Abstract
Gene therapy has broad prospects as an effective treatment for some cancers and hereditary diseases. However, DNA and siRNA are easily degraded in vivo because of their biological activities as macromolecules, and they need the effective transmembrane delivery carrier Selecting the appropriate carrier for delivery will allow nucleic acid molecules to reach their site of action and enhance delivery efficiency. Currently used nucleic acid delivery vectors can be divided into two major categories: viral and non-viral vectors. Viral carrier transport efficiency is high, but there are safety issues. Non-viral vectors have attracted attention because of their advantages such as low immunogenicity, easy production, and non-tumorigenicity. The construction of safe, effective, and controllable vectors is the focus of current gene therapy research. This review presents the current types of nucleic acid delivery vehicles, which focuses on comparing their respective advantages and limitations, and proposes a novel delivery system, RNTs, a novel nanomolecular material, introducing the characteristics and nucleic acid delivery process of RNTs and their latest applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
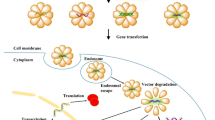
A nucleic acid delivery system is a carrier system capable of encapsulating and delivering exogenous genetic material into specific cells. The exogenous genetic material that is delivered into cells [DNA or small interfering (siRNA)] can be effectively expressed, and the delivery vector itself has low toxicity and does not interfere with the expression of genetic material. The pathway of in vivo mRNA delivery is presented in Fig. 1. Vectors need to extravasate from the bloodstream to reach target tissues, which requires certain characteristics of particles and specific ligands, and mediate cell entry and endosomal escape by specific ligands and pivotal components of the carrier (Meng et al. 2017).
Nucleic acid delivery vectors are classified as biological vectors (mainly viral vectors) and non-biological vectors (mainly chemical substances). The virus itself has the ability to infect cells, which can be easily introduced into target cells as a carrier of nucleic acid molecules; however, the preparation process of viral vectors is complicated, the size of the foreign gene carried is limited, and there is immunogenicity. These issues limit the clinical applications of viral vectors. Most non-viral vectors are compounds that are positively charged under physiological conditions, such as cationic polymers and cationic liposomes. They enter target cells through the principle of charge interaction combined with the negatively charged nucleic acids and through endocytosis. Their preparation process is relatively simple and they have no immunogenicity; however, such vectors are less efficient to introduce foreign genes than viral vectors.
1.1 Viral vectors
Viral vectors are a common tool in molecular biology research and can deliver genetic material into cells. Current viruses that are used as vectors include retroviruses, lentiviruses, poxviruses, vaccinia viruses, rubella viruses, and adenoviruses. Genetically engineered adenovirus and adenovirus-associated virus (AAV) are two common viral vectors. They are suitable for a wide range of hosts and can be targeted by gene recombination (Lai et al. 2002), which can carry a relatively large exogenous gene fragment for transient expression and are not integrated into the host genome. These recombinant viral vectors are widely used in gene therapy of tumors (Roth and Cristiano 1997; Choi et al. 2012) and other diseases.
Cardiac gene therapy using an AAV as a vector is becoming a new platform for treating and even curing currently intractable heart disease. Clinical trials and studies (Chamberlain et al. 2017) have demonstrated significant advances in the treatment of heart failure in preclinical animal models by AAV gene therapy. Therefore, this treatment approach may change the present treatments of heart disease.
Duchenne muscular dystrophy (DMD) is an incurable X-linked muscle wasting disease caused by a mutation in the dystrophin gene. Clinical studies have shown that gene therapy by a highly functional dystrophin gene and recombinant adeno-associated virus (rAAV) vectors is an attractive strategy for the treatment of DMD. Systemic rAAV-cMD1 transmission in large animal models of DMD is highly safe and effective (Le Guiner et al. 2017).
In neovascular age-related macular degeneration (nAMD), long term expression of an anti-vascular endothelial growth factor (VEGF) protein by gene therapy can improve the clinical burden of chronic intravitreal therapy. A previous study (Moore et al. 2017) has shown that AAV vectors can transduce retinal pigment epithelial cells, and early phase application of AAV2-delivered pigment epithelium-derived factor genes has been used for treatment of advanced nAMD.
Viral vectors are widely used as gene therapy delivery systems, and their transfection and expression efficiencies are high. Many clinical reports have confirmed that viral vectors are indeed an effective delivery tool. However, there are still some disadvantages of viral vectors. Their immunogenicity and potential risk of mutation are major issues, making them an unavoidable potential safety hazard. Moreover, viral vectors lack targeting and have a limited length of inserted DNA. Such problems need to be resolved by further study. Therefore, the emergence of various non-viral vectors has gradually become the mainstream of gene delivery vectors.
