and architectures with computer assistance and the creation of extracellular matrix (ECM)-like habitats for cells are combined in 3D bioprinting techniques. The main use of bioprinting is to regenerate or restore wounded and damaged tissues by creating implantable organs and tissues. The medical and cosmetic sectors stand to gain greatly from the technique. The ability of bioprinting has not yet been thoroughly examined in fields like patient-specific cell placement and bio-robotics. But the question arises, with the right application of bioinks and other methodologies, can bioprinting change biomedicine and biomedical engineering, or is it only useful in research settings. This comprehensive study covers current approaches and potential future developments in bioprinting, with a focus on the potential application of 3D bioprinting in other medical disciplines and regenerative medicine. In this chapter, potential uses for experimental systems and techniques that are currently being developed for drug delivery, cancer research, and the bioprinting of various types of tissues are examined. This assessment also explores potential future opportunities for the efficient redesign of technical and medical procedures through the use of bioprinting.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
12.1 Introduction
With the advent of bioprinting technologies, tissue engineering has merged science fiction and reality in the modern era of automation and technology [1]. Bioprinting is an adaption of additive (3D) printing that uses layer-by-layer deposition of biological materials and biologically active substances, such as cells, growth hormones, and other substances. This technique allows for the creation of 3D models of tissues and organs that closely resemble their human counterparts in terms of structure [2]. By setting up preset places and structures in computer-aided design software (CAD), the architecture is methodically finished. How effective the bioprinting method is can be determined by the value of the structures produced. The three primary factors to take into account while creating a product are cellular interactions, bioactive chemicals, and the bioprinting apparatus [3]. The primary biomaterial used in 3D printing, called bioink, is formed of cells that are meant to be deposited. These cells are often contained in a carrier matrix that attempts to replicate the physical and biochemical environment of native tissues in order to improve cell adhesion, proliferation, and differentiation [4].
In the last 10 years, the bioprinting sector has made tremendous achievements, and 3D bioprinting is currently one of the most intriguing and promising technologies with the potential to have a significant impact on a range of medical applications. If scientists could use bioprinting technology to create living, de novo organs like the heart, liver, kidneys, lungs, and skin, the need for organ transplants would be decreased. Taking cells directly from the patient would also prevent immune system assault and organ rejection. Another creative industrial use of 3D bioprinting is found in the pharmaceutical industry. The market for 3D bioprinting is anticipated to reach USD 1647 million [4] by 2024 as a result of the development of 3D bioprinters and biomaterials as well as their use in the pharmaceutical and cosmetics sectors [5, 6]. We briefly addressed the most cutting-edge bioprinting techniques in this chapter, along with other essential elements important to the application of 3D bioprinting to construct 3D tissues and organs. With a focus on the potential for creating in vitro models as tools for drug discovery in the pharmaceutical industry, we also discuss some of the significant challenges and fascinating opportunities that 3D bioprinting technologies present for developing realistic tissue and organs across a variety of market segments.
12.2 History
The concept of 3D-printed organs and bioprinting was first explored in 1996. When Dr. Gabor Forgacs discovered that biological components adhere together and have liquid-like qualities during experiments with Charles Hull (who later founded 3D Systems), the general public started to exhibit interest in bioprinted items that use ingredients extracted directly from patient tissues. Early studies employed synthetic scaffolds, but after investigation into a new technique called Dynamic Optical Projection Stereolithography (DOPsL), it was discovered that biological tissues, like blood cells, could be printed in a matter of seconds.
12.2.1 Which Organs Have Been Previously Bioprinted?
There have not been any additional reports of 3D-printed organs since the first human blood vessel was made. Minor components such as arteries, bones, cartilage, tissues, and veins may have been printed for implantation, but since regenerative medicine professionals prefer to have a thorough understanding of the technology before moving forward, they are currently waiting for new case studies before thinking about printing more complex organs.
12.3 3D Bioprinting
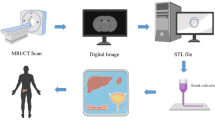
Using a suitable imaging tool, such as computed tomography (CT) or magnetic resonance imaging (MRI), to ascertain the anatomical makeup of the target tissue is the first stage in the bioprinting process. The picture is then transformed using specialist software into a CAD drawing of cross-sectional layers so that the printing machine may add the layers one at a time. The bioprinting device then uses a specific printing method, such as inkjet 3D bioprinting, micro-extrusion 3D bioprinting, laser-assisted 3D bioprinting, or stereolithography, along with a combination of printing materials, such as scaffold and bioink, and other additive factors to produce the tissue (Fig. 12.1). Precision, stability, and tissue viability vary via various methods. In order to replicate the in vivo conditions required to maintain tissue viability during the maturation process, the generated tissue is then further processed in a bioreactor.
Schematic representation of the bioprinting process [7]
12.3.1 Techniques for 3D Bioprinting
The basis for 3D bioprinting technology was laid by conventional 2D printing on paper and then by 3D printing of non-biological materials. Therefore, it is not surprising that the engineering component has more advanced technology than bioink material technology. However, because bioink was first developed for printing on non-biological materials, each printing method still has a number of limitations regarding material compatibility when using other building materials instead of bioink [8,9,10].
12.3.1.1 Micro-Extrusion 3D Bioprinting
It is a regularly used pressure-assisted technique for printing non-biological materials. Typically, a computer-controlled robotic arm uses a pneumatic or mechanical (piston or screw-driven) approach to provide pressure to a nozzle in order to disperse the selected bioinks for the bioprinting process. Most frequently, a glass or plastic cartridge is used to hold the bioinks. The bioink is ejected via the nozzle in the form of a thin filament and deposited on the substrate based on a CAD design that regulates the location and course of nozzle movement to produce the tissue in the required 3D shape. This technology, which developed from conventional 3D printing, has been demonstrated to be the most efficient approach to manufacture large-scale constructs because of its structural soundness, making it more suitable for scaling up for organ manufacturing [11]. Rapid printing also allows for the use of a wide variety of bioinks, including both scaffold-based and scaffold-free bioinks. The resolution of this technique is just 100 m though [12]. Due to the relatively high extrusion pressure via the nozzle, the bioink components are subjected to experience extreme shear stress, which might induce cellular viability loss and tissue structure deformation [13].
12.3.1.2 Inkjet 3D Bioprinting
Because of its structural soundness, this technology, which evolved from traditional 3D printing, has been shown to be the most effective method for producing large-scale structures, making it more ideal for scaling up for organ manufacturing [11]. Additionally, a range of bioinks, including scaffold-based and scaffold-free bioinks, can be used with rapid printing. However, this method only has a 100 m resolution [12]. The bioink components are subjected to significant shear stress as a result of the relatively high extrusion pressure through the nozzle, which could result in cellular viability loss and tissue structural distortion. Eventually, the vapor bubble swiftly bursts and expels a bioink droplet as a result of the pressure-induced expansion it experiences [14]. These temperature changes have little effect on the survival of cells since the processes only last a few microseconds. It has been determined if the bioprinted cells can keep their genetic, phenotypic, and functional characteristics [15]. In contrast, a voltage pulse in a piezoelectric inkjet bioprinter causes the piezoelectric actuator to change form. Then, the volume of the fluid chamber containing the bioink abruptly changes, releasing a droplet [16]. The viability of the cells could be maintained even after the surgery without any issues [17]. Similar principles govern how electrostatic actuators work. When an electrical pulse is supplied between the pressure plate and an electrode, the pressure plate flexes. About 80–95% yield in the cells created utilizing the thermal, piezoelectric, and electrostatic inkjet bioprinting techniques demonstrated a robust cell viability. After the voltage pulse is stopped, the pressure plate reverts to its original form, expelling a bioink droplet.
