Abstract
Breast cancer is one of the most common types of malignancy diagnosed among females worldwide. Due to its heterogenous nature, the type of treatment to be decided is a very crucial thing to treat the disease. Chemotherapy has become the mainstay of breast cancer treatment. The increased understanding of breast cancer made it possible to develop more intelligent therapies that could effectively target the disease and react to its milieu. This was made possible by advancements in molecular biology and pharmacology. The two most important and predominant physiological processes in tissue homeostasis are mammalian cell division and death. Dysregulated cell cycle has become one of the main hallmarks of any kind of cancer, including breast cancer. Among the various regulators of cell cycle, cyclin-dependent kinases play a major role in its regulation. The role of CDKs along with their cyclin partners in breast cancer has become one of the main target in various studies. The dysregulated CDK expression in BC patients affected both overall survival (OS) and relapse-free survival (RFS). Studies on enrichment demonstrated the importance of CDKs in neoplastic processes and suggested that regulating CDKs in conjunction with traditional medications might be a promising option to treat BC patients.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
8.1 Introduction
Breast cancer is one of the most aggressive and lethal types of transformation among women (Mehraj et al. 2021a, b; Mir 2021). However, the enormous improvements in screening techniques, early diagnosis, and treatment discoveries are to blame for the increasing survival rate (Mehraj et al. 2022a, b, c, d, e; Mir 2022). In this crucial area of pharmaceutical business research over the past ten years, there have been numerous acquisitions (Qayoom et al. 2021). The increased understanding of breast cancer made it possible to develop more intelligent therapies that could effectively target the disease and react to its milieu. This was made possible by advancements in molecular biology and pharmacology. Depending upon the intrinsic gene expression profiling, there are five main subtypes of breast cancer (BC): Luminal A, the most common subtype, maybe PR positive, ER positive or negative, HER2 negative, and EGFR and CK5/6 negative; luminal B (ER- or PR-positive and HER2-positive); basal-like (ER-, PR-, and HER2-negative, cytokeratin 5/6-positive, and/or epidermal growth factor receptor (EGFR) (Johnson et al. 2021; Mehraj et al. 2022a, b, c, d, e). These BC subtypes were classified according to distinct structural design, biological characteristics, prognosis, and clinical stages. The targeted medication has shown success in treating approximately 77% of BC patients who are receptor-positive (Hurvitz et al. 2013; Mir et al. 2020; Ghafouri et al. 2022). Unfortunately, because of the lack of appropriate tailored treatment, 15–25% of TNBC patients have poor outcomes. For patients with TNBC and the majority of BC, surgery combined with chemotherapy and radiotherapy is frequently advised (Al-Mahmood et al. 2018; Mehraj et al. 2022a, b, c, d, e). To enhance prognosis, stop cancer from progressing, and create effective medicines, early diagnosis, accurate therapy, and prognosis are critically required. Currently, one exciting field of such research is the function of cell-cycle regulation (Sofi et al. 2022a, b).
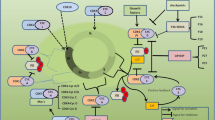
The two most important and predominant physiological processes in tissue homeostasis are cell division and apoptosis. The cell-cycle process has four distinct ordered phases and is referred to as G1 phase, S phase, G2 phase, and M phase. Various cell cycle checkpoints are present that regulate transition from one phase to another to prevent chromosomal aberrations (Malumbres and Barbacid 2009; Mehraj et al. 2021a, b). Availability of growth factors and various intrinsic and extrinsic signals determine whether a cell will go for division or not. Otherwise, the cell will enter into a non-dividing phase in G1 called G0 phase. There is one checkpoint called restriction checkpoint in G1, passing of which guarantees cell division. Numerous cyclins and cyclin-dependent kinases (CDKs), a class of serine/threonine kinases, control the cell cycle (Mehraj et al. 2022a, b, c, d, e). To stabilize, activate, and phosphorylate CDKs in the designated phases, they assemble complexes with cyclins (Brown et al. 2015) (Fig. 8.1). By phosphorylating the target genes, such as the tumor suppressor protein retinoblastoma, the synthesis of cyclin/CDKs regulates the course of the cell cycle (Rb) (Thu et al. 2018). DNA damage usually leads to the inhibition of CDKs. CDK inhibitors (CKIs) are divided into two families namely INK4/6 family (including INK4a, INK4b, INK4c, INK4d) and CIP/KIP family (including p21, p27, and p57). These inhibitors control activation or inhibition of CDKs. The SCF, E3ubiquitin ligase and the anaphase-promoting complex/cyclosome (APC/C) control synthesis and degradation of multiple cyclins. Any type of genetic or epigenetic change in cell cycle regulatory proteins will lead to malignant transformations like breast cancer (Zhou et al. 2016). This represents a frontier in medical sciences for developing artificial CDK inhibitors as antitumor treatments (Gupta et al. 2019).
Using TCGA BRCA datasets accessible on the UCSC XENA and Gepia2 Web servers, we investigated the expression of CDKs in clinical samples of BC patients in this chapter. Here, we report that BC has a considerable deregulation in CDK expression. Additionally, it was discovered that unregulated CDK expression in BC patients affected both OS and RFS. Studies on enrichment demonstrated the importance of CDKs in neoplastic processes and suggested that regulating CDKs in conjunction with traditional medications might be a promising option to treat BC patients.
8.2 Expression Profiles of CDKs in Molecular Subtypes of Breast Cancer
Using UALCAN to evaluate the highly deregulated CDK expression patterns across various BC subclasses, age groups, and ethnicities, it was discovered that TNBC patients express high levels of CDK1, followed by Her2 enriched and luminal subtypes. Additionally, CDK1 was discovered to be substantially upregulated in women between the ages of 20 and 40 and of African-American origin (Fig. 8.2). Asian women and breast cancers that were enriched in HER2 had significantly lower levels of CDK18. However, there were no appreciable differences in CDK11A expression patterns among different BC subclasses, age, groups, or nationalities.