1.2 Non-viral vectors
1.2.1 Cationic liposomes
Cationic liposomes have been used in the transfection of nucleic acids for decades and are the most effective and least cytotoxic non-viral gene delivery vectors (Felgner et al. 1987; Ewert et al. 2002) that include Dc-Chol/DOPE, cationic lipid bilayers, liposome DOTAP, lipofectine, and pH-sensitive liposomes.
1.2.2 Delivery method
At physiological pH, the head group of positively charged lipids interacts with the phosphate groups of negatively charged nucleic acids to form a sandwich structure, in which a plurality of liposome particles enclose nucleic acid molecules. Some positively charged complexes are endocytosed or fused with cell membranes by charge-to-charge interactions. In endosomal environments, the neutral lipids in cationic liposomes undergo a conformational change that results in the release of complexes into the cytoplasm, thereby avoiding the destruction of nucleic acids by lysosomes (Hafez et al. 2001). Nucleic acids released into the cytoplasm unlock binding to cationic liposomes at a certain time and enter the nucleus at the time of mitosis to express the genes they carry. The liposomal structure is presented in Fig. 2 (Kauffman et al. 2016).
Liposomal structure (Kauffman et al. 2016)
1.2.3 Current research progress
Gao and Huang (1991) used a series of cholesterol derivatives of positively charged lipids for nucleic acid transfection in 1991. Among them, DC-Chol is useful because of its high delivery efficiency and low toxicity. Subsequently, cationic liposomes of DC-Chol combined with neutral lipid DOPE were successfully applied in a series of gene delivery experiments including experimental animals and humans (Hui et al. 1997; Villaret et al. 2002).
In 2016, Chen et al. (2016) found that liposomal vectors had an excellent loading capacity and protective effect on siRNA. The in vitro cell transfection efficiency was almost the same as commercially available Lipofectamine 2000. They developed folate-decorated cationic liposomes (fc-LPs) for hypoxia-inducible factor-1α (HIF-1α) siRNA delivery and evaluated the potential of such siRNA/liposome complexes for malignant melanoma (MM) therapy. The study demonstrated that siRNA-fc-LPs substantially reduced the production of HIF-1α-associated proteins and induced apoptosis of hypoxia-tolerant melanoma cells, which might be applicable as a siRNA delivery vehicle to systemically or topically treat MM.
Ojeda et al. (2016) used three ionizable glycerol-based cationic lipids containing a primary amine group (lipid 1), triglycine group (lipid 2), and dimethylamino ethyl pendent group (lipid 3) as polar head groups, which were a part of niosomes. The structure is presented in Fig. 3. Upon addition of the pCMS-EGFP plasmid, nioplexes were obtained at various cationic lipid/DNA ratios (w/w) and then transfected in different cell types. In vivo experiments of the rat retina showed that after intravitreal and subretinal injections together with cerebral cortex administration, niosome formulations based on lipid 3 had promising transfection efficiencies. These results provide new insights into the development of non-viral vectors based on cationic lipids and their applications for efficient delivery of genetic material to the retina and brain.
Chemical structures of cationic lipids. Lipid 1: 2,3-Bis (tetradecyloxy) propan-1-amine. Lipid 2: 2-Amino-N-(2,3-bis (tetradecyloxy)propyl)carbamoyl)methyl) carbamoyl)methyl) acetamide. Lipid 3: 1-(2-Dimethylaminoethyl)-3-[2,3-di (tetrade-.coxy)propyl] urea (Ojeda et al. 2016).
To enhance transfection efficiency, Tao et al. used protamine as a DNA-condensing agent to form liposome-protamine-DNA (LPD) ternary complexes. They found that the LPD complexes had a high transfection efficiency and low cytotoxicity in vitro, and that the LPD vector enhanced the transfection efficiency of CLs. HepG2 cells were found to have very low expression levels of miR-145. Therefore, they optimized a liposome-based delivery system for efficient delivery of miR-145 into cancer cells. This may provide the foundation for further research into the use of miR-145 in anticancer therapeutics (Tao et al. 2016). Their experimental results are presented in Fig. 4.
a Expression of miR-145 in various cell lines. b Transfection efficiency of LPD complexes in HepG2 cells. c Following transfection of HepG2 cells, their proliferation was determined by MTT assays. d RT-qPCR confirmation of miR-145 overexpression at 48 h following transfection of HepG2 cells. e RT-qPCR analysis of miR-145 gene expression in HepG2 cells. *P < 0.05; **P < 0.01; ***P < 0.0001 (all compared with the control group) (Tao et al. 2016)
1.2.4 Existing problems
Cationic liposomes have low cytotoxicity, structural diversity, controllable water and lipid solubilities, a proper density and positive charge distribution, high transfer efficiency, and potential targeting functions that make them an effective means of gene delivery by a novel non-viral vector. Among the current four L-arabinosyl cationic glycolipids (Ara-DiC12MA, Ara-DiC14MA, Ara-DiC16MA, and Ara-DiC18MA), Ara-DiC16MA liposomes have good transfection efficiency in HEK293, PC-3, and Mat cells. In addition, they maintain low cytotoxicity and a better uptake capacity in vitro, suggesting that Ara-DiC16MA liposomes will be an effective vector for the delivery of low toxicity genes (Li et al. 2017). However, only a small portion of the nucleic acid can enter the nucleus after entering the cytoplasm via the endocytic pathway. Because the volume is too large to enter the nucleus through the nuclear pore complex, it depends on the temporary disappearance of the nuclear membrane during mitosis. Thus, how to effectively facilitate liposome entry into the nucleus has become a major issue for their nucleic acid delivery.
