Abstract
CDKs require modulatory subunits to bind them, known as cyclins, to exert their effects. The latter are formed and destroyed at various cell cycle stages in a specific and timely manner, thus regulating the cell cycle properly. Specific complexes of CDK-cyclins are deregulated frequently by mutations associated with tumors, and either unscheduled cell cycle re-entry or continuous proliferation is witnessed due to this deregulation. Furthermore, these two features are seen in most human cancer cells. During the synthesis or formation of DNA and segregation of chromosomes, the checkpoints monitor the proper progression through the cell cycle and sense any defects. Through the regulation of CDK activity, these activated checkpoints lead to the arrest of cell cycle. However, any dysregulation in the CDKs will hamper this normal modulation during the cell cycle, and as such, agents that inhibit these CDKs are of significant importance. The CDK4/6 inhibitors are very effective at preventing the progression of cell cycle and proliferation of tumor cell, and the frequently employed CDK4/6 inhibitors are Abemaciclib, Ribociclib, Trilaciclib, and Palbociclib. The CDK4/6 inhibitors are very effective in BC treatment, and several inhibitors are being tested at different phases of clinical trials nowadays. This chapter highlights the applications of these inhibitors, their success in breast cancer treatment along with their undesirable effects on BC patients.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
11.1 Introduction
A group of serine/threonine kinases known to modulate the cell cycle is the various cyclins and cyclin-dependent kinases (CDKs). To activate, phosphorylate, and stabilize the cyclin-dependent kinases, these lead to the formation of complexes with cyclins in particular cell cycle phases (Malumbres 2014; Nie et al. 2019). In the case of humans, several loci are known to code for cyclin-dependent kinases and cyclins (Malumbres and Barbacid 2005). Nevertheless, only a few of these encoded CDKs and cyclins are directly participating in cell cycle regulation, and the same includes:
-
1.
CDK2, CDK4, and CDK6 are the three interphases CDKs.
-
2.
CDK1, a mitotic CDK.
-
3.
And the other ten cyclins are from four distinct classes (A, B, D, and E type cyclins).
CDKs require modulatory subunits to bind them, known as cyclins, to exert their effects. The latter are formed and destroyed at various cell cycle stages in a specific and timely manner, thus regulating the cell cycle properly (Fig. 11.1).
11.2 Dysregulation of CDKs
Specific complexes of CDK-cyclins are deregulated frequently by mutations associated with tumors, and either unscheduled cell cycle re-entry or continuous proliferation is witnessed due to this deregulation. Furthermore, these two features are seen in most human tumor cells (Malumbres and Barbacid 2001). During the synthesis or formation of DNA and segregation of chromosomes, the checkpoints monitor the proper progression through the cell cycle and sense any defects. Moreover, through the regulation of CDK activity, these activated checkpoints lead to the arrest of the cell cycle (Mir et al. 2022a, b, c, d, e). The purpose of arresting the cell cycle is to provide the time for repairing the defects in the cells properly to hamper their advancement to the daughter cells that will be formed. The endogenous genotoxic agents and the exogenous products could lead to broad changes in the DNA molecule and DNA damage checkpoints help protect the cells from such attacks. Furthermore, when the alterations occur, these by a signaling pathway get sensed leading to CDK inhibition and eventually causing the arrest of cell cycle (Malumbres and Barbacid 2009). The cells may undergo programmed cell death (apoptosis) or enter senescence if the repair process is ineffective due to enormous DNA damage resulting from defects in checkpoints or impaired repair machinery due to genetic defects in the same processes cycle (Mir et al. 2022a, b, c, d, e).
On the flip side, the accumulating changes in DNA may lead to GIN (genomic instability) which leads to the transformation of these cells and thus oncogenesis (Kastan and Bartek 2004). The unscheduled proliferation, the chromosomal instability (CIN), and the genomic instability (GIN) are the primary three defects in the cell cycle. They are mediated by faulty regulation of cyclin-dependent kinesis either directly or indirectly (Mehraj et al. 2021). The chromosomal separation is controlled by SAC (the spindle assembly checkpoint) after the DNA duplication. This signaling process regulates the activity of CDK1 and hinders any defects in the segregation of chromosomes (Kops et al. 2005; Malumbres and Barbacid 2009). On similar grounds, impaired SAC (spindle assembly checkpoint) could lead to an unequal inheritance of DNA. If not repaired, it could aid in tumor progression due to the accumulation of CIN (numerical chromosomal abbreviations). The A-type cyclins are known to activate CDK1 towards the interphase end in order to assist the mitosis onset. After the nuclear envelope is degraded, the A-type cyclins are dissolved to assist in the CDK1-cyclin B complexes formation, which drives the cells through mitosis cycle (Mir et al. 2022a, b, c, d, e).
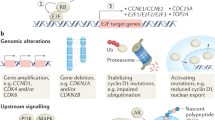
Given their significant role in the progression of cell cycle, the downregulation of CDKs would end up in defective homeostasis in specific tissues. On the other hand, by initiating the untimely division in cells (progenitor or stem cells), the hyperactivated CDKs could also aid in the development of tumors (Malumbres and Barbacid 2009). Breast cancer can result from many causes, including mutations in DNA repair genes, tumor suppressor genes (p53), and the proto-oncogenes like HER-2, c-myc, as well as cyclin D (Gerger et al. 2007). When BRCA1 and BRCA2 genes are dysregulated, the chance of breast cancer increases. These are known to perform multiple functions, including obstruction of cell cycle progression at the S-phase by halting the rb and possibly CDK2 (Rahman and Stratton 1998; Hashemi et al. 2019) (Fig. 11.2).
