Abstract
Microwave Incinerated Rice Husk Ash (MIRHA), produced from the rice husk was proven as one of the low-cost materials that can be used as an adsorbent for heavy metal in aqueous solution. Used engine oil is one of the wastes that contain heavy metals, metals and other contaminants. Most are non- biodegradable and can harm living creatures and human being if not properly disposed. The aim of this research was to study the feasibility of using MIRHA as adsorbent for the removal of heavy metal, metals and other contaminants from used engine oil. The chemical composition of the used engine oil was determined based on ASTM D6595. A series of jar tests were conducted by varying the contact time at fixed concentration of MIRHA and varying the concentration of MIRHA at specified contact time. All the samples were conducted in triplicates. It was observed that the optimum contact time for zinc, calcium and boron removals at fixed concentration of adsorbent of 625,000 mg/L of MIRHA were found to be 4, 4 and 24 h, respectively. The percentage removals of zinc, calcium, and boron were found to be 38.24, 29.39 and 52.27 %, respectively. It was also observed that the optimum adsorbent dosage for Zn, Ca and B removals were at 450,000 mg/L of MIRHA with percentage removals of 55.12, 29.33 and 62.11 %, respectively.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
- Used engine oil
- Metals
- Heavy metals and contaminants
- Adsorption
- Microwave incinerated rice husk ash (MIRHA)
1 Introduction
Wastes are unwanted material or any substance which constitutes a scrap material or an effluent or other unwanted surplus substances arising from the application of any process. The process may come from the industrial, commercial, household or any other process that has no economical demand and necessary to be disposed of properly [1].
Approximately 1.35 billion gallons of used oil are generated yearly and approximately 800 million gallons are collected by recyclers for reuse. Hence, only 59 % of the used engine oil were reused and 41 % were dumped away to the environment [2]. Used engine oil are composed of heavy metals that can caused hazards. The formation of hazards resulted from various additives used in its manufacture and from heavy metal contaminants picked up from internal combustion of engines. Used engine oil poured into household drains or directly onto the ground may lead into waterways and groundwater cause pollution [3]. According to Chevron, used motor oils do not present a significant inhalation health hazard. However, it can present a problem if they come in contact with the eyes or skin, triggering an allergic skin reaction or eye irritation. Long-term effects from repeated contact include a higher risk of developing skin cancer [4].
Rice Husk is a commonly available agriculture waste in Malaysia. It is estimated that, in South East Asia approximately 150 million tonnes (25 % of world production) of rice husk are being produced annually, 95 % of the production were used within the region and the remaining 5 % was exported to other countries [5]. Meanwhile, in Malaysia it is estimated that rice husk takes account for about 20 % of the whole rice produced [6]. According to the statistics compiled by the Department of Statistics Malaysia, [7] the production of Malaysia paddy in 2010 is 2,464,831 tonnes. Therefore, it is estimated that more than 400,000 tonnes of rice husk produced annually in Malaysia. The potential use of rice husk ash in the adsorption of metal ions has been widely investigated [8–10]. Microwave Incinerated Rice Husk Ash (MIRHA) obtained from burning the rice husk at controlled temperature using microwave incinerator have been studied and can be utilized as alternative adsorbent for removal of metal ions [11, 12].
Adsorption is considered as an efficient and economically feasible method to remove or treat heavy metals present in used engine oil. The use of rice husk as adsorbents is an option for cheap and readily available sources than activated carbon, since rice husk are one of the agricultural waste which are abundant in Malaysia. The aim of this research was to study the feasibility of using MIRHA as an adsorbent for the removal of heavy metal and other contaminants from used engine oil.
2 Methodology
2.1 Preparation of Microwave Incinerated Rice Husk Ash
Rice Husk was collected from BERNAS Rice Mill in Seberang Perak, Malaysia. The rice husk was burned at 500 °C (MIRHA) temperature for 8 h. Table 1 illustrates the chemical composition of MIRHA [13].
2.2 Source of Raw Used Engine Oil and Initial Analysis
Raw used engine oil was collected from several car service centres in Perak, Malaysia. All of the raw used engine oil was mixed and the initial elemental analysis of raw used engine oil was analyzed. Table 2 illustrates the elemental analysis of raw used engine oil.
