Abstract
Gene therapy has garnered a lot of interest in the recent past for the treatment of various life-threatening genetic diseases. Gene therapy involves using genes that should be delivered to the infected cells to treat diseases. The materials used for gene delivery play a vital role in successful gene therapy. The common viruses used in gene delivery are adenovirus, adeno-associated virus, vaccinia virus, retrovirus, etc. This chapter will discuss the different viral and nonviral vectors that are used in gene delivery. Furthermore, lipid-, polymer-, and peptide-based gene delivery methods are discussed in this chapter. The physical method of gene delivery uses various techniques such as electroporation, sonoporation, needle injection, hydroboration, and magneto-fiction. In the future, standard DNA and RNA molecular techniques can be used as the principal mode of treatment in biomedical applications.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
14.1 Introduction
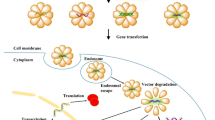
Targeted gene delivery is the way of delivering genes to cells, tissues, and organs through local or systemic blood circulation. This enables the interaction of genes directly on the sites of the intended diseases and produces therapeutic benefits. By enhancing therapeutic molecular activity at certain regions while lowering toxic side effects at normal sites, this selective delivery keeps the systemic effect to a lower level. This type of delivery is used in the therapy known as gene therapy (Zhou et al. 2017; Han et al. 2016; Kuang et al. 2017; Liu et al. 2016; Wang et al. 2016; Chen et al. 2017; Saha et al. 2017; Kemp et al. 2016). Gene therapy has earned considerable interest over the last 20 years as a favorable future treatment of choice for major diseases like cancer, AIDS, cardiovascular or neuronal disorders, as well as hereditary single gene abnormalities. For gene therapy to be effective, the therapeutic gene must be delivered to the infected cells of the patient (Mulligan 1993; Verma et al. 2000; Wirth et al. 2013). By injecting a gene into a patient’s cell, gene therapy may allow medical practitioners to treat disorders without the use of medications or surgery. Numerous techniques of therapy are being investigated by some scientists and medical professionals, including (1) substituting a disease-causing mutated gene with a healthy gene, (2) “knocking out” or inactivating a mutated gene, and (3) inserting new genes into the cells to help defend against the ailments. The interaction between the gene delivery system and the target cell must be thoroughly understood to develop an efficient gene delivery system. A plasmid-based gene expression system that controls a gene’s activity within the target cell, a gene that encodes a particular therapeutic protein, and a gene delivery system that regulates the transfer of the gene expression plasmid to a particular site inside the body include the three elements that form the gene delivery systems (Han et al. 2000; Mahato et al. 1999) (Fig. 14.1).
Since the first human gene therapy study was launched in 1990 (Blaese et al. 1995; Basarkar and Singh 2007), there has been massive interest in delivery materials for better gene delivery. The availability of reliable and secure carrier for the delivery of genes plays a vital role in gene delivery systems.
14.2 Gene Delivery Materials
The materials used for gene delivery are categorized into three types:

14.2.1 Viral Vectors
These are the vectors in which viruses act as delivery systems that deliver genes to the target cells. Depending on their capacity to inject their DNA into host cells, these vectors deliver genes. These capitalize on a virus’s capacity to replicate its own genetic material. Because the shape of the virus prevents DNA liposome breakdown, hence viruses are an excellent method for delivering genes (Kamimura et al. 2011; Van Nies et al. 2018; Singh et al. 2017). Viral-based vectors were first developed as a transgene expression technique in the 1980s. In order to protect chimpanzees against hepatitis B, the vaccinia virus was utilized as a vaccine vector in 1984 (Moss et al. 1984). Seventy percent of all gene therapy clinical trials conducted globally as of 2015 involved viral vectors for gene delivery (Ako-Adounvo et al. 2017). The replication gene of viral vectors used for the delivery of genes is removed during genetic engineering and replaced with the therapeutic gene (gene of interest) (Thomas et al. 2003). This approach retained the virus’s capacity to infect host cells (Basarkar and Singh 2007).
The main characteristics to be considered for choosing a viral vector are as follows:
-
1.
Vector should be safe to be handled.
-
2.
Vector should show less toxic effects in infected cells.
-
3.
Vector should be stable enough to ensure the repeatability of the work.
-
4.
Vector should be capable of being altered for cell-type specificity.
-
5.
Vector should be able to integrate marker genes for facilitating identification.
-
6.
Vector should be capable of carrying significant foreign genes.
-
7.
Vector should be effective in the transducing and transfecting process (Ako-Adounvo et al. 2017).
Viral vectors can be classified into three categories.

14.2.1.1 DNA-Based Viral Vectors for Gene Delivery
DNA-based viral vectors that deliver genes generally last longer and are incorporated into the genes. Viral vectors are used in DNA-based gene delivery systems to transfer genetic material to the host cells. The genetic components are effectively delivered to the host cell by the viral vectors (Wivel and Wilson 1998). Plasmids delivering transgenes for gene therapy are among the viral vectors based on the DNA for the delivery of genes (Crooke 1998). The group of materials known as DNA-based viral vectors has evolved as a promising option for gene carriers for gene therapy in a variety of diseases, including cardiovascular disorders, neurological diseases like Parkinson’s and Alzheimer’s disease, AIDS, and cancer (Stull and Szoka 1995; Patil et al. 2005a).
Some of the main viral vectors based on the DNA used for delivery of genes are as follows:
14.2.1.1.1 Adenovirus
In 1953, human adenoid tissue cultures yielded adenoviruses, which are linear double-stranded and non-enveloped DNA viruses (Rowe et al. 1953; Campos and Barry 2007; Majhen and Ambriović-Ristov 2006) (Fig. 14.2). Adenoviruses can be genetically modified to have a very large capacity for the introduction of transgenes due to their large viral genomes (36–38 kb). The high-capacity “gutless” Helper-dependent adenovirus, for instance, may deliver 37 kb of the transgene (Kamimura et al. 2011; Volpers and Kochanek 2004). Although adenoviral vector systems have a high transgenic capacity, the host immunological response and subsequent transitory expression of gene severely restrict their usage as delivery systems (Kamimura et al. 2011).
14.2.1.1.2 Poxvirus
Poxvirus is a desirable option for immune-based cancer treatment because of the absence of viral integration into the host cellular genome, the maximum size (25 kb) of the gene insert, and it elicits high immune activation. One of the main examples of poxvirus is the vaccinia virus.
14.2.1.1.3 Vaccinia Virus
All cells are infected by the vaccinia virus, but even after multiple injections, the tumor immune response is not completely suppressed by the host immunological response to the vector (Fig. 14.3). The administration of vaccinia in immunocompromised cancer patients is made possible by the availability of attenuated viruses, and the data suggests that this carrier improves tumor immune rejection. The major adverse effects were moderate flulike symptoms that lasted for 1–2 days and localized skin rashes/irritation at the injection site that typically lasted 4–5 days. The immune response at the cellular level and the clinical response were not correlated (Gardlík et al. 2005; Ayllón Barbellido et al. 2008).
14.2.1.1.4 Adeno-Associated Virus
These viruses are having properties like targeting non-proliferating cells, having discrete genome insertion sites, exhibiting little immunogenicity, and having no known toxicity (Fig. 14.4). Using an AAV vector, “suicide” gene therapy was demonstrated to be effective in oral cancer cells. Additionally, antisense or ribozyme genes have been effectively transferred using AAV vectors in preclinical models of cancer. It has been shown that AAV vectors can successfully transduce CD34+ blood cells, the brain, and the liver.
There are numerous limitations in using the adeno-associated virus as a viral vector.
-
1.
Some cells need to be infected at very high multiplicities.
-
2.
The AAV genome is compact, with only space for an additional 4.8 kb of DNA.
-
3.
Since effective packing cells have not yet been devised, the manufacturing of viral particles is still extremely labor-intensive (Gardlík et al. 2005; Kay et al. 1997; Biçeroğlu and Memiş 2005; Mulherkar 2001; Xi and Grandis 2003; Zhou et al. 2004).
14.2.1.1.5 Herpes Simplex Virus
It is a virus that is large in size with a broad range of actions that show continued gene expression from protracted infection (Fig. 14.5). The HSV poses slight chances of insertional mutagenesis since it stays outside the nucleus (episomal). HSV type 1 (HSV-1) strains form the basis for the majority of herpes virus vectors. This double-stranded DNA virus has various unique characteristics, such as the capacity to stay dormant in tissues and to revive at an infection site. HSV-1 replicates once it has infected a cell, leading to cell and infection of neighboring cells. Furthermore, HSV-1 is a widespread human infection that infrequently results in serious illnesses. HSV vectors can quickly and effectively transmit genes while accommodating significant amounts of foreign DNA.
