Abstract
Although lacking mechanistic explanations, different poles of permanent magnets could generate different effects on living organisms has been claimed decades ago, especially in the field of magnetotherapy. In recent years, several studies have confirmed that different magnetic field directions could indeed induce some differential effects in biological systems, including tumor inhibition, blood glucose level regulation, etc. However, it has been a neglected factor by most researchers in the past, which has led to many inconsistent experimental results in the literature. This chapter aims to systematically compare and summarize the biological effects induced by static magnetic fields of different directions. We also discuss about the possible mechanisms, which currently is still largely a mystery. We hope researchers in this field can pay attention to the static magnetic field directions so that they can clearly describe the field direction and/or distributions information in their studies, which will help clarify some confusions and reduce inconsistencies for future investigations.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
2.1 Introduction
On the one hand, the planet Earth itself can be seen as a big magnet with north and south poles, but how magnetoreceptive animals sense the Earth’s magnetic field direction is still an open question. On the other hand, effects of magnetic field on human health have been noticed for a long time, which will be discussed in more details in Chaps. 8 and 15 of this book, but magnetic field direction-induced differential bioeffects have been neglected by most researchers in this field. However, the different poles of permanent magnets have been frequently brought up by people in magnetotherapy for their observed differences. However, the scientific experimental validations or theoretical explanations are both lacking until recent few years. In this chapter, we will summarize reported studies in the literature, which have demonstrated that static magnetic field (SMF) direction is indeed a very important reason that has caused many experimental inconsistencies in MF-induced bioeffects.
2.2 Magnetic Poles vs. Magnetic Field Directions
There are some reports in the magnetotherapy field stating that different poles of a permanent magnet would have differential effects on human bodies. The most famous claim was brought up by Dr. Albert Roy Davis and Walter C. Rawls Jr., who published a very interesting book “Magnetism and its effects on the living systems” in 1974. In this book, they claimed that the North (N) pole and South (S) pole of the magnet could have dramatically different effects on living systems. According to their book, the original finding was actually from an “earthworm incident” in 1936, in which the earthworms had eaten through the one side of the cardboard container near the S pole while the earthworms in the other container near N pole did not. The magnetic flux density was around 3000 Gauss (0.3 T) in this “earthworm incident.” Further analysis revealed that the earthworms near the S pole were “one-third larger, longer in length and larger in diameter and were extremely active.” In this book, they also described many interesting findings about the differential effects of N vs. S magnetic pole on biological processes, such as the ripen speed of green tomatoes, radish seed germination, small animals, as well as cancers. Overall, they think the N pole is the “negative energy pole,” which arrests life growth and/or development, while the S pole is the “positive energy pole” that increases growth and development. Although their claims have not been scientifically proven, there are many other no-scientific reports supporting the Davis and Rawls’s claims. However, since no illustration, picture or other data was provided in their book about these experiments, the relative locations of the earthworms or other samples they tested near the magnets are unclear. Not much experimental details are available either.
After reading this interesting but puzzling book, we have a lot of questions in mind. Does the magnetic pole really matter for living organisms? If it does, is it really because of the “magnetic pole” per se, or it is because of the magnetic field direction? Does the North or South pole magnet could generate the same effects when they were placed on the top vs. bottom, or at the side of the samples? Does MF direction affect some specific aspect of biological activities?