8.3 Expression Analysis of CDKs in Breast Cancer
CDKs are important regulatory enzymes that play their role in cell cycle along with their cyclin partners (Asghar et al. 2015). The dysregulation of different CDKs is a hallmark of any cancer, including breast cancer. Most of the CDKs are highly overexpressed, while as some of them are downregulated in breast cancer (Ramachandiran et al. 2002). For instance, CDK1 is highly upregulated in breast cancer, while as CDK11A and CDK18 are highly downregulated (Table 8.1). Using different online portals, the expression profiles of various CDKs reveal that CDKs are highly upregulated in breast cancer. Among the various CDKs, CDK1 is of utmost importance, as it is the universal master kinase, that is conserved from yeast to humans (Sofi et al. 2022a, b). The expression profiles of various CDKs using UCSC XENA revealed the dysregulation of CDKs in breast cancer (Fig. 8.3) (Goldman et al. 2020).
Further, the dysregulation is highest in case of CDK1, as is evident by the fold change of 2.84 and a p-value of 2.82E-184 (Table 8.1).
8.4 CDK Expression and Various Clinicopathological Parameters
Age, ethnicity, tumor subtypes, and many other pathological characteristics are linked to CDK dysregulation. The relationship between common clinical-pathological traits in breast cancer patients and significantly disrupted CDKs—especially CDK1, CDK x11A, and CDK18—was investigated using the bc-GenEXMiner database (Jézéquel et al. 2012). Breast tumors (ER 235 and PR-negative) were shown to have considerably higher CDK1 expression than those with hormone receptors (p value-0.0001). On the other hand, breast tumors that were HER2-enriched had higher levels of CDK1 expression (p value-0.0001). It was found that greater CDK1 expression was associated with SBR3 in terms of SBR grade. Furthermore, patients with mutant p53 exhibited considerably higher amounts of CDK1 mRNA than those with wild-type p53. Patients with ER-negative breast cancer had significantly lower CDK11A expression than those with HER2-enriched tumors, who had much higher CDK11A expression.
However, there was little correlation with PR, SBR 246 grade, or p53 status and CDK11A expression. Contrarily, CDK18 was found to be markedly downregulated in breast cancer patients who expressed hormone receptors (ER, & PR), indicating a close association with hormone receptor negativity. In BC patients with HER2 amplification, CDK18 mRNA levels were found to be low, and they were found to be correlated with SBR3 grade. It was revealed that CDK18 expression and p53 status are closely related in BC patients with wild-type 53 (Fig. 8.4).
8.5 Protein-Protein Interaction of CDKs in Breast Cancer
The STRING v11 was used to construct related proteins using CDK-1, CDK11a, and CDK18 as input genes (Szklarczyk et al. 2015). The results found that CDKs interact with numerous other genes crucial to the development of breast cancer (Gupta et al. 2019; Ding et al. 2020; Sun et al. 2020). The most significant proteins that play a substantial role in the carcinogenesis of breast cancer among these interacting proteins are CCNA2, CCNE1, and CCNA1 (Fig. 8.5).
8.6 Gene Ontology of CDKs
Using the FunRich program, a Gene Oncology (GO) enrichment study was carried out to determine the functional categories and distinctive biological characteristics of CDKs (Kuleshov et al. 2016). Cellular component (CC), biological process (BP), molecular function (MF), and biological pathway (BP) analysis of GO terms were filtered (Fig. 8.6). At a p-value of 0.05 or below, the top 10 GO words of the related genes in BP, CC, MF, and BP were deemed significant (Figure 8.6a–d). The GO keywords cell communication, ontology; BP, kinase binding, ontology; MF, cyclin-dependent protein kinase holoenzyme complex, ontology; CC, and cell cycle, ontology; BP were all implicated in the functions of these genes. The biological pathway analysis revealed that the genes linked with CDKs, which are connected to the FOXM1 transcription factor network, the regulation of retinoblastoma protein, and the E2F transcription factor network, were highly enriched for the cell cycle (Figure 8.6d). As a result, it is evident from the gene ontology that CDKs control various vital cellular functions that are crucial to the development of breast cancer.
8.7 Prognostic Significance of CDKs in Breast Cancer
Breast cancer patients who have dysregulated CDKs had lower relapse-free survival and overall survival rates. It is interesting to note that there is a theory that breast cancer patients’ prognosis may be influenced by the precise activity of CDK1 and CDK2 (Sofi et al. 2022a, b). Treatment of breast cancer patients with various chemotherapeutics has demonstrated that particular CDK1 and CDK2 activity can be utilized to forecast how well these patients would respond to chemotherapy (Li et al. 2021). Similar to this, Kim and colleagues have demonstrated that patients who have high CDK1 and CDK2 specific activity had poor 5-year relapse-free survival. They also demonstrated how patients with breast cancer can be divided into low-risk and high-risk categories based on this score. As a result, it is hypothesized that assessing CDK1 and CDK2 specific activity may be useful for predicting the course of disease (Kim et al. 2012) . Furthermore, it has been proposed that the distinct CDK1 and CDK2 activities can predict breast cancer patients’ paclitaxel sensitivity (Kim et al. 2012). An in vitro sensitivity to paclitaxel cannot perfectly predict the same response in an in vivo setting, according to research by Nakayama and colleagues. Paclitaxel was effective in treating cancer cells in vitro with high specific activity of CDK1, and cancer cells with high specific activity of CDK2 prior to therapy. As a result, it is possible to predict drug sensitivity in vivo by assessing CDK2-specific activity prior to therapy and CDK1 specific activity following treatment with paclitaxel (Nakayama et al. 2009). Breast cancer cells are also resistant to other anti-cancer medications, including the antiestrogen tamoxifen, which slows the proliferation of cancer cells by blocking CDK2,4,6. Inducing apoptosis in both resistant and sensitive cell lines by silencing CDK1 and CDK2 with siRNA molecules or purine-based inhibitors (NU2058 and NU6102) can also reduce the proliferation of tumor cells (Martin et al. 2005). The Japanese cohort has also been used to show the predictive relevance of CDK1-CDK2-specific activity in hormone receptor-positive and node-negative breast cancer patients. The findings revealed that while tumor size, HER2, age, and histologic grade did not significantly correlate with tumor recurrence, specifically CDK1 and CDK2 activity did (Kim et al. 2008). Five genes, including CDK1, CCNB2, ACACB, PPARG, and MAD2L1, have been demonstrated by Ding and colleagues to be involved in regulation and serve as potential diagnostic indicators for ductal carcinoma in situ (Ding et al. 2017). It is interesting to note that the elimination of CDK1 is recommended as a valuable anticipatory biomarker to assess the effectiveness of anti-cancer therapy. Chemotherapy has been shown by Galindo-Moreno and colleagues to accelerate CDK1 degradation in MCF7 cancer cells through p62/HDAC6-mediated autophagy (Galindo-Moreno et al. 2017).