The specific CDK inhibitors do not act on multiple CDKs, unlike dual and pan-CDK inhibitors, which are directed against two and more than two types of CDKs, respectively (Mehraj et al. 2022a, b, c, d). The CDK4/6 inhibitors are very significant at preventing the progression of cell cycle and proliferation of tumor cell, and the frequently employed agents include Palbociclib, Abemaciclib, Ribociclib, and Trilaciclib, which are all CDK4/6 inhibitors. These inhibitors are very efficient for BC treatment, and several such drugs are being tested at phases I and II of clinical trials nowadays (Wu et al. 2020). Breast cancer subjects presenting with advanced-stage and metastatic types of breast cancer could be cured using such inhibitors as Abemaciclib (Table 11.1).
11.3 Functioning of CDK/Cyclins in the Cell Cycle
Through the target gene phosphorylation like the Rb, a tumor suppressor protein, this complex of cyclin/cyclin-dependent kinases takes control over the cell cycle progression. The active mitogenic signals are known to activate cyclins/cyclin-dependent kinases, and in reaction to the damage of DNA, the Cyclins/CDKs get inhibited via activated cell cycle checkpoints (Otto and Sicinski 2017). The cyclin-dependent kinase inhibitors (CDKIs) modulate the Cyclin/CDKs antagonistically. Examples include the CIP/KIPs as well as the CDK4(INK4) protein inhibitor (Asghar et al. 2015). In addition, Skp1-Cul1-F-box-protein (SCF) complex and anaphase-promoting complex/cyclosome (APC/C) also have a part to play in regulating the mitotic protein expression that impacts the cell cycle transitions, and these are E3 ubiquitin ligases (Sivakumar and Gorbsky 2015; Zhou et al. 2016a, b; Senft et al. 2018).
11.4 Types of CDKs
The cyclin-dependent kinases can be categorized into two types (Fig. 11.3)
-
1.
CDKs associated with cell cycle include CDK1, CDK2, CDK4, and CDK6.
Characteristics: These CDKs are known to moderate the progression and cell cycle phases directly.
-
2.
CDKs associated with transcription include CDK7, CDK8, CDK9, CDK12, and CDK13.
Characteristics: These CDKs are known to phosphorylate the carboxy-terminal domain (CTD) of the DNA-directed RNA polymerase II subunit (RPB1) of RNA Pol II and another target. The exact mechanism concerning the transcription is not fully elucidated (Asghar et al. 2015).
11.5 Role of CDKs in Breast Cancer
The uncontrolled cellular proliferation manifests as one of the cancer hallmarks (Mehraj et al. 2022a, b, c, d). The same occurs through the cell cycle checkpoint disabling and overriding several safeguards involved with the cyclin/CDKs dysregulation or impairment. Moreover, multiple studies revealed the participation of Cyclin/CDKs dysregulation in various BC phenotypes, as indicated in Table 11.2.
In many solid cancers like BC, uncontrolled cell proliferation is witnessed as a result of cell cycle (Sofi et al. 2022a, b, c) dysregulation and the genetic changes in the proteins involved in cell cycle regulation (Hanahan and Weinberg 2011). The cyclin-dependent kinases, along with their partners and their biological functions in case of breast cancer, are given in Table 11.3.
It has been seen that CDK10, along with its interacting partner Cyclin M typically modulates ETS2 transcription but not via RNAPII phosphorylation, and its attributed roles in breast cancer are the correspondence with metastasis in case of lymph node and resistance witnessed in case of endocrine therapy (Iorns et al. 2008; You et al. 2015; Guen et al. 2017). The CDK11, along with its interacting partner Cyclin L modulates the splicing and transcription of RNA, programmed cell death as well as autophagy, and in breast cancer, this complex partner with growth and angiogenesis, proliferation, and programmed cell death, too (Loyer et al. 2008; Chi et al. 2015; Zhou et al. 2016a, b; dos Santos Paparidis and Canduri 2018; Khan et al. 2022a, b). CDK19 and its interacting partner Cyclin C function as a paralog of CDK8, with akin function to CDK8, although appear to execute some different functions and the attributed role in breast cancer is chemoresistance; furnishes potential targets for enhancing chemotherapy (Galbraith et al. 2013; Zheng et al. 2019). In hepatitis B virus-driven transformation, CDK15/CyclinY takes a part, in the case of breast cancer associated with metastasis and cell invasion (Shiraishi et al. 2014; Li et al. 2019).
11.6 Need for CDK Inhibitors for Use in BC Treatment
Given the role of CDKs and their dysregulation in BC, several CDK inhibitors have been formed, some of which are FDA approved for their use in clinical settings. Some are being designed and examined as anticancer drugs at various phases of clinical trials (Sánchez-Martínez et al. 2019). Studies on the human BC mouse models indicate that CyclinD1-CDK4/6 axis stimulation ends up with a tumorigenic phenotype and plays a part in the maintenance and initiation of tumorigenesis in HER2+ breast cancer (Dukelow et al. 2015). The ER-negative and PR-negative status in TNBC is indicated by the Cyclin E overexpression and corresponds to a poor prognostic marker in TNBC (Jabbour-Leung et al. 2016; Mintoo et al. 2021). The absence of INK4 and CIP/KIP family proteins, coupled with CDK4/6 overexpression, has been witnessed in the case of breast cancer clinically (Asghar et al. 2015). Research has revealed that different subtypes of breast cancer display different molecular alterations for cell cycle checkpoints (Mir et al. 2022a, b, c, d, e).