Apparently, to answer these questions, it is necessary to perform carefully designed and well controlled studies to test their claims. For example, in a previous study done by our group in 2018, we set up the experiments to expose cells to 0.2–0.5 T SMFs by facing different magnetic poles to the cells to provide different MF directions, as well as the same MF direction, but the cells are facing different magnetic poles (Fig. 2.1). The surface magnetic flux density of the neodymium permanent magnets we used in the experiments is ~0.5 T, with the N or S pole facing different directions. The “N-down” means that the N pole is at the bottom of the sample. And the “S-up” means that the S pole is at the top of the sample. Therefore, the “N-down” and “S-up” both provided vertically upward SMF direction (Fig. 2.1a). The “N-up” and “S-down” both provided vertically downward SMF direction (Fig. 2.1b). In addition, the “N-right” and “S-left” both provided horizontal MF directions but with different magnetic poles facing the samples (Fig. 2.1c), which also mimic most MRI machines in hospitals that provide horizontal MFs on patients. By setting up SMFs in these ways, multiple parameters could be tested side-by-side (Fig. 2.1d, e). We found that the cell numbers were reduced after 2-day treatment of upward direction SMFs, but not by downward direction SMFs in two lung cancer cell lines, A549 and PC9 cells (Tian et al. 2018). This confirms that different SMF directions indeed have distinct cellular effects. However, there was no difference for the “N-right” and “S-left,” which indicates that the magnetic pole per se did not make a difference. Therefore, these results indicate that it is the magnetic field direction, but not the magnetic pole that generates differences on biological samples, at least for this type of cellular experiments.
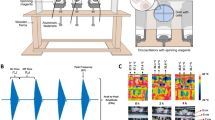
Experimental setup to differentiate bioeffects of magnetic field direction vs. magnetic poles. (a–c) Illustrations of experimental setup. Black arrows indicate magnetic field direction. The SMF was provided by placing the cell culture plate on the center of a 6 cm × 5 cm × 3.5 cm neodymium permanent magnet (measured surface magnetic field intensity is 0.4–0.5 T), with the North (N) or South (S) pole facing up. The control group was placed with at least 30–40 cm away from the magnet with a measured magnetic field intensity background of 0.9 Gs, which was 5000-fold lower than the 0.5 T experimental groups. (d) Experimental setup. (e) Information about the magnetic field strength and field direction in each experimental condition. [Reprinted with permission from (Tian et al. 2018)]
2.3 Bioeffects Induced by Different Magnetic Poles/Field Directions
We did a thorough literature searching to answer the question about whether different magnetic poles/field directions can really induce different bioeffects. Most studies we found were done in the last 10 years, including the ones from our group. Here we summarize and analyze all published studies we can find that involved SMFs of different directions (or magnetic poles) at organism level (Table. 2.1) or cellular level (Table. 2.2). Although it is clear that magnetic field direction can often cause differential effects on some aspects of living organism, no explicit rules can be concluded at current stage yet. It should also be mentioned that we did not include SMF studies that used a single SMF direction in their study, or implanted magnets in animals in a specific way, which cannot provide side-by-side comparison between different magnetic poles/field directions. However, it is interesting that all studies we found about SMF directions are vertically upward vs. downward.
2.3.1 Bioeffects of Different Direction Static Magnetic Fields in Living Organisms
In recent few years, an increasing number of studies have been conducted to investigate whether and how SMFs directions can affect living organisms, but the results are not very consistent. We summarize and compare reported results on SMFs in upward and downward directions and classified them into: “upward ≠ downward” and “upward = downward” based on whether SMFs in upward and downward directions have similar bioeffect on the living organisms (Table. 2.1).
Among the 26 relevant studies, 14 of them revealed differences between upward and downward directions. Although the research subjects, magnetic field parameters and the investigated experimental indicators are very diverse, which led to a large variation in these reports. However, it is interesting that there are multiple studies indicating that the upward direction SMFs might have more beneficial effects than the downward direction SMFs. For example, the upward direction SMF (0.01–0.5 T, 6 h/day, for 38 days) exposure inhibited GIST-T1 tumor growth in nude mice by 19.3% while the downward SMF did not produce significant effect (Tian et al. 2018). Moreover, Yang et al. found that the upward 9.4 T SMF for 88 h significantly inhibited A549 tumor growth (tumor growth inhibition = 41%), but not in the downward 9.4 T SMF (Yang et al. 2021). Furthermore, the upward SMF treatment significantly increased the distance traveled and average speed of Tenebrio (insects) (Todorovic et al. 2013) and increased plant root growth in Arabidopsis young seedlings (Jin et al. 2019).