Herpes simplex virus has some limitations, such as the following:
-
1.
Poor transfection efficiency.
-
2.
Large genome size.
-
3.
The function of HSV is confined due to its high affinity toward neuronal cells. However, some researchers are taking advantage of this limitation to target the neurons (Ayllón Barbellido et al. 2008; Gardlik et al. 2011; Roman et al. 2011).
14.2.1.2 RNA-Based Viral Vectors for Gene Delivery
Infectious RNA transcripts can now be directly translated by RNA-based viral vectors for the delivery of genes. Gene delivery with RNA is typically temporary and not permanent. Oncoretro-viral vectors, human foamy virus, and lentiviral vectors are RNA-based viral vectors for delivery of genes used in the treatment of genetic diseases. The advanced system provides negative-strand RNA templates with RNA-dependent polymerase complexes (Mogler and Kamrud 2015). Patients who received transplantation for AIDS-related lymphoma have used lentiviral vector-altered CD34(+) cells as RNA-based gene delivery methods for HIV (DiGiusto et al. 2010).
14.2.1.2.1 Retrovirus
One of the main RNA-based viral vectors for the delivery of genes is a retrovirus. Retroviruses are RNA-based viruses that are single-stranded that have genomes that can accommodate transgenes up to 10 kb in size (Barquinero et al. 2004; Daniel and Smith 2008) and can contain genes up to 7–11 kb in size (Kamimura et al. 2011; Barquinero et al. 2004). The nuclear pores of proliferating cells do not act as barriers to retroviral vectors, which are very successful in dividing cells (Nayerossadat et al. 2012; Bushman 2007). Additionally, retroviruses are highly useful for interventions that favor permanent gene transfer (Anson 2004; Mancheño-Corvo and Martín-Duque 2006). However, in addition to their potential for pathogenicity and immunogenicity, retroviral vectors also carry the risk of mutation because of how efficiently they incorporate into the DNA of the host cell (Basarkar and Singh 2007; Anson 2004). Additionally, target cells may become randomly infected when retroviral vector systems are used (Yi et al. 2011).
The limitations of retrovirus are as follows:
-
1.
Less vector titer
-
2.
Lesser transfection efficiency exhibited in in vitro studies
-
3.
Particle instability
-
4.
Difficult to transduce nondividing postmitotic cells
It is important to remember that each viral vector system has specific advantages and disadvantages, requiring a distinct study to determine the applications for which each is most appropriate (Mancheño-Corvo and Martín-Duque 2006; Siemens et al. 2003).
14.2.1.3 Oncolytic Viral Vectors for Gene Delivery
These viruses are used as a novel type of treatment for diseases related to cancer. Lately, they have emphasized oncology in an attempt to improve the effectiveness of their treatment interventions (Howells et al. 2017). The benefits and drawbacks of the many modifications made to them to improve infectivity and therapeutic safety for the interaction of tumor cells and oncolytic viruses (OVs) have been discussed. Through the development of T cells expressing IL-18R or IL-12R2, oncolytic adenoviruses co-expressing IL-18 and IL-12 enhance tumor-specific immunity (Choi et al. 2011). The ultimate objective is to develop a virus that can successfully multiply inside the host, find a specific target, and kill cancerous cells. Adenovirus-induced decorin expression triggers p53 activation and mitochondrial apoptosis, which kill cancer cells (Yoon et al. 2017). With the support of gene therapy, oncolytic adenovirus vectors provide a promising treatment option for cancer (Choi et al. 2015). IFN- and TNF-producing T cells that express IL-23 and p35 are activated to provide antitumor immunity. According to studies, cytokine immune-gene therapy is one of the most effective treatments for cancer (Choi et al. 2013; Hernandez-Gea et al. 2013; El-Aneed 2004; Baban et al. 2010).
14.2.2 Nonviral Vectors
In substitution for typical viral-based vectors, the nonviral vector strategy was developed. Lipids, polymers, and peptides are nonviral vector approaches explored for gene delivery (Godbey and Mikos 2001; Zhi et al. 2013; Eliyahu et al. 2005).

14.2.2.1 Lipid-Based Gene Delivery
Currently, the nonviral vector that has received the most emphasis is lipid-based gene delivery, which has shown promise for controlling cellular gene expression in both research and therapeutic contexts. They self-build with DNA having a negative charge to produce a cationic-charged complex, which leads to the production of a complex of plasmid DNA and cationic lipids (Wasungu and Hoekstra 2006). They are cationic at normal pH and are made up of a neutral lipid or cholesterol and cationic lipid (de Ilarduya et al. 2010). DNA is delivered into the cytoplasm when lipoplex comes in contact with cellular plasma membrane and internalize into the cell through endocytosis. This causes the lipoplex to become unstable. However, the delivery of other medicinal macromolecules has been the predominant use for anionic liposomes (Mayhew and Papajadjopoulos 1983).
They are classified into two types:

14.2.2.1.1 Cationic Lipid-Based Gene Delivery
Researchers have been working on designing and examining cationic lipid formulations for effective gene transfer since 1983; [1,2-bis(oleoyloxy)-3-(trimethylammonio)propane] (DOTAP) (Leventis and Silvius 1990), N-[1-(2,3-dioleyloxy)propyl]-N,N,N-trimethylammonium chloride (DOTMA) (Felgner et al. 1987), 3β[N-(N′,N′-dimethylaminoethane)-carbamoyl] cholesterol (DC-Chol) (Gao and Huang 1991), and dioctadecylamido glycylspermine (DOGS) (Behr et al. 1989) are the common agents for cationic lipids. To facilitate endolysosomal escape, cationic lipids are typically combined with the neutral lipid dioleoylphosphatidylethanolamine (DOPE) (Farhood et al. 1995).
14.2.2.1.1.1 N-[1-(2, 3-Dioleyloxy)Propyl]-N,N,N-Trimethylammonium Chloride (DOTMA)
N-[1-(2, 3-dioleyloxy)propyl]-N,N,N-trimethylammonium chloride, or DOTMA, was used as a lipofectin. DOTMA was combined in a 1:1 ratio with a neutral lipid called dioleoylphosphatidylethanolamine (DOPE) to enhance the transfection efficacy of lipofectin (Fig. 14.6). One of the early developed, extensively studied, and widely available cationic lipids for the delivery of genes was DOTMA. In an attempt to increase transfection efficiency and decrease toxicity, various research groups prepared modified DOTMA by altering its key functional components, such as linker, its head group, hydrocarbon chains, and linkage bonds (Vaheri and Pagano 1965; Ren et al. 2000). It was discovered that the cytotoxicity linked to the synthesized monovalent lipids depended on the density of the plated cells and the lipids’ structural properties (Vaheri and Pagano 1965).
N-[1-(2, 3-dioleyloxy)propyl]-N,N,N-trimethylammonium chloride (DOTMA) (Vaidya et al. 2022)
14.2.2.1.1.2 2,3-Dioleyloxy-N[2(Sperminecarbaxamido)Ethyl]-N,N-Dimethyl-1-Propaminium Trifluoroacetate (DOSPA)
Cationic lipid developed as a derivative of DOTMA is 2,3-dioleyloxy-N[2(sperminecarboxamido)ethyl]-N,N-dimethyl-1-propanaminium trifluoroacetate, or DOSPA. DOSPA and DOTMA are almost similar in structure (Fig. 14.7). The main distinction between them is that DOSPA contains a spermine functional group that is linked to the hydrophobic chains by a peptide bond, enabling more effective DNA packing (Jain et al. 1989).
2,3-Dioleyloxy-N[2(sperminecarbaxamido)ethyl]-N,N-dimethyl-1-propaminium trifluoroacetate (Li et al. 2015)
14.2.2.1.1.3 N-[1-(2,3-Dioleyloxy)-Propyl]-N,N,N-Trimethylammonium Chloride (DOTAP)
N-[1-(2,3-dioleyloxy)-propyl]-N,N,N-trimethylammonium chloride (DOTAP) (Felgner et al. 1987) is the most well-researched lipid which has been used to in vivo genetically modify a number of animal organs (Zhu et al. 1993) (Fig. 14.8). The key distinction among DOTMA and DOTAP is that DOTAP has ester bonds instead of ether bonds connecting the backbones, which can be hydrolyzed to help break down lipids and lessen toxicity. Using a cationic lipid complex containing cholesterol and DOTAP, high tumor selectivity can be achieved as per the experimental studies. When compared to liver tissue, this approach yields a minimum of ten times more expression for every milligram of tumor tissue (Wang et al. 2013).