Inkjet bioprinting techniques show promise since they enable high-resolution printing and have fine control over the ejection of droplets and pico-liter sized ink droplets.
12.3.1.3 Laser-Assisted 3D Bioprinting (LAB)
Laser-assisted 3D bioprinting (LAB) is another non-contact printing technique. The laser-assisted bioprinter’s ribbon has an absorbent coating, such as gold, on it. When a laser pulse is directed and travels through the transparent ribbon, a hydrogel droplet is induced and eventually transmitted to the receiving substrate. The final structure is constructed layer by layer by repeating this process utilizing laser pulses to form a jet. This technology, which enables cells to be deposited at a density of up to 108 cells/mL with a single-cell resolution and at a high pace, enables high-throughput cell and biomaterial patterning [18,19,20]. Additionally, cell transfer and selection are made possible by the real-time cell monitoring offered by laser-assisted printing. However, the extreme heat generated by the laser beam might damage the printed tissue’s cells and reduce their vitality.
12.3.1.4 Stereolithography-Based Bioprinting (SLB)
Photopolymerizable liquid polymers are utilized in stereolithography-based bioprinting (SLB). A predefined pattern of laser or UV light is focused on the polymers, causing the polymers to cross-link and harden. Each polymerization cycle generates a little layer of the structure, and this polymerization cycle is then repeated to create the 3D structure layer by layer. The main advantages of this technique over alternative methods are its high resolution and lack of extremely high shear stress. Cells can be harmed by the cross-linking process, which exposes them to strong UV light (Table 12.1).
12.3.1.5 Acoustic Bioprinting
The acoustic bioprinting process shields the biomaterials from destructive stress, such as heat, high voltage, high pressure, and any kind of shear stress. Droplets are released through a nozzle using a gentle acoustic field [29]. Mild acoustic waves, however, cannot expel viscous or highly cell-concentrated bioink droplets. Few research have been conducted on this methodology.
12.4 Bioinks
The most challenging obstacle still standing in the way of the advancement of 3D bioprinting technology is the production of printing biomaterials, sometimes referred to as “bioink,” which is a crucial component of this technology. The ideal properties for bioink must meet both the physical and biological material criteria in order to enable cellular activity comparable to that observed in real creatures, such as cellular proliferation, differentiation, migration, and maturation. The physical properties include viscosity, structural toughness, printing ability, degradation, and functionality. Examples of biological properties include bioactivity, cytocompatibility, and biocompatibility [30]. The most crucial aspect of the bioprinting procedure, the bioink viscosity, needs to be constantly tuned in order to regulate the bioink flow, cell encapsulation efficiency, and tissue structure stability. To meet the requirements of various printing processes, there were several bioink formulas available. Hydrogels are intriguing possibilities for the creation of bioinks due to their biocompatibility, low cytotoxicity, hydrophilicity, and ability to build networks of polymers that allow them to adopt ECM with a similar structure [31]. Self-organizing systems exist in organs and tissues. During the embryonic development process, cells engage in biological self-assembly and self-organization without the use of external guidance or guiding structures [32]. In contrast, the complexity of the cell microenvironment is greatly decreased or absent in vitro, which causes distinct cell fusion and delayed aggregation. In bioprinting, biocompatible scaffolds are frequently used to give structural support for cells to attach, grow inside, differentiate into, and eventually produce the tissue on (Fig. 12.2).
Organ and tissue 3D bioprinting. Schematic image of printing methods. (a) LIFT-based (b) SLA-based (c) Inkjet-based (d) Extrusion-based printing system [33]
Studies revealed that a scaffold’s integrin configuration has a significant impact on stem cell development [34]. Replicating a certain tissue type’s natural environment encourages stem cells to differentiate into that lineage. The use of appropriate biomaterials to support the biological components is essential for the creation of 3D heterogeneous tissue architectures. The most popular biomaterials for live-cell printing are hydrogels, because of their biocompatibility and capacity to take on a structure resembling that of the ECM [35]. The ultimate goal of bioprinting-based tissue engineering is to somewhat replicate the tissue/organs that grow during embryonic development. However, this unique strategy is still too far from the in vivo equivalents’ level of complexity, which is produced by many specialized cells and dynamic extracellular matrix (ECM) composition [36]. In order to print tissue constructions, bioink is a solution of one biomaterial or a combination of many biomaterials (for example, in the form of a hydrogel). Bioink encapsulates the appropriate cell types throughout the printing process. Natural or artificial biomaterials, or a combination of the two, are used to create bioinks (Table 12.2).
In order to create tissue that closely matches it is in vivo counterpart in terms of both form and function, it is crucial to maximize the biological, mechanical, and rheological properties of bioinks. For diverse uses and cell types, several bioinks can be required. There are a number of basic qualities that should be considered while choosing a bioink, including the following:
12.4.1 Viscosity
The bioink matrix should fit in all bioprinting stages, both as a fluid during cell encapsulation and as a solid following dispensing, according to the bioprinting procedure [38].
12.4.2 Gelation and Stabilization Processes
The viability and print resolution may be affected by how the bioink solidifies after extrusion. The bioink should be cell-safe and still have tissue-matching qualities after printing, and the process should be rapid. Ionic, thermal, stereocomplex, photocrosslinking, enzymatic, and click chemistry are just a few of the gelation techniques that may be used, and the characteristics and composition of the bioink material can also affect these techniques [39, 40].
12.4.3 Biocompatibility
Hydrophilic and cell-adhesive materials encourage cell survival and growth. Furthermore, the use of natural or synthetic bioinks has a significant influence on how biological interactions proceed. There is batch-to-batch variability even though natural-based bioinks can endure challenging manufacturing conditions (such high temperatures and organic solvents). On the other hand, batch-to-batch unpredictability is solved by synthetic polymers, which have a tremendous potential for large-scale manufacturing but lack the natural cell attachment sites [41].
12.4.4 Mechanical Characteristics
When the mechanical properties of their surrounding environment, such as matrix elasticity are altered, cells can adapt their behavior [42]. The ability to control cell activity inside the printed tissue construct, such as their form and rate of proliferation, is therefore possible by modifying the mechanical characteristics of bioinks. This is important for the growth of a functional tissue. Another important mechanical property is shear-thinning, a non-Newtonian property that implies a reduction in viscosity as the shear rate increases and leads to rearrangement of the polymer chain. For instance, Chen et al. [43] combined a shear-thinning hybrid bioink which was created by combining hard gellan gum, flexible sodium alginate, bioactive thixotropic magnesium phosphate-based gel, and thixotropic TMP-BG. The mechanical, rheological, and bioactive characteristics of the bioink were improved for printability and cell survival.
