Abstract
Exposing body tissue, in vivo, to a magnetic field promotes metabolic alterations in the cell membrane’s permeability and in the apoptosis phenomenon. This aim of the study was to investigate magnetic field interactions in the process of tissue repair in rats. Twenty-four male Wistar rats, weighing 200–350 g, were assigned to one of the three different groups: Control (without exposure to the magnetic field), South Pole (with exposure to the South magnetic field), and North Pole (with exposure to the north magnetic field). The intensity of the magnetic field used was 1,600 G. All the animals were anesthetized and immobilized on a surgical board in order to receive circular wounds. The size of the wounds was measured by a milimetric paquimeter. For the histological study, the tissues were fixed in paraffin and colored with hematoxylin and eosin. Wound size data were submitted to one-way analysis of variance (ANOVA) and to the test of Student–Newman–Keuls when appropriated. The results of day 5 (F 2,23:F 3,84; P < 0.05), day 10 (F 2,23:F 8,89; P < 0.05), and day 15 (F 2,23:F 7,88; P < 0.05) revealed a significant reduction between the size of the wounds of both North and South groups when compared to Control group. Our data suggest that chronic exposure to a magnetic field of 1,600 G can accelerate the speed of tissue repair in rats.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Patients with restricted mobility due to a long bed rest can develop skin wounds, scars or decubitus, and ulcers [3]. These lesions can be treated by several professionals in the health field, depending on its size. Big lesions demand a lot of medical attention, especially because they often happen during emergencies; however, smaller lesions can be treated differently by physiotherapists, nurses, and other professionals in the health field [34]. Physiotherapists use helium–neon laser (HeNe) to treat scars in general. This equipment produces beneficial effects in several processes of healing, facilitating the repair of the tissue and its cellular functions [2]. However, the magnetic field is another resource that has been studied for a long time and its effects in the human biological system have been providing a satisfactory alternative to such treatments [32].
The great majority of effects that the magnetic field promotes in the human body depend on the cellular structures’ sensibility, because some are more sensitive than others. In the vascular system, e.g., the magnetic field promotes modifications in macrocirculation and microcirculation, consequently increasing blood flow [13] and changes in arterial pressure [26]. In the skeletal system, the magnetic field increases osteoblasts maturation during its exposition in the first two stages of bone lesion repair [6]. In the nervous system, the effects of the magnetic field are related to increased sensibility in cortical excitatory stages [16], as well as in the baroreceptor system [12]. The effects of the magnetic field in internal organs of rats are related to alterations in its density [5]. The effects of the magnetic field in the hormonal system depend on the area that has been exposed to it; although there are reported effects in pancreatic secretion [15], there is an absence of effects reported in the pineal gland melatonin liberation [17] and in the cytokine production [14].
In the ionic systems, the magnetic field produces a significant effect in the movement flow, without collisions [11], and a small increase in the calcium concentration, changing the cell response process [1]. Regarding its effect on the internal cellular environment, the magnetic field promotes alterations in metabolic reactions, permeability of cell membrane [33], and cell apoptosis [30].
Another recent study by Fedrowitz et al. [10] showed evidences that exposure to magnetic field increases the proliferation of epithelial cells in the mammary gland.
As the magnetic field influences several metabolic responses in the human body as mentioned above, an investigation of its interactions in acceleration of tissue repair and its physiological effects seems to be of great importance.
Materials and methods
Animals and surgery
Twenty-four naïve male rats (Wistar) weighing 200–300 g were used in accordance with the Ethics Committee for animal and human research of the University of Franca (protocol number 098/003). The animals were housed in a colony room with food and water ad libitum throughout the 15 days of the experiment. They were maintained on a 12-h light/12-h dark cycle (lights on at 7 a.m.) at 23 ± 1°C. Each animal was anesthetized with thionembutal (5 mg/kg, i.p.) and immobilized on a surgical table. Once the anesthetic took effect, tricotomia procedure and asepsis of the area took place before the surgery itself. The surgery consisted of making a standardized circular wound, placed on the middle of the back of each animal to approximately 20 mm off the base of the cranium, using tongs and surgical scissors [31]. A milimetric paquimeter was used (DIGIMESS) to measure wound size, as soon as the lesion was produced and every 5 days after that until the last day of the experiment (day 15). After the surgery, the animals were housed in groups of four animals per cage.