11.7 Involvement of Other CDKs
It has also been seen that the overexpression of CDK2 ends up with overexpression of cyclin E and cyclin A, which are its partners—in the case of breast cancer (Santo et al. 2015; Singh et al. 2017). Roles of CDK1 in mitotic progression have been observed along with the overexpression of Cyclin A2 and B1 (Aaltonen et al. 2009). With the help of A- and B-type cyclins, the CDK1 kinase modulates the centrosome cycle and the mitotic onset and represents one of the central modulators of mitosis (Mir et al. 2022a, b, c, d, e). The CDK1 activity is shut down after the successful condensation of chromosomes and their alignment at the metaphase plate in order to permit the segregation of sister chromatid via separase or separin activation. The decondensation of chromosomes, nuclear envelope reformation, and the process of cytokinesis all require this inactivation of CDK1 (Potapova et al. 2006). A study has shown that in the absence of CDK12 protein, there has been much improvement in the triple-negative breast cancer phenotype due to CDK12 loss leading to DNA repair defects (Naidoo et al. 2018). Also, one of the valuable therapies is the inhibition of CDK7 for triple-negative breast cancer patients. It has been witnessed in TNBC that the CDK7 brings about transcriptional addition to a significant gene cluster (Wang et al. 2015; Wadhwa et al. 2020). Due to their participation in sustaining the growth of cancer cells, the CDKs and Cyclins are good targets for treating breast cancers (Mir et al. 2022a, b, c, d, e).
11.8 Types of CDK Inhibitors
The inhibitors directed against CDKs are categorized either as Non-selective or Selective, i.e., either pan-inhibitors or against one single cyclin-dependent kinase, solely based on meticulousness against the CDKs (Ding et al. 2020). Various drugs that are CDK inhibitors in action have entered breast cancer clinical trials and are known to target cell modulators in the cancerous cells, thus furnishing a therapeutic window (Ding et al. 2020; Mir et al. 2022a, b, c, d, e).
11.9 Pan-Inhibitors for BC Treatment
All pan-CDK inhibitors act non-specifically. For example, the targets of Seliciclib (a pan-CDK inhibitor) are CDK1, CDK2, CDK5, CDK7, and CDK9 (53). Several undesirable effects/toxicities have been witnessed due to the use of various pan-CDK inhibitors, including fatigue, myelosuppression, nausea, abnormalities in the liver, vomiting, nerve dysfunction, GIT effects, and for these agents lack of predictive biomarkers for the BC patients (Mehraj et al. 2022a, b, c, d). Thus, these collapsed before phase second trials. The undesirable effects are shown in Fig. 11.4. Some examples of early pan-CDK inhibitors are given in Table 11.4. All pan-CDK inhibitors are non-specific.
Obtained from rohitukine (a chromone alkaloid) is a semi-synthetic flavonoid which represents one of the examples of first-generation inhibitors is flavopiridol. It exerts its anticancer effects by inhibiting CDK1, 2, 4, 6, 7, and 9 (Sedlacek et al. 1996; Shapiro 2006). Flavopiridol in G1 and G2 phases leads to cell cycle arrest and is also known to induce cytotoxicity by blocking CDK7 and CDK9 and c-MYC transcription (Canavese et al. 2012). Another example from the pan-CDK inhibitors is provided by Dinaciclib, which is known to inhibit CDK1, CDK2, CDK5, and CDK9 with excellent Rb phosphorylation inhibitory potency, thus showing a better therapeutic index in comparison with the flavopiridol (Asghar et al. 2015).
11.10 Specific CDK Inhibitors for BC Treatment
For treating ER+ /HER2- advanced and metastatic BC, both FDA and European medicines agency approved CDK4/6 selective inhibitors (Abemaciclib, Palbociclib, and Ribociclib) displaying inhibition of growth in ER+ BC in a dose-dependent manner. The three drugs have the property of binding the CDK4 and CDK6 ATP binding pocket and thus are ATP-competitive drugs and are small molecules. In the ATP binding cleft, these display particular types of interactivity with the residues (Asghar et al. 2015). Due to the resistance in ER+ breast cancer patients induced by the endocrine therapy, the CDK inhibitor development came into focus. The general mechanism of action is shown in Fig. 11.5.
11.11 CDK4/6 Inhibitors and their Mechanism of Action
CDK4/6 inhibitors (palbociclib, ribociclib, and abemaciclib) hinder the CDK4/6 activation, causing cell cycle arrest at the G1 phase of the cell cycle. The complex CDK4/6-CyclinD is responsible for Rb (the tumor suppressor gene) phosphorylation and inhibition of its product. When phosphorylation of Rb occurs, the G1 to S-phase proceeds smoothly, DNA replicates, and mitosis usually occurs. However, when blocked by these inhibitors, the process is hampered, and the cell cycle is arrested. The Palbociclib mechanism of action is shown in Fig. 11.6. In both preclinical and clinical trials for Estrogen Receptor-positive BC, the inhibitors (for CDK4/6) were approved effectively when combined with the anti-estrogen therapies (Sobhani et al. 2019). Various clinical trials are being carried out to access the inhibitors, which are specific to CDK4/6 in BC (Mir et al. 2022a, b, c, d, e).
11.11.1 Palbociclib
The first CDK4/6 inhibitor that got the approval for treating breast cancer is Palbociclib. This is a potent, small-molecule selective inhibitor of CDK4/6 and is administered orally (Im et al. 2019). It has been observed that the human breast cancer cell line displays varied sensitivity based on its phenotype towards palbociclib. Also, the ER- breast cancer cells with basal-like and triple-negative breast cancer histology show less palbociclib sensitivity than the ER+ breast cancer cell lines with luminal features (Finn et al. 2016a, b; Asghar et al. 2017). Palbociclib and Abemaciclib have been marked for the second-line therapy with Fulvestrant and these display very low efficacy against CDK1, 2, 7, and 9 (Chen et al. 2009). It has also been predicted that the presence of a functional Rb protein is essential for palbociclib. Hampering the Rb phosphorylation causes the arrest of the cell cycle in G1 phase (Dean et al. 2010). Palbociclib works synergistically in combination with tamoxifen and trastuzumab and efficiently suppresses the ER+ breast cancer cell line proliferation (Finn et al. 2015). In many cancers that occur in humans, the CDK4’s (Cyclin-dependent kinase 4) overexpression has been found, including the breast cancer, and palbociclib has been approved by FDA for its treatment due to its specific CDK4 inhibition (Mehraj et al. 2021). This dual inhibitor for CDK4/6 in the case of BC was approved as it specifically shows its inhibitory effect on HER2- breast cancer/ER+ breast cancer (Finn et al. 2016a, b; Finn et al. 2016a, b).