2.3 General Experimental Procedure
The chemical composition of the used engine oil was determined based on ASTM D6595 (Standard Test Method for determination of wear metals and contaminants in used lubricating oils or used hydraulic fluids by rotating disc electrode atomic emission spectrometry). Two sets of jar tests were conducted using the used engine oil. The final chemical composition or wear metals of the used engine oil after the jar tests were measured based on ASTM D6595 procedure. For each metal, optimum contact time and optimum dosage of MIRHA to be added for the adsorption process was evaluated.
2.4 Procedure on Effect of Contact Time at Fixed Adsorbent Dosage
In this jar test, a constant concentration of MIRHA at 625,000 mg/L were stirred with 800 mL of used engine oil at contact times of 4, 8, 14 and 24 h. Three jars were prepared for each contact time.
2.5 Procedure on Effect of Adsorbent Dosage (Concentration) at Fixed Contact Time
In this jar test, the adsorbent (MIRHA) was varied at 0.20, 0.28, 0.36, 0.40, 0.44, 0.52, 0.60 kg and stirred with 800 mL of used engine oil at a constant contact time of 24 h. Three jars were prepared for each weight of adsorbent.
3 Results and Discussion
3.1 Effect of Contact Time at Fixed Adsorbent Dosage
Based on the experiment, total of 21 metals which consist of Fe, Cr, Pb, Cu, Sn, Al Ni, Ag, Si, B, Na, Mg, Ca, Ba, P, Zn, Mo, Ti, V, Mn, Cd were found in raw used engine oil. Out of these, 10 types of metals were recognized to be significantly affected by MIRHA. The metals adsorbed were Fe, Al, Si, B, Na, Mg, Ca, P, Zn, and Mn. Table 3 show the removals for the 10 elements. It was observed that some elements increased in concentration while some are removed. The elements increased in concentration due to their presence in the adsorbent (MIRHA). The elements that were significantly adsorbed were Ca, Zn and B.
3.2 Effect of Adsorbent Dosage at Fixed Contact Time
Based on the experiment, total of 21 elements of metals which consist of Fe, Cr, Pb, Cu, Sn, Al Ni, Ag, Si, B, Na, Mg, Ca, Ba, P, Zn, Mo, Ti, V, Mn, Cd were found in raw used engine oil. Out of these 10 metals were significantly affected by MIRHA. The metals affected were Fe, Al, Si, B, Na, Mg, Ca, P, Zn, and Mn. Table 4 show the behaviour for the 10 affected elements at various adsorbent dosage. It was observed that some of the elements increased in concentration while some are removed through adsorption. The elements that were significantly adsorbed were Ca, Zn and B.
3.3 Removal Efficiency of Zn, Ca and B at Fixed Adsorbent Dosage (625,000 Mg/L) and Variation of Contact Time
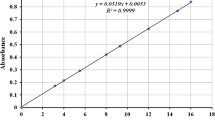
From the 10 affected elements it was observed that only Zn, Ca, B were significantly adsorbed by MIRHA. The removals of the metals through adsorption of MIRHA was plotted in Fig. 1. It was also observed that the optimum contact time for zinc, calcium and boron removals at fixed concentration of adsorbent of 625,000 mg/L of MIRHA were found to be 4, 4 and 24 h, respectively. The removals of Zn, Ca, and B were found to be 38.24, 29.39 and 52.27 %, respectively.
3.4 Removal Efficiency of Zn, Ca and B at Fixed Contact Time and Variation of Adsorbent Dosage
From the 10 affected elements it was observed that only Zn, Ca, B were significantly adsorbed by MIRHA. The removals of the metals through adsorption of MIRHA was plotted in Fig. 2. It was also observed that the optimum adsorbent dosage for zinc, calcium and boron removals were at 450,000 mg/L of MIRHA with removals of 55.12, 29.33 and 62.11 %, respectively.