8.8 Role of CDKs in Breast Cancer
Uncontrolled cell growth that results in tumor clonality, in which a single cell replicates improperly, is a major contributor to the development of both benign and malignant tumors (Thu et al. 2018). In the context of CDKs and breast cancer, both varieties of tumors can be considered. Breast cancer metastasis would indicate a malignant tumor, which is considerably harder to remove locally than a benign tumor (Redig and McAllister 2013; Mehraj et al. 2022a, b, c, d, e). In order to restore control of the cell cycle, CDK inhibition has become a potential strategy. Cancer cells have been stopped in their tracks in the G1 phase of the cell cycle by inhibition of specific CDK pathways (Wenzel and Singh 2018). The specificity of CDKs is one characteristic that makes this a workable option. Cyclins are particular to their linked CDKs, a complexity illustrated by the fact that various cell types can go through mitosis at various periods (Sofi et al. 2022a, b). Globally, breast cancer affects people, primarily but not exclusively women. Unfortunately, patients’ resistance to targeted and non-targeted medicines frequently leads to the failure of existing treatments. In light of this, CDK research has continued, with a focus on CDK4/6 (Deng et al. 2018). Cyclin D1 overexpression is linked to this specific CDK. Research on CDKs, such as CDK4 and CDK6, is being done to find strategies to block their activity and prevent the formation of malignant tissue without harming healthy tissue (Zhang et al. 2020a, b). Additionally, this method of treating breast cancer offers a potential means of avoiding cytotoxic anticancer drugs or chemotherapy-induced alopecia (CIA), a side effect of chemotherapy. Inhibiting CDKs, specifically CDK2, lowers the likelihood that such drugs may divide the epithelium of hair follicles, which results in hair loss (Ding et al. 2020). Treatments for breast cancer that involve CDK inhibition and an understanding of CDK function are safer and more efficient.
Due to their connections to breast cancer development, CDKs can also be employed to treat breast cancer by controlling proliferation with CDK inhibitors. For instance, based on the presence of hormone receptors in breast cancer cells that have undergone biopsy, breast cancer might be either hormone receptor positive or negative. The proliferation of cancer cells is fueled by hormone receptors like estrogen receptors; therefore, understanding this is crucial when treating breast cancer (Sofi et al. 2022a, b).
Because they prevent the growth of these receptors, selective CDK4/6 inhibitors have been demonstrated to be effective in treating breast tumors that are estrogen receptor-positive (Kang et al. 2014). In order to actively contribute to DNA replication and, by extension, the advancement of a cell through the cell cycle, CDK4/6 interacts with cyclin D. Breast cancer could arise if cells divide uncontrolled in the absence of such regulation. In other situations, cyclin D1, an oncogene that encodes cyclin D1, exhibits aberrant behavior, which promotes its overexpression and controls the transition from G1 to S (Mohammadizadeh et al. 2013). This would result in a lack of regulation in CDKs and an unregulated cell cycle because the inactivation of CDKs includes declining amounts of cyclin. Because it is overexpressed in a significant portion of cases, Cyclin D1 is intimately linked to breast cancer. The overexpression of the CCND1 gene is associated with the overexpression of the estrogen receptor (ER), both of which contribute significantly to the proliferation of cancer cells (Eeckhoute et al. 2006). The tumor suppressor protein pRb may become phosphorylated as a result of cyclin D1 activating CDKs, which then frees transcription factors like E2F that promote DNA synthesis (He et al. 2014). As a result of increased proliferation, breast cancer’s pathophysiology is influenced. Cyclin D1 levels are influenced by CCND1 expression and can be utilized to provide patients with a more precise prognosis. Because they prevent the growth of these receptors, selective CDK4/6 inhibitors have been demonstrated to be effective in treating breast tumors that are estrogen receptor positive. In order to actively contribute to DNA replication and, by extension, the advancement of a cell through the cell cycle, CDK4/6 interact with cyclin D (Murphy and Dickler 2015). Breast cancer could arise if cells divide uncontrolled in the absence of such regulation. In other situations, cyclin D1, an oncogene that encodes cyclin D1, exhibits aberrant behavior, which promotes its overexpression and controls the transition from G1 to S (Eeckhoute et al. 2006). This would result in a lack of regulation in CDKs and an unregulated cell cycle because the inactivation of CDKs includes declining amounts of cyclin. Because it is overexpressed in a significant portion of cases, Cyclin D1 is intimately linked to breast cancer. The overexpression of the CCND1 gene is associated with the overexpression of the estrogen receptor (ER), both of which contribute significantly to the proliferation of cancer cells (Eeckhoute et al. 2006). The tumor suppressor protein pRb may become phosphorylated as a result of cyclin D1 activating CDKs, which then frees transcription factors like E2F that promote DNA synthesis. As a result of increased proliferation, breast cancer’s pathophysiology is influenced. According to a mouse study, cyclin D1-deficient animals did not grow mammary carcinomas even when the ErbB-2 oncogene was turned on, which would typically cause the growth of cancer cells. These results provide strong evidence that cyclin D1 plays a key role in breast cancer when combined with the frequent overexpression of cyclin D1 in human breast cancer. Furthermore, a study that was conducted from January 2016 to June 2017 and involved numerous breast tumor patients discovered that enhanced cyclin D1 levels were present in 60% of cases and that given its relationship to other common breast cancer markers, cyclin D1 is a good prognostic indicator for the disease. Cyclin D1 can be overexpressed as a result of CCND1, which can improve prognosis. Cyclin E is another cyclin that may contribute to the emergence of breast cancer. Despite the fact that it is less frequently overexpressed than cyclin D1, the breakdown of its pathways results in the buildup of other products that disrupt the cell cycle. While cyclin E, like cyclin D1, can phosphorylate rPb to promote proliferation, it can also cause a cell to enter the S phase without the aid of rPb or E2F (Ding et al. 2020). In contrast to cyclin E overexpression, which causes breast cancer with higher rates of proliferation and worse outcomes, cyclin D1 overexpression is more frequently found in breast cancer(Ding et al. 2020). In both instances, overexpression of the cyclin does not coincide with overexpression of the gene, suggesting that changes in the breakdown pathway are most likely the cause of the issue (Ding et al. 2020).