Experimental
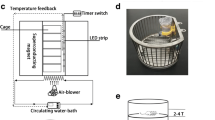
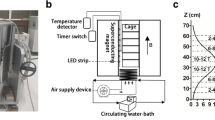
After the surgery, the animals were assigned to one of the three groups: Control (no exposure to the magnetic field, n = 8), South Pole (exposed to the South magnetic field, n = 8), and North Pole (exposed to the North magnetic field, n = 8). For the North and South groups (experimental groups), circular magnets were placed under each cage for 15 days, 24 h a day in an open-field protocol (Fig. 1). The animals in the Control group did not have any magnets type or sham magnet under their cages. All the groups were maintained separately during the whole experiment. Other factors such as brightness and temperature were the same for all groups.
Magnetic field
The magnetic field consisted of a magnet made of Barium Ferrite (BaFe12O19) measuring 220 mm of diameter by 20-mm thickness (Keybass Speakers, Brazil). A GaussMeter (Model TMAG-1T, GLOBALMAG, Brazil) was used to measure the surface intensity of 1,600 G (0.16 T) on the magnetic external extremity. To determine the medium force of the magnetic field on the animals, we measured it on the cage floor and at 70 mm of height, which was the calculated average of the height reached by the animals in the position of four paws in the cage floor (Fig. 2).
Histology
On day 15, the animals were killed with a thionembutal overdose, and tissue samples from the ulceration were collected. These tissues were kept in containers filled with a formalin solution at 10%, cut into 5-μm thick sections, and stained with hematoxylin and eosin.
Statistical analysis
Macroscopic data (size of the wounds) are reported as means ± S.E.M. Data were analyzed by means of analysis of variance. Student–Newman–Keuls post hoc comparisons were used when significant overall F values were obtained. Significant level was set at P < 0.05. To obtain a better visualization of the speed of repair between the groups, the data were transformed in percentages.
Results
Macroscopic analysis
After the production of the wounds, there were no statistically significant differences in wound size within each of the three groups when compared to each other (F 2,23:F 0,79; P = 0.46) (Start bar in Fig. 3).
As shown in Fig. 3, there was a significant reduction in wound size both in the North and South groups when compared with Control on day 5 (F 2,23:F 3,84; P < 0.05), day 10 (F 2,23:F 8,89; P < 0.05), and day 15 (F 2,23:F 7,88; P < 0.05). As seen in Fig. 4, the South group showed doubled healing speed when compared with Control.
Histological analysis
The Control group showed formation of hemato-fibrinous scab, lateral necrosis areas in the lesion borders, presence of intense inflammatory exudate composed by neutrophiles and macrophages in the center of the lesion, formation of granulation tissue, discreet formation of new blood vessels, and absence of multinucleated giant cells or epithelioid cells (Fig. 5). The South group showed hemato-fibrinous adjacent scab with weak adhesion, intense inflammatory exudate composed by macrophages, and discreet amount of fibrina between the hemato-fibrinous scab and the granulation tissue. The granulation tissue was formed by good amount of new blood vessels, fibroblasts, and main disorganized extracellular, however, there was presence of multinucleated giant cells and epithelioid cells, besides discreet mitotic activity of the cells in the germinative layer (basal) of the epidermis (Fig. 6).