1.3 Cationic polyplexes
Currently, the widely used cationic polyplexes include polyethylenimine PEI, polylysine PLL, poly 4-hydroxy-L-proline ester, and PEI/PLA complex. Among them, PEI is a new type of cationic polymer that has been recently used in the study of gene carriers. It has shown an excellent transfection ability in many gene therapy studies. The polycation structure of PEI is very suitable for packaging nucleic acids. Each of the three PEI monomers has a protonated amino nitrogen atom, and multimeric PEI is called a “proton sponge” (Curiel and Douglas 2002). These properties allow PEI to effectively protect the nucleic acid against degradation in the acidic environment of the endosome. In addition, PEI has a certain ability to destabilize the endosome membrane (Lieber et al. 1997). In the PEI/PLA complex, PLA may act as a peptide that facilitates the release of the complex from the endosome into the cytoplasm as soon as possible, reducing its destruction in the lysosomal environment.
For gene therapy of cancer, there have been many reports on the use of cationic polymers as delivery vehicles to efficiently deliver siRNAs to target cells in vivo. A collagen hydrogel has been used as a carrier to test the feasibility of Id1-targeting siRNA for local and sustained gastric cancer inhibition in vivo. To enhance siRNA delivery, PEI was used for plaque modification. The results showed that the PEI-added collagen hydrogel carrier promoted delivery of Id1-siRNA to target cells, prolonged the silencing effect, and further inhibited tumor growth in vitro and in vivo (Peng et al. 2016).
Activation of the HIV-1-infected host autophagy protein Beclin 1 is an important mechanism to control HIV replication and virus-induced inflammatory responses in microglia. In 2017, Rodriguez et al. (2017) studied the biodistribution and efficacy of non-invasive intranasal delivery of the Beclin 1 gene in adult mouse brains using PEI as a gene carrier. Intranasal delivery of siRNA targeted to Beclin1 significantly depleted target protein expression levels in brain tissue without evidence of toxicity. Moreover, binding of the siRNA to the PEI polymer was confirmed by Raman spectroscopy. These results indicate that intranasal drug delivery allows direct delivery of PEI-siRNAs to the central nervous system, which provides an effective method of silencing gene-mediated therapy in HIV-infected brains.
In 2016, Wang et al. (2016) replaced the primary amino group of PEI with a neutral hydrazide group, resulting in a new type of neutral polymer that not only showed good biocompatibility and cell internalization efficiency in vitro, but also allowed tissue uptake in the zebrafish heart. Their experimental results showed that the conversion of conventional branched PEI to this new type of non-viral carrier neutral polymer enables the delivery of siRNA molecules to be more neutral, stable, and efficient, and the resulting functional delivery system can further expand the development of siRNA therapeutics for the treatment of cardiovascular diseases.
Cationic polyplexes have the potential to be a good nucleic acid delivery vehicle, but their inherent cytotoxicity cannot be ignored. At present, the study of the exact intracellular process of cationic polymer-mediated transfection is still in its infancy. Furthermore, effectively reducing their cytotoxicity is a major issue in current research.
1.4 Peptide protein delivery vector
Polypeptides are used as delivery vehicles, which can be used both as modifiers for drug carriers and as main components of carriers. The use of polypeptide-modified cationic polymers can increase the tissue targeting, cell membrane permeability, and biological activity of nucleic acid molecules. As a carrier, they are connected to the drug in a covalent bond form with high stability and biodegradable, no toxicity, and good biocompatibility in vivo.
In 2017, Wan and Dai (2017) combined a multifunctional peptide with a lipid to form a complex, namely lipid 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE) with well-defined synthetic multifunctional peptide binding. The development of this delivery system has been optimized for efficient gene delivery to breast cancer cells. The peptide/lipid hybrid system is a preferred candidate for delivery of DNA or siRNA into breast cancer cells, which can be used for the targeted treatment of Bcl-2-overexpressing breast cancer.
Peptide-mediated nucleic acid drug delivery systems can significantly improve the transmembrane properties and targeting of drugs, reduce their toxic and side effects, and enhance therapeutic efficacy, which have broad application prospects in drug-targeted delivery. However, their preparation process as well as off-target and toxic side effects of peptide carriers are issues that need to be resolved for the application of nucleic acid drug delivery systems.
