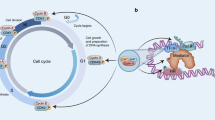
Abstract
Presently, breast cancer (BC) is one of the most common malignancies diagnosed and the leading cause of tumor-related deaths among women worldwide. Cell cycle dysregulation is one of the hallmarks of cancer, resulting in uncontrolled cell proliferation. Cyclin-dependent kinases (CDKs) are central to the cell cycle control system, and deregulation of these kinases leads to the development of malignancies, including breast cancer. CDKs and cyclins have been reported as crucial components involved in tumor cell proliferation and metastasis. Given the aggressive nature, tumor heterogeneity, and chemoresistance, there is an urgent need to explore novel targets and therapeutics to manage breast cancer effectively. Inhibitors targeting CDKs modulate the cell cycle, thus throwing light upon their therapeutic aspect where the progression of tumor cells could be inhibited. This article gives a comprehensive account of CDKs in breast cancer progression and metastasis and recent developments in the modulation of CDKs in treating malignancies. We have also explored the expression pattern and prognostic significance of CDKs in breast cancer patients. The article will also shed light on the Implications of CDK inhibition and TGF-β signaling in breast cancer.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer is the most prevalent cancer diagnosed worldwide [1,2,3,4,5]. According to the GLOBOCAN 2020 data, the incidence rate of BC is highest compared to the other ten most common types of cancer. Globally, the no of new cancer cases has reached a total count of 19.3 in 2020. With an anticipated 2.3 million new cases (11.7%), female breast cancer has surpassed lung cancer as the most often diagnosed malignancy, followed by lung (11.4%), colorectal (10.0%), prostate (7.3%), and stomach (5.6%). With a projected 1.8 million fatalities (18%), lung cancer remained the top cause of cancer death, followed by colorectal (9.4%), liver (8.3%), stomach (7.7%), and female breast (6.9%) cancers. In transitioned vs transitional countries, the overall incidence was 2-3 fold greater for both sexes, although mortality varied twofold for males and slightly for women. Female breast and cervical cancer death rates, on the other hand, were much higher in transitioning nations than in transitioned countries (15.0 vs 12.8 per 100,000 and 12.4 vs 5.2 per 100,000, respectively) [6]. The overall survival of BC patients having early-stage, non-metastatic BC stands at 70–80% [7]. Due to its heterogeneity, BC possesses various morphological descriptions, differences in their profiles concerning their immune-histology, and a specific pattern of distinct molecular subgroups [8]. Based on the gene expression patterns and molecular portraits, BC has been classified into four subtypes: luminal A (Lum A), Luminal B (Lum B), HER2-enriched (HER2+), and triple-negative breast cancer (TNBC) [9, 10]. Among these subtypes, TNBC is the most aggressive one due to the loss of all three receptors, i.e., ER, PR, and HER2 receptors [8, 11]. This heterogeneity of breast tumors has to be considered for any treatment approach. The treatment strategies presently available for BC patients include radiotherapy, chemotherapy, surgery, targeted therapy, and a combinatorial approach [10, 12, 13]. Recent advances in diagnosis and therapeutic strategies have significantly increased the overall survival of BC patients [14, 15]. However, breast tumors demonstrate tumor relapse and therapeutic resistance due to the inherent genetic instability of tumor cells and tumor-stroma cross talks [16,17,18]. Thus, there is an urgent need to explore novel therapeutic targets and treatment strategies for effective breast cancer treatment.
Among the various hallmarks of cancer, increased cell proliferation is one of the most critical aspects that need to be considered [19, 20]. The proliferation of cells is regulated by the cell cycle, and there are well-defined regulatory mechanisms that regulate the cell cycle. The cell cycle is controlled by various cyclins and cyclin Dependent Kinases (CDKs). The importance of cyclins and CDKs in the cell cycle was revealed in a study of fission yeast, which demonstrated the significance of complex Cdc2 (CDK1) [21]. CDKs belong to the serine-threonine kinases regulated by cyclins and phosphorylate several downstream targets during cell cycle progression [21]. Cell division during an individual’s life span and development occurs only at specific places and at a specific time and divides the cell’s content accurately [22]. The cell cycle checkpoints maintain the coherence, integrity, and maintenance of every step in the cell cycle [22]. Breast cancer, like many other cancers, involves increased proliferation of cells, which results from the disruption in the cell cycle regulation by dysregulated expression and activation of CDKs [23, 24]. CDK dysregulation during BC leads to the uncontrolled proliferation of cancer cells, thus maintaining BC’s progression and other factors [21]. Studies have revealed that BC is associated with dysregulation of several Cyclin/CDKs, and their dysregulation has also been observed in breast tumor heterogeneity [25]. Targeting CDKs in BC has been explored, demonstrating significant inhibition of tumor growth with several therapeutic regimens at the clinical stage. In this review, we have shed light on the role of CDKs in the cell cycle, their importance in BC progression and metastasis, their prognostic significance, and the therapeutic implication of CDK inhibition in BC.
Cyclin-dependent kinases and cell cycle
The expansion and division of cells are regulated by a series of events known as the cell cycle. The cell cycle comprises of mainly four phases, namely the G1 phase (growth phase-1), S phase (DNA replication phase), G2 phase (growth phase-2), and M phase (mitotic phase). All these phases are regulated by a set of CDK/cyclin complexes that assist the cell’s progress through these phases. CDKs throughout the process of cell division remain the same while the cell regulates the expression of different cyclins depending upon the stage of the cell cycle [26]. The structure of CDKs consists of two parts: cyclin and kinase. Protein kinase, an enzyme, possesses a highly conserved structure [22]. Cyclin binding to the CDKs triggers the activation of the kinases, and their expression is highly regulated in the multi-step process of the cell cycle. Crystallographic investigations have shed light on the transition of CDKs from inactive to an active form and vice versa [27]. The binding of cyclins induces a conformational change in the catalytic cleft of the kinase with the inherent conformational flexibility. Because of this flexibility, CDKs can exhibit a diverse range of responses in the presence of various growth regulatory signals [27]. CDKs are serine/threonine kinases that phosphorylate several proteins in response to the intrinsic and extrinsic signal, regulating several aspects of cell growth and division [28]. The specificity of CDKs is revealed through the association of specific cyclin with specific CDK (Fig. 1). For instance, CDK2 can form a complex with both cyclin E and cyclin A and acts distinctly upon binding to different cyclins [29]. This demonstrates that the specific CDK having a specific structure possesses a specific purpose in the cell cycle [21]. Besides, the CDK-cyclin complex regulates the stabilization, phosphorylation, and activation of CDKs in distinct cell cycle phases [22]. The importance of this complex lies in the fact that these complexes are crucial for the progression of the cell cycle in terms of phosphorylation of various target genes, like retinoblastoma protein, a tumor suppressor protein. A switch occurs between the inhibition and activation of these cyclins/CDKs in response to the mitogenic signals and checkpoints, respectively [22]. The CKIs (Cyclin-dependent kinase inhibitors), such as INK4 proteins and CIP/KIP (CDK-interacting protein/kinase inhibitory proteins), negatively regulate cyclin/CDKs (Fig. 1) [30]. Also, the cell cycle transition phases are controlled by various mitotic proteins like APC/C (anaphase-promoting complex/cyclosome) and SCF (Skp1–Cul1–F-box-protein) [31,32,33]. The disruption in any one of the above mechanisms can elevate the proliferation of cells by dysregulating the cell cycle, which is a hallmark of several cancers, including BC [9].