14.2.2.1.1.4 3[N-(N′,N′-Dimethylaminoethane)-Carbamoyl]Cholesterol (DC-Chol)
3[N-(N′,N′-dimethylaminoethane)-carbamoyl]cholesterol was discovered in 1991 (Gao and Huang 1991) (Fig. 14.9). Unlike DOTAP and DOTMA, DC-Chol has a tertiary amine group that may assist to prevent lipoplex aggregation and promote increased expression of transgene (Ajmani and Hughes 1999).
3[N-(N′,N′-dimethylaminoethane)-carbamoyl]cholesterol (DC-Chol) (Vaidya et al. 2022)
14.2.2.1.1.5 Di-Octadecyl-Amido-Glycyl-Spermine (DOGS)
DOGS, also known as di-octadecyl-amido-glycyl-spermine, is marketed as transfectam and has similar characteristics to DOSPA (Fig. 14.10). Both DOGS and DOSPA have 2 18-carbon alkyl chains in addition to a spermine group. These two are different from one another since DOSPA contains a quaternary amine. Contrarily, DOGS lacks quaternary amines; instead, it has saturated chains and is connected to the head group by a peptide linkage. Numerous cell lines have been transfected using DOGS. The delivery of the chloramphenicol acetyltransferase (CAT) reporter plasmid without any cytotoxic effects was demonstrated using DOGS (Behr et al. 1989).
Di-octadecyl-amido-glycyl-spermine (DOGS) (Zhi et al. 2018)
14.2.2.1.2 Anionic Lipid-Based Gene Delivery
Plasmid DNA, anionic lipids, and cations constitute the components of anionic lipoplexes (Srinivasan and Burgess 2009). Gene delivery using anionic lipids is often not very desirable or effective. Phospholipids like phosphatidylglycerol, phosphatidylserine, and phosphatidic acid, which are naturally present in biological membranes, are often employed as anionic lipids in gene delivery. As a nonviral means of delivery, DNA encapsulation into anionic and neutral liposomes has also been investigated. Owing to electrostatic repulsive interactions that exist among an anionic head group of the lipids and the phosphate backbone of DNA, an anionic liposome cannot effectively attach to anionic DNA. To use anionic lipids for cell-specific targeting, DNA must be enclosed. However, the DNA matrix’s size and shape restrict the uses of its encapsulated form (Ledley 1995).
A few number of cell types, including hippocampal neurons and CHO cells, have been recorded for gene delivery using a variety of anionic liposomes (Ledley 1995; Patil et al. 2004, 2005b). Nevertheless, despite extremely positive and hopeful outcomes, our total understanding of anionic lipofection is still restricted due to numerous challenges, few of which will definitely test our scientific acumen. The absence of reproducibility is one of the primary causes. Furthermore, because of their systematic administration, it is linked to undesirable side effects from nonspecific immune system cytotoxicity that results in a variety of unfavorable consequences.
14.2.2.2 Polymer-Based Gene Delivery
In an attempt to overcome the immunogenic and carcinogenic issues related with viral vectors, carriers based on the polymer for the delivery of genes have been designed as their alternative. Due to its tremendous scope for the design of safe and reliable vectors, it has garnered a lot of interest over the past two decades (Kang et al. 2012).
Researchers studying gene delivery methods have investigated the possible advantages of polymeric vehicles for genes in cationic biopolymers including chitosan derivatives and liposomes (Ginn et al. 2018; Hulin-Curtis et al. 2016). Polymeric materials can conceal the DNA of negative charges and compress the big genes into smaller tiny molecules when used with cationic polymers for gene delivery. The polyplex is the complex of cationic polymer-based nucleic acid. One important targeting delivery system for gene therapy is the gene complex. The majority of studies concentrate on the impact of ligands that are attached to the DNA complex by covalent bonding. The targeted ligands can combine with the cationic polymers. Poly(l-lactide) is a polymer that can be a potential alternative that is frequently used to link the targeted ligands (Stone 2010; Manno et al. 2006; Kabanov and Kabanov 1995; Buwalda et al. 2012). Under a wide range of circumstances, compaction occurs during cationic polymer condensation of plasmid DNA. Condensing agents mainly utilized are multivalent cationic polymers (Bloomfield 1991, 1996).
One of the main classes of cationic polymers used as condensing agent is polyamidoamine (PAMAM) dendrimer.
Polyamidoamine Dendrimers (PAMAM Dendrimers)
A group of highly branched cationic polymers known as polyamidoamine dendrimers may condense DNA and deliver it to a number of cell types with minimal cytotoxic effects (Kukowska-Latallo et al. 1996). These are circular polymers that are highly branched and are frequently used in transfer of genes using nonviral vectors.
The most widely utilized dendrimers for the delivery of genes using nonviral vectors are the 6-generation Starburst™ PAMAM dendrimers, either in fragmented or intact form. The first polyamidoamine (PAMAM) dendrimers were developed by Tomalia et al. (1985) (Fig. 14.11). The process used to form this type of starburst dendrimer involves adding methyl acrylate by Michael addition to an original core (such as ethylenediamine or ammonia) that has a number of branching points at its center. Next, the resulting esters are amidated with ethylenediamine. Later investigations made use of PAMAM-OH dendrimers with hydroxyl ends (Fig. 14.11b). PAMAMOH that had been neutralized on the surface showed advantages, including less toxicity and lower transfection efficiency (Lee et al. 2003). l-Arginine was used to modify these dendrimers by forming degradable ester linkages (Fig. 14.11c). In fact, the l-arginine ester-grafted PAMAM-OH G4 had a higher transfection and reduced toxic effects than the earlier l-arginine amide-grafted PAMAM G4 (Nam et al. 2009).
The primary benefit of the fractured dendrimer’s structural design is the presence of highly dense amine at the molecule’s periphery, which facilitates effective condensation of nucleic acids while releasing up the amino group present inside for a proton sponge throughout endolysosomal acidification, facilitating most effective endosomal escape. In comparison with the intact polymer, these dendrimers exhibit much increased (>50-fold) levels of expression of reporter gene. This finding’s cause is unknown, although it is possible that one of the key causes is that the polymer’s enhanced flexibility and improved capacity for complexing with DNA serve to promote gene expression (Tang et al. 1996). Researchers have looked at the tertiary structures that resemble noncondensing plasmid DNA in complexes with less hydrophobized stearyl-poly(l-lactide) (Kim et al. 1998a). The process of adding hydrophilic components, such as or hydrophobic stearyl chains, dextran, polyethylene glycol, hydrophobic stearyl chains, or hyaluronic acid, has however been facilitated by DNA condensation (Toncheva et al. 1998; Maruyama et al. 1997a; Katayose and Kataoka 1997; Kim et al. 1997).
Polymer-based gene delivery has been divided into three categories:

14.2.2.2.1 Polysaccharide-Based Gene Delivery Systems
On the basis of grafted oligoamine residues for natural polysaccharides, a range of biodegradable cationic polymers were identified for the delivery of genes (Azzam et al. 2002). The grafting concept enables 3D contact with anionic surfaces of double-stranded DNA chains by attaching side chain oligomers to hydrophilic polysaccharides, which are branched or linear in nature. The polysaccharide-based gene delivery technology suggests that the cationic polysaccharide’s structure has a key impact in the transfection activity for delivery of genes. The delivery of compounds such as plasmids and oligonucleotides through mucosa had been explored using colloidal polysaccharide particles (Janes et al. 2001). Nanoparticles made of natural polysaccharides have also been investigated as methods of delivering drugs and genes (Liu et al. 2008). The mechanism for manufacturing polysaccharide-based nanoparticles included electrolyte complexing, ionic and covalent cross-linking, and hydrophobic polysaccharide self-assembly. Natural polysaccharides like chitosan have been evaluated as vehicles for drugs or genes (Morris et al. 2010).
14.2.2.2.1.1 Chitosan
It is a biodegradable polysaccharide that is linear in nature comprising of N-acetyl-d-glucosamine and b-1,4-linked d-glucosamine residues. Owing to its non-allergenicity, biodegradability, biocompatibility, mucoadhesive property, and great binding with DNA, it is one of the highly reported naturally produced cationic gene polymers used in nonviral gene transfer. In a series of experiments using both experimental animals and humans, chitosan displayed little cytotoxicity and increased transfection effectiveness (Rao and Sharma 1997; Aspden et al. 1997).