11.11.2 Ribociclib
The drug is orally administered and displays high potency with bioavailability and inhibits CDK4/6 selectivity is Ribociclib. This drug does not display significant activity against CDK2 and CDK1 (Sobhani et al. 2019). This drug also inhibits the Rb + cell lines via phosphorylation of Rb inhibition in the case of BC, leading to cell cycle arrest in these tumor cells, as seen in the case of palbociclib (Chen et al. 2008; Sofi et al. 2022a, b, c). Many trials are being carried out to explore the ribociclib efficiency when combined with other drugs or agents to treat breast cancer (Mir et al. 2022a, b, c, d, e).
11.11.3 Abemaciclib
Another drug that inhibits CDK4/6 is Abemaciclib, which is orally administered. It causes a decrease in cell number and halts cancer cells’ proliferation via inhibition of Rb’s phosphorylation coupled with the arrest of cell cycle at the G1 phase (Im et al. 2019). A recent study revealed that the inhibitor Abemaciclib could enhance the activation of T-cells and also up-regulated the antigen presentation genes expression in human BC cells (Schaer et al. 2018). In addition, due to a better understanding of CDKs in various subtypes of breast cancer, their mode of inhibition, their side effects, and resistance, many new CDKs are being explored (Mir et al. 2022a, b, c, d, e).
11.11.4 Other CDK Inhibitors
PL (piperlongumine) is a novel CDK inhibitor discovered by Jeong et al. This inhibitor is known to hamper migration and cell proliferation in the case of ER+ breast cancer. The PL is a natural product, and it is obtained from pepper. It hinders the CDK1 and CDK4/6 expression levels and ends up arresting the cell cycle at the G2/M phase to stop tumorigenesis (Jeong et al. 2019). The highly selective dual inhibitor SR-4835: It was revealed by Quereda et al. that this inhibitor acts on CDK12 and CDK13. It can stop cell proliferation in triple-negative breast cancer (Quereda et al. 2019). According to the study’s upshot (Mir 2015, Li et al. 2020), in the case of BC, the SR-4835 led to the immunogenic death of cell, thus adding to the antitumor function of PD1-PD-L1 immune checkpoint therapy (Qayoom et al. 2021, Mehraj et al. 2022a, b, c, d). Panduratin A (PA) posses several health benefits, including anti-inflammatory, anti-oxidant, and antibacterial, in addition to its anticancer activity. It has been seen that PA leads to the block of the cell cycle in G0/G1 phase by suppressing the expression of CDK4 and cyclin D1 (Liu et al. 2018, Sofi et al. 2022a, b, c). Vanicoside B is phenylpropanoyl sucrose derived from flavonoid glycoside and has been shown to act as a chemopreventive agent (Sofi et al. 2022a, b, c). The vanicoside B could inhibit the CDK8-mediated signaling pathway expression as well as lead to the onset of arrest in the cell cycle in HCC38 and MDA-MB-231 cells as reported previously (Kim et al. 2019). A role in the modulation of cellular invasion, proliferation as well as migration is played by protein phosphatase Mg2+/Mn2+-dependent 1A (PPM1A) by decreasing retinoblastoma and CDK phosphorylation in the case of TNBC. PPM1A belongs to the Ser/Thr protein phosphatase 2C family (Mazumdar et al. 2019). It has been noted in the study that when the Roscovitine, a pan-CDK inhibitor, is administered sequentially preceding doxorubicin, it is synthetically fatal in triple-negative breast cancer cells (Mehraj et al. 2022a, b, c, d). This inhibitor, when administered, blocks the cell cycle in the G2/M phase, preparing them for DNA damage. It was observed that this combined treatment approach led to an enhancement in DNA double-stranded breaks and lowered the protein recruitment, necessary for homologous recombination compared to the solo treatment by doxorubicin (Mir et al. 2022a, b, c, d, e). It was also witnessed that by employing this combination therapy, tumor volume showed a reduction, and an elevated survival was observed compared to the solo drug or related treatment in the case of xenograft studies (Jabbour-Leung et al. 2016, Mir et al. 2022a, b, c, d, e). This inhibitors of CDK1, 2, 5, 7, and 9 (Zhang et al. 2018, Nie et al. 2019) surfaced as the prime orally available drug from this group to become part of the clinical trials due to its relative success in the preclinical stage, where its success led to the onset of apoptosis in tumor cells (MacCallum et al. 2005, Shapiro 2006, Galons et al. 2010, Mehraj et al. 2022a, b, c, d). CDK1 shows the involvement in the homologous recombination DNA double-stranded break repair pathway. The cyclin-dependent kinase activity is needed for removing the DNA double-stranded breaks to produce single stands during the homologous recombination by recruiting endonucleases Sae2 or CtlP, respectively, in yeast and mammalian cells (Ira et al. 2004, Huertas and Jackson 2009). CDK activity has also been recruited for recruiting and associating the BRCA1 to the MRN [Mre11-Rad50-Nbs1] complex during homologous recombination (Chen et al. 2008; Mir et al. 2022a, b, c, d, e). The inhibition of CDK using the Roscovitine decreased the RPA34, a homologous recombination downstream protein in sarcoma cells that had previously received radiation treatment therapy due to the inefficacy of producing the single stands (Jazayeri et al. 2006; Mir et al. 2020). Therefore weakening homologous recombination by inhibiting the cyclin-dependent kinases could furnish plans to increase the cell sensitivity to chemotherapy in case of TNBC (Jabbour-Leung et al. 2016; Mir et al. 2022a, b, c, d, e). It has also been witnessed that there is high CDK11 expression in the case of triple-negative breast cancer, liposarcoma, and multiple myeloma (Jia et al. 2014; Zhou et al. 2015; Sofi et al. 2022a, b, c). Indirectly the CDK7 may modulate the transcription by phosphorylating and modulating transcription factors like ER and Androgen receptors, both of which have a significant role in breast cancer and prostate cancer, the hormone-driven cancers (Asturias 2004; Compe and Egly 2012; Sainsbury et al. 2015). The co-amplification of CDK12 with ERBB2/HER2 oncogene has been witnessed in subsets of breast cancer (Naidoo et al. 2018). It was revealed through the proteomic analysis that the CDK12 amplification is associated with increased phosphorylation of CDK12, indicating that CDK12 could act as a vital therapeutic target in case of HER2-amplified breast cancers (Mertins et al. 2016; Paculová and Kohoutek 2017). It has already been established via multiple studies and trials that CDKs, particularly CDK4/6inhibitors, enhance the treatment efficiency in combination with hormone therapy than HT alone (Turner et al. 2015; Le Saux et al. 2017).