Moreover, several enzymes involved in metabolic pathways, including DNA replication or other cell cycle processes, are regulated by CDKs via distinct phosphorylation of crucial enzymes [34]. The association of CDKs with cyclins is of utmost importance, as the latter assists in activating CDKs, and the former phosphorylates the cyclins for the cell cycle phases [35]. CDKs, in association with their cyclin partners, play a significant role in regulating the cell cycle.
Cell cycle, CDKs, and cancer
Cell cycle deregulation is a classic hallmark of cancers, including BC. In several malignancies, dysregulation of the CDK–cyclin complex disrupts the cell cycle coordination and leads to continuous proliferation of cancer cells [36, 37]. The unsuitable activation of CDKs associated with the amplification and overexpression of cyclin gene, cellular mislocalization or premature cyclin expression, and the inhibition of INK4 or CIP/KIP family leads to the dysregulation in the cell cycle, thus resulting in cancer [38]. Several studies have revealed that cancer cells mainly lose these inhibitory mechanisms that regulate the cell cycle. The inactivation of the tumor suppressor genes like Rb and TP53 (p53) gene or the upregulation of oncogenes affects the cyclin/CDK upregulation, resulting in an uncontrollable cell cycle progression and proliferation [38]. The cell cycle is negatively regulated by several tumor suppressor genes, such as the Retinoblastoma (Rb) gene, which in its unphosphorylated state acts as a transcription repressor for E2F. The phosphorylation of pRb protein by CDK4/6/cyclin D complex leads to the release of pRb protein from E2F and leads to the transcription of several genes, including DNA polymerase α, cyclin E, and A responsible for G1/S transition by E2F (Fig. 2). pRb protein in its hypophosphorylated state remains active and arrests the cells in the resting G0 phase by binding to the E2F transcription factor and blocking the expression of genes vital for G1 to S transition. Dysregulation in the CDK/cyclin complex leads to the modulation in the phosphorylation state of Rb protein, altering the role of Rb protein and leading to uncontrolled growth of cells [38].
Role of Rb protein in the cell cycle: A In its activated state, p53 leads to cell cycle arrest in those cells which are having DNA damage, B The phosphorylation of pRb protein by CDK4/6/cyclin D complex leads to the release of pRb protein from E2F and thereby leads to the transcription of important genes responsible for G1/S transition by E2F, C In cancer cells increase in CDK4/6/cyclin D leads to the of Rb protein hyperphosphorylation, thus leading to uncontrolled cell division
Similarly, p53 is another tumor suppressor gene whose unregulated activation leads to cancer progression [39]. In its activated state, p53 leads to cell cycle arrest in cells with DNA damage. Inactivation of p53 is frequently observed in several types of cancer cells [39]. The inactivation of the tumor suppressor genes like the Rb gene or the upregulation of oncogenes affects the cyclin/CDK upregulation, resulting in an uncontrollable cell cycle progression and proliferation [38, 40].
CDKs in BC progression
Breast cancer is one of the most common malignancies among women and the leading cause of tumor-related deaths worldwide [7]. Several CDKs have been attributed to the development and regulation of breast tumorigenicity, and inhibiting the overexpressed CDKs reduces tumor burden and metastasis [41]. One of the most crucial CDK/Cyclin complexes is CDK4/6/Cyclin D which plays a significant role in the initiation and development of various cancers, including BC [42]. Several studies have revealed that cyclin D is overexpressed in early and metastatic BC. The expression of both cyclin D1 and CDK4 is significantly high in lum B (58% and 25%, respectively) and HER2 BC (38% and 24%, respectively), moderate in lum A (29% and 14%, respectively), and lowest in TNBCs [24].
Moreover, cyclin D1/CDK4/6 is the crucial player contributing to RB protein phosphorylation resulting in the proliferation of cells and thus assisting in the BC tumor progression [7]. Furthermore, several other CDKs have been found deregulated in cancer, including BC. For instance, CDK2 is upregulated in BC, resulting in overexpression of its cognates viz cyclin E and A [43]. The fundamental role of CDK2 in a cell cycle is that it acts as a core regulator in the cell cycle and is functional from late G1 up to the end of the S phase [44]. The activation is done by its cyclin partners, namely cyclins E1or E2 and A2. CDK2 displays various regulatory functions such as phosphorylating RB protein, SMAD3, and several other proteins that regulate DNA synthesis [45]. The studies have suggested that many cancers have been accompanied by overexpression of CDK2 and are associated with cancer cell proliferation [45]. The study conducted by Xiangming He and co-workers revealed that CDK2 plays a significant role in BC initiation and progression [46]. It was further analyzed that inhibiting CDK2 effectively deaccelerates BC cell proliferation.
In addition to the above, the most critical CDK for maintaining the regulation of cell cycle development in a large number of mammalian cells is CDK1 [47]. CDK1 is a universal master kinase and is an essential regulatory CDK of mitosis. Ligation of CDK1 with cyclins A and B mediates the S-G2 and G2-M transition. CDK1 acts as a crucial CDK regulating the cell cycle through phosphorylation and dephosphorylation. Activation of CDK1 plays a significant role in apoptosis induction [48]. Dysregulation of CDK1 can lead to chromosomal instability, vigorous tumor growth, and a high proliferation rate of cancerous cells [49]. CDK1 is also overexpressed in several cancers, including BC [50]. The studies have revealed that inhibiting CDK1 either with a single drug or with a combination of drugs is associated with practical anti-cancer effects, thus throwing light on the prognostic significance of CDK1 in BC. Also, CDK1 inhibition has been proposed as a promising therapeutic strategy for MYC-dependent BC patients [50].
While CDK1, CDK2, and CDK4/6 directly influence the modulation in the cell cycle progression, other CDKs like CDK7, CDK8, and CDK9 play their role in transcription (Table 1) [50]. CDK7 acts both as a kinase and a subpart of the transcription factor TFIIH, which regulates transcription and DNA repair [21]. The activation of CDK7 is done by MAT1 and cyclin H, and it shows its role in the cell cycle by phosphorylating other CDKS in the cell [51]. CDK7 has a more significant correlation with the TNBC subtype than other BC subtypes [52]. Another research has also revealed that inhibition of CDK7 in HER2+ will show much sensitivity compared to the ER+ BC [52]. The study done by Li Tang and co-workers revealed that CDK7 is Upregulated in BC. It was further analyzed that CDK7 expression levels are considerably higher in lobular BC, ductal BC, and invasive ductal BC. The study showed a significant correlation between high CDK7 levels and poor prognosis [53]. Besides CDK7, dysregulation of CDK8 has been associated with various cancers, including HER2-enriched BC [54, 55]. Furthermore, CDK9 is also showing its dysregulation in BC. The studies have pointed out that CDK9 inhibitors retard the growth of BC cells, thus highlighting the role of CDK9 in BC progression [56,57,58].
From the above discussion, it is clear that BC progression is associated with the dysregulation of various CDKs that play a significant role in the cell cycle progression. Thus, these CDKs can be used as biomarkers for detecting BC. Targeting CDKs can be a potent anti-cancer marker for the treatment of BC, thus throwing light on the prognostic value of CDKs in BC.