1.5 Other delivery vehicles
1.5.1 Cyclodextrin polymers
Cyclodextrin is a sugar polymer with good biocompatibility, but the use of cyclodextrin carriers in vivo is limited by instability and aggregation at high salt concentrations. A cyclodextrin derivative can be used directly as a gene delivery vector, or as a linker or modifier for the construction of other gene delivery vectors. It can also be used for gene delivery in the form of a pseudopolyrotaxane or polyrotaxane. A study has shown (Hu-Lieskovan et al. 2005) that complexes of cyclodextrin polymers containing transferrin and siRNAs can inhibit the growth of metastatic Ewing’s sarcoma in mice.
In 2016, Jiang et al. (2016) developed a supramolecular host-polycation gene delivery system based on poly-β-cyclodextrin (PCD) and azobenzene-terminated polycations. In vitro experiments showed that photo-sensitive supramolecular polycationic polymers (PCD/Az-LPDM/DNA and PCD/Az-BPDM/DNA) have high transfection efficiency and low cytotoxicity. After UV irradiation, as more DNA is transferred and released inside the cell nucleus, the transfection efficiency of the photosensitizing supramolecular complex is increased significantly. Therefore, light-responsive supramolecular host-guest systems containing azobenzene-terminated cationic polymers and PCDs are promising gene carriers.
In 2015, Li et al. (2015) developed a pH-sensitive arginine-modified glucan gene vector. α-Cyclodextrin has an excellent cell-penetrating ability when modified with arginine (CDR). They chose glucan as the backbone and studies have shown that CPP-based complexes with shelling polysaccharides may be promising non-viral gene delivery vectors.
Although cyclodextrin has a very broad application prospect in the field of gene delivery, there are still problems such as poor stability because of dilution in blood circulation, a weak escape ability from endosomes, and low transfection efficiency.
1.5.2 Cell-penetrating peptides (CPP)
Cell-penetrating peptides are short peptides capable of carrying macromolecules into a cell, which themselves have the ability to actively cross the cell membrane. This transmembrane-crossing capability is not dependent on classical endocytosis. Carriers that have been used for intracellular delivery of drugs can effectively deliver therapeutic substances such as DNA, siRNA, small molecule drugs, proteins, and micelles into cells to exert therapeutic effects.
In 2016, Fan et al. (2017) studied the effects of the sodium iodide transporter (NIS) cell-penetrating peptide on I-131 radiotherapy of thyroid cancer. They combined the HIV-1 TAT peptide (cell-penetrating peptide, dTAT) and established a nanoparticle carrier (dTAT NP). dTAT NP-transfected TPC-1 cells were used as a model to study the delivery efficiency of this cell-penetrating strategy for tumor-targeted gene delivery.
However, because the application of CPP as a delivery vehicle is complex and difficult to control, the issues of uptake efficiency, bioavailability, and toxicity need to be studied further.
1.5.3 Chitosan
Chitosan is a newly developed second-generation non-viral vector consisting of cationic polymers, cationic liposomes, and nucleic acids. The liposome/polymer/pDNA ternary complex has a tightly compressed spherical structure and is polymorphic. The reason for the high transfection efficiency of this novel composite carrier may be the precompression of DNA by chitosan, which reduces the particle size of the carrier and improves the binding ability to DNA. Spherical composites have high stability due to low surface energy (Davis 2002). Compared with PEI, chitosan has strong biological matching low immunogenicity and cytotoxicity (Lee et al. 2003), and high DNA compressibility. Compared with liposomes alone, liposomal transfection efficiency is low and can cause cytotoxicity such as cell shrinkage and reduce mitosis (Mi-Kyung et al. 2005). The constructed chitosan/liposomal/pDNA ternary complex vector has the characteristics of high transfection efficiency and low toxicity, and is a novel non-viral vector for gene therapy.
1.5.4 Polyamide amine (PAMAM)
Because of the advantages of simple synthesis and commercialization, PAMAM has become the most widely studied gene carrier for dendrimers. Studies have shown that PAMAM promotes the entry of DNA into cells and facilitates DNA escape from lysosomes. Modified PAMAM has a higher transfection rate and is less toxic. Bielinska et al. (1997) found that complexes of PAMAM and DNA protect DNA from nuclease degradation. However, there are problems with delivery efficiency as well as cytotoxicity.
1.6 Nanomaterial delivery systems
Based on the advantages and disadvantages of the various abovementioned gene delivery vectors and problems that still to be solved at present, further research on new delivery vectors has become important in the field of life sciences. In recent years, with the continuous development of nanotechnology, nanomaterials have provided more options to deliver nucleic acids into target cells.