14.2.2.2.2 Gene Delivery Systems Based on Polyethyleneimine
In most cases, polyamines that turn cationic under physiologic conditions are the cationic polymers used for gene complexation. Due to their great complex stability, polyethyleneimines (PEIs), which were initially proposed by Boussif et al. (1995), are one of the most extensively researched and regarded as the reference of nonviral vectors for gene delivery. Compared to other polycations like PLL, PEIs provide a transfection that is significantly more effective and resistant to nuclease degradation. It might be because PEIs form more compact and effective complexations and have higher charge densities. Amino groups present in the PEIS have the ability to increase their ability to act as buffers. These result in the rupture of lysosome and then permeate into the cytoplasm through the effect of the proton sponge (Benjaminsen et al. 2013; Luu et al. 2012). The other functional molecules can receive some buffering capacity enhancement from PEI. PEI conjugated poly(cystamine bis(acrylamide)-diaminohexane) [poly(CBA-DAH)] was designed to improve transfection efficiency and reduce weight ratio. Multiple disulfides constitute poly(CBA-DAH), which are broken down in the cytoplasm by an intracellular reducing agent like glutathione (GSH) (Doss et al. 2013; Hong et al. 2006; Oupický and Li 2014; Wen et al. 2011). The triple peptide that constitutes GSH was formed in the cytosol from precursor amino acids (Chakravarthi et al. 2006).
The improved buffering ability of other compounds including imidazole, histidine, and PEI was also demonstrated in other investigations (Bello Roufaï and Midoux 2001; Pack et al. 2000; Pires et al. 2011; Yang et al. 2008; Zhang et al. 2015). Additionally, water-soluble, branching poly(ethylenimine)-cholesterol lipo-polymers for gene transport were produced (Wang et al. 2002). Gene delivery uses altered linear polyethylenimine-cholesterol conjugates for DNA complexation (Furgeson et al. 2003). A gene delivery approach using polyethylenimine-cholesterol/DNA complexes which are linear in nature had been investigated for tumor effectiveness and biodistribution (Furgeson et al. 2004). A biodegradable gene carrier made of polyethyleneimine with acid-labile links had been developed and evaluated (Kim et al. 2005). Gene delivery technologies consisting of reducible poly(amidoethylenimine)s have been developed and evaluated (Christensen et al. 2006, 2007; Jeong et al. 2007, 2010).
14.2.2.2.3 Poly(l-Lysine)-Based Gene Delivery Systems
The main cationic polymer used for the transfer of genes is poly(l-lysine) (PLL). PLL falls under the category of cationic-charged polymers at neutral pH. A hydrophilic cationic amino group is mainly found in PLL. PLL has the capacity to join with DNA to build a polyelectrolyte complex. In physiologic conditions, PLL (pKa 10.5) is protonated, which ionically interacts with DNA’s negatively charged phosphate groups and builds a polyelectrolyte complex of nanoparticulate range (Laemmli 1975). To facilitate the co-adsorption of plasmid DNA, PLL-based replica particles are cross-linked using a homo-bifunctional linker have been developed for the delivery of genes (Zhang et al. 2010). For the in vivo delivery of genes to the liver, poly-l-lysine/DNA polyplexes have been stabilized (Kwoh et al. 1999). There have been reports on the development and evaluation of poly-l-lysine-based carriers for the delivery of genes (Choi et al. 2000). Modified poly(l-lysine) such as N-terminal modified poly(l-lysine) antibody conjugate is utilized as a vehicle for delivering specific genes in endothelial cells present in the lungs of the mouse (Trubetskoy et al. 1992). Terplex DNA delivery systems and DNA nanoparticle carriers with grafted poly(l-lysine) polysaccharide copolymers were initially developed and recognized as a carrier of genes (Kim et al. 1998a; Maruyama et al. 1997b). For gene delivery systems, terplex delivery systems and polyethylene glycol grafted poly(l-lysine) were also designed (Kim et al. 1998b; Choi et al. 1998a,b, 1999). In the area of biomedical applications, clinical evaluations are now being conducted (Park et al. 2006; Meel et al. 2016; Bodles-Brakhop et al. 2009; Pulkkanen and Yla-Herttuala 2005; Rainov 2000; Young et al. 2006; Yoshida et al. 2004; Weichselbaum et al. 2002; Amer 2014; Jayant et al. 2016; Nam et al. 2015).
14.2.2.3 Peptide-Based Gene Delivery
Genomic carriers based on peptides provide a number of advantageous properties. With the aid of the naturally occurring amino acids ornithine, arginine, and lysine, which provide positive charges, electrostatic interaction can condense the pDNA (Plank et al. 1999) and perform a variety of other functions, including endosomal escape and receptor-targeted delivery. Oligolysine peptides are a substitute for heterogeneous polylysine peptides that include lysines of a fixed length (l-lysine). It is also possible to carry out site-specific adjustments because of the specific structure. According to studies, pDNA can be compacted by oligolysine molecules with 13 or more lysine monomers (Wadhwa et al. 1997), and a peptide with 18 lysines can form stable polyplexes with pDNA that are guarded against breakdown (Adami et al. 1998).
Cross-linking has been shown to significantly enhance the stability of DNA complexes. In order to establish bioreversible disulfide linkages through oxidation, cysteine has been added to the peptide sequence. They studied modifications of the Trp-Lys20 peptide by replacing one to four of the lysines with cysteines. The best transfection efficiency was demonstrated by a peptide containing two terminal cysteines (McKenzie et al. 2000a). Similar transfection effectiveness was attained using reduced lysine chains that contained only two terminal cysteines and four lysines (McKenzie et al. 2000b).
Fusogenic peptides, such as amphipathic peptides with large amounts of basic amino acids, like Tat (Fawell et al. 1994), melittin (Boeckle et al. 2006; Chen et al. 2006), KALA (Wyman et al. 1997), and others (Lehto et al. 2010; El Andaloussi et al. 2011), were used to mediate endosomal escape as an alternate choice to the mechanism of proton sponge (Zorko and Langel 2005). These peptides can damage endosomes by interacting with their lipid membrane. Numerous polymers, cationic peptides, or other gene delivery vehicles were combined with fusogenic peptides.
14.2.3 Physical Method of Gene Delivery
The physical method of transferring genes is not vector-mediated and does not require carriers.
It involves various methods such as electroporation, sonoporation, photoporation, magneto-fection, needle injection, hydroporation, and gene guns.
-
Electroporation: This method involves the use of electromagnetic pulse, which helps in the formation of pores in the membrane of the cell to enable the genetic materials into the cell.
-
Sonoporation: This technique involves the usage of sound waves for the creation of pores in the membrane of the cell for the movement of genetic materials into the cell.
-
Photoporation: The method of photoporation helps in the formation of pores in the cell membrane by using a laser pulse for entering the genetic materials into the cell.
-
Magneto-fection: It involves utilizing magnetic particles that have complexed with DNA and an external magnetic field to concentrate nucleic acid particles inside target cells.
-
Needle injection: This method involves the usage of needles to directly inject genetic materials into the cell.
-
Hydroporation: This is the technique that involves the use of the hydrodynamic capillary effect to alter the permeability of the cell membrane for the entry of genetic materials into the cell (Sung and Kim 2018).
14.3 Conclusion
In recent years, gene therapy has garnered significant interest and can be used as a promising treatment of choice for chronic conditions caused by genetic abnormalities. Gene therapy is a type of treatment in which genes are mainly used for the treatment of diseases. In gene therapy, components used for gene delivery are the essential components for the treatment of genetic diseases. The major aim of the research on gene delivery systems is to design proper vectors for the treatment of serious life-threatening diseases like cancer, Alzheimer’s, and AIDS. Several types of materials used in gene delivery systems are summarized and discussed here.
As all we know, viral vectors are playing a vital role in clinical trials of gene delivery such as adenovirus, herpes simplex virus, adeno-associated virus, retrovirus, etc. These vectors are efficient to carry the genome into target cells. On the other hand, nonviral vectors are also gaining attention in gene therapy. The materials utilized for nonviral vectors are lipids, peptides, and polymers. There are different categories of lipids such as anionic lipids and cationic lipids which are used for the delivery of genes. This lipid-based gene delivery acts as a promising approach to altering gene expression at a cellular level in therapeutic and research applications. Polymeric gene delivery systems have gained interest in basic research and clinical applications. These gene delivery systems have several merits such as cost-effectiveness, easy scale-up production, and easy modulation of DNA loading capacity. However, materials that are stable and monodisperse in size must be used for the effective delivery of genes. Hence the main advantages of gene delivery systems should be assessed carefully to reach the desired target by building an effective system with proper biodistribution to first-pass organs and rapid clearance of complexes for efficient gene delivery. In the future, our main research has to focus on DNA and RNA molecular techniques to become the main treatment in the biomedical field.