CDKs in breast cancer metastasis
Breast cancer metastasis significantly impacts BC patients’ mortality and survival rates. BC metastasis is the leading cause of mortality among BC patients [17]. Almost 20–30% of early-stage BC patients develop distant metastasis, and approximately 90% of BC deaths occur due to the issues of metastatic BC [59]. Thus, it becomes crucial to throw light on the role of CDKs in BC metastasis. BC metastasis often expands to the organs like the brain, liver, lungs, and bones. The aberrant alterations in cyclins and their CDKs lead to continuous cell division and the migration of tumor cells to other organs [60]. The BC cells could be slothful. However, some carcinogens, such as cyclin D1 and CDK4/6. could lead to a systemic response, resulting in the metastasis of BC cells [61]. This depicts the dual function of cyclins that can interact with the CDKs and create a more aggressive nature of this disease. The studies have revealed the role of CDK4/6 in Epithelial-Mesenchymal Transition (EMT), which is a characteristic feature of cancer metastasis [62, 63]. The research was done by Zhen and co-workers and revealed the role of CDK4/6 in BC metastasis [64]. CDK5 has been studied for its role in TGF-β1 induced EMT in BC progression [65]. Similarly, Adrian and co-workers also revealed the role of cyclin F in EMT [63]. Thus, CDKs show their significant role in BC metastasis, thereby enhancing the aggressiveness of this disease among BC patients.
Expression pattern and prognostic significance of CDKs in BC
The bioinformatics analysis of CDK expression in BC patients had revealed that the CDKs are highly deregulated in BC patients [66,67,68] (Table 2). Additionally, the expression pattern of various CDKs in BC was analyzed using UCSC XENA online database [69]. We utilized the TCGA dataset of breast cancer and grouped patients into normal, solid tissue, and primary tumors. The expression pattern was analyzed, and a heat map of the given CDKs was generated. It was concluded that CDK1, CDK2, CDK4, CDK7, and CDK8 show high overexpression in primary tumors as compared to the CDK6 and CDK9 which shows low expression in primary tumors (Fig. 3). Also, various other studies have revealed the dysregulation of many CDKs that contribute to the progression of BC [21].
As discussed above, several elegant studies have established the role of CDKs in BC progression, stemness, and metastasis [70]. Additionally, the expression pattern of CDKs in BC is related to the prognosis of BC patients. We analyzed the prognostic significance of highly deregulated CDKs in BRCA patients using a Kaplan–Meier plotter. The Kaplan–Meier plotter (https://kmplot.com/) is a web resource with gene expression data and information regarding the survival of BC patients [71]. Breast cancer patients were divided into two cohorts based on the median expression of CDKs, defined as high and low expression groups. The results revealed that BC patients with low mRNA levels of CDK1 and CDK4, exhibited better Overall Survival (OS) (Fig. 4). The other deregulated CDKs did not show significant association with OS. These results demonstrate that targeting of CDK1 and CDK4 in BC can potentially bear fruitful results and also their modulation in combination with conventional therapies may be a promising approach in effective management of breast cancer patients.
Expression pattern of cyclin-dependent kinases is associated with overall survival (OS). A BC patients with low mRNA levels of CDK1 show better Overall Survival (OS) with HR = 1.44 and log-rank p-value of 0.00016. B Breast cancer patients with low mRNA levels of CDK2 does not show significant association with Overall Survival (OS) (HR = 1.05 and log-rank p-value of 0.6). C Breast cancer patients with low mRNA levels of CDK4 show better Overall Survival (OS) (HR = 1.27 and log-rank p-value of 0.014). D Breast cancer patients with low mRNA levels of CDK6 does not show significant association with Overall Survival (OS) (HR = 1.01 and log-rank p-value of 0.92). E Breast cancer patients with low mRNA levels of CDK7 does not show significant association with Overall Survival (OS) (HR = 0.93 and log-rank p-value of 0.42). F Breast cancer patients with low mRNA levels of CDK8 does not show significant association with Overall Survival (OS) (HR = 0.91 and log-rank p-value of 0.49). G Breast cancer patients with low mRNA levels of CDK9 does not show significant association with Overall Survival (OS) (HR = 1.12 and log-rank p-value of 0.22)
CDK inhibition in BC: therapeutic implications
CDKs and their cyclins are promising targets for treating BC due to their function of supporting tumor cell proliferation. There has been significant progress in BC treatment because of the innovations and efficient medications during the last decade. In terms of the above CDKs, various studies have analyzed the role of certain drugs used as inhibitors against specific CDK. CDK inhibitors have been categorized into non-selective pan-inhibitors and selective inhibitors based upon their specificity toward a particular CDK [42]. These inhibitors have been analyzed during various clinical studies of BC. CDK inhibitors target the malignant cells by interfering with their cell cycle regulators (Fig. 5), thus throwing light upon their therapeutic aspect where the progression of tumor cells could be retarded with an endurable impact on the normal cells [21].
CDK inhibitors
1st generation CDK inhibitors
Generally, first-generation inhibitors belong to Pan-CDK inhibitors, with most of them non-specific toward CDKs. One of the classic pan-CDK inhibitors highly studied among 1st generation CDK inhibitors is Flavopiridol. Phase I and phase II studies have demonstrated that Flavopiridol exhibits the most negligible single-agent potency and displays specific toxicities like neutropenia [72]. For instance, Flavopiridol in MBC exhibit significantly greater rates of neutropenia [73]. Other Pan-CDK inhibitors are UCN 01 and R-Rescovitine (Cyclacel, seliciclib) (Table 3). UCN-01 is involved in arresting cells at the G1/S phase, activation of P21, and pRb hypo phosphorylation. Besides this, UCN 01 exhibits several toxicities like arrhythmia, hyperglycemia, and pulmonary dysfunction in phase 1 clinical studies [74, 75].
2nd generation CDK inhibitors
The problem with the 1st generation of CDK inhibitors lies with their potency, safety, and endurability. They are not specific against their target CDKs, thus resulting in various side effects. In terms of clinical potency, safety, and tolerance, CDK inhibitors have mainly shown disappointing results. One of the significant drawbacks of 1st generation inhibitors is that they lack particularity toward the target kinases, which are responsible for their unpredictable and severe side effect profiles. Also, 2nd generation CDK inhibitors were discovered in the late 1990s and early 2000s that exhibited specific inhibition toward a particular CDK [70].
CDK4/6 specific inhibitors
There are many inhibitors of CDK4/6 that have been studied in clinical trials. One such inhibitor is Palbociclib. Palbociclib was approved by US FDA on 3 February 2015, being used as 1st line treatment in advanced post-menopausal ER+ and HER2− BC. This CDK4/6 inhibitor is taken orally, possesses IC50 of 0.01 μM in vitro conditions, and exhibits high specificity when examining other 36 kinases, including CDK2 (IC50 > 5 μM) (Table 4) [76]. Studies revealed that Palbociclib acts specifically to target CDK4/6 only. It shows a well-defined inhibition of BC cell proliferation, associated with complete G1 arrest and pRb protein dephosphorylation and retards the E2F-dependent gene expression [77]. Phase 1st clinical trials of Palbociclib displayed excellent bioavailability, usually mild to average unfavorable event profile, and significant dose-limiting toxicities associated mainly with myelosuppression [78]. One study demonstrated the inhibitory effect on the growth of various BC cell lines with different molecular characteristics. It was observed that Lum, HER2+, or ER+ featured BC cell lines showed greater sensitivity than the non-luminal ones that showed more excellent resistance toward Palbociclib [79]. This study also revealed the synergistic effect of Palbociclib in combination with tamoxifen or trastuzumab in ER+ and HER2+ cell lines, respectively. Also, Palbociclib exhibited activity in those cell lines resistant to tamoxifen, thus revealing that it could be clinically effective in ER+ BC, which is hormone-resistant. Besides Palbociclib, other CDK4/6 inhibitors present in early clinical trials include Abemaciclib and Ribiciclib, both of which have entered phase III clinical trials based on the palbociclib experience [42].