1.6.1 Traditional Nanomaterials
When a material is nanoscale (0.1–100 nm), special properties will occur. Nanomaterials are different from the original composition of atoms, molecules, and macroscopic materials. They can easily enter organisms and provide enormous advantages to establish drug delivery systems. Various nanomaterials of interest to researchers include liposomes (Li and Szoka 2007; Yoshizawa et al. 2008), polymer nanostructures (Gary et al. 2007; Pridgen et al. 2007), nanogold (Patel et al. 2008; Giljohann et al. 2009), silicon nanomaterials (Slowing et al. 2008), quantum dots (Li et al. 2011), magnetic nanoparticles (Medarova et al. 2007; Dobson 2006), and carbon nanotubes (Bianco et al. 2005) that have become candidate materials for nucleic acid drug delivery systems because of their unique properties.
The many advantages of a nanobiotechnology gene therapy vector systems provide a new technology platform for targeted, intelligent delivery and efficient and controllable expression of target genes in gene therapy, which have good application prospects. With the in-depth development of nanobiotechnology and new nanobiomaterials, safe, efficient, and controllable targeted nano-gene vectors will greatly improve the efficacy of gene therapy.
1.6.2 Novel Nanomolecular delivery technology
A rosette nanotube (RNT) is a novel nanomolecule suitable for multiple drug delivery. It has a good transmembrane transport capacity. Structurally, an RNT is formed by a self-arranged supramolecular mechanism, the basic structure of which is a paired connection of guanine and cytosine. RNTs have hollow channels that can be combined with drugs to form a packaging system where drugs are packaged to complete drug delivery. Moreover, its capsular shape can effectively protect unstable miRNAs and prevent their decomposition (Journeay et al. 2008a).
The tubular structure of an RNT consists of primary structure lysine side chains and secondary structure hydrogen bonds under a hydrophobic interaction, and it contains a hydrophobic group that can be neutral or negatively charged. The molecules form a complex that better carries oligonucleotide molecules through the plasma membrane. The basic mechanism of an RNT is shown in Fig. 5 (Chen et al. 2011).
The single unit of K1 (G-C motif conjugated to a lysine amino acid) self-assembles into a rosette ring (lower left) that stacks up to form a stable RNT (right) (Chen et al. 2011).
The characteristics of an RNT are as follows: 1. RNTs are easy to synthesize and their hydrophobicity causes rapid self-assembly to form a ring-shaped nanotube; 2. The biomimetic structure of nanomaterials makes them easy to adhere to cells; 3. The amino acid side chains can be regulated. During use, the delivery function and targeting effect can be adjusted by changing the amino acid side chains; 4. Their function can be enhanced by the spatial distribution of the side chains. These characteristics make RNTs an efficient and reliable cell delivery vehicle.
Because of the hydrophobic nature of the RNT outer layer, it has fairly good biocompatibility and cell affinity, and has been successfully applied to the delivery of hydrophobic drugs (Qiu et al. 2014). Chen and colleagues focused on transmembrane transport of tumor-targeted drugs and oligonucleotides by RNT materials. With the rise of tissue engineering techniques for treatment of intervertebral disc degeneration, methods are being developed to regulate the apoptosis of chondrocytes in intervertebral disc tissue by various target genes (Li et al. 2017). Apoptosis of nucleus pulposus cells of the disc is regulated by miRNAs through triggering translational inhibition or RNA degradation to control gene expression (Zheng et al. 2015; Zhou et al. 2017; Mogensen et al. 2012). We speculated that the process of disc degeneration can be controlled by specifically regulating miRNAs that play a role in disc degeneration. Therefore, we assumed that RNTs and oligonucleotides could form a nano-column structure, which is called an RNT-siRNA complex, to compete with miRNA through RNT complex loading technology. Its effective delivery would control the occurrence and development of disc degeneration.
RNTs are produced synthetically (Fenniri et al. 2001) and are assembled under physiological conditions. They have been shown to have low toxicity (Journeay et al. 2008b, 2008c) in vitro and in vivo. RNTs with a lysine side chain undergo spontaneous self-assembly under physiological conditions as shown in Fig. 6 (Chen et al. 2010).
RNTs with a lysine side chain undergo spontaneous self-assembly under physiological conditions (Chen et al. 2010)
2 Summary
Target genes, vectors, and target cells are the three elements of gene therapy, and the construction of safe, effective, and controllable delivery vectors is crucial for gene therapy. With the initial completion of the human genome project and the advances in molecular virology and materials science, based on the original viral vector, new non-viral gene delivery vectors are emerging.