In contrast, there are also some studies indicating that the downward direction SMFs improved the living organism status more significantly than in the upward direction (Table. 2.1). For example, three separate studies used a downward SMF of ~0.1 T to efficiently decrease blood glucose levels in T2D (Yu et al. 2021) and in T1D mice (unpublished data), as well as effectively alleviate alcohol-induced liver damage and lipid accumulation, and improve liver function (Song et al. 2021). Meanwhile, our group also found that a ~0.1 T downward SMF improved the multiple diabetic complications, but not in 0.1 T upward SMF (Yu et al. 2021) (unpublished data). More specifically, two studies in spontaneously hypertensive rats found that the anxious-like behavior (Tasic et al. 2021) and heart rate (Tasic et al. 2017) could be improved by downward direction of 16 mT SMF, but not upward SMF. There are also a few other studies that investigated the influences of different SMF directions on plants (Jin et al. 2019) and gut microbiota (Yu et al. 2021).
In the meantime, there are also 12 studies that show no significant difference between upward and downward direction SMFs. For example, 16 mT (Djordjevich et al. 2012) and 128 mT (Milovanovich et al. 2016) SMF exposures altered the hematological parameters and biological changes of Swiss Webster mice, but no difference was induced by different SMF directions. San et al. found that the thrombosis was significantly ameliorated in both upward and downward groups of BALB/c mice exposed to 1.4–46 mT SMFs (San et al. 2001).
The discrepancy about the different effects of SMFs direction on living organisms could be resulted from multiple aspects, including research subject, SMF devices that generated different magnetic flux density and distributions (Fig. 2.2), as well as experimental procedures, including exposure time and assay time-points. Further systematic studies are needed to get more in-depth information.
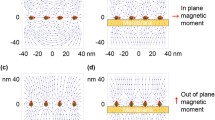
Examples of static magnetic fields of different directions and distributions used on living organisms. (a) A diagram of the two magnetic plates that provide upward and downward SMFs and (b) the MF distribution 1 cm above the magnetic plates, where the mice bodies locate. Reprinted with permission from (Yu et al. 2021). (c) The device consists of ten plates. Each plate contains 8 cylindrical permanent magnets. Moreover, the magnets were placed next to each other with the same orientation. The whole cage was placed on the magnetic plate. (d) The magnetic field strength is ~15 mT at 2 cm above the magnetic plate, where the mice wound located. (e) Devices with different directions of magnetic plates and magnetic field intensities at the positions of the mice in each exposure condition are provided and measured. [Reprinted from (Feng et al. 2022). Open access]
2.3.2 Bioeffects of Different SMF Directions at Cellular Level
Similar to living organism, the cellular studies of SMFs of different field directions also produced seemingly inconsistent results, which is reasonable to some extent because the cellular experiments have more variable parameters than that in vivo. Again, we summarize and compare reported results and categorize them into “upward ≠ downward” or “upward = downward” (Table. 2.2).
Among the 12 relevant studies, 7 of them showed differential effects while 5 of them did not. Among the 7 studies that showed differential effects, 4 of them showed that the upward direction SMF has more significant effects (Coletti et al. 2007; Tian et al. 2018; Yang et al. 2020, 2021) and 3 of them reported that the downward direction has more significant effects (Song et al. 2021; Yu et al. 2021; Feng et al. 2022). As we have introduced in Chap. 1 of this book, cell type, cell density, and MF treatment time could all contribute to the differences in cellular experiments. Moreover, as we mentioned in Fig. 2.2, the SMF distributions on different SMF devices can be very different. For example, for SMFs provided by square shaped permanent magnet of the same size, the SMF direction and distributions can be very different (Fig. 2.3).