CDK1/2 specific inhibitors
CDK1/2 inhibitors which mainly target these CDKs include Roscovitine, Ro-3306, and Dinaciclib [80]. It should be noted that none of the three has been approved by FDA. Roscovitine is an inhibitor of various CDKs like CDK1, 2, 5, 7, and 9 and inhibits CDKs by competing directly at ATP binding sites [81]. The inhibitory effect of Roscovitine has been studied on a diverse range of cancer cell lines, and it was evaluated that this particular inhibitor inhibits the growth of all tested cancer cell lines by arresting their cell cycle with an average IC50 of approximately 15 µM [82]. Based upon the time, dose, or cell line, Roscovitine arrests the cells in G0, G1, S, or G2/M phase by inhibiting CDKs directly [82]. Roscovitine has entered phase I clinical trial along with sapacitabine in BC, Pancreatic cancer, and ovarian cancer patients (NCT00999401). The adverse impacts of Roscovitine include increased levels of transaminase and bilirubin, abdominal pain, hyperglycemia, and neutropenia [80]. Another CDK1/2 inhibitor is Ro-3306 which inhibits the transition from G2 to M and leads to the apoptosis of tumor cells with prolonged exposure [83, 84]. One of the CDK inhibitors that shows dual nature in terms of inhibition is Dinaciclib (SCH727965). The studies have revealed that Dinaciclib inhibits HR repair and sensitizes multiple myeloma cells toward the veliparib, a PARP inhibitor [80]. Clinical trials involving only dinaciclib have displayed adverse impacts like diarrhea, nausea, hypotension, fatigue, and vomiting [80].
Novel CDK inhibitors
The present investigation of new CDK inhibitors against the CDKs and better understanding of different subgroups of BC and their adverse impacts have led to more interest in discovering some novel CDK inhibitors. In this regard, various researchers have analyzed many inhibitors against CDKs. Jeong and co-workers revealed the inhibitory role of piperlongumine (PL) against the cell proliferation and migration of ER+ BC cells and thereby retards the tumor progression [85]. SR-4835, analyzed by Quereda and co-workers, can inhibit specifically CDK13 and CDK12 and retards the cell proliferation in TNBC [86]. Pandurate A (PA), possessing many properties, including anti-cancer activity, arrests the cell cycle during the G0/G1 phase by explicitly inhibiting the dose-dependent CDK4/cyclin D1 complex [87]. Vanicoside B, besides possessing many inhibitory roles, inhibits selectively CDK8 and arrests the cell cycle in HCC38 and MDA-MB-231cells [88]. The Fomes fomentarius ethanol extract (FFE) specifically inhibits CDK2, SKP2, and cyclin A/E, resulting in apoptosis and retardation in MDA-MB-231 cell migration [89]. Recent studies have demonstrated that MTH-3 (a water-soluble bis(hydroxymethyl) alkanoate curcuminoid derivative) arrests the G2/M phase in MDA-MB-231 cells by inhibiting the CDK1 expression [90]. Furthermore, (5, 7, 8-trihydroxyflavone (NOR-wogonin) can specifically inhibit the growth of TNBC cell lines such as MDA-MB-231 in comparison to the non-tumorigenic BC lines like MCF-10A by inhibiting CDK1 expression [91]. In addition to this, a plant compound, namely Galangi, possesses anti-cancer properties and retards the progression of MCF-7 cells, and leads to apoptosis by inhibiting CDK4,2 and 1, resulting in cell cycle arrest [92]. Resveratrol is another inhibitor that can lead to cell cycle arrest by inhibiting specific CDKs like CDK2,4 and 6 [93]. One more inhibitor, namely Icariin, has been shown to retard the expression of CDK2 and CDK4 in those MCF7/TAM cell lines which are resistant to tamoxifen-resistant [94]. AZD1775, Tyrosine kinase WEE1 inhibitor, combined with AZD6738, results in cell death [95]. One specific CDK1 inhibitor, namely, methyl2-(-5-fluoro-2-hydroxyphenyl)-1H-benzo imidazole-5-carboxylate (MBIC) is associated with p53 expression and can result in cell death [96].
Summing up the contention, BC is a challenging disease that can be treated with the CDK mentioned above inhibitors in combination with other drugs resulting in a synergistic effect in terms of treatment and good results. For instance, the studies have revealed that the synergistic effect of palbociclib/paclitaxel in inhibiting cell growth and enhancing cell death is much better than the monotherapy treatments [97]. Thus, the combined effect of both CDK inhibitors and other drugs will be more effective in cell cycle arrest, cell death, and slowing down cell proliferation.
CDK inhibition and TGF-β signaling in breast cancer
TGF-β signaling is one of the central pathways involved in Breast Cancer pathogenesis. This signaling pathway plays a dual role in Breast Cancer, as it assists in tumor progression and tumor suppression [98]. The well-known fact about the TGF-β signaling pathway is that it switches its function according to the stages of BC; it acts as a tumor promoter in the late stages of BC and tumor suppressor in the early stages of BC [98]. All the essential metabolic pathways, i.e. cell proliferation, growth, and metastasis, are influenced by the TGF-β signaling pathway in BC [99]. An overexpression of TGF-β receptors usually characterizes breast tumors. These receptors need to get suppressed for treating BC patients [100]. TGF-β signaling pathway maintains its tumor-suppressive state in healthy cells by promoting several regulatory processes, such as cell cycle regulation and apoptosis [101]. Canonical TGF-β signaling involves the transcription factor SMAD3, phosphorylated to promote tumor suppression. This tumor-suppressive state can be inhibited during BC progression (Fig. 6). The overexpression of CDK2/4 in BC inhibits the tumor-suppressive nature of SMAD3, thus leading to tumor progression. This throws light on CDK-mediated SMAD 3 phosphorylation leading to BC progression. The CDKi, such as CDK2/4 inhibitors, play a decisive role in reversing this CDK-mediated protumorigenic function of TGF-β signaling, thus serving as a brake in Breast tumor progression [98].
Role of CDK inhibition and TGF-β signaling pathway in breast tumor microenvironment
Breast Tumor Environment is associated with a wide range of cells, including Cancer-Associated Fibroblasts that potentially lead to the EMT in BC cells. This EMT transition is generally regulated by the TGF-β signaling pathway [102]. TGF-β signaling also activates several immune cells that switch from anti-tumorigenic phenotype to pro-tumorigenic phenotype, thus leading to an increase in BC aggressiveness [103]. The inhibition of CDKs influences the tumor immune microenvironment so that the pro-tumor environment reverses into an antitumor environment [98]. The studies have revealed that CDK inhibition can enhance the anti-cancerous immune response and effectively overcome the immunosuppressive functions of the TGF-β signaling pathway [98]. Several studies have observed that blockade of TGF-β signaling through CDK inhibition can augment the potency of checkpoint blockade therapy, thus showing the synergistic effect of CDK inhibitor therapy and immune checkpoint blockade therapy [98]. For example, the antitumor effect of CDK4/6 inhibitors has potentially affected the efficacy of anti-PD1 immunotherapy synergistically. Thus CDKi shows its significance in cancer immunotherapy by inducing the immunogenic effects that ultimately result in immune surveillance (Fig. 7) [104].