Treatment Advancements Given that CDKs are known to have a role in controlling checkpoints and cell division, CDK pathways have long been of interest in the treatment of breast cancer. The goal is to identify the source of the cell cycle anomaly and create a remedy that will enable cells to reestablish control over proliferation. In the past, using CDK inhibitors to create medicines showed great promise, but it was plainly not a simple fix. Novel research has indicated that there is more possibility to understand the association between CDKs and breast cancer as a result of the introduction of new medications and combination therapies. It has been discovered that CDK 4/6 and CDK 4/6 inhibitors in particular regulate pathways important for the growth of tumor cells in breast cancer. Cyclin E and the E2F proteins participate in a positive feedback loop that phosphorylates and then hyper phosphorylates RB in the cyclin D1-CDK4 pathways. The phosphorylation of RB can be stopped by CDK 4/6 inhibitors, which causes the cell to be arrested in the G1 phase (Pernas et al. 2018). In some situations, controlling the cyclin D-CDK 4/6-retinoblastoma pathway may be preferable. For instance, endogenous proteins in cells, including the INK4 proteins, can reduce CDK 4/6 activity by attaching to the enzyme’s catalytic subunits. However, the gene that codes for p16, one of these proteins, is deleted in some cells. As a result, CDK 4/6 activity is elevated at a baseline level, which presumably makes these cells more vulnerable to treatment with CDK 4/6 inhibitor (Knudsen and Witkiewicz 2016). Since ER+/HER2-positive breast cancer is the most often diagnosed kind, numerous therapies have been created that particularly target pathways in this subtype (Knudsen and Witkiewicz 2016). Treatments for this type of breast cancer are frequently less effective over time since it has a higher probability of recurrence. The purpose of CDK 4/6 inhibitors is to stimulate the RB tumor suppressor response; however, for unexplained reasons, they have also been proven to enhance breast cancer prognosis. Despite the fact that this might be connected to less well-studied breast cancer subgroups, these responses to CDK 4/6 inhibition aid in preventing recurrences in the future. Endocrine therapy and CDK 4/6 inhibition are complementary therapies that have been shown to reduce the development of tumor cells. Unexpectedly, the use of CDK 4/6 inhibitors led to the activation of genes that may have aided in continued cell development. Fortunately, it has been demonstrated that using endocrine medication blocks this reaction, reducing cycle D1 activity (Knudsen and Witkiewicz 2016). This role of endocrine therapy coupled with CDK 4/6 inhibitors is very important since one round of tumor cell development would result in another round of growth. Palbociclib, Ribociclib, and abemaciclib are three CDK 4/6 inhibitors that have received FDA approval and are each intended to complement a different type of therapy (Asghar et al. 2015). For the treatment of ER+/HER2-metastatic breast cancer, fulvestrant was specifically approved for use in conjunction with palbociclib. Because their mechanical pathways are redundant, metastatic tumors frequently respond rather effectively to combination therapy, as shown in Table 8.2.
A 2017 study that looked at all three inhibitors discovered that they were significant patient options because of their low toxicity but high efficacy and oral dosing. The effectiveness, safety, and pharmacology of palbociclib used in conjunction with endocrine therapy were closely examined in 2018; the findings revealed that this combinatorial therapeutic approach increased standard of life and progression-free survival in patients with metastatic breast cancer (Yu et al. 2006). Palbociclib was the only CDK4/6 inhibitor still in use in 2019 whose results from clinical studies enhanced overall survival (Fig. 8.7) (Ding et al. 2020). Promising findings from phase II clinical trials provided researchers cause to trust in the efficacy of palbociclib when it entered phase III trials to be examined with adjuvant endocrine therapy. In Phase II, postmenopausal, treatment-nave women with ER+/HER2-metastatic breast cancer either received just letrozole treatment or a letrozole and palbociclib combination treatment (Serra et al. 2019). Palbociclib has been shown to have clinical activity when used alone, and it also showed therapeutic activity when combined with the same endocrine medication administered to individuals sparingly before the condition progressed (Qayoom et al. 2020; Orbaugh et al. 2016).