Some commonly used permanent magnet-based magnetic devices in cellular studies. (a–f) Cell culture plates were placed at different direction magnet device. Black arrows indicate magnetic field direction. The SMF was provided by neodymium permanent magnet with upward and downward direction of 0.5 T magnet (a, b), 0.5 T magnet assembly (c, d), and 1.0 T magnet assembly (e, f) magnetic field intensity, with N or S pole upward (Tian et al. 2018; Wang et al. 2018). Magnetic field distribution in the cell exposure area, 1 mm, 5 mm, and 10 mm above the magnetic plates. Left part of the figures was adapted from the above-mentioned refs with permission. The magnetic flux distribution scans on the right were performed by a magnet analyzer (LakeShore 475 DSP Gaussmeter)
Therefore, as mentioned in Chap. 1 of this book, multiple details should be considered when performing these SMF experiments, including the sample distance from the magnet surface, SMF flux density distribution, material composition and dimension of the magnet, magnet polar configuration, and duration of magnet application, etc. For example, as shown in Fig. 2.3, the magnetic flux densities at 1 mm from the surface of the three type magnets were 4700–4980 Gs, 4190–4890 Gs, and 9630–10,330 Gs, respectively; at 5 mm from the surface of the magnets, the magnetic flux densities dropped to 3520–3890 Gs, 2180–2450 Gs, and 4370–5120 Gs, respectively; at 10 mm from the surface of the magnets, the magnetic flux densities further decreased to 2420–3090 Gs, 1050–1120 Gs, and 2120–2450 Gs. The differences in magnetic flux density and spatial SMF distribution arrangement resulted in significant differences in the values of magnetic flux density. Therefore, people in this field should start to accurately measure their SMFs by using a magnet analyzer, which can provide accurate 3D information about the magnetic flux density and distributions (Fig. 2.3).
2.4 Possible Mechanisms
As introduced in Chap. 1, various factors could result in these differential effects of SMF in vivo and in vitro, including SMF flux density, gradient, exposure time, cell type, etc. From above-mentioned animal and cellular studies about SMFs of different directions in this chapter, it is obvious that the field direction is also a key factor for some specific bioeffects. Although the mechanisms behind the observed results are still not completely understood, there are some studies have tried to address them and provided some important clues.
First of all, magnetic field can control the state of electrons, manipulate the unpaired electrons in free radicals, which provides a theoretical basis for cellular reactive oxygen species (ROS) regulation by SMF (Timmel et al. 1999; Ikeya and Woodward 2021). However, the exact effects of SMFs on cellular ROS levels are highly variable in different studies (Wang and Zhang 2017), which will be systematically summarize in Chap. 6. Although some studies proposed that changed cellular ROS formation can be affected by SMF directions (Sullivan et al. 2011; Djordjevich et al. 2012; De Luka et al. 2016; Milovanovich et al. 2016; Naarala et al. 2017; Tasic et al. 2017, 2021; Liu et al. 2019; Song et al. 2021; Yang et al. 2021; Yu et al. 2021), there is no physical explanation for this yet. This is probably due to the complexed ROS generating and clearing system in living organisms and cells.
Secondly, there is a difference between adherent cells vs. suspended cells in SMF direction-induced effects, probably due to shape anisotropy. Our previous study indicated that the upward or downward SMFs had a significant effect on the cell number in multiple types of adherent cells, while there seems to be no difference for suspended cells in the liquid cell culture medium (Tian et al. 2018). This is probably due to the fact that adherent cells are fixed in position so that they will have a shape anisotropy and directional preference. In contrast, suspended cells are round in shape and can rotate freely in the liquid medium, which make them independent of directions.
Thirdly, DNA synthesis has been shown to be differentially regulated by SMFs of different directions. Since DNA is negatively charged and undergoes fast rotation to get winding and unwinding during DNA replication in living cells, the externally applied SMF will affect the DNA movement through Lorentz force. We combined theoretical calculation and cellular experiments to show that the upward and downward SMFs have differential effects on the DNA rotation and supercoil in cells (Fig. 2.4) (Yang et al. 2020), which resulted in differential effects on DNA synthesis. Specifically, the upward moderate to high SMFs can inhibit DNA synthesis by Lorentz forces exerted on the negatively charged moving DNA (Yang et al. 2020, 2021). This theory is actually consistent with other results that have shown the cell proliferation inhibition effects of upward SMFs (Zhang et al. 2017; Tian et al. 2018; Wang and Zhang 2019; Yang et al. 2021).