The interaction between TGFβ signaling and CDK inhibition leads to the change in Breast Tumor microenvironment. The inhibition of CDKs influences the tumor immune microenvironment in such a way that the protumor environment reverses into antitumor environment. CDK inhibition can enhance the anti-cancerous immune response and effectively overcome the immunosuppressive functions of TGF-β signaling pathway
Conclusion and future prospects
Breast cancer is one of the most threatened types of cancer and is associated with the dysregulation of many CDKs. The dysregulation of many CDKs and their cyclins leads to an increase in the progression of BC. These dysregulated CDKs can be targeted by specific CDK inhibitors, which will inhibit a particular CDK and thus will help in retarding the progression of the disease. In this aspect, various CDK inhibitors have been discovered, some of them have been approved by FDA, and some are still in clinical trials. CDK inhibitors have also been used in the combinatorial approach to have much more potency and efficacy than monotherapy approaches. CDK4/6 have been explored very much, and their combination with other drugs can become promising anticancer agents. With this understanding of CDKs, advances in CDK inhibition therapy have been developed. Ribociclib, Palbociclib, and abemaciclib are three FDA-approved CDK 4/6 inhibitors that have been evaluated as monotherapies and in combination with an adjuvant endocrine therapy like letrozole and fulvestrant. The positive results promote using these drugs to treat ER+/HER2− metastatic breast cancer in the future. CDK4/6 inhibition has been well-tolerated and effective in a clinical environment, with enhanced overall survival relative to placebo groups and higher PFS and CBR. While the results of using these treatments on ER+ BC patients indicate clear advantages over more harmful treatments such as chemotherapy and radiation, there are still challenges, such as endocrine therapy resistance. Neutropenia and weariness, typical side effects in patients taking these medications, can often be addressed symptomatically and without further complications. Developing a unique combinatorial approach, including CDK inhibitors, can significantly change BC treatment strategy. The novel CDK inhibitors have made an immense contribution to this field. However, the complaints of resistance and higher costs need to be addressed. Many novel approaches need to be evolved that may inhibit various targets in the common signal pathways, thereby highlighting the therapeutic significance of BC. For instance, MRX34(MiR-34 mimic) inhibits transcripts of numerous cell cycle genes and has come into phase I clinical trial. Further studies are required to target CDKs uniquely, considering all the unfavorable events and using them with other therapeutic drugs to treat BC best.
Abbreviations
- BC:
-
Breast cancer
- Lum A:
-
Luminal A
- HER2+ :
-
Human epidermal growth factor-2
- Lum B:
-
Luminal B
- CDK:
-
Cyclin-dependent kinase
- OS:
-
Overall survival
- EMT:
-
Epithelial-mesenchymal transition
References
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA A Cancer J Clin. 2021;71(1):7–33.
Mir MA, Qayoom H, Mehraj U, Nisar S, Bhat B, Wani NA. Targeting different pathways using novel combination therapy in triple negative breast Cancer. Curr Cancer Drug Targets. 2020;20(8):586–602.
Mehraj U, Aisha S, Sofi S, Mir MA. Expression pattern and prognostic significance of baculoviral inhibitor of apoptosis repeat-containing 5 (BIRC5) in breast cancer: a comprehensive analysis. Adv Cancer Biol-Metastasis. 2022. https://doi.org/10.1016/j.adcanc.2022.100037.
Mir M. Combination therapies and their effectiveness in breast cancer treatment. New York: Nova Science Publishers; 2021. https://doi.org/10.52305/WXJL6770.
Jan S, Qayoom H, Mehraj U, Mir M. Therapeutic options for breast cancer. In: Mir MA, editor. Combination therapies and their effectiveness in breast cancer treatment. New York: Nova Science Publishers; 2021. https://doi.org/10.52305/TILJ1241.
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49.
Harbeck N. Breast cancer is a systemic disease optimally treated by a multidisciplinary team. Nat Rev Dis Primers. 2020;6(1):1–2.
Mir M, Jan S, Mehraj U. Triple-negative breast cancer—an aggressive subtype of breast cancer. In: Combinational therapy in triple negative breast cancer. New York: Elsevier; 2022. p. 1–35.
Feng Y, Spezia M, Huang S, Yuan C, Zeng Z, Zhang L, Ji X, Liu W, Huang B, Luo W. Breast cancer development and progression: risk factors, cancer stem cells, signaling pathways, genomics, and molecular pathogenesis. Genes Dis. 2018;5(2):77–106.
Duffy MJ, Walsh S, McDermott EW, Crown J. Biomarkers in breast cancer: where are we and where are we going? Adv Clin Chem. 2015;71:1–23.
Jan S, Mir M. Therapeutic landscape of metaplastic breast cancer. In: Mir MA, editor. Combination therapies and their effectiveness in breast cancer treatment. New York: Nova Science Publishers; 2021.
Mehraj U, Dar AH, Wani NA, Mir MA. Tumor microenvironment promotes breast cancer chemoresistance. Cancer Chemother Pharmacol. 2021. https://doi.org/10.1007/s00280-020-04222-w.
Mir M, Jan S, Mehraj U. Novel biomarkers in triple-negative breast cancer-role and perspective (Chapter-2). New York: Elsevier; 2022. p. 36–72.
Mir M, Sofi S, Qayoom H. The interplay of immunotherapy, chemotherapy, and targeted therapy in tripple negative breast cancer (TNBC) Chapter-6. New York: Elsevier; 2022. p. 201–44.
Mir M, Sofi S, Qayoom H. Different drug delivery approaches in combinational therapy in TNBC (Chapter-8). New York: Elsevier; 2022. p. 278–311.
Nounou MI, ElAmrawy F, Ahmed N, Abdelraouf K, Goda S, Syed-Sha-Qhattal H. Breast cancer: conventional diagnosis and treatment modalities and recent patents and technologies. Breast Cancer. 2015. https://doi.org/10.4137/BCBCR.S29420.
Mehraj U, Ganai RA, Macha MA, Hamid A, Zargar MA, Bhat AA, Nasser MW, Haris M, Batra SK, Alshehri B. The tumor microenvironment as driver of stemness and therapeutic resistance in breast cancer: New challenges and therapeutic opportunities. Cell Oncol. 2021. https://doi.org/10.1007/s13402-021-00634-.
Mir M, Sofi S, Qayoom H. Targeting biologically specific molecules in triple negative breast canceR (TNBC) Chapter-7. New York: Elsevier; 2022. p. 245–77.
Qayoom H, Bhat BA, Mehraj U, Mir MA. Rising trends of cancers in kashmir valley: distribution pattern, incidence and causes. J Oncol Res Treat. 2020;5(150):2.
Mir MA, Mehraj U. Double-crosser of the immune system: macrophages in tumor progression and metastasis. Curr Immunol Rev. 2019;15(2):172–84.
Lu Y. The role of cyclin-dependent kinases on the metastasis of breast cancer. Novel Approach Cancer Study. 2020. https://doi.org/10.31031/NACS.2020.04.000594.
Ding L, Cao J, Lin W, Chen H, Xiong X, Ao H, Yu M, Lin J, Cui Q. The roles of cyclin-dependent kinases in cell-cycle progression and therapeutic strategies in human breast cancer. Int J Mol Sci. 2020;21(6):1960.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74.
Koboldt D, Fulton R, McLellan M, Schmidt H, Kalicki-Veizer J, McMichael J, Fulton L, Dooling D, Ding L, Mardis E. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490(7418):61–70.
Nicolini A, Ferrari P, Duffy MJ. Prognostic and predictive biomarkers in breast cancer: Past, present and future. Semin Cancer Biol. 2018;52:56–73.