A number of organic and synthetic biomaterials have been employed as bioinks. Bioink has recently been created using materials that are sensitive, dynamic, and supramolecular. Morgan et al. provide a comprehensive summary of the most recent discoveries in bioinks, including their mechanical properties and dynamic nature [44]. The potential for modifying synthetic polymers to increase bioactivity is enormous. However, when they decay, they may emit toxic byproducts and lose mechanical properties. Furthermore, self-assembling peptides are prospective biomaterials for constructing 3D scaffolds with structural and mechanical properties similar to extracellular matrices. Cofino et al. [45], for example, optimized methylcellulose and RAD16-I-based biomaterials to build 3D predefined structures to generate bioink with controllable viscosity. The resulting objects have exceptional shape fidelity and stability. Standardized bioink formulations are desperately needed to enable usage in a variety of bioprinting applications.
12.5 Applications of 3D Bioprinting (Fig. 12.3)
12.5.1 Organ Transplantation Using 3D Bioprinting
Building on the huge success of printing industrial prototypes for prostheses and surgical equipment, 3D bioprinting makes excellent progress in generating thick live cellular structures as a transitional stage toward organ-level complexity. Despite the problems associated with the linked biology and engineering, bioprinting offers huge potential in whole-organ printing with an excellent hierarchical organization of cells and creating tissue blocks in a 3D microenvironment. Cells from either patient or adult stem cells are employed to make a bioink capable of producing live tissues. These components are held together by a dissolvable gel or scaffold that may support cells and mold them into the correct form to achieve the intended function. Current advanced imaging technology, like as CT, permits the development of exact CAD models for 3D printing to ensure a flawless fit into the targeted tissue [46, 47]. There have been reports in recent years of the development of several types of thick tissues in a range of forms, with the eventual objective of printing full organs or body parts for organ donation.
Applications of 3D bioprinting [7]
Long donor search durations and immunological rejection of the transplanted organ can be avoided by extracting stem cells from transplant recipients and printing them into replacement organs. Recent advances in 3D tissue bioprinting have resulted in organ-level structures such as bone, cornea, cartilage, hearts, and skin [48]. Zhou et al. [49] produced a patient-specific ear-shaped cartilage using expanded microtia chondrocytes and a biodegradable scaffold. Five individuals with microtia had their cartilage 3D-printed, and the results were satisfactory in terms of appearance. Researchers have demonstrated thick, vascularized cardiac patches as well as a cellularized human heart with natural architecture. Pluripotent stem cells were generated from omental tissue samples of patients and subsequently differentiated into cardiomyocytes and endothelial cells. The bioink was made by mixing the two cell types separately with hydrogels for the heart tissue and blood vessels. The functionality of functional vascularized patches was demonstrated to work in line with the patient’s anatomy (Fig. 12.4). In order to replace damaged (such as burnt) skin and heal skin ulcers and wounds, much study was undertaken on the skin among the many human tissues. Baltazar et al. [50] described an implantable multilayered vascularized 3D-printed skin transplant. The dermis of the skin was created using a bioink composed of human foreskin dermal fibroblasts, human endothelial cells, and human placental pericytes suspended in rat-tail type I collagen. After that, the epidermis was generated by printing with a second bioink that included human foreskin keratinocytes. In this arrangement, while endothelial cells and pericytes self-assembled into linked microvascular networks, keratinocytes formed a multilayered skin barrier that seemed to speed keratinocyte development.
3D printed human organs: making future transplant wait list shorter [48]
12.5.2 Organ Models Printed in 3D for Drug Discovery
The translational medical research community is actively attempting to move its focus away from animal models and toward more complex human qualities and circumstances. Unfortunately, while traditional in vitro models are robust and well suited for high-throughput research due to their simplicity, they have little biological relevance to the intricate biological tissues of the human body, resulting in a significant technological difference between lab models and industry/clinic adoptable models. Bioprinting using high-resolution vascularized tissue opens the door to the production of biomimetic habitats and structures that facilitate in vivo interactions between cells and their environs. Bioprinted tissue would be a useful tool for developing physiologically correct in vitro human organ models for drug toxicity studies and disease modeling that precisely reproduce the physiologically complicated human. Because it requires a significant number of distinct cell types to make a medically relevant heterotypic tissue, organotypic bioprinting is often a costly technology for large-scale and high-throughput experiments. Furthermore, a hypoxic environment may develop in the artificial tissue due to inadequate delivery of cell nourishment into the tissue’s core in the absence of high-resolution vascularization, which ensures long-term survival. These disadvantages can be solved by creating small in vitro tissue models, sometimes known as “organs-on-a-chip,” by combining bioprinting and microfluidic technology. Different organotypic tissues, for example, can be printed concurrently on a segmented microfluidic chip and linked via a vascular network (perfusion channels) to create a “human-on-a-chip” with numerous organs. It is becoming commonly accepted that 3D tissue models are better than 2D counterparts and more biologically relevant. These tissue models are also immune from rigorous ethical issues, making them an appealing choice for many associated industries. They have not yet been adequately verified for toxicity prediction. Systemic validation and standardization are required to guarantee that these powerful models have the potential benefit for high-throughput drug development. Several companies and start-ups have created 3D tissue in vitro models for toxicity assessment and disease modeling in recent years. Organovo Inc., for example, created a customizable bioprinting method to manufacture tissues in various shapes, such as microscale tissues on multi-well tissue culture plates. For example, human primary hepatocytes, hepatic stellate cells, and endothelial cells were used to bioprint liver-like tissue architectures. The liver damage that leads to fibrosis was one of the effects of amethotrexate and thioacetamide exposure that was monitored using a tissue model [51]. To learn more about Kupffer cells’ impact on the injury/fibrogenic response to cytokine and pharmacological stimuli, they were added to a separate study [52].
The rapid advancement of bioprinting technology and the widespread use of 3D bioprinter modalities have generated unprecedented interest in using this technology to produce in vitro models for pharmacological research. To develop a neuronal micro-physiological system, Bowser and Moore [53] employed 3D bioprinting technology based on spheroid and magnetic spheres. Spinal cord spheroids made of magnetic nanoparticles are arranged in a three-dimensional hydrogel framework using magnetically assisted bioprinting. The structures had long-range projections that mimicked the architecture observed in vivo as well as small-scale cell-to-cell interactions. Zhuang et al. [54] used an integrated ultraviolet (UV) curing system with extrusion-based bioprinting to allow layer-by-layer UV curing of bioprinted photo-curable GelMA-based hydrogels. By using this technique, cell-laden structures with a high aspect ratio and stability may be made without the need of reinforcing elements like poly(−caprolactone) (PCL) polymer. The research by Heinrich et al. also shown how to construct miniature brains consisting of glioblastoma and macrophages to test therapeutic approaches that focus on the interaction between these two cell types. A hybrid 3D cell-printing system that uses both inkjet- and extrusion-based dispensing modules was developed in order to manufacture a 3D facsimile of human skin inside a transwell system. Polycaprolactone was utilized to produce a collagen-based construct by extrusion-based printing, and keratinocytes were equally distributed across the engineered dermis using an inkjet-based dispensing module. In addition, to mimic the composition and functioning of the original intestinal tissue, human primary intestinal epithelial cells, and myofibroblasts were employed to bioprint 3D intestinal tissue. The tissue model showed significant morphological and physiological characteristics, including a polarized epithelium with close connections and the expression of CYP450 enzymes.