References
Adami RC, Collard WT, Gupta SA, Kwok KY, Bonadio J, Rice KG (1998) Stability of peptide-condensed plasmid DNA formulations. J Pharm Sci 87(6):678–683
Ajmani PS, Hughes JA (1999) 3β [N-(N′, N′-Dimethylaminoethane)-carbamoyl] cholesterol (DC-Chol)-mediated gene delivery to primary rat neurons: characterization and mechanism. Neurochem Res 24(5):699–703
Ako-Adounvo AM, Marabesi B, Lemos RC, Patricia A, Karla PK (2017) Drug and gene delivery materials and devices. In: Emerging nanotechnologies for diagnostics, drug delivery and medical devices, pp 375–392. https://doi.org/10.1016/B978-0-323-42978-8.00015-2
Amer MH (2014) Gene therapy for cancer: present status and future perspective. Mol Cell Therap 2(1):1–9. https://doi.org/10.1186/2052-8426-2-27
Anson DS (2004) The use of retroviral vectors for gene therapy-what are the risks? A review of retroviral pathogenesis and its relevance to retroviral vector-mediated gene delivery. Genet Vaccines Ther 2(1):1–3. https://doi.org/10.1186/1479-0556-2-9
Aspden TJ, Mason JD, Jones NS, Lowe J, Skaugrud Ø, Illum L (1997) Chitosan as a nasal delivery system: the effect of chitosan solutions on in vitro and in vivo mucociliary transport rates in human turbinates and volunteers. J Pharm Sci 86(4):509–513
Ayllón Barbellido S, Campo Trapero J, Cano Sánchez J, Perea García MA, Escudero-Castaño N, Bascones MA (2008) Gene therapy in the management of oral cancer: review of the literature. Med Oral Patol Oral Cir Bucal 13(1):E15–E21
Azzam T, Eliyahu H, Shapira L, Linial M, Barenholz Y, Domb AJ (2002) Polysaccharide-oligoamine based conjugates for gene delivery. J Med Chem 45(9):1817–1824
Baban CK, Cronin M, O’Hanlon D, O’Sullivan GC, Tangney M (2010) Bacteria as vectors for gene therapy of cancer. Bioeng Bugs 1(6):385–394
Barquinero J, Eixarch H, Perez-Melgosa M (2004) Retroviral vectors: new applications for an old tool. Gene Ther 11(1):S3–S9
Basarkar A, Singh J (2007) Nanoparticulate systems for polynucleotide delivery. Int J Nanomedicine 2(3):353
Behr JP, Demeneix B, Loeffler JP, Perez-Mutul J (1989) Efficient gene transfer into mammalian primary endocrine cells with lipopolyamine-coated DNA. Proc Natl Acad Sci 86(18):6982–6986
Bello Roufaï M, Midoux P (2001) Histidylated polylysine as DNA vector: elevation of the imidazole protonation and reduced cellular uptake without change in the polyfection efficiency of serum stabilized negative polyplexes. Bioconjug Chem 12(1):92–99
Benjaminsen RV, Mattebjerg MA, Henriksen JR, Moghimi SM, Andresen TL (2013) The possible “proton sponge” effect of polyethylenimine (PEI) does not include change in lysosomal pH. Mol Ther 21(1):149–157
Biçeroğlu S, Memiş A (2005) Gene therapy: applications in interventional radiology. Diagn Interv Radiol 11(113):8
Blaese RM, Culver KW, Miller AD, Carter CS, Fleisher T, Clerici M, Shearer G, Chang L, Chiang Y, Tolstoshev P, Greenblatt JJ (1995) T lymphocyte-directed gene therapy for ADA–SCID: initial trial results after 4 years. Science 270(5235):475–480
Bloomfield VA (1991) Condensation of DNA by multivalent cations: considerations on mechanism. Biopolymers 31(13):1471–1481
Bloomfield VA (1996) DNA condensation. Curr Opin Struct Biol 6(3):334–341
Bodles-Brakhop AM, Heller R, Draghia-Akli R (2009) Electroporation for the delivery of DNA-based vaccines and immunotherapeutics: current clinical developments. Mol Ther 17(4):585–592
Boeckle S, Fahrmeir J, Roedl W, Ogris M, Wagner E (2006) Melittin analogs with high lytic activity at endosomal pH enhance transfection with purified targeted PEI polyplexes. J Control Release 112(2):240–248
Boussif O, Lezoualc’h F, Zanta MA, Mergny MD, Scherman D, Demeneix B, Behr JP (1995) A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc Natl Acad Sci 92(16):7297–7301
Bushman FD (2007) Retroviral integration and human gene therapy. J Clin Invest 117(8):2083–2086
Buwalda SJ, Dijkstra PJ, Feijen J (2012) Poly (ethylene glycol)–poly (l-lactide) star block copolymer hydrogels crosslinked by metal–ligand coordination. J Polym Sci A Polym Chem 50(9):1783–1791
Campos SK, Barry MA (2007) Current advances and future challenges in adenoviral vector biology and targeting. Curr Gene Ther 7(3):189–204
Chakravarthi S, Jessop CE, Bulleid NJ (2006) The role of glutathione in disulphide bond formation and endoplasmic-reticulum-generated oxidative stress. EMBO Rep 7(3):271–275
Chen CP, Kim JS, Steenblock E, Liu D, Rice KG (2006) Gene transfer with poly-melittin peptides. Bioconjug Chem 17(4):1057–1062
Chen D, Yang D, Dougherty CA, Lu W, Wu H, He X, Cai T, Van Dort ME, Ross BD, Hong H (2017) In vivo targeting and positron emission tomography imaging of tumor with intrinsically radioactive metal–organic frameworks nanomaterials. ACS Nano 11(4):4315–4327
Choi YH, Liu F, Kim JS, Choi YK, Park JS, Kim SW (1998a) Polyethylene glycol-grafted poly-L-lysine as polymeric gene carrier. J Control Release 54(1):39–48
Choi YH, Liu F, Park JS, Kim SW (1998b) Lactose-poly (ethylene glycol)-grafted poly-L-lysine as hepatoma cell-targeted gene carrier. Bioconjug Chem 9(6):708–718
Choi YH, Liu F, Choi JS, Kim SW, Park JS (1999) Characterization of a targeted gene carrier, lactose-polyethylene glycol-grafted poly-L-lysine, and its complex with plasmid DNA. Hum Gene Ther 10(16):2657–2665
Choi JS, Joo DK, Kim CH, Kim K, Park JS (2000) Synthesis of a barbell-like triblock copolymer, poly (L-lysine) dendrimer-block-poly (ethylene glycol)-block-poly (L-lysine) dendrimer, and its self-assembly with plasmid DNA. J Am Chem Soc 122(3):474–480
Choi IK, Lee JS, Zhang SN, Park J, Lee KM, Sonn CH, Yun CO (2011) Oncolytic adenovirus co-expressing IL-12 and IL-18 improves tumor-specific immunity via differentiation of T cells expressing IL-12Rβ2 or IL-18Rα. Gene Ther 18(9):898–909
Choi IK, Li Y, Oh E, Kim J, Yun CO (2013) Oncolytic adenovirus expressing IL-23 and p35 elicits IFN-γ-and TNF-α-co-producing T cell-mediated antitumor immunity. PLoS One 8(7):e67512. https://doi.org/10.1371/journal.pone.0067512
Choi JW, Lee YS, Yun CO, Kim SW (2015) Polymeric oncolytic adenovirus for cancer gene therapy. J Control Release 219:181–191. https://doi.org/10.1016/jconrel.2015.10.009
Christensen LV, Chang CW, Kim WJ, Kim SW, Zhong Z, Lin C, Engbersen JF, Feijen J (2006) Reducible poly (amido ethylenimine) s designed for triggered intracellular gene delivery. Bioconjug Chem 17(5):1233–1240
Christensen LV, Chang CW, Yockman JW, Conners R, Jackson H, Zhong Z, Feijen J, Bull DA, Kim SW (2007) Reducible poly (amido ethylenediamine) for hypoxia-inducible VEGF delivery. J Control Release 118(2):254–261
Crooke ST (1998) An overview of progress in antisense therapeutics. Antisense Nucleic Acid Drug Dev 8(2):115–122
Daniel R, Smith JA (2008) Integration site selection by retroviral vectors: molecular mechanism and clinical consequences. Hum Gene Ther 19(6):557–568