According to a study on the Indian population (Lakkavalli et al. 2021), hormone therapy was relatively more effective in combination with CDK4/6 inhibitors with tolerable side effects among the HR-positive advanced breast cancer patients (Mir and Mehraj 2019). The study’s upshot revealed that the palbociclib, when combined with the hormone therapy, resulted in extended PFS (progression-free survival) compared to the HT alone among those women who presented with the HR-positive metastatic breast cancer (Khan et al. 2022a, b).
11.11.5 Side Effects of CDK4/6 Inhibitors
The CDK4/CDK6 inhibitors Palbociclib, Ribociclib, and Abemaciclib are being used in clinical settings and are employed in the case of BC which is hormone receptor-positive [ER and/or PR expressing]. Improved progression-free survival (PFS) and overall survival (OS) have been witnessed (Finn et al. 2016a, b; Sledge Jr et al. 2017). However, various undesirable effects are also witnessed in BC patients, and developing more specific targets with fewer side effects is the need of the hour. The following side effects have been witnessed in BC patients from CDK4/6 Inhibitors that are approved by FDA (Ettl 2019). The various side effects are listed in Fig. 11.6, and the common side effects observed in the case of all the three approved drugs have been mentioned separately in Fig. 11.7.
11.12 Summary
The active participation of CDKs in coordinating and modulating the cell division provides vast scope for further research to elucidate the process of BC development, especially its metastasis. In this direction, multiple therapeutic implications of CDKs in breast cancer have been explored. Several drugs that help inhibit CDKs, for example, specific CDK4/6 inhibitors, have been successfully developed and used in clinical settings today. Similarly, dual inhibitors, as well as pan-CDK inhibitors, have been explored for treating breast cancer. However, pan-CDK inhibitors have shown antitumor activities in the case of BC patients, given the non-specific mode of action coupled with the undesirable effects on BC patients (Mir et al. 2022a, b, c, d, e). Many such inhibitors failed at different clinical trial phases, and their use in actual clinical practice was not approved. Conclusion: There is a need to reduce the undesirable effects, and the combination of therapy with other drugs may help develop an effective treatment for BC patients. Several such inhibitors are being tested at different phases of clinical trials and can find their uses in treating BC in actual clinical settings (Mir et al. 2022a, b, c, d, e).
11.13 Further Readings
For more insights about the topic, we would suggest detailed findings from the books of Mir MA (2022) https://doi.org/10.1016/C2021-0-02565-7, https://doi.org/10.1016/C2022-0-00074-X and Mir MA (2021) https://doi.org/10.52305/WXJL6770, and from the cancer.net website on the following mentioned below links,
-
https://www.cancer.net/cancer-types/breast-cancer/types-treatment
-
https://www.jmedsciences.com/doi/JMEDS/pdf/10.5005/jp-journals-10045-00138
For diagrammatic illustrations, descriptive tables, Lazzeroni (2012) http://www.eurekaselect.com/article/49928
See video links on over all status of cancer, its various types, current new treatment possible options available.