Thu KL, Soria-Bretones I, Mak TW, Cescon DW. Targeting the cell cycle in breast cancer: towards the next phase. Cell Cycle. 2018;17(15):1871–85.
Martínez-Alonso D, Malumbres M. Mammalian cell cycle cyclins. Semin Cell Dev Biol. 2020;107:28–35.
Swaffer MP, Jones AW, Flynn HR, Snijders AP, Nurse P. CDK substrate phosphorylation and ordering the cell cycle. Cell. 2016;167(7):1750–61.
Barnum KJ, O’Connell MJ. Cell cycle regulation by checkpoints. In: Cell cycle control. New York: Springer; 2014. p. 29–40.
Asghar U, Witkiewicz AK, Turner NC, Knudsen ES. The history and future of targeting cyclin-dependent kinases in cancer therapy. Nat Rev Drug Discovery. 2015;14(2):130–46.
Sivakumar S, Gorbsky GJ. Spatiotemporal regulation of the anaphase-promoting complex in mitosis. Nat Rev Mol Cell Biol. 2015;16(2):82–94.
Zhou Z, He M, Shah AA, Wan Y. Insights into APC/C: from cellular function to diseases and therapeutics. Cell Div. 2016;11(1):1–18.
Senft D, Qi J, Ze’ev AR. Ubiquitin ligases in oncogenic transformation and cancer therapy. Nat Rev Cancer. 2018;18(2):69–88.
Solaki M, Ewald JC. Fueling the cycle: CDKs in carbon and energy metabolism. Frontiers in cell and developmental biology. 2018;6:93.
Lim S, Kaldis P. Cdks, cyclins and CKIs: roles beyond cell cycle regulation. Development. 2013;140(15):3079–93.
Lee Y, Lahens NF, Zhang S, Bedont J, Field JM, Sehgal A. G1/S cell cycle regulators mediate effects of circadian dysregulation on tumor growth and provide targets for timed anticancer treatment. PLoS Biol. 2019;17(4): e3000228.
Hafeez S, Urooj M, Saleem S, Gillani Z, Shaheen S, Qazi MH, Naseer MI, Iqbal Z, Ansari SA, Haque A. BAD, a Proapoptotic protein, Escapes ERK/RSK phosphorylation in Deguelin and siRNA-treated Hela cells. PLoS ONE. 2016;11(1): e0145780.
Wenzel ES, Singh ATK. Cell-cycle checkpoints and aneuploidy on the path to cancer. In Vivo. 2018;32(1):1–5.
Yue X, Zhao Y, Xu Y, Zheng M, Feng Z, Hu W. Mutant p53 in cancer: accumulation, gain-of-function, and therapy. J Mol Biol. 2017;429(11):1595–606.
Mir MA, Hamdani SS, Sheikh BA, Mehraj U. Recent advances in metabolites from medicinal plants in cancer prevention and treatment. Curr Immunol Rev. 2019;15(2):185–201.
Bashour SI, Doostan I, Keyomarsi K, Valero V, Ueno NT, Brown PH, Litton JK, Koenig KB, Karuturi M, Abouharb S. Rapid breast cancer disease progression following cyclin dependent kinase 4 and 6 inhibitor discontinuation. J Cancer. 2017;8(11):2004–9.
Finn RS, Aleshin A, Slamon DJ. Targeting the cyclin-dependent kinases (CDK) 4/6 in estrogen receptor-positive breast cancers. Breast Cancer Res. 2016;18(1):1–11.
Santo L, Siu KT, Raje N. Raje N Targeting cyclin-dependent kinases and cell cycle progression in human cancers. Semin Oncol. 2015;42:788–800.
Zardavas D, Pondé N, Tryfonidis K. CDK4/6 blockade in breast cancer: current experience and future perspectives. Expert Opin Investig Drugs. 2017;26(12):1357–72.
Tadesse S, Anshabo AT, Portman N, Lim E, Tilley W, Caldon CE, Wang S. Targeting CDK2 in cancer: challenges and opportunities for therapy. Drug Discovery Today. 2020;25(2):406–13.
He X, Xiang H, Zong X, Yan X, Yu Y, Liu G, Zou D, Yang H. CDK2-AP1 inhibits growth of breast cancer cells by regulating cell cycle and increasing docetaxel sensitivity in vivo and in vitro. Cancer Cell Int. 2014;14(1):1–10.
Santamaría D, Barrière C, Cerqueira A, Hunt S, Tardy C, Newton K, Cáceres JF, Dubus P, Malumbres M, Barbacid M. Cdk1 is sufficient to drive the mammalian cell cycle. Nature. 2007;448(7155):811–5.
Malumbres M, Barbacid M. Cell cycle, CDKs and cancer: a changing paradigm. Nat Rev Cancer. 2009;9(3):153–66.
Barascu A, Besson P, Le Floch O, Bougnoux P, Jourdan M-L. CDK1-cyclin B1 mediates the inhibition of proliferation induced by omega-3 fatty acids in MDA-MB-231 breast cancer cells. Int J Biochem Cell Biol. 2006;38(2):196–208.
Izadi S, Nikkhoo A, Hojjat-Farsangi M, Namdar A, Azizi G, Mohammadi H, Yousefi M, Jadidi-Niaragh F. CDK1 in breast cancer: implications for theranostic potential. Anti-Cancer Agents Med Chem (Formerly Current Medicinal Chemistry-Anti-Cancer Agents). 2020;20(7):758–67.
Patel H, Abduljabbar R, Lai C-F, Periyasamy M, Harrod A, Gemma C, Steel JH, Patel N, Busonero C, Jerjees D. Expression of CDK7, cyclin H, and MAT1 is elevated in breast cancer and is prognostic in estrogen receptor-positive breast cancer. Clin Cancer Res. 2016;22(23):5929–38.
Wang Y, Zhang T, Kwiatkowski N, Abraham BJ, Lee TI, Xie S, Yuzugullu H, Von T, Li H, Lin Z. CDK7-dependent transcriptional addiction in triple-negative breast cancer. Cell. 2015;163(1):174–86.
Li B, Chonghaile TN, Fan Y, Madden SF, Klinger R, O’Connor AE, Walsh L, O’Hurley G, Udupi GM, Joseph J. Therapeutic rationale to target highly expressed CDK7 conferring poor outcomes in triple-negative breast cancer. Can Res. 2017;77(14):3834–45.
Knab VM, Gotthardt D, Klein K, Grausenburger R, Heller G, Menzl I, Prinz D, Trifinopoulos J, List J, Fux D. Triple-negative breast cancer cells rely on kinase-independent functions of CDK8 to evade NK-cell-mediated tumor surveillance. Cell Death Dis. 2021;12(11):1–12.
Crown J. CDK8: a new breast cancer target. Oncotarget. 2017;8(9):14269–70. https://doi.org/10.18632/oncotarget.15354.
Schlafstein AJ, Withers AE, Rudra S, Danelia D, Switchenko JM, Mister D, Harari S, Zhang H, Daddacha W, Ehdaivand S. CDK9 expression shows role as a potential prognostic biomarker in breast cancer patients who fail to achieve pathologic complete response after neoadjuvant chemotherapy. Int J Breast Cancer. 2018. https://doi.org/10.1155/2018/6945129.
Del Re M, Bertolini I, Crucitta S, Fontanelli L, Rofi E, De Angelis C, Diodati L, Cavallero D, Gianfilippo G, Salvadori B. Overexpression of TK1 and CDK9 in plasma-derived exosomes is associated with clinical resistance to CDK4/6 inhibitors in metastatic breast cancer patients. Breast Cancer Res Treat. 2019;178(1):57–62.