Phase III testing of palbociclib included testing it not just with letrozole but also with fulvestrant. The data from these trials revealed that CDK 4/6 inhibitors especially palbociclib in combination with either letrozole or fulvestrant improved median progression-free survival (mPFS) by more than 10 months and showed signs of reversing endocrine resistance in patients (Malorni et al. 2018). According to a 2019 study from the Siteman Cancer Center, resistance to CDK 4/6 inhibitors can happen via a variety of routes since ER+/HER2-breast cancer might be incalculable due to its molecular heterogeneity. As a result, various patients may require tailored treatment plans (Xi et al. 2019). For instance, patients who had previously been identified as being sensitive to endocrine therapy reacted to a palbociclib and fulvestrant combination treatment with a longer overall survival than the placebo and fulvestrant treatment (Turner et al. 2018). Fulvestrant has been demonstrated to nullify some of the negative side effects of selective estrogen receptor modulators like tamoxifen when used as adjuvant therapy (Johnston and Cheung 2010). Ribociclib is a primary combination medication licenced for use in the treatment of ER+/HER2-breast cancer especially patients of bone-only diseases, visceral metastasis, de novo diseases, and prior therapy (Hortobagyi 2018). Ribociclib was examined in phase III trials and proved to be an effective treatment in these subgroups. Neutropenia, leukopenia, abnormal liver function tests, infections, and vomiting were the most frequently reported adverse events (AEs) in these clinical trials; however, when ribociclib and letrozole were tested, the safety profile of the combination treatment was consistent across all subgroups, and mPFS and media clinical benefit response (mCBR) were higher in the ribociclib group compared to the placebo group (Hortobagyi 2018). In comparison to endocrine therapy alone, ribociclib was found to notably improve OS in a study of 672 patients when given in conjunction with tamoxifen or goserelin (Im et al. 2019). The estimated overall survival rate in the ribociclib group at 42 months was 70.2%, with a 95% confidence interval (CI) of 63.5 and 76.0. Estimated overall survival in the placebo group was 46.0%, with a 95% confidence interval (CI) of 32.0 to 58.9. Regarding the adverse effects, studies on the toxicity of both palbociclib and Ribociclib—even when used together to better understand their drug-drug interactions—have been conducted (Bellet et al. 2019). This is in part due to CDK4/6 inhibitors’ capacity to be used over extended periods of time. This study examines how to control the adverse effects of each medication. Abemaciclib, a CDK4/6 inhibitor and the third and most recently created therapy, stops the advancement of the cell cycle by obstructing the phosphorylation of the retinoblastoma tumor suppressor protein (Palumbo et al. 2019). Because it may be administered orally to patients constantly and as monotherapy, abemaciclib is seen to be a good alternative (Martin and Goldstein 2018). Abemaciclib response rates in clinical trials ranged from 19.7% to 59.0% with significantly higher patient mPFS. Additionally, it showed palbociclib and ribociclib-like AEs (Horie et al. 1992). As seen in Table 8.3, all three therapies have demonstrated neutropenia and leukopenia as AEs, although they differ in other ways. For example, fatigue restricted the dosages of abemaciclib rather than neutropenia necessitating lower dosages. Abemaciclib has a bigger effect on the body than the other two therapies, maybe as a result of its higher CDK 4 specificity (Martin and Goldstein 2018). Abemaciclib treatment outcomes were observed in, a phase III clinical stage combining fulvestrant and abemaciclib. Out of the 669 recruited patients, 25.3% were found to be resistant to main endocrine therapy; the group receiving abemaciclib and fulvestrant had a clinical benefit rate of 72.2% and mPFS of 16.4 months compared to the placebo group’s 56.1% and 9.3 months. Abemaciclib has also been investigated in relation to particular ER+/HER2-breast cancer characteristics, such as liver metastasis, CNS metastasis, and quicker tumor shrinkage. Additionally, this phase III setting viz.: MONARCH 2 demonstrated that those who received abemaciclib treatment postponed chemotherapy (Sledge et al. 2020).
8.9 Combination Therapy of CDK Inhibitors and PD1-PDL1 Antibodies
Cancer immunotherapy has become a potent and successful method of cancer treatment thanks to decades of research. Dr. Honjo discovered PD1 (programmed death receptor 1) and showed that T cells express PD1 in 1992. Dr. Chen discovered PDL1 (B7-H1) in 1999 and showed that immune and tumor cells express PDL1 at high levels. T cell death is induced by the interaction between PDL1 and PD1, and lymphocyte activation is adversely regulated. Therefore, inhibiting PD1-PDL1 immunological checkpoints encourages T cell activation, which helps T cells have a lethal effect on tumor cells. Even while blocking PD1-PDL1 immune checkpoints has been clinically effective in treating a number of malignancies, the majority of cancer patients still did not benefit from immunotherapy. Additionally, drug resistance could develop while treating PD1-PDL1 with targeted therapy. As a result, numerous studies have been carried out to determine how combination therapy tactics can increase the responsiveness of cancer patients to immunotherapy. Some CDK inhibitors can improve the anti-tumor immune response, according to recent studies. Some CDK inhibitors have shown strong anti-tumor effectiveness in preclinical and clinical trials when combined with PD1-PDL1 immunotherapy.
8.9.1 Dinaciclib Enhances Anti-PD1 Mediated Tumor Suppression
As previously mentioned, dinaciclib, a powerful CDK inhibitor of CDK1, 2, 5, 9, and 12, can cause apoptosis in a variety of tumor cells. According to Hossain et al., combined therapy using Dinaciclib and an anti-PD1 antibody had significant anti-tumor activity. Combination therapy has the potential to increase anti-tumor immune response and promote tumor regression since it can activate DC and trigger T cell infiltration. Additionally, Dinaciclib can cause immunogenic cell death (ICD) in conjunction with anti-PD1 antibodies to transform tumor cells into endogenous vaccines (Hossain et al. 2018). Together, these studies have opened up new possibilities for addressing pan-CDK inhibitor toxicity and side effects, which expands the range of potential applications for these drugs.
8.9.2 CDK4/6 Inhibitors Augment the Anti-Tumor Efficacy of PD1-PDL1 Immune Checkpoint Blockade
Fundamental cell cycle regulators CDK4 and CDK6, which are necessary for the onset and development of different cancers, are CDK4 and CDK6 (Qayoom et al. 2022). A number of solid cancers have shown notable activity against CDK4/6 pharmacological inhibitors. Goel et al. discovered that CDK4/6 inhibitors not only cause tumor cell cycle arrest but also foster anti-tumor immunity in rat tumor model research (Goel et al. 2017). On the one hand, CDK4/6 inhibitors promote the expression of endogenous retroviral elements in tumor cells, stimulating the synthesis of type III interferons while also improving the presentation of tumor antigens. Conversely, CDK4/6 inhibitors significantly reduce the growth of regulatory T cells. Treatment with CDK4/6 inhibitors dramatically increases the clearance of tumor cells mediated by cytotoxic T cells based on these two functions. Theoretically, this study supported the use of CDK4/6 inhibitors and PD1-PDL1 antibodies in combination therapy.