https://www.sciencedirect.com/science/article/pii/S205970292032278X
The readers can have a look upon the following video YouTube links for the better understanding of the chapter:
References
Aaltonen K et al (2009) High cyclin B1 expression is associated with poor survival in breast cancer. Br J Cancer 100(7):1055–1060
Asghar U et al (2015) The history and future of targeting cyclin-dependent kinases in cancer therapy. Nat Rev Drug Discov 14(2):130–146
Asghar US et al (2017) Single-cell dynamics determines response to CDK4/6 inhibition in triple-negative breast CancerCDK4/6 inhibition in triple-negative breast cancer. Clin Cancer Res 23(18):5561–5572
Asturias FJ (2004) RNA polymerase II structure, and organization of the preinitiation complex. Curr Opin Struct Biol 14(2):121–129
Canavese M et al (2012) Cyclin dependent kinases in cancer: potential for therapeutic intervention. Cancer Biol Ther 13(7):451–457
Cao T et al (2017) CDK3, target of miR-4469, suppresses breast cancer metastasis via inhibiting Wnt/β-catenin pathway. Oncotarget 8(49):84917–84927
Chen L et al (2008) Cell cycle-dependent complex formation of BRCA1· CtIP· MRN is important for DNA double-strand break repair. J Biol Chem 283(12):7713–7720
Chen Y-J et al (2009) A conserved phosphorylation site within the forkhead domain of FoxM1B is required for its activation by cyclin-CDK1. J Biol Chem 284(44):30695–30707
Chi Y et al (2015) Critical role of CDK11p58 in human breast cancer growth and angiogenesis. BMC Cancer 15(1):1–10
Compe E, Egly J-M (2012) TFIIH: when transcription met DNA repair. Nat Rev Mol Cell Biol 13(6):343–354
Crown J (2017) CDK8: a new breast cancer target. Oncotarget 8(9):14269
Dean JL et al (2010) Therapeutic CDK4/6 inhibition in breast cancer: key mechanisms of response and failure. Oncogene 29(28):4018–4032
Del Re M et al (2019) Overexpression of TK1 and CDK9 in plasma-derived exosomes is associated with clinical resistance to CDK4/6 inhibitors in metastatic breast cancer patients. Breast Cancer Res Treat 178(1):57–62
Ding L et al (2020) The roles of cyclin-dependent kinases in cell-cycle progression and therapeutic strategies in human breast cancer. Int J Mol Sci 21(6):1960
Dorand RD et al (2016) Cdk5 disruption attenuates tumor PD-L1 expression and promotes antitumor immunity. Science 353(6297):399–403
Dukelow T et al (2015) CDK4/6 inhibitors in breast cancer. Anti-Cancer Drugs 26(8):797–806
Ettl J (2019) Management of adverse events due to cyclin-dependent kinase 4/6 inhibitors. Breast care 14(2):86–92
Finn RS et al (2015) The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol 16(1):25–35
Finn RS et al (2016a) Targeting the cyclin-dependent kinases (CDK) 4/6 in estrogen receptor-positive breast cancers. Breast Cancer Res 18(1):1–11
Finn RS et al (2016b) Palbociclib and letrozole in advanced breast cancer. N Engl J Med 375(20):1925–1936
Firestein R et al (2008) CDK8 is a colorectal cancer oncogene that regulates β-catenin activity. Nature 455(7212):547–551
Galbraith MD et al (2013) HIF1A employs CDK8-mediator to stimulate RNAPII elongation in response to hypoxia. Cell 153(6):1327–1339
Galons H et al (2010) Cyclin-dependent kinase inhibitors: a survey of recent patent literature. Expert Opin Ther Pat 20(3):377–404
Gerger A et al (2007) A multigenic approach to predict breast cancer risk. Breast Cancer Res Treat 104(2):159–164
Guen VJ et al (2017) The awakening of the CDK10/Cyclin M protein kinase. Oncotarget 8(30):50174
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144(5):646–674
Hashemi V et al (2019) The role of DEAD-box RNA helicase p68 (DDX5) in the development and treatment of breast cancer. J Cell Physiol 234(5):5478–5487
Horiuchi D et al (2012) MYC pathway activation in triple-negative breast cancer is synthetic lethal with CDK inhibition. J Exp Med 209(4):679–696
Huertas P, Jackson SP (2009) Human CtIP mediates cell cycle control of DNA end resection and double strand break repair. J Biol Chem 284(14):9558–9565
Im S-A et al (2019) Overall survival with ribociclib plus endocrine therapy in breast cancer. N Engl J Med 381(4):307–316
Iorns E et al (2008) Identification of CDK10 as an important determinant of resistance to endocrine therapy for breast cancer. Cancer Cell 13(2):91–104
Ira G et al (2004) DNA end resection, homologous recombination and DNA damage checkpoint activation require CDK1. Nature 431(7011):1011–1017
Jabbour-Leung NA et al (2016) Sequential combination therapy of CDK inhibition and doxorubicin is synthetically lethal in p53-mutant triple-negative breast CancerCDK inhibition sensitizes p53-mutated cells to doxorubicin. Mol Cancer Ther 15(4):593–607
Jazayeri A et al (2006) ATM-and cell cycle-dependent regulation of ATR in response to DNA double-strand breaks. Nat Cell Biol 8(1):37–45
Jeong CH et al (2019) Piperlongumine induces cell cycle arrest via reactive oxygen species accumulation and IKKβ suppression in human breast cancer cells. Antioxidants 8(11):553
Jia B et al (2014) Cyclin-dependent kinase 11 (CDK11) is crucial in the growth of liposarcoma cells. Cancer Lett 342(1):104–112
Kastan MB, Bartek J (2004) Cell-cycle checkpoints and cancer. Nature 432(7015):316–323
Khan SU et al (2022a) Understanding the cell survival mechanism of anoikis-resistant cancer cells during different steps of metastasis. Clin Exp Metastasis
Khan SU et al (2022b) Activation of lysosomal mediated cell death in the course of autophagy by mTORC1 inhibitor. Sci Rep 12(1):1–13
Kim D et al (2019) Antitumor activity of vanicoside B isolated from Persicaria dissitiflora by targeting CDK8 in triple-negative breast cancer cells. J Nat Prod 82(11):3140–3149