Although many tissues and organ models have been created, the complexity needed to produce replacement tissues and organ models that are medically correct has not yet been obtained or sufficiently characterized. A range of cell types participate in homeostasis and tissue development in vivo in biological systems with linked tissues and organs. Due to the inherent complexity of these systems, simulating the structure and physiology of interrelated human tissues and animal models to enable tracking the physiological processes is difficult. To accomplish differentiation into the needed phenotypes, it is still unknown how much biomimicry of human physiology is necessary or whether all cellular subpopulations must be utilized. Recent advancements in 3D bioprinting technology may provide definite answers to these crucial questions. For example, multimaterial deposition systems may be employed to create intricate heterogeneous cellular architectures. This makes it possible to incorporate vascular and neuronal networks into the in vitro models’ structure, accurately simulating the complexity of numerous tissue and organ systems. However, to achieve this ambitious aim, further in-depth study is needed. In addition, combining bioprinting methods with other technologies such as imaging, bioreactor technology, organs-on-a-chip (OOC), artificial intelligence (AI), and semiconductors would enhance tissue engineering’s capabilities and speed up the field’s progress toward the production of organs and tissues for a variety of applications.
12.5.3 Tumor Modeling
A tumor microenvironment must be produced via 3D bioprinting so that tumor cells may interact with adjacent stromal cells including endothelium and fibroblasts as well as an extracellular matrix in order to appropriately study tumor growth, monitoring, and therapy response. The conventional techniques for mimicking tumors or cancer are monolayer cell cultures or animal models. However, the complicated connections observed in live tissue are absent from monolayer cultures. On the other hand, animal models could respond and react differently from human tissue. Analysis of intracellular molecular interactions, intercellular connections, enzyme kinetics, changes in protein expression, metastatic progression, etc. may be done using a bioprinted tumor model for specific tissues. Zhou et al. examined the dissemination of breast cancer in bone tissue by fabricating biomimetic bone structures using a table-top commercial stereolithographic printer. In order to establish a 3D structure of bone tissue and examine the relationships, breast cancer cells (BrCas) were planted in vitro in this work [55]. Another study used a bioprinted mini-brain to display how glioblastoma multiforme developed in terms of interactions between glioblastoma cells and glioblastoma-associated macrophages [56]. The results of this study indicated that drugs may be developed to stop the communication between these cells in order to inhibit tumor development.
One of the most difficult elements of cancer modeling is simulating the angiogenesis of tumor cells in the absence of vascular networks. Angiogenesis is a pivotal step in the development of cancer because it is essential for the transportation of nutrients and oxygen to the cells [57]. Vasculature can be created via bioprinting techniques, as was previously said, if the right bioinks and growth factors are used. Using patient-derived GBM cells and human endothelial cells in a collagen matrix, Lee et al. may have printed a physiological glioma-vascular niche model to better understand cell–cell interactions and the effects of microenvironmental factors on glioblastoma multiforme [58]. Gelatin and collagen were used to make fluidic vascular channels. In a recent research, Suarez-Martinez et al. examined cancer cell migration, proliferation, and function during microvascular network growth using a bioprinted tumor microenvironment model of mouse breast cancer cells [59]. A bioprinted hydrogel-based vascular flow device was created by Hynes et al. [60] in another recent work as a unique approach to comprehending the progression of cancer. Bone marrow suppression, gastrointestinal distress, liver toxicity, urinary system toxicity, renal toxicity, cardiotoxicity, and neurotoxicity are among the adverse effects of widely recommended cancer therapies. Heterotypic drug interactions were performed on macrophages inside the channels and bioprinted breast cancer cells covered in peptide-conjugated alginate fibers to evaluate the therapeutic effectiveness and toxicity. HER2-positive breast cancer cells embedded in adipose-derived mesenchymal stem cells (ADMSCs) were employed in a new bioprinted model to assess doxorubicin and look into treatment resistance.
On the basis of a biomimetic ECM consisting of adipose cells, endothelial cells, and mammary fibroblasts produced from MSCs, the chemotherapeutic effects of tamoxifen on breast cancer were evaluated. To gauge the effectiveness of the medication, the adenosine triphosphate (ATP) luciferase test was utilized. Temozolomide (TMZ), an anti-cancer drug, and the angiogenic inhibitor sunitinib were both studied in a tumor microenvironment.
The printed tissue was multicellular tumor spheroids of glioblastoma cells on a blood artery layer composed of fibroblasts and endothelial cells. Sun et al. employed HepG2 cells to construct a model of hepatocellular carcinoma in a different recent work to carry out pharmacological pharmacodynamics research [61]. As bioprinting technology develop, it may soon be possible to construct more intricate biological systems and examine how diseases interact with surrounding tissue as well as at the cellular level.
12.6 In Situ Bioprinting
One of the intriguing applications of 3D bioprinting is the direct patterning of de novo tissue onto the appropriate location of the body, such as chronic skin wounds or bone abnormalities. Medical imaging may be used to adjust the architecture of printed tissue to match the wound or defect, allowing heterotypic cellular structures, hydrogels, and soluble components to be correctly deposited inside the faults. This procedure, also referred to as in situ bioprinting or intraoperative bioprinting (IOB), would shorten the distance between the host and implant interfaces and provide clearly defined structures within areas of irregular topographies during the healing process, which can successfully recruit desired cells from surrounding tissues where the patient’s body acts as a natural bioreactor [62]. There have been fewer attempts than with the other apps mentioned above. In a recent proof-of-concept work, Albana et al. [63] precisely distributed autologous/allogeneic dermal fibroblasts and epidermal keratinocytes into a damaged region in mice, resembling the layered skin structure. Excisional wounds that were bioprinted using layers of autologous dermal fibroblasts and epidermal keratinocytes in a hydrogel carrier showed faster re-epithelialization, reduced contraction, and rapid wound closure. These results showed that in situ skin bioprinting is viable and revealed potential applications for it in the regeneration of various body parts. Successful in situ bioprinting techniques that enable cell extraction from a small biopsy might dramatically accelerate cell therapy-based healing. Zhao and Xu [64] developed a micro-bioprinting technology that was linked to an endoscope to enable bioprinting within the human body. In order to print tissues with extreme accuracy, they also employed printed circuit micro-electro-mechanical system methods. Using gelatin-alginate hydrogels, human gastric epithelium, and smooth muscle cells as bioinks, two-layer tissue scaffolds were printed in a stomach model to reproduce the anatomical structure of the stomach. Researchers patterned endothelial cells into a mouse calvaria bone defect that was filled with mesenchymal stem cells that contained collagen and vascular endothelial growth factor using laser-assisted bioprinting (LAB). This method allowed for organized microvascular networks to enter bone defects and has a promising vascularization rate for in situ pre-vascularization that aids bone regeneration.