4 Conclusion
From the study, it can be concluded that MIRHA has the ability to adsorbed metals such as Zn, Ca, and B in used engine oil. The removals of these metals will further improve in recycling of used engine oil as a potential superplasticizer in concrete.
References
R.G.P. Hawkins, H.S. Shaw, The Practical Guide to Waste Management Law: With a List of Abbreviations and Acronyms, Useful Websites and Relevant Legislation (Thomas Telford, London, 2004)
Utah Department of Quality, Division of Solid and Hazardous Waste. Used Oil Section: Used Oil Statistics. Retrieved Feb 2012, from http://www.hazardouswaste.utah.gov/Used_Oil_Section/UsedOilStatistics.htm (n.d.)
S.C. Chin, N. Shafiq, M.F. Nuruddin, Effects of used engine oil in reinforced concrete beams: the structural behaviour. Paper presented at the proceedings of world academy of science, engineering and technology (2012)
Chevron, Used Motor Oils. Retrieved Feb 2012, from http://psglubricants.chevron.com/PSG%5CChevron_ProdSafetyGds%5C7a_UsedMotorOils_Chevron.pdf (2007)
E. Mutert, T.H. Fairhurst, Developments in rice production in Southeast Asia. Better Crops Int. 15, 12–17 (2002)
T.G. Chuah, A. Jumasiah, I. Azni, S. Katayon, S.Y. Thomas Choong, Rice husk as a potentially low-cost biosorbent for heavy metal and dye removal: an overview. Desalination, 175(3), 305–316 (2005)
Malaysia, Department of Statistics. Paddy Data Series. Retrieved Feb 2012, from http://www.statistics.gov.my/portal/download_Economics/files/DATA_SERIES/2011/pdf/08Padi.pdf (2012)
M. Ajmal, R. Ali Khan Rao, S. Anwar, J. Ahmad, R. Ahmad, R, Adsorption studies on rice husk: removal and recovery of Cd (II) from wastewater. Bioresour. Technol. 86(2), 147–149 (2003)
S. Mohan, G. Sreelakshmi, Fixed bed column study for heavy metal removal using phosphate treated rice husk. J. Hazard. Mater. 153(1), 75–82 (2008)
V.C. Srivastava, I.D. Mall, I.M. Mishra, Competitive adsorption of cadmium (II) and nickel (II) metal ions from aqueous solution onto rice husk ash. Chem. Eng. Process. 48(1), 370–379 (2009)
M.H. Isa, S.R.M. Kutty, S.R. Mohd, N.M.D. Hussin, A. Malakahmad, Cadmium (Cd) Removal from Aqueous Solution Using Microwave Incinerated Rice Husk Ash (MIRHA) (2010)
N.A. Johan, S.R.M. Kutty, M.H. Isa, N.S. Muhamad, H. Hashim, Adsorption of copper by using microwave incinerated rice husk ash (MIRHA). Int. J. Civil Environ. Eng. 3, 211–215 (2011)
M.F. Nuruddin, N. Shafiq, N.L.M. Kamal, The effects of types of rice husk ash on the porosity of concrete. Paper presented at the APSEC-EACEF 2009, Awana Porto Malai, Langkawi (2009)
Acknowledgments
The authors would like also forward his deepest appreciation and extend their acknowledgement to the Universiti Teknologi PETRONAS and Cradle Fund Sdn. Bhd. for providing the funding and resources and to all personals who have given supports for the accomplishments of this research.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer Science+Business Media Singapore
About this paper
Cite this paper
Habib, A.I., Kutty, S.R.M., Shafiq, N., Nuruddin, M.F. (2015). Adsorption of Metals (Zn, Ca and B) in Used Engine Oil by Using Microwave Incinerated Rice Husk Ash (MIRHA). In: Hassan, R., Yusoff, M., Alisibramulisi, A., Mohd Amin, N., Ismail, Z. (eds) InCIEC 2014. Springer, Singapore. https://doi.org/10.1007/978-981-287-290-6_94
Download citation
DOI: https://doi.org/10.1007/978-981-287-290-6_94
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-287-289-0
Online ISBN: 978-981-287-290-6
eBook Packages: EngineeringEngineering (R0)