Another CDK4/6 inhibitor that has received clinical approval for the treatment of HR+ breast cancer is abemaciclib. According to a recent study by Schaer et al., Abemaciclib therapy can boost the expression of antigen presentation genes in breast cancer cells and can stimulate human T cell activation (Schaer et al. 2018). According to another research, abemaciclib monotherapy can boost T cell inflammation and slow the growth of tumors. Abemaciclib and anti-PDL1 antibody combination therapy can promote immune memory and tumor eradication. These findings indicated that Abemaciclib and an anti-PDL1 antibody could be used in combination therapy to successfully activate both innate and adaptive immune response. When used in combination, abemaciclib and an anti-PDL1 antibody have shown tremendous promise for use in clinical settings.
Zhang et al. looked into the regulating mechanisms of PDL1 expression and stability because the effectiveness of PDL1 antibody therapy depends on the protein abundance of PDL1 (Zhang et al. 2020a, b). They discovered that CDK4 participates in the control of PDL1. Another study further demonstrated the extraordinary anti-tumor effectiveness of the combination therapy using CDK4/6 inhibitors and anti-PDL1 antibodies (Deng et al. 2018). Together, our results support the therapeutic utility of combination therapies using CDK4/6 inhibitors and anti-PD1-PDL1 antibodies. Another combination therapy is now being tested (Dai et al. 2003; Gao et al. 2004; Lin et al. 2010; Kruse et al. 2011), in addition to the combination of CDK4/6 inhibitors and PDL1 antibodies. Future cancer treatments are predicted to heavily rely on combination therapies.
8.9.3 Other Combination Therapies
Neoantigen load and tumor T cell infiltration and clonal growth are reported to be enhanced in CDK12 mutant patients (Wu et al. 2018). Immune checkpoint treatment may be beneficial for a subclass of metastatic castration-resistant prostate cancer (mCRPC) defined by CDK12 inactivation (Antonarakis 2018). In fact, patients with metastatic prostate cancer who had CDK12 deficiency underwent a phase ll clinical trial (ClinicalTrials.gov Identifier: NCT03570619). Immune-checkpoint inhibitors, nivolumab, and ipilimumab were given to these individuals, followed by nivolumab monotherapy. SR-4835, a CDK12 and CDK13 selective inhibitor, was also found to trigger immunogenic cell death, which improved the anti-tumor effectiveness of PD1-PD-L1 immune checkpoint therapy in breast cancer, according to a recent study (Li et al. 2020). Additionally, the CDK7 and CDK9 inhibitors YKL-5-124 and MC18029 are being investigated in combination therapy with the previously mentioned PD1/PD-L1 (Zhang et al. 2018; Zhang et al. 2020a, b). These results suggested that PD1-PDL1 immunotherapy in combination with CDK12 inhibitors will be a successful cancer treatment method.
8.10 Summary
Breast cancer is a type of cancer that involves the dysregulation of many genes. CDKs are the key players that are dysregulated in breast cancer patients. Breast cancer patients who have dysregulated CDKs had lower relapse-free survival and overall survival rates. The dysregulation of different CDKs in breast cancer has become a target for the treatment of breast cancer patients. The expression profiles of various CDKs using UCSC XENA revealed the dysregulation of CDKs in breast cancer. The breast tumors that are HER2-enriched have higher levels of CDK1 expression. Further, greater CDK1 expression is associated with SBR3 in terms of SBR grade. Also, CDKs interact with numerous other genes crucial to the development of breast cancer. Gene ontology also revealed that CDKs control various vital cellular functions that are crucial to the development of breast cancer. Due to their connections to breast cancer development, CDKs can also be employed to treat breast cancer by controlling proliferation with CDK inhibitors. Therefore, regulating CDKs in conjunction with traditional medications might be a promising option to treat BC patients.
8.11 Further Reading
The readers can further read about the role of CDKs in breast cancer by going through the following papers
For more incites about the topic we would suggest detailed findings from the books of (Mir 2022) https://doi.org/10.1016/C2021-0-02565-7, https://doi.org/10.1016/C2022-0-00074-X (Mir 2021) https://doi.org/10.52305/WXJL6770. Also, the readers can have a look upon the following visual presentations for the better conceptual understanding of CDKs and their role in breast cancer.