In situ bioprinting, as opposed to in vitro printing, is a contact-based technique that necessitates close consideration of bioink properties, bioprinter setup, and cleaning. For instance, in extrusion-based bioprinting, the printing tip could interfere with the region around the fault and result in collateral damage. The bioinks used for in situ bioprinting often need to be biocompatible with fast cross-linkability in order to allow for shorter operating times and to retain the integrity of bioprinted constructs. Numerous biomaterials show great potential for these applications, including collage, fibrinogen, gelatin methacrylamide (GelMA), hyaluronic acid methacrylate (HAMA), and poly (ethylene glycol). Since it takes living tissue more than 10 days to go through angiogenesis, vascularization is a highly challenging process, particularly for in situ bioprinting. Utilizing oxygen-generating biomaterials or oxygen-filled microparticles that may be bioprinted inside of the bioink might give a temporary oxygen supply prior to angiogenesis. Another technique creates sacrificial porosity structures inside the bioprinted tissue using mesh filaments.
12.7 Microfluidics and Organ-on-a-Chip Converge with Bioprinting
Recent bioprinting research has taken use of well-proven microfluidic technology to develop bioprinting devices that allow precision dispensing of low-viscosity bioink in a well-defined template under very controlled circumstances [65, 66]. Microfluidic dispensing technique is used in several commercial bioprinters. As an example, Aspect Biosystems developed the RX1TM bioprinter, which has precise motion and pressure control as well as high-speed microscale resolution. The RX1TM bioprinter was used to create 3D neural tissues using hiPSC-derived neural aggregates, according to Abelseth et al. [67]. The exact manipulation of the microstructures and microarchitecture of tissue constructs would be made feasible by complex 3D-shaped scaffolds, enabling the development of various tissues and organs as in vitro drug discovery models. As a bottom-up strategy, organ-on-a-chip and organoids are miniature in vitro models of human organs that are produced by spatially immobilizing various types of living cells to produce heterogeneous functional structures within a prefabricated chip and scaffold. Multiorgans-on-a-chip, which are more complex heterogeneous tissue structures, might also be made using this technique. Tissue engineering might significantly benefit from the use of 3D printing technology in this specific discipline. Other tiny organ models, such as those of the heart, kidney, liver, and vasculature, have recently been realized (i.e., printed). Human cell-derived organoids are efficient instruments for disease modeling, drug screening, and individualized treatment. By employing these organoids as the building blocks for 3D bioprinting, it would be able to scale up the deposition of these tissue structures. Maloney et al. described an immersion printing technique to bioprint tissue organoids in 96-well plates (Fig. 12.5) [68, 69].
Steps for 3D-bioprinting organ-on-a-chip [68]
To maintain a spherical shape, a hydrogel made of hyaluronic acid and collagen is bioprinted into a viscous gelatin solution. This prevents the bioink from interacting with the well walls. Reid et al. [70] created tumoroid arrays using 3D bioprinting to study the carcinogenesis and microenvironmental redirection of breast cancer cells. It has been shown that the formation of tumoroids in 3D collagen gels is much improved by the use of bioprinting technology. This technique also makes it possible to precisely create tumoroid arrays and co-print cancer cells with epithelial cells to create chimeric organoids. By merging 3D bioprinting, 3D cell culture, microfluidics, and organ-on-a-chip, it offers a great lot of promise to enable the integration of several organoids inside a single system with tiny footprints and greater biosensing capacity.
12.8 3D Bioprinting for Meat without Animals
It has been shown that the formation of tumoroids in 3D collagen gels is much improved by the use of bioprinting technology. This technique also makes it possible to precisely create tumoroid arrays and co-print cancer cells with epithelial cells to create chimeric organoids. By merging 3D bioprinting, 3D cell culture, microfluidics, and organ-on-a-chip, it offers a great lot of promise to enable the integration of several organoids inside a single system with tiny footprints and greater biosensing capacity. However, because to high production costs, public neophobia may restrict its economic viability in the near future [71]. Skeletal muscles, together with adipocytes, fibroblasts, and endothelial cells that provide the meat its nutritional value, make up the majority of typical edible meat. Myosatellite cells, which serve as the primary adult stem cells for muscle, have emerged as the most promising cell type for the in vitro process of developing muscle tissues [72]. Myosatellites are separated from a biopsy taken from a suitable animal, and they are cultivated in the best culture conditions with a consistent supply of nutrients and growth hormones to encourage the development of multinuclear myotubes. Myotube maturation, ongoing differentiation, and further development by fusing new myoblasts result in the formation of muscle fibers (Fig. 12.6). A scaffold that encourages cell development is a crucial part of tissue engineering. Similar to this, a pliable scaffold that is easy to detach from the completed meat product, enables contraction, and optimizes medium diffusion is required for myoblast development [73]. Alternatively, the scaffolding material needs to be composed of natural ingredients and be eatable. Finding low-cost, large-quantity food-grade culture medium is a big challenge for IVM. Animal-derived sera have long been a standard component of cell culture medium. While using this system, concerns over rules and morality are raised. Alternately, plant-based growth medium might take the place of the divisive animal-based growth media. For in vitro meat to be accepted by consumers, its nutritional value must be on par with or higher than that of regular meat. It should be emphasized that in vitro meat can be supplemented with necessary elements like vitamins and minerals [74].
Schematic of the in vitro meat production process [7]
The primary technological challenge facing the IVM sector is scaling up the product for commercialization. Since lab-grown beef is currently fairly pricey, its marketability is diminished. The advancement of bioreactor technology, however, has caused prices to decline in recent years, which is optimistic for commercialization. The primary obstacles keeping the IVM technology from scaling up are the high cost of the microcarriers and culture medium as well as the absence of an adequate large-scale bioreactor for mass manufacturing. Finally, before the general public embraces IVM, regulatory standards and laws that reassure customers and reduce mistrust among start-ups functioning in the field are required.
12.9 Limitations and Challenges of 3D Bioprinting Technology
The ultimate aim of 3D bioprinting is to develop a method that can generate intricate, functioning organs in 3D, which may subsequently be utilized as a source for tissue grafts, organ transplants, and drug testing models as an alternative for using animals. This technology is still in its early stages, but it is progressing swiftly because of the extensive research being done in areas like printing engineering, tissue engineering, and cell sciences. The scaling up of bioprinting structures to functional tissues is still hampered by a number of significant barriers, despite the significant developments and countless discoveries. The ultimate aim of 3D bioprinting is to develop a method that can generate intricate, functioning organs in 3D, which may subsequently be utilized as a source for tissue grafts, organ transplants, and drug testing models as an alternative for using animals. This technology is still in its early stages, but it is progressing swiftly because of the extensive research being done in areas like printing engineering, tissue engineering, and cell sciences. The scaling up of bioprinting structures to functional tissues is still hampered by a number of significant barriers, despite the significant developments and countless discoveries [75, 76].