Cranked DNA motion and the magnetic Lorentz forces. (Left and right) side view of DNA, (middle) top view of DNA cross section. For downward and upward SMFs, the Lorentz force (FL) of negatively charged DNA has different directions. F0 is an endogenous centripetal force determining DNA rotation. Arrows indicate rotation direction. [Illustration courtesy of Ding Joe Wang, based on reference (Yang et al. 2020). Open access]
Fourthly, there may be some link between these SMF direction-induced bioeffects with the earth magnetic field. It is known that the earth magnetic field is a quasi-SMF that is static for most of the time, but can be affected by solar storm. The directions of earth magnetic field at the southern hemisphere and northern hemisphere are opposite. However, the earth magnetic field is much weaker than the SMFs used in currently reported studies. Whether and how these factors are linked are completely unknown.
Additionally, although the physical mechanisms about the differential bioeffects caused by SMFs of different directions are still lacking, there are some biological experimental evidences that revealed some potential aspects for people to invest in the future. For example, our study shows that the iron metabolism was differentially affected by the upward vs. downward SMFs in diabetic mice. We found that the ~100 mT SMF of a downward direction alone could improve pancreas function by regulating iron metabolism and ROS production. Meanwhile, the downward SMF, but not the upward SMF, markedly restored the Bacteroidetes population and reversed the iron complex outer membrane receptor gene reduction in the mice gut microbiota, and reduced iron deposition in the pancreas (Yu et al. 2021).
Lastly, although there is still no evidence yet, we hypothesis that there might be some ferromagnetic or paramagnetic components in cells that behave like tiny magnets, which will change direction when external SMF applies (Fig. 2.5). The orientation of these components will trigger differential downstream signal transduction pathways in cells and/or various cellular processes. However, since the biological system is very complicated, we currently have only very limited information about the magnetism of materials inside our bodies, which will be reviewed in the next chapter of this book. With the help of recently developed techniques, we will be able to get a better understanding of the magnetism of biomolecules, cells, and tissue in the near future, which is actually one of the main research focuses of our group.
2.5 Summary and Future Perspectives
From current experimental evidences collected, we can conclude that it is the magnetic field direction, but not the magnetic pole, that have induced differential effects. In fact, it is clear that multiple studies have demonstrated that field direction could influence the biological responses at both cellular and living organism level. It is known that research in the literature about magnetic field bioeffects is filled with experimental discrepancies. Here we show that this is not only due to the differences in biological sample types, magnetic field types, flux density, and gradient, but also field direction. We encourage people in this field accurately measure their SMFs using equipment such as a magnet analyzer, which can provide detailed and accurate 3D information about their magnetic device. Although the exact mechanisms about most of the SMF direction-induced bioeffect differences are still unclear, these results alert people that they should pay attention to the magnetic field direction used in their biological studies. Moreover, it is worth to mention that currently some researches related to magnetic therapy as well as the biological effect studies about MFs are not well described or properly controlled. There are multiple other factors that have led to the large variations in the clinical or research work about the SMFs. Meanwhile, these researches need replication and we hope we can make great advancement after we have the proper knowledge of the magnetic field and biological systems, which will improve the current status of magnetic therapy. It indicates a potential to use different magnetic field direction in the future development of SMFs as a new physical therapy modality.