Kops GJPL et al (2005) On the road to cancer: aneuploidy and the mitotic checkpoint. Nat Rev Cancer 5(10):773–785
Lakkavalli RK et al (2021) Metastatic hormone receptor-positive breast cancer in CDK 4/6 era: an outcome audit. J Cancer Res Ther 17(4):994
Le Saux O et al (2017) Assessment of multiple endocrine therapies for metastatic breast cancer in a multicenter national observational study. American Society of Clinical Oncology
Li B et al (2017) Therapeutic rationale to target highly expressed CDK7 conferring poor outcomes in triple-negative breast CancerCDK7 expression and function in triple-negative breast cancer. Cancer Res 77(14):3834–3845
Li S et al (2019) PA28α/β promote breast cancer cell invasion and metastasis via down-regulation of CDK15. Front Oncol 9:1283
Li Y et al (2020) CDK12/13 inhibition induces immunogenic cell death and enhances anti-PD-1 anticancer activity in breast cancer. Cancer Lett 495:12–21
Liu Q et al (2018) Panduratin a inhibits cell proliferation by inducing G0/G1 phase cell cycle arrest and induces apoptosis in breast cancer cells. Biomol Ther 26(3):328
Loyer P et al (2008) Characterization of cyclin L1 and L2 interactions with CDK11 and splicing factors: influence of cyclin L isoforms on splice site selection. J Biol Chem 283(12):7721–7732
MacCallum DE et al (2005) Seliciclib (CYC202, R-Roscovitine) induces cell death in multiple myeloma cells by inhibition of RNA polymerase II-dependent transcription and down-regulation of Mcl-1. Cancer Res 65(12):5399–5407
Malumbres M (2014) Cyclin-dependent kinases. Genome Biol 15(6):1–10
Malumbres M, Barbacid M (2001) To cycle or not to cycle: a critical decision in cancer. Nat Rev Cancer 1(3):222–231
Malumbres M, Barbacid M (2005) Mammalian cyclin-dependent kinases. Trends Biochem Sci 30(11):630–641
Malumbres M, Barbacid M (2009) Cell cycle, CDKs and cancer: a changing paradigm. Nat Rev Cancer 9(3):153–166
Marais A et al (2010) Cell cycle-dependent regulation of the forkhead transcription factor FOXK2 by CDK· cyclin complexes. J Biol Chem 285(46):35728–35739
Mazumdar A et al (2019) The phosphatase PPM1A inhibits triple negative breast cancer growth by blocking cell cycle progression. NPJ Breast Cancer 5(1):1–11
Mehraj U et al (2021) Tumor microenvironment promotes breast cancer chemoresistance. Cancer Chemother Pharmacol 87(2):147–158
Mehraj U et al (2022a) Expression pattern and prognostic significance of baculoviral inhibitor of apoptosis repeat-containing 5 (BIRC5) in breast cancer: a comprehensive analysis. Adv Cancer Biol Metastasis 4:100037
Mehraj U et al (2022b) Expression pattern and prognostic significance of chemokines in breast cancer: an integrated bioinformatics analysis. Clin Breast Cancer
Mehraj U et al (2022c) Adapalene and doxorubicin synergistically promote apoptosis of TNBC cells by hyperactivation of the ERK1/2 pathway through ROS induction. Front Oncol 12
Mehraj U, et al. (2022d) Expression pattern and prognostic significance of CDKs in breast cancer: An integrated bioinformatic study." Cancer Biomarkers(Preprint): 1–15
Mertins P et al (2016) Proteogenomics connects somatic mutations to signalling in breast cancer. Nature 534(7605):55–62
Mintoo M et al (2021) A rohitukine derivative IIIM-290 induces p53 dependent mitochondrial apoptosis in acute lymphoblastic leukemia cells. Mol Carcinog 60(10):671–683
Mir MA (2015) Developing costimulatory molecules for immunotherapy of diseases. Academic Press
Mir MA, Mehraj U (2019) Double-crosser of the immune system: macrophages in tumor progression and metastasis. Curr Immunol Rev 15(2):172–184
Mir MA et al (2020) Targeting different pathways using novel combination therapy in triple negative breast cancer. Curr Cancer Drug Targets 20(8):586–602
Mir MA et al (2022a) Chapter1 - triple-negative breast cancer - an aggressive subtype of breast cancer. In: Mir M (ed) Combinational therapy in triple negative breast cancer. Academic Press, pp 1–28
Mir MA et al (2022b) Chapter 3—current therapeutics and treatment options in TNBC. In: Mir M (ed) Combinational therapy in triple negative breast cancer. Academic Press, pp 61–94
Mir MA et al (2022c) Chapter 8—different drug delivery approaches in combinational therapy in TNBC. In: Mir M (ed) Combinational therapy in triple negative breast cancer. Academic Press, pp 201–230
Mir MA et al. (2022d) Immuno-onco-metabolism and Therapeutic Resistance. Immuno-Oncology Crosstalk and Metabolism, Springer: 45–89
Mir MA et al (2022e) Chapter 4—conventional adjuvant chemotherapy in combination with surgery, radiotherapy, and other specific targets. In: Mir M (ed) Combinational therapy in triple negative breast cancer. Academic Press, pp 95–120
Naidoo K et al (2018) Evaluation of CDK12 protein expression as a potential novel biomarker for DNA damage response–targeted therapies in breast cancer. Mol Cancer Ther 17(1):306–315
NavaneethaKrishnan S et al (2018) Loss of Cdk5 in breast cancer cells promotes ROS-mediated cell death through dysregulation of the mitochondrial permeability transition pore. Oncogene 37(13):1788–1804
Nemet J et al (2014) The two faces of Cdk8, a positive/negative regulator of transcription. Biochimie 97:22–27
Nie L et al (2019) CDK2-mediated site-specific phosphorylation of EZH2 drives and maintains triple-negative breast cancer. Nat Commun 10(1):1–15
Otto T, Sicinski P (2017) Cell cycle proteins as promising targets in cancer therapy. Nat Rev Cancer 17(2):93–115
Paculová H, Kohoutek J (2017) The emerging roles of CDK12 in tumorigenesis. Cell Div 12(1):1–10