The current limitation of 3D printing resolution, which is 20 m, whereas the blood capillary can be as small as 3 m, makes it difficult to fabricate blood capillaries. To generate vascularized human tissue, several potential techniques are being used. For instance, adding angiogenic growth factors to bioinks to encourage the formation of blood vessels after printing. Benmeridja et al. suggested using micro-vascularized units as building blocks in conjunction with 3D bioprinting [48]. In this study, human umbilical vein endothelial cells (HUVEC) and adipose-derived stem cells (ADS) were cocultured to produce dense, viable adipose tissue spheroids with a capillary-like network. Using a microfluidic device, vasculogenesis was promoted in a new way, although the hydrogels used to do so do not support cell–cell interactions and have an effect on the stability of phenotypic traits. It is still not viable to create functioning vasculature in time to sustain the bioprinted tissue because of the vascular network’s complexity and small size.
Biomaterials are essential in 3D bioprinting because they support the structural and functional properties of the printed tissue and preserve structural integrity and biocompatibility during tissue printing and maturation. The printed materials now available on the market, however, cannot completely replicate the original ECM compositions that support the cellular structure. It is crucial to develop novel printable biomaterials that can be handled mechanically by cells and can be produced with living cells. Finding enough cells is a challenging undertaking since tissue printing requires a large number of cells. Since bioprinting would affect stem cell development at various stages of the process, a stem cell source would be the most promising choice. Another limitation at the moment is the 3D bioprinting industry’s low throughput and high cost. All current approaches need manual cell seeding and bioink loading, which is essential for high-throughput manufacture of 3D objects. 3D organoids will probably be used in future large-scale drug discovery technologies. To create organoids, however, microscopic tissue culture plates are still employed.
12.10 Conclusion and Outlook for the Future
Enormous of developments in tissue engineering, it is now possible to regenerate de novo tissue or organs in vitro for the first time in the history of medicine. Despite many challenges, the successful demonstration of printed tissue architectures over the past 10 years points to a very exciting and promising technique that deserves additional study in a number of medical and industrial application areas. As tissue engineering is multidisciplinary, systematic research on bioink optimization, bioreactor engineering, and cell culture environment by engineers, scientists, and doctors is crucial to enabling high-throughput production that is connected to efficient screening tests. It is vital to develop printing systems that can swiftly print hybrid materials (bioinks) with sustained biocompatibility and repeatability since the structure of live tissue and organs is so intricate. Combining bioprinting technologies with other enabling techniques such as 3D cell culture, bioreactor technology, microfluidics, and organ-on-a-chip may be able to achieve this. With the help of this technology, now the vast gap between the lab and the factory can someday be reduced, allowing for the fulfillment of clinical and industrial demands as well as the advancement of regenerative medicine and enhanced drug development [77]. Advanced bio-fabrication technologies underpin this technology. 3D bioprinting might signal a paradigm shift for the twenty-first century in a number of biological sectors. To accelerate the development of this technology and realize this ambition, the scientific and technical communities must effectively collaborate to exchange information.
References
Singh S, Choudhury D, Yu F, Mironov V, Naing MW (2020) In situ bioprinting—bioprinting from benchside to bedside? Acta Biomater 101:14–25
Vyas D, Udyawar D (2019) A review on current state of art of bioprinting. In: Kumar L, Pandey P, Wimpenny D (eds) 3D printing and additive manufacturing technologies. Springer, Singapore, pp 195–201. https://doi.org/10.1007/978-981-13-0305-0_17
Yilmaz B, Tahmasebifar A, Baran ET (2019) Bioprinting technologies in tissue engineering. In: Advances in biochemical engineering/biotechnology. Springer, pp 279–319
Cho DW, Kim BS, Jang J, Gao G, Han W, Singh N (2019) 3D bioprinting techniques. In: 3D bioprinting. Springer, Cham, pp 25–29. https://doi.org/10.1007/978-3-030-32222-9_4
Kala D, Sharma TK, Gupta S, Verma V, Thakur A, Kaushal A, Trukhanov AV, Trukhanov SV (2021) Graphene oxide nanoparticles modified paper electrode as a biosensing platform for detection of the htrA gene of O. tsutsugamushi. Sensors 21:4366
Kačarević ŽP, Rider P, Alkildani S, Retnasingh S, Smeets R, Jung O, Ivanišević Z, Barbeck M (2018) An introduction to 3D bioprinting: possibilities, challenges and future aspects. Materials 11(11):2199
Ramadan Q, Zourob M (2021) 3D bioprinting at the frontier of regenerative medicine, pharmaceutical, and food industries. Frontiers 2:607648
Singh A, Wan F, Yadav K, Salvi A, Thakur P, Thakur A (2023) Synergistic effect of ZnO nanoparticles with Cu2+ doping on antibacterial and photocatalytic activity. Inorg Chem Commun 157:11425
Thakur A, Verma R, Wan F, Ravelo B, Edelman I, Ovchinnikov S, Thakur P (2023) Investigation of structural, elastic and magnetic properties of Cu2+ ions substituted cobalt nanoferrite. J Magn Magn Mater 581:170980
Dababneh A, Ozbolat IT (2014) Bioprinting technology: A current state-of-the-art review. Pubmed 136(6):061016
Leberfinger AN, Dinda S, Wu YC, Koduru SV, Ozbolat V, Ravnic DJ, Ozbolat IT (2019) Bioprinting functional tissues. Acta Biomater 95:32–49
Malda J, Visser J, Melchels FP, Groll J, Hennink WE, Dhert WJ, Hutmacher DW (2013) 25th anniversary article: engineering hydrogels for biofabrication. Adv Mater 25(36):5011–5028
Ozbolat IT, Yu Y (2013) Bioprinting toward organ fabrication: challenges and future trends. IEEE Trans Biomed Eng 60(3):691–699
Yilmaz B, Tahmasebifar A, Baran ET (2019) Bioprinting technologies in tissue engineering. In: Silva A, Moreira J, Lobo J, Almeida H (eds) Current applications of pharmaceutical biotechnology. Springer, Cham, pp 279–319