The above examples are several kinds of delivery vectors commonly used in gene therapy. In biological nucleic acid delivery systems, biological carriers (mainly viral carriers) and non-biological carriers have their respective advantages and disadvantages. Viral vectors are widely used, and their transfection and expression efficiencies are high, but the viral vectors themselves have immunogenicity and the potential risk of mutation, and there are potential safety hazards. Cationic liposomes have been used in the transfection of nucleic acids for decades and are one of the most effective and least cytotoxic non-viral gene delivery methods in current research. However, there is no effective nuclear entry mechanism and their transfection efficiency has not been improved. Both PEI and PEI/PLA complexes can efficiently deliver siRNA into the cytoplasm, but their cytotoxicity cannot be ignored. Peptide-mediated nucleic acid drug delivery systems can significantly improve the transmembrane permeability and targeting of drugs, reduce their toxic and side effects, and enhance therapeutic efficacy, which have broad application prospects in drug-targeted delivery systems. However, the preparation process as well as off-target and toxic side effects of peptide carriers are issues that need to be resolved in the application of nucleic acid drug delivery systems. Cyclodextrins have good biocompatibility, but there are still problems such as poor stability by dilution in blood circulation, a weak endosomal escape ability, and low transfection efficiency. The chitosan/liposomal/pDNA ternary complex vector has the characteristics of high efficiency and low toxicity in transfected cells, and is a potential novel non-viral vector for gene therapy. New nanomaterial delivery methods have gradually become the mainstream. The numerous advantages of nanobiotechnology gene therapy vector systems provide a new technology platform for the targeted, intelligent delivery and efficient and controllable expression of target genes in gene therapy, which have great application prospects. Some of the abovementioned carriers are listed in Table 1 for comparison.
References
A. Bianco, K. Kostarelos, M. Prato, Applications of carbon nanotubes in drug delivery. Curr. Opin. Chem. Biol. 9, 674–679 (2005)
A.U. Bielinska, J.F. Kukowska-Latallo, J.R. Baker, The interaction of plasmid DNA with polyamidoamine dendrimers: Mechanism of complex formation and analysis of alterations induced in nuclease sensitivity and transcriptional activity of the complexed DNA. Gene Structure and Expression 1353(2), 180–190 (1997)
K. Chamberlain, J.M. Riyad, T. Weber, et al., Cardiac gene therapy with adeno-associated, virus-based vectors. Curr. Opin. Cardiol. 4, 140–152 (2017)
Y. Chen, B. Bilgen, et al., Self-Assembled Rosette Nanotube/Hydrogel Composites For Cartilage Tissue Engineering. Tissue Engineering: Part C 16(6) (2010)
Y. Chen, S. Song, Z. Yan, et al., Self-assembled rosette nanotubes encapsulate and slowly release dexamethasone. Int. J. Nanomedicine 6, 1035–1044 (2011)
Z. Chen, T. Zhang, B. Wu, X. Zhang, Insights into the therapeutic potential of hypoxia-inducible factor-1α small interfering rNa in malignant melanoma delivered via folate-decorated cationic liposomes. Int. J. Nanomedicine 11, 991–1002 (2016)
J.W. Choi, J.S. Lee, S.W. Kim, et al., Evolution of oncolytic adenovirus for cancer treatment. Adv. Drug Deliv. Rev. 64, 720–729 (2012)
D.T. Curiel, J.T. Douglas, Adenoviral vectors for gene therapy (Academic Press, San Diego, 2002), pp. 329–348
M.E. Davis, Non-viral gene delivery systems. Curr OpinChem Biol 13, 28–131 (2002)
J. Dobson, Gene therapy progress and prospects: Magnetic nanoparticle-based gene delivery. Gene Ther. 13, 283–287 (2006)
K. Ewert, A. Ahmad, H.M. Evans, et al., Efficient synthesis and cell-transfection properties of a new multivalent cationic lipid for nonviral gene delivery. J. Med. Chem. 45, 5023–5029 (2002)
Y.X. Fan, Z.X. Liang, Q.Z. Liu, et al., Cell penetrating peptide of sodium-iodide symporter effect on the I-131 radiotherapy on thyroid cancer. Exp Ther Med 13(3), 989–994 (2017)
P.L. Felgner, T.R. Gadek, M. Holm, et al., Lipofection: A highly efficient, lipid-mediated DNA-transfection procedure. Proc. Natl. Acad. Sci. U. S. A. 84, 7413–7417 (1987)
H. Fenniri, P. Mathivanan, K.L. Vidale, et al., Helical rosette nano-tubes: Design, self-assembly and characterization. J. Am. Chem. Soc. 123(16), 3854–3855 (2001)