References
Al-Mahmood S et al (2018) Metastatic and triple-negative breast cancer: challenges and treatment options. Drug Deliv Transl Res 8(5):1483–1507
Antonarakis ES (2018) Cyclin-dependent kinase 12, immunity, and prostate cancer. N Engl J Med 379(11):1087–1089
Asghar U et al (2015) The history and future of targeting cyclin-dependent kinases in cancer therapy. Nat Rev Drug Discov 14(2):130–146
Bellet M et al (2019) Palbociclib and ribociclib in breast cancer: consensus workshop on the management of concomitant medication. Ther Adv Med Oncol 11:1758835919833867
Brown NR et al (2015) CDK1 structures reveal conserved and unique features of the essential cell cycle CDK. Nat Commun 6(1):1–12
Dai Y et al (2003) Proteasome inhibitors potentiate leukemic cell apoptosis induced by the cyclin-dependent kinase inhibitor flavopiridol through a SAPK/JNK-and NF-κB-dependent process. Oncogene 22(46):7108–7122
Deng J et al (2018) CDK4/6 inhibition augments antitumor immunity by enhancing T-cell activation. Cancer Discov 8(2):216–233
Ding L et al (2020) The roles of cyclin-dependent kinases in cell-cycle progression and therapeutic strategies in human breast cancer. Int J Mol Sci 21(6):1960
Ding Z-H et al (2017) Docking of CDK1 with antibiotic drugs revealed novel therapeutic value in breast ductal cancer in situ. Oncotarget 8(37):61998
Eeckhoute J et al (2006) A cell-type-specific transcriptional network required for estrogen regulation of cyclin D1 and cell cycle progression in breast cancer. Genes Dev 20(18):2513–2526
Galindo-Moreno M et al (2017) Both p62/SQSTM1-HDAC6-dependent autophagy and the aggresome pathway mediate CDK1 degradation in human breast cancer. Sci Rep 7(1):1–10
Gao N et al (2004) Contribution of disruption of the nuclear factor-κB pathway to induction of apoptosis in human leukemia cells by histone deacetylase inhibitors and flavopiridol. Mol Pharmacol 66(4):956–963
Ghafouri SR et al (2022) Recently approved treatment options for patients with metastatic triple-negative and HER2-neu-positive breast cancer. J Investig Med 70(6):1329–1341
Goel S et al (2017) CDK4/6 inhibition triggers anti-tumour immunity. Nature 548(7668):471–475
Goldman MJ et al (2020) Visualizing and interpreting cancer genomics data via the Xena platform. Nat Biotechnol 38(6):675–678
Gupta P et al (2019) CDK inhibitors as sensitizing agents for cancer chemotherapy. Protein kinase inhibitors as sensitizing agents for chemotherapy. Elsevier, pp 125–149
He J et al (2014) Prognosis of lymph node-negative breast cancer: association with clinicopathological factors and tumor associated gene expression. Oncol Lett 8(4):1717–1724
Horie K et al (1992) Immunohistochemical localization of androgen receptor in the human ovary throughout the menstrual cycle in relation to oestrogen and progesterone receptor expression. Hum Reprod 7(2):184–190
Hortobagyi GN (2018) Ribociclib for the first-line treatment of advanced hormone receptor-positive breast cancer: a review of subgroup analyses from the MONALEESA-2 trial. Breast Cancer Res 20(1):1–11
Hossain DMS et al (2018) Dinaciclib induces immunogenic cell death and enhances anti-PD1–mediated tumor suppression. J Clin Invest 128(2):644–654
Hurvitz SA et al (2013) Current approaches and future directions in the treatment of HER2-positive breast cancer. Cancer Treat Rev 39(3):219–229
Im S-A et al (2019) Overall survival with ribociclib plus endocrine therapy in breast cancer. N Engl J Med 381(4):307–316
Jézéquel P et al (2012) Bc-GenExMiner: an easy-to-use online platform for gene prognostic analyses in breast cancer. Breast Cancer Res Treat 131(3):765–775
Johnson KS et al (2021) Molecular subtypes of breast cancer: a review for breast radiologists. J Breast Imaging 3(1):12–24
Johnston SJ, Cheung KL (2010) Fulvestrant-a novel endocrine therapy for breast cancer. Curr Med Chem 17(10):902–914
Kang J et al (2014) Targeting cyclin-dependent kinase 1 (CDK1) but not CDK4/6 or CDK2 is selectively lethal to MYC-dependent human breast cancer cells. BMC Cancer 14(1):1–13
Kim SJ et al (2008) Determination of the specific activity of CDK1 and CDK2 as a novel prognostic indicator for early breast cancer. Ann Oncol 19(1):68–72
Kim SJ et al (2012) Recurrence risk score based on the specific activity of CDK1 and CDK2 predicts response to neoadjuvant paclitaxel followed by 5-fluorouracil, epirubicin and cyclophosphamide in breast cancers. Ann Oncol 23(4):891–897
Knudsen ES, Witkiewicz AK (2016) Defining the transcriptional and biological response to CDK4/6 inhibition in relation to ER+/HER2-breast cancer. Oncotarget 7(43):69111
Kruse U et al (2011) Chemoproteomics-based kinome profiling and target deconvolution of clinical multi-kinase inhibitors in primary chronic lymphocytic leukemia cells. Leukemia 25(1):89–100
Kuleshov MV et al (2016) Enrichr: a comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res 44(W1):W90–W97
Li N et al (2021) Expression and prognostic value of transcription-associated cyclin-dependent kinases in human breast cancer. Aging (Albany NY) 13(6):8095
Li Y et al (2020) CDK12/13 inhibition induces immunogenic cell death and enhances anti-PD-1 anticancer activity in breast cancer. Cancer Lett 495:12–21
Lin TS et al (2010) Flavopiridol, fludarabine, and rituximab in mantle cell lymphoma and indolent B-cell lymphoproliferative disorders. J Clin Oncol 28(3):418
Malorni L et al (2018) Palbociclib as single agent or in combination with the endocrine therapy received before disease progression for estrogen receptor-positive, HER2-negative metastatic breast cancer: TREnd trial. Ann Oncol 29(8):1748–1754
Malumbres M, Barbacid M (2009) Cell cycle, CDKs and cancer: a changing paradigm. Nat Rev Cancer 9(3):153–166
Martin JM, Goldstein LJ (2018) Profile of abemaciclib and its potential in the treatment of breast cancer. Onco Targets Ther 11:5253
Martin LA et al (2005) The anti-estrogen ICI 182,780, but not tamoxifen, inhibits the growth of MCF7 breast cancer cells refractory to long-term estrogen deprivation through downregulation of ER and IGF signalling. Breast Cancer Res 7(2):1–2
Mehraj U et al (2021a) Tumor microenvironment promotes breast cancer chemoresistance. Cancer Chemother Pharmacol 87(2):147–158