Potapova TA et al (2006) The reversibility of mitotic exit in vertebrate cells. Nature 440(7086):954–958
Pozo K et al (2013) The role of Cdk5 in neuroendocrine thyroid cancer. Cancer Cell 24(4):499–511
Qayoom H. et al. (2021). Integrating immunotherapy with chemotherapy: a new approach to drug repurposing. Drug Repurposing-Molecular Aspects and Therapeutic Applications, IntechOpen
Quereda V et al (2019) Therapeutic targeting of CDK12/CDK13 in triple-negative breast cancer. Cancer Cell 36(5):545–558
Rahman N, Stratton MR (1998) The genetics of breast cancer susceptibility. Annu Rev Genet 32:95
Sainsbury S et al (2015) Structural basis of transcription initiation by RNA polymerase II. Nat Rev Mol Cell Biol 16(3):129–143
Sánchez-Martínez C et al (2019) Cyclin dependent kinase (CDK) inhibitors as anticancer drugs: recent advances (2015–2019). Bioorg Med Chem Lett 29(20):126637
Santamaría D et al (2007) Cdk1 is sufficient to drive the mammalian cell cycle. Nature 448(7155):811–815
Santo L, Siu KT, Raje N (2015) Targeting Cyclin-Dependent Kinases and Cell Cycle Progression in Human Cancers. Semin Oncol 42(6):788–800
dos Santos Paparidis NF, Canduri F (2018) The emerging picture of CDK11: genetic, functional and medicinal aspects. Curr Med Chem 25(8):880–888
Schaer DA et al (2018) The CDK4/6 inhibitor Abemaciclib induces a T cell inflamed tumor microenvironment and enhances the efficacy of PD-L1 checkpoint blockade. Cell Rep 22(11):2978–2994
Schlafstein AJ et al (2018) CDK9 expression shows role as a potential prognostic biomarker in breast cancer patients who fail to achieve pathologic complete response after neoadjuvant chemotherapy. Int J Breast Cancer 2018
Sedlacek H et al (1996) Flavopiridol (L86 8275; NSC 649890), a new kinase inhibitor for tumor therapy. Int J Oncol 9(6):1143–1168
Senft D et al (2018) Ubiquitin ligases in oncogenic transformation and cancer therapy. Nat Rev Cancer 18(2):69–88
Shapiro GI (2006) Cyclin-dependent kinase pathways as targets for cancer treatment. J Clin Oncol 24(11):1770–1783
Shiraishi Y et al (2014) Integrated analysis of whole genome and transcriptome sequencing reveals diverse transcriptomic aberrations driven by somatic genomic changes in liver cancers. PLoS One 9(12):e114263
Singh U et al (2017) Design of novel 3-pyrimidinylazaindole CDK2/9 inhibitors with potent in vitro and in vivo antitumor efficacy in a triple-negative breast cancer model. J Med Chem 60(23):9470–9489
Sivakumar S, Gorbsky GJ (2015) Spatiotemporal regulation of the anaphase-promoting complex in mitosis. Nat Rev Mol Cell Biol 16(2):82–94
Sledge GW Jr et al (2017) MONARCH 2: abemaciclib in combination with fulvestrant in women with HR+/HER2− advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol 35(25):2875–2884
Sobhani N et al (2019) Updates on the CDK4/6 inhibitory strategy and combinations in breast cancer. Cell 8(4):321
Sofi S et al (2022a) Targeting cyclin-dependent kinase 1 (CDK1) in cancer: molecular docking and dynamic simulations of potential CDK1 inhibitors. Med Oncol 39(9):1–15
Sofi S et al (2022b) Cyclin-dependent kinases in breast cancer: expression pattern and therapeutic implications. Med Oncol 39(6):106
Sofi S et al (2022c) Cyclin-dependent kinases in breast cancer: expression pattern and therapeutic implications. Med Oncol 39(6):1–16
Sotillo R et al (2001a) Wide spectrum of tumors in knock-in mice carrying a Cdk4 protein insensitive to INK4 inhibitors. EMBO J 20(23):6637–6647
Sotillo R et al (2001b) Invasive melanoma in Cdk4-targeted mice. Proc Natl Acad Sci 98(23):13312–13317
Turner NC et al (2015) Palbociclib in hormone-receptor–positive advanced breast cancer. N Engl J Med 373(3):209–219
Wadhwa B et al (2020) AKT isoforms have discrete expression in triple negative breast cancers and roles in cisplatin sensitivity. Oncotarget 11(45):4178
Wang Y et al (2015) CDK7-dependent transcriptional addiction in triple-negative breast cancer. Cell 163(1):174–186
Wu Y et al (2020) Current therapeutic progress of CDK4/6 inhibitors in breast cancer. Cancer Manag Res 12:3477
You Y et al (2015) Decreased CDK10 expression correlates with lymph node metastasis and predicts poor outcome in breast cancer patients-a short report. Cell Oncol 38(6):485–491
Zhang X et al (2018) Rhomboid domain-containing protein 1 promotes breast cancer progression by regulating the p-Akt and CDK2 levels. Cell Commun Signal 16(1):1–15
Zhang Z et al (2017) HuR promotes breast cancer cell proliferation and survival via binding to CDK3 mRNA. Biomed Pharmacother 91:788–795
Zheng P et al (2019) Long noncoding RNA CASC2 promotes paclitaxel resistance in breast cancer through regulation of miR-18a-5p/CDK19. Histochem Cell Biol 152(4):281–291
Zhou Y et al (2015) Cyclin-dependent kinase 11p110 (CDK11p110) is crucial for human breast cancer cell proliferation and growth. Sci Rep 5(1):1–13
Zhou Y et al (2016a) The emerging roles and therapeutic potential of cyclin-dependent kinase 11 (CDK11) in human cancer. Oncotarget 7(26):40846
Zhou Z et al (2016b) Insights into APC/C: from cellular function to diseases and therapeutics. Cell Div 11(1):1–18
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Mir, M.A., Haq, B.U. (2023). Therapeutic Implications of CDKs in Breast Cancer. In: Mir, M. (eds) Therapeutic potential of Cell Cycle Kinases in Breast Cancer. Springer, Singapore. https://doi.org/10.1007/978-981-19-8911-7_11
Download citation
DOI: https://doi.org/10.1007/978-981-19-8911-7_11
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-8910-0
Online ISBN: 978-981-19-8911-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)