Xu T, Zhao W, Zhu J, Albanna MZ, Yoo JJ, Atala A (2013) Complex heterogeneous tissue constructs containing multiple cell types prepared by inkjet printing technology. Biomaterials 34(1):130–139
Singh M, Haverinen HM, Dhagat P, Jabbour GE (2010) Inkjet printing-process and its applications. Adv Mater 22(6):673–685
Li X, Chen J, Liu B, Wang X, Ren D, Xu T (2018) 3D printing and biofabrication. Springer eBooks, pp 283–301
Verma R, Thakur P, Sun AC, Thakur A (2023) Investigation of structural, microstructural and electrical characteristics of hydrothermally synthesized Li0.5-0.5xCoxFe2.5-0.5xO4 ferrite nanoparticles. Phys B: Cond Mater 661:414926
Kala D, Sharma TK, Gupta S, Nagraik R, Verma V, Thakur A, Kaushal A (2020) AuNPs/CNF-modified DNA biosensor for early and quick detection of O. tsutsugamushi in patients suffering from scrub typhus. 3 Biotech 10:446
Guillotin B, Souquet A, Catros S, Surdo S, Pippenger BE, Bellance S, Bareille R, Rémy M, Bordenave L, Amédée J, Guillemot F (2010) Laser assisted bioprinting of engineered tissue with high cell density and microscale organization. Biomaterials 31(28):7250–7256
Shim J, Lee J, Kim JM, Cho D (2012) Bioprinting of a mechanically enhanced three-dimensional dual cell-laden construct for osteochondral tissue engineering using a multi-head tissue/organ building system. J Micromech Microeng 22(8):085014
Bhise NS, Manoharan V, Massa S, Tamayol A, Ghaderi M, Miscuglio M, Lang Q, Zhang Y, Shin SR, Calzone G, Annabi N, Shupe T, Bishop CE, Atala A, Dokmeci MR, Khademhosseini A (2016) A liver-on-a-chip platform with bioprinted hepatic spheroids. Biofabrication 8(1):014101
Boland T, Xu T, Damon B, Cui X (2006) Application of inkjet printing to tissue engineering. Biotechnol J 1(9):910–917
Derby B (2008) Bioprinting: inkjet printing proteins and hybrid cell-containing materials and structures. J Mater Chem 18(47):5717
Schiele NR, Corr DT, Huang Y, Raof NA, Xie Y, Chrisey DB (2010) Laser-based direct-write techniques for cell printing. Biofabrication 2(3):032001
Chan VWS, Zorlutuna P, Jeong JM, Kong H, Bashir R (2010) Three-dimensional photopatterning of hydrogels using stereolithography for long-term cell encapsulation. Lab Chip 10(16):2062
Elomaa L, Pan C, Shanjani Y, Malkovskiy AV, Seppälä J, Yang Y (2015) Three-dimensional fabrication of cell-laden biodegradable poly (ethylene glycol-co-depsipeptide) hydrogels by visible light stereolithography. J Mater Chem B 3(42):8348–8358
Jamee R, Araf Y, Naser IB, Promon SK (2021) The promising rise of bioprinting in revolutionalizing medical science: advances and possibilities. Regenerat Therapy 18:133–145
Demirci U, Montesano G (2007) Single cell epitaxy by acoustic picolitre droplets. Lab Chip 7(9):1139
Chang RPH, Nam J, Sun W (2008) Effects of dispensing pressure and nozzle diameter on cell survival from solid freeform fabrication–based direct cell writing. Tissue Eng 14(1):41–48
Shakeel A, Singh A, Das S, Suhag D, Sharma AK, Rajput SK, Mukherjee M (2017) Synthesis and morphological insight of new biocompatible smart hydrogels. J Polym Res 24:1–10
Mironov V, Visconti RP, Kasyanov V, Forgacs G, Drake CL, Markwald RR (2009) Organ printing: tissue spheroids as building blocks. Biomaterials 30(12):2164–2174
Kim JM, Kong JS, Han W, Kim BY, Cho D (2020) 3D cell printing of tissue/organ-mimicking constructs for therapeutic and drug testing applications. Int J Mol Sci 21(20):7757
Prowse AB, Chong F, Gray PH, Munro TP (2011) Stem cell integrins: implications for ex-vivo culture and cellular therapies. Stem Cell Res 6(1):1–12
Murphy SD, Skardal A, Atala A (2013) Evaluation of hydrogels for bio-printing applications. J Biomed Mater Res A 101A(1):272–284
Kaushik S, Gandhi S, Chauhan M, Ma S, Das S, Ghosh D, Chandrasekharan A, Alam B, Parmar AS, Sharma A, Santhoshkumar T, Suhag D (2020) Water-templated, polysaccharide-rich bioartificial 3D microarchitectures as extra-cellular matrix bioautomatons. ACS Appl Mater Interfaces 12(18):20912–20921
Sears NA, Seshadri DR, Dhavalikar P, Cosgriff-Hernandez E (2016) A review of three-dimensional printing in tissue engineering. Tissue Eng Part B Rev 22(4):298–310
Highley CB, Rodell CB, Burdick JA (2015) Direct 3D printing of shear-thinning hydrogels into self-healing hydrogels. Adv Mater 27(34):5075–5079
Chahar D, Thakur P, Sun AC, Thakur A (2023) Investigation of structural, electrical and magnetic properties of nickel substituted Co-Zn nanoferrites. J Mater Sci Mater Electron 34:901
Kang HW, Lee JS, Ko KI, Kengla C, Yoo JJ, Atala A (2016) A 3D bioprinting system to produce human-tissue constructs with structural integrity. Nat Biotechnol 2016(34):313–322
Carrow JK, Kerativitayanan P, Jaiswal M, Lokhande G, Gaharwar AK (2015) Polymers for bioprinting. Elsevier eBooks, pp 229–248
Engler AJ, Sen S, Sweeney HL, Discher DE (2006) Matrix elasticity directs stem cell lineage specification. Cell 126(4):677–689
Chen YY, Xiong X, Liu X, Cui R, Wang C, Zhao G, Zhi W, Lu M, Duan K, Weng J, Qu S, Ge J (2020) 3D bioprinting of shear-thinning hybrid bioinks with excellent bioactivity derived from gellan/alginate and thixotropic magnesium phosphate-based gels. J Mater Chem B 8(25):5500–5514
Morgan FJ, Moroni L, Baker MJ (2020) Dynamic bioinks to advance bioprinting. Adv Healthc Mater 9(15):1901798
Cofino C, Pérez-Amodio S, Semino CE, Engel E, Mateos-Timoneda MA (2019) Development of a self-assembled peptide/methylcellulose-based bioink for 3D bioprinting. Macromol Mater Eng 304(11):1900353
Verma R, Thakur P, Chauhan A, Jasrotia R, Thakur A (2023) A review on MXene and its composite for electromagnetic interference (EMI) shielding applications. Carbon 208:170–190
Vignesh U, Mehrotra D, Dichen A, V., & Howlader, D. (2017) Three dimensional reconstruction of late post traumatic orbital wall defects by customized implants using CAD-CAM, 3D stereolithographic models: A case report. J Oral Biol Craniofac Res 7(3):212–218
Benmeridja L, De Moor L, De Maere E, Vanlauwe F, Ryx M, Tytgat L, Vercruysse C, Dubruel P, Van Vlierberghe S, Blondeel P, Declercq H (2020) High-throughput fabrication of vascularized adipose microtissues for 3D bioprinting. J Tissue Eng Regen Med 14(6):840–854