Photomicrograph (H&E) of histological skin cuts in Wistar rats (Control group). a Absence of hemato-fibrinous scab, great amount of fibrin, and hemacite (arrow) in the center of the lesion. b Formation of disorganized granulation tissue, discreet neoformation of blood vessels (white arrow), accentuated fibroblasts proliferation, and main extracellular in disorganized structure (black arrow)
Photomicrograph (H&E) of histological skin cuts in Wistar rats (South Pole group). a Presence of hemato-fibrinous scab (black arrow) with tendency to separate the adjacent tissue, great amount of fibrin, and inflammatory exudate (white arrow). b Presence of intense inflammatory exudate (black arrow) composed by neutrophils and macrophages in the center of the lesion and right below the hemato-fibrinous scab. Discreet mitotic cell activity in the germinative layer of the epidermis (white arrow). c Presence of multinucleated giant cells with characteristics of epithelioid cells (arrows)
The North group showed absence of hemato-fibrinous scab and inflammatory exudate, substitution of the granulation tissue for dense conjunctive tissue, organized distribution of the fibroblasts and of the main collagen in the dermis, and wide mitotic activity of cells in the germinative layer (basal) of the epidermis with initial presence of these cells in the damaged area (Fig. 7).
Photomicrograph (H&E) of histological skin cuts in Wistar rats (North Pole group). a Presence of cells of the germinative layer (basal) of the epidermis with wide mitotic activity and beginning of the occupation of the damaged area by epidermis cells (arrow). b Substitution of the granulation tissue for conjunctive dense tissue, main collagen, and fibroblasts with organized distribution. Discreet neovascularization (arrow)
Discussion
Several studies have shown that tissue exposure to a strong magnetic field can inhibit the growth of cells in vitro [1, 22, 24, 29] and also during fetal development in rats [7].
In humans, inhibiting effects of magnetic field have been reported; although a magnetic field of 0.5 T (8 h of exposure) was shown to inhibit fibroblasts [22], a stronger magnetic field (up to 6.3 T) has been shown to inhibit T lymphocytes growth (up to 60 h of exposure) [24].
Increase in recaptation rate and fibroblast synthesis in newborns rats due to a long exposure (up to 10 days) to a magnetic field of 0.6 T has also been shown [23], as well as a significant reduction in total number of viable cells in melanoma cultures, carcinoma in ovaries, and lymphoma after the exposure to a magnetic field of 7.0 T for 64 h [29].
Other studies have shown that magnetic field exposure increases proliferation of cells such as epithelial cells and linfocites in vitro [18, 21].
The results reported in our study, namely the magnetic field exposure, increase the speed in tissue repair and improve histological response when compared to unexposed controls, parallel reports in which magnetic field exposure increases activity of cellular proliferation [18, 21]. Our histological analysis indicates the reduction and improvement in quality of inflammatory exudate, formation of multinucleus cells, and Langhans cells, and the presence of early cellular mitotic activity in the germinative layer of the epidermis.
Another interesting finding in our study is the tendency of difference in healing speed observed between the two experimental groups. During the first 10 days of experiment, the South group showed a small increase in speed when compared with the North group. Even though the difference was not statistically significant, there is a need for further investigation because the number of animals in each group is relatively small.
After day 10, the North group showed increase in that speed of healing but again they were not statistically significant.
Histological analysis showed that the healing quality was better in the tissues exposed to the North Pole magnetic field. A better quality of healing in our study is theorized based on the absence or very little amount of inflammatory exudate, substitution of granulation tissue for dense conjunctive tissue with fibroblasts and main collagen of organized distribution, and ample mitotic activity of cells in the germinative layer of the epidermis as seen in other studies [10, 18, 21].
As other studies using high magnetic potency showed inhibition of cell growth/proliferation [22, 24], the intensity of the field could be responsible for such difference. Another possible explanation for the increase in repair speed seen in our results could be the inhibition of cellular apoptosis. The study of Fanelli et al. [9] has shown that static magnetic fields of different intensities can decrease the extension of cellular death for apoptosis induced by several agents in different cellular systems in humans. Such decrease is not related to change in cell death (necrosis) or retardation of the process, instead it seems to be related to increasing the survival of undamaged cells and to the re-application of the damaged cells by apoptogenic agents.
Other studies investigated the interference of magnetic fields in cellular metabolism, altering rates of cellular transcription [20], and genes [28], finding a small decrease in rate of spontaneous cellular death in vitro [8] and changes in the plasmatic membrane [27] due to a larger influx of Ca2 in the membrane [19].