de Ilarduya CT, Sun Y, Düzgüneş N (2010) Gene delivery by lipoplexes and polyplexes. Eur J Pharm Sci 40(3):159–170
DiGiusto DL, Krishnan A, Li L, Li H, Li S, Rao A, Mi S, Yam P, Stinson S, Kalos M, Alvarnas J (2010) RNA-based gene therapy for HIV with lentiviral vector–modified CD34+ cells in patients undergoing transplantation for AIDS-related lymphoma. Sci Transl Med 2(36):36ra43
Doss C, Debottam S, Debajyoti C (2013) Glutathione-responsive nano-transporter-mediated siRNA delivery: silencing the mRNA expression of Ras. Protoplasma 250(3):787–792
El Andaloussi S, Lehto T, Mäger I, Rosenthal-Aizman K, Oprea II, Simonson OE, Sork H, Ezzat K, Copolovici DM, Kurrikoff K, Viola JR (2011) Design of a peptide-based vector, PepFect6, for efficient delivery of siRNA in cell culture and systemically in vivo. Nucleic Acids Res 39(9):3972–3987
El-Aneed A (2004) Current strategies in cancer gene therapy. Eur J Pharmacol 498(1–3):1–8
Eliyahu H, Barenholz Y, Domb AJ (2005) Polymers for DNA delivery. Molecules 10(1):34–64
Farhood H, Serbina N, Huang L (1995) The role of dioleoyl phosphatidylethanolamine in cationic liposome mediated gene transfer. Biochim Biophys Acta 1235(2):289–295
Fawell S, Seery J, Daikh Y, Moore C, Chen LL, Pepinsky B, Barsoum JA (1994) Tat-mediated delivery of heterologous proteins into cells. Proc Natl Acad Sci 91(2):664–668
Felgner PL, Gadek TR, Holm M, Roman R, Chan HW, Wenz M, Northrop JP, Ringold GM, Danielsen M (1987) Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc Natl Acad Sci 84(21):7413–7417
Furgeson DY, Chan WS, Yockman JW, Kim SW (2003) Modified linear polyethylenimine-cholesterol conjugates for DNA complexation. Bioconjug Chem 14(4):840–847
Furgeson DY, Yockman JW, Janat MM, Kim SW (2004) Tumor efficacy and biodistribution of linear polyethylenimine-cholesterol/DNA complexes. Mol Ther 9(6):837–845
Gao X, Huang L (1991) A novel cationic liposome reagent for efficient transfection of mammalian cells. Biochem Biophys Res Commun 179(1):280–285
Gardlík R, Pálffy R, Hodosy J, Lukács J, Turna J, Celec P (2005) Vectors and delivery systems in gene therapy. Med Sci Monit 11(4):110–121
Gardlik R, Celec P, Bernadic M (2011) Targeting angiogenesis for cancer (gene) therapy. Bratisl Lek Listy 112(8):428–434
Ginn SL, Amaya AK, Alexander IE, Edelstein M, Abedi MR (2018) Gene therapy clinical trials worldwide to 2017: an update. J Gene Med 20(5):e3015
Godbey WT, Mikos AG (2001) Recent progress in gene delivery using non-viral transfer complexes. J Control Release 72(1–3):115–125
Han SO, Mahato RI, Sung YK, Kim SW (2000) Development of biomaterials for gene therapy. Mol Ther 2(4):302–317
Han L, Zhang Y, Lu X, Wang K, Wang Z, Zhang H (2016) Polydopamine nanoparticles modulating stimuli-responsive PNIPAM hydrogels with cell/tissue adhesiveness. ACS Appl Mater Interfaces 8(42):29088–29100
Hernandez-Gea V, Toffanin S, Friedman SL, Llovet JM (2013) Role of the microenvironment in the pathogenesis and treatment of hepatocellular carcinoma. Gastroenterology 144(3):512–527
Hong R, Han G, Fernández JM, Kim BJ, Forbes NS, Rotello VM (2006) Glutathione-mediated delivery and release using monolayer protected nanoparticle carriers. J Am Chem Soc 128(4):1078–1079
Howells A, Marelli G, Lemoine NR, Wang Y (2017) Oncolytic viruses—interaction of virus and tumor cells in the battle to eliminate cancer. Front Oncol 7:195. https://doi.org/10.3389/fonc.2017.00195
Hulin-Curtis SL, Uusi-Kerttula H, Jones R, Hanna L, Chester JD, Parker AL (2016) Evaluation of CD46 re-targeted adenoviral vectors for clinical ovarian cancer intraperitoneal therapy. Cancer Gene Ther 23(7):229–234
Jain S, Zon G, Sundaralingam M (1989) Base only binding of spermine in the deep groove of the A-DNA octamer d (GTGTACAC). Biochemistry 28(6):2360–2364
Janes KA, Calvo P, Alonso MJ (2001) Polysaccharide colloidal particles as delivery systems for macromolecules. Adv Drug Deliv Rev 47(1):83–97
Jayant RD, Sosa D, Kaushik A, Atluri V, Vashist A, Tomitaka A, Nair M (2016) Current status of non-viral gene therapy for CNS disorders. Expert Opin Drug Deliv 13(10):1433–1445. https://doi.org/10.1080/17425247.2016.1188802
Jeong JH, Christensen LV, Yockman JW, Zhong Z, Engbersen JF, Kim WJ, Feijen J, Kim SW (2007) Reducible poly (amido ethylenimine) directed to enhance RNA interference. Biomaterials 28(10):1912–1917
Jeong JH, Kim SH, Christensen LV, Feijen J, Kim SW (2010) Reducible poly (amido ethylenimine)-based gene delivery system for improved nucleus trafficking of plasmid DNA. Bioconjug Chem 21(2):296–301
Kabanov AV, Kabanov VA (1995) DNA complexes with polycations for the delivery of genetic material into cells. Bioconjug Chem 6(1):7–20
Kamimura K, Suda T, Zhang G, Liu D (2011) Advances in gene delivery systems. Pharmaceut Med 25(5):293–306
Kang HC, Huh KM, Bae YH (2012) Polymeric nucleic acid carriers: current issues and novel design approaches. J Control Release 164(3):256–264
Katayose S, Kataoka K (1997) Water-soluble polyion complex associates of DNA and poly (ethylene glycol)-poly (l-lysine) block copolymer. Bioconjug Chem 8(5):702–707
Kay MA, Liu D, Hoogerbrugge PM (1997) Gene therapy. Proc Natl Acad Sci U S A 94:12744–12746
Kemp JA, Shim MS, Heo CY, Kwon YJ (2016) “Combo” nanomedicine: co-delivery of multi-modal therapeutics for efficient, targeted, and safe cancer therapy. Adv Drug Deliv Rev 98:3–18
Kim JS, Maruyama A, Akaike T, Kim SW (1997) In vitro gene expression on smooth muscle cells using a terplex delivery system. J Control Release 47(1):51–59
Kim JS, Maruyama A, Akaike T, Kim SW (1998a) Terplex DNA delivery system as a gene carrier. Pharm Res 15(1):116–121
Kim JS, Kim BI, Maruyama A, Akaike T, Kim SW (1998b) A new non-viral DNA delivery vector: the terplex system. J Control Release 53(1–3):175–182
Kim YH, Park JH, Lee M, Kim YH, Park TG, Kim SW (2005) Polyethylenimine with acid-labile linkages as a biodegradable gene carrier. J Control Release 103(1):209–219
Kuang Y, Zhang K, Cao Y, Chen X, Wang K, Liu M, Pei R (2017) Hydrophobic IR-780 dye encapsulated in cRGD-conjugated solid lipid nanoparticles for NIR imaging-guided photothermal therapy. ACS Appl Mater Interfaces 9(14):12217–12226
Kukowska-Latallo JF, Bielinska AU, Johnson J, Spindler R, Tomalia DA, Baker JR Jr (1996) Efficient transfer of genetic material into mammalian cells using Starburst polyamidoamine dendrimers. Proc Natl Acad Sci 93(10):4897–4902
Kwoh DY, Coffin CC, Lollo CP, Jovenal J, Banaszczyk MG, Mullen P, Phillips A, Amini A, Fabrycki J, Bartholomew RM, Brostoff SW (1999) Stabilization of poly-L-lysine/DNA polyplexes for in vivo gene delivery to the liver. Biochim Biophys Acta 1444(2):171–190
Laemmli UK (1975) Characterization of DNA condensates induced by poly (ethylene oxide) and polylysine. Proc Natl Acad Sci 72(11):4288–4292
Ledley FD (1995) Nonviral gene therapy: the promise of genes as pharmaceutical products. Hum Gene Ther 6(9):1129–1144