X. Gao, L. Huang, A novel cationic liposome reagent for efficient transfection of mammaliam cells. Biochem. Biophys. Res. Commun. 179, 280–285 (1991)
D.J. Gary, N. Puri, Y.Y. Won, Polymer-based si RNA delivery: Perspectives on the fundamental and phenomenological distinctions from polymer-based DNA delivery. J. Control. Release 121, 64–73 (2007)
D.A. Giljohann, D.S. Seferos, A.E. Prigodich, et al., Gene regulation with polyvalent si RNA-nanoparticle conjugates. Am. Chem. Soc. 131, 2072–2073 (2009)
I.M. Hafez, N. Maurer, P.R. Cullis, On the mechanism whereby cationic lipids promote intracellular delivery of polynucleic acids. Gene Ther. 8, 1188–1196 (2001)
K.M. Hui, P.T. Ang, L. Huang, et al., Phase I study of immunotherapy of cutaneous metastases of human carcinoma using allogeneic and xenogeneic MHC DNA-liposome complexes. Gene Ther. 4(8), 783–790 (1997)
S. Hu-Lieskovan, J.D. Heidel, D.W. Bartlett, Ea ta. Triche, sequence-specific knockdown of EWS-FLI1 by targeted, nonviral delivery of small interfering RNA inhibits tumor growth in a murine model of metastatic ewing's sarcoma. Cancer Res. 65(19), 8984–8992 (2005)
Q. Jiang, Y. Zhang, R. Zhuo, et al., Supramolecular host-guest polycationic gene delivery system based on poly (cyclodextrin) and azobenzene-terminated polycations. Colloids Surf. B: Biointerfaces 1(147), 25–35 (2016)
W.S. Journeay, S.S. Suri, J.G. Moralez, et al., Rosette nanotubes show low acute pulmonary toxicity in vivo. Int. J. Nanomedicine 3(3), 373–384 (2008a)
W.S. Journeay, S.S. Suri, J.G. Moralez, et al., Rosette nano-tubes show low acute pulmonary toxicity in vivo. Int. J. Nanomedicine 3(3), 373–383 (2008b)
W.S. Journeay, S.S. Suri, J.G. Moralez, et al., Low infamma-tory activation by self-assembling rosette nanotubes in human Calu-3 pulmonary epithelial cells. Small. 4(6), 817–823 (2008c)
K.J. Kauffman, M.J. Webber, D.G. Anderson, J. Control. Release 240, 227–234 (2016)
C.M. Lai, Y.K. Lai, P.E. Rakoczy, Adenovirus and adeno-associated virus vectors. DNA Cell Biol. 21, 895–913 (2002)
C. Le Guiner, L. Servais, M. Montus, et al., Long-term microdystrophin gene therapy is effective in a canine model of Duchenne muscular dystrophy. Nat. Commun. 8, 161–165 (2017)
C.H. Lee, Y.H. Ni, C.C. Chen, et al., Synergistic effect ofpolyethylenimine and cationic liposomes in nucleic acid de-livery to human cancer cells. Biochim. Biophys. Acta 1611, 55–62 (2003)
Y.L. Li, X. Duan, L.H. Jing, et al., Quantum dot-antisense oligonucleotide conjugates for multifunctional gene transfection, mRNA regulation, and tracking of biological processes. Biomaterials 32, 1923–1931 (2011)
B. Li, W. Guo, F. Zhang, et al., Synthesis and evaluation of L-arabinose-based cationic glycolipids as effective vectors for pDNA and siRNA in vitro. PLoS One 12(7), 180–276 (2017)
W. Li, Y. Liu, J. Du, et al., Cell penetrating peptide-based polyplexes shelled with polysaccharide to improve stability and gene transfection. Nanoscale 7(18), 8476–8488 (2015)
W.J. Li, F.C. Szoka, Lipid-based nanoparticles for nucleic acid delivery. Pharm. Res. 24, 438–449 (2007)
P. Li, R. Zhang, Y. Gan, et al., Effects of osteogenic protein-1 on intervertebral disc regeneration: A systematic review of animal studies. Biomed. Pharmacother. 88, 260–266 (2017)
A. Lieber, C.Y. He, L. Meuse, et al., The role of Kupffer cell activation and viral gene expression in early liver toxicity after infusion of recombination adenovirus vectors. J. Virol. 71, 8798–8807 (1997)
Z. Medarova, W. Pham, C. Farrar, et al., In vivo imaging of si RNA delivery and silencing in tumors. Nat. Med. 13, 372–377 (2007)
Z. Meng, J. O’Keeffe-Ahern, J. Lyu, et al., A new developing class of gene delivery: Messenger RNA-based. Biomater. Sci. (2017) https://doi.org/10.1039/C7BM00712D
L. Mi-Kyung, C. Soo-Kyung, C. Woo-Jeong, et al., The use ofchitosan as a condensing agent to enhance emulsion-mediatedgene transfer. Biomaterials 26, 2147–2156 (2005)
M.S. Mogensen, K. Scheibye-Alsing, P. Karlskov-Mortensen, et al., Validation of Genome-wide Intervertebral Disk Calcification Associations in Dachshund and Further Investigation of the Chromosome 12 Susceptibility Locus. Front. Genet. 3, 225 (2012)
N.A. Moore, P. Bracha, R.M. Hussain, et al., Gene therapy for age-related macular degeneration. Expert. Opin. Biol. Ther. 20, 1–10 (2017)