Mehraj U, Qayoom H, Mir MA. Prognostic significance and targeting tumor-associated macrophages in cancer: new insights and future perspectives. Breast Cancer. 2021. https://doi.org/10.1007/s12282-021-01231-2.
Yazici H, Akin B. Molecular genetics of metastatic breast cancer. In: Lasfar A, Cohen-Solal K, editors. Tumor progression and metastasis. London: IntechOpen; 2019.
Qureshi MFH, Shah M, Lakhani M, Abubaker ZJ, Mohammad D, Farhan H, Zia I, Tafveez R, Khan ST, Rubina G. Gene signatures of cyclin-dependent kinases: a comparative study in naïve early and advanced stages of lung metastasis breast cancer among pre-and post-menopausal women. Genes Cancer. 2021;12:1.
Redig AJ, McAllister SS. Breast cancer as a systemic disease: a view of metastasis. J Intern Med. 2013;274(2):113–26.
Padhye A, Konen J, Rodriguez BL, Fradette J, Ochieng J, Diao L, Wang J, Lu W, Solis L, Batra H. Targeting CDK4 overcomes EMT-mediated tumor heterogeneity and therapeutic resistance in KRAS mutant lung cancer. JCI Insight. 2021. https://doi.org/10.1172/jci.insight.148392.
Krajewski A, Gagat M, Mikołajczyk K, Izdebska M, Żuryń A, Grzanka A. Cyclin F downregulation affects epithelial-mesenchymal transition increasing proliferation and migration of the A-375 melanoma cell line. Cancer Manage Res. 2020;12:13085.
Zhang Z, Li J, Ou Y, Yang G, Deng K, Wang Q, Wang Z, Wang W, Zhang Q, Wang H. CDK4/6 inhibition blocks cancer metastasis through a USP51-ZEB1-dependent deubiquitination mechanism. Signal Transduct Target Ther. 2020;5(1):1–13.
Liang Q, Li L, Zhang J, Lei Y, Wang L, Liu D-X, Feng J, Hou P, Yao R, Zhang Y. CDK5 is essential for TGF-β1-induced epithelial-mesenchymal transition and breast cancer progression. Sci Rep. 2013;3(1):1–13.
Li N, Zheng S, Xue Z, Xiong Z, Zou Y, Tang Y, Wei W-D, Yang L. Expression and prognostic value of transcription-associated cyclin-dependent kinases in human breast cancer. Aging (Albany, NY). 2021;13(6):8095.
Boström P, Söderström M, Palokangas T, Vahlberg T, Collan Y, Carpen O, Hirsimäki P. Analysis of cyclins A, B1, D1 and E in breast cancer in relation to tumour grade and other prognostic factors. BMC Res Notes. 2009;2(1):1–8.
Murad H, Hawat M, Ekhtiar A, AlJapawe A, Abbas A, Darwish H, Sbenati O, Ghannam A. Induction of G1-phase cell cycle arrest and apoptosis pathway in MDA-MB-231 human breast cancer cells by sulfated polysaccharide extracted from Laurencia papillosa. Cancer Cell Int. 2016;16(1):1–11.
Goldman MJ, Craft B, Hastie M, Repečka K, McDade F, Kamath A, Banerjee A, Luo Y, Rogers D, Brooks AN, Zhu J, Haussler D. Visualizing and interpreting cancer genomics data via the Xena platform. Nat Biotechnol. 2020;38(6):675–8. https://doi.org/10.1038/s41587-020-0546-8.
Zhang M, Zhang L, Hei R, Li X, Cai H, Wu X, Zheng Q, Cai C. CDK inhibitors in cancer therapy, an overview of recent development. Am J Cancer Res. 2021;11(5):1913.
Györffy B, Lanczky A, Eklund AC, Denkert C, Budczies J, Li Q, Szallasi Z. An online survival analysis tool to rapidly assess the effect of 22,277 genes on breast cancer prognosis using microarray data of 1,809 patients. Breast Cancer Res Treat. 2010;123(3):725–31.
Jessen BA, Lee L, Koudriakova T, Haines M, Lundgren K, Price S, Nonomiya J, Lewis C, Stevens GJ. Peripheral white blood cell toxicity induced by broad spectrum cyclin-dependent kinase inhibitors. J Appl Toxicol. 2007;27(2):133–42.
Fornier MN, Rathkopf D, Shah M, Patil S, O’Reilly E, Tse AN, Hudis C, Lefkowitz R, Kelsen DP, Schwartz GK. Phase I dose-finding study of weekly docetaxel followed by Flavopiridol for patients with advanced solid tumors. Clin Cancer Res. 2007;13(19):5841–6.
Sausville EA, Arbuck SG, Messmann R, Headlee D, Bauer KS, Lush RM, Murgo A, Figg WD, Lahusen T, Jaken S. Phase I trial of 72-hour continuous infusion UCN-01 in patients with refractory neoplasms. J Clin Oncol. 2001;19(8):2319–33.
Kortmansky J, Shah MA, Kaubisch A, Weyerbacher A, Yi S, Tong W, Sowers R, Gonen M, O’Reilly E, Kemeny N. Phase I trial of the cyclin-dependent kinase inhibitor and protein kinase C inhibitor 7-hydroxystaurosporine in combination with Fluorouracil in patients with advanced solid tumors. J Clin Oncol. 2005;23(9):1875–84.
Toogood PL, Harvey PJ, Repine JT, Sheehan DJ, VanderWel SN, Zhou H, Keller PR, McNamara DJ, Sherry D, Zhu T. Discovery of a potent and selective inhibitor of cyclin-dependent kinase 4/6. J Med Chem. 2005;48(7):2388–406.
Fry DW, Harvey PJ, Keller PR, Elliott WL, Meade M, Trachet E, Albassam M, Zheng X, Leopold WR, Pryer NK. Specific inhibition of cyclin-dependent kinase 4/6 by PD 0332991 and associated antitumor activity in human tumor xenografts. Mol Cancer Ther. 2004;3(11):1427–38.
Schwartz GK, LoRusso PM, Dickson MA, Randolph SS, Shaik MN, Wilner KD, Courtney R, O’Dwyer PJ. Phase I study of PD 0332991, a cyclin-dependent kinase inhibitor, administered in 3-week cycles (Schedule 2/1). Br J Cancer. 2011;104(12):1862–8.
Finn RS, Crown JP, Boer K, Lang I, Parikh RJ, Breazna A, Ho SN, Kim ST, Randolph S, Slamon DJ. 100O results of a randomized phase 2 study of Pd 0332991, a cyclin-dependent kinase (Cdk) 4/6 inhibitor, in combination with letrozole vs letrozole alone for first-line treatment of ER+/Her2-advanced breast cancer (BC). Ann Oncol. 2012;23:ii43.
Lin ZP, Zhu Y-L, Ratner ES. Targeting cyclin-dependent kinases for treatment of gynecologic cancers. Front Oncol. 2018;8:303.
Vassilev LT, Tovar C, Chen S, Knezevic D, Zhao X, Sun H, Heimbrook DC, Chen L. Selective small-molecule inhibitor reveals critical mitotic functions of human CDK1. Proc Natl Acad Sci. 2006;103(28):10660–5.
Cicenas J, Kalyan K, Sorokinas A, Stankunas E, Levy J, Meskinyte I, Stankevicius V, Kaupinis A, Valius M. Roscovitine in cancer and other diseases. Ann Transl Med. 2015;3(10):135.