Mehraj U et al (2021b) The tumor microenvironment as driver of stemness and therapeutic resistance in breast cancer: new challenges and therapeutic opportunities. Cell Oncol:1–21
Mehraj U et al (2022a) Expression pattern and prognostic significance of baculoviral inhibitor of apoptosis repeat-containing 5 (BIRC5) in breast cancer: a comprehensive analysis. Adv Cancer Biol-Metastasis 100037
Mehraj U et al (2022b) Expression pattern and prognostic significance of chemokines in breast cancer: an integrated bioinformatics analysis. Clin Breast Cancer
Mehraj U et al (2022c) Cryptolepine targets TOP2A and inhibits tumor cell proliferation in breast cancer cells-an in vitro and in silico study. Anti Cancer Agents Med Chem
Mehraj U et al (2022d) Expression pattern and prognostic significance of CDKs in breast cancer: an integrated bioinformatic study. Cancer Biomarkers(Preprint):1–15
Mehraj U et al (2022e) Adapalene inhibits the growth of triple-negative breast cancer cells by S-phase arrest and potentiates the antitumor efficacy of GDC-0941. Front Pharmacol 13:958443
Mir MA et al (2020) Targeting different pathways using novel combination therapy in triple negative breast cancer. Curr Cancer Drug Targets 20(8):586–602
Mir MA (2021) Combination therapies and their effectiveness in breast cancer treatment. Nova Biomedical Science Publishers USA, pp 1–411. https://doi.org/10.52305/WXJL6770. Book ISBN: 978–1–68507-195-0
Mir MA (2022) Role of tumor microenvironment in breast cancer and targeted therapies, 1st edn. Elsevier Inc. Publishers USA, pp 1–350. https://doi.org/10.1016/C2022-0-00074-X. ISBN: 9780443186974
Mohammadizadeh F et al (2013) Role of cyclin D1 in breast carcinoma. J Res Med Sci 18(12):1021
Murphy CG, Dickler MN (2015) The role of CDK4/6 inhibition in breast cancer. Oncologist 20(5):483–490
Nakayama S et al (2009) Prediction of paclitaxel sensitivity by CDK1 and CDK2 activity in human breast cancer cells. Breast Cancer Res 11(1):1–10
Orbaugh K et al (2016) Palbociclib plus letrozole for the treatment of metastatic breast cancer: an illustrative case scenario. J Adv Pract Oncol 7(5):550
Palumbo A et al (2019) Abemaciclib: the newest CDK4/6 inhibitor for the treatment of breast cancer. Ann Pharmacother 53(2):178–185
Pernas S et al (2018) CDK4/6 inhibition in breast cancer: current practice and future directions. Ther Adv Med Oncol 10:1758835918786451
Qayoom H, Bhat BA, Mehraj U, Mir MA (2020) Rising trends of cancers in Kashmir valley: distribution pattern, incidence and causes. J Oncol Res Treat 5(150):2
Qayoom H et al (2021) An insight into the cancer stem cell survival pathways involved in chemoresistance in triple-negative breast cancer. Future Oncol 17(31):4185–4206
Qayoom H et al (2022) Expression patterns and therapeutic implications of CDK4 across multiple carcinomas: a molecular docking and MD simulation study. Med Oncol 39(10):1–13
Ramachandiran S et al (2002) Mitogen-activated protein kinases contribute to reactive oxygen species-induced cell death in renal proximal tubule epithelial cells. Chem Res Toxicol 15(12):1635–1642
Redig AJ, McAllister SS (2013) Breast cancer as a systemic disease: a view of metastasis. J Intern Med 274(2):113–126
Schaer DA et al (2018) The CDK4/6 inhibitor abemaciclib induces a T cell inflamed tumor microenvironment and enhances the efficacy of PD-L1 checkpoint blockade. Cell Rep 22(11):2978–2994
Serra F et al (2019) Palbociclib in metastatic breast cancer: current evidence and real-life data. Drugs Context 8
Sledge GW et al (2020) The effect of abemaciclib plus fulvestrant on overall survival in hormone receptor–positive, ERBB2-negative breast cancer that progressed on endocrine therapy—MONARCH 2: a randomized clinical trial. JAMA Oncol 6(1):116–124
Sofi S et al (2022a) Targeting cyclin-dependent kinase 1 (CDK1) in cancer: molecular docking and dynamic simulations of potential CDK1 inhibitors. Med Oncol 39(9):1–15
Sofi S et al (2022b) Cyclin-dependent kinases in breast cancer: expression pattern and therapeutic implications. Med Oncol 39(6):1–16
Sun B et al (2020) Inhibition of the transcriptional kinase CDK7 overcomes therapeutic resistance in HER2-positive breast cancers. Oncogene 39(1):50–63
Szklarczyk D et al (2015) STRING v10: protein–protein interaction networks, integrated over the tree of life. Nucleic Acids Res 43(D1):D447–D452
Thu KL et al (2018) Targeting the cell cycle in breast cancer: towards the next phase. Cell Cycle 17(15):1871–1885
Turner NC et al (2018) Overall survival with palbociclib and fulvestrant in advanced breast cancer. N Engl J Med 379(20):1926–1936
Wenzel ES, Singh ATK (2018) Cell-cycle checkpoints and aneuploidy on the path to cancer. In Vivo 32(1):1–5
Wu Y-M et al (2018) Inactivation of CDK12 delineates a distinct immunogenic class of advanced prostate cancer. Cell 173(7):1770–1782
Xi J et al (2019) Retrospective analysis of treatment patterns and effectiveness of palbociclib and subsequent regimens in metastatic breast cancer. J Natl Compr Cancer Netw 17(2):141–147
Yu Q et al (2006) Requirement for CDK4 kinase function in breast cancer. Cancer Cell 9(1):23–32
Zhang H et al (2018) Targeting CDK9 reactivates epigenetically silenced genes in cancer. Cell 175(5):1244–1258
Zhang H et al (2020a) CDK7 inhibition potentiates genome instability triggering anti-tumor immunity in small cell lung cancer. Cancer Cell 37(1):37–54
Zhang Z et al (2020b) CDK4/6 inhibition blocks cancer metastasis through a USP51-ZEB1-dependent deubiquitination mechanism. Signal Transduct Target Ther 5(1):1–13
Zhou Z et al (2016) Insights into APC/C: from cellular function to diseases and therapeutics. Cell Div 11(1):1–18
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Mir, M.A., Sofi, S., Ishfaq, P.M. (2023). CDK Dysregulation in Breast Cancer: A Bioinformatics Analysis. In: Mir, M. (eds) Therapeutic potential of Cell Cycle Kinases in Breast Cancer. Springer, Singapore. https://doi.org/10.1007/978-981-19-8911-7_8
Download citation
DOI: https://doi.org/10.1007/978-981-19-8911-7_8
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-8910-0
Online ISBN: 978-981-19-8911-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)