E. Ojeda, G. Puras, M. Agirre, J. Zarate, S. Grijalvo, R. Eritja, G. Martinez-Navarrete, C. Soto-Sánchez, A. Diaz-Tahoces, M. Aviles-Trigueros, E. Fernández, J.L. Pedraz, The influence of the polar head-group of synthetic cationic lipidson the transfection efficiency mediated by niosomes in rat retina and brain. Biomaterials 77, 267–279 (2016)
P.C. Patel, D.A. Giljohann, D.S. Seferos, et al., Peptide antisense nanoparticles. Proc. Natl. Acad. Sci. U. S. A. 105, 17222–17226 (2008)
H. Peng, H. Yang, L. Song, et al., Sustained delivery of siRNA/PEI complex from in situ forming hydrogels potently inhibits the proliferation of gastric cancer. Exp Clin Cancer Res 31, 35–57 (2016)
E.M. Pridgen, R. Langer, O.C. Farokhzad, Biodegradable, polymeric nanoparticle delivery systems for cancer therapy. Nanomed 2, 669–680 (2007)
W. Qiu, S.L. Li, W.L. Deng, et al., Strain sensor of carbon nanotubes in microscale:From model to metrology. Sci. World J. 2014, 406154 (2014)
M. Rodriguez, J. Lapierre, C.R. Ojha, et al., Intranasal drug delivery of small interfering RNA targeting Beclin1 encapsulated with polyethylenimine (PEI) in mouse brain to achieve HIV attenuation. Sci. Rep. 7(1), 18–62 (2017)
J.A. Roth, R.J. Cristiano, Gene therapy for cancer: What have we done and where are we going? Natl Cancer Inst 89, 21–39 (1997)
I.I. Slowing, J.L. Vivero-Escoto, C.W. Wu, et al., Mesoporous silica nanoparticles as controlled release drug delivery and gene transfection carriers. Adv. Drug Deliv. Rev. 60, 1278–1288 (2008)
J. Tao, W.-F. Ding, X.-H. Che, Y.-C. Chen, F. Chen, X.-D. Chen, X.-L. Ye, S.-B. Xiong, Optimization of a cationic liposome-based gene delivery system for the application of miR-145 in anticancer therapeutics. Int. J. Mol. Med. 37, 1345–1354 (2016)
D. Villaret, B. Glisson, D. Kenady, et al., A multicenter phase II study of tg DCC-E1A for the intratumoral treatment of patients with recurrent head and neck squamous cell carcinoma. Head Neck 24(7), 661–669 (2002)
Y. Wan, W. Dai, R.J. Nevagi, et al., Multifunctional peptide-lipid nanocomplexes for efficient targeted delivery of DNA and siRNA into breast cancer cells. Acta Biomater. 1(59), 257–268 (2017)
F. Wang, L. Gao, L.-Y. Meng, A neutralized noncharged polyethylenimine-Based system for efficient delivery of siRNA into heart without toxicity. Mater. Interfaces 8(49), 33529–33538 (2016)
T. Yoshizawa, Y. Hattori, M. Hakoshima, et al., Folate-linked lipid-based nanoparticles for synthetic si RNA delivery in KB tumor xenografts. Pharm Biopharm 70, 718–725 (2008)
J. Zheng, J. Yang, K. Zhao, et al., Low expression of microRNA-143 is related to degenerative scoliosis possibly by regulation of cyclooxygenase-2 expression. Int. J. Clin. Exp. Med. 8(3), 4140–4145 (2015)
X. Zhou, L. Chen, S. Grad, et al., The roles and perspectives of microRNAs as biomarkers for intervertebral disc degeneration. J. Tissue Eng. Regen. Med. (2017)
Acknowledgments
The authors thank Guo Xiong and Zhang Feng for reviewing the manuscript. We thank M. Arico from Liwen Bianji, Edanz Group China (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript. This review was supported by grants from the National Nature Science Foundation of China (No. 81171761), Shaanxi Natural Science Foundation (No. 2017SF-088), and Shaanxi Provincial Department of Education Fund (No: 18JK0667). The funding sources had no role in this publication.
Author information
Authors and Affiliations
Contributions
Y.J. and Z.L.X. were responsible for study conception and design, acquisition of data, analysis and interpretation of data, and drafting the manuscript. L.J.Z. critically revised the manuscript for important intellectual content. H.J. and B.X. were responsible for the analysis and interpretation of data. S.F. was responsible for study conception and design, acquisition, analysis and interpretation of data, and critical revision of the manuscript for important intellectual content. All authors read and approved the final version of the manuscript and had full access to all of the data in the study.
Corresponding author
Ethics declarations
Conflict of interest
There are no financial or personal relations with other people or organizations, which could inappropriately bias this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jiao, Y., Xia, Z.L., Ze, L.J. et al. Research Progress of nucleic acid delivery vectors for gene therapy. Biomed Microdevices 22, 16 (2020). https://doi.org/10.1007/s10544-020-0469-7
Published:
DOI: https://doi.org/10.1007/s10544-020-0469-7