References
Bertolino G, Braga AD, Rosa KDLD, Brito LCD, de Araujo JE (2006) Macroscopic and histological effects of magnetic field exposition in the process of tissue reparation in Wistar rats. Arch Dermatol Res 298(3):121–126
Bertolino G, De Araujo FLB, Souza HCD, Coimbra NC, De Araujo JE (2013a) Neuropathology and behavioral impairments after bilateral global ischemia surgery and exposure to static magnetic field: evidence in the motor cortex, the hippocampal CA1 region and the neostriatum. Int J Radiat Biol 89(8):595–601
Bertolino G, Souza HCD, de Araujo JE (2013b) Neuropathology and behavioral impairments in Wistar rats with a 6-OHDA lesion in the substantia nigra compacta and exposure to a static magnetic field. Electromagn Biol Med 32(4):527–535
Coletti D, Teodori L, Albertini MC, Rocchi M, Pristera A, Fini M, Molinaro M, Adamo S (2007) Static magnetic fields enhance skeletal muscle differentiation in vitro by improving myoblast alignment. Cytometry A 71(10):846–856
De Luka SR, Ilic AZ, Jankovic S, Djordjevich DM, Cirkovic S, Milovanovich ID, Stefanovic S, Veskovic-Moracanin S, Ristic-Djurovic JL, Trbovich AM (2016) Subchronic exposure to static magnetic field differently affects zinc and copper content in murine organs. Int J Radiat Biol 92(3):140–147
Djordjevich DM, De Luka SR, Milovanovich ID, Jankovic S, Stefanovic S, Veskovic-Moracanin S, Cirkovic S, Ilic AZ, Ristic-Djurovic JL, Trbovich AM (2012) Hematological parameters’ changes in mice subchronically exposed to static magnetic fields of different orientations. Ecotoxicol Environ Saf 81:98–105
Dua HS, Singh A, Gomes JAP, Laibson PR, Donoso LA, Tyagi S (1996) Vortex or whorl formation of cultured human corneal epithelial cells induced by magnetic fields. Eye 10:447–450
Elahee KB, Poinapen D (2006) Effects of static magnetic fields on growth of Paramecium caudatum. Bioelectromagnetics 27(1):26–34
Feng C, Yu B, Song C, Wang J, Zhang L, Ji X, Wang Y, Fang Y, Liao Z, Wei M, Zhang X (2022) Static magnetic fields reduce oxidative stress to improve wound healing and alleviate diabetic complications. Cells 11(3):443
Giorgetto C, Silva ECM, Kitabatake TT, Bertolino G, de Araujo JE (2015) Behavioural profile of Wistar rats with unilateral striatal lesion by quinolinic acid (animal model of Huntington disease) post-injection of apomorphine and exposure to static magnetic field. Exp Brain Res 233(5):1455–1462
Ikeya N, Woodward JR (2021) Cellular autofluorescence is magnetic field sensitive. Proc Natl Acad Sci U S A 118(3):e2018043118
Jin Y, Guo W, Hu XP, Liu MM, Xu X, Hu FH, Lan YH, Lv CK, Fang YW, Liu MY, Shi TL, Ma SS, Fang ZC, Huang JR (2019) Static magnetic field regulates Arabidopsis root growth via auxin signaling. Sci Rep 9:14384
Liu XL, Liu ZM, Liu ZN, Zhang SJ, Bechkoum K, Clark M, Ren LQ (2019) The effects of bio-inspired electromagnetic fields on normal and cancer cells. J Bionic Eng 16(5):943–953
Milovanovich ID, Cirkovic S, De Luka SR, Djordjevich DM, Ilic AZ, Popovic T, Arsic A, Obradovic DD, Opric D, Ristic-Djurovic JL, Trbovich AM (2016) Homogeneous static magnetic field of different orientation induces biological changes in subacutely exposed mice. Environ Sci Pollut Res 23(2):1584–1597
Naarala J, Kesari KK, McClure I, Chavarriaga C, Juutilainen J, Martino CF (2017) Direction-dependent effects of combined static and ELF-magnetic fields on cell proliferation and superoxide radical production. Biomed Res Int 2017:5675086
San J, Yang Z, Xia W, Zhang C (2001) Influence of uneven constant magnetic field on thrombosis and plasminogen activator in mice. Chin J Physiol 24(6):325–327