Lee JH, Lim YB, Choi JS, Lee Y, Kim TI, Kim HJ, Yoon JK, Kim K, Park JS (2003) Polyplexes assembled with internally quaternized PAMAM-OH dendrimer and plasmid DNA have a neutral surface and gene delivery potency. Bioconjug Chem 14(6):1214–1221
Lehto T, Abes R, Oskolkov N, Suhorutšenko J, Copolovici DM, Mäger I, Viola JR, Simonson OE, Ezzat K, Guterstam P, Eriste E (2010) Delivery of nucleic acids with a stearylated (RxR) 4 peptide using a non-covalent co-incubation strategy. J Control Release 141(1):42–51
Leventis R, Silvius JR (1990) Interactions of mammalian cells with lipid dispersions containing novel metabolizable cationic amphiphiles. Biochim Biophys Acta 1023(1):124–132
Li L, He ZY, Wei XW, Gao GP, Wei YQ (2015) Challenges in CRISPR/CAS9 delivery: potential roles of nonviral vectors. Hum Gene Ther 26(7):452–462
Liu Z, Jiao Y, Wang Y, Zhou C, Zhang Z (2008) Polysaccharides-based nanoparticles as drug delivery systems. Adv Drug Deliv Rev 60(15):1650–1662
Liu LX, Li BX, Wang QY, Dong ZP, Li HM, Jin QM, Hong H, Zhang J, Wang Y (2016) An integrative folate-based metal complex nanotube as a potent antitumor nanomedicine as well as an efficient tumor-targeted drug carrier. Bioconjug Chem 27(12):2863–2873
Luu QP, Shin JY, Kim YK, Islam MA, Kang SK, Cho MH, Choi YJ, Cho CS (2012) High gene transfer by the osmotic polysorbitol-mediated transporter through the selective caveolae endocytic pathway. Mol Pharm 9(8):2206–2218
Mahato RI, Smith LC, Rolland A (1999) Pharmaceutical perspectives of nonviral gene therapy. Adv Genet 41:95–156
Majhen D, Ambriović-Ristov A (2006) Adenoviral vectors—how to use them in cancer gene therapy? Virus Res 119(2):121–133
Mancheño-Corvo P, Martín-Duque P (2006) Viral gene therapy. Clin Transl Oncol 8(12):858–867
Manno CS, Pierce GF, Arruda VR, Glader B, Ragni M, Rasko JJ, Ozelo MC, Hoots K, Blatt P, Konkle B, Dake M (2006) Successful transduction of liver in hemophilia by AAV-factor IX and limitations imposed by the host immune response. Nat Med 12(3):342–347
Maruyama A, Katoh M, Ishihara T, Akaike T (1997a) Comb-type polycations effectively stabilize DNA triplex. Bioconjug Chem 8(1):3–6
Maruyama A, Ishihara T, Kim JS, Kim SW, Akaike T (1997b) Nanoparticle DNA carrier with poly (L-lysine) grafted polysaccharide copolymer and poly (D, L-lactic acid). Bioconjug Chem 8(5):735–742
Mayhew E, Papajadjopoulos D (1983) Therapeutic applications of liposomes. In: Ostro MJ (ed) Liposomes. Marcel Dekker, New York
McKenzie DL, Kwok KY, Rice KG (2000a) A potent new class of reductively activated peptide gene delivery agents. J Biol Chem 275(14):9970–9977
McKenzie DL, Smiley E, Kwok KY, Rice KG (2000b) Low molecular weight disulfide cross-linking peptides as nonviral gene delivery carriers. Bioconjug Chem 11(6):901–909
Meel RV, Vehmeijer LJ, Kok RJ, Storm G, van Gaal EV (2016) Ligand-targeted particulate nanomedicines undergoing clinical evaluation: current status. Intracellular Deliv III:163–200
Mogler MA, Kamrud KI (2015) RNA-based viral vectors. Expert Rev Vaccines 14(2):283–312. https://doi.org/10.1586/14760584.2015.979798
Morris GA, Kök SM, Harding SE, Adams GG (2010) Polysaccharide drug delivery systems based on pectin and chitosan. Biotechnol Genet Eng Rev 27(1):257–284
Moss B, Smith GL, Gerin JL, Purcell RH (1984) Live recombinant vaccinia virus protects chimpanzees against hepatitis B. Nature 311(5981):67–69
Mulherkar R (2001) Gene therapy for cancer. Curr Sci 81:555–560
Mulligan RC (1993) The basic science of gene therapy. Science 260(5110):926–923
Nam HY, Nam K, Hahn HJ, Kim BH, Lim HJ, Kim HJ, Choi JS, Park JS (2009) Biodegradable PAMAM ester for enhanced transfection efficiency with low cytotoxicity. Biomaterials 30(4):665–673
Nam K, Jung S, Nam JP, Kim SW (2015) Poly (ethylenimine) conjugated bioreducible dendrimer for efficient gene delivery. J Control Release 220:447–455
Nayerossadat N, Maedeh T, Ali PA (2012) Viral and nonviral delivery systems for gene delivery. Adv Biomed Res 1. https://doi.org/10.4103/2277-9175.98152
Oupický D, Li J (2014 Jul) Bioreducible polycations in nucleic acid delivery: past, present, and future trends. Macromol Biosci 14(7):908–922
Pack DW, Putnam D, Langer R (2000) Design of imidazole-containing endosomolytic biopolymers for gene delivery. Biotechnol Bioeng 67(2):217–223
Park TG, Jeong JH, Kim SW (2006) Current status of polymeric gene delivery systems. Adv Drug Deliv Rev 58(4):467–486
Patil SD, Rhodes DG, Burgess DJ (2004) Anionic liposomal delivery system for DNA transfection. AAPS J 6(4):13–22
Patil SD, Rhodes DG, Burgess DJ (2005a) DNA-based therapeutics and DNA delivery systems: a comprehensive review. AAPS J 7(1):E61–E77
Patil SD, Rhodes DG, Burgess DJ (2005b) Biophysical characterization of anionic lipoplexes. Biochim Biophys Acta 1711(1):1
Pires LR, Oliveira H, Barrias CC, Sampaio P, Pereira AJ, Maiato H, Simões S, Pêgo AP (2011) Imidazole-grafted chitosan-mediated gene delivery: in vitro study on transfection, intracellular trafficking and degradation. Nanomedicine 6(9):1499–1512
Plank C, Tang MX, Wolfe AR, Szoka FC (1999) Branched cationic peptides for gene delivery: role of type and number of cationic residues in formation and in vitro activity of DNA polyplexes. Hum Gene Ther 10(2):319–332
Pulkkanen KJ, Yla-Herttuala S (2005) Gene therapy for malignant glioma: current clinical status. Mol Ther 12(4):585–598
Rainov NG (2000) A phase III clinical evaluation of herpes simplex virus type 1 thymidine kinase and ganciclovir gene therapy as an adjuvant to surgical resection and radiation in adults with previously untreated glioblastoma multiforme. Hum Gene Ther 11(17):2389–2401. https://doi.org/10.1089/104303400750038499
Rao SB, Sharma CP (1997) Use of chitosan as a biomaterial: studies on its safety and hemostatic potential. J Biomed Mater Res 34(1):21–28
Ren T, Song YK, Zhang G, Liu D (2000) Structural basis of DOTMA for its high intravenous transfection activity in mouse. Gene Ther 7(9):764–768
Roman G, Ima D, YH CJ. (2011) Approaches in gene therapy of cancer and cardiovascular diseases. In: Gene therapy applications. IntechOpen. Available from: http://www.intechopen.com/source/pdfs/17760/InTechApproaches_in_gene_therapy_of_cancer_and_cardiovascular_diseases.pdf. Last accessed on 6 Sept 2022
Rowe WP, Huebner RJ, Gilmore LK, Parrott RH, Ward TG (1953 Dec) Isolation of a cytopathogenic agent from human adenoids undergoing spontaneous degeneration in tissue culture. Proc Soc Exp Biol Med 84(3):570–573
Saha A, Mohanta SC, Deka K, Deb P, Devi PS (2017) Surface-engineered multifunctional Eu: Gd2O3 nanoplates for targeted and pH-responsive drug delivery and imaging applications. ACS Appl Mater Interfaces 9(4):4126–4141
Siemens DR, Crist S, Austin JC, Tartaglia J, Ratliff TL (2003) Comparison of viral vectors: gene transfer efficiency and tissue specificity in a bladder cancer model. J Urol 170(3):979–984