Johnson N, Li Y-C, Walton ZE, Cheng KA, Li D, Rodig SJ, Moreau LA, Unitt C, Bronson RT, Thomas HD. Compromised CDK1 activity sensitizes BRCA-proficient cancers to PARP inhibition. Nat Med. 2011;17(7):875–82.
Xia Q, Cai Y, Peng R, Wu G, Shi Y, Jiang W. The CDK1 inhibitor RO3306 improves the response of BRCA-proficient breast cancer cells to PARP inhibition. Int J Oncol. 2014;44(3):735–44.
Jeong CH, Ryu H, Kim DH, Cheng WN, Yoon JE, Kang S, Han SG. Piperlongumine induces cell cycle arrest via reactive oxygen species accumulation and IKKβ suppression in human breast cancer cells. Antioxidants. 2019;8(11):553.
Quereda V, Bayle S, Vena F, Frydman SM, Monastyrskyi A, Roush WR, Duckett DR. Therapeutic targeting of CDK12/CDK13 in triple-negative breast cancer. Cancer Cell. 2019;36(5):545–58.
Liu Q, Cao Y, Zhou P, Gui S, Wu X, Xia Y, Tu J. Panduratin A inhibits cell proliferation by inducing G0/G1 phase cell cycle arrest and induces apoptosis in breast cancer cells. Biomolecules & therapeutics. 2018;26(3):328.
Kim D, Wang CY, Hu R, Lee JY, Luu T-T-T, Park H-J, Lee SK. Antitumor activity of vanicoside B isolated from Persicaria dissitiflora by targeting CDK8 in triple-negative breast cancer cells. J Nat Prod. 2019;82(11):3140–9.
Lee S-OK, Lee M-H, Lee K-R, Lee E-O, Lee H-J. Fomes fomentarius ethanol extract exerts inhibition of cell growth and motility induction of apoptosis via targeting AKT in human breast cancer MDA-MB-231 cells. Int J Mol Sci. 2019;20(5):1147.
Chang L-C, Hsieh M-T, Yang J-S, Lu C-C, Tsai F-J, Tsao J-W, Chiu Y-J, Kuo S-C, Lee K-H. Effect of bis (hydroxymethyl) alkanoate curcuminoid derivative MTH-3 on cell cycle arrest, apoptotic and autophagic pathway in triple-negative breast adenocarcinoma MDA-MB-231 cells: An in vitro study. Int J Oncol. 2018;52(1):67–76.
Abd El-Hafeez AA, Khalifa HO, Mahdy EAM, Sharma V, Hosoi T, Ghosh P, Ozawa K, Montano MM, Fujimura T, Ibrahim ARN. Anticancer effect of nor-wogonin (5, 7, 8-trihydroxyflavone) on human triple-negative breast cancer cells via downregulation of TAK1, NF-κB, and STAT3. Pharmacol Rep. 2019;71(2):289–98.
Liu D, You P, Luo Y, Yang M, Liu Y. Galangin induces apoptosis in MCF-7 human breast cancer cells through mitochondrial pathway and phosphatidylinositol 3-kinase/Akt inhibition. Pharmacology. 2018;102(1–2):58–66.
Zhang W, Jiang H, Chen Y, Ren F. Resveratrol chemosensitizes adriamycin-resistant breast cancer cells by modulating miR-122-5p. J Cell Biochem. 2019;120(9):16283–92.
Cheng X, Tan S, Duan F, Yuan Q, Li Q, Deng G. Icariin induces apoptosis by suppressing autophagy in tamoxifen-resistant breast cancer cell line MCF-7/TAM. Breast Cancer. 2019;26(6):766–75.
Jin J, Fang H, Yang F, Ji W, Guan N, Sun Z, Shi Y, Zhou G, Guan X. Combined inhibition of ATR and WEE1 as a novel therapeutic strategy in triple-negative breast cancer. Neoplasia. 2018;20(5):478–88.
Hasanpourghadi M, Pandurangan AK, Karthikeyan C, Trivedi P, Mustafa MR. Mechanisms of the antitumor activity of Methyl 2-(-5-fluoro-2-hydroxyphenyl)-1 H-benzo [d] imidazole-5-carboxylate against breast cancer in vitro and in vivo. Oncotarget. 2017;8(17):28840.
Cretella D, Fumarola C, Bonelli M, Alfieri R, La Monica S, Digiacomo G, Cavazzoni A, Galetti M, Generali D, Petronini PG. Pre-treatment with the CDK4/6 inhibitor palbociclib improves the efficacy of paclitaxel in TNBC cells. Sci Rep. 2019;9(1):1–11.
Decker JT, Ma JA, Shea LD, Jeruss JS. Implications of TGFβ signaling and CDK inhibition for the treatment of breast cancer. Cancers. 2021;13(21):5343.
Buck MB, Knabbe C. TGF-beta signaling in breast cancer. Ann N Y Acad Sci. 2006;1089(1):119–26.
Decker JT, Kandagatla P, Wan L, Bernstein R, Ma JA, Shea LD, Jeruss JS. Cyclin E overexpression confers resistance to trastuzumab through noncanonical phosphorylation of SMAD3 in HER2+ breast cancer. Cancer Biol Ther. 2020;21(11):994–1004.
Zhao Y, Ma J, Fan Y, Wang Z, Tian R, Ji W, Zhang F, Niu R. TGF-β transactivates EGFR and facilitates breast cancer migration and invasion through canonical Smad3 and ERK/Sp1 signaling pathways. Mol Oncol. 2018;12(3):305–21.
Yu Y, Xiao CH, Tan LD, Wang QS, Li XQ, Feng YM. Cancer-associated fibroblasts induce epithelial–mesenchymal transition of breast cancer cells through paracrine TGF-β signalling. Br J Cancer. 2014;110(3):724–32.
Zhang F, Wang H, Wang X, Jiang G, Liu H, Zhang G, Wang H, Fang R, Bu X, Cai S. TGF-β induces M2-like macrophage polarization via SNAIL-mediated suppression of a pro-inflammatory phenotype. Oncotarget. 2016;7(32):52294.
Baas M, Besançon A, Goncalves T, Valette F, Yagita H, Sawitzki B, Volk H-D, Waeckel-Enée E, Rocha B, Chatenoud L. TGFβ-dependent expression of PD-1 and PD-L1 controls CD8+ T cell anergy in transplant tolerance. eLife. 2016;5:e08133.
Acknowledgements
The authors would like to thank Jammu Kashmir Science Technology and Innovation Council (JKST&IC), Department of Science and Technology, Govt of J&K.
Funding
The work was supported by Research Grant sanctioned to Manzoor Ahmad Mir by Jammu Kashmir Science Technology and Innovation Council (JKST&IC), Department of Science and Technology, Govt of J&K vide Grant NO. JKST&IC/SRE/885-87. The authors would also like to thank Almaarefa University Riyadh, Saudi Arabia, for providing support (TUMA-2021-1) to this study.
Author information
Authors and Affiliations
Contributions
MAM initiated the study and designed the plan. SS wrote the manuscript and designed the figures and tables. UM, SA, HQ, AA, SMBA & MAM revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sofi, S., Mehraj, U., Qayoom, H. et al. Cyclin-dependent kinases in breast cancer: expression pattern and therapeutic implications. Med Oncol 39, 106 (2022). https://doi.org/10.1007/s12032-022-01731-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-022-01731-x