Song C, Chen H, Yu B, Zhang L, Wang J, Feng C, Yang X, Tian X, Fan Y, Ji X, Wang H, Xie C, Zhang X (2021) A magnetic field prevents alcoholic liver disease by reducing oxidative stress. BioRxiv 2021:471564. https://doi.org/10.1101/2021.12.07.471564
Sullivan K, Balin AK, Allen RG (2011) Effects of static magnetic fields on the growth of various types of human cells. Bioelectromagnetics 32(2):140–147
Sztafrowski D, Suchodolski J, Muraszko J, Sigler K, Krasowska A (2019) The influence of N and S poles of static magnetic field (SMF) on Candida albicans hyphal formation and antifungal activity of amphotericin B. Folia Microbiol 64(6):727–734
Tasic T, Djordjevic DM, De Luka SR, Trbovich AM, Japundzic-Zigon N (2017) Static magnetic field reduces blood pressure short-term variability and enhances baro-receptor reflex sensitivity in spontaneously hypertensive rats. Int J Radiat Biol 93(5):527–534
Tasic T, Lozic M, Glumac S, Stankovic M, Milovanovich I, Djordjevich DM, Trbovich AM, Japundzic-Zigon N, De Luka SR (2021) Static magnetic field on behavior, hematological parameters and organ damage in spontaneously hypertensive rats. Ecotoxicol Environ Saf 207:111085
Tian XF, Wang DM, Zha M, Yang XX, Ji XM, Zhang L, Zhang X (2018) Magnetic field direction differentially impacts the growth of different cell types. Electromagn Biol Med 37(2):114–125
Timmel CR, Hore PJ, McLauchlan KA, Brocklehurst B (1999) The effects of weak static magnetic fields on the yields of radical recombination reactions. Abstr Pap Am Chem Soc 217:U287
Todorovic D, Markovic T, Prolic Z, Mihajlovic S, Raus S, Nikolic L, Janac B (2013) The influence of static magnetic field (50 mT) on development and motor behaviour of Tenebrio (Insecta, Coleoptera). Int J Radiat Biol 89(1):44–50
Wang HZ, Zhang X (2017) Magnetic fields and reactive oxygen species. Int J Mol Sci 18(10):2175
Wang H, Zhang X (2019) ROS reduction does not decrease the anticancer efficacy of X-ray in two breast cancer cell lines. Oxid Med Cell Longev 2019:3782074
Wang DM, Wang Z, Zhang L, Li ZY, Tian XF, Fang J, Lu QY, Zhang X (2018) Cellular ATP levels are affected by moderate and strong static magnetic fields. Bioelectromagnetics 39(5):352–360
Yang X, Li Z, Polyakova T, Dejneka A, Zablotskii V, Zhang X (2020) Effect of static magnetic field on DNA synthesis: the interplay between DNA chirality and magnetic field left-right asymmetry. FASEB Bioadv 2(4):254–263
Yang X, Song C, Zhang L, Wang J, Yu X, Yu B, Zablotskii V, Zhang X (2021) An upward 9.4 T static magnetic field inhibits DNA synthesis and increases ROS-P53 to suppress lung cancer growth. Transl Oncol 14(7):101103
Yu B, Liu J, Cheng J, Zhang L, Song C, Tian X, Fan Y, Lv Y, Zhang X (2021) A static magnetic field improves iron metabolism and prevents high-fat-diet/streptozocin-induced diabetes. Innovations 2(1):100077
Zhang L, Ji X, Yang X, Zhang X (2017) Cell type- and density-dependent effect of 1 T static magnetic field on cell proliferation. Oncotarget 8(8):13126–13141
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Yu, B., Zhang, X. (2023). Static Magnetic Field Direction-Induced Differential Biological Effects. In: Zhang, X. (eds) Biological Effects of Static Magnetic Fields. Springer, Singapore. https://doi.org/10.1007/978-981-19-8869-1_2
Download citation
DOI: https://doi.org/10.1007/978-981-19-8869-1_2
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-8868-4
Online ISBN: 978-981-19-8869-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)