Multicellular organisms eliminate the unnecessary cells through apoptosis, an intrinsic cellular mechanism that drives healthy cells to their auto-elimination. This happens in physiological conditions as well as in response to mutagenic lesions [4].
After a lesion, cells that are severely damaged die passively through necrosis; others that were only slightly damaged die through apoptosis [25]. The increase of repair speed in our results might be related to the fact that we did not verify the presence of extensive areas of necrosis in the treated groups. The magnetic filed used in this study could be promoting the contention of programs of cell death through apoptosis in several slightly damaged cells because the untreated group showed cell death through that process. These results indicate the need for further investigation.
References
Aldinucci C, Garcia JB, Palmi M, Sgaragli G, Benocci A, Meini A, Pessina F, Rossi C, Bonechi C, Pessina GP (2003) The effect of strong static magnetic field o lymphocytes. Bioelectromagnetics 24:109–117
Carvalho PTC, Mazzer N, Corazza AV, Raduam RM (2001) The effects of low laser therapy on cutaneous wounds in rats with experimental diabetes mellitus. Fisioterapia Brasil 2:241–246
Carvalho PTC, Silva RR, Silva RJ (2001) Microbiological study in vitro of the bacterial growth after application of the HeNe laser in ulcers of decubitus with bacterial infection. Fisioterapia Brasil 2:183–188
Cotran RS, Kumar V, Collins T (1999) Robbins pathologic basis of disease, 6th edn. Saunders, Philadelphia
Dawson TW, Caputa K, Stuchly MA (2002) Electric fields induced in humans and rodents by 60 Hz magnetic fields. Phys Med Biol 47:2561–2568
Diniz P, Shomura K, Soejima K, Ito G (2002) Effects of pulsed electromagnetic field (PEMF) stimulation on bone tissue like formation are dependent on the maturation stages of the osteoblasts. Bioelectromagnetics 23:398–405
Elbetieha A, Al-Akhras MA, Darmani H (2002) Long-term exposure of male and female mice to 50 Hz magnetic field: effects on fertility. Bioelectromagnetics 23:168–172
Eremenko T, Esposito C, Pasquarelli A, Pasquali E, Volpe P (1997) Cell-cycle kinetics of friend erythroleukemia cells in a magnetically shielded room and in a low-frequency/low-intensity magnetic field. Bioelectromagnetics 18:58–66
Fanelli C, Coppola S, Barone R, Colussi C, Gualandi G, Volpe P, Ghibelli L (1999) Magnetic fields increase cell survival by inhibiting apoptosis via modulation of Ca2 influx. FASEB J 13:95–102
Fedrowitz M, Westermann J, Loscher W (2002) Magnetic field exposure increases cell proliferation but does not affect melatonin levels in the mammary gland of female Sprague–Dawley rats. Cancer Res 62:1356–1363
Giudice ED, Fleischmann M, Preparata G, Talpo G (2002) On the unreasonable effects of ELF magnetic fields upon a system of ions. Bioelectromagnetics 23:522–530
Gmitrov J, Ohkubo C (2002) Verapamil protective effect on natural and artificial magnetic field cardiovascular impact. Bioelectromagnetics 23:531–541
Gmitrov J, Ohkubo C, Okano H (2002) Effect of 0.25 T static magnetic field on microcirculation in rabbits. Bioelectromagnetics 23:224–229
Ikeda K, Shinmura Y, Mizoe H, Yoshizawa H, Yoshida A, Kanao S, Sumitani H, Hasebe S, Motomura T, Yamakawa T, Mizuno F, Otaka Y, Hirose H (2003) No effects of extremely low frequency magnetic fields found on cytotoxic activities and cytokine production of human peripheral blood mononuclear cells in vitro. Bioelectromagnetics 24:21–31
Kobierska AL, Cieslar G, Sieron A, Grzybek H (2002) Influence of alternating extremely low frequency ELF magnetic field on structure and function of pancreas in rats. Bioelectromagnetics 23:49–58