Singh BN, Gupta VK, Chen J, Atanasov AG (2017) Organic nanoparticle-based combinatory approaches for gene therapy. Trends Biotechnol 35(12):1121–1124
Srinivasan C, Burgess DJ (2009) Optimization and characterization of anionic lipoplexes for gene delivery. J Control Release 136(1):62–70
Stone D (2010) Novel viral vector systems for gene therapy. Viruses 2(4):1002–1007
Stull RA, Szoka FC Jr (1995) Antigene, ribozyme and aptamer nucleic acid drugs: progress and prospects. Pharm Res 12(4):465–483
Sung YK, Kim SW (2018) The practical application of gene vectors in cancer therapy. Integrat Cancer Sci Therap 5:1–5
Tang MX, Redemann CT, Szoka FC (1996) In vitro gene delivery by degraded polyamidoamine dendrimers. Bioconjug Chem 7(6):703–714
Thomas CE, Ehrhardt A, Kay MA (2003) Progress and problems with the use of viral vectors for gene therapy. Nat Rev Genet 4(5):346–358
Tomalia DA, Baker H, Dewald J, Hall M, Kallos G, Martin S, Roeck J, Ryder J, Smith P (1985) A new class of polymers: starburst-dendritic macromolecules. Polym J 17(1):117–132
Toncheva V, Wolfert MA, Dash PR, Oupicky D, Ulbrich K, Seymour LW, Schacht EH (1998) Novel vectors for gene delivery formed by self-assembly of DNA with poly (L-lysine) grafted with hydrophilic polymers. Biochim Biophys Acta 1380(3):354–368
Trubetskoy VS, Torchilin VP, Kennel SJ, Huang L (1992) Use of N-terminal modified poly (L-lysine)-antibody conjugate as a carrier for targeted gene delivery in mouse lung endothelial cells. Bioconjug Chem 3(4):323–327
Vaheri A, Pagano JS (1965) Infectious poliovirus RNA: a sensitive method of assay. Virology 27(3):434–436
Vaidya S, Dangi A, Panda K, Jeengar MK (2022) Therapeutic potential of cationic liposome-based nucleic acid delivery for the treatment of cancer and various human ailments. Mol Biol 11:315
Van Nies P, Westerlaken I, Blanken D, Salas M, Mencía M, Danelon C (2018) Self-replication of DNA by its encoded proteins in liposome-based synthetic cells. Nat Commun 9(1):1–2
Verma IM, Naldini L, Kafri T, Miyoshi H, Takahashi M, Blömer U, Somia N, Wang L, Gage FH (2000) Gene therapy: promises, problems and prospects. In: Genes and resistance to disease. Springer, Berlin, pp 147–157
Volpers C, Kochanek S (2004) Adenoviral vectors for gene transfer and therapy. J Gene Med 6(S1):S164–S171
Wadhwa MS, Collard WT, Adami RC, McKenzie DL, Rice KG (1997) Peptide-mediated gene delivery: influence of peptide structure on gene expression. Bioconjug Chem 8(1):81–88
Wang DA, Narang AS, Kotb M, Gaber AO, Miller DD, Kim SW, Mahato RI (2002) Novel branched poly (ethylenimine)-cholesterol water-soluble lipopolymers for gene delivery. Biomacromolecules 3(6):1197–1207
Wang Y, Su HH, Yang Y, Hu Y, Zhang L, Blancafort P, Huang L (2013) Systemic delivery of modified mRNA encoding herpes simplex virus 1 thymidine kinase for targeted cancer gene therapy. Mol Ther 21(2):358–367
Wang S, Zhao X, Wang S, Qian J, He S (2016) Biologically inspired polydopamine capped gold nanorods for drug delivery and light-mediated cancer therapy. ACS Appl Mater Interfaces 8(37):24368–24384
Wasungu L, Hoekstra D (2006) Cationic lipids, lipoplexes and intracellular delivery of genes. J Control Release 116(2):255–264
Weichselbaum RR, Kufe DW, Hellman S, Rasmussen HS, King CR, Fischer PH, Mauceri HJ (2002) Radiation-induced tumour necrosis factor-α expression: clinical application of transcriptional and physical targeting of gene therapy. Lancet Oncol 3(11):665–671
Wen HY, Dong HQ, Xie WJ, Li YY, Wang K, Pauletti GM, Shi DL (2011) Rapidly disassembling nanomicelles with disulfide-linked PEG shells for glutathione-mediated intracellular drug delivery. Chem Commun 47(12):3550–3552
Wirth T, Parker N, Ylä-Herttuala S (2013) History of gene therapy. Gene 525(2):162–169
Wivel NA, Wilson JM (1998) Methods of gene delivery. Hematol Oncol Clin North Am 12(3):483–501
Wyman TB, Nicol F, Zelphati O, Scaria PV, Plank C, Szoka FC (1997) Design, synthesis, and characterization of a cationic peptide that binds to nucleic acids and permeabilizes bilayers. Biochemistry 36(10):3008–3017
Xi S, Grandis JR (2003) Gene therapy for the treatment of oral squamous cell carcinoma. J Dent Res 82(1):11–16
Yang Y, Xu Z, Chen S, Gao Y, Gu W, Chen L, Pei Y, Li Y (2008) Histidylated cationic polyorganophosphazene/DNA self-assembled nanoparticles for gene delivery. Int J Pharm 353(1–2):277–282
Yi Y, Jong Noh M, Hee LK (2011) Current advances in retroviral gene therapy. Curr Gene Ther 11(3):218–228
Yoon AR, Hong J, Yun CO (2017) Adenovirus-mediated decorin expression induces cancer cell death through activation of p53 and mitochondrial apoptosis. Oncotarget 8(44):76666
Yoshida J, Mizuno M, Wakabayashi T (2004) Interferon-β gene therapy for cancer: basic research to clinical application. Cancer Sci 95(11):858–865
Young LS, Searle PF, Onion D, Mautner V (2006) Viral gene therapy strategies: from basic science to clinical application. J Pathol 208(2):299–318. https://doi.org/10.1002/path.1896
Zhang X, Oulad-Abdelghani M, Zelkin AN, Wang Y, Haîkel Y, Mainard D, Voegel JC, Caruso F, Benkirane-Jessel N (2010) Poly (L-lysine) nanostructured particles for gene delivery and hormone stimulation. Biomaterials 31(7):1699–1706
Zhang X, Duan Y, Wang D, Bian F (2015) Preparation of arginine modified PEI-conjugated chitosan copolymer for DNA delivery. Carbohydr Polym 122:53–59
Zhi D, Zhang S, Cui S, Zhao Y, Wang Y, Zhao D (2013) The headgroup evolution of cationic lipids for gene delivery. Bioconjug Chem 24(4):487–519
Zhi D, Bai Y, Yang J, Cui S, Zhao Y, Chen H, Zhang S (2018) A review on cationic lipids with different linkers for gene delivery. Adv Colloid Interf Sci 253:117–140
Zhou HS, Liu DP, Liang CC (2004) Challenges and strategies: the immune responses in gene therapy. Med Res Rev 24(6):748–761
Zhou Y, Chen M, Zhuo Y, Chai Y, Xu W, Yuan R (2017) In situ electrodeposited synthesis of electrochemiluminescent Ag nanoclusters as signal probe for ultrasensitive detection of Cyclin-D1 from cancer cells. Anal Chem 89(12):6787–6793
Zhu N, Liggitt D, Liu Y, Debs R (1993) Systemic gene expression after intravenous DNA delivery into adult mice. Science 261(5118):209–211
Zorko M, Langel Ü (2005) Cell-penetrating peptides: mechanism and kinetics of cargo delivery. Adv Drug Deliv Rev 57(4):529–545
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Manohar, S.K., Gowrav, M.P., Gangadharappa, H.V. (2023). Materials for Gene Delivery Systems. In: Sheikh, F.A., Majeed, S., Beigh, M.A. (eds) Interaction of Nanomaterials With Living Cells. Springer, Singapore. https://doi.org/10.1007/978-981-99-2119-5_14
Download citation
DOI: https://doi.org/10.1007/978-981-99-2119-5_14
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-99-2118-8
Online ISBN: 978-981-99-2119-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)