Zhou G, Jiang H, Yin Z, Liu Y, Zhang Q, Zhang C, Pan B, Zhou J, Zhou X, Sun H, Li D, He A, Zhang Z, Zhang W, Liu W, Cao Y (2018) In vitro regeneration of patient-specific ear-shaped cartilage and its first clinical application for auricular reconstruction. EBioMedicine 28:287–302
Baltazar T, Mulligan DC, Catarino CM, Xie CB, Kirkiles-Smith NC, Lee VS, Hotta SYK, Dai G, Xu X, Ferreira FC, Saltzman WM, Pober JS, Karande P (2020) Three dimensional bioprinting of a vascularized and Perfusable skin graft using human keratinocytes, fibroblasts, pericytes, and endothelial cells. Tissue Eng A 26(5–6):227–238
Norona LM, Nguyen DG, Gerber DE, Presnell SC, LeCluyse EL (2016) Editor’s highlight: modeling compound-induced fibrogenesis in vitro using three-dimensional bioprinted human liver tissues. Toxicol Sci 154(2):354–367
Norona LM, Nguyen DG, Gerber DE, Presnell SC, Mosedale M, Watkins PB (2019) Bioprinted liver provides early insight into the role of Kupffer cells in TGF-β1 and methotrexate-induced fibrogenesis. PLoS One 14(1):e0208958
Bowser DA, Moore M (2019) Biofabrication of neural microphysiological systems using magnetic spheroid bioprinting. Biofabrication 12(1):015002
Zhuang P, Ng WL, An J, Chua CK, Tan LP (2019) Layer-by-layer ultraviolet assisted extrusion-based (UAE) bioprinting of hydrogel constructs with high aspect ratio for soft tissue engineering applications. PLoS One 14(6):e0216776
Zhou X, Zhu W, Nowicki M, Miao S, Cui H, Holmes B et al (2016) 3D bioprinting a cell-laden bone matrix for breast cancer metastasis study. ACS Appl Mater Interfaces 8(44):30017–30026
Heinrich MA, Bansal R, Lammers T, Zhang YS, Schiffelers RM, Prakash J (2019) 3D-Bioprinted mini-brain: a glioblastoma model to study cellular interactions and therapeutics. Adv Mater 31(14):e1806590
Yadav P, Bhaduri A, Thakur A (2023) Manganese oxide nanoparticles: an insight into structure, synthesis and applications. ChemBioEng 10(4):510–528
Lee VS, Dai G, Zou H, Yoo S (2015) Generation of 3-D glioblastoma-vascular niche using 3-D bioprinting
Suarez-Martinez AD, Sole-Gras M, Dykes SS, Wakefield ZR, Bauer KT, Lampejo A, Siemann DW, Huang Y, Murfee WL (2020) A novel tumor microenvironment model that combines bioprinting and tissue culture to investigate cancer cell and microvascular interactions. FASEB J 34(S1):1
Hynes WF, Pepona M, Robertson C, Alvarado J, Dubbin K, Triplett M et al (2020) Examining metastatic behavior within 3D bioprinted vasculature for the validation of a 3D computational flow model. Sci Adv 6(35):eabb3308
Sun L, Yang H, Wang Y, Zhang X, Jin B, Xie F et al (2020) Application of a 3D bioprinted hepatocellular carcinoma cell model in antitumor drug research. Front Oncol 10:878
Lee VS, Dai G (2017) Printing of three-dimensional tissue analogs for regenerative medicine. Ann Biomed Eng 45(1):115–131
Albanna M, Binder KW, Murphy SD, Kim J, Qasem S, Zhao W, Tan J, El-Amin IB, Dice D, Marco J, Green JL, Xu T, Skardal A, Holmes JF, Jackson JE, Atala A, Yoo JJ (2019) In situ bioprinting of autologous skin cells accelerates wound healing of extensive excisional full-thickness wounds. Sci Rep 9(1):1856
Zhao W, Xu T (2020) Preliminary engineering for in situ in vivo bioprinting: a novel micro bioprinting platform for in situ in vivo bioprinting at a gastric wound site. Biofabrication 12(4):045020
Petrov D, Edelman I, Thakur A, Thakur P, Sukhachev A, Ovchinnikov S (2023) Correlation between magnetic and electric properties in the series of CoxZn1-xFe2O4 nanoparticles. JETP Lett 117:765–768
Costantini M, Testa S, Mozetic P, Barbetta A, Fuoco C, Fornetti E, Tamiro F, Bernardini S, Jaroszewicz J, Swieszkowski W, Trombetta M, Castagnoli L, Seliktar D, Garstecki P, Cesareni G, Cannata S, Rainer A, Gargioli C (2017) Microfluidic-enhanced 3D bioprinting of aligned myoblast-laden hydrogels leads to functionally organized myofibers in vitro and in vivo. Biomaterials 131:98–110
Abelseth E, Abelseth L, De La Vega LEF, Beyer S, Wadsworth SC, Willerth SM (2019) 3D printing of neural tissues derived from human induced pluripotent stem cells using a fibrin-based bioink. ACS Biomater Sci Eng 5(1):234–243
Carvalho V, Gonçalves IC, Lage TCC, Rodrigues RO, Minas G, Teixeira SR, Moita AS, Hori T, Kaji H, Lima R (2021) 3D printing techniques and their applications to organ-on-a-Chip platforms: A systematic review. Sensors 21(9):3304
Maloney EK, Clark CT, Sivakumar H, Yoo K, Aleman J, Rajan SAP, Forsythe S, Mazzocchi A, Laxton AW, Tatter SB, Strowd RE, Votanopoulos KI, Skardal A (2020) Immersion bioprinting of tumor organoids in multi-well plates for increasing chemotherapy screening throughput. Micromachines 11(2):208
Reid JL, Palmer X, Mollica PA, Northam N, Sachs PC, Bruno R (2019) A 3D bioprinter platform for mechanistic analysis of tumoroids and chimeric mammary organoids. Sci Rep 9(1):7466
Bhat ZF, Kumar S, Fayaz H (2015) In vitro meat production: challenges and benefits over conventional meat production. J Integr Agric 14(2):241–248
Kadim IT, Mahgoub O, Baqir S, Faye B, Purchas RW (2015) Cultured meat from muscle stem cells: a review of challenges and prospects. J Integr Agric 14(2):222–233
Engler AJ, Griffin MA, Sen S, Bönnemann CG, Sweeney HL, Discher DE (2004) Myotubes differentiate optimally on substrates with tissue-like stiffness. J Cell Biol 166(6):877–887
Young JF, Therkildsen M, Ekstrand B, Che BN, Larsen MV, Oksbjerg N, Stagsted J (2013) Novel aspects of health promoting compounds in meat. Meat Sci 95(4):904–911
Agarwal S, Saha S, Balla VK, Pal A, Barui A, Bodhak S (2020) Current developments in 3D bioprinting for tissue and organ regeneration–a review. Front Mech Eng 6:90
Taneja S, Thakur P, Kumar D, Slimani Y, Thakur A (2023) Comparison of RE doping (Nd and Sm) on structural and magnetic properties of nickel-zinc-bismuth nanoferrites. J Rare Earths
Thakur P, Thakur A (2022) Nanomaterials, their types and properties. In: Thakur A, Thakur P, Khurana SP (eds) Synthesis and applications of nanoparticles. Springer, Singapore
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Verma, R. et al. (2023). Bioprinting for Therapeutics. In: Suhag, D., Thakur, A., Thakur, P. (eds) Integrated Nanomaterials and their Applications. Springer, Singapore. https://doi.org/10.1007/978-981-99-6105-4_12
Download citation
DOI: https://doi.org/10.1007/978-981-99-6105-4_12
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-99-6104-7
Online ISBN: 978-981-99-6105-4
eBook Packages: MedicineMedicine (R0)