Kowalski T, Silny J, Buchner H (2002) Current density threshold for the stimulation of neurons in the motor cortex area. Bioelectromagnetics 23:421–428
Kurokawa Y, Nitta H, Imai H, Kabuto M (2003) Acute exposure to 50 Hz magnetic fields with harmonics and transient components: lack of effects on nighttime hormonal secretion in men. Bioelectromagnetics 24:12–20
Lacy-Hulbert A, Metcalfe JC, Hesketh R (1998) Biological responses to electromagnetic fields. FASEB J 12:395–420
Liburdy RP (1992) Calcium signaling in lymphocytes and ELF field: evidence for an electric field metric and a site of interaction involving the calcium ion channel. FEBS Lett 301:53–59
Liburdy RP, Callahan DE, Harland J, Dunham E, Sloma TR, Yaswen P (1993) Experimental evidence for 60 Hz magnetic fields operating through the signal transduction cascade: effects on calcium influx and c-myc mRNA induction. FEBS Lett 334:301–308
Loscher W, Liburdy RP (1998) Animal and cellular studies on carcinogenic effects of low frequency (50/60-Hz) magnetic fields. Mutat Res 410:185–220
Malinin GI, Gregory WD, Morelli L, Sharma VK, Houck JC (1976) Evidence of morphological and physiological transformation of mammalian cells by strong magnetic fields. Science 194:844–846
McDonald F (1993) Effect of static magnetic fields on osteoblasts and fibroblasts in vitro. Bioelectromagnetics 14:187–196
Norimura T, Imada H, Kunugita N Yoshida N, Nikaido M (1993) Effects of strong magnetic fields on cell growth and radiation response of human T-lymphocytes in culture. J UOEH 15:103–112
Nosseri C, Coppola S, Ghibelli L (1994) Possible involvement of poly(ADP-ribosyl) polymerase in triggering stress-induced apoptosis. Exp Cell Res 212:367–373
Okano H, Ohkubo C (2003) Anti-pressor effects of whole body exposure to static magnetic field on pharmacologically induced hypertension in conscious rabbits. Bioelectromagnetics 24:139–147
Paradisi S, Donelli G, Santini MT, Straface E, Malorni W (1993) A 50 Hz magnetic field induces structural and biophysical changes in membranes. Bioelectromagnetics 14:247–255
Phillips JL, Haggren W, Thomas WJ, Ishida-Jones T, Adey WR (1992) Magnetic field-induced changes in specific gene transcription. Biochim Biophys Acta 1132:140–144
Raylman RR, Clavo AC, Wahl RL (1996) Exposure to strong static magnetic fields slows the growth of human cancer cells in vitro. Bioelectromagnetics 17:358–363
Robison JG, Pendleton AR, Monson KO, Murray BK, O’Neill KL (2002) Decreased DNA repair rates and protection from heat induced apoptosis mediated by electromagnetic field exposure. Bioelectromagnetics 23:106–112
Soares JH, Tardivo JP, Goldenderg S, Katz S, Muora LAR (1989) Aspectos morfológicos e histométrcos das feridas cutânesa de ratos após irradiação com o laser de Hélio-Neônio. Acta Cir Bras 4:56–60
Tenforde TS (2003) The wonders of magnetism. Bioelectromagnetics 24:3–11
Traitcheva N, Angelova P, Radeva M, Berg H (2003) ELF fields and photooxidation yielding lethal effects on cancer cells. Bioelectromagnetics 24:148–150
Whiting WC, Zernicke RF (1998) Biomecânia da lesão musculoesquelética. Guanabara Koogan, Rio de Janeiro
Acknowledgment
We would like to thank Keybass Speakers for the Magnets (Barium Ferrite). Guilherme Bertolino, Aldo de Freitas Braga, and Kelline de Oliveira Lima de Couto Rosa are part of the graduation program in physiotherapy.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bertolino, G., de Freitas Braga, A., de Oliveira Lima do Couto Rosa, K. et al. Macroscopic and histological effects of magnetic field exposition in the process of tissue reparation in Wistar rats. Arch Dermatol Res 298, 121–126 (2006). https://doi.org/10.1007/s00403-006-0667-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00403-006-0667-z